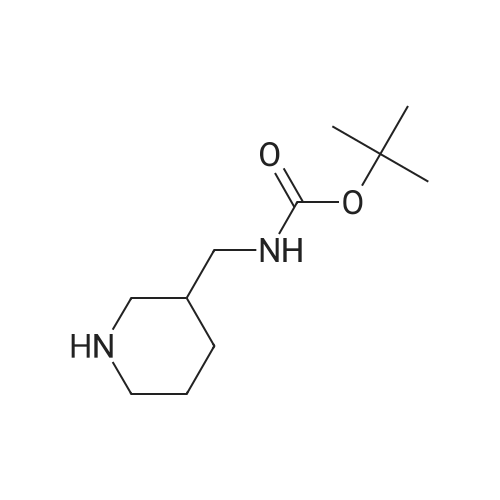
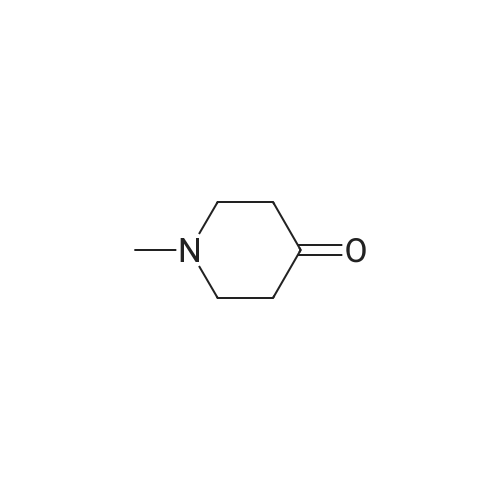
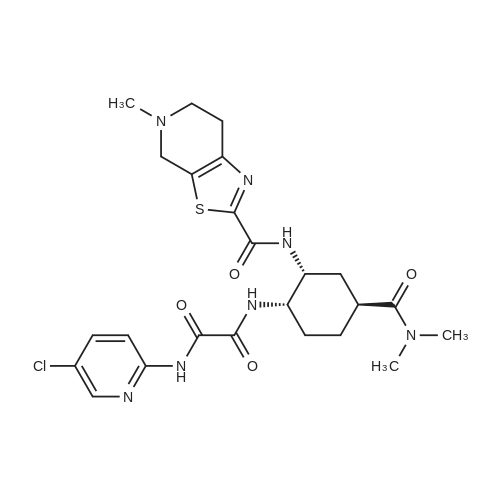
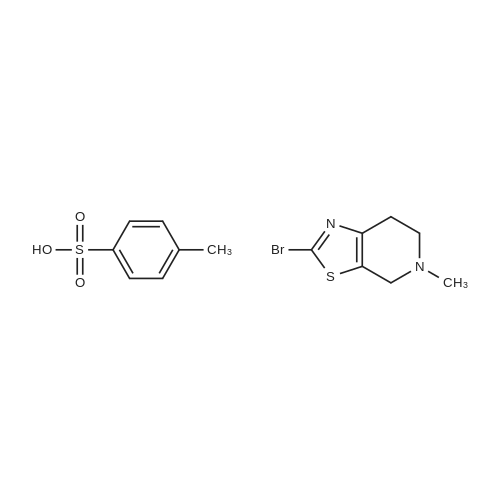
| 85.13% |
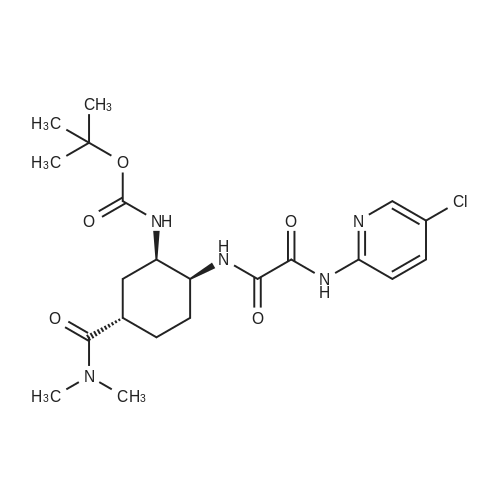
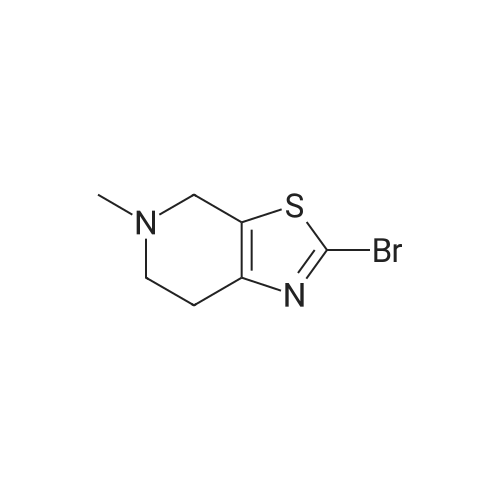
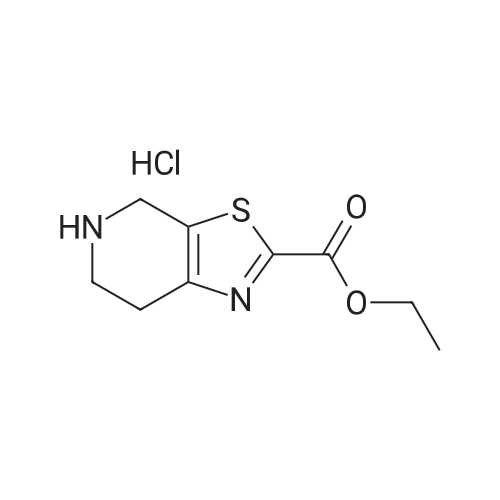
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With hydrogenchloride; lithium hydroxide monohydrate In propan-2-one at 0.25 - 0.3℃; for 2h;
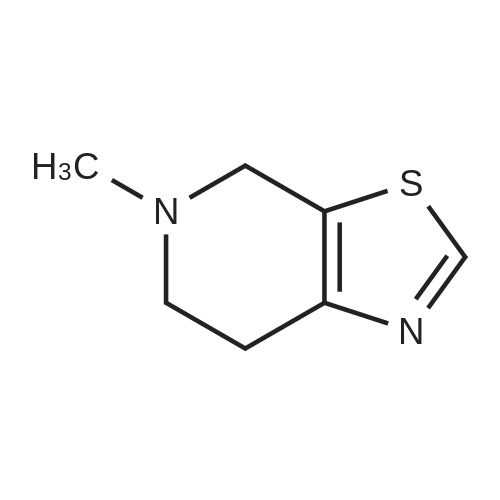
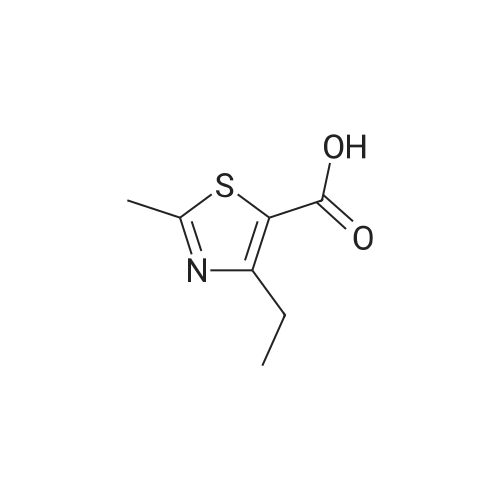
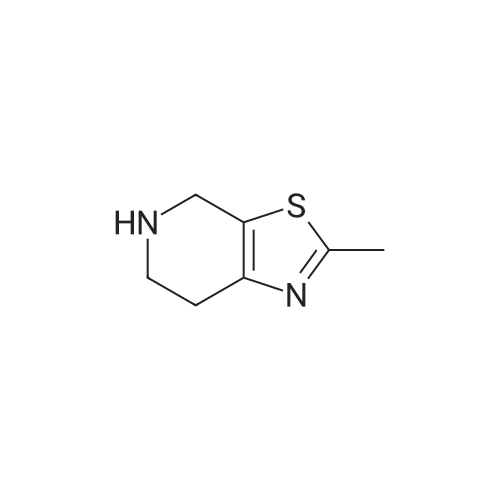
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; dicyclohexyl-carbodiimide In dichloromethane at 0.35 - 0.4℃; for 0.5h; |
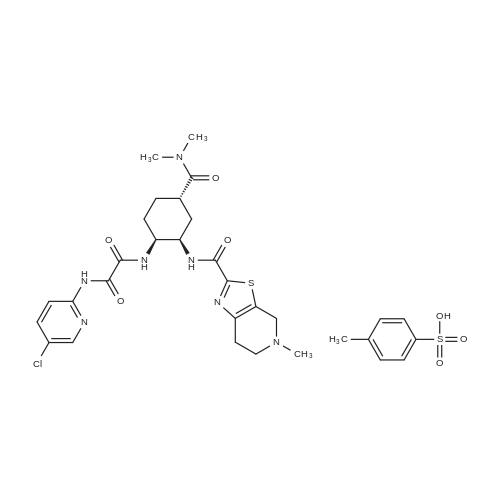
3E Example 3E: Preparation of Edoxaban Free base (compound of formula I-A) directly from Carbamic acid, N-[(1R, 2S, 5S)-2-[[2-[(5-chloro-2-pyridinyl) amino]-2-oxoacetyl] amino] -5- [(dime thy lamino) carbonyl] cyclohexyl]-l, 1- dimethylethyl ester (compound of formula IV):
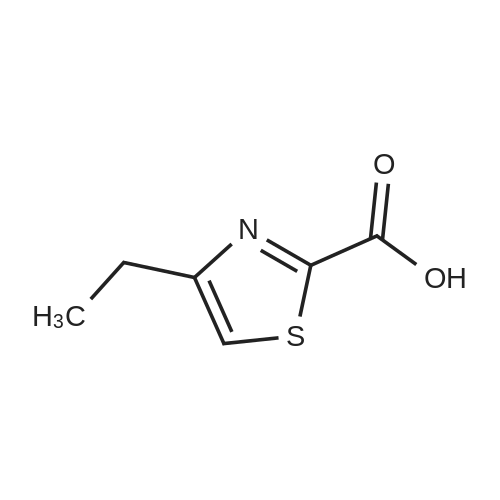
The compound of formula IV (25.0g) obtained from example IB was added to acetone(250 mL) and concentrated hydrochloric acid (50 mL) was then added to it. The reaction mass was then stirred for about 2 hours at about 25°C to 30°C. After completion of the reaction mass, filtered the slurry mass, washed with acetone and suck dried. The obtained wet cake was suspended in methylene dichloride (250 mL) and added 5-Methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride (compound of formula III, 15.05g, diisopropyl ethyl amine (34.5g), N,N- dicyclohexyl carbodiimide (DCC) ( 16.5 lg) and 1-hydroxybenzotriazole (HOBt, 3.62g). The reaction mass was heated to about 35°C to 40°C for about 5 hours. After completion ,the reaction mass was cooled to about 0°C to 5°C for about 1 hour, filtered. The combined filtrate was washed with 5% aq. NaHCCL followed by brine solution. The solvent was distilled to obtain the crude product (compound of formula I-A) to which methanol (200 mL) was added and heated to 60°C to 65 °C for about 1 hour then cooled to 25 °C to 30°C for 1 hour, filtered, washed with methanol to obtained wet cake which was dried in tray drier at 40°C to 45 °C to obtain compound of formula I- A (24.90g, 85.13%), HPLC purity 99.47. |
|
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 20℃; for 2h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With benzotriazol-1-ol; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride; triethylamine In acetonitrile at 0 - 20℃; for 17h; |
2
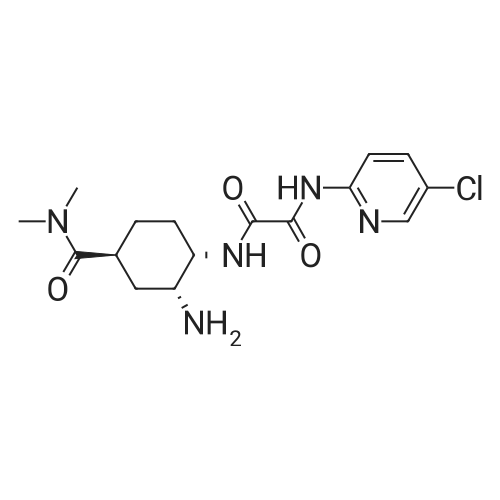
Referential Example 2: N-(5-Chloropyridin-2-yl)-N'-[(1S,2R,4S)-4-(dimethylcarbamoyl)-2-[(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl]ethanediamide Methanesulfonic acid (66 mL) was added at room temperature to a suspension of t-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate (95.1 g) in acetonitrile (1,900 mL), and the mixture was stirred at the same temperature for 2 hours. Triethylamine (155 mL), 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride (52.5 g), 1-hydroxybenzotriazole (33.0 g), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (46.8 g) were added to the reaction mixture under ice-cooling, and the mixture was stirred at room temperature for 16 hours. Triethylamine and water were added thereto, followed by stirring under ice-cooling for 1 hour. The formed crystals were recovered by filtration, to thereby yield 103.2 g of the title compound. 1H-NMR(CDCl3)δ:1.60-1.98(3H,m),2.00-2.16(3H,m),2.52(3H,s), 2.78-2.90(3H,m),2.92-2.98(2H,m),2.95(3H,s),3.06(3H,s), 3.69(1H,d,J=15.4Hz),3.75(1H,d,J=15.4Hz),4.07-4.15(1H,m), 4.66-4.72(1H,m),7.40(1H,dd,J=8.8,0.6Hz), 7.68(1H,dd,J=8.8,2.4Hz),8.03(1H,d,J=7.8Hz), 8.16(1H,dd,J=8.8,0.6Hz),8.30(1H,dd,J=2.4,0.6Hz),9.72(1H,s). |
|
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 20℃; for 2h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With benzotriazol-1-ol; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride; triethylamine In acetonitrile at 20℃; for 16h; Cooling with ice; |
6
(Reference Example 6) N1-(5-Chloropyridin-2-yl)-N2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide (A) Methanesulfonic acid (66 ml) was added to a suspension of tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate (8) (95.1 g) in acetonitrile (1900 ml) at room temperature, and the mixture was stirred at this temperature for 2 hours. To the reaction solution, triethylamine (155 ml), 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride (10) (52.5 g), 1-hydroxybenzotriazole (33.0 g), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (46.8 g) were added under ice cooling, and the mixture was stirred at room temperature for 16 hours. Triethylamine and water were added thereto, and the mixture was stirred for 1 hour under ice cooling. Then, crystals were collected by filtration to obtain the title compound (A) (103.2 g). 1H-NMR (CDCl3) δ: 1.60-1.98 (3H, m), 2.00-2.16 (3H, m), 2.52 (3H, s), 2.78-2.90 (3H, m), 2.92-2.98 (2H, m), 2.95 (3H, s), 3.06 (3H, s), 3.69 (1H, d, J=15.4 Hz), 3.75 (1H, d, J=15.4 Hz), 4.07-4.15 (1H, m), 4.66-4.72 (1H, m), 7.40 (1H, dd, J=8.8, 0.6 Hz), 7.68 (1H, dd, J=8.8, 2.4 Hz), 8.03 (1H, d, J=7.8 Hz), 8.16 (1H, dd, J=8.8, 0.6 Hz), 8.30 (1H, dd, J=2.4, 0.6 Hz), 9.72 (1H, s). MS (ESI) m/z: 548 (M+H)+. |
|
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 20℃; for 2h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With benzotriazol-1-ol; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride; triethylamine In acetonitrile at 20℃; for 16h; Cooling with ice; |
4
(Reference Example 4) N1-(5-Chloropyridin-2-yl)-N2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide (A) Methanesulfonic acid (66 ml) was added to a suspension of tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbanyl)cyclohexyl]carbamate (1) (95.1 g) in acetonitrile (1900 ml) at room temperature, and the mixture was stirred at this temperature for 2 hours. To the reaction solution, triethylamine (155 ml), 5-methyl-4,5,6,7-tetrahydro[1,3]thiazalo[5,4-c]pyridine-2-carboxylic acid hydrochloride (8) (52.5 g), 1-hydroxybenzotriazole (33.0 g), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (46.8 g) were added under ice cooling, and the mixture was stirred at room temperature for 16 hours. Triethylamine and water were added thereto, and the mixture was stirred for 1 hour under ice cooling. Then, crystals were collected by filtration to obtain the title compound (103.2 g). 1H-NMR (CDCI3) δ: 1.60-1.98 (3H, m), 2.00-2.16 (3H, m), 2.52 (3H, s), 2.78-2.90 (3H, m), 2.92-2.98 (2H, m), 2.95 (3H, s), 3.06 (3H, s), 3.69 (1H, d, J=15.4 Hz), 3.75 (1H, d, J=15.4 Hz), 4.07-4.15 (1H, m), 4.66-4.72 (1H, m), 7.40 (1H, dd, J=8.8, 0.6 Hz), 7.68 (1H, dd, J=8.8, 2.4 Hz), 8.03 (1H, d, J=7.8 Hz), 8.16 (1H, dd, J=8.8, 0.6 Hz), 8.30 (1H, dd, J=2.4, 0.6 Hz), 9.72 (1H, s). MS (ESI) m/z: 548 (M+H)+. |
| 103.2 g |
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 20℃; for 2h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With benzotriazol-1-ol; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride; triethylamine In acetonitrile at 20℃; for 16h; Cooling with ice; |
6 (Reference Example 6) N1-(5-Chloropyridin-2-yl)-N2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[(5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide (X) (production method described in the pamphlet of International Publication No. WO 2007/032498)
Methanesulfonic acid (66 ml) was added to a suspension of tert-butyl [(1R,2,S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate (5) (95.1 g) in acetonitrile (1900 ml) at room temperature, and the mixture was stirred at this temperature for 2 hours. To the reaction solution, triethylamine (155 ml), 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyrzdine-2-carboxylic acid hydrochloride (8) (52.5 g), 1-hydroxybenzotriazole (33.0 g), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (46.8 g) were added under ice cooling, and the mixture was stirred at room temperature for 16 hours. Triethylamine and water were added thereto, and the mixture was stirred for 1 hour under ice cooling. Then, crystals were collected by filtration to obtain the title compound (X) (103.2 g). 1H-NMR (CDCl3) δ : 1.60-1.98 (3H, m), 2.00-2.16 (3H, m), 2.52 (3H, s), 2.78-2.90 (3H, m), 2.92-2.98 (2H, m), 2.95 (3H, s), 3.06 (3H, s), 3.69 (1H, d, J = 15.4 Hz), 3.75 (1H, d, J = 15.4 Hz), 4.07-4.15 (1H, m), 4.66-4.72 (1H, m), 7.40 (1H, dd, J = 8.8, 0.6 Hz), 7. 68 (1H, dd, J = 8.8, 2.4 Hz), 8.03 (1H, d, J = 7.8 Hz), 8.16 (1H, dd, J = 8.8, 0.6 Hz), 8.30 (1H, dd, J = 2. 4, 0.6 Hz), 9.72 (1H, s). MS (ESI) m/z: 548 (M+H)+. |
| 103.2 g |
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 20℃; for 2h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With benzotriazol-1-ol; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride; triethylamine In acetonitrile at 20℃; for 17h; Cooling with ice; |
3 Referential Example 3 N1- (5-Chloropyridin-2-yl) -N2- [ (IS, 2R, 4S) -4- (dimethylcarbamoyl) -2 - { [ (5-methyl-4 , 5,6, 7-tetrahydro [1,3] thiazolo [5 , 4-c] yridin-2 -yl ) carbonyl] amino} cyclohexyl] ethanediamide (Edoxaban)
Referential Example 3 N1- (5-Chloropyridin-2-yl) -N2- [ (IS, 2R, 4S) -4- (dimethylcarbamoyl) -2 - { [ (5-methyl-4 , 5,6, 7-tetrahydro [1,3] thiazolo [5 , 4-c] yridin-2 -yl ) carbonyl] amino} cyclohexyl] ethanediamide (Edoxaban) Methanesulfonic acid (66 mL) was added at room temperature to a suspension of ' tert-butyl [ (1R, 2S, 5S) -2- ( { [ (5-chloropyridin-2-yl) amino] (oxo)acet yl }amino) -5- (dimethylaminocarbonyl) cyclohexyl] carbamate (95.1 g) in acetonitrile (1,900 mL) , and the mixture was stirred at the same temperature for 2 hours. Triethylamine (155 mL) , 5-methyl-4 ,5,6, 7-tetrahydro [1,3] thiazolo [5 , 4-c] pyridine-2 -carbox ylic acid hydrochloride (52.5 g) , 1 -hydroxybenzotriazole (33.0 g) , and l-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride (46.8 g) were added to the reaction mixture under ice-cooling, and the mixture was stirred at room temperature for 16 hours. Triethylamine and water were added thereto, followed by stirring under ice-cooling for 1 hour. The formed crystals were recovered by filtration, to thereby yield 103.2 g of the title compound. |
| 103.2 g |
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 20℃; for 2h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride; triethylamine In acetonitrile at 20℃; for 16h; |
5 Reference Example 5N’-(5-Chloropyridin-2-yl)-N2-[(1 S,2R,45)-4-(dim- ethylcarbamoyl)-2-{ [(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl]ethanediamide [edoxaban] (X)
10098] tert-Hutyl[(1R,25,55)-2-([(5-chloropyridin-2-yl)amino] (oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cy-clohexyl]carbamate (12) (95.1 g) was suspended in acetonitrile (1900 mE), and methanesulfonic acid (66 mE) was addedto the suspension at room temperature. The obtained mixturewas stirred at the same temperature as described above for 2hours. While the reaction mixture was stirred under coolingon ice, triethylamine (155 mE), 5-methyl-4,5,6,7-tetrahydro[1 ,3]thiazolo[5,4-c]pyridine-2-carboxylate hydrochloride(52.5 g), 1-hydroxybenzotriazole (33.0 g), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (46.8 g)were added to the mixture. The reaction mixture was stirred atroom temperature for 16 hours. Triethylamine and water wereadded to the reaction mixture, and the obtained mixture wasthen stirred under cooling on ice for 1 hout A precipitatedcrystal was collected by filtration and was dried to obtain103.2 g of the title product. |
| 103.2 g |
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 20℃; for 2h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With benzotriazol-1-ol; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride; triethylamine In lithium hydroxide monohydrate; acetonitrile at 20℃; for 17h; |
10 N1-(5-chloropyridin-2-yl)-N2-((1S,2R,4S)-4-[(dimethylamino)carbonyl]-2-[(5-methyl-4,5,6,7-tetrahydrothiazolone[5,4-c]pyridin-2-yl)carbonyl]amino}cyclohexyl)ethanediamide
The tert-butyl [. (1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl} amino)-5-(dimethylaminocarbonyl)cyclohexyl carbamate (A-9) (95.1g), to the acetonitrile (1900 ml) suspension of a under a room temperature, Methanesulfonic acid (66 ml) was added and it stirred at a temperature as it is for 2 hours. The bottom of ice-cooling to reaction mixture, triethylamine (155 ml), 5-methyl-4,5,6,7-tetrahydro [1,3]thiazolo [5,4-c]pyridin-2-carboxylic acid hydrochloride (52.5g), 1-hydroxybenzotriazol (33.0g) and a 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (46.8g) were added, and it stirred at the room temperature for 16 hours. Triethylamine and water were added, the crystal was separated the bottom of ice-cooling, and after 1-hour stirring, and title compound (X) (103.2g) was obtained. |
| 125.3 g |
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 25℃; for 2h;
Stage #2: With triethylamine In acetonitrile at 10℃; for 0.166667h;
Stage #3: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With benzotriazol-1-ol; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride In acetonitrile at 25℃; for 18h; |
2 Example 2 N- (5-Chloropyridin-2-yl)-N '- ((1S, 2R, 4S)-4 - [(dimethylamino) carbonyl] -2 - [(5-methyl-4,5,6,7-tetrahydrothiazole[5,4-c] pyridin-2-yl) carbonyl] amino} cyclohexyl) ethanediamide, Preparation of Compound (1)
A 10 L reaction flask was charged with 2560 mL of acetonitrile, 128.0 g of (1R, 2S, 5S) -2 - ({2- [5-chloropyridin-Yl] -2-oxoacetyl} amino) -5 - [(dimethylamino) carbonyl] cyclohexylcarbamate was added with stirring and 131.4 g of methanesulfonic acid was added with stirring at 25 ± 2 ° C. The reaction was incubated for 2 h , TLC no raw material point, cooling, 10.2 ° C was added 152.2g triethylamine, stirred 10min, followed by addition of 70.6g 5-Methyl-4,5,6,7-tetrahydrothiazolo [5,4-c] pyridine-2-carboxylic acid hydrochloride,40.7 g of 1-hydroxybenzotriazole and 62.9 g1-ethyl-3- (3-dimethylaminopropyl) carbodiimide hydrochloride,Warming to 25 , 25 ± 2 reaction 18h. TLC detection of raw material point. Developing solvent: dichloromethane: methanol = 10: 1.After the reaction, lowering the temperature to 10 °C, add saturated aqueous solution of sodium bicarbonate, 10 ± 2 °C insulation crystallization 1h, filtered, the filter cake washing, 45 °C drying by blowing 12h, shall 125.3g white solid |
| 103.2 g |
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 20℃; for 2h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With triethylamine In acetonitrile at 20℃; for 16h; Cooling with ice; |
4
{Tert-Butyl [(1R, 2S, 5S) -2-({(5-chloropyridin-2-yl) amino} (oxo) acetyl) amino] -5-dimethylaminocarbonyl in acetonitrile (1900 ml)Cyclohexyl] carbamate(95.1g), Methanesulfonic acid (66 ml) is added at room temperature,The mixture was stirred at room temperature for 2 hours,The reaction solution, trimethylamine (155 ml),5-Methyl-4,5,6,7-tetrahydro [1,3] thiazo [5,4-c] pyridine-2-carboxylic acid hydrochloride (46.8 g) was added under ice cooling, and the mixture was added. Stirred at room temperature for 16 hours.Triethylamine and water were added thereto, and the mixture was stirred under ice cooling for 1 hour. Thereafter, the crystals were collected by filtration to obtain the title compound (103.2 g). |
| 103.2 g |
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With methanesulfonic acid In acetonitrile at 20℃; for 2h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With benzotriazol-1-ol; N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride; triethylamine In acetonitrile at 20℃; for 16h; Cooling with ice; |
1-2; 1-15 Refer to the technical scheme disclosed in patent US8686189B2, selectN-(5-chloropyridin-2-yl)-N'-[(1S,2R,4S)-4-(N,N-dimethylformylamino)-2-(5-methyl-4, 5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxamide)cyclohexyl]oxalamide as a raw material,Prepare compound (1):
At room temperature, toN-(5-Chloropiperidin-2-yl)-N'-[(1S,2R,4S)-4-(N,N-dimethylformylamino)-2-(amino tert-butoxycarbonyl ) Cyclohexyl] oxamide (95.1g) in acetonitrile (1900ml) suspension was added with methanesulfonic acid (66ml),Stir at this temperature for 2 hours.Under ice cooling, triethylamine (155ml), 4,5,6,7-tetrahydro-5-methyl-thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride ( 52.5g), 1-hydroxybenzotriazole (33.0g), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (46.8g), stirred at room temperature for 16 hours . Triethylamine and water were added, and after stirring for 1 hour under ice cooling, the crystals were filtered out to obtain 103.2 g of compound (1) |
|
Stage #1: tert-butyl [(1R,2S,5S)-2-([(5-chloropyridin-2-yl)amino](oxo)acetyl}amino)-5-(dimethylaminocarbonyl)cyclohexyl]carbamate With hydrogenchloride In dichloromethane; lithium hydroxide monohydrate at 20℃; for 1.16h;
Stage #2: 5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride With N-[3-(N,N-dimethylamino)-propyl]-N'-ethyl-carbodiimide hydrochloride; triethylamine In ethanol at 20℃; |
1-2 Example 1
(5-chloropyridin-2-yl)-N2-((1S,2R,4S)-2-[(tert-Butoxycarbonyl)Amino]-4-[(dimethylamino) carbonyl]-cyclohexyl)oxalamide (Formula 2) 11.0 g is added to MC 146.3g and stirred at room temperature. 4.9 g of 35% hydrochloric acid is added, and the mixture is stirred until the reaction is completed, and when the reaction is completed, it is completely concentrated. TEA 7.1g and ethanol 39.5g were added to the concentrate and stirred at room temperature. 4,5,6,7-tetrahydro-5-methylthiazolo[5,4-c]pyridine-2-carboxylic acid hydrochloride (Formula 4) 6.1g and EDCI 5.5g are added and stirred until the reaction is complete. 292.6 g of MC and 220 mL of water are added, stirred, and the MC layer is first separated. After the separation, 146.3 g of MC was added to the water layer again, stirred, and then the layers were separated a second time. 110 mL of water is added to the entire MC layer separated from the first and second water layers, stirred, and then the third layer is separated again. After the tertiary MC layer was concentrated, 35.1 g of MC, 9.2 g of ethanol, and 6 mL of water were added to the concentrate and stirred. Add 5.7 g of benzenesulfonic acid and stir at room temperature for about 10 minutes. After adding 110 mL of ethyl acetate to the stirred reaction solution, the mixture was stirred for 1 hour. After cooling the mixed solution in which crystals are formed by the stirring to 0-5° C., the mixture is stirred again for 1 hour to completely form crystals from the mixed solution. The resulting crystals were filtered and dried under vacuum at 50° C. for 12 hours to obtain the title crystals (yield: 89%, purity: 99.980%) |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping