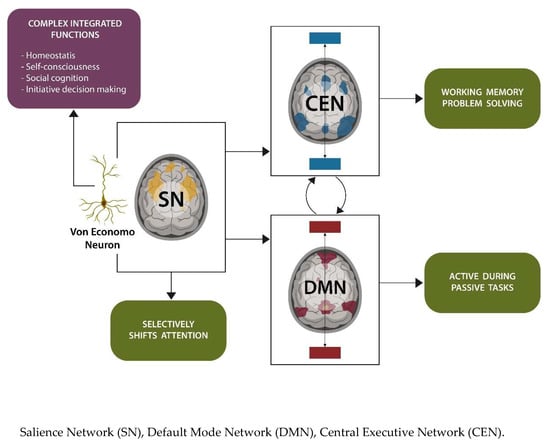
Insomnia in Forensic Detainees: Is Salience Network the Common Pathway for Sleep, Neuropsychiatric, and Neurodegenerative Disorders?
Abstract
:Highlights
- SN dysfunction is the common denominator of insomnia, schizophrenia (SCZ), and frontotemporal dementia behavioral variant (bvFTD).
- The diagnosis of bvFTD is often missed or misdiagnosed in forensic institutions.
- To ensure adequate placement and treatment planning, courts and clinicians require education to differentiate bvFTD from SCZ.
Abstract
1. Introduction
1.1. Salienve Network in Sleep and Neuropathology
1.2. Salience Network in Frontotemporal Dementia Behavioral Variant
1.3. Sleep and Glial Cells
2. Mitochondria and Aryl Hydrocarbon Receptor
2.1. Mitochondria-Protective Treatments
2.2. Membrane Lipid Replacement (MLR)
2.3. Phenazines and Phenothiazine Derivatives
2.4. Natural Antioxidants
Natural Antioxidant Foods
2.5. Mitochondrial Transfer and Transplantation
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sateia, M.J.; Doghramji, K.; Hauri, P.J.; Morin, C.M. Evaluation of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 2000, 23, 243–308. [Google Scholar] [CrossRef]
- Dewa, L.H.; Thibaut, B.; Pattison, N.; Campbell, S.J.; Woodcock, T.; Aylin, P.; Archer, S. Treating insomnia in people who are incarcerated: A feasibility study of a multi-component treatment pathway. Sleep Adv. 2024, 5, zpae003. [Google Scholar] [CrossRef]
- Talih, F.; Ajaltouni, J.; Ghandour, H.; Abu-Mohammad, A.S.; Kobeissy, F. Insomnia in hospitalized psychiatric patients: Prevalence and associated factors. Neuropsychiatr. Dis. Treat. 2018, 14, 969–975. [Google Scholar] [CrossRef]
- Levichkina, E.V.; Busygina, I.I.; Pigareva, M.L.; Pigarev, I.N. The Mysterious Island: Insula and Its Dual Function in Sleep and Wakefulness. Front. Syst. Neurosci. 2021, 14, 592660. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, H.; Wang, L.; Zhong, Q.; Lei, Y.; Yang, L.; Zeng, L.L.; Zhou, Z.; Hu, D.; Yang, Z. Impact of 36 h of total sleep depri-vation on resting-state dynamic functional connectivity. Brain Res. 2018, 1688, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Wylie, K.P.; Tregellas, J.R. The role of the insula in schizophrenia. Schizophr. Res. 2010, 123, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Fathy, Y.Y.; Hoogers, S.E.; Berendse, H.W.; van der Werf, Y.D.; Visser, P.J.; de Jong, F.J.; van de Berg, W.D. Differential insular cortex sub-regional atrophy in neurodegenerative diseases: A systematic review and meta-analysis. Brain Imaging Behav. 2019, 14, 2799–2816. [Google Scholar] [CrossRef] [PubMed]
- Koutsouleris, N.; Pantelis, C.; Velakoulis, D.; McGuire, P.; Dwyer, D.B.; Urquijo-Castro, M.-F.; Paul, R.; Dong, S.; Popovic, D.; Oeztuerk, O.; et al. Exploring Links Between Psychosis and Frontotemporal Dementia Using Multimodal Machine Learning: Dementia Praecox Revisited. JAMA Psychiatry 2022, 79, 907–919. [Google Scholar] [CrossRef]
- Triarhou, L.C. The percipient observations of Constantin von Economo on encephalitis lethargica and sleep disruption and their lasting impact on contemporary sleep research. Brain Res. Bull. 2006, 69, 244–258. [Google Scholar] [CrossRef]
- Allman, J.M.; Tetreault, N.A.; Hakeem, A.Y.; Manaye, K.F.; Semendeferi, K.; Erwin, J.M.; Park, S.; Goubert, V.; Hof, P.R. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct. Funct. 2010, 214, 495–517. [Google Scholar] [CrossRef]
- Berg, M.T.; Rogers, E.M.; Lei, M.-K.; Simons, R.L. Losing Years Doing Time: Incarceration Exposure and Accelerated Biological Aging among African American Adults. J. Health Soc. Behav. 2021, 62, 460–476. [Google Scholar] [CrossRef]
- Kaiksow, F.A.; Brown, L.; Merss, K.B. Caring for the Rapidly Aging Incarcerated Population: The Role of Policy. J. Gerontol. Nurs. 2023, 49, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, E.; Gaughran, F.; Smith, S. Schizophrenia as segmental progeria. J. R. Soc. Med. 2011, 104, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Killilea, D.W.; Wong, S.L.; Cahaya, H.S.; Atamna, H.; Ames, B.N. Iron accumulation during cellular senescence. Ann. N. Y. Acad. Sci. 2004, 1019, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, P.J.; Mena, N.P.; Núñez, M.T. The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front. Pharmacol. 2014, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Carvalhas-Almeida, C.; Cavadas, C.; Álvaro, A.R. The impact of insomnia on frailty and the hallmarks of aging. Aging Clin. Exp. Res. 2022, 35, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.E.; Prather, A.A. Sleep and biological aging: A short review. Curr. Opin. Endocr. Metab. Res. 2021, 18, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna-Żydecka, K.; Jamioł-Milc, D.; Borecki, K.; Stachowska, E.; Zabielska, P.; Kamińska, M.; Karakiewicz, B. The Prevalence of Insomnia and the Link between Iron Metabolism Genes Polymorphisms, TF rs1049296 C>T, TF rs3811647 G>A, TFR rs7385804 A>C, HAMP rs10421768 A>G and Sleep Disorders in Polish Individuals with ASD. Int. J. Environ. Res. Public Health 2020, 17, 400. [Google Scholar] [CrossRef]
- Nacarino-Palma, A.; Rico-Leo, E.M.; Campisi, J.; Ramanathan, A.; González-Rico, F.J.; Rejano-Gordillo, C.M.; Ordiales-Talavero, A.; Merino, J.M.; Fernández-Salguero, P.M. Aryl hydrocarbon receptor blocks aging-induced senescence in the liver and fibroblast cells. Aging 2022, 14, 4281–4304. [Google Scholar] [CrossRef]
- Panda, S.K.; Peng, V.; Sudan, R.; Antonova, A.U.; Di Luccia, B.; Ohara, T.E.; Fachi, J.L.; Grajales-Reyes, G.E.; Jaeger, N.; Trsan, T.; et al. Repression of the aryl-hydrocarbon receptor prevents oxidative stress and ferroptosis of intestinal intraepithelial lymphocytes. Immunity 2023, 56, 797–812.e4. [Google Scholar] [CrossRef]
- Hwang, H.J.; Dornbos, P.; Steidemann, M.; Dunivin, T.K.; Rizzo, M.; LaPres, J.J. Mitochondrial-targeted aryl hydrocarbon receptor and the impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin on cellular respiration and the mitochondrial proteome. Toxicol. Appl. Pharmacol. 2016, 304, 121–132. [Google Scholar] [CrossRef]
- Dietrich-Muszalska, A.; Kontek, B. Lipid peroxidation in patients with schizophrenia. Psychiatry Clin. Neurosci. 2010, 64, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, J.; Qu, C.; Yang, L.; Wu, X.; Wang, S.; Yang, T.; Liu, H.; Fang, Y.; Sun, P. Identification of Ferroptosis-Related Genes in Schizophrenia Based on Bioinformatic Analysis. Genes 2022, 13, 2168. [Google Scholar] [CrossRef] [PubMed]
- Gulec, M.; Ozkol, H.; Selvi, Y.; Tuluce, Y.; Aydin, A.; Besiroglu, L.; Ozdemir, P.G. Oxidative stress in patients with primary insomnia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 37, 247–251. [Google Scholar] [CrossRef]
- Liang, H.; Van Remmen, H.; Frohlich, V.; Lechleiter, J.; Richardson, A.; Ran, Q. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem. Biophys. Res. Commun. 2007, 356, 893–898. [Google Scholar] [CrossRef]
- Seibt, T.M.; Proneth, B.; Conrad, M. Role of GPX4 in ferroptosis and its pharmacological implication. Free Radic. Biol. Med. 2018, 133, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Koumura, T.; Iwamoto, R.; Matsuoka, M.; Terauchi, R.; Yasuda, S.; Shiraya, T.; Watanabe, S.; Aihara, M.; Imai, H.; et al. Mitochondrial glutathione peroxidase 4 is indispensable for photoreceptor development and survival in mice. J. Biol. Chem. 2022, 298, 101824. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Fer-roptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Carroll, J.E.; Esquivel, S.; Goldberg, A.; Seeman, T.E.; Effros, R.B.; Dock, J.; Olmstead, R.; Breen, E.C.; Irwin, M.R. Insomnia and Telomere Length in Older Adults. Sleep 2016, 39, 559–564. [Google Scholar] [CrossRef]
- Schnack, H.G.; van Haren, N.E.; Nieuwenhuis, M.; Hulshoff Pol, H.E.; Cahn, W.; Kahn, R.S. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am. J. Psychiatry 2016, 173, 607–616. [Google Scholar] [CrossRef]
- Porterfield, V.; Khan, S.S.; Foff, E.P.; Koseoglu, M.M.; Blanco, I.K.; Jayaraman, S.; Lien, E.; McConnell, M.J.; Bloom, G.S.; Lazo, J.S.; et al. A three-dimensional dementia model reveals spontaneous cell cycle re-entry and a senescence-associated secretory phenotype. Neurobiol. Aging 2020, 90, 125–134. [Google Scholar] [CrossRef]
- De Lorenzo, B.H.; Marchioro, L.d.O.; Greco, C.R.; Suchecki, D. Sleep-deprivation reduces NK cell number and function mediated by β-adrenergic signalling. Psychoneuroendocrinology 2015, 57, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, N.; Leboyer, M.; Bouleau, A.; Hamdani, N.; Richard, J.R.; Boukouaci, W.; Ching-Lien, W.; Godin, O.; Bengoufa, D.; Le Corvoisier, P.; et al. Natural killer cells in first-episode psychosis: An innate immune signature? Mol. Psychiatry 2021, 26, 5297–5306. [Google Scholar] [CrossRef]
- Huang, A.; Shinde, P.V.; Huang, J.; Senff, T.; Xu, H.C.; Margotta, C.; Häussinger, D.; Willnow, T.E.; Zhang, J.; Pandyra, A.A.; et al. Progranulin prevents regulatory NK cell cytotoxicity against antiviral T cells. J. Clin. Investig. 2019, 4, e129856. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R. Sleep disruption induces activation of inflammation and heightens risk for infectious disease: Role of impairments in thermoregulation and elevated ambient temperature. Temperature 2022, 10, 198–234. [Google Scholar] [CrossRef]
- Bright, F.; Werry, E.L.; Dobson-Stone, C.; Piguet, O.; Ittner, L.M.; Halliday, G.M.; Hodges, J.R.; Kiernan, M.C.; Loy, C.T.; Kassiou, M.; et al. Neuroinflammation in frontotemporal dementia. Nat. Rev. Neurol. 2019, 15, 540–555. [Google Scholar] [CrossRef]
- Vallée, A. Neuroinflammation in Schizophrenia: The Key Role of the WNT/β-Catenin Pathway. Int. J. Mol. Sci. 2022, 23, 2810. [Google Scholar] [CrossRef]
- Nesci, S.; Spagnoletta, A.; Oppedisano, F. Inflammation, Mitochondria and Natural Compounds Together in the Circle of Trust. Int. J. Mol. Sci. 2023, 24, 6106. [Google Scholar] [CrossRef] [PubMed]
- Andrieux, P.; Chevillard, C.; Cunha-Neto, E.; Nunes, J.P.S. Mitochondria as a Cellular Hub in Infection and Inflammation. Int. J. Mol. Sci. 2021, 22, 11338. [Google Scholar] [CrossRef]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef]
- Gollihue, J.; Norris, C. Astrocyte mitochondria: Central players and potential therapeutic targets for neurodegenerative diseases and injury. Ageing Res. Rev. 2020, 59, 101039. [Google Scholar] [CrossRef] [PubMed]
- Boas, S.M.; Joyce, K.L.; Cowell, R.M. The NRF2-Dependent Transcriptional Regulation of Antioxidant Defense Pathways: Relevance for Cell Type-Specific Vulnerability to Neurodegeneration and Therapeutic Intervention. Antioxidants 2021, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Downar, J.; Crawley, A.P.; Mikulis, D.J.; Davis, K.D. A multimodal cortical network for the detection of changes in the sensory environment. Nat. Neurosci. 2000, 3, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.; Vann, S.D. The cognitive thalamus as a gateway to mental representations. J. Neurosci. 2018, 39, 3–14. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef]
- Ueno, D.; Matsuoka, T.; Kato, Y.; Ayani, N.; Maeda, S.; Takeda, M.; Narumoto, J. Individual Differences in Interoceptive Accuracy Are Correlated with Salience Network Connectivity in Older Adults. Front. Aging Neurosci. 2020, 12, 592002. [Google Scholar] [CrossRef]
- Blessing, W.W.; Blessing, E.M.; Mohammed, M.; Ootsuka, Y. Clozapine, chlorpromazine and risperidone dose-dependently reduce emotional hyperthermia, a biological marker of salience. Psychopharmacology 2017, 234, 3259–3269. [Google Scholar] [CrossRef]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.; Nana, A.L.; Toller, G.; Brown, J.A.; Deng, J.; Staffaroni, A.; Kim, E.-J.; Hwang, J.-H.L.; Li, L.; Park, Y.; et al. Salience Network Atrophy Links Neuron Type-Specific Pathobiology to Loss of Empathy in Frontotemporal Dementia. Cereb. Cortex 2020, 30, 5387–5399. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F.; Anderson, E.; Shapira, J.S. An investigate von of moral judgement in frontotemporal dementia. Cogn. Behav. Neurol. 2005, 18, 193–197. [Google Scholar] [CrossRef]
- Mendez, M.F. The neurobiology of moral behavior: Review and neuropsychiatric implications. CNS Spectr. 2009, 14, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F. Behavioral Variant Frontotemporal Dementia. Continuum 2022, 28, 702–725. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, E.; Fuller, P.M. The Sleep-Promoting Ventrolateral Preoptic Nucleus: What Have We Learned over the Past 25 Years? Int. J. Mol. Sci. 2022, 23, 2905. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Nardone, S.; Grace, K.P.; Venner, A.; Cristofolini, M.; Bandaru, S.S.; Sohn, L.T.; Kong, D.; Mochizuki, T.; Viberti, B.; et al. Orexin neurons inhibit sleep to promote arousal. Nat. Commun. 2022, 13, 4163. [Google Scholar] [CrossRef] [PubMed]
- Inutsuka, A.; Yamanaka, A. The regulation of sleep and wakefulness by the hypothalamic neuropeptide orexin/hypocretin. Nagoya J. Med. Sci. 2013, 75, 29–36. [Google Scholar]
- Yin, D.; Dong, H.; Wang, T.-X.; Hu, Z.-Z.; Cheng, N.-N.; Qu, W.-M.; Huang, Z.-L. Glutamate Activates the Histaminergic Tuberomammillary Nucleus and Increases Wakefulness in Rats. Neuroscience 2019, 413, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Konadhode, R.R.; Pelluru, D.; Shiromani, P.J. Neurons containing orexin or melanin concentrating hormone reciprocally regulate wake and sleep. Front. Syst. Neurosci. 2015, 8, 244. [Google Scholar] [CrossRef]
- Chung, S.; Weber, F.; Zhong, P.; Tan, C.L.; Nguyen, T.N.; Beier, K.T.; Hörmann, N.; Chang, W.-C.; Zhang, Z.; Do, J.P.; et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 2017, 545, 477–481. [Google Scholar] [CrossRef]
- Bandarabadi, M.; Li, S.; Aeschlimann, L.; Colombo, G.; Tzanoulinou, S.; Tafti, M.; Becchetti, A.; Boutrel, B.; Vassalli, A. Inactivation of hypocretin receptor-2 signaling in dopaminergic neurons induces hyperarousal and enhanced cognition but impaired inhibitory control. Mol. Psychiatry 2023. online ahead of print. [Google Scholar] [CrossRef]
- Wu, S.; Gao, C.; Han, F.; Cheng, H. Histamine H1 receptor in basal forebrain cholinergic circuit: A novel target for the negative symptoms of schizophrenia? Neurosci. Bull. 2022, 38, 558–560. [Google Scholar] [CrossRef]
- Grady, F.S.; Boes, A.D.; Geerling, J.C. A Century Searching for the Neurons Necessary for Wakefulness. Front. Neurosci. 2022, 16, 930514. [Google Scholar] [CrossRef]
- Kerkhofs, M.; Lavie, P. Frédéric Bremer 1892–1982: A pioneer in sleep research. Sleep Med. Rev. 2000, 4, 505–514. [Google Scholar] [CrossRef]
- Fuller, P.; Sherman, D.; Pedersen, N.P.; Saper, C.B.; Lu, J. Reassessment of the structural basis of the ascending arousal system. J. Comp. Neurol. 2010, 519, 933–956, Erratum in J. Comp. Neurol. 2011, 519, 3817. [Google Scholar] [CrossRef] [PubMed]
- Lavie, P. The sleep theory of Constantin von Economo. J. Sleep Res. 1993, 2, 175–178. [Google Scholar] [CrossRef]
- Vyas, A.; De Jesus, O. Von Economo Encephalitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Rosen, D. Asleep: The Forgotten Epidemic That Remains One of Medicine’s Greatest Mysteries. J. Clin. Sleep Med. 2010, 6, 299. [Google Scholar] [CrossRef]
- Cortelli, P.; Perani, D.; Parchi, P.; Grassi, F.; Montagna, P.; De Martin, M.; Castellani, R.; Tinuper, P.; Gambetti, P.; Lugaresi, E.; et al. Cerebral metabolism in fatal familial insomnia: Relation to duration, neuropathology, and distribution of protease-resistent prion protein. Neurology 1997, 49, 126–133. [Google Scholar] [CrossRef]
- Gallassi, R.; Morreale, A.; Montagna, P.; Cortelli, P.; Avoni, P.; Castellani, R.; Gambetti, R.; Lugaresi, E. Fatal familial insomnia: Behavioral and cognitive features. Neurology 1996, 46, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Sturm, V.E.; Brown, J.A.; Hua, A.Y.; Lwi, S.J.; Zhou, J.; Kurth, F.; Eickhoff, S.B.; Rosen, H.J.; Kramer, J.H.; Miller, B.L.; et al. Network Architecture Underlying Basal Autonomic Outflow: Evidence from Frontotemporal Dementia. J. Neurosci. 2018, 38, 8943–8955. [Google Scholar] [CrossRef] [PubMed]
- Mallikarjun, P.K.; Lalousis, P.A.; Dunne, T.F.; Heinze, K.; Reniers, R.L.; Broome, M.R.; Farmah, B.; Oyebode, F.; Wood, S.J.; Upthegrove, R. Aberrant salience network functional connectivity in auditory verbal hallucinations: A first episode psychosis sample. Transl. Psychiatry 2018, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Cracco, L.; Appleby, B.S.; Gambetti, P. Fatal familial insomnia and sporadic fatal insomnia. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 153, pp. 271–299. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Li, W.; Xiao, L.; Huo, X.; Ding, J.; Sun, T. Is the insula linked to sleep? A systematic review and narrative synthesis. Heliyon 2022, 8, e11406. [Google Scholar] [CrossRef]
- Hehr, A.; Huntley, E.D.; Marusak, H.A. Getting a Good Night′s Sleep: Associations Between Sleep Duration and Par-ent-Reported Sleep Quality on Default Mode Network Connectivity in Youth. J. Adolesc. Health 2023, 72, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zou, G.; Shao, Y.; Chen, J.; Li, Y.; Liu, J.; Yao, P.; Zhou, S.; Xu, J.; Hu, S.; et al. Increased connectivity of the anterior cingulate cortex is associated with the tendency to awakening during N2 sleep in patients with insomnia disorder. Sleep 2022, 46, zsac290. [Google Scholar] [CrossRef] [PubMed]
- Guldenmund, P.; Demertzi, A.; Boveroux, P.; Boly, M.; Vanhaudenhuyse, A.; Bruno, M.-A.; Gosseries, O.; Noirhomme, Q.; Brichant, J.-F.; Bonhomme, V.; et al. Thalamus, brainstem and salience network connectivity changes during propofol-induced sedation and unconsciousness. Brain Connect. 2013, 3, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, L.; Zhou, Z.; Xu, K.; Zhang, L.; Liu, X.; Tan, X.; Zhang, J.; Ye, X.; Gao, J.; et al. Functional Connectivity of Anterior Insula Predicts Recovery of Patients with Disorders of Consciousness. Front. Neurol. 2018, 9, 1024. [Google Scholar] [CrossRef]
- Mashour, G.A. Anesthetizing the Self: The Neurobiology of Humbug. Anesthesiology 2016, 124, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, B.-Z.; Zhang, Y.; Pan, B.; Gao, Y.-H.; Zhan, H.; Liu, Y.; Shao, Y.-C.; Zhang, X. Altered insula-prefrontal functional connectivity correlates to decreased vigilant attention after total sleep deprivation. Sleep Med. 2021, 84, 187–194. [Google Scholar] [CrossRef]
- Li, C.; Dong, M.; Yin, Y.; Hua, K.; Fu, S.; Jiang, G. Aberrant Effective Connectivity of the Right Anterior Insula in Primary Insomnia. Front. Neurol. 2018, 9, 317. [Google Scholar] [CrossRef]
- Chen, M.C.; Chiang, W.-Y.; Yugay, T.; Patxot, M.; Ozcivit, I.B.; Hu, K.; Lu, J. Anterior Insula Regulates Multiscale Temporal Organization of Sleep and Wake Activity. J. Biol. Rhythm. 2016, 31, 182–193. [Google Scholar] [CrossRef]
- Palaniyappan, L.; White, T.; Liddle, P. The concept of salience network dysfunction in schizophrenia: From neuroimaging observations to therapeutic opportunities. Curr. Top. Med. Chem. 2012, 12, 2324–2338. [Google Scholar] [CrossRef]
- Huang, H.; Chen, C.; Rong, B.; Wan, Q.; Chen, J.; Liu, Z.; Zhou, Y.; Wang, G.; Wang, H. Resting-state functional connectivity of salience network in schizophrenia and depression. Sci. Rep. 2022, 12, 11204. [Google Scholar] [CrossRef]
- He, X.; Qin, W.; Liu, Y.; Zhang, X.; Duan, Y.; Song, J.; Li, K.; Jiang, T.; Yu, C. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. 2013, 35, 3446–3464. [Google Scholar] [CrossRef] [PubMed]
- Putcha, D.; Ross, R.S.; Cronin-Golomb, A.; Janes, A.C.; Stern, C.E. Salience and Default Mode Network Coupling Predicts Cognition in Aging and Parkinson’s Disease. J. Int. Neuropsychol. Soc. 2016, 22, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Day, G.S.; Farb, N.A.S.; Tang-Wai, D.F.; Masellis, M.; Black, S.E.; Freedman, M.; Pollock, B.G.; Chow, T.W. Salience Network Resting-State Activity: Prediction of Frontotemporal Dementia Pro-gression. JAMA Neurol. 2013, 70, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.M.; Rogers, B.P.; Blackford, J.U.; Heckers, S.; Woodward, N.D. Insula functional connectivity in schizophrenia. Schizophr. Res. 2020, 220, 69–77. [Google Scholar] [CrossRef]
- Adams, R.; David, A.S. Patterns of anterior cingulate activation in schizophrenia: A selective review. Neuropsychiatr. Dis. Treat. 2007, 3, 87–101. [Google Scholar] [CrossRef]
- Nana, A.L.; Sidhu, M.; Gaus, S.E.; Hwang, J.-H.L.; Li, L.; Park, Y.; Kim, E.-J.; Pasquini, L.; Allen, I.E.; Rankin, K.P.; et al. Neurons selectively targeted in frontotemporal dementia reveal early stage TDP-43 pathobiology. Acta Neuropathol. 2018, 137, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Zago, S.; Scarpazza, C.; Difonzo, T.; Arighi, A.; Hajhajate, D.; Torrente, Y.; Sartori, G. Behavioral Variant of Frontotemporal Dementia and Homicide in a Historical Case. J. Am. Acad. Psychiatry Law 2021, 49, 219–227. [Google Scholar] [PubMed]
- Nilsson, C.; Waldö, M.L.; Nilsson, K.; Santillo, A.; Vestberg, S. Age-related incidence and family history in frontotemporal dementia: Data from the swedish dementia registry. PLoS ONE 2014, 9, e94901. [Google Scholar] [CrossRef]
- Hendriks, S.; Peetoom, K.; Bakker, C.; van der Flier, W.M.; Papma, J.M.; Koopmans, R.; Verhey, F.R.J.; de Vugt, M.; Köhler, S.; Withall, A.; et al. Global Prevalence of Young-Onset Dementia: A Systematic Review and Meta-analysis. JAMA Neurol. 2021, 78, 1080–1090. [Google Scholar] [CrossRef]
- Onyike, C.U.; Diehl-Schmid, J. The epidemiology of frontotemporal dementia. Int. Rev. Psychiatry 2013, 25, 130–137. [Google Scholar] [CrossRef]
- Diehl-Schmid, J.; Perneczky, R.; Koch, J.; Nedopil, N.; Kurz, A. Guilty by suspicion? Criminal behavior in frontotemporal lobar degeneration. Cogn. Behav. Neurol. 2013, 26, 73–77. [Google Scholar] [CrossRef]
- Krause, M.; Theiss, C.; Brüne, M. Ultrastructural Alterations of Von Economo Neurons in the Anterior Cingulate Cortex in Schizophrenia. Anat. Rec. 2017, 300, 2017–2024. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, Y.J.; Lee, B.H.; Lee, P.; Park, J.H.; Seo, S.W.; Jeong, Y. Behavioral Reserve in Behavioral Variant Frontotemporal Dementia. Front. Aging Neurosci. 2022, 14, 875589. [Google Scholar] [CrossRef]
- Vohryzek, J.; Cabral, J.; Vuust, P.; Deco, G.; Kringelbach, M.L. Understanding brain states across spacetime informed by whole-brain modelling. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2022, 380, 20210247. [Google Scholar] [CrossRef]
- Nathani, M.; Jaleel, V.; Turner, A.; Dirvonas, C.; Suryadevara, U.; Tandon, R. When you hear hoofbeats, think horses and zebras: The importance of a wide differential when it comes to frontotemporal lobar degeneration. Asian J. Psychiatry 2019, 47, 101875. [Google Scholar] [CrossRef] [PubMed]
- Herbert, B.M.; Herbert, C.; Pollatos, O. On the Relationship Between Interoceptive Awareness and Alexithymia: Is Interoceptive Awareness Related to Emotional Awareness? J. Personal. 2011, 79, 1149–1175. [Google Scholar] [CrossRef]
- Quadt, L.; Critchley, H.D.; Garfinkel, S.N. The neurobiology of interoception in health and disease. Ann. N. Y. Acad. Sci. 2018, 1428, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Cauda, F.; Geminiani, G.C.; Vercelli, A. Evolutionary appearance of von Economo’s neurons in the mammalian cerebral cortex. Front. Hum. Neurosci. 2014, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- López-Ojeda, W.; Hurley, R.A. Von Economo Neuron Involvement in Social Cognitive and Emotional Impairments in Neuropsychiatric Disorders. J. Neuropsychiatry Clin. Neurosci. 2022, 34, 302–306. [Google Scholar] [CrossRef]
- Hodge, R.D.; Miller, J.A.; Novotny, M.; Kalmbach, B.E.; Ting, J.T.; Bakken, T.E.; Aevermann, B.D.; Barkan, E.R.; Berkowitz-Cerasano, M.L.; Cobbs, C.; et al. Transcriptomic evidence that von Economo neurons are regionally specialized extratelencephalic-projecting excitatory neurons. Nat. Commun. 2020, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, A.A.; Lin, L.-C.; Nana, A.L.; Gaus, S.E.; Seeley, W.W. Von Economo Neurons and Fork Cells: A Neurochemical Signature Linked to Monoaminergic Function. Cereb. Cortex 2016, 28, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.A. Monoamines: Dopamine, Norepinephrine, and Serotonin, Beyond Modulation, “Switches” That Alter the State of Target Networks. Neuroscientist 2020, 28, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Valli, M.; Cho, S.S.; Uribe, C.; Masellis, M.; Chen, R.; Mihaescu, A.; Strafella, A.P. VMAT2 availability in Parkinson’s disease with probable REM sleep behaviour disorder. Mol. Brain 2021, 14, 165. [Google Scholar] [CrossRef] [PubMed]
- Broese, M.; Riemann, D.; Hein, L.; Nissen, C. α-Adrenergic Receptor Function, Arousal and Sleep: Mechanisms and Therapeutic Implications. Pharmacopsychiatry 2012, 45, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Gaus, R.; Popal, M.; Heinsen, H.; Schmitt, A.; Falkai, P.; Hof, P.R.; Schmitz, C.; Vollhardt, A. Reduced cortical neuron number and neuron density in schizophrenia with focus on area 24: A post-mortem case–control study. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 273, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, M.; Minthon, L.; Passant, U.; Blennow, K.; Wallin, A. Decreased monoamine metabolites in frontotemporal dementia and Alzheimer’s disease. Neurobiol. Aging 1998, 19, 379–384. [Google Scholar] [CrossRef]
- Levenson, J.C.; Kay, D.B.; Buysse, D.J. The Pathophysiology of Insomnia. Chest 2015, 147, 1179–1192. [Google Scholar] [CrossRef]
- Zhou, M.; Kiyoshi, C.M. Astrocyte syncytium: A functional reticular system in the brain. Neural Regen. Res. 2019, 14, 595–596. [Google Scholar] [CrossRef]
- Garofalo, S.; Picard, K.; Limatola, C.; Nadjar, A.; Pascual, O.; Tremblay, M.E. Role of Glia in the Regulation of Sleep in Health and Disease. Compr. Physiol. 2020, 10, 687–712. [Google Scholar] [CrossRef] [PubMed]
- Que, M.; Li, Y.; Wang, X.; Zhan, G.; Luo, X.; Zhou, Z. Role of astrocytes in sleep deprivation: Accomplices, resisters, or bystanders? Front. Cell. Neurosci. 2023, 17, 1188306. [Google Scholar] [CrossRef] [PubMed]
- Mader, S.; Brimberg, L. Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease. Cells 2019, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Valenti, D.; Vacca, R.A.; Moro, L.; Atlante, A. Mitochondria Can Cross Cell Boundaries: An Overview of the Biological Relevance, Pathophysiological Implications and Therapeutic Perspectives of Intercellular Mitochondrial Transfer. Int. J. Mol. Sci. 2021, 22, 8312. [Google Scholar] [CrossRef] [PubMed]
- Leow, D.M.-K.; Cheah, I.K.-M.; Fong, Z.W.-J.; Halliwell, B.; Ong, W.-Y. Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 5498. [Google Scholar] [CrossRef] [PubMed]
- Asami, T.; Bouix, S.; Whitford, T.J.; Shenton, M.E.; Salisbury, D.F.; McCarley, R.W. Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. Neuroimage 2012, 59, 986–996. [Google Scholar] [CrossRef]
- Hill, V.M.; O’connor, R.M.; Sissoko, G.B.; Irobunda, I.S.; Leong, S.; Canman, J.C.; Stavropoulos, N.; Shirasu-Hiza, M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018, 16, e2005206. [Google Scholar] [CrossRef]
- Patergnani, S.; Bonora, M.; Ingusci, S.; Previati, M.; Marchi, S.; Zucchini, S.; Perrone, M.; Wieckowski, M.R.; Castellazzi, M.; Pugliatti, M.; et al. Antipsychotic drugs counteract autophagy and mitophagy in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2021, 118, e2020078118. [Google Scholar] [CrossRef]
- Hirata, Y.; Cai, R.; Volchuk, A.; Steinberg, B.E.; Saito, Y.; Matsuzawa, A.; Grinstein, S.; Freeman, S.A. Lipid peroxidation increases membrane tension, Piezo1 gating, and cation permeability to execute ferroptosis. Curr. Biol. 2023, 33, 1282–1294.e5. [Google Scholar] [CrossRef]
- Heckers, S.; Konradi, C. Hippocampal neurons in schizophrenia. J. Neural Transm. 2002, 109, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Padurariu, M.; Ciobica, A.; Mavroudis, I.; Fotiou, D.; Baloyannis, S. Hippocampal neuronal loss in the CA1 and CA3 areas of Alz-heimer’s disease patients. Psychiatr. Danub. 2012, 24, 152–158. [Google Scholar] [PubMed]
- Zhang, P.; Li, Y.-X.; Zhang, Z.-Z.; Yang, Y.; Rao, J.-X.; Xia, L.; Li, X.-Y.; Chen, G.-H.; Wang, F. Astroglial Mechanisms Underlying Chronic Insomnia Disorder: A Clinical Study. Nat. Sci. Sleep 2020, 12, 693–704. [Google Scholar] [CrossRef]
- Bellesi, M.; de Vivo, L.; Chini, M.; Gilli, F.; Tononi, G.; Cirelli, C. Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J. Neurosci. 2017, 37, 5263–5273. [Google Scholar] [CrossRef]
- Vilalta, A.; Brown, G.C. Neurophagy, the phagocytosis of live neurons and synapses by glia, contributes to brain development and disease. FEBS J. 2017, 285, 3566–3575. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Whitehurst, T.; Howes, O. The role of mitochondria in the pathophysiology of schizophrenia: A critical review of the evidence focusing on mitochondrial complex one. Neurosci. Biobehav. Rev. 2021, 132, 449–464. [Google Scholar] [CrossRef]
- Beaupre, L.M.M.; Brown, G.M.; Braganza, N.A.; Kennedy, J.L.; Gonçalves, V.F. Mitochondria’s role in sleep: Novel insights from sleep deprivation and restriction studies. World J. Biol. Psychiatry 2021, 23, 1–13. [Google Scholar] [CrossRef]
- Richardson, R.B.; Mailloux, R.J. Mitochondria Need Their Sleep: Redox, Bioenergetics, and Temperature Regulation of Circadian Rhythms and the Role of Cysteine-Mediated Redox Signaling, Uncoupling Proteins, and Substrate Cycles. Antioxidants 2023, 12, 674. [Google Scholar] [CrossRef]
- Ene, H.M.; Karry, R.; Farfara, D.; Ben-Shachar, D. Mitochondria play an essential role in the trajectory of adolescent neurodevelopment and behavior in adulthood: Evidence from a schizophrenia rat model. Mol. Psychiatry 2022, 28, 1170–1181. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.-G.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Heo, M.J.; Suh, J.H.; Lee, S.H.; Poulsen, K.L.; An, Y.A.; Moorthy, B.; Hartig, S.M.; Moore, D.D.; Kim, K.H. Aryl hydrocarbon receptor maintains hepatic mitochondrial homeostasis in mice. Mol. Metab. 2023, 72, 101717. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Hospital, C.; Tête, A.; Brial, F.; Benoit, L.; Koual, M.; Tomkiewicz, C.; Kim, M.J.; Blanc, E.B.; Coumoul, X.; Bortoli, S. Mitochondrial Dysfunction as a Hallmark of Environmental Injury. Cells 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Heath-Pagliuso, S.; Rogers, W.J.; Tullis, K.; Seidel, S.D.; Cenijn, P.H.; Brouwer, A.; Denison, M.S. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 1998, 37, 11508–11515. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Slominski, R.M.; Song, Y.; Qayyum, S.; Placha, W.; Janjetovic, Z.; Kleszczyński, K.; Atigadda, V.; Song, Y.; et al. Melatonin and Its Metabolites Can Serve as Agonists on the Aryl Hydrocarbon Receptor and Peroxisome Proliferator-Activated Receptor Gamma. Int. J. Mol. Sci. 2023, 24, 15496. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-X.; Wang, C.; Krager, S.L.; Bottum, K.M.; Tischkau, S.A. Aryl hydrocarbon receptor activation attenuates Per1 gene induction and influences circadian clock resetting. Toxicol. Sci. 2013, 132, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Kempf, A. Mitochondrial control of sleep. Curr. Opin. Neurobiol. 2023, 81, 102733. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-D.; Helleberg, H.; Rannug, U.; Rannug, A. Rapid and transient induction of CYP1A1 gene expression in human cells by the tryptophan photoproduct 6-formylindolo(3,2-b)carbazole. Chem. Biol. Interact. 1998, 110, 39–55. [Google Scholar] [CrossRef]
- Ziv-Gal, A.; Flaws, J.A.; Mahoney, M.M.; Miller, S.R.; Zacur, H.A.; Gallicchio, L. Genetic polymorphisms in the aryl hydrocarbon receptor-signaling pathway and sleep disturbances in middle-aged women. Sleep Med. 2013, 14, 883–887. [Google Scholar] [CrossRef]
- Frau-Méndez, M.A.; Fernández-Vega, I.; Ansoleaga, B.; Tech, R.B.; Tech, M.C.; del Rio, J.A.; Zerr, I.; Llorens, F.; Zarranz, J.J.; Ferrer, I. Fatal familial insomnia: Mitochondrial and protein synthesis machinery decline in the mediodorsal thalamus. Brain Pathol. 2016, 27, 95–106. [Google Scholar] [CrossRef]
- Kishikawa, J.-I.; Inoue, Y.; Fujikawa, M.; Nishimura, K.; Nakanishi, A.; Tanabe, T.; Imamura, H.; Yokoyama, K. General anesthetics cause mitochondrial dysfunction and reduction of intracellular ATP levels. PLoS ONE 2018, 13, e0190213. [Google Scholar] [CrossRef]
- Wei, H. The role of calcium dysregulation in anesthetic-mediated neurotoxicity. Anesth. Analg. 2011, 113, 972–974. [Google Scholar] [CrossRef]
- Lee, H.-G.; Choi, S.-I.; Park, S.-K.; Park, S.-J.; Kim, N.-H.; Choi, E.-K. Alteration of glutathione metabolism and abnormal calcium accumulation in the mitochondria of hamster brain infected with scrapie agent. Neurobiol. Aging 2000, 21, 151. [Google Scholar] [CrossRef]
- Glatzel, M.; Sepulveda-Falla, D. Losing sleep over mitochondria: A new player in the pathophysiology of fatal familial insomnia. Brain Pathol. 2016, 27, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Park, S.-Y. Prion peptide-mediated calcium level alteration governs neuronal cell damage through AMPK-autophagy flux. Cell Commun. Signal. 2020, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, S.; Matarrese, P.; Ascione, B.; Ricci-Vitiani, L.; Pallini, R.; Villani, V.; Pace, A.; Paggi, M.G.; Abbruzzese, C. Chlorpromazine induces cytotoxic autophagy in glioblastoma cells via endoplasmic reticulum stress and unfolded protein response. J. Exp. Clin. Cancer Res. 2021, 40, 347. [Google Scholar] [CrossRef] [PubMed]
- Barreca, M.L.; Iraci, N.; Biggi, S.; Cecchetti, V.; Biasini, E. Pharmacological Agents Targeting the Cellular Prion Protein. Pathogens 2018, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Korth, C.; May, B.C.H.; Cohen, F.E.; Prusiner, S.B. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc. Natl. Acad. Sci. USA 2001, 98, 9836–9841. [Google Scholar] [CrossRef] [PubMed]
- Khattar, K.E.; Safi, J.; Rodriguez, A.-M.; Vignais, M.-L. Intercellular Communication in the Brain through Tunneling Nanotubes. Cancers 2022, 14, 1207. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gerdes, H.-H. Transfer of mitochondria via tunneling nanotubes rescues apoptotic PC12 cells. Cell Death Differ. 2015, 22, 1181–1191. [Google Scholar] [CrossRef]
- Savaskan, N.E.; Borchert, A.; Bräuer, A.U.; Kuhn, H. Role for glutathione peroxidase-4 in brain development and neuronal apoptosis: Specific induction of enzyme expression in reactive astrocytes following brain injury. Free Radic. Biol. Med. 2007, 43, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Mauri, S.; Favaro, M.; Bernardo, G.; Mazzotta, G.M.; Ziviani, E. Mitochondrial autophagy in the sleeping brain. Front. Cell Dev. Biol. 2022, 10, 956394. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Owens, G.C.; Crossin, K.L.; Edelman, D.B. Serotonin stimulates mitochondrial transport in hippocampal neurons. Mol. Cell. Neurosci. 2007, 36, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Nana, A.L.; Hepker, M.; Hwang, J.-H.L.; Gaus, S.E.; Spina, S.; Cosme, C.G.; Gan, L.; Grinberg, L.T.; Geschwind, D.H.; et al. Preferential tau aggregation in von Economo neurons and fork cells in frontotemporal lobar degeneration with specific MAPT variants. Acta Neuropathol. Commun. 2019, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Kato, T. The other, forgotten genome: Mitochondrial DNA and mental disorders. Mol. Psychiatry 2001, 6, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Course, M.M.; Wang, X. Transporting mitochondria in neurons. F1000Research 2016, 5, 1735. [Google Scholar] [CrossRef]
- Nuñez, M.T.; Chana-Cuevas, P. New Perspectives in Iron Chelation Therapy for the Treatment of Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 109. [Google Scholar] [CrossRef]
- Kupershmidt, L.; Youdim, M.B.H. The Neuroprotective Activities of the Novel Multi-Target Iron-Chelators in Models of Alzheimer’s Disease, Amyotrophic Lateral Sclerosis and Aging. Cells 2023, 12, 763. [Google Scholar] [CrossRef]
- Calderon-Montaño, J.M.; Burgos-Morón, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Du, Y.-Y.; Sun, T.; Yang, Q.; Liu, Q.-Q.; Li, J.-M.; Yang, L.; Luo, L.-X. Therapeutic Potential of Kaempferol against Sleep Deprivation-Induced Cognitive Impairment: Modulation of Neuroinflammation and Synaptic Plasticity Disruption in Mice. ACS Pharmacol. Transl. Sci. 2023, 6, 1934–1944. [Google Scholar] [CrossRef]
- Saleem, A.; Ain, Q.U.; Akhtar, M.F. Alternative Therapy of Psychosis: Potential Phytochemicals and Drug Targets in the Management of Schizophrenia. Front. Pharmacol. 2022, 13, 895668. [Google Scholar] [CrossRef]
- Zhou, M.; Ren, H.; Han, J.; Wang, W.; Zheng, Q.; Wang, D. Protective Effects of Kaempferol against Myocardial Ischemia/Reperfusion Injury in Isolated Rat Heart via Antioxidant Activity and Inhibition of Glycogen Synthase Kinase-3β. Oxidative Med. Cell. Longev. 2015, 2015, 481405. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Roh, M.-S. Glycogen Synthase Kinase-3 (GSK3) in Psychiatric Diseases and Therapeutic Interventions. Curr. Drug Targets 2006, 7, 1421–1434. [Google Scholar] [CrossRef]
- Mohammad, M.K.; Al-Masri, I.M.; Taha, M.O.; Al-Ghussein, M.A.; AlKhatib, H.S.; Najjar, S.; Bustanji, Y. Olanzapine inhibits glycogen synthase kinase-3beta: An investigation by docking simulation and experimental validation. Eur. J. Pharmacol. 2008, 584, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Shilovsky, G.A.; Putyatina, T.S.; Morgunova, G.V.; Seliverstov, A.V.; Ashapkin, V.V.; Sorokina, E.V.; Markov, A.V.; Skulachev, V.P. A Crosstalk between the Biorhythms and Gatekeepers of Longevity: Dual Role of Glycogen Synthase Kinase-3. Biochemistry 2021, 86, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Dozza, B.; Smith, M.A.; Perry, G.; Tabaton, M.; Strocchi, P. Regulation of glycogen synthase kinase-3beta by products of lipid peroxidation in human neuroblastoma cells. J. Neurochem. 2004, 89, 1224–1232. [Google Scholar] [CrossRef]
- Agmon, E.; Solon, J.; Bassereau, P.; Stockwell, B.R. Modeling the effects of lipid peroxidation during ferroptosis on membrane properties. Sci. Rep. 2018, 8, 5155. [Google Scholar] [CrossRef]
- Ohlendorf, B.; Schulz, D.; Erhard, A.; Nagel, K.; Imhoff, J.F. Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a streptomyces species. J. Nat. Prod. 2012, 75, 1400–1404. [Google Scholar] [CrossRef]
- Foster, D.J.; Bryant, Z.K.; Conn, P.J. Targeting muscarinic receptors to treat schizophrenia. Behav. Brain Res. 2021, 405, 113201. [Google Scholar] [CrossRef]
- Wang, X.; Abbas, M.; Zhang, Y.; Elshahawi, S.I.; Ponomareva, L.V.; Cui, Z.; Van Lanen, S.G.; Sajid, I.; Voss, S.R.; Shaaban, K.A.; et al. Baraphenazines A–G, Divergent Fused Phenazine-Based Metabolites from a Himalayan Streptomyces. J. Nat. Prod. 2019, 82, 1686–1693. [Google Scholar] [CrossRef]
- van Wezel, G.P.; McKenzie, N.L.; Nodwell, J.R. Chapter 5 Applying the genetics of secondary metabolism in model actino-mycetes to the discovery of new antibiotics. In Methods in Enzymology, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 458. [Google Scholar]
- Cha, J.W.; Lee, S.I.; Kim, M.C.; Thida, M.; Lee, J.W.; Park, J.-S.; Kwon, H.C. Pontemazines A and B, phenazine derivatives containing a methylamine linkage from Streptomyces sp. UT1123 and their protective effect to HT-22 neuronal cells. Bioorganic Med. Chem. Lett. 2015, 25, 5083–5086. [Google Scholar] [CrossRef]
- Krishnaiah, M.; Rodriguez de Almeida, N.; Udumula, V.; Song, Z.; Chhonker, Y.S.; Abdelmoaty, M.M.; Aragao do Nascimento, V.; Murry, D.J.; Conda-Sheridan, M. Synthesis, biological evaluation, and metabolic stability of phenazine derivatives as antibacterial agents. Eur. J. Med. Chem. 2018, 143, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Lavaggi, M.L.; Aguirre, G.; Boiani, L.; Orelli, L.; García, B.; Cerecetto, H.; González, M. Pyrimido[1,2-a]quinoxaline 6-oxide and phenazine 5,10-dioxide derivatives and related compounds as growth inhibitors of Trypanosoma cruzi. Eur. J. Med. Chem. 2008, 43, 1737–1741. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Shindo, K.; Yamagishi, Y.; Matsuoka, M.; Kawai, H.; Mochizuki, J. Phenazoviridin, a novel free radical scavenger from Streptomyces sp. taxonomy, fermentation, isolation, structure elucidation and biological properties. J. Antibiot. 1993, 46, 1485–1493. [Google Scholar] [CrossRef]
- Laxmi, M.; Bhat, S.G. Characterization of pyocyanin with radical scavenging and antibiofilm properties isolated from Pseudomonas aeruginosa strain BTRY1. 3 Biotech 2016, 6, 27. [Google Scholar] [CrossRef]
- Alatawneh, N.; Meijler, M.M. Unraveling the Antibacterial and Iron Chelating Activity ofN-Oxide Hydroxy-Phenazine natural Products and Synthetic Analogs againstStaphylococcus aureus. Isr. J. Chem. 2023, 63, e202200112. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Armistead, G.; Rosa, C.A.; Anderson, A.; Patil, R.; Cornett, E.M.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Phenothiazines and their Evolving Roles in Clinical Practice: A Narrative Review. Health Psychol. Res. 2022, 10, 38930. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, A.S.B.; Zanjani, A.A.H.; Klenow, M.B.; Mularski, A.; Sønder, S.L.; Lund, F.W.; Boye, T.L.; Dias, C.; Bendix, P.M.; Simonsen, A.C.; et al. Phenothiazines alter plasma membrane properties and sensitize cancer cells to injury by inhibiting annexin-mediated repair. J. Biol. Chem. 2021, 297, 101012. [Google Scholar] [CrossRef]
- Boonnoy, P.; Jarerattanachat, V.; Karttunen, M.; Wong-Ekkabut, J. Bilayer Deformation, Pores, and Micellation Induced by Oxidized Lipids. J. Phys. Chem. Lett. 2015, 6, 4884–4888. [Google Scholar] [CrossRef]
- Wu, C.-H.; Bai, L.-Y.; Tsai, M.-H.; Chu, P.-C.; Chiu, C.-F.; Chen, M.Y.; Chiu, S.-J.; Chiang, J.-H.; Weng, J.-R. Pharmacological exploitation of the phenothiazine antipsychotics to develop novel antitumor agents–A drug repurposing strategy. Sci. Rep. 2016, 6, 27540. [Google Scholar] [CrossRef]
- Voronova, O.; Zhuravkov, S.; Korotkova, E.; Artamonov, A.; Plotnikov, E. Antioxidant Properties of New Phenothiazine Derivatives. Antioxidants 2022, 11, 1371. [Google Scholar] [CrossRef]
- Keynes, R.G.; Karchevskaya, A.; Riddall, D.; Griffiths, C.H.; Bellamy, T.C.; Chan, A.W.E.; Selwood, D.L.; Garthwaite, J. N10-carbonyl-substituted phenothiazines inhibiting lipid peroxidation and associated nitric oxide consumption powerfully protect brain tissue against oxidative stress. Chem. Biol. Drug Des. 2019, 94, 1680–1693. [Google Scholar] [CrossRef]
- Iuga, C.; Campero, A.; Vivier-Bunge, A. Antioxidant vs. prooxidant action of phenothiazine in a biological environment in the presence of hydroxyl and hydroperoxyl radicals: A quantum chemistry study. RSC Adv. 2015, 5, 14678–14689. [Google Scholar] [CrossRef]
- Yue, Y.; Kong, L.; Wang, J.; Li, C.; Tan, L.; Su, H.; Xu, Y. Regional Abnormality of Grey Matter in Schizophrenia: Effect from the Illness or Treatment? PLoS ONE 2016, 11, e0147204. [Google Scholar] [CrossRef]
- Martínez, A.; Ibarra, I.A.; Vargas, R. A quantum chemical approach representing a new perspective concerning agonist and antagonist drugs in the context of schizophrenia and Parkinson’s disease. PLoS ONE 2019, 14, e0224691. [Google Scholar] [CrossRef]
- Goode-Romero, G.; Dominguez, L.; Martínez, A. Electron Donor–Acceptor Properties of Different Muscarinic Ligands: On the Road to Control Schizophrenia. J. Chem. Inf. Model. 2021, 61, 5117–5124. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.C.; Andreasen, N.C.; Ziebell, S.; Pierson, R.; Magnotta, V. Long-term antipsychotic treatment and brain volumes: A longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry 2011, 68, 128–137. [Google Scholar] [CrossRef]
- Engwa, G.A.; Ayuk, E.L.; Igbojekwe, B.U.; Unaegbu, M. Potential Antioxidant Activity of New Tetracyclic and Pentacyclic Nonlinear Phenothiazine Derivatives. Biochem. Res. Int. 2016, 2016, 9896575. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Currais, A.; Soriano-Castell, D.; Schubert, D.; Maher, P. Natural products targeting mitochondria: Emerging therapeutics for age-associated neurological disorders. Pharmacol. Ther. 2021, 221, 107749. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Drużga, A.; Katarzyna, J.; Skonieczna-Żydecka, K. Antioxidant Potential of Curcumin—A Meta-Analysis of Randomized Clinical Trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.-D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Geng, Y.-N.; Jiang, J.-D.; Kong, W.-J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid. Based Complement. Altern. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Frankel, E.N. The antioxidant and nutritional effects of tocopherols, ascorbic acid and beta-carotene in relation to processing of edible oils. Bibl. Nutr. Dieta 1989, 43, 297–312. [Google Scholar] [CrossRef]
- Liu, D.; Gao, Y.; Liu, J.; Huang, Y.; Yin, J.; Feng, Y.; Shi, L.; Meloni, B.P.; Zhang, C.; Zheng, M.; et al. Intercellular mitochondrial transfer as a means of tissue revitalization. Signal Transduct. Target. Ther. 2021, 6, 65. [Google Scholar] [CrossRef]
- Katrangi, E.; D’Souza, G.; Boddapati, S.V.; Kulawiec, M.; Singh, K.K.; Bigger, B.; Weissig, V. Xenogenic transfer of isolated murine mitochondria into human rho0 cells can improve respiratory function. Rejuvenation Res. 2007, 10, 561–570. [Google Scholar] [CrossRef]
- Pacak, C.A.; Preble, J.M.; Kondo, H.; Seibel, P.; Levitsky, S.; del Nido, P.J.; Cowan, D.B.; McCully, J.D. Actin-dependent mitochondrial internalization in cardiomyocytes: Evidence for rescue of mitochondrial function. Biol. Open 2015, 4, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Schneider, R.T.; McConnell, M.J. Mitochondrial Transfer from Astrocytes to Neurons following Ischemic Insult: Guilt by Association? Cell Metab. 2016, 24, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Pour, P.A.; Hosseinian, S.; Kheradvar, A. Mitochondrial transplantation in cardiomyocytes: Foundation, methods, and outcomes. Am. J. Physiol. Cell Physiol. 2021, 321, C489–C503. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.B.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; del Nido, P.J.; McCully, J.D. Transit and fusion of exogenous mitochondria in human heart cells. Nat. Sci. Rep. 2017, 7, 17450. [Google Scholar] [CrossRef]
- Zhang, T.-G.; Miao, C.-Y. Mitochondrial transplantation as a promising therapy for mitochondrial diseases. Acta Pharm. Sin. B 2023, 13, 1028–1035. [Google Scholar] [CrossRef]
- Scheiblich, H.; Dansokho, C.; Mercan, D.; Schmidt, S.V.; Bousset, L.; Wischhof, L.; Eikens, F.; Odainic, A.; Spitzer, J.; Griep, A.; et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell 2021, 184, 5089–5106.e21. [Google Scholar] [CrossRef]
- Geng, Z.; Guan, S.; Wang, S.; Yu, Z.; Liu, T.; Du, S.; Zhu, C. Intercellular mitochondrial transfer in the brain, a new perspec-tive for targeted treatment of central nervous system diseases. CNS Neurosci. Ther. 2023, 29, 3121–3135. [Google Scholar] [CrossRef]
- Ren, D.; Zheng, P.; Zou, S.; Gong, Y.; Wang, Y.; Duan, J.; Deng, J.; Chen, H.; Feng, J.; Zhong, C.; et al. GJA1-20K Enhances Mitochondria Transfer from Astrocytes to Neurons via Cx43-TnTs After Traumatic Brain Injury. Cell. Mol. Neurobiol. 2021, 42, 1887–1895. [Google Scholar] [CrossRef]




| Antioxidants | Source | References |
|---|---|---|
| Lycopene | Grape skin, guava, grapefruit, blueberries, tomatoes | [193] |
| Apigenin | Cabbage, blueberries, acai berries | [194] |
| Phenolic acid | Oilseeds, cereals, grains | [195] |
| Curcumin | chicken, beef, tofu, vegetables | [196] |
| Epigallocatechin gallate | Apples, blackberries, broad beans, cherries, black grapes, pears, raspberries, and chocolate | [197] |
| Berberine | Oregon grape, phellodendron, and tree turmeric. | [198] |
| Quercetin | Fruits, apples, onions, parsley, sage, tea, and red wine | [199] |
| Kempferol | Fruits and vegetables. | [200] |
| Tocopherols | Oilseed, cereals, eggs, deary products | [201] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfera, A.; Thomas, K.A.; Ogunjale, I.A.; Jafri, N.; Bota, P.G. Insomnia in Forensic Detainees: Is Salience Network the Common Pathway for Sleep, Neuropsychiatric, and Neurodegenerative Disorders? J. Clin. Med. 2024, 13, 1691. https://doi.org/10.3390/jcm13061691
Sfera A, Thomas KA, Ogunjale IA, Jafri N, Bota PG. Insomnia in Forensic Detainees: Is Salience Network the Common Pathway for Sleep, Neuropsychiatric, and Neurodegenerative Disorders? Journal of Clinical Medicine. 2024; 13(6):1691. https://doi.org/10.3390/jcm13061691
Chicago/Turabian StyleSfera, Adonis, Kyle A. Thomas, Isaac A. Ogunjale, Nyla Jafri, and Peter G. Bota. 2024. "Insomnia in Forensic Detainees: Is Salience Network the Common Pathway for Sleep, Neuropsychiatric, and Neurodegenerative Disorders?" Journal of Clinical Medicine 13, no. 6: 1691. https://doi.org/10.3390/jcm13061691