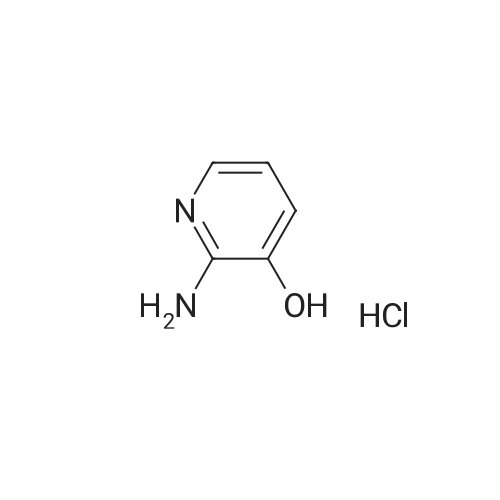
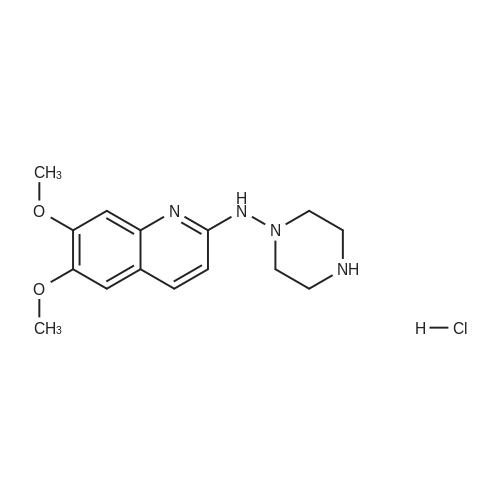
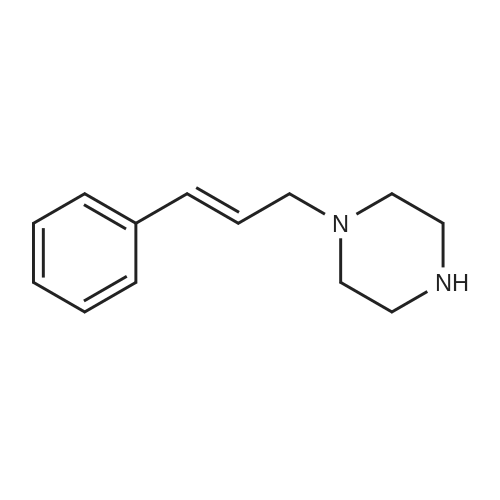
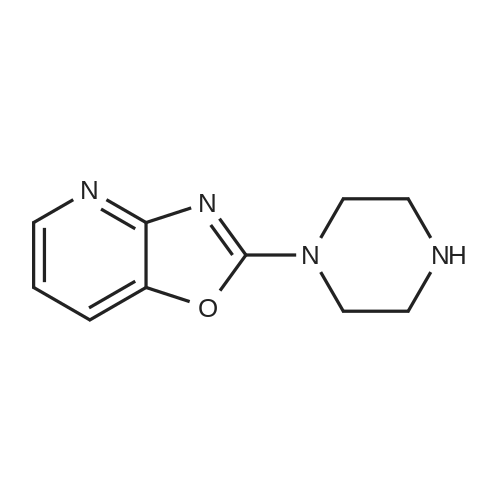
| 27% |
With potassium carbonate; In N,N-dimethyl-formamide; |
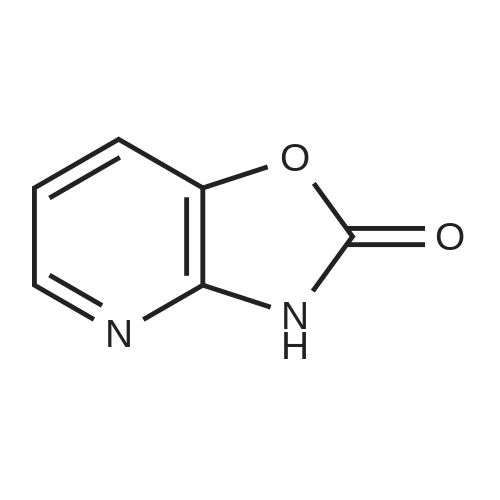
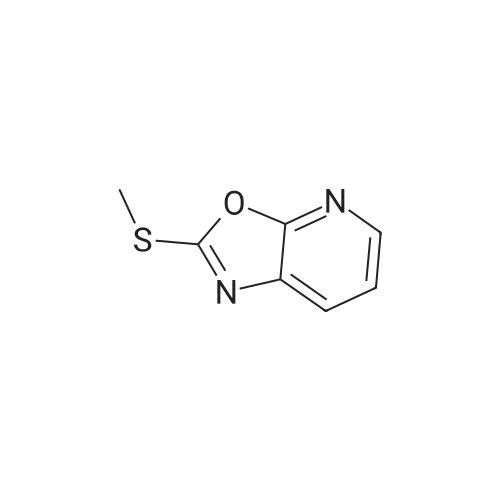
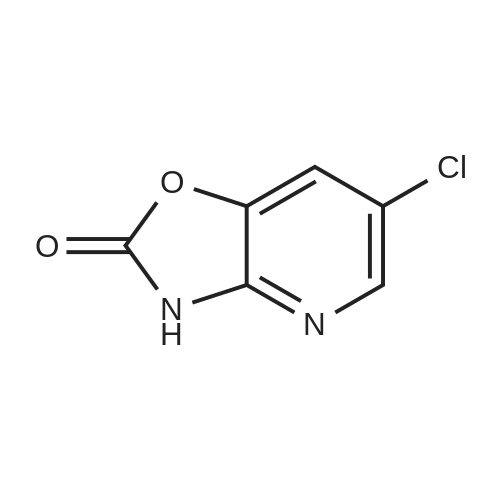
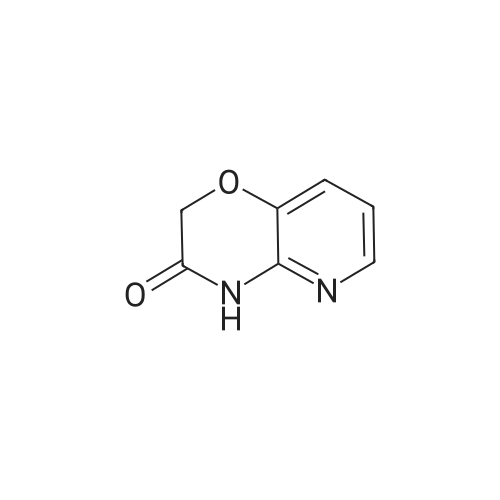
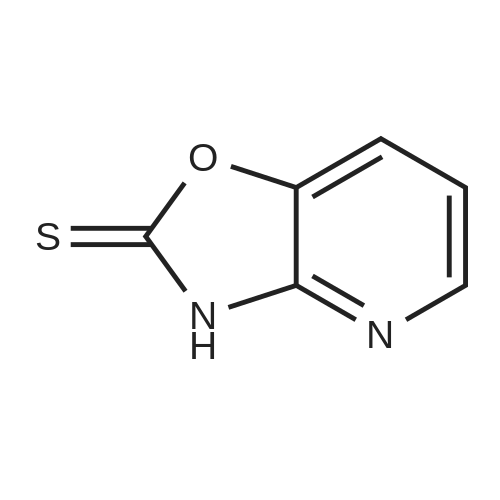
Example 1 Production of 6-(oxazolo[4,5-b]pyridin-2-ylthio)-N-(2,6-diisopropylphenyl)hexanamide Potassium carbonate (64 mg, 0.47 mmol) and 18-crown-6 (11 mg, 0.04 mmol) were added to a solution of <strong>[53052-06-5]2-mercaptooxazolo[4,5-b]pyridine</strong> (64 mg, 0.42 mmol) and 6-bromo-N-(2,6-diisopropylphenyl)hexanamide (150 mg, 0.42 mmol) in DMF (3 ml), and the resulting mixture was stirred at 80 C. for4 hours. After there action solution was diluted with water, the organic layer was extracted with ethyl acetate. The organic layer was washed with water and dried over anhydrous magnesium sulfate, from which the solvents were distilled off. The residue was purified by silica gel column chromatography (elution solvents: hexane:ethyl acetate=2:1); the resulting crystal was recrystallized from ethyl acetate-hexane, to recover the objective compound of 49 mg (at a yield of 27%) as a colorless needle-like crystal. Melting Point: 94-95 C. IR (KBr) cm-1: 3230, 2965, 1646, 1497, 1403. 1H-NMR (d6-DMSO) delta: 1.14 (12H, d, J=6.8 Hz), 1.52-1.68 (2H, m), 1.68-1.82 (2H, m), 1.82-1.97 (2H, m), 2.33-2.45 (2H, m), 3.11 (2H, sept, J=6.8 Hz), 3.43 (2H, t, J=7.0 Hz), 7.12 (1H, d, J=8.1 Hz), 7.12 (1H, d, J=6.6 Hz), 7.22 (1H, dd, J=8.1, 6.6 Hz), 7.31 (1H, dd, J=8.1, 4.8 Hz), 7.98 (1H, dd, J=8.1, 1.5 Hz), 8.42 (1H, d, J=4.8 Hz), 8.72 (1H, br s). EIMS m/z (relative intensity): 425 (M+), 407 (100). Elementary Analysis: C24H31N3O2S Required: C, 67.73; H, 7.34; N, 9.87; S, 7.53. Found: C, 67.68; H, 7.33; N, 9.86; S, 7.57. |
| 27% |
With 18-crown-6 ether; potassium carbonate; In N,N-dimethyl-formamide; at 80.0℃; for 4h; |
To a stirred solution of oxazolo[4,5-b]-pyridine-2-thiol (21a) (64?mg, 0.42?mmol) and 2e (150?mg, 0.42?mmol) in DMF (3 mL) were added K2CO3 (64?mg, 0.47?mmol) and 18-crown-6 (11?mg, 0.04?mmol). The reaction mixture was stirred at 80 C for 4?h and diluted with water and AcOEt. The organic layer was washed with water and dried over MgSO4. After filtration, the solvent was concentrated in vacuo. The residue was purified by silica gel column chromatography, and eluted with AcOEt/n-hexane (1:2) to give a solid, which was recrystallized from AcOEt/n-hexane to afford 22a (49?mg, 27%) as colorless needles. Mp 94-95?C; IR (KBr) cm-1: 3230, 2965, 1646, 1497, 1403; 1H-NMR (DMSO-d6) delta: 1.14 (12H, d, J?=?6.8?Hz), 1.52-1.68 (2H, m), 1.68-1.82 (2H, m), 1.82-1.97 (2H, m), 2.33-2.45 (2H, m), 3.11 (2H, sept, J?=?6.8?Hz), 3.43 (2H, t, J?=?7.0?Hz), 7.11 (1H, d, J?=?8.1?Hz), 7.12 (lH, d, J?=?6.6?Hz), 7.22 (lH, dd, J?=?8.1, 6.6?Hz), 7.31 (1H, dd, J?=?8.1, 4.8?Hz), 7.98 (1H, dd, J?=?8.1, 1.5?Hz), 8.42 (1H, d, J?=?4.8?Hz), 8.72 (lH, br s); EIMS m/z: 425 (M+); Anal. Calcd for C24H31N3O2S: C, 67.73; H, 7.34; N 9.87; S, 7.53. Found: C, 67.68; H, 7.33; N, 9.86; S, 7.57. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping