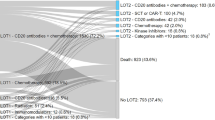
Abstract
Introduction
The use of erythropoiesis-stimulating agents (ESAs) for treatment of chemotherapy-induced anemia (CIA) has been linked to potential negative health effects. Additionally, research has identified disparities in ESA utilization for CIA treatment. This study examines (1) health disparities in ESA use and (2) whether reimbursement (Medicare National Coverage Determination [NCD]) or regulatory (Risk Evaluation and Mitigation Strategy [REMS]) policies impacted disparities.
Methods
In a retrospective cohort study (2006–2018) among 1,747,889 patients with cancer in the United States receiving myelosuppressive chemotherapy at age ≥ 65 years, differences in ESA use for CIA were estimated using generalized estimating equation models, adjusting for policy periods, demographic characteristics, and clinical factors extracted from Medicare claims data.
Results
After controlling for covariates, ESA use was higher among Black, female, and urban patients in all policy periods, but these disparities decreased significantly following the NCD. The gap continued to close through the REMS period. Disparities in ESA use across geographic regions were modest, and ESA use disparities across socioeconomic characteristics (area deprivation index or dual Medicare/Medicaid eligibility) were not observed.
Conclusions
Being female, Black, or an urban resident was associated with higher ESA use for CIA in older patients with cancer. Both NCD and REMS implementation helped reduce disparities. REMS release was not observed to contribute to racial, sex, and rural–urban disparities in ESA use for CIA.


Similar content being viewed by others
References
Institute of Medicine Committee on U, Eliminating R, Ethnic Disparities in Health C. In: Smedley BD, Stith AY, Nelson AR, editors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington (DC): The National Academies Press; 2003.
Hershman DL, Buono DL, Malin J, et al. Patterns of use and risks associated with erythropoiesis-stimulating agents among medicare patients with cancer. JNCI J Natl Cancer Inst. 2009;101(23):1633–41.
Hershman DL, Neugut AI, Shim JJ, et al. Erythropoiesis-stimulating agent use after changes in medicare reimbursement policies. J Oncol Pract. 2014;10(4):264–9.
Li M, Schulz R, Chisholm-Burns M, et al. Racial/ethnic and gender disparities in the use of erythropoiesis-stimulating agents and blood transfusions: cancer management under Medicare’s reimbursement policy. J Manag Care Spec Pharm. 2020;26(11):1477–86.
Stafkey-Mailey D, Bennett C, Dickson M. Racial/ethnic disparities of erythropoiesis stimulating agent use among the insured poor: pre- and post-safety advisories. Blood. 2010;116(21):395.
Hedenus M, Adriansson M, Miguel JS, Kramer MH, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122(3):394–403.
Leyland-Jones B, Bondarenko I, Nemsadze G, Smirnov V, et al. A randomized, open-label, multicenter, phase III study of epoetin alfa versus best standard of care in anemic patients with metastatic breast cancer receiving standard chemotherapy. Am J Clin Oncol. 2016;34(11):1197–207.
Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. Am J Clin Oncol. 2005;23(25):5960–72.
Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108(2):317–25.
Untch M, Fasching P, Bauerfeind I. A randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF with a standard dosed epirubicin/cyclophosphamide followed by paclitaxel±darbepoetin alfa in primary breast cancer: a preplanned interim analysis of efficacy at surgery. Program and Abstracts of the 44th American Society of Clinical Oncology Annual Meeting; 2008; 2008.
Centers for Medicare & Medicare Services. National Coverage Determination (NCD) for Erythropoiesis Stimulating Agents (ESAs) in Cancer and Related Neoplastic Conditions (110.21). 2018. https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDid=322&ncdver=1. Accessed 24 Feb 2019.
U.S. Food & Drug Administration. ESA REMS Approval Letter. 2010.
U.S. Food & Drug Administration. Information for Epogen/Procrit (Epoetin alfa). 2017. https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm541173.htm. Accessed 24 Feb 2019
United States Department of Human Services of Health and Human Services. Phase I report: Recommendations for the framework and format of Healthy People 2020; 2020.
Vega A, Zhang R, Wong H-L, et al. Trends in erythropoiesis-stimulating agent use and blood transfusions for chemotherapy-induced anemia throughout FDA’s risk evaluation and mitigation strategy lifecycle. Pharmacoepidemiol Drug Saf. 2021;30(5):626–35.
University of Wisconsin School of Medicine and Public Health. Area Deprivation Index v2.0. 2015. https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed 7 May 2020.
Chu E, DeVita Jr VT. Physicians' cancer chemotherapy drug manual 2020. Jones & Bartlett Learning; 2019.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med care. 2005:1130-9.
Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. 2004;7(2):127–50.
Turenne MN, Cope EL, Porenta S, et al. Has dialysis payment reform led to initial racial disparities in anemia and mineral metabolism management? J Am Soc Nephrol. 2015;26(3):754–64.
Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer. 2021;124(2):315–32.
Esnaola NF, Ford ME. Racial differences and disparities in cancer care and outcomes: where’s the rub? Surg Oncol Clin N Am. 2012;21(3):417–37 (viii).
Chu K, Miller B, Springfield S. Measures of racial/ethnic health disparities in cancer mortality rates and the influence of socioeconomic status. J Natl Med Assoc. 2007;99:1092–100 (102).
Singh GK, Williams SD, Siahpush M, et al. Socioeconomic, rural–urban, and racial inequalities in US cancer mortality: part I-all cancers and lung cancer and part II-colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011: 107497.
Gawade PL, Berlin JA, Henry DH, et al. Changes in the use of erythropoiesis-stimulating agents (ESAs) and red blood cell transfusion in patients with cancer amidst regulatory and reimbursement changes. Pharmacoepidemiol Drug Saf. 2017;26(11):1357–66.
Ryta A, Chmielewski M, Debska-Slizien A, et al. Impact of gender and dialysis adequacy on anaemia in peritoneal dialysis. Int Urol Nephrol. 2017;49(5):903–8.
Lacson E Jr, Rogus J, Teng M, et al. The association of race with erythropoietin dose in patients on long-term hemodialysis. Am J Kidney Dis. 2008;52(6):1104–14.
Kaufman JS. Racial differences in erythropoietin responsiveness. Am J Kidney Dis. 2008;52(6):1035–8.
Hoque S, Chen BJ, Schoen MW, et al. End of an era of administering erythropoiesis stimulating agents among Veterans Administration cancer patients with chemotherapy-induced anemia. PLoS One. 2020;15(6): e0234541.
Jeffries N, Zaslavsky AM, Roux AVD, et al. methodological approaches to understanding causes of health disparities. Am J Public Health. 2019;109(S1):S28–33.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Author contributions
Conceptualization: all authors; Methodology: Hui-Lee Wong, Rongmei Zhang, Bradley Lufkin, Yuhui Feng, An-Chi Lo, Manzi Ngaiza, Michael Wernecke, Qin Ryan, Amarilys Vega, David Graham; Resources: Hui-Lee Wong, Michael Wernecke, Thomas MaCurdy, David Graham; Formal analysis and investigation: All authors; Writing—original draft preparation: Hui-Lee Wong, Rongmei Zhang, Bradley Lufkin, Yuhui Feng, An-Chi Lo, Manzi Ngaiza, Michael Wernecke, Qin Ryan, Amarilys Vega, David Graham; Writing—review and editing: all authors; Study supervision: Hui-Lee Wong, Bradley Lufkin, Michael Wernecke, Thomas MaCurdy, David Graham.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy and ethical restrictions.
Ethics approval
This is a retrospective observational study. No IRB approval is required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Funding
This study was funded through an intra-agency agreement between the Centers for Medicare & Medicaid Services and the US Food and Drug Administration.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wong, HL., Zhang, R., Lufkin, B. et al. Disparities in erythropoiesis-stimulating agent use after changes in medicare reimbursement and implementation of a risk evaluation and mitigation strategy. Drugs Ther Perspect 39, 29–39 (2023). https://doi.org/10.1007/s40267-022-00969-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-022-00969-9