Abstract
The Adriatic Sea is one of the largest areas of occurrence of shared small pelagic stocks and the most fished area of the Mediterranean Sea, which is in turn one of the most exploited basins of the world. The variations in the stable isotope contents (δ15N and δ13C) were determined for three small pelagic fishes (i.e., Engraulis encrasicolus, Sardina pilchardus, and Sprattus sprattus, respectively known as anchovies, sardines and sprats) collected across the western side of the basin. Our data allowed to determine the width and features of their trophic niches, to assess potential overlap or resource partitioning among them, and likely anticipate species adaptation to future climate change scenarios. Moreover, variations in stable isotope contents were correlated to both resource availability (i.e., mesozooplankton) and environmental variables. The high productivity and in turn the high resource availability of the basin, especially in the northern part, resulted in favor of the resource partitioning that occurs in each sub-area of the Adriatic Sea among the three species. Medium-sized specimens of the three species mostly fed on small zooplankton, while adult sprats relied on large copepods and those of sardines and anchovies also consumed large portion of phytoplankton, confirming the high trophic plasticity of these two dominants small pelagic species. However, considering that anchovies have the greatest degree of trophic diversity compared with the other two species, they could be the most adapted to changing feeding conditions. The increase in sea temperatures that are reducing primary production and in turn zooplankton abundances, coupled with even more frequent extreme meteorologic events could exacerbate the competition for trophic resources among pelagic mesopredators, and could lead to more notable stocks’ fluctuations and unpredictable wasp-waist effects.
Similar content being viewed by others
Introduction
Small pelagic fishes represent most of the fish biomass in pelagic ecosystems but, despite this, their trophic level is usually dominated by only few species1. In the Mediterranean Sea, small pelagics are mostly represented by the European anchovy (Engraulis encrasicolus, Linnaeus, 1758), European sardine (Sardina pilchardus, Walbaum, 1792), European sprat (Sprattus sprattus, Linnaeus, 1758) and round sardinella (Sardinella aurita, Valenciennes, 1847).
This study is focused on sprats, anchovies, and sardines. Anchovy and sardine are widely distributed in the Eastern Atlantic Ocean and are common in the Mediterranean and Black Sea2, while sprat is mostly confined to the northeast Atlantic, and in the Mediterranean is concentrated in the Gulf of Lions, the Northern Adriatic, and the Black Sea2. In the Western Adriatic Sea, where this study was conducted, they share the same distribution area (Fig. 1A,B).
(A) Map of the sampling sites surveyed in 2019 for GSA 17 and GSA 18. Black lines represent transects for acoustic data sampling; orange dots are the hauls where small pelagic fish were collected. (B) Distribution maps of fish biomass (in tons, t) as derived by acoustic data from MEDIAS 2019 survey for Sprattus sprattus (on the left, black symbols), Sardina pilchardus (in the center, blue symbols), and Engraulis encrasicolus (on the right, green symbols). This figure was originally created with QGis version 3.28.8 (QGIS Development Team, 2023. QGIS Geographic Information System, Open Source Geospatial Foundation Project, http://qgis.osgeo.org.
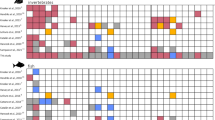
In the Mediterranean basin, catches of anchovies and sardines represent the 52.5% of total landings3. Sprats instead play a minor role, being important only for the Northern Adriatic and the Gulf of Lions2,4. Despite anchovies and sardines are managed by the General Fisheries Commission for the Mediterranean (GFCM) through multiannual plans in the Adriatic Sea, small pelagic fish stocks can experience strong fluctuations that could make it difficult to keep the fishing effort at sustainable levels. Indeed, as suggested by the most recent stock assessments5,6, both anchovies and sardines are currently overfished in the Adriatic Sea, with a more severe toll on sardines (Fig. 2A). On the other hand, sprat has very limited landings, and according to an experimental stock assessment for 20187 is quite underexploited (Fig. 2B and Table 1). The analysis of long-term data series8 showed an increase of anchovies in the Northwestern Adriatic Sea (Geographical Sub-Area, GSA 17, data from 1976 to 2019) and a quite stable trend in the Southern Adriatic Sea (GSA 18, data from 1987 to 2019), while both sardines and sprats decreased in the whole basin (Fig. 3). Opposite biomass contractions observed between anchovies and sardines in the Adriatic Sea in the last 40 years were attributed to regime shifts9.
Biomass data expressed as tons per nm2 for anchovy (green), sardine (blue) and sprat (black) in (A) GSA 17 West and (B) GSA18 West. Data were adapted from Leonori et al.8.
Small pelagics mainly feed on phytoplankton and micro/mesozooplankton10, resulting in high abundance especially in nutrient-rich upwelling regions10, where their significant abundance and success have been attributed to the flexibility of their feeding behavior11,12. A recent study conducted along the Adriatic Sea showed a higher mesozooplankton biomass and abundance in the Northern Adriatic, strongly influenced by Po River inputs, where13 the community is dominated by the calanoids Acartia clausi and Oithona similis, cladocerans (mostly Evadne spinifera), copepodites, and gastropod larvae. Conversely, the Central and the Southern Adriatic Sea are characterized by an “oceanic” community, with a higher abundance of typical offshore carnivorous zooplankton such as tunicates, chaetognaths, siphonophores and large copepods such as Euchaeta spp.13.
Small pelagics are planktivorous species, diurnal predators14 and with ontogenetic shift in the diet from copepod developmental stages (eggs, nauplii, meta-nauplii and copepodites) in fish larvae to small copepods in juveniles, while adults may switch from preying on larger copepods and other mesozooplankton, to filter-feeding (see Table S1). Grazing on phytoplankton has been rarely reported in anchovies, and it seems to occur only when mesozooplankton is limiting15. On the contrary, phytoplankton appears to be an important food item in sardines and sprats14,15,16, especially with increasing size14,17,18. Feeding ecology of anchovy and sardine has been already investigated in the Adriatic through visual identification of gut content. Some of these studies conducted in the eastern basin report a dietary overlap between small pelagic species18, while few SIA data from the same area point out to no overlap19. Due to their intermediate trophic level, small pelagic fishes can play a crucial role in many ecosystems, exerting a negative top-down control on plankton abundance, but also a positive bottom-up control on top predators. However, since small pelagic populations show extensive fluctuations under intensive exploitation, changes in productivity or climate changes, modifying the structure, and functioning of marine ecosystems, they often exert a less predictable ‘wasp-waist’ control20,21. Therefore, characterizing better food webs structure and niche overlap has become particularly important since the rise of ecosystem-based management, which aims to create a sustainable exploitation strategy that protects the ecosystem and the goods it provides.
Recently, most of the studies assessing food-web structure and function of pelagic communities, use stable isotope analyses (SIA)11,12, in addition to traditional stomach contents analyses or more advanced genetic tools such as metabarcoding11 or Compound Specific-SIA22. Stable isotope values increase with trophic level due to selective metabolic fractionation, that leads to a preferential loss of lighter isotopes during respiration (carbon) and excretion (nitrogen). Nitrogen δ15N and carbon δ13C are the most common isotopes measured in trophic ecology studies23. The increase of δ15N averages about 3‰ per trophic level, so it can be used to determine the trophic position of the consumers along the food web24,25. On the contrary, δ13C increases below 1‰ per trophic step, and can be used to track the origin of organic matter (pelagic vs. benthic, terrigenous vs. marine)24,25. Stable isotope analyses give a time-integrated picture of fish diet and allow to evaluate the relative contribution of various food sources in consumer’s diet, intraspecific trophic relationships as a response to ontogeny, neighboring-linked connectivity, migration, reproduction, and changes in environmental features26,27,28. However, SIA alone also fails to differentiate between two food sources with overlapping isotope values and among ecological niches of species that have the same isotopic niche29. This can be partially solved through Bayesian mixing models to estimate errors concerning turnover rates and isotope ratios of putative food sources30.
In this study, using SIA and literature data on the well-known diets of sardines and anchovies and new original data on stomach contents for sprats, we deepen our knowledge about the feeding ecology and the trophic niche overlap among these three species across the Western Adriatic Sea, the most productive and exploited basin within the Mediterranean31. Stomach content analysis is a well-known method to study the trophic ecology of fish and provides a snapshot of the diet32 but does not allow to identify high-digestible prey and to understand the real proportion of assimilated prey.
More precisely, in this study we aimed at (1) assessing changes in feeding habits of the three species from the northern to the southern Adriatic basin, also considering variations in the diet with increasing size; (2) analyzing resource partitioning and trophic niche overlap; and (3) relating these changes and patterns with resource availability and variations of environmental variables. Finally, as anchovies and sardines are widely distributed in both coastal and offshore waters, while sprats are confined to the neritic zone33, for the two former species we also tested the hypothesis of 4) trophic changes across an inshore-offshore gradient.
Results
Diet composition of Sprattus sprattus based on stomach content analysis
Fifty-eight specimens were individually analyzed to depict sprat’s diet in the area. This sample size was considered sufficient according to the cumulative curve analysis (Fig. S1), as the asymptote was reached at 22 stomachs (containing 18 different items). Sprats mostly fed on small copepods (Microcalanus sp., Calanus sp., and Acartia clausi) and cladocerans (Table S2a), and changes in the diet according to size were just below the level of significance (pseudo-F1,56 = 2.29, p = 0.04). Indeed, SIMPER test did not evidence differences in most typifying species according to size, being Microcalanus sp., Calanus sp., cladocerans and Acartia sp. the taxa that most contributed to sprats’ diet in both medium and large specimens (Table S2b). The stomach fullness (F) of sprats was greater for medium-sized specimens from both the Northern Adriatic (NA) and the Southern Adriatic (SA) (F = 1.90 ± 0.49 for NA and F = 2.18 ± 0.73 for SA, respectively) than for large specimens (F = 1.47 ± 0.39, only available from NA).
Stable isotopes composition of small pelagics
δ15N values of the 81 sprats analyzed varied from 8.4 to 11.3‰ (mean value 9.7 ± 0.6‰) and δ13C values from − 20.8 to − 18.1‰ (mean value − 19.8 ± 0.6‰). A total of 189 anchovies were analyzed, with δ15N values ranging from 6.6 to 12.0‰, (mean value of 9.1 ± 1.3‰) and δ13C from − 20.9 to − 18.1‰ (mean value = − 19.0 ± 0.5‰). For sardines, 138 specimens were analyzed, with δ15N values ranging from 6.3 to 10.9‰ (mean value 8.91 ± 0.9‰) and δ13C from − 22.4 to − 18.7‰ (mean value = − 19.9 ± 0.8‰) (Table 2).
The Pearson correlation of δ15N to fish total length (TL) is non-significant (p > 0.05) for sardines, and significant for sprats and anchovies (p < 0.001) (positive and negative correlations, respectively) (Fig. 4 and Table 3). δ13C was significantly (p < 0.001) and negatively correlated with TL in sprat, while correlations were positive and significant (p < 0.001) for sardine and anchovy (Fig. 4 and Table 3). Correlation values for each species at sub-area level are reported in Table S3. The correlation of δ15N vs. fish length was never significant for sprat. For sardines, correlation was negative and significant in CA and in the Southern Adriatic Sea (SA), and it was always negative and significant for anchovies in all sub-basins. δ13C–TL correlations were positive and significant in Northern Adriatic Sea (NA) for sardines, and positive and always significant for anchovies.
δ15N vs TL (Total Length) (left) and δ13C vs TL (right) scatterplots of (A) Sprattus sprattus, (B) Sardina pilchardus and (C) Engraulis encrasicolus, with the polynomial or linear relationship reported with fish length for anchovy, sardine and sprat in the NA (North Adriatic), CA (Central Adriatic) and SA (South Adriatic) sub areas.
To comply with the aims of the study, we carried out statistical analyses to identify the resource partitioning among specimens of different species and size. The isotopic composition of the three species varied significantly for the factors ‘species’, ‘size’, and their interactions according to the PERMANOVA main tests at both multivariate and univariate levels (Table S4a, design 1). Pairwise comparison on the interaction term for pairs of levels of factor species showed significant differences between all pairs considered for both medium and large specimens, with the only exceptions of the pairwise on δ15N values between medium-sized sprat and anchovy and on δ15N values between large-sized sardine and anchovy (Table S4b). Similarly, when examining differences of size within the same species, all combinations were significant, i.e., for all species the isotopic composition between medium and large-sized individuals varied significantly, at multivariate or univariate level, with the only exception of δ15N values in sprats and sardines (medium and large specimens) (Table S4c).
Still the isotopic composition of small pelagics varied among the three species in the different sub-basins (Table S4a, design 2). Within NA, significant variations were detected between all pairs of species, except for δ15N values of anchovy and sprat (Table S5b), and the δ13C values of sardine and sprat. In CA, significant variations were detected between anchovy and sardine, except for δ15N values. In SA, all pairs of comparisons were significant apart the δ15N values of anchovy and sardine and the δ13C values of anchovy and sprat.
Finally, to depict better the resource partitioning among anchovy and sardine across an inshore-offshore gradient, the PERMANOVA main test carried out considering a two-factor design (‘species’ and ‘inshore vs. offshore areas’) highlighted significant differences in the isotopic composition of anchovy and sardine collected in inshore vs. offshore areas both at univariate and multivariate levels, but not for the interaction of the term ‘species × inshore vs. offshore areas’ at univariate level considering δ13C contents (Table S6a, design 3). The two species varied significantly for the variable(s) considered in inshore areas (Table S6b), but not for δ15N in offshore areas. At species level the isotopic composition of both anchovy and sardine differed significantly between inshore and offshore (Table S6c).
Linking isotope composition with environmental variables and resource availability
Environmental variables drive resources availability for small pelagics. Different resources and environmental variables were found to drive small pelagics’ isotopic signals and thus, the assimilated proportion of food sources. In sprats, the dissolved oxygen concentration was the main explanatory variable, accounting for 20% of the variance (Table 4a). Other environmental variables such as turbidity and salinity added less to the explained variance (1–3%) and they were not significant. Similarly, in sardines, the diet was mainly driven by resource availability, i.e., omnivore zooplankton (both groups, those with preference for carnivory and those for herbivory) which together explained 16% of the total variance. However, also adding the other variables, both environmental (salinity and temperature) and biological (abundance of carnivore zooplankton) the total explained variance was only 22% (Table 4b), being in addition not significant. Finally, the anchovy diet seemed to be mainly controlled by environmental variables (mostly salinity and temperature, but also turbidity, fluorescence, and dissolved oxygen) which together accounted for 49% of the total variance (Table 4c).
Mixing models and niche width by standard ellipse areas corrected for small sample size (SEAC)
The Bayesian mixing model SIMMR provided the proportional contribution of each food source to the diet of the three species. In NA, the main contribution to the diet of S. sprattus was given by Acartia sp. and Decapoda larvae (53 and 41% of proportional contribute, respectively). In SA, the main assimilated source was the Particulate Organic Matter (hereafter, POM) (52%), followed by large Copepoda (22%). Minor contributions were given by phytoplankton (9%), Copepoda of the family Clauso-Paracalanidae (9%) and Pleuromamma abdominalis (8%) (Fig. 5).
Posterior probabilities for the proportional source contribution to Sprattus sprattus–Sardina pilchardus–Engraulis encrasicolus diets from SIMMR output for Northern Adriatic Sea, Central Adriatic Sea and Southern Adriatic Sea. Phyto1 and Phyto2 are the SI values of phytoplankton from Northern Adriatic36, POM_NA is the SI of the Particulate Organic Matter (POM) from the Northern Adriatic37. Macroaggregates are the stable isotope (SI) signatures of macroaggregates from the Northern Adriatic36.
POM gave the main contribution to the diet of sardines in NA (58%), followed by Decapoda larvae (37%). In CA, POM and macroaggregates from NA mostly contributed to the diet of sardines (45 and 42% of proportional contribution, respectively). Minor contribution was given by Brachyuran larvae (8%). In SA, POM gave 52% of the proportional contribution to the diet of sardine, followed by Decapoda larvae that contributed 34%. Minor contribution was given by Thaliacea (9%) (Fig. 5).
In NA, POM and macroaggregates gave the main contribution to the diet of anchovy (47% of the proportional contribute from both the sources). Macroaggregates and POM were the main sources in CA (49 and 40% of proportional contribution, respectively). In SA, macroaggregates contributed to the diet of anchovy for 44% while 34% of contribution derived from POM. Minor contributions were given by phytoplankton (9%), Thaliacea (7%) and P. abdominalis (6%) (Fig. 5).
Standard ellipses showed that sprat has the widest δ13C variability and anchovy the smallest one (Fig. 6). Anchovy has the widest δ15N variability. Additionally, Layman metrics indicated that sprat had the smallest SEAC (1.2‰2), stretched along the x-axis, while anchovy and sardine showed a similar SEAC (around 2‰2), being that of anchovy stretched along the y-axis (Fig. 6 and Table S7). In NA, anchovies show the widest SEAC. In CA and SA, sardines have thew widest SEAC (Table S7).
δ13C–δ15N scatterplot with total area (TA, broken-line areas) and standard ellipses corrected for small sample size population (SEAC, solid-line areas) of the three species (p interval = 0.4) in (A) the Adriatic Sea; (B) the North Adriatic Sea; (C) the Central Adriatic Sea and (D) the South Adriatic Sea.
Sprats showed the highest mean distance to the centroid (CD = 0.6 for sprat vs CD = 0.54 for anchovy and CD = 0.3 for sardine), which is a proxy of trophic diversity. Sprat and anchovy’s SEACs do not overlap, while a partial overlap is displayed between the SEACs of sardine and anchovy (0.32) and between sprat and sardine (0.23) (Fig. 6).
According to the Post equation for the trophic position (TP) estimation, the three small pelagics were all positioned at the third trophic level, with TPs ranging from 3.3 in E. encrasicolus from SA to 3.9 in S. sprattus from the same area. The food web of the Adriatic Sea seemed to be better represented by a continuum of trophic levels rather than discrete ones (see Fig. 7) with “ancillary” small pelagics29, located at the TP 4, followed by small tunnids34, and potentially preyed by large pelagic species such as the swordfish, Xiphias gladius (authors’ unpublished data), and the bottlenose dolphin, Tursiops truncatus, the apex predators of the pelagic food web of the Western Adriatic Sea35.
Scatterplot of isotopic data for zooplankton, sprat, sardine, anchovy, large pelagic fish and bottlenose dolphin. Data for large pelagics are from Sicilian waters and have been previously corrected with respect to a common baseline (i.e., comparing the stable isotope signature of herbivore-omnivore copepods from Rumolo et al.38 with our own data) and specifically, δ13C–δ15N values for Thunnus thynnus are from Sarà and Sarà34 and are related to specimens of 95–130 cm TL; data for Euthynnus alletteratus, Sarda sarda and Xiphias gladius are from Fanelli unpublished data and corresponds to specimens of the following dimensions: E. alletteratus 39.5–41 cm TL; S. sarda: 55–58 cm TL, and X. gladius: 200–203 cm Fork length. Data for Tursiops truncatus are from Fortibuoni et al.35. Tursiops truncatus was redrawn from https://oceanomaredelphis.org/bottlenose-dolphin/. All the fishes were redrawn from images available at: https://en.wikipedia.org/wiki/Atlantic_bluefin_tuna#/media/File:Bluefin-big.jpg; https://it.wikipedia.org/wiki/Engraulis#/media/File:Engraulis_encrasicolus_Gervais_flipped.jpg; https://species.wikimedia.org/wiki/Sardina_pilchardus#/media/File:Sardina_pilchardus_Gervais.jpg; https://it.wikipedia.org/wiki/Sprattus_sprattus#/media/File:Sprattus_sprattus_Gervais.jpg; https://en.wikipedia.org/wiki/Atlantic_bonito#/media/File:Sarda_sarda.jpg; https://it.wikipedia.org/wiki/Euthynnus_alletteratus#/media/File:Euall_u0.gif; https://it.wikipedia.org/wiki/Xiphias_gladius#/media/File:Xiphias_gladius1.jpg; https://arrankoba.com/wp-content/uploads/2022/06/trachurus_trachurus.jpg; https://www.colapisci.it/PescItalia/pisces/Carangiformes/Carangidae/maggiore/pic/Trachurus%20mediterraneus-fr.jpg; https://www.fisheries.noaa.gov/s3/2021-04/640x427-Atlantic-chub-mackerel-FNL_NB_W.jpg; https://it.wikipedia.org/wiki/Scomber#/media/File:Scomber_scombrus.png.
Discussion
Our study allowed to assess the resource partitioning and trophic niche overlap among sprat Sprattus sprattus, sardine Sardina pilchardus and anchovy Engraulis encrasicolus in different sub-areas of the Adriatic Sea and at different life stages. The results of the study emphasize the feeding plasticity of these pelagic species as previously observed for anchovy in the Adriatic Sea39, and in other areas of the Mediterranean such as the Gulf of Lions40 and the Sicily Strait16.
Our results allowed us to define the trophic habits of sprat in the western side of the Adriatic Sea. Here, sprats mainly feed on calanoid copepods of the genus Microcalanus and Calanus, two taxa that characterized more than half of the diet of this species in both the Northern and Southern Adriatic. However, it seems that these taxa are an important food source even if they are not the most abundant during the sampling period13, highlighting a specialist feeding behavior of sprats41,42,43,44. These SCA results are consistent with those of previous studies41. The species seemed to have a quite constant diet as demonstrated in other studies conducted in the Black Sea42, in the Gulf of Lions43 and in the Bay of Biscay44.
Concerning SIA data, sprats showed an increase of δ15N values with increasing total length, pointing out to an ontogenetic shift in the diet as observed with SCA for sardine and anchovy of Algerian waters and the Northern Adriatic39,45. Ontogenetic shifts in a species’ trophic level reduce intra-competition for feeding and probably allows larger individuals to sustain better energy consumption facing the spawning period. In the Adriatic Sea, sprats spawn between November and April41. Larger sprats caught during MEDIAS surveys (June–July) seemed to be specialized in capturing larger prey, probably recovering energies lost during reproductive period, while smaller specimens likely rely more on particulate organic matter or algal material due to a lesser filter-feeding capacity43.
Additionally, in the Adriatic Sea, ontogenetic changes in sprats’ diet likely occur thanks to the high availability of food (e.g., zooplankton abundance13), as also observed in another highly productive area, the North Chilean Patagonia, where a similar δ15N pattern was reported for Sprattus fuegensis46, suggesting that high primary productivity supports in turn high food availability and, thus, allows to larger and smaller sprat specimens to avoid intra-specific overlapping of trophic niches with a more selective feeding compared to that in areas with lower food availability46. On the other hand, the strong decrease in δ13C with increasing size suggests that in the northern Adriatic larger animals move more inshore, reaching their coastal spawning grounds41. The lower δ13C values of coastal areas in the Western Adriatic Sea is due to the freshwater inputs by the Po River discharge, as observed in other Mediterranean areas47.
Conversely, the δ15N trend of sardine and anchovy best fitted with a polynomial distribution, thus suggesting an ontogenetic shift in their trophic habits as already observed for anchovy in Northern Adriatic39 and for sardine in the Algerian Sea39,45, and in Galicia48. Here, this was interpreted as a dietary shift to phytoplankton consumption in larger fishes, thanks to the acquisition of filter feeding ability following the gill rakers development49. The consumption on a wide array of diatoms was recently revealed for species collected in the western Mediterranean through metabarcoding11. The increasing trend of δ13C with size in both species pointed out to an inshore-offshore displacement with growth43,50. Such offshore movements to more oligotrophic areas, with marine phytoplankton being the main carbon source, was already observed for sardines in the Gulf of Lions43. Although anchovy is generally considered to be more zoophagous than sardine14,50, our results are quite unexpected as large specimens of anchovy in the Adriatic Sea seemed to prefer to assume phytoplankton, a feeding behavior that could be validated in the future through metabarcoding11. This trend was observed in the co-generic Engraulis capensis in South Africa51, suggesting also for this species a shift from zooplankton to phytoplankton assumption according to resource availability38, but also to the acquisition of filter feeding ability. This preference for phytoplankton could be also supported by the declining trend in mesozooplankton observed since 2000 in the Northern and Central Adriatic (authors’ unpublished data).
Small pelagics have high turn-over rates and short life history52 and although any experimental study is available for these or similar species, we can assume the incorporation rate is at the scale of days/weeks and not months, as for larger and slow-growth species53. In this study, we used environmental and biological variables collected simultaneously to fish samples, thus introducing potential bias due to the isotopic incorporation rate of the species, i.e., the time required by an organism to acquire the isotopic composition of its new diet54, which can be highly variable depending on a species turn-over rate, environmental conditions, the physiological state of the animal, etc.55. The variables here used, although with some caveats, can mirror the situation of few weeks before, considering that the typical summer conditions have already been established during the time of the sampling (end of June–July)56.
The analyses conducted in our study showed that the δ15N and δ13C content of sprat was mainly dependent on water oxygen concentration, which in turn is one of the limiting factors for the survival and the growth of zooplankton, and one of the driving factors of zooplanktonic communities' composition throughout the Adriatic Sea13. The isotopic signals of sardines were mostly driven by resource availability rather than environmental variables, which were found instead determinant for larval fish survival57, and fish population dynamics58. Such results are in contrast with observations on sardines from the Bay of Biscay44,50 where no clear link was found between food resource availability and fish diets. In the Adriatic the temporal fluctuations in the abundance of sprats and sardines could be driven by food availability. Therefore, being their abundance dependent to variations in mesozooplankton communities, the monitoring of this last should be essential for the appropriate management of the two species. Indeed, sprats and sardines showed a declining trend in the last 40 years8 in agreement with the general decline in zooplankton abundance described above (Authors’ unpubl. data). On the other hand, in anchovy, the δ15N and δ13C content was mostly controlled by environmental variables such as salinity and temperature, and the species showed an increasing trend in the abundance in the last 40 years in the Northern-central Adriatic Sea8. Thus, for this species climatic factors control its abundance’s fluctuations, rather than its trophic ecology.
Even if for sardine and anchovy further studies are needed to couple SIA with other analyses (e.g., visual stomach content characterization, DNA metabarcoding etc.) to define better their diets, especially for the microscopic components, such as diatoms, the Bayesian mixing model SIMMR allowed us to determine the food sources that were mainly assimilated in the tissues of the three species. The obtained results highlighted that in the Northern Adriatic, sprat preferentially assimilates zooplanktonic items like Acartia sp., and Decapoda larvae belonging to higher trophic levels. In Southern Adriatic Sea, sprat prefers to assimilate macroaggregates of Particulate Organic Carbon (POC) and POM, but still feeds on copepods. POC and POM are easily assumed, so probably in the more oligotrophic Southern Adriatic, sprat does not invest too much energy in catching calanoid copepods. The species here is at the southernmost boundary of its distribution33, and moreover this is an ultra-oligotrophic area, so on one hand the species could rely preferentially on easy-to-find resources, investing less energy in an unsuitable area, and on the other hand here zooplankton abundance is lower. The same trend could be highlighted for sardines that in all the sub-areas mainly assimilate POM, followed by Decapoda larvae in the North and South Adriatic Sea and phytoplanktonic macroaggregates in the Central Adriatic. Anchovy seemed to mainly assume POM and macroaggregates in all the sub-basins. This species is usually more zoophagous than sardine, but our results demonstrate that in the Adriatic Sea, at least in early summer, anchovies mostly rely on phytoplankton like previously observed in other studies59. Data about δ15N and δ13C contents showed how these species share a similar trophic position, based on comparable δ15N values, with some degrees of separation in δ13C values, meaning that they minimize dietary overlap by recurring to different carbon sources43. Accordingly, the three species have similar niche’s width and SEAC, but very different δ15N and δ13C ranges, confirming that anchovy is the species with the widest δ15N range and sprat that with the greatest diversity of basal resources. Through this differentiation of δ13C values of basal resources, trophic niches of the three species in the Adriatic Sea do not overlap. A higher diet overlap was instead observed in the Gulf of Lions43, and in the Spanish Mediterranean for anchovy, sardine and round sardinella60. The high productivity of the Adriatic basin likely determines a good resource partitioning, as enough food is available for the species to achieve their optimum fitness44. As suggested for the Gulf of Lions, the increase of sea surface temperature in the Adriatic basin61 and the whole Mediterranean Sea62 is expected to drive changes in distribution and increase in the competition for food among small pelagics63.
Furthermore, the combined scatterplot of δ15N and δ13C values of small pelagic fishes with those of zooplankton, large pelagic fishes, and dolphins (i.e., Tursiops truncatus) allowed to stress the central role of anchovies, sardines, and sprats in pelagic ecosystems, being located between zooplankton and larger predators. However, this central role seems to be shared with the so called “ancillary” small pelagics such as Trachurus spp. and Scomber spp.29. Such food web structure could drastically change in a climate change scenario with persistent high level of fishing pressures64. Small pelagics are indeed sensitive to environmental fluctuations63, and this could be amplified by extreme meteorological events, with cascading effects up and down the food web65. Further, the northward expansion of temperate species observed in different areas of the Mediterranean Sea, such as round sardinella33 that is able to effectively adapt its feeding activity switching to filter feeding when its preferred food source (i.e., the krill) is scarce, or obtaining their ingested biomass from other large prey like jellyfish and siphonophores as they increase in abundance60, may lead to stronger inter-specific competition.
Since sardine and sprat showed a decreasing trend in their abundances across the last 40 years, which could be influenced also by resource availability, a regular monitoring of their resources (i.e., mesozooplankton) should have been done consistently for a correct management of such resources within the ecosystem approach to fisheries. Based on our results, the species with the most adaptative diet in the Adriatic Sea seems to be anchovy that, in all the sub-basins, are likely able to feed on prey belonging to a wider range of trophic levels, thus allowing to encompass diets shifts due to changes in zooplankton community composition caused by climate changes.
Materials and methods
Ethical statement
Ethical review and approval were waived for this study, due to fish specimens’ collection being authorized by the MEDIAS project as part of annual research surveys under the EU Fisheries Data Collection Framework (EC 665/2008), all involving lethal sampling. The procedures we used did not include animal experimentation. The care and use of collected animals complied with animal welfare guidelines, laws and regulations set by the Italian Government.
We confirm the study is reported in accordance with ARRIVE guidelines.
Study area
The Adriatic Sea is an elongated semi-enclosed basin, with its major axis in the northwest–southeast direction, located in the central Mediterranean, between the Italian peninsula and the Balkans (Fig. 1A). It is 800 km long and 150–200 km wide. The North Adriatic is very shallow, with an average bottom depth of 35 m and a maximum depth of 70 m. The Middle Adriatic has a maximum depth of 100 m, except for the Jabuka-Pomo Pit (maximum depth 280 m). In these two areas the eastern part has deeper bottoms, with high and rocky shores, while the western part is shallower, with low sandy shores56,66. Despite the differences, these areas share many bioecological features, and for stock assessment purpose they were grouped as GSA 17, according to the GFCM. The southern part of the Adriatic Sea, GSA 18, is deeper with a wide depression of 1200 m deep. Here, the Otranto channel, which is 800 m deep, acts as an exchange area for water masses with the Mediterranean Sea56,66.
Despite being only the 5% of the total Mediterranean surface area, the Adriatic Sea produces about 15% of total Mediterranean landings (and 53–54% of Italian landings), with a fish production density of 1.5 t/km2, three times the Mediterranean estimate66. Three main factors are responsible of such impressive productivity feature: river runoff, shallow depths, and oceanographic structure. By providing nutrients, rivers inputs favor phytoplanktonic blooms, thus causing a bottom-up effect through the whole food web. The wide extension of the continental shelf, together with high environmental variability, favors a short trophic chain67, that improves the efficiency of energy transfer from lower trophic levels to higher ones68. Moreover, the structure of the basin allows water to be mixed during winter, especially in the northern and central Adriatic, transferring nutrients from sediments to the water column. However, the same condition can be responsible of water stratification, harmful algal blooms, mucilage, dystrophy and anoxia phenomena during summer66.
Sampling strategy
Samples were collected on board R/V “G. Dallaporta” in June–July 2019, during the acoustic survey MEDIAS 2019, in the Adriatic Sea69, within the framework of the EU MEDIAS (Mediterranean International Acoustic Surveys) action that coordinates the acoustic surveys performed in the Mediterranean to assess the biomass and spatial distribution of small pelagics in the target areas8. According to the MEDIAS protocol70 annual hydroacoustic surveys are conducted from June to September. Summer coincides with the peak reproductive period of anchovy and the peak recruitment period of sardine2,15.
Simultaneously, pelagic fishes were collected through a pelagic trawl (10 m vertical opening and 12 m horizontal opening, with mesh size 18 mm), equipped with a wireless SIMRAD ITI system that allowed to gather information on the correct opening of the net and on entering fishes during trawling. Hauls of ca. 30 min were performed during both daytime and nighttime, covering as evenly as possible the target area, both inshore and offshore, also considering acoustic data on fish aggregation position in the water column8.
Once onboard, fish were sorted, counted, measured (total length—TL—in cm) and weighted (wet weight in g). Since sardines, anchovy and sprats were the main target, 10 individuals per length class (0.5 cm) of each species were frozen at − 20 °C for further laboratory analysis. During the survey, also zooplankton sampling was performed along the acoustic transects, using a 200 µm mesh-size WP2 net, with a circular mouth of 57 cm diameter and 2.6 m long and equipped with a flowmeter. The net was towed vertically, with a towing speed of 1 m/s, starting from 3 m above the bottom till the surface. All samples were sorted, and specimens analyzed to the lowest taxonomic level possible, and the wet weight was calculated. All data were then standardized to the filtered volume of water recorded for each haul. More abundant/representative groups, as resulted after sorting and identifying each sample, were analyzed for determining stable isotopes contents13.
After the survey was completed, a set of fishing hauls was selected to be used for this study. The whole Western Adriatic (GSA 17 and GSA 18) has been divided into three different sub-areas13, mainly based on oceanographic characteristics: (1) the Northern Adriatic (NA) encompassing the northern part of the GSA 17 characterized by shallow waters, including the Po river mouth, up to the Conero Promontory (hauls 8–24 in Fig. 1A); (2) the Central Adriatic (CA) encompassing the lower part of the GSA 17 up to the Gargano Promontory (hauls 27–39) and (3) the Southern Adriatic (SA) including the whole GSA 18, characterized by the presence of the South Adriatic Pit and the Otranto Channel (hauls 40–46).
In selected hauls, for each species, three individuals per 0.5 bin were chosen for SIA, according to length-frequency distributions (Fig. S2).
Stomach contents analysis of Sprattus sprattus
As information on S. sprattus diet from literature was only available for the Eastern Adriatic Sea41, to integrate data on SIA for mixing models (see below), the SCA on 58 specimens was carried out. Specimens were thus dissected, and stomach contents identified to the lowest possible taxonomic level. To assess sample size adequacy, the cumulative number of analyzed stomachs was plotted against the mean cumulative number of the different prey species71. This was done by using the “species-accumulation” plot in PRIMER6&PERMANOVA+, under 9999 permutations and using bootstrapping. Sample size was considered sufficient when the cumulative prey species curve reached an asymptote, with further changes in the cumulative number of prey items observed < 0.1. The fullness index, as a proxy of feeding intensity, was measured as the ratio of stomach content weight to body weight28,32,39.
A PERMANOVA test (Permutational Multivariate Analysis of Variance)72 was run only on factor size (fixed, two levels, juveniles and adults) on the Bray–Curtis resemblance matrix of 4th root-transformed biomass (prey weight) data, given the low number of specimens analyzed from the SA. A SIMPER test was also run to identify the most typifying species of the diet of sprat for each size class. All statistical analyses were run using the software PERMANOVA+ for PRIMER72,73.
Samples preparation for stable isotope analyses
For fishes, a small sample of white muscle close to the dorsal fin, from selected specimens (81 sprats ranging from 7 to 13 cm TL, 189 anchovies from 4 to 15.5 cm TL, 138 sardines from 7.5 to 16 cm TL), was oven-dried for 24 h at 60 °C, weighted, between 0.5 and 1.3 mg, and placed into tin capsules, that were then put in a numbered rack. Samples were analyzed through an elemental analyzer (Thermo Flash EA 1112) for the determination of total carbon and nitrogen, and then analyzed for δ13C and δ15N in a continuous-flow isotope-ratio mass spectrometer (Thermo Delta Plus XP) at the Laboratory of Stable Isotopes Ecology of the University of Palermo (Italy). Stable isotope ratio was expressed, in relation to international standards (atmospheric N2 and PeeDee Belemnite for δ15N and δ13C, respectively), as:
where R = 13C/12C or 15N/14N. Analytical precision based on standard deviations of internal standards (International Atomic Energy Agency IAEA-CH-6; IAEA-NO-3; IAEA-N-2) ranged from 0.10 to 0.19‰ for δ13C and 0.02 to 0.08‰ for δ15N.
Since lipids can alter the values of δ13C72, samples with high lipid concentration can be defatted to avoid 13C depletion. However, lipid extraction can alter δ15N values and thus complicating sample preparation and reducing samples availability, a crucial point when analyzing small animals. For these reasons, δ13C of samples rich in lipids was normalized according to Post equation74 for sardines, anchovies and zooplankton, and to Kiljunen equation75 for sprats. C/N ratio was used as a proxy of lipid content, because their values are strongly related in animals74. In particular, the normalization was applied to samples with a C/N ratio > 3, according to74. Post’s equation was widely used and allowed us to compare our data with other similar studies12.
SIA data analyses
Intra-population variation in the feeding habits explained by body size is a frequent determinant of fish trophodynamics76. δ15N and δ13C relationships with size (as total length, TL) for the three species, were explored using Pearson correlation, at basin scale and also at sub-area level.
After analyzing the length frequency distribution for each species (Fig. S2), all specimens were assigned to two main size classes, i.e., medium, and large. For S. sprattus and E. encrasicolus medium-sized individuals were those smaller than 9.5 cm (Total Length, TL), while for S. pilchardus the threshold between medium and large was 10.5 cm TL.
According to the objectives, three different experimental designs were used for testing: (i) differences in the isotopic composition of the three species according to the size class (medium vs. large), named design 1; (ii). resource partitioning among the three species across the different sub-areas (design 2) and iii. resource partitioning among the anchovy and sardine across an inshore-offshore gradient (design 3), as for mesozooplankton in13 (Table 5). This approach was selected because it allowed pairwise comparisons among fixed factors, otherwise impossible with nested designs, due to the unbalanced distribution of the species, i.e., sprat only occurring in NA and SA, and in SA only medium-sized specimens were collected, similarly medium-size sardine only occurred in NA and only inshore at SA.
Differences were tested by means of PERMANOVA72 tests on the Euclidean resemblance matrix of untransformed univariate (δ15N or δ13C, separately) and bivariate (δ15N–δ13C) matrices.
Correlation with biological (zooplankton abundance) and environmental data
To identify the biological (i.e., resource availability) and environmental drivers of the trophic ecology, as by SIA, of the small pelagics in the Adriatic basin, stable isotope data of the three species separately were correlated to zooplankton abundance (i.e., number of taxa recorded at each haul, as detailed in Section 2.2) by trophic group, as by13, and to environmental variables, as described below. Environmental data, obtained by CTD casts carried out close to the sampling hauls13, were pressure (dbar, decibar), temperature (°C), fluorescence (µg/l), turbidity (NTU), dissolved oxygen (expressed as ml/l and saturation percentage), salinity and density (kg/m3). Biological and environmental data were first tested for collinearity among variables by using a Draftsman plot, with fluorescence, Dissolved O2 concentration (DO, ml/l), % of O2 saturation and turbidity data being Log (x + 1)-transformed to fit a linear distribution in the Draftsman plot. Finally, a DistLM (Distance based linear models72) was run with temperature, fluorescence, turbidity, dissolved oxygen and salinity as environmental variables, and the abundances of omnivore-carnivore, omnivore-herbivore and carnivore zooplankton as resource availability, using “step-wise” as selection procedure and “AIC (Akaike Information Criterion)” as selection criterion.
Bayesian mixing models and trophic level estimates
A Bayesian model with “SIMMR” package (Stable Isotope Mixing Models in R)77 was run to estimate the potential food sources for the three species separately under the software R 4.0.578. Before running the model, the isotopic values of the sources and the three fishes were plotted, applying the correct trophic enrichment factors (TEFs) to potential sources to build the mixing polygons79. As TEFs, we used a value of 1.3 ± 0.1‰ for δ13C80 and 3.3 ± 0.2‰ for δ15N81. According to80, the first value was the best estimate of δ13C for consumers analyzed as muscle tissue, while the second is the δ15N specifically estimated for zooplanktivorous species, based on a scaled framework approach81. The sources used in the mixing model were selected among those highlighted as dominant in literature (for sardine and anchovy, see Suppl. Table S1) and SCA results (for sprat, see Table S2), and that allowed to construct the best mixing plot79. Stable isotope (SI) signatures of the sources used to construct the best mixing polygon were taken from literature (Table S8). We used POM, macroaggregates and phytoplankton isotopic values from the Northern Adriatic Sea to run the mixing models in the three basins, also in the Central and Southern Adriatic, as other values of POM available from literature82 did not allow to close the mixing polygons. Furthermore, the use of POM_NA is justifiable because cascading phenomena of dense shelf waters from the Northern Adriatic occur periodically in the area and are found to affect zooplanktonic communities in the Southern basin83. The SI signatures of zooplanktonic species were taken from the dataset of a previous study13. Macroaggregates are the SI signatures of macroaggregates from the Northern Adriatic36, POM_NA is the SI of POM (Particulate Organic Matter) from the Northern Adriatic37, Phyto1 and Phyto2 are the SI values of phytoplankton from the Northern Adriatic36. Consistently with the best mixing polygons determined for the three species of fishes collected in the different sub-areas (Fig. S3), we used the selected sources to run SIMMR models, specifically, for each of the three sub-areas.
The SIBER package (Stable Isotope Bayesian Ellipses in R 3.5.3)84 was then used to calculate TA and SEAC (respectively, Total Convex Hull Area and Standard Ellipse Areas corrected for small sample size)85 and standard ellipse areas (p interval = 0.40 to encompass the 40% of our data) for the three species. Moreover, δ15N range (NR), δ13C range (CR), and the mean distance to centroid (CD), that is considered a proxy for estimating trophic diversity, were calculated for all the species84,85. The overlap of the SEAC of the three different species was calculated through the function “maxLikOverlap” included in SIBER package.
Additionally, the trophic level of the three species in the different sub-areas was estimated according to24 as: ((δ15Ni − δ15NPC)/TEF) + λ.
where δ15Ni is the δ15N value of the taxon considered, δ15NPC is the δ15N values of a primary consumer, i.e., an herbivore or a filter feeder, used as baseline of the food web, TEF is the trophic enrichment factor which is considered varying between 2.5486 and 3.424, and here is assumed to be 3.3 as above81, and λ is the trophic position of the baseline, which is 2 in our case. Here, we used three different values as baselines for the food web of the three sub-areas, specifically the average values of FF-HERB taxa for each sub-area, as from Fanelli et al.13.
Finally, the pelagic food web of the Adriatic Sea was depicted by plotting δ13C and δ15N mean values of primary producers, mesozooplankton, small pelagics and large pelagic fishes, from our own published13 and unpublished data, and from literature35,36,37,82.
Data availability
Data can be requested from the corresponding author upon reasonable request.
References
FAO-GFCM. The State of Mediterranean and Black Sea Fisheries 2018 172 (FAO-GFCM, 2018).
Palomera, I. et al. Small pelagic fish in the NW Mediterranean Sea: An ecological review. Prog. Oceanogr. 74, 377–396. https://doi.org/10.1016/j.pocean.2007.04.012 (2007).
FAO. The State of Mediterranean and Black Sea Fisheries 2020 (General Fisheries Commission for the Mediterranean, 2020). https://doi.org/10.4060/cb2429en.
Grbec, B., Dulcic, J. & Morovic, M. Long-term changes in landings of small pelagic fish in the eastern Adriatic-possible influence of climate oscillations over the Northern Hemisphere. Climate Res. 20, 241–252. https://doi.org/10.3354/cr020241 (2002).
Angelini, S. et al. Stock Assessment Form Small Pelagics—Anchovy—GSA 17 & 18. https://www.fao.org/gfcm/data/safs/en. (2021).
Cikes-Kec, V. et al. Assessment Form Small Pelagics—Sardine—GSA 17 & 18. https://www.fao.org/gfcm/data/safs/en. (2021).
Angelini, S. et al. Understanding the dynamics of ancillary pelagic species in the Adriatic Sea. Front. Mar. Sci. https://doi.org/10.3389/fmars.2021.728948 (2021).
Leonori, I. et al. History of hydroacoustic surveys of small pelagic fish species in the European Mediterranean Sea. Mediterranean Mar. Sci. https://doi.org/10.12681/mms.26001 (2021).
Grbec, B. et al. Climate regime shifts and multi-decadal variability of the Adriatic Sea pelagic ecosystem. Acta Adriat. 56, 47–66 (2015).
Fréon, P., Cury, P., Shannon, L. & Roy, C. Sustainable exploitation of small pelagic fish stocks challenged by environmental and ecosystem changes: A review. Bull. Mar. Sci. 76, 385–462 (2005).
Bachiller, E. et al. A trophic latitudinal gradient revealed in anchovy and sardine from the Western Mediterranean Sea using a multi-proxy approach. Sci. Rep. 10, 17598. https://doi.org/10.1038/s41598-020-74602-y (2020).
Albo-Puigserver, M., Navarro, J., Coll, M., Layman, C. A. & Palomera, I. Trophic structure of pelagic species in the northwestern Mediterranean Sea. J. Sea Res. 117, 27–35. https://doi.org/10.1016/j.seares.2016.09.003 (2016).
Fanelli, E., Menicucci, S., Malavolti, S., De Felice, A. & Leonori, I. Mesoscale variations in the assemblage structure and trophodynamics of mesozooplankton communities of the Adriatic basin (Mediterranean Sea). Biogeosciences https://doi.org/10.5194/bg-2021-240 (2022).
Borme, D. Ecologia Trofica dell’acciuga, Engraulis encrasicolus, in Adriatico settentrionale (Università degli Studi di Trieste, 2006).
Morello, E. B. & Arneri, E. Anchovy and sardine in the Adriatic Sea: An ecological review. Oceanogr. Mar. Biol. Annu. Rev. 47, 209–256 (2009).
Rumolo, P. et al. Trophic relationships between anchovy (Engraulis encrasicolus) and zooplankton in the Strait of Sicily (Central Mediterranean sea): A stable isotope approach. Hydrobiologia 821, 41–56. https://doi.org/10.1007/s10750-017-3334-9 (2018).
Borme, D., Tirelli, V. & Palomera, I. Feeding habits of European pilchard late larvae in a nursery area in the Adriatic Sea. J. Sea Res. 78, 8–17. https://doi.org/10.1016/j.seares.2012.12.010 (2013).
Hure, M. & Mustać, B. Feeding ecology of Sardina pilchardus considering co-occurring small pelagic fish in the eastern Adriatic Sea. Mar. Biodivers. https://doi.org/10.1007/s12526-020-01067-7 (2020).
Zorica, B. et al. Diet Composition and isotopic analysis of nine important fisheries resources in the Eastern Adriatic Sea (Mediterranean). Front. Mar. Sci. https://doi.org/10.3389/fmars.2021.609432 (2021).
Cury, P. Small pelagics in upwelling systems: patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 57, 603–618. https://doi.org/10.1006/jmsc.2000.0712 (2000).
Coll, M., Santojanni, A., Palomera, I. & Arneri, E. Food-web changes in the Adriatic Sea over the last three decades. Mar. Ecol. Prog. Ser. 381, 17–37. https://doi.org/10.3354/meps07944 (2009).
Giménez, J. et al. Trophic position variability of European sardine by compound-specific stable isotope analyses. Can. J. Fish. Aquat. Sci. 80, 761–770. https://doi.org/10.1139/cjfas-2022-0192 (2023).
Bearhop, S., Adams, C. E., Waldron, S., Fueller, R. A. & MacLeod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 73, 1007–1012. https://doi.org/10.1111/j.0021-8790.2004.00861.x (2004).
Post, D. Using stable isotopes to estimate trophic position: Models, methods and assumptions. Ecology 83, 703–718 (2002).
Caut, S., Angulo, E. & Courchamp, F. Variation in discrimination factors (Δ15N and Δ13C): the effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 46, 443–453. https://doi.org/10.1111/j.1365-2664.2009.01620.x (2009).
Fanelli, E. et al. Seasonal trophic ecology and diet shift in the common sole Solea solea in the Central Adriatic Sea. Animals https://doi.org/10.3390/ani12233369 (2022).
Fanelli, E., Cartes, J. E., Papiol, V., López-Pérez, C. & Carrassón, M. Long-term decline in the trophic level of megafauna in the deep Mediterranean Sea: A stable isotopes approach. Clim. Res. 67, 191–207. https://doi.org/10.3354/cr01369 (2016).
Fanelli, E., Badalamenti, F., D’Anna, G., Pipitone, C. & Romano, C. Trophodynamic effects of trawling on the feeding ecology of pandora, Pagellus erythrinus, off the northern Sicily coast (Mediterranean Sea). Mar. Freshw. Res. 61, 408–417 (2010).
Da Ros, Z. et al. Resource partitioning among “ancillary” pelagic fishes (Scomber spp., Trachurus spp.) in the Adriatic Sea. Biology https://doi.org/10.3390/biology12020272 (2023).
da Silveira, E. L. et al. Methods for trophic ecology assessment in fishes: A critical review of stomach analyses. Rev. Fish. Sci. Aquacult. 28, 71–106. https://doi.org/10.1080/23308249.2019.1678013 (2020).
Micheli, F. et al. Cumulative human impacts on Mediterranean and Black Sea marine ecosystems: Assessing current pressures and opportunities. PLoS ONE 8, e79889. https://doi.org/10.1371/journal.pone.0079889 (2013).
Hyslop, E. Stomach contents analysis: A review of methods and their application. J. Fish Biol. 17, 411–429. https://doi.org/10.1111/j.1095-8649.1980.tb02775.x (1980).
De Felice, A. et al. Environmental drivers influencing the abundance of round sardinella (Sardinella aurita) and European sprat (Sprattus sprattus) in different areas of the Mediterranean Sea. Mediterranean Mar. Sci. https://doi.org/10.12681/mms25933 (2021).
Sarà, G. & Sarà, R. Feeding habits and trophic levels of bluefin tuna Thunnus thynnus of different size classes in the Mediterranean Sea. J. Appl. Ichthyol. 23, 122–127. https://doi.org/10.1111/j.1439-0426.2006.00829.x (2007).
Fortibuoni, T. et al. Evidence of butyltin biomagnification along the Northern Adriatic food-web (Mediterranean Sea) elucidated by stable isotope ratios. Environ. Sci. Technol. 47, 3370–3377. https://doi.org/10.1021/es304875b (2013).
Faganeli, J. et al. Carbon and nitrogen isotope composition of particulate organic matter in relation to mucilage formation in the northern Adriatic Sea. Mar. Chem. 114, 102–109. https://doi.org/10.1016/j.marchem.2009.04.005 (2009).
Berto, D. et al. Stable carbon and nitrogen isotope ratios as tools to evaluate the nature of particulate organic matter in the Venice lagoon. Estuar. Coast. Shelf Sci. 135, 66–76. https://doi.org/10.1016/j.ecss.2013.06.021 (2013).
Rumolo, P. et al. Spatial variations in feeding habits and trophic levels of two small pelagic fish species in the central Mediterranean Sea. Mar. Environ. Res. 115, 65–77. https://doi.org/10.1016/j.marenvres.2016.02.004 (2016).
Borme, D., Tirelli, V., Brandt, S. B., Fonda Umani, S. & Arneri, E. Diet of Engraulis encrasicolus in the northern Adriatic Sea (Mediterranean): Ontogenetic changes and feeding selectivity. Mar. Ecol. Progress Ser. 392, 193–209. https://doi.org/10.3354/meps08214 (2009).
Costalago, D., Garrido, S. & Palomera, I. Comparison of the feeding apparatus and diet of European sardines Sardina pilchardus of Atlantic and Mediterranean waters: Ecological implications. J. Fish Biol. 86, 1348–1362. https://doi.org/10.1111/jfb.12645 (2015).
Tičina, V., Vidjak, O. & Kačič, I. Feeding of adult sprat, Sprattus sprattus, during spawning season in the Adriatic Sea. Ital. J. Zool. 67, 307–311. https://doi.org/10.1080/11250000009356329 (2000).
Oven, L. S., Shevchenko, N. F. & Giragosov, V. E. Size-age composition, feeding, and reproduction of Sprattus sprattus phalericus (Clupeidae) in different sites of the Black Sea. J. Ichthyol. 37, 769–778 (1997).
Le Bourg, B. et al. Trophic niche overlap of sprat and commercial small pelagic teleosts in the Gulf of Lions (NW Mediterranean Sea). J. Sea Res. 103, 138–146. https://doi.org/10.1016/j.seares.2015.06.011 (2015).
Bachiller, E. & Irigoien, X. Trophodynamics and diet overlap of small pelagic fish species in the Bay of Biscay. Mar. Ecol. Prog. Ser. 534, 179–198. https://doi.org/10.3354/meps11375 (2015).
Bacha, M. & Amara, R. Spatial, temporal and ontogenetic variation in diet of anchovy (Engraulis encrasicolus) on the Algerian coast (SW Mediterranean). Estuar. Coast. Shelf Sci. 85, 257–264. https://doi.org/10.1016/j.ecss.2009.08.009 (2009).
Montecinos, S., Castro, L. R. & Neira, S. Stable isotope (δ13C and δ15N) and trophic position of Patagonian sprat (Sprattus fuegensis) from the Northern Chilean Patagonia. Fish. Res. 179, 139–147. https://doi.org/10.1016/j.fishres.2016.02.014 (2016).
Darnaude, A. M., Salen-Picard, C., Polunin, N. V. & Harmelin-Vivien, M. L. Trophodynamic linkage between river runoff and coastal fishery yield elucidated by stable isotope data in the Gulf of Lions (NW Mediterranean). Oecologia 138, 325–332. https://doi.org/10.1007/s00442-003-1457-3 (2004).
Bode, A., Carrera, P. & Lens, S. The pelagic foodweb in the upwelling ecosystem of Galicia (NW Spain) during spring: natural abundance of stable carbon and nitrogen isotopes. ICES J. Mar. Sci. 60, 11–22. https://doi.org/10.1006/jmsc.2002.1326 (2003).
Costalago, D. & Palomera, I. Feeding of European pilchard (Sardina pilchardus) in the northwestern Mediterranean: From late larvae to adults. Sci. Mar. 78, 41–54. https://doi.org/10.3989/scimar.03898.06D (2014).
Chouvelon, T. et al. Trophic ecology of European sardine Sardina pilchardus and European anchovy Engraulis encrasicolus in the Bay of Biscay (north-east Atlantic) inferred from δ13C and δ15N values of fish and identified mesozooplanktonic organisms. J. Sea Res. 85, 277–291. https://doi.org/10.1016/j.seares.2013.05.011 (2014).
King, D. P. F. & Macleod, P. Comparison of the food and the filtering mechanism of Pilchard sardinops ocellata and anchovy Engraulis capensis of South West Africa, 1971–1972. (1976).
Albo-Puigserver, M. et al. Changes in life history traits of small pelagic fish in the Western Mediterranean Sea. Front. Mar. Sci. https://doi.org/10.3389/fmars.2021.570354 (2021).
Rodde, C. et al. Variations in isotope incorporation rates and trophic discrimination factors of carbon and nitrogen stable isotopes in scales from three European sea bass (Dicentrarchus labrax) populations. J. Exp. Mar. Biol. Ecol. https://doi.org/10.1016/j.jembe.2020.151468 (2020).
Martínez del Rio, C. & Carleton, S. A. How fast and how faithful: The dynamics of isotopic incorporation into animal tissues. J. Mammal. 93, 353–359. https://doi.org/10.1644/11-mamm-s-165.1 (2012).
Carter, W. A., Bauchinger, U. & McWilliams, S. R. The importance of isotopic turnover for understanding key aspects of animal ecology and nutrition. Diversity https://doi.org/10.3390/d11050084 (2019).
Artegiani, A. et al. The Adriatic sea general circulation. Part I: Air–sea interactions and water mass structure. J. Phys. Oceanogr. 27, 1492–1514 (1996).
Costalago, D. et al. Ecological understanding for fishery management: Condition and growth of anchovy late larvae during different seasons in the Northwestern Mediterranean. Estuar. Coast. Shelf Sci. 93, 350–358. https://doi.org/10.1016/j.ecss.2011.05.005 (2011).
Beaugrand, G. & Reid, P. C. Long-term changes in phytoplankton, zooplankton and salmon related to climate. Glob. Change Biol. 9, 801–817. https://doi.org/10.1046/j.1365-2486.2003.00632.x (2003).
Bulgakova, Y. V. Diurnal dynamics of feeding in anchovy, Engraulis encrasicolus, and factors determining it. J. Ichthyol. 33, 395–400 (1993).
Bachiller, E. et al. Trophic niche overlap between round sardinella (Sardinella aurita) and sympatric pelagic fish species in the Western Mediterranean. Ecol. Evol. 11, 16126–16142. https://doi.org/10.1002/ece3.8293 (2021).
Bonacci, O., Patekar, M., Pola, M. & Roje-Bonacci, T. Analyses of climate variations at four meteorological stations on remote islands in the Croatian part of the Adriatic Sea. Atmosphere https://doi.org/10.3390/atmos11101044 (2020).
Pisano, A. et al. New Evidence of Mediterranean climate change and variability from sea surface temperature observations. Remote Sens. https://doi.org/10.3390/rs12010132 (2020).
Ramirez, F. et al. SOS small pelagics: A safe operating space for small pelagic fish in the western Mediterranean Sea. Sci. Total Environ. 756, 144002. https://doi.org/10.1016/j.scitotenv.2020.144002 (2021).
Pennino, M. G. et al. Current and future influence of environmental factors on small pelagic fish distributions in the Northwestern Mediterranean Sea. Front. Mar. Sci. https://doi.org/10.3389/fmars.2020.00622 (2020).
Fauchald, P., Skov, H., Skern-Mauritzen, M., Johns, D. & Tveraa, T. Wasp-waist interactions in the North Sea ecosystem. PLoS ONE 6, e22729. https://doi.org/10.1371/journal.pone.0022729 (2011).
Marini, M., Bombace, G. & Iacobone, G. Il Mare Adriatico e le sue Risorse (Palermo, 2017).
Pimm, S. L. Food Webs 1–11 (Springer, 1982).
Vander Zanden, M. J. & Fetzer, W. W. Global patterns of aquatic food chain length. Oikos 116, 1378–1388 (2007).
Leonori, I. et al. Piano di Lavoro Nazionale Raccolta Dati Alieutici 2017–2019 EC-DCR—MIPAAF Anno 2019. Sezione Campagne di ricerca in mare Moduli MEDIAS GSA 17 e GSA 18—Relazione Tecnica. (CNR—IRBIM, 2020).
MEDIAS. Common Protocol for the MEDiterranean International Acoustic Survey (MEDIAS), MEDIAS Handbook. http://www.medias-project.eu/medias/website. (2019).
Ferry, L. & Cailliet, G. Sample size and data analysis: Are we characterizing and comparing diet properly. Feed. Ecol. Nutr. Fish 1, 71–80 (1996).
Anderson, M., Gorley, R. N. & Clarke, K. PERMANOVA+ for Primer: Guide to Software and Statistical Methods. (2008).
Clarke, K. R. & Gorley, R. N. PRIMER V6: User Manual/Tutorial. (PRIMER-E, 2006).
Post, D. M. et al. Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152, 179–189. https://doi.org/10.1007/s00442-006-0630-x (2007).
Kiljunen, M. et al. A revised model for lipid-normalizing δ13C values from aquatic organisms, with implications for isotope mixing models. J. Appl. Ecol. 43, 1213–1222. https://doi.org/10.1111/j.1365-2664.2006.01224.x (2006).
Galván, D. E., Sweeting, C. J. & Reid, W. D. K. Power of stable isotope techniques to detect size-based feeding in marine fishes. Mar. Ecol. Prog. Ser. 407, 271–278. https://doi.org/10.3354/meps08528 (2010).
Parnell, A. C. simmr: A Stable Isotope Mixing Model. R Package Version 0.4.5. https://CRAN.R-project.org/package=simmr (2021).
R Development Core Team. R: A Language and Environment for Statistical Computing. (R Foundation for Statistical Computing, 2021). http://www.R-project.org.
Phillips, D. L. et al. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 92, 823–835. https://doi.org/10.1139/cjz-2014-0127 (2014).
McCutchan, J. H. Jr., Lewis, W. M. Jr., Kendall, C. & McGrath, C. C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390. https://doi.org/10.1034/j.1600-0706.2003.12098.x (2003).
Hussey, N. E. et al. Rescaling the trophic structure of marine food webs. Ecol. Lett. 17, 239–250. https://doi.org/10.1111/ele.12226 (2014).
Langone, L. et al. Dynamics of particles along the western margin of the Southern Adriatic: Processes involved in transferring particulate matter to the deep basin. Mar. Geol. 375, 28–43. https://doi.org/10.1016/j.margeo.2015.09.004 (2016).
Conese, I., Fanelli, E., Miserocchi, S. & Langone, L. Food web structure and trophodynamics of deep-sea plankton from the Bari Canyon and adjacent slope (Southern Adriatic, central Mediterranean Sea). Prog. Oceanogr. 175, 92–104. https://doi.org/10.1016/j.pocean.2019.03.011 (2019).
Jackson, A. L., Inger, R., Parnell, A. C. & Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x (2011).
Layman, C. A., Quattrochi, J. P., Peyer, C. M. & Allgeier, J. E. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 10, 937–944. https://doi.org/10.1111/j.1461-0248.2007.01087.x (2007).
Vanderklift, M. A. & Ponsard, S. Sources of variation in consumer-diet delta 15N enrichment: A meta-analysis. Oecologia 136, 169–182. https://doi.org/10.1007/s00442-003-1270-z (2003).
Acknowledgements
The authors wish to thank the crew of the R/V “G. Dallaporta” and all the scientific personnel on board for their invaluable support during the MEDIAS 2019 survey. EF thanks Miss Marion Rivot for helping in stomach content analysis of Sprattus sprattus. EF also thanks ISOAD project funded by the Polytechnic University of Marche and CONISMA contract for D.4, for supporting part of the SIA costs. This study has been mostly supported by the MEDIAS project within the Data Collection Framework programs of the EU and the Directorate of Fishery of the Italian national Ministry of Agriculture, Food and Forestry Policies. This study represents partial fulfillment of the requirements for the PhD thesis of S. Menicucci, within the international PhD program “Innovative technologies and Sustainable use of Mediterranean Sea Fishery and Biological Resources” (FishMed; www.FishMed-PhD.org) at the University of Bologna, Italy”.
Author information
Authors and Affiliations
Contributions
E.F. and I.L. conceived the study; I.L., and A.D.F. designed the experimental survey. A.D.F., I.B., G.C., S.Me. and S.Ma. collected the samples; G.C. performed the trawls to obtain the samples; S.Me. prepared the samples for SIA and run the preliminary analysis; Z.d.R. analyzed the SIA data and run the SIA-based models and metrics; E.F. wrote the first draft of the manuscript with the support of S.Me. and Z.d.R.; All authors have contributed to write previous versions and approved the final, submitted version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fanelli, E., Da Ros, Z., Menicucci, S. et al. The pelagic food web of the Western Adriatic Sea: a focus on the role of small pelagics. Sci Rep 13, 14554 (2023). https://doi.org/10.1038/s41598-023-40665-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-40665-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.