Effects of the Molecular Structure of Starch in Foods on Human Health
Abstract
:1. Introduction
2. Starch Functional Properties and Structural Features
2.1. Functional Properties of Starch-Based Foods and Human Health
2.2. Distributions
2.3. The Size of a Starch Molecule in Solution
2.4. Measuring Distributions Related to Starch Structure
2.5. Interpreting Experimental CLDs
2.6. Two-Dimensional Distributions
2.7. Distributions of Whole Starch Molecules
3. Biosynthesis, Measurement and Fitting of the CLD
3.1. Enzymes Controlling the CLD
3.2. Fitting Observed CLDs to Biosynthesis-Based Models
4. Relationships between Starch Fine Structure and Health-Related Properties
4.1. Relationship between Molecular Fine Structure of Starch and Digestibility
4.2. Relationship between Starch Molecular Fine Structure and RVA Profile
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franco, C.M.L.; Wong, K.-S.; Yoo, S.-H.; Jane, J.-L. Structural and functional characteristics of selected soft wheat starches. Cereal Chem. 2002, 79, 243–248. [Google Scholar] [CrossRef]
- Cai, C.H.; Lin, L.S.; Man, J.M.; Zhao, L.X.; Wang, Z.F.; Wei, C.X. Different Structural Properties of High-Amylose Maize Starch Fractions Varying in Granule Size. J. Agric. Food Chem. 2014, 62, 11711–11721. [Google Scholar] [CrossRef] [PubMed]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Chemistry, structure, functionality and applications of rice starch. J. Cereal Sci. 2016, 70, 291–300. [Google Scholar] [CrossRef]
- Lee, E.S.; Shin, H.; Seo, J.M.; Nam, Y.D.; Lee, B.H.; Seo, D.H. Effects of raw potato starch on body weight with controlled glucose delivery. Food Chem. 2018, 256, 367–372. [Google Scholar] [CrossRef]
- Hizukuri, S.; Takeda, Y.; Yasuda, M.; Suzuki, A. Multi-branched nature of amylose and the action of debranching enzymes. Carbohydr. Res. 1981, 94, 205–213. [Google Scholar] [CrossRef]
- Jane, J.L.; Kasemsuwan, T.; Leas, S.; Zobel, H.; Robyt, J.F. Anthology of starch granule morphology by scanning electron microscopy. Starch-Stärke 1994, 46, 121–129. [Google Scholar] [CrossRef]
- Vilaplana, F.; Meng, D.; Hasjim, J.; Gilbert, R.G. Two-dimensional macromolecular distributions reveal detailed architectural features in high-amylose starches. Carbohydr. Polym. 2014, 113, 539–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Li, H.; Zou, W.; Tao, K.; Zhu, J.; Gilbert, R.G. Using starch molecular fine structure to understand biosynthesis-structure-property relations. Trends Food Sci. Technol. 2019, 86, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Bertoft, E. Understanding Starch Structure: Recent Progress. Agronomy 2017, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Hanashiro, I.; Sakaguchi, I.; Yamashita, H. Branched Structures of Rice Amylose Examined by Differential Fluorescence Detection of Side-chain Distribution. J. Appl. Glycosci. 2013, 60, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Takeda, Y.; Shitaozono, T.; Hizukuri, S. Molecular Structure of Corn Starch. Starch-Stärke 1988, 40, 51–54. [Google Scholar] [CrossRef]
- Myllärinen, P.; Autio, K.; Schulman, A.H.; Poutanen, K. Heat-induced structural changes of small and large barley starch granules. J. Inst. Brew. 1998, 104, 343–349. [Google Scholar] [CrossRef]
- Jane, J.; Xu, A.; Radosavljevic, M.; Seib, P.A. Location of amylose in normal starch granules. I. Susceptibility of amylose and amylopectin to cross-linking reagents. Cereal Chem. 1992, 69, 405–409. [Google Scholar]
- Magallanes-Cruz, P.A.; Flores-Silva, P.C.; Bello-Perez, L.A. Starch Structure Influences Its Digestibility: A Review. J. Food Sci. 2017, 82, 2016–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mua, J.P.; Jackson, D.S. Relationships between Functional Attributes and Molecular Structures of Amylose and Amylopectin Fractions from Corn Starch. J. Agric. Food Chem. 1997, 45, 3848–3854. [Google Scholar] [CrossRef]
- Patindol, J.; Wang, Y.-J. Fine structures of starches from long-grain rice cultivars with different functionality. Cereal Chem. 2002, 79, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Fujita, N. Thermal and rheological characteristics of mutant rice starches with widespread variation of amylose content and amylopectin structure. Food Hydrocoll. 2017, 62, 83–93. [Google Scholar] [CrossRef]
- Chi, C.D.; Li, X.X.; Huang, S.X.; Chen, L.; Zhang, Y.P.; Li, L.; Miao, S. Basic principles in starch multi-scale structuration to mitigate digestibility: A review. Trends Food Sci. Technol. 2021, 109, 154–168. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, L.; Liu, W.; Liu, Q.; Wang, F.; Zhang, H.; Hu, H.; Blecker, C. Physicochemical and Structural Characterization of Potato Starch with Different Degrees of Gelatinization. Foods 2021, 10, 1104. [Google Scholar] [CrossRef]
- Suklaew, P.O.; Chusak, C.; Adisakwattana, S. Physicochemical and Functional Characteristics of RD43 Rice Flour and Its Food Application. Foods 2020, 9, 1912. [Google Scholar] [CrossRef]
- Grace, N.C.F.; Jeyakumar Henry, C. The Physicochemical Characterization of Unconventional Starches and Flours Used in Asia. Foods 2020, 9, 182. [Google Scholar] [CrossRef] [Green Version]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M.; et al. Resistant Starch: Promise for Improving Human Health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champagne, E.T.; Bett, K.L.; Vinyard, B.T.; McClung, A.M.; Barton, F.E.; Moldenhauer, K.; Linscombe, S.; McKenzie, K. Correlation between cooked rice texture and Rapid Visco Analyses measurements. Cereal Chem. 1999, 76, 764–771. [Google Scholar] [CrossRef]
- Nakamura, S.; Katsura, J.; Maruyama, Y.; Ohtsubo, K.i. Evaluation of Hardness and Retrogradation of Cooked Rice Based on Its Pasting Properties Using a Novel RVA Testing. Foods 2021, 10, 987. [Google Scholar] [CrossRef]
- Yanjie, X.; Yining, Y.; Shuhong, O.; Xiaoliang, D.; Hui, S.; Shukun, J.; Shichen, S.; Jinsong, B. Factors affecting sensory quality of cooked japonica rice. Rice Sci. 2018, 25, 330–339. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Gilbert, R.G. The effects of starch molecular fine structure on thermal and digestion properties of rice starch. Foods 2022, 11, 4012. [Google Scholar] [CrossRef]
- Ells, L.J.; Seal, C.J.; Kettlitz, B.; Bal, W.; Mathers, J.C. Postprandial glycaemic, lipaemic and haemostatic responses to ingestion of rapidly and slowly digested starches in healthy young women. Br. J. Nutr. 2005, 94, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Englyst, K.N.; Vinoy, S.; Englyst, H.N.; Lang, V. Glycaemic index of cereal products explained by their content of rapidly and slowly available glucose. Br. J. Nutr. 2003, 89, 329–339. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Franceschi, S.; Hamidi, M.; Marchie, A.; Jenkins, A.L.; Axelsen, M. Glycemic index: Overview of implications in health and disease. Am. J. Clin. Nutr. 2002, 76, 266S–273S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seal, C.J.; Daly, M.E.; Thomas, L.C.; Bal, W.; Birkett, A.M.; Jeffcoat, R.; Mathers, J.C. Postprandial carbohydrate metabolism in healthy subjects and those with type 2 diabetes fed starches with slow and rapid hydrolysis rates determined in vitro. Br. J. Nutr. 2003, 90, 853–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolever, T.M.S. Carbohydrate and the regulation of blood glucose and metabolism. Nutr. Rev. 2003, 61, S40–S48. [Google Scholar] [CrossRef]
- Axelsen, M.; Arvidsson Lenner, R.; Lönnroth, P.; Smith, U. Breakfast glycaemic response in patients with type 2 diabetes: Effects of bedtime dietary carbohydrates. Eur. J. Clin. Nutr. 1999, 53, 706–710. [Google Scholar] [CrossRef] [Green Version]
- Golay, A.; Koellreutter, B.; Bloise, D.; Assal, J.-P.; Würsch, P. The effect of muesli or cornflakes at breakfast on carbohydrate metabolism in type 2 diabetic patients. Diabetes Res. Clin. Pract. 1992, 15, 135–141. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Hudson, G.J.; Cummings, J.H. Measurement of resistant starch in vitro and in vivo. Br. J. Nutr. 1996, 75, 749–755. [Google Scholar] [CrossRef] [Green Version]
- Afandi, F.A.; Wijaya, C.H.; Faridah, D.N.; Suyatma, N.E.; Jayanegara, A. Evaluation of Various Starchy Foods: A Systematic Review and Meta-Analysis on Chemical Properties Affecting the Glycemic Index Values Based on In Vitro and In Vivo Experiments. Foods 2021, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Gourineni, V.; Stewart, M.L.; Wilcox, M.L.; Maki, K.C. Nutritional Bar with Potato-Based Resistant Starch Attenuated Post-Prandial Glucose and Insulin Response in Healthy Adults. Foods 2020, 9, 1679. [Google Scholar] [CrossRef]
- Benton, D.; Nabb, S. Carbohydrate, Memory, and Mood. Nutr. Rev. 2003, 61, S61–S67. [Google Scholar] [CrossRef] [Green Version]
- Campfield, L.A.; Smith, F.J. Blood Glucose Dynamics and Control of Meal Initiation: A Pattern Detection and Recognition Theory. Physiol. Rev. 2003, 83, 25–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, J. Glucostatic Mechanism of Regulation of Food Intake. N. Engl. J. Med. 1953, 249, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.D.; Shrestha, A.K.; Arcot, J. Physicochemical properties and digestibility of eleven Vietnamese rice starches with varying amylose contents. Food Funct. 2016, 7, 3599–3608. [Google Scholar] [CrossRef]
- Jane, J.L.; Chen, J.F. Effect of amylose molecular size and amylopectin branch chain length on paste properties of starch. Cereal Chem. 1992, 69, 60–65. [Google Scholar]
- Jacobs, H.; Eerlingen, R.C.; Delcour, J.A. Factors Affecting the Visco-Amylograph and Rapid Visco-Analyzer Evaluation of the Impact of Annealing on Starch Pasting Properties. Starch-Stärke 1996, 48, 266–270. [Google Scholar] [CrossRef]
- Jones, R.G.; Kahovec, J.; Stepto, R.; Wilks, E.S.; Hess, M.; Kitayama, T.; Metanomski, W.V. Compendium of Polymer Terminology and Nomenclature. IUPAC Recommendations 2008; Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar] [CrossRef] [Green Version]
- Kostanski, L.K.; Keller, D.M.; Hamielec, A.E. Size-exclusion chromatography—A review of calibration methodologies. J. Biochem. Biophys. Methods 2004, 58, 159–186. [Google Scholar] [CrossRef] [PubMed]
- Clay, P.A.; Gilbert, R.G. Molecular weight distributions in free-radical polymerizations. 1. Model development and implications for data interpretation. Macromolecules 1995, 28, 552–569. [Google Scholar] [CrossRef]
- O’Shea, M.G.; Samuel, M.S.; Konik, C.M.; Morell, M.K. Fluorophore-assisted carbohydrate electrophoresis (FACE) of oligosaccharides—Efficiency of labelling and high-resolution separation. Carbohydr. Res. 1998, 307, 1–12. [Google Scholar] [CrossRef]
- Wu, A.C.; Li, E.; Gilbert, R.G. Exploring extraction/dissolution procedures for analysis of starch chain-length distributions. Carbohydr. Polym. 2014, 114, 36–42. [Google Scholar] [CrossRef]
- Grubisic, Z.; Rempp, P.; Benoit, H. Universal calibration for gel permeation chromatography. J. Polym. Sci. Polym. Lett. Ed. 1967, 5, 753–759. [Google Scholar] [CrossRef]
- Gaborieau, M.; Gilbert, R.G.; Gray-Weale, A.; Hernandez, J.M.; Castignolles, P. Theory of multiple detection size exclusion chromatography of complex branched polymers. Macromol. Theory Simul. 2007, 16, 13–28. [Google Scholar] [CrossRef]
- Vilaplana, F.; Gilbert, R.G. Characterization of branched polysaccharides using multiple-detection size separation techniques. J. Sep. Sci. 2010, 33, 3537–3554. [Google Scholar] [CrossRef]
- Cave, R.A.; Seabrook, S.A.; Gidley, M.J.; Gilbert, R.G. Characterization of starch by size-exclusion chromatography: The limitations imposed by shear scission. Biomacromolecules 2009, 10, 2245–2253. [Google Scholar] [CrossRef]
- Baumgarten, J.L.; Busnel, J.P.; Meira, G.R. Band broadening in size exclusion chromatography of polymers. State of the art and some novel solutions. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 1967–2001. [Google Scholar] [CrossRef]
- Busnel, J.P.; Foucault, F.; Denis, L.; Lee, W.; Chang, T. Investigation and interpretation of band broadening in size exclusion chromatography. J. Chromatog. A 2001, 930, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Schnöll-Bitai, I.; Mader, C. How much does band broadening adulterate results deduced from chromatograms measured by size-exclusion chromatography really? J. Chromatog. A 2006, 1137, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Jane, J. Quantitative analysis of debranched amylopectin by hpaec-pad with a postcolumn enzyme reactor. J. Liq. Chromatogr. Relat. Technol. 1997, 20, 297–310. [Google Scholar] [CrossRef]
- Pfister, B.; Lu, K.J.; Eicke, S.; Feil, R.; Lunn, J.E.; Streb, S.; Zeeman, S.C. Starch granule biosynthesis in Arabidopsis is abolished by removal of all debranching enzymes but restored by the subsequent removal of an endoamylaseGenetic evidence that chain length and branch point distributions are linked determinants of starch granule formation in arabidopsis. Plant Physiol. 2014, 165, 1457–1474. [Google Scholar]
- Wu, A.C.; Morell, M.K.; Gilbert, R.G. A parameterized model of amylopectin synthesis provides key insights into the synthesis of granular starch. PLoS ONE 2013, 8, e65768. [Google Scholar] [CrossRef]
- Nada, S.S.; Zou, W.; Li, C.; Gilbert, R.G. Parameterizing amylose chain-length distributions for biosynthesis-structure-property relations. Anal. Bioanal. Chem. 2017, 409, 6813–6819. [Google Scholar] [CrossRef]
- Whistler, R.L.; Doane, W.M. Characterization of intermediary fractions of high-amylose corn starches. Cereal Chem. 1961, 38, 251–255. [Google Scholar]
- Gray-Weale, A.; Gilbert, R.G. General description of the structure of branched polymers. J. Polym. Sci. Part A Polym. Chem. Ed. 2009, 47, 3914–3930. [Google Scholar] [CrossRef]
- Vilaplana, F.; Gilbert, R.G. Two-dimensional size/branch length distributions of a branched polymer. Macromolecules 2010, 43, 7321–7329. [Google Scholar] [CrossRef]
- Vilaplana, F.; Gilbert, R.G. Analytical methodology for multidimensional size/branch-length distributions for branched glucose polymers using off-line 2-dimensional size-exclusion chromatography and enzymatic treatment. J. Chromatogr. A 2011, 1218, 4434–4444. [Google Scholar] [CrossRef]
- Malik, M.I.; Pasch, H. Field-flow fractionation: New and exciting perspectives in polymer analysis. Progess Polym. Sci. 2016, 63, 42–85. [Google Scholar] [CrossRef]
- Ciric, J.; Rolland-Sabate, A.; Guilois, S.; Loos, K. Characterization of enzymatically synthesized amylopectin analogs via asymmetrical flow field flow fractionation. Polymer 2014, 55, 6271–6277. [Google Scholar] [CrossRef]
- Perez-Rea, D.; Bergenstahl, B.; Nilsson, L. Development and evaluation of methods for starch dissolution using asymmetrical flow field-flow fractionation. Part I: Dissolution of amylopectin. Anal. Bioanal. Chem. 2015, 407, 4315–4326. [Google Scholar] [CrossRef] [PubMed]
- Perez-Rea, D.; Bergenståhl, B.; Nilsson, L. Development and evaluation of methods for starch dissolution using asymmetrical flow field-flow fractionation. Part II: Dissolution of amylose. Anal. Bioanal. Chem. 2015, 408, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Rolland-Sabate, A.; Guilois, S.; Grimaud, F.; Lancelon-Pin, C.; Roussel, X.; Laguerre, S.; Vikso-Nielsen, A.; Putaux, J.L.; D’Hulst, C.; Potocki-Veronese, G.; et al. Characterization of hyperbranched glycopolymers produced in vitro using enzymes. Anal. Bioanal. Chem. 2014, 406, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Okita, T.W.; Nakata, P.A.; Anderson, J.M.; Sowokinos, J.; Morell, M.; Preiss, J. The subunit structure of potato tuber ADPglucose pyrophosphorylase. Plant Physiol. 1990, 93, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Okita, T.W. Is there an alternative pathway for starch synthesis? Plant Physiol. 1992, 100, 560–564. [Google Scholar] [CrossRef] [Green Version]
- Fujita, N.; Yoshida, M.; Kondo, T.; Saito, K.; Utsumi, Y.; Tokunaga, T.; Nishi, A.; Satoh, H.; Park, J.-H.; Jane, J.-L.; et al. Characterization of SSIIIa-Deficient Mutants of Rice: The Function of SSIIIa and Pleiotropic Effects by SSIIIa Deficiency in the Rice Endosperm. Plant Physiol. 2007, 144, 2009–2023. [Google Scholar] [CrossRef] [Green Version]
- Fujita, N.; Satoh, R.; Hayashi, A.; Kodama, M.; Itoh, R.; Aihara, S.; Nakamura, Y. Starch biosynthesis in rice endosperm requires the presence of either starch synthase I or IIIa. J. Exp. Bot. 2011, 62, 4819–4831. [Google Scholar] [CrossRef] [Green Version]
- Jeon, J.S.; Ryoo, N.; Hahn, T.R.; Walia, H.; Nakamura, Y. Starch biosynthesis in cereal endosperm. Plant Physiol. Biochem. 2010, 48, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, F.; Xu, S.; Chu, X.; Mukai, Y.; Yamamoto, M.; Ali, S.; Rampling, L.; Kosar-Hashemi, B.; Rahman, S.; et al. The structural organisation of the gene encoding class II starch synthase of wheat and barley and the evolution of the genes encoding starch synthases in plants. Funct. Integr. Genom. 2003, 3, 76–85. [Google Scholar] [CrossRef]
- Guan, H.; Li, P.; Imparl-Radosevich, J.; Preiss, J.; Keeling, P. Comparing the properties of Escherichia coli branching enzyme and maize branching enzyme. Arch. Biochem. Biophys. 1997, 342, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.H.; Baunsgaard, L.; Blennow, A. Intermediary glucan structures formed during starch granule biosynthesis are enriched in short side chains, a dynamic pulse labeling approach. J. Biol. Chem. 2002, 277, 20249–20255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, N.; Kubo, A.; Suh, D.-S.; Wong, K.-S.; Jane, J.-L.; Ozawa, K.; Takaiwa, F.; Inaba, Y.; Nakamura, Y. Antisense inhibition of isoamylase alters the structure of amylopectin and the physicochemical properties of starch in rice endosperm. Plant Cell Physiol. 2003, 44, 607–618. [Google Scholar] [CrossRef] [Green Version]
- Fujita, N. Starch biosynthesis in rice endosperm. Agri-Biosci. Monogr. 2014, 4, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M.; Crofts, N.; Oitome, N.F.; Fujita, N. Analyses of starch biosynthetic protein complexes and starch properties from developing mutant rice seeds with minimal starch synthase activities. BMC Plant Biol. 2018, 18, 59. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.; Ying, Y.; Zhou, X.; Xu, Y.; Wu, P.; Xu, F.; Pang, Y. Relationships among starch biosynthesizing protein content, fine structure and functionality in rice. Carbohydr. Polym. 2020, 237, 116118. [Google Scholar] [CrossRef]
- Crofts, N.; Iizuka, Y.; Abe, N.; Miura, S.; Kikuchi, K.; Matsushima, R.; Fujita, N. Rice Mutants Lacking Starch Synthase I or Branching Enzyme IIb Activity Altered Starch Biosynthetic Protein Complexes. Front. Plant Sci. 2018, 9, 1817. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; You, H.; Zhang, O.; Xiang, X. Genetic Effects of Soluble Starch Synthase IV-2 and It with ADPglucose Pyrophorylase Large Unit and Pullulanase on Rice Qualities. Rice 2020, 13, 46. [Google Scholar] [CrossRef]
- Sun, C.; Sathish, P.; Ahlandsberg, S.; Jansson, C. Analyses of isoamylase gene activity in wild-type barley indicate its involvement in starch synthesis. Plant Mol. Biol. 1999, 40, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-H.; Matheson, N.K. Structures of the amylopectins of waxy, normal, amylose-extender, and wx:ae genotypes and of the phytoglycogen of maize. Carbohydr. Res. 1993, 243, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.C.; Gilbert, R.G. Molecular weight distributions of starch branches reveal genetic constraints on biosynthesis. Biomacromolecules 2010, 11, 3539–3547. [Google Scholar] [CrossRef]
- Nakamura, Y. Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: Rice endosperm as a model tissue. Plant Cell Physiol. 2002, 43, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Dian, W.; Jiang, H.; Chen, Q.; Liu, F.; Wu, P. Cloning and characterization of the granule-bound starch synthase II gene in rice: Gene expression is regulated by the nitrogen level, sugar and circadian rhythm. Planta 2003, 218, 261–268. [Google Scholar] [CrossRef]
- Umemoto, T.; Yano, M.; Satoh, H.; Shomura, A.; Nakamura, Y. Mapping of a gene responsible for the difference in amylopectin structure between japonica-type and indica-type rice varieties. Theor. Appl. Genet. 2002, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.K.; Kosar-Hashemi, B.; Cmiel, M.; Samuel, M.S.; Chandler, P.; Rahman, S.; Buleon, A.; Batey, I.L.; Li, Z. Barley sex6 mutants lack starch synthase IIa activity and contain a starch with novel properties. Plant J. 2003, 34, 173–185. [Google Scholar] [CrossRef]
- Yamamori, M.; Fujita, S.; Hayakawa, K.; Matsuki, J.; Yasui, T. Genetic elimination of a starch granule protein, SGP-1, of wheat generates an altered starch with apparent high amylose. Theor. Appl. Genet. 2000, 101, 21–29. [Google Scholar] [CrossRef]
- Nakamura, Y. Rice starch biotechnology: Rice endosperm as a model of cereal endosperms. Starch-Starke 2018, 70, 1600375. [Google Scholar] [CrossRef]
- Smith, A.M. The biosynthesis of starch granules. Biomacromolecules 2001, 2, 335–341. [Google Scholar] [CrossRef]
- Hanashiro, I.; Itoh, K.; Kuratomi, Y.; Yamazaki, M.; Igarashi, T.; Matsugasako, J.-I.; Takeda, Y. Granule-bound starch synthase I is responsible for biosynthesis of extra-long unit chains of amylopectin in rice. Plant Cell Physiol. 2008, 49, 925–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirose, T.; Terao, T. A comprehensive expression analysis of the starch synthase gene family in rice (Oryza sativa L.). Planta 2004, 220, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Vrinten, P.L.; Nakamura, T. Wheat granule-bound starch synthase I and II are encoded by separate genes that are expressed in different tissues. Plant Physiol. 2000, 122, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Ozaki, H.; Okada, K.; Hori, H.; Takeda, Y.; Mitsui, T. Introduction of Wx transgene into rice wx mutants leads to both high- and low-amylose rice. Plant Cell Physiol. 2003, 44, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.G.; van de Wal, M.H.B.J.; Visser, R.G.F. Progress in understanding the biosynthesis of amylose. Trends Plant Sci. 1998, 3, 462–467. [Google Scholar] [CrossRef]
- Denyer, K.; Clarke, B.; Hylton, C.; Tatge, H.; Smith, A.M. The elongation of amylose and amylopectin chains in isolated starch granules. Plant J. 1996, 10, 1135–1143. [Google Scholar] [CrossRef]
- Nishi, A.; Nakamura, Y.; Tanaka, N.; Satoh, H. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol. 2001, 127, 459–472. [Google Scholar] [CrossRef]
- Wang, Y.J.; White, P.; Pollak, L.; Jane, J. Characterization of Starch Structures of 17 Maize Endosperm Mutant Genotypes with Oh43 Inbred Line Background. Cereal Chem. 1993, 70, 171–179. [Google Scholar]
- Sissons, M.; Sestili, F.; Botticella, E.; Masci, S.; Lafiandra, D. Can Manipulation of Durum Wheat Amylose Content Reduce the Glycaemic Index of Spaghetti? Foods 2020, 9, 693. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, W.; Zhang, C.; Zhu, Y.; Xu, J.; Li, E.; Gilbert, R.G.; Liu, Q. New insights into amylose and amylopectin biosynthesis in rice endosperm. Carbohydr. Polym. 2020, 230, 115656. [Google Scholar] [CrossRef]
- Li, H.; Yu, W.; Dhital, S.; Gidley, M.J.; Gilbert, R.G. Starch-branching enzymes contributing to amylose and amylopectin fine structure in wheat. Carbohydr. Polym. 2019, 224, 115185. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, C.; Xu, J.; Gilbert, R.G.; Liu, Q. Identification of structure-controlling rice biosynthesis enzymes. Biomacromolecules 2021, 22, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Yoshida, M.; Asakura, N.; Ohdan, T.; Miyao, A.; Hirochika, H.; Nakamura, Y. Function and characterization of starch synthase I using mutants in rice. Plant Physiol. 2006, 140, 1070–1084. [Google Scholar] [CrossRef] [Green Version]
- Hanashiro, I.; Higuchi, T.; Aihara, S.; Nakamura, Y.; Fujita, N. Structures of Starches from Rice Mutants Deficient in the Starch Synthase Isozyme SSI or SSIIIa. Biomacromolecules 2011, 12, 1621–1628. [Google Scholar] [CrossRef]
- Ryoo, N.; Yu, C.; Park, C.S.; Baik, M.Y.; Park, I.; Cho, M.H.; Bhoo, S.; An, G.; Hahn, T.R.; Jeon, J.S. Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.). Plant Cell Rep. 2007, 26, 1083–1095. [Google Scholar] [CrossRef]
- Li, H.; Dhital, S.; Slade, A.; Yu, W.; Gilbert, R.G.; Gidley, M.J. Altering starch branching enzymes in wheat generates high-amylose starch with novel molecular structure and functional properties. Food Hydrocoll. 2019, 92, 51–59. [Google Scholar] [CrossRef]
- Sawada, T.; Itoh, M.; Nakamura, Y. Contributions of Three Starch Branching Enzyme Isozymes to the Fine Structure of Amylopectin in Rice Endosperm. Front. Plant Sci. 2018, 9, 1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, B.; Zhang, C.; Zhang, Y.; Du, S.; Xi, M.; Song, F.; Ni, J.; Luo, Z.; Ni, D. Effects of different Wx alleles on amylopectin molecular structure and enzymatic hydrolysis properties of rice starch. Int. J. Food Prop. 2018, 21, 2772–2784. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Yang, C.; Zhu, J.; Zhang, L.; Bai, Y.; Li, E.; Gilbert, R.G. Competition between granule bound starch synthase and starch branching enzyme in starch biosynthesis. Rice 2019, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Crofts, N.; Itoh, A.; Abe, M.; Miura, S.; Oitome, N.F.; Bao, J.S.; Fujita, N. Three major nucleotide polymorphisms in the Waxy gene correlated with the amounts of extra-long chains of amylopectin in rice cultivars with S or L-type amylopectin. J. Appl. Glycosci. 2019, 66, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.C.; Gilbert, R.G. APCLDFIT; Brisbane, Australia, 2013. [Google Scholar]
- Chung, H.-J.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Ao, Z.H.; Simsek, S.; Zhang, G.Y.; Venkatachalam, M.; Reuhs, B.L.; Hamaker, B.R. Starch with a slow digestion property produced by altering its chain length, branch density, and crystalline structure. J. Agric. Food Chem. 2007, 55, 4540–4547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Sofyan, M.; Hamaker, B.R. Slowly digestible state of starch: Mechanism of slow digestion property of gelatinized maize starch. J. Agric. Food Chem. 2008, 56, 4695–4702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ao, Z.; Hamaker, B.R. Nutritional property of endosperm starches from maize mutants: A parabolic relationship between slowly digestible starch and amylopectin fine structure. J. Agric. Food Chem. 2008, 56, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Wolf, B.W.; Garleb, K.A.; Choe, Y.S.; Humphrey, P.M.; Maki, K.C. Pullulan Is a Slowly Digested Carbohydrate in Humans. J. Nutr. 2003, 133, 1051–1055. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Garcia, M.; Kormpa, A.; van der Maarel, M. The glycogen of Galdieria sulphuraria as alternative to starch for the production of slowly digestible and resistant glucose polymers. Carbohydr. Polym. 2017, 169, 75–82. [Google Scholar] [CrossRef]
- Lehmann, U.; Robin, F. Slowly digestible starch‚ its structure and health implications: A review. Trends Food Sci. Technol. 2007, 18, 346–355. [Google Scholar] [CrossRef]
- Guraya, H.S.; James, C.; Champagne, E.T. Effect of Cooling, and Freezing on the Digestibility of Debranched Rice Starch and Physical Properties of the Resulting Material. Starch-Stärke 2001, 53, 64–74. [Google Scholar] [CrossRef]
- Guraya, H.S.; James, C.; Champagne, E.T. Effect of Enzyme Concentration and Storage Temperature on the Formation of Slowly Digestible Starch from Cooked Debranched Rice Starch. Starch-Stärke 2001, 53, 131–139. [Google Scholar] [CrossRef]
- Gong, B.; Cheng, L.; Gilbert, R.G.; Li, C. Distribution of short to medium amylose chains are major controllers of in vitro digestion of retrograded rice starch. Food Hydrocoll. 2019, 96, 634–643. [Google Scholar] [CrossRef]
- Han, X.-Z.; Hamaker, B.R. Amylopectin Fine Structure and Rice Starch Paste Breakdown. J. Cereal Sci. 2001, 34, 279–284. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Capitani, T.; Trzasko, P.; Jeffcoat, R. Molecular Structure of a Low-Amylopectin Starch and Other High-Amylose Maize Starches. J. Cereal Sci. 1998, 27, 289–299. [Google Scholar] [CrossRef]
- Lu, S.; Chen, L.-N.; Lii, C.-Y. Correlations between the fine structure, physicochemical properties, and retrogradation of amylopectins from Taiwan rice varieties. Cereal Chem. 1997, 74, 34–39. [Google Scholar] [CrossRef]
- Srichuwong, S.; Isono, N.; Mishima, T.; Hisamatsu, M. Structure of lintnerized starch is related to X-ray diffraction pattern and susceptibility to acid and enzyme hydrolysis of starch granules. Int. J. Biol. Macromol. 2005, 37, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, A.; Flanagan, B.M.; Shrestha, A.K.; Gidley, M.J.; Gilbert, E.P. Molecular rearrangement of starch during in vitro digestion: Toward a better understanding of enzyme resistant starch formation in processed starches. Biomacromolecules 2008, 9, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, Z.; Deng, B.; Gilbert, R.G.; Sullivan, M.A. The effect of high-amylose resistant starch on the glycogen structure of diabetic mice. Int. J. Biol. Macromol. 2022, 200, 124–131. [Google Scholar] [CrossRef]
- Yu, S.; Du, D.; Wu, A.; Bai, Y.; Wu, P.; Li, C.; Gilbert, R.G. Effects of non-starch genetic modifications on starch structure and properties. Foods 2020, 9, 222. [Google Scholar] [CrossRef] [Green Version]






| Isoform | Enzyme Set | CLD Control | DP Range |
|---|---|---|---|
| SSI | Ap enzyme set i, Am enzyme set i, Am enzyme set ii | Ap short chains, Am short and intermediate–long chains | 6–24, 100–500; 500–1500 |
| SSII-1 | Ap enzyme set iii | Ap intermediate chains | 28–58 |
| SSII-2 | Ap enzyme set i, Ap enzyme set iii | Ap short and intermediate chains | 6–24; 28–58 |
| SSII-3 | Ap enzyme set i, Ap enzyme set iii, Ap enzyme set v, Am enzyme set i | Ap short, intermediate and long chains, Am short chains | 6–24; 28–58; 100–500 |
| SSIII-1 | Ap enzyme set iii, Am enzyme set i, Am enzyme set ii | Ap intermediate chains, Am short and intermediate–long chains | 28–58; 100–500; 500–1500 |
| SSIII-2 | Ap enzyme set i, Ap enzyme set iii, Am enzyme set i, Am enzyme set ii | Ap short and intermediate chains, Am short and intermediate–long chains | 6–24; 28–58; 100–500; 500–1500 |
| SSIV-1 | Ap enzyme set i, Ap enzyme set iii | Ap short and intermediate chains | 6–24; 28–58 |
| SSIV-2 | Ap enzyme set i | Ap short chains | 6–24 |
| SBEI | Ap enzyme set i, Am enzyme set ii | Ap short chains, Am intermediate–long chains | 6–24; 500–1500 |
| SBEIIa | Am enzyme set i, Am enzyme set ii | Am short and intermediate–long chains | 100–500; 500–1500 |
| SBEIIb | Am enzyme set i, Am enzyme set ii | Am short and intermediate–long chains | 100–500; 500–1500 |
| GBSSI | Ap enzyme sets i, iii and v, Am enzyme sets i and ii | Ap short, intermediate and long chains, Am short and long chains | 6–24; 28–58; 68–78; 100–500; 500–1500 |
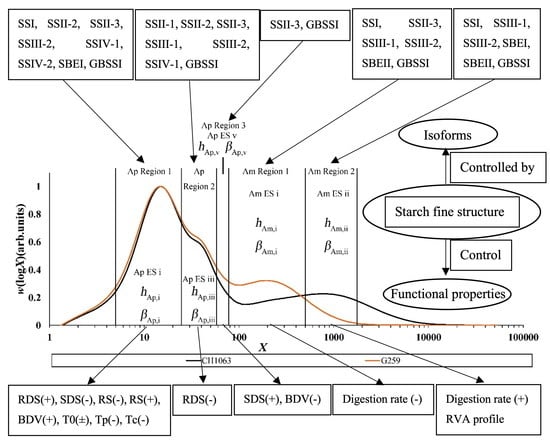
| DP Region [103] | Isoform [103] | Digestibility (Crops) | RVA Properties | |
|---|---|---|---|---|
| DP 6~12 | Ap short chains, Ap ES 1 | SSI, SSII-2, SSII-3, SSIII-2, SSIV-1, SSIV-2, SBEI, GBSSI | RDS(+), SDS(–), RS(–) [113] (Rice, Maize) | BDV(+) [123,124,125] |
| DP 12~24 | RS(+) [126] (Maize, Canna, Potato, Yam) | BDV(+) [123,124,125] | ||
| DP 24~40 | Ap intermediate chains, Ap ES 2 | SSII-1, SSII-2, SSII-3, SSIII-1, SSIII-2, SSIV-1, GBSSI | RDS(–) [113,127] (Rice, Maize) | Unknown |
| DP 40~100 | Ap long chains, Ap ES 3 | SSII-3, GBSSI | SDS(+) [115] (Rice, Maize) | BDV(–) [123,124,125] |
| DP 100~500 | Am short chains, Am ES 1 | SSI, SSII-3, SSIII-1, SSIII-2, SBEII, GBSSI | Digestion rate(–) [119,120,121] (Rice) | Unknown |
| DP 500~1000 | Am intermediate chains, Am ES 2 | SSI, SSIII-1, SSIII-2, SBEI, SBEII, GBSSI | Digestion rate(+) [122] (Rice) | RVA profile [41] |
| DP 1000~1500 | Am long chains, Am ES 2 | Unknown | Unknown | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Bai, Y.; Gilbert, R.G. Effects of the Molecular Structure of Starch in Foods on Human Health. Foods 2023, 12, 2263. https://doi.org/10.3390/foods12112263
Zhu J, Bai Y, Gilbert RG. Effects of the Molecular Structure of Starch in Foods on Human Health. Foods. 2023; 12(11):2263. https://doi.org/10.3390/foods12112263
Chicago/Turabian StyleZhu, Jihui, Yeming Bai, and Robert G. Gilbert. 2023. "Effects of the Molecular Structure of Starch in Foods on Human Health" Foods 12, no. 11: 2263. https://doi.org/10.3390/foods12112263