Abstract
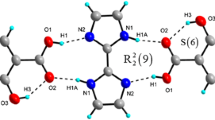
The structure of ammonium hydrogensquarate squaric acid monohydrate has been determined by single crystal X-ray diffraction. The compound crystallizes in the monoclinic space group C2/c and exhibits a 3D network with molecules linked by intermolecular interactions with participation of the H2Sq, HSq−, NH4 +, and H2O species. The HSq− anion and the neutral H2Sq form a strong head-to-tail dimer through O–H···O hydrogen bonding with lengths of 2.587 and 2.494 Å (protected space between numeral and unit). The layers are connected by ammonium cations and water molecules in a plane through the O···N (2.950, 2.978, 3.036 Å) and O···O (2.953, 2.781 Å) bonds. Another such layer is connected to the NH4 + cation in the adjacent plane through bifurcated N–H···O hydrogen-bonding to form a double layer (NH···O bond lengths are 3.036, 2.978, 2.857, 2.909, 2.958, and 2.742 Å, respectively). The IR-band assignment of the compound was achieved using the polarized IR-spectroscopy of oriented colloids in a nematic host. Theoretical ab initio calculations were performed and achieved with a view to explain the IR-bands of the H2Sq.HSq− motif.








Similar content being viewed by others
References
Gilli G, Bertolasi V, Gilli P, Ferretti V (2001) Acta Cryst B57:859
Bertolasi V, Gilli P, Ferretti V, Gilli G (2001) Acta Cryst B57:591
Karle I, Ranganathan D, Haridas V (1996) J Am Chem Soc 118:7128
Mathew S, Paul G, Shivasankar K, Choudhury A, Rao CNR (2002) J Mol Struct 641:263
Kolev T, Glavcheva Z, Petrova R, Angelova O (2000) Acta Cryst C56:110
Kolev T, Glavcheva Z, Stahl R, Preut H, Bleckmann P, Radomirska V (1997) Acta Cryst C53:IUC9700010
Yeşilel OZ, Odabaşoğlu M, Büyükgüngör O (2007) J Mol Struct, in press
Kolev T (2007) J Mol Struct, in press
Georgopoulos SL, Diniz R, Rodrigues BL, Yoshida MI, de Oliveira LFC (2006) J Mol Struct 794:63
Georgopoulos SL, Diniz R, Rodrigues BL, Yoshida MI, de Oliveira LFC (2005) J Mol Struct 753:147
Georgopoulos SL, Diniz R, Rodrigues BL, de Oliveira LFC (2005) J Mol Struct 741:61
Gonçalves NS, Noda LK, Neto AMP, Santos PS, Mutarelli SR, Sala O (2003) J Mol Struct 645:185
de Oliveira LFC, Santos PS (1992) J Mol Struct 269:85
de Oliveira LFC, Santos PS (1991) J Mol Struct 245:215
Ivanova BB, Arnaudov MG, Bontchev PR (2004) Spectrochim Acta 60A(4):855
Ivanova BB, Tsalev DL, Arnaudov MG (2006) Talanta 69:822
Ivanova BB, Simeonov VD, Arnaudov MG, Tsalev DL (2007) Spectrochim Acta 67A:66
Koleva BB, Kolev T, Simeonov V, Spassov T, Spiteller M (2007) Coll Int. submitted
Sheldrick GM (1997) SHELXS-97, Program for Crystal Structure Solution, University of Göttingen
Sheldrick GM (1997) SHELXL-97, Program for Crystal Structure Refinement, University of Göttingen
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas Ö, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Salvador P, Dannenberg JJ, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komáromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98. Gaussian, Inc., Pittsburgh, PA
Zhurko GA, Zhurko DA (2005) ChemCraft: Tool for treatment of chemical data, Lite version build 08 (freeware)
Evans JC (1960) Spectrochim Acta 16:994
Evans JC (1961) Spectrochim Acta 17:129
Evans JC (1962) Spectrochim Acta 18:507
Acknowledgments
The author Bojidarka B. Koleva wishes to thank the Alexander von Humboldt Foundation for the Fellowship. The authors Tsonko Kolev and Michael Spiteller wish to thank the DAAD for a grant within the priority program “Stability Pact South-Eastern Europe” and the Alexander von Humboldt Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolev, T., Seidel, R.W., Koleva, B.B. et al. Crystal structure and spectroscopic properties of ammonium hydrogensquarate squaric acid monohydrate. Struct Chem 19, 101–107 (2008). https://doi.org/10.1007/s11224-007-9257-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-007-9257-8