Intracerebral Electrophysiological Recordings to Understand the Neural Basis of Human Face Recognition
Abstract
:1. Introduction: Human Face Recognition Investigated with Intracerebral Recordings
- (1)
- Studies are performed in large groups of implanted patients. In mapping studies of face recognition described below, all patients with at least one electrode in the ventral occipito-temporal cortex (VOTC, including the occipital lobe (OCC), Posterior Temporal Lobe (PTL), and Anterior Temporal Lobe (ATL) (Figure 1C)) are included, with samples of up to, e.g., N = 121 patients for some studies [37].
- (2)
- Patients are well characterized in neuropsychological functions, in particular regarding their ability to recognize face identity [38]. Unfortunately, this critical issue is neglected most of the time in iEEG studies of face recognition or other cognitive functions in general.
- (3)
- The use of face and nonface stimuli that are carefully controlled to eliminate or minimize the contributions of unreliable low-level sensory features. A ‘natural’ control to isolate high-level face recognition processes (e.g., through stimulus inversion or variability of low-level sensory features in the stimuli grouped in single condition) is often preferred to artificial procedures of global normalization or elimination of visual cues that degrade visual stimuli and may paradoxically increase low-level confounds by making local cues highly salient (e.g., [39]).
- (4)
- The use of tasks that are relatively easy for the patients, with implicit measures (i.e., tasks that do not require explicit recognition of faces).
- (5)
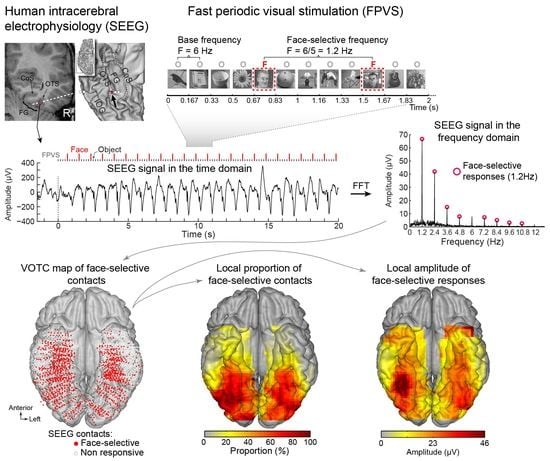
- Finally, a key component of this research program is the presentation of stimuli at periodic (i.e., fixed frequency) rates, relatively rapidly, e.g., six images/second during stimulation sequences of about 1 min. Given the periodic stimulation mode, the brain’s electrophysiological responses to the stimulation can be measured and quantified in the frequency domain. This fast periodic visual stimulation (FPVS) approach, is also referred to as “steady-state visual evoked potentials” (SSVEP; [40]) or “frequency-tagging” [41]. However, besides referring to the type of response rather than the approach, SSVEP is a loaded term, which suggests that there is an inherent difference between SSVEP and standard event-related potentials (ERPs) (e.g., see [42]). In reality, an SSVEP is no more than an ERP evoked periodically and expressed in the frequency-domain. When the temporal distance between periodically evoked ERPs is too small, they tend to overlap to the point where the response may appear as an externally-evoked “oscillation” [43]. This creates substantial confusion, with the frequent claim that SSVEP is an oscillatory brain response (with the same frequency as the flickering stimulus) that results from “entrainment” of spontaneous EEG oscillations (e.g., [44,45,46,47,48,49]). This interpretation is speculative and not necessary. The most parsimonious account of the fast periodic responses (i.e., superimposition with or without temporal overlap of ERPs; [43,50,51] is preferred here). Regardless, this approach provides objectivity in the identification of the response of interest (i.e., exactly at the frequency known by the experimenter) and its quantification (amplitude in the frequency-domain exactly at the stimulation frequency). The technique is also associated with high sensitivity (i.e., high signal-to-noise ratio, SNR), which is important even when recording large responses inside the brain (as compared to weaker responses in scalp recordings) (see [52,53] for reviews).
2. A Cartography of Human Face Recognition with SEEG
3. Putting Findings into Context

4. Implications for Understanding Face Recognition
5. Human Specificity
6. Contrasting Categories
6.1. Face-Selective vs. House-Selective Responses
6.2. Face-Selective vs. Word-Selective Responses
7. Face Identity Recognition
8. Summary, Conclusions, and Perspectives
8.1. Spatial Maps of Human Face Recognition
8.2. General Implications
8.3. Limitations and Challenges for Future Studies

Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruce, V.; Young, A. Understanding Face Recognition. Br. J. Psychol. 1986, 77, 305–327. [Google Scholar] [CrossRef] [PubMed]
- McNeill, D. The Face: A Natural History; Back Bay Books: New York, NY, USA, 2000; ISBN 9780316588126. [Google Scholar]
- O’Toole, A.J.; Vetter, T.; Blanz, V. Three-Dimensional Shape and Two-Dimensional Surface Reflectance Contributions to Face Recognition: An Application of Three-Dimensional Morphing. Vision Res. 1999, 39, 3145–3155. [Google Scholar] [CrossRef] [Green Version]
- Valentine, T.; Lewis, M.B.; Hills, P.J. Face-Space: A Unifying Concept in Face Recognition Research. Q. J. Exp. Psychol. (Hove) 2016, 69, 1996–2019. [Google Scholar] [CrossRef] [Green Version]
- Carey, S. Becoming a Face Expert. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1992, 335, 93–95. [Google Scholar] [CrossRef] [Green Version]
- Hills, P.J.; Lewis, M.B. The Development of Face Expertise: Evidence for a Qualitative Change in Processing. Cogn. Dev. 2018, 48, 1–18. [Google Scholar] [CrossRef]
- Golarai, G.; Liberman, A.; Grill-Spector, K. Experience Shapes the Development of Neural Substrates of Face Processing in Human Ventral Temporal Cortex. Cereb. Cortex 2017, 27, 1229–1244. [Google Scholar] [CrossRef] [Green Version]
- Mondloch, C.J.; Geldart, S.; Maurer, D.; Le Grand, R. Developmental Changes in Face Processing Skills. J. Exp. Child Psychol. 2003, 86, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B. What Makes Us Human? Face Identity Recognition. In Routledge Handbook of Neurosemiotics; Garcia, A.M., Ibanez, A., Eds.; Routledge: New York, NY, USA, 2022; pp. 325–345. [Google Scholar]
- Rossion, B.; Taubert, J. What Can We Learn about Human Individual Face Recognition from Experimental Studies in Monkeys? Vision Res. 2019, 157, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Ramon, M.; Bobak, A.K.; White, D. Super-Recognizers: From the Lab to the World and Back Again. Br. J. Psychol. 2019, 110, 461–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.; Duchaine, B.; Nakayama, K. Super-Recognizers: People with Extraordinary Face Recognition Ability. Psychon. Bull. Rev. 2009, 16, 252–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrmann, M.; Avidan, G. Congenital Prosopagnosia: Face-Blind from Birth. Trends Cogn. Sci. 2005, 9, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B. Prosopdysgnosia? What Could It Tell Us about the Neural Organization of Face and Object Recognition? Cogn. Neuropsychol. 2018, 35, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Yovel, G.; Wilmer, J.B.; Duchaine, B. What Can Individual Differences Reveal about Face Processing? Front. Hum. Neurosci. 2014, 8, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barragan-Jason, G.; Cauchoix, M.; Barbeau, E.J. The Neural Speed of Familiar Face Recognition. Neuropsychologia 2015, 75, 390–401. [Google Scholar] [CrossRef]
- Barragan-Jason, G.; Lachat, F.; Barbeau, E.J. How Fast Is Famous Face Recognition? Front. Psychol. 2012, 3, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crouzet, S.M.; Thorpe, S.J. Fast Saccades toward Faces: Face Detection in Just 100 Ms. J. Vis. 2010, 10, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, S.; Martinez, A.M. Wait, Are You Sad or Angry? Large Exposure Time Differences Required for the Categorization of Facial Expressions of Emotion. J. Vis. 2013, 13, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacques, C.; Arripe, O.; Rossion, B. The Time Course of the Inversion Effect during Individual Face Discrimination. J. Vis. 2007, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ramon, M.; Caharel, S.; Rossion, B. The Speed of Recognition of Personally Familiar Faces. Perception 2011, 40, 437–449. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Rossion, B. A Robust Neural Familiar Face Recognition Response in a Dynamic (Periodic) Stream of Unfamiliar Faces. Cortex 2020, 132, 281–295. [Google Scholar] [CrossRef]
- Visconti di Oleggio Castello, M.; Gobbini, M.I. Familiar Face Detection in 180 Ms. PLoS ONE 2015, 10, e0136548. [Google Scholar] [CrossRef]
- Mandler, G. Recognizing: The Judgment of Previous Occurrence. Psychol. Rev. 1980, 87, 252–271. [Google Scholar] [CrossRef]
- Rossion, B.; Retter, T.L. Face Perception. In The Cognitive Neurosciences, 6th ed.; Poeppel, D., Mangun, G.R., Gazzaniga, M.S., Eds.; The MIT Press: Cambridge, MA, USA, 2020; pp. 129–139. [Google Scholar]
- Axmacher, N. Intracranial EEG: A Guide for Cognitive Neuroscientists; Springer: Cham, Switzerland, 2003. [Google Scholar]
- Johnson, E.L.; Kam, J.W.Y.; Tzovara, A.; Knight, R.T. Insights into Human Cognition from Intracranial EEG: A Review of Audition, Memory, Internal Cognition, and Causality. J. Neural Eng. 2020, 17, 51001. [Google Scholar] [CrossRef] [PubMed]
- Wyler, A.R.; Ojemann, G.A.; Lettich, E.; Ward, A.A.J. Subdural Strip Electrodes for Localizing Epileptogenic Foci. J. Neurosurg. 1984, 60, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.J.; Gupta, D.; Brunner, P.; Gunduz, A.; Adamo, M.A.; Ritaccio, A.; Schalk, G. Recording Human Electrocorticographic (ECoG) Signals for Neuroscientific Research and Real-Time Functional Cortical Mapping. J. Vis. Exp. 2012, e3993. [Google Scholar] [CrossRef] [Green Version]
- Talairach, J.; Bancaud, J. Stereotaxic Approach to Epilepsy. In Progress in Neurological Surgery; Karger: Basel, Switzerland, 1973; pp. 297–354. ISBN 0079-6492. [Google Scholar]
- Cardinale, F.; Cossu, M.; Castana, L.; Casaceli, G.; Schiariti, M.P.; Miserocchi, A.; Fuschillo, D.; Moscato, A.; Caborni, C.; Arnulfo, G.; et al. Stereoelectroencephalography: Surgical Methodology, Safety, and Stereotactic Application Accuracy in 500 Procedures. Neurosurgery 2013, 72, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Harary, M.; Cosgrove, G.R. Jean Talairach: A Cerebral Cartographer. Neurosurg. Focus 2019, 47, E12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazoyer, B. In Memoriam: Jean Talairach (1911-2007): A Life in Stereotaxy. Hum. Brain Mapp. 2008, 29, 250–252. [Google Scholar] [CrossRef]
- Bourdillon, P.; Ryvlin, P.; Isnard, J.; Montavont, A.; Catenoix, H.; Mauguière, F.; Rheims, S.; Ostrowsky-Coste, K.; Guénot, M. Stereotactic Electroencephalography Is a Safe Procedure, Including for Insular Implantations. World Neurosurg. 2017, 99, 353–361. [Google Scholar] [CrossRef]
- Salado, A.L.; Koessler, L.; De Mijolla, G.; Schmitt, E.; Vignal, J.-P.; Civit, T.; Tyvaert, L.; Jonas, J.; Maillard, L.G.; Colnat-Coulbois, S. SEEG Is a Safe Procedure for a Comprehensive Anatomic Exploration of the Insula: A Retrospective Study of 108 Procedures Representing 254 Transopercular Insular Electrodes. Oper. Neurosurg. 2018, 14, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zilles, K.; Palomero-Gallagher, N.; Amunts, K. Development of Cortical Folding during Evolution and Ontogeny. Trends Neurosci. 2013, 36, 275–284. [Google Scholar] [CrossRef]
- Jacques, C.; Jonas, J.; Colnat-Coulbois, S.; Maillard, L.; Rossion, B. Low and High Frequency Intracranial Neural Signals Match in the Human Associative Cortex. eLife 2022, 11, e76544. [Google Scholar] [CrossRef]
- Volfart, A.; Jonas, J.; Maillard, L.; Busigny, T.; Rossion, B.; Brissart, H. Typical Visual Unfamiliar Face Individuation in Left and Right Mesial Temporal Epilepsy. Neuropsychologia 2020, 147, 107583. [Google Scholar] [CrossRef] [PubMed]
- Rousselet, G.; Husk, J.S.; Bennett, P.J.; Sekuler, A.B. Time Course and Robustness of ERP Object and Face Differences. J. Vis. 2008, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Regan, D. Some Characteristics of Average Steady-State and Transient Responses Evoked by Modulated Light. Electroencephalogr. Clin. Neurophysiol. 1966, 20, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Srinivasan, R.; Russell, D.P.; Edelman, G.M. Investigating Neural Correlates of Conscious Perception by Frequency-Tagged Neuromagnetic Responses. Proc. Natl. Acad. Sci. USA 1998, 95, 3198–3203. [Google Scholar] [CrossRef] [Green Version]
- Regan, D. Comparison of Transient and Steady-State Methods. Ann. N. Y. Acad. Sci. 1982, 388, 45–71. [Google Scholar] [CrossRef]
- Retter, T.L.; Rossion, B. Uncovering the Neural Magnitude and Spatio-Temporal Dynamics of Natural Image Categorization in a Fast Visual Stream. Neuropsychologia 2016, 91, 9–28. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, T.A.; Gross, J.; Paterson, G.; Rusch, T.; Sack, A.T.; Thut, G. Alpha-Band Rhythms in Visual Task Performance: Phase-Locking by Rhythmic Sensory Stimulation. PLoS ONE 2013, 8, e60035. [Google Scholar] [CrossRef]
- Herrmann, C.S. Human EEG Responses to 1-100 Hz Flicker: Resonance Phenomena in Visual Cortex and Their Potential Correlation to Cognitive Phenomena. Exp. Brain Res. 2001, 137, 346–353. [Google Scholar] [CrossRef]
- Keitel, C.; Quigley, C.; Ruhnau, P. Stimulus-Driven Brain Oscillations in the Alpha Range: Entrainment of Intrinsic Rhythms or Frequency-Following Response? J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 10137–10140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Notbohm, A.; Kurths, J.; Herrmann, C.S. Modification of Brain Oscillations via Rhythmic Light Stimulation Provides Evidence for Entrainment but Not for Superposition of Event-Related Responses. Front. Hum. Neurosci. 2016, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Nuttall, R.; Jäger, C.; Zimmermann, J.; Archila-Melendez, M.E.; Preibisch, C.; Taylor, P.; Sauseng, P.; Wohlschläger, A.; Sorg, C.; Dowsett, J. Evoked Responses to Rhythmic Visual Stimulation Vary across Sources of Intrinsic Alpha Activity in Humans. Sci. Rep. 2022, 12, 5986. [Google Scholar] [CrossRef]
- Zoefel, B.; Ten Oever, S.; Sack, A.T. The Involvement of Endogenous Neural Oscillations in the Processing of Rhythmic Input: More Than a Regular Repetition of Evoked Neural Responses. Front. Neurosci. 2018, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Prieto, E.; Van Belle, G.; Liu-Shuang, J.; Norcia, A.M.; Rossion, B. The 6 Hz Fundamental Stimulation Frequency Rate for Individual Face Discrimination in the Right Occipito-Temporal Cortex. Neuropsychologia 2013, 51, 2863–2875. [Google Scholar] [CrossRef]
- Gaume, A.; Vialatte, F.; Dreyfus, G. Transient Brain Activity Explains the Spectral Content of Steady-State Visual Evoked Potentials. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2014, 2014, 688–692. [Google Scholar] [CrossRef]
- Rossion, B.; Retter, T.L.; Liu-Shuang, J. Understanding Human Individuation of Unfamiliar Faces with Oddball Fast Periodic Visual Stimulation and Electroencephalography. Eur. J. Neurosci. 2020, 52, 4283–4344. [Google Scholar] [CrossRef]
- Norcia, A.M.; Appelbaum, L.G.; Ales, J.M.; Cottereau, B.R.; Rossion, B. The Steady-State Visual Evoked Potential in Vision Research: A Review. J. Vis. 2015, 15, 1–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossion, B.; Jacques, C.; Liu-shuang, J. Fast Periodic Presentation of Natural Images Reveals a Robust Face-Selective Electrophysiological Response in the Human Brain. J. Vis. 2015, 15, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossion, B.; Boremanse, A. Robust Sensitivity to Facial Identity in the Right Human Occipito-Temporal Cortex as Revealed by Steady-State Visual-Evoked Potentials. J. Vis. 2011, 11, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekow, D.; Baudouin, J.-Y.; Brochard, R.; Rossion, B.; Leleu, A. Rapid Neural Categorization of Facelike Objects Predicts the Perceptual Awareness of a Face (Face Pareidolia). Cognition 2022, 222, 105016. [Google Scholar] [CrossRef]
- Quek, G.L.; Rossion, B. Category-Selective Human Brain Processes Elicited in Fast Periodic Visual Stimulation Streams Are Immune to Temporal Predictability. Neuropsychologia 2017, 104, 182–200. [Google Scholar] [CrossRef]
- Hauk, O.; Rice, G.E.; Volfart, A.; Magnabosco, F.; Ralph, M.A.L.; Rossion, B. Face-Selective Responses in Combined EEG/MEG Recordings with Fast Periodic Visual Stimulation (FPVS). Neuroimage 2021, 242, 118460. [Google Scholar] [CrossRef] [PubMed]
- Retter, T.L.; Rossion, B.; Schiltz, C. Harmonic Amplitude Summation for Frequency-Tagging Analysis. J. Cogn. Neurosci. 2021, 33, 2372–2393. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.; Jacques, C.; Liu-shuang, J.; Brissart, H.; Colnat-coulbois, S.; Maillard, L.; Rossion, B. A Face-Selective Ventral Occipito-Temporal Map of the Human Brain with Intracerebral Potentials. Proc. Natl. Acad. Sci. USA 2016, 113, E4088–E4097. [Google Scholar] [CrossRef] [Green Version]
- Rossion, B. Understanding Face Perception by Means of Human Electrophysiology. Trends Cogn. Sci. 2014, 18, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Regan, D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine; Elsevier: New York, NY, USA, 1989. [Google Scholar]
- Jacques, C.; Retter, T.L.; Rossion, B. A Single Glance at Natural Face Images Generate Larger and Qualitatively Different Category-Selective Spatio-Temporal Signatures than Other Ecologically-Relevant Categories in the Human Brain. Neuroimage 2016, 137, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B.; Prieto, E.A.; Boremanse, A.; Kuefner, D.; Van Belle, G. A Steady-State Visual Evoked Potential Approach to Individual Face Perception: Effect of Inversion, Contrast-Reversal and Temporal Dynamics. Neuroimage 2012, 63, 1585–1600. [Google Scholar] [CrossRef]
- Zhen, Z.; Yang, Z.; Huang, L.; Kong, X.; Wang, X.; Dang, X.; Huang, Y.; Song, Y.; Liu, J. Quantifying Interindividual Variability and Asymmetry of Face-Selective Regions: A Probabilistic Functional Atlas. Neuroimage 2015, 113, 13–25. [Google Scholar] [CrossRef] [Green Version]
- Grill-Spector, K.; Weiner, K.S.; Kay, K.N.; Gomez, J. The Functional Neuroanatomy of Human Face Perception. Annu. Rev. Vis. Sci. 2017, 3, 167–196. [Google Scholar] [CrossRef]
- Kanwisher, N.; McDermott, J.; Chun, M.M. The Fusiform Face Area: A Module in Human Extrastriate Cortex Specialized for Face Perception. J. Neurosci. 1997, 17, 4302–4311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herreras, O. Local Field Potentials: Myths and Misunderstandings. Front. Neural Circuits 2016, 10, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Gentile, F.; Rossion, B. Fast Periodic Stimulation (FPS): A Highly Effective Approach in FMRI Brain Mapping. Brain Struct. Funct. 2018, 223, 2433–2454. [Google Scholar] [CrossRef]
- Allison, T.; Ginter, H.; McCarthy, G.; Nobre, A.C.; Puce, A.; Luby, M.; Spencer, D.D. Face Recognition in Human Extrastriate Cortex. J. Neurophysiol. 1994, 71, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Allison, T.; Puce, A.; Spencer, D.D.; McCarthy, G.; Belger, A. Electrophysiological Studies of Human Face Perception. I: Potential Generated in Occiptotemporal Cortex by Face and Non-Face Stimuli. Cereb. Cortex 1999, 9, 415–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, G.; Puce, A.; Belger, A.; Allison, T. Electrophysiological Studies of Human Face Perception. II: Response Properties of Face-Specific Potentials Generated in Occipitotemporal Cortex. Cereb. Cortex 1999, 9, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Puce, A.; Allison, T.; McCarthy, G. Electrophysiological Studies of Human Face Perception. III: Effects of Top-down Processing on Face-Specific Potentials. Cereb. Cortex 1999, 9, 445–458. [Google Scholar] [CrossRef] [Green Version]
- Sergent, J.; Ohta, S.; Macdonald, B. Functional Neuroanatomy of Face and Object Processing—A Positron Emission Tomography Study. Brain 1992, 115, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Puce, A.; Allison, T.; Asgari, M.; Gore, J.C.; McCarthy, G. Differential Sensitivity of Human Visual Cortex to Faces, Letterstrings, and Textures: A Functional Magnetic Resonance Imaging Study. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 5205–5215. [Google Scholar] [CrossRef] [Green Version]
- Puce, A.; Allison, T.; Gore, J.C.; McCarthy, G. Face-Sensitive Regions in Human Extrastriate Cortex Studied by Functional MRI. J. Neurophysiol. 1995, 74, 1192–1199. [Google Scholar] [CrossRef]
- McCarthy, G.; Puce, A.; Gore, J.C.; Allison, T. Face-Specific Processing in the Human Fusiform Gyrus. J. Cogn. Neurosci. 1997, 9, 605–610. [Google Scholar] [CrossRef]
- Halgren, E.; Baudena, P.; Heit, G.; Clarke, J.M.; Marinkovic, K.; Clarke, M. Spatio-Temporal Stages in Face and Word Processing. I. Depth-Recorded Potentials in the Human Occipital, Temporal and Parietal Lobes. J. Physiol. 1994, 88, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Barbeau, E.J.; Taylor, M.J.; Regis, J.; Marquis, P.; Chauvel, P.; Liégeois-Chauvel, C. Spatio Temporal Dynamics of Face Recognition. Cereb. Cortex 2008, 18, 997–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engell, A.D.; McCarthy, G. The Relationship of Gamma Oscillations and Face-Specific ERPs Recorded Subdurally from Occipitotemporal Cortex. Cereb. Cortex 2011, 21, 1213–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engell, A.D.; McCarthy, G. Face, Eye, and Body Selective Responses in Fusiform Gyrus and Adjacent Cortex: An Intracranial EEG Study. Front. Hum. Neurosci. 2014, 8, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachaux, J.-P.; Axmacher, N.; Mormann, F.; Halgren, E.; Crone, N.E. High-Frequency Neural Activity and Human Cognition: Past, Present and Possible Future of Intracranial EEG Research. Prog. Neurobiol. 2012, 98, 279–301. [Google Scholar] [CrossRef] [Green Version]
- Vidal, J.R.; Ossandón, T.; Jerbi, K.; Dalal, S.S.; Minotti, L.; Ryvlin, P.; Kahane, P.; Lachaux, J.-P. Category-Specific Visual Responses: An Intracranial Study Comparing Gamma, Beta, Alpha, and ERP Response Selectivity. Front. Hum. Neurosci. 2010, 4, 195. [Google Scholar] [CrossRef] [Green Version]
- Boring, M.J.; Silson, E.H.; Ward, M.J.; Richardson, R.M.; Fiez, J.A.; Baker, C.I.; Ghuman, A.S. Multiple Adjoining Word- And Face-Selective Regions in Ventral Temporal Cortex Exhibit Distinct Dynamics. J. Neurosci. 2021, 41, 6314–6327. [Google Scholar] [CrossRef] [PubMed]
- Kadipasaoglu, C.M.; Conner, C.R.; Whaley, M.L.; Baboyan, V.G.; Tandon, N. Category-Selectivity in Human Visual Cortex Follows Cortical Topology: A Grouped IcEEG Study. PLoS ONE 2016, 11, e0157109. [Google Scholar] [CrossRef] [Green Version]
- Lachaux, J.-P.; George, N.; Tallon-Baudry, C.; Martinerie, J.; Hugueville, L.; Minotti, L.; Kahane, P.; Renault, B. The Many Faces of the Gamma Band Response to Complex Visual Stimuli. Neuroimage 2005, 25, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Richardson, R.M.; Ghuman, A.S. Posterior Fusiform and Midfusiform Contribute to Distinct Stages of Facial Expression Processing. Cereb. Cortex 2019, 29, 3209–3219. [Google Scholar] [CrossRef]
- Jacques, C.; Witthoft, N.; Weiner, K.S.; Foster, B.L.; Rangarajan, V.; Hermes, D.; Miller, K.J.; Parvizi, J.; Grill-spector, K. Corresponding ECoG and FMRI Category-Selective Signals in Human Ventral Temporal Cortex. Neuropsychologia 2016, 83, 14–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergent, J. Face Perception and the Right Hemisphere. In Thought without Language; Weiskrantz, L., Ed.; Oxford University Press: Oxford, UK, 1988; pp. 108–131. [Google Scholar]
- Rossion, B.; Lochy, A. Is Human Face Recognition Lateralized to the Right Hemisphere Due to Neural Competition with Left-Lateralized Visual Word Recognition? A Critical Review. Brain Struct. Funct. 2022, 227, 599–629. [Google Scholar] [CrossRef]
- Parvizi, J.; Jacques, C.; Foster, B.L.; Withoft, N.; Rangarajan, V.; Weiner, K.S.; Grill-Spector, K. Electrical Stimulation of Human Fusiform Face-Selective Regions Distorts Face Perception. J. Neurosci. 2012, 32, 14915–14920. [Google Scholar] [CrossRef] [Green Version]
- Puce, A.; Allison, T.; Spencer, S.S.; Spencer, D.D.; McCarthy, G. Comparison of Cortical Activation Evoked by Faces Measured by Intracranial Field Potentials and Functional MRI: Two Case Studies. Hum. Brain Mapp. 1997, 5, 298–305. [Google Scholar] [CrossRef]
- Rossion, B.; Dricot, L.; Devolder, A.; Bodart, J.M.; Crommelinck, M.; De Gelder, B.; Zoontjes, R. Hemispheric Asymmetries for Whole-Based and Part-Based Face Processing in the Human Fusiform Gyrus. J. Cogn. Neurosci. 2000, 12, 793–802. [Google Scholar] [CrossRef]
- Ojemann, J.G.; Akbudak, E.; Snyder, A.Z.; McKinstry, R.C.; Raichle, M.E.; Conturo, T.E. Anatomic Localization and Quantitative Analysis of Gradient Refocused Echo-Planar FMRI Susceptibility Artifacts. Neuroimage 1997, 6, 156–167. [Google Scholar] [CrossRef]
- Wandell, B.A. The Neurobiological Basis of Seeing Words. Ann. N. Y. Acad. Sci. 2011, 1224, 63–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossion, B.; Jacques, C.; Jonas, J. Mapping Face Categorization in the Human Ventral Occipito-Temporal Cortex with Direct Neural Intracranial Recordings. Ann. N. Y. Acad. Sci. 2018, 1426, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Volfart, A.; Yan, X.; Maillard, L.; Colnat-Coulbois, S.; Hossu, G.; Rossion, B.; Jonas, J. Intracerebral Electrical Stimulation of the Right Anterior Fusiform Gyrus Impairs Human Face Identity Recognition. Neuroimage 2022, 250, 118932. [Google Scholar] [CrossRef] [PubMed]
- Weiner, K.S.; Grill-Spector, K. Sparsely-Distributed Organization of Face and Limb Activations in Human Ventral Temporal Cortex. Neuroimage 2010, 52, 1559–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiner, K.S.; Zilles, K. The Anatomical and Functional Specialization of the Fusiform Gyrus. Neuropsychologia 2016, 83, 48–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovács, G. Getting to Know Someone: Familiarity, Person Recognition, and Identification in the Human Brain. J. Cogn. Neurosci. 2020, 32, 2205–2225. [Google Scholar] [CrossRef]
- Rajimehr, R.; Young, J.C.; Tootell, R.B.H. An Anterior Temporal Face Patch in Human Cortex, Predicted by Macaque Maps. Proc. Natl. Acad. Sci. USA 2009, 106, 1995–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Metoki, A.; Smith, D.V.; Medaglia, J.D.; Zang, Y.; Benear, S.; Popal, H.; Lin, Y.; Olson, I.R. Multimodal Mapping of the Face Connectome. Nat. Hum. Behav. 2020, 4, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Weiner, K.S.; Grill-Spector, K. The Evolution of Face Processing Networks. Trends Cogn. Sci. 2015, 19, 240–241. [Google Scholar] [CrossRef] [Green Version]
- Lafer-sousa, R.; Conway, B.R.; Kanwisher, N.G. Color-Biased Regions of the Ventral Visual Pathway Lie between Face- and Place-Selective Regions in Humans, as in Macaques. J. Neurosci. 2016, 36, 1682–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chance, S.A.; Sawyer, E.K.; Clover, L.M.; Wicinski, B.; Hof, P.R.; Crow, T.J. Hemispheric Asymmetry in the Fusiform Gyrus Distinguishes Homo Sapiens from Chimpanzees. Brain Struct. Funct. 2013, 218, 1391–1405. [Google Scholar] [CrossRef]
- Mehler, J.; Morton, J.; Jusczyk, P.W. On Reducing Language to Biology. Cogn. Neuropsychol. 1984, 1, 83–116. [Google Scholar] [CrossRef]
- Uttal, W.R. The New Phrenology: The Limits of Localizing Cognitive Processes in the Brain; Life and mind: Philosophical issues in biology and psychology; The MIT Press: Cambridge, MA, USA, 2001; ISBN 0-262-21017-7. (Hardcover). [Google Scholar]
- Searle, J.R. Minds, Brains and Science (1984 Reith Lectures); Harvard University Press: Cambridge, MA, USA, 1984. [Google Scholar]
- Marr, D. Vision: A Computational Investigation into the Human Representation and Processing of Visual Information; W. H. Free.; W. H. Freeman and Co: San Francisco, CA, USA, 1982. [Google Scholar]
- Freiwald, W.A.; Tsao, D.Y. Functional Compartmentalization and Viewpoint Generalization within the Macaque Face-Processing System. Science 2010, 330, 845–851. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Rosen, E.; Zeffiro, T.; Vanmeter, J.; Blanz, V.; Riesenhuber, M. Evaluation of a Shape-Based Model of Human Face Discrimination Using FMRI and Behavioral Techniques. Neuron 2006, 50, 159–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiCarlo, J.J.; Cox, D.D. Untangling Invariant Object Recognition. Trends Cogn. Sci. 2007, 11, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. Network Memory. Trends Neurosci. 1997, 20, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Sugden, N.A.; Mohamed-Ali, M.I.; Moulson, M.C. I Spy with My Little Eye: Typical, Daily Exposure to Faces Documented from a First-Person Infant Perspective. Dev. Psychobiol. 2014, 56, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Haxby, J.V.; Gobbini, M.I.; Furey, M.L.; Ishai, A.; Schouten, J.L.; Pietrini, P. Distributed and Overlapping Representations of Faces and Objects in Ventral Temporal Cortex. Science 2001, 293, 2425–2430. [Google Scholar] [CrossRef] [Green Version]
- Huth, A.G.; Nishimoto, S.; Vu, A.T.; Gallant, J.L. A Continuous Semantic Space Describes the Representation of Thousands of Object and Action Categories across the Human Brain. Neuron 2012, 76, 1210–1224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passingham, R. How Good Is the Macaque Monkey Model of the Human Brain? Curr. Opin. Neurobiol. 2009, 19, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preuss, T.M. Taking the Measure of Diversity: Comparative Alternatives to the Model-Animal Paradigm in Cortical Neuroscience. Brain. Behav. Evol. 2000, 55, 287–299. [Google Scholar] [CrossRef]
- Krug, K.; Parker, A. The Neural Events That Change Perception. Neuroforum 2019, 24, A31–A39. [Google Scholar] [CrossRef] [Green Version]
- Gross, C.G.; Rocha-Miranda, C.E.; Bender, D.B. Visual Properties of Neurons in Inferotemporal Cortex of the Macaque. J. Neurophysiol. 1972, 35, 96–111. [Google Scholar] [CrossRef]
- Desimone, R. Face-Selective Cells in the Temporal Cortex of Monkeys. J. Cogn. Neurosci. 1991, 3, 1–8. [Google Scholar] [CrossRef]
- Barraclough, N.E.; Perrett, D.I. From Single Cells to Social Perception. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2011, 366, 1739–1752. [Google Scholar] [CrossRef] [PubMed]
- Hesse, J.K.; Tsao, D.Y. The Macaque Face Patch System: A Turtle’s Underbelly for the Brain. Nat. Rev. Neurosci. 2020, 21, 695–716. [Google Scholar] [CrossRef]
- Freiwald, W.A. The Neural Mechanisms of Face Processing: Cells, Areas, Networks, and Models. Curr. Opin. Neurobiol. 2020, 60, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Perrett, D.I.; Rolls, E.T.; Caan, W. Visual Neurones Responsive to Faces in the Monkey Temporal Cortex. Exp. Brain Res. 1982, 47, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Issa, E.B.; DiCarlo, J.J. Precedence of the Eye Region in Neural Processing of Faces. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 16666–16682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taubert, J.; Van Belle, G.; Vanduffel, W.; Rossion, B.; Vogels, R. Neural Correlate of the Thatcher Face Illusion in a Monkey Face-Selective Patch. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 9872–9878. [Google Scholar] [CrossRef] [Green Version]
- Tsao, D.Y. A Cortical Region Consisting Entirely of Face-Selective Cells. Science 2006, 311, 670–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taubert, J.; Japee, S.; Murphy, A.P.; Tardiff, C.T.; Koele, E.A.; Kumar, S.; Leopold, D.A.; Ungerleider, L.G. Parallel Processing of Facial Expression and Head Orientation in the Macaque Brain. J. Neurosci. Off. J. Soc. Neurosci. 2020, 40, 8119–8131. [Google Scholar] [CrossRef]
- Tsao, D.Y.; Moeller, S.; Freiwald, W.A. Comparing Face Patch Systems in Macaques and Humans. Proc. Natl. Acad. Sci. USA 2008, 105, 19514–19519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinsk, M.A.; Arcaro, M.; Weiner, K.S.; Kalkus, J.F.; Inati, S.J.; Gross, C.G.; Kastner, S. Neural Representations of Faces and Body Parts in Macaque and Human Cortex: A Comparative FMRI Study. J. Neurophysiol. 2009, 101, 2581–2600. [Google Scholar] [CrossRef] [PubMed]
- Laurent, M.A.; Audurier, P.; De Castro, V.; Gao, X.; Durand, J.B.; Jonas, J.; Rossion, B.; Cottereau, B.R. Towards an Optimization of Functional Localizers in Non-Human Primate Neuroimaging with (FMRI) Frequency-Tagging.
- Janssens, T.; Zhu, Q.; Popivanov, I.D.; Vanduffel, W. Probabilistic and Single-Subject Retinotopic Maps Reveal the Topographic Organization of Face Patches in the Macaque Cortex. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 10156–10167. [Google Scholar] [CrossRef]
- Bell, A.H.; Malecek, N.J.; Morin, E.L.; Hadj-bouziane, F.; Tootell, R.B.H.; Ungerleider, L.G. Relationship between Functional Magnetic Resonance Imaging-Identified Regions and Neuronal Category Selectivity. J. Neurosci. 2011, 31, 12229–12240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Tsao, D.Y. The Code for Facial Identity in the Primate Brain. Cell 2017, 169, 1013–1028.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, M.P.; Yamane, S. Sparse Population Coding of Faces in the Inferotemporal Cortex. Science 1992, 256, 1327–1331. [Google Scholar] [CrossRef]
- Leopold, D.A.; Bondar, I.V.; Giese, M.A. Norm-Based Face Encoding by Single Neurons in the Monkey Inferotemporal Cortex. Nature 2006, 442, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Bruce, V. Changing Faces: Visual and Non-Visual Coding Processes in Face Recognition. Br. J. Psychol. 1982, 73, 105–116. [Google Scholar] [CrossRef]
- Parr, L.A.; Heintz, M.; Pradhan, G. Rhesus Monkeys (Macaca Mulatta) Lack Expertise in Face Processing. J. Comp. Psychol. 2008, 122, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Griffin, J.W. Quantifying the Face Inversion Effect in Nonhuman Primates: A Phylogenetic Meta-Analysis. Anim. Cogn. 2020, 23, 237–249. [Google Scholar] [CrossRef]
- Koba, R.; Izumi, A. Sex Categorization of Conspecific Pictures in Japanese Monkeys (Macaca Fuscata). Anim. Cogn. 2006, 9, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ohshiba, N. Nihonnzaru Ni Yoru Doushutakotai No Seibennbetu (Japanese Macaques’ Sex Discrimination of Their Conspecifics). Reichorui Kenkyu 1995, 11, 179–186. [Google Scholar]
- Herculano-Houzel, S. The Human Advantage: A New Understanding of How Our Brain Became Remarkable; MIT Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Rilling, J.K.; Seligman, R.A. A Quantitative Morphometric Comparative Analysis of the Primate Temporal Lobe. J. Hum. Evol. 2002, 42, 505–533. [Google Scholar] [CrossRef] [PubMed]
- Bryant, K.L.; Glasser, M.F.; Li, L.; Bae, J.J.; Jacquez, N.J.; Preuss, T.M. Organization of Extrastriate and Temporal Cortex in Chimpanzees Compared to Humans and Macaques. Cortex 2019, 118, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Voorhies, W.I.; Li, X.; Raghuram, I.; Palomero-Gallagher, N.; Zilles, K.; Sherwood, C.C.; Hopkins, W.D.; Weiner, K.S. Sulcal Morphology of Ventral Temporal Cortex Is Shared between Humans and Other Hominoids. Sci. Rep. 2020, 10, 17132. [Google Scholar] [CrossRef]
- Bryant, K.L.; Preuss, T.M. A Comparative Perspective on the Human Temporal Lobe. In Digital Endocasts: From Skulls to Brains; Bruner, E., Ogihara, N., Tanabe, H.C., Eds.; Springer: Tokyo, Japan, 2018; pp. 239–258. ISBN 978-4-431-56582-6. [Google Scholar]
- Yovel, G.; Freiwald, W.A. Face Recognition Systems in Monkey and Human: Are They the Same Thing? F1000Prime Rep. 2013, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.-C.; Yen, C.C.; Ciuchta, J.L.; Papoti, D.; Bock, N.A.; Leopold, D.A.; Silva, A.C. Functional Mapping of Face-Selective Regions in the Extrastriate Visual Cortex of the Marmoset. J. Neurosci. 2015, 35, 1160–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heywood, C.A.; Cowey, A. The Role of the “face-Cell” Area in the Discrimination and Recognition of Faces by Monkeys. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 1992, 335, 31–38. [Google Scholar] [CrossRef]
- Epstein, R.A.; Vass, L.K. Neural Systems for Landmark-Based Wayfinding in Humans. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2014, 369, 20120533. [Google Scholar] [CrossRef]
- Hagen, S.; Jacques, C.; Maillard, L.; Colnat-Coulbois, S.; Rossion, B.; Jonas, J. Spatially Dissociated Intracerebral Maps for Face- and House-Selective Activity in the Human Ventral Occipito-Temporal Cortex. Cereb. Cortex 2020, 30, 4026–4043. [Google Scholar] [CrossRef]
- Bastin, J.; Vidal, J.R.; Bouvier, S.; Perrone-Bertolotti, M.; Bénis, D.; Kahane, P.; David, O.; Lachaux, J.-P.; Epstein, R.A. Temporal Components in the Parahippocampal Place Area Revealed by Human Intracerebral Recordings. J. Neurosci. 2013, 33, 10123–10131. [Google Scholar] [CrossRef] [Green Version]
- Kret, M.E.; Tomonaga, M. Getting to the Bottom of Face Processing. Species-Specific Inversion Effects for Faces and Behinds in Humans and Chimpanzees (Pan Troglodytes). PLoS ONE 2016, 11, e0165357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, P.; Liu, T.T.; Cruz, F.; Pereira, A. The Mechanisms Supporting Holistic Perception of Words and Faces Are Not Independent. Mem. Cognit. 2022, 136, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Martelli, M.; Majaj, N.J.; Pelli, D.G. Are Faces Processed like Words? A Diagnostic Test for Recognition by Parts. J. Vis. 2005, 5, 58–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrmann, M.; Plaut, D.C. Hemispheric Organization for Visual Object Recognition: A Theoretical Account and Empirical Evidence. Perception 2020, 49, 373–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrmann, M.; Plaut, D.C. A Vision of Graded Hemispheric Specialization. Ann. N. Y. Acad. Sci. 2015, 1359, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Kriegeskorte, N.; Formisano, E.; Sorger, B.; Goebel, R. Individual Faces Elicit Distinct Response Patterns in Human Anterior Temporal Cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 20600–20605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehaene, S.; Cohen, L.; Morais, J.; Kolinsky, R. Illiterate to Literate: Behavioural and Cerebral Changes Induced by Reading Acquisition. Nat. Rev. Neurosci. 2015, 16, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Dehaene, S.; Pegado, F.; Braga, L.W.; Ventura, P.; Nunes Filho, G.; Jobert, A.; Dehaene-Lambertz, G.; Kolinsky, R.; Morais, J.; Cohen, L. How Learning to Read Changes the Cortical Networks for Vision and Language. Science 2010, 330, 1359–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, H.D. The Role of the Right Hemisphere in Face Perception. In Functions of the Right Cerebral Hemisphere; Young, A., Ed.; Academic Press: London, UK, 1983; pp. 33–64. [Google Scholar]
- Edelman, G.M. Neural Darwinism: The Theory of Neuronal Group Selection; Basic Book: New York, NY, USA, 1987. [Google Scholar]
- Merzenich, M.; Recanzone, G.; Jenkins, W.M.; Allard, T.T.; Nudo, R.J. Cortical Representational Plasticity. In Neurobiology of Neo-Cortex; Rakic, P., Singer, W., Eds.; Wiley: New York, NY, USA, 1988; pp. 41–67. [Google Scholar]
- Gainotti, G. Is There a Causal Link between the Left Lateralization of Language and Other Brain Asymmetries? A Review of Data Gathered in Patients with Focal Brain Lesions. Brain Sci. 2021, 11, 1644. [Google Scholar] [CrossRef] [PubMed]
- Pinel, P.; Lalanne, C.; Bourgeron, T.; Fauchereau, F.; Poupon, C.; Artiges, E.; Le Bihan, D.; Dehaene-Lambertz, G.; Dehaene, S. Genetic and Environmental Influences on the Visual Word Form and Fusiform Face Areas. Cereb. Cortex 2015, 25, 2478–2493. [Google Scholar] [CrossRef] [Green Version]
- Lochy, A.; Van Belle, G.; Rossion, B. A Robust Index of Lexical Representation in the Left Occipito-Temporal Cortex as Evidenced by EEG Responses to Fast Periodic Visual Stimulation. Neuropsychologia 2015, 66, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Hagen, S.; Lochy, A.; Jacques, C.; Maillard, L.; Colnat-Coulbois, S.; Jonas, J.; Rossion, B. Dissociated Face- and Word-Selective Intracerebral Responses in the Human Ventral Occipito-Temporal Cortex. Brain Struct. Funct. 2021, 226, 3031–3049. [Google Scholar] [CrossRef]
- Retter, T.L.; Jiang, F.; Webster, M.A.; Rossion, B. All-or-None Face Categorization in the Human Brain. Neuroimage 2020, 213, 116685. [Google Scholar] [CrossRef] [PubMed]
- Lochy, A.; Jacques, C.; Maillard, L.; Colnat-Coulbois, S.; Rossion, B.; Jonas, J. Selective Visual Representation of Letters and Words in the Left Ventral Occipito-Temporal Cortex with Intracerebral Recordings. Proc. Natl. Acad. Sci. USA 2018, 115, E7595–E7604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubota, E.C.; Joo, S.J.; Huber, E.; Yeatman, J.D. Word Selectivity in High-Level Visual Cortex and Reading Skill. Dev. Cogn. Neurosci. 2019, 36, 100593. [Google Scholar] [CrossRef]
- Sheehan, M.J.; Nachman, M.W. Morphological and Population Genomic Evidence That Human Faces Have Evolved to Signal Individual Identity. Nat. Commun. 2014, 5, 4800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, A.M.; Kramer, R.S.S.; Ritchie, K.L.; Jenkins, R. Identity From Variation: Representations of Faces Derived From Multiple Instances. Cogn. Sci. 2016, 40, 202–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, R.; Dowsett, A.J.; Burton, A.M. How Many Faces Do People Know? Proceed. Biol. Sci. 2018, 285, 20181319. [Google Scholar] [CrossRef] [Green Version]
- Caharel, S.; Ramon, M.; Rossion, B. Face Familiarity Decisions Take 200 Msec in the Human Brain: Electrophysiological Evidence from a Go/No-Go Speeded Task. J. Cogn. Neurosci. 2014, 26, 81–95. [Google Scholar] [CrossRef]
- Rossion, B. Damasio’s Error—Prosopagnosia with Intact within-Category Object Recognition. J. Neuropsychol. 2018, 12, 357–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce, V.; Henderson, Z.; Newman, C.; Burton, A.M. Matching Identities of Familiar and Unfamiliar Faces Caught on CCTV Images. J. Exp. Psychol. Appl. 2001, 7, 207–218. [Google Scholar] [CrossRef]
- Megreya, A.M.; Burton, A.M. Unfamiliar Faces Are Not Faces: Evidence from a Matching Task. Mem. Cognit. 2006, 34, 865–876. [Google Scholar] [CrossRef] [Green Version]
- Young, A.W.; Burton, A.M. Are We Face Experts? Trends Cogn. Sci. 2018, 22, 100–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hancock, P.J.; Bruce, V.V.; Burton, A.M. Recognition of Unfamiliar Faces. Trends Cogn. Sci. 2000, 4, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Freire, A.; Lee, K.; Symons, L.A. The Face-Inversion Effect as a Deficit in the Encoding of Configural Information: Direct Evidence. Perception 2000, 29, 159–170. [Google Scholar] [CrossRef]
- Yin, R.K. Looking at Upside-down Faces. J. Exp. Psychol. Percept. Perform. 1969, 81, 141–145. [Google Scholar] [CrossRef]
- Rossion, B. Picture-Plane Inversion Leads to Qualitative Changes of Face Perception. Acta Psychol. (Amst) 2008, 128, 274–289. [Google Scholar] [CrossRef]
- Liu-Shuang, J.; Norcia, A.M.; Rossion, B. An Objective Index of Individual Face Discrimination in the Right Occipito-Temporal Cortex by Means of Fast Periodic Oddball Stimulation. Neuropsychologia 2014, 52, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Meissner, C.A.; Brigham, J.C. Thirty Years of Investigating the Own-Race Bias in Memory for Faces: A Meta-Analytic Review. Psychol. Public Policy Law 2001, 7, 3–35. [Google Scholar] [CrossRef]
- Rossion, B.; Michel, C. An Experience-Based Holistic Account of the Other-Race Face Effect. In Oxford Handbook of Face Perception; Calder, A.J., Rhodes, G., Johnson, M.H., Haxby, J.V., Eds.; Oxford University Press: Oxford, UK, 2011; ISBN 9780199559053. [Google Scholar]
- Schwartz, L.; Yovel, G. The Roles of Perceptual and Conceptual Information in Face Recognition. J. Exp. Psychol. Gen. 2016, 145, 1493–1511. [Google Scholar] [CrossRef] [Green Version]
- Jacques, C.; Rossion, B.; Volfart, A.; Brissart, H.; Colnat-Coulbois, S.; Maillard, L.; Jonas, J. The Neural Basis of Rapid Unfamiliar Face Individuation with Human Intracerebral Recordings. Neuroimage 2020, 221, 117174. [Google Scholar] [CrossRef] [PubMed]
- Dzhelyova, M.; Jacques, C.; Dormal, G.; Michel, C.; Schiltz, C.; Rossion, B. High Test-Retest Reliability of a Neural Index of Rapid Automatic Discrimination of Unfamiliar Individual Faces. Vis. Cogn. 2019, 27, 127–141. [Google Scholar] [CrossRef]
- de Heering, A.; Rossion, B.; Maurer, D. Developmental Changes in Face Recognition during Childhood: Evidence from Upright and Inverted Faces. Cogn. Dev. 2012, 27, 17–27. [Google Scholar] [CrossRef]
- Mazard, A.; Schiltz, C.; Rossion, B. Recovery from Adaptation to Facial Identity Is Larger for Upright than Inverted Faces in the Human Occipito-Temporal Cortex. Neuropsychologia 2006, 44, 912–922. [Google Scholar] [CrossRef]
- Gilaie-Dotan, S.; Gelbard-Sagiv, H.; Malach, R. Perceptual Shape Sensitivity to Upright and Inverted Faces Is Reflected in Neuronal Adaptation. Neuroimage 2010, 50, 383–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yovel, G.; Kanwisher, N. The Neural Basis of the Behavioral Face-Inversion Effect. Curr. Biol. 2005, 15, 2256–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haxby, J.V.; Ungerleider, L.G.; Clark, V.P.; Schouten, J.L.; Hoffman, E.A.; Martin, A. The Effect of Face Inversion on Activity in Human Neural Systems for Face and Object Perception. Neuron 1999, 22, 189–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenthal, G.; Sporns, O.; Avidan, G. Stimulus Dependent Dynamic Reorganization of the Human Face Processing Network. Cereb. Cortex 2017, 27, 4823–4834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, M.G.; Park, J.; Gonzalez, R.; Polk, T.A.; Gehrke, A.; Knaffla, S.; Jonides, J. Evaluating Functional Localizers: The Case of the FFA. Neuroimage 2010, 50, 56–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitcher, D.; Duchaine, B.; Walsh, V.; Yovel, G.; Kanwisher, N. The Role of Lateral Occipital Face and Object Areas in the Face Inversion Effect. Neuropsychologia 2011, 49, 3448–3453. [Google Scholar] [CrossRef]
- Edelman, G.M. Biochemistry and the Sciences of Recognition. J. Biol. Chem. 2004, 279, 7361–7369. [Google Scholar] [CrossRef] [Green Version]
- Devlin, J.T.; Russell, R.P.; Davis, M.H.; Price, C.J.; Wilson, J.; Moss, H.E.; Matthews, P.M.; Tyler, L.K. Susceptibility-Induced Loss of Signal: Comparing PET and FMRI on a Semantic Task. Neuroimage 2000, 11, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, J.A.; Koski, J.E.; Olson, I.R. More Than Meets the Eye: The Merging of Perceptual and Conceptual Knowledge in the Anterior Temporal Face Area. Front. Hum. Neurosci. 2016, 10, 189. [Google Scholar] [CrossRef] [Green Version]
- Axelrod, V.; Yovel, G. The Challenge of Localizing the Anterior Temporal Face Area: A Possible Solution. Neuroimage 2013, 81, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Binney, R.J.; Hoffman, P.; Lambon Ralph, M.A. Mapping the Multiple Graded Contributions of the Anterior Temporal Lobe Representational Hub to Abstract and Social Concepts: Evidence from Distortion-Corrected FMRI. Cereb. Cortex 2016, 26, 4227–4241. [Google Scholar] [CrossRef] [PubMed]
- Embleton, K.V.; Haroon, H.A.; Morris, D.M.; Ralph, M.A.L.; Parker, G.J.M. Distortion Correction for Diffusion-Weighted MRI Tractography and FMRI in the Temporal Lobes. Hum. Brain Mapp. 2010, 31, 1570–1587. [Google Scholar] [CrossRef]
- Halai, A.D.; Welbourne, S.R.; Embleton, K.; Parkes, L.M. A Comparison of Dual Gradient-Echo and Spin-Echo FMRI of the Inferior Temporal Lobe. Hum. Brain Mapp. 2014, 35, 4118–4128. [Google Scholar] [CrossRef] [PubMed]
- Persichetti, A.S.; Denning, J.M.; Gotts, S.J.; Martin, A. A Data-Driven Functional Mapping of the Anterior Temporal Lobes. J. Neurosci. 2021, 41, 6038–6049. [Google Scholar] [CrossRef] [PubMed]
- Simmons, W.K.; Reddish, M.; Bellgowan, P.S.F.; Martin, A. The Selectivity and Functional Connectivity of the Anterior Temporal Lobes. Cereb. Cortex 2010, 20, 813–825. [Google Scholar] [CrossRef] [Green Version]
- Haxby, J.V.; Hoffman, E.A.; Gobbini, M.I. The Distributed Human Neural System for Face Perception. Trends Cogn. Sci. 2000, 4, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Wiley: Oxford, UK, 1949. [Google Scholar]
- Chen, X.; Liu, X.; Parker, B.J.; Zhen, Z.; Weiner, K.S. Functionally and Structurally Distinct Fusiform Face Area(s) in over 1000 Participants. Neuroimage 2023, 265, 119765. [Google Scholar] [CrossRef] [PubMed]
- Duchaine, B.; Yovel, G. A Revised Neural Framework for Face Processing. Annu. Rev. Vis. Sci. 2015, 1, 393–416. [Google Scholar] [CrossRef] [Green Version]
- Gershman, S.J. Just Looking: The Innocent Eye in Neuroscience. Neuron 2021, 109, 2220–2223. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wen, M.; Sun, M.; Rossion, B. A Genuine Interindividual Variability in Number and Anatomical Localization of Face-Selective Regions in the Human Brain. Cereb. Cortex 2022, 32, 4834–4856. [Google Scholar] [CrossRef]
- Rossion, B.; Hanseeuw, B.; Dricot, L. Defining Face Perception Areas in the Human Brain: A Large-Scale Factorial FMRI Face Localizer Analysis. Brain Cogn. 2012, 79, 138–157. [Google Scholar] [CrossRef]
- Jonas, J.; Rossion, B. Intracerebral Electrical Stimulation to Understand the Neural Basis of Human Face Identity Recognition. Eur. J. Neurosci. 2021, 54, 4197–4211. [Google Scholar] [CrossRef] [PubMed]
- Hécaen, H.; Angelergues, R.; Bernhardt, C.; Chiarelli, J. Essai de Distinction Des Modalités Cliniques de l’agnosie Des Physionomies. Rev. Neurol. (Paris) 1957, 96, 125–144. [Google Scholar] [PubMed]
- Lochy, A.; de Heering, A.; Rossion, B. The Non-Linear Development of the Right Hemispheric Specialization for Human Face Perception. Neuropsychologia 2019, 126, 10–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringo, J.L.; Doty, R.W.; Demeter, S.; Simard, P.Y. Time Is of the Essence: A Conjecture That Hemispheric Specialization Arises from Interhemispheric Conduction Delay. Cereb. Cortex 1994, 4, 331–343. [Google Scholar] [CrossRef]
- Rogers, L.J. Brain Lateralization and Cognitive Capacity. Animals 2021, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J.; Schalk, G.; Hermes, D.; Ojemann, J.G.; Rao, R.P.N. Spontaneous Decoding of the Timing and Content of Human Object Perception from Cortical Surface Recordings Reveals Complementary Information in the Event-Related Potential and Broadband Spectral Change. PLoS Comput. Biol. 2016, 12, e1004660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anzellotti, S.; Fairhall, S.L.; Caramazza, A. Decoding Representations of Face Identity That Are Tolerant to Rotation. Cereb. Cortex 2014, 24, 1988–1995. [Google Scholar] [CrossRef]
- Nestor, A.; Plaut, D.C.; Behrmann, M. Unraveling the Distributed Neural Code of Facial Identity through Spatiotemporal Pattern Analysis. Proc. Natl. Acad. Sci. USA 2011, 108, 9998–10003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, I.; Tarr, M.J.; Moylan, J.; Skudlarski, P.; Gore, J.C.; Anderson, A.W. The Fusiform “Face Area” Is Part of a Network That Processes Faces at the Individual Level. J. Cogn. Neurosci. 2000, 12, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ewbank, M.P.; Andrews, T.J. Differential Sensitivity for Viewpoint between Familiar and Unfamiliar Faces in Human Visual Cortex. Neuroimage 2008, 40, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.L.; Camp, N.P.; Gomez, J.; Natu, V.S.; Grill-Spector, K.; Eberhardt, J.L. Neural Adaptation to Faces Reveals Racial Outgroup Homogeneity Effects in Early Perception. Proc. Natl. Acad. Sci. USA 2019, 116, 14532–14537. [Google Scholar] [CrossRef] [Green Version]
- Schiltz, C.; Sorger, B.; Caldara, R.; Ahmed, F.; Mayer, E.; Goebel, R.; Rossion, B. Impaired Face Discrimination in Acquired Prosopagnosia Is Associated with Abnormal Response to Individual Faces in the Right Middle Fusiform Gyrus. Cereb. Cortex 2006, 16, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Rossion, B.; Dricot, L.; Goebel, R.; Busigny, T. Holistic Face Categorization in Higher Order Visual Areas of the Normal and Prosopagnosic Brain: Toward a Non-Hierarchical View of Face Perception. Front. Hum. Neurosci. 2011, 4, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamberger, M.J.; Cole, J. Language Organization and Reorganization in Epilepsy. Neuropsychol. Rev. 2011, 21, 240–251. [Google Scholar] [CrossRef]
- Drane, D.L.; Ojemann, J.G.; Phatak, V.; Loring, D.W.; Gross, R.E.; Hebb, A.O.; Silbergeld, D.L.; Miller, J.W.; Voets, N.L.; Saindane, A.M.; et al. Famous Face Identification in Temporal Lobe Epilepsy: Support for a Multimodal Integration Model of Semantic Memory. Cortex 2013, 49, 1648–1667. [Google Scholar] [CrossRef] [Green Version]
- Gainotti, G. Different Patterns of Famous People Recognition Disorders in Patients with Right and Left Anterior Temporal Lesions: A Systematic Review. Neuropsychologia 2007, 45, 1591–1607. [Google Scholar] [CrossRef]
- Gainotti, G.; Marra, C. Differential Contribution of Right and Left Temporo-Occipital and Anterior Temporal Lesions to Face Recognition Disorders. Front. Hum. Neurosci. 2011, 5, 55. [Google Scholar] [CrossRef] [Green Version]
- Crane, J.; Milner, B. Do I Know You? Face Perception and Memory in Patients with Selective Amygdalo-Hippocampectomy. Neuropsychologia 2002, 40, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Race, E.; LaRocque, K.F.; Keane, M.M.; Verfaellie, M. Medial Temporal Lobe Contributions to Short-Term Memory for Faces. J. Exp. Psychol. Gen. 2013, 142, 1309–1322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, J.; Brissart, H.; Hossu, G.; Colnat-Coulbois, S.; Vignal, J.-P.; Rossion, B.; Maillard, L. A Face Identity Hallucination (Palinopsia) Generated by Intracerebral Stimulation of the Face-Selective Right Lateral Fusiform Cortex. Cortex 2018, 99, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Crone, N.E.; Miglioretti, D.L.; Gordon, B.; Lesser, R.P. Functional Mapping of Human Sensorimotor Cortex with Electrocorticographic Spectral Analysis. II. Event-Related Synchronization in the Gamma Band. Brain 1998, 121 Pt 1, 2301–2315. [Google Scholar] [CrossRef] [Green Version]
- Hermes, D.; Miller, K.J.; Vansteensel, M.J.; Aarnoutse, E.J.; Leijten, F.S.S.; Ramsey, N.F. Neurophysiologic Correlates of FMRI in Human Motor Cortex. Hum. Brain Mapp. 2012, 33, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.J.; Leuthardt, E.C.; Schalk, G.; Rao, R.P.N.; Anderson, N.R.; Moran, D.W.; Miller, J.W.; Ojemann, J.G. Spectral Changes in Cortical Surface Potentials during Motor Movement. J. Neurosci. 2007, 27, 2424–2432. [Google Scholar] [CrossRef] [Green Version]
- Nir, Y.; Fisch, L.; Mukamel, R.; Gelbard-Sagiv, H.; Arieli, A.; Fried, I.; Malach, R. Coupling between Neuronal Firing Rate, Gamma LFP, and BOLD FMRI Is Related to Interneuronal Correlations. Curr. Biol. 2007, 17, 1275–1285. [Google Scholar] [CrossRef] [Green Version]
- Ray, S.; Crone, N.E.; Niebur, E.; Franaszczuk, P.J.; Hsiao, S.S. Neural Correlates of High-Gamma Oscillations (60–200 Hz) in Macaque Local Field Potentials and Their Potential Implications in Electrocorticography. J. Neurosci. 2008, 28, 11526–11536. [Google Scholar] [CrossRef] [Green Version]
- Winawer, J.; Kay, K.N.; Foster, B.L.; Rauschecker, A.M.; Parvizi, J.; Wandell, B.A. Asynchronous Broadband Signals Are the Principal Source of the BOLD Response in Human Visual Cortex. Curr. Biol. 2013, 23, 1145–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukamel, R.; Gelbard, H.; Arieli, A.; Hasson, U.; Fried, I.; Malach, R. Coupling between Neuronal Firing, Field Potentials, and FMRI in Human Auditory Cortex. Science 2005, 309, 951–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzhelyova, M.; Jacques, C.; Rossion, B. At a Single Glance: Fast Periodic Visual Stimulation Uncovers the Spatio-Temporal Dynamics of Brief Facial Expression Changes in the Human Brain. Cereb. Cortex 2017, 27, 4106–4123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rekow, D.; Baudouin, J.-Y.; Rossion, B.; Leleu, A. An Ecological Measure of Rapid and Automatic Face-Sex Categorization. Cortex 2020, 127, 150–161. [Google Scholar] [CrossRef]
- Schalk, G.; Kapeller, C.; Guger, C.; Ogawa, H.; Hiroshima, S.; Lafer-sousa, R. Facephenes and Rainbows: Causal Evidence for Functional and Anatomical Specificity of Face and Color Processing in the Human Brain. Proc. Natl. Acad. Sci. USA 2017, 114, 12285–12290. [Google Scholar] [CrossRef] [Green Version]
- Rangarajan, V.; Hermes, D.; Foster, B.L.; Weiner, K.S.; Jacques, C.; Grill-Spector, K.; Parvizi, J. Electrical Stimulation of the Left and Right Human Fusiform Gyrus Causes Different Effects in Conscious Face Perception. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 12828–12836. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.J.; Davidesco, I.; Megevand, P.; Lado, F.A.; Malach, R.; Mehta, A.D. Tuning Face Perception with Electrical Stimulation of the Fusiform Gyrus. Hum. Brain Mapp. 2017, 38, 2830–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, S.C.; Jo, S.; Park, K.M.; Joo, E.Y.; Lee, M.-J.; Hong, S.C.; Hong, S.B. Interaction between the Electrical Stimulation of a Face-Selective Area and the Perception of Face Stimuli. Neuroimage 2013, 77, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.; Descoins, M.; Koessler, L.; Colnat-Coulbois, S.; Sauvée, M.; Guye, M.; Vignal, J.-P.; Vespignani, H.; Rossion, B.; Maillard, L. Focal Electrical Intracerebral Stimulation of a Face-Sensitive Area Causes Transient Prosopagnosia. Neuroscience 2012, 222, 281–288. [Google Scholar] [CrossRef]
- Jonas, J.; Rossion, B.; Krieg, J.; Koessler, L.; Colnat-Coulbois, S.; Vespignani, H.; Jacques, C.; Vignal, J.P.; Brissart, H.; Maillard, L. Intracerebral Electrical Stimulation of a Face-Selective Area in the Right Inferior Occipital Cortex Impairs Individual Face Discrimination. Neuroimage 2014, 99, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.; Rossion, B.; Brissart, H.; Frismand, S.; Jacques, C.; Hossu, G.; Colnat-Coulbois, S.; Vespignani, H.; Vignal, J.P.; Maillard, L. Beyond the Core Face-Processing Network: Intracerebral Stimulation of a Face-Selective Area in the Right Anterior Fusiform Gyrus Elicits Transient Prosopagnosia. Cortex 2015, 72, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Rossion, B. Twenty Years of Investigation with the Case of Prosopagnosia PS to Understand Human Face Identity Recognition. Part I: Function. Neuropsychologia 2022, 173, 108278. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.; Rossion, B. What Are the Contributions and Challenges of Direct Intracranial Electrical Stimulation in Human Cognitive Neuroscience? In Intracranial EEG. A Guide for Cognitive Neuroscientists; Axmacher, N., Ed.; Springer: Cham, Switzerland, 2023; ISBN 978-3-031-20912-3. [Google Scholar]
- Rey, H.G.; Fried, I.; Quian Quiroga, R. Timing of Single-Neuron and Local Field Potential Responses in the Human Medial Temporal Lobe. Curr. Biol. 2014, 24, 299–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, I.; Rutishauser, U.; Cerf, M.; Kreiman, G. Single Neuron Studies of the Human Brain: Probing Cognition; The MIT Press: Cambridge, MA, USA, 2014; ISBN 9780262027205. [Google Scholar]
- Quiroga, R.Q.; Reddy, L.; Kreiman, G.; Koch, C.; Fried, I. Invariant Visual Representation by Single Neurons in the Human Brain. Nature 2005, 435, 1102–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quiroga, R.Q. Concept Cells: The Building Blocks of Declarative Memory Functions. Nat. Rev. Neurosci. 2012, 13, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, V.; Rozier, C.; Malkinson, T.S.; Lehongre, K.; Adam, C.; Lambrecq, V.; Navarro, V.; Naccache, L. Face-Selective Neurons in the Vicinity of the Human Fusiform Face Area. Neurology 2019, 92, 197–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axelrod, V.; Rozier, C.; Malkinson, T.S.; Lehongre, K.; Adam, C.; Lambrecq, V.; Navarro, V.; Naccache, L. Face-Selective Multi-Unit Activity in the Proximity of the FFA Modulated by Facial Expression Stimuli. Neuropsychologia 2022, 170, 108228. [Google Scholar] [CrossRef] [PubMed]
- Decramer, T.; Premereur, E.; Uytterhoeven, M.; Van Paesschen, W.; van Loon, J.; Janssen, P.; Theys, T. Single-Cell Selectivity and Functional Architecture of Human Lateral Occipital Complex. PLoS Biol. 2019, 17, e3000280. [Google Scholar] [CrossRef] [Green Version]
- Khuvis, S.; Yeagle, E.M.; Norman, Y.; Grossman, S.; Malach, R.; Mehta, A.D. Face-Selective Units in Human Ventral Temporal Cortex Reactivate during Free Recall. J. Neurosci. 2021, 41, 3386–3399. [Google Scholar] [CrossRef]
- Chaure, F.J.; Rey, H.G.; Quian Quiroga, R. A Novel and Fully Automatic Spike-Sorting Implementation with Variable Number of Features. J. Neurophysiol. 2018, 120, 1859–1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]





















| SEEG Advantages | SEEG Weaknesses |
|---|---|
| Recordings in humans (in vivo) Direct recording of neural activity (i.e., not a metabolic correlate) Inside grey matter, including sulci (SEEG) High spatial and temporal resolution Possibility of focal electrical stimulation No major SNR fluctuations (i.e., signal drop due to artifacts) Complex datasets varying in space/time/frequency | Invasive Rare (typically small N) Potential brain lesions or malformations in epileptic patients, long-term epileptic seizures Integrity of brain function in the patients Few electrode locations (limited sampling) Electrode locations based on clinical purposes Variability across patients in electrode positions Complex datasets varying in space/time/frequency |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossion, B.; Jacques, C.; Jonas, J. Intracerebral Electrophysiological Recordings to Understand the Neural Basis of Human Face Recognition. Brain Sci. 2023, 13, 354. https://doi.org/10.3390/brainsci13020354
Rossion B, Jacques C, Jonas J. Intracerebral Electrophysiological Recordings to Understand the Neural Basis of Human Face Recognition. Brain Sciences. 2023; 13(2):354. https://doi.org/10.3390/brainsci13020354
Chicago/Turabian StyleRossion, Bruno, Corentin Jacques, and Jacques Jonas. 2023. "Intracerebral Electrophysiological Recordings to Understand the Neural Basis of Human Face Recognition" Brain Sciences 13, no. 2: 354. https://doi.org/10.3390/brainsci13020354