1. Introduction
Immunosuppressive drugs, such as cyclosporine A (CsA), tacrolimus, and rapamycin, demonstrate undesirable side-effects upon prolonged application [
1]. The search for new drugs devoid of side-effects, particularly peptides from natural sources and their synthetic analogs, represents a challenge for medical chemistry. Isolated in 1959 from linen seeds, a strongly hydrophobic cyclic nonapeptide, cyclolinopeptide A (CLA) [
2], was described as a strong immunosuppressor with a potency comparable to that of CsA [
3]. The mechanism of action of CLA was proposed to be similar to that of CsA, since CLA was shown to form a complex with cyclophilin A, leading to inactivation of calcineurin [
4,
5]. CLA suppressed both the humoral and cellular immune response and graft-versus-host reaction; prolonged acceptance of alogeneic skin grafts; ameliorated the symptoms of post-adjuvant polyarthritis in rats and hemolytic anemia in New Zealand black mice; and, as in the case of CsA, diminished IL-1 and IL-2 production [
6]. However, high hydrophobicity of CLA was one of the obstacles for potential application of the compound in therapy. Synthesis of new analogs of CLA aimed to improve their bioaccessibility and activity led to the following conclusions. Linear CLA analogs, bearing alanine residue in successive positions of the peptide chain, also demonstrated suppressive activities [
6]. Of interest, the activity of linear CLA analogs gradually decreased with shortening of the peptide chain from the N-end and, at the same time, displaying an increase of activity for C-terminal tetra- and tripeptides [
7]. The introduction of a single, hydrophilic threonine residue into the CLA molecule did not result in improved solubility in water. However, an improvement in solubility could be achieved by introducing a sulfonic group in the “
para” position of the aromatic ring of one or two phenyloalanine residues without loss of biological activity [
8,
9]. In addition, Pro-Pro-Phe-Phe or Pro-Phe-Phe CLA fragments seemed to be responsible for the immunosuppressive activity [
10,
11]. Further studies aimed to confirm the role of the
cis-peptide bond between proline residues. Therefore, we synthesized a series of analogs where this bond was replaced with a 1,5-disubstituted tetrazole ring as a proper mimetic of amide bonds in the
cis configuration. The obtained peptides exhibited strong immunosuppressive activities. It could be also concluded that the Pro-Pro-Phe-Phe fragment was crucial for the biological activity of CLA [
12].
In order to improve solubility of CLA in aqueous milieu, we performed syntheses of analogs where leucine residues in position 5 and/or 8 were replaced with its hydroxymethyl analog. The obtained peptides, despite diminution of the biological activity by 25% as compared with native CLA, were characterized by a better (four-fold) solubility in water [
13]. A replacement of proline residues by ß
2-isoproline and ß
3-homoproline [
14] allowed us to obtain a series of nine CLA analogs of particularly strong inhibitory properties, comparable to CsA, in the cellular immune response. The majority of these analogs were practically devoid of cell toxicity.
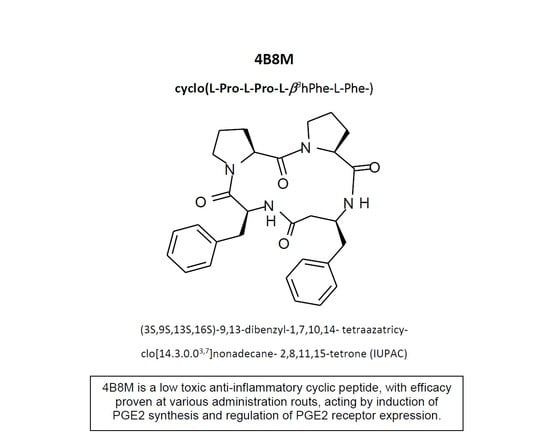
The aim of this study was to present, for the first time, an evaluation of the immunosuppressive activities of a modified cyclic tetrapeptide Pro-Pro-Phe-β
3hoPhe (denoted as 4B8M) using mouse and human cells in in vitro models as well as in vivo models of the immune response in mice. Appropriate reference compounds or commercially available therapeutics were used in the experimental models. In addition, we attempted to establish a plausible mechanism of action using molecular techniques, cytofluorometric assays, and in vivo models. Due to the enormous volume of the experimental data, we present only a short overview of the results in the main body of text, whereas preliminary in vitro data, a description of additional experiments, and most of the histological documentation are contained in the
Supplementary Materials.
2. Materials and Methods
2.1. Mice
CBA, BALB/c, and C57Bl/6 mice of both sexes, 8–12 weeks old, weighing 19–22 g, delivered by the Breeding Centre of Laboratory Animals at the Institute of Occupational Medicine, Łódź, Poland, were used for the study. Mice were housed in a cage at 21–22 °C with a 12/12-h light/dark cycle and had free access to commercial laboratory chow and filtered tap water. The local ethics committee at the Institute of Immunology and Experimental Therapy, Wrocław, Poland, approved the study (permissions #41/2008 19 November 2008, 61/2009 18 November 2009, 62/2009 18 November 2009, 22/2010 19 May 2010, 52/2010 17 November 2010, 21/2012 18 April 2012, 10/2013 20 March, 2013, and 4/2014 15 January, 2014.
2.2. Reagents
Cyclosporin A (CsA) (Sandimmun, Neoral, Sandoz, Basel, Switzerland) in ampoules, RPMI-1640 medium (Cibi/Life Technologies, Inchinnan, UK), fetal calf serum (FCS), serum-free keratinocyte medium, bovine pituitary extract derived from Gibco Thermo Fisher Scientific (Waltham, MA, USA), glutamate, HEPES, sodium pyruvate, antibiotic and antimycotic solution, cyclolinopeptide (CLA), lipopolysaccharide (LPS) from Escherichia coli 0:111, concanavalin A (Con A), phytohemagglutinin A (PHA), ovalbumin (OVA), indomethacin, aspirin (acetylsalicylic acid), MTT (93-[4-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), oxazolone, salicylic acid, toluene diisocyanate (TDI), recombinant Epidermal Growth Factor (EGF), carrageenan (C-1013, Lot 102 K0871), AH23848 (A8227, Lot 090M4618V) (EP4 receptor antagonist), L-798106 (L4545, Lot 020M4617V) (EP3 receptor antagonist), Giemsa and May-Grünwald reagents, hematoxylin, eosin, and toluidine blue were derived from Sigma-Aldrich (St. Louis, MO, USA); acetone was from Acros Organics, Poznań, Poland; and isotonic solution of sodium chloride buffered with phosphates (PBS) was from the Laboratory of General Chemistry, Institute of Immunology and Eperimental Therapy, Wrocław. Narcotan® (halotane) and Pentasa® (5-aminosalicylic acid (5-ASA)) were from Ferring-Leciva (Jesenice, Czech Republic). Hydrocortisonum® (hydrocortisone acetate) was from Jelfa (Jelenia Góra, Poland), Dexaven® (dexamethasone) was from Polfa-Warszawa (Warszawa, Poland), Protopic® (tacrolimus) 0.1% ointment was from Astellas (Dublin, Ireland), Elidel® (pimecrolimus) 1% creme was from Novartis, and DMSO (dimethylsulfoxide) was from Honeywell-Fluka, Toruń, Poland. Culture plates and flasks were purchased from Corning Incorporated (Tewksbury, MA, USA), and Nunc Lab-Tek II Chamber Slides were purchased from Thermo Scientific (Rockford, IL, USA). Cells were grown at 37 °C in 5% CO2 (NuAire, Plymouth, MN, USA). A Trypsin-EDTA (ethylenediaminetetraacetic acid, disodium salt) solution (Institute of Immunology and Experimental Therapy, Wrocław) was used to collect the cells.
The peptide 4B8M was synthesized and delivered by one of us (J.Z.), Łódź University of Technology. Synthesis of the peptide was described in a US patent [
15]. The peptide was initially dissolved in DMSO and subsequently in the culture medium at a concentration of 1 mg/mL. The peptide solution was stored at −20 °C until use. For topical administration, the peptide was dissolved by means of 1 h stirring in a 50:50 weight mixture of Vaseline and paraffin (obtained from a drug store). A microscopic evaluation showed no presence of crystals in the mixture containing up to 1% of the peptide.
2.3. Cell cultures for Molecular Studies
Jurkat T cells and peripheral blood mononuclear cells (PBMC) cells were cultured in a RPMI-1640 medium supplemented with 5% (Jurkat) or 10% (PBMC) FCS, glutamate, HEPES, sodium pyruvate, and an antibiotic and antimycotic solution. KERTr cells were cultured in a serum-free keratinocyte medium supplemented with 20 µg/mL bovine pituitary extract and 20 ng/mL recombinant EGF. For the cell culture studies, 4B8M was suspended in DMSO for 20 mg/mL. Then, it was diluted slowly in warm RPMI-1640 media supplemented with 10% of FCS and 5% of DMSO to obtain a working stock solution of 4B8M (500 µg/mL).
2.4. Cytofluorimetry
For cytofluorimetric analyses, the cells were seeded at densities of 2.5 × 105/well and treated for 24 h. After 24 h, cells were collected and incubated with appropriate antibodies. For Intercellular Adhesion Molecule 1 (ICAM-1) expression fluorescein isothiocyanate (FITC)-conjugated anti-ICAM-1 antibody (Santa Cruz, Dallas, TX, USA; 15.2 clone, 1:25) or FITC-conjugated normal mouse IgG1 (Santa Cruz) for 30 min in room temperature (RT) were used.
For intracellular cyclooxygenase (COX)-1 and COX-2 expression, the Intracellular Flow Cytometry (FCM) System (Santa Cruz) was used according to the supplier’s instructions. Cells were incubated with phycoerythrin (PE)-conjuga–ted anti-COX-1, anti-COX-2 or normal rabbit IgG isotype control antibodies (all Santa Cruz, 1:50, 1 h, 4 °C). Washed cells were collected and analyzed on a FACS Callibur cytofluorimeter (Becton Dickinson Biosciences, Franklin Lakes, NJ, USA) and WinMDI 2.9 software.
2.5. Western Blotting
Whole cell lysates were prepared using a cold radioimmunoprecipitation (RIPA) buffer supplemented with a SigmaFAST Protease Inhibitor Cocktail (Sigma-Aldrich, St. Luis, MS, USA). The cell lysates were then sonicated using a Sonopuls HD 2070 ultrasonic homogenizer (Bandelin, Berlin, Germany). Protein concentration was determined by the Pierce bicinchoninic acid assay (BCA) Protein Assay Kit (Thermo Fisher Scientifific, Waltham, MA, USA). Protein lysates were separated by SDS-PAGE using 12% resolving gels and transferred (semi-dry, 1 mA/cm2) to a poly(vinylidene fluoride) PVDF membrane (0.45 µm pore size; Merck Millipore, Burlington, MA, USA). Membranes were blocked with 1% casein (Sigma-Aldrich) for 1 h at RT, washed with TBST (20 mM Tris, 150 mM NaCl, 0.1% Tween 20 (BioShop Canada, Burlington, NO, Canada)), and subsequently incubated overnight with a primary antibody at 4 °C. After probing with an horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at RT, proteins of interest were detected using enhanced chemiluminescence (ECL), Western Blotting Substrate (Pierce, Thermo Fisher Scientifific, Waltham, MA, USA). The following antibodies were used in this study: EP1 goat polyclonal IgG (L-15, Sc-22646, 2 μg/mL), EP2 goat polyclonal IgG (E-13, Sc-22196, 1 μg/mL), EP3 rabbit polyclonal IgG (H-200, Sc-20676, 2 μg/mL), EP4 rabbit polyclonal IgG (H-160 Sc-20677, 2 μg/mL) (all Santa Cruz), and secondary polyclonal goat anti-rabbit immunoglobulins/HRP (Dako, Brookings, SD, USA, P0448, 1:2000) and polyclonal rabbit anti-goat immunoglobulins/HRP (Dako, P0449, 1:1000).
2.6. Toxicity Test
Mouse splenocytes at a density of 2 × 10
5/100 µL/well, resuspended in the culture medium, were cultured for 24 h in a cell culture incubator with the preparations at the indicated concentrations. Cell survival was determined by the MTT colorimetric method [
16].
2.7. Colorimetric MTT Assay for Cell Growth and Kill
Briefly, 25 µL of MTT (5 mg/mL) stock solution was added per well at the end of cell incubation and the plates were incubated for 3 h in a cell culture incubator. Then, 100 µL of the extraction buffer (20% SDS with 50% DMF [dimethylformamide], pH 4.7) was added. After additional overnight incubation, the optical density (OD) was measured at 550 nm with the reference wavelength of 630 nm in a Dynatech 5000 spectrophotometer. The results are given as a percentage of viable cells as compared with appropriate DMSO or controls.
2.8. Carrageenan Foot Pad Test
CBA 3-month-old male mice were used for the test. The reaction was initiated by interaction of carrageenan (a complex glycan) with skin mastocytes, which release histamine and other mediators to attract phagocytes, such as macrophages and neutrophils, constituting cell infiltration accounting for skin edema with a peak intensity after 3–4 h. The mice were injected with 4B8M (250 µg/mouse) intraperitoneally (i.p.) 1 h before carrageenan administration. PGE receptor antagonists AH23848 (EP4 receptor antagonist) and L-798106 (EP3 receptor antagonist) were given at doses of 40 µg/mouse intramuscularly (i.m.) 45 min following 4B8M, and 2% carrageenan solution in 0.9% NaCl was administered subcutaneously (s.c.) into hind foot pads. The footpad thickness was measured after 3 h with a spring caliper with an accuracy of 0.05 mm (Mitutoyo, Kawasaki, Japan).
2.9. Contact Sensitivity to Oxazolone
The test was performed as described by others [
17], with some modifications. The reaction was initiated by uptake of oxazolone (the antigen) by skin dendritic cells, which migrate then to draining lymph nodes for antigen presentation to T cells. A second exposure to the antigen attracts antigen-specific T cells to the site of antigen deposition by means of chemokines and initiates an effectual phase of this reaction by attraction of neutrophils for antigen elimination. The accompanying inflammatory processes increase edema of the tissue (auricles) with a peak response at 24 h following antigen challenge (the delayed type hypersensitivity). Mice (6 or 7 per experimental group) were shaved on the abdomen, and after 24 h, 100 µL of 0.5% oxazolone in acetone was applied topically. 4B8M peptide was applied as an ointment at a concentration of 0.07–0.1%, topically, on both sides of the ears. The contact sensitivity reaction was elicited 5 days later by application of 50 µL of 1% oxazolone in acetone on both sides of the ears. Reference compounds (Hydrocortisonum
®, Protopic
® and Elidel
®) were used in commercially available forms in a similar fashion. The compounds were applied 24 h after elicitation of contact sensitivity. The ear edema was measured 24 h later using a spring caliper. The results were presented as an antigen-specific increase of the ear thickness, i.e., the ear thickness of background (BG) mice (given only the eliciting dose of the antigen) was subtracted.
2.10. Inflammatory Skin Reaction to Toluene Diisocyanate (TDI)
The test was performed as described elsewhere [
18] with minor modifications. This type of antigen predisposes mice to developing cutaneous inflammatory responses dependent on development of antibody response to TDI. Mice, treated topically with subsequent doses of TDI gradually develop higher levels of antibodies. After a sufficient concentration of antibodies is attained, a challenge with antigen (TDI) elicits an inflammatory reaction, beginning with response at 4 h and developing thereafter, generated by antigen-antibody complexes affecting permeability of capillary blood vessels and attracting neutrophils. The mice were shaved on the abdomen (2 × 2 cm area), and after 24 h, 100 µL of 3% TDI in acetone was applied over 3 consecutive days (the sensitizing dose of the antigen). After 14 days, the reaction was elicited by application of 50 µL of 0.3% TDI (the eliciting dose of antigen) on both sides of the ears. The procedure was repeated 5 times every 3 days, on days 14, 17, 20, 23, and 27. Twenty-four hours after each challenge with TDI, the ear thickness was measured with a spring caliper, and 24 h after the last elicitation of the reaction (28th day of the test), the parameters of the inflammatory reaction were determined. 4B8M peptide was applied in the form of a 0.1% ointment, topically, on both sides of the ears (total volume of 50-100 µL per one ear), 1 h following each challenge with TDI. The reference drugs (Protopic
® and Elidel
®) were applied in a similar way.
2.11. Ovalbumin (OVA)-Induced Pleurisy
The previously described method was used [
19]. The immunization of mice with OVA preferentially elicits humoral immune response, characterized by the presence of antigen-specific Th2 type cells and IgE antibodies. A second challenge with OVA in an intrapleural cavity triggers an inflammatory response in pleural cavity and lungs initiated by cytokines (IL-5) and mediators from degranulated eosinophils and mastocytes. The mice were sensitized i.p. with 50 μg of OVA in Maalox (aluminii hydroxydum 3.5 g, magnesi hydroxydum 4.0 g in 100 mL; Rhone-Poulenc Rorer, Paris, France) as an adjuvant. After 14 days, the mice were injected with 12.5 μg OVA in 50 μL of 0.9% NaCl into the pleural cavity by means of a syringe with a 2-mm needle length. Mice from the BG group were given only a second (eliciting) dose of OVA; 2 h before administration of the eliciting dose of the antigen, the mice were given per os (by gastic gavage) 250 μg of 4B8M in olive oil. The reference compounds—dexamethasone (125 μg/mouse) and indomethacin (250 μg/mouse)—were also given per os. In another experiment, the mice were given 4B8M peptide at a dose of 250 μg/mouse i.p. On the next day, mice were subjected to halothane anesthesia and bled via the retroorbital plexus, followed by cervical dislocation. The draining lymph nodes and exudates from the inflamed pleural cavity were also isolated.
2.12. Dextran Sulfate-Induced Colitis
The test was performed according to a previously described method [
20], with some modifications. This model mimics colitis described in patients of still unknown etiology and has no immune specific basis. Rather, a destruction of mucosal colonic membrane and disintegration of crypts results from a direct cytotoxic effect of dextran sulfate on colonic mucosa. C57Bl/6 mice were given a 4% solution of dextran sulfate in tap drinking water acidified to pH 3.5 (with hydrochloric acid to limit growth of
Pseudomonas species) for 6 days. Then, the mice were given only the acidified water. The tetrapeptide 4B8M was administered to mice intragastrically, by means of a stomach tube, at a dose of 200 µg in 0.2 mL, daily on days 1–5. Pentasa
® was given in the same way at a dose of 1.5 mg (0.15 mL). The control group received an appropriately diluted DMSO solution (as in 4B8M preparation). On day 12 of the experiment, the mice were subjected to halothane anesthesia and bled via the retroorbital plexus, followed by cervical dislocation. The thymuses and colon were isolated.
2.13. Determination of Thymocyte and Lymph Node Cell Number and Viability
Thymuses, spleen, and draining lymph nodes were isolated and placed in disposable Petri dishes with sterile, cold PBS. The cells were released from the lymphatic organs by passing them through a nylon mesh. Then, the cells were resuspended in PBS containing 0.2% trypan blue. The numbers of viable and dead cells were counted in a Bürker hemocytometer in a light microscope. The total number of cells, number of viable and dead cells, and percentage of viable and dead cells were shown. The mean values ± standard error (SE) for each group without subtraction of BG values were shown.
2.14. Analysis of Peripheral Blood Cell Picture
The mice were subjected to halothane anesthesia, and blood was obtained from the retroorbital plexus. The blood smears were prepared on microscopic glasses. After drying out, the smears were stained with Giemsa and May-Grünwald reagents. The smears were subsequently evaluated at 1000× magnification (in immersion oil) in a Nicon ATC 2000 microscope. Up to 100 cells were counted per glass/preparation. The results were presented as a percentage of main cell types in the peripheral blood (mature neutrophils and neutrophil precursors—bands, eosinophils, lymphocytes, and monocytes). Mean values for each group were shown.
2.15. Determination of Cell Composition in the Pleural Exudates
The exudates from pleural cavities were obtained by adding with a pipette to each cavity 1 mL of 0.9% NaCl and aspiration of the liquid to tubes; 20 µL of the cell suspension was diluted 20× with Turk’s solution, and cells were counted in Bürker’s hemocytometer. Then, the exudates were centrifuged and supernatants saved at −20 °C for cytokine determination. From the cell pellets, smears were prepared on microscopic glasses, stained with Giemsa and May-Grünwald reagents, and inspected in 1000× magnification (in immersion oil). The following cell types were taken into account: mastocytes, monocytes, macrophages, lymphocytes, basophils, eosinophils, neutrophils, and immature forms of neutrophils (bands). The results were presented as a percentage of each cell type in the exudates cell population.
2.16. Histological Analysis
The mice auricles, skin fragments, lungs, and colons were fixed in 4.0% neutral buffered formalin for 48 h, dehydrated in an alcohol series, cleared in xylene, and embedded in paraffin. Paraffin blocks were sliced in a microtome (Microm HM310, Walldorf, Germany) into 6-µm sections. The paraffin sections were stained with hematoxylin and eosin (H&E) and with toluidine blue in order to detect mastocytes in the mice skin. The histological and quantitative (in case of mice auricles) analysis was conducted in a light microscope Nikon Eclipse 80i with the aid of imagine software NIS-Elements at 400× magnification. The number of neutrophils, macrophages, lymphocytes, and mast cells in the connective tissue of auricles in random high-power fields (HPF, area 0.071 mm2) was estimated. For each studied group, 25 determinations of the abovementioned cell types were performed. Mean values for each group without subtraction of the background values were shown.
2.17. Statistics
Each experimental group consisted of 7–8 mice, and each experiment was repeated 3–4 times. The results are presented as mean values ± SE (standard error). A Brown–Forsyth’s test was used to determine the homogeneity of variance between groups. In addition, the occurrence of dependencies between group means and variances were checked. When the above assumptions (normal or symmetric distribution of variables, homogeneity of variance, and lack of dependencies between means and variances) were completed, an analysis of variance (one-way ANOVA) was applied, followed by post hoc comparisons with Tukey’s test to estimate the significance of the differences between groups. Nonparametric data were evaluated with the Kruskal–Wallis’ (K-W) analysis of variance, as indicated in the text. Significance was determined at p < 0.05. A statistical analysis was performed using STATISTICA 7 for Windows.
4. Discussion
In the preliminary investigations (presented in more detail in the
Supplementary Materials), we found that the cyclic peptide 4B8M showed antiproliferative and immunosuppressive properties. Although the antiproliferative effects of the peptide were weaker as compared with CsA and CLA, a clear advantage of the peptide over the reference compounds was a negligible toxicity against mouse splenocytes, even at high doses (
Figure 1). The inhibition of LPS-inducible TNF-α production and stimulation of IL-6 activity in human whole blood cultures suggested anti-inflammatory properties (
Figure S3). The findings were similar to previous observations on the inhibitory actions of CLA on IL-1 production and post-adjuvant polyarthritis in rats [
3] and suggested that the peptide shared the anti-inflammatory properties of native, parent CLA. Interestingly, comparison of the suppressive effects of the peptide and CsA in the models of humoral (
Figure S4) and cellular (
Figure S5) immune responses in vivo revealed that these types of immune responses were more strongly inhibited by the peptide. These differences could be due to a better bioavailability of the peptide in vivo in comparison with CsA, the reference compound, as well as related to different mechanisms of action.
The investigation demonstrated a high efficacy of 4B8M in amelioration of the contact sensitivity to oxazolone in mice that surpassed the effects of the reference preparations (
Figure 3 and
Figure 4,
Figures S6 and S10). In this experimental model, Protopic
® (tacrolimus) exhibited an undesirable action. The application of tacrolimus in atopic dermatitis is generally well-tolerated but not devoid of side-effects such as skin irritation, particularly in the first stage of therapy [
23]. Elidel
® (pimecrolimus), on the other hand, is less irritable [
24] and permeates to a lesser degree the circulation of patients as compared to tacrolimus [
25]. However, both topical tacrolimus and pimecrolimus transiently induced mast cell degranulation in mouse skin [
26], which may account for their decreased efficacy in diminishing skin inflammation, observed in our study, in comparison to 4B8M. Also consistent with the transient pro-inflammatory properties of tacrolimus were our observations on the high mortality of lymphocytes in the draining lymph nodes (
Figure S6), increased skin vessel permeability, and high numbers of infiltrating neutrophils in the ear connective tissue (
Figure S10). In addition, topical calcineurin inhibitors disrupt the skin barrier, which may lead to susceptibility to infection [
27]. Another limitation of pimecrolimus is that its action is mostly restricted to the effectual phase of contact sensitivity [
28].
4B8M also very effectively suppressed the cutaneous, humoral immune response to TDI (
Figure 5 and
Figure 6,
Figures S12–S17). Unlike the peptide, the reference compounds showed a differential action, consistent with the literature. Tacrolimus had a significant effect on allergic skin inflammation in mice [
29]. Pimecrolimus, in turn, was weakly effective in rats in inhibiting anti-KLH (keyhole limpet hemocyanin) antibodies, in contrast to tacrolimus and CsA [
30]. Moreover, no significant improvement was achieved following application of pimecrolimus in the case of facial skin changes [
31].
4B8M also proved to be very effective in diminishing symptoms of nonspecific skin irritation by salicylic acid (
Figures S18–S23). In this model, tacrolimus was less efficacious by showing some cytotoxicity, as also reported by others in a similar mouse model [
26]. Sensitizing properties of tacrolimus were also found in acute skin inflammation in mice [
32]. Our unpublished results did not reveal any sensitizing properties of 4B8M when the peptide was applied topically to mice, daily, for a long period.
In the air pouch model of carrageenan-induced inflammation, the peptide was more efficient, as compared to the reference drugs, in diminishing symptoms of inflammation (
Figures S24–S29). The model is considered to be a clinical equivalent of rheumatoid arthritis [
33]. 4B8M more effectively normalized the cell composition of circulating blood, activity of mastocytes in the air pouch exudates, and the granuloma layer thickness, as compared with the reference compounds (indomethacin, aspirin, and dexamethasone).
Therapeutic action of 4B8M on chemically induced colitis was comparable with that of 5-ASA (
Figure 7 and
Figure 8,
Figures S30 and S31). We confirmed another finding [
34] that the high IL-1β level in the colon reflects a good health condition (as evaluated by the presence of physiologic microflora) of naive mice. That level was lower in control mice, and the peptide and, to a lesser degree, 5-ASA reversed that decrease in IL-1β content (
Figure S30). On the other hand, the histological analysis of the colon revealed some toxicity of 5-ASA, in contrast to 4B8M (
Figure S31). That was confirmed by our monitoring of the mouse condition since 5-ASA-treated mice sometimes had blood in the stool and diarrhea.
The 4B8M peptide effectively inhibited symptoms of allergic pleural and lung inflammation induced by OVA in the analyzed parameters (
Figure 9,
Figure 10 and
Figure 11 and
Figures S32–S35). Of importance, 4B8M administered at a higher dose in olive oil by intragastric gavage was also preventive, suggesting its potential therapeutic use in asthma.
In all experimental models, mice demonstrated increased content of myelocytic cells in their circulation including immature forms of neutrophils and eosinophils. This effect was probably due to the release of endogenous steroids in response to injury related stress [
21,
35] and effects of some acute phase proteins, such as lactoferrin, which induce corticosterone in operated mice [
36]. Therefore, the application of dexamethasone in some models such as carrageenan inflammation and pleurisy (
Figure 9 and
Figure S26) had no effect on the altered blood cell composition. In the case of hydrocortisone applied in the model of contact sensitivity to oxazolone, the drug even caused a high distortion of blood cell composition (
Figure S9) despite good therapeutic efficacy (
Figures S7 and S8).
The results derived from the above described models led us to conclude that 4B8M has a decisive anti-inflammatory property, so our investigation on its mechanism of action should be focused on analyzing the genes associated with inflammatory processes. In our preliminary experiments (not shown), a potential effect of the peptide on the expression of 38 genes was confirmed. Of interest, as many as 15 of these were directly linked to the metabolism of arachidonoic acid and at least 10 genes may be indirectly associated with the metabolism of arachidonoic acid, prostaglandins, and prostacyclins. Among 12 proteins involved in regulating the inflammatory process, based on real-time PCR analysis, the peptide lowered LPS-induced EP1 and EP3 receptor cell expression on PBMC and Jurkat T cells. The expression of EP2 and EP4 receptors was, however, not affected. In addition, 4B8M lowered the expression of ICAM-1, the receptor of cell adhesion, and induced expression of COX-2 on keratinocytes. The above described phenomena induced by the peptide were helpful in explaining its effects in the described experimental models since the actions of PGE2 differ depending on the type of EP receptors used [
37]. In general, transduction of signals by PGE2 via EP1 and EP3 receptors results in inflammatory reactions whereas engagement of EP2 and EP4 receptors is anti-inflammatory. The ability of 4B8M to induce Cox-2 expression, which leads to PGE2 synthesis, may explain its anti-inflammatory properties in several models. Cox-2 was implicated in the inhibition of inflammatory states by lowering ICAM-1 expression [
38] and in the suppression of TNF α-induced chemokine production by keratinocytes [
39]. In fact, the inhibition of ICAM-1 expression by the peptide was also found in this investigation. The inhibition by PGE2 of ICAM-1 expression induced by IL-18 in human monocytes involved the EP2/EP4 signaling pathway [
22]. The inhibition of ICAM-1 expression on epidermal cells by 4B8M indicates that the effective suppression of skin inflammation may be due to inhibition of inflammatory cell infiltration. Of interest, the expression of these receptors was not affected by the peptide in our study. The suppressive effect of PGE2 via EP2 and EP4 receptors was also confirmed in the case of dendritic cells in which the expression of major histocompatibility complex class II antigens (MHC class II) was downregulated [
40]. The above described effects of PGE2 are in agreement in our results from antigen-specific and nonspecific immune responses, where both recognition and presentation of antigens by antigen presenting cells may be inhibited by the peptide. In addition, the inhibition of cell-to-cell contact by lowering ICAM-1 expression may explain the suppressive effect of the peptide when administered during full blown inflammation.
Our preliminary studies demonstrated that the peptide inhibited LPS-induced TNF α but stimulated IL-6 production (
Figure S3). This finding is in agreement with data demonstrating that, in LPS-treated mouse neutrophils, PGE2 inhibited TNF α production via EP2 receptors whereas IL-6 production was enhanced [
41]. The suppressive effect of 4B8M on TNF α production provides a good explanation for the inhibition of the inflammatory processes where this cytokine plays a key role. Our assumption that the peptide activity involves induction of PGE2 production could be further supported by the fact that PGE2 can inhibit growth of cells dependent on the availability of IL-2 or IL-4 such as CTLL-2 [
42] because 4B8M inhibited growth of this line in our study (
Figure S36). Again, this effect was dependent on the involvement of EP4 receptors, the expression of which was not affected (downregulated) by the peptide. The results obtained in experimental colitis are also in agreement with our notion that the protective action of 4B8M is mediated via the EP4 receptor. The expression of EP4 receptor mRNA was significantly elevated after treatment with dextran sulfate in a rodent model, and a selective agonist of EP4 (ONO-AE1-329) decreased the pathological changes of colitis [
43]. The application of an EP4 agonist was also effective in amelioration of LPS-elicited changes in rat nasal epithelium and in cultured human airway epithelial cells [
44] as well as in a model of chemotaxis applying human peripheral blood eosinophils [
45]. Thus, a similar mechanism could play a role in amelioration by 4B8M of OVA-specific induction of pleurisy.
More direct evidence for involvement of EP receptors in diminishing inflammatory reactions by the peptide was delivered in the model of carrageenan-induced foot pad inflammation where the application of specific EP receptor antagonists abolished the protective action of the peptide. Whereas the effectiveness of blocking the EP4 antagonist was not disputable, the effective block of the EP3 receptor was rather surprising. However, in some models, treatment with COX-2 and EP3 inhibitors significantly reversed the obesity-associated adipose tissue inflammatory gene and protein expressions [
46]. Provided that proinflammatory processes mediated by PGE2 engage EP3 receptors [
47], it was not surprising that increased levels of EP3 receptors following treatment with LPS were lowered by 4B8M.