Abstract
Protein translation is a tightly regulated cellular process that is essential for gene expression and protein synthesis. The deregulation of this process is increasingly recognized as a critical factor in the pathogenesis of various human diseases. In this review, we discuss how deregulated translation can lead to aberrant protein synthesis, altered cellular functions, and disease progression. We explore the key mechanisms contributing to the deregulation of protein translation, including functional alterations in translation factors, tRNA, mRNA, and ribosome function. Deregulated translation leads to abnormal protein expression, disrupted cellular signaling, and perturbed cellular functions- all of which contribute to disease pathogenesis. The development of ribosome profiling techniques along with mass spectrometry-based proteomics, mRNA sequencing and single-cell approaches have opened new avenues for detecting diseases related to translation errors. Importantly, we highlight recent advances in therapies targeting translation-related disorders and their potential applications in neurodegenerative diseases, cancer, infectious diseases, and cardiovascular diseases. Moreover, the growing interest lies in targeted therapies aimed at restoring precise control over translation in diseased cells is discussed. In conclusion, this comprehensive review underscores the critical role of protein translation in disease and its potential as a therapeutic target. Advancements in understanding the molecular mechanisms of protein translation deregulation, coupled with the development of targeted therapies, offer promising avenues for improving disease outcomes in various human diseases. Additionally, it will unlock doors to the possibility of precision medicine by offering personalized therapies and a deeper understanding of the molecular underpinnings of diseases in the future.
Similar content being viewed by others
Introduction
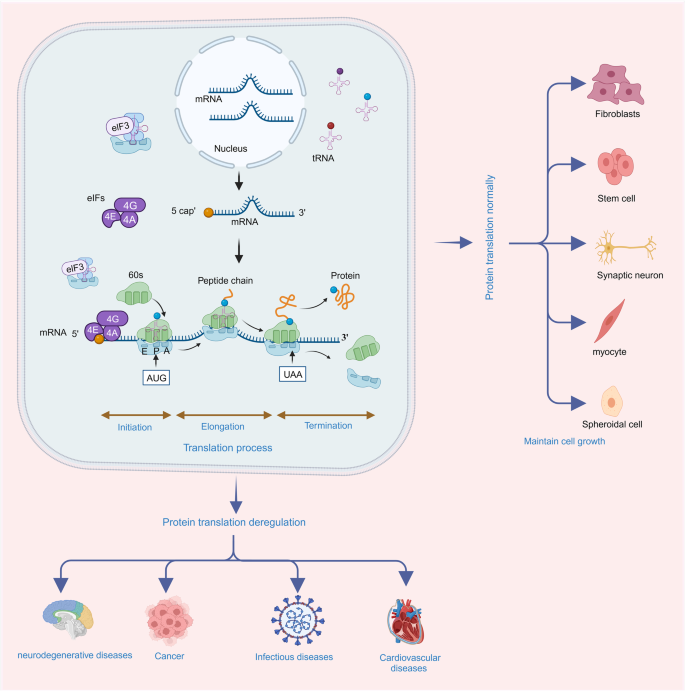
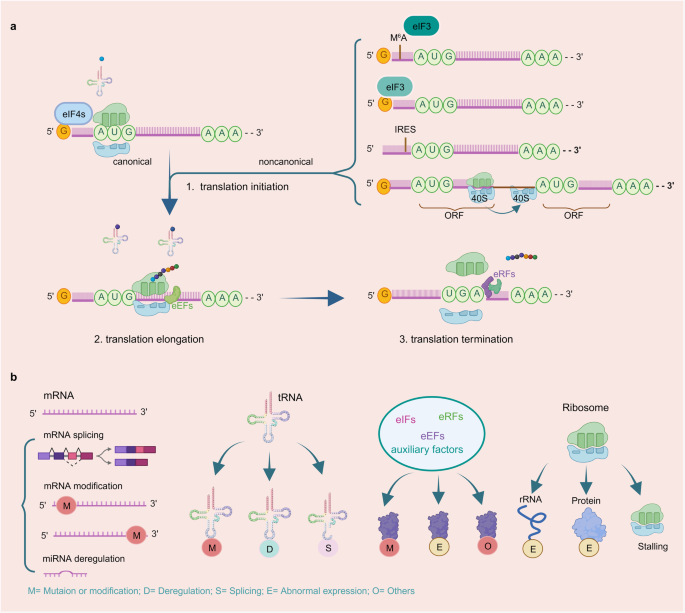
Protein translation, also known as protein synthesis, is a fundamental biological process that involves the conversion of the nucleotide sequence in mRNA into a specific sequence of amino acids, forming a functional protein. This process is essential for all living organisms and plays a central role in gene expression, allowing cells to produce the proteins necessary for their structure, function, and regulation. Protein translation consists of several key steps, including initiation, elongation, and termination (Fig. 1). Initiation typically involves the small ribosomal subunit binding to the mRNA, guided by initiation factors, and scanning for the start codon (usually AUG). Once the start codon is recognized, the large ribosomal subunit joins, and protein synthesis begins with transfer RNA (tRNA) molecules bringing in amino acids to build the growing polypeptide chain. During elongation, tRNA molecules bring in amino acids that match the codons on mRNA. The ribosome moves in a 5’ to 3’ direction along the mRNA, and the tRNA that was previously in the A (aminoacyl) site is moved to the P (peptidyl) site and then the E (exit) site. This allows the next codon on mRNA to enter the A site continuing elongation and peptide bond formation. During termination, release factors (RFs) recognize the stop codon and bind to the A site of the ribosome. This binding triggers the hydrolysis of the bond between the completed polypeptide chain and the final tRNA in the P site, resulting in release of polypeptide from the ribosome.
Protein translation deregulation and its related human disease. Protein translation includes three processes of initiation, elongation and termination. With the participation of ribosome, mRNA, tRNA, and translation related factors, the protein translation process enrolls in orderly to synthesis the nascent peptides accurately, thus, maintaining the cell proliferation and differentiation accurately. When this process is deregulated, the abundance, stability or functions of translated peptides alter and the cell fates run into disease states. Protein translation deregulation leads to neurodegenerative diseases, cancer, infectious diseases, cardiovascular diseases and other diseases
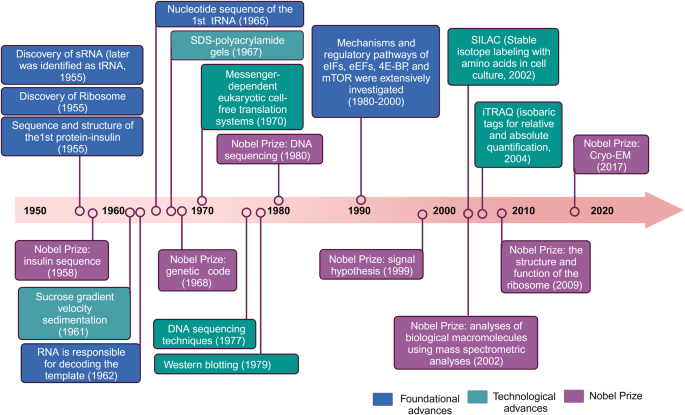
Researches focusing on protein translation has been studied over the last decades (Fig. 2).1 Before the 1950s, research addressing physiological questions of protein translation was largely descriptive in nature.2 In the 1950s, the discovery of tRNA and the characterization of ribosome laid the foundation for understanding the intricate molecular machinery involved in translation.3,4,5 In 1955, Sanger determined the sequence and structure of the first protein (bovine insulin), earning him the Nobel Prize in chemistry three years later.6 Advancements in molecular biology and biochemistry during the 1960s–1970s, led to the elucidation of the genetic code, translation factors and other components of the eukaryotic translation system.7,8 For instance, in 1962, chemical modifications of amino acids substantiated that the RNA component was responsible for decoding the template.9 Subsequently, in 1965, the complete nucleotide sequence of the first nucleic acid, alanine transfer RNA, was determined.10 Three years later, this achievement led to the recognition for the interpretation of the genetic code and its function in protein synthesis.11 Several technological and foundational advancements emerged during this time frame, including Sucrose gradient velocity sedimentation (1961),12 SDS-polyacrylamide gels (1967),13 Western blotting (1979),14 and messenger-dependent eukaryotic cell-free translation systems (1970),15 which researchers began to extensively utilize. Furthermore, the successful application of DNA sequencing techniques in 1977 led to its recognition with the Nobel Prize in 1980.16 During the 1980s–1990s, appreciation for mechanistic and regulatory pathways expands rapidly.17 For example, detailed investigations into the signal pathways involving translation initiation factors, elongation factors, 4E-BP, and mTOR were conducted extensively during this period.1 Moreover, the signal hypothesis, proposing that proteins possess intrinsic signals governing their transport and localization within the cell, was discovered and subsequently awarded the Nobel Prize in Physiology or Medicine in 1999.18 Since then, significant progress has been made in structural biology with the determination of high-resolution structures of ribosomes and translation-related complexes that shed light on the detailed mechanisms of translation.19,20 The Nobel Prize was awarded in 2002 for the identification and structure analyses of biological macromolecules using mass spectrometric analyses, in 2009 for studies of the structure and function of the ribosome, and in 2017 for the development of cryo-electron microscopy, allowing high-resolution structure determination of biomolecules in solution. Furthermore, the advent of genomics and proteomics has enabled researchers to explore translation on a global scale, uncovering the complexity of translation regulation and its role in various cellular processes and diseases.21,22,23
Protein translation deregulation refers to the disruption or alteration of the normal process of protein synthesis, resulting in aberrant protein production or impaired regulation of protein expression.24,25 It encompasses various mechanisms that can affect different stages of translation. In this process, deregulation can occur at the expression level through mutation or modification of mRNA, tRNA, translation factors, ribosomes, or regulatory elements.25,26,27 These deregulations can impair fidelity of translation and increase the occurrence of translation errors such as incorrect amino acid incorporation or premature termination, leading to the synthesis of defective or non-functional proteins and deregulated protein localization.27 Translation deregulation can be induced by various factors including epigenetic modifications, genetic mutations, deregulated translation factors or regulatory proteins, alterations in mRNA stability or localization, as well as environmental and cellular stress conditions.28,29,30
Protein translation deregulation, with its various underlying mechanisms affecting translation, has far-reaching consequences in human diseases, such as neurodegenerative diseases, cancer, infectious diseases, cardiovascular diseases (CVDs) (Fig. 1).31,32,33,34,35,36 The dysregulation can alter protein expression, abnormal protein isoforms, or impaired protein quality control. The dysregulated expression of specific proteins can disrupt normal cellular processes, signaling pathways, and molecular networks, contributing to disease pathogenesis.37 Deregulation of protein translation can result in the production of aberrant protein isoforms with altered sequences, truncations or modifications.33 These abnormal isoforms may have altered functions, loss of regulatory control or gain of toxic properties. When protein translation is dysregulated, the load of misfolded or aberrant proteins may overwhelm the cellular quality control machinery leading to the accumulation of toxic protein aggregates and proteinopathies.38 Overall, deregulation of protein translation plays a significant role in human disease by influencing protein expression, function, and cellular homeostasis. Elucidating the mechanisms of protein translation deregulation in specific diseases can provide insights into disease pathogenesis and guide the development of novel therapeutic strategies.
Mechanisms of protein translation deregulation
Regulation of translation initiation
Translation initiation is a highly regulated process that governs the initiation of protein synthesis in cells. It involves the assembly of the translation machinery at the start codon of mRNA, typically AUG (methionine) in eukaryotes. Depending on the initiation model, translation initiation can be classified into canonical and noncanonical modes (Fig. 3a). The canonical translation initiation process commences with the recognition and subsequent binding of the 5’-cap (m7GpppN) domain of mRNA by the eukaryotic initiation factor (eIF)4E complex (comprising eIF4E, eIF4G, and eIF4A) in a cap-dependent manner. Upon binding to the cap, eIF4E recruits the 43 S ribosomal subunit to the 5’ end of the mRNA, thus activating mRNA translation. This subunit is formed through the interaction of an eIF2•Met-tRNAi•GTP ternary complex and the 40 S ribosome complex. After recruitment, the 43 S ribosomal subunit scans along the mRNA in a 5’ to 3’ direction until it recognizes the AUG start codon. Subsequently, joining of the 60 S ribosomal subunit forms an elongation-competent ribosome (80 S) for peptide elongation contribution.17,39,40,41 While mechanisms underlying noncanonical translation initiation vary in terms categories involving different eIFs, they share common features such as cap recognition and ribosome scanning manner as well as other conditions. Noncanonical translation initiations currently known include N(6)-methyl adenosine (m6A) translation initiation, internal ribosome entry sites (IRESs)-mediated translation initiation, eIF3d translation initiation, and ribosome shunting initiation (Fig. 3a).42 m6A modification is commonly found in both 3’ UTR and 5’ UTR regions of eukaryotic mRNAs.43 In the 5’ UTR, m6A modification can facilitate translation independently of 5’ cap-binding proteins, particularly in response to cellular stress.44 Specifically, a single m6A modification in the 5’ UTR directly interacts with eIF3, thereby independently recruiting 43 S complex for translation initiation even in the absence of the cap-binding factor eIF4E. Selectively inhibition of adenosine methylation reduces translation efficiency of mRNAs harboring m6A in their 5’ UTRs. For example, elevated levels of m6A in the Hsp70 mRNA control its cap-independent translation when cells experience heat shock.45 In the eIF3d mediated translation initiation, eIF3d, a subunit of the eIF3 complex, has a cap-binding activity, which allows it to recognize the mRNA cap structure. eIF3d can operate cap-dependent translation initiation pathway independently of eIF4E, which was previously considered essential for cap recognition.46,47 IRES-mediated translation initiation refers to a mechanism by which certain mRNA molecules, often found in viruses, can initiate protein synthesis within an eukaryotic cell without relying on the traditional cap-dependent translation initiation.48,49,50 IRESs can interact directly with the ribosome and other initiation factors, allowing the ribosome to directly access the start codon without the need for scanning from the 5’ cap. This enables the rapid initiation of protein synthesis, which is crucial for viruses to hijack cellular translation machinery to produce their own proteins within host cells. Ribosome shunting initiation is often observed in plant viruses.51,52 Unlike the typical scanning mechanism where ribosomes start translation at the 5’ cap and move along the mRNA in a linear fashion until they find the start codon, ribosome shunting allows the 40 S to bypass certain sections of mRNA and directly jump or “shunt” to a specific downstream start codon, promoting the initiation of protein translation process.
The mechanism of protein translation process and protein translation deregulation manners. a Process of protein translation. 1. Translation initiation: The canonical translation initiation starts with recognizing and binding with 5′-cap domain of the mRNA by eIF4E complex in a cap dependent manner. After binding with cap, the mRNA translation is activated and recruits the 43 S ribosomal subunit to the 5’ end of the mRNA. Upon the 43 S ribosomal subunit scanning and recognizing the AUG start codon in the mRNA, 60 S ribosomal subunit is recruited and forms an 80 S ribosome complex to contribute for peptides elongation. noncanonical translation initiation vary in the aspects of eIFs categories, the cap recognition manner and the other conditions. The currently known noncanonical translation initiation includes m6A translation initiation, eIF3d translation initiation, IRESs-mediated translation initiation and ribosome shunting. 2. Translation elongation: In this process, the ribosome moves along the mRNA in the 5’ to 3’ direction with the attending of eEFs, the aminoacyl-tRNA in the A site forms a peptide bond with the growing polypeptide chain attached to the tRNA in the P site. The uncharged tRNA shifts from the P site to the E site and the peptidyl-tRNA from the A site to the P site. Then the uncharged tRNA in the E site is released from the ribosome, making way for the next aminoacyl-tRNA to enter the A site and repeat the process. 3. Translation termination: when ribosome complex recognizes a stop codon, termination is triggered. This process is mediated by the release factors eRF1 and eRF3. eRF1 regulates the nascent polypeptide release from the P-site peptidyl-tRNA, whereas eRF3 enhances polypeptide release. b Protein translation deregulation mechanism in mRNA, tRNA, translation factors and ribosome. mRNA: alternative splicing, mutation or modification in 5’ or 3’ UTR of mRNA. tRNA: mutation or modification of tRNA, tRNA deregulation and abnormal splicing. Translational factors: mutation, modification, abnormal expression and other variations. Ribosome: mutation or abnormal expression of ribosome components, ribosome stalling and so on
According to the specific combination and interplay manner, these regulatory mechanisms can vary depending on the cellular stress, developmental stage, viral infections and other conditions.51,53,54 During translational initiation process in cells, mRNA serves as a template, tRNA functions as an amino acid transporter, ribosomal rRNA provides the translation sites and eukaryotic initiation factors, along with other auxiliary proteins work collaboratively to tightly regulate protein synthesis initiation and ensure precise control of gene expression.33 Protein expression dysregulation and post-translational modifications and mutations of initiation-related proteins frequently lead to the inhibition of the translation initiation process.55,56,57 Deregulation of tRNA, including altered tRNA expression, tRNA modifications, tRNA aminoacylation defects, tRNA splicing and maturation defects can contribute to cellular dysfunction and disease.58,59,60 Abnormal expression or mutations of rRNA and ribosome proteins in the ribosome cause aberrant ribosome biogenesis, impairing ribosome functions and inhibiting the translation process.61,62 Deregulations in ribosome such as RPS19, RPS14 and others can lead to a spectrum of diseases including Diamond–Black fan anemia, myelodysplastic syndrome and bone and skeletal abnormalities.36,63,64 In eukaryotes, the presence of a 5’ cap structure on mRNA is crucial for translation initiation. Modifications in 5’ and 3’ UTRs as well as deregulation of enzymes involved in cap formation or cap-binding proteins can affect the integrity or availability of the 5’ cap.65,66,67 This disruption can interfere with the recruitment of translation initiation factors like eIF4E and impair the assembly of the translation initiation complex. Besides, dysregulated miRNA expression, modification or aberrant alternative splicing of mRNA, can lead to abnormal translation inhibition or activation of specific genes.42,68,69,70 These small RNA molecules target specific mRNAs, such as eIFs, splicing factors and upstream regulators, to affect mRNA secondary structure and modulate their expression. By binding to the 3’ or 5’ UTRs of target mRNAs, miRNAs can inhibit their translations or promote their degradation.
Regulation of elongation
Deregulation of elongation, the process by which ribosomes moving along the mRNA during protein synthesis, can significantly impact translation efficiency, fidelity, and protein production (Fig. 3a). The regulation of the protein elongation involves modifications of elongation factors (eEFs), errors in aminoacyl-tRNA selection, tRNA mutation, ribosome stalling, modification of ribosome itself, as well as environmental or cellular stress conditions (Fig. 3b).71,72
Based on the deregulation mechanisms, such as post modification or mutation, eEFs can disrupt their normal function, leading to defects in elongation.73,74 Impaired eEF2 activity can result in reduced ribosome translocation, leading to slower translation rates and potentially affecting protein folding, localization or function.75 Deregulation of alternative mRNA splicing encodes abnormal protein isoforms that disturb the biosynthesis of EEF1B2, thus promoting the progression of diseases in eukaryotes.76 Furthermore, modifications of mRNA, such as methylation or methyl adenosine, disrupt tRNA selection and decoding in the elongation process.77,78 Aminoacyl-tRNAs are selected and delivered to the ribosome based on codon-anticodon recognition. Therefore, deregulation of this process can lead to errors in aminoacyl-tRNA selection and incorporation of incorrect amino acids into the growing polypeptide chain,79 resulting in defective or non-functional proteins that contribute to cellular dysfunction or disease. Additionally, mutation of tRNA is reported to cause ribosome stalling leading to premature polypeptide release and neurodegeneration.80 Ribosome stalling, a phenomenon where ribosomes become temporarily trapped on the mRNA template, can result in translation errors, premature termination, or formation of abnormal protein structures.81,82 This can be caused by various factors, including mRNA secondary structures, codon repeats, rare codons, mRNA damage or limitations in the availability of specific eEFs. Additionally, modifications to ribosomal proteins could modulate the rate of ribosome movement during elongation process.83,84,85 Changes in ribosome speed can influence protein folding, co-translational modifications or interactions with molecular chaperones, which impact the quality and functionality of the synthesized proteins.61 Furthermore, various environmental or cellular stress conditions such as oxidative stress, heat shock, nutrient deprivation or viral infection can influence elongation dynamics. Stress-induced deregulation of eEFs, ribosome modifications or the availability of aminoacyl-tRNAs can affect translation elongation and lead to ribosome pausing or changes in the ribosome composition,86 thus impacting protein synthesis and cellular adaptation to stress.87
Deregulation of elongation can have profound implications on protein synthesis and cellular function. It can result in translation errors, protein misfolding, and alterations in protein abundance or quality, leading to cellular dysfunction, disease pathogenesis or cellular responses to stress. Investigating the mechanisms of elongation deregulation can offer valuable insights into disease processes and potential therapeutic targets.
Regulation of termination
During the termination process, when the stop codon (UAG, UGA, or UAA) of mRNA enters the ribosomal A-site, the protein releasing factor complex eRF1/eRF3•GTP binds to the A-site instead of activated amino acid tRNA, thereby inducing the termination of protein synthesis (Fig. 3a).88 Deregulation of termination, which represents the final stage of protein synthesis, can significant impact translation fidelity and functional proteins production. Abnormal termination occurs due to dysregulated read manner of termination codon and alterations in 3’ UTR of mRNA, ribosome and modifications of termination factors (Fig. 3b).
Normally, termination of translation occurs upon ribosome encountering a stop codon in the mRNA sequence. However, premature termination codons (PTCs) can sometimes be bypassed, and translation continues beyond the intended stop site. This phenomenon, known as PTC readthrough or nonsense suppression, can be induced by various factors, including specific genetic mutations, ribosomal context, or the presence of suppressor tRNAs.89,90 PTC readthrough can lead to the synthesis of elongated or abnormal proteins that could potentially alter their function or stability.90,91 Additionally, changes in regulatory elements within the 3’ UTR of mRNA can influence translation termination efficiency and lead to deregulated termination, aberrant protein synthesis or altered protein levels.92 Ribosome stalling at stop codons is also possible during the process of translation termination.93 Stalling can be caused by mRNA secondary structures, cis-acting sequences, or interactions with specific factors. Ribosome stalling at termination codons can result in the accumulation of incomplete polypeptides, triggering cellular quality control mechanisms such as the nonsense-mediated mRNA decay pathway or the ribosome-associated protein quality control pathway. Furthermore, post-translational modifications of termination factors, such as eRFs or ribosomal proteins, can influence their activity or interactions during termination.94,95,96 Alterations in the modification patterns of termination factors may impact their functions and subsequently affect termination efficiency and fidelity. For instance, phosphorylation of eRF1 or eRF3 has been shown to modulate their interactions with the ribosome or other translation factors, thereby impact termination dynamics.88,97
Furthermore, deregulation of termination can lead to the production of truncated or abnormal proteins, disrupting protein homeostasis and impacting cellular function. This phenomenon may contribute to disease pathogenesis by generating non-functional or toxic proteins, eliciting cellular stress responses, or interfering normal protein-protein interactions. Therefore, targeting deregulation of termination can provide potential intervention therapeutic for human diseases.
Human diseases associated with protein translation deregulation
Neurodegenerative diseases
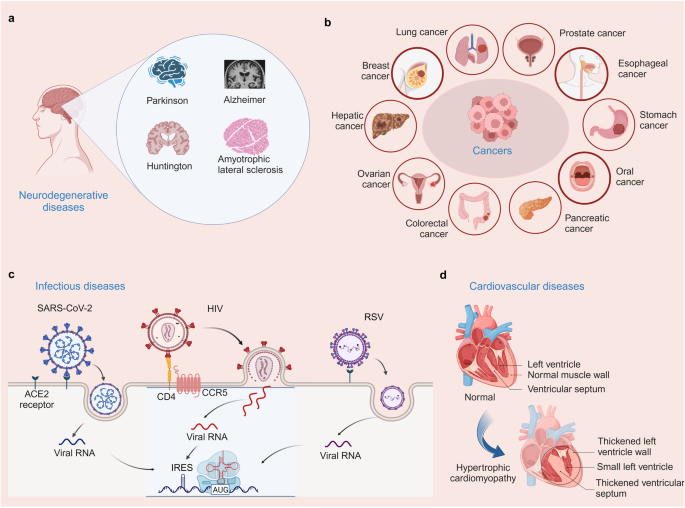
Neurodegenerative diseases encompass a group of debilitating disorders characterized by the progressive loss of neurons in the central nervous system. These diseases, including Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), and Huntington’s disease (HD), impose a significant burden on global healthcare systems (Fig. 4a).98 Despite extensive research efforts, the precise causes and mechanisms underlying these disorders remain elusive. This section provides an overview of the association between protein translation deregulation and neurodegenerative diseases, with a special focus on mRNA, tRNA, translation factors, and ribosome aspects. The development of novel drugs targeting these translation regulators have provided compelling evidence in neurodegenerative diseases.99,100,101,102 However, most of these disorders progress exhibit rapid progression and currently lack effective treatments capable of halting or reversing disease advancement. Available therapies mainly focus on symptom management or offer only a modest extension to lifespan. This underscores the urgent need to identify compounds that can regulate the affected factors, with the aim of alleviating translational defects or protein accumulation.
Human diseases associated with protein translation deregulation. a Neurodegenerative diseases (Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and amyotrophic lateral sclerosis) associated with protein translation deregulation. b Cancers (including lung cancer, breast cancer, liver cancer, ovarian cancer, colon cancer, pancreatic cancer, oral cancer, stomach cancer, esophageal cancer, and prostate cancer) associated with protein translation deregulation. c Infectious diseases (SARS-CoV-2, HIV, RSV) associated with protein translation deregulation. d Cardiovascular diseases associated with protein translation deregulation (Hypertrophic cardiomyopathy)
Accumulation of evidence showed that chronic dysregulation of cellular processes leads to the gradual build-up of subtle cytotoxic effects, ultimately resulting in premature death of dopaminergic neurons.103 Several cellular processes have been extensively studied including protein folding, mitochondrial physiology, membrane physiology, vesicular transport, gene transcription, protein degradation and autophagy.104 Recent studies have revealed the involvement of several PD-related proteins in protein translation processes. Abnormal aggregation of α-synaptic nuclear proteins may also occur during the onset of PD and is closely related to protein deregulation. A notable example is eIF4G1, which has been linked to both PD.105 and Lewy body dementia.106 eIF4G1 is a translation initiation factor that facilitates the recruitment of ribosomes and tRNAs to the 5’ cap structure of mRNA by acting as a scaffold in the eIF4F translation initiation complex.107 Additionally, studies have shown the relevance of tRNA enzymes in neurological disorders. Furthermore, a recent study showed an association between a single nucleotide polymorphism in the mitochondrial translation initiation factor 3 gene and PD risk.108 Translation factor activity is regulated by signaling pathways like PI3K, mTOR, and MAPKs that modulate general translation factors or factors influencing mRNA transport or stability.109,110 For example, the deregulated mTOR pathway in PD controls translation proteins including eIF4E inhibitor 4E-BP, eEF2 kinase, and ribosomal S6K.111 ALS is a progressive neurodegenerative disease characterized by loss of motor neurons leading to muscle weakness. Most cases of ALS are sporadic, some similar familial cases exist clinically. When eIF2α is phosphorylated, the global protein synthesis process is attenuated.112 The unfolded protein response sensor PERK is activated by high levels of misfolded proteins to phosphorylate and globally inhibit the translation factor eIF2α.113 Dysregulated levels of eIF2α and other cellular stress biomarkers have been observed in specimens from ALS patients and disease models.114 Modulation of eIF2α phosphorylation or upstream factors has emerged as a potential therapeutic approach. eIF2α undergoes phosphorylation by the guanine nucleotide exchanger eIF2B, leading to inhibition of eIF2B activity by phospho-eIF2α.115 eIF2α can be phosphorylated by both PERK and eIF2B. Halliday et al. conducted a screening for safe compounds targeting eIF2α phosphorylation and found that trazodone hydrochloride (a licensed antidepressant) and dibenzoyl methane could reverse p-eIF2α induced translational repression without affecting eIF2α levels. These compounds were able to rescue deficits and provide neuroprotection in vitro as well as in mouse models of prion disease and tauopathy.99 As the most prevalent neurodegenerative disorder, AD is characterized aberrant accumulation of misfolded proteins in the endoplasmic reticulum (ER).116 In AD, aggregates are composed of the amyloid-β (Aβ) peptide and tau protein, which form extracellular amyloid plaques and intracellular neurofibrillary tangles (NFT), respectively. Although these insoluble aggregates are classical histopathological hallmarks of AD, a substantial body of evidence indicates that soluble oligomeric forms of Aβ (AβOs) and tau (TauOs) are the most neurotoxic forms, inducing brain oxidative stress, mitochondrial damage, deregulation of intracellular signaling pathways, synapse failure and memory deficits.112 The key histological findings in AD encompass the accumulation of amyloid precursor protein (APP) and its cleavage into amyloid beta by β-site APP-cleaving enzyme 1 (BACE1). Amyloid beta aggregation forms extracellular plaques and intracellular neurofibrillary tangles. Additionally, the accumulation of tau protein is also gaining attention as a significant feature of AD.117 Increased phosphorylation of eIF2α has been observed in postmortem samples from sporadic AD patients and transgenic mouse models.118 In 2016, a study reported that Gastrodin suppressed BACE1 expression in the hippocampi of Tg2576 AD mice under oxidative stress by inhibiting the PKR/eIF2α signaling pathway. Furthermore, Gastrodin improved learning and memory while ameliorating oxidative stress in the hippocampi of Tg2576 mice overexpressing the Swedish mutation of the amyloid precursor protein, suggesting it may be a potential candidate of AD treatment.119 Genetic or pharmacological inhibition of eIF2α phosphorylation can restore memory and prevent neurodegeneration.120 HD is a progressive and fatal neurodegenerative disorder. The pathogenesis of HD is attributed to an expanded CAG trinucleotide repeat in the HTT gene encoding huntingtin protein. This leads to an abnormally elongated polyglutamine tract within the mutant huntingtin protein, which elicits cytotoxicity and neural cell death through both gain-of-function and loss-of-function mechanisms, ultimately leading to the characteristic clinical manifestations of HD.121 In terms of protein translation interventions for Huntington’s disease, potential strategies include targeting Huntington’s DNA and RNA as well as promoting protein clearance.121,122,123
Collectively, understanding the intricate relationship between protein translation and neuronal function is essential for the development of effective therapeutic interventions.
Cancers
In recent years, there has been growing evidence of deregulation in protein translation, which plays a crucial role in the development and progression of various cancers (Fig. 4b). In this section, we provide an overview of protein translation deregulation in different cancers based on the alteration of mRNA, tRNA, translation factors, and ribosome aspects (Table 1).
First, depletion of leucyl-tRNA synthetase reduces the abundance of specific leucine tRNAs, thereby affecting leucine codon-dependent translation and promoting tumor formation and proliferation in breast cancer.124 Recent studies have demonstrated dysregulation of tRNA expression levels, particularly tRNA-Leu and tRNA-Tyr, in breast cancer.125,126 The dysregulation of RPL15, a gene that encodes a component of the large ribosomal subunit, significantly affects the protein synthesis process in circulating tumor cells, leading to the accumulation of proliferation and epithelial markers in these cells. Deregulation of RPL15 expression in ribosomes leads to enhanced translation of cell cycle regulator proteins, thereby promoting tumor metastasis.127 Deregulation of translation factors, such as eIF2, eIF4A, eEF2, and eEF2K, is consistently observed in breast cancer.128,129,130,131,132,133 Furthermore, deregulation of the mechanistic target of rapamycin (mTOR) signaling pathway extensively affects protein translation, metabolism, and proliferation in breast cancer.134,135 Relatedly, in colorectal cancer, alterations in translation factors, including increased expression levels of eIFs, contribute to enhanced protein synthesis and upregulation of key factors involved in cell proliferation, tumor growth, metastasis, and oxaliplatin resistance.136,137 Dysregulated translation also impacts therapy response and development of resistance in colorectal cancer.138 Epigenetic loss of tRNA-yW Synthesizing Protein 2 increases guanosine hypomodification of tRNA, inducing ribosome frameshift and leading to the translation of oncogenic genes.139 M6A modifications of mRNAs result in abnormal translation in colorectal carcinogenesis, affecting various aspects of colorectal cancer.140,141 Alterations in rRNA and ribosomal protein biogenesis are closely associated with colorectal cancer cell growth.142 In PI3K mutant tumors, ribosomal components are significantly upregulated in an mTOR-dependent manner.143
Besides, abnormal expression of translation factors, such as eIF4E, is associated with enhanced cap-dependent translation initiation and increased protein synthesis in non-small cell lung cancer.144 Dysregulated translation promotes cell proliferation, survival, and therapy resistance in lung cancer.145,146 Mutations in tRNA disrupt its secondary structure and post-transcriptional modifications, affecting protein synthesis in lung cancer.147 Relatedly, prostate cancer cells and patient samples exhibit notable changes in translation factors such as eIF4A, eIF4E, and eEF1A.148,149,150 Specifically, eIF4E, which is under the regulation of Heat shock protein 27 (Hsp27), assumes a critical role in enhancing cell survival and fostering resistance to therapies.121,151,152 This identifies eIF4E as a promising therapeutic target for advanced prostate cancer. The disruption of translation initiation processes, leading to heightened protein synthesis of components involved in cell growth, survival, and androgen receptor signaling, actively drives the progression of prostate cancer.153 Additionally, the aberrant ribosomal biosynthesis of PIM1 and ribosomal small subunit protein 7 leads to ribosomal stress, thereby promoting tumor progression within prostate cancer.154 Dysregulation of translation factors, such as eIF5A, eEF1, and eIF4E, contributes to enhanced protein synthesis and upregulation of factors involved in cell proliferation, invasion, and metastasis in pancreatic cancer.155,156,157,158,159 Methylation of tRNA by the RNA methyltransferase METTL8 plays an important role in the protein translation process of pancreatic cancer.160 Moreover, enhanced ribosome biogenesis contributes to the cell proliferation of RAS-induced or Wnt-dependent pancreatic cancer.161,162 Targeting mTORC1/2 has been shown to decrease downstream proteins, thus overcoming adaptive resistance to KRAS and MEK in pancreatic cancer.163 Overexpression of eEF1a, eIF3b, and eIF4a indicates a worse outcome for cancer patients in gastric cancer.164,165,166 Dysregulation of tRNA-derived fragments (tRFs) can displace RNA binding proteins and alter protein translation, making them potential diagnostic and prognostic biomarkers for gastric cancer.167 The distribution of L22 ribosomal protein in the nucleus and cytoplasm has been positively associated with gastric cancer proliferation.168 Additionally, in liver cancer, modification and abnormal expression of eIFs are closely associated with the development of hepatocellular carcinoma induced by chronic hepatitis C or chronic hepatitis B.169 The m6A modification in the 5’ UTR of PDK4 promotes hepatoma tumor growth by binding with eEF2.170 Furthermore, deregulation of tRNA modifications can affect PPARδ translation and trigger cholesterol synthesis in the liver tumorigenesis.171 Overexpression of small nucleolar RNA H/ACA box leads to hyperactive ribosome biogenesis and disrupts the nuclear location of ribosomal proteins RPL5 and RPL11, resulting in the ubiquitylation and degradation of p53.172 Translation factors such as eIF3H, eEF1A, and eEF2 have been reported to promote proliferation of esophageal cancer cells.173,174,175 Deregulation of N7-methylguanosine tRNA modification (m7G) has been found to be essential for the tumorigenesis process of esophageal cancer.176 Alterations in microRNA have been observed to lead to aberrant expression and translation of mRNA, thereby promoting cancer cell proliferation, invasion, and metastasis.177,178,179 Phosphorylation of ribosomal protein S6 has also been found to be closely related to the progression of esophageal cancer.180 In addition to the above-mentioned cancers, protein translation deregulation is also involved in the tumorigenesis process of ovarian cancer and oral cancer.163,181
Overall, elucidating the mechanisms of protein translation deregulation in cancer is crucial for the development of targeted therapies. Strategies aimed at translation factors, upstream modification targets of mRNA, tRNA, and ribosomes, or global translational control exhibit promising in impeding tumor growth, overcoming therapy resistance, and improving cancer treatment outcomes.
Infectious diseases
In infectious diseases, protein translation deregulation is a common characteristic, and is observed playing a crucial role in their pathogenesis (Fig. 4c). This dysregulation involves various aspects, such as alterations in translation initiation,182 changes in translation elongation,183 and the involvement of regulatory factors and signaling pathways, all of which contribute to the abnormal synthesis of proteins. Dysregulation of translation initiation can result in the preferential synthesis of viral proteins, enabling the pathogen to evade host immune surveillance.184 Similarly, alterations in translation elongation can impact the synthesis of host defense proteins, compromising immune responses. Additionally, the involvement of regulatory factors and signaling pathways further modulates protein translation, influencing disease outcomes. Therefore, targeting translation initiation and eEFs, regulatory factors, or signaling pathways involved in protein translation deregulation may offer potential therapeutic strategies.
Viral reproduction is contingent on viral protein synthesis that relies on the host ribosomes. As such, viruses have evolved remarkable strategies to hijack the host translational apparatus in order to favor viral protein production and to interfere with cellular innate defenses.185 COVID-19 is a viral inflammatory disease primarily affecting the lungs. SARS-CoV-2 replication in the lungs induces inflammatory and immune responses, leading to respiratory issues, systemic effects, and lung damage.186 SARS-CoV-2 viral proteins interact with the host translation machinery. Inhibitors targeting translation have shown potent antiviral effects against SARS-CoV-2. Plitidepsin, for example, has been found to exhibit antiviral activity against SARS-CoV-2 by inhibiting eEF1A.183 Another study by Sidharth Jain et al. discovered that C16 is a dual inhibitor of EIF2AK and SARS-CoV-2N, exhibiting strong antiviral activity.187 Viruses evade the innate immune response by suppressing the production or activity of cytokines such as type I interferons (IFNs). The SARS-CoV-2 NSP2 protein impedes Ifnb1 mRNA translation by hijacking the GIGYF2/4EHP complex. This evades the innate immune response and promotes viral replication.188 miRNAs and tRFs have emerged as important elements in controlling viral replication and host responses. Studies have shown that certain tRFs are highly over-expressed in liver biopsies from patients with chronic hepatitis and hepatocellular carcinoma.189 Furthermore, in the case of human respiratory syncytial virus (RSV), which commonly causes bronchiolitis and pneumonia in infants, RSV infection leads to the abundant production of 30-nt tRFs known as tRNA-derived stress-induced RNAs (tiRNAs).190 These tiRNAs have been found to bind to apolipoprotein E receptor 2 (APOER2) and suppress its expression, thereby promoting RSV replication.191 Similarly, tRF3 has been shown to target the HIV-1 virus through RNA interference, and its cellular prevalence is positively correlated with HIV proliferation.192 Modulating translation initiation or elongation can disrupt viral protein synthesis, restore host defense protein synthesis, and enhance immune responses. For example, the discovery of oxazole-benzenesulfonamides has shown promise in inhibiting HIV-1 replication by disrupting the RT-eEF1A interaction, offering potential anti-HIV drugs.193 Certain pathogens can exploit the host cell’s unfolded protein response (UPR) pathway to exert beneficial effects. For instance, Plasmodium and Newcastle disease virus activate UPR to promote host cell apoptosis, facilitating pathogen release; hepatitis C virus manipulates UPR signaling to reprogram host cell metabolism, optimizing the intracellular environment for its replication.182 Meanwhile, UPR activation can also increase inflammatory cytokine expression and apoptosis in host cells, eliciting immune responses. Therefore, effector molecules like YopJ in Plasmodium and NS5A in hepatitis C virus can inhibit UPR, helping pathogens evade immune surveillance.194 The pathogen-host interplay at the translational level impacts disease outcomes. Elucidating these complex interactions hold promise for treating infections.
Cardiovascular diseases
Emerging evidence suggests that protein translation deregulation plays a critical role in the development and progression of CVDs (Fig. 4d).195 Although specific CVDs and their associated cardiometabolic abnormalities have distinct pathophysiological and clinical manifestations, they often share common traits, including disruption of proteostasis resulting in accumulation of unfolded or misfolded proteins in the ER.185 Preliminary findings indicate that protein translation deregulation contributes to the pathogenesis of CVDs through multiple mechanisms. Cardiac gene expression is extensively controlled at the translational level in a process-specific manner. Analysis of 80 human hearts uncovered extensive translational regulation of cardiac gene expression, including inefficient translation termination, leading to identification of many novel microproteins with diverse cellular functions.195 Dysregulated translation leads to altered expression of key proteins involved in cardiac contractility, endothelial function, and vascular remodeling. Moreover, the deregulation affects the synthesis of proteins implicated in oxidative stress, inflammation, and lipid metabolism, all of which are implicated in CVDs. Additionally, aberrant translation can disrupt protein quality control mechanisms, resulting in the accumulation of misfolded proteins and cellular dysfunction.196 Recent research has shown that the binding of TIP30 to the eEF1A prevents its interaction with its essential co-factor eEF1B2, thereby inhibiting translational elongation. This suggests that TIP30 could be therapeutically targeted to counteract cardiac hypertrophy.197 Stimulating PI3K and inhibiting PTEN, which degrades inositol 3, 4, 5-trisphosphate, increases cardiac cell size. This is also seen with a constitutively active Akt mutant, along with increased phosphorylation of 4EBP1 and S6K1.198 Administration of rapamycin attenuates the cardiac hypertrophy resulting from Akt expression, further demonstrating the involvement of mTOR as the mediating effector.199 Additionally, understanding the molecular mechanisms underlying protein translation deregulation in CVDs may facilitate the development of novel diagnostic biomarkers and personalized treatment strategies. Further research is warranted to elucidate the precise mechanisms involved and explore potential therapeutic interventions for CVDs.
Techniques used to study protein translation deregulation
In the course of research, protein translation deregulation is inseparable from the utilization of various techniques that provide valuable insights into the molecular basis of this process. Ribosome profiling,200 mass spectrometry-based proteomics.201 and single-cell translation profiling.202 each possess unique advantages and limitations. Integrating these techniques enable a comprehensive understanding of protein translation deregulation (Fig. 5). Continued advancements in these techniques, along with their integration with other omics approaches, will undoubtedly contribute to further unraveling the intricate mechanisms underlying the deregulation.
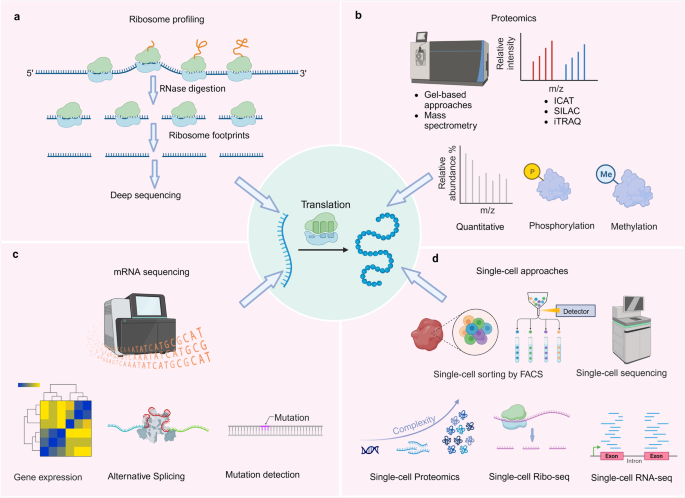
Techniques used to study protein translation deregulation. a Ribosome profiling, a technique used to study protein translation deregulation. b Mass spectrometry-based proteomics, including protein quantification, phosphorylation quantification, and methylation quantification, used to study protein translation deregulation. c mRNA sequencing, including gene expression, alternative splicing, and mutation detection, used to study protein translation deregulation. d Single-cell approaches, including single-cell proteomics, single-cell ribosome profiling, and single-cell transcriptomics, used to study protein translation deregulation
Ribosome profiling
Ribosome profiling is a technique that involves sequencing ribosome footprints, which are short fragments of mRNA (Fig. 5a).203 These footprints are protected from nuclease digestion because they are physically enclosed by the ribosome. These footprints are then converted into a library of DNA fragments for analysis by next-generation sequencing.204 Ribosome profiling enables precise measurement of translation levels, overcoming limitations of traditional methods.205 By capturing ribosome-protected mRNA fragments, ribosome profiling provides valuable insights into translation initiation, elongation, and termination. This innovative approach allows for meticulous monitoring of each stage of protein synthesis in vivo and facilitates quantification of protein synthesis rates across the entire proteome. Moreover, ribosome profiling has provided invaluable insights into the mechanism of action of various anticancer drugs that specifically target the translational apparatus.206 For instance, rocaglates selectively kill cancer cells by targeting eIF4A.207 Ribosome profiling has revealed that these drugs specifically inhibit translation of certain mRNAs.208 Unlike conventional eIF4A inhibitors, rocaglates clamp eIF4A onto polypurine RNA sequences, acting as roadblocks to translation initiation.209 By employing this technique, researchers have been able to precisely examine the effects of these drugs on protein synthesis within cancer cells. Ribosome profiling has enabled the observation changes in translation dynamics and identification of alterations in the ribosome occupancy on specific mRNA transcripts upon drug treatment. This information has shed light on the specific targets and pathways affected by these anticancer drugs, allowing for a better understanding of their mode of action. Moreover, they facilitate the annotation of the protein coding potential of genomes, the examination of localized protein synthesis, and the exploration of co-translational folding and targeting phenomena. The wealth of data generated by ribosome profiling possesses an unparalleled quantitative nature, presenting an unprecedented opportunity to investigate and model intricate cellular processes.210
Mass spectrometry (MS)-based proteomics
In recent years, mass spectrometry proteomics has emerged as a powerful tool for investigating dynamic changes in protein translation and identifying key players (Fig. 5b). By pulse-labeling nascent peptide chains with heavy amino acid isotopes (SILAC) or click-reactive amino acids/puromycin, mass spectrometry approaches can assess protein dynamics like degradation and synthesis. Quantitative proteomics with mass spectrometry can compare overall protein levels between healthy and diseased cells/tissues, revealing which proteins are over/under produced due to defects in translation.211 Chemical labeling and pulse-chase assays tracked by mass spectrometry can monitor the kinetics of protein synthesis and degradation, uncovering abnormalities in translation disorders.212 This sensitive method of proteomics also reveals the role of eIF2 pathways in regulating translation for cellular survival under stress.213 Additionally, proteomics analysis enhances our understanding of the drug reaction of eIF4A targeting compound zotatifin.214 Moreover, integrating MS proteomics with other omics approaches is crucial for gaining a comprehensive understanding of protein translation dysregulation. Through the identification and quantification of differentially translated proteins, as well as the characterization of translational regulatory mechanisms, mass spectrometry-based proteomics provides valuable insights into the molecular basis of protein translation deregulation.215,216 Integration with other omics data further enhances our understanding of this complex process. Continued advancements in mass spectrometry technology and data analysis methods will undoubtedly contribute to further unraveling the intricate mechanisms underlying the deregulation.
mRNA sequencing
mRNA sequencing is a powerful technique for profiling the transcriptome and has emerged as a valuable tool for investigating protein translation deregulation (Fig. 5c). Moreover, we highlight the importance of integrating mRNA sequencing with other omics approaches to gain a comprehensive understanding of translation deregulation.217 Bioinformatic analysis of mRNA sequences can identify motifs or structures that result in ribosome stalling during faulty translation. Through mRNA sequencing, mutations in the Kozak sequence, which plays a role in protein translation, can be identified.218 Additionally, mRNA sequencing enables identification of the 5’ UTRs of eukaryotic mRNAs involved in eukaryotic translation regulation.219 Investigation into the molecular basis of protein translation deregulation necessitates a thorough comprehension of the transcriptome. By enabling high sensitivity and accuracy in profiling the entire transcriptome, mRNA sequencing has revolutionized the field of transcriptomics. It offers valuable insights into the molecular basis of the deregulated protein through identification of differentially expressed genes, detection of alternative splicing events, characterization of translational efficiency, and discovery of translational regulatory elements. Integration with other omics approaches further enhances our understanding of this complex process. Ongoing improvements in mRNA sequencing technology and data analysis methods will undoubtedly contribute to further unraveling the intricate mechanisms underlying protein translation deregulation.
Single-cell approaches
Traditional bulk RNA sequencing methods provide an average measurement of gene expression across a population of cells, masking the heterogeneity that exists within individual cells. Single-cell approaches have emerged as powerful tools for dissecting the intricate dynamics of protein translation at the single-cell level (Fig. 5d). Single-cell ribosome sequencing (scRibo-seq) combines nuclease foot-printing, small-RNA library construction and size enrichment to measure translation dynamics in individual cells.217 Single-cell proteomics has provided valuable information about protein translation dynamics during cellular differentiation.220 Single-cell RNA-seq can define cell-to-cell heterogeneity in gene expression, revealing how translation defects may arise in subpopulations of cells. Single-cell ribosome profiling quantifies translation at the codon level in individual cells, uncovering cell-specific translational dysregulation.221 The use of single-cell approaches to study protein translation deregulation has revolutionized our understanding of cellular heterogeneity and dynamics. These techniques have the potential to uncover novel therapeutic targets and biomarkers for various diseases. However, several challenges need to be addressed, including the limited sensitivity and throughput of current methods, as well as the integration of multi-omics data. Future advancements in single-cell technologies, such as the development of high-throughput single-cell proteomics methods and the integration of transcriptomic and proteomic data will further enhance our understanding of translation deregulation.
Therapeutic strategies targeting protein translation deregulation
Targeting translation initiation
Extensive research has shown that eIFs play a crucial role in modulating translation and are closely associated with various diseases. In particular, targeting eIFs has emerged as a promising strategy for therapeutic interventions against cancer, as supported by current studies.222
Recent studies have revealed that different subunits of eIF3 exhibit varying expression patterns in tumors and are believed to have either oncogenic or tumor suppressor functions.223 The eIF3a subunit has been implicated in various cellular processes including the cell cycle, DNA synthesis/repair, differentiation, fibrosis, and tumorigenesis.224 Increased expression of eIF3a has been associated with the maintenance of malignant phenotypes in numerous types of tumor.222 eIF3a is highly expressed in lung cancer tissues and influences lung cancer patient response to platinum chemotherapy by regulating DNA repair protein expression.225 Additionally, the presence of anti-eIF3a autoantibodies has been identified as a potential diagnostic biomarker for hepatocellular carcinoma.226 eIF3a regulates HIF1α-dependent glycolytic metabolism in hepatocellular carcinoma cells through translational regulation, and is involved in tumor metabolism.223 Given its significant role in carcinogenesis, eIF3a has emerged as a promising therapeutic target for inhibiting tumor proliferation. The small molecule NCE22 has demonstrated cytotoxicity against tumor cells in vitro by acting as an inhibitor of eIF3a.227 Ongoing studies are currently investigating the potential clinical applications and benefits of eIF3 subunits for patients. EIF3b expression has been found to be associated with the prognosis of bladder and prostate cancer, suggesting that targeting eIF3b could open up new possibilities for cancer therapeutics.228 The role of eIF3e in cancer is controversial. Elevated levels of eIF3e correlate with prolonged progression-free survival in tamoxifen-treated breast cancer patients. Additionally, mutations in eIF3e contribute to the malignant phenotype of mammary epithelial cells.229 Conversely, low eIF3m expression is an unfavorable indicator, associating with reduced overall, relapse-free, and post-progression survival in breast cancer and colon adenocarcinoma patients.230 EIF3h is upregulated in various cancers, including breast cancer, hepatocellular carcinoma, lung cancer, and colon cancer.231 In vitro studies have demonstrated that the beta-carboline derived from harmine, CM16, targets eIF3h and exhibits anti-cancer effects.232 Conversely, eIF3f acts as a tumor suppressor in cancers like melanoma and pancreatic cancer. Overexpressing eIF3f inhibits cell proliferation and induces apoptosis.233 In lymphoma models, inhibiting eIF4A and translation can restore chemosensitivity to doxorubicin.234 eIF4F inhibition may also decrease PD-L1 expression and stimulate anti-tumor immunity in melanoma.235 eIF4A inhibition with silvestrol suppresses Sin1 translation and attenuates invasion in colon carcinoma.236
In addition, the mRNA 5’ cap-binding protein, eIF4E, plays a crucial role in facilitating mRNA translation and promoting the translation of oncogenic mRNAs, including cyclin D3, vascular endothelial growth factor, and Mcl-1.237 Furthermore, increased eIF4E activity reduces tumor cell sensitivity to BRAF inhibitors.238 The eIF4F complex also associates with resistance to BRAF and MEK inhibitors in BRAF V600-mutant melanoma, colon, and thyroid cancer cells.239 Small molecule inhibitors targeting the eIF4E-eIF4G interaction domain, like Quabain and Perillyl alcohol, have been investigated.153 These eIF4E inhibitors mimic the eIF4G or 4E-BP1 domain to bind eIF4E. A high-throughput approach identified the eIF4F inhibitor 4EGI-1, which disrupts eIF4F and inhibits cap-dependent translation. 4EGI-1 has shown anti-tumor activity by inhibiting proliferation and inducing apoptosis in Jurkat T-ALL and A549 cancer cell lines in vitro.240 Moreover, Ribavirin, an eIF4E inhibitor, has shown striking improvements as monotherapy in an 11-patient clinical trial for acute myeloid leukemia.241
Targeting translation elongation
Elongation involves tRNA entering, peptide bond formation, and ribosome translocation along mRNA.71 In mammals, eEF1A, similar to bacterial EF-Tu, binds aminoacyl-tRNA in a GTP-dependent manner and guides it to the ribosomal A-site. GTP hydrolysis by eEF1A occurs upon codon recognition between mRNA and tRNA. eEF1A-GDP is recycled to eEF1A-GTP with help from the eEF1B (α, δ, γ subunits) guanine exchange factor.219 Understanding the intricacies of translation elongation are essential for unraveling the mechanisms of protein synthesis.
In the context of cancer, inhibiting translation elongation can selectively impact rapidly dividing cancer cells, leading to growth arrest or apoptosis.242 In neurodegenerative disorders, enhancing translation elongation can facilitate the production of functional proteins, thereby mitigating disease progression.243 Moreover, modulation of translation elongation can be utilized to combat viral infections by disrupting the synthesis machinery of viral proteins.244 These findings underscore the broad therapeutic implications of targeting translation elongation. This tightly regulated process is orchestrated by a complex interplay of various factors, including ribosomes, tRNAs, and eEFs. Current elongation inhibitors primarily target the elongation factors eEF1 and eEF2, as well as the ribosomal A-, P- or E-sites.245 Mutations in mitochondrial elongation factors EF-Tumt, EF-Tsmt, EF-Gmt and their cytosolic counterparts eEF1A, eEF1B, eEF2 associate with human diseases affecting the central nervous system.246
Targeting translation termination
Targeting strategies for translation termination initiation holds great promise for therapeutic intervention. In eukaryotes, eRF1 and eRF3 are the primary RFs responsible for recognizing stop codons and promoting peptide release.247 Dysregulation of these factors can result in premature termination or readthrough of stop codons, leading to abnormal protein synthesis. Small molecules that selectively target translation termination initiation factors have shown promise as potential therapeutics. For instance, compounds that enhance eRF1 binding to stop codons can promote efficient termination, preventing premature termination or readthrough.88 Conversely, inhibitors that disrupt eRF1 or eRF3 function can be employed to modulate translation termination initiation and restore protein synthesis. PF846 inhibits translation termination by slowing elongation and trapping the nascent chain on the ribosome, suppressing the catalytic activity of the peptidyl transferase center (PTC) that is normally stimulated by eukaryotic release factor 1 (eRF1).248 SRI-37240 and SRI-41315 promote cystic fibrosis transmembrane conductance regulator (CFTR) nonsense mutation suppression by prolonging translational pause and reducing eRF1 abundance, synergistic with aminoglycosides.249
Preclinical and clinical studies of translation-targeted therapies
Translation-targeted strategies have garnered significant attention due to their ability to modulate protein synthesis and their potential for precision medicine.250 Preclinical studies have demonstrated their efficacy in various disease models, including cancer, neurodegenerative disorders, and CVDs.36
By specifically targeting the translational machinery, these therapies offer a unique and targeted treatment approach. In the field of cancer research, translation-targeted therapies have shown promise in inhibiting tumor growth.244 Similarly, in neurodegenerative disorders, these therapies have been shown to modulate protein synthesis and alleviate disease pathology.99 Additionally, translation-targeted therapies have exhibited potential in CVDs by targeting specific proteins involved in cardiac remodeling. Clinical trials evaluating these therapies have demonstrated favorable safety profiles, tolerability, and preliminary efficacy. As an illustration, clinical trial for cancer treatment showed a translation-targeted therapy’s safety and preliminary evidence of antitumor activity (Table 2).251 While translation-targeted therapies offer a promising avenue for precision medicine by directly modulating protein synthesis, several challenges need to be addressed for successful translation into clinical practice. These challenges include off-target effects, drug resistance, and delivery strategies, which require further investigation. Additionally, identifying optimal therapeutic targets and developing personalized treatment approaches are crucial for maximizing the efficacy of translation-targeted therapies. Preclinical and clinical studies have provided valuable insights into the development and application of translation-targeted therapies. Further research is warranted to optimize therapeutic strategies, overcome challenges, and improve patient outcomes.
Conclusion and future perspectives
Dysregulation of translation can lead to abnormal protein expression, altered protein isoforms, and disrupted cellular functions, which are often associated with various human diseases. Here, we summarize the deregulation of protein translation based on the alterations in tRNA, mRNA, ribosome and related translation factors. This provides new insights for classifying the deregulations of protein translation in human diseases, such as neurodegenerative diseases, cancer, infectious diseases and CVDs. We also discuss the challenges regarding candidate targets and their related inhibitors that have been evaluated in pre-clinical study. With the advancements in techniques used to study protein translation, mutations, modifications or other disorders in translation elements will be identified in the future researches. However, effective and actionable biomarker targeting translation process and its related inhibitors are still lacking in the clinical for human disease. Continued efforts are needed to discover novel targets and biomarkers focusing on alterations of translation factors, tRNA, mRNA and ribosomes implicated in disease pathogenesis. In addition, further research on rare genetic diseases caused by mutations in translational factors or ribosomal proteins can deepen our understanding of translation mechanisms and offer potential therapeutic approaches for more prevalent diseases. Furthermore, targeting patient-specific alterations in translation which are detected by integrating genomic, transcriptomic, and proteomic data can improve treatment outcomes by tailoring treatments to individual patients based on their specific translation deregulation profiles and holds promise for precision medicine and personalized treatments.
Translation deregulation, with its intricate molecular interactions, reveals the inner mechanics of cellular processes and carries significant consequences for health and disease. The ability to modulate this mechanism, specifically targeting stages such as initiation, elongation, or termination, facilitates the emergence of previously unattainable precision medicine approaches. This research calls for the creation of an advanced approach to engage with cellular processes, facilitating the development of individualized treatment strategies and deepening our understanding of molecular biology. In this domain, the complexities involved in the cellular protein synthesis process pose both a challenge and an opportunity, paving the way for innovative therapeutic methods and a future where diseases are confronted at their cellular foundation.
Overall, a collaborative, multidisciplinary approach that integrates genomics, proteomics, bioinformatics, and functional studies will be essential for advancing our understanding of protein translation deregulation and its implications in human disease. By addressing these future research directions, we can pave a way towards more effective and personalized treatments, ultimately making significant progress in combating various diseases and improving global health outcomes.
References
Tahmasebi, S., Sonenberg, N., Hershey, J. W. B. & Mathews, M. B. Protein Synthesis and Translational Control: A Historical Perspective. Cold Spring Harb. Perspect. Biol. 11, a035584 (2019).
Daly, M. M. & Mirsky, A. E. Formation of protein in the pancreas. J. Gen. Physiol. 36, 243–254 (1952).
Beskow, G. & Hultin, T. The incorporation of C14-L-leucine into rat liver proteins in vitro visualized as a two-step reaction. Exp. Cell Res. 11, 664–666 (1956).
Hoagland, M. B., Stephenson, M. L., Scott, J. F., Hecht, L. I. & Zamecnik, P. C. A soluble ribonucleic acid intermediate in protein synthesis. J. Biol. Chem. 231, 241–257 (1958).
Palade, G. E. A small particulate component of the cytoplasm. J. Biophys. Biochem. Cytol. 1, 59–68 (1955).
Sanger, F. The structure of insulin. Bull. Soc. Chim. Biol. (Paris). 37, 23–35 (1955).
Jacob, F. & Monod, J. Genetic regulatory mechanisms in the synthesis of proteins. J. Mol. Biol. 3, 318–356 (1961).
Crick, F. H. Codon-anticodon pairing: the wobble hypothesis. J. Mol. Biol. 19, 548–555 (1966).
Chapeville, F. et al. On the role of soluble ribonucleic acid in coding for amino acids. Proc. Natl Acad. Sci. USA 48, 1086–1092 (1962).
Holley, R. W. et al. Structure Of A Ribonucleic Acid. Science 147, 1462–1465 (1965).
Lagerkvist, U. The 1968 Nobel prize in physiology or medicine. The genetic code and its translation. Lakartidningen 65, 4373–4381 (1968).
Green, M. H. & Hall, B. D. A comparison of the native and derived 30S and 50S ribosomes of Escherichia coli. Biophys. J. 1, 517–523 (1961).
Shapiro, A. L., Viñuela, E. & Maizel, J. V. J. Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem. Biophys. Res. Commun. 28, 815–820 (1967).
Towbin, H., Staehelin, T. & Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA 76, 4350–4354 (1979).
Mathews, M. & Korner, A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur. J. Biochem. 17, 328–338 (1970).
Sanger, F., Nicklen, S. & Coulson, A. R. DNA sequencing with chain-terminating inhibitors. Proc. Natl Acad. Sci. USA 74, 5463–5467 (1977).
Hershey, J. W. B., Sonenberg, N. & Mathews, M. B. Principles of Translational Control. Cold Spring Harb. Perspect. Biol. 11, a032607 (2019).
Nałecz, K. A. The 1999 Nobel Prize for physiology or medicine. Neurologia i neurochirurgia Pol. 34, 233–242 (2000).
Gemmer, M. et al. Visualization of translation and protein biogenesis at the ER membrane. Nature 614, 160–167 (2023).
Kišonaitė, M., Wild, K., Lapouge, K., Ruppert, T. & Sinning, I. High-resolution structures of a thermophilic eukaryotic 80S ribosome reveal atomistic details of translocation. Nat. Commun. 13, 476 (2022).
An, H., Ordureau, A., Körner, M., Paulo, J. A. & Harper, J. W. Systematic quantitative analysis of ribosome inventory during nutrient stress. Nature 583, 303–309 (2020).
Vistain, L. F. & Tay, S. Single-Cell Proteomics. Trends Biochem. Sci. 46, 661–672 (2021).
Tang, Q. & Chen, X. Nascent Proteomics: Chemical Tools for Monitoring Newly Synthesized Proteins. Angew. Chem. Int. Ed. Engl. 62, e202305866 (2023).
Holland, M. L. Epigenetic Regulation of the Protein Translation Machinery. EBioMedicine 17, 3–4 (2017).
Ribas de Pouplana, L., Santos, M. A. S., Zhu, J.-H., Farabaugh, P. J. & Javid, B. Protein mistranslation: friend or foe? Trends Biochem. Sci. 39, 355–362 (2014).
Orellana, E. A., Siegal, E. & Gregory, R. I. tRNA dysregulation and disease. Nat. Rev. Genet. 23, 651–664 (2022).
Inada, T. Quality controls induced by aberrant translation. Nucleic Acids Res. 48, 1084–1096 (2020).
Lee, C.-H. et al. A Regulatory Response to Ribosomal Protein Mutations Controls Translation, Growth, and Cell Competition. Dev. Cell 46, 456–469.e4 (2018).
Chee, N. T., Lohse, I. & Brothers, S. P. mRNA-to-protein translation in hypoxia. Mol. Cancer 18, 49 (2019).
Ivanova, I. G., Park, C. V. & Kenneth, N. S. Translating the Hypoxic Response-the Role of HIF Protein Translation in the Cellular Response to Low Oxygen. Cells 8, 114 (2019).
Taymans, J.-M., Nkiliza, A. & Chartier-Harlin, M.-C. Deregulation of protein translation control, a potential game-changing hypothesis for Parkinson’s disease pathogenesis. Trends Mol. Med. 21, 466–472 (2015).
Feng, W. & Feng, Y. MicroRNAs in neural cell development and brain diseases. Sci. China Life Sci. 54, 1103–1112 (2011).
Song, P., Yang, F., Jin, H. & Wang, X. The regulation of protein translation and its implications for cancer. Signal Transduct. Target. Ther. 6, 68 (2021).
Seo, H., Jeon, L., Kwon, J. & Lee, H. High-Precision Synthesis of RNA-Loaded Lipid Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 12, e2203033 (2023).
Ghosh, A. & Shcherbik, N. Effects of Oxidative Stress on Protein Translation: Implications for Cardiovascular Diseases. Int. J. Mol. Sci. 21, 2661 (2020).
Tahmasebi, S., Khoutorsky, A., Mathews, M. B. & Sonenberg, N. Translation deregulation in human disease. Nat. Rev. Mol. Cell Biol. 19, 791–807 (2018).
Sim, E. U.-H., Lee, C.-W. & Narayanan, K. The roles of ribosomal proteins in nasopharyngeal cancer: culprits, sentinels or both. Biomark. Res. 9, 51 (2021).
Austin, R. C. The unfolded protein response in health and disease. Antioxid. redox Signal. 11, 2279–2287 (2009).
Merrick, W. C. & Pavitt, G. D. Protein Synthesis Initiation in Eukaryotic Cells. Cold Spring Harb. Perspect. Biol. 10, a033092 (2018).
Jackson, R. J., Hellen, C. U. T. & Pestova, T. V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 (2010).
Bhat, M. et al. Targeting the translation machinery in cancer. Nat. Rev. Drug Discov. 14, 261–278 (2015).
James, C. C. & Smyth, J. W. Alternative mechanisms of translation initiation: An emerging dynamic regulator of the proteome in health and disease. Life Sci. 212, 138–144 (2018).
Dominissini, D. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485, 201–206 (2012).
Zhou, J. et al. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594 (2015).
Meyer, K. D. et al. 5’ UTR m(6)A Promotes Cap-Independent Translation. Cell 163, 999–1010 (2015).
Lee, A. S., Kranzusch, P. J., Doudna, J. A. & Cate, J. H. D. eIF3d is an mRNA cap-binding protein that is required for specialized translation initiation. Nature 536, 96–99 (2016).
Ma, S., Liu, J.-Y. & Zhang, J.-T. eIF3d: A driver of noncanonical cap-dependent translation of specific mRNAs and a trigger of biological/pathological processes. J. Biol. Chem. 299, 104658 (2023).
Yokoyama, T. et al. HCV IRES Captures an Actively Translating 80S Ribosome. Mol. Cell 74, 1205–1214.e8 (2019).
Petrov, A., Grosely, R., Chen, J., O’Leary, S. E. & Puglisi, J. D. Multiple Parallel Pathways of Translation Initiation on the CrPV IRES. Mol. Cell 62, 92–103 (2016).
Colussi, T. M. et al. Initiation of translation in bacteria by a structured eukaryotic IRES RNA. Nature 519, 110–113 (2015).
Stern-Ginossar, N., Thompson, S. R., Mathews, M. B. & Mohr, I. Translational Control in Virus-Infected Cells. Cold Spring Harb. Perspect. Biol. 11, a033001 (2019).
Kwan, T. & Thompson, S. R. Noncanonical Translation Initiation in Eukaryotes. Cold Spring Harb. Perspect. Biol. 11, a032672 (2019).
Wek, R. C. Role of eIF2α Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb. Perspect. Biol. 10, a032870 (2018).
Anisimova, A. S. et al. Multifaceted deregulation of gene expression and protein synthesis with age. Proc. Natl Acad. Sci. USA 117, 15581–15590 (2020).
Young-Baird, S. K., Shin, B.-S. & Dever, T. E. MEHMO syndrome mutation EIF2S3-I259M impairs initiator Met-tRNAiMet binding to eukaryotic translation initiation factor eIF2. Nucleic Acids Res. 47, 855–867 (2019).
Stolfi, C. et al. A functional role for Smad7 in sustaining colon cancer cell growth and survival. Cell Death Dis. 5, e1073 (2014).
Puri, P. et al. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology 134, 568–576 (2008).
Breuss, M. W. et al. Autosomal-Recessive Mutations in the tRNA Splicing Endonuclease Subunit TSEN15 Cause Pontocerebellar Hypoplasia and Progressive Microcephaly. Am. J. Hum. Genet. 99, 228–235 (2016).
Karaca, E. et al. Human CLP1 mutations alter tRNA biogenesis, affecting both peripheral and central nervous system function. Cell 157, 636–650 (2014).
Schaffer, A. E. et al. CLP1 founder mutation links tRNA splicing and maturation to cerebellar development and neurodegeneration. Cell 157, 651–663 (2014).
Cassaignau, A. M. E., Cabrita, L. D. & Christodoulou, J. How Does the Ribosome Fold the Proteome? Annu. Rev. Biochem. 89, 389–415 (2020).
Watanabe, S. et al. A Mutation in the 16S rRNA Decoding Region Attenuates the Virulence of Mycobacterium tuberculosis. Infect. Immun. 84, 2264–2273 (2016).
Mills, E. W. & Green, R. Ribosomopathies: There’s strength in numbers. Science 358, 6363 (2017).
Narla, A. & Ebert, B. L. Ribosomopathies: human disorders of ribosome dysfunction. Blood 115, 3196–3205 (2010).
Wang, J. et al. Rapid 40S scanning and its regulation by mRNA structure during eukaryotic translation initiation. Cell 185, 4474–4487.e17 (2022).
de la Parra, C. et al. A widespread alternate form of cap-dependent mRNA translation initiation. Nat. Commun. 9, 3068 (2018).
Sakharov, P. A., Smolin, E. A., Lyabin, D. N. & Agalarov, S. C. ATP-Independent Initiation during Cap-Independent Translation of m(6)A-Modified mRNA. Int. J. Mol. Sci. 22, 3662 (2021).
Mengardi, C. et al. microRNAs stimulate translation initiation mediated by HCV-like IRESes. Nucleic Acids Res. 45, 4810–4824 (2017).
Shi, H., Chai, P., Jia, R. & Fan, X. Novel insight into the regulatory roles of diverse RNA modifications: Re-defining the bridge between transcription and translation. Mol. Cancer 19, 78 (2020).
Kamhi, E., Raitskin, O., Sperling, R. & Sperling, J. A potential role for initiator-tRNA in pre-mRNA splicing regulation. Proc. Natl Acad. Sci. USA 107, 11319–11324 (2010).
Dever, T. E., Dinman, J. D. & Green, R. Translation Elongation and Recoding in Eukaryotes. Cold Spring Harb. Perspect. Biol. 10, a032649 (2018).
Hu, Z. et al. Ssd1 and Gcn2 suppress global translation efficiency in replicatively aged yeast while their activation extends lifespan. Elife 7, e35551 (2018).
Kaul, G., Pattan, G. & Rafeequi, T. Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem. Funct. 29, 227–234 (2011).
Jørgensen, R., Merrill, A. R. & Andersen, G. R. The life and death of translation elongation factor 2. Biochem. Soc. Trans. 34, 1–6 (2006).
Mönkemeyer, L. et al. Chaperone Function of Hgh1 in the Biogenesis of Eukaryotic Elongation Factor 2. Mol. Cell 74, 88–100.e9 (2019).
He, C., Guo, J., Tian, W. & Wong, C. C. L. Proteogenomics Integrating Novel Junction Peptide Identification Strategy Discovers Three Novel Protein Isoforms of Human NHSL1 and EEF1B2. J. Proteome Res. 20, 5294–5303 (2021).
Choi, J. et al. N(6)-methyladenosine in mRNA disrupts tRNA selection and translation-elongation dynamics. Nat. Struct. Mol. Biol. 23, 110–115 (2016).
Choi, J. et al. 2’-O-methylation in mRNA disrupts tRNA decoding during translation elongation. Nat. Struct. Mol. Biol. 25, 208–216 (2018).
Zhang, L., Wang, Y., Dai, H. & Zhou, J. Structural and functional studies revealed key mechanisms underlying elongation step of protein translation. Acta Biochim. Biophys. Sin. (Shanghai). 52, 749–756 (2020).
Ishimura, R. et al. RNA function. Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration. Science 345, 455–459 (2014).
Wu, C. C.-C., Peterson, A., Zinshteyn, B., Regot, S. & Green, R. Ribosome Collisions Trigger General Stress Responses to Regulate Cell Fate. Cell 182, 404–416.e14 (2020).
Schuller, A. P. & Green, R. Roadblocks and resolutions in eukaryotic translation. Nat. Rev. Mol. Cell Biol. 19, 526–541 (2018).
Padmanabhan, P. K. et al. Genetic depletion of the RNA helicase DDX3 leads to impaired elongation of translating ribosomes triggering co-translational quality control of newly synthesized polypeptides. Nucleic Acids Res. 49, 9459–9478 (2021).
Han, P. et al. Genome-wide Survey of Ribosome Collision. Cell Rep. 31, 107610 (2020).
Bao, C. et al. mRNA stem-loops can pause the ribosome by hindering A-site tRNA binding. Elife 9, e55799 (2020).
Rodnina, M. V. & Wintermeyer, W. Protein Elongation, Co-translational Folding and Targeting. J. Mol. Biol. 428, 2165–2185 (2016).
Richter, J. D. & Coller, J. Pausing on Polyribosomes: Make Way for Elongation in Translational Control. Cell 163, 292–300 (2015).
Hellen, C. U. T. Translation Termination and Ribosome Recycling in Eukaryotes. Cold Spring Harb. Perspect. Biol. 10, a032656 (2018).
Azimi, A. et al. Targeting CDK 2 overcomes melanoma resistance against BRAF and Hsp90 inhibitors. Mol. Syst. Biol. 14, e7858 (2018).
Lueck, J. D. et al. Engineered transfer RNAs for suppression of premature termination codons. Nat. Commun. 10, 822 (2019).
García-Rodríguez, R. et al. Premature termination codons in the DMD gene cause reduced local mRNA synthesis. Proc. Natl Acad. Sci. USA 117, 16456–16464 (2020).
Arefeen, A., Liu, J., Xiao, X. & Jiang, T. TAPAS: tool for alternative polyadenylation site analysis. Bioinformatics 34, 2521–2529 (2018).
Ma, C. et al. Mechanistic insights into the alternative translation termination by ArfA and RF2. Nature 541, 550–553 (2017).
Xu, W. et al. Dynamic control of chromatin-associated m(6)A methylation regulates nascent RNA synthesis. Mol. Cell 82, 1156–1168.e7 (2022).
Beißel, C. et al. Translation termination depends on the sequential ribosomal entry of eRF1 and eRF3. Nucleic Acids Res. 47, 4798–4813 (2019).
Baradaran-Heravi, A. et al. Effect of small molecule eRF3 degraders on premature termination codon readthrough. Nucleic Acids Res. 49, 3692–3708 (2021).
Beißel, C., Grosse, S. & Krebber, H. Dbp5/DDX19 between Translational Readthrough and Nonsense Mediated Decay. Int. J. Mol. Sci. 21, 1085 (2020).
Dugger, B. N. & Dickson, D. W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 9, a028035 (2017).
Halliday, M. et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain 140, 1768–1783 (2017).
Halliday, M. et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 6, e1672 (2015).
Sidrauski, C., McGeachy, A. M., Ingolia, N. T. & Walter, P. The small molecule ISRIB reverses the effects of eIF2α phosphorylation on translation and stress granule assembly. Elife 4, e05033 (2015).
Wong, Y. L. et al. eIF2B activator prevents neurological defects caused by a chronic integrated stress response. Elife 8, e42940 (2019).
Kulkarni, A. et al. Proteostasis in Parkinson’s disease: Recent development and possible implication in diagnosis and therapeutics. Ageing Res. Rev. 84, 101816 (2023).
Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 27, 954–963 (2021).
Chartier-Harlin, M.-C. et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am. J. Hum. Genet. 89, 398–406 (2011).
Fujioka, S. et al. Sequence variants in eukaryotic translation initiation factor 4-gamma (eIF4G1) are associated with Lewy body dementia. Acta Neuropathol. 125, 425–438 (2013).
Sonenberg, N. & Dever, T. E. Eukaryotic translation initiation factors and regulators. Curr. Opin. Struct. Biol. 13, 56–63 (2003).
Behrouz, B. et al. Mitochondrial translation initiation factor 3 polymorphism and Parkinson’s disease. Neurosci. Lett. 486, 228–230 (2010).
Tain, L. S. et al. Rapamycin activation of 4E-BP prevents parkinsonian dopaminergic neuron loss. Nat. Neurosci. 12, 1129–1135 (2009).
Fabbri, L., Chakraborty, A., Robert, C. & Vagner, S. The plasticity of mRNA translation during cancer progression and therapy resistance. Nat. Rev. Cancer 21, 558–577 (2021).
Liu, S. & Lu, B. Reduction of protein translation and activation of autophagy protect against PINK1 pathogenesis in Drosophila melanogaster. PLoS Genet 6, e1001237 (2010).
Donnelly, N., Gorman, A. M., Gupta, S. & Samali, A. The eIF2α kinases: their structures and functions. Cell. Mol. Life Sci. 70, 3493–3511 (2013).
Di Conza, G. & Ho, P.-C. ER Stress Responses: An Emerging Modulator for Innate Immunity. Cells 9, 695 (2020).
Zheng, W. et al. C9orf72 regulates the unfolded protein response and stress granule formation by interacting with eIF2α. Theranostics 12, 7289–7306 (2022).
Wuerth, J. D. et al. eIF2B as a Target for Viral Evasion of PKR-Mediated Translation Inhibition. MBio 11, e00976–20 (2020).
Hoozemans, J. J. M. et al. The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am. J. Pathol. 174, 1241–1251 (2009).
Tzioras, M., McGeachan, R. I., Durrant, C. S. & Spires-Jones, T. L. Synaptic degeneration in Alzheimer disease. Nat. Rev. Neurol. 19, 19–38 (2023).
Kim, H.-S. et al. Swedish amyloid precursor protein mutation increases phosphorylation of eIF2alpha in vitro and in vivo. J. Neurosci. Res. 85, 1528–1537 (2007).
Zhang, J.-S. et al. Gastrodin suppresses BACE1 expression under oxidative stress condition via inhibition of the PKR/eIF2α pathway in Alzheimer’s disease. Neuroscience 325, 1–9 (2016).
Trinh, M. A. et al. Brain-specific disruption of the eIF2α kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep. 1, 676–688 (2012).
Karaki, S., Andrieu, C., Ziouziou, H. & Rocchi, P. The Eukaryotic Translation Initiation Factor 4E (eIF4E) as a Therapeutic Target for Cancer. Adv. Protein Chem. Struct. Biol. 101, 1–26 (2015).
Tabrizi, S. J., Ghosh, R. & Leavitt, B. R. Huntingtin Lowering Strategies for Disease Modification in Huntington’s Disease. Neuron 101, 801–819 (2019).
Tabrizi, S. J. et al. Potential disease-modifying therapies for Huntington’s disease: lessons learned and future opportunities. Lancet Neurol. 21, 645–658 (2022).
Passarelli, M. C. et al. Leucyl-tRNA synthetase is a tumour suppressor in breast cancer and regulates codon-dependent translation dynamics. Nat. Cell Biol. 24, 307–315 (2022).
Goodarzi, H. et al. Modulated Expression of Specific tRNAs Drives Gene Expression and Cancer Progression. Cell 165, 1416–1427 (2016).
Huang, S.-Q. et al. The dysregulation of tRNAs and tRNA derivatives in cancer. J. Exp. Clin. Cancer Res. 37, 101 (2018).
Ebright, R. Y. et al. Deregulation of ribosomal protein expression and translation promotes breast cancer metastasis. Science 367, 1468–1473 (2020).
Hung, Y.-W. et al. Extracellular arginine availability modulates eIF2α O-GlcNAcylation and heme oxygenase 1 translation for cellular homeostasis. J. Biomed. Sci. 30, 32 (2023).
Sengupta, S., Sevigny, C. M., Bhattacharya, P., Jordan, V. C. & Clarke, R. Estrogen-Induced Apoptosis in Breast Cancers Is Phenocopied by Blocking Dephosphorylation of Eukaryotic Initiation Factor 2 Alpha (eIF2α) Protein. Mol. Cancer Res. 17, 918–928 (2019).
Ju, Y., Ben-David, Y., Rotin, D. & Zacksenhaus, E. Inhibition of eEF2K synergizes with glutaminase inhibitors or 4EBP1 depletion to suppress growth of triple-negative breast cancer cells. Sci. Rep. 11, 9181 (2021).
Meric-Bernstam, F. et al. Aberrations in translational regulation are associated with poor prognosis in hormone receptor-positive breast cancer. Breast Cancer Res 14, R138 (2012).
González-Ortiz, A. et al. eIF4A/PDCD4 Pathway, a Factor for Doxorubicin Chemoresistance in a Triple-Negative Breast Cancer Cell Model. Cells 11, 4069 (2022).
Varone, E. et al. Endoplasmic reticulum oxidoreductin 1-alpha deficiency and activation of protein translation synergistically impair breast tumour resilience. Br. J. Pharmacol. 179, 5180–5195 (2022).
Sridharan, S. & Basu, A. Distinct Roles of mTOR Targets S6K1 and S6K2 in Breast Cancer. Int. J. Mol. Sci. 21, 1199 (2020).
Anderson, G. R. et al. PIK3CA mutations enable targeting of a breast tumor dependency through mTOR-mediated MCL-1 translation. Sci. Transl. Med. 8, 369ra175 (2016).
Chen, Z.-H. et al. Eukaryotic initiation factor 4A2 promotes experimental metastasis and oxaliplatin resistance in colorectal cancer. J. Exp. Clin. Cancer Res. 38, 196 (2019).
Mei, C. et al. eIF3a Regulates Colorectal Cancer Metastasis via Translational Activation of RhoA and Cdc42. Front. cell Dev. Biol. 10, 794329 (2022).
Zhang, K. et al. N(6)-methyladenosine-mediated LDHA induction potentiates chemoresistance of colorectal cancer cells through metabolic reprogramming. Theranostics 12, 4802–4817 (2022).
Rosselló-Tortella, M. et al. Epigenetic loss of the transfer RNA-modifying enzyme TYW2 induces ribosome frameshifts in colon cancer. Proc. Natl Acad. Sci. Usa. 117, 20785–20793 (2020).
Shen, C. et al. m(6)A-dependent glycolysis enhances colorectal cancer progression. Mol. Cancer 19, 72 (2020).
Song, P. et al. β-catenin represses miR455-3p to stimulate m6A modification of HSF1 mRNA and promote its translation in colorectal cancer. Mol. Cancer 19, 129 (2020).
Nait Slimane, S. et al. Ribosome Biogenesis Alterations in Colorectal Cancer. Cells 9, 2361 (2020).
Davoli, T. et al. Functional genomics reveals that tumors with activating phosphoinositide 3-kinase mutations are dependent on accelerated protein turnover. Genes Dev. 30, 2684–2695 (2016).
Yin, J. et al. Association of positively selected eIF3a polymorphisms with toxicity of platinum-based chemotherapy in NSCLC patients. Acta. Pharmacol. Sin. 36, 375–384 (2015).
Kong, T. et al. eIF4A Inhibitors Suppress Cell-Cycle Feedback Response and Acquired Resistance to CDK4/6 Inhibition in Cancer. Mol. Cancer Ther. 18, 2158–2170 (2019).
Sobol, A. et al. Amyloid precursor protein (APP) affects global protein synthesis in dividing human cells. J. Cell. Physiol. 230, 1064–1074 (2015).
Wang, L., Chen, Z.-J., Zhang, Y.-K. & Le, H.-B. The role of mitochondrial tRNA mutations in lung cancer. Int. J. Clin. Exp. Med. 8, 13341–13346 (2015).
Wang, C. et al. Epigenetic regulation of EIF4A1 through DNA methylation and an oncogenic role of eIF4A1 through BRD2 signaling in prostate cancer. Oncogene 41, 2778–2785 (2022).
Stoyanova, T. et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. Proc. Natl Acad. Sci. USA. 110, 20111–20116 (2013).
Sun, Y. et al. Up-regulation of eEF1A2 promotes proliferation and inhibits apoptosis in prostate cancer. Biochem. Biophys. Res. Commun. 450, 1–6 (2014).
Andrieu, C. et al. Heat shock protein 27 confers resistance to androgen ablation and chemotherapy in prostate cancer cells through eIF4E. Oncogene 29, 1883–1896 (2010).
Ziouziou, H. et al. Nucleoside-Lipid-Based Nanoparticles for Phenazine Delivery: A New Therapeutic Strategy to Disrupt Hsp27-eIF4E Interaction in Castration Resistant Prostate Cancer. Pharmaceutics 13, 623 (2021).
Ramamurthy, V. P., Ramalingam, S., Kwegyir-Afful, A. K., Hussain, A. & Njar, V. C. O. Targeting of protein translation as a new treatment paradigm for prostate cancer. Curr. Opin. Oncol. 29, 210–220 (2017).
Zhang, C. et al. Kinase PIM1 promotes prostate cancer cell growth via c-Myc-RPS7-driven ribosomal stress. Carcinogenesis 40, 202 (2019).
Xu, C., Hu, D. & Zhu, Q. eEF1A2 promotes cell migration, invasion and metastasis in pancreatic cancer by upregulating MMP-9 expression through Akt activation. Clin. Exp. Metastasis 30, 933–944 (2013).
Strnadel, J. et al. eIF5A-PEAK1 Signaling Regulates YAP1/TAZ Protein Expression and Pancreatic Cancer Cell Growth. Cancer Res. 77, 1997–2007 (2017).
Golob-Schwarzl, N. et al. New Pancreatic Cancer Biomarkers eIF1, eIF2D, eIF3C and eIF6 Play a Major Role in Translational Control in Ductal Adenocarcinoma. Anticancer Res. 40, 3109–3118 (2020).
Ma, X., Li, B., Liu, J., Fu, Y. & Luo, Y. Phosphoglycerate dehydrogenase promotes pancreatic cancer development by interacting with eIF4A1 and eIF4E. J. Exp. Clin. Cancer Res. 38, 66 (2019).
Hashimoto, S. et al. ARF6 and AMAP1 are major targets of KRAS and TP53 mutations to promote invasion, PD-L1 dynamics, and immune evasion of pancreatic cancer. Proc. Natl Acad. Sci. USA. 116, 17450–17459 (2019).
Schöller, E. et al. Balancing of mitochondrial translation through METTL8-mediated m(3)C modification of mitochondrial tRNAs. Mol. Cell 81, 4810–4825.e12 (2021).
Azman, M. S. et al. An ERK1/2-driven RNA-binding switch in nucleolin drives ribosome biogenesis and pancreatic tumorigenesis downstream of RAS oncogene. EMBO J. 42, e110902 (2023).
Madan, B. et al. Temporal dynamics of Wnt-dependent transcriptome reveal an oncogenic Wnt/MYC/ribosome. axis. J. Clin. Invest. 128, 5620–5633 (2018).
Brown, W. S. et al. Overcoming Adaptive Resistance to KRAS and MEK Inhibitors by Co-targeting mTORC1/2 Complexes in Pancreatic Cancer. Cell reports. Medicine 1, 100131 (2020).
Gao, C. et al. High intratumoral expression of eIF4A1 promotes epithelial-to-mesenchymal transition and predicts unfavorable prognosis in gastric cancer. Acta Biochim. Biophys. Sin. (Shanghai). 52, 310–319 (2020).
Yang, S. et al. Overexpression of eukaryotic elongation factor 1 alpha-2 is associated with poorer prognosis in patients with gastric cancer. J. Cancer Res. Clin. Oncol. 141, 1265–1275 (2015).
Wang, L. et al. EIF3B is associated with poor outcomes in gastric cancer patients and promotes cancer progression via the PI3K/AKT/mTOR signaling pathway. Cancer Manag. Res. 11, 7877–7891 (2019).
Yu, X. et al. tRNA-derived fragments: Mechanisms underlying their regulation of gene expression and potential applications as therapeutic targets in cancers and virus infections. Theranostics 11, 461–469 (2021).
Cheng, J. et al. L22 ribosomal protein is involved in dynamin-related protein 1-mediated gastric carcinoma progression. Bioengineered 13, 6650–6664 (2022).
Golob-Schwarzl, N. et al. New liver cancer biomarkers: PI3K/AKT/mTOR pathway members and eukaryotic translation initiation factors. Eur. J. Cancer 83, 56–70 (2017).
Li, Z. et al. N(6)-methyladenosine regulates glycolysis of cancer cells through PDK4. Nat. Commun. 11, 2578 (2020).
Wang, Y. et al. N(1)-methyladenosine methylation in tRNA drives liver tumourigenesis by regulating cholesterol metabolism. Nat. Commun. 12, 6314 (2021).
Cao, P. et al. Germline Duplication of SNORA18L5 Increases Risk for HBV-related Hepatocellular Carcinoma by Altering Localization of Ribosomal Proteins and Decreasing Levels of p53. Gastroenterology 155, 542–556 (2018).
Wu, W. et al. TOPK promotes the growth of esophageal cancer in vitro and in vivo by enhancing YB1/eEF1A1 signal pathway. Cell Death Dis. 14, 364 (2023).
Guo, X. et al. EIF3H promotes aggressiveness of esophageal squamous cell carcinoma by modulating Snail. Stab. J. Exp. Clin. Cancer Res. 39, 175 (2020).
Jia, X. et al. Toosendanin targeting eEF2 impedes Topoisomerase I & II protein translation to suppress esophageal squamous cell carcinoma growth. J. Exp. Clin. Cancer Res. 42, 97 (2023).
Han, H. et al. N(7)-methylguanosine tRNA modification promotes esophageal squamous cell carcinoma tumorigenesis via the RPTOR/ULK1/autophagy axis. Nat. Commun. 13, 1478 (2022).
Phatak, P. et al. MicroRNA-141-3p regulates cellular proliferation, migration, and invasion in esophageal cancer by targeting tuberous sclerosis complex 1. Mol. Carcinog. 60, 125–137 (2021).
Sharma, P. & Sharma, R. miRNA-mRNA crosstalk in esophageal cancer: From diagnosis to therapy. Crit. Rev. Oncol. Hematol. 96, 449–462 (2015).
Zhang, X. et al. Circular RNAs and esophageal cancer. Cancer Cell Int 20, 362 (2020).
Kim, S.-H., Jang, Y. H., Chau, G. C., Pyo, S. & Um, S. H. Prognostic significance and function of phosphorylated ribosomal protein S6 in esophageal squamous cell carcinoma. Mod. Pathol. J. U. S. Can. Acad. Pathol. Inc. 26, 327–335 (2013).
Liu, T. et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 48, 3816–3831 (2020).
Li, Y. et al. eIF2α-CHOP-BCl-2/JNK and IRE1α-XBP1/JNK signaling promote apoptosis and inflammation and support the proliferation of Newcastle disease virus. Cell Death Dis. 10, 891 (2019).
White, K. M. et al. Plitidepsin has potent preclinical efficacy against SARS-CoV-2 by targeting the host protein eEF1A. Science 371, 926–931 (2021).
Chigbu, D. I. et al. Virus Infection: Host−Virus Interaction and Mechanisms of Viral Persistence. Cells 8, e05033 (2019).
Ye, H., Robak, L. A., Yu, M., Cykowski, M. & Shulman, J. M. Genetics and Pathogenesis of Parkinson’s Syndrome. Annu. Rev. Pathol. 18, 95–121 (2023).
Wang, D. et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020).
Jain, S. et al. RNASeq profiling of COVID19-infected patients identified an EIF2AK2 inhibitor as a potent SARS-CoV-2 antiviral. Clin. Transl. Med. 12, e1098 (2022).
Xu, Z. et al. SARS-CoV-2 impairs interferon production via NSP2-induced repression of mRNA translation. Proc. Natl Acad. Sci. USA. 119, e2204539119 (2022).
Selitsky, S. R. et al. Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C. Sci. Rep. 5, 7675 (2015).
Wang, Q. et al. Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol. Ther. 21, 368–379 (2013).
Deng, J. et al. Respiratory Syncytial Virus Utilizes a tRNA Fragment to Suppress Antiviral Responses Through a Novel Targeting Mechanism. Mol. Ther. 23, 1622–1629 (2015).
Yeung, M. L. et al. Pyrosequencing of small non-coding RNAs in HIV-1 infected cells: evidence for the processing of a viral-cellular double-stranded RNA hybrid. Nucleic Acids Res 37, 6575–6586 (2009).
Rawle, D. J. et al. Oxazole-Benzenesulfonamide Derivatives Inhibit HIV-1 Reverse Transcriptase Interaction with Cellular eEF1A and Reduce Viral Replication. J. Virol. 93, e00239–19 (2019).
Shrestha, N. et al. Eukaryotic Initiation Factor 2 (eIF2) signaling regulates proinflammatory cytokine expression and bacterial invasion. J. Biol. Chem. 287, 28738–28744 (2012).
Heesch et al. The Translational Landscape of the Human Heart. Cell 178, 242–260 (2019).
Zhang, G. et al. Unfolded Protein Response as a Therapeutic Target in Cardiovascular Disease. Curr. Top. Med. Chem. 19, 1902–1917 (2020).
Grund, A. et al. TIP 30 counteracts cardiac hypertrophy and failure by inhibiting translational elongation. EMBO Mol. Med. 11, 1–20 (2019).
Oudit, G. Y. & Penninger, J. M. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc. Res. 82, 250–260 (2009).
Zhou, H., Dickson, M. E., Kim, M. S., Bassel-Duby, R. & Olson, E. N. Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes. Proc. Natl Acad. Sci. USA 112, 11864–11869 (2015).
Fremin, B. J., Nicolaou, C. & Bhatt, A. S. Simultaneous ribosome profiling of hundreds of microbes from the human microbiome. Nat. Protoc. 16, 4676–4691 (2021).
Meissner, F., Geddes-McAlister, J., Mann, M. & Bantscheff, M. The emerging role of mass spectrometry-based proteomics in drug discovery. Nat. Rev. Drug Discov. 21, 637–654 (2022).
Vera, M., Biswas, J., Senecal, A., Singer, R. H. & Park, H. Y. Single-Cell and Single-Molecule Analysis of Gene Expression Regulation. Annu. Rev. Genet. 50, 267–291 (2016).
Ingolia, N. T., Brar, G. A., Rouskin, S., McGeachy, A. M. & Weissman, J. S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 7, 1534–1550 (2012).
McGlincy, N. J. & Ingolia, N. T. Transcriptome-wide measurement of translation by ribosome profiling. Methods 126, 112–129 (2017).
Ingolia, N. T., Ghaemmaghami, S., Newman, J. R. S. & Weissman, J. S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324, 218–223 (2009).
Chu, J. & Pelletier, J. Therapeutic Opportunities in Eukaryotic Translation. Cold Spring Harb. Perspect. Biol. 10, a032995 (2018).
Santagata, S. et al. Tight coordination of protein translation and HSF1 activation supports the anabolic malignant state. Science 341, 1238303 (2013).
Wolfe, A. L. et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature 513, 65–70 (2014).
Iwasaki, S., Floor, S. N. & Ingolia, N. T. Rocaglates convert DEAD-box protein eIF4A into a sequence-selective translational repressor. Nature 534, 558–561 (2016).
Cheng, Z. et al. Pervasive, Coordinated Protein-Level Changes Driven by Transcript Isoform Switching during Meiosis. Cell 172, 910–923.e16 (2018).
Becher, I. et al. Pervasive Protein Thermal Stability Variation during the Cell Cycle. Cell 173, 1495–1507.e18 (2018).
Beller, N. C., Lukowski, J. K., Ludwig, K. R. & Hummon, A. B. Spatial Stable Isotopic Labeling by Amino Acids in Cell Culture: Pulse-Chase Labeling of Three-Dimensional Multicellular Spheroids for Global Proteome Analysis. Anal. Chem. 93, 15990–15999 (2021).
Klann, K., Tascher, G. & Münch, C. Functional Translatome Proteomics Reveal Converging and Dose-Dependent Regulation by mTORC1 and eIF2α. Mol. Cell 77, 913–925.e4 (2020).
Ho, J. J. D. et al. Proteomics reveal cap-dependent translation inhibitors remodel the translation machinery and translatome. Cell Rep. 37, 109806 (2021).
Messner, C. B. et al. The proteomic landscape of genome-wide genetic perturbations. Cell 186, 2018–2034.e21 (2023).
Angel, T. E. et al. Mass spectrometry-based proteomics: existing capabilities and future directions. Chem. Soc. Rev. 41, 3912–3928 (2012).
Akirtava, C. & McManus, C. J. Control of translation by eukaryotic mRNA transcript leaders-Insights from high-throughput assays and computational modeling. Wiley Interdiscip. Rev. RNA 12, e1623 (2021).
Roos, D. & de Boer, M. Mutations in cis that affect mRNA synthesis, processing and translation. Biochim. Biophys. acta Mol. basis Dis. 1867, 166166 (2021).
Leppek, K., Das, R. & Barna, M. Functional 5’ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 19, 158–174 (2018).
Deng, Q., Ramsköld, D., Reinius, B. & Sandberg, R. Single-cell RNA-seq reveals dynamic, random monoallelic gene expression in mammalian cells. Science 343, 193–196 (2014).
Trevino, A. E. et al. Chromatin and gene-regulatory dynamics of the developing human cerebral cortex at single-cell resolution. Cell 184, 5053–5069.e23 (2021).
Yin, J.-Y., Dong, Z., Liu, Z.-Q. & Zhang, J.-T. Translational control gone awry: a new mechanism of tumorigenesis and novel targets of cancer treatments. Biosci. Rep. 31, 1–15 (2011).
Miao, B. et al. eIF3a mediates HIF1α-dependent glycolytic metabolism in hepatocellular carcinoma cells through translational regulation. Am. J. Cancer Res. 9, 1079–1090 (2019).
Koromilas, A. E., Lazaris-Karatzas, A. & Sonenberg, N. mRNAs containing extensive secondary structure in their 5’ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J. 11, 4153–4158 (1992).
Yin, J.-Y. et al. Effect of eIF3a on response of lung cancer patients to platinum-based chemotherapy by regulating DNA repair. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 17, 4600–4609 (2011).
Heo, C.-K. et al. Serum anti-EIF3A autoantibody as a potential diagnostic marker for hepatocellular carcinoma. Sci. Rep. 9, 11059 (2019).
Yin, J.-Y., Zhang, J.-T., Zhang, W., Zhou, H.-H. & Liu, Z.-Q. eIF3a: A new anticancer drug target in the eIF family. Cancer Lett. 412, 81–87 (2018).
Wang, H. et al. Translation initiation factor eIF3b expression in human cancer and its role in tumor growth and lung colonization. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 19, 2850–2860 (2013).
Umar, A. et al. Identification of a putative protein profile associated with tamoxifen therapy resistance in breast cancer. Mol. Cell. Proteom. 8, 1278–1294 (2009).
Han, W. et al. Roles of eIF3m in the tumorigenesis of triple negative breast cancer. Cancer Cell Int. 20, 141 (2020).
Lee, A. S. Y., Kranzusch, P. J. & Cate, J. H. D. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature 522, 111–114 (2015).
Carvalho, A. et al. A harmine-derived beta-carboline displays anti-cancer effects in vitro by targeting protein synthesis. Eur. J. Pharmacol. 805, 25–35 (2017).
Shi, J. et al. Decreased expression of eukaryotic initiation factor 3f deregulates translation and apoptosis in tumor cells. Oncogene 25, 4923–4936 (2006).
Bordeleau, M.-E. et al. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J. Clin. Invest. 118, 2651–2660 (2008).
Cerezo, M. et al. Translational control of tumor immune escape via the eIF4F-STAT1-PD-L1 axis in melanoma. Nat. Med. 24, 1877–1886 (2018).
Wang, Q. et al. Tumor suppressor Pdcd4 attenuates Sin1 translation to inhibit invasion in colon carcinoma. Oncogene 36, 6225–6234 (2017).
Topisirovic, I., Svitkin, Y. V., Sonenberg, N. & Shatkin, A. J. Cap and cap-binding proteins in the control of gene expression. Wiley Interdiscip. Rev. RNA 2, 277–298 (2011).
Zhan, Y. et al. The role of eIF4E in response and acquired resistance to vemurafenib in melanoma. J. Invest. Dermatol. 135, 1368–1376 (2015).
Boussemart, L. et al. eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature 513, 105–109 (2014).
Moerke, N. J. et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell 128, 257–267 (2007).
Urtishak, K. A. et al. Targeting EIF4E signaling with ribavirin in infant acute lymphoblastic leukemia. Oncogene 38, 2241–2262 (2019).
Tavares, C. D. J., Devkota, A. K., Dalby, K. N. & Cho, E. J. Application of Eukaryotic Elongation Factor-2 Kinase (eEF-2K) for Cancer Therapy: Expression, Purification, and High-Throughput Inhibitor Screening. Methods Mol. Biol. 1360, 19–33 (2016).
Wojciechowska, M., Olejniczak, M., Galka-Marciniak, P., Jazurek, M. & Krzyzosiak, W. J. RAN translation and frameshifting as translational challenges at simple repeats of human neurodegenerative disorders. Nucleic Acids Res 42, 11849–11864 (2014).
Gupta, A. & Bansal, M. RNA-mediated translation regulation in viral genomes: computational advances in the recognition of sequences and structures. Brief. Bioinform. 21, 1151–1163 (2020).
Brönstrup, M. & Sasse, F. Natural products targeting the elongation phase of eukaryotic protein biosynthesis. Nat. Prod. Rep. 37, 752–762 (2020).
Leprivier, G. et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell 153, 1064–1079 (2013).
Jackson, R. J., Hellen, C. U. T. & Pestova, T. V. Termination and post-termination events in eukaryotic translation. Adv. Protein Chem. Struct. Biol. 86, 45–93 (2012).
Li, W., Chang, S. T.-L., Ward, F. R. & Cate, J. H. D. Selective inhibition of human translation termination by a drug-like compound. Nat. Commun. 11, 4941 (2020).
Sharma, J. et al. A small molecule that induces translational readthrough of CFTR nonsense mutations by eRF1 depletion. Nat. Commun. 12, 4358 (2021).
Coleman, L. J. et al. Combined analysis of eIF4E and 4E-binding protein expression predicts breast cancer survival and estimates eIF4E activity. Br. J. Cancer 100, 1393–1399 (2009).
Liberale, L., Montecucco, F., Schwarz, L., Lüscher, T. F. & Camici, G. G. Inflammation and cardiovascular diseases: lessons from seminal clinical trials. Cardiovasc. Res. 117, 411–422 (2021).
Assouline, S. et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood 114, 257–260 (2009).
Hong, D. S. et al. A phase 1 dose escalation, pharmacokinetic, and pharmacodynamic evaluation of eIF-4E antisense oligonucleotide LY2275796 in patients with advanced cancer. Clin. Cancer Res. J. Am. Assoc. Cancer Res. 17, 6582–6591 (2011).
Khoury, H. J. et al. Omacetaxine mepesuccinate in patients with advanced chronic myeloid leukemia with resistance or intolerance to tyrosine kinase inhibitors. Leuk. Lymphoma 56, 120–127 (2015).
Chen, X. et al. A noncanonical function of EIF4E limits ALDH1B1 activity and increases susceptibility to ferroptosis. Nat. Commun. 13, 6318 (2022).
Naineni, S. K. et al. A comparative study of small molecules targeting eIF4A. RNA 26, 541–549 (2020).
Zhang, W. et al. The eIF4A Inhibitor Silvestrol Blocks the Growth of Human Glioblastoma Cells by Inhibiting AKT/mTOR and ERK1/2 Signaling Pathway. J. Oncol. 2022, 4396316 (2022).
Dong, Z. & Zhang, J.-T. EIF3 p170, a mediator of mimosine effect on protein synthesis and cell cycle progression. Mol. Biol. Cell 14, 3942–3951 (2003).
Boyce, M. et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005).
Hagner, P. R., Schneider, A. & Gartenhaus, R. B. Targeting the translational machinery as a novel treatment strategy for hematologic malignancies. Blood 115, 2127–2135 (2010).
Devkota, A. K. et al. Investigating the kinetic mechanism of inhibition of elongation factor 2 kinase by NH125: evidence of a common in vitro artifact. Biochemistry 51, 2100–2112 (2012).
Ashour, A. A. et al. Targeting elongation factor-2 kinase (eEF-2K) induces apoptosis in human pancreatic cancer cells. Apoptosis 19, 241–258 (2014).
Acknowledgements
This work was supported by the National Natural Science Foundations of China [No. 82073075]; the Central Plains Science and Technology Innovation Leading Talents [No. 224200510015]; and Natural Science Foundation of Henan [No. 222102310029]. We’d like to express our gratitude to Dr. Fred Bogott for the language revision. The figures in this manuscript were generated from BioRender.com.
Author information
Authors and Affiliations
Contributions
X.C.J. and X.Y.H. wrote the original review. X.C.J. and X.Y.H. created all original figures. C.T.H. and J.L. collected the data. Z.G.D. and K.D.L. revised the review and provided editorial assistance. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jia, X., He, X., Huang, C. et al. Protein translation: biological processes and therapeutic strategies for human diseases. Sig Transduct Target Ther 9, 44 (2024). https://doi.org/10.1038/s41392-024-01749-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-024-01749-9