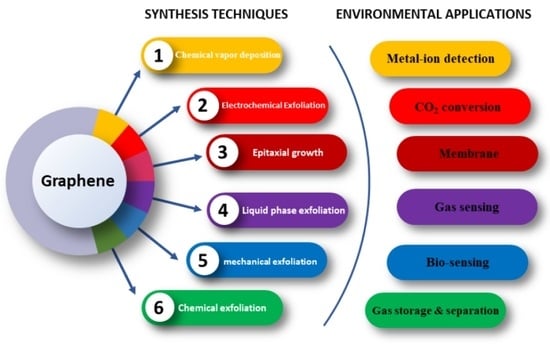
Graphene Synthesis Techniques and Environmental Applications
Abstract
:1. Introduction
2. Graphene Synthesis
2.1. Chemical Exfoliation
2.2. Mechanical Exfoliation
2.3. Electrochemical Exfoliation
- Water electrolysis and the production of oxygen and hydroxyl radicals.
- The movement of hydroxyl and oxygen radicals and opening of graphite edges.
- Intercalation of the species of electrolyte and, subsequently, gas formation for the expansion of graphite.
2.4. Liquid-Phase Exfoliation
2.5. Epitaxial Growth
2.6. Chemical Vapor Deposition (CVD)
2.7. Trend Analysis of Different Graphene Synthesis Techniques
3. Applications of Graphene
3.1. Gas Sensing Applications
3.2. Membrane Applications
3.3. Metal Ions Detection Applications
3.4. CO2 Conversion Applications
3.5. Trend Analysis Graphene Use in Environmental Applications
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Randviir, E.P.; Brownson, D.A.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent progress in graphene/polymer nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A.; Wilberforce, T.; Sayed, E.T. Application of graphene in energy storage device—A review. Renew. Sustain. Energy Rev. 2021, 135, 110026. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Ruoff, R.S.; Bielawski, C.W. From conception to realization: An historial account of graphene and some perspectives for its future. Angew. Chem. Int. Ed. 2010, 49, 9336–9344. [Google Scholar] [CrossRef]
- Shareena, T.P.D.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A review on graphene-based nanomaterials in biomedical applications and risks in environment and health. Nano-Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef]
- Schafhaeutl, C. Ueber die verbindungen des kohlenstoffes mit silicium, eisen und anderen metallen, welche die verschiedenen gallungen von roheisen, stahl und schmiedeeisen bilden. J. Prakt. Chem. 1840, 21, 129–157. [Google Scholar] [CrossRef] [Green Version]
- Wallace, P.R. The band theory of graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Boehm, H.; Clauss, A.; Fischer, G.; Hofmann, U. Dünnste kohlenstoff-folien. Z. Nat. B 1962, 17, 150–153. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef]
- Kumar, N.; Salehiyan, R.; Chauke, V.; Botlhoko, O.J.; Setshedi, K.; Scriba, M.; Masukume, M.; Ray, S.S. Top-down synthesis of graphene: A comprehensive review. FlatChem 2021, 27, 100224. [Google Scholar] [CrossRef]
- Gadipelli, S.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2015, 69, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Fal, V.; Colombo, L.; Gellert, P.; Schwab, M.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Selvaraj, V.; Krishnan, H. Synthesis of graphene encased alumina and its application as nanofluid for cooling of heat-generating electronic devices. Powder Technol. 2020, 363, 665–675. [Google Scholar] [CrossRef]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.-B. Design, synthesis, and characterization of graphene–nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Shi, H.; Das, P.; Qin, J.; Li, Y.; Wang, X.; Su, F.; Wen, P.; Li, S.; Lu, P.; et al. The chemistry and promising applications of graphene and porous graphene materials. Adv. Funct. Mater. 2020, 30, 1909035. [Google Scholar] [CrossRef]
- Tao, Y.; Sui, Z.-Y.; Han, B.-H. Advanced porous graphene materials: From in-plane pore generation to energy storage applications. J. Mater. Chem. A 2020, 8, 6125–6143. [Google Scholar] [CrossRef]
- Karthik, V.; Selvakumar, P.; Kumar, P.S.; Vo, D.-V.N.; Gokulakrishnan, M.; Keerthana, P.; Elakkiya, V.T.; Rajeswari, R. Graphene-based materials for environmental applications: A review. Environ. Chem. Lett. 2021, 19, 3631–3644. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Chen, G.; Fan, C.; Liu, X. Recent progress in the transfer of graphene films and nanostructures. Small Methods 2021, 5, 2100771. [Google Scholar] [CrossRef]
- Du, Y.; Xu, X.; Liu, Q.; Bai, L.; Hang, K.; Wang, D. Identification of organic pollutants with potential ecological and health risks in aquatic environments: Progress and challenges. Sci. Total Environ. 2021, 806, 150691. [Google Scholar] [CrossRef]
- Lai, J.Y.; Ngu, L.H. The production cost analysis of oil palm waste activated carbon: A pilot-scale evaluation. Greenh. Gases Sci. Technol. 2020, 10, 999–1026. [Google Scholar] [CrossRef]
- Andonovic, B.; Ademi, A.; Grozdanov, A.; Paunović, P.; Dimitrov, A.T. Enhanced model for determining the number of graphene layers and their distribution from X-ray diffraction data. Beilstein J. Nanotechnol. 2015, 6, 2113–2122. [Google Scholar] [CrossRef]
- Ruammaitree, A.; Nakahara, H.; Akimoto, K.; Soda, K.; Saito, Y. Determination of non-uniform graphene thickness on SiC (0 0 0 1) by X-ray diffraction. Appl. Surf. Sci. 2013, 282, 297–301. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the nature of defects in graphene by Raman spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.L.; Campos, J.L.E.; Fernandes, T.F.; Rocha, J.N.; Machado, L.R.; Soares, E.M.; Miquita, D.R.; Miranda, H.; Rabelo, C.; Neto, O.P.V.; et al. Raman spectroscopy analysis of number of layers in mass-produced graphene flakes. Carbon 2020, 161, 181–189. [Google Scholar] [CrossRef]
- Caicedo, F.M.C.; López, E.V.; Agarwal, A.; Drozd, V.; Durygin, A.; Hernandez, A.F.; Wang, C. Synthesis of graphene oxide from graphite by ball milling. Diam. Relat. Mater. 2020, 109, 108064. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Khasnabis, S.; Kumar, V.; Nath, B.; Adiga, V.; Naik, T.S.S.K.; Subramanian, S.; Kumare, V.; Singh, J.; et al. Sustainable removal of Cr (VI) using graphene oxide-zinc oxide nanohybrid: Adsorption kinetics, isotherms and thermodynamics. Environ. Res. 2022, 203, 111891. [Google Scholar] [CrossRef]
- Yan, Y.; Chu, Y.; Khan, M.A.; Xia, M.; Shi, M.; Zhu, S.; Lei, W.; Wang, F. Facile immobilization of ethylenediamine tetramethylene-phosphonic acid into UiO-66 for toxic divalent heavy metal ions removal: An experimental and theoretical exploration. Sci. Total Environ. 2021, 806, 150652. [Google Scholar] [CrossRef]
- Lyon, C.; Saupe, E.E.; Smith, C.J.; Hill, D.J.; Beckerman, A.P.; Stringer, L.C.; Marchant, R.; McKay, J.; Burke, A.; O’Higgins, P.; et al. Climate change research and action must look beyond 2100. Glob. Change Biol. 2022, 28, 349–361. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.V.; Jeevanantham, S.; Harikumar, P.; Priyanka, G.; Devakirubai, D.R.A. A comprehensive review on sources, analysis and toxicity of environmental pollutants and its removal methods from water environment. Sci. Total Environ. 2021, 812, 152456. [Google Scholar] [CrossRef]
- Karimifard, S.; Moghaddam, M.R.A. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640–641, 772–797. [Google Scholar] [CrossRef]
- Cosgrove, S.; Jefferson, B.; Jarvis, P. Application of activated carbon fabric for the removal of a recalcitrant pesticide from agricultural run-off. Sci. Total Environ. 2022, 815, 152626. [Google Scholar] [CrossRef]
- Shi, H.; Dai, Z.; Sheng, X.; Xia, D.; Shao, P.; Yang, L.; Luo, X. Conducting polymer hydrogels as a sustainable platform for advanced energy, biomedical and environmental applications. Sci. Total Environ. 2021, 786, 147430. [Google Scholar] [CrossRef]
- Hao, M.; Qiu, M.; Yang, H.; Hu, B.; Wang, X. Recent advances on preparation and environmental applications of MOF-derived carbons in catalysis. Sci. Total Environ. 2020, 760, 143333. [Google Scholar] [CrossRef]
- Ursueguía, D.; Díaz, E.; Ordóñez, S. Metal-Organic Frameworks (MOFs) as methane adsorbents: From storage to diluted coal mining streams concentration. Sci. Total Environ. 2021, 790, 148211. [Google Scholar] [CrossRef]
- Li, M.; Kuang, S.; Kang, Y.; Ma, H.; Dong, J.; Guo, Z. Recent advances in application of iron-manganese oxide nanomaterials for removal of heavy metals in the aquatic environment. Sci. Total Environ. 2022, 819, 153157. [Google Scholar] [CrossRef]
- Feng, L.; Qin, Z.; Huang, Y.; Peng, K.; Wang, F.; Yan, Y.; Chen, Y. Boron-, sulfur-, and phosphorus-doped graphene for environmental applications. Sci. Total Environ. 2019, 698, 134239. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Yun, Y.-S. Synthesis and environmental applications of graphene oxide/layered double hydroxides and graphene oxide/MXenes: A critical review. Sep. Purif. Technol. 2022, 297, 121518. [Google Scholar] [CrossRef]
- Ghany, N.A.A.; Elsherif, S.A.; Handal, H.T. Revolution of graphene for different applications: State-of-the-art. Surf. Interfaces 2017, 9, 93–106. [Google Scholar] [CrossRef]
- Gutiérrez-Cruz, A.; Ruiz-Hernández, R.; Vega-Clemente, J.F.; Luna-Gazcón, D.G.; Campos-Delgado, J. A review of top-down and bottom-up synthesis methods for the production of graphene, graphene oxide and reduced graphene oxide. J. Mater. Sci. 2020, 35, 76–89. [Google Scholar] [CrossRef]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb Carbon: A Review of Graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, J.; Huang, L. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon 2019, 143, 610–640. [Google Scholar] [CrossRef]
- Al-Rawashdeh, N.A.; Allabadi, O.; Aljarrah, M.T. Photocatalytic activity of graphene oxide/zinc oxide nanocomposites with embedded metal nanoparticles for the degradation of organic dyes. ACS Omega 2020, 5, 28046–28055. [Google Scholar] [CrossRef]
- Madurani, A.; Suprapto, S.; Machrita, N.I.; Bahar, S.L.; Illiya, W.; Kurniawan, F. Progress in graphene synthesis and its application: History, challenge and the future outlook for research and industry. ECS J. Solid State Sci. Technol. 2020, 9, 093013. [Google Scholar] [CrossRef]
- Cai, X.; Jiang, Z.; Zhang, X.; Zhang, X. Effects of tip sonication parameters on liquid phase exfoliation of graphite into graphene nanoplatelets. Nanoscale Res. Lett. 2018, 13, 241. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.; Lopez-Mercado, J.; Carreon, M.G.; Lopez-Miranda, A.; Carreon, M.L. Plasma synthesis of graphene from mango peel. ACS Omega 2018, 3, 455–463. [Google Scholar] [CrossRef]
- Prekodravac, J.; Kepic, D.; Colmenares, J.C.; Giannakoudakis, D.A.; Jovanovic, S.P. A comprehensive review on selected graphene synthesis methods: From electrochemical exfoliation through rapid thermal annealing towards biomass pyrolysis. J. Mater. Chem. C 2021, 9, 6722–6748. [Google Scholar] [CrossRef]
- Torres-Mendieta, R.; Ventura-Espinosa, D.; Sabater, S.; Lancis, J.; Mínguez-Vega, G.; Mata, J.A. In situ decoration of graphene sheets with gold nanoparticles synthetized by pulsed laser ablation in liquids. Sci. Rep. 2016, 6, 30478. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Fernández, G.; Boulanger, N.; Nordenström, A.; Iakunkov, A.; Talyzin, A.; Carriazo, D.; Mysyk, R. Ball-milling-enhanced capacitive charge storage of activated graphene in aqueous, organic and ionic liquid electrolytes. Electrochim. Acta 2021, 370, 137738. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Ryzhkov, S.A.; Kirilenko, D.A.; Ulin, N.V.; Baidakova, M.V.; Shnitov, V.V.; Pavlov, S.I.; Chumakov, R.G.; Stolyarova, D.Y.; Besedina, N.A. From graphene oxide towards aminated graphene: Facile synthesis, its structure and electronic properties. Sci. Rep. 2020, 10, 6902. [Google Scholar] [CrossRef] [Green Version]
- Yoon, K.-Y.; Dong, G. Liquid-phase bottom-up synthesis of graphene nanoribbons. Mater. Chem. Front. 2019, 4, 29–45. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Primers 2021, 1, 5. [Google Scholar] [CrossRef]
- Shang, W.; Cai, T.; Zhang, Y.; Liu, D.; Liu, S. Facile one pot pyrolysis synthesis of carbon quantum dots and graphene oxide nanomaterials: All carbon hybrids as eco-environmental lubricants for low friction and remarkable wear-resistance. Tribol. Int. 2018, 118, 373–380. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, S.; Berry, J.; Zhang, Q.; Gebhardt, J.; Parkin, W.M.; Avila, J.; Yi, H.; Chen, C.; Hurtado-Parra, S.; et al. Large-area epitaxial growth of curvature-stabilized ABC trilayer graphene. Nat. Commun. 2020, 11, 546. [Google Scholar] [CrossRef] [Green Version]
- Sahu, T.K.; Ranjan, P.; Kumar, P. Chemical exfoliation synthesis of boron nitride and molybdenum disulfide 2D sheets via modified Hummers’ method. Emergent Mater. 2021, 4, 645–654. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Huang, Y.; Ma, Y.; Wan, X.; Chen, Y. Controlled synthesis of few-layered graphene sheets on a large scale using chemical exfoliation. Carbon 2010, 48, 2367–2371. [Google Scholar] [CrossRef]
- Abakumov, O.; Bychko, I.; Trypolskii, A. Structural characteristics of graphene oxide reduced by hydrazine and hydrogen. Theor. Exp. Chem. 2021, 57, 289–296. [Google Scholar] [CrossRef]
- Misra, S.; Katiyar, N.K.; Kumar, A.; Goel, S.; Biswas, K. Nanofabrication route to achieve sustainable production of next generation defect-free graphene: Analysis and Characterisation. Nanofabrication 2021, 6, 36–43. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Dey, B.; Sarkhel, G.; Bag, D.S.; Choudhury, A. Exfoliated graphene reinforced polybenzimidazole nanocomposites with high dielectric permittivity at low percolation threshold. J. Mol. Struct. 2018, 1177, 491–498. [Google Scholar] [CrossRef]
- Gebreegziabher, G.; Asemahegne, A.; Ayele, D.; Dhakshnamoorthy, M.; Kumar, A. One-step synthesis and characterization of reduced graphene oxide using chemical exfoliation method. Mater. Today Chem. 2019, 12, 233–239. [Google Scholar] [CrossRef]
- Betancur, N.; Ornelas-Soto, A.; Garay-Tapia, F.; Pérez, Á.; Salazar, A.; García, A.G. A general strategy for direct synthesis of reduced graphene oxide by chemical exfoliation of graphite. Mater. Chem. Phys. 2018, 218, 51–61. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Wu, W.; Liu, T.; Liu, Y.; Guo, B.; Zhang, R. One-step chemical exfoliation of graphite to ∼100% few-layer graphene with high quality and large size at ambient temperature. Chem. Eng. J. 2018, 355, 181–185. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Shen, Z. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Zhang, Y.; Small, J.P.; Pontius, W.V.; Kim, P. Fabrication and electric-field-dependent transport measurements of mesoscopic graphite devices. Appl. Phys. Lett. 2005, 86, 073104. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef] [Green Version]
- Knieke, A.; Berger, A.; Peukert, W. Graphene production with stirred media mills. MRS Online Proc. Libr. 2010, 1259. [Google Scholar] [CrossRef]
- Sumdani, M.; Islam, M.; Yahaya, A.; Safie, S. Recent advances of the graphite exfoliation processes and structural modification of graphene: A review. J. Nanoparticle Res. 2021, 23, 253. [Google Scholar] [CrossRef]
- Posudievsky, O.Y.; Khazieieva, O.A.; Cherepanov, V.V.; Koshechko, V.G.; Pokhodenko, V.D. High yield of graphene by dispersant-free liquid exfoliation of mechanochemically delaminated graphite. J. Nanopart. Res. 2013, 15, 2046. [Google Scholar] [CrossRef]
- Lv, Y.; Yu, L.; Jiang, C.; Chen, S.; Nie, Z. Synthesis of graphene nanosheet powder with layer number control via a soluble salt-assisted route. RSC Adv. 2014, 4, 13350–13354. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, M.; Yang, M.; Zhang, Z.; Yu, J.; Zhang, Y.; Cheng, W.; Li, X. High-yield production of few-layer graphene via new-fashioned strategy combining resonance ball milling and hydrothermal exfoliation. Nanomaterials 2020, 10, 667. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.; Navik, R.; Liu, Z.; Xiang, Q.; Zhao, Y. Scalable massive production of defect-free few-layer graphene by ball-milling in series with shearing exfoliation in supercritical CO2. J. Supercrit. Fluids 2021, 181, 105496. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samorì, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-concentration, surfactant-stabilized graphene dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef]
- Htwe, Y.; Chow, W.; Suda, Y.; Thant, A.; Mariatti, M. Effect of electrolytes and sonication times on the formation of graphene using an electrochemical exfoliation process. Appl. Surf. Sci. 2019, 469, 951–961. [Google Scholar] [CrossRef]
- Pingale, D.; Owhal, A.; Katarkar, A.S.; Belgamwar, S.U.; Rathore, J.S. Facile synthesis of graphene by ultrasonic-assisted electrochemical exfoliation of graphite. Mater. Today Proc. 2021, 44, 467–472. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Sui, X.; Riaz, M.A.; Xu, M.; Wei, L.; Chen, Y. Synthesis of graphene materials by electrochemical exfoliation: Recent progress and future potential. Carbon Energy 2019, 1, 173–199. [Google Scholar] [CrossRef]
- Prakoso, B.; Mab, Y.; Stephanie, R.; Hawari, N.H.; Suendo, V.; Judawisastra, H.; Zong, Y.; Liu, Z.; Sumboja, A. Facile synthesis of battery waste-derived graphene for transparent and conductive film application by an electrochemical exfoliation method. RSC Adv. 2020, 10, 10322–10328. [Google Scholar] [CrossRef] [PubMed]
- Anurag, K.; Kumar, S. Synthesis of graphene through electrochemical exfoliation technique in aqueous medium. Mater. Today Proc. 2021, 44, 2695–2699. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Eslami-Farsani, R.; Torabian, M.; Amousa, N. Recent advances in one-pot functionalization of graphene using electrochemical exfoliation of graphite: A review study. Synth. Met. 2020, 269, 116549. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, C.K.; Sarkar, C.K.; Roy, S. Facile synthesis of multi-layer graphene by electrochemical exfoliation using organic solvent. Nanotechnol. Rev. 2018, 7, 497–508. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Nasir, H.; Honey, S. Liquid-phase exfoliation of graphene in organic solvents with addition of picric acid. Nano Hybrids Compos. 2021, 33, 47–60. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Zhao, Y.; Sun, K.; Vieira, C.L.; Jia, Z.; Cui, C.; Wang, Z.; Walsh, A.; Huang, S. Method of ultrasound-assisted liquid-phase exfoliation to prepare graphene. Ultrason. Sonochem. 2019, 58, 104630. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Liu, H.; Zhang, X. SMA-assisted exfoliation of graphite by microfluidization for efficient and large-scale production of high-quality graphene. Nanomaterials 2019, 9, 1653. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, G.R.; Iakimov, T.; Yakimova, R. Epitaxial graphene on SiC: A review of growth and characterization. Crystals 2016, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Chen, J.; Wu, X.-J.; Zhang, H. Epitaxial growth of hybrid nanostructures. Nat. Rev. Mater. 2018, 3, 17089. [Google Scholar] [CrossRef]
- Mishra, N.; Boeckl, J.; Motta, N.; Iacopi, F. Graphene growth on silicon carbide: A review. Phys. Status Solidi 2016, 213, 2277–2289. [Google Scholar] [CrossRef]
- Krieger, M.; Weber, H.B. Epitaxial graphene on silicon carbide as a tailorable metal–semiconductor interface. Wide Bandgap Semicond. Power Electron. Mater. Devices Appl. 2021, 1, 249–270. [Google Scholar]
- Beshkova, M.; Hultman, L.; Yakimova, R. Device applications of epitaxial graphene on silicon carbide. Vacuum 2016, 128, 186–197. [Google Scholar] [CrossRef] [Green Version]
- Sutter, P.W.; Flege, J.-I.; Sutter, E.A. Epitaxial graphene on ruthenium. Nat. Mater. 2008, 7, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Deng, X.; Li, M.; Wang, Y.; Mao, K.; Yang, Y.; Zhang, M. Preparation of large area graphene on SiC (0 0 0− 1) by moderate vacuum technology. J. Cryst. Growth 2020, 555, 125968. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical vapour deposition of graphene—Synthesis, characterisation, and applications: A review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Karu, E.; Beer, M. Pyrolytic formation of highly crystalline graphite films. J. Appl. Phys. 1966, 37, 2179–2181. [Google Scholar] [CrossRef]
- Land, T.; Michely, T.; Behm, R.; Hemminger, J.; Comsa, G. STM investigation of single layer graphite structures produced on Pt (111) by hydrocarbon decomposition. Surf. Sci. 1992, 264, 261–270. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.-T.; Xu, Y. Chemical Vapour Deposition: An Integrated Engineering Design for Advanced Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Yen, C.-C.; Chang, Y.-C.; Tsai, H.-C.; Woon, W.-Y. Nucleation and growth dynamics of graphene grown through low power capacitive coupled radio frequency plasma enhanced chemical vapor deposition. Carbon 2019, 154, 420–427. [Google Scholar] [CrossRef]
- Van Nang, L.; Kim, D.-O.; Trung, T.N.; Arepalli, V.K.; Kim, E.-T. Understanding the growth kinetics of graphene on Cu and Fe2O3 using inductively-coupled plasma chemical vapor deposition. Appl. Microsc. 2017, 47, 13–18. [Google Scholar] [CrossRef]
- Zhai, Z.; Shen, H.; Chen, J.; Li, X.; Jiang, Y. Direct growth of nitrogen-doped graphene films on glass by plasma-assisted hot filament CVD for enhanced electricity generation. J. Mater. Chem. A 2019, 7, 12038–12049. [Google Scholar] [CrossRef]
- Othman, M.; Ritikos, R.; Rahman, S.A. Growth of plasma-enhanced chemical vapour deposition and hot filament plasma-enhanced chemical vapour deposition transfer-free graphene using a nickel catalyst. Thin Solid Film. 2019, 685, 335–342. [Google Scholar] [CrossRef]
- Bo, Z.; Yang, Y.; Chen, J.; Yu, K.; Yan, J.; Cen, K. Plasma-enhanced chemical vapor deposition synthesis of vertically oriented graphene nanosheets. Nanoscale 2013, 5, 5180–5204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura, H.; Jia, L.; Ohashi, Y.; Kondo, H.; Ishikawa, K.; Tsutsumi, T.; Hayashi, T.; Takeda, K.; Sekine, M.; Hori, M. Control of sp2-C cluster incorporation of amorphous carbon films grown by H-radical-injection CH4/H2 plasma-enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 2019, 58, 030912. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lin, W.-H.; Tseng, W.-S.; Chen, C.-C.; Rossman, G.; Chen, C.-D.; Wu, Y.-S.; Yeh, N.-C. Direct growth of mm-size twisted bilayer graphene by plasma-enhanced chemical vapor deposition. Carbon 2020, 156, 212–224. [Google Scholar] [CrossRef]
- Muñoz, R.; Martínez, L.; López-Elvira, E.; Munuera, C.; Huttel, Y.; García-Hernández, M. Direct synthesis of graphene on silicon oxide by low temperature plasma enhanced chemical vapor deposition. Nanoscale 2018, 10, 12779–12787. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Yang, X.; Ta, H.Q.; Hasan, M.; Bachmatiuk, A.; Tokarska, K.; Trzebicka, B.; Fu, L.; Rummeli, M.H. Graphene transfer methods: A review. Nano Res. 2021, 14, 3756–3772. [Google Scholar] [CrossRef]
- Park, B.-J.; Choi, J.-S.; Eom, J.-H.; Ha, H.; Kim, H.Y.; Lee, S.-H.; Shin, H.; Yoon, S.-G. Defect-free graphene synthesized directly at 150 C via chemical vapor deposition with no transfer. ACS Nano 2018, 12, 2008–2016. [Google Scholar] [CrossRef]
- Shams, S.S.; Zhang, R.; Zhu, J. Graphene synthesis: A Review. Mater. Sci. Pol. 2015, 33, 566–578. [Google Scholar] [CrossRef] [Green Version]
- Amiri, M.; Naraghi, G.; Ahmadi, M.; Soleymaniha, M.; Shanbedi, M. A review on liquid-phase exfoliation for scalable production of pure graphene, wrinkled, crumpled and functionalized graphene and challenges. FlatChem 2018, 8, 40–71. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-phase exfoliation of graphene: An overview on exfoliation media, techniques, and challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog. Energy Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Cooper, D.R.; D’Anjou, B.; Ghattamaneni, N.; Harack, B.; Hilke, M.; Horth, A.; Majlis, N.; Massicotte, M.; Vandsburger, L.; Whiteway, E.; et al. Experimental review of graphene. Int. Sch. Res. Not. 2012, 2012, 501686. [Google Scholar] [CrossRef] [Green Version]
- Nisa, M.-U.; Nadeem, N.; Yaseen, M.; Iqbal, J.; Zahid, M.; Abbas, Q.; Mustafa, G.; Shahid, I. Applications of graphene-based tungsten oxide nanocomposites: A review. J. Nanostructure Chem. 2022, 1–30. [Google Scholar] [CrossRef]
- Jiříčková, O.; Jankovský, Z.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Floraki, A.; Sapountzis, A.; Vernardou, D. APCVD graphene-based composite electrodes for Li-Ion batteries. Energies 2022, 15, 926. [Google Scholar] [CrossRef]
- Sang, M.; Shin, J.; Kim, K.; Yu, K.J. Electronic and thermal properties of graphene and recent advances in graphene based electronics applications. Nanomaterials 2019, 9, 374. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Chai, Y. Design and applications of graphene-based flexible and wearable physical sensing devices. 2D Mater. 2020, 8, 022001. [Google Scholar] [CrossRef]
- Carvalho, F.; Kulyk, B.; Fernandes, A.J.; Fortunato, E.; Costa, F.M. A review on the applications of graphene in mechanical transduction. Adv. Mater. 2021, 34, e2101326. [Google Scholar] [CrossRef]
- Ghosal, K.; Sarkar, K. Biomedical applications of graphene nanomaterials and beyond. ACS Biomater. Sci. Eng. 2018, 4, 2653–2703. [Google Scholar] [CrossRef] [PubMed]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical applications of graphene-based structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokaya, V.; Srimaneepong, P.; Thunyakitpisal, J.; Qin, V.; Rosa, V.; Sapkota, J. Potential Applications of Graphene-Based Nanomaterials in Biomedical, Dental, and Implant Applications. In Advances in Dental Implantology Using Nanomaterials and Allied Technology Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 77–105. [Google Scholar]
- Padinjareveetil, K.K.; Padil, V.V.T.; Černík, M. Graphene oxide-based nanocomposite and their biomedical applications. Nanoeng. Biomater. Biomed. Appl. 2022, 2, 427–456. [Google Scholar]
- Liu, J.; Bao, S.; Wang, X. Applications of graphene-based materials in sensors: A review. Micromachines 2022, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, M. Applications of Graphene-Based Materials in Sensors; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2020; Volume 20, p. 3196. [Google Scholar]
- Li, Y.; Jiao, J.; Wu, Q.; Song, Q.; Xie, W.; Liu, B. Environmental applications of graphene oxide composite membranes. Chin. Chem. Lett. 2022, 33, 5001–5012. [Google Scholar] [CrossRef]
- Cheng, C.; Liang, Q.; Yan, M.; Liu, Z.; He, Q.; Wu, T.; Luo, S.; Pan, Y.; Zhao, C.; Liu, Y. Advances in preparation, mechanism and applications of graphene quantum dots/semiconductor composite photocatalysts: A review. J. Hazard. Mater. 2022, 424, 127721. [Google Scholar] [CrossRef]
- Shabbir, M.; Raza, Z.A.; Shah, T.H.; Tariq, M.R. Recent progress in graphenes: Synthesis, covalent functionalization and environmental applications. J. Nanostructure Chem. 2022, 1–19. [Google Scholar] [CrossRef]
- Raya, H.; Kzar, H.; Mahmoud, Z.H.; Ahmed, A.A.; Ibatova, A.Z.; Kianfar, E. A review of gas sensors based on carbon nanomaterial. Carbon Lett. 2021, 32, 339–364. [Google Scholar] [CrossRef]
- Tian, W.; Liu, X.; Yu, W. Research progress of gas sensor based on graphene and its derivatives: A review. Appl. Sci. 2018, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Xie, T.; Wang, Q.; Wallace, R.M.; Gong, C. Understanding and optimization of graphene gas sensors. Appl. Phys. Lett. 2021, 119, 013104. [Google Scholar] [CrossRef]
- Fan, J.; Li, H.; Hu, H.; Niu, Y.; Hao, R.; Umard, A.; Mabkhoot, M.S.; Alsaiarid, A.; Wang, Y. An insight into improvement of room temperature formaldehyde sensitivity for graphene-based gas sensors. Microchem. J. 2021, 160, 105607. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Wang, Y. Strategies for the performance enhancement of graphene-based gas sensors: A review. Talanta 2021, 235, 122745. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.M.; Ibrahim, S.; Rozali, S. Advances on graphene-based gas sensors for acetone detection based on its physical and chemical attributes. J. Mater. Res. 2022, 37, 405–423. [Google Scholar] [CrossRef]
- Mendoza, D.; Califrer, I.J.; Freire, F.L., Jr. Strain in twisted bilayer graphene grown by chemical vapour deposition on Ni surfaces. Appl. Surf. Sci. 2021, 544, 148884. [Google Scholar] [CrossRef]
- Machac, P.; Cichon, S.; Lapcak, L.; Fekete, L. Graphene prepared by chemical vapour deposition process. Graphene Technol. 2020, 5, 9–17. [Google Scholar] [CrossRef]
- Yang, W.; Gan, L.; Li, H.; Zhai, T. Two-dimensional layered nanomaterials for gas-sensing applications. Inorg. Chem. Front. 2016, 3, 433–451. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, S.J.; Kim, Y.J.; Shim, Y.S.; Kim, S.Y.; Hong, B.H.; Jang, H.W. Self-activated transparent all-graphene gas sensor with endurance to humidity and mechanical bending. ACS Nano 2015, 9, 10453–10460. [Google Scholar] [CrossRef]
- Leenaerts, O.; Partoens, B.; Peeters, F. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wang, X.; Li, X.; Guo, Z.; Zhou, X.; Wu, Y. The effects of amino substituents on the enhanced ammonia sensing performance of PcCo/rGO hybrids. RSC Adv. 2018, 8, 41280–41287. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Panes-Ruiz, L.A.; Croy, A.; Löffler, M.; Khavrus, V.; Bezugly, V.; Cuniberti, G. Highly sensitive room temperature ammonia gas sensor using pristine graphene: The role of biocompatible stabilizer. Carbon 2021, 173, 262–270. [Google Scholar] [CrossRef]
- Chen, B.; Yao, C.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Yoo, J.; Park, H.B. Effect of hydrogen peroxide on properties of graphene oxide in Hummers method. Carbon 2019, 141, 515–522. [Google Scholar] [CrossRef]
- Alamdarlo, V.; Solookinejad, G.; Zahakifar, F.; Jalal, M.R.; Jabbari, M. Synthesis of graphene oxide and functionalized graphene oxide using improved hummers method for the adsorption of lead from aqueous solutions. J. Water Wastewater 2021, 32, 108–121. [Google Scholar]
- Love, H.; Nazemi, E.; El-Masri, K.; Ambrose, M.; Freund, S.; Emadi, A. A review on advanced sensing materials for agricultural gas sensors. Sensors 2021, 21, 3423. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-C.; Chen, Y.-Y.; Wu, T.-T. A room temperature surface acoustic wave hydrogen sensor with Pt coated ZnO nanorods. Nanotechnology 2009, 20, 065501. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, C.; Contini, G.; Fratoddi, I.; Irrera, S.; Pertici, P.; Scavia, G.; Russo, M.V. Nanostructured organometallic polymer and palladium/polymer hybrid: Surface investigation and sensitivity to relative humidity and hydrogen in surface acoustic wave sensors. Nanotechnology 2007, 18, 125504. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Ma, R.-H.; Li, L.-S.; Fan, L.; Yang, Y.-T.; Zhang, S.-Y. A room-temperature ultrasonic hydrogen sensor based on a sensitive layer of reduced graphene oxide. Sci. Rep. 2021, 11, 2404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Kaur, A. Chemiresistive gas sensors based on thermally reduced graphene oxide for sensing sulphur dioxide at room temperature. Diam. Relat. Mater. 2020, 109, 108039. [Google Scholar] [CrossRef]
- Galstyan, V.; Ponzoni, A.; Kholmanov, I.; Natile, M.M.; Comini, E.; Nematov, S.; Sberveglieri, G. Reduced graphene oxide–TiO2 nanotube composite: Comprehensive study for gas-sensing applications. ACS Appl. Nano Mater. 2018, 1, 7098–7105. [Google Scholar] [CrossRef]
- Bhati, V.S.; Kumar, M.; Banerjee, R. Gas sensing performance of 2D nanomaterials/metal oxide nanocomposites: A review. J. Mater. Chem. C 2021, 9, 8776–8808. [Google Scholar] [CrossRef]
- Niu, F.; Shao, Z.-W.; Gao, H.; Tao, L.-M.; Ding, Y. Si-doped graphene nanosheets for NOx gas sensing. Sens. Actuators B Chem. 2021, 328, 129005. [Google Scholar] [CrossRef]
- Aquatar, O.; Bhatia, U.; Rayalu, S.S.; Krupadam, R.J. Reduced graphene oxide-MnO2 nanocomposite for CO2 capture from flue gases at elevated temperatures. Sci. Total Environ. 2021, 816, 151522. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Debarnot, D.; Poncin-Epaillard, F. Polyaniline as a new sensitive layer for gas sensors. Anal. Chim. Acta 2003, 475, 1–15. [Google Scholar] [CrossRef]
- Huang, D.; Yang, Z.; Li, X.; Zhang, L.; Hu, J.; Su, Y.; Hu, N.; Yin, G.; He, D.; Zhang, Y. Three-dimensional conductive networks based on stacked SiO2@ graphene frameworks for enhanced gas sensing. Nanoscale 2017, 9, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Hu, J.; Su, Y.; Zhou, Z.; Cai, B.; Tao, Z.; Huo, T.; Hu, N.; Zhang, Y. Highly repeatable and sensitive three-dimensional γ-Fe2O3@ reduced graphene oxide gas sensors by magnetic-field assisted assembly process. Sens. Actuators B Chem. 2020, 306, 127546. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical biosensors based on conducting polymers: A review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Al-Hartomy, A.; Khasim, S.; Roy, A.; Pasha, A. Highly conductive polyaniline/graphene nano-platelet composite sensor towards detection of toluene and benzene gases. Appl. Phys. A 2019, 125, 12. [Google Scholar] [CrossRef]
- Farooqi, B.A.; Ashraf, A.; Farooq, U.; Ayub, K. Comparative study on sensing abilities of polyaniline and graphene polyaniline composite sensors toward methylamine and ammonia. Polym. Adv. Technol. 2020, 31, 3351–3360. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Fauzi, N.I.M. Sensing methods for hazardous phenolic compounds based on graphene and conducting polymers-based materials. Chemosensors 2021, 9, 291. [Google Scholar] [CrossRef]
- Karouei, S.F.H.; Moghaddam, H.M.; Niavol, S.S. Characterization and gas sensing properties of graphene/polyaniline nanocomposite with long-term stability under high humidity. J. Mater. Sci. 2021, 56, 4239–4253. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wang, Y.; Li, X. Humidity-enabled ionic conductive trace carbon dioxide sensing of nitrogen-doped Ti3C2T x MXene/polyethyleneimine composite films decorated with reduced graphene oxide nanosheets. Anal. Chem. 2020, 92, 16033–16042. [Google Scholar] [CrossRef] [PubMed]
- Vasseghian, Y.; Hosseinzadeh, S.; Khataee, A.; Dragoi, E.-N. The concentration of persistent organic pollutants in water resources: A global systematic review, meta-analysis and probabilistic risk assessment. Sci. Total Environ. 2021, 796, 149000. [Google Scholar] [CrossRef] [PubMed]
- Abascal, L.; Gómez-Coma, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2021, 810, 152233. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, T.P.; Sampath, P.V.; Maliyekkal, S.M. A critical review of uranium contamination in groundwater: Treatment and sludge disposal. Sci. Total Environ. 2022, 825, 153947. [Google Scholar] [CrossRef]
- Kim, L.; Thanh, N.T.; Toan, P.V.; Minh, H.V.T.; Kumar, P. Removal of arsenic in groundwater using Fe (III) oxyhydroxide coated sand: A case study in Mekong Delta, Vietnam. Hydrology 2022, 9, 15. [Google Scholar] [CrossRef]
- Vu, C.T.; Wu, T. Enhanced slow sand filtration for the removal of micropollutants from groundwater. Sci. Total Environ. 2022, 809, 152161. [Google Scholar] [CrossRef]
- Spoială, C.-I.; Ilie, D.; Ficai, A.; Andronescu, E. Chitosan-based nanocomposite polymeric membranes for water purification—A review. Materials 2021, 14, 2091. [Google Scholar] [CrossRef]
- Li, C.; Yang, J.; Zhang, L.; Li, S.; Yuan, Y.; Xiao, X.; Fan, X.; Song, C. Carbon-based membrane materials and applications in water and wastewater treatment: A review. Environ. Chem. Lett. 2021, 19, 1457–1475. [Google Scholar] [CrossRef]
- Hursthouse, A.; Menzies, B.; Kelly, S.; Mirzaeian, M.; McPherson, W.; Wood, D. WEEE collection and CRM recovery trials: Piloting a holistic approach for Scotland. Glob. NEST J. 2018, 20, 712–718. [Google Scholar]
- Zhang, Z.; Simon, A.; Abetz, C.; Held, M.; Höhme, A.; Schneider, E.S.; Segal-Peretz, T.; Abetz, V. Hybrid organic–inorganic–organic isoporous membranes with tunable pore sizes and functionalities for molecular separation. Adv. Mater. 2021, 33, 2105251. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, A.; Ciacchi, L.C.; Wei, G. Recent advances in nanoporous membranes for water purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadarshini, S.; Tay, W.; Hong, L. Zeolite composite membranes with a nanoporous fluorinated carbonaceous sheath for organic solvent filtration. ACS Appl. Nano Mater. 2021, 4, 2783–2794. [Google Scholar] [CrossRef]
- Joshi, R.; Alwarappan, S.; Yoshimura, M.; Sahajwalla, V.; Nishina, Y. Graphene oxide: The new membrane material. Appl. Mater. Today 2015, 1, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Giri, K.; Cordeiro, M.N.D. Heavy metal ion separation from industrial wastewater using stacked graphene Membranes: A molecular dynamics simulation study. J. Mol. Liq. 2021, 338, 116688. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Sun, J.; Wang, T.; Zeng, L.; Jiang, G. Graphene and graphene oxide sheets supported on silica as versatile and high-performance adsorbents for solid-phase extraction. Angew. Chem. 2011, 123, 6035–6039. [Google Scholar] [CrossRef]
- Liu, Q.; Shi, J.; Jiang, G. Application of graphene in analytical sample preparation. TrAC Trends Anal. Chem. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Ahmad, F.; Husain, M.; Khan, R.A. Graphene oxide lamellar membrane with enlarged inter-layer spacing for fast preconcentration and determination of trace metal ions. RSC Adv. 2021, 11, 11889–11899. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, J.; Gao, C.; Van der Bruggen, B.; Shen, Q.; Shao, H.; Shen, J. Preparation of high-flux nanoporous solvent resistant polyacrylonitrile membrane with potential fractionation of dyes and Na2SO4. Ind. Eng. Chem. Res. 2017, 56, 11967–11976. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, H.; Bai, J.; Liu, J.; Zhang, Y. A porous graphene composite membrane intercalated by halloysite nanotubes for efficient dye desalination. Desalination 2017, 420, 145–157. [Google Scholar] [CrossRef]
- Guan, K.; Zhao, D.; Zhang, M.; Shen, J.; Zhou, G.; Liu, G.; Jin, W. 3D nanoporous crystals enabled 2D channels in graphene membrane with enhanced water purification performance. J. Membr. Sci. 2017, 542, 41–51. [Google Scholar] [CrossRef]
- Huang, L.; Huang, S.; Venna, S.R.; Lin, H. Rightsizing nanochannels in reduced graphene oxide membranes by solvating for dye desalination. Environ. Sci. Technol. 2018, 52, 12649–12655. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, A.; Dixit, K.; Tripathi, K.M. Magnetic Nanoparticles: Application in the Removal of Next-Generation Pollutants from Wastewater. In New Trends in Emerging Environmental Contaminants; Springer: Berlin/Heidelberg, Germany, 2022; pp. 287–310. [Google Scholar]
- Yan, J.; Li, R. Simple and low-cost production of magnetite/graphene nanocomposites for heavy metal ions adsorption. Sci. Total Environ. 2021, 813, 152604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, J.; Lu, T.; Tang, G.; Wu, S.; Ma, W.; Huang, C. Robust, functionalized reduced graphene-based nanofibrous membrane for contaminated water purification. Chem. Eng. J. 2021, 404, 126347. [Google Scholar] [CrossRef]
- Qian, Y.; Shang, J.; Liu, D.; Yang, G.; Wang, X.; Chen, C.; Kou, L.; Lei, W. Enhanced ion sieving of graphene oxide membranes via surface amine functionalization. J. Am. Chem. Soc. 2021, 143, 5080–5090. [Google Scholar] [CrossRef]
- Sheikh, M.; Pazirofteh, M.; Dehghani, M.; Asghari, M.; Rezakazemi, M.; Valderrama, C.; Cortina, J.-L. Application of ZnO nanostructures in ceramic and polymeric membranes for water and wastewater technologies: A review. Chem. Eng. J. 2020, 391, 123475. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Gopal, S.; Nambikkattu, J.; Rambabu, K.; Aboulella, A.M.; Wickramasinghe, S.R.; Banat, F. Recent developments in porous ceramic membranes for wastewater treatment and desalination: A review. J. Environ. Manag. 2021, 293, 112925. [Google Scholar] [CrossRef]
- Kallem, P.; Bharath, G.; Rambabu, K.; Srinivasakannan, C.; Banat, F. Improved permeability and antifouling performance of polyethersulfone ultrafiltration membranes tailored by hydroxyapatite/boron nitride nanocomposites. Chemosphere 2021, 268, 129306. [Google Scholar] [CrossRef]
- Vatanpour, V.; Keskin, B.; Mehrabani, S.A.N.; Karimi, H.; Arabi, N.; Behroozi, A.H.; Shokrollahi-Far, A.; Gul, B.Y.; Koyuncu, I. Investigation of boron nitride/silver/graphene oxide nanocomposite on separation and antibacterial improvement of polyethersulfone membranes in wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 107035. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, D.; Liang, R.-P.; Qiu, J.-D. Graphene-based optical nanosensors for detection of heavy metal ions. TrAC Trends Anal. Chem. 2018, 102, 280–289. [Google Scholar] [CrossRef]
- Sang, S.; Li, D.; Zhang, H.; Sun, Y.; Jian, A.; Zhang, Q.; Zhang, W. Facile synthesis of AgNPs on reduced graphene oxide for highly sensitive simultaneous detection of heavy metal ions. RSC Adv. 2017, 7, 21618–21624. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Y.; Mubarak, N.; Abdullah, E.; Nizamuddin, S.; Khalid, M. Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals—A review. J. Ind. Eng. Chem. 2018, 66, 29–44. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M.; Saleviter, S. Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors 2019, 19, 3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrinha, Á.; Oliveira, T.M.; Ribeiro, F.W.; Morais, S.; Correia, A.N.; de Lima-Neto, P. Advantages and Limitations of Functionalized Graphene-Based Electrochemical Sensors for Environmental Monitoring. In Functionalized Nanomaterial-Based Electrochemical Sensors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 487–520. [Google Scholar]
- Lu, M.; Deng, Y.; Luo, Y.; Lv, J.; Li, T.; Xu, J.; Chen, S.-W.; Wang, J. Graphene aerogel–metal–organic framework-based electrochemical method for simultaneous detection of multiple heavy-metal ions. Anal. Chem. 2018, 91, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef]
- Gao, C.; Yu, X.Y.; Xu, R.X.; Liu, J.H.; Huang, X.-J. AlOOH-reduced graphene oxide nanocomposites: One-pot hydrothermal synthesis and their enhanced electrochemical activity for heavy metal ions. ACS Appl. Mater. Interfaces 2012, 4, 4672–4682. [Google Scholar] [CrossRef]
- Akhtar, M.; Tahir, A.; Zulfiqar, S.; Hanif, F.; Warsi, M.F.; Agboola, P.O.; Shakir, I. Ternary hybrid of polyaniline-alanine-reduced graphene oxide for electrochemical sensing of heavy metal ions. Synth. Met. 2020, 265, 116410. [Google Scholar] [CrossRef]
- De Acha, N.; Elosúa, C.; Corres, J.M.; Arregui, F.J. Fluorescent sensors for the detection of heavy metal ions in aqueous media. Sensors 2019, 19, 599. [Google Scholar] [CrossRef] [Green Version]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to improve the adsorption properties of graphene-based adsorbent towards heavy metal ions and their compound pollutants: A review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef]
- Bie, C.; Yu, H.; Cheng, B.; Ho, W.; Fan, J.; Yu, J. Design, fabrication, and mechanism of nitrogen-doped graphene-based photocatalyst. Adv. Mater. 2021, 33, 2003521. [Google Scholar] [CrossRef]
- Battaglia, V.; Compagnone, A.; Bandino, A.; Bragadin, M.; Rossi, C.A.; Zanetti, F.; Colombatto, S.; Grillo, M.A.; Toninello, A. Cobalt induces oxidative stress in isolated liver mitochondria responsible for permeability transition and intrinsic apoptosis in hepatocyte primary cultures. Int. J. Biochem. Cell Biol. 2009, 41, 586–594. [Google Scholar] [CrossRef]
- Karovic, O.; Tonazzini, I.; Rebola, N.; Edström, E.; Lövdahl, C.; Fredholm, B.B.; Daré, E. Toxic effects of cobalt in primary cultures of mouse astrocytes: Similarities with hypoxia and role of HIF-1α. Biochem. Pharmacol. 2007, 73, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, W.; Fen, Y.; Saleviter, S.; Chanlek, N.; Nakajima, H.; Abdullah, J.; Yusof, N. X-ray photoelectron spectroscopy analysis of chitosan–graphene oxide-based composite thin films for potential optical sensing applications. Polymers 2021, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Keets, K.A.; Cole, E.B.; Morris, A.J.; Sivasankar, N.; Teamey, K.; Lakkaraju, P.S.; Bocarsly, A.B. Analysis of pyridinium catalyzed electrochemical and photoelectrochemical reduction of CO2: Chemistry and economic impact. Indian J. Chem. 2012, 51, 1284–1297. [Google Scholar]
- Mohammed, S.; Gill, A.R.; Alsafadi, K.; Hijazi, O.; Kumar, K.; Moh, Y.; Afzal, A.H.; Khan, H.; Marina, S.I.; Cabral-Pinto, M.S.; et al. An overview of greenhouse gases emissions in Hungary. J. Clean. Prod. 2021, 314, 127865. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef]
- Wang, D.; Leung, Y.; Xuan, J. Modeling of a microfluidic electrochemical cell for CO2 utilization and fuel production. Appl. Energy 2013, 102, 1057–1062. [Google Scholar] [CrossRef]
- Lim, S.L.; Lee, L.H.; Wu, T.Y. Sustainability of using composting and vermicomposting technologies for organic solid waste biotransformation: Recent overview, greenhouse gases emissions and economic analysis. J. Clean. Prod. 2016, 111, 262–278. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Zhao, Q.-F.; Liang, X.-M.; Wang, X.-L.; Ma, F.-X.; Suo, B.-B.; Zou, W.-L.; Han, H.-X.; Song, Q.; Wu, Q.; et al. Computational studies of electrochemical CO2 reduction on chalcogen doped Cu4 cluster. Int. J. Hydrogen Energy 2018, 43, 9935–9942. [Google Scholar] [CrossRef]
- Yu, K.M.K.; Curcic, I.; Gabriel, J.; Tsang, S.C.E. Recent advances in CO2 capture and utilization. ChemSusChem. 2008, 1, 893–899. [Google Scholar] [CrossRef]
- Koytsoumpa, I.; Bergins, C.; Kakaras, E. The CO2 economy: Review of CO2 capture and reuse technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Figueroa, D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The US Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Ma, J.; Sun, N.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. A short review of catalysis for CO2 conversion. Catal. Today 2009, 148, 221–231. [Google Scholar] [CrossRef]
- Li, Y.; Chan, S.H.; Sun, Q. Heterogeneous catalytic conversion of CO2: A comprehensive theoretical review. Nanoscale 2015, 7, 8663–8683. [Google Scholar] [CrossRef] [PubMed]
- Aldana, P.A.U.; Ocampo, F.; Kobl, K.; Louis, B.; Thibault-Starzyk, F.; Daturi, M.; Bazin, P.; Thomas, S.; Roger, A.C. Catalytic CO2 valorization into CH4 on Ni-based ceria-zirconia. Reaction mechanism by operando IR spectroscopy. Catal. Today 2013, 215, 201–207. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. State of the art and perspectives in catalytic processes for CO2 conversion into chemicals and fuels: The distinctive contribution of chemical catalysis and biotechnology. J. Catal. 2016, 343, 2–45. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, D.; Lin, C.; Zhu, Y.; Shen, Y.; Zhang, J.; Han, X.; Zhang, L.; Xia, Z. Catalytic mechanisms and design principles for single-atom catalysts in highly efficient CO2 conversion. Adv. Energy Mater. 2019, 9, 1902625. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Shown, I.; Wei, H.-Y.; Chang, Y.-C.; Du, H.-Y.; Lin, Y.-G.; Tseng, C.-A.; Wang, C.-H.; Chen, L.-C.; Lin, Y.-C.; et al. Graphene oxide as a promising photocatalyst for CO2 to methanol conversion. Nanoscale 2013, 5, 262–268. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, P.; Sharma, O.P.; Jain, S.L.; Khatri, O.P. Reduced graphene oxide–CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B Environ. 2016, 181, 352–362. [Google Scholar] [CrossRef]
- An, X.; Li, K.; Tang, J. Cu2O/reduced graphene oxide composites for the photocatalytic conversion of CO2. ChemSusChem 2014, 7, 1086–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whang, S.; Lim, J.; Choi, M.S.; Lee, J.; Lee, H. Heterogeneous catalysts for catalytic CO2 conversion into value-added chemicals. BMC Chem. Eng. 2019, 1, 9. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, T.; Sun, L.; Nsanzimana, J.M.; Fisher, A.C.; Wang, X. Selective electrochemical reduction of CO2 to ethylene on nanopores-modified copper electrodes in aqueous solution. ACS Appl. Mater. Interfaces 2017, 9, 32782–32789. [Google Scholar] [CrossRef] [PubMed]
- Zarandi, F.; Rezaei, B.; Ghaziaskar, H.S.; Ensafi, A.A. Electrochemical conversion of CO2 to methanol using a glassy carbon electrode, modified by Pt@ histamine-reduced graphene oxide. Int. J. Hydrogen Energy 2019, 44, 30820–30831. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Cao, F.; Ma, Y.; Qu, Y. Catalytic behavior of graphene oxides for converting CO2 into cyclic carbonates at one atmospheric pressure. ACS Sustain. Chem. Eng. 2018, 6, 4204–4211. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Cai, Q. Pyrrolic-nitrogen doped graphene: A metal-free electrocatalyst with high efficiency and selectivity for the reduction of carbon dioxide to formic acid: A computational study. Phys. Chem. Chem. Phys. 2016, 18, 5491–5498. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hou, X.; Ma, C.; Tan, T. Nitrogen-doped graphenes as efficient electrocatalysts for the selective reduction of carbon dioxide to formate in aqueous solution. Green Chem. 2016, 18, 3250–3256. [Google Scholar] [CrossRef]
- Sun, X.; Kang, X.; Zhu, Q.; Ma, J.; Yang, G.; Liu, Z.; Han, B. Very highly efficient reduction of CO2 to CH4 using metal-free N-doped carbon electrodes. Chem. Sci. 2016, 7, 2883–2887. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Liu, M.; Sharma, P.P.; Yadav, R.M.; Ma, L.; Yang, Y.; Zou, X.; Zhou, X.-D.; Vajtai, R.; Yakobson, B.I.; et al. Incorporation of nitrogen defects for efficient reduction of CO2 via two-electron pathway on three-dimensional graphene foam. Nano Lett. 2016, 16, 466–470. [Google Scholar] [CrossRef]
- Naushad, M. (Ed.) A New Generation Material Graphene: Applications in Water Technology; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Minale, M.; Gu, Z.; Guadie, A.; Kabtamu, D.M.; Li, Y.; Wang, X. Application of graphene-based materials for removal of tetracyclines using adsorption and photocatalytic-degradation: A review. J. Environ. Manag. 2020, 276, 111310. [Google Scholar] [CrossRef]

























| Technique | Advantages | Disadvantages | Ref |
|---|---|---|---|
| Chemical vapour deposition |
|
| [11] |
| Chemical exfoliation |
|
| [111] |
| Electrochemical exfoliation |
|
| [50] |
| Epitaxial growth |
|
| [69,91] |
| Liquid phase exfoliation |
|
| [112,113] |
| Mechanical exfoliation |
|
| [69,114] |
| Technique | No of Layers | Size | Mobility (cm2v−1S−1) |
|---|---|---|---|
| Exfoliation | 1 to 10+ | 1 mm | 15,000 |
| Thermal SiC | 1 to 4 | 50 µm | 2000 |
| Ni-CVD | 1 to 4 | 1 cm | 3700 |
| Cu-CVD | 1 | 65 cm | 16,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Yadav, A.; Olabi, A.G. Graphene Synthesis Techniques and Environmental Applications. Materials 2022, 15, 7804. https://doi.org/10.3390/ma15217804
Abbas Q, Shinde PA, Abdelkareem MA, Alami AH, Mirzaeian M, Yadav A, Olabi AG. Graphene Synthesis Techniques and Environmental Applications. Materials. 2022; 15(21):7804. https://doi.org/10.3390/ma15217804
Chicago/Turabian StyleAbbas, Qaisar, Pragati A. Shinde, Mohammad Ali Abdelkareem, Abdul Hai Alami, Mojtaba Mirzaeian, Arti Yadav, and Abdul Ghani Olabi. 2022. "Graphene Synthesis Techniques and Environmental Applications" Materials 15, no. 21: 7804. https://doi.org/10.3390/ma15217804