Abstract
Mucinolytic bacteria modulate host–microbiota symbiosis and dysbiosis through their ability to degrade mucin O-glycans. However, how and to what extent bacterial enzymes are involved in the breakdown process remains poorly understood. Here we focus on a glycoside hydrolase family 20 sulfoglycosidase (BbhII) from Bifidobacterium bifidum, which releases N-acetylglucosamine-6-sulfate from sulfated mucins. Glycomic analysis showed that, in addition to sulfatases, sulfoglycosidases are involved in mucin O-glycan breakdown in vivo and that the released N-acetylglucosamine-6-sulfate potentially affects gut microbial metabolism, both of which were also supported by a metagenomic data mining analysis. Enzymatic and structural analysis of BbhII reveals the architecture underlying its specificity and the presence of a GlcNAc-6S-specific carbohydrate-binding module (CBM) 32 with a distinct sugar recognition mode that B. bifidum takes advantage of to degrade mucin O-glycans. Comparative analysis of the genomes of prominent mucinolytic bacteria also highlights a CBM-dependent O-glycan breakdown strategy used by B. bifidum.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Atomic coordinates and structure factors of GlcNAc-6S-complexed and PUGNAc-6S-complexed BbhII proteins from B. bifidum JCM 1254 have been deposited in the Protein Data Bank under accession numbers 7WDT and 7WDU, respectively. The sequences of 16S rRNA V3–V4 variable regions of fecal microbiotas of mice and humans have been deposited in the DNA Data Bank of Japan under accession numbers DRA013515 and DRA013516, respectively. Source data are provided with this paper.
Code availability
No custom code was used in this study.
References
Johansson, M. E. V. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Bergstrom, K. et al. Proximal colon-derived O-glycosylated mucus encapsulates and modulates the microbiota. Science 370, 467–472 (2020).
Desai, M. S. et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167, 1339–1353 (2016).
Pudlo, N. A. et al. Symbiotic human gut bacteria with variable metabolic priorities for host mucosal glycans. mBio 6, e01282–15 (2015).
Pichler, M. J. et al. Butyrate producing colonic Clostridiales metabolise human milk oligosaccharides and cross feed on mucin via conserved pathways. Nat. Commun. 11, 3285 (2020).
Belzer, C. et al. Microbial metabolic networks at the mucus layer lead to diet-independent butyrate and vitamin B12 production by intestinal symbionts. mBio 8, e00770–17 (2017).
Yamada, T. et al. Mucin O-glycans facilitate symbiosynthesis to maintain gut immune homeostasis. EBioMedicine 48, 513–525 (2019).
Robbe, C., Capon, C., Coddeville, B. & Michalski, J.-C. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem. J. 384, 307–316 (2004).
Tailford, L. E., Crost, E. H., Kavanaugh, D. & Juge, N. Mucin glycan foraging in the human gut microbiome. Front. Genet. 6, 81 (2015).
Wright, D. P., Knight, C. G., Parkar, S. G., Christie, D. L. & Roberton, A. M. Cloning of a mucin-desulfating sulfatase gene from Prevotella strain RS2 and its expression using a Bacteroides recombinant system. J. Bacteriol. 182, 3002–3007 (2000).
Praharaj, A. B., Dehury, B., Mahapatra, N., Kar, S. K. & Behera, S. K. Molecular dynamics insights into the structure, function, and substrate binding mechanism of mucin desulfating sulfatase of gut microbe Bacteroides fragilis. J. Cell. Biochem. 119, 3618–3631 (2018).
Luis, A. S. et al. A single sulfatase is required to access colonic mucin by a gut bacterium. Nature 598, 332–337 (2021).
Drula, E. et al. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 50, D571–D577 (2022).
Rho, J. et al. A novel mechanism for desulfation of mucin: identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J. Bacteriol. 187, 1543–1551 (2005).
Katoh, T. et al. Identification and characterization of a sulfoglycosidase from Bifidobacterium bifidum implicated in mucin glycan utilization. Biosci. Biotechnol. Biochem. 81, 2018–2027 (2017).
Katoh, T. et al. Enzymatic adaptation of Bifidobacterium bifidum to host glycans, viewed from glycoside hydrolyases and carbohydrate-binding modules. Microorganisms 8, 481 (2020).
Turroni, F. et al. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc. Natl Acad. Sci. USA 107, 19514–19519 (2010).
Milani, C. et al. Unveiling bifidobacterial biogeography across the mammalian branch of the tree of life. ISME J. 11, 2834–2847 (2017).
Kim, M. J. et al. Safety evaluations of Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI. Int. J. Mol. Sci. 19, 1422 (2018).
Barbeyron, T. et al. Matching the diversity of sulfated biomolecules: creation of a classification database for sulfatases reflecting their substrate specificity. PLoS ONE 11, e0164846- (2016).
Bäckhed, F. et al. Dynamics and stabilization of the human gut microbiome in the first year of life. Cell Host Microbe 17, 690–703 (2015).
Howard, B. M., Ekborg, A. N., Taylor, E. L., Weiner, M. R. & Hutcheson, W. S. Genomic analysis and initial characterization of the chitinolytic system of Microbulbifer degradans strain 2-40. J. Bacteriol. 185, 3352–3360 (2003).
Luis, A. S. et al. Sulfated glycan recognition by carbohydrate sulfatases of the human gut microbiota. Nat. Chem. Biol. 18, 841–849 (2022).
Holm, L. DALI and the persistence of protein shape. Protein Sci. 29, 128–140 (2020).
Mark, B. L. et al. Crystallographic evidence for substrate-assisted catalysis in a bacterial β-hexosaminidase. J. Biol. Chem. 276, 10330–10337 (2001).
Shinya, S. et al. Mechanism of chitosan recognition by CBM32 carbohydrate-binding modules from a Paenibacillus sp. IK-5 chitosanase/glucanase. Biochem. J. 473, 1085–1095 (2016).
Greig, I. R., Zahariev, F. & Withers, S. G. Elucidating the nature of the Streptomyces plicatus β-hexosaminidase-bound intermediate using ab initio molecular dynamics simulations. J. Am. Chem. Soc. 130, 17620–17628 (2008).
Lonhienne, T. et al. Cloning, sequences, and characterization of two chitinase genes from the antarctic Arthrobacter sp. strain TAD20: isolation and partial characterization of the enzymes. J. Bacteriol. 183, 1773–1779 (2001).
Knapp, S. et al. NAG-thiazoline, an N-acetyl-β-hexosaminidase inhibitor that implicates acetamido participation. J. Am. Chem. Soc. 118, 6804–6805 (1996).
Beer, D., Maloisel, J.-L., Rast, D. M. & Vasella, A. Synthesis of 2-acetamido-2-deoxy-d-gluconhydroximolactone- and chitobionhydroximolactone-derived N-phenylcarbamates, potential inhibitors of β-N-acetylglucosaminidase. Helv. Chim. Acta 73, 1918–1922 (1990).
Horsch, M., Hoesch, L., Vasella, A. & Rast, D. M. N-Acetylglucosaminono-1,5-lactone oxime and the corresponding (phenylcarbamoyl)oxime. Novel and potent inhibitors of β-N-acetylglucosaminidase. Eur. J. Biochem. 197, 815–818 (1991).
Katayama, T. et al. Molecular cloning and characterization of Bifidobacterium bifidum 1,2-α-l-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J. Bacteriol. 186, 4885–4893 (2004).
Ficko-Blean, E. & Boraston, A. B. N-acetylglucosamine recognition by a family 32 carbohydrate-binding module from Clostridium perfringens NagH. J. Mol. Biol. 390, 208–220 (2009).
Abbott, D. W., Eirín-López, J. M. & Boraston, A. B. Insight into ligand diversity and novel biological roles for family 32 carbohydrate-binding modules. Mol. Biol. Evol. 25, 155–167 (2008).
Teh, A.-H., Sim, P.-F. & Hisano, T. Structural basis for binding uronic acids by family 32 carbohydrate-binding modules. Biochem. Biophys. Res. Commun. 533, 257–261 (2020).
Viborg, A. H. et al. A subfamily roadmap of the evolutionarily diverse glycoside hydrolase family 16 (GH16). J. Biol. Chem. 294, 15973–15986 (2019).
Crouch, L. I. et al. Prominent members of the human gut microbiota express endo-acting O-glycanases to initiate mucin breakdown. Nat. Commun. 11, 4017 (2020).
Tailford, L. E. et al. Discovery of intramolecular trans-sialidases in human gut microbiota suggests novel mechanisms of mucosal adaptation. Nat. Commun. 6, 7624 (2015).
Martens, E. C., Chiang, H. C. & Gordon, J. I. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4, 447–457 (2008).
Arzamasov, A. et al. Human milk oligosaccharide utilization in intestinal bifidobacteria is governed by global transcriptional regulator NagR. mSystems 7, e00343–22 (2022).
Terrapon, N. et al. PULDB: the expanded database of Polysaccharide Utilization Loci. Nucleic Acids Res. 46, D677–D683 (2018).
Gotoh, A. et al. Sharing of human milk oligosaccharides degradants within bifidobacterial communities in faecal cultures supplemented with Bifidobacterium bifidum. Sci. Rep. 8, 13958 (2018).
Egan, M., Jiang, H., O’Connell Motherway, M., Oscarson, S. & van Sinderen, D. Glycosulfatase-encoding gene cluster in Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 82, 6611–6623 (2016).
Bell, A. et al. Elucidation of a sialic acid metabolism pathway in mucus-foraging Ruminococcus gnavus unravels mechanisms of bacterial adaptation to the gut. Nat. Microbiol 4, 2393–2404 (2019).
Nishiyama, K. et al. Evaluation of bifidobacterial adhesion to acidic sugar chains of porcine colonic mucins. Biosci. Biotechnol. Biochem. 78, 1444–1451 (2014).
Yasui, K. et al. Improvement of bacterial transformation efficiency using plasmid artificial modification. Nucleic Acids Res. 37, e3 (2009).
Kozakai, T., Shimofusa, Y., Nomura, I. & Suzuki, T. Construction of a reporter system for bifidobacteria using chloramphenicol acetyltransferase and its application for evaluation of promoters and terminators. Biosci. Microbiota Food Health 40, 115–122 (2021).
Sakanaka, M. et al. Evolutionary adaptation in fucosyllactose uptake systems supports bifidobacteria-infant symbiosis. Sci. Adv. 5, eaaw7696 (2019).
Kumagai, T., Katoh, T., Nix, D. B., Tiemeyer, M. & Aoki, K. In-gel β-elimination and aqueous-organic partition for improved O- and sulfoglycomics. Anal. Chem. 85, 8692–8699 (2013).
Asakuma, S. et al. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 286, 34583–34592 (2011).
Minamisawa, T. & Hirabayashi, J. Fragmentations of isomeric sulfated monosaccharides using electrospray ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 19, 1788–1796 (2005).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Segata, N. et al. Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60 (2011).
Wood, D. E., Lu, J. & Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 20, 257 (2019).
Camacho, C. et al. BLAST+: architecture and applications. BMC Bioinformatics 10, 421 (2009).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300 (1995).
Liu, J., Shikhman, A. R., Lotz, M. K. & Wong, C. H. Hexosaminidase inhibitors as new drug candidates for the therapy of osteoarthritis. Chem. Biol. 8, 701–711 (2001).
Sakurama, H. et al. Lacto-N-biosidase encoded by a novel gene of Bifidobacterium longum subspecies longum shows unique substrate specificity and requires a designated chaperone for its active expression. J. Biol. Chem. 288, 25194–25206 (2013).
Yamada, C. et al. Molecular insight into evolution of symbiosis between breast-fed infants and a member of the human gut microbiome Bifidobacterium longum. Cell Chem. Biol. 24, 515–524 (2017).
Acknowledgements
This study was partly supported by JSPS-KAKENHI (17K17820 and 19K05789 to T. Katoh and 21H02116 to T. Katayama); a grant-in-aid from the Institution for Fermentation, Osaka (2015 to T. Katoh); and a JSPS Research Fellowship (17J08530 to A.G. and 21J15883 to H.T.). We thank T. Arakawa (The University of Tokyo) for helping with X-ray data collection and inhibition kinetics assay; the staff of the Photon Factory and SPring-8 (proposal no. 2019B2556) for X-ray data collection; S. Nagao (Nagao Midwife Clinic) for helping with infant stool collection; F. Sato, K. Ifuku and T. Nakano (Kyoto University) for MALDI-TOF/MS instrument maintenance; J. Wada (Kyoto Integrated Science and Technology Bio-Analysis Center) for LC–MS/MS technical assistance; M. Yamaguchi (Wakayama University) for providing pNP-β-GlcNAc-3S and pNP-β-GlcNAc-3,4diS; and A. Yoshimi (Kyoto University) for technical assistance on sugar analysis. M.D.W. and K.A.S. thank the facilities and the scientific and technical assistance of Microscopy Australia at the Centre for Microscopy, Characterisation and Analysis, University of Western Australia, a facility funded by the university and by State and Federal Commonwealth Governments. M.D.W. is supported by a Research Training Program Scholarship provided by the Australian Federal Government and the University of Western Australia.
Author information
Authors and Affiliations
Contributions
T. Katoh and T. Katayama conceived the project and designed the experiments. T. Katoh, T.M. and M.A. performed glycomic analysis and enzyme characterization. A.G., T. Katoh, M.A., M.N.O., H.S., H.T., I.K. and T. Katayama conducted animal experiments and microbiota analysis. H.T. performed monosaccharide analysis. J.H. collected infant samples and managed the metadata. H.T. and M.S. constructed a bbhII mutant of B. bifidum. C.Y., T. Kashima and S.F. determined the protein structures and the inhibition constants. A.Y. and M.N. are responsible for isothermal titration calorimetry analysis. H.A. constructed the full-length BbhII expression plasmid. M.D.W. and K.A.S. synthesized inhibitors. K.N. prepared PCM. A.H., M.S.D. and T. Katoh performed metagenomic data mining analysis. T. Katoh, K.A.S., S.F. and T. Katayama drafted and edited the manuscript. All authors discussed the data and contributed to the completion of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Employment of M.N.O. and M.S. at Kyoto University is, in part, supported by Morinaga Milk Industry Co., Ltd. Employment of H.S. at Kyoto University is supported by Noster, Inc. The other authors declare no conflicts of interest.
Peer review
Peer review information
Nature Chemical Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
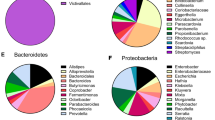
Extended Data Fig. 1 16S rRNA gene-based mouse faecal microbiota analysis.
a, Relative abundances of bacterial taxa at the family level. Faeces of PBS- and Bb-administered conventional mice (n = 5/group) at day 0 and day 5 were used for the microbiota analysis. b, The LDA score was calculated using LEfSe algorithm. Relative abundances of > 0.1% were used for the analysis.
Extended Data Fig. 2 16S rRNA gene-based microbiota analysis of human faecal suspensions incubated in the absence and presence of GlcNAc-6S.
a, Relative abundances of bacterial taxa at the family level. Faecal samples obtained from 5 individuals were used for cultivation. Microbiotas were analysed pre- and post 24 h cultivation in the absence (none-added) and presence (GlcNAc-6S-added) of 10 mM GlcNAc-6S. b, Analysis of β-diversity among the samples, based on weighted UniFrac distance metrics. c, The LEfSe analysis at the species level comparing between microbiotas of the none-added and GlcNAc-6S-added faecal suspensions post 24 h incubation. Relative abundances of > 0.1% were used for the analysis.
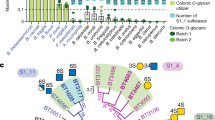
Extended Data Fig. 3 Phylogenetic tree constructed using characterised GH20 members and sulfoglycosidase homologues.
Amino acid sequences of characterised GH20 enzymes (CAZy database)13, uncharacterised BbhII homologues of >40% identity (WP_172192827.1, WP_153878949.1, CRH87835.1, WP_206666329.1, WP_125968884.1, and WP_076060111.1), and an uncharacterised Sgl homologue of > 40% identity (AAO75563.1) were aligned by clustal omega with the tree constructed with FigTree v1.4.4. BbhII and Sgl clades are indicated. The reported substrate specificities for the homologues are indicated by circles with different colors. The sources of the homologues are also shown by different colors. The sequences classified into the BbhII and Sgl clades were used for analysing a deposited human metagenomic dataset21 (Fig. 1i and Extended Data Fig. 4).
Extended Data Fig. 4 Correlation analysis between the abundance of sulfoglycosidase (BbhII or Sgl)-specific reads and the abundance of each bacterial species-specific reads in a deposited metagenomic dataset.
Bacterial species whose abundances show statistically significant correlations (q < 0.05) with the abundance of bbhII homologues (a), sgl homologues (b, c), in the metagenomic dataset21 are shown. Relative abundances of species- and gene-specific reads were calculated as described in the Methods section and used for two-tailed Spearman’s rank correlation analysis. The reads of eighty mother (a, b)-unweaning infant (c) pair samples at 4 months post-delivery were used for the analysis.
Extended Data Fig. 5 Confirmation of bbhII disruption in B. bifidum, heterologous expression of BbhII in B. longum, and recombinant protein preparation.
a, Schematic representation of the bbhII gene inactivation by a single crossover recombination event. Primers used for the construction of a suicide vector (Pr-MS955 and Pr-MS956) are shown (Supplementary Table 9). The numbers of B. bifidum cells in mouse intestines and the bbhII gene in human faeces were determined by qPCR with a primer pair of bbhIIrt-P2-F and bbhIIrt-P2-R (Fig. 1h, Fig. 2b, and Supplementary Table 1). b, Western blot analysis examining the expression of BbhII in B. bifidum. The cell-free extracts prepared from B. bifidum WT and bbhII mutant cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by the detection using anti-BbhII antibodies. As a loading control, the expression of galacto-N-biose/lacto-N-biose I-binding protein (GLBP) was detected with anti-GLBP antibodies50. The images obtained in a single experiment are shown. These two strains were used for mono-colonisation of germ-free mice. c, GlcNAc-6S-releasing activity of cell-free extracts prepared from B. bifidum WT and bbhII mutant cells. pNP-β-GlcNAc-6S was used at the concentration of 2 mM. Data are mean ± SD of three independent assays, represented by the bars and whiskers. d and e, The results of SDS-PAGE of purified BbhII variants used for GlcNAc-6S-releasing assay (d) and ELISA and ITC analysis (e). The images of the gels obtained in a single experiment are shown. f, Heterologous expression of BbhII. Cell-free extracts prepared from recombinant B. longum strains harboring BbhII variant genes on plasmids (WT, W183A, and ∆CBM, Supplementary Fig. 3a) were separated by SDS-PAGE, followed by the detection using anti-BbhII antibodies. GLBP was used as the loading control. A representative image obtained in duplicate experiments is shown with essentially the same results obtained. The recombinant cells were used for examining the GlcNAc-6S-releasing activity from PGM O-glycans. g, The results of SDS-PAGE of purified BbhII-His6 variants (WT, W183A, and ∆CBM, Supplementary Fig. 3a). The image of the gel obtained in a single experiment is shown.
Extended Data Fig. 6 Degradation of GAGs by B. bifidum.
Heparan sulfate (HS), keratan sulfate (KS), chondroitin sulfate A (CS), and hyaluronan (HA) (0.4% each) were incubated with B. bifidum cell suspensions (equivalent to OD600 = 0.4) for 24 h at 37 °C, and the reaction mixtures were analysed by thin-layer chromatography. The data obtained in a single experiment are shown. PGM was used as a positive control. Standard sugars used are Fuc, GlcNAc, GalNAc, Gal, GlcNAc-6S, and NeuAc.
Extended Data Fig. 7 Identification of BbhII-susceptible and resistant O-glycan structures of PCM.
PCM was incubated in the absence and presence of purified BbhII (WTc-His6). O-glycans were then analysed with MALDI-TOF/MS. a, The representative full-mass profiles (m/z 400–2400) of non-sulfated (left) and sulfated (right), permethylated O-glycan fractions obtained from non-treated (upper panels) and BbhII-treated (lower panels) PCM. The ion peaks shown in red are the externally added standards: LNFP I* (m/z 1100.5) and sulfo-Lewisa trisaccharide** (m/z 780.4). The analysis was conducted in technical triplicate. b, A Volcano plot comparing non-treated with BbhII-treated PCM O-glycans. Fold-changes of the estimated glycan amounts and their q-values were plotted. The q-values are the adjusted p-values obtained by multiple t-tests with false discovery rate correction with Q = 5% with the mean ± s.d. of three independent experiments used for evaluation. c, A MS/MS spectrum of the most abundant, BbhII-susceptible sulfated glycan at m/z 1041 obtained in a. The deduced O-glycan structure is shown with its fragmentation pattern. Glycan symbols are shown in inset.
Extended Data Fig. 8 BbhII-resistant O-glycan structures of PCM.
a and b, Data obtained in Extended Data Fig. 7 were further analysed here. a, A MS/MS spectrum of the BbhII-resistant peak at m/z 1246 obtained from non-treated PCM. Two predicted glycan structures are shown with their fragmentation patterns. b, MS/MS spectra of the m/z 1491 peaks obtained from non-treated PCM (upper panel) and BbhII-treated PCM (lower panel). The proposed BbhII-susceptible and resistant glycan structures are shown with their fragmentation patterns. The peaks and m/z values of the characteristic fragment ions formed from a predicted BbhII-susceptible glycan are shown in red (upper MS/MS profile). These peaks were not formed when the m/z 1491 peak obtained from BbhII-treated sample was subjected to MS/MS analysis (lower MS/MS profile). Glycan symbols are shown in inset.
Extended Data Fig. 9 Biochemical analyses of BbhII.
a,b, Inhibition of BbhII-catalysed reaction by the synthesised inhibitors. S-v plots (left panels) and Lineweaver-Burk plots (right panels) of pNP-β-GlcNAc-6S hydrolysis by BbhII WTc-His6 in the absence and presence of PUGNAc-6S (a) and NAGT-6S (b). Inhibitor concentrations are shown in the insets. The kinetic parameters were calculated by curve-fitting experimental data to the Michaelis-Menten equation with competitive inhibition with the equation used for fitting shown. The results obtained from a single experiment were used for calculating the parameters. c,d, ITC analysis of BbhII CBM32 N-domain. Thermograms and binding isotherms obtained for pNP-β-GlcNAc-6S (left) and pNP-β-GlcNAc (right) are shown in the top and bottom panels, respectively. WT (c) and W183A (d) CBM-His6 were used for the analysis. The concentrations and c-value are shown in the insets. Values of association constant (Ka), enthalpy of binding (∆H), and binding stoichiometry (n) are expressed with the standard errors from a single fit to one set of sites model. Dissociation constants (Kd) were calculated from the reciprocal of Ka. The Gibbs free energy change (∆G0) and the entropy change (∆S0) were calculated from the equations ∆G0 = − RTlnKa and T∆S0 = ∆H − ∆G0, respectively (R, gas constant; T, absolute temperature). The results obtained from a single technical replicate were used for calculating the parameters.
Extended Data Fig. 10 Possible association between the abundance of muc-CBMs and the prevalence of muc-GHs in the prominent mucinolytic gut microbes.
Exploratory analysis examining effect size and significance of presence and absence of possible muc-GHs on the distribution of possible muc-CBMs in the genomes of selected mucinolytic bacterial species was performed by NMDS, followed by a PERMANOVA with 9,999 iterations. NMDS on the distribution of muc-CBMs was used for ordination based on Bray-Curtis distances. R2 and P values are shown in the table. The colors are: blue, B. bifidum; yellow, Clostridium perfringens; pink, Akkermansia muciniphila; green, Bacteroides caccae; purple, Bacteroides fragilis; orange, Bacteroides thetaiotaomicron; khaki, Prevotella melaninogenica; and gray, Ruminococcus gnavus.
Supplementary information
Supplementary Information
Supplementary Figs. 1–7 and Supplementary Tables 1–9
Supplementary Source Data 1
Statistical source data for Supplementary Fig. 1a,b
Source data
Source Data Fig. 1
Statistical source data for Fig. 1f–i.
Source Data Fig. 2
Statistical source data for Fig. 2b–e.
Source Data Fig. 5
Statistical source data for Fig. 5c–f.
Source Data Extended Data Fig./Table 5
Raw data for Extended Data Fig. 5c and uncropped or unprocessed images for Extended Data Fig. 5b,d–g.
Source Data Extended Data Fig./Table 6
Uncropped image for Extended Data Fig. 6.
Source Data Extended Data Fig./Table 9
Raw data for Extended Data Fig. 9a–d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Katoh, T., Yamada, C., Wallace, M.D. et al. A bacterial sulfoglycosidase highlights mucin O-glycan breakdown in the gut ecosystem. Nat Chem Biol 19, 778–789 (2023). https://doi.org/10.1038/s41589-023-01272-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01272-y