Plant-Derived Bioactive Compounds in the Management of Neurodegenerative Disorders: Challenges, Future Directions and Molecular Mechanisms Involved in Neuroprotection
Abstract
:1. Introduction
2. Methodology
3. Plants and Their Bioactive Compounds in Averting the Pathogenesis of Neurodegenerative Disorders
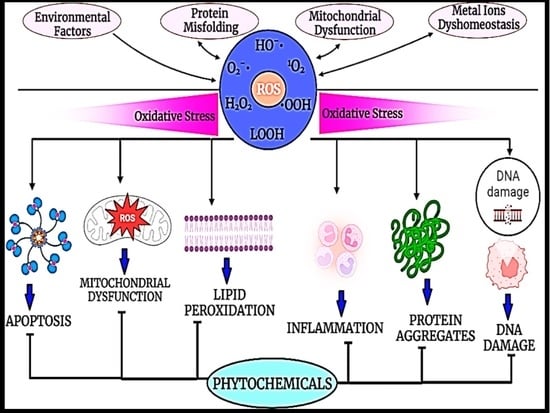
3.1. Exploring Antioxidant, Anti-Acetylcholinesterase, and Anti-Butyrylcholinesterase Activity of Plants and Their Compounds in Neurodegenerative Disorders
3.2. Exploring Anti-Apoptotic and Anti-Inflammatory Activities of Plants and Their Compounds in Neurodegenerative Disorders
3.3. Exploring Anti-Amyloid β (Aβ) Activity of Plants and Their Bioactive Compounds in Neurodegenerative Disorders
| Plant | Plant Part/Extract | Type of Study | Effects | Ref. |
|---|---|---|---|---|
| Viccinium genus (blueberries) | Fruits and leaves (methanol/water/formic acid 60:37:3 v/v/v) | In vivo (C57BL/6 mice) | Protected microglia cells, curtail the signs of neuroinflammation. | [19] |
| Acacia dealbata | Flower Ethanol/water | In vitro | Antioxidant Anti-AChE | [20] |
| Sophora secundiflora and Sophora tomentosa | Leaves Ethyl acetate and methanol | In vivo (Rats) | Antioxidant Anti-AChE | [21] |
| Piper divaricatum | Leaves Essential oil | In vitro | Anti-AChE | [22] |
| Lavandula stoechas | Aerial parts Methanol | In vivo (Swiss albino mice) | Antioxidant Anti-AChE | [23] |
| Elatostema papillosum | Leaves Methanol | In vivo (Wistar albino rats) | Anti-AChE Anti-BChE Antioxidant | [24] |
| Evolvulus alsinoides | Leaves Methanol and water | In vitro (SH-SY5Y cell-line) | Antioxidant Anti-AChE | [25] |
| Psychotria calocarpa | Leaves Methanol | In vitro and in vivo (Swiss albino mice) | Antioxidant | [26] |
| Morus alba | Leaves Water | In vivo (Swiss albino mice) | Anti-AChE, Anti-BChE Antioxidant | [27] |
| Bauhinia coccinea | Stems Ethanol | In vitro (HT22 neuronal cell line) | Anti-AChE Anti-BChE Antioxidant anti-amyloid-β (Aβ) | [28] |
| Enhydra fluctuans | Stems and leaves Chloroform | In vivo (Swiss albino mice) | Anti-AChE, Anti-BChE Antioxidant | [29] |
| Dillenia suffruticosa | Leaves Methanol | In vitro (Caenorhabditis elegans) | Anti-AChE Anti-BChE Antioxidant | [30] |
| Rosmarinus officinalis | Whole plant Ethanol, ethyl acetate and water | In vitro | Anti-AChE Antioxidant | [31] |
| Origanum vulgare | Aerial parts Ethanol | In vitro | Antioxidant Anti-apoptotic | [33] |
| Bacopa floribunda | Leaves Ethanol/water | In vivo (BALB/c mice) | Suppression of oxidative stress, neuroinflammation, and microgliosis | [34] |
| Cyperus rotundus and Zingiber officinale | Aerial parts Ethanol/methanol | In vivo (Wistar rats) | Reduced oxidative stress and AChE levels | [36] |
| Typha domingensis | Whole dried plant parts Methanol and hexane | In vitro | Anti-AChE Anti-BChE Antioxidant | [37] |
| Annona cherimola | Fruits Methanol | In vitro | Anti-AChE | [38] |
| Syzygium antisepticum | Leaves Ethanol/methanol | In vitro | Anti-AChE Antioxidant | [39] |
| Dracaena reflexa | Aerial parts and roots Methanol, butanol, and hexane | In vitro | Anti-tyrosinase Anti-AChE, Antioxidant | [40] |
| Solanum macrocarpon (L.) | Leaves Methanol and ethyl acetate | In vitro | Anti-AChE Antioxidant | [42] |
| Bruguiera gymnorhiza (L.) | Leaves and roots Water | In vitro | Anti-tyrosinase Anti-AChE Anti-BChE Antioxidant | [44] |
| Artemisia scoparia, Artemisia | Leaves Water | In vitro | Anti-BChE | [45] |
| Mentha pulegium (L.) | Whole plant parts Water and methanol | In vitro | Anti-AChE, Anti-BChE Antioxidant | [46] |
| Lawsonia inermis | Fruits Methanol and ethyl acetate | In vitro | Anti-BChE Antioxidant | [47] |
| Ferula ammoniacum | Aerial parts Ethanol and methanol | In vivo (Swiss albino mice) | Anti-AChE Anti-BChE Antioxidant | [48] |
| Ginkgo biloba | Fruits Ethanol, butanol, and dichloromethane | In vitro | Antioxidant | [49] |
| Salvia eriophora | Leaves Methanol and water | In vitro | Anti-AChE Anti-BChE Antioxidant | [51] |
| Origanum majorana, Origanum onites, Origanum syriacum, Origanum hirtum | Whole plant Ethanol | In vitro | Anti-AChE Anti-BChE | [52] |
| Folium perseae | Leaves Ethanol | In vitro | Antioxidant | [53] |
| Petroselinum crispum | Leaves Water | In vivo (Wistar albino rats) | Anti-AChE Antioxidant Anti-apoptotic | [58] |
| Guazuma ulmifolia, Limonium brasiliense, Paullinia cupana, Poincianella pluviosa, Stryphnodendron adstringens and Trichilia catigua | Crude plant extract and ethyl acetate extract | In vitro (SH-SY5Y cell-line) | Anti-AChE, Antioxidant | [60] |
| Ocimum basilicum (L.) | Leaves Methanol and water | In vivo (mice) | Anti-AChE Antioxidant Anti-apoptotic | [61] |
| Stenocereus pruinosus | Aerial parts Methanol | In vitro | Anti-amyloid | [95] |
| Pandanus amaryllifolius | Leaves Crude alcoholic extract | In vitro (SH-SY5Y cell-line) | Anti-amyloid β | [98] |
| Carthamus tinctorius (L.) and Taraxacum coreanum | Dry seeds Water | In vivo (mice) | Inhibit β-secretase and γ-secretase activity in Aβ25–35-infused mice. | [99] |
| Cirsium japonicum | Aerial parts Ethanol | In vivo (mice) | Antioxidant activity and attenuated lipid peroxidation and NO production. | [101] |
| Phytocompound | Structure | Plant | Study | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| β-caryophyllene |  | Cannabis sativa | In vivo (Swiss albino mice) | Neuroprotection by abrogating apoptosis through increased expression of bcl-2 and TrkB and suppression of bax and caspase-3 | [55] |
| Sinensetin |  | Citrus sinensis | In vitro (SH-SY5Y cell-line) | In vivo and in vitro anti-inflammatory, antioxidant, and antiapoptotic activities against amyloid beta (Aβ25–35)-induced neurotoxicity | [56] |
| Stigmasterol |  | Calotropis gigantean | In vitro (SH-SY5Y cell-line) | Hampered apoptosis induction by suppressing ROS production and upregulated bcl-2 and FoX3a and catalase | [57] |
| Rosmarinic acid and ursolic acid |  | Clinopodium revolutum | In vivo (BALB/c mice) | Improves spatial and recognition memory | [63] |
| Gastrodin |  | Gastrodia elata | In vivo (rats) | Prevented apoptosis induction by down-regulation of bax and alleviated autophagy by inhibiting beclin-1 and LC3-II | [64] |
| Quercetin |  | Citrus plants | In vivo (mice) | Inhibited cell death and degeneration by down-regulation of IL-6 and TNF-α | [66] |
| Asiaticoside |  | Centella asiatica | In vivo (Sprague-Dawley rats) | Elevated beclin-1 expression and decreased mTOR phosphorylation | [68] |
| Rosiridin |  | Rhodiola rosea | In vivo (Wistar rats) | Anti-inflammatory, antioxidant, and anti-apoptotic | [69] |
| Rehmannioside A |  | Glutinous rehmannia | In vivo (Sprague-Dawley rats) | Anti-inflammatory, antioxidant, and anti-apoptotic | [71] |
| Tilianin |  | Dracocephalum moldavica | In vivo (Sprague-Dawley rats) | Anti-neurodegenerative, antioxidant, and anti-apoptotic | [73] |
| Kaempferol |  | Camellia sinensis | In vivo (Wistar rats) | Anti-inflammatory, antioxidant, and anti-apoptotic | [74] |
| Marinoid J |  | Morinda lucida | In vivo (Sprague-Dawley rats) | Reducing MDA level and NO activity | [75] |
| Morin |  | Moraceae family | In vivo (Sprague-Dawley rats) | Neuroprotection by antioxidation, anti-aggregation, and anti-inflammatory mechanism | [76] |
| Glycyrrhizic acid |  | Glycyrrhiza glabra | In vivo (Sprague-Dawley rats) | Antioxidant activity by inhibition of ROS production and cyt-c activity | [79] |
| Tyrosol and hydroxytyrosol |  | Olea europaea | In vivo (APP/PS1 mice) | Anti-AβO aggregation and inhibition of caspase-3 activation | [80] |
| Hydroxytyrosol |  | Olea europaea | In vivo (APP/PS1 mice) | Reverse the deregulation of JAK2/STAT3, PI3K/Akt, ERK-MAPK, and JNK-p38 signalings | [81] |
| Honokiol |  | Magnolia officinalis | In vivo (mice) | Suppress apoptosis and neuronal damage in CA1 region of hippocampus and inhibit ROS production through attenuation of NF-κB signaling pathway | [82] |
| Ferrulic acid |  | Commelinid plants | In vivo (mice) | Anti-AβO, diminished cognitive impairment and exerted antioxidant effects by activating Nrf2 | [83,84] |
| Ferrulic acid |  | - | In vivo (Sprague-Dawley rats) | Antioxidant and neuroprotection against Aβ1–42-induced neurotoxicity | [85,86] |
| Gastrodin |  | - | In vivo (Sprague-Dawley rats) | Suppresses deposition of Aβ1–40 and Aβ1–42 plaques in plasma and hippocampus of 2-VO rats by inhibiting phosphorylation of tau and amyloid β | [87] |
| Naringenin |  | Citrus plants | In vitro (Neuro2a cells) In vivo (mice) | Neuroprotection against Aβ1–42 evoked neurotoxicity by restoring AMPK level | [88] |
| Pocahemiketone A | - | Pogostemon cablin | In vitro (SH-SY5Y cell line) | Targets NLRP3-dependent pyroptosis and oxidative stress | [89] |
| Myricetin |  | Vitis vivifera | In vitro (SH-SY5Y cell line) and in vivo (mice) | Anti-apoptotic and anti-AChE | [90] |
| Sulforaphane |  | Cruciferae family | In vivo (C57BL/6 mice) | Neuroprotection | [91] |
| Procyanidin B3 |  | Elaeagnus glabra f. oxyphylla | In vitro | Anti-Aβ aggregation effects | [92] |
| Procyanidin B4 |  | Elaeagnus glabra f. oxyphylla | In vitro | Anti-Aβ aggregation effects | [92] |
| Helichrysoside |  | Elaeagnus glabra f. oxyphylla | In vitro | Anti-Aβ aggregation effects | [92] |
| Rosmarinic acid |  | Salvia fruticosa | In vitro (SH-SY5Y cell-line) | Neuroprotection against Aβ1–42 induced neurotoxicity by down-regulating GSK3β and β-secretase | [93] |
| Coumestrol |  | Glycine max | In vitro | Anti-Aβ aggregation and selective inhibition of monoamine oxidase activation | [96] |
| Ginsenoside F1 |  | Aralia nudicaulis | In vivo (APPswe/PSEN1dE9 double-transgenic mice) and In vitro (Neuro2a and SH-SY5Y cell lines) | Anti-Aβ aggregation | [97] |
| β-caryophyllene |  | - | In vitro (NSC-34 cells) | Antioxidant and anti-apoptotic activities by inhibiting Aβ aggregation | [100] |
| α-bisabolol |  | Gochnatia polymorpha | In vitro (NSC-34 cells) | Antioxidant and anti-apoptotic activities by inhibiting Aβ aggregation | [100] |
| Curcumin |  | Curcuma longa | In vitro (SH-SY5Y cell-line) | Antioxidant activity by inhibited ROS generation | [102] |
| Resveratrol |  | Vaccinium species | In vitro (SH-SY5Y cell-line) | Antioxidant activity by inhibited ROS generation | [102] |
4. Plant-Derived Bioactive Compounds and Combinatorial Approaches for the Management of Neurodegenerative Disorders
5. Plants and Phytochemicals under Clinical Trials
6. Conclusions, Challenges, and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arvanitakis, Z.; Bennett, D.A. What is dementia? JAMA 2019, 322, 1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendriks, S.; Peetoom, K.; Bakker, C.; Van Der Flier, W.M.; Papma, J.M.; Koopmans, R.; Verhey, F.R.; De Vugt, M.; Köhler, S.; Young-Onset Dementia Epidemiology Study Group; et al. Global prevalence of young-onset dementia: A systematic review and meta-analysis. JAMA Neurol. 2021, 78, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, J.; Abu-Hanna, A.; de Rooij, S.E.; Smidt, N. Association between dementia parental family history and mid-life modifiable risk factors for dementia: A cross-sectional study using propensity score matching within the Lifelines cohort. BMJ Open 2021, 11, e049918. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Govindu, M.; Srivastava, S. Relationship between chewing tobacco, smoking, consuming alcohol and cognitive impairment among older adults in India: A cross-sectional study. BMC Geriatr. 2021, 21, 85. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Vernuccio, L.; Catanese, G.; Inzerillo, F.; Salemi, G.; Barbagallo, M. Nutrition, physical activity, and other lifestyle factors in the prevention of cognitive decline and dementia. Nutrients 2021, 13, 4080. [Google Scholar] [CrossRef]
- Cho, E.; Shin, J.; Kang, B.; Kim, S.; Hwang, S.; Kwon, E.; Heo, S.J. Risk Factors of Sleep Disturbance in Older Adults With Dementia: An Actigraphy-Based Validation Study. Innov. Aging 2021, 5 (Suppl. 1), 651. [Google Scholar]
- Gao, R.; Wang, R.; Chen, C. Age at diabetes onset and subsequent risk of dementia. JAMA 2021, 326, 871. [Google Scholar] [CrossRef]
- Nordestgaard, L.T.; Christoffersen, M.; Afzal, S.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Triglycerides as a shared risk factor between dementia and atherosclerotic cardiovascular disease: A study of 125 727 individuals. Clin. Chem. 2021, 67, 245–255. [Google Scholar] [CrossRef]
- Killin, L.O.; Starr, J.M.; Shiue, I.J.; Russ, T.C. Environmental risk factors for dementia: A systematic review. BMC Geriatr. 2016, 16, 175. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Ducharme, S.; Dols, A.; Laforce, R.; Devenney, E.; Kumfor, F.; Van Den Stock, J.; Dallaire-Théroux, C.; Seelaar, H.; Gossink, F.; Vijverberg, E.; et al. Recommendations to distinguish behavioural variant frontotemporal dementia from psychiatric disorders. Brain 2020, 143, 1632–1650. [Google Scholar] [CrossRef]
- Minoshima, S.; Mosci, K.; Cross, D.; Thientunyakit, T. Brain [F-18] FDG PET for clinical dementia workup: Differential diagnosis of Alzheimer’s disease and other types of dementing disorders. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2021; Volume 51, pp. 230–240. [Google Scholar]
- Rege, S.; Carnahan, R.M.; Johnson, M.L.; Chen, H.; Holmes, H.M.; Aparasu, R.R. Antipsychotic Initiation Among Older Dementia Patients Using Cholinesterase Inhibitors: A National Retrospective Cohort Study. Drugs Aging 2021, 38, 493–502. [Google Scholar] [CrossRef]
- Khan, A.; Jahan, S.; Alshahrani, S.; Alshehri, B.M.; Sameer, A.S.; Arafah, A.; Ahmad, A.; Rehman, M.U. Phytotherapeutic agents for neurodegenerative disorders: A neuropharmacological review. In Phytomedicine; Academic Press: Cambridge, MA, USA, 2021; pp. 581–620. [Google Scholar]
- Yadav, M.; Sehrawat, N.; Singh, M.; Upadhyay, S.K.; Aggarwal, D.; Sharma, A.K. Cardioprotective and hepatoprotective potential of citrus flavonoid naringin: Current status and future perspectives for health benefits. Asian J. Biol. Life Sci. 2020, 9, 1–5. [Google Scholar] [CrossRef]
- Khan, T.; Ali, M.; Khan, A.; Nisar, P.; Jan, S.A.; Afridi, S.; Shinwari, Z.K. Anticancer plants: A review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulat, M.; Khan, F.; Muluneh, G.; Pandita, A. Phytochemical profile and antimicrobial effects of different medicinal plant: Current knowledge and future perspectives. Curr. Tradit. Med. 2020, 6, 24–42. [Google Scholar] [CrossRef]
- Sun, C.; Liu, Y.; Zhan, L.; Rayat, G.R.; Xiao, J.; Jiang, H.; Li, X.; Chen, K. Anti-diabetic effects of natural antioxidants from fruits. Trends Food Sci. Technol. 2021, 117, 3–14. [Google Scholar] [CrossRef]
- Debnath-Canning, M.; Unruh, S.; Vyas, P.; Daneshtalab, N.; Igamberdiev, A.U.; Weber, J.T. Fruits and leaves from wild blueberry plants contain diverse polyphenols and decrease neuroinflammatory responses in microglia. J. Funct. Foods 2020, 68, 103906. [Google Scholar] [CrossRef]
- Paula, V.; Pedro, S.I.; Campos, M.G.; Delgado, T.; Estevinho, L.M.; Anjos, O. Special Bioactivities of Phenolics from Acacia dealbata L. with Potential for Dementia, Diabetes and Antimicrobial Treatments. Appl. Sci. 2022, 12, 1022. [Google Scholar] [CrossRef]
- Aly, S.H.; Elissawy, A.M.; Fayez, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Neuroprotective effects of Sophora secundiflora, Sophora tomentosa leaves and formononetin on scopolamine-induced dementia. Nat. Prod. Res. 2021, 35, 5848–5852. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; da Cruz, J.N.; Silva, S.G.; da Costa, W.A.; de Sousa, S.H.B.; Bezerra, F.W.F.; Teixeira, E.; da Silva, N.J.N.; de Aguiar Andrade, E.H.; Neto, A.M.D.J.C.; et al. Phytochemical profile, antioxidant activity, inhibition of acetylcholinesterase and interaction mechanism of the major components of the Piper divaricatum essential oil obtained by supercritical CO2. J. Supercrit. Fluids 2019, 145, 74–84. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Ahmad, M. Lavandula stoechas (L.) a very potent antioxidant attenuates dementia in scopolamine induced memory deficit mice. Front. Pharmacol. 2018, 9, 1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Reza, A.S.M.; Hossain, M.S.; Akhter, S.; Rahman, M.; Nasrin, M.; Uddin, M.; Sadik, G.; Khurshid Alam, A.H.M. In vitro antioxidant and cholinesterase inhibitory activities of Elatostemapapillosum leaves and correlation with their phytochemical profiles: A study relevant to the treatment of Alzheimer’s disease. BMC Complement. Altern. Med. 2018, 18, 123. [Google Scholar] [CrossRef] [Green Version]
- MettupalayamKaliyannanSundaramoorthy, P.; KilavanPackiam, K. In vitro enzyme inhibitory and cytotoxic studies with Evolvulusalsinoides (Linn.) Linn. Leaf extract: A plant from Ayurveda recognized as Dasapushpam for the management of Alzheimer’s disease and diabetes mellitus. BMC Complement. Med. Ther. 2020, 20, 129. [Google Scholar]
- Bristy, T.A.; Barua, N.; Montakim Tareq, A.; Sakib, S.A.; Etu, S.T.; Chowdhury, K.H.; Jyoti, M.A.; Aziz, M.A.I.; Reza, A.S.M.A.; Caiazzo, E.; et al. Deciphering the Pharmacological Properties of Methanol Extract of Psychotria calocarpa Leaves by In Vivo, In Vitro and In Silico Approaches. Pharmaceuticals 2020, 13, 183. [Google Scholar] [CrossRef]
- Islam, Z.; Islam, J.; Tony, S.R.; Anjum, A.; Ferdous, R.; Roy, A.K.; Hossain, S.; Salam, K.A.; Nikkon, F.; Hossain, K.; et al. Mulberry leaves juice attenuates arsenic-induced neurobehavioral and hepatic disorders in mice. Food Sci. Nutr. 2022, 10, 4360–4370. [Google Scholar] [CrossRef]
- Kim, Y.J.; Sohn, E.; Lim, H.S.; Kim, Y.; Kim, J.H.; Jeong, S.J. Simultaneous Quantification of Four Marker Compounds in Bauhinia coccinea Extract and Their Potential Inhibitory Effects on Alzheimer’s Disease Biomarkers. Plants 2021, 10, 702. [Google Scholar] [CrossRef]
- Lopa, S.S.; Al-Amin, M.; Hasan, M.; Ahammed, M.; Islam, K.M.; Alam, A.H.M.; Tanaka, T.; Sadik, M. Phytochemical analysis and cholinesterase inhibitory and antioxidant activities of Enhydra fluctuans relevant in the management of Alzheimer’s disease. Int. J. Food Sci. 2021, 2021, 8862025. [Google Scholar] [CrossRef]
- Abubakar, S.; Khor, B.K.; Khaw, K.Y.; Murugaiyah, V.; Chan, K.L. Cholinesterase inhibitory potential of Dillenia suffruticosa chemical constituents and protective effect against Aβ− induced toxicity in transgenic Caenorhabditis elegans model. Phytomedicine Plus 2021, 1, 100022. [Google Scholar] [CrossRef]
- Kamli, M.R.; Sharaf, A.A.M.; Sabir, J.S.; Rather, I.A. Phytochemical Screening of Rosmarinus officinalis L. as a Potential Anticholinesterase and Antioxidant–Medicinal Plant for Cognitive Decline Disorders. Plants 2022, 11, 514. [Google Scholar] [CrossRef]
- Impellizzeri, D.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; Di Paola, R.; et al. Açai Berry Mitigates Vascular Dementia-Induced Neuropathological Alterations Modulating Nrf-2/Beclin1 Pathways. Cells 2022, 11, 2616. [Google Scholar] [CrossRef]
- de Torre, M.P.; Cavero, R.Y.; Calvo, M.I. Anticholinesterase Activity of Selected Medicinal Plants from Navarra Region of Spain and a Detailed Phytochemical Investigation of Origanum vulgare L. ssp. vulgare. Molecules 2022, 27, 7100. [Google Scholar] [CrossRef] [PubMed]
- Oyeleke, M.B.; Owoyele, B.V. Saponins and flavonoids from Bacopa floribunda plant extract exhibit antioxidant and anti-inflammatory effects on amyloid beta 1-42-induced Alzheimer’s disease in BALB/c mice. J. Ethnopharmacol. 2022, 288, 114997. [Google Scholar] [CrossRef] [PubMed]
- Masondo, N.A.; Stafford, G.I.; Aremu, A.O.; Makunga, N.P. Acetylcholinesterase inhibitors from southern African plants: An overview of ethnobotanical, pharmacological potential and phytochemical research including and beyond Alzheimer’s disease treatment. South Afr. J. Bot. 2019, 120, 39–64. [Google Scholar] [CrossRef]
- Sutalangka, C.; Wattanathorn, J. Neuroprotective and cognitive-enhancing effects of the combined extract of Cyperus rotundus and Zingiber officinale. BMC Complement. Altern. Med. 2017, 17, 135. [Google Scholar] [CrossRef] [Green Version]
- Dilshad, R.; Ahmad, S.; Aati, H.Y.; Al-qahtani, J.H.; Sherif, A.E.; Hussain, M.; Ghalloo, B.A.; Tahir, H.; Basit, A.; Ahmed, M. Phytochemical profiling, in vitro biological activities, and in-silico molecular docking studies of Typha domingensis. Arab. J. Chem. 2022, 15, 104133. [Google Scholar] [CrossRef]
- Galarce-Bustos, O.; Pavón, J.; Henríquez-Aedo, K.; Aranda, M. Detection and identification of acetylcholinesterase inhibitors in Annona cherimola Mill. by effect-directed analysis using thin-layer chromatography-bioassay-mass spectrometry. Phytochem. Anal. 2019, 30, 679–686. [Google Scholar] [CrossRef]
- Mangmool, S.; Kunpukpong, I.; Kitphati, W.; Anantachoke, N. Antioxidant and anticholinesterase activities of extracts and phytochemicals of Syzygiumantisepticum leaves. Molecules 2021, 26, 3295. [Google Scholar] [CrossRef]
- Ghalloo, B.A.; Khan, K.-u.-R.; Ahmad, S.; Aati, H.Y.; Al-Qahtani, J.H.; Ali, B.; Mukhtar, I.; Hussain, M.; Shahzad, M.N.; Ahmed, I. Phytochemical Profiling, In Vitro Biological Activities, and In Silico Molecular Docking Studies of Dracaena reflexa. Molecules 2022, 27, 913. [Google Scholar] [CrossRef]
- Laws, J.S., III; Smid, S.D. Evaluating Cannabis Sativa L.’s neuroprotection potential: From bench to bedside. Phytomedicine 2022, 107, 154485. [Google Scholar] [CrossRef]
- Idowu, G.P.; Obuotor, E.M.; Onajobi, F.D. In vitro and in silico investigation of cholinesterase inhibition and anti-radical properties of Solanum macrocarpon leaf extracts: A preliminary anti-Alzheimer’s study. Alzheimer’s Dement. 2021, 17, e049605. [Google Scholar] [CrossRef]
- Zeng, P.; Liu, Y.C.; Wang, X.M.; Ye, C.Y.; Sun, Y.W.; Su, H.F.; Qiu, S.W.; Li, Y.N.; Wang, Y.; Wang, Y.C.; et al. Targets and mechanisms of Alpinia oxyphylla Miquel fruits in treating neurodegenerative dementia. Front. Aging Neurosci. 2022, 14, 1394. [Google Scholar] [CrossRef]
- Bibi Sadeer, N.; Sinan, K.I.; Cziáky, Z.; Jekő, J.; Zengin, G.; Jeewon, R.; Abdallah, H.H.; AlDhaheri, Y.; Eid, A.H.; Mahomoodally, M.F. Towards the Pharmacological Validation and Phytochemical Profiling of the Decoction and Maceration of Bruguiera gymnorhiza (L.) Lam.—A Traditionally Used Medicinal Halophyte. Molecules 2022, 27, 2000. [Google Scholar] [CrossRef]
- Khan, F.A.; Khan, N.M.; Ahmad, S.; Nasruddin; Aziz, R.; Ullah, I.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A. Phytochemical Profiling, Antioxidant, Antimicrobial and Cholinesterase Inhibitory Effects of Essential Oils Isolated from the Leaves of Artemisia scoparia and Artemisia absinthium. Pharmaceuticals 2022, 15, 1221. [Google Scholar] [CrossRef]
- Politeo, O.; Bektašević, M.; Carev, I.; Jurin, M.; Roje, M. Phytochemical composition, antioxidant potential and cholinesterase inhibition potential of extracts from Mentha pulegium L. Chem. Biodivers. 2018, 15, e1800374. [Google Scholar] [CrossRef]
- Balaei-Kahnamoei, M.; Saeedi, M.; Rastegari, A.; Shams Ardekani, M.R.; Akbarzadeh, T.; Khanavi, M. Phytochemical analysis and evaluation of biological activity of Lawsoniainermis Seeds related to Alzheimer’s disease. Evid.-Based Complement. Altern. Med. 2021, 2021, 5965061. [Google Scholar] [CrossRef]
- Nazir, N.; Nisar, M.; Zahoor, M.; Uddin, F.; Ullah, S.; Ullah, R.; Ansari, S.A.; Mahmood, H.M.; Bari, A.; Alobaid, A. Phytochemical analysis, in vitro anticholinesterase, antioxidant activity and in vivo nootropic effect of Ferula ammoniacum (Dorema ammoniacum) D. Don. in scopolamine-induced memory impairment in mice. Brain Sci. 2021, 11, 259. [Google Scholar] [CrossRef]
- Alishir, A.; Kim, K.H. Antioxidant Phenylpropanoid Glycosides from Ginkgo biloba Fruit and Identification of a New Phenylpropanoid Glycoside, Ginkgopanoside. Plants 2021, 10, 2702. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Sritalahareuthai, V.; Jom, K.N.; Jongruaysup, B.; Tabtimsri, S.; Pruesapan, K.; Thangsiri, S.; Inthachat, W.; Siriwan, D.; Charoenkiatkul, S.; et al. Comparison of Phytochemicals, Antioxidant, and In Vitro Anti-Alzheimer Properties of Twenty-Seven Morus spp. Cultivated in Thailand. Molecules 2020, 25, 2600. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Kılıç, Ö.; Taslimi, P.; Gören, A.C.; Gülçin, İ. Phytochemical content, antioxidant activity, and enzyme inhibition effect of Salvia eriophora Boiss. & Kotschy against acetylcholinesterase, α-amylase, butyrylcholinesterase, and α-glycosidase enzymes. J. Food Biochem. 2019, 43, e12776. [Google Scholar]
- Gök, H.N.; Luca, S.V.; Ay, S.T.; Komsta, Ł.; Salmas, R.E.; Orhan, I.E.; Skalicka-Woźniak, K. Profiling the annual change of the neurobiological and antioxidant effects of five Origanum species in correlation with their phytochemical composition. Food Chem. 2022, 368, 130775. [Google Scholar] [CrossRef]
- Polat Kose, L.; Bingol, Z.; Kaya, R.; Goren, A.C.; Akincioglu, H.; Durmaz, L.; Koksal, E.; Alwasel, S.H.; Gülçin, İ. Anticholinergic and antioxidant activities of avocado (Folium perseae) leaves–phytochemical content by LC-MS/MS analysis. Int. J. Food Prop. 2020, 23, 878–893. [Google Scholar] [CrossRef]
- Gill, I.; Kaur, S.; Kaur, N.; Dhiman, M.; Mantha, A.K. Phytochemical ginkgolide B attenuates amyloid-β 1-42 induced oxidative damage and altered cellular responses in human neuroblastoma SH-SY5Y cells. J. Alzheimer’s Dis. 2017, 60, S25–S40. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, H.V.; Venkatakrishna, K.; Gouthamchandra, K.; Reethi, B.; Naveen, P.; Lingaraju, H.B.; Shyamprasad, K. A standardized black pepper seed extract containing β-caryophyllene improves cognitive function in scopolamine-induced amnesia model mice via regulation of brain-derived neurotrophic factor and MAPK proteins. J. Food Biochem. 2021, 45, e13994. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Z.; Tang, X.; Wang, Y.; Chen, R.; Ji, H. Sinensetin Attenuates Amyloid Beta25-35-Induced Oxidative Stress, Inflammation, and Apoptosis in SH-SY5Y Cells Through the TLR4/NF-κB Signaling Pathway. Neurochem. Res. 2021, 46, 3012–3024. [Google Scholar] [CrossRef]
- Pratiwi, R.; Nantasenamat, C.; Ruankham, W.; Suwanjang, W.; Prachayasittikul, V.; Prachayasittikul, S.; Phopin, K. Mechanisms and neuroprotective activities of stigmasterol against oxidative stress-induced neuronal cell death via sirtuin family. Front. Nutr. 2021, 8, 648995. [Google Scholar] [CrossRef]
- Sener, G.; Karakadıoglu, G.; Ozbeyli, D.; Ede, S.; Yanardag, R.; Sacan, O.; Aykac, A. Petroselinum crispum extract ameliorates scopolamine-induced cognitive dysfunction: Role on apoptosis, inflammation and oxidative stress. Food Sci. Hum. Wellness 2022, 11, 1290–1298. [Google Scholar] [CrossRef]
- Kumar, K.H.; Khanum, F. Hydroalcoholic extract of Cyperus rotundus ameliorates H2O2-induced human neuronal cell damage via its anti-oxidative and anti-apoptotic machinery. Cell. Mol. Neurobiol. 2013, 33, 5–17. [Google Scholar] [CrossRef]
- Sereia, A.L.; de Oliveira, M.T.; Baranoski, A.; Marques, L.L.M.; Ribeiro, F.M.; Isolani, R.G.; de Medeiros, D.C.; Chierrito, D.; Lazarin-Bidoia, D.; Zielinski, A.A.F.; et al. In vitro evaluation of the protective effects of plant extracts against amyloid-beta peptide-induced toxicity in human neuroblastoma SH-SY5Y cells. PLoS ONE 2019, 14, e0212089. [Google Scholar] [CrossRef]
- Singh, V.; Kaur, K.; Kaur, S.; Shri, R.; Singh, T.G.; Singh, M. Trimethoxyflavones from Ocimum basilicum L. leaves improve long term memory in mice by modulating multiple pathways. J. Ethnopharmacol. 2022, 295, 115438. [Google Scholar] [CrossRef]
- Jia, Y.; Li, Z.; Wang, T.; Fan, M.; Song, J.; Lv, P.; Jin, W. Shikonin Attenuates Chronic Cerebral Hypoperfusion-Induced Cognitive Impairment by Inhibiting Apoptosis via PTEN/Akt/CREB/BDNF Signaling. Evid.-Based Complement. Altern. Med. 2021, 2021, 5564246. [Google Scholar] [CrossRef]
- Mirza, F.J.; Amber, S.; Hassan, D.; Ahmed, T.; Zahid, S. Rosmarinic acid and ursolic acid alleviate deficits in cognition, synaptic regulation and adult hippocampal neurogenesis in an Aβ1-42-induced mouse model of Alzheimer’s disease. Phytomedicine 2021, 83, 153490. [Google Scholar] [CrossRef]
- Liu, B.; Gao, J.M.; Li, F.; Gong, Q.H.; Shi, J.S. Gastrodin attenuates bilateral common carotid artery occlusion-induced cognitive deficits via regulating Aβ-related proteins and reducing autophagy and apoptosis in rats. Front. Pharmacol. 2018, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, E.; Yang, H.; Chen, Y.; Tao, L.; Xu, Y.; Chen, T.; Shen, X. Gastrodin Ameliorates Cognitive Dysfunction in Vascular Dementia Rats by Suppressing Ferroptosis via the Regulation of the Nrf2/Keap1-GPx4 Signaling Pathway. Molecules 2022, 27, 6311. [Google Scholar] [CrossRef]
- Olayinka, J.; Eduviere, A.; Adeoluwa, O.; Fafure, A.; Adebanjo, A.; Ozolua, R. Quercetin mitigates memory deficits in scopolamine mice model via protection against neuroinflammation and neurodegeneration. Life Sci. 2022, 292, 120326. [Google Scholar] [CrossRef]
- Hong, Y.; Choi, Y.-H.; Han, Y.-E.; Oh, S.-J.; Lee, A.; Lee, B.; Magnan, R.; Ryu, S.Y.; Choi, C.W.; Kim, M.S. Central Administration of Ampelopsin A Isolated from Vitis vinifera Ameliorates Cognitive and Memory Function in a Scopolamine-Induced Dementia Model. Antioxidants 2021, 10, 835. [Google Scholar] [CrossRef]
- Guo, M.; Xu, J.; Wang, S.; Dong, B. Asiaticoside reduces autophagy and improves memory in a rat model of dementia through mTOR signaling pathway regulation. Mol. Med. Rep. 2021, 24, 645. [Google Scholar] [CrossRef]
- Afzal, M.; Alzarea, S.I.; Alharbi, K.S.; Alzarea, A.I.; Alenezi, S.K.; Alshammari, M.S.; Alquraini, A.H.; Kazmi, I. Rosiridin Attenuates Scopolamine-Induced Cognitive Impairments in Rats via Inhibition of Oxidative and Nitrative Stress Leaded Caspase-3/9 and TNF-α Signaling Pathways. Molecules 2022, 27, 5888. [Google Scholar] [CrossRef]
- Dolanbay, S.N.; Kocanci, F.G.; Aslim, B. Neuroprotective effects of allocryptopine-rich alkaloid extracts against oxidative stress-induced neuronal damage. Biomed. Pharmacother. 2021, 140, 111690. [Google Scholar] [CrossRef]
- Sun, M.; Shen, X.; Ma, Y. Rehmannioside A attenuates cognitive deficits in rats with vascular dementia (VD) through suppressing oxidative stress, inflammation and apoptosis. Biomed. Pharmacother. 2019, 120, 109492. [Google Scholar] [CrossRef]
- Zhu, J.D.; Wang, J.J.; Zhang, X.H.; Yu, Y.; Kang, Z.S. Panax ginseng extract attenuates neuronal injury and cognitive deficits in rats with vascular dementia induced by chronic cerebral hypoperfusion. Neural Regen. Res. 2018, 13, 664. [Google Scholar] [CrossRef]
- Jiang, H.; Ashraf, G.M.; Liu, M.; Zhao, K.; Wang, Y.; Wang, L.; Xing, J.; Alghamdi, B.S.; Li, Z.; Liu, R. Tilianin ameliorates cognitive dysfunction and neuronal damage in rats with vascular dementia via p-CaMKII/ERK/CREB and ox-CaMKII-dependent MAPK/NF-κB pathways. Oxidative Med. Cell. Longev. 2021, 2021, 6673967. [Google Scholar] [CrossRef] [PubMed]
- Kouhestani, S.; Jafari, A.; Babaei, P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen. Res. 2018, 13, 1827. [Google Scholar] [PubMed]
- Yi, X.X.; Li, J.Y.; Tang, Z.Z.; Jiang, S.; Liu, Y.H.; Deng, J.G.; Gao, C.H. Marinoid J, a phenylglycoside from Avicennia marina fruit, ameliorates cognitive impairment in rat vascular dementia: A quantitative iTRAQ proteomic study. Pharm. Biol. 2020, 58, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- El-Gazar, A.A.; Soubh, A.A.; Mohamed, E.A.; Awad, A.S.; El-Abhar, H.S. Morin post-treatment confers neuroprotection in a novel rat model of mild repetitive traumatic brain injury by targeting dementia markers, APOE, autophagy and Wnt/β-catenin signaling pathway. Brain Res. 2019, 1717, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-B.; Kim, S.-H.; Uhm, S.-H.; Kim, D.-K.; Lee, N.-S.; Jeong, Y.-G.; Sung, N.-Y.; Kim, D.-S.; Han, I.-J.; Yoo, Y.-C.; et al. Perilla frutescens Leaf Extract Attenuates Vascular Dementia-Associated Memory Deficits, Neuronal Damages, and Microglial Activation. Curr. Issues Mol. Biol. 2022, 44, 257–272. [Google Scholar] [CrossRef]
- Taheri, S.; Khalifeh, S.; Shajiee, H.; Ashabi, G. Dietary uptake of Salvia macilenta extract improves Nrf2 antioxidant signaling pathway and diminishes inflammation and apoptosis in amyloid beta-induced rats. Mol. Biol. Rep. 2021, 48, 7667–7676. [Google Scholar] [CrossRef]
- Sathyamoorthy, Y.; Kaliappan, K.; Nambi, P.; Radhakrishnan, R. Glycyrrhizic acid renders robust neuroprotection in rodent model of vascular dementia by controlling oxidative stress and curtailing cytochrome-c release. Nutr. Neurosci. 2020, 23, 955–970. [Google Scholar] [CrossRef]
- Peng, Y.; Hou, C.; Yang, Z.; Li, C.; Jia, L.; Liu, J.; Tang, Y.; Shi, L.; Li, Y.; Long, J.; et al. Hydroxytyrosol mildly improve cognitive function independent of APP processing in APP/PS1 mice. Mol. Nutr. Food Res. 2016, 60, 2331–2342. [Google Scholar] [CrossRef]
- Qin, C.; Hu, S.; Zhang, S.; Zhao, D.; Wang, Y.; Li, H.; Peng, Y.; Shi, L.; Xu, X.; Wang, C.; et al. Hydroxytyrosol Acetate Improves the Cognitive Function of APP/PS1 Transgenic Mice in ERβ-dependent Manner. Mol. Nutr. Food Res. 2021, 65, 2000797. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Ni, C.; Song, G. Honokiol attenuates oligomeric amyloid β1-42-induced Alzheimer’s disease in mice through attenuating mitochondrial apoptosis and inhibiting the nuclear factor kappa-B signaling pathway. Cell. Physiol. Biochem. 2017, 43, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.J.; Jung, J.S.; Kim, T.K.; Hasan, M.A.; Hong, C.W.; Nam, J.S.; Song, D.K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PloS ONE 2013, 8, e55774. [Google Scholar] [CrossRef] [Green Version]
- Sultana, R.; Ravagna, A.; Mohmmad-Abdul, H.; Calabrese, V.; Butterfield, D.A. Ferulic acid ethyl ester protects neurons against amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity: Relationship to antioxidant activity. J. Neurochem. 2005, 92, 749–758. [Google Scholar] [CrossRef]
- Picone, P.; Bondi, M.L.; Picone, P.; Bondi, M.L.; Montana, G.; Bruno, A.; Pitarresi, G.; Giammona, G.; Di Carlo, M. Ferulic acid inhibits oxidative stress and cell death induced by Ab oligomers: Improved delivery by solid lipid nanoparticles. Free. Radic. Res. 2009, 43, 1133–1145. [Google Scholar] [CrossRef]
- Shi, R.; Zheng, C.B.; Wang, H.; Rao, Q.; Du, T.; Bai, C.; Xiao, C.; Dai, Z.; Zhang, C.; Chen, C.; et al. Gastrodin alleviates vascular dementia in a 2-VO-vascular dementia rat model by altering amyloid and tau levels. Pharmacology 2020, 105, 386–396. [Google Scholar] [CrossRef]
- Ahsan, A.U.; Sharma, V.L.; Wani, A.; Chopra, M. Naringenin upregulates AMPK-mediated autophagy to rescue neuronal cells from β-amyloid (1–42) evoked neurotoxicity. Mol. Neurobiol. 2020, 57, 3589–3602. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Zhao, H.Y.; Ma, C.; Huang, L.; Liu, J.; Guo, L.; Peng, C.; Xiong, L. Pocahemiketone A, a Sesquiterpenoid Possessing a Spirocyclic Skeleton with a Hemiketal Endoperoxide Unit, Alleviates Aβ25–35-Induced Pyroptosis and Oxidative Stress in SH-SY5Y Cells. Org. Lett. 2022, 24, 4734–4738. [Google Scholar] [CrossRef]
- Liu, M.; Guo, H.; Li, Z.; Zhang, C.; Zhang, X.; Cui, Q.; Tian, J. Molecular level insight into the benefit of myricetin and dihydromyricetin uptake in patients with Alzheimer’s diseases. Front. Aging Neurosci. 2020, 12, 601603. [Google Scholar] [CrossRef]
- Zhang, R.; Miao, Q.W.; Zhu, C.X.; Zhao, Y.; Liu, L.; Yang, J.; An, L. Sulforaphane ameliorates neurobehavioral deficits and protects the brain from amyloid β deposits and peroxidation in mice with Alzheimer-like lesions. Am. J. Alzheimer’s Dis. Other Dement. 2015, 30, 183–191. [Google Scholar] [CrossRef]
- Kim, Y.J.; Sohn, E.; Kim, J.-H.; Na, M.; Jeong, S.-J. Catechol-Type Flavonoids from the Branches of Elaeagnus glabra f. oxyphylla Exert Antioxidant Activity and an Inhibitory Effect on Amyloid-β Aggregation. Molecules 2020, 25, 4917. [Google Scholar]
- Gürbüz, P.; Dokumacı, A.H.; Gündüz, M.G.; Perez, C.; Göger, F.; Paksoy, M.Y.; Yerer, M.B.; Demirezer, L.Ö. In vitro biological activity of Salvia fruticosa Mill. infusion against amyloid β-peptide-induced toxicity and inhibition of GSK-3β, CK-1δ, and BACE-1 enzymes relevant to Alzheimer’s disease. Saudi Pharm. J. 2021, 29, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Iyaswamy, A.; Krishnamoorthi, S.K.; Zhang, H.; Sreenivasmurthy, S.G.; Zhu, Z.; Liu, J.; Su, C.F.; Guan, X.J.; Wang, Z.Y.; Cheung, K.H.; et al. Qingyangshen mitigates amyloid-β and Tau aggregate defects involving PPARα-TFEB activation in transgenic mice of Alzheimer’s disease. Phytomedicine 2021, 91, 153648. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Shimoyama, T.; Kawazu, R.; Sasaki, H.; Koyama, K.; Takahashi, K.; Kinoshita, K. Amyloid β aggregation inhibitory activity of triterpene saponins from the cactus Stenocereuspruinosus. J. Nat. Med. 2021, 75, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Kim, B.-R.; Cho, M.L.; Kim, T.-S.; Im, S.; Han, S.; Jeong, J.-W.; Jung, H.A.; Choi, J.S. Phytoestrogen Coumestrol Selectively Inhibits Monoamine Oxidase-A and Amyloid β Self-Aggregation. Nutrients 2022, 14, 3822. [Google Scholar] [CrossRef]
- Yun, Y.J.; Park, B.H.; Hou, J.; Oh, J.P.; Han, J.H.; Kim, S.C. Ginsenoside F1 Protects the Brain against Amyloid Beta-Induced Toxicity by Regulating IDE and NEP. Life 2022, 12, 58. [Google Scholar] [CrossRef]
- Tan, M.A.; Ishikawa, H.; An, S.S.A. Pandanus amaryllifolius Exhibits In vitro Anti-Amyloidogenic Activity and Promotes Neuroprotective Effects in Amyloid-β-Induced SH-SY5Y Cells. Nutrients 2022, 14, 3962. [Google Scholar] [CrossRef]
- He, M.; Kim, J.; Park, C.; Cho, E. Herbal Mixture of Carthamus tinctorius L. Seed and Taraxacum coreanum Attenuates Amyloid Beta-Induced Cognitive Dysfunction In vivo. Foods 2022, 11, 142. [Google Scholar] [CrossRef]
- Laws, J.S., III; Shrestha, S.; Smid, S.D. Cannabis terpenes display variable protective and anti-aggregatory actions against neurotoxic β amyloid in vitro: Highlighting the protective bioactivity of α-bisabolol in motorneuronal-like NSC-34 cells. NeuroToxicology 2022, 90, 81–87. [Google Scholar] [CrossRef]
- Pang, Q.Q.; Kim, J.H.; Choi, J.M.; Song, J.L.; Lee, S.; Cho, E.J. Cirsium japonicum var. Maackii Improves Cognitive Impairment under Amyloid Beta25-35-Induced Alzheimer’s Disease Model. BioMed Res. Int. 2022, 2022, 4513998. [Google Scholar] [CrossRef]
- Yu, H.; Yamashita, T.; Hu, X.; Bian, Z.; Hu, X.; Feng, T.; Tadokoro, K.; Morihara, R.; Abe, K. Protective and anti-oxidative effects of curcumin and resveratrol on Aβ-oligomer-induced damage in the SH-SY5Y cell line. J. Neurol. Sci. 2022, 441, 120356. [Google Scholar] [CrossRef]
- Batarseh, Y.S.; Kaddoumi, A. Oleocanthal-rich extra-virgin olive oil enhances donepezil effect by reducing amyloid-β load and related toxicity in a mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2018, 55, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Zhou, G.S.; Tan, Y.J.; Tao, H.J.; Chen, J.Q.; Pu, Z.J.; Ma, J.Y.; She, W.; Kang, A.; et al. Studies of the anti-amnesic effects and mechanisms of single and combined use of donepezil and ginkgo ketoester tablet on scopolamine-induced memory impairment in mice. Oxidative Med. Cell. Longev. 2019, 2019, 8636835. [Google Scholar] [CrossRef] [PubMed]
- Yancheva, S.; Ihl, R.; Nikolova, G.; Panayotov, P.; Schlaefke, S.; Hoerr, R.; GINDON Study Group. Ginkgo biloba extract EGb 761®, donepezil or both combined in the treatment of Alzheimer’s disease with neuropsychiatric features: A randomised, double-blind, exploratory trial. Aging Ment. Health 2009, 13, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G. Modulatory effect of caffeic acid on cholinesterases inhibitory properties of donepezil. J. Complement. Integr. Med. 2018, 15, 20170016. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Marathe, A.; Manjrekar, P.A.; Joy, T.; Ullal, S.; Pai, M.M.; Murlimanju, B.V. Comparison of malondialdehyde levels and superoxide dismutase activity in resveratrol and resveratrol/donepezil combination treatment groups in Alzheimer’s disease induced rat model. 3 Biotech 2021, 11, 329. [Google Scholar] [CrossRef]
- Obafemi, T.O.; Owolabi, O.V.; Omiyale, B.O.; Afolabi, B.A.; Ojo, O.A.; Onasanya, A.; Adu, I.A.; Rotimi, D. Combination of donepezil and gallic acid improves antioxidant status and cholinesterases activity in aluminum chloride-induced neurotoxicity in Wistar rats. Metab. Brain Dis. 2021, 36, 2511–2519. [Google Scholar] [CrossRef]
- Liu, Q.F.; Choi, H.; Son, T.; Kim, Y.M.; Kanmani, S.; Chin, Y.W.; Kim, S.N.; Kim, K.K.; Kim, K.W.; Koo, B.S. Co-Treatment with the Herbal Medicine SIP3 and Donepezil Improves Memory and Depression in the Mouse Model of Alzheimer’s Disease. Curr. Alzheimer Res. 2022, 19, 246–263. [Google Scholar]
- Pattanashetti, L.A.; Taranalli, A.D.; Parvatrao, V.; Malabade, R.H.; Kumar, D. Evaluation of neuroprotective effect of quercetin with donepezil in scopolamine-induced amnesia in rats. Indian J. Pharmacol. 2017, 49, 60. [Google Scholar]
- Kong, X.-P.; Ren, H.-Q.; Liu, E.Y.L.; Leung, K.-W.; Guo, S.-C.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. The Cholinesterase Inhibitory Properties of Stephaniae Tetrandrae Radix. Molecules 2020, 25, 5914. [Google Scholar] [CrossRef]
- Oboh, G.; Ogunsuyi, O.B.; Olonisola, O.E. Does caffeine influence the anticholinesterase and antioxidant properties of donepezil? Evidence from in vitro and in vivo studies. Metab. Brain Dis. 2017, 32, 629–639. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Hallak, J.E. Effects of the natural β-carboline alkaloid harmine, a main constituent of ayahuasca, in memory and in the hippocampus: A systematic literature review of preclinical studies. J. Psychoact. Drugs 2017, 49, 1–10. [Google Scholar] [CrossRef]
- He, D.; Wu, H.; Wei, Y.; Liu, W.; Huang, F.; Shi, H.; Zhang, B.; Wu, X.; Wang, C. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur. J. Pharmacol. 2015, 768, 96–107. [Google Scholar] [CrossRef]
- Castillo, W.O.; Palomino, N.V.; Takahashi, C.S.; Giuliatti, S. Genistein and galantamine combinations decrease β-amyloid peptide (1–42)–induced genotoxicity and cell death in SH-SY5Y cell line: An in vitro and in silico approach for mimic of Alzheimer’s Disease. Neurotox. Res. 2020, 38, 691–706. [Google Scholar] [CrossRef]
- Thancharoen, O.; Limwattananon, C.; Waleekhachonloet, O.; Rattanachotphanit, T.; Limwattananon, P.; Limpawattana, P. Ginkgo biloba extract (EGb761), cholinesterase inhibitors, and memantine for the treatment of mild-to-moderate Alzheimer’s disease: A network meta-analysis. Drugs Aging 2019, 36, 435–452. [Google Scholar] [CrossRef]
- Callizot, N.; Campanari, M.L.; Rouvière, L.; Jacquemot, G.; Henriques, A.; Garayev, E.; Poindron, P. Huperzia serrata Extract ‘NSP01’with Neuroprotective Effects-Potential Synergies of Huperzine A and Polyphenols. Front. Pharmacol. 2021, 12, 681532. [Google Scholar] [CrossRef]
- Shao, Z.Q. Comparison of the efficacy of four cholinesterase inhibitors in combination with memantine for the treatment of Alzheimer’s disease. Int. J. Clin. Exp. Med. 2015, 8, 2944. [Google Scholar]
- Zhang, L.; Cao, H.; Wen, J.; Xu, M. Green tea polyphenol (−)-epigallocatechin-3-gallate enhances the inhibitory effect of huperzine A on acetylcholinesterase by increasing the affinity with serum albumin. Nutr. Neurosci. 2009, 12, 142–148. [Google Scholar] [CrossRef]
- Ahmad, I.; Swaroop, A.; Bagchi, D. A synergistic combination of Huperzine A, Convolvulus pluricaulis and Celastruspaniculatus promote cognitive function and health. FASEB J. 2018, 32, 656–657. [Google Scholar] [CrossRef]
- Robinson, H. The Gut Brain Axis: Impact of Dietary Fiber on a Murine Model of Multiple Sclerosis. Ph.D. Dissertation, University of British Columbia, Vancouver, BC, Canada, 2019. [Google Scholar]
- Patricio, F.; Morales-Andrade, A.A.; Patricio-Martínez, A.; Limón, I.D. Cannabidiol as a therapeutic target: Evidence of its neuroprotective and neuromodulatory function in Parkinson’s disease. Front. Pharmacol. 2020, 11, 595635. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoaib, S.; Ansari, M.A.; Fatease, A.A.; Safhi, A.Y.; Hani, U.; Jahan, R.; Alomary, M.N.; Ansari, M.N.; Ahmed, N.; Wahab, S.; et al. Plant-Derived Bioactive Compounds in the Management of Neurodegenerative Disorders: Challenges, Future Directions and Molecular Mechanisms Involved in Neuroprotection. Pharmaceutics 2023, 15, 749. https://doi.org/10.3390/pharmaceutics15030749
Shoaib S, Ansari MA, Fatease AA, Safhi AY, Hani U, Jahan R, Alomary MN, Ansari MN, Ahmed N, Wahab S, et al. Plant-Derived Bioactive Compounds in the Management of Neurodegenerative Disorders: Challenges, Future Directions and Molecular Mechanisms Involved in Neuroprotection. Pharmaceutics. 2023; 15(3):749. https://doi.org/10.3390/pharmaceutics15030749
Chicago/Turabian StyleShoaib, Shoaib, Mohammad Azam Ansari, Adel Al Fatease, Awaji Y. Safhi, Umme Hani, Roshan Jahan, Mohammad N. Alomary, Mohd Nazam Ansari, Nabeel Ahmed, Shadma Wahab, and et al. 2023. "Plant-Derived Bioactive Compounds in the Management of Neurodegenerative Disorders: Challenges, Future Directions and Molecular Mechanisms Involved in Neuroprotection" Pharmaceutics 15, no. 3: 749. https://doi.org/10.3390/pharmaceutics15030749