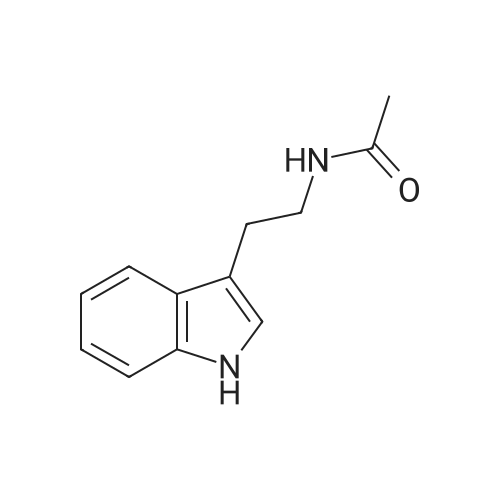
| 71% |
With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 20℃; for 3h; Inert atmosphere; |
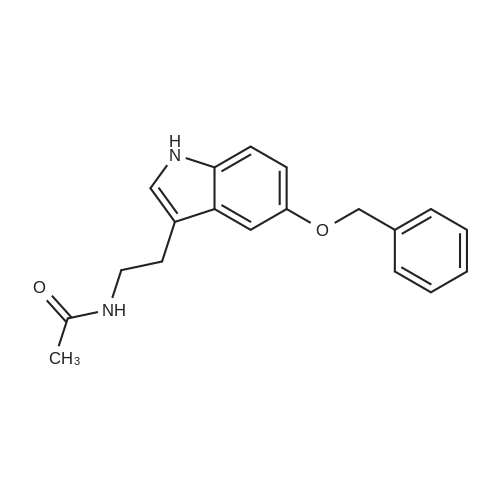
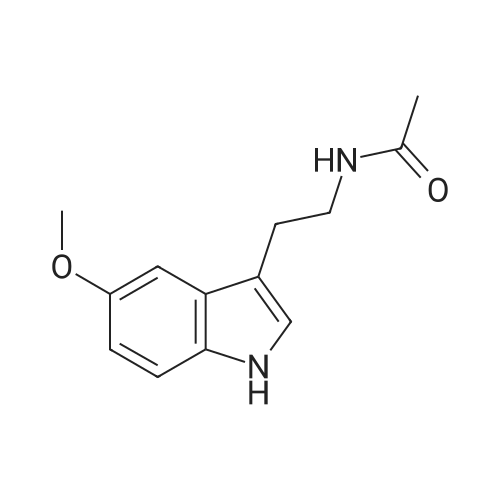
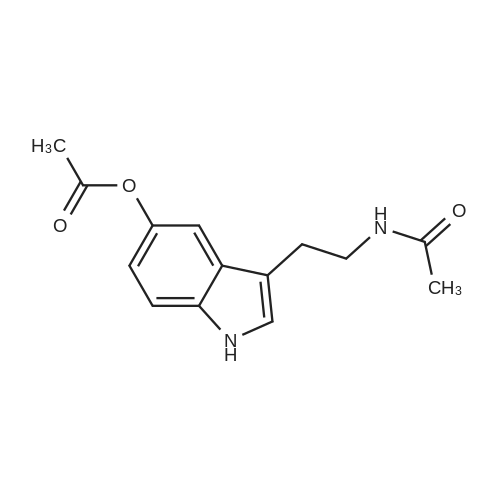
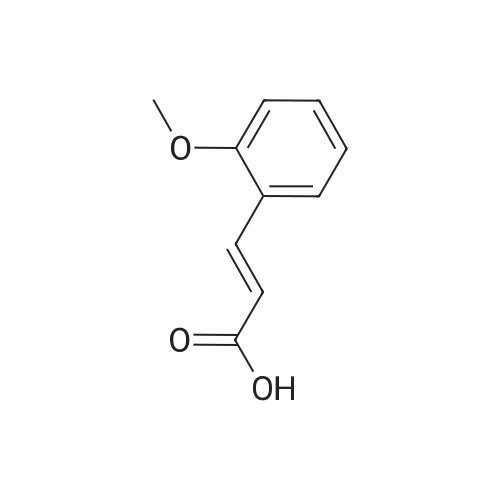
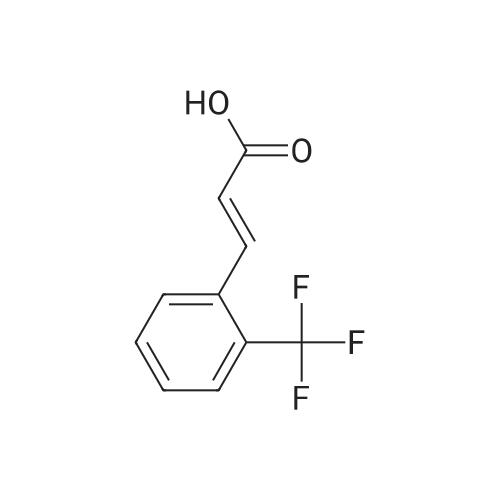
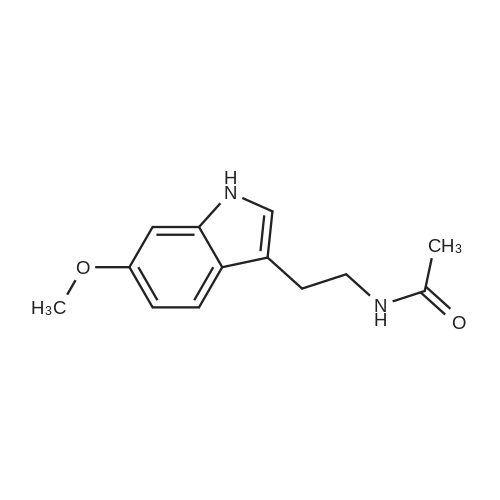
4 Example 4: Preparation of 3-(2-acetamidoethyl)-1H-indol-5-yl (E)-3-(2-methoxyphenyl)acrylate (Compound 4)
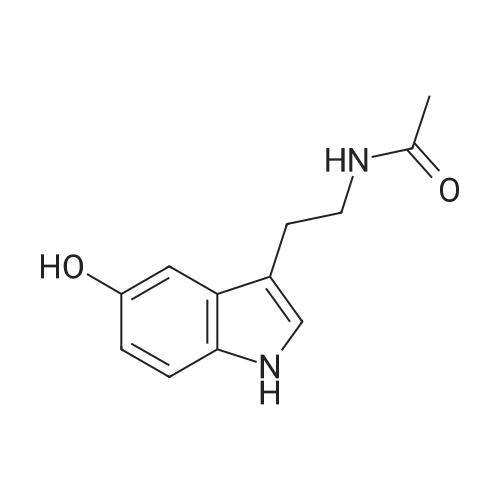
To a solution of N-(2-(5-hydroxy-1H-indol-3-yl)ethyl)acetamide (1 eq) and Et3N (1.5 eq) in CH2Cl2 (0.057 M) under argon, a solution of the corresponding acrylic acid (1.2 eq) and HATU (1.5 eq) in dry CH2Cl2 (0.057 M) was added dropwise. The resulting solution was stirred at room temperature. When the reaction was finished (TLC) it was quenched with distilled water and allowed to stir for 15 min. Thereafter, the mixture was extracted three times with CH2Cl2 (3 x 20 mL). The organic layer was washed with brine, filtrated and dried over anhydrous MgSO4. The resulting solid was purified by flash chromatography on silica gel using CH2Cl2/MeOH mixtures as eluent. Following general procedure A, N-(2-(5-hydroxy-1H-indol-3-yl)ethyl)acetamide (50 mg, 0.23 mmol) and Et3N (48.0 μL, 0.34 mmol) in CH2Cl2 (5 mL), (E)-3-(2-methoxyphenyl)acrylic acid (48.8 mg, 0.27 mmol) and HATU (129.3 mg, 0.34 mmol) in CH2Cl2 (5 mL), 3 h, flash chromatography on silica gel (CH2Cl2-MeOH 0-2 %) to afford compound 4 as a white solid (59 mg, 71 % yield); MP: 163-165 °C; IR (KBr) v 3366, 2961, 2924, 1715, 1634, 1487, 1294, 1252, 1158, 765; 1H NMR (300 MHz, DMSO-d6) δH 10.93 (1H, Sbr, NH), 8.07 (1H, d, J = 16.2 Hz, 3'-H), 7.91 (1H, Sbr, NH), 7.81 (1H, dd, J6"-5" = 7.5 Hz, J6''-4''= 1.6 Hz, 6"-H), 7.46 (1H, ddd, J4''-3'' = 8.5 Hz, J4''-5''= 7.5 Hz, J4''-6" = 1.6 Hz, 4"-H), 7.36 (1H, d, J = 8.6 Hz, 7-H), 7.31 (1H, d, J = 2.2 Hz, 4-H), 7.23 (1H, d, J = 2.2 Hz, 2-H), 7.13 (1H, d, J3"-4" = 8.5 Hz, 3"-H), 7.03 (1H, m, 5"-H), 6.93-6.83 (2H, m, 2'-H, 6-H), 3.90 (3H, s, OCH3), 3.37-3.23 (2H, m, CH2CH2NHCOCH3), 2.79 (2H, t, J = 7.3 Hz, CH2CH2NHCOCH3), 1.80 (3H, s, CH2CH2NHCOCH3); 13C NMR (75 MHz, DMSO-d6) δC 169.0, 166.0, 158.0, 143.2, 140.5, 134.0, 132.3, 128.9, 127.3, 124.1, 122.1, 120.7, 117.7, 115.3, 112.2, 111.8, 111.6, 110.4, 55.7, 39.5, 25.1, 22.6; HRMS (ES+) mass calcd. for C22H22N2O4 378.1580; found [(M+H)+] 379.1669; found [(M+Na)+] 401.1483; found [(2M+Na)+] 779.3040; Anal. calcd. for C22H22N2O4: C: 69.83 %; H: 5.86 %; N: 7.40 %; found: C: 69.78 %; H: 5.86 %; N: 7.25 %. H NMR (300 MHz, DMSO-d6) δH 10.93 (1H, Sbr, NH), 8.07 (1H, d, J = 16.2 Hz, 3'-H), 7.91 (1H, Sbr, NH), 7.81 (1H, dd, J6"-5" = 7.5 Hz, J6"-4" = 1.6 Hz, 6"-H), 7.46 (1H, ddd, J4''-3" = 8.5 Hz, J4"-5" = 7.5 Hz, J4''-6" = 1.6 Hz, 4"-H), 7.36 (1H, d, J = 8.6 Hz, 7-H), 7.31 (1H, d, J = 2.2 Hz, 4-H), 7.23 (1H, d, J = 2.2 Hz, 2-H), 7.13 (1H, d, J3"-4" = 8.5 Hz, 3"-H), 7.03 (1H, m, 5"-H), 6.93-6.83 (2H, m, 2'-H, 6-H), 3.90 (3H, s, OCH3), 3.37-3.23 (2H, m, CH2CH2NHCOCH3), 2.79 (2H, t, J = 7.3 Hz, CH2CH2NHCOCH3), 1.80 (3H, s, CH2CH2NHCOCH3); 13C NMR (75 MHz, DMSO-d6) δC 169.0, 166.0, 158.0, 143.2, 140.5, 134.0, 132.3, 128.9, 127.3, 124.1, 122.1, 120.7, 117.7, 115.3, 112.2, 111.8, 111.6, 110.4, 55.7, 39.5, 25.1, 22.6; HRMS (ES+) mass calcd. for C22H22N2O4 378.1580; found [(M+H)+] 379.1669; found [(M+Na)+] 401.1483; found [(2M+Na)+] 779.3040; Anal. calcd. for C22H22N2O4: C: 69.83 %; H: 5.86 %; N: 7.40 %; found: C: 69.78 %; H: 5.86 %; N: 7.25 %. |

 Chemistry
Chemistry
 Pharmaceutical Intermediates
Pharmaceutical Intermediates
 Inhibitors/Agonists
Inhibitors/Agonists
 Material Science
Material Science














 For Research Only
For Research Only
 110K+ Compounds
110K+ Compounds
 Competitive Price
Competitive Price
 1-2 Day Shipping
1-2 Day Shipping