Abstract
Skeletal muscle is dynamically controlled by the balance of protein synthesis and degradation. Here we discover an unexpected function for the transcriptional repressor B cell lymphoma 6 (BCL6) in muscle proteostasis and strength in mice. Skeletal muscle-specific Bcl6 ablation in utero or in adult mice results in over 30% decreased muscle mass and force production due to reduced protein synthesis and increased autophagy, while it promotes a shift to a slower myosin heavy chain fibre profile. Ribosome profiling reveals reduced overall translation efficiency in Bcl6-ablated muscles. Mechanistically, tandem chromatin immunoprecipitation, transcriptomic and translational analyses identify direct BCL6 repression of eukaryotic translation initiation factor 4E-binding protein 1 (Eif4ebp1) and activation of insulin-like growth factor 1 (Igf1) and androgen receptor (Ar). Together, these results uncover a bifunctional role for BCL6 in the transcriptional and translational control of muscle proteostasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All sequencing data generated in this study are publicly available in the GEO repository SuperSeries GSE190956. We also utilized publicly accessible RNA-seq and microarray data from GEO repository entries GSE65936, GSE112204, GSE107470 and GSE14710, as well as the mm10 reference assembly for the mouse genome. Source data are provided with this paper.
Code availability
No custom code was used.
References
Sartori, R., Romanello, V. & Sandri, M. Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat. Commun. 12, 330 (2021).
Cohen, S., Nathan, J. A. & Goldberg, A. L. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat. Rev. Drug Discov. 14, 58–74 (2015).
Saxton, R. A. & Sabatini, D. M. mTOR signaling in growth, metabolism, and disease. Cell 169, 361–371 (2017).
Bodine, S. C. et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708 (2001).
Brunet, A. et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96, 857–868 (1999).
McPherron, A. C., Lawler, A. M. & Lee, S. J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387, 83–90 (1997).
Goodman, C. A. & Hornberger, T. A. New roles for Smad signaling and phosphatidic acid in the regulation of skeletal muscle mass. F1000Prime Rep. 6, 20 (2014).
Rossetti, M. L., Steiner, J. L. & Gordon, B. S. Androgen-mediated regulation of skeletal muscle protein balance. Mol. Cell. Endocrinol. 447, 35–44 (2017).
Ye, B. H. et al. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science 262, 747–750 (1993).
Kerckaert, J. P. et al. LAZ3, a novel zinc-finger encoding gene, is disrupted by recurring chromosome 3q27 translocations in human lymphomas. Nat. Genet. 5, 66–70 (1993).
Dent, A. L., Shaffer, A. L., Yu, X., Allman, D. & Staudt, L. M. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 276, 589–592 (1997).
Albagli-Curiel, O. et al. Increased expression of the LAZ3 (BCL6) proto-oncogene accompanies murine skeletal myogenesis. Differentiation 64, 33–44 (1998).
Parikh, H. et al. Molecular correlates for maximal oxygen uptake and type 1 fibers. Am. J. Physiol. Endocrinol. Metab. 294, E1152–E1159 (2008).
Haberland, M., Montgomery, R. L. & Olson, E. N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 10, 32–42 (2009).
Yamamoto, H. et al. NCoR1 is a conserved physiological modulator of muscle mass and oxidative function. Cell 147, 827–839 (2011).
Ramachandran, K. et al. Dynamic enhancers control skeletal muscle identity and reprogramming. PLoS Biol. 17, e3000467 (2019).
Hnisz, D. et al. Super-enhancers in the control of cell identity and disease. Cell 155, 934–947 (2013).
Senagolage, M. D. et al. Loss of transcriptional repression by BCL6 confers insulin sensitivity in the setting of obesity. Cell Rep. 25, 3283–3298 (2018).
Miniou, P. et al. Gene targeting restricted to mouse striated muscle lineage. Nucleic Acids Res. 27, e27 (1999).
McCarthy, J. J., Srikuea, R., Kirby, T. J., Peterson, C. A. & Esser, K. A. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet. Muscle 2, 8 (2012).
Wang, G. et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat. Med. 24, 770–781 (2018).
Goncalves, M. D. et al. Fenofibrate prevents skeletal muscle loss in mice with lung cancer. Proc. Natl Acad. Sci. USA 115, E743–E752 (2018).
Tseng, Y. C. et al. Preclinical investigation of the novel histone deacetylase inhibitor AR-42 in the treatment of cancer-induced cachexia. J. Natl Cancer Inst. 107, djv274 (2015).
Wang, Y. & Pessin, J. E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 16, 243–250 (2013).
Ciciliot, S., Rossi, A. C., Dyar, K. A., Blaauw, B. & Schiaffino, S. Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 45, 2191–2199 (2013).
Zhang, Y., Laz, E. V. & Waxman, D. J. Dynamic, sex-differential STAT5 and BCL6 binding to sex-biased, growth hormone-regulated genes in adult mouse liver. Mol. Cell. Biol. 32, 880–896 (2012).
Lin, G., LaPensee, C. R., Qin, Z. S. & Schwartz, J. Reciprocal occupancy of BCL6 and STAT5 on growth hormone target genes: contrasting transcriptional outcomes and promoter-specific roles of p300 and HDAC3. Mol. Cell. Endocrinol. 395, 19–31 (2014).
Klover, P., Chen, W., Zhu, B. M. & Hennighausen, L. Skeletal muscle growth and fiber composition in mice are regulated through the transcription factors STAT5a/b: linking growth hormone to the androgen receptor. FASEB J. 23, 3140–3148 (2009).
Brooks, S. A. & Blackshear, P. J. Tristetraprolin (TTP): interactions with mRNA and proteins, and current thoughts on mechanisms of action. Biochim. Biophys. Acta 1829, 666–679 (2013).
White, M. R. & Garcin, E. D. The sweet side of RNA regulation: glyceraldehyde-3-phosphate dehydrogenase as a noncanonical RNA-binding protein. Wiley Interdiscip. Rev. RNA 7, 53–70 (2016).
Kejiou, N. S. et al. Pyruvate Kinase M (PKM) binds ribosomes in a poly-ADP ribosylation dependent manner to induce translational stalling. Nucleic Acids Res. 51, 6461–6478 (2023).
Richter, J. D. CPEB: a life in translation. Trends Biochem. Sci. 32, 279–285 (2007).
Yang, C. & Carrier, F. The UV-inducible RNA-binding protein A18 (A18 hnRNP) plays a protective role in the genotoxic stress response. J. Biol. Chem. 276, 47277–47284 (2001).
Kawagishi, H. et al. Hzf regulates adipogenesis through translational control of C/EBPalpha. EMBO J. 27, 1481–1490 (2008).
Mandal, S., Mandal, A., Johansson, H. E., Orjalo, A. V. & Park, M. H. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc. Natl Acad. Sci. USA 110, 2169–2174 (2013).
Dever, T. E. & Ivanov, I. P. Roles of polyamines in translation. J. Biol. Chem. 293, 18719–18729 (2018).
Hatzi, K. et al. A hybrid mechanism of action for BCL6 in B cells defined by formation of functionally distinct complexes at enhancers and promoters. Cell Rep. 4, 578–588 (2013).
Sommars, M. A. et al. Dynamic repression by BCL6 controls the genome-wide liver response to fasting and steatosis. Elife 8, e43922 (2019).
Barish, G. D. et al. Bcl-6 and NF-kappaB cistromes mediate opposing regulation of the innate immune response. Genes Dev. 24, 2760–2765 (2010).
Li, S. et al. Cistrome-GO: a web server for functional enrichment analysis of transcription factor ChIP–seq peaks. Nucleic Acids Res. 47, W206–W211 (2019).
Cardenas, M. G. et al. Rationally designed BCL6 inhibitors target activated B cell diffuse large B cell lymphoma. J. Clin. Invest. 126, 3351–3362 (2016).
Langer, H. T. et al. Muscle atrophy due to nerve damage is accompanied by elevated myofibrillar protein synthesis rates. Front. Physiol. 9, 1220 (2018).
Emery, P. W., Edwards, R. H., Rennie, M. J., Souhami, R. L. & Halliday, D. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. BMJ 289, 584–586 (1984).
Rennie, M. J. et al. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin. Sci. 63, 519–523 (1982).
Bederman, I. R., Dufner, D. A., Alexander, J. C. & Previs, S. F. Novel application of the ‘doubly labeled’ water method: measuring CO2 production and the tissue-specific dynamics of lipid and protein in vivo. Am. J. Physiol. Endocrinol. Metab. 290, E1048–E1056 (2006).
Bederman, I. R. et al. Chronic hindlimb suspension unloading markedly decreases turnover rates of skeletal and cardiac muscle proteins and adipose tissue triglycerides. J. Appl. Physiol. 119, 16–26 (2015).
Goodman, C. A. et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 25, 1028–1039 (2011).
Ingolia, N. T., Ghaemmaghami, S., Newman, J. R. & Weissman, J. S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324, 218–223 (2009).
Ju, J. S., Varadhachary, A. S., Miller, S. E. & Weihl, C. C. Quantitation of ‘autophagic flux’ in mature skeletal muscle. Autophagy 6, 929–935 (2010).
Barth, S., Glick, D. & Macleod, K. F. Autophagy: assays and artifacts. J. Pathol. 221, 117–124 (2010).
Sartori, R. et al. BMP signaling controls muscle mass. Nat. Genet. 45, 1309–1318 (2013).
Winbanks, C. E. et al. The bone morphogenetic protein axis is a positive regulator of skeletal muscle mass. J. Cell Biol. 203, 345–357 (2013).
Tabebordbar, M. et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell 184, 4919–4938 (2021).
Sonenberg, N. & Hinnebusch, A. G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136, 731–745 (2009).
Madsen, J. G. S. et al. Integrated analysis of motif activity and gene expression changes of transcription factors. Genome Res. 28, 243–255 (2018).
Amoasii, L. et al. NURR1 activation in skeletal muscle controls systemic energy homeostasis. Proc. Natl Acad. Sci. USA 116, 11299–11308 (2019).
Tontonoz, P. et al. The orphan nuclear receptor Nur77 is a determinant of myofiber size and muscle mass in mice. Mol. Cell. Biol. 35, 1125–1138 (2015).
Hendrickse, P. & Degens, H. The role of the microcirculation in muscle function and plasticity. J. Muscle Res. Cell Motil. 40, 127–140 (2019).
Zhang, W., Liu, Y. & Zhang, H. Extracellular matrix: an important regulator of cell functions and skeletal muscle development. Cell Biosci. 11, 65 (2021).
Pereyra, A. S. et al. Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation. Mol. Metab. 59, 101456 (2022).
McKinsey, T. A., Zhang, C. L. & Olson, E. N. Control of muscle development by dueling HATs and HDACs. Curr. Opin. Genet Dev. 11, 497–504 (2001).
Moresi, V. et al. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 143, 35–45 (2010).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Sun, L. et al. DiVenn: an interactive and integrated web-based visualization tool for comparing gene lists. Front. Genet. 10, 421 (2019).
Tripathi, S. et al. Meta- and orthogonal integration of influenza ‘OMICs’ data defines a role for UBR4 in virus budding. Cell Host Microbe 18, 723–735 (2015).
Metsalu, T. & Vilo, J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 43, W566–W570 (2015).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
McLen, C. Y. et al. GREAT improves functional interpretation of cis-regulatory regions. Nat. Biotechnol. 28, 495–501 (2010).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10–12 (2011).
Zhong, Y. et al. RiboDiff: detecting changes of mRNA translation efficiency from ribosome footprints. Bioinformatics 33, 139–141 (2017).
Liu, Q., Shvarts, T., Sliz, P. & Gregory, R. I. RiboToolkit: an integrated platform for analysis and annotation of ribosome profiling data to decode mRNA translation at codon resolution. Nucleic Acids Res. 48, W218–W229 (2020).
Acknowledgements
We thank N. Hay (University of Illinois Chicago), L. Goodyear (Joslin Diabetes Center, Harvard), C. He (Northwestern University), J. Bass (Northwestern University), D. Chakravarti (Northwestern University) and J. Madsen (University of Southern Denmark) for helpful advice and discussion. We thank Northwestern University’s NU-Seq Core, Comprehensive Metabolic Core and Mouse Histology and Phenotyping Laboratory (supported by NCI P30-CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center) for services. This research was supported by computational resources provided by the Quest high-performance computing facility at Northwestern University. Figures 1d, 2a, 6c and 8 were created with BioRender.com. This work was funded by Merit Review Award #I01BX004898 from the United States (U.S.) Department of Veterans Affairs (to G.D.B.), National Institutes of Health (NIH) grants R01DK108987 (to G.D.B.) and T32 GM008061 (to E.F.) and Pfizer Aspire award WI183795 (to G.D.B.). M.Q. is supported by NIH grants R56HL158531, R01HL166356-01, R03DK130908-01A1 and R01AG078174-01 and CCHMC grants RIP, CCRF Endowed Scholarship and HI Translational Funds. D.A. is supported by the Searle Scholar Program (grant no. SSP-2023-102). E.M.M. is supported by NIH grants R01HL061322 and R01NS047726. The article contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Author information
Authors and Affiliations
Contributions
Experiments were designed by G.D.B., K.R., C.R.F., A.R.D., I.R.B. and E.M.M. Experiments were performed by K.R., C.R.F., M.A.S., M.Q., Y.O., E.F., J.C.W., M.D.S., C.G.T., E.P. and I.R.B. Data analysis was done by K.R., C.R.F., M.A.S., E.F., G.D.B., N.L., I.R.B., D.A., N.J.W. and M.Q. The paper was written by K.R., C.R.F., D.A. and G.D.B. with input from the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Metabolism thanks Stuart Phillips, Marco Sandri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Yanina-Yasmin Pesch, in collaboration with the Nature Metabolism team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Bcl6 controls the development of skeletal muscle mass.
(a) qPCR expression of Bcl6 and myogenic markers in primary myoblasts and myotubes relative to the housekeeping gene Rn45s (n = 4 per group). Myoblast vs myotube P-value for Bcl6 = 0.0124, Mb = 0.0153, Mef2c = 0.0146 by unpaired, two-tailed t-test with Welch correction. (b) Western blot and densitometry of BCL6 and total (Memcode) protein in primary myoblasts and myotubes (n = 4/group). Myoblast vs myotube P = 0.0036 by unpaired, two-tailed t-test with Welch correction. (c) Western blot and densitometry of BCL6 and Actin protein in heart from Bcl6fl/fl and Bcl6MKO mice (n = 3/group). (d) Total body weight, fat, and lean mass in n = 7 Bcl6fl/fl and n = 10 Bcl6MKO female mice at 8 weeks of age. Bcl6fl/fl vs Bcl6MKO lean mass P = 0.0184 by unpaired, two-tailed t-test with Welch correction. (e) Tissue weights of quadriceps (quad), gastrocnemius (gastroc), tibialis anterior (TA), extensor digitorum longus (EDL) and soleus from female Bcl6fl/fl and Bcl6MKO mice at 16 weeks of age (n = 7/group). Unpaired, two-tailed t-test with Welch correction was performed and all p-values can be found in the source data file. All data are represented as mean + SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001.
Extended Data Fig. 2 Inducible deletion of Bcl6 in adult mice results in muscle loss.
(a, b) Serial measurements of total body, lean, and fat mass in (a) n = 6 Cre+oil, n = 9 Cre-tmx and n = 9 Bcl6i-MKO male and (b) n = 6 Cre+oil, n = 8 Cre-tmx and n = 8 Bcl6i-MKO female mice starting at 8 weeks of age prior to (pre) and up to 12 weeks after treatment. Two-way ANOVA with Tukey’s multiple comparisons test was performed. (c, d) Weights of quadriceps (quad), gastrocnemius (gastroc), tibialis anterior (TA), extensor digitorum longus (EDL) and soleus from Cre+oil, Cre-tmx and Bcl6i-MKO (c) male and (d) female mice 12 weeks after treatment (n = same as (a,b)). One-way ANOVA with Dunnett’s multiple comparisons test was performed. (e) Tissue weights in n = 4 ad lib chow fed and n = 5 48-hour fasted C57BL/6 male mice. (f) qPCR of Bcl6 normalized to 36b4 in muscles from fed and 48-hour fasted mice (n = 3/group). (g) Western blot and densitometry of BCL6 and Actin in n = 4 fed and n = 5 from 48 hour fasted mouse quadriceps. Unpaired, two-tailed t-test with Welch correction was performed in (e-g). (h) Bcl6 levels in published cancer-associated cachexia syndrome (CACS) models. GEO accession numbers are listed. Control vs C26 colon cancer P = 0.0008 by unpaired, two-tailed t-test with Welch correction (n = 3/group). Boxplot shows min to max values with line at the mean. (i) Bcl6 expression in C2C12 myotubes exposed to 33% colon-26 carcinoma (C26) conditioned versus control media for 8 hours (n = 3/group; unpaired, two-tailed t-test with Welch correction was performed) and corresponding protein blots after 8 or 24 hours. (j) Bcl6 expression in C2C12 myotubes treated with cytokines (n = 3/group), growth hormone (n = 2/group) and glucocorticoids (n = 3/group) for 8 hours. Unpaired, two-tailed t-test with Welch correction and one-way ANOVA with Dunnett’s multiple comparisons were performed. Exact p-values can be found in the source data file. All data in (a-g) and (i-j) are represented as mean + SEM. δ p < 0.05, δδ p < 0.01 for Cre+oil vs Cre-tmx; # p < 0.05, ## p < 0.01, ### p < 0.001 for Cre+oil vs Bcl6i-MKO; * p < 0.05, ** p < 0.01, *** p < 0.001 for Cre-tmx vs Bcl6i-MKO or fed vs fasted or vehicle vs treated myotubes.
Extended Data Fig. 3 Bcl6 controls an oxidative to glycolytic shift in myofibres.
(a) Representative fluorescent antibody-stained images of four myosin heavy chain isoforms in quadriceps from Cre- tmx and Bcl6i-MKO mice (left). MYH7 (type 1 fiber) is blue, MYH2 (type 2a fiber) is green, MYH1 (type 2x fiber) is black, and MYH4 (type 2b fiber) is red. Quantification of fiber types (right) expressed as percentage of total (n = 3/group). Cre- tmx vs Bcl6i-MKO type 2a fiber P = 0.0374 by unpaired, two-tailed t-test with Welch correction. (b) qPCR DNA ratios of mitochondrial genes Cox1 and Cytb over nuclear genes Gcg and Hbb in quadriceps from Bcl6fl/fl and Bcl6MKO males (n = 7/group). (c) Western blot and densitometry of VDAC and total (Licor) protein in quadriceps from n = 6 Bcl6fl/fl and n = 7 Bcl6MKO males. (d) Protein levels of mitochondrial complex subunits and total (Memcode) protein in quadriceps from Bcl6fl/fl and Bcl6MKO mice. Western blot (left) and protein densitometry (right) are shown. (n = 4/group) Bcl6fl/fl vs Bcl6MKO complex II P = 0.0139 by unpaired, two-tailed t-test with Welch correction. (e, f) Cross-sectional areas of specific fiber types determined from fluorescent antibody-stained sections in quadriceps from (e) Bcl6fl/fl and Bcl6MKO mice (n = 3/group) and (f) Cre- tmx and Bcl6i-MKO mice (n = 3/group). Type 2b fiber in Bcl6fl/fl vs Bcl6MKO P = 0.003 and in Cre− tmx vs Bcl6i-MKO P = 0.0385 by unpaired, two-tailed t-test with Welch correction. All data are represented as mean + SEM. * p < 0.05, ** p < 0.01.
Extended Data Fig. 4 Muscle loss due to Bcl6 deletion is associated with reduced muscle function.
(a, b) Absolute (left) and lean body mass normalized (right) forelimb grip strength measured in (a) 13-week old n = 7 Bcl6fl/fl and n = 10 Bcl6MKO female mice and (b) n = 6 Cre+ oil, n = 8 Cre- tmx and n = 8 Bcl6i-MKO female mice twelve weeks after tamoxifen or oil treatment. Whiskers in the boxplot show min to max values with line at the median and box representing the first to third quartile values. (c) Maximum tetanic force for the tibialis anterior (TA) muscle from 13-week old n = 3 Bcl6fl/fl and n = 2 Bcl6MKO female mice (P-value for maximum force = 0.0311 by unpaired, two-tailed t-test with Welch correction). (d, e) Distance and time run on a treadmill to exhaustion in (d) 13-week old n = 15 Bcl6fl/fl and n = 14 Bcl6MKO male mice and (e) Cre- tmx and Bcl6i-MKO female mice twelve weeks after tamoxifen treatment (n = 12/group). (f, g) Light phase, dark phase and total activity in (f) 13-week old Bcl6fl/fl and Bcl6MKO male mice (n = 5/group) and (g) Cre- tmx and Bcl6i-MKO male mice 1.5 weeks after tamoxifen treatment (n = 5/group). All data in (c-g) are represented as mean + SEM. * p< 0.05.
Extended Data Fig. 5 BCL6 directly controls the expression of anabolic regulators.
(a) Principal component analysis of RNA-seq from n = 5 Bcl6i-MKO and n = 4 tamoxifen-treated controls (Cre- tmx) or n = 4 corn oil-treated controls (Cre+ oil). (b) Venn diagram depicting overlap between differentially expressed (DE) genes in muscle Stat5 knockout (Stat5MKO) mice (GEO series GSE14710) compared to muscle Bcl6i-MKO mice. (c) Quadrant plot of log2 fold change in gene expression in Stat5MKO/controls versus Bcl6i-MKO/Cre- tmx controls. Spearman correlation coefficient rs = 0.03. (d) Scatterplot (left) of normalized H3K27ac tag counts and boxplot (right) showing quantification of H3K27ac tag densities within 1kb of BCL6 peaks in Cre- tmx and Bcl6i-MKO quadriceps (n = 3/group; P = 3.5e−10 by unpaired, two-tailed Mann Whitney test). Whiskers in the boxplot show min to max values with line at the median and box representing the first to third quartile values. (e) Receiver operator curve (ROC) of the association between differential expression in Bcl6-ablated muscle and BCL6 binding peaks. (f) UCSC browser tracks of RNAseq in quadriceps from Cre+ oil, Cre- tmx and Bcl6i-MKO mice, H3K27ac ChIP-seq in quadriceps from Cre- tmx and Bcl6i-MKO mice 1 week after oil or tamoxifen treatment, nascent RNA-seq in quadriceps from mice treated with DMSO or FX1 for 3 hours, and BCL6 ChIP-seq track in quadriceps from C57BL/6 animals along the Mstn, Ar, and Smox genes. (g) qPCR expression of Bcl6, Akt1, Eif4ebp1, Igf1 and Ar in quadriceps from males (n = 5/group) and females (n = 4 Bcl6fl/fl and n = 5 Bcl6MKO mice). Two-way ANOVA followed by Tukey’s multiple comparisons test was performed and all individual p-values can be found in the source data file. (h, i) Western blot and densitometry of (h) BCL6 and actin (i) 4EBP1 and total protein (licor) in quadriceps from male and female n = 3 Bcl6fl/fl and n = 4 Bcl6MKO mice. For BCL6, P(male Bcl6fl/fl vs Bcl6MKO) = 0.005, P(male Bcl6fl/fl vs female Bcl6MKO) = 0.005, P(male Bcl6MKO vs female Bcl6fl/fl) = 0.0299, P(female Bcl6fl/fl vs Bcl6MKO) = 0.0305 and for 4EBP1, P(male Bcl6fl/fl vs Bcl6MKO) = 0.0034, P(male Bcl6fl/fl vs female Bcl6MKO) = 0.0199 by two-way ANOVA followed by Tukey’s multiple comparisons. All data in (g-i) are represented as mean + SEM. * p < 0.05, ** p < 0.01 and *** p < 0.001.
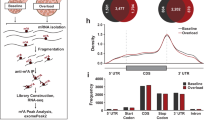
Extended Data Fig. 6 Loss of Bcl6 reduces the rate of muscle protein synthesis.
(a) Heatmap of ribosome protected fragment (RPF) sizes in sequencing libraries of tamoxifen-treated Bcl6i-MKOmRiboTag and mRiboTag mice. (b) Pie chart distribution of mapped RPF reads. (c) Empirical cumulative distribution frequency (Ecdf) plots of the translation efficiencies (natural logarithm of TE) in quadriceps for all genes. Bcl6i-MKO mRiboTag vs mRiboTag P = 2.2e-16 by two-sided Kolmogorov-Smirnov testing (d) Metagene distribution plot showing normalized ribo-seq RPF reads from the TSS to TTS for all genes (left) and genes with increased TE (right). (e) Western blot and densitometry of ubiquitin protein and actin in quadriceps from Cre- tmx and Bcl6i-MKO males 1.5 weeks after tamoxifen treatment (n = 4/group). (f) EIF4E co-immunoprecipitation and western blots for EIF4G (top) and 4EBP1 (bottom) in gastrocnemius from Cre- tmx and Bcl6i-MKO males 1 week after tamoxifen treatment (n = 2/group). (g) Western blot and densitometry of p4EBP1, Non-phospho 4EBP1, Total 4EBP1, and total (Memcode) protein in quadriceps from 10 week old Bcl6fl/fl and Bcl6MKO males (n = 4/group). P(Bcl6fl/fl vs Bcl6MKO) for p4EBP1 = 0.0009, total 4EBP1 = 0.0004, and nonP 4EBP1 = 0.0002 by unpaired, two-tailed t-test with Welch correction. (h) qPCR of protein synthesis and degradation regulators in n = 4 Cre+ oil, n = 4 Cre- tmx and n = 5 Bcl6i-MKO male mice 7 days after treatment. One-way ANOVA with Dunnett’s multiple comparisons test showed that P(Cre+ oil vs Bcl6i-MKO) for Eif4ebp1 = 0.0086, Mstn = 0.0018, Igf1 = 0.0332 and Ar = 0.0420; P(Cre- tmx vs Bcl6i-MKO) for Eif4ebp1 = 0.0163, Mstn = 0.0011, Igf1 = 0.0140 and Ar = 0.0330. (i, j) Analysis of Eif4ebp1 knockdown efficiency in muscles (n = 3 mice/group for scramble and Eif4ebp1 shRNA1 and n = 2 mice/group for Eif4ebp1 shRNA2). (i) qPCR of Eif4ebp1 and (j) western blot and densitometry of 4EBP1 in Cre- tmx and Bcl6i-MKO male mice transduced with scramble (black border), Eif4ebp1 shRNA1 (green border), or Eif4ebp1 shRNA2 (yellow border) and treated with tamoxifen one week after viral infection. Tissues were analyzed eight weeks later. All bar graph data in (e, g-j) are represented as mean + SEM. # p < 0.05, ## p < 0.01 for Cre+oil vs Bcl6i-MKO and * p < 0.05, ** p < 0.01, *** p < 0.001 for Cre-tmx vs Bcl6i-MKO and Bcl6fl/fl vs Bcl6MKO.
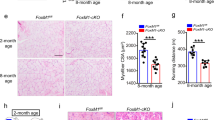
Extended Data Fig. 7 Chronic Bcl6 loss evokes compensatory signaling to maintain muscle mass.
(a) SUnSET analysis of puromycin incorporation into quadriceps (top) in Cre- tmx and Bcl6i-MKO mice 12 weeks after tamoxifen treatment. Puromycin labeling relative to total protein (Licor) by densitometry (bottom) (n = 3/group; P = 0.0017 by unpaired, two-tailed t-test with Welch correction). (b) Western blot and densitometry in quadriceps from Cre- tmx and Bcl6i-MKO males 15 weeks after tamoxifen treatment for LC3, 3 days after colchicine or vehicle treatment. (n = 3/group; Two-way ANOVA followed by Tukey’s multiple comparisons test was performed. P (Veh-Cre- tmx vs Col-Cre- tmx) = 0.0174; P (Veh-Cre- tmx vs Col-Bcl6i-MKO) = 0.0005; P (Veh-Bcl6i-MKO vs Col-Bcl6i-MKO) = 0.0083.) Flux is the difference in average LC3-II levels in colchicine minus vehicle (bottom right). (c) Western blot and densitometry of ubiquitin and total (Licor) protein in quadriceps from Cre- tmx and Bcl6i-MKO males 12 weeks after tamoxifen treatment (n = 4/group). (d) Quadrant plot of log2 fold change in gene expression in Bcl6i-MKO/Cre- tmx 12 weeks after tamoxifen treatment (chronic) versus Bcl6i-MKO/Cre- tmx 1 week after tamoxifen treatment (acute). (e) Ontology analysis of differential genes unique in chronic Bcl6 deletion. Cumulative hypergeometric statistical testing was used. (f) Log p-value versus Pearson correlation plotted for causal transcription factors identified by IMAGE that were unique to either acute or chronic Bcl6 deletion. Pink = high, green = medium, and gray = low confidence factors. Factors in acute dataset are shown as circles and factors in chronic are squares. (g) Causal transcription factors with opposing Pearson correlations to gene expression in acute versus chronic Bcl6 deletion. Acute = green circle, chronic = orange square. (h-l) Western blot and densitometry of (h) pSMAD1/5/8, Total SMAD1, and total (Licor) protein, (i) pFOXO1, Total FOXO1, and Actin, (j) p4EBP1, Non-phospho 4EBP1, Total 4EBP1, and Actin, (k) pAKT, Total AKT, and Actin and (l) pS6, Total S6, and Actin in quadriceps from Cre- tmx and Bcl6i-MKO males 12 week after tamoxifen treatment (n = 4/group). Unpaired, two-tailed t-test with Welch correction and all individual p-values can be found in the source data file. All bar graph data in (a-c, h-l) are represented as mean + SEM. * p < 0.05 and ** p < 0.01.
Supplementary information
Supplementary Information
Supplementary Table 1. List of qPCR primers.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Ramachandran, K., Futtner, C.R., Sommars, M.A. et al. Transcriptional programming of translation by BCL6 controls skeletal muscle proteostasis. Nat Metab 6, 304–322 (2024). https://doi.org/10.1038/s42255-024-00983-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s42255-024-00983-3