The Impact of Progressive Pulmonary Fibrosis in Systemic Sclerosis–Associated Interstitial Lung Disease
Abstract
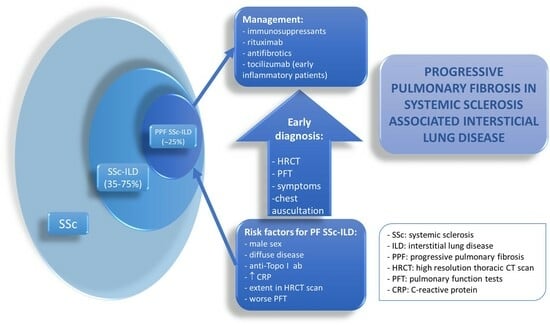
:1. Introduction
2. Identification of Patients with Progressive Fibrosing SSc-ILD and Diagnosis
3. Definition of PPF
4. Risk Factors for Progressive SSc-ILD
5. PPF Treatment in SSc-ILD Patients
6. Future Research Agenda
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffmann-Vold, A.M.; Fretheim, H.; Halse, A.K.; Seip, M.; Bitter, H.; Wallenius, M.; Garen, T.; Salberg, A.; Brunborg, C.; Midtvedt, Ø.; et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am. J. Respir. Crit. Care Med. 2019, 200, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Walker, U.A.; Tyndall, A.; Czirjak, L.; Denton, C.; Farge-Bancel, D.; Kowal-Bielecka, O.; Müller-Ladner, U.; Bocelli-Tyndall, C.; Matucci-Cerinic, M. Clinical risk assessment of organ manifestations in systemic sclerosis: A report from the EULAR Scleroderma Trials and Research group database. Ann. Rheum. Dis. 2007, 66, 754–763. [Google Scholar] [CrossRef]
- Bruni, C.; Campochiaro, C.; de Vries-Bouwstra, J.K. Interstitial Lung Disease: How Should Therapeutics Be Implemented? Rheum. Dis. Clin. N. Am. 2023, 49, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Vold, A.M.; Allanore, Y.; Alves, M.; Brunborg, C.; Airó, P.; Ananieva, L.P.; Czirják, L.; Guiducci, S.; Hachulla, E.; Li, M.; et al. Progressive interstitial lung disease in patients with systemic sclerosis-associated interstitial lung disease in the EUSTAR database. Ann. Rheum. Dis. 2021, 80, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Cottin, V. Treatment of progressive fibrosing interstitial lung diseases: A milestone in the management of interstitial lung diseases. Eur. Respir. Rev. 2019, 28, 190109. [Google Scholar] [CrossRef]
- Tashkin, D.P.; Roth, M.D.; Clements, P.J.; Furst, D.E.; Khanna, D.; Kleerup, E.C.; Goldin, J.; Arriola, E.; Volkmann, E.R.; Kafaja, S.; et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): A randomised controlled, double-blind, parallel group trial. Lancet Respir. Med. 2016, 4, 708–719. [Google Scholar] [CrossRef]
- Distler, O.; Highland, K.B.; Gahlemann, M.; Azuma, A.; Fischer, A.; Mayes, M.D.; Raghu, G.; Sauter, W.; Girard, M.; Alves, M.; et al. Nintedanib for systemic sclerosis associated interstitial lung disease. N. Engl. J. Med. 2019, 380, 2518–2528. [Google Scholar] [CrossRef]
- Maher, T.M.; Tudor, V.A.; Saunders, P.; Gibbons, M.A.; Fletcher, S.V.; Denton, C.P.; Hoyles, R.K.; Parfrey, H.; Renzoni, E.A.; Kokosi, M.; et al. Rituximab versus intravenous cyclophosphamide in patients with connective tissue disease-associated interstitial lung disease in the UK (RECITAL): A double-blind, double-dummy, randomised, controlled, phase 2b trial. Lancet Respir. Med. 2023, 11, 45–54. [Google Scholar] [CrossRef]
- Khanna, D.; Lin, C.J.F.; Furst, D.E.; Goldin, J.; Kim, G.; Kuwana, M.; Allanore, Y.; Matucci-Cerinic, M.; Distler, O.; Shima, Y.; et al. Tocilizumab in systemic sclerosis: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2020, 8, 963–974. [Google Scholar] [CrossRef]
- Hoffmann-Vold, A.-M.; Maher, T.M.; Philpot, E.E.; Ashrafzadeh, A.; Barake, R.; Barsotti, S.; Bruni, C.; Carducci, P.; Carreira, P.E.; Castellví, I.; et al. The identification and management of interstitial lung disease in systemic sclerosis: Evidence-based European consensus statements. Lancet Rheumatol. 2020, 2, e71–e83. [Google Scholar] [CrossRef]
- Roofeh, D.; Khanna, D. Management of systemic sclerosis: The first five years. Curr. Opin Rheumatol. 2020, 32, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Wijsenbeek, M.; Kreuter, M.; Olson, A.; Fischer, A.; Bendstrup, E.; Wells, C.D.; Denton, C.P.; Mounir, B.; Zouad-Lejour, L.; Quaresma, M.; et al. Progressive fibrosing interstitial lung diseases: Current practice in diagnosis and management. Curr. Med. Res. Opin. 2019, 35, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Distler, O.; Assassi, S.; Cottin, V.; Cutolo, M.; Danoff, S.K.; Denton, C.P.; Distler, J.H.W.; Hoffmann-Vold, A.M.; Johnson, S.R.; Müller Ladner, U.; et al. Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur. Respir. J. 2020, 55, 1902026. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Scirè, C.A.; Talarico, R.; Airo, P.; Alexander, T.; Allanore, Y.; Bruni, C.; Codullo, V.; Dalm, V.; De Vries-Bouwstra, J.; et al. Systemic sclerosis: State of the art on clinical practice guidelines. RMD Open 2018, 4 (Suppl. 1), e000782. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Hughes, M.; Gak, N.; Vila, J.; Buch, M.H.; Chakravarty, K.; BSR and BHPR Standards, Guidelines and Audit Working Group. BSR and BHPR guideline for the treatment of systemic sclerosis. Rheumatology 2016, 55, 1906–1910. [Google Scholar] [CrossRef]
- Suliman, Y.A.; Dobrota, R.; Huscher, D.; Nguyen-Kim, T.D.; Maurer, B.; Jordan, S.; Speich, R.; Frauenfelder, T.; Distler, O. Brief report: Pulmonary function tests: High rate of false-negative results in the early detection and screening of scleroderma-related interstitial lung disease. Arthritis Rheumatol. 2015, 67, 3256–3261. [Google Scholar] [CrossRef]
- Bernstein, E.J.; Jaafar, S.; Assassi, S.; Domsic, R.T.; Frech, T.M.; Gordon, J.K.; Broderick, R.J.; Hant, F.N.; Hinchcliff, M.E.; Shah, A.A.; et al. Performance characteristics of pulmonary function tests for the detection of interstitial lung disease in adults with early diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2020, 72, 1892–1896. [Google Scholar] [CrossRef]
- Khanna, D.; Lescoat, A.; Roofeh, D.; Bernstein, E.J.; Kazerooni, E.A.; Roth, M.D.; Martinez, F.; Flaherty, K.R.; Denton, C.P. Systemic sclerosis-associated interstitial lung disease: How to incorporate two food and drug administration-approved therapies in clinical practice. Arthritis Rheumatol. 2022, 74, 13–27. [Google Scholar] [CrossRef]
- De Lauretis, A.; Sestini, P.; Pantelidis, P.; Hoyles, R.; Hansell, D.M.; Goh, N.S.; Zappala, C.J.; Visca, D.; Maher, T.M.; Denton, C.P.; et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J. Rheumatol. 2013, 40, 435–446. [Google Scholar] [CrossRef]
- Elhai, M.; Hoffmann-Vold, A.M.; Avouac, J.; Pezet, S.; Cauvet, A.; Leblond, A.; Fretheim, H.; Garen, T.; Kuwana, M.; Molberg, Ø.; et al. Performance of Candidate Serum Biomarkers for Systemic Sclerosis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2007, 56, 1685–1693. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Tashkin, D.P.; Kuwana, M.; Li, N.; Roth, M.D.; Charles, J.; Hant, F.N.; Bogatkevich, G.S.; Akter, T.; Kim, G.; et al. Progression of Interstitial Lung Disease in Systemic Sclerosis: The Importance of Pneumoproteins Krebs von den Lungen 6 and CCL18. Arthritis Rheumatol. 2019, 71, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Goh, N.S.; Hoyles, R.K.; Denton, C.P.; Hansell, D.M.; Renzoni, E.A.; Maher, T.M.; Nicholson, A.G.; Wells, A.U. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol. 2017, 69, 1670–1678. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Richeldi, L.; Thomson, C.C.; Inoue, Y.; Johkoh, T.; Kreuter, M.; Lynch, D.A.; Maher, T.M.; Martinez, F.J.; et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2022, 205, e18–e47. [Google Scholar] [CrossRef]
- Flaherty, K.R.; Wells, A.U.; Cottin, V.; Devaraj, A.; Walsh, S.L.F.; Inoue, Y.; Richeldi, L.; Kolb, M.; Tetzlaff, K.; Stowasser, S.; et al. Nintedanib in Progressive Fibrosing Interstitial Lung Diseases. N. Engl. J. Med. 2019, 381, 1718–1727. [Google Scholar] [CrossRef]
- Cottin, V.; Hirani, N.A.; Hotchkin, D.L.; Nambiar, A.M.; Ogura, T.; Otaola, M.; Skowasch, D.; Park, J.S.; Poonyagariyagorn, H.K.; Wuyts, W.; et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur. Respir. Rev. 2018, 27, 180076. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Mittoo, S.; Aggarwal, R.; Proudman, S.M.; Dalbeth, N.; Matteson, E.L.; Brown, K.; Flaherty, K.; Wells, A.U.; Seibold, J.R.; et al. Connective Tissue Disease-associated Interstitial Lung Diseases (CTD-ILD)—Report from OMERACT CTD-ILD Working Group. J. Rheumatol. 2015, 42, 2168–2171. [Google Scholar] [CrossRef]
- Peoples, C.; Medsger, T.A., Jr.; Lucas, M.; Rosario, B.L.; Feghali-Bostwick, C.A. Gender differences in systemic sclerosis: Relationship to clinical features, serologic status and outcomes. J. Scleroderma Relat. Disord. 2016, 1, 177–240. [Google Scholar] [CrossRef]
- Behr, J.; Prasse, A.; Kreuter, M.; Johow, J.; Rabe, K.F.; Bonella, F.; Bonnet, R.; Grohe, C.; Held, M.; Wilkens, H.; et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 476–486. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Tashkin, D.P.; Silver, R.; Feghali-Bostwick, C.A.; Assassi, S.; Baker Frost, D.; Leng, M.; Wilhalme, H.; Kim, G.H.; Goldin, J.; et al. Sex differences in clinical outcomes and biological profiles in systemic sclerosis-associated interstitial lung disease: A post-hoc analysis of two randomised controlled trials. Lancet Rheumatol. 2022, 4, e668–e678. [Google Scholar] [CrossRef]
- Morgan, N.D.; Shah, A.A.; Mayes, M.D.; Domsic, R.T.; Medsger, T.A., Jr.; Steen, V.D.; Varga, J.; Carns, M.; Ramos, P.S.; Silver, R.M.; et al. Clinical and serological features of systemic sclerosis in a multicenter African American cohort: Analysis of the genome research in African American scleroderma patients clinical database. Medicine 2017, 96, e8980. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Steen, V.; Li, N.; Roth, M.D.; Clements, P.J.; Furst, D.E.; Assassi, S.; Khanna, D.; Kim, G.J.; Goldin, J.; et al. Racial disparities in systemic sclerosis: Short- and long-term outcomes among African American participants of SLS I and II. ACR Open Rheumatol. 2021, 3, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Salazar, G.A.; Kuwana, M.; Wu, M.; Estrada-Y-Martin, R.M.; Ying, J.; Charles, J.; Mayes, M.D.; Assassi, S. KL-6 but not CCL-18 is a predictor of early progression in systemic sclerosis-related interstitial lung disease. J. Rheumatol. 2018, 45, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; Wilhalme, H.; Assassi, S.; Kim, G.H.J.; Goldin, J.; Kuwana, M.; Tashkin, D.P.; Roth, M.D. Combining Clinical and Biological Data to Predict Progressive Pulmonary Fibrosis in Patients with Systemic Sclerosis Despite Immunomodulatory Therapy. ACR Open Rheumatol. 2023, 5, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, C.; Hoffmann-Vold, A.M.; Avouac, J.; Henes, J.; de Vries-Bouwstra, J.; Smith, V.; Siegert, E.; Airò, P.; Oksel, F.; Pellerito, R.; et al. Sex influence on outcomes of patients with systemic sclerosis-associated interstitial lung disease: A EUSTAR database analysis. Rheumatology 2023, 62, 2483–2491. [Google Scholar] [CrossRef]
- Moinzadeh, P.; Kuhr, K.; Siegert, E.; Mueller-Ladner, U.; Riemekasten, G.; Günther, C.; Kötter, I.; Henes, J.; Blank, N.; Zeidler, G.; et al. Registry of the German Network for Systemic Scleroderma. Older age onset of systemic sclerosis—Accelerated disease progression in all disease subsets. Rheumatology 2020, 59, 3380–3389. [Google Scholar] [CrossRef]
- Nihtyanova, S.I.; Schreiber, B.E.; Ong, V.H.; Rosenberg, D.; Moinzadeh, P.; Coghlan, J.G.; Wells, A.U.; Denton, C.P. Prediction of pulmonary complications and long-term survival in systemic sclerosis. Arthritis Rheumatol. 2014, 66, 1625–1635. [Google Scholar] [CrossRef]
- Volkmann, E.R. Natural history of systemic sclerosis–related interstitial lung disease: How to identify a progressive fibrosing phenotype. J. Scleroderma Relat. Disord. 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Winstone, T.A.; Assayag, D.; Wilcox, P.G.; Dunne, J.V.; Hague, C.J.; Leipsic, J.; Collard, H.R.; Ryerson, C.J. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: A systematic review. Chest 2014, 146, 422–436. [Google Scholar] [CrossRef]
- Le Gouellec, N.; Duhamel, A.; Perez, T.; Hachulla, A.L.; Sobanski, V.; Faivre, J.B.; Morell-Dubois, S.; Lambert, M.; Hatron, P.Y.; Hachulla, E.; et al. Predictors of lung function test severity and outcome in systemic sclerosis-associated interstitial lung disease. PLoS ONE 2017, 12, e0181692. [Google Scholar] [CrossRef]
- Takei, R.; Arita, M.; Kumagai, S.; Ito, Y.; Tokioka, F.; Koyama, T.; Saito, R.; Nishimura, K.; Tokumasu, H.; Ishida, T. Radiographic fibrosis score predicts survival in systemic sclerosis-associated interstitial lung disease. Respirology 2018, 23, 385–391. [Google Scholar] [CrossRef]
- Lee, J.S. The role of gastroesophageal reflux and microaspiration in idiopathic pulmonary fibrosis. Clin. Pulm. Med. 2014, 21, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, J.N.; Carlson, D.A.; Hinchcliff, M.; Carns, M.A.; Aren, K.A.; Lee, J.; Pandolfino, J.E. The association between systemic sclerosis disease manifestations and esophageal high resolution manometry parameters. Neurogastroenterol. Motil. 2016, 28, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Ryu, J.H.; Elicker, B.M.; Lydell, C.P.; Jones, K.D.; Wolters, P.J.; King, T.E., Jr.; Collard, H.R. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 184, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Elhaj, M.; Charles, J.; Pedroza, C.; Liu, X.; Zhou, X.; Estrada-Y-Martin, R.M.; Gonzalez, E.B.; Lewis, D.E.; Draeger, H.T.; Kim, S.; et al. Can serum surfactant protein D or CC-chemokine ligand 18 predict outcome of interstitial lung disease in patients with early systemic sclerosis? J. Rheumatol. 2013, 40, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Odani, T.; Yasuda, S.; Ota, Y.; Fujieda, Y.; Kon, Y.; Horita, T.; Kawaguchi, Y.; Atsumi, T.; Yamanaka, H.; Koike, T. Up-regulated expression of HLA-DRB5 transcripts and high frequency of the HLA-DRB5*01:05 allele in scleroderma patients with interstitial lung disease. Rheumatology 2012, 51, 1765–1774. [Google Scholar] [CrossRef]
- Usategui, A.; Municio, C.; Arias-Salgado, E.G.; Martín, M.; Fernández-Varas, B.; Del Rey, M.J.; Carreira, P.; González, A.; Criado, G.; Perona, R.; et al. Evidence of telomere attrition and a potential role for DNA damage in systemic sclerosis. Immun. Ageing 2022, 19, 7. [Google Scholar] [CrossRef]
- Jung, S.M.; Park, K.S.; Kim, K.J. Integrative analysis of lung molecular signatures reveals key drivers of systemic sclerosis-associated interstitial lung disease. Ann. Rheum. Dis. 2022, 81, 108–116. [Google Scholar] [CrossRef]
- Molina-Molina, M.; Castellví, I.; Valenzuela, C.; Ramirez, J.; Rodríguez Portal, J.A.; Franquet, T.; Narváez, J. Management of progressive pulmonary fibrosis associated with connective tissue disease. Expert. Rev. Respir. Med. 2022, 16, 765–774. [Google Scholar] [CrossRef]
- Kowal-Bielecka, O.; Fransen, J.; Avouac, J.; Becker, M.; Kulak, A.; Allanore, Y.; Distler, O.; Clements, P.; Cutolo, M.; Czirjak, L.; et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1327–1339. [Google Scholar] [CrossRef]
- Denton, C.P.; De Lorenzis, E.; Roblin, E.; Goldman, N.; Alcacer-Pitarch, B.; Blamont, E.; Buch, M.; Carulli, M.; Cotton, C.; Del Galdo, F.; et al. Management of systemic sclerosis: British Society for Rheumatology guideline scope. Rheumatol. Adv. Pract. 2023, 7, rkad022. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Tashkin, D.P.; Li, N.; Roth, M.D.; Khanna, D.; Hoffmann-Vold, A.M.; Kim, G.; Goldin, J.; Clements, P.J.; Furst, D.E.; et al. Mycophenolate mofetil versus placebo for systemic sclerosis-related interstitial lung disease: An analysis of scleroderma lung studies I and II. Arthritis Rheumatol. 2017, 69, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Elashoff, R.; Clements, P.J.; Goldin, J.; Roth, M.D.; Furst, D.E.; Arriola, E.; Silver, R.; Strange, C.; Bolster, M.; et al. Cyclophosphamide versus placebo in scleroderma lung disease. N. Engl. J. Med. 2006, 354, 2655–2666. [Google Scholar] [CrossRef] [PubMed]
- Tashkin, D.P.; Elashoff, R.; Clements, P.J.; Roth, M.D.; Furst, D.E.; Silver, R.M.; Goldin, J.; Arriola, E.; Strange, C.; Bolster, M.B.; et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am. J. Respir. Crit. Care Med. 2007, 176, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Roofeh, D.; Lin, C.J.F.; Goldin, J.; Kim, G.H.; Furst, D.E.; Denton, C.P.; Huang, S.; Khanna, D.; focuSSced Investigators. Tocilizumab prevents progression of early systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol. 2021, 73, 1301–1310. [Google Scholar] [CrossRef]
- Khanna, D.; Denton, C.P. Integrating new therapies for systemic sclerosis associated lung fibrosis in clinical practice. Lancet Respir. Med. 2021, 9, 560–562. [Google Scholar] [CrossRef]
- Sullivan, K.M.; Goldmuntz, E.A.; Keyes-Elstein, L.; McSweeney, P.A.; Pinckney, A.; Welch, B.; Mayes, M.D.; Nash, R.A.; Crofford, L.J.; Eggleston, B.; et al. Myeloablative Autologous Stem-Cell Transplantation for Severe Scleroderma. N. Engl. J. Med. 2018, 378, 35–47. [Google Scholar] [CrossRef]
- Ciaffi, J.; van Leeuwen, N.M.; Boonstra, M.; Kroft, L.J.M.; Schouffoer, A.A.; Ninaber, M.K.; Huizinga, T.W.J.; de Vries-Bouwstra, J.K. Evolution of systemic sclerosis associated interstitial lung disease one year after hematopoietic stem cell transplantation or cyclophosphamide. Arthritis Care Res. 2022, 74, 433–441. [Google Scholar] [CrossRef]
- Pradère, P.; Tudorache, I.; Magnusson, J.; Savale, L.; Brugiere, O.; Douvry, B.; Reynaud-Gaubert, M.; Claustre, J.; Borgne, A.L.; Holm, A.M.; et al. Lung transplantation for scleroderma lung disease: An international, multicenter, observational cohort study. J. Heart Lung Transplant. 2018, 37, 903–911. [Google Scholar] [CrossRef]
- Perelas, A.; Silver, R.M.; Arrossi, A.V.; Highland, K.B. Systemic sclerosis-associated interstitial lung disease. Lancet Respir. Med. 2020, 8, 304–320. [Google Scholar] [CrossRef]
- Farina, N.; Benanti, G.; De Luca, G.; Palmisano, A.; Peretto, G.; Tomassetti, S.; Giorgione, V.; Forma, O.; Esposito, A.; Danese, S.; et al. The Role of the Multidisciplinary Health Care Team in the Management of Patients with Systemic Sclerosis. J. Multidiscip. Healthc. 2022, 15, 815–824. [Google Scholar] [CrossRef]
- Cottin, V.; Martinez, F.J.; Jenkins, R.G.; Belperio, J.A.; Kitamura, H.; Molina-Molina, M.; Tschoepe, I.; Coeck, C.; Lievens, D.; Costabel, U. Safety and tolerability of nintedanib in patients with progressive fibrosing interstitial lung diseases: Data from the randomized controlled INBUILD trial. Respir. Res. 2022, 23, 85. [Google Scholar] [CrossRef] [PubMed]

| Diagnostic Criteria | Definition of Progression | Time Period Progression is Assessed | ||
|---|---|---|---|---|
| Lung Function | Symptoms | Chest CT | ||
| Pirfenidone in progressive non-IPF ILD (RELIEF trial) [28] | FVC ≥ 5% decline (absolute) | Within up to 24 months | ||
| Nintedanib in progressive non-IPF ILD (INBUILD trial) [24] | FVC ≥ 10% decline (relative); or FVC 5–10% decline and worsening of respiratory symptoms or increased extent of fibrosis on HRCT; or worsening of respiratory symptoms and increased extent of fibrosis on HRCT | Within 24 months | ||
| ATS/ERS/JRS/ALAT clinical practice guidelines [23] | At least two of the following three criteria occurred within the past year:
|
|
| Within 1 year of follow-up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-López, M.; Carreira, P.E. The Impact of Progressive Pulmonary Fibrosis in Systemic Sclerosis–Associated Interstitial Lung Disease. J. Clin. Med. 2023, 12, 6680. https://doi.org/10.3390/jcm12206680
Martín-López M, Carreira PE. The Impact of Progressive Pulmonary Fibrosis in Systemic Sclerosis–Associated Interstitial Lung Disease. Journal of Clinical Medicine. 2023; 12(20):6680. https://doi.org/10.3390/jcm12206680
Chicago/Turabian StyleMartín-López, María, and Patricia E. Carreira. 2023. "The Impact of Progressive Pulmonary Fibrosis in Systemic Sclerosis–Associated Interstitial Lung Disease" Journal of Clinical Medicine 12, no. 20: 6680. https://doi.org/10.3390/jcm12206680