Adsorption of Ethylenediaminetetraacetic Acid on a Gel-Type Ion-Exchange Resin for Purification of Liquid Waste Containing Cs Ions
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Instrumentation
2.2. Determination of EDTA Concentration
2.3. Batch Adsorption
3. Results and Discussion
3.1. Characterization of the IERs
3.2. Effect of Adsorption Time in Pure EDTA Solution
3.3. Effect of pH in Pure EDTA Solution
3.4. Effect of the Amount of Adsorbent in Pure EDTA Solution
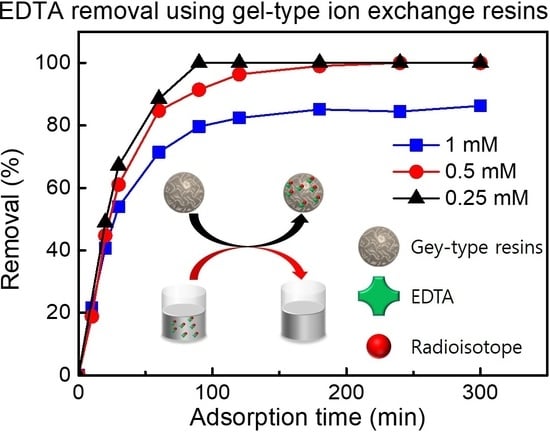
3.5. Effect of Adsorption Time in a Solution Mixed with EDTA and Cs Ions
3.6. Adsorption Kinetics
3.7. Adsorption Isotherms
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jardine, P.M.; Taylor, D.L. Fate and transport of ethylenediaminetetraacetate chelated contaminants in subsurface environments. Geoderma 1995, 67, 125–140. [Google Scholar] [CrossRef]
- Mahmoudian, M.; Nozad, E.; Hosseinzadeh, M. Characterization of EDTA functionalized graphene oxide/polyethersulfone (FGO/PES) nanocomposite membrane and using for elimination of heavy metal and dye contaminations. Polym. Korea 2018, 42, 434–445. [Google Scholar] [CrossRef]
- Hishinuma, N.Y.; Kaji, R.; Akimoto, H.; Nakajima, F.; Mor, T.; Kamo, T.; Arikawa, Y.; Nozawa, S. Reversible binding of NO to Fe(II)–EDTA. Bull. Chem. Soc. Jpn. 1979, 52, 2863–2865. [Google Scholar] [CrossRef]
- Sada, E.; Kumazawa, H. Individual and simultaneous absorption of dilute NO and SO2 in aqueous slurries of MgSO3 with Fe (II)–EDTA. Ind. Eng. Chem. Process Des. Dev. 1980, 19, 377–382. [Google Scholar] [CrossRef]
- Yih, S.M.; Lii, C.W. Simultaneous absorption of nitric oxide and sulphur dioxide in FeII–EDTA solutions in a packed absorber-stripper unit. J. Chem. Eng. 1989, 42, 145–152. [Google Scholar]
- Gambardella, F.; Winkelman, J.G.M.; Heeres, H.J. Experimental and modelling studies on the simultaneous absorption of NO and O2 in aqueous iron chelate solutions. Chem. Eng. Sci. 2006, 61, 6880–6891. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, W.R.; Wu, Z.B. Simultaneous absorption of NO and SO2 by Fe (II)–EDTA combined with Na2SO3 solution. J. Chem. Eng. 2007, 132, 227–232. [Google Scholar] [CrossRef]
- William, J.C.; Randy, D.C.; Kevin, E.O. Organics in mixed nuclear wastes: Gamma-radiolytic degradation of chelating and complexing agents. In Environmental Applications of Ionizing Radiation; Anthony, P.T., Ed.; John Wiley & Sons: New York, NY, USA, 1998; pp. 429–449. [Google Scholar]
- Hladký, E.; Blažek, J.; Majerský, D.; Řeháček, V. Decontamination and Decommissioning of Nuclear Facilities; TECDOC-511; IAEA: Vienna, Austria, 1989. [Google Scholar]
- Rahman, R.O.A.; Ibrahium, H.A.; Hung, Y.-T. Liquid radioactive wastes treatment: A review. Water 2011, 3, 551–565. [Google Scholar] [CrossRef]
- Sinha, P.K.; Amalraj, R.V.; Krishnasamy, V. Flocculation studies on freshly precipitated copper ferrocyanide for the removal of caesium from radioactive liquid waste. Waste Manag. 1993, 13, 341–350. [Google Scholar] [CrossRef]
- Hinck, M.L.; Ferguson, J.; Puhaakka, J. Resistance of EDTA and DTPA to aerobic biodegradation. Water Sci. Technol. 1997, 35, 25–31. [Google Scholar] [CrossRef]
- Brauch, H.J.; Schullerer, S. Behaviour of ethylenediaminetetraacetate (EDTA) and nitrilotriacetate (NTA) in drinking water treatement. Vom Wasser 1987, 69, 155–164. [Google Scholar]
- Rubio, J.; Matijevic, E. Interaction of metal hydrous oxides with chelating agents I. β-FeOOH-EDTA. J. Colloid Interface Sci. 1979, 68, 408–421. [Google Scholar] [CrossRef]
- Bowers, A.R.; Huang, C.P. Adsorption characteristics of polyacetic aminoacids onto hydrous γ-Al2O3. J. Colloid Interface Sci. 1985, 106, 197–215. [Google Scholar] [CrossRef]
- Ryczkowski, J. FT-IR study of the adsorption of some complexones and of EDTA alkaline salts into alumina. Vib. Spectrosc. 2000, 22, 55–62. [Google Scholar] [CrossRef]
- Nowack, B.; Sigg, L. Adsorption of EDTA and metal-EDTA complexes onto goethite. J. Colloid Interface Sci. 1996, 177, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, H.; Lan, H.; Liu, H.; Qu, J. Adsorption of Cu(II)–EDTA chelates on tri-ammonium-functionalized mesoporous silica from aqueous solution. Sep. Sci. Technol. 2013, 117, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.-S.; Yang, X.-J.; Mao, Y.-P.; Chen, Y.; Long, X.-L.; Yuan, W.-K. Adsorption of EDTA on activated carbon from aqueous solutions. J. Hazard. Mater. 2011, 185, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Sillanpää, M.E.T.; Yavari, Z.; Savadkoohi, M.; Ghadiri, K.; Mahvi, A.H. Adsorption of EDTA from aqueous solution using HDTMA-modified zeolite. Middle-East J. Sci. Res. 2015, 23, 2232–2245. [Google Scholar]
- Li, Z.H.; Bowman, R.S. Sorption of perchloroethylene by surfactant-modified zeolite as controlled by surfactant loading. Environ. Sci. Technol. 1998, 32, 2278–2282. [Google Scholar] [CrossRef]
- Guelat, B.; Khalaf, R.; Lattuada, M.; Costioli, M.; Morbidelli, M. Protein adsorption on ion exchange resins and monoclonal antibodycharge variant modulation. J Chromatogr. A 2016, 1447, 82–91. [Google Scholar]
- Zhang, J.; Zhu, C.; Zhou, F.; Ma, L. Adsorption behavior and kinetics for L-valine separation from aqueous solution using ion exchange resin. React. Funct. Polym. 2018, 130, 51–60. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, W.; Zhang, Z.; Yang, Z.; Wang, Y. Adsorption of three pharmaceuticals on two magnetic ion-exchange resins. J. Environ. Sci. 2015, 31, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zuo, P.; Luo, J.; Singh, R.P. Adsorption behavior of benzenesulfonic acid by novel weakly basic anion exchange resins. J. Environ. Sci. 2017, 54, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, D.; Beiser, N.; Regenbrecht, C.; Zirbes, M.; Waldvogel, S.R. Adsorption and separation of black liquor-derived phenol derivatives using anion exchange resins. Sep. Purif. Technol. 2017, 181, 8–17. [Google Scholar] [CrossRef]
- Parvazinia, M.; Garcia, S.; Maroto-Valer, M. CO2 capture by ion exchange resins as amine functionalised adsorbents. Chem. Eng. J. 2018, 331, 335–342. [Google Scholar] [CrossRef]
- Chiarle, S.; Ratto, M.; Rovatti, M. Mercury removal from water by ion exchange resins adsorption. Water Res. 2000, 34, 2971–2978. [Google Scholar] [CrossRef]
- Shen, S.; Guishen, L.; Pan, T.; He, J.; Guo, Z. Selective adsorption of Pt ions from chloride solutions obtained by leaching chlorinated spent automotive catalysts on ion exchange resin Diaion WA21J. J. Colloid Interface Sci. 2011, 364, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Ezzeldin, H.A.; Apblett, A.; Foutch, G.L. Synthesis and properties of anion exchangers derived from chloromethyl styrene codivinylbenzene and their use in water treatment. Int. J. Polym. Sci. 2010, 2010, 684051. [Google Scholar] [CrossRef]
- Gokulakrishnan, N.; Pandurangan, A.; Sinha, P.K. Removal of decontaminating agent ethylenediaminetetraacetic acid from aqueous solution by an effective mesoporous Al-MCM-41 molecular sieves. Ind. Eng. Chem. Res. 2006, 45, 5326–5331. [Google Scholar] [CrossRef]
- Krishnan, K.A.; Sreejalekshmi, K.G.; Varghese, S.; Anirudhan, T.S. Removal of EDTA from aqueous solutions using activated carbon prepared from rubber wood sawdust: Kinetic and equilibrium modeling. Clean–Soil Air Water 2010, 38, 361–369. [Google Scholar] [CrossRef]
- Ho, Y.-S.; Mckay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Ho, Y.S. McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–469. [Google Scholar] [CrossRef]
- Asthana, A.; Verma, R.; Singh, A.K.; Susan, M.A.B.H.; Adhikari, R. Silver nanoparticle entrapped calcium-alginate beads for Fe (II) removal via adsorption. Macromol. Symp. 2016, 366, 42–51. [Google Scholar] [CrossRef]
- Cho, E.; Kim, J.; Park, C.W.; Lee, K.-W.; Lee, T.S. Chemically bound Prussian blue in sodium alginate hydrogel for enhanced removal of Cs ions. J. Hazard. Mater. 2018, 360, 243–249. [Google Scholar] [CrossRef] [PubMed]















| C0 (mmol/L) | qexp (mmol/g) | Pseudo-first-order | Pseudo-second-order | |||||
|---|---|---|---|---|---|---|---|---|
| k1 (min−1) | qe,cal (mmol/g) | R2 | k2 (g mmol−1 min−1) | qe,cal (mmol/g) | h (mmol g−1 min−1) | R2 | ||
| 1 | 0.41 | 0.0204 | 0.2503 | 0.9768 | 0.1301 | 0.4347 | 0.0074 | 0.9925 |
| 0.5 | 0.25 | 0.0310 | 0.3150 | 0.9487 | 0.1226 | 0.2808 | 0.0042 | 0.9721 |
| 0.25 | 0.13 | 0.0932 | 0.5636 | 0.9630 | 0.6827 | 0.1310 | 0.0611 | 0.9943 |
| Isotherm Models | Isotherm Constants | Value |
|---|---|---|
| Langmuir | qmax (mmol/g) | 0.4725 |
| b (L/mmol) | 15.130 | |
| R2 | 0.9988 | |
| Freundlich | 1/n (L/mmol) | 0.6494 |
| KF (mmol/g) | 1.081 | |
| R2 | 0.9721 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Park, C.W.; Lee, K.-W.; Lee, T.S. Adsorption of Ethylenediaminetetraacetic Acid on a Gel-Type Ion-Exchange Resin for Purification of Liquid Waste Containing Cs Ions. Polymers 2019, 11, 297. https://doi.org/10.3390/polym11020297
Kim J, Park CW, Lee K-W, Lee TS. Adsorption of Ethylenediaminetetraacetic Acid on a Gel-Type Ion-Exchange Resin for Purification of Liquid Waste Containing Cs Ions. Polymers. 2019; 11(2):297. https://doi.org/10.3390/polym11020297
Chicago/Turabian StyleKim, Jongho, Chan Woo Park, Kune-Woo Lee, and Taek Seung Lee. 2019. "Adsorption of Ethylenediaminetetraacetic Acid on a Gel-Type Ion-Exchange Resin for Purification of Liquid Waste Containing Cs Ions" Polymers 11, no. 2: 297. https://doi.org/10.3390/polym11020297