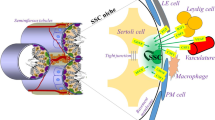
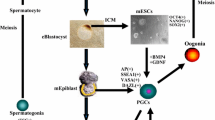
Abstract
Purpose
Spermatogonial stem cells (SSCs) are the source for the mature male gamete. SSC technology in humans is mainly focusing on preserving fertility in cancer patients. Whereas in livestock, it is used for mining the factors associated with male fertility. The review discusses the present status of SSC biology, methodologies developed for in vitro culture, and challenges ahead in establishing SSC technology for the propagation of superior germplasm with special reference to livestock.
Method
Published literatures from PubMed and Google Scholar on topics of SSCs isolation, purification, characterization, short and long-term culture of SSCs, stemness maintenance, epigenetic modifications of SSCs, growth factors, and SSC cryopreservation and transplantation were used for the study.
Result
The fine-tuning of SSC isolation and culture conditions with special reference to feeder cells, growth factors, and additives need to be refined for livestock. An insight into the molecular mechanisms involved in maintaining stemness and proliferation of SSCs could facilitate the dissemination of superior germplasm through transplantation and transgenesis. The epigenetic influence on the composition and expression of the biomolecules during in vitro differentiation of cultured cells is essential for sustaining fertility. The development of surrogate males through gene-editing will be historic achievement for the foothold of the SSCs technology.
Conclusion
Detailed studies on the species-specific factors regulating the stemness and differentiation of the SSCs are required for the development of a long-term culture system and in vitro spermatogenesis in livestock. Epigenetic changes in the SSCs during in vitro culture have to be elucidated for the successful application of SSCs for improving the productivity of the animals.
Graphical abstract



Similar content being viewed by others
References
Hai Y, Hou J, Liu Y, Liu Y, Yang H, Li Z, He Z. The roles and regulation of Sertoli cells in fate determinations of spermatogonial stem cells and spermatogenesis. Semin Cell Dev Biol. 2014;29:66–75.
Chen SR, Liu YX. Regulation of spermatogonial stem cell self-renewal and spermatocyte meiosis by Sertoli cell signaling. Reproduction. 2015;149(4):R159-67.
de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121(3):347. https://doi.org/10.1530/rep.0.1210347.
Thibier M, Wagner HG. World statistics for artificial insemination in cattle. Livest Prod Sci. 2002;74(2):203–12.
Giassetti MI, Ciccarelli M, Oatley JM. Spermatogonial stem cell transplantation: insights and outlook for domestic animals. Annu Rev Anim Biosci. 2019;7:385–401. https://doi.org/10.1146/annurev-animal-020518-115239.
Honaramooz A, Yang Y. Recent advances in application of male germ cell transplantation in farm animals. Vet Med Int. 2011;657860:1–9.
Abbasi H, Hosseini SM, Hajian M, Nasiri Z, Bahadorani M, Tahmoorespur M, Nasiri MR, Nasr-Esfahani MH. Lentiviral vector-mediated transduction of goat undifferentiated spermatogonia. Anim Reprod Sci. 2015;163:10–7 (https://www.sciencedirect.com/science/article/pii/S0378432015300130).
González R, Tang L, Dobrinski I. Application of spermatogonial transplantation in agricultural animals. In: Oatley J., Griswold M. (eds) The biology of mammalian spermatogonia. Springer, New York, NY; 2017. pp. 343–377. https://doi.org/10.1007/978-1-4939-7505-1_14.
Sahare MG, Suyatno Imai H. Recent advances of in vitro culture systems for spermatogonial stem cells in mammals. Reprod Med Biol. 2018;17(2):134–42. https://doi.org/10.1002/rmb2.12087.
Binsila BK, Selvaraju S, Somashekar L, Archana SS, Arangasamy A, Ravindra JP, Bhatta R. Molecular advances in semen quality assessment and improving fertility in bulls—a review. Indian J Anim Reprod. 2018;39:1–10 (https://www.researchgate.net/profile/Sarvpreet_Ghuman/publication/318453930_1_).
Kim Y, Selvaraj V, Dobrinski I, Lee H, Mcentee MC, Travis AJ. Recipient preparation and mixed germ cell isolation for spermatogonial stem cell transplantation in domestic cats. J Androl. 2006;27(2):248–56. https://doi.org/10.2164/jandrol.05034.
Sato T, Katagiri K, Yokonishi T, Kubota Y, Inoue K, Ogonuki N, Matoba S, Ogura A, Ogawa T. In vitro production of fertile sperm from murine spermatogonial stem cell lines. Nat Commun. 2011;2(1):472. 10.1038/ncomms1478.
Kurita K, Burgess SM, Sakai N. Transgenic zebrafish produced by retroviral infection of in vitro-cultured sperm. PNAS. 2004;101(5):1263–7 (www.pnas.orgcgidoi10.1073pnas.0304265101).
Giudice M, De Michele F, Poels J, Vermeulen M, Wyns C. Update on fertility restoration from prepubertal spermatogonial stem cells: how far are we from clinical practice? Stem Cell Res. 2017;21:171–7 (https://www.sciencedirect.com/science/article/pii/S1873506117300107).
Ciccarelli M, Giassetti MI, Miao D, Oatley MJ, Robbins C, Lopez-Biladeau B, Waqas MS, Tibary A, Whitelaw B, Lillico S, Park CH. Donor-derived spermatogenesis following stem cell transplantation in sterile NANOS2 knockout males. Proc Natl Acad Sci. 2020;117(39):24195–204.
Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11(5):715–26 (https://www.sciencedirect.com/science/article/pii/S1934590912004754).
Oatley JM. Spermatogonial stem cell biology in the bull: development of isolation, culture, and transplantation methodologies and their potential impacts on cattle production. Soc Reprod Fertil Suppl. 2010;67:133–43 (https://pubmed.ncbi.nlm.nih.gov/21755668/).
Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. PNAS. 2004;101(47):16489–94 (www.pnas.orgcgidoi10.1073pnas.0407063101).
He Z, Kokkinaki M, Jiang J, Dobrinski I, Dym M. Isolation, characterization, and culture of human spermatogonia. Biol Reprod. 2010;82(2):363–72 (https://academic.oup.com/biolreprod/article-abstract/82/2/363/2557980).
Valli H, Gassei K, Orwig KE. Stem cell therapies for male infertility: Where are we now and where are we going? In: Carrell D., Schlegel P., Racowsky C., Gianaroli L. (eds) Biennial review of infertility. Springer, Cham. 2015. pp. 17–39. https://doi.org/10.1007/978-3-319-17849-3_3.
Wu Y, Zhou H, Fan X, Zhang Y, Zhang M, Wang Y, Xie Z, Bai M, Yin Q, Liang D, Tang W. Correction of a genetic disease by CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Cell Res. 2015;25(1):67–79. https://doi.org/10.1038/cr.2014.160.
Goel S, Reddy N, Mandal S, Fujihara M, Kim SM, Imai H. Spermatogonia-specific proteins expressed in prepubertal buffalo (Bubalus bubalis) testis and their utilization for isolation and in vitro cultivation of spermatogonia. Theriogenology. 2010;74(7):1221–32 (https://www.sciencedirect.com/science/article/pii/S0093691X10002797).
Mahla RS, Reddy N, Goel S. Spermatogonial stem cells (SSCs) in buffalo (Bubalus bubalis) testis. PLoS One. 2012;7(4):e36020. https://doi.org/10.1371/journal.pone.0036020.
Pramod RK, Mitra A. in vitro culture and characterization of spermatogonial stem cells on Sertoli cell feeder layer in goat (Capra hircus). J Assist Reprod Genet. 2014;31(8):993–1001. https://doi.org/10.1007/s10815-014-0277-1.
Paul RK, Bahire SV, Kumar D. Isolation and biochemical characterization of ovine spermatogonial stem cells. Indian J Small Rumin (The). 2017;23(2):186. https://doi.org/10.5958/0973-9718.2017.00041.1.
Lee K, Lee W, Kim J, Yoon M, Kim N, Uhm S, Kim D, Chung H, Song H. Characterization of GFR α-1-positive and GFR α-1-negative spermatogonia in neonatal Pig testis. Reprod Domest Anim. 2013;48(6):954–60 (https://onlinelibrary.wiley.com/doi/abs/10.1111/rda.12193).
Sahare M, Otomo A, Komatsu K, Minami N, Yamada M, Imai H. The role of signaling pathways on proliferation and self-renewal of cultured bovine primitive germ cells. Reprod Med Biol. 2015;14(1):17–25. https://doi.org/10.1007/s12522-014-0189-x.
Zheng Y, Zhang Y, Qu R, He Y, Tian X, Zeng W. Spermatogonial stem cells from domestic animals: progress and prospects. Reproduction. 2014;147(3):R65–74. https://doi.org/10.1530/REP-13-0466.
Clermont Y. Spermatogenesis in man: a study of the spermatogonial population. Fertil Steril. 1966;17(6):705–21.
Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290(2):193–200. https://doi.org/10.1016/0027-5107(93)90159-d.
Marshall GR, Plant TM. Puberty occurring either spontaneously or induced precociously in rhesus monkey (Macaca mulatta) is associated with a marked proliferation of Sertoli cells. Biol Reprod. 1996;54(6):1192–9.
Paniagua R, Codesal J, Nistal M, Rodriguez MC, Santamaría L. Quantification of cell types throughout the cycle of the human seminiferous epithelium and their DNA content. J Anat Embryol. 1987;176(2):225–30.
Narenji Sani R, Tajik P, Yousefi MH, Movahedin M, Qasemi-Panahi B, Shafiei S, Ahmadi Hamedani M. Follicle stimulating hormone increases spermatogonial stem cell colonization during in vitro co-culture. Vet Res Forum. 2013;4(1):37–41 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4293895/).
Binsila KB, Selvaraju S, Ghosh SK, Parthipan S, Archana SS, Arangasamy A, Prasad JK, Bhatta R, Ravindra JP. Isolation and enrichment of putative spermatogonial stem cells from ram (Ovis aries) testis. Anim Reprod Sci. 2018;196:9–18 (https://www.sciencedirect.com/science/article/pii/S0378432017309430).
Han SY, Gupta MK, Uhm SJ, Lee HT. Isolation and in vitro culture of pig spermatogonial stem cell. Asian-Aust J AnimSci. 2009;22(2):187–93 (www.ajas.info).
Yang Y, Yarahmadi M, Honaramooz A. Development of novel strategies for the isolation of piglet testis cells with a high proportion of gonocytes. Reprod Fertil Dev. 2010;22(7):1057. https://doi.org/10.1071/RD09316.
Bryant JM, Meyer-Ficca ML, Dang VM, Berger SL, Meyer RG. Separation of spermatogenic cell types using STA-PUT velocity sedimentation. J Vis Exp. 2013;80:e50648 (https://www.jove.com/video/50648/separation-spermatogenic-cell-types-using-sta-put-velocity).
Bellvé AR. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113.
Liu Y, Niu M, Yao C, Hai Y, Yuan Q, Liu Y, Guo Y, Li Z, He Z. Fractionation of human spermatogenic cells using STA-PUT gravity sedimentation and their miRNA profiling. Sci Rep. 2015;5(1):1–1.
Bellve AR, Cavicchia JC, Millette CF, O’brien DA, Bhatnagar YM, Dym M. Spermatogenic cells of the prepuberal mouse isolation and morphological characterization. Int J Cell Biol. 1977;74(1):68–85.
Habas K, Brinkworth MH, Anderson D. Diethylstilbestrol induces oxidative DNA damage, resulting in apoptosis of spermatogonial stem cells in vitro. Toxicology. 2017;382:117–21.
Shah MA, Xu C, Wu S, Zhao W, Luo H, Yi C, Liu W, Cai X. Isolation and characterization of spermatogenic cells from cattle, yak and cattleyak. Anim Reprod Sci. 2018;193:182–90.
Chen X, Che D, Zhang P, Li X, Yuan Q, Liu T, Guo J, Feng T, Wu L, Liao M, He Z. Profiling of miRNAs in porcine germ cells during spermatogenesis. Reproduction. 2017;154(6):789–98.
Hofmann MC, Braydich-Stolle L, Dym M. Isolation of male germ-line stem cells; influence of GDNF. Dev Biol. 2005;279(1):114–24.
Ogawa T, Ohmura M, Tamura Y, Kita K, Ohbo K, Suda T, Kubota Y. Derivation and morphological characterization of mouse spermatogonial stem cell lines. Arch Histol Cytol. 2004;67(4):297–306. https://doi.org/10.1679/aohc.67.297.
Koruji M, Shahverdi A, Janan A, Piryaei A, Lakpour MR, Sedighi MA. Proliferation of small number of human spermatogonial stem cells obtained from azoospermic patients. J Assist Reprod Genet. 2012;29(9):957–67.
Bahadorani M, Hosseini SM, Abedi P, Hajian M, Afrough M, Azhdari Tafti Z, Azizi H, Hosseini SE, Vahdati A, Baharvand H, Nasr-Esfahani MH. Comparative immunohistochemical analysis of VASA, PLZF and THY1 in goats and sheep suggests that these markers are also conserved in these species. J Cytol Histol. 2011;2(6):126.
Bahadorani M, Hosseini SM, Abedi P, Hajian M, Afrough M, Azhdari Tafti Z, Azizi H, Hosseini SE, Vahdati A, Baharvand H, Nasr-Esfahani MH. Comparative immunohistochemical analysis of VASA, PLZF and THY1 in goats and sheep suggests that these markers are also conserved in these species. J Cytol istol. 2011;2(6):126.
Aponte PM, De Rooij DG. Biomanipulation of bovine spermatogonial stem cells. Anim Reprod (AR). 2018;5(1):16–22.
Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Bühring HJ, Mattheus U, Mack A. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456(7220):344–9 (https://www.nature.com/articles/nature07404).
Huang YH, Chin CC, Ho HN, Chou CK, Shen CN, Kuo HC, Ling TY. Pluripotency of mouse spermatogonial stem cells maintained by IGF-1-dependent pathway. FASEB J. 2009;23(7):2076–87. https://doi.org/10.1096/fj.08-121939.
Bai Y, Zhu C, Feng M, Wei H, Li L, Tian X, Zhao Z, Liu S, Ma N, Zhang X, Shi R. Previously claimed male germline stem cells from porcine testis are actually progenitor Leydig cells. Stem Cell Res Ther. 2018;9(1):1–5.
Jafarnejad A, Aminafshar M, Zandi M, Sanjabi MR, Kashan NE. Optimization of in vitro culture and transfection condition of bovine primary spermatogonial stem cells. S Afr J Anim Sci. 2018;48(1):108–16.
Wang J, Cao H, Xue X, Fan C, Fang F, Zhou J, Zhang Y, Zhang X. Effect of vitamin C on growth of caprine spermatogonial stem cells in vitro. Theriogenology. 2014;81(4):545–55.
Sharma A, Shah SM, Saini N, Mehta P, Kumar BB, Dua D, Singh MK, Singla SK, Palta P, Manik RS, Chauhan MS. Optimization of serum-free culture conditions for propagation of putative buffalo (Bubalus bubalis) spermatogonial stem cells. Cell Reprogram. 2019;21(1):1.
Aponte P, Soda T, Van De Kant H, de Rooij DG. Basic features of bovine spermatogonial culture and effects of glial cell line-derived neurotrophic factor. Theriogenology. 2006;65(9):1828–47 (https://www.sciencedirect.com/science/article/pii/S0093691X05004498).
Aponte P, Soda T, Teerds KJ, Mizrak SC, van de Kant HJ, de Rooij DG. Propagation of bovine spermatogonial stem cells in vitro. Reproduction. 2008;136(5):543–57 (https://rep.bioscientifica.com/view/journals/rep/136/5/543.xml).
Giassetti MI, Goissis MD, de Barros FRO, Bruno AH, Assumpcao MEOA, Visintin JA. Comparison of diverse differential plating methods to enrich bovine spermatogonial cells. Reprod Domest Anim. 2016;51(1):26–32. https://doi.org/10.1111/rda.12641.
Heidari B, Rahmati-Ahmadabadi M, Akhondi MM, Zarnani AH, Jeddi-Tehrani M, Shirazi A, Behzadi B. Isolation identification and culture of goat spermatogonial stem cells using c-kit and PGP95 markers. J Assist Reprod Genet. 2012;29(10):1029–38. https://doi.org/10.1007/s10815-012-9828-5.
Goel S, Sugimoto M, Minami N, Yamada M, Kume S, Imai H. Identification, isolation, and in vitro culture of porcine gonocytes. Biol Reprod. 2007;77(1):127–37 (https://academic.oup.com/biolreprod/article-abstract/77/1/127/2629746).
Kim YH, Choi YR, Kim BJ, Jung SE, Kim SM, Jin JH, Yun MH, Kim SU, Kim YH, Hwang S, Pang MG. GDNF family receptor alpha 1 is a reliable marker of undifferentiated germ cells in bulls. Theriogenology. 2019;132:172–81.
Shinohara T, Avarbock MR, Brinster RL. Beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. PNAS. 1999;96(10):5504–9. https://doi.org/10.1073/pnas.96.10.5504.
Oatley MJ, Kaucher AV, Racicot KE, Oatley JM. Inhibitor of DNA binding 4 is expressed selectively by single spermatogonia in the male germline and regulates the self-renewal of spermatogonial stem cells in mice. Biol Reprod. 2011;85(2):347–56.
Guo Y, Hai Y, Gong Y, Li Z, He Z. Characterization, isolation, and culture of mouse and human spermatogonial stem cells. J Cell Physiol. 2014;229(4):407–13.
Zhou W, Shao H, Zhang D, Dong J, Cheng W, Wang L, Teng Y, Yu Z. PTEN signaling is required for the maintenance of spermatogonial stem cells in mouse, by regulating the expressions of PLZF and UTF1. Cell Biosci. 2015;5(1):1.
Gassei K, Valli H, Orwig KE. Whole-mount immunohistochemistry to study spermatogonial stem cells and spermatogenic lineage development in mice, monkeys, and humans. InStem Cells and Tissue Repair. New York, NY: Humana Press; 2014. p. 193–202.
Jung H, Roser JF, Yoon M. UTF1 a putative marker for spermatogonial stem cells in stallions. PLoS One. 2014;9(10):e108825.
Lee WY, Lee KH, Heo YT, Kim NH, Kim JH, Kim JH, Moon SH, Chung HJ, Yoon MJ, Song H. Transcriptional coactivator undifferentiated embryonic cell transcription factor 1 expressed in spermatogonial stem cells: a putative marker of boar spermatogonia. Anim Reprod Sci. 2014;150(3–4):115–24.
Fayomi AP, Orwig KE. Spermatogonial stem cells and spermatogenesis in mice, monkeys and men. Stem Cell Res. 2018;29:207–14.
Bedford-Guaus S, Kim S, Mulero L, Vaquero J, Morera C, Adan-Milanes R, Veiga A, Raya A. Molecular markers of putative spermatogonial stem cells in the domestic cat. Reprod Domest Anim. 2017;52:177–86. https://doi.org/10.1111/rda.12819.
Goel S, Fujihara M, Tsuchiya K, Takagi Y, Minami N, Yamada M, Imai H. Multipotential ability of primitive germ cells from neonatal pig testis cultured in vitro. Reprod Fertil Dev. 2009;21(5):696–708.
Borjigin U, Davey R, Hutton K, Herrid M. Expression of promyelocytic leukaemia zinc-finger in ovine testis and its application in evaluating the enrichment efficiency of differential plating. Reprod Fertil Dev. 2010;22(5):733–42 (http://www.publish.csiro.au/rd/RD09237).
Valli H, Sukhwani M, Dovey SL, Peters KA, Donohue J, Castro CA, Chu T, Marshall GR, Orwig KE. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril. 2014;102(2):566-580e7. https://doi.org/10.1016/j.fertnstert.2014.04.036.
Herrid M, Davey RJ, Hutton K, Colditz IG, Hill JR. A comparison of methods for preparing enriched populations of bovine spermatogonia. Reprod Fertil and Dev. 2009;21(3):393–9 (http://www.publish.csiro.au/rd/RD08129).
Zheng Y, He Y, An J, Qin J, Wang Y, Zhang Y, Tian X, Zeng W. THY1 is a surface marker of porcine gonocytes. Reprod Fertil Deve. 2014;26(4):533. https://doi.org/10.1071/RD13075.
Reding SC, Stepnoski AL, Cloninger EW, Oatley JM. THY1 is a conserved marker of undifferentiated spermatogonia in the pre-pubertal bull testis. Reproduction. 2010;139(5):893–903. https://doi.org/10.1530/REP-09-0513.
Abbasi H, Tahmoorespur M, Hosseini S, Nasiri Z, Bahadorani M, Hajian M, Nasiri MR, Nasr-Esfahani MH. THY1 as a reliable marker for enrichment of undifferentiated spermatogonia in the goat. Theriogenology. 2013;80(8):923–32 (https://www.sciencedirect.com/science/article/pii/S0093691X13002914).
Wu J, Song W, Zhu H, Niu Z, Mu H, Lei A, Yang C, Peng S, Li X, Li G, Hua J. Enrichment and characterization of Thy1-positive male germline stem cells (mGSCs) from dairy goat (Capra hircus) testis using magnetic microbeads. Theriogenology. 2013;80(9):1052–60. https://doi.org/10.1016/j.theriogenology.2013.08.003.
Cai Y, Wang J, Zou K. The progresses of spermatogonial stem cells sorting using fluorescence-activated cell sorting. Stem Cell Rev Rep. 2020;16(1):94–102.
von Schönfeldt V, Krishnamurthy H, Foppiani L, Schlatt S, von Schönfeldt V, Krishnamurthy H, Foppiani L, Schlatt S. Magnetic cell sorting is a fast and effective method of enriching viable spermatogonia from djungarian hamster, mouse, and marmoset monkey Testes1. Biol Reprod. 1999;61(3):582–9. https://doi.org/10.1095/biolreprod61.3.582.
Zhang R, Sun J, Zou K. Advances in isolation methods for spermatogonial stem cells. Stem Cell Rev Rep. 2016;12(1):15–25.
Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci USA. 2000;97(15):8346–51. https://doi.org/10.1073/pnas.97.15.8346.
Kent Hamra F, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. PNAS. 2005;102(48):17430–5 (www.pnas.orgcgidoi10.1073pnas.0508780102).
Piravar Z, Jeddi-Tehrani M, Sadeghi MR, Mohazzab A, Eidi A, Akhondi MM. In vitro culture of human testicular stem cells on feeder-free condition. J Reprod Infertil. 2013;14(1):17–22 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3719359/).
Tiptanavattana N, Techakumphu M, Tharasanit T. Simplified isolation and enrichment of spermatogonial stem-like cells from pubertal domestic cats (Felis catus). J Vet Med Sci. 2015;77(11):1347–53. https://doi.org/10.1292/jvms.15-0207.
Scarpino S, Rita Morena A, Petersen C, Fröysa B, Söder O, Boitani C. A rapid method of Sertoli cell isolation by DSA lectin, allowing mitotic analyses. Mol Cell Endocrinol. 1998;146(1–2):121–7. https://doi.org/10.1016/S0303-7207(98)00190-7.
Bahadorani M, Hosseini S, Abedi P, Hajian M, Hosseini S, Vahdati A, Baharvand H, Nasr-Esfahani MH. Short-term in-vitro culture of goat enriched spermatogonial stem cells using different serum concentrations. J Assist Reprod Genet. 2012;29(1):39–46. https://doi.org/10.1007/s10815-011-9687-5.
Sharma V, Saini S, Aneja B, Kumar A, Thakur A, Bajwa KK, Kumar S, Mohanty AK, Malakar D. 180 Increasing GfrA1-positive spermatogonial stem cell population of goat. Reprod Fertil Dev. 2018;30(1):230. https://doi.org/10.1071/RDv30n1Ab180.
Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2005;69(2):612–6 (https://academic.oup.com/biolreprod/article-abstract/69/2/612/2713013).
Kim YH, Kang HG, Kim BJ, Jung SE, Karmakar PC, Kim SM, Hwang S, Ryu BY. Enrichment and in vitro culture of spermatogonial stem cells from pre-pubertal monkey testes. J Tissue Eng Regen Med. 2017;14(5):557–66. https://doi.org/10.1007/s13770-017-0058-x.
Ahmad S, Xiao Y, Han L, Hua H, Riaz H, Liang A, Yang LG. Isolation, identification and enrichment of type a spermatogonia from the testis of Chinese cross-bred buffaloes (swamp × river). Reprod Domest Anim. 2013;48(3):373–81. https://doi.org/10.1111/j.1439-0531.2012.02159.x.
Oatley MJ, Kaucher AV, Yang QE, Waqas MS, Oatley JM. Conditions for long-term culture of cattle undifferentiated spermatogonia. Biol Reprod. 2016;95(1):14–14. https://doi.org/10.1095/biolreprod.116.139832.
Yang Y, Honaramooz A. Efficient purification of neonatal porcine gonocytes with Nycodenz and differential plating. Reprod Fertil Dev. 2011;23(3):496–505. https://doi.org/10.1071/RD10042.
Zhu H, Liu C, Li M, Sun J, Song W, Hua J. Optimization of the conditions of isolation and culture of dairy goat male germline stem cells (mGSC). Anim Reprod Sci. 2013;137(1–2):45–52. https://doi.org/10.1016/j.anireprosci.2012.12.005.
Kitamura Y, Minami N, Yamada M, Imai H. 192 Culture conditions supporting long-term expansion of bovine spermatogonial stem cells isolated from adult and immature testes. Reprod Fertil Dev. 2018;30(1):236–236 (http://www.publish.csiro.au/rd/fulltext/RDv30n1Ab192).
Nasiri Z, Hosseini SM, Hajian M, Abedi P, Bahadorani M, Baharvand H, Nasr-Esfahani MH. Effects of different feeder layers on short-term culture of prepubertal bovine testicular germ cells In-vitro. Theriogenology. 2012;77(8):1519–28. https://doi.org/10.1016/j.theriogenology.2011.11.019.
Aponte PM, Van Bragt MPA, De Rooij DG, Van Pelt AMM. Spermatogonial stem cells: characteristics and experimental possibilities. APMIS. 2005;113(11–12):727–42. https://doi.org/10.1111/j.1600-0463.2005.apm_302.x.
Fujihara M, Kim S, Minami N, Yamada M, Imai H. Characterization and in vitro culture of male germ cells from developing bovine testis. J Reprod Dev. 2011;57(3):355–64 (https://www.jstage.jst.go.jp/article/jrd/advpub/0/advpub_10-185M/_article/-char/ja/).
Kubota H, Brinster RL. Transplantation and culture of spermatogonial stem cells. In The Biology of Mammalian Spermatogonia, Springer New York, NY. 2017; 271–300. https://doi.org/10.1007/978-1-4939-7505-1_11
Helsel A, Oatley MJ, Oatley JM. Glycolysis-optimized conditions enhance maintenance of regenerative integrity in mouse spermatogonial stem cells during long-term culture. Stem Cell Rep. 2017;8(5):1430–41 (https://www.sciencedirect.com/science/article/pii/S2213671117301108).
Ryu BY, Kubota H, Avarbock MR, Brinster RL. Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat. PNAS. 2005;102(40):14302–7. https://doi.org/10.1073/pnas.0506970102.
Kanatsu-Shinohara M, Inoue K, Ogonuki N, Morimoto H, Ogura A, Shinohara T. Serum-and feeder-free culture of mouse germline stem cells. Biol Reprod. 2011;84(1):97–105 (https://academic.oup.com/biolreprod/article-abstract/84/1/97/2530290).
Aponte PM. Spermatogonial stem cells: current biotechnological advances in reproduction and regenerative medicine. World J Stem Cells. 2015;7(4):669 (https://www.ncbi.nlm.nih.gov/pmc/articles/pmc4444608/).
Medrano JV, Rombaut C, Simon C, Pellicer A, Goossens E. Human spermatogonial stem cells display limited proliferation in vitro under mouse spermatogonial stem cell culture conditions. Fertil Steril. 2016;106(6):1539-1549.e8. https://doi.org/10.1016/j.fertnstert.2016.07.1065.
Oatley Jon M, de Avila DM, Reeves JJ, McLean DJ. success. Biol Reprod. 2004;70(3):625–31. https://doi.org/10.1095/biolreprod.103.022483.
Izadyar F, den Ouden K, Creemers LB, Posthuma G, Parvinen M, De Rooij DG. Proliferation and differentiation of bovine type A spermatogonia during long-term culture. Biol Reprod. 2003;68(1):272–81 (https://academic.oup.com/biolreprod/article-abstract/68/1/272/2683752).
Kanatsu-Shinohara M, Ogonuki N, Matoba S, Morimoto H, Ogura A, Shinohara T. Improved serum-and feeder-free culture of mouse germline stem cells. Biol Reprod. 2014;91(4):88–1 (https://academic.oup.com/biolreprod/article-abstract/91/4/88, 1-11/2434297).
Binsila BK, Selvaraju S, Ghosh SK, Ramya L, Arangasamy A, Ranjithkumaran R, Bhatta R. EGF, GDNF, and IGF-1 influence the proliferation and stemness of ovine spermatogonial stem cells in vitro. J Assist Reprod Genet. 2020;37(10):2615–30.
Kuijk EW, Colenbrander B, Roelen BA. The effects of growth factors on in vitro-cultured porcine testicular cells. Reproduction. 2009;138(4):721–31 (https://rep.bioscientifica.com/view/journals/rep/138/4/721.xml).
Tian R, Yang S, Zhu Y, Zou S, Li P, Wang J, Zhu Z, Huang Y, He Z, Li Z. VEGF/VEGFR2 signaling regulates germ cell proliferation in vitro and promotes mouse testicular regeneration in vivo. Cells Tissues Organs. 2016;201(1):1–13.
Yang F, Whelan EC, Guan X, Deng B, Wang S, Sun J, Avarbock MR, Wu X, Brinster RL. FGF9 promotes mouse spermatogonial stem cell proliferation mediated by p38 MAPK signalling. Cell Prolif. 2021;54(1):e12933.
Gharenaz NM, Movahedin M, Mazaheri Z. Three-dimensional culture of mouse spermatogonial stem cells using a decellularised testicular scaffold. Cell J (Yakhteh). 2020;21(4):410.
Tanaka T, Kanatsu-Shinohara M, Lei Z, Rao CV, Shinohara T. The luteinizing hormone-testosterone pathway regulates mouse spermatogonial stem cell self-renewal by suppressing WNT5A expression in Sertoli cells. Stem Cell Rep. 2016;7(2):279–91. https://doi.org/10.1016/j.stemcr.2016.07.005.
Lim JJ, Sung SY, Kim HJ, Song SH, Hong JY, Yoon TK, Kim JK, Kim KS, Lee DR. Long-term proliferation and characterization of human spermatogonial stem cells obtained from obstructive and non-obstructive azoospermia under exogenous feeder-free culture conditions. Cell Prolif. 2010;43(4):405–17. https://doi.org/10.1111/j.1365-2184.2010.00691.x.
Caires KC, de Avila J, McLean DJ. Vascular endothelial growth factor regulates germ cell survival during establishment of spermatogenesis in the bovine testis. Reproduction. 2009;138(4):667.
Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119(7):1001–12 (https://www.sciencedirect.com/science/article/pii/S0092867404010578).
Hofmann MC. Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol Cell Endocrinol. 2008;288(1–2):95–103 (https://www.sciencedirect.com/science/article/pii/S0303720708001536).
Oatley Jon M, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J Biol Chem. 2007;282(35):25842–51. https://doi.org/10.1074/jbc.M703474200.
He Z, Jiang J, Kokkinaki M, Dym M. Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through Smad2/3 and Oct-4 activation. Stem Cells. 2009;27(10):2580–90.
Lord T, Oatley JM. Regulation of spermatogonial stem cell maintenance and self-renewal The Biology of Mammalian Spermatogonia. New York: Springer; 2017. p. 91–129. https://doi.org/10.1007/978-1-4939-7505-1_5.
Li L, Wang M, Wang M, Wu X, Geng L, Xue Y, Wei X, Jia Y, Wu X. A long non-coding RNA interacts with Gfra1 and maintains survival of mouse spermatogonial stem cells. Cell Death Dis. 2016;7(3): e2140. https://doi.org/10.1038/cddis.2016.24.
Kala S, Kaushik R, Singh KP, Kadam PH, Singh MK, Manik RS, Chauhan MS. in vitro culture and morphological characterization of prepubertal buffalo (Bubalus bubalis) putative spermatogonial stem cell. J Assist Reprod Genet. 2012;29(12):1335–42. https://doi.org/10.1007/s10815-012-9883-y.
Kadam P, Kala S, Agrawal H, Singh KP, Singh MK, Chauhan MS, Palta P, Singla SK, Manik RS. Effects of glial cell line-derived neurotrophic factor, fibroblast growth factor 2 and epidermal growth factor on proliferation and the expression of some genes in buffalo. Reprod Fertil Dev. 2013;25(8):1149–57 (http://www.publish.csiro.au/rd/rd12330).
Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y. Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev. 2002;113(1):29–39. https://doi.org/10.1016/S0925-4773(02)00004-7.
Abé K, Eto K, Abé SI. Epidermal growth factor mediates spermatogonial proliferation in newt testis. Reprod Biol Endocrinol. 2008;6(1):1–3.
Wang KM, Zhang DL, Mi YL, Zeng WD, Zhang CQ. Promoting effects of epidermal growth factor and prostaglandin E1 on the proliferation of mouse spermatogonia. Chin J Cell Biol. 2008;30:537–40.
Chen JX, Xu LL, Wang XC, Qin HY, Wang JL. Involvement of c-Src/STAT3 signal in EGF-induced proliferation of rat spermatogonial stem cells. Mol Cell Biochem. 2011;358(1):67–73.
Yu RJ, Xu SF. Mechanism of epidermal growth factor effects on spermatogonial stem cells. Life Sci Res. 2010;14(1):6–9 (http://en.cnki.com.cn/Article_en/CJFDTotal-SMKY201001004.htm).
Zhang DL, Mi YL, Wang KM, Zeng WD, Zhang CQ. Effects of follicle-stimulating hormone and epidermal growth factor on proliferation of mouse spermatogonial cells. Chinese J Cell Biol. 2007;29:565–8 (https://ncbi.nlm.nih.gov/pmc/articles/PMC4293895/).
Hu JH, Zhang WC, Wang P, Fan ZG, Jiang ZL, Li QW, Qiang Z. Effects of growth-promoting factors on proliferation of mouse spermatogonial stem cells SSCs in vitro. Afr J Biotechnol. 2012;11(14):3482–9. https://doi.org/10.5897/AJB10.1242.
Lee KH, Lee WY, Kim DH, Lee SH, Do JT, Park C, Kim JH, Choi YS, Song H. Vitrified canine testicular cells allow the formation of spermatogonial stem cells and seminiferous tubules following their xenotransplantation into nude mice. Sci Rep. 2016;6(1):21919. https://doi.org/10.1038/srep21919.
Wu Z, Falciatori I, Molyneux LA, Richardson TE, Chapman KM, Hamra FK. Spermatogonial culture medium: an effective and efficient nutrient mixture for culturing Rat spermatogonial stem cells. Biol Reprod. 2009;81(1):77–86. https://doi.org/10.1095/biolreprod.108.072645.
Sahare M, Kim SM, Otomo A, Komatsu K, Minami N, Yamada M, Imai H. Factors supporting long-term culture of bovine male germ cells. Reprod Fertil and Dev. 2016;28(12):2039. https://doi.org/10.1071/RD15003.
Lenhard T, Schober A, Suter-Crazzolara C, Unsicker K. Fibroblast growth factor-2 requires glial-cell-line-derived neurotrophic factor for exerting its neuroprotective actions on glutamate-lesioned hippocampal neurons. Mol Cell Neurosci. 2002;20(2):181–97.
Wang S, Wang X, Wu Y, Han C. IGF-1R signaling is essential for the proliferation of cultured mouse spermatogonial stem cells by promoting the G2/M progression of the cell cycle. Stem Cells Dev. 2015;24(4):471–83. https://doi.org/10.1089/scd.2014.0376.
Kuo YC, Au HK, Hsu JL, Wang HF, Lee CJ, Peng SW, Lai SC, Wu YC, Ho HN, Huang YH. IGF-1R promotes symmetric self-renewal and migration of alkaline phosphatase+ germ stem cells through HIF-2α-OCT4/CXCR4 loop under hypoxia. Stem Cell Rep. 2018;10(2):524–37 (https://www.sciencedirect.com/science/article/pii/S2213671117305519).
Qasemi Panahi B, Tajik P, Movahedin M, Moghaddam G, Geranmayeh MH. Study of insulin-like growth factor 1 effects on bovine type A spermatogonia proliferation and viability. Turk J Vet Anim Sci. 2014;38:693–8. https://doi.org/10.3906/vet-1402-70.
Bahadorani M, Hosseini SM, Abedi P, Abbasi H, Nasr-Esfahani MH. Glial cell line-derived neurotrophic factor in combination with insulin-like growth factor 1 and basic fibroblast growth factor promote in vitro culture of goat spermatogonial stem cells. Growth Factors. 2015;33(3):181–91. https://doi.org/10.3109/08977194.2015.1062758.
Yao J, Zuo H, Gao J, Wang M, Wang D, Li X. The effects of IGF-1 on mouse spermatogenesis using an organ culture method. Biochem Biophys Res Commun. 2017;491(3):840–7. https://doi.org/10.1016/j.bbrc.2017.05.125.
Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. https://doi.org/10.1038/336688a0.
Mirzapour T, Movahedin M, Tengku Ibrahim TA, Koruji M, Haron AW, Nowroozi MR, Rafieian SH. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia. 2012;44:41–55.
Rastegar T, Habibi Roudkenar M, Parvari S, Baazm M. The interaction between Sertoli cells and luekemia inhibitory factor on the propagation and differentiation of spermatogonial stem cells in vitro. Iran J Reprod Med. 2015;13(11):679–86.
Morimoto H, Yamamoto T, Miyazaki T, Ogonuki N, Ogura A, Tanaka T, Kanatsu-Shinohara M, Yabe-Nishimura C, Zhang H, Pommier Y, Trumpp A. An interplay of NOX1-derived ROS and oxygen determines the spermatogonial stem cell self-renewal efficiency under hypoxia. Genes Dev. 2021;35(3–4):250–60.
Goodyear S, Brinster R. Culture and expansion of primary undifferentiated spermatogonial stem cells. Cold Spring Harbor Protocols. 2017; 2017(4): pdb-prot094193.
Shams A, Eslahi N, Movahedin M, Izadyar F, Asgari H, Koruji M. Future of spermatogonial stem cell culture: application of nanofiber scaffolds. Curr Stem Cell Res Ther. 2017;12(7):544–53. https://doi.org/10.2174/1574888X12666170623095457.
Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, Huleihel M, Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Hum Reprod. 2009;15(9):521–9. https://doi.org/10.1093/molehr/gap052.
Murdock MH, David S, Swinehart IT, Reing JE, Tran K, Gassei K, Orwig KE, Badylak SF. Human testis extracellular matrix enhances human spermatogonial stem cell survival in vitro. Tissue Eng - Part A. 2019;25(7–8):663–76. https://doi.org/10.1089/ten.tea.2018.0147.
Izadyar F, Matthijs-Rijsenbilt JJ, Den Ouden K, Creemers LB, Woelders H, De Rooij DG. Development of a cryopreservation protocol for type A spermatogonia. J Androl. 2002;23(4):537–45. https://doi.org/10.1002/j.1939-4640.2002.tb02276.x.
Herrid M, Olejnik J, Jackson M, Suchowerska N, Stockwell S, Davey R, Hutton K, Hope S, Hill JR. Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol Reprod. 2009;81(5):898–905 (https://academic.oup.com/biolreprod/article-abstract/81/5/898/2557874).
Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, Hovatta O. Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod. 2005;20(6):1676–87.
Whaley D, Damyar K, Witek RP, Mendoza A, Alexander M, Lakey JR. Cryopreservation: an overview of principles and cell-specific considerations. Cell Transplant. 2021;30:0963689721999617.
Yong KW, Laouar L, Elliott JA, Jomha NM. Review of nonpermeating cryoprotectants as supplements for vitrification of mammalian tissues. Cryobiology. 2020;96:1–11.
Kim KJ, Lee YA, Kim BJ, Kim YH, Kim BG, Kang HG, Jung SE, Choi SH, Schmidt JA, Ryu BY. Cryopreservation of putative pre-pubertal bovine spermatogonial stem cells by slow freezing. Cryobiology. 2015;70(2):175–83 (https://www.sciencedirect.com/science/article/pii/S0011224015000358).
Zeng W, Snedaker AK, Megee S, Rathi R, Chen F, Honaramooz A, Dobrinski I. Preservation and transplantation of porcine testis tissue. Reprod Fertil Dev. 2009;21(3):489–97. https://doi.org/10.1071/rd08235.
Ha SJ, Kim BG, Lee YA, Kim YH, Kim BJ, Jung SE, Pang MG, Ryu BY. Effect of antioxidants and apoptosis inhibitors on cryopreservation of murine germ cells enriched for spermatogonial stem cells. PLoS One. 2016;11(8):e0161372.
Amidi F, Rashidi Z, Khosravizadeh Z, Khodamoradi K, Talebi A, Navid S, Abbasi M. Antioxidant effects of quercetin in freeze-thawing process of mouse spermatogonial stem cells. Asian Pac J Reprod. 2019;8(1):7.
Pukazhenthi BS, Nagashima J, Travis AJ, Costa GM, Escobar EN, França LR, Wildt DE. Slow freezing, but not vitrification supports complete spermatogenesis in cryopreserved, neonatal sheep testicular xenografts. PLoS One. 2015;10(4):e0123957.
Lee YA, Kim YH, Ha SJ, Kim KJ, Kim BJ, Kim BG, Choi SH, Kim IC, Schmidt JA, Ryu BY. Cryopreservation of porcine spermatogonial stem cells by slow-freezing testis tissue in trehalose. J Anim Sci. 2014;92(3):984–95.
Radaelli MR, Almodin CG, Minguetti-Câmara VC, Cerialli PM, Nassif AE, Gonçalves AJ. A comparison between a new vitrification protocol and the slow freezing method in the cryopreservation of prepubertal testicular tissue. JBRA Assist Reprod. 2017;21(3):188.
Han S, Zhao L, Yang C, Xu J, Yao C, Huang C, Zhang H, Ji Z, Luo J, Guo Y, Hong Y. Vitrification with microinjection of single seminiferous tubules: an efficient cryopreservation approach for limited testicular tissue. Reprod Biomed Online. 2021. https://doi.org/10.1016/j.rbmo.2021.06.026.
Bebbere D, Pinna S, Nieddu S, Natan D, Arav A, Ledda S. Gene expression analysis of ovine prepubertal testicular tissue vitrified with a novel cryodevice (E. Vit). J Assist Reprod Genet. 2019;36(10):2145–54.
Lima DB, da Silva TF, Aquino-Cortez A, Leiva-Revilla J, da Silva LD. Vitrification of testicular tissue from prepubertal cats in cryotubes using different cryoprotectant associations. Theriogenology. 2018;110:110–5.
Peng Z, Peng-fei H, Ya-guang T, He H, Gui-xue Z. Isolation, purification and cryopreservation of cells from neonatal bovine testis. J Northeast Agric. 2013;20(1):37–42.
Qasemi-Panahi B, Movahedin M, Moghaddam G, Tajik P, Koruji M, Ashrafi-Helan J, Rafat SA. Isolation and proliferation of spermatogonial cells from Ghezel sheep. Avicenna J Med Biotechnol. 2018;10(2):93.
Pan C, Yu S, Zhang P, Wang B, Zhu Z, Liu Y, Zeng W. Effect of sucrose on cryopreservation of pig spermatogonial stem cells. J Integr Agric. 2017;16(5):1120–9. https://doi.org/10.1016/S2095-3119(16)61489-2.
Zhu WQ, Cai NN, Jiang Y, Yang R, Shi JZ, Zhu CL, Zhang BY, Tang B, Zhang XM. Survivable potential of germ cells after trehalose cryopreservation of bovine testicular tissues. Cryobiology. 2021;101:105–14.
Jiang Y, Zhu WQ, Zhu XC, Cai NN, Yang R, Cai H, Zhang XM. Cryopreservation of calf testicular tissues with knockout serum replacement. Cryobiology. 2020;92:255–7.
Costa GM, Avelar GF, Lacerda SM, Figueiredo AF, Tavares AO, Rezende-Neto JV, Franca LR. Horse spermatogonial stem cell cryopreservation: feasible protocols and potential biotechnological applications. Cell Tissue Res. 2017;370(3):489–500. https://doi.org/10.1007/s00441-017-2673-1.
Kaneko H, Kikuchi K, Nakai M, Somfai T, Noguchi J, Tanihara F, Ito J, Kashiwazaki N. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PloS one. 2013;8(7):e70989.
Jung SE, Jin JH, Ahn JS, Kim YH, Yun MH, Kim SH, Kim BJ, Ryu BY. Effect of serum replacement on murine spermatogonial stem cell cryopreservation. Theriogenology. 2021;159:165–75.
Mahdi NS, Azarbani F, Pirnia A, Abbaszadeh A, Gholami M. The effect of caffeic acid on spermatogonial stem cell-type a cryopreservation. Rep Biochem Mol Biol. 2018;7(1):85.
Aliakbari F, Heidari M, Hossini MA, Hosseini J. Increasing of post-freezing quality of Spermatogonial Stem Cells after pretreatment by vitamin E. Men’s Health J. 2019;3(1):e1–e1.
Aliakbari F, Gilani MA, Amidi F, Baazm M, Korouji M, Izadyar F, Yazdekhasti H, Abbasi M. Improving the efficacy of cryopreservation of spermatogonia stem cells by antioxidant supplements. Cell Reprogram. 2016;18(2):87–95.
Shabani H, Zandi M, Ofogi H, Sanjabi MR, Pajooh KH. The effect of combining vitamin E and C on the viability improvement of transfected ovine spermatogonial stem cells after cryopreservation and thawing. Turk J Vet Anim Sci. 2017;41(5):648–55.
Jung S-E, Oh H-J, Ahn J-S, Kim Y-H, Kim B-J, Ryu B-Y. Antioxidant or apoptosis inhibitor supplementation in culture media improves post-thaw recovery of murine spermatogonial stem cells. Antioxidants. 2021;10(5):754. https://doi.org/10.3390/antiox10050754.
Feng TY, Li Q, Ren F, Xi HM, Lv DL, Li Y, Hu JH. Melatonin protects goat spermatogonial stem cells against oxidative damage during cryopreservation by improving antioxidant capacity and inhibiting mitochondrial apoptosis pathway. Oxid Med Cell Longev 2020. https://doi.org/10.1155/2020/5954635.
Honaramooz A, Behboodi E, Megee SO, Overton SA, Galantino-Homer H, Echelard Y, Dobrinski I. Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol Reprod. 2003;69(4):1260–4 (https://academic.oup.com/biolreprod/article-abstract/69/4/1260/2712666).
Oatley JM, de Avila DM, McLean DJ, Griswold MD, Reeves JJ. Transplantation of bovine germinal cells into mouse testes. J Anim Sci. 2002;80(7):1925–31. https://doi.org/10.2527/2002.8071925x.
Ciccarelli M, Giassetti MI, Miao D, Oatley MJ, Robbins C, Lopez-Biladeau B, Waqas MS, Tibary A, Whitelaw B, Lillico S, Park CH. Donor-derived spermatogenesis following stem cell transplantation in sterile NANOS2 knockout males. Proc Natl Acad Sci. 2020;117(39):24195–204.
Park KE, Kaucher AV, Powell A, Waqas MS, Sandmaier SE, Oatley MJ, Park CH, Tibary A, Donovan DM, Blomberg LA, Lillico SG, Whitelaw CB, Mileham A, Telugu BP, Oatley JM. Generation of germline ablated male pigs by CRISPR/Cas9 editing of the NANOS2 gene. Sci Rep. 2017;7(1):40176. https://doi.org/10.1038/srep40176.
Oatley JM. Recent advances for spermatogonial stem cell transplantation in livestock. Reprod Fertil Dev. 2018;30(1):44–9.
Mirzapour T, Tengku Ibrahim TA, Movahedin M, Nowroozi MR. Morphological and ultrastructural studies of human spermatogonial stem cells from patients with maturation arrest. Andrologia. 2017;49(7):e12700.
Dym M, Kokkinaki M, He Z. Spermatogonial stem cells: mouse and human comparisons. Birth Defects Res C Embryo Today. 2009;87(1):27–34.
Aoshima K, Baba A, Makino Y, Okada Y. Establishment of alternative culture method for spermatogonial stem cells using knockout serum replacement. PLoS ONE. 2013;8(10):e77715. https://doi.org/10.1371/journal.pone.0077715.
Niu Z, Mu H, Zhu H, Wu J, Hua J. p38 MAPK pathway is essential for self-renewal of mouse male germline stem cells (mGSCs). Cell Prolif. 2017;50(1):e12314. https://doi.org/10.1111/cpr.12314.
Shinohara Takashi, Kazuki K, Ogonuki N, Morimoto H, Matoba S, Hiramatsu K, Honma K, Suzuki T, Hara T, Ogura A, Oshimura M, Kanatsu-Shinohara M, Kazuki Y. Transfer of a mouse artificial chromosome into spermatogonial stem cells generates transchromosomic mice. Stem Cell Rep. 2017;9(4):1180–91. https://doi.org/10.1016/j.stemcr.2017.08.012.
Niu Z, Zheng L, Wu S, Mu H, Ma F, Song W, Zhu H, Wu J, He X, Hua J. Ras/ERK1/2 pathway regulates the self-renewal of dairy goat spermatogonia stem cells. Reproduction. 2015;149(5):445–52.
Chassot AA, Le Rolle M, Jourden M, Taketo MM, Ghyselinck NB, Chaboissier MC. Constitutive WNT/CTNNB1 activation triggers spermatogonial stem cell proliferation and germ cell depletion. Dev Biol. 2017;426(1):17–27 (https://www.sciencedirect.com/science/article/pii/S0012160616304717).
Li Y, Zhang Y, Zhang X, Sun J, Hao J. BMP4/Smad signaling pathway induces the differentiation of mouse spermatogonial stem cells via upregulation of Sohlh2. Anat Rec. 2014;297(4):749–57. https://doi.org/10.1002/ar.22891.
Cui N, Hao G, Zhao Z, Wang F, Cao J, Yang A. MicroRNA-224 regulates self-renewal of mouse spermatogonial stem cells via targeting DMRT1. J Cell Mol Med. 2016;20(8):1503–12. https://doi.org/10.1111/jcmm.12838.
Li J, Liu X, Hu X, Tian GG, Ma W, Pei X, Wang Y, Wu J. MicroRNA-10b regulates the renewal of spermatogonial stem cells through Kruppel-like factor 4. Cell Biochem Funct. 2017;35(3):184–91. https://doi.org/10.1002/cbf.3263.
Liu SS, Maguire EM, Bai YS, Huang L, Liu Y, Xu L, Fauzi I, Zhang SQ, Xiao Q, Ma NF. A novel regulatory axis, CHD1L-MicroRNA 486-matrix metalloproteinase 2, controls spermatogonial stem cell properties. Mol Cell Biol, 2019; 39(4):e00357–18. https://doi.org/10.1128/MCB.00357-18.
Meshorer E, Misteli T. Chromatin in pluripotent embryonic stem cells and differentiation. Nat Rev Mol Cell Biol. 2006;7(7):540–6. https://doi.org/10.1038/nrm1938.
Phillips BT, Gassei K, Orwig KE. Spermatogonial stem cell regulation and spermatogenesis. Philos Trans R Soc B: Biol Sci. 2010;365(1546):1663–78. https://doi.org/10.1098/rstb.2010.0026.
Dean W. Altered imprinted gene methylation and expression in completely ES cell-derived mouse fetuses: association with aberrant phenotypes. Development. 1998;125(12):2273–82. https://dev.biologists.org/content/125/12/2273.short.
Bahadur G. Ethics of testicular stem cell medicine. Hum Reprod. 2004;19(12):2702–10. https://doi.org/10.1093/humrep/deh538.
Weber M, Schübeler D. Genomic patterns of DNA methylation: targets and function of an epigenetic mark. Curr Opin Cell Biol. 2007;19(3):273–80. 10.1016/j.ceb.2007.04.011.
Lee J, Shinohara T. Epigenetic modifications and self-renewal regulation of mouse germline stem cells. Cell Res. 2011;21(8):1164. https://www.nature.com/articles/cr2011111.
Venkatesh S, Workman JL. Histone exchange, chromatin structure and the regulation of transcription. Nat Rev Mol Cell Biol. 2015;16(3):178–89. https://doi.org/10.1038/nrm3941.
Kubo N, Toh H, Shirane K, Shirakawa T, Kobayashi H, Sato T, Sone H, Sato Y, Tomizawa SI, Tsurusaki Y, Shibata H. DNA methylation and gene expression dynamics during spermatogonial stem cell differentiation in the early postnatal mouse testis. BMC Genomics. 2015;16(1):624. https://doi.org/10.1186/s12864-015-1833-5.
Hammoud SS, Low DH, Yi C, Carrell DT, Guccione E, Cairns BR. Chromatin and transcription transitions of mammalian adult germline stem cells and spermatogenesis. Cell Stem Cell. 2014;15(2):239–53. https://doi.org/10.1016/j.stem.2014.04.006.
Bao JQ, Bedford MT. Epigenetic regulation of the histone-to-protamine transition during spermiogenesis. Reproduction. 2016;151(5):55–70 (https://www.ncbi.nlm.nih.gov/pmc/articles/pmc4896072/).
Liu SS, Bai YS, Feng L, Dong WW, Li Y, Xu LP, Ma NF. Identification of CHD1L as an important regulator for spermatogonial stem cell survival and self-renewal. Stem cells Int. 2016;4069543:1–12. https://doi.org/10.1155/2016/4069543.
Niu Z, Goodyear SM, Rao S, Wu X, Tobias JW, Avarbock MR, Brinster RL. MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. PNAS. 2011;108(31):12740–5. https://doi.org/10.1073/pnas.1109987108.
Goertz MJ, Wu Z, Gallardo TD, Hamra FK, Castrillon DH. Foxo1 is required in mouse spermatogonial stem cells for their maintenance and the initiation of spermatogenesis. J Clin Invest. 2011;121(9):3456–66.
Yamaji M, Jishage M, Meyer C, Suryawanshi H, Der E, Yamaji M, Garzia A, Morozov P, Manickavel S, McFarland HL, Roeder RG. DND1 maintains germline stem cells via recruitment of the CCR4–NOT complex to target mRNAs. Nature. 2017;543(7646):568–72.
Niimi Y, Imai A, Nishimura H, Yui K, Kikuchi A, Koike H, Saga Y, Suzuki A. Essential role of mouse Dead end1 in the maintenance of spermatogonia. Dev Biol. 2019;445(1):103–12.
Suzuki A, Niimi Y, Shinmyozu K, Zhou Z, Kiso M, Saga Y. Dead end1 is an essential partner of NANOS 2 for selective binding of target RNAs in male germ cell development. EMBO reports. 2016;17(1):37–46.
Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436(7053):1030–4.
Zheng L, Zhai Y, Li N, Ma F, Zhu H, Du X, Li G, Hua J. The modification of Tet1 in male germline stem cells and interact with PCNA, HDAC1 to promote their self-renewal and proliferation. Sci Rep. 2016;6:37414.
Zhou W, Shao H, Zhang D, Dong J, Cheng W, Wang L, Teng Y, Yu Z. PTEN signaling is required for the maintenance of spermatogonial stem cells in mouse, by regulating the expressions of PLZF and UTF1. Cell Biosci. 2015;5(1):42.
Raju P, Nyamsuren G, Elkenani M, Kata A, Tsagaan E, Engel W, Adham IM. Pelota mediates gonocyte maturation and maintenance of spermatogonial stem cells in mouse testes. Reproduction. 2015;149(3):213–21.
Hu K, Zhang J, Liang M. LncRNA AK015322 promotes proliferation of spermatogonial stem cell C18–4 by acting as a decoy for microRNA-19b-3p. In Vitro Cell Dev Biol Anim. 2017;53(3):277–84.
Zhou F, Yuan Q, Zhang W, Niu M, Fu H, Qiu Q, Mao G, Wang H, Wen L, Wang H, Lu M. MiR-663a stimulates proliferation and suppresses early apoptosis of human spermatogonial stem cells by targeting NFIX and regulating cell cycle. Mol Ther-Nucleic Acids. 2018;12:319–36.
Fu H, Zhang W, Yuan Q, Niu M, Zhou F, Qiu Q, Mao G, Wang H, Wen L, Sun M, Li Z. PAK1 promotes the proliferation and inhibits apoptosis of human spermatogonial stem cells via PDK1/KDR/ZNF367 and ERK1/2 and AKT pathways. Mol Ther-Nucleic Acids. 2018;12:769–86.
He Z, Jiang J, Kokkinaki M, Tang L, Zeng W, Gallicano I, Dobrinski I, Dym M. MiRNA-20 and mirna-106a regulate spermatogonial stem cell renewal at the post-transcriptional level via targeting STAT3 and Ccnd1. Stem Cells. 2013;31(10):2205–17.
Ma F, Zhou Z, Li N, Zheng L, Wu C, Niu B, Tang F, He X, Li G, Hua J. Lin28a promotes self-renewal and proliferation of dairy goat spermatogonial stem cells (SSCs) through regulation of mTOR and PI3K/AKT. Sci Rep. 2016;6:38805.
Ma F, Du X, Wei Y, Zhou Z, Clotaire DZJ, Li N, Peng S, Li G, Hua J. LIN28A activates the transcription of NANOG in dairy goat male germline stem cells. J Cell Physiol. 2019;234(6):8113–21.
Zheng L, Zhu H, Tang F, Mu H, Li N, Wu J, Hua J. The Tet1 and histone methylation expression pattern in dairy goat testis. Theriogenology. 2015;83(7):1154–61.
Mu H, Li N, Wu J, Zheng L, Zhai Y, Li B, Song W, Wang J, Zhu H, Li G, Hua J. PLZF-induced upregulation of CXCR4 promotes dairy goat male germline stem cell proliferation by targeting Mir146a. J Cell Biochem. 2016;117(4):844–52.
Song W, Mu H, Wu J, Liao M, Zhu H, Zheng L, He X, Niu B, Zhai Y, Bai C, Lei A. miR-544 regulates dairy goat male germline stem cell self-renewal via targeting PLZF. J Cell Biochem. 2015;116(10):2155–65.
Zhu H, Zheng L, Wang L, Tang F, Hua J. MiR-302 enhances the viability and stemness of male germline stem cells. Reprod Domest Anim. 2018;53(6):1580–8.
Lee R, Lee WY, Park HJ, Ha WT, Woo JS, Lee JH, Hong K, Song H. Stage-specific expression of DDX4 and c-kit at different developmental stages of the porcine testis. Anim Reprod Sci. 2018;190:18–26.
Sharma M, Srivastava A, Fairfield HE, Bergstrom D, Flynn WF, Braun RE. Identification of EOMES-expressing spermatogonial stem cells and their regulation by PLZF. Elife. 2019;8:e43352.
Acknowledgements
The authors thank the Director, ICAR-National Institute of Animal Nutrition and Physiology, Indian Council of Agricultural Research, Ministry of Agriculture, Govt. of India, Bengaluru, India, for providing the necessary facilities to undertake the work. This research work is supported by the DST-SERB funded project (ECR/2018/001304), Department of Science and Technology, Government of India. Dr. S. Selvaraju is supported by ICAR-National Fellow Project, ICAR, Ministry of Agriculture, Government of India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Binsila, B., Selvaraju, S., Ranjithkumaran, R. et al. Current scenario and challenges ahead in application of spermatogonial stem cell technology in livestock. J Assist Reprod Genet 38, 3155–3173 (2021). https://doi.org/10.1007/s10815-021-02334-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02334-7