Abstract
During mechanical thrombectomy in the anterior cerebral circulation, thrombus embolization resulting in Willisian collateral failure may lead to critical stroke outcomes due to a shutdown of leptomeningeal collaterals. We hypothesized that the outcomes of dynamic Willisian collateral failure (DWF), induced during mechanical thrombectomy, would be associated with grave outcomes. We evaluated this hypothesis in consecutive patients, between January 2011 and May 2016, who underwent mechanical thrombectomy for anterior circulation occlusions, with an onset-to-puncture of 24 h. Patients with initial Willisian collateral failure (IWF) were identified first, with remaining patients classified into the DWF and Willisian collateral sparing (WCS) groups. Comparative and multivariable analyses were performed to predict grave outcomes (3-month modified Rankin Scale score of 5–6). Among 567 patients, 37 were in the IWF group, 38 in the DWF group, and 492 in the WCS group. Compared to the WCS and DWF groups, the IWF group had a higher baseline National Institute of Health Stroke Scale score and lower Alberta Stroke Program Early CT Score. The prevalence of grave outcomes was similarly high in the IWF (48.6%) and DWF (47.4%) groups, but lower in the WCS group (22.0%; p < 0.001). IWF and DWF were independent risk factors for a grave outcome.
Similar content being viewed by others
Introduction
Mechanical thrombectomy for cerebral infarcts resulting from large vessel occlusions (LVOs) is a very effective treatment1, which aims to salvage cells within the penumbra and arrest infarct growth. However, there can be potential complications, such as access site complications, hemorrhagic complications, device-related complications, and embolization of thrombus resulting in new ischemic events2. Embolization of thrombus occurs in up to 6% of mechanical procedures, resulting in infarct growth2. In these cases, failure of Willisian collaterals is thought to result in fast infarct growth and poor clinical outcomes.
Willisian collateral failure often occurs at the initial stroke event (initial Willisian collateral failure [IWF]) due to complex ICA terminus occlusions (CTO)3. It can occur in an internal carotid artery (ICA) terminus occlusion when thrombus occludes the A2 trunk or distal branches of the anterior cerebral artery (ACA) and/or the P2 segment or distal branches of the posterior cerebral artery (PCA) in combination with occlusion in the middle cerebral artery (MCA). CTO can also functionally occur without involving the ICA terminus when embolus lodges itself in neighboring major intracranial vessels concomitantly (e.g., respective occlusions of the A2 trunk of the ACA and M1 segments of the MCA). Such Willisian collateral failure may also occur during thrombectomy due to thrombus manipulation or distal embolization. Theoretically, such an event would result in near-complete shutdown of leptomeningeal collaterals in the affected hemisphere, potentially harming the patient. The frequency and influence of such dynamic Willisian collateral failure (DWF) have not been previously reported.
Thus, in this study, our aim was to evaluate the effect of DWF on grave clinical outcomes for an LVO of the anterior cerebral circulation and to evaluate factors associated with good clinical outcomes after DWF. Identification of these factors could guide a future bailout plan during DWF to protect against increasing stroke severity.
Methods
Patient enrollment and evaluation
Patients were retrospectively identified from the Acute Stroke due to Intracranial Atherosclerotic occlusion and Neurointervention—Korean Retrospective (ASIAN KR) registry4,5. Between January 2011 and May 2016, 720 patients who underwent endovascular treatment (EVT) for an acute ischemic stroke caused by intracranial and/or extracranial LVO were identified. From these patients, those who underwent EVT for an LVO of the anterior circulation (intracranial ICA and M1 segment of the MCA) within 24 h of stroke onset were included in this study. The type of EVT procedure performed was at the discretion of the treating physician, with direct aspiration and stent retrieval primarily used in most cases6,7,8,9.
A 3-month modified Rankin Scale (mRS) score of 0–2 or no change, compared to the premorbid mRS score, was classified as a good outcome, with a 3-month mRS score of 5–6 classified as a grave outcome. After de-identification and blinding of clinical data, assessment of laboratory images was performed to ensure consistent grading and to eliminate the possibility of bias.
On preprocedural diffusion-weighted images (DWIs), the infarct volume was evaluated by manually outlining the DWI hyperintense lesions using the NordicICE semi-automated software (NordicNeuroLab, Bergen, Norway) (J.W.C.). Successful reperfusion was defined as a modified Treatment In Cerebral Ischemia (mTICI) grade of 2b (more than 50% reperfusion of target downstream territory) or 3 (complete reperfusion)10. Post-procedural hemorrhagic transformations were classified based on the criteria defined by the European Cooperative Acute Stroke Study as none, hemorrhagic infarct type 1 or 2, or parenchymal hematoma type 1 or 211. The post-procedural subarachnoid hemorrhage was graded using the modified Fisher grading system12. A parenchymal hematoma type 2 or Fisher grade 3 or 4 subarachnoid hemorrhage was considered as serious hemorrhagic complications.
Conventional leptomeningeal collateral circulation was assessed using single-phase or dynamic computed tomography angiography (CTA). Two different collateral grading systems were applied: the Miteff scoring system13 for CTA in general and the modified ASITN/SIR system for dynamic CTA, according to the criteria proposed by Higashida et al.14 Collateral circulation was subsequently categorized as poor (Miteff grades 1 and 2; ASITN/SIR grades 0, 1, and 2) or good (Miteff grade 3; ASITN/SIR grades 3 and 4). Collateral circulation could not be graded by CTA in cases of intracranial ICA occlusions with sparing of the ICA bifurcation due to the presence of antegrade flow, rather than a retrograde flow from collaterals.
Statement of ethics
The data collection protocol was approved by the institutional review board of each participating hospital and implemented in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. The need for written informed consent was waived given the retrospective design of the study. All data included in this study are available upon reasonable request to the corresponding author.
Definition of DWF and patient grouping
Changes in occlusion were evaluated by procedural angiography, where all changes that occurred from after the initial CTA or magnetic resonance angiography (MRA) up till the end of the EVT procedure (S.J.L.) were included. Following this analysis, patients were classified into three groups, namely the IWF, DWF, and Willisian collateral sparing (WCS) groups (Fig. 1). IWF was first classified according to pre-procedure CTA or MRA if a CTO pattern or its equivalent was observed3. In brief, an acute ICA terminus occlusion or an M1 occlusion combined with: (1) occlusion of the ipsilateral A2 or more distal segment; (2) occlusion of the fetal-type ipsilateral posterior cerebral artery, was considered IWF. In addition, an ICA terminus occlusion with: (1) insufficient contralateral Willisian collateral blood supply via the anterior communicating artery due to contralateral agenesis of the A1 segment, or; (2) contralateral ICA occlusion, was classified as IWF. Patients who presented with (1) conventional M1 occlusion (M1O), (2) simple ICA T occlusions (STO), or (3) isolated ICA I occlusion (IIO) with sparing of the ICA bifurcation, were considered to be spared of IWF. In these patients, DWF was defined as an embolization during EVT resulting in occlusion of another major intracranial artery without resolution of the initial occlusion. In this DWF type, a new occlusion of the ACA A2, fetal PCA, or both occurred in combination with a functional M1 occlusion, causing a near complete shutdown of blood flow. DWF was classified regardless of final occlusion status. Patients who did not present with IWF or did not experience DWF were classified in the WCS group. Various patterns could be included in the WCS group, as long as there was no Willisian collateral failure; absence of embolization, distal embolization within the same arterial bed, and occlusion of another territory after complete reperfusion of the initial territory were all classified WCS.
A flowchart of patient grouping based on the dynamic Willisian collateral status. All patients with an intracranial anterior circulation LVO were first classified as (A) IWF if they presented with an acute occlusion of the ICA terminus or M1 segment of the MCA in combination with occlusion of the ipsilateral A2 segment or occlusion of the ipsilateral fetal-PCA. Also, an ICA terminus occlusion with agenesis or severe hypoplasia of the contralateral A1 segment or occlusion of the contralateral ICA occlusion were classified as IWF. In the rest anterior circulation occlusions with preserved Willisian collateral flow, patients were classified as (B) DWF if a new occlusion of the A2 segment of the ACA, fetal PCA, or both occurred without resolution of the original M1 or intracranial ICA occlusion during EVT. The classification of DWF was made regardless of the final reperfusion outcomes, which is why the term ‘dynamic’ was used. Patients who did not experience DWF or IWF were classified as (C) WCS. DWF, dynamic Willisian collateral failure; EVT, endovascular treatment; ICA, internal carotid artery; IIO, isolated intracranial internal carotid artery occlusion; IWF, initial Willisian collateral failure; LVO, large vessel occlusion; MCA, middle cerebral artery; M1O, middle cerebral artery M1 occlusion; PCA; posterior cerebral artery; STO, simple internal carotid artery terminus occlusion; WCS, Willisian collateral sparing.
Statistical analysis
Clinical characteristics, imaging and endovascular therapy findings, and outcomes were compared between the IWF, DWF, and WCS groups. Univariate analysis was performed using the chi-squared (χ2) test for categorical variables. For continuous variables, an analysis of variance with a Bonferroni post-hoc analysis was used for comparison between three groups, with Student’s t-test for comparison between two groups. The effect of Willisian collateral failure on the risk of grave functional outcomes was further analyzed using a multiple logistic regression analysis, adjusting for clinically significant variables. In the DWF subgroup, patients with good and poor functional outcomes were compared. To identify procedural factors associated with a good outcome in this subgroup, a multiple logistic regression analysis was performed on variables presumed to be clinically significant.
Data are presented as the mean ± standard deviation, median [interquartile range], or number (%) as appropriate for the data type and distribution. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics (version 22; IBM Corp., Armonk, NY, USA).
Results
Occlusion changes during the procedure
Among the 720 patients identified in the ASIAN KR database, 567 presented with primary occlusion of the intracranial ICA and MCA M1, within 24 h of symptom onset. The distribution of the type of occlusion was as follows: 37 (6.5%) IWF, 38 (6.7%) DWF, and 492 (86.8%) WCS. Among patients in the DWF group, 37 had a new occlusion into ACA, and 1 into PCA.
Effect of Willisian collateral failure on functional outcomes and its predictors
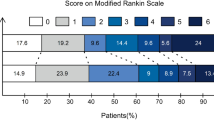
Comparisons between the IWF, DWF, and WCS groups are reported in Table 1. The National Institutes of Health Stroke Scale (NIHSS) score was higher and the Alberta Stroke Program Early CT Score (ASPECTS) lower in the IWF group than in the WCS group (IWF versus WCS, p < 0.005, post hoc Bonferroni test), with no difference in scores between the DWF and WCS groups. The reperfusion procedure time was the longest for the DWF group (DWF vs. WCS, p = 0.007, post hoc Bonferroni test). The rate of successful recanalization was slightly lower for the DWF group (70.3% vs. 60.5% vs. 77.6%, p = 0.041), while the rate of complete TICI grade 3 reperfusion was significantly lower for the DWF group (2.6%) compared to the rate for the IWF (18.9%) and WCS (33.3%) groups (p < 0.001 for overall TICI grades). The rate of a grave outcome was higher in the IWF (48.6%) and DWF (47.4%) groups than in the WCS (22.0%) group (p < 0.001). Of note, patients with an IWF presented with a large baseline infarct volume (51 [20–210] ml) and ended up with a large final infarct volume (109 [15–229] ml), while patients in the DWF group presented with a smaller baseline infarct volume (11 [5–43] ml) but a large final infarct volume (97 [23–167] ml). Patients in the WCS group presented with a small baseline infarct volume (11 [5–31] ml) and a smaller final infarct volume (28 [11–85] ml) (baseline infarct volume, p < 0.001; and final infarct volume, p < 0.001). The rate of significant hemorrhagic complications was higher in the IWF group than in the DWF and WCS groups (35.1% vs. 10.5% and 9.6%, respectively, p < 0.001). On multivariable analysis, both IWF (odds ratio [OR]: 2.44, 95% confidence interval [CI]: [1.15–5.20], p = 0.020) and DWF (OR: 2.82, 95% CI: [1.34–5.95], p = 0.007) were associated with a grave outcome, with the OR adjusted by age, sex, premorbid mRS, admission NIHSS score, onset-to-puncture time, intravenous thrombolytic administration, and final successful reperfusion (Table 2. model 1). When baseline DWI infarct volume was further included as a covariate, only DWF (OR: 3.21, 95% CI: [1.38–7.49], p < 0.001) was significantly associated with grave outcomes (Table 2. model 2). An example of a DWF resulting in a large infarct volume, regardless of the final reperfusion status, and poor outcomes is depicted in Fig. 2.
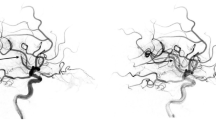
An example of dynamic Willisian collateral failure during endovascular therapy. (A) Preprocedural imaging reveals preservation of the Willisian collaterals by the anterior communicating artery (yellow arrow) and shows a small infarct volume. (B) Angiography of the contralateral ICA, before the procedure, confirms the patency of the leptomeningeal collaterals of the ipsilateral anterior cerebral artery (blue triangle). During the procedure, DWF occurs, and a new embolization into the A2 segment of the ACA (orange arrowheads) abruptly resulting in a near-complete leptomeningeal collateral shutdown. (C) Despite complete reperfusion achieved on final angiography, a large final infarct volume was induced, due to DWF, which was associated with poor outcomes. ACA, anterior cerebral artery; DWF, dynamic Willisian collateral failure; EVT, endovascular treatment; ICA, internal carotid artery; MCA, middle cerebral artery; mRS, modified Rankin Scale; mTICI, modified thrombolysis in cerebral ischemia.
Predictors of DWF
As for predicting factors for DWF, a simple ICA T occlusion or isolated intracranial ICA occlusion as a primary occlusion type was the only predictor—among the 530 patients without IWF, DWF occurred in 23 of 167 patients (13.8%) with STO, 5 of 37 patients (13.5%) with an IIO, and 10 of 326 patients (3.1%) with an M1 occlusion (p < 0.001). Intravenous administration of tissue plasminogen activator, presence of tandem occlusions, type of reperfusion procedure (such a balloon guide catheter), and primary reperfusion modality were not predictive DWF.
Factors associated with a good outcome in the DWF group
Within the DWF subgroup, the comparison of characteristics of patients that achieved good and poor outcomes is shown in Table 3. Patients with good outcomes despite DWF, compared to those with a poor outcome, had a lower rate of diabetes mellitus (8.3% vs. 46.2%, respectively, p = 0.022), atrial fibrillation (25.0% vs. 65.4%, respectively, p = 0.020), and higher rate of tandem ICA occlusions (25.0% vs. 3.8%, respectively, p = 0.048). Follow-up DWI volume was also much smaller in the good compared to poor outcome group (26 [9–95] ml vs. 77 [147–184] ml, respectively, p = 0.006). While the rate of successful reperfusion was only marginally significant at 83.3% for patients with a good outcome compared to 50.0% for those with a poor outcome (p = 0.051), the rate of successful reperfusion of newly embolized territory was significantly higher in the good outcome group (91.7%) than in the poor outcome group (30.8%, p < 0.001), with shorter procedure time (53 ± 27 min vs. 113 ± 55 min, respectively, p < 0.001). In the multivariable analysis, total procedure time (inverse correlation, OR: 0.94, 05% CI: [0.90–0.99], p = 0.012) and reperfusion of the newly embolized vascular territory (OR: 21.42, 95% CI: [1.20–383.30], p = 0.037) were independent predictors of a good outcome, along with age and successful reperfusion as covariates (Table 4).
Discussion
The principal finding of this study is that clinical outcomes of anterior circulation LVOs were strongly influenced by the Willisian collaterals, even when it occurs during the reperfusion process. DWF occurs in approximately 7% of all anterior circulation LVOs and were mostly associated with embolization of thrombus to the ACA. DWF resulted in grave outcomes comparable to IWF, which is likely due to the rapid growth of infarct volume. Good outcomes despite DWF was associated with reduced procedure time and reperfusion of the newly embolized vascular territory.
Unlike previous studies, we measured presence of embolic complications by whether Willisian collateral failure occurred or not. While some studies have evaluated the effect of embolization to the ACA15 or distal embolization in the same territory16, the mechanistic rationale was not based on the patency of Willisian collaterals. Our findings indicate that DWF has a critical effect on outcomes in contrast to other embolic events that do not, such as distal embolizations16. Simultaneous occlusion of the MCA and ACA or PCA results in near-total impairment of collateral flow to the affected hemisphere, resulting in a rapid infarct growth rapidly exceeding the threshold for good clinical outcomes even with successful reperfusion. Moreover, in the presence of DWF, a grade 3 mTICI reperfusion appears to be difficult to accomplish, which is another factor associated with poor observed outcomes.
When DWF occurred during the procedure, shorter procedure time and reperfusion of the newly embolized vascular territory, which mostly occurred in the main branch of the ACA, were predictors of good clinical outcomes. The specific importance of reperfusion of the ACA has been previously reported. In a retrospective review of 105 recanalization procedures for M1 occlusions, ACA occlusion occurred in 11.4% of the cases, with the resultant ACA infarcts limiting post-stroke motor recovery15. Endovascular recanalization of major ACA branches reduced the burden of infarcts with no adverse events15. Generally, reperfusion of the ACA is avoided because of its smaller arterial caliber and the risk of procedural complications. However, according to the limited clinical evidence available, the recanalization rate is reported to be high and periprocedural complications are rare17,18. Considering these factors, reperfusion of the ACA may be pursued in feasible patients as a bailout therapy for DWF.
In our dataset, we could not find any preprocedural variables that predicted DWF, other than the location of the initial occlusion, and no specific reperfusion modality could prevent such failure from occurring. DWF more commonly occurred in STO and IIO types of occlusion than M1 occlusions, likely due to the large thrombus burden. Factors such as intravenous administration of tissue plasminogen activator, which was previously reported to cause a pre-interventional change of occlusion site resulting in worsening of perfusion (due to an effect on collaterals)19, or the use of balloon guide catheters, known to reduce distal embolization20, was not predictive of DWF. However, taking into consideration the grave consequences of DWF, the authors advocate the use of the balloon guide catheters21 or other methods to improve first-pass effect22.
The outcome of IWF in this study is consistent with those in previous studies, even with modern thrombectomy procedures. The outcome of carotid terminus occlusions was once considered to be grave due to the low rate of reperfusion and high rate of hemorrhagic transformations23. However, advances in thrombectomy techniques, including the use of longer stent retrievers24, large-bore aspiration catheters25, and balloon guide catheters26, as well as the use of combination modalities27, have led to a high rate of reperfusion for carotid terminus occlusions. With these improvements in EVT outcomes, ICA terminus occlusions can now usually be categorized along with MCA M1 occlusions under the term anterior circulation LVO. However, there is still a population where outcomes are universally grave despite best EVT. These patients may be candidates for combining EVT with future therapeutic advances, such as neuroprotection or hypothermia28.
The limitations of our study need to be mentioned. First, MR imaging was not performed in a small number of patients, with infarct growth only measured in patients who underwent follow-up MR imaging. However, follow-up MR imaging could not be performed in deceased patients or in patients with a medically grave condition due to a large infarct. Accordingly, the increase in infarct size in the DWF group was likely to have been under-estimated than over-estimated. Second, predictors of good outcome despite DWF were only evaluated in a small number of patients, possibly resulting in lower predictive power. In this regard, the corresponding results should be confirmed in larger prospective registry data. Third, CTA collateral grades were not included in the multivariable analysis for two reasons; conventional leptomeningeal collateral scores cannot be applied to IIO and the grade was available in only about half of the total study sample. Thus, the added benefit of evaluating Willisian collateral failure over conventional leptomeningeal collateral grading could not be shown in this study. Fourth, the results of this study could not identify the factors associated with an increased risk of DWF, except for the location of the initial occlusion. It remains to be determined if advances in reperfusion techniques, such as the use of the Solumbra technique29 or others, may reduce the rate of DWF.
Conclusion
In EVT of LVOs of the anterior circulation, DWF most commonly occurred by concurrent occlusion of the previously uninvolved ACA and was significantly associated with grave outcomes along with IWF. As good outcomes despite DWF are associated with total procedure time and reperfusion of the newly embolized vascular territory, rapid reperfusion of the ACA may be considered when feasible.
References
Nogueira, R. G. & Ribo, M. Endovascular treatment of acute stroke. Stroke 50, 2612–2618. https://doi.org/10.1161/STROKEAHA.119.023811 (2019).
Balami, J. S., White, P. M., McMeekin, P. J., Ford, G. A. & Buchan, A. M. Complications of endovascular treatment for acute ischemic stroke: Prevention and management. Int. J. Stroke Off. J. Int. Stroke Soc. 13, 348–361. https://doi.org/10.1177/1747493017743051 (2018).
Lee, S. U. et al. Differentiating carotid terminus occlusions into two distinct populations based on Willisian collateral status. J. Stroke 18, 179–186. https://doi.org/10.5853/jos.2015.01529 (2016).
Lee, J. S. et al. Temporal changes in care processes and outcomes for endovascular treatment of acute ischemic stroke: retrospective registry data from three Korean centers. Neurointervention 13, 2–12. https://doi.org/10.5469/neuroint.2018.13.1.2 (2018).
Lee, J. S. et al. Prognosis of acute intracranial atherosclerosis-related occlusion after endovascular treatment. J. Stroke 20, 394–403. https://doi.org/10.5853/jos.2018.01627 (2018).
Kang, D. H. et al. Direct thrombus retrieval using the reperfusion catheter of the penumbra system: forced-suction thrombectomy in acute ischemic stroke. AJNR Am. J. Neuroradiol. 32, 283–287. https://doi.org/10.3174/ajnr.A2299 (2011).
Roth, C. et al. Stent-assisted mechanical recanalization for treatment of acute intracerebral artery occlusions. Stroke J. Cereb. Circ. 41, 2559–2567. https://doi.org/10.1161/strokeaha.110.592071 (2010).
Saver, J. L. et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet (London, England) 380, 1241–1249. https://doi.org/10.1016/S0140-6736(12)61384-1 (2012).
Nogueira, R. G. et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet (London, England) 380, 1231–1240. https://doi.org/10.1016/S0140-6736(12)61299-9 (2012).
Tomsick, T. et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am. J. Neuroradiol. 29, 582–587. https://doi.org/10.3174/ajnr.A0843 (2008).
Fiorelli, M. et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke J. Cereb. Circ. 30, 2280–2284 (1999).
Fisher, C. M., Kistler, J. P. & Davis, J. M. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 6, 1–9. https://doi.org/10.1227/00006123-198001000-00001 (1980).
Miteff, F. et al. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain J. Neurol. 132, 2231–2238. https://doi.org/10.1093/brain/awp155 (2009).
Higashida, R. T. et al. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke 34, e109-137. https://doi.org/10.1161/01.STR.0000082721.62796.09 (2003).
Kurre, W. et al. Frequency and relevance of anterior cerebral artery embolism caused by mechanical thrombectomy of middle cerebral artery occlusion. AJNR Am. J. Neuroradiol. 34, 1606–1611. https://doi.org/10.3174/ajnr.A3462 (2013).
Mazur, M. D., Kilburg, C., Park, M. S. & Taussky, P. Patterns and clinical impact of angiographically visible distal emboli during thrombectomy with solitaire for acute ischemic stroke. Neurosurgery 78, 242–250. https://doi.org/10.1227/NEU.0000000000001135 (2016).
Pfaff, J. et al. Mechanical thrombectomy of distal occlusions in the anterior cerebral artery: recanalization rates, periprocedural complications, and clinical outcome. AJNR Am. J. Neuroradiol. 37, 673–678. https://doi.org/10.3174/ajnr.A4594 (2016).
Uno, J. et al. Mechanical thrombectomy for acute anterior cerebral artery occlusion. World Neurosurg. 120, e957–e961. https://doi.org/10.1016/j.wneu.2018.08.196 (2018).
Kaesmacher, J. et al. Rates and quality of preinterventional reperfusion in patients with direct access to endovascular treatment. Stroke 49, 1924–1932. https://doi.org/10.1161/STROKEAHA.118.021579 (2018).
Lee, D. H. et al. Effective use of balloon guide catheters in reducing incidence of mechanical thrombectomy related distal embolization. Acta Neurochir. 159, 1671–1677. https://doi.org/10.1007/s00701-017-3256-3 (2017).
Yoo, A. J. & Andersson, T. Thrombectomy in acute ischemic stroke: challenges to procedural success. J. Stroke 19, 121–130. https://doi.org/10.5853/jos.2017.00752 (2017).
Zaidat, O. O. et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke 49, 660–666. https://doi.org/10.1161/STROKEAHA.117.020315 (2018).
Lee, J. S. et al. Comparison of the incidence of parenchymal hematoma and poor outcome in patients with carotid terminus occlusion treated with intra-arterial urokinase alone or with combined IV rtPA and intra-arterial urokinase. AJNR Am. J. Neuroradiol. 33, 175–179. https://doi.org/10.3174/ajnr.A2722 (2012).
Zaidat, O. O. et al. Impact of stent retriever size on clinical and angiographic outcomes in the STRATIS stroke thrombectomy registry. Stroke 50, 441–447. https://doi.org/10.1161/STROKEAHA.118.022987 (2019).
Delgado Almandoz, J. E. et al. Larger ACE 68 aspiration catheter increases first-pass efficacy of ADAPT technique. J. Neurointervent. Surg. 11, 141–146. https://doi.org/10.1136/neurintsurg-2018-013957 (2019).
Nguyen, T. N. & Zaidat, O. O. Balloon guide catheter in large-vessel occlusion stroke therapy. Radiology 284, 607–608. https://doi.org/10.1148/radiol.2017170375 (2017).
Lee, J. S. et al. The combined use of mechanical thrombectomy devices is feasible for treating acute carotid terminus occlusion. Acta Neurochir. 155, 635–641. https://doi.org/10.1007/s00701-013-1649-5 (2013).
Choi, M. H. et al. The clinical usefulness of targeted temperature management in acute ischemic stroke with malignant trait after endovascular thrombectomy. Neurocrit. Care https://doi.org/10.1007/s12028-020-01069-0 (2020).
Humphries, W. et al. Distal aspiration with retrievable stent assisted thrombectomy for the treatment of acute ischemic stroke. J. Neurointervent. Surg. 7, 90–94. https://doi.org/10.1136/neurintsurg-2013-010986 (2015).
Acknowledgements
This work was partly supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP) (NRF-2018R1A2B6007094; J.S.L.), and (NRF No. 2014R1A5A2010008: S.I.S.).
Author information
Authors and Affiliations
Contributions
S.J.L. and J.S.L. contributed to the conception and design of the study, acquisition and analysis of data, and preparation of the manuscript. Y.H.H., S.I.S., J.M.H., J.W.C., D.H.K., Y.W.K., Y.S.K., J.H.H., J.Y., and C.H.K. contributed to acquisition and analysis of data, and critical revision of the article for important intellectual content. B.O. and A.D. contributed to interpretation of data and critical revision of the article for important intellectual content. All authors approved the manuscript. There were no other contributors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, SJ., Hwang, YH., Hong, J.M. et al. Predictors and prognoses of Willisian collateral failure during mechanical thrombectomy. Sci Rep 10, 20874 (2020). https://doi.org/10.1038/s41598-020-77946-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77946-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.