Abstract
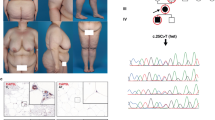
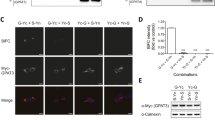
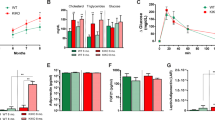
Phospholipase A/acyltransferase 3 (PLAAT3) is a phospholipid-modifying enzyme predominantly expressed in neural and white adipose tissue (WAT). It is a potential drug target for metabolic syndrome, as Plaat3 deficiency in mice protects against diet-induced obesity. We identified seven patients from four unrelated consanguineous families, with homozygous loss-of-function variants in PLAAT3, who presented with a lipodystrophy syndrome with loss of fat varying from partial to generalized and associated with metabolic complications, as well as variable neurological features including demyelinating neuropathy and intellectual disability. Multi-omics analysis of mouse Plaat3−/− and patient-derived WAT showed enrichment of arachidonic acid-containing membrane phospholipids and a strong decrease in the signaling of peroxisome proliferator-activated receptor gamma (PPARγ), the master regulator of adipocyte differentiation. Accordingly, CRISPR–Cas9-mediated PLAAT3 inactivation in human adipose stem cells induced insulin resistance, altered adipocyte differentiation with decreased lipid droplet formation and reduced the expression of adipogenic and mature adipocyte markers, including PPARγ. These findings establish PLAAT3 deficiency as a hereditary lipodystrophy syndrome with neurological manifestations, caused by a PPARγ-dependent defect in WAT differentiation and function.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data generated and analyzed in this study are included in the article. Mouse RNA-seq data have been deposited in the Gene Expression Omnibus at NCBI (GSE233433). The mouse mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD038815. The human and mouse lipidomics data are available as Supplementary Data 1–3 (Plaat3 mouse lipidomics.html; Plaat3 mouse mediator lipidomics.html; PLAAT3 human lipidomics.html). For reasons of privacy, clinical patient sequencing data are not publicly available. Source data are provided with this paper.
Code availability
All multi-omics-related software used in this study are published and cited either in the main text or Methods. No custom code was used for data processing or analysis of the transcriptomics, proteomics or lipidomics datasets. Data analysis approaches using published software packages are described in the Methods and Supplementary Notes. For patients‘ privacy reasons, the Seqplorer codes for variant calling and filtering won’t be publicly available.
References
Pang, X. Y. et al. Structure/function relationships of adipose phospholipase A2 containing a Cys–His–His catalytic triad. J. Biol. Chem. 287, 35260–35274 (2012).
Duncan, R. E., Sarkadi-Nagy, E., Jaworski, K., Ahmadian, M. & Sul, H. S. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA). J. Biol. Chem. 283, 25428–25436 (2008).
Uyama, T., Jin, X. H., Tsuboi, K., Tonai, T. & Ueda, N. Characterization of the human tumor suppressors TIG3 and HRASLS2 as phospholipid-metabolizing enzymes. Biochim. Biophys. Acta 1791, 1114–1124 (2009).
Uyama, T. et al. The tumor suppressor gene H-Rev107 functions as a novel Ca2+-independent cytosolic phospholipase A1/2 of the thiol hydrolase type. J. Lipid Res. 50, 685–693 (2009).
Jaworski, K. et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat. Med. 15, 159–168 (2009).
Hussain, I. & Garg, A. Lipodystrophy syndromes. Endocrinol. Metab. Clin. North Am. 45, 783–797 (2016).
Jéru, I. Genetics of lipodystrophy syndromes. Presse Med. 50, 104074 (2021).
Brown, R. J. et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J. Clin. Endocrinol. Metab. 101, 4500–4511 (2016).
Letourneau, L. R. & Greeley, S. A. W. Congenital forms of diabetes: the β-cell and beyond. Curr. Opin. Genet. Dev. 50, 25–34 (2018).
Sollier, C. et al. Lipodystrophic syndromes: from diagnosis to treatment. Ann. Endocrinol. (Paris) 81, 51–60 (2020).
Plagnol, V. et al. A robust model for read count data in exome sequencing experiments and implications for copy number variant calling. Bioinformatics 28, 2747–2754 (2012).
Raman, L., Dheedene, A., De Smet, M., Van Dorpe, J. & Menten, B. WisecondorX: improved copy number detection for routine shallow whole-genome sequencing. Nucleic Acids Res. 47, 1605–1614 (2019).
Sobreira, N., Schiettecatte, F., Valle, D. & Hamosh, A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 36, 928–930 (2015).
Hummasti, S., Hong, C., Bensinger, S. J. & Tontonoz, P. HRASLS3 is a PPARγ-selective target gene that promotes adipocyte differentiation. J. Lipid Res. 49, 2535–2544 (2008).
Wang, Z. et al. LncPLAAT3-AS regulates PLAAT3-mediated adipocyte differentiation and lipogenesis in pigs through miR-503-5p. Genes (Basel) 14, 161 (2023).
Michaud, A. et al. Expression of genes related to prostaglandin synthesis or signaling in human subcutaneous and omental adipose tissue: depot differences and modulation by adipogenesis. Mediators Inflamm. 2014, 451620 (2014).
Morishita, H. et al. Organelle degradation in the lens by PLAAT phospholipases. Nature 592, 634–638 (2021).
Wishart, D. S. et al. HMDB 5.0: the human metabolome database for 2022. Nucleic Acids Res. 50, D622–d31 (2022).
Qin, Q. et al. Lisa: inferring transcriptional regulators through integrative modeling of public chromatin accessibility and ChIP–seq data. Genome Biol. 21, 32 (2020).
Cataldi, S., Costa, V., Ciccodicola, A. & Aprile, M. PPARγ and diabetes: beyond the genome and towards personalized medicine. Curr. Diab. Rep. 21, 18 (2021).
Lefterova, M. I., Haakonsson, A. K., Lazar, M. A. & Mandrup, S. PPARγ and the global map of adipogenesis and beyond. Trends Endocrinol. Metab. 25, 293–302 (2014).
Zhang, K., Chen, X., Zhang, P. & Liu, G. Perilipin2 is an earlier marker than perilipin1 for identifying adipocyte regeneration in fat grafts. Aesthet. Surg. J. 41, Np646–NP652 (2021).
Olzmann, J. A. & Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 20, 137–155 (2019).
Kaushik, S. & Cuervo, A. M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 17, 759–770 (2015).
Ge, K. et al. Alternative mechanisms by which mediator subunit MED1/TRAP220 regulates peroxisome proliferator-activated receptor γ-stimulated adipogenesis and target gene expression. Mol. Cell. Biol. 28, 1081–1091 (2008).
Gorwood, J. et al. SIV infection and the HIV proteins tat and nef induce senescence in adipose tissue and human adipose stem cells, resulting in adipocyte dysfunction. Cells 9, 854 (2020).
Ahmadian, M. et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat. Med. 19, 557–566 (2013).
Mory, P. B. et al. Atypical generalized lipoatrophy and severe insulin resistance due to a heterozygous LMNA p.T10I mutation. Arq. Bras. Endocrinol. Metabol. 52, 1252–1256 (2008).
Gautheron, J. et al. Loss of thymidine phosphorylase activity disrupts adipocyte differentiation and induces insulin-resistant lipoatrophic diabetes. BMC Med. 20, 95 (2022).
Payne, F. et al. Mutations disrupting the Kennedy phosphatidylcholine pathway in humans with congenital lipodystrophy and fatty liver disease. Proc. Natl Acad. Sci. USA 111, 8901–8906 (2014).
Mann, J. P. & Savage, D. B. What lipodystrophies teach us about the metabolic syndrome. J. Clin. Invest. 129, 4009–4021 (2019).
Barroso, I. et al. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature 402, 880–883 (1999).
Capel, E. et al. MFN2-associated lipomatosis: clinical spectrum and impact on adipose tissue. J. Clin. Lipidol. 12, 1420–1435 (2018).
Sollier, C. et al. LIPE-related lipodystrophic syndrome: clinical features and disease modeling using adipose stem cells. Eur. J. Endocrinol. 184, 155–168 (2021).
Gautheron, J. et al. EPHX1 mutations cause a lipoatrophic diabetes syndrome due to impaired epoxide hydrolysis and increased cellular senescence. eLife 10, e68445 (2021).
Hernandez-Quiles, M., Broekema, M. F. & Kalkhoven, E. PPARγ in metabolism, immunity, and cancer: unified and diverse mechanisms of action. Front. Endocrinol. (Lausanne) 12, 624112 (2021).
Kim, Y. G., Lou, A. C. & Saghatelian, A. A metabolomics strategy for detecting protein-metabolite interactions to identify natural nuclear receptor ligands. Mol. Biosyst. 7, 1046–1049 (2011).
Civelek, E. & Ozen, G. The biological actions of prostanoids in adipose tissue in physiological and pathophysiological conditions. Prostaglandins Leukot. Essent. Fatty Acids 186, 102508 (2022).
Hajnal, A., Klemenz, R. & Schäfer, R. Subtraction cloning of H-rev107, a gene specifically expressed in H-ras resistant fibroblasts. Oncogene 9, 479–490 (1994).
Rochford, J. J. Mouse models of lipodystrophy and their significance in understanding fat regulation. Curr. Top. Dev. Biol. 109, 53–96 (2014).
Le Lay, S., Magré, J. & Prieur, X. Not enough fat: mouse models of inherited lipodystrophy. Front. Endocrinol. (Lausanne) 13, 785819 (2022).
King, E. A., Davis, J. W. & Degner, J. F. Are drug targets with genetic support twice as likely to be approved? Revised estimates of the impact of genetic support for drug mechanisms on the probability of drug approval. PLoS Genet. 15, e1008489 (2019).
Nelson, M. R. et al. The support of human genetic evidence for approved drug indications. Nat. Genet. 47, 856–860 (2015).
Slifer, S. H. PLINK: key functions for data analysis. Curr. Protoc. Hum. Genet. 97, e59 (2018).
Richards, S. et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 17, 405–424 (2015).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Rausch, T. et al. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics 28, i333–i339 (2012).
Layer, R. M., Chiang, C., Quinlan, A. R. & Hall, I. M. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol. 15, R84 (2014).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
DePristo, M. A. et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 43, 491–498 (2011).
Cingolani, P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly (Austin) 6, 80–92 (2012).
Cingolani, P. et al. Using Drosophila melanogaster as a model for genotoxic chemical mutational studies with a new program, SnpSift. Front. Genet. 3, 35 (2012).
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 33, 290–295 (2015).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Kanehisa, M., Goto, S., Sato, Y., Furumichi, M. & Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 40, D109–D114 (2012).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Maia, T. M. et al. Simple peptide quantification approach for MS-based proteomics quality control. ACS Omega 5, 6754–6762 (2020).
Chiva, C. et al. QCloud: a cloud-based quality control system for mass spectrometry-based proteomics laboratories. PLoS ONE 13, e0189209 (2018).
Zhang, X. et al. Proteome-wide identification of ubiquitin interactions using UbIA-MS. Nat. Protoc. 13, 530–550 (2018).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Acknowledgements
The authors would like to thank the patients and families who participated in this study and the GenomEast facility (IGBMC) for exome sequencing in patient 4. We thank M. Baetens for in-depth CNV-seq analysis. We also thank L. Müller and P. Pellet for their technical help in genetic analyses in families 3 and 4. J.G. is funded by the Fondation pour la Recherche Médicale (FRM; ARF20170938613 and EQU202003010517), the Société Francophone du Diabète (SFD; R19114DD), the Mairie de Paris (Emergences—R18139DD) and the Agence Nationale de la Recherche (ANR-21-CE18-0002-01). B.D. is supported by an Odysseus type 1 Grant of the Research Foundation Flanders (G0H8318N) and a starting grant from Ghent University Special Research Fund (01N10319). N.M. is supported by the Exploratory Research for Advanced Technology (ERATO) research funding program of the Japan Science and Technology Agency (JPMJER1702) and a Grant-in-Aid for Specially Promoted Research from the Japan Society for the Promotion of Science (22H04919). The Program for Undiagnosed Diseases (UD-PrOZA) is supported by the Spearhead Research Policy Program and the Fund for Innovation from the Ghent University Hospital. The Neuromendeliome Study (to C.D., Strasbourg, France) was financially supported by Agence de la Biomédecine (France). C.V. and M.A. are supported by institutional funding from Inserm, Sorbonne Université, Assistance-Publique Hôpitaux de Paris, by the Fondation pour la Recherche Médicale (grant EQU201903007868), and by the Association Française des Lipodystrophies (AFLIP), through a donation to Association Robert-Debré pour la Recherche Médicale (ARDRM). D.H. would like to thank S. van Sprang and the whole team at the Academia Belgica, Center for History, Arts and Sciences in Rome, Italy (https://www.academiabelgica.it), for supporting the writing of this manuscript. The authors of this study are members of the European Reference Network for Rare Neurological Diseases (ERN-RND to D.H. and B.D.), the Solve-RD Consortium (N.S., D.H., B.P. and B.D) and the European Reference Network on Rare Endocrine Conditions (Endo-ERN, Project ID 739527 to C.V.). For more information about the ERNs and the EU health strategy, visit http://ec.europa.eu/health/ern. For more information about Solve-RD, visit https://solve-rd.eu.
Author information
Authors and Affiliations
Consortia
Contributions
N.S., D.H., J.G., S.E.C., C.D., I.J. and B.D. wrote the manuscript. N.S., J.G., I.J. and B.D. designed the study and performed the main analyses. N.S., S.E.C., D.H., M.V., S.N., M.T., C.V., N.V.D., F.R.C., J.A.U., J.C., W.T., B.D. and S.C. were involved in phenotyping and clinical follow-up of the patients. N.S., E.B., T.R., G.D., E.D., B.F., F.A., S.K., P.H., C.D., B.P., I.J. and B.D. were involved in genotyping of the patients. S.D. and F.I. performed the proteomics analysis. J.G. designed the CRISPR–Cas9-mediated PLAAT3 KO cellular model. M.A. provided technical support for the cell experiments. N.S. and B.D. collected adipose tissue biopsy specimens. F.O. and N.M. provided WAT from Plaat3−/− and Plaat3+/− mice. J.V.D. and C.V.H. performed histological analyses of human and mouse WAT. N.S., D.H., J.G., I.J. and B.D. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Robert Semple, David Savage and Rebecca Brown for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes, Supplementary Figs. 1–4 and Supplementary Tables 1–8.
Supplementary Data 1
Lipidomics report of Plaat3−/− and Plaat3+/− mice.
Supplementary Data 2
Mediator lipidomics report of Plaat3−/− and Plaat3+/− mice.
Supplementary Data 3
Lipidomics report of PLAAT3 patients and normal controls.
Source data
Source Data Fig. 5
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Schuermans, N., El Chehadeh, S., Hemelsoet, D. et al. Loss of phospholipase PLAAT3 causes a mixed lipodystrophic and neurological syndrome due to impaired PPARγ signaling. Nat Genet 55, 1929–1940 (2023). https://doi.org/10.1038/s41588-023-01535-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01535-3
This article is cited by
-
Identification of genomic characteristics and selective signals in Guizhou black goat
BMC Genomics (2024)
-
Phospholipid biosynthetic pathways and lipodystrophies: a novel syndrome due to PLAAT3 deficiency
Nature Reviews Endocrinology (2024)