Synthesis, Microstructure, and Electrical Conductivity of Eutectic Composites in MF2–RF3 (M = Ca, Sr, Ba; R = La–Nd) Systems
Abstract
:1. Introduction
2. Materials and Methods
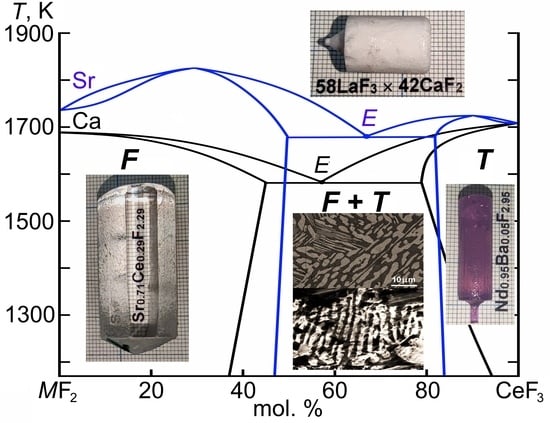
2.1. Composites Synthesis
2.2. X-ray Difraction (XRD) Phase Analysis
2.3. Optical and Scanning Electron Microscopy
2.4. Electrical Conductivity Measurements
3. Results and Discussion
3.1. XRD Characterization of Eutectic Composites
3.2. Characterization of Composites by Optical and SEM Microscopy
3.3. Temperature Dependence of the Ionic Conductivity of Composites
3.4. Anisotropy of the Ionic Conductivity of Composites
3.5. The Mechanism of Fluorine-Ionic Conductivity in pRF3 × qMF2 Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uvarov, N.F. Composite solid electrolytes: Resent advances and design strategies. J. Solid State Electrochem. 2011, 15, 367–389. [Google Scholar] [CrossRef]
- Sorokin, N.I. Superionic transport in fluoride composites and glasses. Russ. J. Electrochem. 2004, 40, 569–577. [Google Scholar] [CrossRef]
- Llorca, J.; Orera, V.M. Directionally solidified eutectic ceramic oxides. Prog. Mater. Sci. 2006, 51, 711–809. [Google Scholar] [CrossRef]
- Pawlak, D.A.; Turczynski, S.; Gajc, M.; Kolodziejak, K.; Diduszko, R.; Rozniatowski, K.; Smalc, J.; Vendik, I. Metamaterials: How far are we from making metamaterials by self-organization? The microstructure of highly anisotropic particles with an SSR-like geometry. Adv. Funct. Mater. 2010, 20, 1116–1124. [Google Scholar] [CrossRef]
- Ashbrook, R.L. Directionally solidified ceramic eutectics. J. Am. Ceram. Soc. 1977, 60, 428–435. [Google Scholar] [CrossRef]
- Hunt, J.D.; Lu, S.Z. Crystallization of eutectics, monotectics and peritectics. In Handbook of Crystal Growth; Hurle, D.T.J., Ed.; North–Holland Elseiver Science: Amsterdam, The Netherlands; New York, NY, USA, 1994; Volume 2, Part B, pp. 1111–1166. [Google Scholar]
- Akamatsu, S.; Plapp, M. Eutectic and peritectic solidification patterns. Curr. Opin. Solid State Mater. Sci. 2016, 20, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Zhang, Z.; Zhao, J.; Sun, D.; Wang, F. Orientation and microstructure evolution of Al-Al2Cu regular eutectic lamellar bifurcating in an abruptly changing velocity under directional solidification. Materials 2020, 13, 1004. [Google Scholar] [CrossRef] [Green Version]
- Semenenko, V.E.; Kovtun, G.P. Influence of rolling on the stability of the compositional microstructure of eutectic alloys. Voprosy Atomnoy Nauki i Tekhniki 2002, 1, 148–150. (In Russian) [Google Scholar]
- Gus’kov, A.P. Stability of the interphase boundary during the crystallization of eutectics. Tech. Phys. Lett. 2001, 27, 480–483. [Google Scholar] [CrossRef]
- Gus’kov, A.; Orlov, A. Dependence of period of macrostructures on kinetic parameters under directed crystallization. Comput. Mater. Sci. 2002, 24, 93–98. [Google Scholar] [CrossRef]
- Bei, H.; George, E.P. Microstructures and mechanical properties of a directionally solidified NiAl-Mo eutectic alloy. Acta Mater. 2005, 53, 69–77. [Google Scholar] [CrossRef]
- Ohashi, Y.; Yasui, N.; Suzuki, T.; Watanabe, M.; Den, T.; Kamada, K.; Yokota, Y.; Yoshikawa, A. Orientation relationships of unidirectionally aligned GdAlO3/Al2O3 eutectic fibers. J. Eur. Ceram. Soc. 2014, 34, 3849–3857. [Google Scholar] [CrossRef]
- Orera, V.M.; Merino, R.I.; Pardo, J.A.; Larrea, A.; Pena, J.I.; Gonzalez, C.; Poza, P.; Pastor, J.Y.; Llorca, J. Microstructure and physical properties of some oxide eutectic composites processed by directional solidification. Acta Mater. 2000, 48, 4683–4689. [Google Scholar] [CrossRef]
- Cicka, R.; Trnovcova, V.; Starostin, M.Y. Electrical properties of alumina–zirconia eutectic composites. Solid State Ion. 2002, 148, 425–429. [Google Scholar] [CrossRef]
- Trnovcova, V.; Starostin, M.Y.; Cicka, R.; Fedorov, P.P.; Barta, C.; Labas, V.; Sobolev, B.P. Microstructure and fast ionic conduction of inorganic fluoride and oxide eutectic composites prepared from the melt. Solid State Ion. 2000, 136–137, 11–17. [Google Scholar] [CrossRef]
- Semenenko, V.E.; Pylypenko, M.M. The morphology of carbide phases in eutectic alloys created by unidirectional solidification. Vopr. At. Nauk. Tekhniki 2003, 13, 117–121. (In Russian) [Google Scholar]
- Fedorov, P.; Trubitsyn, M.; Trnovtseva, V.; Sobolev, B. Obtaining the eutectic composition in the LiF-PbF2 system. Inorg. Mater. 1992, 28, 1805–1808. [Google Scholar]
- Kim, K.J.; Furuya, Y.; Kamada, K.; Murakami, R.; Kochurikhin, V.V.; Yoshino, M.; Chiba, H.; Kurosawa, S.; Yamaji, A.; Shoji, Y.; et al. Growth and Scintillation Properties of Directionally Solidified Ce:LaBr3/AEBr2 (AE = Mg, Ca, Sr, Ba) Eutectic System. Crystals 2020, 10, 584. [Google Scholar] [CrossRef]
- Cheng, S.; Hunneke, R.E.; Tian, M.; Lukosi, E.; Zhuravleva, M.; Melcher, C.L.; Wu, Y. Self-assembled natLiCl-CeCl3 directionally solidified eutectics for thermal neutron detection. CrystEngComm 2020, 22, 3269–3273. [Google Scholar] [CrossRef]
- Barta, C.; Fendrych, F.; Recker, K.; Triska, A.; Wallrafen, F. On the influence of the crystallization conditions on the microstructure of the directionally solidified eutectic of the LiF-LiYF4 system. Cryst. Res Technol. 1991, 26, 413–424. [Google Scholar] [CrossRef]
- Nishimoto, K.; Yokota, Y.; Kurosawa, S.; Fujimoto, Y.; Kawaguchi, N.; Fukuda, K.; Yoshikawa, A. Crystal growth of LiF/LiYF4 eutectic crystals and their luminescent properties. J. Eur. Ceram. Soc. 2014, 34, 2117–2121. [Google Scholar] [CrossRef]
- Yanagida, T.; Fukuda, K.; Fujimoto, Y.; Kawaguchi, N.; Kurosawa, S.; Yamazaki, A.; Watanabe, K.; Futami, Y.; Yokota, Y.; Pejchal, J.; et al. Eu-doped 6LiF-SrF2 eutectic scintillators for neutron detection. Opt. Mater. 2012, 34, 868–871. [Google Scholar] [CrossRef]
- Hishinuma, K.; Kamada, K.; Kurosawa, S.; Yamaji, A.; Pejchal, J.; Yokoto, Y.; Jhashi, Y.; Yoshikawa, A. LiF/CaF2/LiBaF3 ternary fluoride eutectic scintillator. Jpn. J. Appl. Phys. 2015, 54, 04DH04. [Google Scholar] [CrossRef] [Green Version]
- Trnovcova, V.; Fedorov, P.P.; Barta, C.; Labas, C.V.; Meleshina, V.A.; Sobolev, B.P. Microstructure and physical properties of superionic eutectic composites of the LiF-RF3 (R-rare earth element) system. Solid State Ion. 1999, 119, 173–180. [Google Scholar] [CrossRef]
- Trnovcova, V.; Fedorov, P.P.; Buchinskaya, I.I.; Smatko, V.; Hanic, F. Fast ionic conductivity of PbF2:MF2 (M = Mg, Ba, Cd) and PbF2:ScF3 single crystals and composites. Solid State Ion. 1999, 119, 181–189. [Google Scholar] [CrossRef]
- Wakahara, S.; Furuya, Y.; Yanagida, T.; Yokoto, Y.; Pejchal, J.; Sugiyama, M.; Kawaguchi, N.; Totsuka, D.; Yoshikawa, A. Crystal growth and scintillation properties of Ce-doped sodium calcium lutetium complex fluoride. Opt. Mater. 2012, 34, 729–732. [Google Scholar] [CrossRef]
- Karimov, D.N.; Sorokin, N.I.; Grebenev, V.V.; Ivanova, A.G.; Arkharova, N.A.; Orekhov, A.S.; Sobolev, B.P. 75LiF+25SmF3 eutectic composite and ionic conductivity of SmF3 near the polymorphic α-β transition. Crystallogr. Rep. 2020, 65, 468–472. [Google Scholar] [CrossRef]
- Hartmann, E.; Peller, V.V.; Rogalski, G.I. Electrical conductivity of fluoride eutectic composites. Solid State Ion. 1988, 28–30, 1098–1101. [Google Scholar] [CrossRef]
- Xiang, M.; Zhang, Y.; Lin, H.; Zhu, Y.; Guo, X.; Chen, J.; Li, L. LiBH4-NaX (X = Cl, I) composites with enhanced lithium ionic conductivity. J. Alloys Compd. 2018, 764, 307–313. [Google Scholar] [CrossRef]
- Trnovcova, V.; Fedorov, P.P.; Furar, I. Fluoride solid electrolytes. Russ. J. Electrochem. 2009, 45, 630–639. [Google Scholar] [CrossRef]
- Trnovcova, V.; Barta, C.; Zibrov, I.P.; Fedorov, P.P. Phase relations, microstructure and physical properties of superionic fluoride composites. Mater. Sci. Forum 1991, 76, 13–18. [Google Scholar] [CrossRef]
- Medvedeva, L.V.; Sorokin, N.I.; Vistin’, L.L.; Fedorov, P.P.; Sobolev, B.P. Phase equilibria in the system LiF-SrF2-LaF3 and ionic conductivity of the eutectic electrolyte. Russ. J. Inorg. Chem. 1994, 39, 304–306. [Google Scholar]
- Sorokin, N.I.; Buchinskaya, I.I.; Bystrova, A.A.; Konovalova, V.V.; Sobolev, B.P. Eutectic composition systems NaF-DyF3, NaF-HoF3 and MgF2-ScF3 as ionic conductors. Russ. J. Electrochem. 2005, 41, 900–902. [Google Scholar] [CrossRef]
- Trnovcova, V.; Fedorov, P.P.; Buchinskaya, I.I.; Sobolev, B.P. Ionic conductivity of the PbF2-NaF eutectic composite and PbF2 single crystals doped with alkali fluorides. Inorg. Mater. 1996, 32, 1104–1107. [Google Scholar]
- Buchinskaya, I.I.; Fedorov, P.P.; Sorokin, N.I.; Akchurin, M.S.; Sobolev, B.P. Section Pb0.67Cd0.33F2.00-NaF and the conductivity of the composites. Rus. J. Inorg. Chem. 1996, 41, 166–170. [Google Scholar]
- Trnovcova, V.; Garashina, L.S.; Skubla, A.; Fedorov, P.P.; Cicka, R.; Krivandina, E.A.; Sobolev, B.P. Structural aspects of fast ionic conductivity of rare earth fluorides. Solid State Ion. 2003, 157, 195–201. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Trnovcova, V.; Meleshina, V.A.; Chugunov, V.D.; Sobolev, B.P. Eutectic alloys in PbF2-RF3 systems (R = Ho, Yb, Sc). Inorg. Mater. 1994, 30, 384–388. [Google Scholar]
- Sorokin, N.I.; Sobolev, B.P. Conductivity of a eutectic composite in the SrF2-LaF3 system. Crystallogr. Rep. 1996, 41, 490–493. [Google Scholar]
- Sorokin, N.I.; Karimov, D.N.; Samsonova, N.V.; Ivanova, A.G.; Fedorov, V.A.; Sobolev, B.P. Growth of Sm1−ySryF3−y (0 < y ≤ 0.31) crystals and investigation of their properties. Crystallogr. Rep. 2019, 64, 488–495. [Google Scholar] [CrossRef]
- Sorokin, N.I. Superionic transport in solid fluoride solutions with a fluorite structure. Russ. J. Electrochem. 2006, 42, 744–759. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Sorokin, N.I. Nonstoichiometry in inorganic fluorides: 2. Ionic conductivity of nonstoichiometric M1−xRxF2+x and R1−yMyF3−y crystals (M = Ca, Sr, Ba; R are rare earth elements). Crystallogr. Rep. 2014, 59, 807–830. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Sorokin, N.I.; Bolotina, N.B. Nonstoichiometric single crystals M1−xRxF2+x and R1−yMyF3−y (M-Ca, Sr, Ba; R-rare earth elements) as fluorine-conducting solid electrolytes. In Progress in Fluorine Science; Tressaud, A., Poeppelmeier, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1, Chapter 21; pp. 465–491. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Tkachenko, N.L. Phase diagrams of the BaF2-(Y, Ln)F3 systems. J. Less Common Met. 1982, 85, 155–170. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Fedorov, P.P. Phase Diagrams of the CaF2-(Y, Ln)F3 systems. I. Experimental. J. Less Common Met. 1978, 60, 33–46. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Seiranian, K.B.; Garashina, L.S.; Fedorov, P.P. Phase diagrams of the SrF2-(Y, Ln)F3 Systems. I. X-ray characteristics of phases. J. Solid State Chem. 1979, 28, 51–58. [Google Scholar] [CrossRef]
- Buchinskaya, I.I.; Karimov, D.N.; Sorokin, N.I. La1−yBayF3−y Solid Solution Crystals as an Effective Solid Electrolyte: Growth and Properties. Crystals 2021, 11, 629. [Google Scholar] [CrossRef]
- Bolotina, N.B.; Chernaya, T.S.; Verin, I.A.; Khrykina, O.N.; Sobolev, B.P. Dimorphism of RF3 (R = La-Nd) crystals based on the data of X-ray diffraction studies. Crystallogr. Rep. 2016, 61, 29–34. [Google Scholar] [CrossRef]
- Jackson, K.A.; Hunt, J.D. Lamellar and rod eutectic growth. In Dynamics of Curved Fronts; Pelcé, P., Ed.; Academic Press Inc.: Cambridge, MA, USA, 1988; pp. 363–376. [Google Scholar] [CrossRef]
- Konstantinova, A.F.; Krivandina, E.A.; Karimov, D.N.; Sobolev, B.P. Calculation of the Refractive Indices of M1−xRxF2+x Crystals (M = Ca, Sr, Ba, Cd, Pb; R are Rare Earth Elements). Crystallogr. Rep. 2010, 55, 990–994. [Google Scholar] [CrossRef]
- Glushkova, T.M.; Karimov, D.N.; Krivandina, E.A.; Zmurova, Z.I.; Sobolev, B.P. Nanostructured crystals of fluorite phases Sr1−xRxF2+x (R = Y, La-Lu) and their ordering: Part III. A study of the refractive indices. Crystallogr. Rep. 2009, 54, 603–608. [Google Scholar] [CrossRef]
- Chalmers, B. Principles of Solidification; John Wiley and Sons: Hoboken, NJ, USA; London, UK, 1964; p. 319. [Google Scholar]
- Sorokin, N.I.; Breiter, M.W. Anionic conductivity and thermal stability of single crystals of solid solutions based on barium fluoride. Solid State Ion. 1977, 99, 241–250. [Google Scholar] [CrossRef]
- Sorokin, N.I.; Fominykh, M.V.; Krivandina, E.A.; Zhmurova, Z.I.; Sobolev, B.P. Ion transport in the anion-deficient nonstoichiometric phases La0.95(Ba1−xSrx)0.05F2.95 (0 ≤ x ≤ 1). Phys. Solid State 1998, 40, 604–607. [Google Scholar] [CrossRef]
- Rongeat, C.; Anji Reddy, M.; Witter, R.; Fichtner, M. Nanostructured fluorite-type fluorides as electrolytes for fluoride ion batteries. J. Phys. Chem. C 2013, 117, 4943–4950. [Google Scholar] [CrossRef]
- Sorokin, N.I.; Fedorov, P.P.; Sobolev, B.P. Superionic materials based on lead fluoride. Inorg. Mater. 1997, 33, 1–11. [Google Scholar]
- Sobolev, B.P.; Sorokin, N.I.; Krivandina, E.A.; Zhmurova, Z.I. 293-K conductivity optimization for single crystals of solid electrolytes with tysonite structure (LaF3): I. Nonstoichiometric phases R1−yCayF3−y (R = La-Lu, Y). Crystallogr. Rep. 2014, 59, 550–562. [Google Scholar] [CrossRef]
- Sobolev, B.P.; Sorokin, N.I.; Krivandina, E.A.; Zhmurova, Z.I. Optimization of single crystals of solid electrolytes with tysonite-type structure (LaF3) for conductivity at 293 K: 2. Nonstoichiometric phases R1−yMyF3−y (R = La-Lu, Y.; M = Sr, Ba). Crystallogr. Rep. 2015, 60, 123–129. [Google Scholar] [CrossRef]
- Sorokin, N.I. Crystal-physical model of electrotransfer in the superionic conductor Pb1−xScxF2+x (x = 0.1). Phys. Solid State 2018, 60, 714–718. [Google Scholar] [CrossRef]
- Sorokin, N.I.; Sobolev, B.P. Frequency response of the low-temperature ionic conductivity of single crystals R1−yMyF3−y (R = La-Er, M = Ca, Sr, Ba, Cd). Phys. Solid State 2008, 50, 416–421. [Google Scholar] [CrossRef]
- Breuer, S.; Lunghamer, S.; Kies, A.; Wilkening, M. F anion dynamics in cation-mixed nanocrystalline LaF3: SrF2. J. Mater. Sci. 2018, 53, 13669–13681. [Google Scholar] [CrossRef] [Green Version]
- Chable, J.; Martin, A.G.; Bourdin, A.; Body, M.; Legein, C.; Jouanneaux, A.; Crosnier-Lopez, M.-P.; Galven, C.; Dieudonne, B.; Leblanc, M.; et al. Fluoride solid electrolytes: From microcrystalline to nanostructured tysonite-type La0.95Ba0.05F2.95. J. Alloys Compd. 2017, 692, 980–988. [Google Scholar] [CrossRef]
- Rongeat, C.; Anji Reddy, M.; Witter, R.; Fichtner, M. Solid electrolytes for fluoride ion batteries: Ionic conductivity in polycrystalline tysonite-type fluorides. ACS Appl. Mater. Interfaces 2014, 6, 2103–2110. [Google Scholar] [CrossRef]
- Duvel, A.; Bednarcik, J.; Sepelak, V.; Heitjans, P. Mechanosynthesis of the fast ion conductor Ba1−xLaxF2+x: From the fluorite to the tysonite structure. J. Phys. Chem. C 2014, 118, 7117–7129. [Google Scholar] [CrossRef] [Green Version]
- Sorokin, N.I.; Sobolev, B.P. Correlation between the fluorine ion conductivities of Sr1−xRxF2+x (CaF2 type) and R1−ySryF3−y (LaF3 type) crystals in the SrF2-RF3 systems (R = La-Nd). Phys. Solid State 2019, 61, 2034–2040. [Google Scholar] [CrossRef]
- Karimov, D.N.; Buchinskaya, I.I.; Sorokin, N.I.; Sobolev, B.P. Fluorine-Conducting Composite Electrolyte and Method for Its Preparation. Patent RU 2702905, 14 October 2019. Bulletin No. 29. [Google Scholar]






| MF2–RF3 Systems | Eutectic Compositions * | Lattice Parameters *, Å | Limiting Molar Phase Compositions (x, y) at TE | Mole Phase Ratio (F/T) in Eutectics | |||||
|---|---|---|---|---|---|---|---|---|---|
| mol. % MF2 | TE, K | F-Phase | T-Phase | F-Phase M1−xRxF2+x | T-Phase R1−yMyF3−y | ||||
| a | a | c | |||||||
| CaF2 | LaF3 | 42 | 1584 | 5.709 | 7.054 | 7.314 | 0.46 | 0.23 | 1/0.63 |
| CeF3 | 43 | 1581 | 5.676 | 7.035 | 7.250 | 0.45 | 0.21 | 1/0.55 | |
| PrF3 | 41 | 1573 | 5.650 | 6.982 | 7.217 | 0.43 | 0.22 | 1/0.84 | |
| NdF3 | 40 | 1557 | 5.638 | 6.956 | 7.193 | 0.44 | 0.19 | 1/0.76 | |
| SrF2 | LaF3 | 30 | 1723 | 5.867 | 7.173 | 7.368 | 0.49 | 0.17 | 1/1.61 |
| CeF3 | 34 | 1678 | 5.843 | 7.127 | 7.326 | 0.50 | 0.18 | 1/1.10 | |
| PrF3 | 34 | 1657 | 5.822 | 7.095 | 7.290 | 0.49 | 0.19 | 1/1.28 | |
| NdF3 | 30 | 1635 | 5.800 | 7.077 | 7.274 | 0.50 | 0.19 | 1/1.81 | |
| BaF2 | LaF3 | 32 | 1663 | 6.042 | 7.235 | 7.408 | 0.52 | 0.14 | 1/0.89 |
| CeF3 | 32 | 1596 | 6.012 | 7.203 | 7.378 | 0.53 | 0.16 | 1/0.94 | |
| PrF3 | 33 | 1543 | 6.007 | 7.208 | 7.335 | 0.51 | 0.18 | 1/1.07 | |
| NdF3 | 31 | 1488 | 5.994 | 7.174 | 7.301 | 0.51 | 0.18 | 1/1.38 | |
| Crystallized Eutectic Compositions | Lattice Parameters of the Saturated SSs, Å | Experimental Phase Mass Fraction in the SS Composition | Calculated Limiting Phase Compositions (x, y) | Mass Phase (F/T) Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| F-Phase | T-Phase * | F-Phase | T-Phase | F-Phase M1−xRxF2+x | T-Phase R1−yMyF3−y | |||
| a | a | c | ||||||
| 58LaF3 × 42CaF2 | 5.662 (1) | 4.118 (1) | 7.332 (1) | 0.43(9) | 0.57 (10) | 0.36 | - | 1/1.32 |
| 59PrF3 × 41CaF2 | 5.631 (1) | 4.053 (1) | 7.223 (1) | 0.37 (6) | 0.63 (9) | 0.36 | 0.15 | 1/1.67 |
| 60NdF3 × 40CaF2 | 5.618 (1) | 4.028 (1) | 7.188 (1) | 0.41 (6) | 0.59 (10) | 0.38 | - | 1/1.44 |
| 70NdF3 × 30SrF2 | 5.801 (1) | 4.069 (1) | 7.251 (1) | 0.30 (3) | 0.70 (5) | - | 0.13 | 1/2.33 |
| 68LaF3 × 32BaF2 | 6.037 (1) | 4.186 (1) | 7.419 (1) | 0.464 (1) | 0.536 (1) | 0.54 | 0.17 | 1/1.16 |
| 68CeF3 × 32BaF2 | 6.019 (1) | 4.164 (1) | 7.372 (1) | 0.415 (4) | 0.585 (15) | 0.51 | 0.15 | 1/1.41 |
| 69NdF3 × 31BaF2 | 5.984 (1) | 4.135 (1) | 7.310 (1) | 0.314 (11) | 0.700 (19) | 0.53 | 0.20 | 1/2.23 |
| Composites pRF3 × qMF2 | Temperature Range ΔT, K | Factor A, 105 S·K/cm | Enthalpy Ha, eV | References |
|---|---|---|---|---|
| 59PrF3 × 41CaF2 | 297–537 | 1 | 0.52 | this work |
| 60NdF3 × 40CaF2 | 298–536 | 2.9 | 0.56 | |
| 71LaF3 × 29SrF2 | 293–570 | 12–25 | 0.57–0.60 | [39] |
| 70NdF3 × 30SrF2 | 298–553 | 6.4 | 0.60 | this work |
| 69SmF3 × 31SrF2 | 290–541 | 27 | 0.65 | [40] |
| 68LaF3 × 32BaF2 | 296–535 | 0.7 | 0.49 | this work |
| 68CeF3 × 32BaF2 | 298–536 | 0.65 | 0.50 | |
| 69NdF3 × 31BaF2 | 297–706 | 0.4 | 0.53 |
| Composites | Conductivity σdc, S/cm | References | |
|---|---|---|---|
| 293 K | 500 K | ||
| 58LaF3 × 42CaF2 | 3.5 × 10−7 | – | this work |
| 59PrF3 × 41CaF2 | 4.2 × 10−7 | 1.1 × 10−3 | |
| 60NdF3 × 40CaF2 | 1.75 × 10−7 | 1.3 × 10−3 | |
| 71LaF3 × 29SrF2 | 6 × 10−7–2 × 10−6 | 2 × 10−3–7 × 10−3 | [39] |
| 70NdF3 × 30SrF2 | 1.8 × 10−7 | 1.2 × 10−3 | this work |
| NdF3 × SrF2 | ~1.6 × 10−7 | ~2 × 10−3 | [32] |
| 69SmF3 × 31SrF2 | 6 × 10−8 | 1.5 × 10−3 | [40] |
| 68LaF3 × 32BaF2 | 8.2 × 10−7 | 1.6 × 10−3 | this work |
| 68CeF3 × 32BaF2 | 8.3 × 10−7 | 1.2 × 10−3 | |
| 69NdF3 × 31BaF2 | 1.3 × 10−7 | 4 × 10−4 | |
| NdF3 × BaF2 | ~1 ×10−7 | ~2 × 10−4 | [32] |
| 82LaF3 × 18LiF | 1 × 10−7–2 × 10−7 | 2 × 10−5 | [32] |
| 82LaF3 × 18LiF | 4 × 10−8 | 2.2 × 10−5 | [33] |
| LaF3 × LiF | 4 × 10−8 | 1 × 10−5 | [25,26] |
| PrF3 × LiF | 5 × 10−7 | ~1 × 10−4 | [25,26] |
| 82NdF3 × 18LiF | 1 × 10−7–2 × 10−7 | 4 × 10−5 | [32] |
| 80NdF3 × 20LiF | 2 × 10−7 | 1.2 × 10−4 | [2] |
| NdF3 × LiF | 6 × 10−7 | 8 × 10−5 | [25,26] |
| SmF3 × LiF | 8 × 10−12 | 6 × 10−7 | [25,26] |
| GdF3 × LiF | 4 × 10−8 | 3 × 10−5 | [25,26] |
| ErF3 × LiF | ~1 × 10−9 | 2 × 10−6 | [32] |
| YF3 × LiF | 3 × 10−9 | 3 × 10−6 | [25,26] |
| 75DyF3 × 25NaF | – | 2 × 10−7 | [34] |
| 75HoF3 × 25NaF | – | 4 × 10−7 | [34] |
| 45ScF3 × 55MgF2 | – | 2 × 10−5 | [34] |
| 52HoF3 × 48PbF2 | 7 × 10−9 | 2 × 10−5 | [38] |
| 52YbF3 × 48PbF2 | 3 × 10−11 | 6 × 10−7 | [33] |
| 25ScF3 × 75PbF2 | 1 × 10−4 | 3 × 10−3 | [33] |
| 38LiF × 62PbF2 | 4 × 10−7 | 8 × 10−4 | [18] |
| 31NaF × 69PbF2 | 1.5 × 10−4 | 4 × 10−3 | [35] |
| 34NaF × 66PbF2 | 6 × 10−5 | 5 × 10−3 | [36,53] |
| 40NaF × 60Pb0.67Cd0.33F2 | 4.5 × 10−5 | – | [36] |
| 38NaF × 62Pb0.74Cd0.26F2 | 2.4 × 10−5 | – | [36] |
| NaF × LiF | – | 2 × 10−10–4 × 10−9 | [29] |
| NaF × CaF2 | – | 6 × 10−7–3 × 10−6 | [29] |
| CaF2 × MgF2 | – | 2 × 10−9–1 × 10−8 | [16] |
| LiF × LiBaF3, | – | 5 × 10−8–1 × 10−6 | [16] |
| LiF × LiGdF4 | – | 2 × 10−6 | [25] |
| LiF × LiYF4 | – | 1 × 10−6–5 × 10−6 | [25] |
| LiF × LiLuF4 | – | 6 × 10−7 | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchinskaya, I.I.; Arkharova, N.A.; Ivanova, A.G.; Sorokin, N.I.; Karimov, D.N. Synthesis, Microstructure, and Electrical Conductivity of Eutectic Composites in MF2–RF3 (M = Ca, Sr, Ba; R = La–Nd) Systems. J. Compos. Sci. 2023, 7, 330. https://doi.org/10.3390/jcs7080330
Buchinskaya II, Arkharova NA, Ivanova AG, Sorokin NI, Karimov DN. Synthesis, Microstructure, and Electrical Conductivity of Eutectic Composites in MF2–RF3 (M = Ca, Sr, Ba; R = La–Nd) Systems. Journal of Composites Science. 2023; 7(8):330. https://doi.org/10.3390/jcs7080330
Chicago/Turabian StyleBuchinskaya, Irina I., Natalia A. Arkharova, Anna G. Ivanova, Nikolay I. Sorokin, and Denis N. Karimov. 2023. "Synthesis, Microstructure, and Electrical Conductivity of Eutectic Composites in MF2–RF3 (M = Ca, Sr, Ba; R = La–Nd) Systems" Journal of Composites Science 7, no. 8: 330. https://doi.org/10.3390/jcs7080330