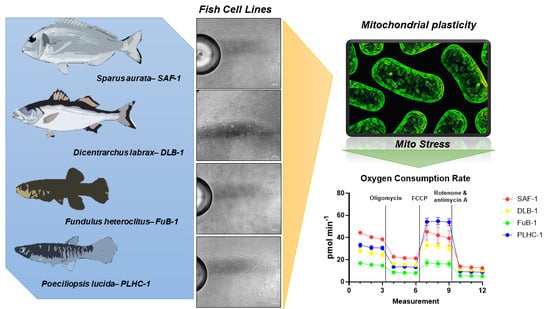
Mitochondrial Metabolism Characterization of Four Different Fish Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Mitochondrial Stress
2.3. Statistical Analysis
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Basu, H.S.; Wilganowski, N.; Robertson, S.; Reuben, J.M.; Cohen, E.N.; Zurita, A.; Ramachandran, S.S.; Xiao, L.L.-C.; Titus, M.; Wilding, G. Prostate Cancer Cells Survive Anti-androgen and Mitochondrial Metabolic Inhibitors by Modulating Glycolysis and Mitochondrial Metabolic Activities. Prostate 2021, 81, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.D.S.; Falcão, K.V.G.; Amaral, I.P.G.; Leite, A.C.R.; Bezerra, R.S. Mitochondria as Targets for Toxicity and Metabolism Research Using Zebrafish. Biochim. Biophys. Acta. Gen. Subj. 2020, 1864, 129634. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, S.E.; Sena, L.A.; Chandel, N.S. Mitochondria in the Regulation of Innate and Adaptive Immunity. Immunity 2015, 42, 406–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, J.S.; Tait, S.W. Mitochondrial DNA in Inflammation and Immunity. EMBO Rep. 2020, 21, e49799. [Google Scholar] [CrossRef] [PubMed]
- Schug, H.; Maner, J.; Hülskamp, M.; Begnaud, F.; Debonneville, C.; Berthaud, F.; Gimeno, S.; Schirmer, K. Extending the Concept of Predicting Fish Acute Toxicity In Vitro to the Intestinal Cell Line RTgutGC. ALTEX 2020, 37, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, M.; Leaver, M.J.; Taggart, J.B.; Casadei, E.; Auslander, M.; Tom, M.; George, S.G. Copper Induces Cu-ATPase ATP7A MRNA in a Fish Cell Line, SAF1. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, C.; García Beltrán, J.M.; Esteban, M.A.; Cuesta, A. In Vitro Effects of Virgin Microplastics on Fish Head-Kidney Leucocyte Activities. Environ. Pollut. 2018, 235, 30–38. [Google Scholar] [CrossRef]
- Trapani, A.; Mandracchia, D.; Di Franco, C.; Cordero, H.; Morcillo, P.; Comparelli, R.; Cuesta, A.; Esteban, M.A. In Vitro Characterization of 6-Coumarin Loaded Solid Lipid Nanoparticles and Their Uptake by Immunocompetent Fish Cells. Colloids Surf. B Biointerfaces 2015, 127, 79–88. [Google Scholar] [CrossRef]
- Almeida, M.; Martins, M.A.; Soares, A.M.V.; Cuesta, A.; Oliveira, M. Polystyrene Nanoplastics Alter the Cytotoxicity of Human Pharmaceuticals on Marine Fish Cell Lines. Environ. Toxicol. Pharmacol. 2019, 69, 57–65. [Google Scholar] [CrossRef]
- García Beltrán, J.M.; Espinosa, C.; Guardiola, F.A.; Esteban, M.Á. Dietary Dehydrated Lemon Peel Improves the Immune but Not the Antioxidant Status of Gilthead Seabream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 64, 426–436. [Google Scholar] [CrossRef]
- Valero, Y.; López-Vázquez, C.; Souto, S.; Olveira, J.G.; Cuesta, A.; Bandín, I. Differential Nervous Necrosis Virus (NNV) Replication in Five Putative Susceptible Cell Lines. Pathogens 2021, 10, 1565. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Bandín, I.; Olveira, J.G.; Esteve-Codina, A.; Gómez-Garrido, J.; Dabad, M.; Alioto, T.; Ángeles Esteban, M.; Cuesta, A. European Sea Bass Brain DLB-1 Cell Line Is Susceptible to Nodavirus: A Transcriptomic Study. Fish Shellfish Immunol. 2019, 86, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Leach, G.J.; Taylor, M.H. The Role of Cortisol in Stress-Induced Metabolic Changes in Fundulus heteroclitus. Gen. Comp. Endocrinol. 1980, 42, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Fangue, N.; Hofmeister, M.; Schulte, P. Intraspecific Variation in Thermal Tolerance and Heat Shock Protein Gene Expression in Common Killifish, Fundulus heteroclitus. J. Exp. Biol. 2006, 209, 2859–2872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvan, M.J.; Gallagher, E.P.; Goksøyr, A.; Hahn, M.E.; Joakim Larsson, D.G. Fish Models in Toxicology. Zebrafish 2007, 4, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Palacios, M.; Almeida, M.; Martins, M.A.; Oliveira, M.; Esteban, M.Á.; Cuesta, A. Establishment of a Brain Cell Line (FuB-1) from Mummichog (Fundulus heteroclitus) and Its Application to Fish Virology, Immunity and Nanoplastics Toxicology. Sci. Total Environ. 2020, 708, 134821. [Google Scholar] [CrossRef]
- Bols, N.C.; Dayeh, V.R.; Lee, L.E.J.; Schirmer, K. Use of Fish Cell Lines in the Toxicology and Ecotoxicology of Fish. Piscine Cell Lines in Environmental Toxicology. Biochem. Mol. Biol. Fishes 2005, 6, 43–84. [Google Scholar] [CrossRef]
- Hua, Q.; Mi, B.; Xu, F.; Wen, J.; Zhao, L.; Liu, J.; Huang, G. Hypoxia-Induced LncRNA-AC020978 Promotes Proliferation and Glycolytic Metabolism of Non-Small Cell Lung Cancer by Regulating PKM2/HIF-1α Axis. Theranostics 2020, 10, 4762–4778. [Google Scholar] [CrossRef]
- Bairoch, A. The Cellosaurus, a Cell-Line Knowledge Resource. J. Biomol. Tech. 2018, 29, 25–38. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Paradyse, A.; Ferrick, D.A.; Murphy, A.N.; Jastroch, M. Analysis and Interpretation of Microplate-Based Oxygen Consumption and PH Data. Methods Enzymol. 2014, 547, 309–354. [Google Scholar] [CrossRef]
- Berntsen, H.H.; Bech, C. Incubation Temperature and Physiological Aging in the Zebra Finch. PLoS ONE 2021, 16, e0260037. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, C.; Manuguerra, S.; Cuesta, A.; Esteban, M.A.; Santulli, A.; Messina, C.M. Sub-Lethal Doses of Polybrominated Diphenyl Ethers Affect Some Biomarkers Involved in Energy Balance and Cell Cycle, via Oxidative Stress in the Marine Fish Cell Line SAF-1. Aquat. Toxicol. 2019, 210, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP Synthesis and Storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.; Yucel, N.; Kim, B.; Arany, Z. Local Mitochondrial ATP Production Regulates Endothelial Fatty Acid Uptake and Transport. Cell Metab. 2020, 32, 309–319.e7. [Google Scholar] [CrossRef]
- Jaber, S.M.; Yadava, N.; Polster, B.M. Mapping Mitochondrial Respiratory Chain Deficiencies by Respirometry: Beyond the Mito Stress Test. Exp. Neurol. 2020, 328, 113282. [Google Scholar] [CrossRef] [PubMed]
- Morcillo, P.; Chaves-Pozo, E.; Meseguer, J.; Esteban, M.Á.; Cuesta, A. Establishment of a New Teleost Brain Cell Line (DLB-1) from the European Sea Bass and Its Use to Study Metal Toxicology. Toxicol. In Vitro 2017, 38, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Plitzko, B.; Loesgen, S. Measurement of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR) in Culture Cells for Assessment of the Energy Metabolism. Bio-Protocol 2018, 8, e2850. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espinosa-Ruiz, C.; Mayor-Lafuente, J.; Esteban, M.Á. Mitochondrial Metabolism Characterization of Four Different Fish Cell Lines. Fishes 2022, 7, 354. https://doi.org/10.3390/fishes7060354
Espinosa-Ruiz C, Mayor-Lafuente J, Esteban MÁ. Mitochondrial Metabolism Characterization of Four Different Fish Cell Lines. Fishes. 2022; 7(6):354. https://doi.org/10.3390/fishes7060354
Chicago/Turabian StyleEspinosa-Ruiz, Cristóbal, Javier Mayor-Lafuente, and M. Ángeles Esteban. 2022. "Mitochondrial Metabolism Characterization of Four Different Fish Cell Lines" Fishes 7, no. 6: 354. https://doi.org/10.3390/fishes7060354