Summary:
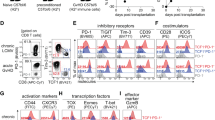
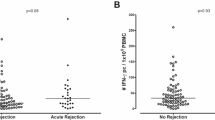
Cytotoxic T lymphocytes (CTLs) are important effector cells of graft-versus-host disease (GVHD) and vascular endothelial cells are target cells of allospecific CTL. A combined assessment of T-cell activation and endothelial injury should result in a specific and sensitive test for GVHD. We examined circulating T lymphocytes for effector molecules involved in CTL-mediated endothelial injury. We analyzed CD4 and CD8 T lymphocytes of 24 long-term survivors of allogeneic stem cell transplantation with or without GVHD, and nine healthy, age-matched controls for signs of CTL activation and endothelial injury. IFN-γ transcript levels in CD8 T cells were significantly elevated in SCT recipients with GVHD compared to patients without GVHD (767 CD3ɛ units/T cell (376–2050) vs 211 CD3ɛ units/T cell (159–274), P=0.01). Fas ligand transcript levels in CD4 T cells were significantly elevated in SCT recipients without GVHD compared to patients with GVHD (20 CD3ɛ units/T cell (0–78) vs 0 CD3ɛ units/T cell (0–0), P=0.01). Von Willebrand factor plasma levels were high in patients with GVHD, but normal in patients without GVHD (209 (186–254) vs 120 (100–141), P=0.0005). This assessment of T-cell activation and endothelial injury results in a sensitive and specific test to identify patients with active chronic GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Majolino I, Saglio G, Scime R et al. High incidence of chronic GVHD after primary allogeneic peripheral blood stem cell transplantation in patients with hematologic malignancies. Bone Marrow Transplant 1996; 17: 555.
Socie G, Stone JV, Wingard JR et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med 1999; 341: 14.
Gratwohl A, Hermans J, Apperley J et al. Acute graft-versus-host disease: grade and outcome in patients with chronic myelogenous leukemia. Working Party Chronic Leukemia of the European Group for Blood and Marrow Transplantation. Blood 1995; 86: 813.
van den Brink MR, Burakoff SJ . Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol 2002; 2: 273.
Roosnek E, Hogendijk S, Zawadynski S et al. The frequency of pretransplant donor cytotoxic T cell precursors with anti-host specificity predicts survival of patients transplanted with bone marrow from donors other than HLA-identical siblings. Transplantation 1993; 56: 691.
Mutis T, Gillespie G, Schrama E et al. Tetrameric HLA class I-minor histocompatibility antigen peptide complexes demonstrate minor histocompatibility antigen-specific cytotoxic T lymphocytes in patients with graft-versus-host disease. Nat Med 1999; 5: 839.
Biedermann BC, Sahner S, Gregor M et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet 2002; 359: 2078.
Shulman HM, Sullivan KM, Weiden PL et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204.
Hamann D, Baars PA, Rep MH et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med 1997; 186: 1407.
Biedermann BC, Pober JS . Human vascular endothelial cells favor clonal expansion of unusual alloreactive CTL. J Immunol 1999; 162: 7022.
Fitzpatrick DR, Shirley KM, McDonald LE et al. Distinct methylation of the interferon gamma (IFN-gamma) and interleukin 3 (IL-3) genes in newly activated primary CD8+ T lymphocytes: regional IFN-gamma promoter demethylation and mRNA expression are heritable in CD44(high)CD8+T cells. J Exp Med 1998; 188: 103.
Callan MF, Tan L, Annels N et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein–Barr virus in vivo. J Exp Med 1998; 187: 1395.
Busch DH, Pilip I, Pamer EG . Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J Exp Med 1998; 188: 61.
Ogg GS, Jin X, Bonhoeffer S et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 1998; 279: 2103.
Callan MF, Fazou C, Yang H et al. CD8(+) T-cell selection, function, and death in the primary immune response in vivo. J Clin Invest 2000; 106: 1251.
Cerny A, McHutchison JG, Pasquinelli C et al. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J Clin Invest 1995; 95: 521.
Strehlau J, Pavlakis M, Lipman M et al. Quantitative detection of immune activation transcripts as a diagnostic tool in kidney transplantation. Proc Natl Acad Sci USA 1997; 94: 695.
Starzl TE, Zinkernagel RM . Antigen localization and migration in immunity and tolerance. N Engl J Med 1998; 339: 1905.
Woywodt A, Streiber F, de Groot K et al. Circulating endothelial cells as markers for ANCA-associated small-vessel vasculitis. Lancet 2003; 361: 206.
Acknowledgements
We thank the medical staff of the Division of Hematology for their support and expert care for the patients included in this study, D Wittwer and I Grilli for excellent technical assistance, J Passweg for critically reading the manuscript and advice on statistical analysis and R Krapf for helpful discussion and support. This study was supported by a grant from the Swiss National Science Foundation (#31-55948), by the Krebsliga Beider, Basel and by the NIH (HL62188).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biedermann, B., Tsakiris, D., Gregor, M. et al. Combining altered levels of effector transcripts in circulating T cells with a marker of endothelial injury is specific for active graft-versus-host disease. Bone Marrow Transplant 32, 1077–1084 (2003). https://doi.org/10.1038/sj.bmt.1704258
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1704258
Keywords
This article is cited by
-
A non-interventional study of microcirculation dynamics in allogeneic hematopoietic cell transplantation survivors compared to controls: evidence of impaired microvascular response regardless of conventional cardiovascular risk factors
Bone Marrow Transplantation (2022)
-
Numerical impairment of nestin+ bone marrow niches in acute GvHD after allogeneic hematopoietic stem cell transplantation for AML
Bone Marrow Transplantation (2015)
-
GVHD after allogeneic haematopoietic SCT for AML: angiogenesis, vascular endothelial growth factor and VEGF receptor expression in the BM
Bone Marrow Transplantation (2013)
-
Replacement of calcineurin inhibitors with daclizumab in patients with transplantation-associated microangiopathy or renal insufficiency associated with graft-versus-host disease
Bone Marrow Transplantation (2006)