Introduction
Global expansion of the human population has resulted in rapid declines of biodiversity (Ceballos et al., Reference Ceballos, Ehrlich and Raven2020). To offset biodiversity losses, reintroductions and translocations are increasingly used to re-establish wildlife populations in areas where they were previously extirpated (Bubac et al., Reference Bubac, Johnson, Fox and Cullingham2019). Large carnivores have experienced dramatic contractions of their geographical range and subsequent population declines in the past 2 centuries (Ripple et al., Reference Ripple, Estes, Beschta, Wilmers, Ritchie and Hebblewhite2014). They are now amongst the most commonly reintroduced taxa (Seddon et al., Reference Seddon, Soorae and Launay2005), with reintroductions aiming to recover parts of their historical ranges and restore ecosystem functions (Ripple et al., Reference Ripple, Estes, Beschta, Wilmers, Ritchie and Hebblewhite2014; Atkins et al., Reference Atkins, Long, Pansu, Daskin, Potter and Stalmans2019).
In South Africa, the historical loss of wildlife, and the potential financial value of large carnivores through ecotourism (Di Minin et al., Reference Di Minin, Fraser, Slotow and MacMillan2013), have led to an increase in reintroductions into fenced protected areas (Hayward et al., Reference Hayward, Adendorff, O'Brien, Sholto-Douglas, Bissett and Moolman2007a). A managed metapopulation approach has resulted in population increases in lions Panthera leo (Ferreira & Hofmeyer, Reference Ferreira and Hofmeyr2014), cheetahs Acinonyx jubatus (Buk et al., Reference Buk, van der Merwe, Marnewick and Funston2018) and African wild dogs Lycaon pictus (Davies-Mostert et al., Reference Davies-Mostert, Mills and Macdonald2015), and has provided opportunities to expand the ranges of large carnivores in various countries. Although pre-release procedures for carnivores have been refined over time to maximize success (e.g. so-called soft releases; Hayward et al., Reference Hayward, Adendorff, O'Brien, Sholto-Douglas, Bissett and Moolman2007a), few studies have evaluated post-reintroduction movements, settlement and their effects on the early establishment of founder populations (Hunter, Reference Hunter1998; Yiu et al., Reference Yiu, Keith, Karczmarski and Parrini2015; Briers-Louw et al., Reference Briers-Louw, Verschueren and Leslie2019).
Successful population establishment requires the settlement of a sufficient number of dispersing individuals that go on to develop a viable population based on the species’ life histories (Hovestadt & Poethke, Reference Hovestadt and Poethke2005). Reintroductions are essentially forced dispersals; translocated animals have to adjust to novel resources and environmental conditions (Berger-Tal & Saltz, Reference Berger-Tal and Saltz2014), and balance a trade-off between exploration and exploitation of their new habitat (Stamps & Swaisgood, Reference Stamps and Swaisgood2007). Translocations may fail if released individuals range over large areas, do not establish individual home ranges or have low survival and reproductive rates (Armstrong & Seddon, Reference Armstrong and Seddon2007; Stamps & Swaisgood, Reference Stamps and Swaisgood2007). Therefore, careful monitoring of early post-release movements and population establishment is crucial to assess reintroduction success (Armstrong & Seddon, Reference Armstrong and Seddon2007).
Cheetahs were extirpated in Malawi by the early 1990s (Purchase & Purchase, Reference Purchase and Purchase2007). In 2015 a public–private partnership between African Parks and the Malawi Department of National Parks and Wildlife to manage Liwonde National Park (hereafter Liwonde) made reintroductions of large carnivores possible. Liwonde was selected to be the first protected area to reintroduce cheetahs in Malawi as it was well protected and harboured an abundant prey base (7,296 small to medium-sized antelopes counted in a 2016 aerial survey; C. Reid, pers. comm., 2019) and depleted competitor populations (an estimated 25 spotted hyenas Crocuta crocuta, and initially no lions or leopards Panthera pardus; C. Reid, pers. comm., 2019). Here, we investigated the early post-release movements, settlement, survival and reproduction of seven cheetahs reintroduced to Liwonde. We expected distances of daily movements to be highest immediately after release, with a decrease over time and subsequent stability. Stabilization of daily movement distances, and movements within a gradually more confined area are indicative of home range development (Börger & Fryxell, Reference Börger, Fryxell, Clobert, Baguette, Benton and Bullock2012) and thus indicate settlement. We also compared survival and reproduction parameters to those of source populations to examine population establishment and early-stage reintroduction success.
Study area
We monitored post-release movements of reintroduced cheetahs in Liwonde National Park in southern Malawi (Fig. 1). The Park covers 548 km2 and its boundary is fenced with a 2.4-m high fence (Bonnox, Johannesburg, South Africa) consisting of eight electric wires. Maximum temperature ranges from 28 °C in July to 40 °C in November, and mean annual rainfall is 944 mm, primarily occurring during December–March (Bhima & Dudley, Reference Bhima and Dudley1996). In the wet season, the perennial Shire River, which runs through the Park, creates extensive lagoons and marshlands on its floodplains. Only a few pools of water remain scattered throughout the Park by September, the peak of the dry season. Dry deciduous woodland with Colophospermum mopane occupies the majority of the Park.
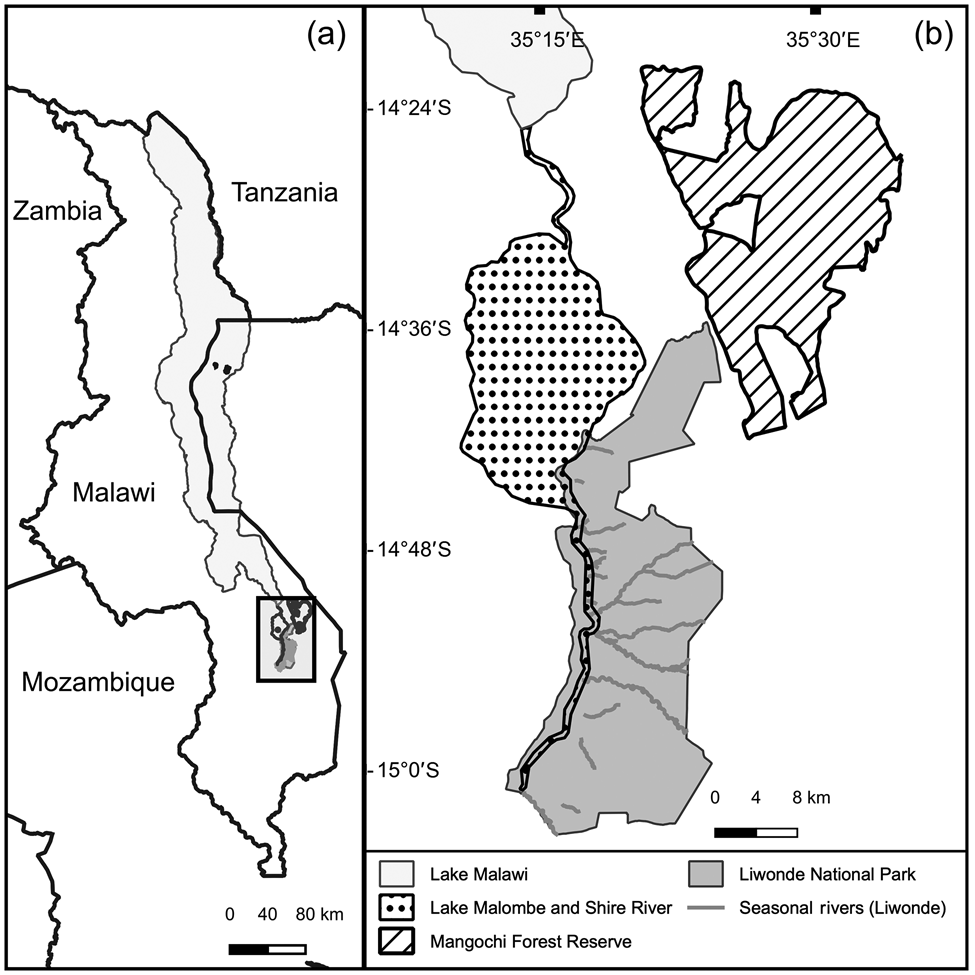
Fig. 1 (a) Location of the study area in Malawi. (b) Liwonde National Park and Mangochi Forest Reserve, and relevant water bodies.
Methods
Pre-release management
A total of seven cheetahs (four males and three females; Table 1) were released in Liwonde in various combinations on four occasions during June 2017–February 2018. To ensure genetic diversity of the founder population, cheetahs were sourced from five protected areas in South Africa through the Endangered Wildlife Trust's Cheetah Metapopulation Project. Prior to release, three females and two males were fitted with Pinnacle LITE GPS satellite collars (465 g; Sirtrack, Hawkes Bay, New Zealand), and one male within a coalition of two related males was fitted with a VHF tracking collar (253 g; African Wildlife Tracking, Pretoria, South Africa). Collars weighed 0.4–1.3% of the animals’ body weight. All individuals were kept in temporary holding enclosures (bomas; 50 × 50 m) for 23–58 days before being released into the reserve. A group of three siblings was held together in a boma, as were two non-sibling individuals, CF1 and CM1. CF1 had been orphaned and subsequently captured for relocation at a young age, and it was hoped the bonding afforded by co-housing with CM1 would facilitate reintroduction success for CF1. Once individuals no longer demonstrated stress behaviours such as pacing, they were released from the boma through coaxing with a final feed, and the gate was closed to ensure they did not return to the boma. Animals were not fed post-release.
Table 1 Biological and translocation details of the seven cheetahs Acinonyx jubatus reintroduced into Liwonde National Park, Malawi, over four reintroduction events during 2017–2018.
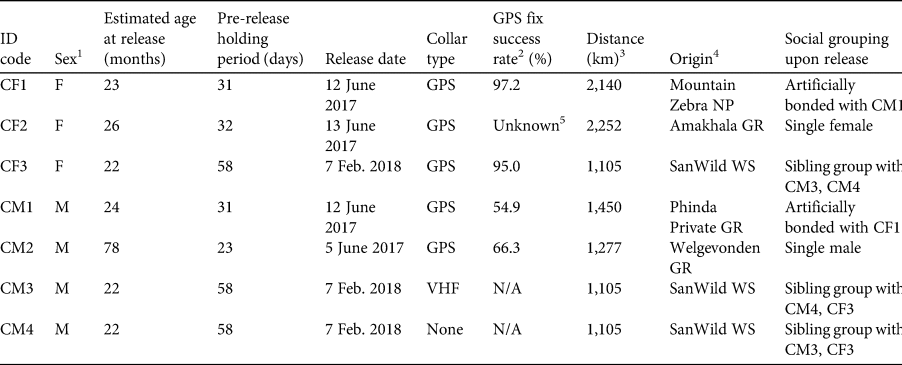
1 F, female; M, male.
2 Per cent of successful fixes of the number of attempted fixes.
3 Euclidian distance from origin to release location.
4 GR, Game Reserve; NP, National Park; WS, Wildlife Sanctuary. All origin locations are in South Africa.
5 Damage to collar prevented the download of data.
Post-release monitoring
We tracked the cheetahs’ post-release movements during June 2017–July 2019. GPS collars collected a minimum of three GPS fixes per day (at 05.00, 06.00, 12.00). Frequency of GPS fixes was increased when animals had cubs or were injured, to monitor them more closely during these periods. To assess survival, we checked physical condition at least twice per week, locating cheetahs through VHF tracking (receiver: Communication Specialists, Orange, USA; H-Type antenna: Telonics, Mesa, USA). We supplemented these observations with opportunistic sightings. We also inspected dens within the first 2 weeks of denning to record location, litter size and cub survival. To minimize disturbance, dens were checked by a single person while the female was hunting, and we did not handle the cubs (Laurenson & Caro, Reference Laurenson and Caro1994). To replace GPS collars before battery depletion, animals were immobilized by chemical capture and then re-fitted with VHF collars (Sirtrack, Hawkes Bay, New Zealand) modified with a long-range geolocation transmitter (LoRa; Smart Parks, Rotterdam, The Netherlands). Re-fitted collars (359 g) facilitated continued checks of physical condition and dens.
Data analysis
We analysed the first year (range 297–365 days) of post-release movements for five cheetahs. Two cheetahs (CM3 and CM4) were omitted from the analysis because of insufficient data from the VHF collars. To ensure consistent sampling across individuals, we selected the GPS fix closest to 12.00 for analysis. Although two mixed-sex groupings were released from the boma, the females separated from their groupings 2 and 19 days post-release, respectively; we therefore analysed each GPS-collared animal as a singleton adult or female with dependent cubs. We conducted all analyses in R 3.5.1 (R Core Team, 2018). We also compared demographic parameters to those documented within source populations of the Cheetah Metapopulation Project.
Post-release exploration
We investigated initial post-release exploration by calculating daily displacement for each individual as the Euclidean distance between two successive locations in a 24-hour cycle using the adehabitatLT package in R (Calenge, Reference Calenge2006). We developed a linear mixed model using the lme4 package in R (Bates et al., Reference Bates, Maechler, Bolker and Walker2015), to assess drivers of post-release exploration. Variables included in the model were sex, age at translocation, duration of the pre-release holding period in Liwonde, and days since release. We included individual identity as a random intercept.
Settlement
For the first year post-release, we calculated progressive home ranges for each of the five cheetahs, to determine the duration of exploratory behaviour and time of settlement (Weise et al., Reference Weise, Lemeris, Munro, Bowden, Venter, van Vuuren and van Vuuren2015a). We defined progressive home ranges as the minimum convex polygon around GPS fixes over a moving window shifting continuously by 1 day. Because of collar failure, we used a moving window of 11 days for the calculation of a minimum convex polygon based on a minimum of five GPS fixes. We used 100% minimum convex polygons as they include potential exploratory forays of interest. We also calculated the net squared displacement between each location and the first location collected post-release to determine site fidelity and settlement (Börger & Fryxell, Reference Börger, Fryxell, Clobert, Baguette, Benton and Bullock2012). We visually assessed the progressive minimum convex polygon and net squared displacement curves to identify when patterns of space use stabilized, indicative of individuals having settled, similar to natal dispersers (Fattebert et al., Reference Fattebert, Robinson, Balme, Slotow and Hunter2015; Weise et al., Reference Weise, Lemeris, Munro, Bowden, Venter, van Vuuren and van Vuuren2015a,Reference Weise, Lemeris, Stratford, van Vuuren, Munro and Crawfordb).
Results
Post-release exploration
Males moved longer daily distances (3.6 ± SD 3.3 km, n = 2) than females (1.2 ± SD 1.2 km, n = 3; β male = 2.5, 95% CI = 1.99–3.14, P < 0.001; Fig. 2). Older individuals moved shorter daily distances than younger ones (β age = −0.008, 95% CI = −0.015–0.002, P = 0.01), and cheetahs moved shorter distances as more time elapsed post-release (β time = −0.005, 95% CI = −0.006–0.004, P < 0.001). Duration of pre-release holding periods did not significantly affect daily movements post-release (Table 2).
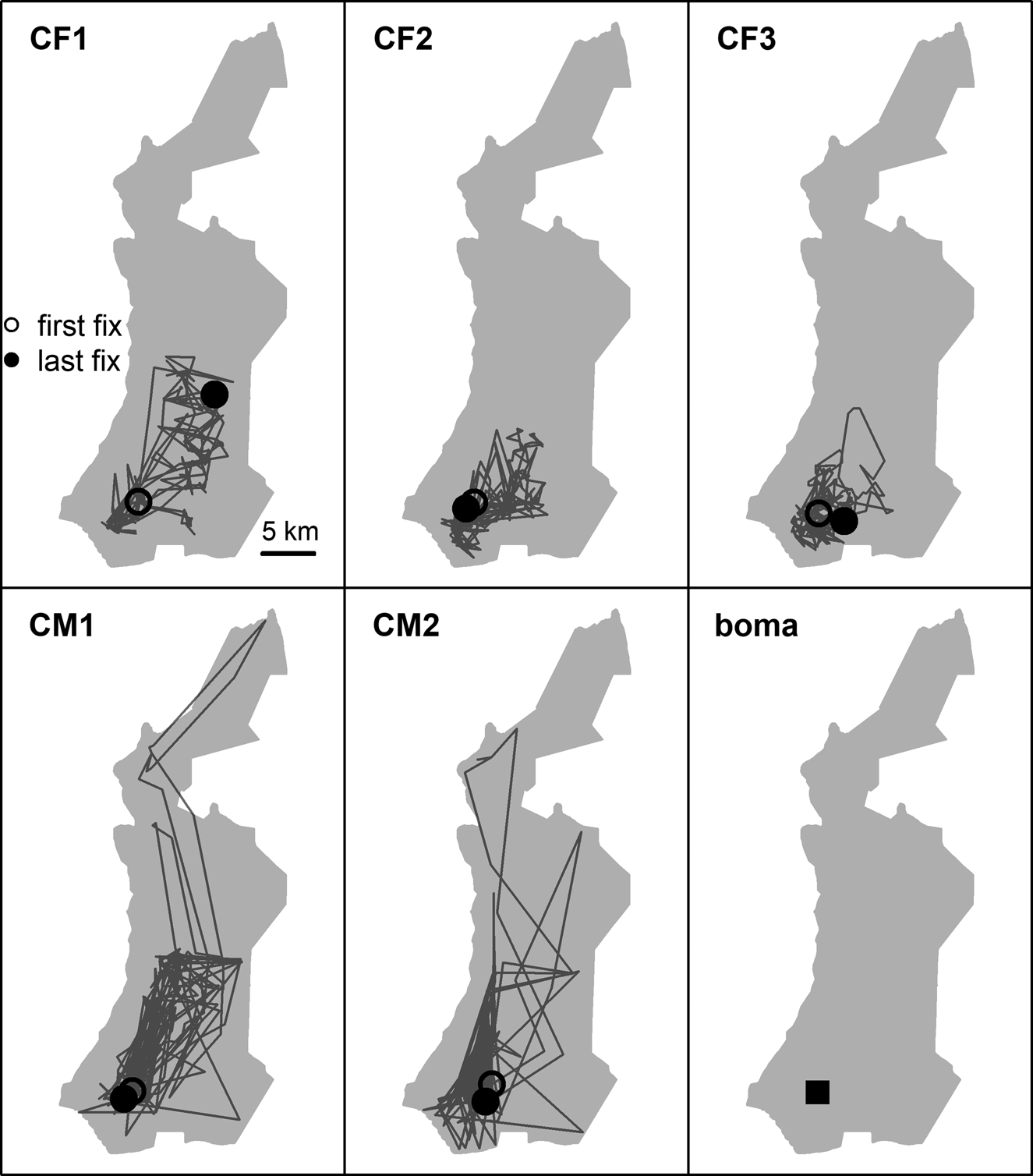
Fig. 2 First year post-release movements of female (CF) and male (CM) cheetahs Acinonyx jubatus reintroduced into Liwonde National Park (shaded area), Malawi, during 2017–2018, and the location of their release site (boma). Only the five individuals fitted with GPS collars are represented.
Table 2 Fixed-effect β coefficients, associated standard errors (SE) and 95% confidence intervals (95% CI) of a linear mixed model investigating factors affecting daily distance moved post-release of five cheetahs reintroduced into Liwonde National Park, Malawi, during 2017–2018. The model was fitted with individual identity as a random intercept. Coefficients for which the 95% CI does not overlap zero are deemed significant.
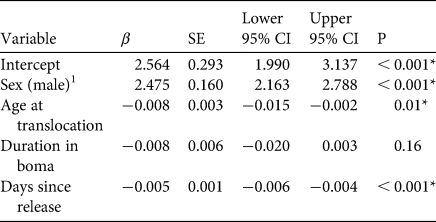
1 Female was the reference category.
* Variables with significant influence.
Settlement
Reintroduced GPS-collared cheetahs (n = 5) developed home ranges and settled on average 103 ± SD 68 days post-release (range 29–174 days). The three females developed home ranges within 3 months post-release (55 ± SD 23 days; 29–73 days; Figs 3 & 4). Before the estimated start of the denning period, both CF1 and CF2 demonstrated a period of early settlement prior to the final settlement phase between 23–44 and 34–55 days post-release, respectively. Males settled 174 days post-release (Figs 3 & 4), after initial extensive exploratory movements (Fig. 2).
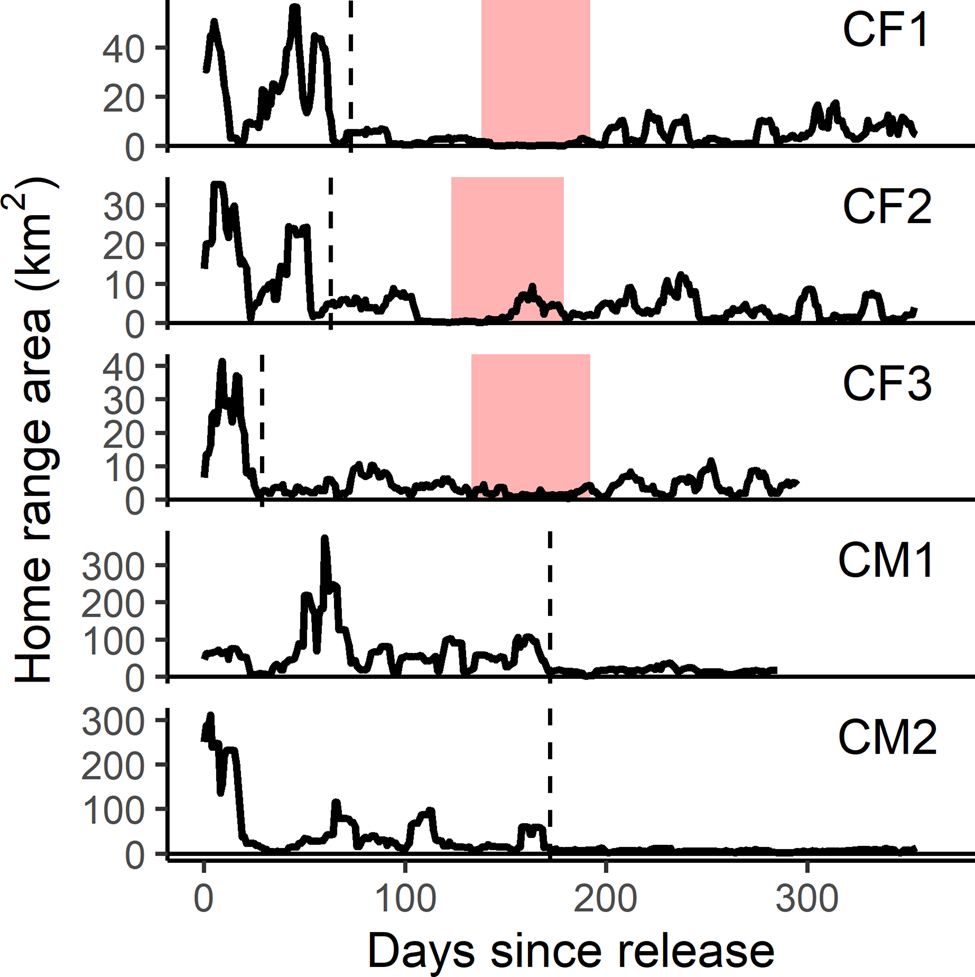
Fig. 3 Home range areas derived from progressive 11-day 100% minimum convex polygons for the first year post-release of female (CF) and male (CM) cheetahs reintroduced to Liwonde National Park, Malawi, during 2017–2018. Shaded bands indicate the known denning periods for females. Dashed line indicates time of settlement for each individual. Note different scale of y-axes between individuals.
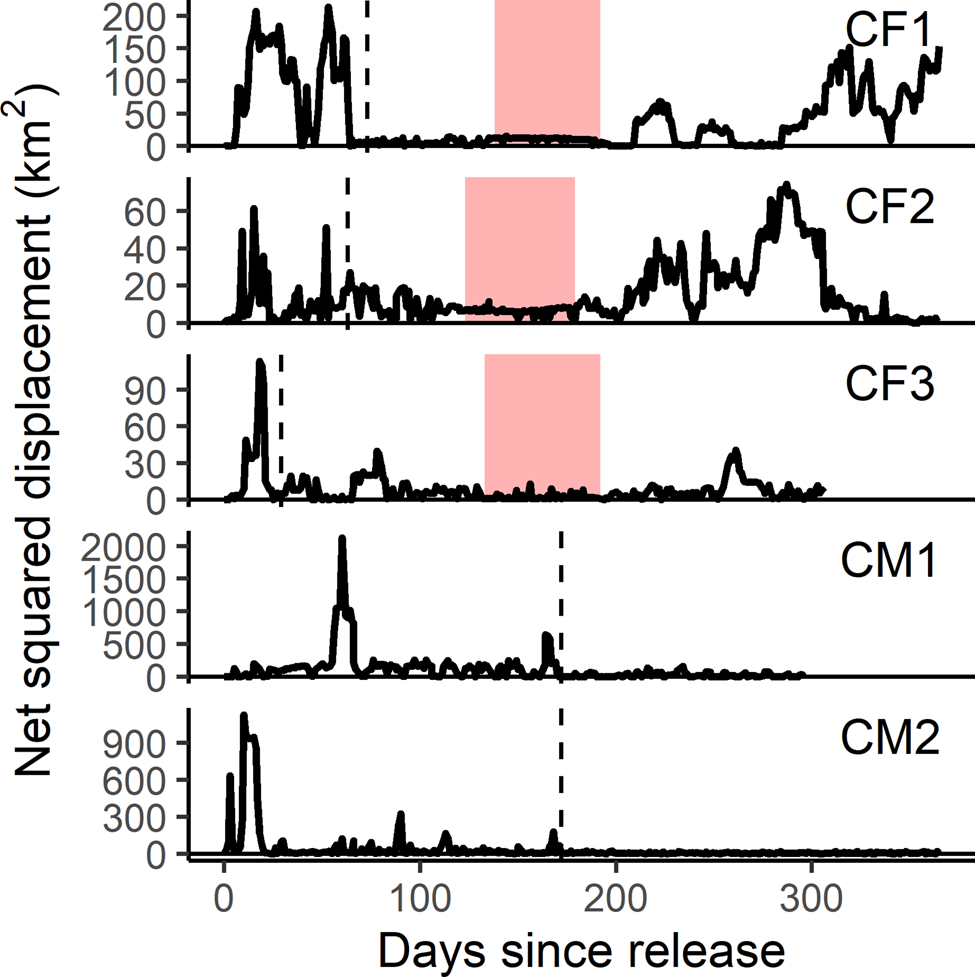
Fig. 4 Net squared displacement curves for the first year post-release of female (CF) and male (CM) cheetahs reintroduced to Liwonde National Park, Malawi, during 2017–2018. Shaded bands indicate the known denning periods for females. Y-axis is representative of the square of the Euclidean distance between each location and the known original location; therefore, a return to zero represents a return to the release site. Dashed line indicates time of settlement for each individual. Note different scale of y-axes between individuals.
Survival and reproduction
Four of the five GPS-collared cheetahs survived their first year post-release. CF3 was killed in a wire snare 307 days post-release, and CM4 and CM3 were recorded missing 85 and 152 days post-release, respectively. All three females gave birth to their first litter after home range development 131 ± SD 7 days post-release (range 123–138 days). Four birthing events were recorded over the 2-year monitoring period. Dens were located within 2.2 ± SD 0.5 km (n = 4) of the boma. Denning lasted 55 ± SD 1 days (53–56 days; n = 4 litters). Mean litter size was 4 ± SD 1.4 cubs (range 3–6; n = 4), which was consistent with mean litter sizes recorded in source populations (4.3–3.3 cubs; Table 3). All litters reached the age of emergence from the den, but cub mortality was highest during the first 6 months, with an overall cub survival of 60%. Of the four recorded litters, six offspring from two litters reached independence, all three offspring of CF3 died following the death of their mother, and one litter with six offspring was still dependent at the time of writing (cubs < 12 months old; Table 3). At the end of the 2-year monitoring period the population consisted of one adult male, two adult females, four subadult males, two subadult females and six dependent cubs.
Table 3 Mean demographic parameters for cheetahs in Liwonde National Park and various South African (SA) reserves in the Cheetah Metapopulation Project (Bissett & Bernard, Reference Bissett and Bernard2011), Pilanesberg National Park (Power et al., Reference Power, van der Merwe, Page-Nicholson, Botha, Dell and Nel2019) and Mun-Ya-Wana Conservancy (Gigliotti et al., Reference Gigliotti, Slotow, Hunter, Fattebert, Sholto-Douglas and Jachowski2020b). Sample sizes are the number of times each event was recorded (i.e. number of litters or number of females). For interbirth interval, the first sample size is for the number of intervals, and the second is the number of females.
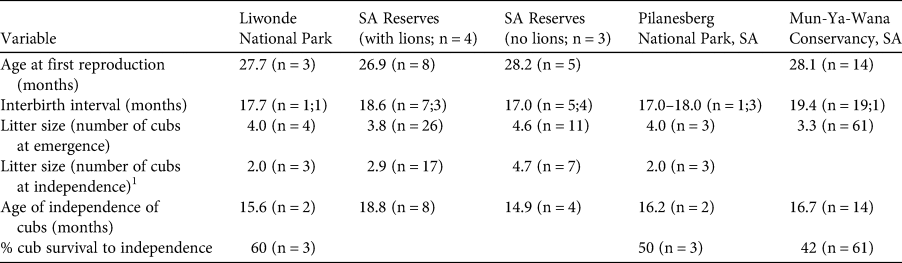
1 Some litters were still dependent on their mothers at the end of these studies, therefore the litter numbers at independence may be fewer than at emergence.
Discussion
Although post-release monitoring is common, analyses of individual settlement and population establishment are rare. This limits our understanding of early post-release movements, the strategies animals use to settle into novel environments, and ultimately reintroduction success (Armstrong & Seddon, Reference Armstrong and Seddon2007). We determined individual settlement post-reintroduction using two methods based on movement and home ranging patterns. We also assessed individual survival and reproduction to assess population establishment and reintroduction success. In Liwonde, reintroduced male cheetahs displayed more extensive exploratory movements than females. Settlement occurred sooner for females than males, with all females developing home ranges before denning and giving birth. All females reproduced within the first few months of release. An overall success rate of 57% was recorded (80% for GPS-collared animals) based on individuals that settled in the reserve, survived at least 1 year, and reproduced (females). Population demographic parameters were similar to those of the source populations, also indicating successful establishment. We therefore deem this reintroduction successful in establishing a breeding population of cheetahs, with anecdotal evidence of second-generation reproduction in 2020 (OS, pers. obs., 2020).
Holding periods
Longer holding periods are thought to decrease post-release movements, thereby increasing survival (Fritts et al., Reference Fritts, Mack, Smith, Murphy, Phillips, Jimenez, Maehr, Noss and Larkin2001; Hayward et al., Reference Hayward, Adendorff, O'Brien, Sholto-Douglas, Bissett and Moolman2007b). They are also important in preventing diseases from spreading, facilitating the formation of social groups and, where applicable, exposing individuals to electrified fences (Hunter, Reference Hunter1998). Holding period duration, however, had no significant effect on post-release movements in Liwonde, congruent with the findings of Weise et al. (Reference Weise, Lemeris, Munro, Bowden, Venter, van Vuuren and van Vuuren2015a). Studies of translocated leopards (Weise et al., Reference Weise, Lemeris, Stratford, van Vuuren, Munro and Crawford2015b) and tigers Panthera tigris (Sarkar et al., Reference Sarkar, Ramesh, Johnson, Sen, Nigam and Gupta2016) also found that the success of releases without a holding period was comparable to that of releases with a holding period.
Time to settlement
Time to settlement in our study was similar to that of translocated cheetahs in Namibia, which settled after 13–190 days post-release (Weise et al., Reference Weise, Lemeris, Munro, Bowden, Venter, van Vuuren and van Vuuren2015a). Males took significantly longer to develop home ranges than females, and their exact time of settlement was more difficult to determine. However, robust inference about such inter-sexual differences is limited by the inherently small sample size of reintroductions. Both males in our study settled and exhibited spatial strategies described in free-roaming populations (Melzheimer et al., Reference Melzheimer, Streif, Wasiolka, Fischer, Thalwitzer and Heinrich2018). CM2 was found to scent-mark often and had a limited, predictable range that overlapped with those of all females, indicating territoriality. In contrast, CM1 travelled across a large area and displayed the occasional foray, which is probably representative of so-called male floating behaviour and subsequent displacement by a territorial male (CM2).
Females exhibited similar levels of net squared displacement pre- and post-denning, but their 11-day home range contracted post-denning, indicative of localized movements that progressively shifted. This also indicates that net squared displacement curves alone are not the best representation of spatial or settlement behaviour for females with dependent cubs. Considering a gestation period of 90–95 days (Bissett & Bernard, Reference Bissett and Bernard2011), all three females released into Liwonde conceived within the first 2 months post-release. This time frame was similar to a female translocated to Pilanesberg National Park, South Africa (Power et al., Reference Power, van der Merwe, Page-Nicholson, Botha, Dell and Nel2019), and sooner than for females translocated into protected areas in Namibia (> 3 months; Weise et al., Reference Weise, Lemeris, Munro, Bowden, Venter, van Vuuren and van Vuuren2015a). Whereas CF1 and CF2 settled after conceiving, CF3 developed a home range prior to conceiving, suggesting that pregnancy may not influence settlement behaviour in all females.
Habitat and competition
In Liwonde, external factors, rather than pre-release management, probably influenced post-release movements and ultimately reintroduction success. Habitat heterogeneity limits kleptoparasitism, enables flexibility in hunting strategies and favours adult and cub survival (Durant, Reference Durant1998; Rostro-Garcia et al., Reference Rostro-Garcia, Kamler and Hunter2015; Gigliotti et al., Reference Gigliotti, Slotow, Hunter, Fattebert, Sholto-Douglas and Jachowski2020a). Translocated cheetahs exhibit wide ranging post-release movements in search of familiar or more suitable habitat conditions (Weise et al., Reference Weise, Lemeris, Munro, Bowden, Venter, van Vuuren and van Vuuren2015a). Liwonde's mixed habitat structure, low density of competitors and abundant prey base provided suitable habitat, including den sites, and probably reduced post-release exploration and positively influenced breeding and settlement for females. Considering the distance of dens to the release site (2.2 ± SD 0.5 km), presence of suitable habitat near the release site probably influenced post-release exploration and time to reproduction for females. Rapid breeding post-release further indicates the influence of suitable environmental conditions on female settlement (Weise et al., Reference Weise, Lemeris, Munro, Bowden, Venter, van Vuuren and van Vuuren2015a).
Conversely, males displayed extensive post-release movements. Male cheetahs exhibit two forms of spatial behavioural patterns: territoriality or floating (Melzheimer et al., Reference Melzheimer, Streif, Wasiolka, Fischer, Thalwitzer and Heinrich2018). It is possible that reintroduction initiated floating behaviour as released males investigated Liwonde for conspecifics, only switching to territorial behaviour and settlement when no resident territory holders were found. Males approached the perimeter fence on numerous occasions during post-release exploration. The fence probably reduced the area available for post-release exploration, which may have influenced time to settlement. In open systems, it has been suggested that males should be released first to prevent extensive exploration, and females should remain in a holding boma as a so-called anchor (Boast et al., Reference Boast, Chelysheva, van der Merwe, Schmidt-Küntzel, Walker, Cilliers and Nyhus2018). However, our findings suggest that post-release movements and reintroduction success of male cheetahs were strongly influenced by natural and physical barriers of the Park, as well as the lack of resident territorial males.
Survival and mortality
Cheetahs generally exhibit lower survival rates during the first year post-release than other large African carnivores (Hayward et al., Reference Hayward, Adendorff, O'Brien, Sholto-Douglas, Bissett and Moolman2007a; Fontúrbel & Simonetti, Reference Fontúrbel and Simonetti2011). Survival in Liwonde 1 year post-release was 80%, which is higher than the average for felid translocations (39 ± SE 6%; Fontúrbel & Simonetti, Reference Fontúrbel and Simonetti2011), higher than in a population hard-released into unfenced protected areas in Namibia (67%; Weise et al., Reference Weise, Lemeris, Munro, Bowden, Venter, van Vuuren and van Vuuren2015a), and similar to releases in some South African fenced reserves (84%; Marnewick et al., Reference Marnewick, Hayward, Cilliers, Somers, Hayward and Somers2009). Even with the loss of CM3 and CM4 (57%), survival in Liwonde was higher than in Matusadona National Park, Zimbabwe (36%; Purchase & Vhurumuku, Reference Purchase and Vhurumuku2005). In addition, cub survival in Liwonde (60%) was higher than that recorded in the South African source population (42–50%; Table 3).
Notably, we recorded no mortalities caused by lions, although lions were reintroduced to Liwonde only 107–354 days after the cheetahs. Lions are a major cause of mortality for cheetahs (Buk et al., Reference Buk, van der Merwe, Marnewick and Funston2018; Gigliotti et al., Reference Gigliotti, Slotow, Hunter, Fattebert, Sholto-Douglas and Jachowski2020b), and it has been recommended that cheetahs intended for release into areas with resident lions have prior knowledge of lions (Hayward et al., Reference Hayward, Adendorff, O'Brien, Sholto-Douglas, Bissett and Moolman2007b). The fact that 57% (n = 4) of the cheetahs were sourced from reserves with resident lions may have contributed to them successfully adapting their behaviour in the presence of lions. Ongoing analyses of habitat use before and after lion reintroduction should provide further insights on this particular aspect.
Reintroduction assessment and perspectives
Reintroduction success can be viewed on three scales: an individual's settlement, the establishment of a population and overall population persistence (Armstrong & Seddon, Reference Armstrong and Seddon2007). If post-release movements and mortality are low, individuals will settle sooner, allowing the population to establish from a small number of founders (e.g. Hayward et al., Reference Hayward, Adendorff, O'Brien, Sholto-Douglas, Bissett and Moolman2007a; Briers-Louw et al., Reference Briers-Louw, Verschueren and Leslie2019). The reintroduction of cheetahs into Liwonde was considered successful in establishing a breeding population, with five individuals developing home ranges and all females breeding. The fact that founder populations in most large carnivore reintroductions are small (Breitenmoser et al., Reference Breitenmoser, Breitenmoser-Wursten, Carbyn, Funk, Gittleman, Funk, Macdonald and Wayne2001) emphasizes the importance of analysing post-release monitoring data, which will facilitate the comparison across multiple case studies and varying factors over time.
Successful establishment is not a guarantee for population persistence. Populations monitored by the Cheetah Metapopulation Project that did not persist in the long term were extirpated, on average, 8.4 years after reintroduction (Buk et al., Reference Buk, van der Merwe, Marnewick and Funston2018). Park management must therefore consider long-term population monitoring to establish persistence, and address factors that may cause extirpation of this small population, which is inherently susceptible to stochastic events such as disease outbreaks (Davidson-Phillips et al., Reference Davidson-Phillips, Davidson-Phillips, Canning, Schroder, Swart and Burger2019). Also, the death of CF3 in an old wire snare set for bushmeat hunting demonstrates that although Liwonde's law enforcement rangers removed > 27,000 snares in the 2 years prior to this reintroduction, snaring remains a problem.
Given the geographical isolation of Liwonde's cheetahs, and the small founder population, inbreeding depression is a significant risk (Frankham, Reference Frankham2010; Naude et al., Reference Naude, Balme, O'Riain, Hunter, Fattebert, Dickerson and Bishop2020). Supplementation of Liwonde's current population with unrelated individuals is strongly recommended, to mimic natural immigration (Ferreira & Hofmeyr, Reference Ferreira and Hofmeyr2014) and ensure the long-term genetic diversification of the population (Gustafson et al., Reference Gustafson, Vickers, Boyce and Ernest2017). This could include individuals from the more recent reintroduction of unrelated cheetahs to Majete Wildlife Reserve, Malawi, through the development of a managed metapopulation node in Malawi.
Conclusion
As the conservation value of protected areas in Malawi has been declining over recent decades because of lack of funding, high levels of poaching and anthropogenic encroachment, certain protected areas in Malawi are now shifting towards fenced systems. This approach aims to curtail offtake of natural resources and edge effect pressures caused by the activities of surrounding communities, thereby opening opportunities for species reintroductions (Packer et al., Reference Packer, Loveridge, Canney, Caro, Garnett and Pfeifer2013). We recommend that future reintroduction projects follow the IUCN guidelines on post-release monitoring (IUCN, 1998), to ascertain the outcome of the reintroduction.
In this study, we documented the initial success of the first reintroduction of cheetahs in Malawi based on individual movement tracking to determine settlement in the reserve, survival and reproduction rates. We determined that a pre-release holding period > 23 days had no effect on post-release movements and suggest that suitable habitat structure and the lack of competitors influenced settlement. We also suspect that fencing contributed to settlement by confining some individuals within the limit of the reintroduction site, despite extensive early exploratory movement, and prevented their dispersal and possibly lowered the risk of mortality in an inhospitable matrix. The success of the reintroduction of cheetahs in Liwonde is therefore not only encouraging for the continued re-establishment of cheetahs across fenced systems in Malawi, but also for the future of range expansion projects across the continent.
Acknowledgements
We thank the staff of Liwonde National Park for their assistance during this study, and Endangered Wildlife Trust for providing guidance post-release. This study was funded by African Parks Liwonde.
Author contributions
Study design: OS, AL, KM; fieldwork: OS; data analysis: OS, JF; writing: all authors.
Conflicts of interest
None.
Ethical standards
This research abided by the Oryx guidelines on ethical standards. Fieldwork was conducted with the relevant clearance from African Parks Liwonde. Movements of cheetahs were conducted with relevant CITES permits (permit no.: 175828; 0000062; 0000079). Ethical clearance for third-party data users was obtained through Stellenbosch University (reference no.: ACU-2018-8311). All intellectual property rights were within the legal requirements of Malawi and monitoring of reintroduced cheetahs was conducted following the IUCN Guidelines for Reintroductions and other Conservation Translocations (IUCN, 1998).











