Advanced Progress in the Role of Adipose-Derived Mesenchymal Stromal/Stem Cells in the Application of Central Nervous System Disorders
Abstract
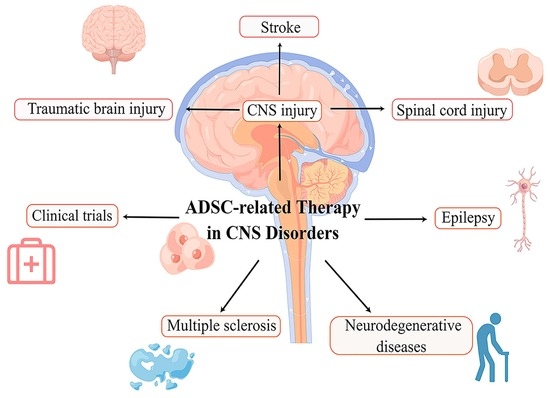
:1. Introduction
2. Search Strategy and Selection Criteria
3. Mechanism of ADMSC-Related Therapy in Central Nervous System
4. ADMSCs and Therapy of CNS Injuries
4.1. ADMSCs-Related Therapy in Stroke
4.2. ADMSCs-Related Therapy in Traumatic Brain Injury
4.3. ADMSCs-Related Therapy in Spinal Cord Injury
5. ADMSCs-Related Therapy of Epilepsy
6. ADMSCs-Related Therapy of Neurodegenerative Diseases
7. ADMSCs-Related Therapy of Multiple Sclerosis
8. The Application of Gene-Modified ADMSC in CNS Disorders
9. The Application of ADMSCs Secretome in CNS Disorders
10. The Prospect of ADMSC Related Therapy and Clinical Trails
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mohr, A.; Zwacka, R. The future of mesenchymal stem cell-based therapeutic approaches for cancer—From cells to ghosts. Cancer Lett. 2018, 414, 239–249. [Google Scholar] [CrossRef]
- Barkholt, L.; Flory, E.; Jekerle, V.; Lucas-Samuel, S.; Ahnert, P.; Bisset, L.; Büscher, D.; Fibbe, W.; Foussat, A.; Kwa, M.; et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy 2013, 15, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Ying, L.; Liang, C.; Zhang, Y.; Wang, J.; Wang, C.; Xia, K.; Shi, K.; Yu, C.; Yang, B.; Xu, H.; et al. Enhancement of nucleus pulposus repair by glycoengineered adipose-derived mesenchymal cells. Biomaterials 2022, 283, 121463. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, H.; Mehdipour, A.; Dianat-Moghadam, H.; Salehi, R.; Khoshfetrat, A.B.; Hassani, A.; Mohammadnejad, D. PCL/Col I-based magnetic nanocomposite scaffold provides an osteoinductive environment for ADSCs in osteogenic cues-free media conditions. Stem Cell Res. Ther. 2022, 13, 143. [Google Scholar] [CrossRef]
- Shi, Z.L.; Fan, Z.Y.; Zhang, H.; Li, S.T.; Yuan, H.; Tong, J.H. Localized delivery of brain-derived neurotrophic factor from PLGA microspheres promotes peripheral nerve regeneration in rats. J. Orthop. Surg. Res. 2022, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Zavan, B.; Vindigni, V.; Gardin, C.; D’Avella, D.; Della Puppa, A.; Abatangelo, G.; Cortivo, R. Neural potential of adipose stem cells. Discov. Med. 2010, 10, 37–43. [Google Scholar]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef]
- López-Díaz de Cerio, A.; Perez-Estenaga, I.; Inoges, S.; Abizanda, G.; Gavira, J.J.; Larequi, E.; Andreu, E.; Rodriguez, S.; Gil, A.G.; Crisostomo, V.; et al. Preclinical Evaluation of the Safety and Immunological Action of Allogeneic ADSC-Collagen Scaffolds in the Treatment of Chronic Ischemic Cardiomyopathy. Pharmaceutics 2021, 13, 1269. [Google Scholar] [CrossRef]
- Bhattacharjee, M.; Escobar Ivirico, J.L.; Kan, H.M.; Shah, S.; Otsuka, T.; Bordett, R.; Barajaa, M.; Nagiah, N.; Pandey, R.; Nair, L.S.; et al. Injectable amnion hydrogel-mediated delivery of adipose-derived stem cells for osteoarthritis treatment. Proc. Natl. Acad. Sci. USA 2022, 119, e2120968119. [Google Scholar] [CrossRef]
- Yamana, H.; Inagaki, A.; Imura, T.; Nakamura, Y.; Nishimaki, H.; Katano, T.; Ohashi, K.; Miyagi, S.; Kamei, T.; Unno, M.; et al. Cotransplantation with Adipose Tissue-Derived Stem Cells Improves Engraftment of Transplanted Hepatocytes. Transplantation 2022, 106, 1963–1973. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.; Ding, Y.; Tan, K.; Ni, W.; Ouyang, W.; Tang, J.; Ding, X.; Zhao, J.; Hao, Y.; et al. IL-6 coaxes cellular dedifferentiation as a pro-regenerative intermediate that contributes to pericardial ADSC-induced cardiac repair. Stem Cell Res. Ther. 2022, 13, 44. [Google Scholar] [CrossRef]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef]
- Lee, M.; Ban, J.J.; Yang, S.; Im, W.; Kim, M. The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res. 2018, 1691, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Tsuchiya, R.; Kosaka, N.; Yoshioka, Y.; Takagaki, K.; Oki, K.; Takeshita, F.; Sakai, Y.; Kuroda, M.; Ochiya, T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013, 3, 1197. [Google Scholar] [CrossRef]
- Lee, M.; Ban, J.J.; Kim, K.Y.; Jeon, G.S.; Im, W.; Sung, J.J.; Kim, M. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem. Biophys. Res. Commun. 2016, 479, 434–439. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, G.; Marchi, A.; Galiè, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef]
- Yun, I.S.; Jeon, Y.R.; Lee, W.J.; Lee, J.W.; Rah, D.K.; Tark, K.C.; Lew, D.H. Effect of human adipose derived stem cells on scar formation and remodeling in a pig model: A pilot study. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2012, 38, 1678–1688. [Google Scholar] [CrossRef]
- Si, Z.; Wang, X.; Sun, C.; Kang, Y.; Xu, J.; Wang, X.; Hui, Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. Biomed. Pharmacother. 2019, 114, 108765. [Google Scholar] [CrossRef]
- Darvishi, M.; Tiraihi, T.; Mesbah-Namin, S.A.; Delshad, A.; Taheri, T. Motor Neuron Transdifferentiation of Neural Stem Cell from Adipose-Derived Stem Cell Characterized by Differential Gene Expression. Cell. Mol. Neurobiol. 2017, 37, 275–289. [Google Scholar] [CrossRef]
- Madhu, V.; Dighe, A.S.; Cui, Q.; Deal, D.N. Dual Inhibition of Activin/Nodal/TGF-β and BMP Signaling Pathways by SB431542 and Dorsomorphin Induces Neuronal Differentiation of Human Adipose Derived Stem Cells. Stem Cells Int. 2016, 2016, 1035374. [Google Scholar] [CrossRef]
- Tang, Y.; He, H.; Cheng, N.; Song, Y.; Ding, W.; Zhang, Y.; Zhang, W.; Zhang, J.; Peng, H.; Jiang, H. PDGF, NT-3 and IGF-2 in combination induced transdifferentiation of muscle-derived stem cells into Schwann cell-like cells. PLoS ONE 2014, 9, e73402. [Google Scholar] [CrossRef]
- Brettschneider, J.; Del Tredici, K.; Lee, V.M.; Trojanowski, J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Reviews. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Griffin, M.; Mosahebi, A.; Butler, P. Systematic review of patient factors affecting adipose stem cell viability and function: Implications for regenerative therapy. Stem Cell Res. Ther. 2017, 8, 45. [Google Scholar] [CrossRef]
- Luo, L.; Hu, D.H.; Yin, J.Q.; Xu, R.X. Molecular Mechanisms of Transdifferentiation of Adipose-Derived Stem Cells into Neural Cells: Current Status and Perspectives. Stem Cells Int. 2018, 2018, 5630802. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Park, J.S.; Jeong, H.S. Neural Differentiation of Human Adipose Tissue-Derived Stem Cells Involves Activation of the Wnt5a/JNK Signalling. Stem Cells Int. 2015, 2015, 178618. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Subbalekha, K.; Sastravaha, P.; Pavasant, P. Notch signalling inhibits the adipogenic differentiation of single-cell-derived mesenchymal stem cell clones isolated from human adipose tissue. Cell Biol. Int. 2012, 36, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, A.J.; Gómez, D.E.; Argibay, P.F. Transcriptional characterization of Wnt and Notch signaling pathways in neuronal differentiation of human adipose tissue-derived stem cells. J. Mol. Neurosci. MN 2011, 44, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Reddy, L.V.K.; Abbas, S.; Mullick, M.; Moghal, E.T.B.; Balakrishna, J.P.; Sen, D. NOTCH Signaling Is Essential for Maturation, Self-Renewal, and Tri-Differentiation of In Vitro Derived Human Neural Stem Cells. Cell. Reprogramming 2017, 19, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, N.; Kushida, S.; Suzuki, K.; Ogura, T.; Notodihardjo, P.V.; Hara, T.; Kusumoto, K. Effects of transforming growth factor-beta1 on cell motility, collagen gel contraction, myofibroblastic differentiation, and extracellular matrix expression of human adipose-derived stem cell. Hum. Cell 2012, 25, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, A.; Ielpi, M.; Gómez, D.; Argibay, P. Differential expression of Shh and BMP signaling in the potential conversion of human adipose tissue stem cells into neuron-like cells in vitro. Gene Expr. 2010, 14, 307–319. [Google Scholar] [CrossRef]
- Xu, F.T.; Li, H.M.; Yin, Q.S.; Cui, S.E.; Liu, D.L.; Nan, H.; Han, Z.A.; Xu, K.M. Effect of ginsenoside Rg1 on proliferation and neural phenotype differentiation of human adipose-derived stem cells in vitro. Can. J. Physiol. Pharmacol. 2014, 92, 467–475. [Google Scholar] [CrossRef]
- Béjot, Y.; Daubail, B.; Giroud, M. Epidemiology of stroke and transient ischemic attacks: Current knowledge and perspectives. Rev. Neurol. 2016, 172, 59–68. [Google Scholar] [CrossRef]
- Guzik, A.; Bushnell, C. Stroke Epidemiology and Risk Factor Management. Continuum 2017, 23, 15–39. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Huang, H.; Lin, F.; Jiang, J.; Chen, Y.; Mei, A.; Zhu, P. Effects of intra-arterial transplantation of adipose-derived stem cells on the expression of netrin-1 and its receptor DCC in the peri-infarct cortex after experimental stroke. Stem Cell Res. Ther. 2017, 8, 223. [Google Scholar] [CrossRef]
- Zhou, F.; Gao, S.; Wang, L.; Sun, C.; Chen, L.; Yuan, P.; Zhao, H.; Yi, Y.; Qin, Y.; Dong, Z.; et al. Human adipose-derived stem cells partially rescue the stroke syndromes by promoting spatial learning and memory in mouse middle cerebral artery occlusion model. Stem Cell Res. Ther. 2015, 6, 92. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, H.; Hu, Y.; Pan, C.; Wu, G.; Li, Q.; Tang, Z. Adipose-derived mesenchymal stem cells stereotactic transplantation alleviate brain edema from intracerebral hemorrhage. J. Cell. Biochem. 2019, 120, 14372–14382. [Google Scholar] [CrossRef]
- Zhao, K.; Li, R.; Gu, C.; Liu, L.; Jia, Y.; Guo, X.; Zhang, W.; Pei, C.; Tian, L.; Li, B.; et al. Intravenous Administration of Adipose-Derived Stem Cell Protein Extracts Improves Neurological Deficits in a Rat Model of Stroke. Stem Cells Int. 2017, 2017, 2153629. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, H.; Jin, M.; Yang, X.; Ji, H.; Jiang, Y.; Zhang, H.; Wu, F.; Wu, G.; Lai, X.; et al. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 47, 864–878. [Google Scholar] [CrossRef]
- Lv, H.; Li, J.; Che, Y. miR-31 from adipose stem cell-derived extracellular vesicles promotes recovery of neurological function after ischemic stroke by inhibiting TRAF6 and IRF5. Exp. Neurol. 2021, 342, 113611. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, H.; Li, L.; Han, J.; Liu, Z.; Chu, M.; Sha, X.; Zhao, J. Anti-CHAC1 exosomes for nose-to-brain delivery of miR-760-3p in cerebral ischemia/reperfusion injury mice inhibiting neuron ferroptosis. J. Nanobiotechnol. 2023, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Aertker, B.M.; Bedi, S.; Cox, C.S., Jr. Strategies for CNS repair following TBI. Exp. Neurol. 2016, 275 Pt 3, 411–426. [Google Scholar] [CrossRef]
- Moss, L.D.; Sode, D.; Patel, R.; Lui, A.; Hudson, C.; Patel, N.A.; Bickford, P.C. Intranasal delivery of exosomes from human adipose derived stem cells at forty-eight hours post injury reduces motor and cognitive impairments following traumatic brain injury. Neurochem. Int. 2021, 150, 105173. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Diao, Y.F.; Wang, J.; Liang, J.; Xu, H.H.; Zhao, M.L.; Zheng, B.; Luan, Z.; Wang, J.J.; Yang, X.P.; et al. Intravenously Infusing the Secretome of Adipose-Derived Mesenchymal Stem Cells Ameliorates Neuroinflammation and Neurological Functioning After Traumatic Brain Injury. Stem Cells Dev. 2020, 29, 222–234. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Ma, B.; Li, N.; Wang, S.; Sun, Z.; Xue, C.; Han, Q.; Wei, J.; Zhao, R.C. MSC-derived exosomes promote recovery from traumatic brain injury via microglia/macrophages in rat. Aging 2020, 12, 18274–18296. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, N.; Acosta, S.A.; Shahaduzzaman, M.; Ishikawa, H.; Shinozuka, K.; Pabon, M.; Hernandez-Ontiveros, D.; Kim, D.W.; Metcalf, C.; Staples, M.; et al. Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: Cell graft biodistribution and soluble factors in young and aged rats. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 313–326. [Google Scholar] [CrossRef]
- Patel, N.A.; Moss, L.D.; Lee, J.Y.; Tajiri, N.; Acosta, S.; Hudson, C.; Parag, S.; Cooper, D.R.; Borlongan, C.V.; Bickford, P.C. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflamm. 2018, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.P.; Silver, J. Neuroscience. Systemically treating spinal cord injury. Science 2015, 348, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Torres-Espín, A.; Hernández, J.; Navarro, X. Gene expression changes in the injured spinal cord following transplantation of mesenchymal stem cells or olfactory ensheathing cells. PLoS ONE 2013, 8, e76141. [Google Scholar] [CrossRef] [PubMed]
- van der Slot, A.J.; Zuurmond, A.M.; Bardoel, A.F.; Wijmenga, C.; Pruijs, H.E.; Sillence, D.O.; Brinckmann, J.; Abraham, D.J.; Black, C.M.; Verzijl, N.; et al. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J. Biol. Chem. 2003, 278, 40967–40972. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, H.; Zhang, K.; Xiao, D.J.; Wang, C.; Wang, Y.S. Adipose-derived stromal cells improve functional recovery after spinal cord injury through TGF-β1/Smad3/PLOD2 pathway activation. Aging 2021, 13, 4370–4387. [Google Scholar] [CrossRef]
- Oliveira, E.; Assunção-Silva, R.C.; Ziv-Polat, O.; Gomes, E.D.; Teixeira, F.G.; Silva, N.A.; Shahar, A.; Salgado, A.J. Influence of Different ECM-Like Hydrogels on Neurite Outgrowth Induced by Adipose Tissue-Derived Stem Cells. Stem Cells Int. 2017, 2017, 6319129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tian, X.; Mo, B.; Xu, H.; Zhang, L.; Huang, L.; Yao, S.; Huang, Z.; Wang, Y.; Xie, H.; et al. Adipose mesenchymal stem cell transplantation alleviates spinal cord injury-induced neuroinflammation partly by suppressing the Jagged1/Notch pathway. Stem Cell Res. Ther. 2020, 11, 212. [Google Scholar] [CrossRef]
- Krueger, E.; Magri, L.M.S.; Botelho, A.S.; Bach, F.S.; Rebellato, C.L.K.; Fracaro, L.; Fragoso, F.Y.I.; Villanova, J.A., Jr.; Brofman, P.R.S.; Popović-Maneski, L. Effects of low-intensity electrical stimulation and adipose derived stem cells transplantation on the time-domain analysis-based electromyographic signals in dogs with SCI. Neurosci. Lett. 2019, 696, 38–45. [Google Scholar] [CrossRef]
- Vialle, E.N.; Fracaro, L.; Barchiki, F.; Dominguez, A.C.; Arruda, A.O.; Olandoski, M.; Brofman, P.R.S.; Kuniyoshi Rebelatto, C.L. Human Adipose-Derived Stem Cells Reduce Cellular Damage after Experimental Spinal Cord Injury in Rats. Biomedicines 2023, 11, 1394. [Google Scholar] [CrossRef]
- Tien, N.L.B.; Hoa, N.D.; Thanh, V.V.; Thach, N.V.; Ngoc, V.T.N.; Dinh, T.C.; Phuong, T.N.T.; Toi, P.L.; Chu, D.T. Autologous Transplantation of Adipose-Derived Stem Cells to Treat Acute Spinal Cord Injury: Evaluation of Clinical Signs, Mental Signs, and Quality of Life. Open Access Maced. J. Med. Sci. 2019, 7, 4399–4405. [Google Scholar] [CrossRef]
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Syvertsen, M.; Nakken, K.O.; Edland, A.; Hansen, G.; Hellum, M.K.; Koht, J. Prevalence and etiology of epilepsy in a Norwegian county-A population based study. Epilepsia 2015, 56, 699–706. [Google Scholar] [CrossRef]
- de la Court, A.; Breteler, M.M.; Meinardi, H.; Hauser, W.A.; Hofman, A. Prevalence of epilepsy in the elderly: The Rotterdam Study. Epilepsia 1996, 37, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Nevitt, S.J.; Sudell, M.; Cividini, S.; Marson, A.G.; Tudur Smith, C. Antiepileptic drug monotherapy for epilepsy: A network meta-analysis of individual participant data. Cochrane Database Syst. Rev. 2022, 4, Cd011412. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Han, Y.; Du, J.; Jin, H.; Zhang, J.; Niu, M.; Qin, J. Alterations of apoptosis and autophagy in developing brain of rats with epilepsy: Changes in LC3, P62, Beclin-1 and Bcl-2 levels. Neurosci. Res. 2018, 130, 47–55. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Pan, X.; Zhang, Y.; Lin, L.; Wu, Y.; Huang, Y.; He, H. Adipose-derived stem cell transplantation improves learning and memory via releasing neurotrophins in rat model of temporal lobe epilepsy. Brain Res. 2021, 1750, 147121. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, M.Y.; Ryu, J.H.; Lee, D.H.; Jeon, B.T.; Roh, G.S.; Kang, S.S.; Kim, H.J.; Cho, G.J.; Choi, W.S. Clusterin interaction with Bcl-xL is associated with seizure-induced neuronal death. Epilepsy Res. 2012, 99, 240–251. [Google Scholar] [CrossRef]
- Xiaoying, G.; Guo, M.; Jie, L.; Yanmei, Z.; Ying, C.; Shengjie, S.; Haiyan, G.; Feixiang, S.; Sihua, Q.; Jiahang, S. CircHivep2 contributes to microglia activation and inflammation via miR-181a-5p/SOCS2 signalling in mice with kainic acid-induced epileptic seizures. J. Cell. Mol. Med. 2020, 24, 12980–12993. [Google Scholar] [CrossRef]
- Gaiottino, J.; Norgren, N.; Dobson, R.; Topping, J.; Nissim, A.; Malaspina, A.; Bestwick, J.P.; Monsch, A.U.; Regeniter, A.; Lindberg, R.L.; et al. Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS ONE 2013, 8, e75091. [Google Scholar] [CrossRef]
- Ebadi, M.; Brown-Borg, H.; Ren, J.; Sharma, S.; Shavali, S.; El ReFaey, H.; Carlson, E.C. Therapeutic efficacy of selegiline in neurodegenerative disorders and neurological diseases. Curr. Drug Targets 2006, 7, 1513–1529. [Google Scholar] [CrossRef]
- Chan, T.M.; Chen, J.Y.; Ho, L.I.; Lin, H.P.; Hsueh, K.W.; Liu, D.D.; Chen, Y.H.; Hsieh, A.C.; Tsai, N.M.; Hueng, D.Y.; et al. ADSC therapy in neurodegenerative disorders. Cell Transplant. 2014, 23, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Sen, D. Mesenchymal Stem Cells as a Source of Dopaminergic Neurons: A Potential Cell Based Therapy for Parkinson’s Disease. Curr. Stem Cell Res. Ther. 2017, 12, 326–347. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Shi, G.; Lei, X.; Huang, Y.; Bai, L.; Qin, C. Effectiveness and mechanisms of adipose-derived stem cell therapy in animal models of Parkinson’s disease: A systematic review and meta-analysis. Transl. Neurodegener. 2021, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.K.; Martinez, T.N.; Ruhn, K.A.; Wrage, P.C.; Keefer, E.W.; Botterman, B.R.; Tansey, K.E.; Tansey, M.G. Autologous transplants of Adipose-Derived Adult Stromal (ADAS) cells afford dopaminergic neuroprotection in a model of Parkinson’s disease. Exp. Neurol. 2008, 210, 14–29. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xing, H.; Wang, Y.; Guo, Y. Exosomes derived from miR-188-3p-modified adipose-derived mesenchymal stem cells protect Parkinson’s disease. Mol. Therapy. Nucleic Acids 2021, 23, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, M.; Jang, S.; Hwang, J.; Kim, B.; Cho, H.H.; Kim, E.; Jeong, H.S. Neuroprotective Effects of the Neural-Induced Adipose-Derived Stem Cell Secretome against Rotenone-Induced Mitochondrial and Endoplasmic Reticulum Dysfunction. Int. J. Mol. Sci. 2023, 24, 5622. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, A.; Darvishi, M.; Amraei, M. Homing of Super Paramagnetic Iron Oxide Nanoparticles (SPIONs) Labeled Adipose-Derived Stem Cells by Magnetic Attraction in a Rat Model of Parkinson’s Disease. Int. J. Nanomed. 2020, 15, 1297–1308. [Google Scholar] [CrossRef]
- Chi, K.; Fu, R.H.; Huang, Y.C.; Chen, S.Y.; Hsu, C.J.; Lin, S.Z.; Tu, C.T.; Chang, L.H.; Wu, P.A.; Liu, S.P. Adipose-derived Stem Cells Stimulated with n-Butylidenephthalide Exhibit Therapeutic Effects in a Mouse Model of Parkinson’s Disease. Cell Transplant. 2018, 27, 456–470. [Google Scholar] [CrossRef]
- Ma, T.; Gong, K.; Ao, Q.; Yan, Y.; Song, B.; Huang, H.; Zhang, X.; Gong, Y. Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer’s disease mice. Cell Transplant. 2013, 22 (Suppl. S1), S113–S126. [Google Scholar] [CrossRef]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Bernard, E.; Pegat, A.; Svahn, J.; Bouhour, F.; Leblanc, P.; Millecamps, S.; Thobois, S.; Guissart, C.; Lumbroso, S.; Mouzat, K. Clinical and Molecular Landscape of ALS Patients with SOD1 Mutations: Novel Pathogenic Variants and Novel Phenotypes. A Single ALS Center Study. Int. J. Mol. Sci. 2020, 21, 6807. [Google Scholar] [CrossRef]
- Shigematsu, K.; Takeda, T.; Komori, N.; Urushihata, N.; Oki, K.; Tahara, K.; Yamagishi, H. Long-term survival of a patient with amyotrophic lateral sclerosis (ALS) who received autologous adipose-derived mesenchymal stem cells. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4086–4090. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Karimi, S.H.; Arab, L.; Sanjari, L.; Mardpour, S.; Azimian, V.; Jarughi, N.; Ghaheri, A.; Hosseini, S.E.; Aghdami, N.; et al. Safety and Efficacy of Allogeneic Adipose Tissue Mesenchymal Stromal Cells in Amyotrophic Lateral Sclerosis Patients, Single-Center, Prospective, Open-Label, Single-Arm Clinical Trial, Long-Term Follow-up. Cell J. 2021, 23, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Liu, J.W.; Chen, B.C.; Jiang, Z.S.; Tu, C.T.; Su, C.H.; Yang, H.H.; Liu, Z.Q.; Deng, Y.C.; Chen, C.Y.; et al. Transplantation of Adipose-Derived Stem Cells Alleviates Striatal Degeneration in a Transgenic Mouse Model for Multiple System Atrophy. Cell Transplant. 2020, 29, 963689720960185. [Google Scholar] [CrossRef]
- Pacheco, R.; Contreras, F.; Zouali, M. The dopaminergic system in autoimmune diseases. Front. Immunol. 2014, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Stepien, A.; Dabrowska, N.L.; Maciagowska, M.; Macoch, R.P.; Zolocinska, A.; Mazur, S.; Siennicka, K.; Frankowska, E.; Kidzinski, R.; Chalimoniuk, M.; et al. Clinical Application of Autologous Adipose Stem Cells in Patients with Multiple Sclerosis: Preliminary Results. Mediat. Inflamm. 2016, 2016, 5302120. [Google Scholar] [CrossRef]
- Hedayatpour, A.; Ragerdi, I.; Pasbakhsh, P.; Kafami, L.; Atlasi, N.; Pirhajati Mahabadi, V.; Ghasemi, S.; Reza, M. Promotion of remyelination by adipose mesenchymal stem cell transplantation in a cuprizone model of multiple sclerosis. Cell J. 2013, 15, 142–151. [Google Scholar] [PubMed]
- Shalaby, S.M.; Sabbah, N.A.; Saber, T.; Abdel Hamid, R.A. Adipose-derived mesenchymal stem cells modulate the immune response in chronic experimental autoimmune encephalomyelitis model. IUBMB Life 2016, 68, 106–115. [Google Scholar] [CrossRef]
- Strong, A.L.; Bowles, A.C.; Wise, R.M.; Morand, J.P.; Dutreil, M.F.; Gimble, J.M.; Bunnell, B.A. Human Adipose Stromal/Stem Cells from Obese Donors Show Reduced Efficacy in Halting Disease Progression in the Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis. Stem Cells 2016, 34, 614–626. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Yousefi, F.; Ebtekar, M.; Soudi, S.; Soleimani, M.; Hashemi, S.M. In vivo immunomodulatory effects of adipose-derived mesenchymal stem cells conditioned medium in experimental autoimmune encephalomyelitis. Immunol. Lett. 2016, 172, 94–105. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Semon, J.A.; Zhang, X.; Pandey, A.C.; Alandete, S.M.; Maness, C.; Zhang, S.; Scruggs, B.A.; Strong, A.L.; Sharkey, S.A.; Beuttler, M.M.; et al. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl. Med. 2013, 2, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Inoue, K.; Suga, H.; Eto, H.; Kato, H.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2008, 34, 1178–1185. [Google Scholar] [CrossRef]
- Bowles, A.C.; Strong, A.L.; Wise, R.M.; Thomas, R.C.; Gerstein, B.Y.; Dutreil, M.F.; Hunter, R.S.; Gimble, J.M.; Bunnell, B.A. Adipose Stromal Vascular Fraction-Mediated Improvements at Late-Stage Disease in a Murine Model of Multiple Sclerosis. Stem Cells 2017, 35, 532–544. [Google Scholar] [CrossRef]
- Nan, D.; Dou, X.; Qi, Y.; Zhang, W.; He, G.; Zhang, X. In Vitro Study of Adipose-Derived Mesenchymal Stem Cells Transduced with Lentiviral Vector Carrying the Brain-Derived Neurotrophic Factor Gene. Int. J. Stem Cells 2020, 13, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Zhang, X.; Ji, L.; Wang, K.; Qiu, Y. Effects of brain-derived neurotrophic factor and neurotrophin-3 on the neuronal differentiation of rat adipose-derived stem cells. Mol. Med. Rep. 2015, 12, 4981–4988. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Yoon, Y.; Kim, A.; Jo, K.R.; Choi, K.U.; Jung, T.; Kim, N.; Son, Y.; Kim, W.H.; Kweon, O.K. Improved Healing after the Co-Transplantation of HO-1 and BDNF Overexpressed Mesenchymal Stem Cells in the Subacute Spinal Cord Injury of Dogs. Cell Transplant. 2018, 27, 1140–1153. [Google Scholar] [CrossRef]
- Tang, L.; Lu, X.; Zhu, R.; Qian, T.; Tao, Y.; Li, K.; Zheng, J.; Zhao, P.; Li, S.; Wang, X.; et al. Adipose-Derived Stem Cells Expressing the Neurogenin-2 Promote Functional Recovery After Spinal Cord Injury in Rat. Cell. Mol. Neurobiol. 2016, 36, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Marin-Bañasco, C.; Benabdellah, K.; Melero-Jerez, C.; Oliver, B.; Pinto-Medel, M.J.; Hurtado-Guerrero, I.; de Castro, F.; Clemente, D.; Fernández, O.; Martin, F.; et al. Gene therapy with mesenchymal stem cells expressing IFN-ß ameliorates neuroinflammation in experimental models of multiple sclerosis. Br. J. Pharmacol. 2017, 174, 238–253. [Google Scholar] [CrossRef]
- Mohammadzadeh, A.; Pourfathollah, A.A.; Shahrokhi, S.; Fallah, A.; Tahoori, M.T.; Amari, A.; Forouzandeh, M.; Soleimani, M. Evaluation of AD-MSC (adipose-derived mesenchymal stem cells) as a vehicle for IFN-β delivery in experimental autoimmune encephalomyelitis. Clin. Immunol. 2016, 169, 98–106. [Google Scholar] [CrossRef]
- Payne, N.L.; Dantanarayana, A.; Sun, G.; Moussa, L.; Caine, S.; McDonald, C.; Herszfeld, D.; Bernard, C.C.; Siatskas, C. Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination. Cell Adhes. Migr. 2012, 6, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wu, J.; Lang, L.; Hu, J.; Tang, H.; Xu, J.; Sun, B. Implantation of glial cell line-derived neurotrophic factor-expressing adipose tissue-derived stromal cells in a rat Parkinson’s disease model. Neurol. Res. 2020, 42, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Stahn, L.; Rasińska, J.; Dehne, T.; Schreyer, S.; Hakus, A.; Gossen, M.; Steiner, B.; Hemmati-Sadeghi, S. Sleeping Beauty transposon system for GDNF overexpression of entrapped stem cells in fibrin hydrogel in a rat model of Parkinson’s disease. Drug Deliv. Transl. Res. 2023, 13, 1745–1765. [Google Scholar] [CrossRef] [PubMed]
- Curtis, T.M.; Hannett, J.M.; Harman, R.M.; Puoplo, N.A.; Van de Walle, G.R. The secretome of adipose-derived mesenchymal stem cells protects SH-SY5Y cells from arsenic-induced toxicity, independent of a neuron-like differentiation mechanism. Neurotoxicology 2018, 67, 54–64. [Google Scholar] [CrossRef]
- Baldassarro, V.A.; Perut, F.; Cescatti, M.; Pinto, V.; Fazio, N.; Alastra, G.; Parziale, V.; Bassotti, A.; Fernandez, M.; Giardino, L.; et al. Intra-individual variability in the neuroprotective and promyelinating properties of conditioned culture medium obtained from human adipose mesenchymal stromal cells. Stem Cell Res. Ther. 2023, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Üçal, M.; Maurer, C.; Etschmaier, V.; Hamberger, D.; Grünbacher, G.; Tögl, L.; Roosen, M.J.; Molcanyi, M.; Vorholt, D.; Hatay, F.F.; et al. Inflammatory Pre-Conditioning of Adipose-Derived Stem Cells with Cerebrospinal Fluid from Traumatic Brain Injury Patients Alters the Immunomodulatory Potential of ADSC Secretomes. J. Neurotrauma 2021, 38, 2311–2322. [Google Scholar] [CrossRef]
- Chiu, T.L.; Baskaran, R.; Tsai, S.T.; Huang, C.Y.; Chuang, M.H.; Syu, W.S.; Harn, H.J.; Lin, Y.C.; Chen, C.H.; Huang, P.C.; et al. Intracerebral transplantation of autologous adipose-derived stem cells for chronic ischemic stroke: A phase I study. J. Tissue Eng. Regen. Med. 2022, 16, 3–13. [Google Scholar] [CrossRef]
- Ramos-Cejudo, J.; Wisniewski, T.; Marmar, C.; Zetterberg, H.; Blennow, K.; de Leon, M.J.; Fossati, S. Traumatic Brain Injury and Alzheimer’s Disease: The Cerebrovascular Link. EBioMedicine 2018, 28, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Oohira, A. Chondroitin sulfate, a major niche substance of neural stem cells, and cell transplantation therapy of neurodegeneration combined with niche modification. Curr. Stem Cell Res. Ther. 2009, 4, 200–209. [Google Scholar] [CrossRef] [PubMed]


| Neurodegenerative Diseases | Animals or Cells Model of Disease | Origin of Graft | Type of Therapy | Summarized Experimental Design | Main Outcomes/Therapeutic Effect | Reference |
|---|---|---|---|---|---|---|
| Parkinson’s disease | 6-OHDA rat model | Autologous ADMSC | ADMSC transplantation | Investigating ability of both naive and differentiated ADMSC autologous graft to protect, repair or restore the function of nigrostriatal pathway was performed on the basis of study of neurohistology, electrophysiology, cell biology, and gene expression. | Produce neuroprotective trophic factors, modulate the microenvironment | [76] |
| Parkinson’s disease | MPTP-induced mice models; MPP+-induced cell models | Adipose obtained from C57BL/6 mice | Injection of miR-188-3p-enriched exosome derived from ADMSC | The level of miR-188-3p was assessed in PD patients. Levels of injury, inflammatory factors and autophagy were evaluated in MPP+-induced cell models and MPTP-induced PD mice models after treated with miR-188-3p-enriched ADMSC-E. | Inhibit autophagy, pyroptosis and promote proliferation by targeting CDK5 and NLRP3 in vitro. Alleviate inflammation and restore neurons in the substantia nigra in vivo. | [77] |
| Parkinson’s disease | ROT-induced SH-SY5Y cells | Adipose obtained from human donors | Injection of NI-ADMSC-SM | Neuroprotective effects of NI-ADMSC-SM on ROT-induced dysfunction in human SH-SY5Y cells was explored by measuring the alternation of damage in endoplasmic reticulum, the mitochondria, and their tethering proteins with molecular biological method. | Restore mitochondrial fusion, mitophagy, and tethering to ER | [78] |
| Parkinson’s disease | 6-OHDA rat model | Adipose obtained from male Sprague–Dawley rats | ADMSC transplantation | Adipose-derived MSCs obtained were immunostained-coating SPION with polyl-lysine hydrobromide and transfecting GFP reporter gene into ADMSCs to explore the role of external magnets in the delivery and homing of stem cells in the target tissue. | Provide a solution for the delivery and homing of transplanted adipose-derived MSC transplantation | [79] |
| Parkinson’s disease | MPTP-induced mice models | Adipose obtained from human donors | ADMSC transplantation | Evaluation of the possible neurogenic effects of BP was performed in vitro, including examination of cell survival and gene expression patterns. After transplanting ADMSCs into the mouse striatum, effects of BP-pretreated ADMSCs in vivo were evaluated by behavioral experiment. | Restore motor abilities. BP stimulation improved the therapeutic effects of transplantation. | [80] |
| Alzheimer’s Disease | APP/PS1 double transgenic AD model mice | Adipose obtained from male Sprague–Dawley rats | ADMSC transplantation | Immunomodulatory effect and mechanism of microglia activation after adipose-derived MSC transplantation was revealed in AD model mice. The effects of ADMSC transplantation on cognitive function of AD mice were studied by behavioral experiments. | Optimize alternative microglial activation, alleviate inflammation and Aβ pathology. Improves cognition, memory, and learning capabilities | [81] |
| Amyotrophic lateral sclerosis | G93A ALS mice model | Subcutaneous adipose obtained from human donors | Neuronal cells of ALS mice were treated with ADMSC-E in vitro | The production and aggregation of SOD1, as well as the mitochondrial function was evaluated after treatment with ADMSC-E for twice in vitro. | Inhibit production and aggregation of intracellular SOD1, alleviate the mitochondrial dysfunction of neuronal cells. | [17] |
| Multiple-system atrophy | MBP Line 1 (MBP1) mice | Human ADMSCs | Intracerebral injection of ADMSCs | The effective dose of ADMSC transplantation was explored, and immunohistochemical and molecular biological methods were used to investigate the mechanism. | Alleviate striatal degeneration and inflammation, improve the nigrostriatal pathway for dopamine, ameliorate cell survival and myelination at caudate-putamen. | [86] |
| CNS Disorders | Gene-Modified ADMSCs | Animal Model of Disease | Origin of Transplanted ADMSCs | Method of Administration | Main Therapeutic Effect | Reference |
|---|---|---|---|---|---|---|
| Spinal cord injury | BDNF-ADMSC | Male SCI dog model | Gluteal subcutaneous fat from healthy beagle dog of age 1.5 years | Two injections into epicenter and one into the center of the injured segment | Induce neuroregeneration | [100] |
| Spinal cord injury | HO1-ADMSC | Male SCI dog model | Gluteal subcutaneous fat from healthy beagle dog of age 1.5 years | Two injections into epicenter and one into the center of the injured segment | Alleviate inflammation | [100] |
| Spinal cord injury | Ngn2-ADMSCs | Female SCI rat model | Female Sprague–Dawley rats | Injection into the lesion epicenter | Differentiated into neurons, promote post-SCI functional recovery | [101] |
| Multiple sclerosis | IFN-β-ADMSCs | Female EAE mice model | Mice | Intravenous injection | Inhibit neuroinflammation | [102] |
| Multiple sclerosis | IFN-β-ADMSCs | Female EAE mice model | Inguinal fat of 6–8 weeks old C57BL/6 female mice | Intraperitoneal injection | Reduce IL-17; induce both IL-10 and Treg, alleviate cell infiltration | [103] |
| Multiple sclerosis | IL-4-ADMSCs | Female EAE mice model | Human ADMSC | Intraperitoneal injection | Reduce the clinical score of EAE | [104] |
| Parkinson’s disease | GDNF-ADMSC | 6-OHDA-induced male SD rat model | Groin subcutaneous adipose tissue of Sprague-Dawley rat | Intrastriatal injection | Differentiate into dopaminergic neuron-like cells; clinical improvement | [105] |
| Parkinson’s disease | GDNF-ADMSC | Mild 6-OHDA hemiparkinson male rat model | Human adipose tissue | Intrastriatal injection | Immunomodulation and neuroprotection; restoration of tyrosine hydroxylase expressing cells | [106] |
| CNS Disorders | Status | Purpose | Enrollment | Intervention Model | Study Start Date | Study Completion Date | Main Outcomes | Reference/NCT Numbers |
|---|---|---|---|---|---|---|---|---|
| Stroke | Completed | To confirm the possible of autologous ADMSCs transplantation of chronic stroke | 3 participants | Single-Group Assignment | 19 October 2017 | 27 November 2018 | All patients improved prominently, and no adverse effects or related safety issue detected within 6 months after treatment. | [110]; NCT02813512 |
| Stroke | Active, not recruiting | To explore the dose of autologous ADMSCs transplantation | 15 participants | Single-Group Assignment | 6 February 2020 | 30 September 2023 | NA | NCT04088149 |
| Stroke | Recruiting | To investigate the safety, tolerability and efficiency of ADMSCs transplantation. | 95 participants | Sequential Assignment | 2 January 2018 | 1 July 2027 | NA | NCT03570450 |
| Traumatic brain injury | Active, not recruiting | To provide primary assessments of the safety, tolerability, and clinical effect of intravenous ADMSCs infusion in post concussion syndrome (PCS). | 20 participants | Parallel Assignment | 1 February 2021 | 31 January 2024 | NA | NCT04744051 |
| Spinal cord injury | Completed | To assess the clinical effects, change of quality of life, and mental signs after autologous ADMSCs transplantation to acute SCI. | 47 participants | Parallel Assignment | NA | NA | Autologous ADMSCs transplantation was safe and provided both functional and post-SCI mental improvement. | [60] |
| Alzheimer’s disease | Recruiting | To evaluate the safety, severe adverse events, tolerability, and therapeutic effect of autologous ADMSCs intracerebroventricular injections in AD patients. | 18 participants | Sequential Assignment | 14 August 2023 | March 2025 | NA | NCT05667649 |
| Multiple Sclerosis | Not yet recruiting | To investigate the safety and efficacy of escalating doses of ADMSCs SCM-010 in patients with secondary progressive multiple sclerosis (SPMS). | 12 participants | Sequential Assignment | 1 February 2023 | 1 February 2024 | NA | NCT03696485 |
| Amyotrophic Lateral Sclerosis | Completed | To evaluate the safety and possible efficiency of ADMSCs treatment in an ALS patient | 1 participant | Single-Group Assignment | January 2015 | January 2016 | NA | NCT02383654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Fan, Y.; Zhang, M. Advanced Progress in the Role of Adipose-Derived Mesenchymal Stromal/Stem Cells in the Application of Central Nervous System Disorders. Pharmaceutics 2023, 15, 2637. https://doi.org/10.3390/pharmaceutics15112637
Wu H, Fan Y, Zhang M. Advanced Progress in the Role of Adipose-Derived Mesenchymal Stromal/Stem Cells in the Application of Central Nervous System Disorders. Pharmaceutics. 2023; 15(11):2637. https://doi.org/10.3390/pharmaceutics15112637
Chicago/Turabian StyleWu, Haiyue, Yishu Fan, and Mengqi Zhang. 2023. "Advanced Progress in the Role of Adipose-Derived Mesenchymal Stromal/Stem Cells in the Application of Central Nervous System Disorders" Pharmaceutics 15, no. 11: 2637. https://doi.org/10.3390/pharmaceutics15112637