Justification of the Reduction Possibility of Sulfur Oxides and Fly Ash Emissions during Co-Combustion of Coal and Waste from Woodworking Enterprises
Abstract
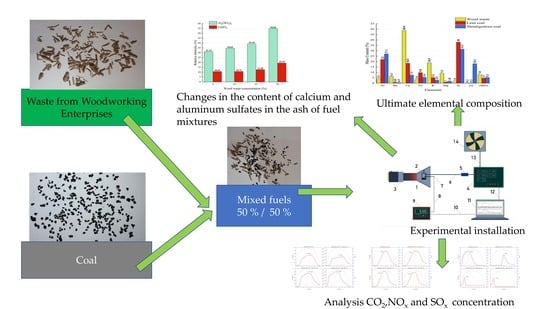
:1. Introduction
2. Materials and Methods of Experimental Research
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IEA Coal-Fuels & Technologies-IEA. Available online: https://www.iea.org/fuels-and-technologies/coal (accessed on 17 March 2021).
- Key World Energy Statistics 2020—Analysis-IEA. Available online: https://www.iea.org/reports/key-world-energy-statistics-2020 (accessed on 17 March 2021).
- British Petroleum. Energy Outlook: 2020 Edition—The Energy Outlook Explores the Forces Shaping the Global Energy Transition out to 2050 and the Key Uncertainties Surrounding that Transition; BP: London, UK, 2020; p. 81. [Google Scholar]
- European Commission. Implementing the SET Plan 2020 Report|SETIS; Publications Office of European Union: Luxembourg, 2020. [Google Scholar]
- Mitchell, S.R.; Harmon, M.E.; O’Connell, K.E.B. Carbon debt and carbon sequestration parity in forest bioenergy production. GCB Bioenergy 2012, 4, 818–827. [Google Scholar] [CrossRef] [Green Version]
- The European Parliament and The Council of The European Union. Directive 2009/28/ec of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:140:0016:0062:en:PDF (accessed on 17 March 2021).
- Ninikas, K.; Hytiris, N.; Emmanuel, R.; Aaen, B. Recovery and Valorisation of Energy from Wastewater Using a Water Source Heat Pump at the Glasgow Subway: Potential for Similar Underground Environments. Resources 2019, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Lee, S.; Chandrasekaran, R.; Yang, Y.; Caballes, M.; Alamu, O.; Chen, G. Electricity Evaluation and Emission Characteristics of Poultry Litter Co-Combustion Process. Appl. Sci. 2019, 9, 4116. [Google Scholar] [CrossRef] [Green Version]
- Ayli, U.E.; Özgirgin, E.; Tareq, M. Solar Chimney Power Plant Performance for Different Seasons under Varying Solar Irradiance and Temperature Distribution. J. Energy Resour. Technol. Trans. ASME 2021, 143, 061303. [Google Scholar] [CrossRef]
- Gebreslassie, M.G. Development and Manufacturing of Solar and Wind Energy Technologies in Ethiopia: Challenges and Policy Implications. Renew. Energy 2020, 168, 107–118. [Google Scholar] [CrossRef]
- Ninikas, K.; Hytiris, N.; Emmanuel, R.; Aaen, B. Heat energy from a shallow geothermal system in Glasgow, UK: Performance evaluation design. Environ. Geotech. 2020, 7, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yang, X.; Luo, Z.; Duan, C.; Song, S. Progress in developments of dry coal beneficiation. Int. J. Coal Sci. Technol. 2014, 1, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Laimon, M.; Mai, T.; Goh, S.; Yusaf, T. Energy sector development: System dynamics analysis. Appl. Sci. 2020, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, E.J.S.; Lea-Langton, A.R.; Jones, J.M.; Williams, A.; Layden, P.; Johnson, R. The impact of fuel properties on the emissions from the combustion of biomass and other solid fuels in a fixed bed domestic stove. Fuel Process. Technol. 2016, 142, 115–123. [Google Scholar] [CrossRef]
- Baxter, L. Biomass-coal co-combustion: Opportunity for affordable renewable energy. Fuel 2005, 84, 1295–1302. [Google Scholar] [CrossRef] [Green Version]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Qian, X.; Xue, J.; Yang, Y.; Lee, S.W. Thermal properties and combustion-related problems prediction of agricultural crop residues. Energies 2021, 14, 4619. [Google Scholar] [CrossRef]
- Casaca, C.; Costa, M. Co-combustion of biomass in a natural gas-fired furnace. Combust. Sci. Technol. 2010, 175, 1953–1977. [Google Scholar] [CrossRef]
- Daian, G.; Ozarska, B. Wood waste management practices and strategies to increase sustainability standards in the Australian wooden furniture manufacturing sector. J. Clean. Prod. 2009, 17, 1594–1602. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Ho, S.H.; Zhang, C.; Tao, F.; Zhang, C.; Chen, W.H. Microalgal Torrefaction for Solid Biofuel Production. Trends Biotechnol. 2020, 38, 1023–1033. [Google Scholar] [CrossRef]
- Ninikas, K.; Mitani, A.; Koutsianitis, D.; Ntalos, G.; Taghiyari, H.R.; Papadopoulos, A.N. Thermal and Mechanical Properties of Green Insulation Composites Made from Cannabis and Bark Residues. J. Compos. Sci. 2021, 5, 132. [Google Scholar] [CrossRef]
- Lim, J.S.; Abdul Manan, Z.; Wan Alwi, S.R.; Hashim, H. A review on utilisation of biomass from rice industry as a source of renewable energy. Renew. Sustain. Energy Rev. 2012, 16, 3084–3094. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Intasit, R.; Cheirsilp, B.; Louhasakul, Y.; Boonsawang, P.; Chaiprapat, S.; Yeesang, J. Valorization of palm biomass wastes for biodiesel feedstock and clean solid biofuel through non-sterile repeated solid-state fermentation. Bioresour. Technol. 2020, 298, 122551. [Google Scholar] [CrossRef]
- Schönnenbeck, C.; Trouvé, G.; Valente, M.; Garra, P.; Brilhac, J.F. Combustion tests of grape marc in a multi-fuel domestic boiler. Fuel 2016, 180, 324–331. [Google Scholar] [CrossRef]
- Mediavilla, I.; Barro, R.; Borjabad, E.; Peña, D.; Fernández, M.J. Quality of olive stone as a fuel: Influence of oil content on combustion process. Renew. Energy 2020, 160, 374–384. [Google Scholar] [CrossRef]
- Skone, T.J.; Littlefield, J.; Eckard, R.; Cooney, G.; Wallace, R.; Marriott, J. Role of Alternative Energy Sources: Pulverized Coal and Biomass Co-Firing Technology Assessment; No. NETL/DOE-2012/1537. National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA; Morgantown, WV, USA; Albany, OR, USA, 2012.
- Liu, P.; Zhu, M.; Leong, Y.K.; Zhang, Y.; Zhang, Z.; Zhang, D. An Experimental Study of the Rheological Properties and Stability Characteristics of Biochar-Algae-Water Slurry Fuels. Energy Procedia 2017, 105, 125–130. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, M.; Wu, H. Fuel properties and ageing of bioslurry prepared from glycerol/methanol/bio-oil blend and biochar. Fuel 2016, 176, 72–77. [Google Scholar] [CrossRef]
- Guo, F.; Zhong, Z. Co-combustion of anthracite coal and wood pellets: Thermodynamic analysis, combustion efficiency, pollutant emissions and ash slagging. Environ. Pollut. 2018, 239, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, G.V.; Yankovsky, S.A.; Tolokolnikov, A.A.; Zenkov, A.V.; Cherednik, I.V. Conditions and characteristics of mixed fuel granules ignition based on coal and finely dispersed wood. Energy 2020, 194, 116896. [Google Scholar] [CrossRef]
- Lauri, P.; Havlík, P.; Kindermann, G.; Forsell, N.; Böttcher, H.; Obersteiner, M. Woody biomass energy potential in 2050. Energy Policy 2014, 66, 19–31. [Google Scholar] [CrossRef]
- Czekała, W.; Bartnikowska, S.; Dach, J.; Janczak, D.; Smurzyńska, A.; Kozłowski, K.; Bugała, A.; Lewicki, A.; Cieślik, M.; Typańska, D.; et al. The energy value and economic efficiency of solid biofuels produced from digestate and sawdust. Energy 2018, 159, 1118–1122. [Google Scholar] [CrossRef]
- Tillman, D.A. Biomass cofiring: The technology, the experience, the combustion consequences. Biomass Bioenergy 2000, 19, 365–384. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.J.; Yu, J.; Jeon, C.H. Improvement in Reactivity and Pollutant Emission by Cofiring of Coal and Pretreated Biomass. Energy Fuels 2019, 33, 4331–4339. [Google Scholar] [CrossRef]
- Zhao, R.; Qin, J.; Chen, T.; Wang, L.; Wu, J. Experimental study on co-combustion of low rank coal semicoke and oil sludge by TG-FTIR. Waste Manag. 2020, 116, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Al-Mansour, F.; Zuwala, J. An evaluation of biomass co-firing in Europe. Biomass Bioenergy 2010, 34, 620–629. [Google Scholar] [CrossRef]
- Agbor, E.; Zhang, X.; Kumar, A. A review of biomass co-firing in North America. Renew. Sustain. Energy Rev. 2014, 40, 930–943. [Google Scholar] [CrossRef]
- Mylläri, F.; Karjalainen, P.; Taipale, R.; Aalto, P.; Häyrinen, A.; Rautiainen, J.; Pirjola, L.; Hillamo, R.; Keskinen, J.; Rönkkö, T. Physical and chemical characteristics of flue-gas particles in a large pulverized fuel-fired power plant boiler during co-combustion of coal and wood pellets. Combust. Flame 2017, 176, 554–566. [Google Scholar] [CrossRef]
- Roni, M.S.; Chowdhury, S.; Mamun, S.; Marufuzzaman, M.; Lein, W.; Johnson, S. Biomass co-firing technology with policies, challenges, and opportunities: A global review. Renew. Sustain. Energy Rev. 2017, 78, 1089–1101. [Google Scholar] [CrossRef]
- Zuwała, J.; Lasek, J. Co-combustion of low-rank coals with biomass. In Low-Rank Coals for Power Generation, Fuel and Chemical Production; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 125–158. ISBN 9780081009291. [Google Scholar]
- Ma, L.; Yu, S.; Chen, X.; Fang, Q.; Yin, C.; Zhang, C.; Chen, G. Combustion interactions in oxy-fuel firing of coal blends: An experimental and numerical study. J. Energy Inst. 2021, 94, 11–21. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Co-combustion of low rank coal/waste biomass blends using dry air or oxygen. Appl. Therm. Eng. 2013, 50, 251–259. [Google Scholar] [CrossRef]
- Krzywański, J.; Czakiert, T.; Muskała, W.; Nowak, W. Modelling of CO2, CO, SO2, O2 and NO x emissions from the oxy-fuel combustion in a circulating fluidized bed. Fuel Process. Technol. 2011, 92, 590–596. [Google Scholar] [CrossRef]
- Yi, Q.; Zhao, Y.; Huang, Y.; Wei, G.; Hao, Y.; Feng, J.; Mohamed, U.; Pourkashanian, M.; Nimmo, W.; Li, W. Life cycle energy-economic-CO2 emissions evaluation of biomass/coal, with and without CO2 capture and storage, in a pulverized fuel combustion power plant in the United Kingdom. Appl. Energy 2018, 225, 258–272. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y. Effect of the Coal Blending Ratio on NOx Formation under Multiple Deep Air-Staged Combustion. Energy Fuels 2020, 34, 853–862. [Google Scholar] [CrossRef]
- Skeen, S.A.; Kumfer, B.M.; Axelbaum, R.L. Nitric oxide emissions during coal and coal/biomass combustion under air-fired and oxy-fuel conditions. Energy Fuels 2010, 24, 4144–4152. [Google Scholar] [CrossRef]
- Coppola, A.; Esposito, A.; Montagnaro, F.; Iuliano, M.; Scala, F.; Salatino, P. The combined effect of H2O and SO2 on CO2 uptake and sorbent attrition during fluidised bed calcium looping. Proc. Combust. Inst. 2019, 37, 4379–4387. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Jankovsky, S.A.; Tolokolnikov, A.A.; Zenkov, A.V. Mechanism of Sulfur and Nitrogen Oxides Suppression in Combustion Products of Mixed Fuels Based on Coal and Wood. Combust. Sci. Technol. 2019, 191, 2071–2081. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Yankovskii, S.A. Conditions and Characteristics in Ignition of Composite Fuels Based on Coal with the Addition of Wood. Therm. Eng. 2019, 66, 133–137. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Huang, W. Influences of igneous intrusions on coal rank, coal quality and adsorption capacity in Hongyang, Handan and Huaibei coalfields, North China. Int. J. Coal Geol. 2011, 88, 135–146. [Google Scholar] [CrossRef]
- Flores, R.M. Coal Composition and Reservoir Characterization. In Coal and Coalbed Gas; Elsevier: Amsterdam, The Netherlands, 2014; pp. 235–299. [Google Scholar]
- Kuznetsov, G.V.; Zenkov, A.V.; Tolokolnikov, A.A.; Cherednik, I.V.; Yankovsky, S.A. Ignition of particles of finely dispersed fuel mixtures based on coal and fine wood. Energy 2020, 220, 119697. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Yankovskii, S.A.; Tolokol’nikov, A.A.; Cherednik, I.V. Mechanism of the Suppression of Sulfur Oxides in the Oxidative Thermolysis Products of Coals upon Their Combustion in a Mixture with Dispersed Wood. Solid Fuel Chem. 2020, 54, 311–317. [Google Scholar] [CrossRef]























| Fuel (Wood/Coal), % | Moisture Content, W a | Ash Content, A d | Volatile Matter Content, V daf | Net Heating Value, Q, MJ/kg | Elemental Composition 1 | ||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | |||||
| wt.% | wt.% | ||||||||
| 100 D | 5.83 | 15.76 | 12.36 | 26.20 | 74.20 | 3.60 | 1.60 | 0.1000 | 20.50 |
| 10 W/90 D | 5.42 | 14.00 | 14.55 | 24.92 | 63.20 | 4.50 | 1.80 | 0.1034 | 30.40 |
| 25 W/75 D | 5.23 | 11.91 | 38.89 | 24.31 | 60.60 | 4.70 | 1.50 | 0.1079 | 33.09 |
| 50 W/50 D | 5.35 | 10.44 | 40.96 | 23.84 | 56.20 | 5.20 | 1 | 0.1179 | 37.48 |
| 100 T | 5.52 | 18.37 | 24.93 | 25.72 | 84.30 | 6.40 | 2.90 | 0.40 | 6.00 |
| 10 W/90 T | 5.42 | 14.24 | 26.46 | 25.60 | 53.50 | 4.10 | 1.40 | 0.15 | 40.85 |
| 25 W/75 T | 5.34 | 13.65 | 28.33 | 25.22 | 52.20 | 4.40 | 1.20 | 0.13 | 42.07 |
| 50 W/50 T | 5.41 | 11.08 | 39.95 | 24.79 | 51.00 | 4.90 | 0.80 | 0.09 | 43.21 |
| 100 W | 5.40 | 0.30 | 80.30 | 21.70 | 58.90 | 6.90 | - | - | 34.20 |
| Element | The Composition of the Mixture | |||
|---|---|---|---|---|
| 100% Coal | 90% Coal/10% Wood | 75% Coal/25% Wood | 50% Coal/50% Wood | |
| SO2,% | Delta | −48.36 | −94.06 | −96.12 |
| Meaning | 6514.5 | 3364 | 387 | 252.6 |
| NOx,% | Delta | −19.16 | −1.38 | −17.32 |
| Meaning | 34554 | 27,934.5 | 34,077 | 28,570.5 |
| CO2,% | Delta | −5.01 | +22.77 | +29.85 |
| Meaning | 20,310,000 | 192,91500 | 26,299,500 | 28,954,260 |
| CO,% | Delta | −39.29 | +48.56 | +70.70 |
| Meaning | 38,671.00 | 23,478.00 | 75,183.00 | 131,977.00 |
| Element | The Composition of the Mixture | |||
|---|---|---|---|---|
| 100% Coal | 90% Coal/10% Wood | 75% Coal/25% Wood | 50% Coal/50% Wood | |
| SO2,% | Delta | −22.46 | −67.52 | −80.24 |
| Meaning | 258.61 | 200.52 | 84.00 | 51.11 |
| NOx,% | Delta | −56.33 | −44.43 | −5.81 |
| Meaning | 382.65 | 167.10 | 212.65 | 360.41 |
| CO2,% | Delta | −72.00 | −40.29 | −23.73 |
| Meaning | 259,895.84 | 72,760.39 | 155,183.33 | 198,214.65 |
| CO,% | Delta, | +13.10 | +48.37 | +61.76 |
| Meaning | 33,047.00 | 38,026.00 | 64,013.00 | 86,429.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yankovsky, S.; Tolokol’nikov, A.; Gorshkov, A.; Misyukova, A.; Kuznetsov, G. Justification of the Reduction Possibility of Sulfur Oxides and Fly Ash Emissions during Co-Combustion of Coal and Waste from Woodworking Enterprises. Appl. Sci. 2021, 11, 11719. https://doi.org/10.3390/app112411719
Yankovsky S, Tolokol’nikov A, Gorshkov A, Misyukova A, Kuznetsov G. Justification of the Reduction Possibility of Sulfur Oxides and Fly Ash Emissions during Co-Combustion of Coal and Waste from Woodworking Enterprises. Applied Sciences. 2021; 11(24):11719. https://doi.org/10.3390/app112411719
Chicago/Turabian StyleYankovsky, Stanislav, Anton Tolokol’nikov, Alexander Gorshkov, Albina Misyukova, and Geniy Kuznetsov. 2021. "Justification of the Reduction Possibility of Sulfur Oxides and Fly Ash Emissions during Co-Combustion of Coal and Waste from Woodworking Enterprises" Applied Sciences 11, no. 24: 11719. https://doi.org/10.3390/app112411719