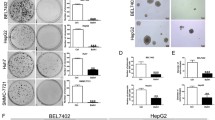
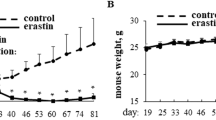
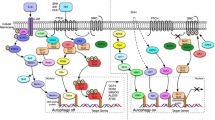
Abstract
Metastasis is the leading cause of death in cancer patients and a major challenging aspect of cancer biology. Various adaptive molecular signaling pathways play a crucial role in cancer metastasis and later in the formation of secondary tumors. Aggressive cancer cells like triple negative breast cancer (TNBCs) are more inclined to undergo metastasis hence having a high recurrence rate and potential of micro-metastasis. Tumor cells in circulation known as circulating tumor cells (CTCs) offer an attractive drug target to treat metastatic disease. Cell cycle regulation and stress response of CTCs in blood has a crucial role in their survival and progression and thus may be considered therapeutically active hotspots. The cyclin D/cyclin-dependent kinase (CDK) pathway regulates cell cycle checkpoints, a process that is frequently dysregulated in cancer cells. Selective CDK inhibitors can limit the phosphorylation of cell cycle regulatory proteins by inducing cell cycle phase arrest, and thus may be an effective therapeutic strategy for aggressive cancer cells in their dividing phase at the primary or secondary site. However, during the floating condition, cancer cells halt their multiplication process and proceed through the various steps of metastasis. Current study showed that a novel CDK inhibitor 4ab induced autophagy and endoplasmic reticulum (ER) stress in agressive cancer cells grown under adherent and floating conditions resulting in paraptosis. Further, our results showed that 4ab efficiently induced cell death in aggressive cancer cells through ER stress-mediated activation of JNK signaling. Additionally, was observed that treatment of 4ab in tumor-bearing mice displayed a significant reduction in tumor burden and micro-metastasis. The outcome of these studies showed that 4ab can be a potential anti-tumor and anti-metastatic agent.
Graphical abstract
Graphical representation of 4ab: image representing the effect of 4ab on death-inducing pathways in aggressive cancer cells. 4ab induces ER stress and activates autophagy leading to vacuolation of there by causing apoptosis in aggressive cancer cells.









Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Susanti NM, Tjahjono DH. Cyclin-dependent kinase 4 and 6 inhibitors in cell cycle dysregulation for breast cancer treatment. Molecules. 2021;26(15):4462. https://doi.org/10.3390/molecules26154462.
Cicenas J, Kalyan K, Sorokinas A, Jatulyte A, Valiunas D, Kaupinis A, Valius M. Highlights of the latest advances in research on CDK inhibitors. Cancers. 2014;6(4):2224–42. https://doi.org/10.3390/cancers6042224.
Adeshakin FO, Adeshakin AO, Afolabi LO, Yan D, Zhang G, Wan X. Mechanisms for modulating anoikis resistance in cancer and the relevance of metabolic reprogramming. Front Oncol. 2021. https://doi.org/10.3389/fonc.2021.626577.
Örd M, Möll K, Agerova A, Kivi R, Faustova I, Venta R, Valk E, Loog M. Multisite phosphorylation code of CDK. Nat Struct Mol Biol. 2019;26(7):649–58. https://doi.org/10.1038/s41594-019-0256-4.
Patterson JO, Basu S, Rees P, Nurse P. CDK control pathways integrate cell size and ploidy information to control cell division. Elife. 2021. https://doi.org/10.7554/eLife.64592.
Shen S, Dean DC, Yu Z, Duan Z. Role of cyclin-dependent kinases (CDKs) in hepatocellular carcinoma: therapeutic potential of targeting the CDK signaling pathway. Hepatol Res. 2019;49(10):1097–108. https://doi.org/10.1111/hepr.13353.
García-Reyes B, Kretz AL, Ruff JP, Von Karstedt S, Hillenbrand A, Knippschild U, Henne-Bruns D, Lemke J. The emerging role of cyclin-dependent kinases (CDKs) in pancreatic ductal adenocarcinoma. Int J Mol Sci. 2018;19(10):3219. https://doi.org/10.3390/ijms19103219.
Ding L, Cao J, Lin W, Chen H, Xiong X, Ao H, Yu M, Lin J, Cui Q. The roles of cyclin-dependent kinases in cell-cycle progression and therapeutic strategies in human breast cancer. Int J Mol Sci. 2020;21(6):1960. https://doi.org/10.3390/ijms21061960.
Malhotra N, Gupta R, Kumar P. Pharmacological relevance of CDK inhibitors in Alzheimer’s disease. Neurochem Int. 2021;148:105115. https://doi.org/10.1016/j.neuint.2021.105115.
Joseph C, Mangani AS, Gupta V, Chitranshi N, Shen T, Dheer Y, Devaraj KB, Mirzaei M, You Y, Graham SL, Gupta V. Cell cycle deficits in neurodegenerative disorders: uncovering molecular mechanisms to drive innovative therapeutic development. Aging Dis. 2020;11(4):946. https://doi.org/10.14336/AD.2019.0923.
Cartwright JA, Lucas CD, Rossi AG. Inflammation resolution and the induction of granulocyte apoptosis by cyclin-dependent kinase inhibitor drugs. Front Pharmacol. 2019;19(10):55. https://doi.org/10.3389/fphar.2019.00055.
Wang P, Xie M, Yang D, Wang F, Chen E. 2020 Integrative multi-omics analysis reveals the landscape of cyclin-dependent kinase (CDK) family genes in pan-cancer, https://doi.org/10.21203/rs.3.rs-24985/v2
Wood DJ, Endicott JA. Structural insights into the functional diversity of the CDK–cyclin family. Open Biol. 2018;8(9):180112. https://doi.org/10.1098/rsob.180112.
Loyer P, Trembley JH. Roles of CDK/Cyclin complexes in transcription and pre-mRNA splicing: cyclins L and CDK11 at the crossroads of cell cycle and regulation of gene expression. In: Seminars in cell & developmental biology. Academic Press; 2020. p. 36–45.
Hassan S, Sajjad N, Khan SU, Gupta S, Bhat MA, Ali R, Ahmad Z, Ganie SA, Hamid R. Dipsacus inermis Wall. Modulates inflammation by inhibiting NF-κB pathway: an in vitro and in vivo study. J Ethnopharmacol. 2020;254:112710. https://doi.org/10.1016/j.jep.2020.112710.
Khan SU, Pathania AS, Wani A, Fatima K, Mintoo MJ, Hamza B, Paddar MA, Bhumika W, Anand LK, Maqbool MS, Mir SA. Activation of lysosomal mediated cell death in the course of autophagy by mTORC1 inhibitor. Sci Rep. 2022;12(1):5052. https://doi.org/10.1038/s41598-022-07955-1.
Singh U, Chashoo G, Khan SU, Mahajan P, Nargotra A, Mahajan G, Singh A, Sharma A, Mintoo MJ, Guru SK, Aruri H. Design of novel 3-pyrimidinylazaindole CDK2/9 inhibitors with potent in vitro and in vivo antitumor efficacy in a triple-negative breast cancer model. J Med Chem. 2017;60(23):9470–89. https://doi.org/10.1021/acs.jmedchem.7b00663.
Bury M, Le Calvé B, Ferbeyre G, Blank V, Lessard F. New insights into CDK regulators: novel opportunities for cancer therapy. Trends Cell Biol. 2021;31(5):331–44. https://doi.org/10.1016/j.tcb.2021.01.010.
Khan SU, Fatima K, Malik F. Understanding the cell survival mechanism of anoikis-resistant cancer cells during different steps of metastasis. Clin Exp Metas. 2022;39(5):715–26. https://doi.org/10.1007/s10585-022-10172-9.
Gao JJ, Cheng J, Bloomquist E, Sanchez J, Wedam SB, Singh H, Amiri-Kordestani L, Ibrahim A, Sridhara R, Goldberg KB, Theoret MR. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: a US food and drug administration pooled analysis. Lancet Oncol. 2020;21(2):250–60. https://doi.org/10.1016/S1470-2045(19)30804-6.
Cidado J, Boiko S, Proia T, Ferguson D, Criscione SW, San Martin M, Pop-Damkov P, Su N, Roamio Franklin VN, Sekhar Reddy Chilamakuri C, D’Santos CS. AZD4573 Is a highly selective CDK9 inhibitor that suppresses MCL-1 and Induces apoptosis in hematologic cancer cells AZD4573, a selective CDK9 inhibitor, targets MCL-1. Clin Cancer Res. 2020;26(4):922–34. https://doi.org/10.1158/1078-0432.CCR-19-1853.
Mintoo M, Khan S, Wani A, Malik S, Bhurta D, Bharate S, Malik F, Mondhe D. A rohitukine derivative IIIM-290 induces p53 dependent mitochondrial apoptosis in acute lymphoblastic leukemia cells. Mol Carcinog. 2021;60(10):671–83. https://doi.org/10.1002/mc.23332.
Odle RI, Walker SA, Oxley D, Kidger AM, Balmanno K, Gilley R, Okkenhaug H, Florey O, Ktistakis NT, Cook SJ. An mTORC1-to-CDK1 switch maintains autophagy suppression during mitosis. Mol Cell. 2020;77(2):228-240.e7. https://doi.org/10.1016/j.molcel.2019.10.016.
Wani A, Al Rihani SB, Sharma A, Weadick B, Govindarajan R, Khan SU, Sharma PR, Dogra A, Nandi U, Reddy CN, Bharate SS. Crocetin promotes clearance of amyloid-β by inducing autophagy via the STK11/LKB1-mediated AMPK pathway. Autophagy. 2021;17(11):3813–32. https://doi.org/10.1080/15548627.2021.1872187.
Khan SU, Fatima K, Aisha S, Hamza B, Malik F. Redox balance and autophagy regulation in cancer progression and their therapeutic perspective. Med Oncol. 2022;40(1):12. https://doi.org/10.1007/s12032-022-01871-0.
Khan SU, Rayees S, Sharma P, Malik F. Targeting redox regulation and autophagy systems in cancer stem cells. Clin Exp Med. 2022;6:1–9. https://doi.org/10.1007/s10238-022-00955-5.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA: Cancer J Clin. 2017;67(3):177–93. https://doi.org/10.3322/caac.21395.
Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH, Neoptolemos JP. Pancreatic cancer. Nat Rev Dis Primers. 2016;2(1):1–22. https://doi.org/10.1038/nrdp.2016.22.
Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605–17. https://doi.org/10.1056/NEJMra0901557.
Mancuso A, Calabrò F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol. 2006;58(3):231–41. https://doi.org/10.1016/j.critrevonc.2006.02.004.
Chand S, O’Hayer K, Blanco FF, Winter JM, Brody JR. The landscape of pancreatic cancer therapeutic resistance mechanisms. Int J Biol Sci. 2016;12(3):273. https://doi.org/10.7150/ijbs.14951.
Conroy T, Bachet JB, Ayav A, Huguet F, Lambert A, Caramella C, Maréchal R, Van Laethem JL, Ducreux M. Current standards and new innovative approaches for treatment of pancreatic cancer. Eur J Cancer. 2016;1(57):10–22. https://doi.org/10.1016/j.ejca.2015.12.026.
Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–64. https://doi.org/10.1016/s0092-8674(02)00625-6.
Kretz AL, Von Karstedt S, Hillenbrand A, Henne-Bruns D, Knippschild U, Trauzold A, Lemke J. Should we keep walking along the trail for pancreatic cancer treatment? Revisiting TNF-related apoptosis-inducing ligand for anticancer therapy. Cancers. 2018;10(3):77. https://doi.org/10.3390/cancers10030077.
Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Chone L, Francois E, Artru P, Biagi JJ, Lecomte T. 2018 Unicancer GI PRODIGE 24/CCTG PA. 6 trial: A multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas.
Saung MT, Zheng L. Current standards of chemotherapy for pancreatic cancer. Clin Ther. 2017;39(11):2125–34. https://doi.org/10.1016/j.clinthera.2017.08.015.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14(2):130–46. https://doi.org/10.1038/nrd4504.
Gonzalez Suarez N, Rodriguez Torres S, Ouanouki A, El Cheikh-Hussein L, Annabi B. EGCG inhibits adipose-derived mesenchymal stem cells differentiation into adipocytes and prevents a STAT3-mediated paracrine oncogenic control of triple-negative breast cancer cell invasive phenotype. Molecules. 2021;26(6):1506. https://doi.org/10.3390/molecules26061506.
Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12(2):106. https://doi.org/10.7497/j.issn.2095-3941.2015.0030.
Ma F, Li H, Wang H, Shi X, Fan Y, Ding X, Lin C, Zhan Q, Qian H, Xu B. Enriched CD44+/CD24− population drives the aggressive phenotypes presented in triple-negative breast cancer (TNBC). Cancer Lett. 2014;353(2):153–9. https://doi.org/10.1016/j.canlet.2014.06.022.
Pickup MW, Owens P, Gorska AE, Chytil A, Ye F, Shi C, Weaver VM, Kalluri R, Moses HL, Novitskiy SV. Development of aggressive pancreatic ductal adenocarcinomas depends on granulocyte colony stimulating factor secretion in carcinoma cellsG-CSF regulation of aggressive PDAC. Cancer Immunol Res. 2017;5(9):718–29. https://doi.org/10.1158/2326-6066.CIR-16-0311.
Mendes GC, Reis PA, Calil IP, Carvalho HH, Aragão FJ, Fontes EP. GmNAC30 and GmNAC81 integrate the endoplasmic reticulum stress-and osmotic stress-induced cell death responses through a vacuolar processing enzyme. Proc Natl Acad Sci USA. 2013;110(48):19627–32. https://doi.org/10.1073/pnas.1311729110.
Lee WJ, Chien MH, Chow JM, Chang JL, Wen YC, Lin YW, Cheng CW, Lai GM, Hsiao M, Lee LM. Corrigendum: Nonautophagic cytoplasmic vacuolation death induction in human PC-3M prostate cancer by curcumin through reactive oxygen species-mediated endoplasmic reticulum stress. Sci Rep. 2017. https://doi.org/10.1038/srep10420.
Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L. Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther. 2004;3(5):551–66. https://doi.org/10.1158/1535-7163.551.3.5.
Nishiumi F, Kawai Y, Nakura Y, Yoshimura M, Wu HN, Hamaguchi M, Kakizawa S, Suzuki Y, Glass JI, Yanagihara I. Blockade of endoplasmic reticulum stress-induced cell death by Ureaplasma parvum vacuolating factor. Cell Microbiol. 2021;12:e13392. https://doi.org/10.1111/cmi.13392.
Ram BM, Ramakrishna G. Endoplasmic reticulum vacuolation and unfolded protein response leading to paraptosis like cell death in cyclosporine: a treated cancer cervix cells is mediated by cyclophilin B inhibition. Biochim et Biophys Acta (BBA) Mol Cell Res. 2014;11:2497–512. https://doi.org/10.1016/j.bbamcr.2014.06.020.
Williams JA, Hou Y, Ni HM, Ding WX. Role of intracellular calcium in proteasome inhibitor-induced endoplasmic reticulum stress, autophagy, and cell death. Pharm Res. 2013;30:2279–89. https://doi.org/10.1007/s11095-013-1139-8.
Fuest M, Willim K, MacNelly S, Fellner N, Resch GP, Blum HE, Hasselblatt P. The transcription factor c-Jun protects against sustained hepatic endoplasmic reticulum stress thereby promoting hepatocyte survival. Hepatology. 2012;55(2):408–18. https://doi.org/10.1002/hep.24699.
Hendrix MJ, Seftor EA, Kirschmann DA, Seftor RE. Molecular biology of breast cancer metastasis molecular expression of vascular markers by aggressive breast cancer cells. Breast Cancer Res. 2000;2(6):1–6. https://doi.org/10.1186/bcr88.
Geng SQ, Alexandrou AT, Li JJ. Breast cancer stem cells: Multiple capacities in tumor metastasis. Cancer Lett. 2014;349(1):1–7. https://doi.org/10.1016/j.canlet.2014.03.036.
Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44(12):2144–51. https://doi.org/10.1016/j.biocel.2012.08.022.
Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–34. https://doi.org/10.1038/nm.4409.
Park SY, Choi JH, Nam JS. Targeting cancer stem cells in triple-negative breast cancer. Cancers. 2019;11(7):965. https://doi.org/10.3390/cancers11070965.
Hua Z, White J, Zhou J. Cancer stem cells in TNBC. In: Seminars in cancer biology. Academic Press; 2022. p. 26–34.
Acknowledgements
We would also like to thank our lab colleagues, particularly Abubakar Wani (St. Jude Children Hospital, USA), Shariqa Aisha (CSIR IIIM, India) for their fruitful discussions, proof read and valuable inputs. We would like to thank CSIR-IIIM for providing publication approval (Institutional Publication ID No. CSIR-IIIM/IPR/00465).
Funding
Funding for F.M. laboratory was provided by the Council of Scientific and Industrial Research (CSIR) India fellowship, a grant from the Department of Biotechnology, Ministry of Science of Technology (DBT) (BT/IN/SWISS/48/FM/2018-19). CSIR-SRF budget head for providing fellowship to S.K.
Author information
Authors and Affiliations
Contributions
FM supervised the study; FM and SK designed the experiments; SK performed most of the experiments and wrote the paper; SK and KF helped in the interpretation of the results; SK and KF repeated most of the experiments; SK draws the graphical abstract; SK and KF performed animal experimentation. US and PP synthesized the molecule.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
The use of experimental animals in this study was approved by the Ethics and Institutional Animal Care and Use, Committees of the Council of Scientific and Industrial Research-Indian Institute of Integrative Medicine (CSIR-IIIM) following guidelines of the Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA). I confirm that all methods were carried out in accordance with relevant guidelines and regulations. I confirm that the study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
The content of this manuscript has not been previously published anywhere and is not under consideration for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, S.U., Fatima, K., Singh, U. et al. Small molecule ‘4ab’ induced autophagy and endoplasmic reticulum stress-mediated death of aggressive cancer cells grown under adherent and floating conditions. Med Oncol 40, 121 (2023). https://doi.org/10.1007/s12032-023-01963-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-01963-5