Abstract
Glioblastoma multiforme (GBM) is a highly aggressive brain tumor characterized by uncontrollable diffusive growth, resistance to chemo- and radiotherapy, and a high recurrence rate leading to a low survival rate of patients with GBM. Due to a large number of signaling pathways regulating GBM pathogenesis, one of the promising directions is development of novel anti-glioblastoma compounds based on natural metabolites capable of affecting multiple targets. Here, we investigated the antitumor potential of the semisynthetic triterpenoid soloxolone tryptamide (STA) against human glioblastoma U87 cells. STA efficiently blocked the growth of U87 cells in 2D and 3D cultures, enhanced adhesiveness of tumor cells, and displayed synergistic cytotoxicity with temozolomide. In silico analysis suggested that the anti-glioblastoma activity of STA can be explained by its direct interaction with EGFR, ERBB2, and AKT1 which play an important role in the regulation of GBM malignancy. Along with direct effect on U87 cells, STA normalized tumor microenvironment in murine heterotopic U87 xenograft model by suppressing the development of immature blood vessels and elastin production in the tumor tissue. Taken together, our results clearly demonstrate that STA can be a novel promising antitumor candidate for GMB treatment.
Similar content being viewed by others
INTRODUCTION
According to the World Health Organization, glioblastoma multiforme (GBM) is one of the most aggressive brain tumors with a prevalence of about one case per 100,000 people per year [1]. Despite extensive treatment including radical resection, tumor bed radiotherapy, and chemotherapy with temozolomide (TMZ), the median overall survival for GBM patients is as little as 15-18 months [2]. This is due to a high malignant potential of glioblastoma cells capable of diffuse infiltrative outgrowth into surrounding tissues and development of resistance to radiotherapy and chemotherapy, which underlies the tendency of GBM to relapse [1]. The low efficacy of GBM treatment is also associated with a very limited arsenal of drugs approved for its treating. For example, the use of the first-line drug TMZ (alkylating agent based on imidazole tetrazine) is limited by the development of resistance to this agent in GBM cells, while administration of the second-line drug bevacizumab (humanized recombinant antibody against vascular endothelial growth factor, VEGF) is hindered by its low ability to penetrate into the tumor tissue [3]. Therefore, a search for new drugs able to pass across the blood–brain barrier and to block proliferation and invasion of GBM cells is crucial for current medicinal chemistry.
A distinctive feature of GBM is its prominent heterogeneity, i.e., the presence in tumor of cell populations differing in the profiles of key signaling pathways and, as results, in their functional states [2]. This property underlies a low efficacy of existing approaches to GBM chemotherapy [4] and suggests the need for creating new anti-glioblastoma drugs capable of affecting multiple targets [5]. One of the most important sources of multitarget pharmacologically active compounds are natural metabolites that are currently studied in both in vitro experiments and in vivo glioblastoma orthotopic models for their ability to suppress GBM cell proliferation and invasiveness [6].
Previously, in a collaborative study with the Laboratory of Pharmacologically Active Substances at the Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of the Russian Academy of Sciences, we developed a semisynthetic derivative of glycyrrhetinic acid, soloxolone tryptamide (STA), which exhibited multiple in vitro antitumor effects in GBM cells. In particular, STA induced mitochondrial stress followed by initiation of apoptosis, abolished the clonogenic potential of tumor cells, and suppressed cell motility and vasculogenic mimicry [7]. Moreover, STA efficiently penetrated through the blood–brain barrier and suppressed the growth of human U87 glioblastoma in the heterotopic xenograft mouse model [7]. As we aimed to obtain a deeper insight into the anti-glioblastoma potential of STA, here we investigated the effect of STA on the growth and adhesive properties of human U87 cells in a 3D culture, analyzed the synergistic cytotoxic activity of STA and TMZ against U87 cells, and evaluated the effect of STA on several tumor microenvironment parameters in the U87 glioblastoma model in vivo. We also used computational biology approaches to identify a set of putative STA targets involved in its anti-glioblastoma action. Based on the data obtained, STA can be considered as a promising drug candidate with a multiple modulating effect on the key parameters determining the high malignancy of GBM.
MATERIALS AND METHODS
Soloxolone tryptamide (STA) was synthesized in the Laboratory of Pharmacologically Active Substances at the Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of the Russian Academy of Sciences, by Dr. O. V. Salomatina according to the previously published method [7]. The crystalline powder of STA was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM and stored at –20°C.
Human GBM U87 cell culture. Human U87 glioblastoma cells obtained from the American Cell Culture Collection (ATCC, USA) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Sigma Aldrich, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA) and antimycotic/antibiotic cocktail (100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.25 µg/ml amphotericin; MP Biomedicals, France). The cell culture was maintained in a humidified incubator at 37°C in 5% CO2 (hereinafter referred to as standard conditions).
Assessment of STA cytotoxicity in a 2D U87 cell culture (MTT assay). U87 cells were seeded in 96-well plates (TPP, Switzerland) at a concentration of 104 cells per well in DMEM supplemented with 10% FBS and incubated for 24 h under standard conditions. Next, the culture medium was replaced with a serum-free medium containing STA (100-800 nM), and the cells were incubated for 24 h followed by adding tetrazolium dye (MTT) solution to each well to a final concentration of 0.5 mg/ml. After 2-hour incubation, the medium was removed and 100 µl of DMSO was added to dissolve formazan crystals. The absorbance of the resulting solution was measured at 570 nm and at the reference wavelength of 620 nm with a Multiscan FC Microplate Photometer (Thermo LabSystems, Finland).
Assessment of STA effect on the growth of U87 cell spheroid. The effect of STA on the growth of U87 cell spheroids was analyzed by culturing cells in plates covered with (i) 1% agarose or (ii) hydrophilic non-ionic neutrally charged hydrogel (hereinafter ultra-low attachment plates) (Corning, USA). U87 cells resuspended in DMEM/10% FBS were seeded into agarose-coated 48-well plates (TPP, Switzerland) and 6-well ultra-low attachment plates at a concentration of 2×103 and 5×105 cells per well, respectively. After incubation for 7 (i) or 4 (ii) days under standard conditions, STA was added to the formed spheroids to a final concentrations of 0.1-2 µM in serum-free DMEM. The spheroids were imaged immediately after adding STA solutions and then every 2-3 days until 28 days of incubation (i) or once every 2 days (ii) with a ZEISS Primo Vert inverted microscope with a built-in ZEISS AxioCam ERc5s camera (Carl Zeiss Microscopy GmbH, Germany). For each experimental group, the area of 4 (i) or 250 (ii) spheroids was measured using the ImageJ software (NIH, USA) and normalized to the average area of control spheroids (U87 cells without added STA).
Assessment of STA effect on the TGF-β-stimulated formation of spheroid-like structures on a U87 cell monolayer. U87 cells resuspended in DMEM/10% FBS were seeded into 96-well plates at a concentration of 5×103 cells per well and cultured overnight under standard conditions. The culture medium was then replaced with a serum-free medium containing TGF-β1 (50 ng/ml) (Prospec, Israel) and STA (200 or 400 µM). After 48 h of incubation, formation of spheroid-like structures was imaged with an EVOS XL Core inverted microscope (Thermo Fisher Scientific, USA). For each experimental and control group, 4 fields of view (FOV) were imaged at 200× magnification using a microscope built-in CMOS camera. For each experimental group, the area of at least 10 spheroid-like structures was measured using the ImageJ software and normalized to the mean area of spheroids in the control group (cells incubated with TGF-β in the absence of STA).
Assessment of STA effect on the adhesion properties of U87 cells (trypsin test). U87 cells were seeded into 96-well plates at a concentration of 104 cells per well in DMEM/10% FBS and cultured overnight under standard conditions. The culture medium was replaced with a serum-free medium containing STA at a concentration of 25-100 nM and the cells were incubated for another 24 h under standard conditions. Next, 30 µl of TrypLETM (Gibco) diluted with phosphate buffered saline (PBS) (1 : 10) was added to the cells. The cells were incubated in a CO2 incubator for 3 min and then washed twice with DMEM/10% FBS. The number of cells remaining attached to the well bottom after washing was assessed in the MTT assay.
Assessment of STA effect on the adhesion of U87 cell to the collagen substrate. U87 cells were seeded into 6-well plates at a concentration of 2.8×105 cells per well in DMEM/10% FBS and incubated overnight under standard conditions. Next, the culture medium was removed and STA (25-100 nM) dissolved in serum-free DMEM medium was added to the cells. The cells were cultured for 24 h under standard conditions, detached from the plate surface with TrypLETM, resuspended in DMEM/10% FBS, and seeded into 96-well plates pre-coated with rat tail collagen (Cell Applications, USA) at a concentration of 105 cells per well. After incubation for 1 h under standard conditions, the cells were washed twice with PBS and the number of attached cells was assessed in the MTT test.
Assessment of the STA and TMZ combined action on U87 cells. U87 cells were seeded into 96-well plates at a concentration of 104 cells per well in DMEM/10% FBS and incubated overnight under standard conditions. The culture medium was then replaced with serum-free DMEM containing STA (0.1-1 µM) and/or TMZ (100-500 µM), and the cells were cultured for 72 h under standard conditions. Next, the number of live U87 cells was assessed in the MTT test. The effect of the STA and TMZ combined application was analyzed using the SynergyFinder web resource (https://synergyfinder.fimm.fi) [8].
Assessment of aldehyde dehydrogenase activity in U87 cells. U87 cells resuspended in DMEM/10% FBS were seeded into 6-well plates (Servicebio, China) at a concentration of 2.8×105 cells per well and incubated overnight under standard conditions. The culture medium was then replaced with serum-free DMEM containing 0.5 or 1 μM STA, and the cells were incubated for 24 h under standard conditions. Next, the activity of aldehyde dehydrogenase (ALDH) was assessed using AldeRed® ALDH Detection Assay kit (Sigma-Aldrich Inc., Germany) according to the manufacturer’s protocol. Briefly, the cells were detached from the plate surface with TrypLETM and resuspended in 1 ml of AldeRed Assay Buffer. AldeRed fluorescent dye (5 µl) was added to each tube; control tubes also contained 5 µl of ALDH inhibitor diethylaminobenzaldehyde (DEAB). After incubation for 45 min under standard conditions, the cells were collected by centrifugation, resuspended in 200 µl of buffer, placed on ice, and analyzed with a NovoCyte flow cytometer (ACEA Biosciences, Inc, USA) using NovoExpress Software (ACEA Biosciences, Inc). For each sample, 10,000 events were collected and analyzed.
In silico identification of putative protein targets of STA. At the first stage, putative protein targets of STA were identified based on the analysis of its structure using the Polypharmacology Browser 2.0 [9] and SwissTargetPrediction [10] chemoinformatics platforms. Next, predicted protein targets were added separately to the list of genes associated with GBM pathogenesis (3,197 genes; ID: C1621958; DisGeNET database) to reconstruct gene association networks using the STRING (Search Tool for Retrieval of Interacting Genes/Proteins) database [11]. The resulting final networks were visualized in the Cytoscape 3.9.1 software and analyzed with the cytoHubba plugin [12]: Degree, MCC, and Betweenness (measures of network gene centrality) were calculated for each primary STA target, and obtained list of STA targets were ranked accordingly to these parameters. The total rank was finally calculated as a mean of target rank for Degree, MCC, and Betweenness.
Next, we used molecular docking to simulate interaction between STA and top targets associated with the glioblastoma gene network (i.e., those characterized by the lowest rank). The crystal structures of EGFR (PDB ID: 8A2D), AKT1 (PDB ID: 3O96), FYN (PDB ID: 2DQ7), and ERBB2 (PDB ID: 3RCD) were retrieved from the RCSB PDB database (https://www.rcsb.org/). Water molecules and cocrystallized ligands (validated inhibitors) were extracted with the Discovery Studio Visualizer v21.1.0, and polar hydrogens and partial atomic charges were added according to the Gasteiger method using AutoDock Tools 1.5.7. The 2D structures of STA and validated inhibitors were 3D converted in the MarvinSketch v22.1 software and their molecular geometry was optimized with Avogadro v1.2.0 (MMFF94 force field). Ligand intramolecular bonds were allowed to rotate freely using AutoDock Tools 1.5.7. Protein–ligand interactions were simulated with AutoDockVina [13] with the following grid box parameters: EGFR – size_x = 22, size_y = 14, size_z = 16, center_x = –1.379, center_y = –9.243, center_z = 18.98; AKT1 – size_x = 14, size_y = 18, size_z = 18, center_x = 8.507, center_y = –7.504, center_z = 10.15; FYN – x = 14, size_y = 14, size_z = 14, center_x = –15.594, center_y = 17.532, center_z = –12.787; ERBB2 – x = 16, size_y = 16, size_z = 14, center_x = 13.147, center_y = 29.114, center_z = 29.114. Docking complexes with tight ligand–protein interactions (ΔG ≤ 7.0 kcal/mol) were visualized in the 3D and 2D formats using Discovery Studio Visualizer v21.1.0 and LigPlot+ v.2.2.7, respectively, to select the structures stabilized by the largest number of hydrogen bonds.
Putative STA protein targets selected by molecular docking were mapped to the human kinome hierarchical tree using the KinMap platform (http://www.kinhub.org/kinmap/). Additionally, GBM pathogenesis-related kinases were highlighted in the reconstructed kinome, in accordance with the Open Targets Platform (https://platform.opentargets.org/).
Laboratory animals. Six- to eight-week-old athymic female Nude mice (Russian National Center for Genetic Resources of Laboratory Animals) were kept at a specific pathogen-free (SPF) animal facility (six animals per cage) in accordance with the standards for keeping SPF animals at the Institute of Cytology and Genetics (ICG), Siberian Branch of the Russian Academy of Science (Novosibirsk, Russia). The mice had an ad libitum access to food and water. Cages, bedding, feed, and water were autoclaved.
Heterotopic GBM xenograft model. A xenograft model with primary tumor nodes was created by using a suspension of U87 glioblastoma cells (2×107 cells/ml) in 0.1 ml of 0.9% sodium chloride and 0.05 ml of Matrigel (Matrigel® Matrix; Corning, USA) that was inoculated subcutaneously into the left flanks of mice. On day 5 after transplantation, the animals were divided into groups (six mice in each): (i) mice receiving intraperitoneal (i.p.) injection of 10% Tween-80 (control) and (ii) mice receiving i.p. injection of STA dissolved in 10% Tween-80 at a dose of 20 mg/kg body weight. All i.p. injections were carried out in a volume of 200 μl 3 times a week (a total of 7 injections). On day 21, the mice were sacrificed by cervical dislocation under isoflurane anesthesia, and developed tumors were sampled for subsequent histology examination.
Histology. Tumor samples were fixed in 10% neutral formalin (BioVitrum, Russia) followed by dehydration in escalating alcohol concentrations, cleared with xylene, and embedded in HISTOMIX paraffin (BioVitrum). Paraffin sections up to 5 µm thick were stained with hematoxylin and eosin. The location and amount of tumor stromal component (elastin fibers) in the sections was assessed by Van Gieson staining [14]. Histology sections were analyzed with an AxioStar Plus microscope equipped with an Axiocam MRc5 camera (Zeiss, Germany).
Tumor morphometric examination. Tumor morphometric analysis of histology sections was performed with a closed test system of 100 points with an area of 3.2×106 μm2 at 200× microscope magnification with a randomly overlaid grid. The volume density of the tumor stromal component and the numerical density of thin-walled blood vessels in the tumor tissue were calculated. The volume density of the analyzed histological structure was calculated as follows: Vv = (Pstructure/Ptest)×100%, where Pstructure is the number of points per structure, and Ptest is the total number of test points (here, 100). The numerical density of the analyzed histological structure was estimated by counting the number of structures within the test field (3.2×106 µm2). A total of 5 FOVs were assessed in each sample for each histological structure (i.e., a total of 30 FOVs were analyzed in each experimental group).
Statistical analysis. The data were analyzed using the GraphPad Prism 8.0.1 software package. The normality of distribution for quantitative parameters was assessed with the Shapiro–Wilk test. The significance of differences between the groups with normal and non-normal distribution was analyzed using unpaired Student’s t-test and non-parametric Mann–Whitney U-test, respectively. The differences were considered significant at p < 0.05.
RESULTS
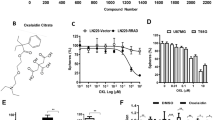
STA efficiently inhibited the growth of U87 cell spheroids. First, we evaluated the cytotoxicity of STA against human U87 GBM cells in vitro (Fig. 1a). At low submicromolar concentrations, STA efficiently suppressed the viability of cultured U87 cell with IC50 (24 h) of ~0.7 μM (Fig. 1b). Considering that cell monolayer (2D culture) does not fully reproduce tumor growth in vivo, i.e., when the cells have different access to nutrients and oxygen depending on their location in the tumor, the antitumor potential of STA was further analyzed in GBM spheroids (3D culture). For this, U87 cell spheroids formed in 1% agarose-coated plates were treated with STA (0.5-4 μM) and their relative area was assessed every 2-4 days for 21 days. The area of spheroids in the control group (in the absence of STA) increased linearly (Fig. 1c), whereas STA inhibited the growth of spheroids in a dose-dependent manner, which became evident starting from day 7 of incubation. By the end of the experiment, STA at the concentrations of 1, 2, and 4 μM significantly reduced the area of spheroids (2.5-, 3-, and 12.1-fold , respectively), compared to the control (Fig. 1c). Inhibition of GBM cell growth by STA in a 3D culture was also verified in an independent experiment with U87 cell spheroids formed on ultra-low attachment plates. It was found that exposure to 2 μM STA for 48 h also resulted in a significantly smaller area of the spheroids (Fig. 1d).
Transforming growth factor beta (TGF-β) plays an essential role in the regulation of GBM malignancy potential by promoting cell stemness, invasion, and metastasis [15]. Taking into account that TGF-β stimulates the growth of glioblastoma spheroids [16], we assessed the effect of STA on the formation of spheroid-like structures in a TGF-β-stimulated U87 cell monolayer. We found that after 3 days, an intact U87 cell monolayer contained a small number of multilayered structures, the size of which increased 5.3-fold after exposure to TGF-β compared with control cells, and this effect was unrelated to the activation of cell proliferation (Fig. 1, e and f). Incubation of TGF-β-stimulated cells with 200 nM STA significantly (2.6-fold) reduced the size of spheroid-like structures compared with the TGF-β-treated control cells. When the concentration of STA was increased to 400 nM, the size of spheroids in the TGF-β-stimulated U87 cells decreased to the level found in untreated control cells (Fig. 1, e and f).
Effect of STA on the growth of human glioblastoma cell. a) Structural formula of STA. b) STA cytotoxicity against U87 cells. c and d) Effect of STA on the growth of U87 cell spheroid in agarose-coated plates (c) and ultra-low attachment plates (d). STA was added to U87 cells followed by estimating spheroid area at various time points (c) or after 48 h of incubation (d) using phase contrast microscopy. e and f) Effect of STA on the assembly of spheroid-like structures in the U87 cell monolayer exposed to TGF-β. Magnification, 200× (e); arrows, spheroid-like structures. The area of spheroids (c, d) and spheroid-like structures (e) was calculated using ImageJ software. ** p < 0.01, *** p < 0.001.
STA promoted adhesion of U87 cells. Having established that STA strongly suppresses proliferation of U87 cells (Fig. 1), we assessed whether STA exhibits the antitumor activity at non-toxic concentrations by evaluating its effect on cell adhesion, since it is known that low cell adhesion is associated with a high metastatic potential of GBM [17].
A monolayer of U87 cells was incubated with STA (25-100 nM) or without it (control) for 24 h followed by 3-min treatment with a diluted solution of TrypLETM (recombinant trypsin). The detached cells were washed out and the number of cells remaining attached to the plate was determined using the MTT assay. STA at all concentrations significantly promoted adhesion of U87 cells in a dose-dependent manner (Fig. 2a). The number of cells remaining attached to the plate in the experimental groups exceeded that in the control group 1.9 to 2.8-fold.
We also investigated the effect of STA on the adhesion U87 cell to a collagen substrate that mimicked the extracellular matrix. For this, U87 cells were incubated in the presence of 25-100 nM STA for 24 h, detached from plate bottom with TrypLETM, and transferred to a rat tail collagen-coated 96-well plate. The cells were incubated on the collagen substrate for 1 h; non-attached cells were washed off, and the number of attached cells was assessed by the MTT assay. Similar to the previous experiment, STA significantly enhanced adhesion of U87 cells, which was maximal after incubation with 50 and 100 nM STA (Fig. 2b).
STA and TMZ synergistically affected the viability of U87 cells. As noted above, TMZ is the first-line drug in GBM therapy. It is a DNA-methylating agent that promotes generation of O6-methylguanine and emergence of DNA double-strand breaks resulting from the DNA mismatch repair, which induces the death of GBM cells [18]. Taking into consideration the importance of TMZ in the GBM treatment regimens, assessment of STA action in combination with TMZ is essential aspect for the development of novel anti-glioblastoma drugs. For this, U87 cells were incubated for 72 h in the presence of various concentrations of STA (0.1-1 μM) and TMZ (100-500 μM) followed by estimation of the number of viable cells by the MTT assay and analysis of the combined action of STA and TMZ using the SynergyFinder 2.0 platform [8]. It was found that TMZ demonstrated a low cytotoxicity toward U87 cells [IC50 (72 h), > 500 μM], whereas STA at a concentration of 1 μM caused almost complete death of tumor cells [IC50 (72 h), 0.4 ± 0.1 μM] (Fig. 3a). A combination of TMZ and STA produced a synergistic effect when STA was used within its IC50 range. The highest δ-index of synergy was 54.1 when U87 cells were treated simultaneously with 0.5 μM STA and 100 μM TMZ, resulting in 94% cell death within 72 h from the start of treatment (Fig. 3b). Interestingly, further increase in the TMZ and STA concentrations resulted in the reduced δ-index (Fig. 3b), which suggests induction of compensatory mechanisms against increasing xenobiotic stress. To verify this hypothesis, we assessed the effect of STA on the activity of ALDH, an enzyme involved in detoxification of antineoplastic drugs and suppression of oxidative stress they trigger [19]. For this, U87 cells were incubated for 24 h with 0.5 or 1 μM STA, stained with AldeRed (fluorescent ALDH substrate), and analyzed by flow cytometry. We found that control cells displayed low ALDH activity (ALDHhigh cells; 0.3%; Fig. 3c), whereas STA at the concentrations of 0.5 and 1 μM increased the content of ALDHhigh cells by 17.5 and 50.7%, respectively. Addition of the selective ALDH inhibitor DEAB to the culture medium markedly reduced the effect of STA, indicating that the rise in the fluorescent signal in the experimental groups of cells was related to the ALDH activity (Fig. 3c).
Synergistic effect of STA and TMZ on U87 glioblastoma cells. a) Combined action of STA and TMZ on the viability of U87 cell. b) The profile of the combined cytotoxic action of STA and TMZ created using the SynergyFinder web resource. c) Effect of STA on the ALDH activity in U87 cells. U87 cells were incubated with STA at various concentrations for 24 h and the ALDH activity was determined using AldeRed® ALDH Detection Assay and flow cytometry.
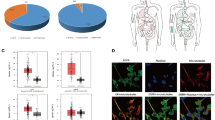
Direct interaction of STA with EGFR, ERBB2, and AKT1 can underlie its anti-glioblastoma activity. Both natural and semisynthetic pentacyclic triterpenoids have a wide range of intracellular molecular targets [20]. To search for probable proteins targets of STA linked to GBM pathogenesis, computational biology approaches were used. At the first stage, a set of putative STA protein targets were predicted based on the STA structure using Polypharmacology Browser 2.0 [9] and SwissTargetPrediction [10] chemoinformatics platforms (Fig. 4a). Based on the key GBM-related genes (according to the DisGeNET), we reconstructed the GBM-associated regulome and incorporated identified target proteins into it, analyzed the network topology, and ranked STA targets according to their interaction with the GBM-associated regulome. The strongest association with GBM pathogenesis among the analyzed proteins was found for the epidermal growth factor receptor (EGFR), serine/threonine protein kinase AKT1, tyrosine protein kinase FYN, and receptor tyrosine protein kinase ERBB2 (also known as HER2) (Fig. 4a). Molecular modeling confirmed that STA is able to interact with EGFR, AKT1, and ERBB2 with low Gibbs free energies (ΔG = –9.2, –7.1 and –7.0 kcal/mol, respectively) in the binding sites for validated inhibitors (Fig. 4b). FYN was excluded from further analysis because the final STA/FYN docking complex was characterized by the binding energy ΔG = –5.3 kcal/mol, which significantly exceeded the threshold value (ΔG ≤ –7.0 kcal/mol) (Fig. 4b). Detailed analysis of the obtained structures showed that STA forms bonds with amino acid residues playing an important role in the functioning of EGFR, AKT1, and ERBB2, in particular, hydrogen bonds with Arg858 in EGFR, Glu85 and Cys296 in AKT1, and Lys753, Asn850, and Asp863 in ERBB2. Identified STA interactions with mentioned proteins were similar to the interactions of corresponding known inhibitors with EGFR, AKT1, and ERBB2 (Fig. 4c). Mapping of the protein targets of STA verified by molecular docking to a phylogenetic tree containing human GBM-associated kinome (reconstructed based on the Open Targets Platform database) independently confirmed the association of EGFR and ERBB2 with GBM pathogenesis (Fig. 4d). Interestingly, although AKT1 is highly integrated into the GBM-specific regulome (Fig. 4a), it was not associated with glioblastoma pathogenesis (Fig. 4d), which might be explained by the difficulties in updating the Open Targets Platform with AKT1 data, because most of the published studies on the relationship between AKT and GBM have been carried out with pan-AKT inhibitors exerting the same effect against different AKT isoforms [21]. Nevertheless, GBM progression seems to be profoundly affected by AKT1, because downregulation of its expression by small interfering RNAs and antisense oligonucleotides significantly reduced proliferation of GBM cell in vitro and in mice model, respectively [22, 23].
Identification of putative protein targets of STA associated with GBM. a) Analysis of integration of potential STA targets in the GBM regulome. The targets of STA were predicted using the Polypharmacology Browser (PPB2) and SwissTargetPrediction (SWT) web resources; their interaction with the GBM-associated regulome was reconstructed based on data retrieved from the DisGeNET and STRING databases and analyzed with the cytoHubba plugin in the Cytoscape software environment. The targets were ranked based on three measures of gene centrality in the gene regulatory network (Degree, MCC, and Betweenness); the rank value was inversely proportional to the target significance in GBM regulation. b) Binding energies for STA and verified AKT1 and EGFR inhibitors determined by molecular docking. c) 3D and 2D images of EGFR and AKT1 docking complexes with STA. Red circles, amino acid residues common for the docking complexes of STA and verified inhibitors with EGFR and AKT1; green dashed lines, hydrogen bonds. d) Human kinome phylogenetic tree reconstructed using the KinMap platform; the interconnection between kinases and GBM pathogenesis was retrieved using the Open Targets Platform database [24].
Therefore, in silico analysis revealed that STA can interact with the active sites of several kinases involved in GBM pathogenesis (EGFR, ERBB2, and AKT1); however, these data have to be verified experimentally.
STA suppressed development of immature thin-walled vessels and decreased the content of connective tissue fibers in the tumor. Earlier, we showed that STA administration (7 intraperitoneal injections at 20 mg/kg body weight) efficiently reduced the growth of peripheral tumors in the heterotopic U87 glioblastoma xenograft model by suppression of proliferative potential of tumor cells and modulation of tumor microenvironment [7]. Considering this effect and ability of STA to block vasculogenic mimicry of U87 cells in vitro [7], we decided to investigate the impact of STA on the angiogenesis rate in the mouse GBM model.
Taking into account a close morphological relationship between tumor vascular and stromal constituents, we analyzed both the content of thin-walled blood vessels and the amount of connective tissue fibers in the tumors of mice with subcutaneously grafted U87 glioblastoma after intraperitoneal injection with STA (20 mg/kg, dissolved in 10% Tween-80). The scheme of the experiment is shown in Fig. 5a.
Histological examination revealed that the U87 glioblastoma tissue contained a lot of tumor-penetrating immature thin-walled blood vessels (Fig. 5b), whose numerical density was significantly (3.4-fold) decreased after STA therapy compared to the control group (Fig. 5c). These data were in complete agreement with our earlier results on the STA potential to block formation of tubular structures in vitro (vasculogenic mimicry) by U87 cells [7]. Moreover, we identified an extensive network of connective tissue fibers, the content of which in the tumor significantly reduced after treatment with STA (Fig. 5d). The volume density of Van Gieson-stained elastic fibers was reduced 2.7-fold in the experimental vs. control group (Fig. 5e). These data agree well with the previously identified capability of STA to reduce the content of collagen fibers in the mouse U87 glioblastoma model [7].
Effect of STA on the content of thin-walled blood vessels and elastic fibers in the xenograft U87 glioblastoma model. a) Scheme of the experiment. b) Tumors stained with hematoxylin and eosin. Magnification, ×200. Black arrows, thin-walled blood vessels. c) The content of thin-walled blood vessels in U87 glioblastoma tissue. Nv, numerical density of blood vessels. d) Van Gieson staining of connective tissue fibers in the tumor; magnification, ×200. e) The content of elastic fibers in U87 glioblastoma tissue. Vv, volume density (%). ** p < 0.01, *** p < 0.001.
DISCUSSION
Because of a high malignancy of GBM resulting from active proliferation, increased motility, predisposition to developing drug resistance, and invasiveness of tumor cells [2], the search and synthesis of compounds than can not only produce direct toxic effects on GBM cells, but also suppress their pro-metastatic phenotype, is one of the promising approaches in creation of new classes of anti-glioblastoma agents [25]. Here, we investigated the anti-glioblastoma potential of STA, a semisynthetic pentacyclic triterpenoid with a cyanoenone pharmacophore in the A ring. STA exhibits multiple pharmacological effects on the tumor progression, inflammation, and tissue damage [20, 26, 27]. We found that the growth of U87 cells in a 3D culture was efficiently inhibited by STA (Fig. 1, c-f), which was the first time when such effect was shown for cyanoenone-containing triterpenoid. Previously, the anti-glioblastoma potential of these compounds has been assessed only in cell monolayers [28-30]. Zhou et al. [31], So et al. [32] and Murad et al. [33] demonstrated that CDDO-Me and CDDO-Im suppressed the growth of spheroids formed by human breast and prostate cancer cells. Hence, the ability of STA to inhibit the growth of U87 cell spheroids is in good agreement with its suppressive effect that we showed earlier in the mouse heterotopic xenograft U87 glioblastoma model [7].
STA also promoted the adhesiveness of U87 cells (Fig. 2), thus confirming its prominent anti-glioblastoma potential. It is known that low adhesiveness of tumor cells is associated with the mesenchymal phenotype and plays an important role in metastasis [34]. Hence, modulation of tumor cell adhesiveness may be a promising approach in the antitumor therapy [35]. On one hand, the ability of STA to increase cell–cell adhesion of U87 cells in the trypsin test (Fig. 2a) is consistent with a similar effect of asiatic acid on the adhesiveness of human lung cancer A549 cells [36]. On the other hand, it contradicts the inhibitory effect of RTA-404 (STA analogue) on the adhesive properties of U87 cells described by Tsai et al. [30]. This discrepancy may be accounted for by significantly different triterpenoid concentrations used in the studies: whereas we tested the effects of STA within a non-toxic concentration range (25-100 nM) (Fig. 2a), the concentration of RTA-404 used by Tsai et al. [30] was 500 nM, which resulted in ~50% cell death. Hence, the decline in the adhesiveness of RTA-404-exposed GBM cells is most likely the result of cell apoptosis [30]. STA also promoted adhesion of U87 cells to the collagen substrate that mimicked the extracellular matrix (Fig. 2b), which again confirmed its anti-glioblastoma potential. Ramaswamy et al. [37] observed a similar effect of U0126, selective ERK1/2 inhibitor with powerful anti-glioblastoma properties, on the adhesion of U87 cells to gelatin.
The synergistic cytotoxic effect elicited by STA and TMZ in the U87 cell model (Fig. 3b), as well as the ability of STA to efficiently accumulate in mouse brain tissues described by us earlier [7], suggest that STA might be introduced into currently used GBM chemotherapy regimens. However, an elevated ALDH activity in the STA-treated U87 cells (Fig. 3c) indicated the need for a careful selection of the optimal STA dose and administration regimen. The activation of ALDH in tumor cells is associated with their cytoprotective response to xenobiotics, electrophilic and oxidative stress [38, 39], while also being a key marker of tumor stem cells (TSCs). Taking into account the presence of an electrophilic Michael acceptor (cyanoenone group) in STA, the stimulating effect of STA on the generation of reactive oxygen species in U87 cells [7], and STA ability to inhibit the growth of U87 cell spheroids (Fig. 1, c-f), which is an important source of CSCs [40], we suggested that ALDH activation observed after exposure to STA (Fig. 3c) resulted from the compensatory mechanisms elicited in response to xenobiotic stress, rather than represented as a marker of enhanced stemness of GBM cells, but this hypothesis needs experimental verification.
Histological examination of U87 glioblastoma sections showed that STA not only affected tumor cells, but also modulated tumor microenvironment by suppressing the growth of local immature thin-walled blood vessels(Fig. 5c). These chaotically branching highly permeable blood vessels create hypoxic foci within the tumor tissue, thus contributing to the emergence of highly metastatic GBM cells with the mesenchymal phenotype accounting for the diffuse glioblastoma growth [41]. The suppressive effect of STA on the thin-walled blood vessels lined not only with endotheliocytes, but also with tumor cells [42] correlates with its blocking effect on the in vitro formation of tube-like structures from U87 cells (vasculogenic mimicry) shown in [7]. Together with the previously revealed STA potential to upregulate expression of α-SMA and CD31 (markers of mature vessels) in U87 glioblastoma tissue in the in vivo xenograft model [7], these data evidence normalization of aberrant angiogenesis in the tumor, which might contribute to the reduction in the volume of hypoxic zones in GBM tissue and, subsequently, elevated tumor sensitivity to chemo- and radiotherapy [43]. Along with the effect on the growth of thin-walled blood vessels, STA decreased the content of connective tissue fibers in the tumor microenvironment (Fig. 5, d and e). Large amounts of connective tissue fibers create a barrier to chemotherapy agents, nutrients, oxygen, and immune cells (immune trapping), thus decreasing the susceptibility of GBM cells to chemo-, radio-, and immunotherapy [44].
Based on computational modeling data, the anti-glioblastoma effects of STA can primarily result from its direct inhibitory effect on EGFR, ERBB2, and AKT1 (Fig. 4, b and c). Accumulated evidence confirms that cyanoenone-bearing triterpenoids efficiently inhibit the EGFR, ERBB2, and AKT1 signaling pathways in various tumor cells [20, 45] including glioblastoma cells [30, 46]. Moreover, Liu et al. [47] experimentally proved that the STA analog CDDO-Me directly blocks the activity of AKT1. Kim et al. [48] reported that CDDO-Me binds with ERBB2. Our recent study revealed that CDDO-Im interacts with the EGFR active site with the binding energy that was lower than the energy observed for the EGFR inhibitor erlotinib [49]. Taking into consideration an essential role played by EGFR, ERBB2, and AKT1 in regulating GBM progression [50-52], in particular, acquisition of mesenchymal phenotype and stemness properties [53, 54], the ability of STA to form complexes with these proteins revealed in in silico studies requires to be further investigated.
CONCLUSION
We found that STA has a prominent anti-glioblastoma potential implemented via direct modulation of GBM cells (suppression of spheroid growth, increase in the cell adhesiveness, and synergistic cytotoxic effect with TMZ), as well as by normalization of tumor microenvironment. The data obtained evidence the need to further investigate the effects of STA on the acquisition of mesenchymal phenotype and stemness properties by GBM cells in order to propose STA as a promising multitarget anti-glioblastoma agent.
Abbreviations
- ALDH:
-
aldehyde dehydrogenase
- GBM:
-
glioblastoma multiforme
- STA:
-
soloxolone tryptamide
- TGF-β:
-
transforming growth factor beta
- TMZ:
-
temozolomide
References
Sanati, M., Binabaj, M. M., Ahmadi, S. S., Aminyavari, S., Javid, H., Mollazadeh, H., Bibak, B., Mohtashami, E., Jamialahmadi, T., Afshari, A. R., and Sahebkar, A. (2022) Recent advances in glioblastoma multiforme therapy: a focus on autophagy regulation, Biomed. Pharmacother., 155, 113740, https://doi.org/10.1016/j.biopha.2022.113740.
Torrisi, F., Alberghina, C., D’Aprile, S., Pavone, A. M., Longhitano, L., Giallongo, S., Tibullo, D., Di Rosa, M., Zappalà, A., Cammarata, F. P., Russo, G., Ippolito, M., Cuttone, G., Li Volti, G., Vicario, N., and Parenti, R. (2022) The hallmarks of glioblastoma: heterogeneity, intercellular crosstalk and molecular signature of invasiveness and progression, Biomedicines, 10, 806, https://doi.org/10.3390/biomedicines10040806.
Cha, G. D., Kang, T., Baik, S., Kim, D., Choi, S. H., Hyeon, T., and Kim, D.-H. (2020) Advances in drug delivery technology for the treatment of glioblastoma multiforme, J. Control. Release, 328, 350-367, https://doi.org/10.1016/j.jconrel.2020.09.002.
Qazi, M. A., Vora, P., Venugopal, C., Sidhu, S. S., Moffat, J., Swanton, C., and Singh, S. K. (2017) Intratumoral heterogeneity: pathways to treatment resistance and relapse in human glioblastoma, Ann. Oncol., 28, 1448-1456, https://doi.org/10.1093/annonc/mdx169.
Sestito, S., Runfola, M., Tonelli, M., Chiellini, G., and Rapposelli, S. (2018) New multitarget approaches in the war against glioblastoma: a mini-perspective, Front. Pharmacol., 9, 874, https://doi.org/10.3389/fphar.2018.00874.
Behl, T., Sharma, A., Sharma, L., Sehgal, A., Singh, S., Sharma, N., Zengin, G., Bungau, S., Toma, M. M., Gitea, D., Babes, E. E., Judea Pusta, C. T., and Bumbu, A. G. (2021) Current perspective on the natural compounds and drug delivery techniques in glioblastoma multiforme, Cancers (Basel), 13, 2765, https://doi.org/10.3390/cancers13112765.
Markov, A. V., Ilyina, A. A., Salomatina, O. V., Sen’kova, A. V., Okhina, A. A., Rogachev, A. D., Salakhutdinov, N. F., and Zenkova, M. A. (2022) Novel soloxolone amides as potent anti-glioblastoma candidates: design, synthesis, in silico analysis and biological activities in vitro and in vivo, Pharmaceuticals, 15, 603, https://doi.org/10.3390/ph15050603.
Ianevski, A., Giri, A. K., and Aittokallio, T. (2020) SynergyFinder 2.0: visual analytics of multi-drug combination synergies, Nucleic Acids Res., 48, W488-W493, https://doi.org/10.1093/nar/gkaa216.
Awale, M. and Reymond, J. L. (2019) Polypharmacology browser PPB2: target prediction combining nearest neighbors with machine learning, J. Chem. Inf. Model., 59, 10-17, https://doi.org/10.1021/acs.jcim.8b00524.
Daina, A., Michielin, O., and Zoete, V. (2019) SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules, Nucleic Acids Res., 47, W357-W364, https://doi.org/10.1093/nar/gkz382.
Szklarczyk, D., Franceschini, A., Wyder, S., Forslund, K., Heller, D., Huerta-Cepas, J., Simonovic, M., Roth, A., Santos, A., Tsafou, K. P., Kuhn, M., Bork, P., Jensen, L. J., and Von Mering, C. (2015) STRING v10: protein–protein interaction networks, integrated over the tree of life, Nucleic Acids Res., 43, D447-D452, https://doi.org/10.1093/nar/gku1003.
Chin, C.-H., Chen, S.-H., Wu, H.-H., Ho, C.-W., Ko, M.-T., and Lin, C.-Y. (2014) cytoHubba: identifying hub objects and sub-networks from complex interactome, BMC Syst. Biol., 8, S11, https://doi.org/10.1186/1752-0509-8-S4-S11.
Trott, O., and Olson, A. J. (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem., 31, 455-461, https://doi.org/10.1002/jcc.21334.
Suhovskih, A. V., Kazanskaya, G. M., Volkov, A. M., Tsidulko, A. Y., Aidagulova, S. V., and Grigorieva, E. V. (2019) Suitability of RNALater solution as a tissue-preserving reagent for immunohistochemical analysis, Histochem. Cell Biol., 152, 239-247, https://doi.org/10.1007/s00418-019-01799-z.
Joseph, J. V., Balasubramaniyan, V., Walenkamp, A., and Kruyt, F. A. E. (2013) TGF-β as a therapeutic target in high grade gliomas – promises and challenges, Biochem. Pharmacol., 85, 478-485, https://doi.org/10.1016/j.bcp.2012.11.005.
Peñuelas, S., Anido, J., Prieto-Sánchez, R. M., Folch, G., Barba, I., Cuartas, I., García-Dorado, D., Poca, M. A., Sahuquillo, J., Baselga, J., and Seoane, J. (2009) TGF-β increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma, Cancer Cell, 15, 315-327, https://doi.org/10.1016/j.ccr.2009.02.011.
Beri, P., Popravko, A., Yeoman, B., Kumar, A., Chen, K., Hodzic, E., Chiang, A., Banisadr, A., Placone, J. K., Carter, H., Fraley, S. I., Katira, P., and Engler, A. J. (2020) Cell adhesiveness serves as a biophysical marker for metastatic potential, Cancer Res., 80, 901-911, https://doi.org/10.1158/0008-5472.CAN-19-1794.
Beltzig, L., Schwarzenbach, C., Leukel, P., Frauenknecht, K. B. M., Sommer, C., Tancredi, A., Hegi, M. E., Christmann, M., and Kaina, B. (2022) Senescence is the main trait induced by temozolomide in glioblastoma cells, Cancers (Basel), 14, 2233, https://doi.org/10.3390/cancers14092233.
Poturnajova, M., Kozovska, Z., and Matuskova, M. (2021) Aldehyde dehydrogenase 1A1 and 1A3 isoforms – mechanism of activation and regulation in cancer, Cell. Signal., 87, 110120, https://doi.org/10.1016/j.cellsig.2021.110120.
Markov, A. V., Zenkova, M. A., and Logashenko, E. B. (2017) Modulation of Tumour-related signaling pathways by natural pentacyclic triterpenoids and their semisynthetic derivatives, Curr. Med. Chem., 24, 1277-1320, https://doi.org/10.2174/0929867324666170112115313.
McDowell, K. A., Riggins, G. J., and Gallia, G. L. (2011) Targeting the AKT pathway in glioblastoma, Curr. Pharm. Des., 17, 2411-2420, https://doi.org/10.2174/138161211797249224.
Mure, H., Matsuzaki, K., Kitazato, K. T., Mizobuchi, Y., Kuwayama, K., Kageji, T., and Nagahiro, S. (2010) Akt2 and Akt3 play a pivotal role in malignant gliomas, Neuro. Oncol., 12, 221-232, https://doi.org/10.1093/neuonc/nop026.
Yoon, H., Kim, D. J., Ahn, E. H., Gellert, G. C., Shay, J. W., Ahn, C.-H., and Lee, Y. B. (2009) Antitumor activity of a novel antisense oligonucleotide against Akt1, J. Cell. Biochem., 108, 832-838, https://doi.org/10.1002/jcb.22311.
Ochoa, D., Hercules, A., Carmona, M., Suveges, D., Gonzalez-Uriarte, A., Malangone, C., Miranda, A., Fumis, L., Carvalho-Silva, D., Spitzer, M., Baker, J., Ferrer, J., Raies, A., Razuvayevskaya, O., Faulconbridge, A., Petsalaki, E., Mutowo, P., Machlitt-Northen, S., Peat, G., McAuley, E., Ong, C. K., Mountjoy, E., Ghoussaini, M., Pierleoni, A., Papa, E., Pignatelli, M., Koscielny, G., Karim, M., Schwartzentruber, J., Hulcoop, D. G., Dunham, I., and McDonagh, E. M. (2021) Open Targets Platform: supporting systematic drug–target identification and prioritisation, Nucleic Acids Res., 49, D1302-D1310, https://doi.org/10.1093/nar/gkaa1027.
Bazan, N. G., Reid, M. M., Flores, V. A. C., Gallo, J. E., Lewis, W., and Belayev, L. (2021) Multiprong control of glioblastoma multiforme invasiveness: blockade of pro-inflammatory signaling, anti-angiogenesis, and homeostasis restoration, Cancer Metastasis Rev., 40, 643-647, https://doi.org/10.1007/s10555-021-09987-x.
Liby, K. T., and Sporn, M. B. (2012) Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease, Pharmacol. Rev., 64, 972-1003, https://doi.org/10.1124/pr.111.004846.
Sen’kova, A. V., Savin, I. A., Odarenko, K. V., Salomatina, O. V., Salakhutdinov, N. F., Zenkova, M. A., and Markov, A. V. (2023) Protective effect of soloxolone derivatives in carrageenan- and LPS-driven acute inflammation: Pharmacological profiling and their effects on key inflammation-related processes, Biomed. Pharmacother., 159, 114231, https://doi.org/10.1016/j.biopha.2023.114231.
Tsai, T.-H., Lieu, A.-S., Huang, T.-Y., Kwan, A.-L., Lin, C.-L., and Hsu, Y.-C. (2021) Induction of mitosis delay and apoptosis by CDDO-TFEA in glioblastoma multiforme, Front. Pharmacol., 12, 756228, https://doi.org/10.3389/fphar.2021.756228.
Tsai, T. H., Hsu, Y. C., Lieu, A. S., Huang, T. Y., Kwan, A. L., and Lin, C. L. (2021) RTA404, an activator of Nrf2, activates the checkpoint kinases and induces apoptosis through intrinsic apoptotic pathway in malignant glioma, J. Clin. Med., 10, 4805, https://doi.org/10.3390/jcm10214805.
Tsai, T.-H., Lieu, A.-S., Wang, Y.-W., Yang, S.-F., Hsu, Y.-C., and Lin, C.-L. (2021) Therapeutic potential of RTA 404 in human brain malignant glioma cell lines via cell cycle arrest via p21/AKT signaling, Biomed Res. Int., 2021, 5552226, https://doi.org/10.1155/2021/5552226.
Zhou, L., Wang, Z., Yu, S., Xiong, Y., Fan, J., Lyu, Y., Su, Z., Song, J., Liu, S., Sun, Q., and Lu, D. (2020) CDDO-Me elicits anti-breast cancer activity by targeting LRP6 and FZD7 receptor complex, J. Pharmacol. Exp. Ther., 373, 149-159, https://doi.org/10.1124/jpet.119.263434.
So, J. Y., Lin, J. J., Wahler, J., Liby, K. T., Sporn, M. B., and Suh, N. (2014) A synthetic triterpenoid CDDO-Im inhibits tumorsphere formation by regulating stem cell signaling pathways in triple-negative breast cancer, PLoS One, 9, e107616, https://doi.org/10.1371/journal.pone.0107616.
Murad, H. Y., Chandra, P. K., Kelly, C. A., Khurana, N., Yu, H., Bortz, E. P., Hong, S. N., Mondal, D., and Khismatullin, D. B. (2022) Pre-exposure to stress-inducing agents increase the anticancer efficacy of focused ultrasound against aggressive prostate cancer cells, Antioxidants, 11, 341, https://doi.org/10.3390/antiox11020341.
Le Bras, G. F., Taubenslag, K. J., and Andl, C. D. (2012) The regulation of cell–cell adhesion during epithelial-mesenchymal transition, motility and tumor progression, Cell Adh. Migr., 6, 365-373, https://doi.org/10.4161/cam.21326.
Janiszewska, M., Primi, M. C., and Izard, T. (2020) Cell adhesion in cancer: beyond the migration of single cells, J. Biol. Chem., 295, 2495-2505, https://doi.org/10.1074/jbc.REV119.007759.
Cui, Q., Ren, J., Zhou, Q., Yang, Q., and Li, B. (2019) Effect of asiatic acid on epithelial-mesenchymal transition of human alveolar epithelium A549 cells induced by TGF-β1, Oncol. Lett., 17, 4285-4292, https://doi.org/10.3892/ol.2019.10140.
Ramaswamy, P., Nanjaiah, N. D., and Borkotokey, M. (2019) Role of MEK-ERK signaling mediated adhesion of glioma cells to extracellular matrix: possible implication on migration and proliferation, Ann. Neurosci., 26, 52-56, https://doi.org/10.5214/ans.0972.7531.260203.
Singh, S., Brocker, C., Koppaka, V., Chen, Y., Jackson, B. C., Matsumoto, A., Thompson, D. C., and Vasiliou, V. (2013) Aldehyde dehydrogenases in cellular responses to oxidative/electrophilicstress, Free Radic. Biol. Med., 56, 89-101, https://doi.org/10.1016/j.freeradbiomed.2012.11.010.
Rodriguez, S. M., Staicu, G.-A., Sevastre, A.-S., Baloi, C., Ciubotaru, V., Dricu, A., and Tataranu, L. G. (2022) Glioblastoma stem cells – useful tools in the battle against cancer, Int. J. Mol. Sci., 23, 4602, https://doi.org/10.3390/ijms23094602.
Wang, X., Zhou, W., Li, X., Ren, J., Ji, G., Du, J., Tian, W., Liu, Q., and Hao, A. (2020) Graphene oxide suppresses the growth and malignancy of glioblastoma stem cell-like spheroids via epigenetic mechanisms, J. Transl. Med., 18, 200, https://doi.org/10.1186/s12967-020-02359-z.
Angara, K., Borin, T. F., and Arbab, A. S. (2017) Vascular mimicry: a novel neovascularization mechanism driving anti-angiogenic therapy (AAT) resistance in glioblastoma, Transl. Oncol., 10, 650-660, https://doi.org/10.1016/j.tranon.2017.04.007.
Viallard, C., and Larrivée, B. (2017) Tumor angiogenesis and vascular normalization: alternative therapeutic targets, Angiogenesis, 20, 409-426, https://doi.org/10.1007/s10456-017-9562-9.
Zheng, R., Li, F., Li, F., and Gong, A. (2021) Targeting tumor vascularization: promising strategies for vascular normalization, J. Cancer Res. Clin. Oncol., 147, 2489-2505, https://doi.org/10.1007/s00432-021-03701-8.
Mohiuddin, E. and Wakimoto, H. (2021) Extracellular matrix in glioblastoma: opportunities for emerging therapeutic approaches, Am. J. Cancer Res., 11, 3742-3754.
Konopleva, M., Zhang, W., Shi, Y. X., McQueen, T., Tsao, T., Abdelrahim, M., Munsell, M. F., Johansen, M., Yu, D., Madden, T., Safe, S. H., Hung, M. C., and Andreeff, M. (2006) Synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest in HER2-overexpressing breast cancer cells, Mol. Cancer Ther., 5, 317-328, https://doi.org/10.1158/1535-7163.MCT-05-0350.
Gao, X., Deeb, D., Jiang, H., Liu, Y., Dulchavsky, S. A., and Gautam, S. C. (2007) Synthetic triterpenoids inhibit growth and induce apoptosis in human glioblastoma and neuroblastoma cells through inhibition of prosurvival Akt, NF-κB and Notch1 signaling, J. Neurooncol., 84, 147-157, https://doi.org/10.1007/s11060-007-9364-9.
Liu, Y., Gao, X., Deeb, D., and Gautam, S. C. (2012) Oleanane triterpenoid CDDO-Me inhibits Akt activity without affecting PDK1 kinase or PP2A phosphatase activity in cancer cells, Biochem. Biophys. Res. Commun., 417, 570-575, https://doi.org/10.1016/j.bbrc.2011.12.007.
Kim, E.-H. H., Deng, C., Sporn, M. B., Royce, D. B., Risingsong, R., Williams, C. R., and Liby, K. T. (2012) CDDO-methyl ester delays breast cancer development in Brca1-mutated mice, Cancer Prev. Res., 5, 89-97, https://doi.org/10.1158/1940-6207.CAPR-11-0359.
Markov, A. V., Odarenko, K. V., Ilyina, A. A., and Zenkova, M. A. (2022) Uncovering the anti-angiogenic effect of semisynthetic triterpenoid CDDO-Im on HUVECs by an integrated network pharmacology approach, Comput. Biol. Med., 141, 105034, https://doi.org/10.1016/J.COMPBIOMED.2021.105034.
Westphal, M., Maire, C. L., and Lamszus, K. (2017) EGFR as a target for glioblastoma treatment: an unfulfilled promise, CNS Drugs, 31, 723-735, https://doi.org/10.1007/s40263-017-0456-6.
Majewska, E., and Szeliga, M. (2017) AKT/GSK3β signaling in glioblastoma, Neurochem. Res., 42, 918-924, https://doi.org/10.1007/s11064-016-2044-4.
Vitanza, N. A., Johnson, A. J., Wilson, A. L., Brown, C., Yokoyama, J. K., Künkele, A., Chang, C. A., Rawlings-Rhea, S., Huang, W., Seidel, K., Albert, C. M., Pinto, N., Gust, J., Finn, L. S., Ojemann, J. G., Wright, J., Orentas, R. J., Baldwin, M., Gardner, R. A., Jensen, M. C., and Park, J. R. (2021) Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis, Nat. Med., 27, 1544-1552, https://doi.org/10.1038/s41591-021-01404-8.
Takashima, Y., Kawaguchi, A., and Yamanaka, R. (2019) Promising prognosis marker candidates on the status of epithelial–mesenchymal transition and glioma stem cells in glioblastoma, Cells, 8, 1312, https://doi.org/10.3390/cells8111312.
Barzegar Behrooz, A., Talaie, Z., Jusheghani, F., Łos, M. J., Klonisch, T., and Ghavami, S. (2022) Wnt and PI3K/Akt/mTOR survival pathways as therapeutic targets in glioblastoma, Int. J. Mol. Sci., 23, 1353, https://doi.org/10.3390/ijms23031353.
Acknowledgments
The authors are grateful to O. V. Salomatina (Vorozhtsov Novosibirsk Institute of Organic Chemistry) for providing STA and A. V. Vladimirova (Institute of Chemical Biology and Fundamental Medicine) for assisting in cell culture maintenance.
Funding
The study was supported by the Russian Science Foundation (Grant no. 19-74-30011, https://rscf.ru/en/project/19-74-30011/) and by the Russian State funded budget project of the Institute of Chemical Biology and Fundamental Medicine, Siberian Branch of the Russian Academy of Sciences, no. 121031300044-5.
Author information
Authors and Affiliations
Contributions
A.V.M. conceived and supervised the study; K.V.O., A.V.S., and A.A.I. conducted the experiments; A.V.M. and A.V.S. discussed the results of the study; A.V.M., K.V.O., and A.V.S. wrote the manuscript; M.A.Z. edited the manuscript and provided project administration.
Corresponding author
Ethics declarations
The authors declare no conflict of interest. All experiments with mice were carried out in accordance with the Directive EU 2010/63/EU of the European Parliament and of the Council of September 22, 2010, on the protection of animals used for scientific purposes and the Rules for working with experimental animals (Order of the Ministry of Health of the USSR no. 755; August 12, 1977). All animal experiments were approved by the Committee on the Ethics of Animal Experiments at the Institute of Cytology and Genetics of the Siberian Branch of the Russian Academy of Sciences (Protocol no. 56; August 10, 2019).
Rights and permissions
Open access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Markov, A.V., Odarenko, K.V., Sen’kova, A.V. et al. Evaluation of the Antitumor Potential of Soloxolone Tryptamide against Glioblastoma Multiforme Using in silico, in vitro, and in vivo Approaches. Biochemistry Moscow 88, 1008–1021 (2023). https://doi.org/10.1134/S000629792307012X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S000629792307012X