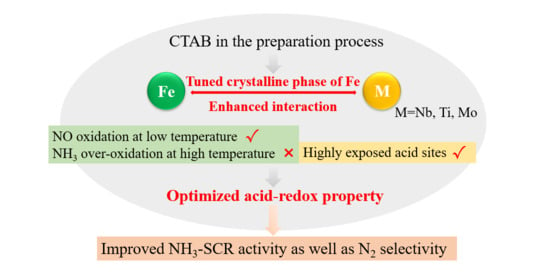
Iron-Based Composite Oxide Catalysts Tuned by CTAB Exhibit Superior NH3–SCR Performance
Abstract
:1. Introduction
2. Results
2.1. NH3–SCR Performance
2.2. Kinetic Studies
2.3. Structural Properties
2.3.1. N2-Physisorption Analysis
2.3.2. XRD Analysis
2.4. Acidity and Redox Ability
2.4.1. Acidic Properties
2.4.2. XPS Analysis
2.4.3. H2-TPR Analysis
2.4.4. Direct Oxidation of NH3 and NO
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Activity Test
4.3. Kinetic Study
4.4. Catalyst Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Han, L.; Cai, S.; Gao, M.; Hasegawa, J.Y.; Wang, P.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Shan, W.; Yu, Y.; Zhang, Y.; He, G.; Peng, Y.; Li, J.; He, H. Theory and practice of metal oxide catalyst design for the selective catalytic reduction of NO with NH3. Catal. Today 2020. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx abatement systems for mobile sources: From three-way to lean burn after-treatment technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef]
- Liu, F.; Yu, Y.; He, H. Environmentally-benign catalysts for the selective catalytic reduction of NOx from diesel engines: Structure-activity relationship and reaction mechanism aspects. Chem. Commun. 2014, 50, 8445–8463. [Google Scholar] [CrossRef] [PubMed]
- Marberger, A.; Ferri, D.; Elsener, M.; Krocher, O. The significance of Lewis acid sites for the selective catalytic reduction of nitric oxide on vanadium-based catalysts. Angew. Chem. Int. Ed. Engl. 2016, 55, 11989–11994. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C.; Feng, Z.; Zheng, L.; Xie, Y.; Hu, T. Selective catalytic reduction of NO with NH3 over iron titanate catalyst: Catalytic performance and characterization. Appl. Catal. B Environ. 2010, 96, 408–420. [Google Scholar] [CrossRef]
- Yang, S.; Li, J.; Wang, C.; Chen, J.; Ma, L.; Chang, H.; Chen, L.; Peng, Y.; Yan, N. Fe–Ti spinel for the selective catalytic reduction of NO with NH3: Mechanism and structure–activity relationship. Appl. Catal. B Environ. 2012, 117–118, 73–80. [Google Scholar] [CrossRef]
- Liu, J.; Meeprasert, J.; Namuangruk, S.; Zha, K.; Li, H.; Huang, L.; Maitarad, P.; Shi, L.; Zhang, D. Facet–activity relationship of TiO2 in Fe2O3/TiO2 nanocatalysts for selective catalytic reduction of NO with NH3: In situ DRIFTs and DFT studies. J. Phys. Chem. C. 2017, 121, 4970–4979. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, N.; Li, Q.; Zhang, Z.; Cao, X.; Zheng, L.; Zeng, Y.; Anderson, J.A. Active site identification and modification of electronic states by atomic-scale doping to enhance oxide catalyst innovation. ACS Catal. 2018, 8, 1399–1404. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, C.; Chen, J.; Tang, X. Self-prevention of well-defined-facet Fe2O3/MoO3 against deposition of ammonium bisulfate in low-temperature NH3-SCR. Environ. Sci. Technol. 2018, 52, 11796–11802. [Google Scholar] [CrossRef]
- Qu, W.; Liu, X.; Chen, J.; Dong, Y.; Tang, X.; Chen, Y. Single-atom catalysts reveal the dinuclear characteristic of active sites in NO selective reduction with NH3. Nat Commun. 2020, 11, 1532. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qu, Z.; Dong, S.; Xie, H.; Tang, C. Superior performance of Fe1-xWxOδ for the selective catalytic reduction of NOx with NH3: Interaction between Fe and W. Environ. Sci. Technol. 2016, 50, 13511–13519. [Google Scholar] [CrossRef]
- Liu, F.; Shan, W.; Lian, Z.; Liu, J.; He, H. The smart surface modification of Fe2O3 by WOx for significantly promoting the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2018, 230, 165–176. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, N.; Li, Q.; Zhang, Z.; Cao, X.; Zheng, L.; Zeng, Y.; Anderson, J.A. Selective catalytic reduction of NO with NH3 over short-range ordered W-O-Fe structures with high thermal stability. Appl. Catal. B Environ. 2018, 229, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Xin, Y.; Wang, X.; Shao, M.; Li, Q.; Ma, X.; Qi, Y.; Zheng, L.; Zhang, Z. Iron-niobium composite oxides for selective catalytic reduction of NO with NH3. Catal. Commun. 2017, 97, 111–115. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Zhang, C. Novel iron titanate catalyst for the selective catalytic reduction of NO with NH3 in the medium temperature range. Chem. Commun. 2008, 17, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- Mou, X.; Zhang, B.; Li, Y.; Yao, L.; Wei, X.; Su, D.S.; Shen, W. Rod-shaped Fe2O3 as an efficient catalyst for the selective reduction of nitrogen oxide by ammonia. Angew. Chem. Int. Ed. Engl. 2012, 51, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Chen, Y.; Huang, Z.; Gao, J.; Zhou, M.; Chen, J.; Li, C.; Ma, Z.; Chen, J.; Tang, X. Active tetrahedral iron sites of γ-Fe2O3 catalyzing NO reduction by NH3. Environ. Sci. Technol. Let. 2017, 4, 246–250. [Google Scholar] [CrossRef]
- Ning, W.; Wang, T.; Chen, H.; Yang, X.; Jin, Y. The effect of Fe2O3 crystal phases on CO2 hydrogenation. PLoS ONE 2017, 12, e0182955. [Google Scholar] [CrossRef] [Green Version]
- Apte, S.K.; Naik, S.D.; Sonawane, R.S.; Kale, B.B.; Baeg, J.O. Synthesis of nanosize-necked structure α- and γ-Fe2O3 and its photocatalytic activity. J. Am. Ceram. Soc. 2007, 90, 412–414. [Google Scholar] [CrossRef]
- Shi, X.; Chu, B.; Wang, F.; Wei, X.; Teng, L.; Fan, M.; Li, B.; Dong, L.; Dong, L. Mn-modified CuO, CuFe2O4 and gamma-Fe2O3 three-phase strong synergistic coexistence catalyst system for NO reduction by CO with wider active window. ACS Appl. Mater. Interfaces 2018, 10, 40509–40522. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Shafi, K.V.P.M.; Loos, K.; Ulman, A.; Lee, Y.; Vogt, T.; Estournès, C. Doping γ-Fe2O3 nanoparticles with Mn(III) suppresses the transition to the α-Fe2O3 structure. J. Am. Chem. Soc. 2003, 125, 11470–11471. [Google Scholar] [CrossRef]
- Machala, L.; Tucek, J.; Zboril, R. Polymorphous transformations of nanometric Iron(III) Oxide: A review. Chem. Mater. 2011, 23, 3255–3272. [Google Scholar] [CrossRef]
- Geng, Y.; Xiong, S.; Li, B.; Peng, Y.; Yang, S. The promotion of H3PW12O40 grafting on NOx abatement over γ-Fe2O3: Performance and reaction mechanism. Ind. Eng. Chem. Res. 2018, 57, 13661–13670. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Hochella, M.F., Jr.; Madden, A.S. Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition. Phys. Chem. Chem. Phys. 2007, 9, 1736–1750. [Google Scholar] [CrossRef]
- Yang, S.; He, H.; Wu, D.; Chen, D.; Liang, X.; Qin, Z.; Fan, M.; Zhu, J.; Yuan, P. Decolorization of methylene blue by heterogeneous Fenton reaction using Fe3−xTixO4 (0 ≤ x ≤ 0.78) at neutral pH values. Appl. Catal. B Environ. 2009, 89, 527–535. [Google Scholar] [CrossRef]
- Lee, J.; Kwak, S.Y. Mn-doped maghemite (gamma-Fe2O3) from metal-organic framework accompanying redox reaction in a bimetallic system: The structural phase transitions and catalytic activity toward NOx removal. ACS Omega 2018, 3, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiong, Z.; Du, Y.; Ning, X.; Li, Z.; He, J.; Qu, X.; Lu, W.; Wu, S.; Tan, L. Promotional effect of tungsten modification on magnetic iron oxide catalyst for selective catalytic reduction of NO with NH3. J. Energy Inst. 2020. [Google Scholar] [CrossRef]
- Bharathi, S.; Nataraj, D.; Seetha, M.; Mangalaraj, D.; Ponpandian, N.; Masuda, Y.; Senthil, K.; Yong, K. Controlled growth of single-crystalline, nanostructured dendrites and snowflakes of α-Fe2O3: Influence of the surfactant on the morphology and investigation of morphology dependent magnetic properties. CrystEngComm 2010, 12, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Chen, X.; Deng, Z.; Li, Y. A CTAB-assisted hydrothermal orientation growth of ZnO nanorods. Mater. Chem. Phys. 2002, 78, 99–104. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Zhang, L.; Cao, Y.; Zou, W.; Li, L.; Ma, K.; Tang, C.; Gao, F.; Dong, L. Improved low temperature NH3-SCR performance of FeMnTiOx mixed oxide with CTAB-assisted synthesis. Chem. Commun. 2015, 51, 3470–3473. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Hou, Y.; Guo, Y.; Liu, Y.; Cui, Y.; Huang, Z. In situ preparation of mesoporous iron titanium catalysts by a CTAB-assisted process for NO reduction with NH3. Appl. Catal. A Gen. 2018, 559, 146–152. [Google Scholar] [CrossRef]
- Ding, S.; Liu, F.; Shi, X.; He, H. Promotional effect of Nb additive on the activity and hydrothermal stability for the selective catalytic reduction of NO with NH3 over CeZrO catalyst. Appl. Catal. B Environ. 2016, 180, 766–774. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; He, H.; Shi, X.; Mo, J.; Wu, Z. Manganese–niobium mixed oxide catalyst for the selective catalytic reduction of NOx with NH3 at low temperatures. Chem. Eng. J. 2014, 250, 390–398. [Google Scholar] [CrossRef]
- Fudong, L.; Hong, H. Structure-activity relationship of iron titanate catalysts in the selective catalytic reduction of NOx with NH3. J. Phys. Chem. C. 2010, 114, 16929–16936. [Google Scholar] [CrossRef]
- Xu, W.; Yu, Y.; Zhang, C.; He, H. Selective catalytic reduction of NO by NH3 over a Ce/TiO2 catalyst. Catal. Commun. 2008, 9, 1453–1457. [Google Scholar] [CrossRef]
- Xu, H.; Liu, J.; Zhang, Z.; Liu, S.; Lin, Q.; Wang, Y.; Dai, S.; Chen, Y. Design and synthesis of highly-dispersed WO3 catalyst with highly effective NH3–SCR activity for NOx abatement. ACS Catal. 2019, 11557–11562. [Google Scholar] [CrossRef]
- Yamada, Y.; Nishida, N. Iron-based nanoparticles and their Mössbauer spectra. Radioisotopes 2019, 68, 125–143. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Guo, L.; Tao, Y.; Wang, Y.B.; Wang, W.D. Synthesis and interfacial structure of nanoparticles γ-Fe2O3 coated with surfactant DBS and CTAB. Nanostruct. Mater. 1999, 11, 487–492. [Google Scholar] [CrossRef]
- Guo, L.; Wu, Z.; Liu, T.; Yang, S. The effect of surface modification on the microstructure and properties of γ-Fe2O3 nanoparticles. Physica E 2000, 8, 199–203. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Meng, X.; Li, Y.; Zhao, J. Structural evolution and characteristics of the phase transformations between α-Fe2O3, Fe3O4 and γ-Fe2O3 nanoparticles under reducing and oxidizing atmospheres. CrystEngComm 2013, 15, 8166–8172. [Google Scholar] [CrossRef]
- Ramos Guivar, J.A.; Sanches, E.A.; Bruns, F.; Sadrollahi, E.; Morales, M.A.; López, E.O.; Litterst, F.J. Vacancy ordered γ-Fe2O3 nanoparticles functionalized with nanohydroxyapatite: XRD, FTIR, TEM, XPS and Mössbauer studies. Appl. Surf. Sci. 2016, 389, 721–734. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Dunlop, D.J.; Berquó, T.S. Morin transition in hematite: Size dependence and thermal hysteresis. Geochem. Geophys. Geosyst. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Cheng, C.; Lin, C.; Chiang, R.; Lin, C.; Lyubutin, I.; Alkaev, E.; Lai, H. Synthesis of monodisperse magnetic iron oxide nanoparticles from submicrometer hematite powders. Cryst. Growth Des. 2008, 8, 877–883. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, W.; An, D.; Wang, X.; Liu, A.; Zou, W.; Tang, C.; Ge, C.; Tong, Q.; Sun, J.; et al. Insight into the SO2 resistance mechanism on γ-Fe2O3 catalyst in NH3-SCR reaction: A collaborated experimental and DFT study. Appl. Catal. B Environ. 2021, 281. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Du, Y.; Wu, X.; Shen, H.; Jing, G. Atomic-scale insights into the nature of active sites in Fe2O3-supported submonolayer WO3 catalysts for selective catalytic reduction of NO with NH3. Chem. Eng. J. 2020, 381, 122668. [Google Scholar] [CrossRef]
- Nguyen, T.P.T.; Yang, K.H.; Kim, M.H.; Hong, Y.S. Selective catalytic reduction of NO by NH3 over Fe2O3-promoted V2O5/TiO2-based catalysts with high Fe2O3-to-V2O5 ratios. Catal. Today 2020. [Google Scholar] [CrossRef]
- Zhu, N.; Shan, W.; Lian, Z.; Zhang, Y.; Liu, K.; He, H. A superior Fe-V-Ti catalyst with high activity and SO2 resistance for the selective catalytic reduction of NO with NH3. J. Hazard. Mater. 2019, 382, 120970. [Google Scholar] [CrossRef]
- Wang, P.; Chen, S.; Gao, S.; Zhang, J.; Wang, H.; Wu, Z. Niobium oxide confined by ceria nanotubes as a novel SCR catalyst with excellent resistance to potassium, phosphorus, and lead. Appl. Catal. B Environ. 2018, 231, 299–309. [Google Scholar] [CrossRef]
- Lian, Z.; Shan, W.; Zhang, Y.; Wang, M.; He, H. Morphology-dependent catalytic performance of NbOx/CeO2 catalysts for selective catalytic reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 12736–12741. [Google Scholar] [CrossRef]
- Liu, F.; Asakura, K.; He, H.; Shan, W.; Shi, X.; Zhang, C. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2011, 103, 369–377. [Google Scholar] [CrossRef]
- Mendiola-Alvarez, S.Y.; Hernández-Ramírez, A.; Guzmán-Mar, J.L.; Maya-Treviño, M.L.; Caballero-Quintero, A.; Hinojosa-Reyes, L. A novel P-doped Fe2O3-TiO2 mixed oxide: Synthesis, characterization and photocatalytic activity under visible radiation. Catal. Today 2019, 328, 91–98. [Google Scholar] [CrossRef]
- Pu, Z.; Liu, Q.; Asiri, A.M.; Luo, Y.; Sun, X.; He, Y. 3D macroporous MoS2 thin film: In situ hydrothermal preparation and application as a highly active hydrogen evolution electrocatalyst at all pH values. Electrochim. Acta. 2015, 168, 133–138. [Google Scholar] [CrossRef]
- Hanawa, T.; Hiromoto, S.; Asami, K. Characterization of the surface oxide film of a Co-Cr-Mo alloy after being located in quasi-biological environments using XPS. Appl. Surf. Sci. 2001, 183, 68–75. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; Du, Y.; Wu, X.; Shen, H.; Jing, G. Alkali-poisoning-resistant Fe2O3/MoO3/TiO2 catalyst for the selective reduction of NO by NH3: The role of the MoO3 safety buffer in protecting surface active sites. Environ. Sci. Technol. 2020, 54, 595–603. [Google Scholar] [CrossRef] [PubMed]
- France, L.J.; Li, W.; Zhang, Y.; Mu, W.; Chen, Z.; Shi, J.; Zeng, Q.; Li, X. A superior Fe-Zr mixed oxide catalyst for the simultaneous reduction of NO and SO2 with CO. Appl. Catal. B Environ. 2020, 269, 118822. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, R.; Wang, M.; Li, Y.; Tong, Y.; Yang, P.; Zhu, Y. Two steps synthesis of CeTiOx oxides nanotube catalyst: Enhanced activity, resistance of SO2 and H2O for low temperature NH3-SCR of NOx. Appl. Catal. B Environ. 2021, 282. [Google Scholar] [CrossRef]
- Xu, Q.; Fang, Z.; Chen, Y.; Guo, Y.; Guo, Y.; Wang, L.; Wang, Y.; Zhang, J.; Zhan, W. Titania-samarium-manganese composite oxide for the low-temperature selective catalytic reduction of NO with NH3. Environ. Sci. Technol. 2020, 54, 2530–2538. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Gao, M.; Feng, C.; Shi, L.; Zhang, D. Fe2O3-CeO2@Al2O3 nanoarrays on Al-mesh as SO2-tolerant monolith catalysts for NOx reduction by NH3. Environ. Sci. Technol. 2019, 53, 5946–5956. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Q.; He, C.; Ma, C.; Cheng, J.; Li, L.; Hao, Z. Investigation of selective catalytic reduction of N2O by NH3 over an Fe–mordenite catalyst: Reaction mechanism and O2 effect. ACS Catal. 2012, 2, 512–520. [Google Scholar] [CrossRef]
- Shu, Y.; Sun, H.; Quan, X.; Chen, S. Enhancement of catalytic activity over the iron-modified Ce/TiO2 catalyst for selective catalytic reduction of NOx with ammonia. J. Phys. Chem. C 2012, 116, 25319–25327. [Google Scholar] [CrossRef]
- Zhu, B.; Zi, Z.; Sun, Y.; Fang, Q.; Xu, J.; Song, W.; Yu, H.; Liu, E. Enhancing low-temperature SCR de-NOx and alkali metal poisoning resistance of a 3Mn10Fe/Ni catalyst by adding Co. Catal. Sci. Technol. 2019, 9, 3214–3225. [Google Scholar] [CrossRef]
- Yang, J.; Ren, S.; Zhou, Y.; Su, Z.; Yao, L.; Cao, J.; Jiang, L.; Hu, G.; Kong, M.; Yang, J.; et al. In situ IR comparative study on N2O formation pathways over different valence states manganese oxides catalysts during NH3–SCR of NO. Chem. Eng. J. 2020, 397, 125446. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Li, J.; Yan, N.; Ma, L.; Chang, H. Low temperature selective catalytic reduction of NO with NH3 over Mn–Fe spinel: Performance, mechanism and kinetic study. Appl. Catal. B Environ. 2011, 110, 71–80. [Google Scholar] [CrossRef]
- Lv, Y.; Yao, W.; Zong, R.; Zhu, Y. Fabrication of wide-range-visible photocatalyst Bi2WO6-x nanoplates via surface oxygen vacancies. Sci. Rep. 2016, 6, 19347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Chen, J.; Bai, B.; Zhao, S.; Hu, F.; Li, J. Bridging the reaction route of toluene total oxidation and the structure of ordered mesoporous Co3O4: The roles of surface sodium and adsorbed oxygen. Catal. Today 2017, 297, 173–181. [Google Scholar] [CrossRef]
- Xu, L.; Niu, S.; Lu, C.; Zhang, Q.; Li, J. Influence of calcination temperature on Fe0.8Mg0.2O catalyst for selective catalytic reduction of NO with NH3. Fuel 2018, 219, 248–258. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Wang, X.; Zou, W.; Cao, Y.; Sun, J.; Tang, C.; Gao, F.; Deng, Y.; Dong, L. Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl. Catal. A Gen. 2015, 505, 235–242. [Google Scholar] [CrossRef]
- Marberger, A.; Elsener, M.; Ferri, D.; Sagar, A.; Schermanz, K.; Krocher, O. Generation of NH3 selective catalytic reduction active catalysts fromdecomposition of supported FeVO4. ACS Catal. 2015, 5, 4180–4188. [Google Scholar] [CrossRef]
- Xin, Y.; Li, H.; Zhang, N.; Li, Q.; Zhang, Z.; Cao, X.; Hu, P.; Zheng, L.; Anderson, J.A. Molecular-level insight into selective catalytic reduction of NOx with NH3 to N2 over a highly efficient bifunctional Va-MnOx catalyst at low temperature. ACS Catal. 2018, 8, 4937–4949. [Google Scholar] [CrossRef] [Green Version]






| Ea (kJ mol−1) | R2 | Reaction Rate at 260 °C (mol m−2 s−1) | |
|---|---|---|---|
| FeNb0.3Ox-C | 34.5 | 0.991 | 9.9 × 10−9 |
| FeNb0.3Ox | 40.0 | 0.993 | 2.9 × 10−9 |
| FeTi0.3Ox-C | 28.0 | 0.993 | 9.5 × 10−9 |
| FeTi0.3Ox | 22.6 | 0.999 | 6.1 × 10−9 |
| FeMo0.3Ox-C | 26.3 | 0.993 | 9.5 × 10−9 |
| FeMo0.3Ox | 26.2 | 0.990 | 7.1 × 10−9 |
| Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | |
|---|---|---|
| FeNb0.3Ox-C | 172 | 0.25 |
| FeNb0.3Ox | 210 | 0.13 |
| FeTi0.3Ox-C | 151 | 0.21 |
| FeTi0.3Ox | 191 | 0.24 |
| FeMo0.3Ox-C | 99 | 0.15 |
| FeMo0.3Ox | 167 | 0.24 |
| NH3 Desorption Amount (μmol g−1) * | NH3 Desorption Amount Normalized by Surface Area (μmol m−2) | Percentage of Weak-Adsorbed NH3 (%) | |
|---|---|---|---|
| FeNb0.3Ox-C | 260 ± 7.0 | 1.5 ± 0.05 | 75.9 ± 1.3 |
| FeNb0.3Ox | 289 ± 7.6 | 1.4 ± 0.07 | 72.8 ± 1.1 |
| FeTi0.3Ox-C | 226 ± 4.7 | 1.5 ± 0.03 | 75.9 ± 0.1 |
| FeTi0.3Ox | 246 ± 12.7 | 1.3 ± 0.08 | 74.2 ± 1.2 |
| FeMo0.3Ox-C | 145 ± 8.3 | 1.5 ± 0.12 | 79.2 ± 1.7 |
| FeMo0.3Ox | 229 ± 15.2 | 1.4 ± 0.08 | 77.9 ± 1.2 |
| Proportion of O | Oα/(Oα + Oβ) | Proportion of Oα | M/Fe | |
|---|---|---|---|---|
| FeNb0.3Ox-C | 49.4% | 33.2% | 16.4% | 0.49 |
| FeNb0.3Ox | 48.5% | 32.6% | 15.8% | 0.43 |
| FeTi0.3Ox-C | 50.7% | 38.7% | 19.6% | 0.72 |
| FeTi0.3Ox | 48.3% | 36.4% | 17.6% | 0.45 |
| FeMo0.3Ox-C | 52.5% | 33.3% | 17.5% | 0.97 |
| FeMo0.3Ox | 49.6% | 35.0% | 17.4% | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Shi, X.; Gao, M.; Liu, J.; Lv, Z.; Wang, Y.; Huo, Y.; Cui, C.; Yu, Y.; He, H. Iron-Based Composite Oxide Catalysts Tuned by CTAB Exhibit Superior NH3–SCR Performance. Catalysts 2021, 11, 224. https://doi.org/10.3390/catal11020224
Zhang W, Shi X, Gao M, Liu J, Lv Z, Wang Y, Huo Y, Cui C, Yu Y, He H. Iron-Based Composite Oxide Catalysts Tuned by CTAB Exhibit Superior NH3–SCR Performance. Catalysts. 2021; 11(2):224. https://doi.org/10.3390/catal11020224
Chicago/Turabian StyleZhang, Wenshuo, Xiaoyan Shi, Meng Gao, Jingjing Liu, Zhihui Lv, Yingjie Wang, Yanlong Huo, Chang Cui, Yunbo Yu, and Hong He. 2021. "Iron-Based Composite Oxide Catalysts Tuned by CTAB Exhibit Superior NH3–SCR Performance" Catalysts 11, no. 2: 224. https://doi.org/10.3390/catal11020224