Abstract
Purpose
Despite the advances in treatment, lung cancer is a global concern and necessitates the development of new treatments. Biguanides like metformin (MET) and artemisinin (ART) have recently been discovered to have anti-cancer properties. As a consequence, in the current study, the anti-cancer effect of MET and ART co-encapsulated in niosomal nanoparticles on lung cancer cells was examined to establish an innovative therapy technique.
Methods
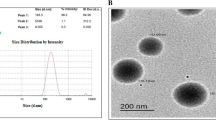
Niosomal nanoparticles (Nio-NPs) were synthesized by thin-film hydration method, and their physicochemical properties were assessed by FTIR. The morphology of Nio-NPs was evaluated with FE-SEM and AFM. The MTT assay was applied to evaluate the cytotoxic effects of free MET, free ART, their encapsulated form with Nio-NPs, as well as their combination, on A549 cells. Apoptosis assay was utilized to detect the biological processes involved with programmed cell death. The arrest of cell cycle in response to drugs was assessed using a cell cycle assay. Following a 48-h drug treatment, the expression level of hTERT, Cyclin D1, BAX, BCL-2, Caspase 3, and 7 genes were assessed using the qRT-PCR method.
Results
Both MET and ART reduced the survival rate of lung cancer cells in the dose-dependent manner. The IC50 values of pure ART and MET were 195.2 μM and 14.6 mM, respectively while in nano formulated form their IC50 values decreased to 56.7 μM and 78.3 μM, respectively. The combination of MET and ART synergistically decreased the proliferation of lung cancer cells, compared to the single treatments. Importantly, the combination of MET and ART had a higher anti-proliferative impact against A549 lung cancer cells, with lower IC50 values. According to the result of Real-time PCR, hTERT, Cyclin D1, BAX, BCL-2, Caspase 3, and Caspase 7 genes expression were considerably altered in treated with combination of nano formulated MET and ART compared to single therapies.
Conclusion
The results of this study showed that the combination of MET and ART encapsulated in Nio-NPs could be useful for the treatment of lung cancer and can increase the efficiency of lung cancer treatment.
Graphical abstract









Similar content being viewed by others
Availability of data and materials
The data and materials that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Zhang Y, et al. Global Patterns and Trends in Lung Cancer Incidence: A Population-Based Study. J Thorac Oncol. 2021;16(6):933–44.
Siegel RL, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Oser MG, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol. 2015;16(4):e165–72.
Zheng M. Classification and Pathology of Lung Cancer. Surg Oncol Clin N Am. 2016;25(3):447–68.
Huang CY, et al. A review on the effects of current chemotherapy drugs and natural agents in treating non-small cell lung cancer. Biomedicine (Taipei). 2017;7(4):23.
Shafiei G et al. Targeted delivery of silibinin via magnetic niosomal nanoparticles: potential application in treatment of colon cancer cells. Front Pharmacol. 2023;14:1174120
Davoudi Z, et al. Molecular target therapy of AKT and NF-kB signaling pathways and multidrug resistance by specific cell penetrating inhibitor peptides in HL-60 cells. Asian Pac J Cancer Prev. 2014;15(10):4353–8.
Abdulzehra S, Jafari-Gharabaghlou D, Zarghami N. Targeted delivery of oxaliplatin via folate-decorated niosomal nanoparticles potentiates resistance reversion of colon cancer cells. Heliyon, 2023;9(11):e21400
Olaku O, White JD. Herbal therapy use by cancer patients: a literature review on case reports. Eur J Cancer. 2011;47(4):508–14.
Luo H, et al. Naturally occurring anti-cancer compounds: shining from Chinese herbal medicine. Chin Med. 2019;14(1):48.
Kamarudin MNA, et al. Metformin in colorectal cancer: molecular mechanism, preclinical and clinical aspects. J Exp Clin Cancer Res. 2019;38(1):1–23.
Yang B, Shi J. Developing new cancer nanomedicines by repurposing old drugs. Angew Chem Int Ed. 2020;59(49):21829–38.
Yu H, et al. The Potential Effect of Metformin on Cancer: An Umbrella Review. Front Endocrinol (Lausanne). 2019;10:617.
Saraei P, et al. The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances. Cancer Manag Res. 2019;11:3295–313.
Gupta G, et al. A clinical update on metformin and lung cancer in diabetic patients. Panminerva Med. 2018;60(2):70–5.
Ghorbanzadeh F, et al. Advanced nano-therapeutic delivery of metformin: potential anti-cancer effect against human colon cancer cells through inhibition of GPR75 expression. Med Oncol. 2023;40(9):255.
Jafari-Gharabaghlou D, et al. Potentiation of Folate-Functionalized PLGA-PEG nanoparticles loaded with metformin for the treatment of breast Cancer: possible clinical application. Mol Biol Rep. 2023;50(4):3023–33.
Jafari-Gharabaghlou D, Jabbari A, Soltani A. 187P Development of a magnetic nanostructure for co-delivery of metformin and silibinin on growth of lung cancer cells: possible action through leptin gene and its receptor regulation. Ann Oncol. 2022;33:S116.
Xiao K, et al. The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: A meta-analysis. J Clin Pharm Ther. 2020;45(4):783–92.
Mohammadinejad S, Jafari-Gharabaghlou D, Zarghami N. Development of PEGylated PLGA Nanoparticles Co-Loaded with Bioactive Compounds: Potential Anticancer Effect on Breast Cancer Cell Lines. Asian Pac J Cancer Prev: APJCP. 2022;23(12):4063.
Zi F, et al. Metformin and cancer: An existing drug for cancer prevention and therapy. Oncol Lett. 2018;15(1):683–90.
Hassani N, et al. The effect of dual bioactive compounds artemisinin and metformin co-loaded in PLGA-PEG nano-particles on breast cancer cell lines: potential apoptotic and anti-proliferative action. Appl Biochem Biotechnol. 2022;194(10):4930–45.
Konstat-Korzenny E et al. Artemisinin and Its Synthetic Derivatives as a Possible Therapy for Cancer. Med Sci (Basel). 2018;6(1):19
Li D, Zhang J, Zhao X. Mechanisms and Molecular Targets of Artemisinin in Cancer Treatment. Cancer Invest. 2021;39(8):675–84.
Kiani BH, et al. Artemisinin and its derivatives: a promising cancer therapy. Mol Biol Rep. 2020;47(8):6321–36.
Alibakhshi A, et al. An update on phytochemicals in molecular target therapy of cancer: potential inhibitory effect on telomerase activity. Curr Med Chem. 2016;23(22):2380–93.
Zhang Q, et al. Artemisinin Derivatives Inhibit Non-small Cell Lung Cancer Cells Through Induction of ROS-dependent Apoptosis/Ferroptosis. J Cancer. 2021;12(13):4075–85.
Mokhtari RB, et al. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022.
Dashti MR, et al. G Protein-Coupled Receptor 75 (GPR75) As a Novel Molecule for Targeted Therapy of Cancer and Metabolic Syndrome. Asian Pac J Cancer Prev. 2023;24(5):1817–25.
Firouzi-Amandi A, et al. Development, characterization, and in vitro evaluation of cytotoxic activity of Rutin loaded PCL-PEG nanoparticles against Skov3 ovarian cancer cell. Asian Pac J Cancer Prev: APJCP. 2022;23(6):1951.
Alagheband Y, et al. Design and fabrication of a dual-drug loaded nano-platform for synergistic anticancer and cytotoxicity effects on the expression of leptin in lung cancer treatment. J Drug Deliv Sci Technol. 2022;73:103389.
Monsen RC, et al. The hTERT core promoter forms three parallel G-quadruplexes. Nucleic Acids Res. 2020;48(10):5720–34.
Hannen R, Bartsch JW. Essential roles of telomerase reverse transcriptase hTERT in cancer stemness and metastasis. FEBS Lett. 2018;592(12):2023–31.
Khosravi-Maharlooei M, et al. Expression pattern of alternative splicing variants of human telomerase reverse transcriptase (hTERT) in cancer cell lines was not associated with the origin of the cells. Int J Mol Cell Med. 2015;4(2):109.
Chen RJ, et al. P53-dependent downregulation of hTERT protein expression and telomerase activity induces senescence in lung cancer cells as a result of pterostilbene treatment. Cell Death Dis. 2017;8(8):e2985.
Barkhordari A, et al. Potential Anti-Cancer Effect of Helenalin as a Natural Bioactive Compound on the Growth and Telomerase Gene Expression in Breast Cancer Cell Line. Asian Pac J Cancer Prev: APJCP. 2023;24(1):133.
Suvarna V, Singh V, Murahari M. Current overview on the clinical update of Bcl-2 anti-apoptotic inhibitors for cancer therapy. Eur J Pharmacol. 2019;862:172655.
Carrington EM, et al. Anti-apoptotic proteins BCL-2, MCL-1 and A1 summate collectively to maintain survival of immune cell populations both in vitro and in vivo. Cell Death Differ. 2017;24(5):878–88.
Zhou M, et al. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 2018;143(4):921–30.
Walsh JG, et al. Executioner caspase-3 and caspase-7 are functionally distinct proteases. Proc Natl Acad Sci. 2008;105(35):12815–9.
Lamkanfi M, Kanneganti T-D. Caspase-7: a protease involved in apoptosis and inflammation. Int J Biochem Cell Biol. 2010;42(1):21–4.
Caglar HO, BirayAvci C. Alterations of cell cycle genes in cancer: unmasking the role of cancer stem cells. Mol Biol Rep. 2020;47(4):3065–76.
Kaldis P. The cdk-activating kinase (CAK): from yeast to mammals. Cell Mol Life Sci CMLS. 1999;55(2):284–96.
Amirsaadat S et al. Potential anti-proliferative effect of nano-formulated curcumin through modulating micro RNA-132, Cyclin D1, and hTERT genes expression in breast cancer cell lines. J Clust Sci. 2023;8:1–10
Kato J-Y, et al. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7(3):331–42.
Bonelli M, et al. Multiple effects of CDK4/6 inhibition in cancer: From cell cycle arrest to immunomodulation. Biochem Pharmacol. 2019;170:113676.
Musgrove EA, et al. Cyclin D as a therapeutic target in cancer. Nat Rev Cancer. 2011;11(8):558–72.
Wagh VD, Deshmukh OJ. Itraconazole Niosomes Drug Delivery System and Its Antimycotic Activity against Candida albicans. ISRN Pharm. 2012;2012:653465.
Arunachalam A, et al. Niosomes: a novel drug delivery system. Int J Novel Trends Pharm Sci. 2012;2(1):25–31.
Bhardwaj P et al. Niosomes: A review on niosomal research in the last decade. J Drug Deliv Sci Technol. 2020;56:101581
Shahbazi R, et al. Design and optimization various formulations of PEGylated niosomal nanoparticles loaded with phytochemical agents: potential anti-cancer effects against human lung cancer cells. Pharmacol Rep. 2023;75(2):442–55.
Ali Hadi Z, et al. Design and Development of Fe3O4@Prussian Blue Nanocomposite: Potential Application in the Detoxification of Bilirubin. Asian Pac J Cancer Prev. 2023;24(8):2809–15.
Ge X, et al. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics. 2019;11(2):55.
Umamaheswari A, et al. Green synthesis of zinc oxide nanoparticles using leaf extracts of Raphanus sativus var. Longipinnatus and evaluation of their anticancer property in A549 cell lines. Biotechnol Rep. 2021;29:e00595.
Gurunathan S, et al. Platinum nanoparticles enhance exosome release in human lung epithelial adenocarcinoma cancer cells (A549): oxidative stress and the ceramide pathway are key players. Int J Nanomed. 2021;16:515.
Amirsaadat S, et al. Metformin and Silibinin co-loaded PLGA-PEG nanoparticles for effective combination therapy against human breast cancer cells. J Drug Deliv Sci Technol. 2021;61:102107.
Alshetaili AS. Gefitinib loaded PLGA and chitosan coated PLGA nanoparticles with magnified cytotoxicity against A549 lung cancer cell lines. Saudi J Biol Sci. 2021;28(9):5065–5073
Guo M et al. Cediranib Induces Apoptosis, G1 Phase Cell Cycle Arrest, and Autophagy in Non-Small-Cell Lung Cancer Cell A549 In Vitro. BioMed Res Int. 2021:5582648
Kis B, et al. Inorganic Element Determination of Romanian Populus nigra L. Buds Extract and In Vitro Antiproliferative and Pro-Apoptotic Evaluation on A549 Human Lung Cancer Cell Line. Pharmaceutics. 2021;13(7):986.
Firouzi-Amandi A, et al. Chrysin-nanoencapsulated PLGA-PEG for macrophage repolarization: Possible application in tissue regeneration. Biomed Pharmacother. 2018;105:773–80.
Hawash M, et al. The impact of filtered water-pipe smoke on healthy versus cancer cells and their neurodegenerative role on AMPA receptor. Drug Chem Toxicol. 2022;45(5):2292–300.
Yoo J, et al. Active targeting strategies using biological ligands for nanoparticle drug delivery systems. Cancers. 2019;11(5):640.
Dang Y, Guan J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater Med. 2020;1:10–9.
Rizvi SA, Saleh AM. Applications of nanoparticle systems in drug delivery technology. Saudi Pharm J. 2018;26(1):64–70.
Momekova DB, Gugleva VE, Petrov PD. Nanoarchitectonics of multifunctional niosomes for advanced drug delivery. ACS Omega. 2021;6(49):33265–73.
Rasul A et al. In vitro characterization and release studies of combined nonionic surfactant-based vesicles for the prolonged delivery of an immunosuppressant model drug. Int J Nanomedicine. 2020;7937–7949.
Solomun JI, et al. Manual versus microfluidic-assisted nanoparticle manufacture: impact of silk fibroin stock on nanoparticle characteristics. ACS Biomater Sci Eng. 2020;6(5):2796–804.
Hajizadeh MR, et al. In vitro cytotoxicity assay of D-limonene niosomes: an efficient nano-carrier for enhancing solubility of plant-extracted agents. Res Pharm Sci. 2019;14(5):448.
Barani M, et al. Evaluation of carum-loaded niosomes on breast cancer cells: Physicochemical properties, in vitro cytotoxicity, flow cytometric, DNA fragmentation and cell migration assay. Sci Rep. 2019;9(1):7139.
Minamisakamoto T, et al. Sequential administration of PEG-Span 80 niosome enhances anti-tumor effect of doxorubicin-containing PEG liposome. Eur J Pharm Biopharm. 2021;169:20–8.
Buchman JT, et al. Understanding nanoparticle toxicity mechanisms to inform redesign strategies to reduce environmental impact. Acc Chem Res. 2019;52(6):1632–42.
Barani M, et al. In vitro and in vivo anticancer effect of pH-responsive paclitaxel-loaded niosomes. J Mater Sci - Mater Med. 2021;32:1–13.
Hajizadeh MR, et al. Diosgenin-loaded niosome as an effective phytochemical nanocarrier: Physicochemical characterization, loading efficiency, and cytotoxicity assay. DARU J Pharm Sci. 2019;27:329–39.
Durak S, et al. Niosomal drug delivery systems for ocular disease—Recent advances and future prospects. Nanomaterials. 2020;10(6):1191.
Jadon PS, et al. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS PharmSciTech. 2009;10:1186–92.
Jadid MFS et al. Enhanced anti-cancer effect of curcumin loaded-niosomal nanoparticles in combination with heat-killed Saccharomyces cerevisiae against human colon cancer cells. J Drug Deliv Sci Technol. 2023;80:104167
Davarpanah F, et al. Magnetic delivery of antitumor carboplatin by using PEGylated-Niosomes. DARU J Pharm Sci. 2018;26:57–64.
Barani M, et al. In silico and in vitro study of magnetic niosomes for gene delivery: The effect of ergosterol and cholesterol. Mater Sci Eng, C. 2019;94:234–46.
El-Ridy MS, et al. Metformin hydrochloride and wound healing: from nanoformulation to pharmacological evaluation. J Liposome Res. 2019;29(4):343–56.
Nazim UM, et al. Activation of autophagy flux by metformin downregulates cellular FLICE–like inhibitory protein and enhances TRAIL-induced apoptosis. Oncotarget. 2016;7(17):23468.
Wu N, et al. Metformin induces apoptosis of lung cancer cells through activating JNK/p38 MAPK pathway and GADD153. Neoplasma. 2011;58(6):482–90.
Zaki NM. Augmented cytotoxicity of hydroxycamptothecin-loaded nanoparticles in lung and colon cancer cells by chemosensitizing pharmaceutical excipients. Drug Deliv. 2014;21(4):265–75.
Kamranvar SA, Rani B, Johansson S. Cell cycle regulation by integrin-mediated adhesion. Cells. 2022;11(16):2521.
Cheong DH, et al. Anti-malarial drug, artemisinin and its derivatives for the treatment of respiratory diseases. Pharmacol Res. 2020;158:104901.
Prasedya ES, et al. Carrageenan delays cell cycle progression in human cancer cells in vitro demonstrated by FUCCI imaging. BMC Complement Altern Med. 2016;16(1):1–9.
Funding
The authors declare that no funds were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Conflict of ınterest
No potential competing interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
40199_2023_495_MOESM1_ESM.tif
Supplementary file1 (TIF 1093 KB) Figure S1. FTIR result of ART, MET, and drug loaded NPs. A) ART, B) MET, C) blank Nio-NPs, D) ANP, E) MNP. Fourier Transform Infrared (FTIR) spectroscopy is a powerful technique used for the characterization and analysis of various materials, including polymeric and biopolymeric materials.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abdulkareem, S.J., Jafari-Gharabaghlou, D., Farhoudi-Sefidan-Jadid, M. et al. Co-delivery of artemisinin and metformin via PEGylated niosomal nanoparticles: potential anti-cancer effect in treatment of lung cancer cells. DARU J Pharm Sci (2024). https://doi.org/10.1007/s40199-023-00495-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40199-023-00495-7