Abstract
Mimicry of receptor functions by designing synthetic receptors would be one of the recently hot research trends in cell engineering. While several types of synthetic receptors have been designed to induce desired cell fates in response to external stimuli, little is known about which receptor type signals more efficiently for inducing a certain cell fate. In this study, we compared the performance of three types of synthetic receptor scaffolds, i.e. myristoylated, cytosolic, and transmembrane types that signal through JAK-dependent phosphorylation of tyrosine motifs to transduce growth signaling. As a result, the phosphorylation levels of JAK and subsequent downstream signaling molecules were significantly maintained in the cytosolic type receptors, leading to more efficient cell growth than the other types. In contrast, the phosphorylation levels of JAK decreased in a motif-dependent manner in the transmembrane type receptors. Although various studies on receptor engineering based on domain or motif engineering have been reported, to our knowledge this study is the first to demonstrate that synthetic receptor scaffolds significantly affect the efficiency of cell fate signals. These findings are important for both receptor biology and receptor engineering, providing guidelines for rationally designing synthetic receptors that can transduce as efficient signaling as possible.
Similar content being viewed by others
Introduction
Cells respond to changes in their extracellular environments through receptors expressed on the plasma membrane or in the cytosol. Hormones and cytokines activate their specific receptors and trigger intracellular signal transduction, which dynamically changes post-translational modifications of various intracellular molecules to induce cell fates such as proliferation, differentiation, migration, death, and even a variety of immune reactions for host defense. One of the recently hot research trends in cell engineering would be mimicry of receptor functions by designing synthetic receptors to enable cells to exert desired functions in response to external stimuli1,2. Synthetic notch (synNotch) receptors realize intercellular communications by arbitrarily coupling transcriptional regulations with external antigen ligands3,4. When expressed on T cells, chimeric antigen receptors (CAR) recognize cancer-specific antigens on the plasma membrane and induce tumor-killing activities through the CD3ζ-based signaling domains5,6.
Thus, it would theoretically be possible to induce desired cell fates in response to any external stimuli with rational design of synthetic receptors. A major aspect of the rational design is how we engineer the signaling domain of synthetic receptors on a domain or motif basis to regulate signaling properties1,2. Another important aspect would be how we choose an appropriate receptor scaffold among those with different structural characteristics. Such synthetic receptor scaffolds could be categorized into two types according to cell membrane permeability of the ligands. One type receives soluble or transmembrane proteins as an extracellular ligand and thus generally spans the plasma membrane. The marked examples are synNotch and CAR, which we have introduced in the previous paragraph. Particularly, CAR-T cells attained high therapeutic effects on CD19-positive B cell lymphoma and have already reached clinical applications6. In addition, we previously developed a variety of cytokine receptor-based cell fate-inducing CARs (cfiCARs) that induce various cell fates including proliferation, differentiation, migration, and apoptosis in response to soluble antigens, depending on the combination of signaling domains and host cells7,8,9,10,11,12,13,14. The other type of the synthetic receptor scaffolds receives membrane-permeable small molecules through a dimerization domain and thus is expressed intracellularly in the cytosol or at the inner leaflet of the plasma membrane with lipid modification. A representative example is a chemical inducer of dimerization (CID) system using an F36V mutant of FK506-binding protein 12 (FKBPF36V), which can control the activation of not only receptors but also various signal transducers and transcription factors in response to a synthetic dimerizer AP1903 or AP2018715,16. Particularly, iCasp9, an inducible suicide gene, has progressed to Phase I clinical trials as a safety switch in cell therapy17. In addition, we previously developed designer receptors that can activate target signaling molecules by appropriately arranging domains and tyrosine motifs18,19,20. Such a designer receptor was also utilized for phenotypic screening of tyrosine motifs that efficiently induce cell proliferation21.
The above-mentioned transmembrane and intracellular receptor types have their own advantages. The transmembrane type has more choices of ligands, and the membrane raft facilitates association between the receptor chains. On the contrary, the intracellular type has a higher degree of conformational freedom in the dimer/oligomer structure after ligand binding22. However, little is known about which receptor type signals more efficiently for inducing a certain cell fate. This is due to the lack of studies that compare the signaling properties of the different receptor types in parallel. Therefore, in this study, we aim to obtain useful knowledge on design strategies for synthetic receptors by systematically comparing the signaling intensity of the different receptor types. To accomplish this, we employ a series of signaling domains with various tyrosine motifs and test the activity of cell growth, which is the most fundamental cell fate.
Results
Designing three types of the synthetic receptor scaffolds
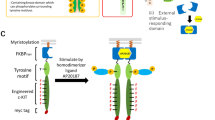
We aimed to systematically compare the signaling properties of the following three types of the synthetic receptor scaffolds (Fig. 1a). A myristoylated (Myr) type encodes an FKBPF36V-based receptor scaffold and is localized at the inner leaflet of the plasma membrane via an N-terminal myristoylation signal sequence. A cytosolic (Cyt) type also encodes the FKBPF36V-based scaffold and is simply expressed in the cytosol without any signal sequences. The Myr and Cyt types are commonly activated by a membrane-permeable dimerizer ligand AP20187. A transmembrane (TM) type encodes anti-fluorescein single-chain Fv and the D2 domain of erythropoietin receptor (EpoRD2) in the extracellular domain and is activated by an oligomeric antigen fluorescein-conjugated BSA (FL-BSA). These three types commonly encode the JAK-binding domain of c-mpl and a tyrosine motif as a signal-transducing domain and a Myc tag at the C-terminus. The tyrosine motif sequences include cell growth-inducing motif sequences selected from a library based on the STAT1-binding motif (#4, #5, #13, #17, #18)21, the intracellular domain of c-mpl, the STAT3-binding motif, and no motif as a negative control (Fig. 1b).
Outline of the chimeric receptor constructs. (a) Illustration of the three-type chimeric receptors. The myristoylated (Myr) and cytoplasmic (Cyt) receptors are activated by a cell-permeable synthetic dimerizer AP20187, whereas the transmembrane (TM) receptor is activated by fluorescein-conjugated BSA (FL-BSA). (b) The amino acid sequence of the tyrosine motifs incorporated in the chimeric receptors. The clones (#4, 5, 13, 17, 18) are derived from a three amino acid-randomized library of the STAT1-binding motif. The randomized positions are indicated as bold and italic. The tyrosine residues are colored in red.
Cyt receptor transduces the most effective growth signaling
The receptor constructs were genetically introduced into murine IL-3-dependent Ba/F3 cells, in which we easily compare the properties of growth signaling induced by the synthetic receptors. First, we confirmed the expression of the synthetic receptors in western blot analysis. As a result, all of the synthetic receptors were expressed with the expected molecular mass (Fig. 2). The expression levels differed among the receptors, but not depending on the types of the receptor scaffolds.
Confirmation of the receptor expression. (a) The estimated molecular mass of each chimeric receptor. (b) Western blotting to detect expression of the chimeric receptors. Blots with Myc tag and GAPDH represent receptor expression levels and loading controls, respectively. Uncropped blot images are provided in Supplementary Information (Supplementary Fig. 3).
Next, we performed a cell growth assay to examine whether the cells expressing the synthetic receptors could induce growth signals in response to the corresponding ligand AP20187 (for the Myr and Cyt types) or FL-BSA (for the TM type). The results showed that in all motif sequences the Cyt type receptors showed higher cell growth levels than the TM type receptors (Fig. 3). When compared with the corresponding Cyt type receptors, TM #13 was non-responsive, TM #4, #5, and mpl were slightly responsive, TM #18 and STAT3 were moderately responsive, and TM #17 was highly responsive and thus comparable to Cyt #17. The Cyt type showed higher growth activity than the Myr type, indicating that membrane localization is not required for growth signaling of the synthetic receptors tested in this study.
Growth assay. Cells were cultured with the indicated concentrations of the ligand AP20187 (for the Myr and Cyt receptors) or FL-BSA (for the TM receptors) for 3 days with the initial cell density of 7.0 × 103 cells/mL. Cell growth levels were measured by a colorimetric assay. The (-) panel indicates the data of parental Ba/F3 cells cultured with each ligand (AP20187 or FL-BSA), which means the background levels. The data are represented as mean ± SE (n = 3, independent experiments).
Signaling analysis revealed the dramatic difference of signaling intensities between Cyt and TM receptors
To explore the reason why the Cyt type receptors transduce stronger growth signals than the TM type receptors, we performed signaling analysis to examine the phosphorylation of signaling molecules in western blotting (Fig. 4). We chose #18 as the highest growth-inducing tyrosine motif clone, mpl as the natural receptor-derived tyrosine motifs, and no motif as a negative control. Consequently, JAK2 and Tyk2, which are the most upstream tyrosine kinases activated by receptor dimerization, exhibited interesting phosphorylation properties. Intriguingly, the cytosolic type showed similar phosphorylation levels of JAK2 and Tyk2 regardless of the presence or absence of the motifs, while the TM type showed the phosphorylation levels of JAK2 and Tyk2 decreased in the presence of the tyrosine motifs. Furthermore, the Cyt type induced higher levels of phosphorylation of downstream growth signaling molecules than the TM type.
Exploring the ligand-dependent activation levels of signaling molecules. Cells were depleted in the absence of IL-3 and stimulated for 15 min without any factors (-) or with 10 nM AP20187 (AP), 10 nM FL-BSA (FL), or 1 ng/mL IL-3 (IL3). The cell lysates were subjected to SDS-PAGE and western blotting. Blots with the antibodies recognizing each whole molecule and GAPDH serve as loading controls. The most right four lanes in which the type and motif are (-) represent parental Ba/F3 cells as stimulation controls. Uncropped blot images are provided in Supplementary Information (Supplementary Fig. 4).
In order to reliably demonstrate that the Cyt type signals stronger than the TM type, we compared the Cyt type and TM type in parallel for #18 and mpl by varying the stimulation time (0, 5, 15, 60, or 240 min) with each ligand. Consequently, the phosphorylation levels of all signaling molecules tested (JAK2, Tyk2, STAT5, MEK and Akt) in the Cyt type were much higher than those in the TM type for all stimulation time periods (Fig. 5a,b). While the phosphorylation levels of STAT5 and Akt decreased in a time-dependent manner, the phosphorylation levels of MEK lasted long and reached the maximum at 240 min in the Cyt type. On the other hand, for no motif, JAK2 and Tyk2 were phosphorylated in similar levels for both Cyt and TM types (Fig. 5c).
Comparing the time-dependent signaling levels between the Cyt and TM receptors. Cells were depleted in the absence of IL-3 and stimulated for the indicated time periods with 10 nM AP20187 (for the Cyt receptors) or 10 nM FL-BSA (for the TM receptors). The cell lysates were subjected to SDS-PAGE and western blotting. Blots with the antibodies recognizing each whole molecule and GAPDH serve as loading controls. The receptor constructs incorporating (a) motif #18, (b) the intracellular domain of c-mpl, and (c) no motif were analyzed. Uncropped blot images are provided in Supplementary Information (Supplementary Figs. 5, 6, and 7).
Thus, the TM type led to motif-dependent decrease of activation levels of the most upstream kinases JAK2 and Tyk2, but the Cyt type did not. This phenomenon was also manifested in the phosphorylation levels of downstream signaling molecules. Consequently, the Cyt type activated signaling with higher intensity and ultimately promoted faster cell growth.
Discussion
In this study, we compared the performance of three types of synthetic receptor scaffolds, i.e. myristoylated (Myr), cytosolic (Cyt), and transmembrane (TM) types that signal through JAK-dependent phosphorylation of tyrosine motifs. As a result, the phosphorylation levels of JAK and subsequent downstream signaling molecules were significantly maintained in the Cyt type receptors, leading to more efficient cell growth than the other types. In contrast, the phosphorylation levels of JAK decreased in a motif-dependent manner in the TM type receptors. Although various studies on receptor engineering based on domain or motif engineering have been reported, to our knowledge this study is the first to demonstrate that synthetic receptor scaffolds significantly affect the efficiency of cell fate signals. This finding would be interesting in both receptor biology and receptor engineering aspects.
The TM type receptors are present in various organelles such as ER, Golgi, and endosomes in the cytoplasm, whereas the ligand (FL-BSA) only acts those localized on the plasma membrane. Therefore, it is intrinsically difficult to precisely adjust the number of dimerized receptors among Cyt, Myr, and TM type receptors. Thus, we simply expressed these receptors in the same way using the same expression vector. Consequently, the Cyt type and the TM type receptors with no motif exhibited similar phosphorylation levels of JAK (Fig. 5c), suggesting that the TM type receptors were not in extremely unfavorable conditions with respect to dimerization. Under this condition, the TM type receptors with the motifs (#18 and mpl) resulted in much weaker phosphorylation levels of downstream signaling molecules than the corresponding Cyt type receptors, which we believe is a fair evaluation.
It has been reported that the Myr type receptors are not completely localized at the membrane, but is in equilibrium between the cytoplasmic fraction (corresponding to the Cyt type) and the membrane-localized fraction23. For example, Myr #13 induced a certain degree of proliferation probably due to the effects of the cytoplasmic fraction (Fig. 3). Therefore, in this paper we mainly discussed the differences in signaling intensities between Cyt and TM types in order to avoid complicated discussions due to ambiguity of the Myr type.
In the signaling analyses, the JAK activation levels decreased tyrosine motif-dependently in the TM type receptors, whereas this phenomenon did not occur in the Cyt type receptors. The results suggest that signaling molecules with tyrosine motif-binding SH2 domains are involved in growth suppression in the TM type receptors. Furthermore, as clearly shown in Fig. 5, the phosphorylation levels of downstream signaling molecules were weaker overall in the TM type, which was correlated with the phosphorylation levels of JAK. Taken together, we could hypothesize that a molecule that has an SH2 domain, can inactivate JAK, and works efficiently on the plasma membrane would be involved in the signaling events of the TM type receptors.
Considering the first two conditions of the above hypothesis, suppressor of cytokine signaling (SOCS) and SH2 domain-containing phosphatases (SHP1 and SHP2) would be candidate signaling molecules that have SH2 domains and can inactivate JAK24. Among these, while SOCS and SHP2 have not been reported to be localized at the plasma membrane, the tyrosine phosphatase SHP1 has been reported to have a lipid raft-binding motif25,26,27, suggesting that SHP1 may be membrane-localized and predominantly act on the TM type receptors.
As a negative regulator, SHP1 preferentially binds to the immunoreceptor tyrosine-based inhibitory motif (ITIM) with a consensus sequence (I/V/L)xYxx(L/V), when the tyrosine residue of the motif is phosphorylated28. When ITIM is present in the signaling domain of receptors, SHP1 dephosphorylates kinases associated with the receptors. Interestingly, the results of the growth assay (Fig. 3) revealed that #13, whose cell growth was extremely suppressed in the TM type, has another tyrosine residue at the Y + 3 position, which generates the completely matched ITIM sequence (VNYHVL). In addition, #4 and #5, whose cell growth was strongly suppressed in the TM type, have YxxL, which matches the latter half of the ITIM sequence (#4: FGYINL, #5: FGYIQL). In addition, the intracellular domain of the natural receptor mpl, whose cell growth was strongly suppressed in the TM type at similar levels to #4 and #5, have two YxxL motifs out of the three motifs (MDYRRL, HSYLPL, LSYWQQ). Of note, the cell growth level of #17 (FGYVTA) was not suppressed in the TM type compared to the Cyt type, which implies that SHP1 may not preferentially bind to the YxxA motif. Since #18 (FGYYLNH) and the STAT3-binding motif (SGYRHQ) have intermediate cell growth levels in the TM type, SHP1 may bind to these motifs at moderate levels.
As the name indicates, ITIM has been considered to be suppressive for receptor signaling. However, our results of #13 suggest that ITIM lost the suppressive functions when located in the cytosol. This is an interesting finding and also importantly suggests that receptors are susceptible to negative regulation by tyrosine phosphatases at the vicinity of the plasma membrane rafts but are released from the negative regulation in the cytosol. Therefore, choosing the Cyt type rather than the TM type would be reasonable to create synthetic receptors with more intensive phosphorylation levels and eventually higher signaling efficiency. Although we have demonstrated the importance of the receptor type for inducing growth of Ba/F3 cells in the present study, this knowledge could also be applied to other cells and cell fates, by which synthetic receptors could be recognized as an increasingly important and promising tool for cell fate regulation with high efficiency.
Materials and methods
Plasmid construction
The chimeric receptor constructs were subcloned into a retroviral transfer plasmid pMK-stuffer-IPTG as described previously21. Owing to the internal ribosomal entry site (I) –puromycin resistance (P) gene cassette, retrovirally transduced cells stably expressing the chimeric receptors can be selected by culturing cells with puromycin. We conducted plasmid construction and sequencing according to standard protocols. The amino acid sequences of the myristoylated, cytosolic, and transmembrane type chimeric receptors are shown in Supplementary Information (Supplementary Fig. 1).
Retroviral transduction
Ba/F3 cells (RIKEN Cell Bank #RCB0805, Ibaraki, Japan) were retrovirally transduced as described previously21. Briefly, retroviral packaging cells were transiently transfected with each of the constructed retroviral transfer plasmids, and the retroviral supernatant was used for transducing Ba/F3 cells on a Retronectin (TakaraBio)-coated plate. The transduced cells were selected in the presence of 1 µg/mL puromycin.
Cell growth assay
Cell growth assay was also performed as described previously21. Briefly, cells cultured with 1 ng/mL of IL-3 (ThermoFisher Scientific) were washed twice with PBS and inoculated into 24-well plates with the indicated concentrations of the ligand AP20187 (TakaraBio) or FL-BSA (Sigma-Aldrich). Viable cell densities were estimated using Cell Counting Kit-8 (Dojindo Laboratories) by measuring absorbance at 450 nm using GloMax Discover Microplate Reader (Promega).
Western blotting
Western blotting was also performed as described previously21. For confirming receptor expression, cells (1.0 × 106 cells) cultured with 1 ng/mL of IL-3 were simply harvested. For the signaling analysis, cells cultured with 1 ng/mL of IL-3 were washed twice with PBS and then cultured without IL-3 for 10–13 h. The depleted cells (1.0 × 106 cells) were stimulated with 10 nM AP20187, 10 nM FL-BSA (FL), or 1 ng/mL IL-3 at 37˚C for the indicated time periods and then harvested. The stimulated cells were treated with 2 mM Na3VO4 in ice-cold PBS to inhibit dephosphorylation. Subsequently, the cells were lysed with 100 μl of lysis buffer containing 1 mM Na3VO4, incubated on ice for 10 min, and centrifuged at 22,300 g for 10 min at 4 ℃. The supernatant was mixed with 33 μl of 4X SDS-PAGE Sample Buffer (Tokyo Chemical Industry) and boiled at 98 ℃ for 5 min. The lysates were subjected to the SDS-PAGE/western blot analysis. The antibodies used in western blotting are listed in Supplementary Information (Supplementary Fig. 2).
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information.
References
Kawahara, M. & Nagamune, T. Engineering of mammalian cell membrane proteins. Curr. Opin. Chem. Eng. 1, 411–417 (2012).
Kawahara, M., Ueda, H. & Nagamune, T. Engineering cytokine receptors to control cellular functions. BIochem. Eng. J. 48, 283–294 (2010).
Morsut, L. et al. Engineering customized cell sensing and response behaviors using synthetic notch receptors. Cell 164, 780–791 (2016).
Roybal, K. T. et al. Precision Tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 164, 770–779 (2016).
Kagoya, Y. et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 24, 352–359 (2018).
Larson, R. C. & Maus, M. V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 21, 145–161 (2021).
Kawahara, M., Hitomi, A. & Nagamune, T. Antigen-responsive regulation of cell motility and migration via the signalobodies based on c-Fms and c-Mpl. Biotechnol. Prog. 30, 411–417 (2014).
Kawahara, M., Hitomi, A. & Nagamune, T. S-Fms signalobody enhances myeloid cell growth and migration. Biotechnol. J. 9, 954–961 (2014).
Kawahara, M. et al. Antigen-mediated migration of murine pro-B Ba/F3 cells via an antibody/receptor chimera. J. Biotechnol. 133, 154–161 (2008).
Nakabayashi, H., Aoyama, S., Kawahara, M. & Nagamune, T. Differentiation signalobody: Demonstration of antigen-dependent osteoclast differentiation from a progenitor cell line. J. Biosci. Bioeng. 122, 357–363 (2016).
Nakabayashi, H., Kawahara, M. & Nagamune, T. Cell-surface expression levels are important for fine-tuning the performance of receptor tyrosine kinase-based signalobodies. Biotechnol. J. 12, 1700441 (2017).
Nakajima, K., Shen, Z., Miura, M., Nakabayashi, H. & Kawahara, M. Sequential control of myeloid cell proliferation and differentiation by cytokine receptor-based chimeric antigen receptors. PLoS One 17, e0279409 (2022).
Tone, Y., Kawahara, M., Kawaguchi, D., Ueda, H. & Nagamune, T. Death signalobody: Inducing conditional cell death in response to a specific antigen. Hum. Gene Ther. Methods 24, 141–150 (2013).
Tsukamoto, T. et al. Chimeric G-CSF receptor-mediated STAT3 activation contributes to efficient induction of cardiomyocytes from mouse induced pluripotent stem cells. Biotechnol. J. 15, e1900052 (2020).
Clackson, T. et al. Redesigning an FKBP–ligand interface to generate chemical dimerizers with novel specificity. Proc. Natl. Acad. Sci. USA 95, 10437–10442 (1998).
Neff, T. & Blau, C. A. Pharmacologically regulated cell therapy. Blood 97, 2535–2540 (2001).
Zhang, P. et al. Phase I trial of inducible caspase 9 T cells in adult stem cell transplant demonstrates massive clonotypic proliferative potential and long-term persistence of transgenic T cells. Clin. Cancer Res. 25, 1749–1755 (2019).
Kongkrongtong, T., Sumigama, Y., Nagamune, T. & Kawahara, M. Reprogramming signal transduction through a designer receptor tyrosine kinase. Commun. Biol. 4, 752 (2021).
Kongkrongtong, T., Zhang, R. & Kawahara, M. Rational design of heterodimeric receptors capable of activating target signaling molecules. Sci. Rep. 11, 16809 (2021).
Nakajima, K., Araki, S. & Kawahara, M. Tailoring minimal synthetic receptors to reconstitute signaling properties through multiple tyrosine motifs. Biochem. Biophys. Res. Commun. 566, 148–154 (2021).
Umene, K., Nagamune, T. & Kawahara, M. Phenotypic screening of signaling motifs that efficiently induce cell proliferation. Sci. Rep. 13, 15639 (2023).
Horikawa, M., Kakiuchi, Y., Kashima, D., Ogawa, K. & Kawahara, M. Thrombopoietin receptor-based protein-protein interaction screening (THROPPIS). Biotechnol. Bioeng. 119, 287–298 (2022).
Resh, M. D. Covalent lipid modifications of proteins. Curr. Biol. 23, R431-435 (2013).
Morris, R., Kershaw, N. J. & Babon, J. J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 27, 1984–2009 (2018).
Fawcett, V. C. J. & Lorenz, U. Localization of Src homology 2 domain-containing phosphatase 1 (SHP-1) to lipid rafts in T lymphocytes: Functional implications and a role for the SHP-1 carboxyl terminus1. J. Immunol. 174, 2849–2859 (2005).
Lorenz, U. SHP-1 and SHP-2 in T cells: Two phosphatases functioning at many levels. Immunol. Rev. 228, 342–359 (2009).
Sankarshanan, M., Ma, Z., Iype, T. & Lorenz, U. Identification of a novel lipid raft-targeting motif in Src homology 2-containing phosphatase 11. J. Immunol. 179, 483–490 (2007).
Daëron, M., Jaeger, S., Du Pasquier, L. & Vivier, E. Immunoreceptor tyrosine-based inhibition motifs: A quest in the past and future. Immunol. Rev. 224, 11–43 (2008).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 21H01735 and 23K17854 (to M.K.).
Author information
Authors and Affiliations
Contributions
K.U. and M.K. conceived the project and designed the overall experiments. K.U. performed the experiments and analyzed the data. K.U. and M.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umene, K., Kawahara, M. Synthetic receptor scaffolds significantly affect the efficiency of cell fate signals. Sci Rep 14, 5801 (2024). https://doi.org/10.1038/s41598-024-56612-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56612-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.