|
In carbon disulfide at -5 - 20℃; for 0.5h; |
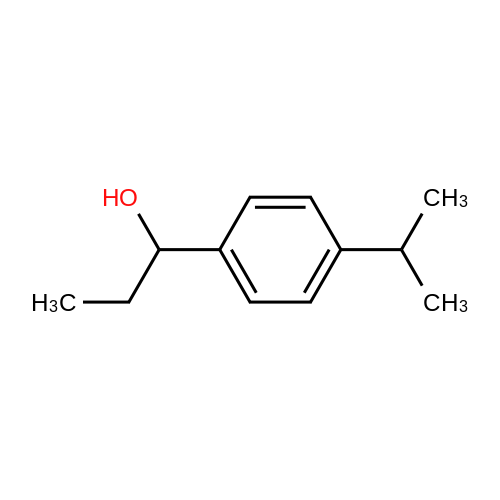
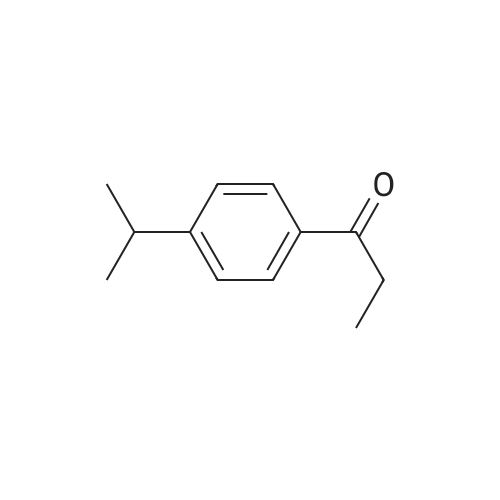
11 1-(4-Isopropylphenyl)propan-1-ol
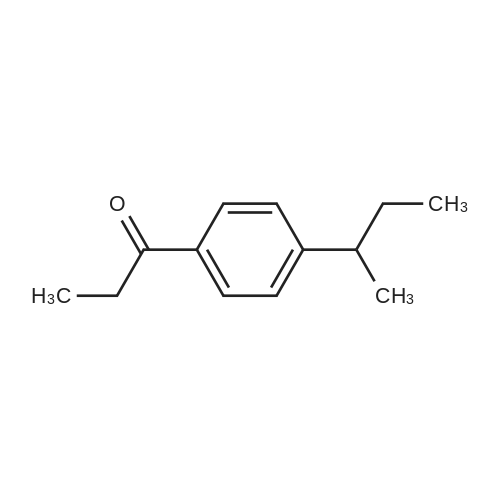
Propionyl chloride (11.6 g, 125 mmol) was dropwise added to a suspension of aluminium chloride (16.7 g, 125 mmol) and cumene (18.0 g, 150 mmol) in carbon disulfide (30 mL) at -5° C., and the mixture was stirred for 30 minutes at room temperature. The reaction mixture was poured into water with ice, and the organic layer was separated, washed with an aqueous saturated sodium hydrogencarbonate and water, dried over magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 1-(4-isopropylphenyl)propan-1-one (24.7 g). Sodium borohydride (1.29 g, 34.2 mmol) was added to a solution of the thus-obtained compound (13.0 g, 68.4 mmol) in ethanol (80 mL) with cooling with ice, and the mixture was stirred for 30 minutes at room temperature. Water was added to the reaction mixture, which was then extracted with ethyl acetate. The organic layer was washed with water, dried over magnesium sulfate, filtered, and concentrated under reduced pressure to obtain the title compound (11.5 g, yield 79%). This was oily.1H-NMR (CDCl3) δ: 0.91 (3H, t, J=7.4 Hz), 1.25 (6H, d, J=7.0 Hz), 1.63-1.92 (2H, m), 1.94 (1H, br s), 2.90 (1H, septet, J=7.0 Hz), 4.47-4.61 (1H, m), 7.16-7.29 (4H, m). |
|
Stage #1: propionyl chloride With aluminum (III) chloride at -10 - -5℃; for 6h;
Stage #2: Isopropylbenzene at -10 - 25℃; for 5h; |
1 Example 1
A) First, 605 g of dichloroethane was charged into a 2000 L four-necked distillation flask, and then 248 g of aluminum chloride was added,Force stirrer and constant temperature water bath, cooling to -10 .B) 176 g of propionyl chloride was dropwise added to the distillation flask, the dropping time was controlled for 5 hours, and the reaction temperature was controlled at -5 ° C,After completion of the dropwise addition, stirring was continued for 1 h.C) keep the kettle temperature at -10 , dropping cumene 218g, drop time control in 4h, the temperature rose to 25 , continue stirring 1h.D) sampling detection, when the content of cumene ≤ 0.5%, the reaction system temperature is reduced to -10 ,Increase the stirring speed Add 650g of water slowly, carry out extraction, termination of the reaction, extraction process, the control reactor temperature <40 .E) Transfer the reaction mixture into a separatory funnel, allow to stand for 1 h, and place the lower layer into the beaker., Add 350g dichloroethane, stir, standing stratified 1h, the lower oil layer into the beaker.F) 300 g of a 10% sodium hydroxide solution was added to the beaker, stirred sufficiently, and the collected oil layer was washed with caustic,Standing stratification 2h, the lower layer of oil into the rotary evaporator, the upper lye layer collection after the next batch of sets.G) Turn on the rotary evaporator switch for heating, raise the temperature to 80 ° C, distill, collect the collected dichloroethaneAfter the application. The resulting kettle liquid is p-isopropylpropiophenone. |
|
Stage #1: propionyl chloride With aluminum (III) chloride In 1,2-dichloro-ethane at -5℃; for 5h;
Stage #2: Isopropylbenzene In 1,2-dichloro-ethane at -5 - 25℃; for 5h; |
1.a; 1.b; 1.c; 1.d; 1.e; 1.f; 1.g
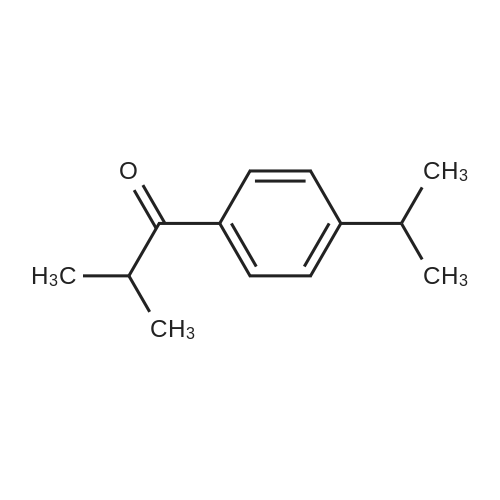
a) Add 300 g of dichloroethane to a 1000 L four-necked distillation flask and pour 125 g of aluminum trichloride to open the magnetic stirrer and the constant temperature water bath and cool to -10 °C. b) 88 g of propionyl chloride was added dropwise to the distillation flask to control the dropping time for 4 h, and the reaction temperature was controlled to -5 °C. After completion of the dropwise addition, stirring was continued for 1 hour. c) continue to keep the kettle temperature at -5 , dropping 10mg of cumene, dropping time control at 4h, after the drop of the kettle temperature increased to 25 °C, continue stirring 1h. d) sampling test, when the cumene content ≤ 0.5%, the reaction system temperature down to -10 , increase the stirring rate, slowly add 320g of water to extract, to terminate the reaction, the extraction process, control the reactor temperature < 40 °C. e) The reaction solution was transferred to a separatory funnel and allowed to stand for 1 h. Place the lower layer into the beaker. The upper layer of water, add dichloroethane 175g, fully stirred, standing stratification 1h, the lower layer into the beaker. f) 150 g of 10% sodium hydroxide solution was added to the beaker and stirred thoroughly. The collected oil layer was subjected to alkali washing, and the mixture was allowed to stand for 2 hours. The lower layer was placed in a rotary evaporator and the upper layer was collected Apply the next batch. g) Turn the rotary evaporator switch to heat, heat up to 80 °C, and collect the collected dichloroethane. The resulting solution is p-isopropylphenylacetone. |
|
Stage #1: propionyl chloride With hydrogenchloride; aluminum (III) chloride In 1,2-dichloro-ethane at -10℃; for 5h; Industrial scale;
Stage #2: Isopropylbenzene In 1,2-dichloro-ethane at -10 - 25℃; for 5h; Industrial scale; |
1
First 295kg dichloroethane was measured through elevated tank, then added to a 1000L of enamel reactor A, and then through the hand hole placed 120kg of aluminum chloride into the reactor, start the Stirrer and the freezer brine valve.The reactor temperature was reduced to -10 ± 2 ° C.Start hydrochloric acid Falling film absorption column to maintain the reactor at 10 ± 2 ° C, by dropping pump added dropwise a 85kg of propionyl chloride to the reaction kettle, and the control dropping time 4h. After the dropwise addition , the stirring was continued for 1h.Continue to maintain the reactor temperature at -10 ± 2 ° C, then by dropping pump added dropwise a 105 kg of Isopropyl benzene to the reaction kettle, and the control dropping time 4h. After the dropwise addition , close the frozen brine valve. The reactor temperature raised to 25 ± 2 ° C, and stirring was continued for 1h.Sampling test, when Isopropyl benzene content ≤ 0.5%, then reaction system temperature lowered to -10 ± 2 ° C, and start the Stirrer and the freezer brine valve. Then slowly added 390kg of clean water for extraction, and terminate the reaction. In the extraction process, the reactor temperature controlled <40°C.The reaction solution was pumped through the material to the bottom of the cone stirred tank, and carried out standing stratification for 2h. The lower layer reservoir placed into the caustic washing stirred tank, the upper aqueous layer was added dichloroethane 300kg, start the stirrer, then stirred for 2h and carried out standing stratification. The lower layer reservoir placed into the caustic washing stirred tank, the upper aqueous layer was discharged into the sewage treatment station for treatment.The 900kg measured clean water was added to sodium hydroxide stirred tank, then through the hand hole placed 100kg sodium hydroxide in sodium hydroxide stirred tank, start the stirrer to prepare a 10% sodium hydroxide solution, then enter into to sodium hydroxide solution into the elevated tank. Through the sodium hydroxide solution elevated tank, the 300kg of 10% of sodium hydroxide solution added to alkali washed stirred tank metering, start the stirrer, collect the collected reservoir to carry out alkali washing, then after stirring for 2h, carried out standing stratification for 2h. The lower layer reservoir placed into the distillation kettle A, the upper layer of alkali washing entered into a alkali washing elevated tank through the material pump to use for the next batch of applications.Open the steam valve on the distillation kettle A for heating, heating to 70 ~ 85 ° C for carrying out atmospheric distillation. the collected dichloroethane entered into dichloroethane elevated tank through the material pump for application. When the tower top temperature reduces or no discharge materials then stop the distillation.As the kettle using the nitrogen pressure the material is pressed to the p-isopropyl propiophenone elevated tank. |
|
With aluminum (III) chloride In dichloromethane at 0℃; for 2h; Sealed tube; Inert atmosphere; |
Typical Procedure for Transformation of Arenes 1 into 5-Alkyl-1-aryltetrazoles 3:
General procedure: To a solution ofcumene 1A (1.0 mmol, 120.0 mg) in CH2Cl2 (2.0 mL) in a 30 mL sealed tube was added acetyl chloride(1.2 mmol, 85.4 μL) at 0 °C. After flash with argon gas, the mixture was stirred for a few minutes.Anhydrous AlCl3 (1.2 mmol, 161.0 mg) was added and the obtained mixture was stirred for 2 h at 0 °C.Then, the reaction mixture was quenched with cooled water (1.0 mL) and stirred for 0.5 h. Afterremoval of the solvent under reduced pressure, MeOH (3.0 mL), NH2OHHCl (1.5 mmol, 107.0 mg), andK2CO3 (1.5 mmol, 207.0 mg) were added. After flash with argon gas, the obtained mixture was stirredfor 16 h at rt. After removal of the solvent under reduced pressure, toluene (3.0 mL), DPPA (2.5 mmol,0.54 mL), and DBU (3.5 mmol, 0.53 mL) were added to the residue. After flash with argon gas, themixture was warmed at 120 °C for 16 h. The mixture was cooled to rt and then, sat. NaHCO3 aq. (15.0mL) was added. The mixture was filtered through Celite, and the filtrate was extracted with AcOEt(20.0 mL ×3). The organic layer was dried over Na2SO4. After removal of the solvent under reducedpressure, the residue was purified by short column chromatography on silica gel (n-hexane/AcOEt,3:1-1:3) to afford 1-(4’-isopropylphenyl)-5-methyl-1H-tetrazole 3Aa in 60% yield (121.1 mg). |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping