E-Selectin, ICAM-1, and ET-1 Biomarkers Address the Concern of the Challenging Diagnosis of Interstitial Lung Disease in Patients with Autoimmune Diseases
Abstract
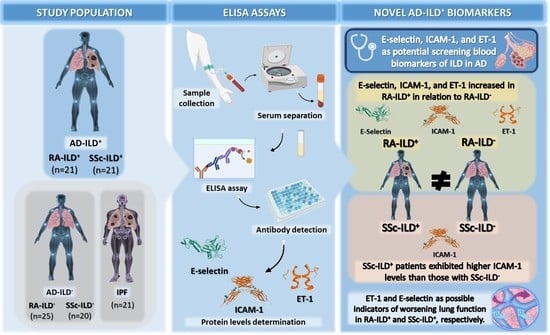
:1. Introduction
2. Results
2.1. E-Selectin, ICAM-1, and ET-1 as Biomarkers for the Presence of ILD in RA Patients
2.2. ICAM-1 Implicated in the Presence of ILD in SSc Patients
2.3. Association of E-Selectin, ICAM-1, and ET-1 with Clinical Characteristics of Patients with RA-ILD+ and SSc-ILD+
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Determination of E-Selectin, ICAM-1, and ET-1 Serum Levels
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murdaca, G.; Colombo, B.M.; Cagnati, P.; Gulli, R.; Spanò, F.; Puppo, F. Endothelial Dysfunction in Rheumatic Autoimmune Diseases. Atherosclerosis 2012, 224, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Steyers, C.M.; Miller, F.J. Endothelial Dysfunction in Chronic Inflammatory Diseases. Int. J. Mol. Sci. 2014, 15, 11324–11349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano-Dourado, L.; Ab’Saber, A.M.; Capelozzi, V.L.; Valeri, C.; Barbas, C.S.V. In Situ Evidence of Pulmonary Endothelial Activation in Patients with Granulomatosis with Polyangiitis and Systemic Sclerosis. Lung 2015, 193, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Andersen, G.N.; Caidahl, K.; Kazzam, E.; Petersson, A.S.; Waldenström, A.; Mincheva-Nilsson, L.; Rantapää-Dahlqvist, S. Correlation between Increased Nitric Oxide Production and Markers of Endothelial Activation in Systemic Sclerosis: Findings with the Soluble Adhesion Molecules E-Selectin, Intercellular Adhesion Molecule 1, and Vascular Cell Adhesion Molecule 1. Arthritis Rheum. 2000, 43, 1085–1093. [Google Scholar] [CrossRef]
- Atzeni, F.; Gerardi, M.C.; Barilaro, G.; Masala, I.F.; Benucci, M.; Sarzi-puttini, P. Interstitial Lung Disease in Systemic Autoimmune Rheumatic Diseases: A Comprehensive Review. Expert. Rev. Clin. Immunol. 2018, 14, 69–82. [Google Scholar] [CrossRef]
- Mathai, S.C.; Danoff, S.K. Management of Interstitial Lung Disease Associated with Connective Tissue Disease. Bmj 2016, 352, h6819. [Google Scholar] [CrossRef]
- Cottin, V.I. Idiopathic Interstitial Pneumonias with Connective Tissue Diseases Features: A Review. Respirology 2016, 21, 245–258. [Google Scholar] [CrossRef]
- Atienza-Mateo, B.; Remuzgo-Martínez, S.; Mora Cuesta, V.M.; Iturbe-Fernández, D.; Fernández-Rozas, S.; Prieto-Peña, D.; Calderón-Goercke, M.; Corrales, A.; Blanco Rodriguez, G.; Gómez-Román, J.J.; et al. The Spectrum of Interstitial Lung Disease Associated with Autoimmune Diseases: Data of a 3.6-Year Prospective Study from a Referral Center of Interstitial Lung Disease and Lung Transplantation. J. Clin. Med. 2020, 9, 1606. [Google Scholar] [CrossRef]
- Bendstrup, E.; Møller, J.; Kronborg-white, S.; Prior, T.S.; Hyldgaard, C. Interstitial Lung Disease in Rheumatoid Arthritis Remains a Challenge for Clinicians. J. Clin. Med. 2019, 8, 2038. [Google Scholar] [CrossRef] [Green Version]
- Khanna, D.; Tashkin, D.P.; Denton, C.P.; Renzoni, E.A.; Desai, S.R.; Varga, J. Etiology, Risk Factors, and Biomarkers in Systemic Sclerosis with Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2020, 201, 650–660. [Google Scholar] [CrossRef]
- Cerro Chiang, G.; Parimon, T. Understanding Interstitial Lung Diseases Associated with Connective Tissue Disease (CTD-ILD): Genetics, Cellular Pathophysiology, and Biologic Drivers. Int. J. Mol. Sci. 2023, 24, 2405. [Google Scholar] [CrossRef] [PubMed]
- McGroder, C.F.; Aaron, C.P.; Bielinski, S.J.; Kawut, S.M.; Tracy, R.P.; Raghu, G.; Barr, R.G.; Lederer, D.J.; Podolanczuk, A.J. Circulating Adhesion Molecules and Subclinical Interstitial Lung Disease: The Multi-Ethnic Study of Atherosclerosis. Eur. Respir. J. 2019, 54, 1900295. [Google Scholar] [CrossRef]
- Ross, B.; D’Orléans-Juste, P.; Giaid, A. Potential Role of Endothelin-1 in Pulmonary Fibrosis: From the Bench to the Clinic. Am. J. Respir. Cell Mol. Biol. 2010, 42, 16–20. [Google Scholar] [CrossRef]
- Takehara, H.; Tada, S.; Kataoka, M.; Matsuo, K.; Ueno, Y.; Ozaki, S.; Miyake, T.; Fujimori, Y.; Yamadori, I.; Harada, M. Intercellular Adhesion Molecule-1 in Patients with Idiopathic Interstitial Pneumonia. Acta Med. Okayama 2001, 55, 205–211. [Google Scholar] [CrossRef]
- Hayashi, S.; Abe, K.; Matsuoka, H.; Goya, S.; Morishita, H.; Mori, M.; Arai, T.; Kida, H.; Nishino, K.; Takeda, Y.; et al. Increased Level of Soluble E-Selectin in the Serum from Patients with Idiopathic Pulmonary Fibrosis. Inflammation 2004, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Atienza-Mateo, B.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Lera-Gómez, L.; Rodriguez-Carrio, J.; Prieto-Peña, D.; Portilla, V.; et al. Angiogenic T Cells: Potential Biomarkers for the Early Diagnosis of Interstitial Lung Disease in Autoimmune Diseases? Biomedicines 2022, 10, 851. [Google Scholar] [CrossRef] [PubMed]
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Fernández-Rozas, S.; Atienza-Mateo, B.; Lera-Gómez, L.; Alonso-Lecue, P.; Rodríguez-Carrio, J.; et al. Endothelial Progenitor Cells as a Potential Biomarker in Interstitial Lung Disease Associated with Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 4098. [Google Scholar] [CrossRef] [PubMed]
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Atienza-mateo, B.; Mora-cuesta, V.M.; Iturbe-Fernández, D.; Lera-Gómez, L.; Prieto-Peña, D.; Portilla, V.; Blanco, R.; et al. Endothelial Progenitor Cells: Relevant Players in the Vasculopathy and Lung Fibrosis Associated with the Presence of Interstitial Lung Disease in Systemic Sclerosis Patients. Biomedicines 2021, 9, 847. [Google Scholar] [CrossRef]
- Pulito-Cueto, V.; Genre, F.; López-Mejías, R.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Portilla, V.; Sebastián Mora-Gil, M.; Ocejo-Vinyals, J.G.; Gualillo, O.; Blanco, R.; et al. Endothelin-1 as a Biomarker of Idiopathic Pulmonary Fibrosis and Interstitial Lung Disease Associated with Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 1275. [Google Scholar] [CrossRef]
- Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Atienza-Mateo, B.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Lera-Gómez, L.; Sebastián Mora-Gil, M.; Prieto-Peña, D.; Portilla, V.; et al. Elevated VCAM-1, MCP-1 and ADMA Serum Levels Related to Pulmonary Fibrosis of Interstitial Lung Disease Associated with Rheumatoid Arthritis. Front. Mol. Biosci. 2022, 9, 1056121. [Google Scholar] [CrossRef]
- Mcmurray, R.W. Adhesion Molecules in Autoimmune Disease. Semin. Arthritis Rheum. 1996, 25, 215–233. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A Master Regulator of Cellular Responses in Inflammation, Injury Resolution, and Tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Sticherling, M. The Role of Endothelin in Connective Tissue Diseases. Rheumatology 2006, 45 (Suppl. S3), iii8–iii10. [Google Scholar] [CrossRef] [Green Version]
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D. Circulating Biologic Markers of Endothelial Dysfunction in Cerebral Small Vessel Disease: A Review. J. Cereb. Blood Flow. Metab. 2016, 36, 72–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltész, P.; Kerekes, G.; Dér, H.; Szücs, G.; Szántó, S.; Kiss, E.; Bodolay, E.; Zeher, M.; Timár, O.; Szodoray, P.; et al. Comparative Assessment of Vascular Function in Autoimmune Rheumatic Diseases: Considerations of Prevention and Treatment. Autoimmun. Rev. 2011, 10, 416–425. [Google Scholar] [CrossRef]
- Spagnolo, P.; Distler, O.; Ryerson, C.J.; Tzouvelekis, A.; Lee, J.S.; Bonella, F.; Bouros, D.; Hoffmann-Vold, A.M.; Crestani, B.; Matteson, E.L. Mechanisms of Progressive Fibrosis in Connective Tissue Disease (CTD)-Associated Interstitial Lung Diseases (ILDs). Ann. Rheum. Dis. 2021, 80, 143–150. [Google Scholar] [CrossRef]
- Azuma, A.; Takahashi, S.; Nose, M.; Araki, K.; Araki, M.; Takahashi, T.; Hirose, M.; Kawashima, H.; Miyasaka, M.; Kudoh, S. Role of E-Selectin in Bleomycin Induced Lung Fibrosis in Mice. Thorax 2000, 55, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, A.; Hasegawa, Y.; Tsuchiya, Y.; Shimokata, K. Expression of Cell Adhesion Molecules in the Lungs of Patients with Idiopathic Pulmonary Fibrosis. Chest 1995, 108, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southcott, A.M.; Hemingway, I.; Lorimer, S.; Sugars, K.; Hellewell, P.G.; Black, C.M.; Jefferys, P.K.; Gearing, A.J.H.; Haskard, D.O.; Du Bois, R.M. Adhesion Molecule Expression in the Lung: A Comparison between Normal and Diffuse Interstitial Lung Disease. Eur. Respir. J. 1998, 11, 91–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizaki, A.; Yanaba, K.; Iwata, Y.; Komura, K.; Ogawa, A.; Akiyama, Y.; Muroi, E.; Hara, T.; Ogawa, F.; Takenaka, M.; et al. Cell Adhesion Molecules Regulate Fibrotic Process via Th1/Th2/Th17 Cell Balance in a Bleomycin-Induced Scleroderma Model. J. Immunol. 2010, 185, 2502–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, C.; Abraham, D.; Renzoni, E.A. Endothelin in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2011, 44, 1–10. [Google Scholar] [CrossRef]
- Swigris, J.J.; Brown, K.K. The Role of Endothelin-1 in the Pathogenesis of Idiopathic Pulmonary Fibrosis. BioDrugs 2010, 24, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uguccioni, M.; Pulsatelli, L.; Grigolo, B.; Facchini, A.; Fasano, L.; Cinti, C.; Fabbri, M.; Gasbarrini, G.; Meliconi, R. Endothelin-1 in Idiopathic Pulmonary Fibrosis. J. Clin. Pathol. 1995, 48, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Saleh, D.; Furukawa, K.; Tsao, M.-S.; Maghazachi, A.; Corrin, B.; Yanagisawa, M.; Barnes, P.J.; Giaid, A. Elevated Expression of Endothelin-1 and Endothelin-Converting Enzyme-1 in Idiopathic Pulmonary Fibrosis: Possible Involvement of Proinflammatory Cytokines. Am. J. Respir. Cell Mol. Biol. 1997, 16, 187–193. [Google Scholar] [CrossRef]
- Hasegawa, M.; Asano, Y.; Endo, H.; Fujimoto, M.; Goto, D.; Ihn, H.; Inoue, K.; Ishikawa, O.; Kawaguchi, Y.; Kuwana, M.; et al. Serum Adhesion Molecule Levels as Prognostic Markers in Patients with Early Systemic Sclerosis: A Multicentre, Prospective, Observational Study. PLoS ONE 2014, 9, e88150. [Google Scholar] [CrossRef]
- Ihn, H.; Sato, S.; Fujimoto, M.; Kikuchi, K.; Kadono, T.; Tamaki, K.; Takehara, K. Circulating Intercellular Adhesion Molecule-1 in the Sera of Patients with Systemic Sclerosis: Enhancement by Inflammatory Cytokines. Br. J. Rheumatol. 1997, 36, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, V.; Patterson, K.A.; Stevens, W.; Wilson, M.; Roddy, J.; Sahhar, J.; Proudman, S.; Hissaria, P.; Nikpour, M. Increased Serum Levels of Adhesion Molecules ICAM-1 and VCAM-1 in Systemic Sclerosis Are Not Specific for Pulmonary Manifestations. Clin. Rheumatol. 2018, 37, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, K.D.; Khatri, A.; Donato, M.; Chang, S.E.; Li, S.; Steen, V.D.; Utz, P.J.; Khatri, P.; Chung, L. Cytokine Signatures Differentiate Systemic Sclerosis Patients at High versus Low Risk for Pulmonary Arterial Hypertension. Arthritis Res. Ther. 2022, 24, 39. [Google Scholar] [CrossRef] [PubMed]
- Iannone, F.; Riccardi, M.T.; Guiducci, S.; Bizzoca, R.; Cinelli, M.; Matucci-Cerinic, M.; Lapadula, G. Bosentan Regulates the Expression of Adhesion Molecules on Circulating T Cells and Serum Soluble Adhesion Molecules in Systemic Sclerosis-Associated Pulmonary Arterial Hypertension. Ann. Rheum. Dis. 2008, 67, 1121–1126. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. Arthritis & Rheumatism 2013 Classification Criteria for Systemic Sclerosis. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic Criteria for Idiopathic Pulmonary Fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef] [PubMed]




| AUC (95% CI) | p | Optimal Cutoff Value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| RA-ILD+ vs. RA-ILD− | |||||
| E-selectin | 0.78 (0.64–0.92) | <0.01 | 74.56 ng/mL | 60.00 | 95.83 |
| ICAM-1 | 0.72 (0.57–0.87) | 0.01 | 451.70 ng/mL | 85.00 | 52.00 |
| ET-1 | 0.77 (0.62–0.91) | <0.01 | 1.02 pg/mL | 57.89 | 86.96 |
| SSc-ILD+ vs. SSc-ILD− | |||||
| ICAM-1 | 0.79 (0.65–0.94) | <0.01 | 484.70 ng/mL | 63.2 | 94.7 |
| E-Selectin Serum Levels | ICAM-1 Serum Levels | ET-1 Serum Levels | ||||
|---|---|---|---|---|---|---|
| Variable | r | p | r | p | r | p |
| Duration of RA (years) | −0.42 | 0.10 | −0.33 | 0.23 | 0.30 | 0.30 |
| CRP (mg/dL) | −0.22 | 0.43 | −0.17 | 0.56 | 0.36 | 0.23 |
| ESR (mm/1st hour) | −0.08 | 0.77 | 0.17 | 0.55 | 0.07 | 0.82 |
| FVC (% predicted) | 0.00 | 0.99 | −0.39 | 0.15 | −0.56 | 0.04 |
| FEV1 (% predicted) | 0.05 | 0.87 | −0.33 | 0.24 | −0.65 | 0.01 |
| DLCO (% predicted) | 0.03 | 0.93 | −0.21 | 0.59 | −0.13 | 0.76 |
| Category | Mean ± SD (ng/mL) | p | Mean ± SD (ng/mL) | p | Mean ± SD (pg/mL) | p |
| RF− | 105.52 ± 39.58 | 0.44 | 674.47 ± 132.40 | 0.31 | 1.27 ± 0.34 | 0.94 |
| RF+ | 73.97 ± 20.23 | 540.51 ± 117.69 | 1.17 ± 0.34 | |||
| ACPA− | 86.65 | 0.74 | 502.00 | 0.64 | 0.87 | 0.28 |
| ACPA+ | 76.77 ± 24.01 | 564.98 ± 129.34 | 1.20 ± 0.33 | |||
| UIP HRCT Pattern | 83.81 ± 26.05 | 0.09 | 545.13 ± 120.36 | 0.99 | 1.19 ± 0.30 | 0.90 |
| NSIP HRCT Pattern | 65.51 ± 14.90 | 539.37 ± 135.50 | 1.17 ± 0.37 | |||
| E-Selectin Serum Levels | ICAM-1 Serum Levels | ET-1 Serum Levels | ||||
|---|---|---|---|---|---|---|
| Variable | r | p | r | p | r | p |
| Duration of SSc (years) | −0.19 | 0.45 | −0.33 | 0.22 | −0.02 | 0.96 |
| CRP (mg/dL) | 0.39 | 0.17 | 0.03 | 0.92 | 0.23 | 0.48 |
| ESR (mm/1st hour) | 0.12 | 0.69 | 0.15 | 0.64 | 0.47 | 0.12 |
| FVC (% predicted) | −0.64 | <0.01 | −0.41 | 0.11 | −0.41 | 0.12 |
| FEV1 (% predicted) | −0.56 | 0.02 | −0.37 | 0.16 | −0.32 | 0.22 |
| DLCO (% predicted) | −0.56 | 0.02 | −0.41 | 0.11 | −0.47 | 0.07 |
| Category | Mean ± SD (ng/mL) | p | Mean ± SD (ng/mL) | p | Mean ± SD (pg/mL) | p |
| ANA− | 65.21 | 0.76 | 534.00 | 0.36 | 0.82 | 0.83 |
| ANA+ | 84.10 ± 37.35 | 511.91 ± 120.47 | 1.42 ± 0.83 | |||
| ACA− | 85.39 ± 36.17 | 0.27 | 522.32 ± 113.70 | 0.19 | 1.37 ± 0.84 | 0.79 |
| ACA+ | 40.69 | 357.00 | 1.66 | |||
| ATA− | 68.46 ± 34.43 | 0.07 | 487.58 ± 118.87 | 0.17 | 1.32 ± 0.88 | 0.72 |
| ATA+ | 97.84 ± 34.11 | 538.70 ± 116.11 | 1.45 ± 0.80 | |||
| UIP HRCT Pattern | 99.69 ± 43.67 | 0.17 | 542.40 ± 130.37 | 0.10 | 1.42 ± 0.59 | 0.33 |
| NSIP HRCT Pattern | 80.93 ± 34.16 | 496.98 ± 113.98 | 1.40 ± 0.89 | |||
| Study Objective Groups | Comparison Groups | ||||
|---|---|---|---|---|---|
| RA-ILD+ n = 21 | SSc-ILD+ n = 21 | RA-ILD− n = 25 | SSc-ILD n = 20 | IPF n = 21 | |
| Sex (women), n (%) | 9 (45.9) | 13 (61.9) | 15 (60.0) | 18 (90.0) | 7 (33.3) |
| Age at study (years), mean ± SD | 66.5 ± 10.1 | 60.3 ± 7.0 | 60.1 ± 11.8 | 56.6 ± 15.4 | 69.2 ± 10.0 |
| Smoking ever, n (%) | 13 (65.0) | 11 (52.4) | 13 (52.0) | 11 (55.0) | 16 (76.2) |
| AD duration (years), mean ± SD | 9.2 ± 10.2 | 10.8 ± 8.3 | 4.1 ± 7.4 | 9.6 ± 8.1 | - |
| CRP (mg/dL), mean ± SD | 1.1 ± 1.1 | 0.7 ± 1.4 | 0.5 ± 0.5 | 0.5 ± 0.5 | - |
| ESR (mm/1st hour), mean ± SD | 22.8 ± 27.2 | 20.1 ± 15.9 | 14.4 ± 12.4 | 17.2 ± 13.4 | - |
| Antibody status | |||||
| RF+, n (%) | 17 (81.0) | - | 11 (44.0) | - | - |
| ACPA+, n (%) | 19 (90.4) | - | 15 (60.0) | - | - |
| ANA+, n (%) | - | 19 (95.0) | - | 18 (90.0) | - |
| ACA+, n (%) | - | 1 (5.0) | - | 9 (45.0) | - |
| ATA (anti-Scl70) +, n (%) | - | 10 (50.0) | - | 4 (20.0) | - |
| Pulmonary function tests | |||||
| FVC (% predicted), mean ± SD | 95.2 ± 24.1 | 88.4 ± 27.1 | 99.2 ± 16.0 | 106.6 ± 15.9 | 84.9 ± 14.7 |
| FEV1 (% predicted), mean ± SD | 92.2 ± 21.0 | 87.3 ± 25.6 | 94.9 ± 22.0 | 101.9 ± 17.8 | 87.3 ± 19.6 |
| DLCO (% predicted), mean ± SD | 43.3 ± 15.9 | 47.5 ± 19.5 | 79.9 ± 20.0 | 71.5 ± 15.3 | 43.6 ± 18.4 |
| HRCT | |||||
| Pulmonary involvement in HRCT, n (%) | 21 (100.0) | 21 (100.0) | 0 (0.0) | 0 (0.0) | 21 (100.0) |
| UIP pattern, n (%) | 11 (52.4) | 3 (14.3) | - | - | 21 (100.0) |
| Probable UIP pattern, n (%) | 2 (9.5) | 3 (14.3) | - | - | 0 (0.0) |
| NSIP pattern, n (%) | 7 (33.3) | 14 (66.7) | - | - | 0 (0.0) |
| Non-NSIP pattern, n (%) | 1 (4.8) | 1 (4.7) | - | - | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulito-Cueto, V.; Remuzgo-Martínez, S.; Genre, F.; Atienza-Mateo, B.; Mora-Cuesta, V.M.; Iturbe-Fernández, D.; Lera-Gómez, L.; Mora-Gil, M.S.; Portilla, V.; Corrales, A.; et al. E-Selectin, ICAM-1, and ET-1 Biomarkers Address the Concern of the Challenging Diagnosis of Interstitial Lung Disease in Patients with Autoimmune Diseases. Int. J. Mol. Sci. 2023, 24, 12518. https://doi.org/10.3390/ijms241512518
Pulito-Cueto V, Remuzgo-Martínez S, Genre F, Atienza-Mateo B, Mora-Cuesta VM, Iturbe-Fernández D, Lera-Gómez L, Mora-Gil MS, Portilla V, Corrales A, et al. E-Selectin, ICAM-1, and ET-1 Biomarkers Address the Concern of the Challenging Diagnosis of Interstitial Lung Disease in Patients with Autoimmune Diseases. International Journal of Molecular Sciences. 2023; 24(15):12518. https://doi.org/10.3390/ijms241512518
Chicago/Turabian StylePulito-Cueto, Verónica, Sara Remuzgo-Martínez, Fernanda Genre, Belén Atienza-Mateo, Víctor M. Mora-Cuesta, David Iturbe-Fernández, Leticia Lera-Gómez, María Sebastián Mora-Gil, Virginia Portilla, Alfonso Corrales, and et al. 2023. "E-Selectin, ICAM-1, and ET-1 Biomarkers Address the Concern of the Challenging Diagnosis of Interstitial Lung Disease in Patients with Autoimmune Diseases" International Journal of Molecular Sciences 24, no. 15: 12518. https://doi.org/10.3390/ijms241512518