- Academic Editor
Background: White matter injury (WMI) in basal ganglia usually induces
long-term disability post intracerebral hemorrhage (ICH). Kv1.3 is an ion channel
expressed in microglia and induces neuroinflammation after ICH. Here, we
investigated the functions and roles of Kv1.3 activation-induced inflammatory
response in WMI and the Kv1.3 blockade effect on microglia polarization after
ICH. Methods: Mice ICH model was constructed by autologous
blood injection. The expression of Kv1.3 was measured using immunoblot, real-time
quantitative polymerase chain reaction (RT-qPCR), and immunostaining assays.
Then, the effect of administration of 5-(4-Phenoxybutoxy) psoralen (PAP-1), a
selectively pharmacological Kv1.3 blocker, was investigated using open field test
(OFT) and basso mouse score (BMS). RT-qPCR, immunoblot, and enzyme-linked
immunosorbent assay (ELISA) were taken to elucidate the expression of
pro-inflammatory or anti-inflammatory factors around hematoma. PAP-1’s function
in regulating microglia polarization was investigated using immunoblot, RT-qPCR,
and immunostaining assays. The downstream PAP-1 signaling pathway was determined
by RT-qPCR and immunoblot. Results: Kv1.3 expression was increased in
microglia around the hematoma significantly after ICH. PAP-1 markedly improved
neurological outcomes and the WMI by reducing pro-inflammatory cytokine
accumulation and upregulating anti-inflammatory factors. Mechanistically, PAP-1
reduces NF-
Intracerebral hemorrhage (ICH) is a kind of life-threatening disease with high morbidity, and nearly every ICH survivor undergoes long-term disabilities [1, 2]. Researchers have suggested that long-term disabilities possibly originate from white matter injury (WMI) in the basal ganglia [3, 4]. Previous studies have shown that white matter integrity is a promotor for locomotion functional recovery [5, 6, 7]. Various strategies, such as the inhibition of ferroptosis with the administration of Dexpramipexole (DPX) [8], upregulation of brain-derived neurotrophic factor (BDNF) by lithium [9], and inactivation of microglia into M1 phenotype using Ambroxol [10], have been identified to accelerate functional recovery through mitigating WMI after ICH. Hence, reducing WMI is a potential strategy to improve post-ICH neurological impairments.
Microglia are the first-line innate immune cells in the central nervous system
(CNS) that respond to exogenous stimuli [11, 12]. Once activated, the microglia
served as both a blessing and a curse cause microglia’s activity is determined by
the composition ratio of two phenotypes, comprising M1-like (microglia that
promote inflammation) and M2-like (microglia that inhibit inflammation)
phenotypes [13]. After ICH, M1 microglia were activated through classical
activation from primary microglia and exaggerating the immune and inflammation
response. M2 microglia were formed through alternative activation of primary
microglia, attenuating inflammation reaction and boosting tissue repair after
injury [11, 13]. The M1-like microglia aim to enhance neuroinflammation and
induce neurocyte death [11, 13]. Cytokines like tumor necrosis factor-
Kv1.3 is a voltage-gated potassium channel that is highly expressed in the CNS
and immune systems [15, 16, 17]. Previous studies have indicated that in
neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s
disease (PD), Kv1.3 is upregulated [15, 16]. With the overexpression of Kv1.3,
microglia are shifted into the M1 phenotype resulting in an increase in
pro-inflammatory factors, including TNF-
Herein, we hypothesized that Kv1.3 blockade alleviated WMI by reducing microglia
M1 phenotype and enhancing microglia M2 phenotype polarization via inhibiting the
NF-
The National Institutes of Health Guide for the Care and Use of Laboratory Animals was followed for all experiments and treatments on animals. And all animal experiments were sanctified and supervised by the Ethics Committee of the Chongqing Southwest Hospital (approval no. AMUWEC20224059). A total of 118 adult C57BL/6J mice (male, 23–26 g, aged between 8–11 weeks, 112 were enrolled in experiments and 6 died) were acquired from the Southwest Hospital experiments animal center. Throughout the experiment, food and water were freely available to the mice. The feeding room has a constant temperature of 22–25 °C and humidity of 55–60%, and 12 hours dark/12 hours light circle.
The mice were stabilized on the stereotactic instrument (No.68001, RWD Life Science Corporation, Shenzhen, Guangdong, China) after proper anesthetization by a mixed 2% isoflurane/air (2–3 L/min). The mice ICH model was constructed according to the previous description [26]. Briefly, after performing a scalp incision and drilling a cranial hole, 16 µL autologous blood from the tail vein was harvested after tail shearing and pumped into the basal ganglia (from bregma: 2.1 mm lateral, 0.9 mm anterior, and 3.0 mm ventral) by a sterilized syringe (No.7000, Hamilton, Bonaduz, Glaubenden, Switzerland) at 2 µL/min. After injecting the blood, the syringe needle was fixed for five min to prevent blood back-flow. An animal surgery temperature control device (No.78600 Zhongshi Corporation, Beijing, China) was used to control mice’s body temperatures during surgery. Then, the weight of each mouse was measured. Mice experiment groups were randomly divided by a mice breeder who is blind to this study by means of a serious of random number. Needle insertion was the only procedure performed on the Sham mice. In the ICH group, 16 µL autologous blood from the tail vein was injected as mentioned. In the ICH + PAP-1 group, mice were further randomly divided by the dosage of PAP-1 (No.HY-10015, MedChemExpress, Monmouth Junction, NJ, USA) (20, 40, and 60 mg/kg/d). The PAP-1 was administrated via intraperitoneal injections immediately after ICH. PAP-1 was dissolved in dimethyl sulfoxide (DMSO) and corn oil with 0.05:0.95 before injection. Each mouse received a 2 mL mixture in one shot daily, and the injections continued for 3 or 7 days according to the experimental design. The ICH group only received the same volume of 2 mL mixed dimethyl sulfoxide and corn oil intraperitoneal injections.
Open field test (OFT) was used for mice behavioral changes evaluation, as described before [27]. Briefly, a field box was evenly divided into four sections adjacent to each other but isolated by a board. Each section was 50 cm square and 50 cm high. Each mouse was placed in one of the chambers for five min allowing free movement. Their activities were video recorded. Then, the chamber was cleaned with purified water and ready for the next test. The resultant videos were then analyzed by a software (VideoTrack, ViewPoint Behaviour Technology, Lyon, Rhone, France). The mean velocity of the movement was then calculated from the software by two examiners blinded to the group’s design.
Basso mouse score (BMS) is a scoring system used to evaluate mice’s joint movement and coordination [28, 29]. Each mouse was placed on a broad experimental table one by one for free movement under the observation of two investigators. After one min of observation, the score from each observer blind to group design was recorded. The scoring scale ranges from 0 to 9, and the significance of each point is described previously [28, 29].
Transmission electron microscopy (TEM) was performed to detect the integrity of
the white matter bundle as described [30]. Briefly, after perfusion, samples (1
mm
Briefly, a double antibody sandwich method was used in this study. The following
enzyme-linked immune-sorbent assay (ELISA) kits were used: Murine TNF-
Brain sections were obtained from the aforementioned freezing microtome and then perforated with 0.3% Triton-X 100 (No.P0090, Beyotime, Shanghai, China)for 30 min. Then, the 5% bovine serum album (BSA) was added for 2 h blocking at RT. Thereafter, the following primary antibodies were incubated at 4 °C overnight: Mouse anti-Iba-1 (1:200, No.ab283319, Abcam, Cambridge, Cambridgeshire, UK); Rabbit anti-CD16 (1:100, No.16559-1-AP, Proteintech, Philadelphia, PA, USA); Rabbit anti-CD206 (1:100, No.18704-1-AP, Proteintech, Philadelphia, PA, USA); Rabbit anti-iNOS (1:100, No.22226-1-AP, Proteintech, Philadelphia, PA, USA); Rabbit anti-Arg-1 (1:100, No.16001-1-AP, Proteintech, Philadelphia, PA, USA); Rabbit anti-Kv1.3 (1:200, No.14079-1-AP, Proteintech, Philadelphia, PA, USA); Mouse anti-MBP (1:200, No.sc-365701, Santa Cruz, Dallas, TX, USA); Rabbit anti-NFH (1:200, No.BM0100, Boster, Wuhan, Hubei, China). After incubation, the sections were washed thrice with PBS; each sample was washed for at least 5 min. Then, the following secondary antibodies were incubated for 2 h at RT: Goat anti-Mouse 488; Goat anti-Mouse 555; Goat anti-Rabbit 488; Goat anti-Rabbit 555. Then, brain sections were washed with PBS and DAPI (4’,6-diamidino-2-phenylindole) was used for nuclear staining for 5 min at RT. Afterwards, the neutral resin was administrated for mounting. A fluorescent microscope (Axioskop2, Carl ZEISS, Weimar, German) was then taken to record the images of the resultant samples. At least 3 independent samples were studied in each group. At least 3 sections were prepared for the evaluation of each sample. The images were investigated by Image J (V.1.8, NIH, Bethesda, MA, USA). At least 3 images of the peri-hematoma area were taken from each section for statistics.
Mouse brain peri-hematoma tissue was extracted from homogenate and centrifuged
at 4 °C, 12000 rpm 30 min after sacrifice. The protein concentration was also
detected by the Bicinchoninic Acid kit above.
Then, the protein samples were mixed with 5
After liquid nitrogen milling, the total RNA of mice hematoma ipsilateral brain
was extracted by Trizol reagent (No.DP451, Tiangen, Beijing, China). Then, an
extraction procedure was performed, followed by an extraction kit manual
(No.DP451, Tiangen, Beijing, China). After RNA concentration measurement and
dilution, RNA reverse-transcription was made by a reverse-transcription kit
(No.RR047A, Takara Bio Inc., Shiga, Japan) on a PCR system (V.1.5, Bio-Rad CFX
manager, Hercules, CA, USA). Thereafter, real-time fluorescence quantitative
polymerase chain reaction (RT-qPCR) was conducted using SYBR-green Premix Ex
Taq™ (No.RR820A, Takara Bio Inc., Shiga, Japan) on the system. In
brief, a total of 25 µL reaction system was added to the 96-well plates
followed by the manual (No.RR820A, Takara Bio Inc., Shiga, Japan) and then cycled
for 41 rounds under conditions prescribed in the manual. Each sample was studied
in triplicate. The threshold cycle (CT) values were collected, and the mRNA level
was calculated by 2
| Target genes | Forward sequence (5′–3′) | Reverse sequence (5′–3′) |
| Kv1.3 | GGGGCATTGCCATTGTGTC | AGGCGGGATAGTCTTTCTCATC |
| Kca3.1 | GCTCAACCAAGTCCGCTTC | GTGATCGGAATCAGCCACAGT |
| Kv1.1 | GTGATGTCGGGGGAGAATGTT | CCGGAGATGTTGATTACTACGC |
| Kv1.5 | TCCGACGGCTGGACTCAATAA | CAGATGGCCTTCTAGGCTGTG |
| Tnf- |
CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| Il-6 | TAGTCCTTCCTACCCCAATTTC | TTGGTCCTTAGCCACTCCTTC |
| Il-1 |
GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| Il-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| Bdnf | TCATACTTCGGTTGCATGAAGG | AGACCTCTCGAACCTGCCC |
| Ncf | CCAGTGAAATTAGGCTCCCTG | CCTTGGCAAAACCTTTATTGGG |
| Cd16 | CAGAATGCACACTCTGGAAGC | GGGTCCCTTCGCACATCAG |
| Cd32 | AGGGCCTCCATCTGGACTG | GTGGTTCTGGTAATCATGCTCTG |
| Inos | GTTCTCAGCCCAACAATACAAGA | GTGGACGGGTCGATGTCAC |
| Cd206 | CTCTGTTCAGCTATTGGACGC | CGGAATTTCTGGGATTCAGCTTC |
| Arg1 | CTCCAAGCCAAAGTCCTTAGAG | AGGAGCTGTCATTAGGGACAT |
| Tgf- |
CTCCCGTGGCTTCTAGTGC | GCCTTAGTTTGGACAGGATCTG |
| P65 | CTTCTGGGCCTTATGTGGAGATC | GGTCCTGTGTAGCCATTGATCTT |
| P50 | GATGGGACTACACCTCTGCATAT | AGGCTCATACGGTTTCCCATTTA |
| Actb | ACTGTCGAGTCGCGTCC | CTGACCCATTCCCACCATCA |
| Gapdh | CCTGGAGAAACCTGCCAAGTA | TCATACCAGGAAATGAGCTTGAC |
RT-qPCR, real-time quantitative polymerase chain reaction.
All data in this study were presented as mean
To determine which subunit of voltage-gated potassium channel plays the dominant role in enhancing neuroinflammation around the post-ICH hemorrhage, the mRNA expression of several subunits belonging to voltage-gated potassium channel was investigated using RT-qPCR (Fig. 1). Results demonstrated an increased expression of Kv1.3 mRNA from day 1 to day 7, especially on day 3, while the Kv1.3 mRNA expression exhibited no notable difference on day 14 between the Sham group and the ICH group (Fig. 2A). Moreover, the expression of other potassium channels exhibited no significant difference, such as Kv1.1, Kv1.5, and Kca3.1, between the two groups (Fig. 2A). Subsequently, the immunoblot bands indicated that in the ICH group, Kv1.3 protein expression was higher than that in the Sham group on day 3 (Fig. 2B,C). Next, the immunostaining images revealed higher optical densities of Kv1.3 in the ICH group than that in the Sham group around hematoma (Fig. 2D,E). Collectively, these results demonstrated that Kv1.3’s expression was markedly enhanced around the hematoma following ICH. The highest expression of Kv1.3 is on day 3 of ICH among the checkpoints of days 1, 3, 7, and 14.
 Fig. 1.
Fig. 1.Schematic illustration showing the experimental design and timeline in the current study. ICH, intracerebral hemorrhage; PAP-1, 5-(4- Phenoxybutoxy) psoralen.
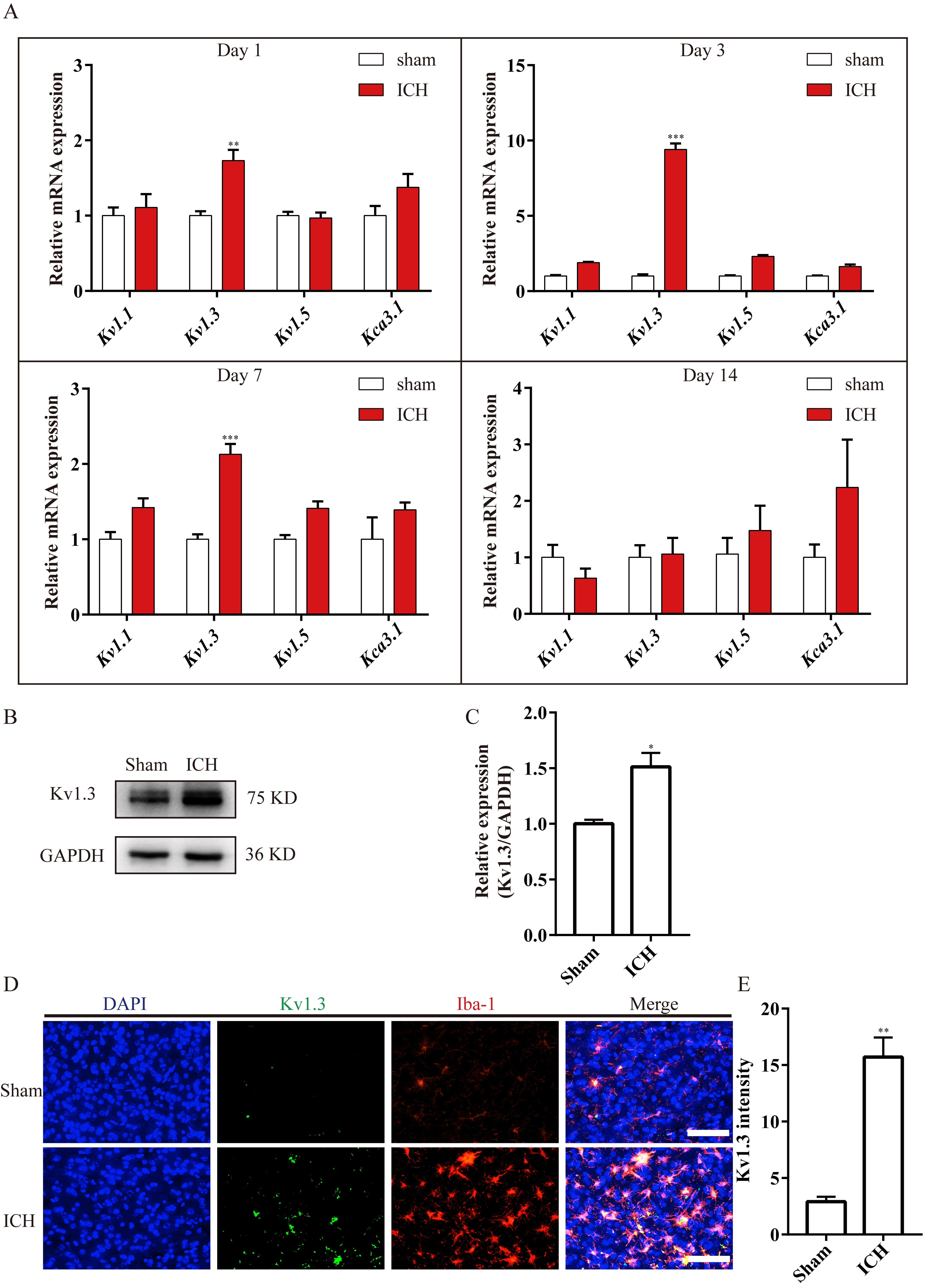 Fig. 2.
Fig. 2.Kv1.3 expression was significantly increased after ICH. (A) Bar
charts illustrating the Kv1.1, Kv1.3, Kv1.5, and
Kca3.1 mRNA expressions in Sham and ICH groups at different time points.
n = 3 each group,
The aforementioned results revealed that Kv1.3 expression was enhanced in microglia in ICH mice. Considering that Kv1.3 blockage using PAP-1 exerts a neuroprotective effect in neurodegenerative diseases and ischemic stroke [15, 16, 19], the different dosages (20, 40, and 60 mg/kg) of PAP-1 were used to test the neuroprotective effect of Kv1.3 blockage on ICH mice. The weight measurement and behavioral tests were conducted to validate the time-point and dosage for further research on days 1, 3, 7, and 14 (Fig. 1). Then the various examinations were performed on day 3 in the current study (Fig. 1).
First, the mice in the ICH + PAP-1 group weighed more than those in the ICH group, and the dosages of 40 and 60 mg/kg showed a better weight gain effect than the dosage of 20 mg/kg from day 3 to 14 (Fig. 3A). Next, the curves collected from BMS indicated that mice that received PAP-1 treatment exhibited higher scores than that in the ICH group, and the scores in ICH + PAP-1 40 mg/kg and ICH + PAP-1 60 mg/kg groups were higher than that in ICH + PAP-1 20 mg/kg group from day 3 to 14 (Fig. 3B). Subsequently, the results obtained from OFT revealed that the mean velocity of mice in OFT in the ICH group decreased significantly after ICH, whereas the same was considerably increased with the application of PAP-1 (Fig. 3C,D). Meanwhile, mice in ICH + PAP-1 40 mg/kg and ICH + PAP-1 60 mg/kg groups showed increased mean velocity than that in ICH + PAP-1 20 mg/kg group (Fig. 3C,D). Collectively, these results indicated that PAP-1 administration improved neurological deficits after ICH. The dosage was 40 mg/kg, and the checkpoint was day 3 for future experiments in the present experiments.
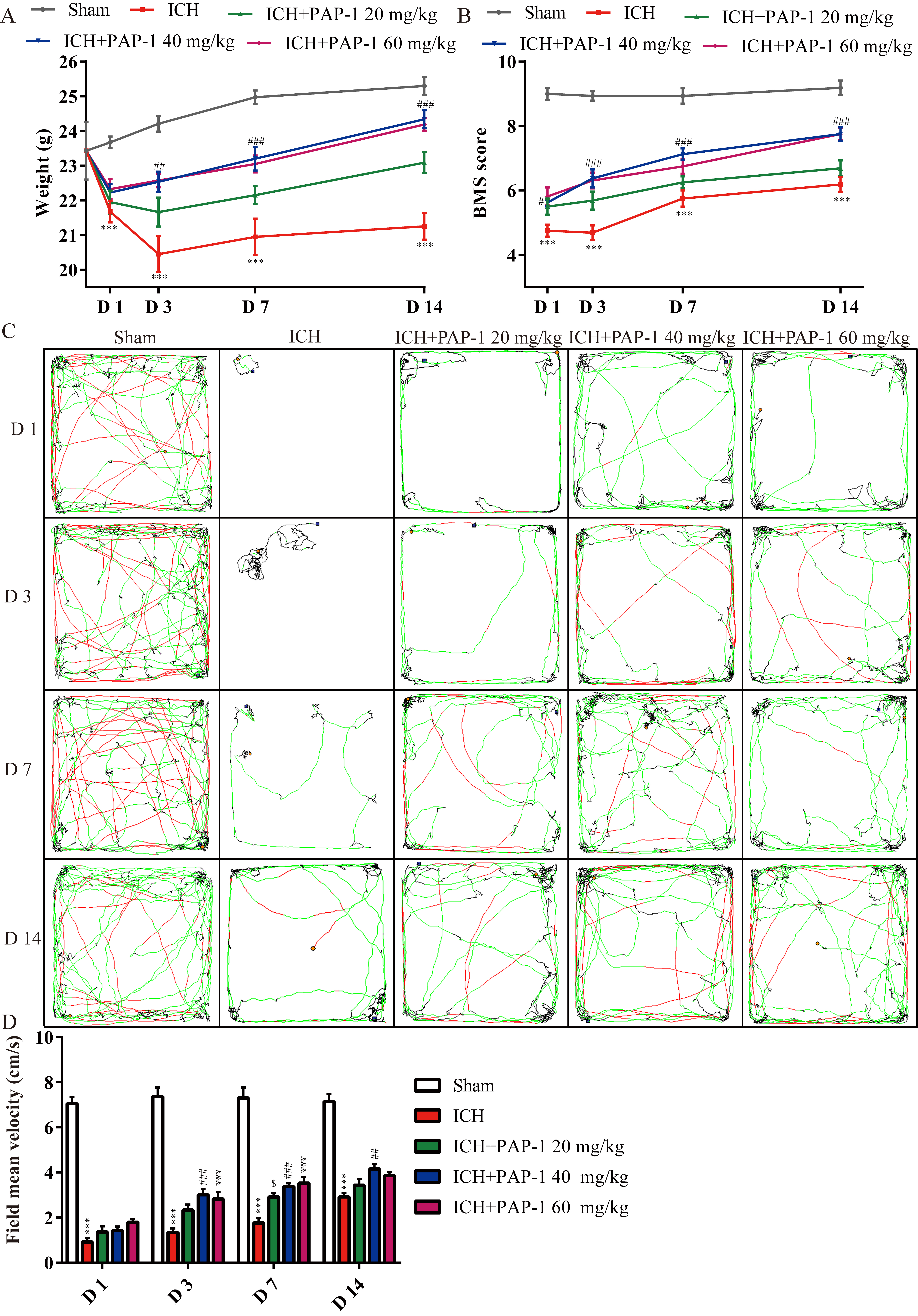 Fig. 3.
Fig. 3.Application of PAP-1 facilitated neurological outcomes after
ICH. (A) Weight variation curves of mice in each group. n = 8 each group,
The integrity of white matter bundles contributes greatly to neurological outcomes after ICH. Results indicated that the effect of PAP-1 administration on WMI was elucidated on day 3 following ICH. The immunostaining results of MBP and NF200 depicted that the optical density of MBP and NF200 decreased around hematoma on day 3 after ICH, while the treatment of PAP-1 partially ameliorated this phenomenon (Fig. 4A–C). Next, the immunoblot results elucidated a downregulated myelin basic protein (MBP) expression in the ICH group, whereas the application of PAP-1 abrogated this effect (Fig. 4D,E). Furthermore, the integrity of the white matter bundle was determined by TEM. As a result, the ICH group had a much greater G-ratio for the myelin sheath. In contrast, PAP-1 treatment markedly decreased this phenomenon (Fig. 4F,G). Combinedly, these results implied that ICH caused WMI, and the application of PAP-1 alleviated ICH-induced WMI in mice.
 Fig. 4.
Fig. 4.Administration of PAP-1 reduced ICH-induced white matter injury.
(A) Immunostaining of MBP (red) and NF200 (green) in each group on day 3 after
ICH. Scale bars: 50 µm. (B) Summarized bar graph depicting MBP expression
from (A). n = 3 each group,
To decipher why PAP-1 administration reduced WMI, a further investigation of
anti-inflammatory and pro-inflammatory mediator levels was conducted. Initially,
the results obtained using RT-qPCR assays on day 3 after ICH corroborated that
Tnf-
 Fig. 5.
Fig. 5.Administration of PAP-1 decreased the pro-inflammatory factors
around hematoma after ICH. (A) Bar charts illustrating Tnf-
Subsequently, the bar charts showed that the mRNA expressions of anti-inflammatory cytokines, including nerve growth factor (Ngf), Il-10, and brain-derived neurotrophic factor (Bdnf), were increased after ICH, and their expressions were notably enhanced with PAP-1 administration (Fig. 6A). Furthermore, immunoblot shows elevated IL-4 and IL-10 protein expressions, and this effect was markedly enhanced with the PAP-1 application on day 3 after ICH (Fig. 6B–D). Thereafter, the ELISA findings demonstrated that the content of IL-4 and IL-10 were prominently increased, and this effect was reinforced with the administration of PAP-1 (Fig. 6E,F). Taken together, these results verified that PAP-1 improved the neuro-inflammatory microenvironment by decreasing the accumulation of pro-inflammatory factors and enhancing the deposition of anti-inflammatory factors on day 3 after ICH.
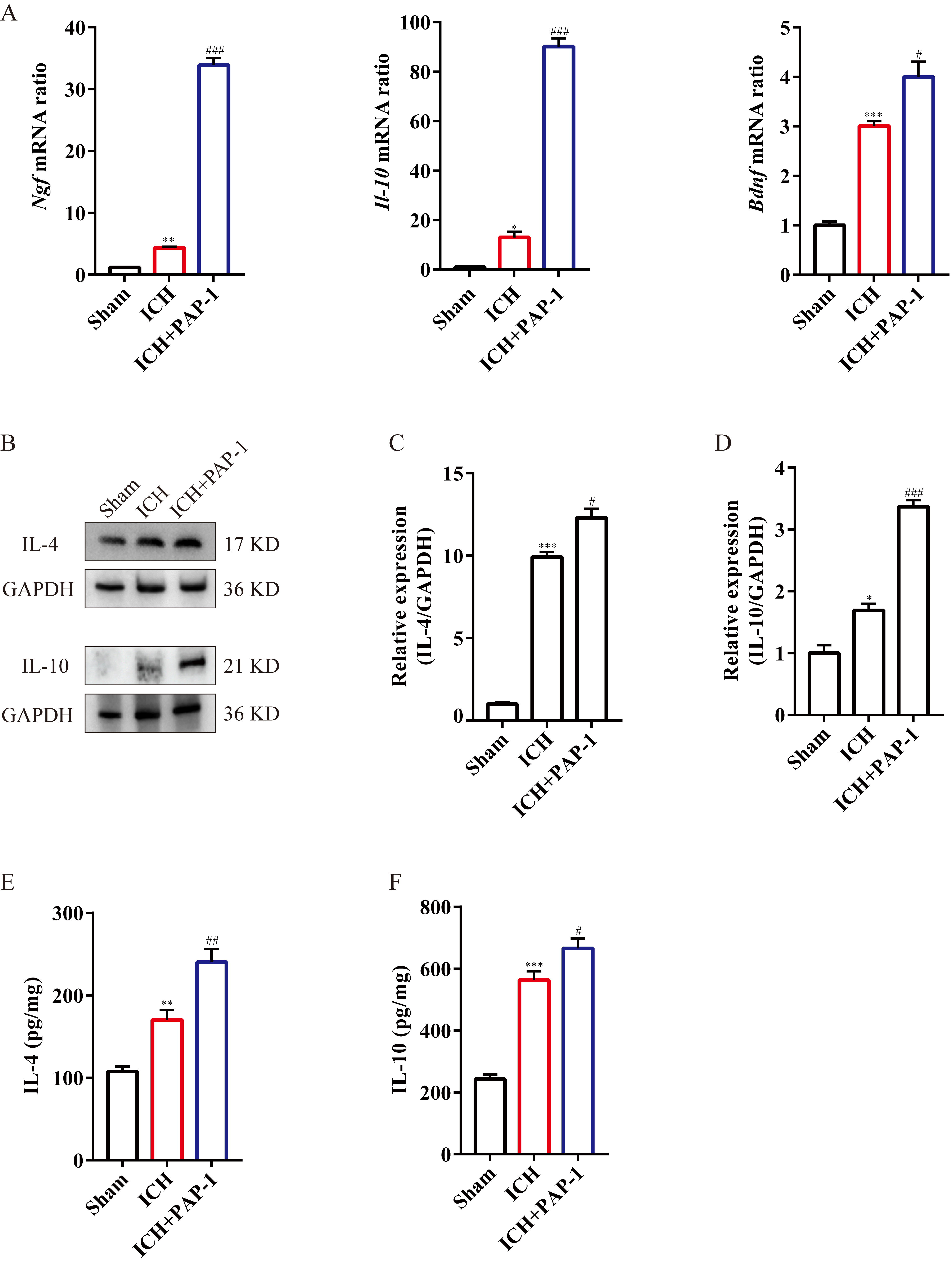 Fig. 6.
Fig. 6.The application of PAP-1 increased the anti-inflammatory
cytokines around hematoma after ICH. (A) Bar charts illustrating Ngf,
Il-10, and Bdnf mRNA expression in each group on day 3 after
ICH. n = 3 each group,
The above findings confirmed that the application of PAP-1 improved the post-ICH
neuro-inflammatory niche. Because the microglia transformation of M1/M2
phenotypes exerted the aforementioned effect, the polarization of microglia was
investigated. First, the results indicated that the M1-microglia markers
Cd16, Cd32, and Inos mRNA expression were
substantially upregulated after ICH, and PAP-1 abrogated this effect on day 3
(Fig. 7A). Then, the immunoblot implied that CD16 and iNOS protein expression
exhibited the same tendency as the mRNA expression level (Fig. 7B–D).
Afterwards, the immunostaining images indicated that the Iba1
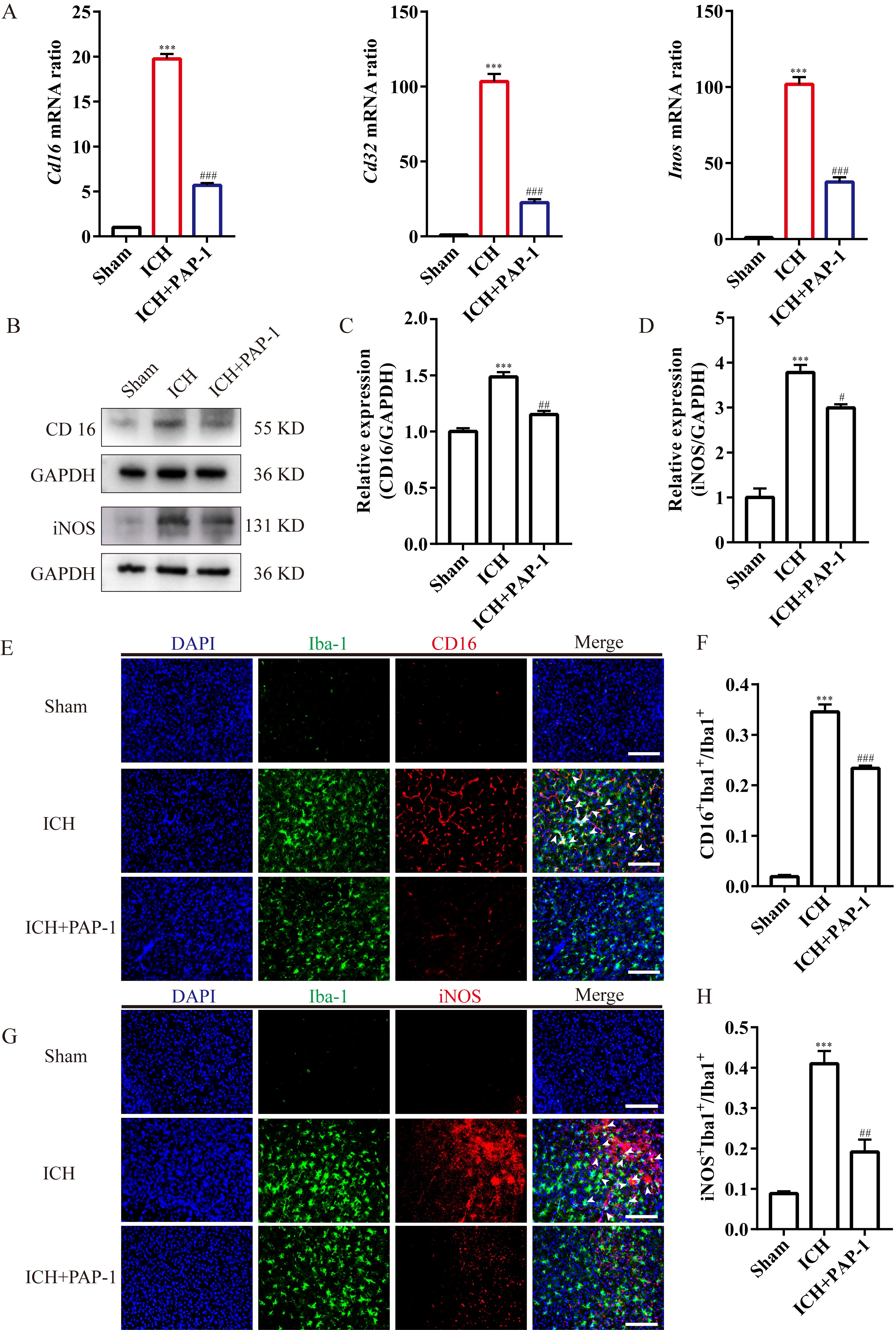 Fig. 7.
Fig. 7.Application of PAP-1 reduced the M1-like microglia around the
hematoma after ICH. (A) Bar charts illustrating Cd16, Cd32, and
Inos mRNA expression in each group on day 3 after ICH. n = 3 each group,
Next, the bar graphs implied that the M2-like microglia markers’ mRNA expression
levels, including Cd206, Arg-1, and Tgf-
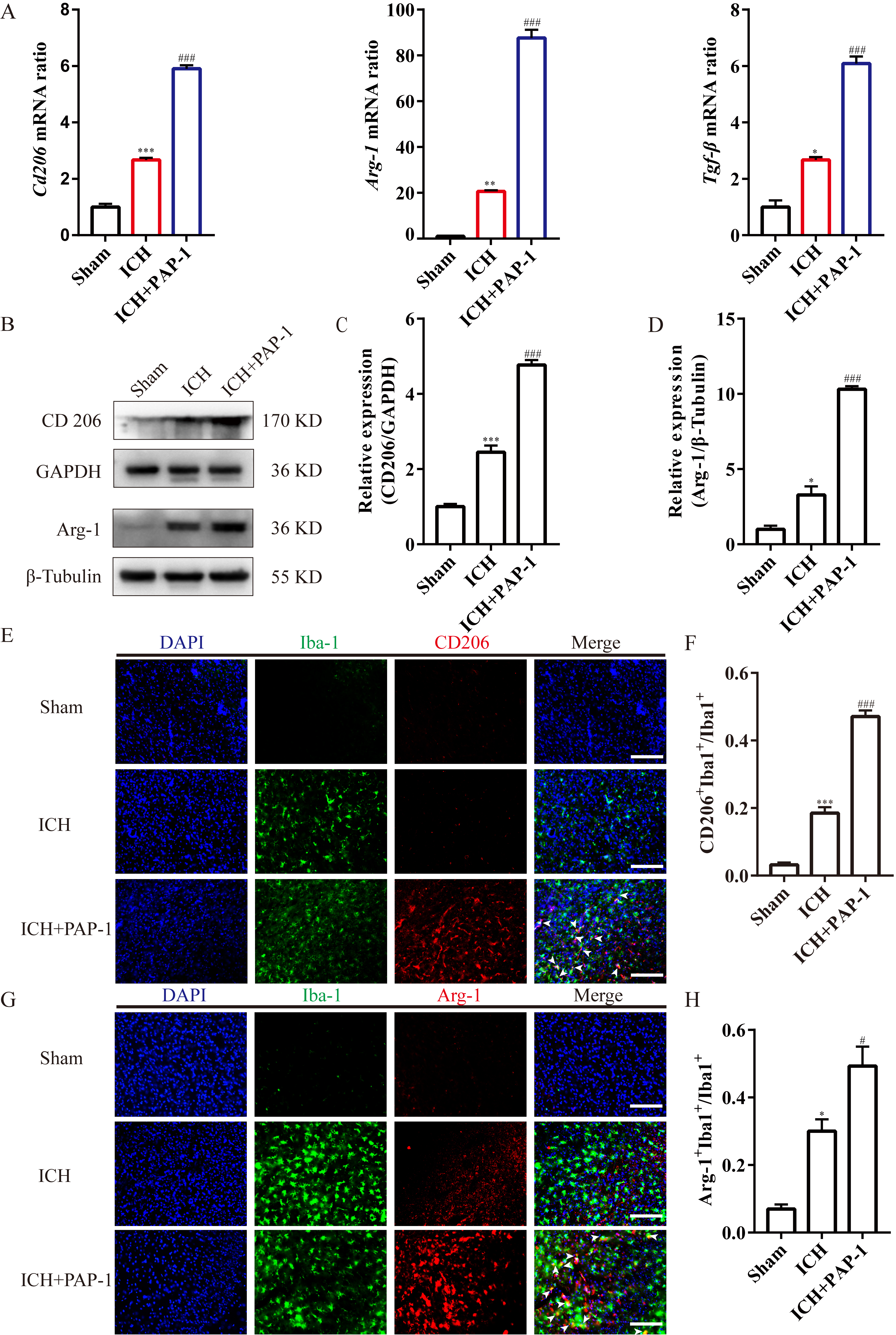 Fig. 8.
Fig. 8.Application of PAP-1 increased the M2-like microglia around the
hematoma after ICH. (A) Bar charts showcasing Cd206, Arg-1, and
Tgf-
In previous studies, the NF-
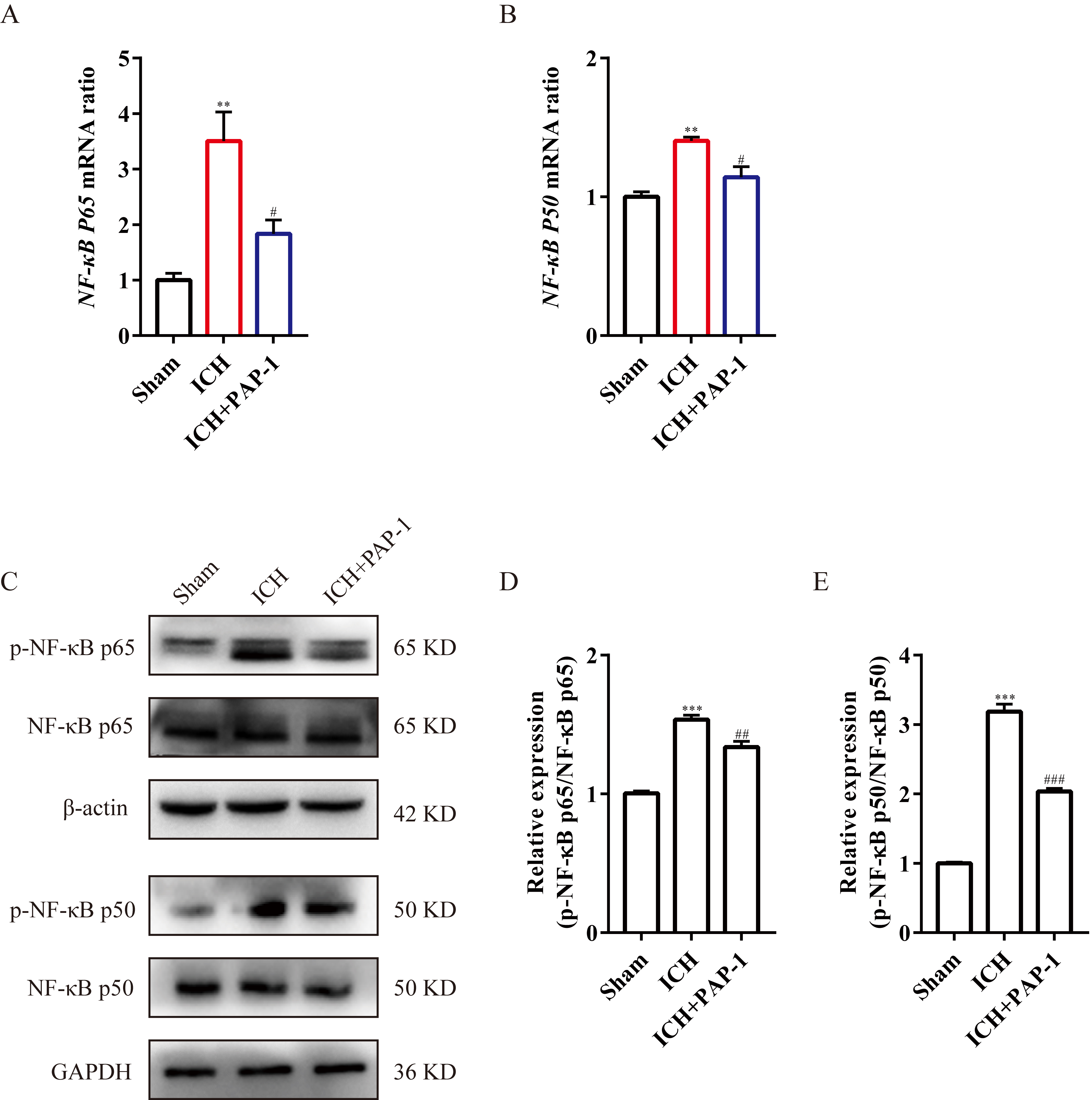 Fig. 9.
Fig. 9.Application of PAP-1 inhibiting NF-
ICH usually causes long-term disability due to WMI in the basal ganglia [34, 35]. Facilitating microglia polarization to M2 phenotype around hematoma is an
underlying and feasible therapeutic strategy for alleviating WMI owing to a
reduced accumulation of pro-inflammatory factors and increased deposition of
anti-inflammatory cytokines [36, 37]. The results in this study illustrated that
Kv1.3 expression increased around hematoma after mice ICH (Fig. 10). Furthermore,
the administration of PAP-1, a pharmacological inhibitor of Kv1.3, markedly
reduced the accumulation of TNF-
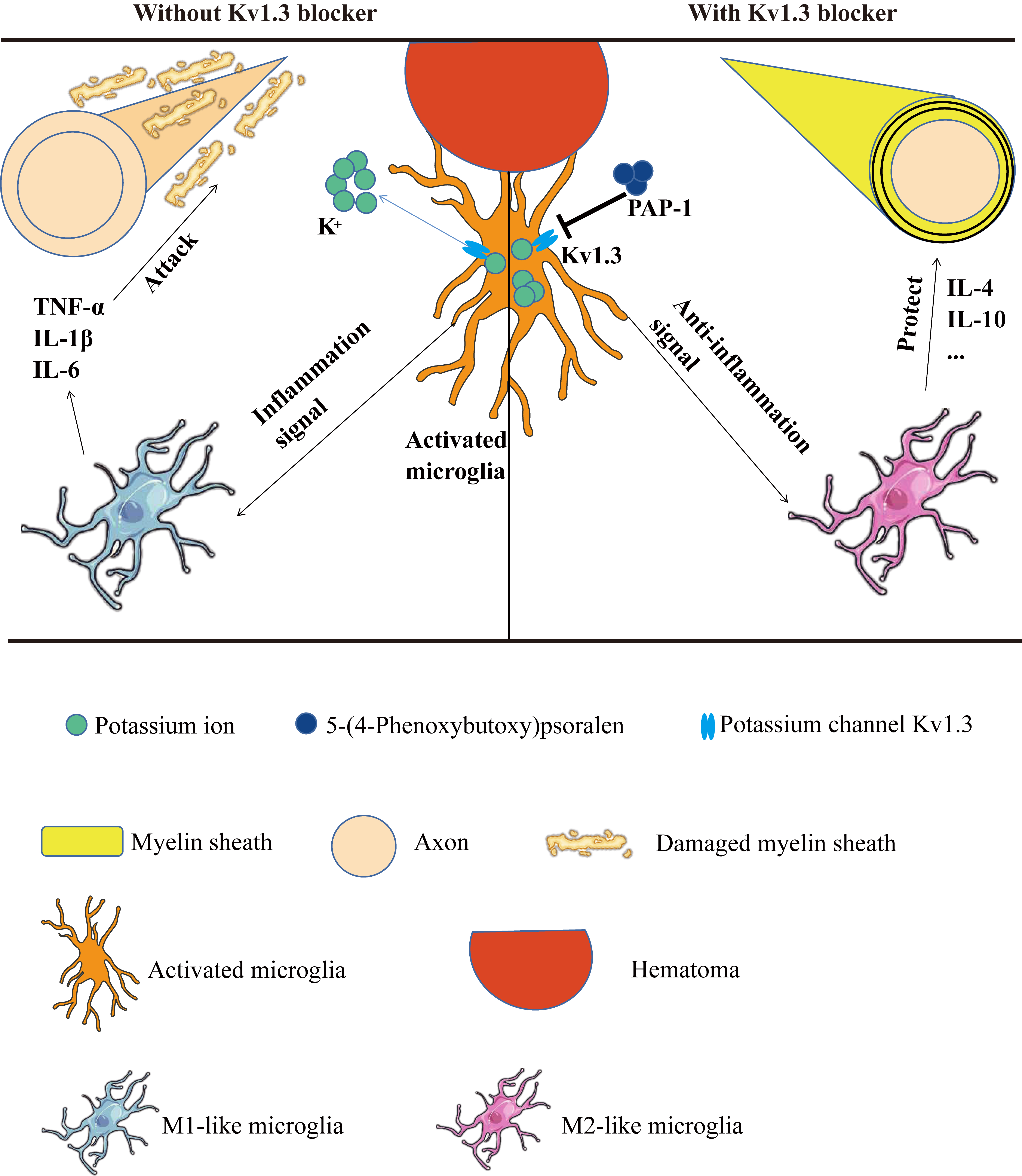 Fig. 10.
Fig. 10.ICH-induced WMI could be inhibited by PAP-1 by reshaping M1/M2
microglia phenotype. WMI, White matter injury; TNF-
After ICH, neuroinflammation begins immediately following hematoma formation,
exerting an active defense to respond to secondary brain injury [38, 39, 40]. It is to
be noted that microglia are the main immune cells responsible for
neuroinflammation [41, 42]. Microglia are activated into an M1-like phenotype
after brain injury and produce pro-inflammatory factors, such as TNF-
Kv1.3 is a subtype of voltage-gated potassium channel responsible for activating
immune cells by regulating membrane potential [16, 17]. Kv1.3 is mainly located
on microglia, astrocytes, and macrophages, playing an evident role in responding
to inflammatory stimuli in several diseases like Type 1 diabetes, multiple
sclerosis (MS), asthma, and rheumatoid arthritis [17, 53, 54, 55]. Furthermore,
studies have shown that Kv1.3 activation is related to the propagation of
neuroinflammation by increasing the secretion of pro-inflammatory cytokines
(IL-1
Targeting Kv1.3 is a potential strategy to maintain the integrity of white
matter bundles in mice ICH models. The reasons for the WMI ameliorated by PAP-1
might be due to several beneficial effects, except for the suppression of
neuroinflammation after ICH. First, Kv1.3 blockade suppresses the NF-
The results of the current study illustrate that the Kv1.3 expression is enhanced in microglia around hematoma after ICH. In addition, Kv1.3 blockade using PAP-1 evidently reduces the accumulation of pro-inflammatory factors and upregulates the deposition of anti-inflammatory and neurotrophic factors by facilitating microglia polarization into M2-like microglia. The present study provides a feasible target for facilitating microglia transformation into an M2-like phenotype and offers evidence that PAP-1 administration bears the potential to ameliorate WMI by suppressing neuroinflammation in ICH treatment.
ICH, intracerebral hemorrhage; WMI, white matter injury; RT-qPCR, real-time
quantitative polymerase chain reaction; PAP-1, 5-(4-Phenoxybutoxy) psoralen; OFT,
open field test; BMS, basso mouse score; ELISA, enzyme-linked immunosorbent
assay; DPX, dexpramipexole; BDNF, brain-derived neurotrophic factor; CNS, the
central nervous system; TNF-
All raw data in this research are available upon reasonable request.
HFG, YY, and HF conceived and designed the study. BW, JC, SHW, and LC performed the ICH model and RT-qPCR. BW, SHW, and YLZ performed the immunoblot blots and immunostaining. BW, XYZ, and TYZ prepared the figures. JZ and CZ analyzed data. YJH conducted TEM. BW and YY prepared the manuscript draft. BW and HFG wrote the paper. HF revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the Ethics Committee of the Southwest Hospital, Third Military Medical University, and Conformed to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (approval no. AMUWEC20224059).
Not applicable.
This work was supported by the National Natural Science Foundation of China (Nos. 82271424, 81802509, and 82001321) and the Natural Science Foundation of Chongqing (2022NSCQ-MSX4518).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
