Impedance Characterization of an LCO-NMC/Graphite Cell: Ohmic Conduction, SEI Transport and Charge-Transfer Phenomenon
Abstract
:1. Introduction
2. Results and Discussion
2.1. Half-Cells Impedance
2.1.1. Negative Electrode (Graphite/Li Half-Cell)
2.1.2. Positive Electrode (LCO-NMC/Li Half-Cell)
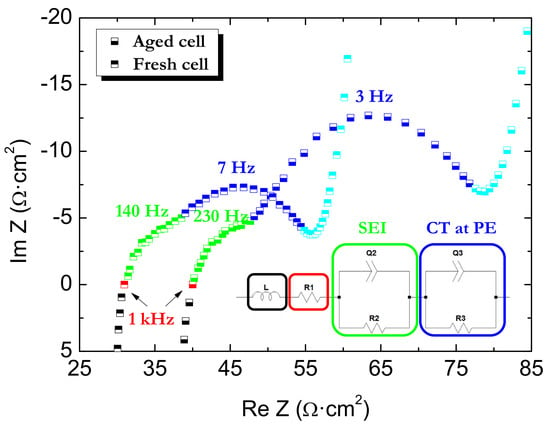
2.2. Full-Cell Impedance
3. Materials and Methods
- (1)
- Cycle-aging processes: Charge and discharge are used to age the samples.
- (2)
- Postmortem analyses: Half-cells composed of cathode—Li and Li—anode.
- (3)
- Characterization: Impedance spectroscopy on a two-electrode configuration for both original cells and half-cells.
3.1. Cell Preparation and Cyclec-Aging Process
3.2. Building Half-Cells
3.3. Impedance Measurements
3.4. State-of-Charge Definition
3.5. Fitting Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A





References
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Choi, N.S.; Chen, Z.; Freunberger, S.A.; Ji, X.; Sun, Y.K.; Amine, K.; Yushin, G.; Nazar, L.F.; Cho, J.; Bruce, P.G. Challenges facing lithium batteries and electrical double-layer capacitors. Angew. Chem. Int. Ed. 2012, 51, 9994–10024. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Marano, V.; Onori, S.; Guezennec, Y.; Rizzoni, G.; Madella, N. Lithium-ion batteries life estimation for plug-in hybrid electric vehicles. In Proceedings of the 2009 IEEE Vehicle Power and Propulsion Conference, Dearborn, MI, USA, 7–10 September 2009; IEEE: Piscataway, NJ, USA, 2009; pp. 536–543. [Google Scholar]
- Abraham, D.P.; Liu, J.; Chen, C.H.; Hyung, Y.E.; Stoll, M.; Elsen, N.; MacLaren, S.; Twesten, R.; Haasch, R.; Sammann, E.; et al. Diagnosis of power fade mechanisms in high-power lithium-ion cells. J. Power Sources 2003, 119–121, 511–516. [Google Scholar] [CrossRef]
- Zavalis, T.G.; Klett, M.; Kjell, M.H.; Behm, M.; Lindström, R.W.; Lindbergh, G. Aging in lithium-ion batteries: Model and experimental investigation of harvested LiFePO4 and mesocarbon microbead graphite electrodes. Electrochim. Acta 2013, 110, 335–348. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Zhang, C.; Zhang, W.; Ma, Z.; Jiang, Y. Lithium-ion battery aging mechanisms and life model under different charging stresses. J. Power Sources 2017, 356, 103–114. [Google Scholar] [CrossRef]
- Prada, E.; Domenico, D.; Creff, Y.; Bernard, J.; Sauvant-Moynot, V.; Huet, F. A Simplified Electrochemical and Thermal Aging Model of LiFePO4-Graphite Li-ion Batteries: Power and Capacity Fade Simulations. J. Electrochem. Soc. 2013, 160, A616–A628. [Google Scholar] [CrossRef]
- Bernard, P.; Martinez, H.; Tessier, C.; Garitte, E.; Franger, S.; Dedryvere, R. Role of Negative Electrode Porosity in Long-Term Aging of NMC//Graphite Li-Ion Batteries. J. Electrochem. Soc. 2015, 162, A7096–A7103. [Google Scholar] [CrossRef]
- Fu, R.; Choe, S.Y.; Agubra, V.; Fergus, J. Development of a physics-based degradation model for lithium ion polymer batteries considering side reactions. J. Power Sources 2015, 278, 506–521. [Google Scholar] [CrossRef]
- Klett, M.; Zavalis, T.G.; Kjell, M.H.; Lindström, R.W.; Behm, M.; Lindbergh, G. Altered electrode degradation with temperature in LiFePO4/mesocarbon microbead graphite cells diagnosed with impedance spectroscopy. Electrochim. Acta 2014, 141, 173–181. [Google Scholar] [CrossRef]
- Berecibar, M.; Gandiaga, I.; Villarreal, I.; Omar, N.; Van Mierlo, J.; Van Den Bossche, P. Critical review of state of health estimation methods of Li-ion batteries for real applications. Renew. Sustain. Energy Rev. 2016, 56, 572–587. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Lewerenz, M.; Münnix, J.; Schmalstieg, J.; Käbitz, S.; Knips, M.; Sauer, D.U. Systematic aging of commercial LiFePO4|Graphite cylindrical cells including a theory explaining rise of capacity during aging. J. Power Sources 2017, 345, 254–263. [Google Scholar] [CrossRef]
- Dees, D.W.; Abraham, D.P.; Lu, W.; Gallagher, K.G.; Bettge, M.; Jansen, A.N. Electrochemical Modeling and Performance of a Lithium- and Manganese-Rich Layered Transition-Metal Oxide Positive Electrode. J. Electrochem. Soc. 2015, 162, 559–572. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Chrobak, T.; Ender, M.; Illig, J.; Klotz, D.; Ivers-Tiffée, E. Studies on LiFePO4 as cathode material using impedance spectroscopy. J. Power Sources 2011, 196, 5342–5348. [Google Scholar] [CrossRef]
- Illig, J.; Ender, M.; Chrobak, T.; Schmidt, J.P.; Klotz, D.; Ivers-Tiffee, E. Separation of Charge Transfer and Contact Resistance in LiFePO4-Cathodes by Impedance Modeling. J. Electrochem. Soc. 2012, 159, A952–A960. [Google Scholar] [CrossRef]
- Abarbanel, D.W.; Nelson, K.J.; Dahn, J.R. Exploring Impedance Growth in High Voltage NMC/Graphite Li-Ion Cells Using a Transmission Line Model. J. Electrochem. Soc. 2016, 163, A522–A529. [Google Scholar] [CrossRef]
- Osaka, T.; Momma, T.; Mukoyama, D.; Nara, H. Proposal of novel equivalent circuit for electrochemical impedance analysis of commercially available lithium ion battery. J. Power Sources 2012, 205, 483–486. [Google Scholar] [CrossRef]
- Olofsson, Y.; Groot, J. Impedance spectroscopy characterisation of automotive NMC/graphite Li-ion cells aged with realistic PHEV load profile Quantification of cell properties vs. temperature at different stages of ageing. In Proceedings of the 2014 IEEE International Electric Vehicle Conference (IEVC), Florence, Italy, 17–19 December 2014; pp. 1–6. [Google Scholar] [CrossRef]
- Prada, E.; Di Domenico, D.; Creff, Y.; Bernard, J.; Sauvant-Moynot, V.; Huet, F. Physics-based modelling of LiFePO4-graphite Li-ion batteries for power and capacity fade predictions: Application to calendar aging of PHEV and EV. In Proceedings of the 2012 IEEE Vehicle Power and Propulsion Conference, Seoul, Korea, 9–12 October 2012; IEEE: Piscataway, NJ, USA, 2012; pp. 301–308. [Google Scholar]
- Heubner, C.; Schneider, M.; Michaelis, A. Investigation of charge transfer kinetics of Li-Intercalation in LiFePO4. J. Power Sources 2015, 288, 115–120. [Google Scholar] [CrossRef]
- Zheng, Y.; He, Y.B.; Qian, K.; Li, B.; Wang, X.; Li, J.; Chiang, S.W.; Miao, C.; Kang, F.; Zhang, J. Deterioration of lithium iron phosphate/graphite power batteries under high-rate discharge cycling. Electrochim. Acta 2015, 176, 270–279. [Google Scholar] [CrossRef]
- Illig, J.; Chrobak, T.; Klotz, D.; Ivers-Tiffée, E. Evaluation of the Rate Determining Processes for LiFePO4 as Cathode Material in Lithium-ION-Batteries. ECS Trans. 2011, 33, 3–15. [Google Scholar] [CrossRef]
- Lin, X.; Perez, H.E.; Mohan, S.; Siegel, J.B.; Stefanopoulou, A.G.; Ding, Y.; Castanier, M.P. A lumped-parameter electro-thermal model for cylindrical batteries. J. Power Sources 2014, 257, 1–11. [Google Scholar] [CrossRef]
- Zhuang, Q.-C.; Qiu, X.-Y.; Xu, S.-D.; Qiang, Y.-H.; Su, S.-G. Diagnosis of Electrochemical Impedance Spectroscopy in Lithium-Ion Batteries. In Lithium Ion Batteries—New Developments; ECS: Wembley, UK, 2012; pp. 189–226. ISBN 978-953-51-0077-5. [Google Scholar]
- Illig, J. Physically Based Impedance Modelling of Lithium-Ion Cells; KIT Scientific Publishing: Karlsruhe, Germany, 2014. [Google Scholar]
- Li, Y.; Bettge, M.; Polzin, B.; Zhu, Y.; Balasubramanian, M.; Abraham, D.P. Understanding Long-Term Cycling Performance of Li1.2Ni0.15Mn0.55Co0.1O2–Graphite Lithium-Ion Cells. J. Electrochem. Soc. 2013, 160, A3006–A3019. [Google Scholar] [CrossRef]
- Choi, Y.M.; Pyun, S.I.; Bae, J.S.; Moon, S.I. Effects of lithium content on the electrochemical lithium intercalation reaction into LiNiO2 and LiCoO2 electrodes. J. Power Sources 1995, 56, 25–30. [Google Scholar] [CrossRef]
- Kao, Y.H.; Tang, M.; Meethong, N.; Bai, J.; Carter, W.C.; Chiang, Y.M. Overpotential-dependent phase transformation pathways in lithium iron phosphate battery electrodes. Chem. Mater. 2010, 22, 5845–5855. [Google Scholar] [CrossRef]
- Klett, M.; Eriksson, R.; Groot, J.; Svens, P.; Ciosek Högström, K.; Lindström, R.W.; Berg, H.; Gustafson, T.; Lindbergh, G.; Edström, K. Non-uniform aging of cycled commercial LiFePO4//graphite cylindrical cells revealed by post-mortem analysis. J. Power Sources 2014, 257, 126–137. [Google Scholar] [CrossRef]
- Bach, T.C.; Schuster, S.F.; Fleder, E.; Müller, J.; Brand, M.J.; Lorrmann, H.; Jossen, A.; Sextl, G.; Müller, J.; Brand, M.J.; et al. Nonlinear aging of cylindrical lithium-ion cells linked to heterogeneous compression. J. Energy Storage 2016, 5, 212–223. [Google Scholar] [CrossRef]
- Costard, J.; Ender, M.; Weiss, M.; Ivers-Tiffée, E. Three-Electrode Setups for Lithium-Ion Batteries. J. Electrochem. Soc. 2017, 164, A80–A87. [Google Scholar] [CrossRef]
- Skoog, S.; David, S. Parameterization of linear equivalent circuit models over wide temperature and SOC spans for automotive lithium-ion cells using electrochemical impedance spectroscopy. J. Energy Storage 2017, 14, 39–48. [Google Scholar] [CrossRef]
- Heins, T.P.; Harms, N.; Schramm, L.-S.; Schröder, U. Development of a new Electrochemical Impedance Spectroscopy Approach for Monitoring the Solid Electrolyte Interphase Formation. Energy Technol. 2016, 4, 1509–1513. [Google Scholar] [CrossRef] [Green Version]
- Howey, D.A.; Mitcheson, P.D.; Yufit, V.; Offer, G.J.; Brandon, N.P. Online measurement of battery impedance using motor controller excitation. IEEE Trans. Veh. Technol. 2014, 63, 2557–2566. [Google Scholar] [CrossRef]
- Momma, T.; Matsunaga, M.; Mukoyama, D.; Osaka, T. AC impedance analysis of lithium ion battery under temperature control. J. Power Sources 2012, 216, 304–307. [Google Scholar] [CrossRef]
- Buchberger, I.H. Electrochemical and Structural Investigations on Lithium-Ion Battery Materials and Related Degradation Processes. Ph.D. Thesis, Technischen Universität München, München, Germany, 2016. [Google Scholar]
- Ovejas, V.J. Determination of the State of Health of Li-Ion Batteries: The Irreversible Entropy Production Approach. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2017. [Google Scholar]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental investigation of the Lithium-Ion battery impedance characteristic at various conditions and aging states and its influence on the application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
- Uhlmann, C.; Illig, J.; Ender, M.; Schuster, R.; Ivers-Tiffée, E. In situ detection of lithium metal plating on graphite in experimental cells. J. Power Sources 2015, 279, 428–438. [Google Scholar] [CrossRef]
- Illig, J.; Schmidt, J.P.; Weiss, M.; Weber, A.; Ivers-Tiffée, E. Understanding the impedance spectrum of 18650 LiFePO4-cells. J. Power Sources 2013, 239, 670–679. [Google Scholar] [CrossRef]
- Howey, D.A.; Yufit, V.; Mitcheson, P.D.; Offer, G.J.; Brandon, N.P. Impedance measurement for advanced battery management systems. In Proceedings of the 2013 World Electric Vehicle Symposium and Exhibition (EVS27), Barcelona, Spain, 17–20 November 2013; pp. 1–7. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. EIS study on the formation of solid electrolyte interface in Li-ion battery. Electrochim. Acta 2006, 51, 1636–1640. [Google Scholar] [CrossRef]
- Martino, F.A. Effect of Electrolyte Concentration on the Capacitance and Mobility of Graphene. Bachelor’s Thesis, Linfield College, McMinnville, OR, USA, 2016. [Google Scholar]
- Brown, M.A.; Goel, A.; Abbas, Z. Effect of Electrolyte Concentration on the Stern Layer Thickness at a Charged Interface. Angew. Chem. Int. Ed. 2016, 55, 3790–3794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G. Simulating the Electrical Double Layer Capacitance. In Proceedings of the 2010 Excerpt from Proceedings of the COMSOL Conference, Boston, MA, USA, 7–9 October 2010. [Google Scholar]
- Arunachala, R.; Makinejad, K.; Athlekar, S.; Jossen, A.; Garche, J. Cycle life characterisation of large format lithium-ion cells. In Proceedings of the 2013 World Electric Vehicle Symposium and Exhibition, Barcelona, Spain, 17–20 November 2013. [Google Scholar]
- Stiaszny, B.; Ziegler, J.C.; Krauß, E.E.; Schmidt, J.P.; Ivers-Tiffée, E. Electrochemical characterization and post-mortem analysis of aged LiMn2O4-Li(Ni0.5Mn0.3Co0.2)O2/graphite lithium ion batteries. Part I: Cycle aging. J. Power Sources 2014, 251, 439–450. [Google Scholar] [CrossRef]
- Lam, L. A Practical Circuit-Based Model for State of Health Estimation of Li-Ion Battery Cells in Electric Vehicles. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2011. [Google Scholar]
- Franck, E.U.; Robinson, R.A.; Stokes, R.H. Electrolyte Solutions: The Measurement and Interpretation of Conductance, Chemical Potential and Diffusion in Solutions of Simple Electrolytes, 2nd ed.; Butterworths: London, UK, 1968; Volume 72, ISBN 048613878X. [Google Scholar]
- Balasundaram, M.; Ramar, V.; Yap, C.; Lu, L.; Tay, A.A.O.; Palani, B. Heat loss distribution: Impedance and thermal loss analyses in LiFePO4/graphite 18650 electrochemical cell. J. Power Sources 2016, 328, 413–421. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Charge and discharge characteristics of a commercial LiCoO2-based 18650 Li-ion battery. J. Power Sources 2006, 160, 1403–1409. [Google Scholar] [CrossRef]
- Gordon, I.A.J.; Grugeon, S.; Takenouti, H.; Tribollet, B.; Armand, M.; Davoisne, C.; Débart, A.; Laruelle, S. Electrochemical Impedance Spectroscopy response study of a commercial graphite-based negative electrode for Li-ion batteries as function of the cell state of charge and ageing. Electrochim. Acta 2017, 223, 63–73. [Google Scholar] [CrossRef]
- Tang, M.H.-M. Side Reactions in Lithium-Ion Batteries. Ph.D. Thesis, UC Berkeley, Berkeley, CA, USA, 2012. [Google Scholar]
- Anderson, A. Surface Phenomena in Li-Ion Batteries. Ph.D. Thesis, Uppsala University, Uppsala, Sweden, 2001. [Google Scholar]
- Zheng, H.; Qu, Q.; Zhu, G.; Liu, G.; Battaglia, V.S.; Zheng, H. Quantitative Characterization of the Surface Evolution for LiNi0.5Co0.2Mn0.3O2/Graphite Cell during Long-Term Cycling. ACS Appl. Mater. Interfaces 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Bio-Logic. Application Note 49 “EIS measurements: Potentio (PEIS) or Galvano (GEIS) mode, that is the question”. 2013. Available online: http://www.bio-logic.net/wp-content/uploads/AN49-Potentio_vs_galvano_EIS.pdf (accessed on 1 July 2018).
- Gamry Instruments EIS-Potentiostatic or Galvanostatic Mode. Available online: https://www.gamry.com/application-notes/EIS/eis-potentiostatic-galvanostatic-mode/ (accessed on 12 July 2017).
- Song, J.Y.; Lee, H.H.; Wang, Y.Y.; Wan, C.C. Two- and three-electrode impedance spectroscopy of lithium-ion batteries. J. Power Sources 2002, 111, 255–267. [Google Scholar] [CrossRef]
- Cogger, N.D.; Evans, N.J. An Introduction to Electrochemical Impedance Measurement. Solartron Anal. Tech. Rep. 1999, 11. [Google Scholar] [CrossRef]
- Murnane, M.; Ghazel, A. A Closer Look at State of Charge (SOC) and State of Health (SOH) Estimation Techniques for Batteries; Analog Devices, Inc.: Norwood, MA, USA, 2017. [Google Scholar]
- Osswald, P.J.; Erhard, S.V.; Noel, A.; Keil, P.; Kindermann, F.M.; Hoster, H.; Jossen, A. Current density distribution in cylindrical Li-Ion cells during impedance measurements. J. Power Sources 2016, 314, 93–101. [Google Scholar] [CrossRef]
- Zheng, Y.; Qian, K.; Luo, D.; Li, Y.; Lu, Q.; Li, B.; He, Y.-B.; Wang, X.; Li, J.; Kang, F. Influence of over-discharge on the lifetime and performance of LiFePO4/graphite batteries. RSC Adv. 2016, 6, 30474–30483. [Google Scholar] [CrossRef]
- Gamry Instruments: “Physical Electrochemistry and Equivalent Circuit Elements part 2”. Available online: https://www.gamry.com/assets/Uploads/resources/The-Basics-of-EIS-Part-2.pdf (accessed on 1 July 2018).
- Feng, X. Nanocarbons for Advanced Energy Storage; Wiley-VCH: Weinheim, Germany, 2015; Volume 1, ISBN 3527336656. [Google Scholar]
- Metrohm Electrochemical Impedance Spectroscopy (EIS) Part 3—Data Analysis; Application Note EIS03; Metrophm: Blackheath, Switzerland, 2011; pp. 1–2.
- Bio-Logic Pseudo capacitance calculation. Sci. Instrum. 2010, 3, 68–70.
- Hirschorn, B.D. Distributed Time-Constant Impedance Responses Interpreted in Terms of Physically Meaningful Properties; University of Florida: Gainesville, FL, USA, 2010. [Google Scholar]
- Chen, W.-W. The Electrical Engineering Handbook; Elsevier Academic Press: San Diego, CA, USA, 2005; ISBN 978-0-12-170960-0. [Google Scholar]














| Cell | Minimum Voltage (V) | Maximum Voltage (V) | C-Rate | Parameter |
|---|---|---|---|---|
| Full cell LCO-NMC/C6 | 3 | 4.3 | C/25 | SoC |
| Half-cell LCO-NMC/Li | 3 | 4.4 | C/25 | Positive electrode (PE) SoC |
| Half-cell C6/Li | 0.00075 | 2.5 | C/25 | Negative electrode (NE) SoC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovejas, V.J.; Cuadras, A. Impedance Characterization of an LCO-NMC/Graphite Cell: Ohmic Conduction, SEI Transport and Charge-Transfer Phenomenon. Batteries 2018, 4, 43. https://doi.org/10.3390/batteries4030043
Ovejas VJ, Cuadras A. Impedance Characterization of an LCO-NMC/Graphite Cell: Ohmic Conduction, SEI Transport and Charge-Transfer Phenomenon. Batteries. 2018; 4(3):43. https://doi.org/10.3390/batteries4030043
Chicago/Turabian StyleOvejas, Victoria Julia, and Angel Cuadras. 2018. "Impedance Characterization of an LCO-NMC/Graphite Cell: Ohmic Conduction, SEI Transport and Charge-Transfer Phenomenon" Batteries 4, no. 3: 43. https://doi.org/10.3390/batteries4030043