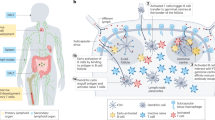
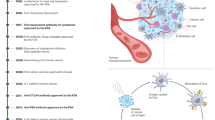
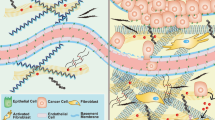
Abstract
Disease progression is usually accompanied by changes in the biochemical composition of cells and tissues and their biophysical properties. For instance, hallmarks of cancer include the stiffening of tissues caused by extracellular matrix remodelling and the softening of individual cancer cells. In this context, accumulating evidence has shown that immune cells sense and respond to mechanical signals from the environment. However, the mechanisms regulating these mechanical aspects of immune surveillance remain partially understood. The growing appreciation for the ‘mechano-immunology’ field has urged researchers to investigate how immune cells sense and respond to mechanical cues in various disease settings, paving the way for the development of novel engineering strategies that aim at mechanically modulating and potentiating immune cells for enhanced immunotherapies. Recent pioneer developments in this direction have laid the foundations for leveraging ‘mechanical immunoengineering’ strategies to treat various diseases. This Review first outlines the mechanical changes occurring during pathological progression in several diseases, including cancer, fibrosis and infection. We next highlight the mechanosensitive nature of immune cells and how mechanical forces govern the immune responses in different diseases. Finally, we discuss how targeting the biomechanical features of the disease milieu and immune cells is a promising strategy for manipulating therapeutic outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Klotter, V. et al. Assessment of pathologic increase in liver stiffness enables earlier diagnosis of CFLD: results from a prospective longitudinal cohort study. PLoS ONE 12, e0178784 (2017).
Medrano, L. M. et al. Elevated liver stiffness is linked to increased biomarkers of inflammation and immune activation in HIV/hepatitis C virus-coinfected patients. AIDS 32, 1095–1105 (2018).
Tomlin, H. & Piccinini, A. M. A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens. Immunology 155, 186–201 (2018).
Martinez-Vidal, L. et al. Causal contributors to tissue stiffness and clinical relevance in urology. Commun. Biol. 4, 1011 (2021).
Mohammadi, H. & Sahai, E. Mechanisms and impact of altered tumour mechanics. Nat. Cell Biol. 20, 766–774 (2018).
Du, H. et al. Tuning immunity through tissue mechanotransduction. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-022-00761-w (2022).
Zhu, C., Chen, W., Lou, J., Rittase, W. & Li, K. Mechanosensing through immunoreceptors. Nat. Immunol. 20, 1269–1278 (2019).
Judokusumo, E., Tabdanov, E., Kumari, S., Dustin, M. L. & Kam, L. C. Mechanosensing in T lymphocyte activation. Biophys. J. 102, L5–L7 (2012).
O’Connor, R. S. et al. Substrate rigidity regulates human T cell activation and proliferation. J. Immunol. 189, 1330–1339 (2012).
Saitakis, M. et al. Different TCR-induced T lymphocyte responses are potentiated by stiffness with variable sensitivity. eLife 6, e23190 (2017).
Blumenthal, D., Chandra, V., Avery, L. & Burkhardt, J. K. Mouse T cell priming is enhanced by maturation-dependent stiffening of the dendritic cell cortex. eLife 9, e55995 (2020). Important work that sheds light on the mechanical aspect of dendritic cell-mediated activation of T cells.
Basu, R. et al. Cytotoxic T cells use mechanical force to potentiate target cell killing. Cell 165, 100–110 (2016). Seminal study that highlights the critical role of mechanical forces in cytotoxic activity of T cells.
Liu, Y. et al. Cell softness prevents cytolytic T-cell killing of tumor-repopulating cells. Cancer Res. 81, 476–488 (2021).
Tello-Lafoz, M. et al. Cytotoxic lymphocytes target characteristic biophysical vulnerabilities in cancer. Immunity 54, 1037–1054.e7 (2021).
Lei, K. et al. Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy. Nat. Biomed. Eng. 5, 1411–1425 (2021). Influential studies (refs. 14,15) that show that stiffening tumour cells through genetic manipulation targeting MRTF or by depleting cholesterol of the cell membrane results in higher vulnerabiliy to T-cell-mediated killing.
Provenzano, P. P. et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38 (2006).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009).
Goetz, J. G. et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146, 148–163 (2011).
Massagué, J. TGFβ in cancer. Cell 134, 215–230 (2008).
Insua‐Rodríguez, J. et al. Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis. EMBO Mol. Med. 10, e9003 (2018).
He, X. et al. Extracellular matrix physical properties govern the diffusion of nanoparticles in tumor microenvironment. Proc. Natl Acad. Sci. USA 120, e2209260120 (2023).
Salmon, H. et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest. 122, 899–910 (2012).
Salnikov, A. V. et al. Lowering of tumor interstitial fluid pressure specifically augments efficacy of chemotherapy. FASEB J. 17, 1756–1758 (2003).
Guck, J. et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J. 88, 3689–3698 (2005).
Plodinec, M. et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 7, 757–765 (2012).
Chen, Y., McAndrews, K. M. & Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 18, 792–804 (2021).
Gensbittel, V. et al. Mechanical adaptability of tumor cells in metastasis. Dev. Cell 56, 164–179 (2021). This review presents the hypothesis that tumour cells adjust their mechanical properties throughout their metastatic journey.
Lv, J. et al. Cell softness regulates tumorigenicity and stemness of cancer cells. EMBO J. 40, e106123 (2021).
Matthews, H. K. et al. Oncogenic signaling alters cell shape and mechanics to facilitate cell division under confinement. Dev. Cell 52, 563–573.e3 (2020).
Young, K. M. et al. Correlating mechanical and gene expression data on the single cell level to investigate metastatic phenotypes. iScience 26, 106393 (2023).
Rianna, C., Radmacher, M. & Kumar, S. Direct evidence that tumor cells soften when navigating confined spaces. Mol. Biol. Cell 31, 1726–1734 (2020).
Regmi, S., Fu, A. & Luo, K. Q. High shear stresses under exercise condition destroy circulating tumor cells in a microfluidic system. Sci. Rep. 7, 39975 (2017).
Moose, D. L. et al. Cancer cells resist mechanical destruction in circulation via rhoa/actomyosin-dependent mechano-adaptation. Cell Rep. 30, 3864–3874.e6 (2020).
Chen, J. et al. Efficient extravasation of tumor-repopulating cells depends on cell deformability. Sci. Rep. 6, 19304 (2016).
Saito, D. et al. Stiffness of primordial germ cells is required for their extravasation in avian embryos. iScience 25, 105629 (2022).
Er, E. E. et al. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat. Cell Biol. 20, 966–978 (2018).
Wen, Z., Zhang, Y., Lin, Z., Shi, K. & Jiu, Y. Cytoskeleton—a crucial key in host cell for coronavirus infection. J. Mol. Cell. Biol. 12, 968–979 (2021).
Paluck, A. et al. Role of ARP2/3 complex-driven actin polymerization in RSV infection. Pathogens 11, 26 (2021).
Kubánková, M. et al. Physical phenotype of blood cells is altered in COVID-19. Biophys. J. 120, 2838–2847 (2021).
Yang, J., Barrila, J., Roland, K. L., Ott, C. M. & Nickerson, C. A. Physiological fluid shear alters the virulence potential of invasive multidrug-resistant non-typhoidal Salmonella typhimurium D23580. npj Microgravity 2, 16021 (2016).
Padron, G. C. et al. Shear rate sensitizes bacterial pathogens to H2O2 stress. Proc. Natl Acad. Sci. USA 120, e2216774120 (2023).
Mikaty, G. et al. Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathog. 5, e1000314 (2009).
Kuo, C. et al. Rhinovirus infection induces extracellular matrix protein deposition in asthmatic and nonasthmatic airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 300, L951–L957 (2011).
Nagy, N. et al. Hyaluronan in immune dysregulation and autoimmune diseases. Matrix Biol. 78–79, 292–313 (2019).
Fingleton, B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta Mol. Cell Res. 1864, 2036–2042 (2017).
Krishnamurty, A. T. & Turley, S. J. Lymph node stromal cells: cartographers of the immune system. Nat. Immunol. 21, 369–380 (2020).
Wynn, T. A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 208, 1339–1350 (2011).
Tschöpe, C. et al. Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat. Rev. Cardiol. 18, 169–193 (2021).
Fabre, T. et al. Identification of a broadly fibrogenic macrophage subset induced by type 3 inflammation. Sci. Immunol. 8, eadd8945 (2023).
de Boer, R. A. et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur. J. Heart Fail. 21, 272–285 (2019).
Liu, F. et al. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 190, 693–706 (2010).
Georges, P. C. et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G1147–G1154 (2007).
Stock, K. F. et al. ARFI-based tissue elasticity quantification in comparison to histology for the diagnosis of renal transplant fibrosis. Clin. Hemorheol. Microcirc. 46, 139–148 (2010).
Gadd, V. L. et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology 59, 1393–1405 (2014).
Mogilenko, D. A., Shchukina, I. & Artyomov, M. N. Immune ageing at single-cell resolution. Nat. Rev. Immunol. 22, 484–498 (2022).
Roman, M. J. et al. Arterial stiffness in chronic inflammatory diseases. Hypertension 46, 194–199 (2005).
Klingberg, F., Hinz, B. & White, E. S. The myofibroblast matrix: implications for tissue repair and fibrosis: the myofibroblast matrix. J. Pathol. 229, 298–309 (2013).
Liu, F. et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L344–L357 (2015).
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C. & Brown, R. A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349–363 (2002).
Munger, J. S. et al. A mechanism for regulating pulmonary inflammation and fibrosis: the integrin αvβ6 binds and activates latent TGF β1. Cell 96, 319–328 (1999).
Santos, A. & Lagares, D. Matrix stiffness: the conductor of organ fibrosis. Curr. Rheumatol. Rep. 20, 2 (2018).
Morvan, M. G. & Lanier, L. L. NK cells and cancer: you can teach innate cells new tricks. Nat. Rev. Cancer 16, 7–19 (2016).
Janeway, C. A. How the immune system works to protect the host from infection: a personal view. Proc. Natl Acad. Sci. USA 98, 7461–7468 (2001).
Dustin, M. L. T-cell activation through immunological synapses and kinapses. Immunol. Rev. 221, 77–89 (2008).
Feng, Y., Zhao, X., White, A. K., Garcia, K. C. & Fordyce, P. M. A bead-based method for high-throughput mapping of the sequence- and force-dependence of T cell activation. Nat. Methods 19, 1295–1305 (2022).
Mordechay, L. et al. Mechanical regulation of the cytotoxic activity of natural killer cells. ACS Biomater. Sci. Eng. 7, 122–132 (2021).
Lei, K., Kurum, A. & Tang, L. Mechanical immunoengineering of T cells for therapeutic applications. Acc. Chem. Res. 53, 2777–2790 (2020). Comprehensive review on recent advances in mechanical immunoengineering and their potential therapeutic applications.
Seghir, R. & Arscott, S. Extended PDMS stiffness range for flexible systems. Sens. Actuators Phys. 230, 33–39 (2015).
Guimarães, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Denisin, A. K. & Pruitt, B. L. Tuning the range of polyacrylamide gel stiffness for mechanobiology applications. ACS Appl. Mater. Interfaces 8, 21893–21902 (2016).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Follain, G. et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat. Rev. Cancer 20, 107–124 (2020).
Baratchi, S. et al. Transcatheter aortic valve implantation represents an anti-inflammatory therapy via reduction of shear stress–induced, piezo-1–mediated monocyte activation. Circulation 142, 1092–1105 (2020).
Serafini, N. et al. The TRPM4 channel controls monocyte and macrophage, but not neutrophil, function for survival in sepsis. J. Immunol. 189, 3689–3699 (2012).
Beningo, K. A. & Wang, Y. Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target. J. Cell Sci. 115, 849–856 (2002).
Sosale, N. G. et al. Cell rigidity and shape override CD47’s ‘self’-signaling in phagocytosis by hyperactivating myosin-II. Blood 125, 542–552 (2015).
Sridharan, R., Cavanagh, B., Cameron, A. R., Kelly, D. J. & O’Brien, F. J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 89, 47–59 (2019).
Hu, Y. et al. Molecular force imaging reveals that integrin-dependent mechanical checkpoint regulates Fcγ-receptor-mediated phagocytosis in macrophages. Nano Lett. 23, 5562–5572 (2023).
Atcha, H. et al. Mechanically activated ion channel Piezo1 modulates macrophage polarization and stiffness sensing. Nat. Commun. 12, 3256 (2021).
Geng, J. et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat. Commun. 12, 3519 (2021).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Rice, A. J. et al. Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 6, e352 (2017).
Oliver-De La Cruz, J. et al. Substrate mechanics controls adipogenesis through YAP phosphorylation by dictating cell spreading. Biomaterials 205, 64–80 (2019).
Meli, V. S. et al. YAP-mediated mechanotransduction tunes the macrophage inflammatory response. Sci. Adv. 6, eabb8471 (2020).
Steinman, R. M. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 30, 1–22 (2012).
Moreau, H. D. et al. Macropinocytosis overcomes directional bias in dendritic cells due to hydraulic resistance and facilitates space exploration. Dev. Cell 49, 171–188.e5 (2019).
Laplaud, V. et al. Pinching the cortex of live cells reveals thickness instabilities caused by myosin II motors. Sci. Adv. 7, eabe3640 (2021).
Barbier, L. et al. Myosin II activity is selectively needed for migration in highly confined microenvironments in mature dendritic cells. Front. Immunol. 10, 747 (2019).
Chabaud, M. et al. Cell migration and antigen capture are antagonistic processes coupled by myosin II in dendritic cells. Nat. Commun. 6, 7526 (2015).
Leithner, A. et al. Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse. J. Cell Biol. 220, e202006081 (2021).
Kang, J.-H. et al. Biomechanical forces enhance directed migration and activation of bone marrow-derived dendritic cells. Sci. Rep. 11, 12106 (2021).
van den Dries, K. et al. Geometry sensing by dendritic cells dictates spatial organization and PGE2-induced dissolution of podosomes. Cell. Mol. Life Sci. 69, 1889–1901 (2012).
Chakraborty, M. et al. Mechanical stiffness controls dendritic cell metabolism and function. Cell Rep. 34, 108609 (2021).
Mennens, S. F. B. et al. Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells. Sci. Rep. 7, 17511 (2017).
Figdor, C. G., van Kooyk, Y. & Adema, G. J. C-type lectin receptors on dendritic cells and langerhans cells. Nat. Rev. Immunol. 2, 77–84 (2002).
Bufi, N. et al. Human primary immune cells exhibit distinct mechanical properties that are modified by inflammation. Biophys. J. 108, 2181–2190 (2015).
Comrie, W. A., Babich, A. & Burkhardt, J. K. F-actin flow drives affinity maturation and spatial organization of LFA-1 at the immunological synapse. J. Cell Biol. 208, 475–491 (2015).
Wang, Y. et al. Dendritic cell Piezo1 directs the differentiation of TH1 and Treg cells in cancer. eLife 11, e79957 (2022).
Valignat, M.-P. et al. Lymphocytes can self-steer passively with wind vane uropods. Nat. Commun. 5, 5213 (2014).
Roy, N. H., MacKay, J. L., Robertson, T. F., Hammer, D. A. & Burkhardt, J. K. Crk adaptor proteins mediate actin-dependent T cell migration and mechanosensing induced by the integrin LFA-1. Sci. Signal. 11, eaat3178 (2018).
Hope, J. M. et al. Fluid shear stress enhances T cell activation through Piezo1. BMC Biol. 20, 61 (2022).
Husson, J., Chemin, K., Bohineust, A., Hivroz, C. & Henry, N. Force generation upon T cell receptor engagement. PLoS ONE 6, e19680 (2011). An elegant use of a biomembrane force probe technique for measuring forces exerted by T cells upon engagement with antigen-presenting cells.
Liu, B., Chen, W., Evavold, B. D. & Zhu, C. Accumulation of dynamic catch bonds between TCR and agonist peptide–MHC triggers T cell signaling. Cell 157, 357–368 (2014).
Thauland, T. J., Hu, K. H., Bruce, M. A. & Butte, M. J. Cytoskeletal adaptivity regulates T cell receptor signaling. Sci. Signal. 10, eaah3737 (2017).
Gaertner, F. et al. WASp triggers mechanosensitive actin patches to facilitate immune cell migration in dense tissues. Dev. Cell 57, 47–62.e9 (2022).
Majedi, F. S. et al. T-cell activation is modulated by the 3D mechanical microenvironment. Biomaterials 252, 120058 (2020).
Wang, H. et al. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb. Perspect. Biol. 2, a002279 (2010).
Bashour, K. T. et al. CD28 and CD3 have complementary roles in T-cell traction forces. Proc. Natl Acad. Sci. USA 111, 2241–2246 (2014).
Hu, K. H. & Butte, M. J. T cell activation requires force generation. J. Cell Biol. 213, 535–542 (2016).
Liu, Y. et al. DNA-based nanoparticle tension sensors reveal that T-cell receptors transmit defined pN forces to their antigens for enhanced fidelity. Proc. Natl Acad. Sci. USA 113, 5610–5615 (2016).
Tabdanov, E. et al. Micropatterning of TCR and LFA-1 ligands reveals complementary effects on cytoskeleton mechanics in T cells. Integr. Biol. 7, 1272–1284 (2015).
Govendir, M. A. et al. T cell cytoskeletal forces shape synapse topography for targeted lysis via membrane curvature bias of perforin. Dev. Cell 57, 2237–2247.e8 (2022).
Wang, M. S. et al. Mechanically active integrins target lytic secretion at the immune synapse to facilitate cellular cytotoxicity. Nat. Commun. 13, 3222 (2022).
Liu, C. S. C. et al. Cutting edge: Piezo1 mechanosensors optimize human T cell activation. J. Immunol. 200, 1255–1260 (2018).
Jin, W. et al. T cell activation and immune synapse organization respond to the microscale mechanics of structured surfaces. Proc. Natl Acad. Sci. USA 116, 19835–19840 (2019).
Kumari, S. et al. Cytoskeletal tension actively sustains the migratory T‐cell synaptic contact. EMBO J. 39, e102783 (2020).
Huby, R. D. J., Weiss, A. & Ley, S. C. Nocodazole inhibits signal transduction by the T cell antigen receptor. J. Biol. Chem. 273, 12024–12031 (1998).
Le Saux, G. et al. Nanoscale mechanosensing of natural killer cells is revealed by antigen-functionalized nanowires. Adv. Mater. 31, 1805954 (2019).
Bhingardive, V. et al. Nanowire based mechanostimulating platform for tunable activation of natural killer cells. Adv. Funct. Mater. 31, 2103063 (2021).
Brumbaugh, K. M. et al. Functional role for Syk tyrosine kinase in natural killer cell-mediated natural cytotoxicity. J. Exp. Med. 186, 1965–1974 (1997).
Matalon, O. et al. Actin retrograde flow controls natural killer cell response by regulating the conformation state of SHP‐1. EMBO J. 37, e96264 (2018).
Garrity, D., Call, M. E., Feng, J. & Wucherpfennig, K. W. The activating NKG2D receptor assembles in the membrane with two signaling dimers into a hexameric structure. Proc. Natl Acad. Sci. USA 102, 7641–7646 (2005).
Friedman, D. et al. Natural killer cell immune synapse formation and cytotoxicity are controlled by tension of the target interface. J. Cell Sci. 134, jcs258570 (2021).
Yanamandra, A. K. et al. PIEZO1-mediated mechanosensing governs NK cell killing efficiency in 3D. Preprint at https://doi.org/10.1101/2023.03.27.534435 (2023).
Wan, Z. et al. B cell activation is regulated by the stiffness properties of the substrate presenting the antigens. J. Immunol. 190, 4661–4675 (2013).
Natkanski, E. et al. B cells use mechanical energy to discriminate antigen affinities. Science 340, 1587–1590 (2013).
Merino-Cortés, S. V. et al. Diacylglycerol kinase ζ promotes actin cytoskeleton remodeling and mechanical forces at the B cell immune synapse. Sci. Signal. 13, eaaw8214 (2020).
Zeng, Y. et al. Substrate stiffness regulates B-cell activation, proliferation, class switch, and T-cell-independent antibody responses in vivo: Cellular immune response. Eur. J. Immunol. 45, 1621–1634 (2015).
Nowosad, C. R., Spillane, K. M. & Tolar, P. Germinal center B cells recognize antigen through a specialized immune synapse architecture. Nat. Immunol. 17, 870–877 (2016).
Jiang, H. & Wang, S. Immune cells use active tugging forces to distinguish affinity and accelerate evolution. Proc. Natl Acad. Sci. USA 120, e2213067120 (2023).
Stanton, R. J. et al. HCMV pUL135 remodels the actin cytoskeleton to impair immune recognition of infected cells. Cell Host Microbe 16, 201–214 (2014).
Pai, R. K., Convery, M., Hamilton, T. A., Boom, W. H. & Harding, C. V. Inhibition of IFN-γ-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 171, 175–184 (2003).
Samassa, F. et al. Shigella impairs human T lymphocyte responsiveness by hijacking actin cytoskeleton dynamics and T cell receptor vesicular trafficking. Cell. Microbiol. 22, e13166 (2020).
Hanč, P. et al. Structure of the complex of F-actin and DNGR-1, a C-type lectin receptor involved in dendritic cell cross-presentation of dead cell-associated antigens. Immunity 42, 839–849 (2015).
Man, S. M. et al. Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc. Natl Acad. Sci. USA 111, 17588–17593 (2014).
Jacobson, E. C. et al. Migration through a small pore disrupts inactive chromatin organization in neutrophil-like cells. BMC Biol. 16, 142 (2018).
Solis, A. G. et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 573, 69–74 (2019).
Robledo-Avila, F. H., Ruiz-Rosado, J., de, D., Brockman, K. L. & Partida-Sánchez, S. The TRPM2 ion channel regulates inflammatory functions of neutrophils during Listeria monocytogenes infection. Front. Immunol. 11, 97 (2020).
Meng, K. P., Majedi, F. S., Thauland, T. J. & Butte, M. J. Mechanosensing through YAP controls T cell activation and metabolism. J. Exp. Med. 217, e20200053 (2020). This study sheds light on T cells sensing the mechanical signals of their environment and tuning their response accordingly.
Al-Aghbar, M. A., Jainarayanan, A. K., Dustin, M. L. & Roffler, S. R. The interplay between membrane topology and mechanical forces in regulating T cell receptor activity. Commun. Biol. 5, 40 (2022).
Wong, V. W. et al. Mechanical force prolongs acute inflammation via T‐cell‐dependent pathways during scar formation. FASEB J. 25, 4498–4510 (2011).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019).
Dustin, M. L. & Long, E. O. Cytotoxic immunological synapses: NK and CTL synapses. Immunol. Rev. 235, 24–34 (2010).
González-Granado, J. M. et al. Nuclear envelope lamin-A couples actin dynamics with immunological synapse architecture and T cell activation. Sci. Signal. 7, ra37 (2014).
González, C. et al. Nanobody-CD16 catch bond reveals NK cell mechanosensitivity. Biophys. J. 116, 1516–1526 (2019).
Fan, J. et al. NKG2D discriminates diverse ligands through selectively mechano‐regulated ligand conformational changes. EMBO J. 41, e107739 (2022).
Tsopoulidis, N. et al. T cell receptor–triggered nuclear actin network formation drives CD4+ T cell effector functions. Sci. Immunol. 4, eaav1987 (2019).
Tamzalit, F. et al. Interfacial actin protrusions mechanically enhance killing by cytotoxic T cells. Sci. Immunol. 4, eaav5445 (2019).
Sanchez, E. E. et al. Apoptotic contraction drives target cell release by cytotoxic T cells. Nat. Immunol. https://doi.org/10.1038/s41590-023-01572-4 (2023).
Händel, C. et al. Cell membrane softening in human breast and cervical cancer cells. N. J. Phys. 17, 083008 (2015).
Huang, B., Song, B. & Xu, C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat. Metab. 2, 132–141 (2020).
Hanna, R. N. et al. Patrolling monocytes control tumor metastasis to the lung. Science 350, 985–990 (2015).
Vyas, M. et al. Natural killer cells suppress cancer metastasis by eliminating circulating cancer cells. Front. Immunol. 13, 1098445 (2023).
Hu, B., Xin, Y., Hu, G., Li, K. & Tan, Y. Fluid shear stress enhances natural killer cell’s cytotoxicity toward circulating tumor cells through NKG2D-mediated mechanosensing. APL Bioeng. 7, 036108 (2023).
Boussommier-Calleja, A. et al. The effects of monocytes on tumor cell extravasation in a 3D vascularized microfluidic model. Biomaterials 198, 180–193 (2019).
Soderquest, K. et al. Monocytes control natural killer cell differentiation to effector phenotypes. Blood 117, 4511–4518 (2011).
Kumar, B. V., Connors, T. J. & Farber, D. L. Human T cell development, localization, and function throughout life. Immunity 48, 202–213 (2018).
Surcel, A. et al. Pharmacological activation of myosin II paralogs to correct cell mechanics defects. Proc. Natl Acad. Sci. USA 112, 1428–1433 (2015).
Mittelheisser, V. et al. Optimal physicochemical properties of antibody–nanoparticle conjugates for improved tumor targeting. Adv. Mater. 34, 2110305 (2022).
Guo, P. et al. Nanoparticle elasticity directs tumor uptake. Nat. Commun. 9, 130 (2018).
Liang, Q. et al. The softness of tumour-cell-derived microparticles regulates their drug-delivery efficiency. Nat. Biomed. Eng. 3, 729–740 (2019).
Chen, X. et al. Nanoparticle-mediated specific elimination of soft cancer stem cells by targeting low cell stiffness. Acta Biomater. 135, 493–505 (2021).
Perez, J. E. et al. Transient cell stiffening triggered by magnetic nanoparticle exposure. J. Nanobiotechnol. 19, 117 (2021).
Liu, Y. X. et al. Single-cell mechanics provides an effective means to probe in vivo interactions between alveolar macrophages and silver nanoparticles. J. Phys. Chem. B 119, 15118–15129 (2015).
Binnewies, M. et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550 (2018).
Hartmann, N. et al. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin. Cancer Res. 20, 3422–3433 (2014).
Kuczek, D. E. et al. Collagen density regulates the activity of tumor-infiltrating T cells. J. Immunother. Cancer 7, 68 (2019).
Sun, X. et al. Tumour DDR1 promotes collagen fibre alignment to instigate immune exclusion. Nature 599, 673–678 (2021).
Di Martino, J. S. et al. A tumor-derived type III collagen-rich ECM niche regulates tumor cell dormancy. Nat. Cancer 3, 90–107 (2021).
Lampi, M. C. & Reinhart-King, C. A. Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Sci. Transl. Med. 10, eaao0475 (2018).
Diop-Frimpong, B., Chauhan, V. P., Krane, S., Boucher, Y. & Jain, R. K. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl Acad. Sci. USA 108, 2909–2914 (2011).
Liu, J. et al. TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc. Natl Acad. Sci. USA 109, 16618–16623 (2012).
Van Cutsem, E. et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J. Clin. Oncol. 38, 3185–3194 (2020).
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
Zhong, Y. et al. Tumor microenvironment‐activatable nanoenzymes for mechanical remodeling of extracellular matrix and enhanced tumor chemotherapy. Adv. Funct. Mater. 31, 2007544 (2021).
Caruana, I. et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 21, 524–529 (2015).
Prescher, J. A., Dube, D. H. & Bertozzi, C. R. Chemical remodelling of cell surfaces in living animals. Nature 430, 873–877 (2004).
Meng, D. et al. In situ activated NK cell as bio‐orthogonal targeted live‐cell nanocarrier augmented solid tumor immunotherapy. Adv. Funct. Mater. 32, 2202603 (2022).
Zhao, Y. et al. Bioorthogonal equipping CAR-T cells with hyaluronidase and checkpoint blocking antibody for enhanced solid tumor immunotherapy. ACS Cent. Sci. 8, 603–614 (2022).
Saatci, O. et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 11, 2416 (2020).
Nicolas-Boluda, A. et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. eLife 10, e58688 (2021).
De Vita, A. et al. Lysyl oxidase engineered lipid nanovesicles for the treatment of triple negative breast cancer. Sci. Rep. 11, 5107 (2021).
Kim, H. Y. et al. Detection of lysyl oxidase activity in tumor extracellular matrix using peptide-functionalized gold nanoprobes. Cancers 13, 4523 (2021).
Kanapathipillai, M. et al. Inhibition of mammary tumor growth using lysyl oxidase-targeting nanoparticles to modify extracellular matrix. Nano Lett. 12, 3213–3217 (2012).
Vennin, C. et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci. Transl. Med. 9, eaai8504 (2017). A compelling demonstration that altering the mechanical features of the tumour environment holds great potential for improving therapies.
Murphy, K. J. et al. Intravital imaging technology guides FAK-mediated priming in pancreatic cancer precision medicine according to Merlin status. Sci. Adv. 7, eabh0363 (2021).
Tran, E. et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J. Exp. Med. 210, 1125–1135 (2013).
Wang, L.-C. S. et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol. Res. 2, 154–166 (2014).
Rurik, J. G. et al. CAR T cells produced in vivo to treat cardiac injury. Science 375, 91–96 (2022).
Correia, A. L. et al. Hepatic stellate cells suppress NK cell-sustained breast cancer dormancy. Nature 594, 566–571 (2021).
Roberts, E. W. et al. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J. Exp. Med. 210, 1137–1151 (2013).
Fujimori, K., Covell, D. G., Fletcher, J. E. & Weinstein, J. N. Modeling analysis of the global and microscopic distribution of immunoglobulin G, F(ab’)2, and Fab in tumors. Cancer Res. 49, 5656–5663 (1989).
Tabdanov, E. D. et al. Engineering T cells to enhance 3D migration through structurally and mechanically complex tumor microenvironments. Nat. Commun. 12, 2815 (2021).
Whitlock, B. Enhancing Cytotoxic T Cell Killing by PTEN Depletion (Weill Cornell Medicine, 2018).
Li, R., Ma, C., Cai, H. & Chen, W. The CAR T‐cell mechanoimmunology at a glance. Adv. Sci. 7, 2002628 (2020).
Chockley, P. J., Ibanez-Vega, J., Krenciute, G., Talbot, L. J. & Gottschalk, S. Synapse-tuned CARs enhance immune cell anti-tumor activity. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01650-2 (2023). This study shows that improving the immunological synapse architecture of CAR-NK cells leads to superior therapeutic efficacy.
Roybal, K. T. et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 164, 770–779 (2016).
Gordon, W. R. et al. Mechanical allostery: evidence for a force requirement in the proteolytic activation of notch. Dev. Cell 33, 729–736 (2015).
Sloas, D. C., Tran, J. C., Marzilli, A. M. & Ngo, J. T. Tension-tuned receptors for synthetic mechanotransduction and intercellular force detection. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01638-y (2023).
Mittelheisser, V. et al. Leveraging immunotherapy with nanomedicine. Adv. Ther. 3, 2000134 (2020).
Perica, K. et al. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano 8, 2252–2260 (2014).
Majedi, F. S. et al. Augmentation of T-cell activation by oscillatory forces and engineered antigen-presenting cells. Nano Lett. 19, 6945–6954 (2019).
Vis, B. et al. Ultrasmall silica nanoparticles directly ligate the T cell receptor complex. Proc. Natl Acad. Sci. USA 117, 285–291 (2020).
Kim, K.-S. et al. Cationic nanoparticle-mediated activation of natural killer cells for effective cancer immunotherapy. ACS Appl. Mater. Interfaces 12, 56731–56740 (2020).
Sim, T. et al. Magneto-activation and magnetic resonance imaging of natural killer cells labeled with magnetic nanocomplexes for the treatment of solid tumors. ACS Nano 15, 12780–12793 (2021).
Liu, Z. et al. Nanoscale optomechanical actuators for controlling mechanotransduction in living cells. Nat. Methods 13, 143–146 (2016).
Farhadi, A., Ho, G. H., Sawyer, D. P., Bourdeau, R. W. & Shapiro, M. G. Ultrasound imaging of gene expression in mammalian cells. Science 365, 1469–1475 (2019).
Wang, X., Chen, X. & Yang, Y. Spatiotemporal control of gene expression by a light-switchable transgene system. Nat. Methods 9, 266–269 (2012).
Pan, Y. et al. Mechanogenetics for the remote and noninvasive control of cancer immunotherapy. Proc. Natl Acad. Sci. USA 115, 992–997 (2018).
González-Bermúdez, B., Guinea, G. V. & Plaza, G. R. Advances in micropipette aspiration: applications in cell biomechanics, models, and extended studies. Biophys. J. 116, 587–594 (2019).
Otto, O. et al. Real-time deformability cytometry: on-the-fly cell mechanical phenotyping. Nat. Methods 12, 199–202 (2015). Introduction of the state-of-the-art and high-throughput RT-DC technology for measuring the mechanical properties of cells.
Gerum, R. et al. Viscoelastic properties of suspended cells measured with shear flow deformation cytometry. eLife 11, e78823 (2022).
Sánchez-Iranzo, H., Bevilacqua, C., Diz-Muñoz, A. & Prevedel, R. A 3D Brillouin microscopy dataset of the in-vivo zebrafish eye. Data Brief. 30, 105427 (2020).
Conrad, C., Gray, K. M., Stroka, K. M., Rizvi, I. & Scarcelli, G. Mechanical characterization of 3D ovarian cancer nodules using Brillouin confocal microscopy. Cell. Mol. Bioeng. 12, 215–226 (2019).
Wu, P.-H. et al. Particle tracking microrheology of cancer cells in living subjects. Mater. Today 39, 98–109 (2020).
Falchuk, K. & Berliner, R. Hydrostatic pressures in peritubular capillaries and tubules in the rat kidney. Am. J. Physiol. 220, 1422–1426 (1971).
Petrie, R. J. & Koo, H. Direct measurement of intracellular pressure. Curr. Protoc. Cell Biol. 63, (2014).
Harlepp, S., Thalmann, F., Follain, G. & Goetz, J. G. Hemodynamic forces can be accurately measured in vivo with optical tweezers. Mol. Biol. Cell 28, 3252–3260 (2017).
Mongera, A. et al. A fluid-to-solid jamming transition underlies vertebrate body axis elongation. Nature 561, 401–405 (2018).
Mongera, A. et al. Mechanics of the cellular microenvironment as probed by cells in vivo during zebrafish presomitic mesoderm differentiation. Nat. Mater. 22, 135–143 (2023).
Vorselen, D. et al. Microparticle traction force microscopy reveals subcellular force exertion patterns in immune cell–target interactions. Nat. Commun. 11, 20 (2020).
Meng, F., Suchyna, T. M. & Sachs, F. A fluorescence energy transfer-based mechanical stress sensor for specific proteins in situ: mechanical stress sensor. FEBS J. 275, 3072–3087 (2008).
Grashoff, C. et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466, 263–266 (2010).
Conway, D. E. et al. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr. Biol. 23, 1024–1030 (2013).
Pan, X. et al. Assessment of cancer cell migration using a viscosity-sensitive fluorescent probe. Chem. Commun. 58, 4663–4666 (2022).
Shimolina, L. E. et al. Imaging tumor microscopic viscosity in vivo using molecular rotors. Sci. Rep. 7, 41097 (2017).
Sack, I. Magnetic resonance elastography from fundamental soft-tissue mechanics to diagnostic imaging. Nat. Rev. Phys. 5, 25–42 (2022).
Soteriou, D. et al. Rapid single-cell physical phenotyping of mechanically dissociated tissue biopsies. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-023-01015-3 (2023).
Acknowledgements
L.T. acknowledges the grant support from Swiss National Science Foundation (315230_204202, IZLCZ0_206035, CRSII5_205930), European Research Council under the ERC grant agreement MechanoIMM (805337), Swiss Cancer Research Foundation (KFS-4600-08-2018), Kristian Gerhard Jebsen Foundation, Anna Fuller Fund, Xtalpi Inc., and EPFL. J.G.G. acknowledges support from INSERM, University of Strasbourg and charities (Ligue contre le Cancer, Fondation ARC pour la recherche sur le cancer, Association Ruban Rose), which also support fellowships to V.M. and V.G. This work was also supported by grants from Plan Cancer to J.G.G. We apologize to the authors whose work could not be cited due to size constraints.
Author information
Authors and Affiliations
Contributions
V.M., V.G., L.B., W.L., L.T. and J.G.G. researched data for the paper. All authors made substantial contributions to the discussion of content, wrote the paper and reviewed and/or edited the paper before submission.
Corresponding authors
Ethics declarations
Competing interests
L.T. is a co-founder, shareholder and advisor for Leman Biotech. The interests of L.T. were reviewed and managed by EPFL. The other authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Claire Hivroz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mittelheisser, V., Gensbittel, V., Bonati, L. et al. Evidence and therapeutic implications of biomechanically regulated immunosurveillance in cancer and other diseases. Nat. Nanotechnol. 19, 281–297 (2024). https://doi.org/10.1038/s41565-023-01535-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-023-01535-8