Highly Selective Synthesis of 6-Glyoxylamidoquinoline Derivatives via Palladium-Catalyzed Aminocarbonylation
Abstract
:1. Introduction
2. Results
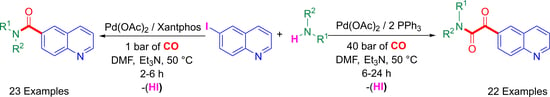
2.1. Optimization Study
2.2. Synthesis of Quinoline-6-glyoxylamides Using Various Amine Nucleophiles
2.3. Selective Synthesis of Quinoline-6-carboxamide Derivatives
2.4. X-ray Cristallographic Study
3. Materials and Methods
3.1. General Procedures
3.2. Aminocarbonylation of 6-Iodoquinoline (1) in the Presence of Various Amine Nucleophiles (a–w) under Atmospheric Carbon Monoxide Pressure
3.3. Aminocarbonylation of 6-Iodoquinoline (1) in the Presence of Various Amine Nucleophiles (a–w) under High Carbon Monoxide Pressure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cornils, B.; Herrmann, W.A.; Beller, M.; Paciello, R. (Eds.) Applied Homogeneous Catalysis with Organometallic Compounds, 3rd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017. [Google Scholar]
- Beller, M.; Wu, X.-F. Transition Metal Catalyzed Carbonylation Reactions: Carbonylative Activation of C-X Bonds; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kollár, L. (Ed.) Modern Carbonylation Methods; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Colquhoun, H.M.; Thompson, D.J.; Carbonylation, M.T. Direct Synthesis of Carbonyl Compounds; Springer: New York, NY, USA, 1991. [Google Scholar]
- Schoenberg, A.; Heck, R.F. Palladium-catalyzed amidation of aryl, heterocyclic, and vinylic halides. J. Org. Chem. 1974, 39, 3327–3331. [Google Scholar] [CrossRef]
- Schoenberg, A.; Bartoletti, I.; Heck, R.F. Palladium-catalyzed carboalcoxilation of aryl, benzyl and vinylic halides. J. Org. Chem. 1974, 39, 3318–3326. [Google Scholar] [CrossRef]
- Schoenberg, A.; Heck, R.F. Palladium-catalyzed formylation of aryl, heterocyclic, and vinylic halides. J. Am. Chem. Soc. 1974, 96, 7761–7765. [Google Scholar] [CrossRef]
- Peng, J.; Qi, X.; Wu, X. Recent Achievements in Carbonylation Reactions: A Personal Account. Synlett 2017, 28, 175–194. [Google Scholar] [CrossRef]
- Wu, X. Palladium-catalyzed carbonylative transformation of aryl chlorides and aryl tosylates. RSC Adv. 2016, 6, 83831–83837. [Google Scholar] [CrossRef]
- Gadge, S.; Bhanage, B. Recent developments in palladium catalysed carbonylation reactions. RSC Adv. 2014, 4, 10367–10389. [Google Scholar] [CrossRef]
- Roy, S.; Gribble, G. Metal-catalyzed amidation. Tetrahedron 2012, 68, 9867–9923. [Google Scholar] [CrossRef]
- Wu, X.; Neumann, H.; Beller, M. Palladium-catalyzed carbonylative coupling reactions between Ar-X and carbon nucleophiles. Chem. Soc. Rev. 2011, 40, 4986–5009. [Google Scholar] [CrossRef]
- Magano, J.; Dunetz, J. Large-Scale Applications of Transition Metal-Catalyzed Couplings for the Synthesis of Pharmaceuticals. Chem. Rev. 2011, 111, 2177–2250. [Google Scholar] [CrossRef] [PubMed]
- Grigg, R.; Mutton, S. Pd-catalysed carbonylations: Versatile technology for discovery and process chemists. Tetrahedron 2010, 66, 5515–5548. [Google Scholar] [CrossRef]
- Brennfuhrer, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Aryl Halides and Related Compounds. Angew. Chem. Int. Ed. 2009, 48, 4114–4133. [Google Scholar] [CrossRef]
- Barnard, C. Palladium-Catalyzed Carbonylation-A Reaction Come of Age. Organometallics 2008, 27, 5402–5422. [Google Scholar] [CrossRef]
- Skoda-Foldes, R.; Kollar, L. Synthetic applications of palladium catalysed carbonylation of organic halides. Curr. Org. Chem. 2002, 6, 1097–1119. [Google Scholar] [CrossRef]
- Kaspar, A.; Reichert, J. Future directions for peptide therapeutics development. Drug Discov. Today 2013, 18, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, V.; Bode, J. Rethinking amide bond synthesis. Nature 2011, 480, 471–479. [Google Scholar] [CrossRef]
- Ghose, A.; Viswanadhan, V.; Wendoloski, J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 2013, 9, 2265–2319. [Google Scholar] [CrossRef] [Green Version]
- Roughley, S.; Jordan, A. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef] [PubMed]
- Wheless, J.; Vazquez, B. Rufinamide: A Novel Broad-Spectrum Antiepileptic Drug. Epilepsy Curr. 2010, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Friis, S.; Skrydstrup, T.; Buchwald, S. Mild Pd-Catalyzed Aminocarbonylation of (Hetero)Aryl Bromides with a Palladacycle Precatalyst. Org. Lett. 2014, 16, 4296–4299. [Google Scholar] [CrossRef] [Green Version]
- Skoda-Földes, R.; Kollár, L. Sustainable Synthesis of Pharmaceuticals: Using Transition Metal Complexes as Catalysts in Carbonylation Reactions in the Synthesis of Pharmaceutically Active Compounds; Pereira, M.M., Calvette, M.J.F., Eds.; RSC Publishing: Cambridge, UK, 2013. [Google Scholar]
- Wu, X.; Neumann, H.; Beller, M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35. [Google Scholar] [CrossRef]
- Alsabeh, P.; Stradiotto, M.; Neumann, H.; Beller, M. Aminocarbonylation of (Hetero)aryl Bromides with Ammonia and Amines using a Palladium/DalPhos Catalyst System. Adv. Synth. Catal. 2012, 354, 3065–3070. [Google Scholar] [CrossRef]
- Begouin, A.; Queiroz, M. Palladium-Catalysed Multicomponent Aminocarbonylation of Aryl or Heteroaryl Halides with [Mo(CO)(6)] and Aryl- or Heteroarylamines Using Conventional Heating. Eur. J. Org. Chem. 2009, 2009, 2820–2827. [Google Scholar] [CrossRef]
- Letavic, M.; Ly, K. Microwave assisted, palladium catalyzed aminocarbonylations of heteroaromatic bromides using solid Mo(CO)(6) as the carbon monoxide source. Tetrahedron Lett. 2007, 48, 2339–2343. [Google Scholar] [CrossRef]
- Ozawa, F.; Yamamoto, A. Double Carbonylation Reactions of Methyl-Palladium(II) and Phenylpalladium(II) Complexes in the Presence of Secondary-Amines Affording Alpha-Keto Amides. Chem. Lett. 1982, 11, 865–868. [Google Scholar] [CrossRef]
- Robello, M.; Barresi, E.; Baglini, E.; Salerno, S.; Taliani, S.; Da Settimo, F. The Alpha Keto Amide Moiety as a Privileged Motif in Medicinal Chemistry: Current Insights and Emerging Opportunities. J. Med. Chem. 2021, 64, 3508–3545. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Bhanage, B. Double Carbonylation Reactions: Overview and Recent Advances. Adv. Synth. Catal. 2020, 362, 3022–3058. [Google Scholar] [CrossRef]
- Du, H.; Ruan, Q.; Qi, M.; Han, W. Ligand-Free Pd-Catalyzed Double Carbonylation of Aryl Iodides with Amines to alpha-Ketoamides under Atmospheric Pressure of Carbon Monoxide and at Room Temperature. J. Org. Chem. 2015, 80, 7816–7823. [Google Scholar] [CrossRef]
- Kahn, K.; Bruice, T. Alpha-ketoamides and alpha-ketocarbonyls: Conformational analysis and development of all-atom OPLS force field. Bioorg. Med. Chem. 2000, 8, 1881–1891. [Google Scholar] [CrossRef]
- Harbeson, S.L.; Abelleira, S.M.; Akiyama, A.; Barrett, R.; Carroll, R.M.; Straub, J.A.; Tkacz, J.N.; Wu, C.C.; Musso, G.F. Stereospecific Synthesis of Peptidyl Alpha-Keto Amides as Inhibitors of Calpain. J. Med. Chem. 1994, 37, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Lescop, C.; Herzner, H.; Siendt, H.; Bolliger, R.; Hennebohle, M.; Weyermann, P.; Briguet, A.; Courdier-Fruh, I.; Erb, M.; Foster, M.; et al. Novel cell-penetrating alpha-keto-amide calpain inhibitors as potential treatment for muscular dystrophy. Bioorg. Med. Chem. Lett. 2005, 15, 5176–5181. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Miyashita, H.; Yamaguchi, M.; Inoue, J.; Nakamura, M. Exploration of orally available calpain inhibitors: Peptidyl alpha-ketoamides containing an amphiphile at P3 site. Bioorg. Med. Chem. 2005, 13, 4473–4484. [Google Scholar] [CrossRef]
- Lubisch, W.; Beckenbach, E.; Bopp, S.; Hofmann, H.; Kartal, A.; Kastel, C.; Lindner, T.; Metz-Garrecht, M.; Reeb, J.; Regner, F.; et al. Benzoylalanine-derived ketoamides carrying vinylbenzyl amino residues: Discovery of potent water-soluble calpain inhibitors with oral bioavailability. J. Med. Chem. 2003, 46, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Matada, B.; Pattanashettar, R.; Yernale, N. A comprehensive review on the biological interest of quinoline and its derivatives. Bioorg. Med. Chem. 2021, 32, 1–25. [Google Scholar] [CrossRef]
- Gonec, T.; Bobal, P.; Sujan, J.; Pesko, M.; Guo, J.; Kralova, K.; Pavlacka, L.; Vesely, L.; Kreckova, E.; Kos, J.; et al. Investigating the Spectrum of Biological Activity of Substituted Quinoline-2-Carboxamides and Their Isosteres. Molecules 2012, 17, 613–644. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Zhaorigetu, B.; Agula, B.; Baiyin, M.; Jia, M. Aminolysis of Aryl Ester Using Tertiary Amine as Amino Donor via C-O and C-N Bond Activations. J. Org. Chem. 2014, 79, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Liao, Y.; Chen, S.; Chen, Y.; Deng, G. Copper-catalyzed efficient direct amidation of 2-methylquinolines with amines. Org. Biomol. Chem. 2015, 13, 6944–6948. [Google Scholar] [CrossRef]
- Zhou, B.; Du, J.; Yang, Y.; Li, Y. Rhodium(III)-Catalyzed Intermolecular Direct Amidation of Aldehyde C H Bonds with N-Chloroamines at Room Temperature. Org. Lett. 2013, 15, 2934–2937. [Google Scholar] [CrossRef] [PubMed]
- Coppa, F.; Fontana, F.; Lazzarini, E.; Minisci, F. A facile, convenient and selective homolytic carbamoylation of heteroaromatic bases. Heterocycles 1993, 36, 2687–2696. [Google Scholar]
- De Risi, C.; Pollini, G.; Zanirato, V. Recent Developments in General Methodologies for the Synthesis of alpha-Ketoamides. Chem. Rev. 2016, 116, 3241–3305. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Taniguchi, T.; Hoshiya, N.; Shuto, S.; Arisawa, M.; Sato, Y. Double carbonylation of aryl iodides with amines under an atmospheric pressure of carbon monoxide using sulfur-modified Au-supported palladium. Green Chem. 2015, 17, 2358–2361. [Google Scholar] [CrossRef]
- Ozawa, F.; Yamagami, I.; Nakano, M.; Fujisawa, F.; Yamamoto, A. Palladium-catalyzed carbonylation of aromatic-aldehydes and hydrocarbons—A novel synthetic route to alpha-keto amides via C-H activation. Chem. Lett. 1989, 18, 125–128. [Google Scholar] [CrossRef]
- Smith, J.; Jones, R.; Booker, G.; Pyke, S. Sequential and Selective Buchwald-Hartwig Amination Reactions for the Controlled Functionalization of 6-Bromo-2-chloroquinoline: Synthesis of Ligands for the Tec Src Homology 3 Domain. J. Org. Chem. 2008, 73, 8880–8892. [Google Scholar] [CrossRef]
- Tsvetkov, A.; Latyshev, G.; Lukashev, N.; Beletskaya, I. The successive substitution of halogens in 4-chloro-6-iodoquinoline by aryl groups in cross-coupling reactions with arylboronic acids. Tetrahedron Lett. 2002, 43, 7267–7270. [Google Scholar] [CrossRef]
- Qi, X.; Jiang, L.; Li, H.; Wu, X. A Convenient Palladium-Catalyzed Carbonylative Suzuki Coupling of Aryl Halides with Formic Acid as the Carbon Monoxide Source. Chem. Eur. J. 2015, 21, 17650–17656. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Li, Y.; Kramer, S.; Skrydstrup, T.; Lian, Z. Silylcarboxylic Acids as Bifunctional Reagents: Application in Palladium-Catalyzed External-CO-Free Carbonylative Cross-Coupling Reactions. Adv. Synth. Catal. 2020, 362, 4078–4083. [Google Scholar] [CrossRef]
- Wu, F.; Peng, J.; Qi, X.; Wu, X. Palladium-Catalyzed Carbonylative Homocoupling of Aryl Iodides for the Synthesis of Symmetrical Diaryl Ketones with Formic Acid. ChemCatChem 2018, 10, 173–177. [Google Scholar] [CrossRef]
- Peng, J.; Wu, F.; Li, C.; Qi, X.; Wu, X. A Convenient and Efficient Palladium-Catalyzed Carbonylative Sonogashira Transformation with Formic Acid as the CO Source. Eur. J. Org. Chem. 2017, 2017, 1434–1437. [Google Scholar] [CrossRef]
- Qi, X.; Ai, H.; Cai, C.; Peng, J.; Ying, J.; Wu, X. A Convenient Palladium-Catalyzed Aminocarbonylation of Aryl Iodides to Primary Amides under Gas-Free Conditions. Eur. J. Org. Chem. 2017, 2017, 7222–7225. [Google Scholar] [CrossRef]
- Barton, P.; Jewsbury, P.; Pease, J. 1,4-Disubstituted Piperidine Derivatives and Their Use a 11betaHSD1 Inhibitors. International Patent Application WO 2004033427 A1, 27 July 2004. [Google Scholar]
- Veale, C.A.; Edwards, P.D.; Jacobs, R.T.; Davenport, T.W.; Warwick, P.J. Modified Pentapeptide Antagonists of the Artial Natriuretic Peptide Clearance Receptor. International Patent Application WO 200061631 A1, 19 October 2000. [Google Scholar]
- Eibl, C.; Tomassoli, I.; Munoz, L.; Stokes, C.; Papke, R.; Gundisch, D. The 3,7-diazabicyclo[3.3.1]nonane scaffold for subtype selective nicotinic acetylcholine receptor (nAChR) ligands. Part 1: The influence of different hydrogen bond acceptor systems on alkyl and (hetero)aryl substituents. Bioorg. Med. Chem. 2013, 21, 7283–7308. [Google Scholar] [CrossRef] [Green Version]
- Ting, P.; Lee, J.; Wu, J.; Umland, S.; Aslanian, R.; Cao, J.; Dong, Y.; Garlisi, C.; Gilbert, E.; Huang, Y.; et al. The synthesis of substituted bipiperidine amide compounds as CCR3 antagonists. Bioorg. Med. Chem. Lett. 2005, 15, 1375–1378. [Google Scholar] [CrossRef]
- Kollar, L.; Varga, M.; Dornyei, A.; Takacs, A. Functionalisation of the uracil ring via palladium-catalysed aminocarbonylation. Tetrahedron 2019, 75, 4632–4639. [Google Scholar] [CrossRef]
- Takacs, A.; Varga, G.; Kardos, J.; Kollar, L. Palladium-catalysed aminocarbonylation of diiodopyridines. Tetrahedron 2017, 73, 2131–2138. [Google Scholar] [CrossRef]
- Takacs, A.; Marosvolgyi-Hasko, D.; Kabak-Solt, Z.; Damas, L.; Rodrigues, F.; Carrilho, R.; Pineiro, M.; Pereira, M.; Kollar, L. Functionalization of indole at C-5 or C-7 via palladium-catalysed double carbonylation. A facile synthesis of indole ketocarboxamides and carboxamide dimers. Tetrahedron 2016, 72, 247–256. [Google Scholar] [CrossRef]
- Szoke, G.; Takacs, A.; Berente, Z.; Petz, A.; Kollar, L. Synthesis of amino-substituted pyridylglyoxylamides via palladium-catalysed aminocarbonylation. Tetrahedron 2016, 72, 3063–3067. [Google Scholar] [CrossRef]
- Szoke, G.; Takacs, A.; Kollar, L. Synthesis of Pyridazine Dicarboxamides via Highly Selective Palladium-catalyzed Aminocarbonylation. J. Heterocycl. Chem. 2016, 53, 2020–2024. [Google Scholar] [CrossRef]
- Takacs, A.; Szilagyi, A.; Acs, P.; Mark, L.; Peixoto, A.; Pereira, M.; Kollar, L. Palladium-catalysed reactions of 8-hydroxy- and 8-benzyloxy-5,7-diiodoquinoline under aminocarbonylation conditions. Tetrahedron 2011, 67, 2402–2406. [Google Scholar] [CrossRef]
- Takacs, A.; Abreu, A.; Peixoto, A.; Pereira, M.; Kollar, L. Synthesis of Ortho-alkoxy-aryl Carboxamides via Palladium-Catalyzed Aminocarbonylation. Synth. Commun. 2009, 39, 1534–1548. [Google Scholar] [CrossRef]
- Takacs, A.; Jakab, B.; Petz, A.; Kollar, L. Homogeneous catalytic aminocarbonylation of nitrogen-containing iodo- heteroaromatics. Synthesis of N-substituted nicotinamide related compounds. Tetrahedron 2007, 63, 10372–10378. [Google Scholar] [CrossRef]
- Takacs, A.; Petz, A.; Jakab, B.; Kollar, L. Aminocarbonylation of 2-iodothiophene: High-yielding synthesis of thiophen-2-yl-glyoxylamides. Lett. Org. Chem. 2007, 4, 590–594. [Google Scholar] [CrossRef]
- Skoda-Foldes, R.; Kollar, L. Palladium-Catalyzed Aminocarbonylation of Iodoalkenes and Iodoarenes. Lett. Org. Chem. 2010, 7, 621–633. [Google Scholar] [CrossRef]
- Kollar, L.; Takacs, A. Novel synthesis of 3-carboxamidolactam derivatives via palladium-catalysed aminocarbonylation. Tetrahedron 2018, 74, 6116–6128. [Google Scholar] [CrossRef]
- Kollar, L.; Erdelyi, A.; Rasheed, H.; Takacs, A. Selective Synthesis of N-Acylnortropane Derivatives in Palladium-Catalysed Aminocarbonylation. Molecules 2021, 26, 1813. [Google Scholar] [CrossRef] [PubMed]
- Csakai, Z.; Skoda-Foldes, R.; Kollar, L. NMR investigation of Pd(II)-Pd(0) reduction in the presence of mono- and ditertiary phosphines. Inorg. Chim. Acta 1999, 286, 93–97. [Google Scholar] [CrossRef]
- Amatore, C.; Carre, E.; Jutand, A.; Mbarki, M.; Meyer, G. Evidence for the Ligation of Palladium(0) Complexes by Acetate Ions: Consequences on the Mechanism of Their Oxidative Addition with Phenyl Iodide and PhPd(OAc)(PPh3)2 as Intermediate in the Heck Reaction. Organometallics 1995, 14, 5605–5614. [Google Scholar] [CrossRef]
- Amatore, C.; Jutand, A.; Mbarki, M. Evidence of the formation of zerovalentpalladium from Pd(OAc)2 and triphenylphosphine. Organometallics 1992, 11, 3009–3013. [Google Scholar] [CrossRef]
- Ozawa, F.; Soyama, H.; Yanagihara, H.; Aoyama, I.; Takino, H.; Izawa, K.; Yamamoto, T.; Yamamoto, A. Palladium-catylalyzed double carbonylation of aryl halides to give alpha-keto amides - Mechanistic studies. J. Am. Chem. Soc. 1985, 107, 3235–3245. [Google Scholar] [CrossRef]
- Gergely, M.; Farkas, R.; Takacs, A.; Petz, A.; Kollar, L. Synthesis of N-picolylcarboxamides via palladium-catalysed aminocarbonylation of iodobenzene and iodoalkenes. Tetrahedron 2014, 70, 218–224. [Google Scholar] [CrossRef]
- Krabbe, S.; Chan, V.; Franczyk, T.; Shekhar, S.; Napolitano, J.; Presto, C.; Simanis, J. Copper-Catalyzed Aerobic Oxidative Amidation of Benzyl Alcohols. J. Org. Chem. 2016, 81, 10688–10697. [Google Scholar] [CrossRef]
- Ning, Y.; Wang, S.; Li, M.; Han, J.; Zhu, C.; Xie, J. Site-specific Umpolung amidation of carboxylic acids via triplet synergistic catalysis. Nat. Commun. 2021, 12, 1–9. [Google Scholar] [CrossRef]
- Piechaczek, J.; Bojarska-Dahlig, H. Monoamine-oxidase inhibitors. VII. Derivatives of quinolinecarboxylic acids. Acta. Pol. Pharm. 1966, 23, 7. [Google Scholar]
- Paul, S.; Roy, P.; Sardar, P.; Majhi, A. Design, Synthesis, and Biophysical Studies of Novel 1,2,3-Triazole-Based Quinoline and Coumarin Compounds. Acs Omega 2019, 4, 7213–7230. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Westrip, S. publCIF: Software for editing, validating and formatting crystallographic information files. J. Appl. Crystallogr. 2010, 43, 920–925. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Riwar, L.; Trapp, N.; Kuhn, B.; Diederich, F. Substituent Effects in Parallel- Displaced π-π Stacking Interactions: Distance Matters. Angew. Chem. Int. Ed. 2017, 56, 11252–11257. [Google Scholar] [CrossRef] [Green Version]
- Janiak, C. A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]








 | ||||||
|---|---|---|---|---|---|---|
| Entry | Amine | Ligand | Temp. (°C) | pCO (bar) | Ratio of the Carbonylated Products (b) | |
| Amide | Ketoamide | |||||
| 1 | Piperidine (b) | PPh3 | 50 | 1 | 63 | 37 |
| 2 | tert-Butylamine (c) (a) | PPh3 | 50 | 1 | 40 | 60 |
| 3 | Piperidine (b) | PPh3 | 30 | 1 | 33 | 66 |
| 4 | Piperidine (b) | PPh3 | 80 | 1 | 90 | 10 |
| 5 | Cyclohexylamine (c) | PPh3 | 80 | 1 | 70 | 30 |
| 6 | Piperidine (b) | PPh3 | 50 | 10 | 25 | 75 |
| 7 | Piperidine (b) | PPh3 | 50 | 40 | 18 | 82 |
| 8 | tert-Butylamine (a) | PPh3 | 50 | 40 | 6 | 94 |
| 9 | Cyclohexylamine (c) | PPh3 | 50 | 40 | 16 | 84 |
| 10 | Piperidine (b) | XantPhos | 50 | 1 | 95 | 5 |
 | ||||
|---|---|---|---|---|
| Entry | Amine | R. Time (h) (b) | Ratio of the Carbonylated Products (c) | |
| Amide | Ketoamide | |||
| 1 | tert-Butylamine (a) | 19 | 6 (2a) | 94 (3a) |
| 2 | Piperidine (b) | 6 | 18 (2b) | 82 (3b) |
| 3 | Cyclohexylamine (c) | 6 | 16 (2c) | 84(3c) |
| 4 | Pyrrolidine (d) | 6 | 30 (2d) | 70 (3d) |
| 5 | N-methylpiperazine (e) | 6 | 6 (2e) | 94 (3e) |
| 6 | Cyclopentylamine (f) | 6 | 14 (2f) | 86 (3f) |
| 7 | Decylamine (g) | 6 | 13 (2g) | 87 (3g) |
| 8 | L-Glycine methyl ester (h) | 7 | 3 (2h) | 97(3h) |
| 9 | L-Alanine methy lester (i) | 6 | 16 (2i) | 84 (3i) |
| 10 | L-Valine methyl ester (j) | 7 | 66 (2j) | 34 (3j) |
| 11 | R-(-)-2-Phenylglycine methyl ester (k) | 24 | 72 (2k) | 28 (3k) |
| 12 | L-Proline methyl ester (l) | 6 | 7 (2l) | 93 (3l) |
| 13 | (L/D)-Serine methyl ester (m) | 6 | 15 (2m) | 85 (3m) |
| 14 | Aniline (n) | 8 | 100 (2n) | 0(3n) |
| 15 | Benzylamine (o) | 8 | 14 (2o) | 86(3o) |
| 16 | 2-(aminomethyl)pyridine (p) | 14 | 33 (2p) | 66(3 p) |
| 17 | 3-(aminomethyl)pyridine (q) | 6 | 3 (2q) | 97(3q) |
| 18 | 4-(aminomethyl)pyridine (r) | 6 | 2 (2r) | 98(3r) |
| 19 | 4-(ethylaminomethyl)pyridine (s) | 24 | 60 (2s) | 40 (3s) |
| 20 | Furfyrlamine (t) | 6 | 5 (2t) | 95(3t) |
| 21 | 2-(Aminomethyl)thiophene (u) | 6 | 2(2u) | 98(3u) |
| 22 | Nortropine (v) | 7 | 37 (2v) | 63(3v) |
| 23 | Propargylamine (w) | 6 | 15 (2w) | 85 (3w) |
| Compounds | (2n) | (2c) | (3g) |
|---|---|---|---|
| Chemical formula | C16H18N2O | 2(C16H12N2O) | C21H28N2O2 |
| Mr | 254.32 | 248.28 | 340.45 |
| Crystal system | Monoclinic | Triclinic | Triclinic |
| Space group | P21 | P ī | P ī |
| Temperature (K) | 294 | 294 | 300 |
| a, b, c (Å) | 5.1616 (4), 6.6616 (5),19.2655 (14) | 5.1930 (4), 9.0314 (7), 27.180 (2) | 5.2074 (9), 11.0289 (18), 16.859 (3) |
| α, β, γ (°) | 90, 92.600 (3), 90 | 98.887 (4), 93.763 (4), 97.281 (4) | 79.495 (10), 86.693 (11), 89.903 (9) |
| V (Å3) | 661.75 (9) | 1244.55 (17) | 950.4 (3) |
| Z | 2 | 4 | 2 |
| Radiation type | Mo Kα | ||
| μ (mm−1) | 0.08 | 0.09 | 0.08 |
| Crystal size(mm) | 0.30 × 0.17 × 0.10 | 0.38 × 0.19 × 0.04 | 0.54 × 0.17 × 0.07 |
| Data collection | |||
| Diffractometer | Bruker D8 VENTURE | ||
| Absorption correction | Multi-scan SADABS2016/2—Bruker AXS area detector scaling and absorption correction | ||
| Tmin, Tmax | 0.91, 0.99 | 0.74, 1.00 | 0.42, 0.99 |
| No. of measured, independent and observed [I > 2σ(I)] reflections 7476, 2725, 2250 41129, 4550, 3249 20248, 3507, 2145 | |||
| Rint | 0.038 | 0.140 | 0.193 |
| (sin θ/λ)max (Å−1) | 0.626 | 0.604 | 0.609 |
| Refinement | |||
| R[F2 > 2s(F2)], wR(F2), S | 0.052, 0.141, 1.15 | 0.099, 0.208, 1.17 | 0.143, 0.359, 1.13 |
| No. of reflections | 2725 | 4550 | 3507 |
| No. of parameters | 176 | 350 | 232 |
| No. of restraints | 2 | 2 | 1 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement | ||
| Δ>max, Δ>min (e Å−3) | 0.51, −0.60 | 0.26, −0.22 | 0.73, −0.59 |
| Crystals | (2c) | (2n) | (3g) | |
|---|---|---|---|---|
| Intermolecular Hydrogen Bonds (N-H)i⋯(O=C)ii | N⋯O/Å | 3.020 | 3.019 | 3.046 |
| N-H⋯O/Å | 2.188 | 2.177 | 2.24 | |
| ∠N-H⋯O/° | 161.23 | 161.81 | 158.34 | |
| Dihedral Angles | ∠CAr-CAr-Cco-N/° | 153.30 | −143.28 | - |
| ∠CAr-CAr-Cco-O/° | 149.08 | 142.77 | 157.15 | |
| ∠O-Cco-Cco-O/° | - | - | 138.86 | |
| Interplanar distances (Å) I(d(centroid-to-centroid)) | 5.162 | 5.193 | 5.207 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chniti, S.; Kollár, L.; Bényei, A.; Takács, A. Highly Selective Synthesis of 6-Glyoxylamidoquinoline Derivatives via Palladium-Catalyzed Aminocarbonylation. Molecules 2022, 27, 4. https://doi.org/10.3390/molecules27010004
Chniti S, Kollár L, Bényei A, Takács A. Highly Selective Synthesis of 6-Glyoxylamidoquinoline Derivatives via Palladium-Catalyzed Aminocarbonylation. Molecules. 2022; 27(1):4. https://doi.org/10.3390/molecules27010004
Chicago/Turabian StyleChniti, Sami, László Kollár, Attila Bényei, and Attila Takács. 2022. "Highly Selective Synthesis of 6-Glyoxylamidoquinoline Derivatives via Palladium-Catalyzed Aminocarbonylation" Molecules 27, no. 1: 4. https://doi.org/10.3390/molecules27010004