Abstract
Purpose
To develop a neuroradiological score in patients with deep cerebral venous thrombosis (DCVT), capable of assessing extension of intracranial changes and venous occlusion at diagnosis; to assess the relationship between neuroradiological and clinical features at follow-up.
Material and methods
In 14 patients with DCVT, we developed 2 score systems on non-enhanced and contrast-enhanced CT: Intracranial Imaging Score (IIS) and Venous Occlusion Imaging Score (VOIS). ISS considers parenchymal venous strokes, hemorrhage, mass effect, and hydrocephalus; VOIS evaluates unilateral or bilateral venous occlusion extension. Modified Rankin Scale (mRS) and vessel recanalization status were assessed at follow-up.
Results
At diagnosis, higher IIS was related to bilateral venous thrombosis involvement (p 0,02; r:0,60), but parenchymal strokes were not related to venous occlusion extension (unilateral or bilateral) (p > 0,05). Moreover, the symptoms’ onset time did not correlate with the severity scores (p > 0,05). At follow-up, 8 out of 14 patients showed good clinical outcomes with complete recanalization and neurological improvement, 1 patient showed a poor neurological outcome, whereas 5 patients died within 1 week. Positive correlations were found between IIS and mRS (p 0,003, r = 0,73), between IIS and vessels’ recanalization status (p 0,002, r = 0,75), and between vessels’ recanalization status and mRS (p < 0,001, r = 0,98).
Conclusion
Neuroradiological scores may enhance diagnostic accuracy, and they may have a predictive significance. In patients with DCVT, although intracranial involvement was not influenced by symptoms’ onset time or extension of venous occlusion, clinical outcome was related to both intracranial involvement and venous recanalization state. Collateral venous drainage status may counterbalance the thrombotic process improving prognosis.
Similar content being viewed by others
Introduction
Cerebral venous thrombosis (CVT) is a serious and often fatal medical condition with an increasing incidence, accounting for about 0.5% of stroke patients and a prevalence of 5 per 1 million [1].
Oral contraceptives/hormone replacement, pregnancy, and puerperium are the most common risk factors related to CVT [2]. During the pandemic, increased risks of CVT have been reported in COVID-19–positive patients [3, 4] and following the ChAdOx1 vaccine [5, 6]. Although the most common clinical presentation involves dural sinuses, CVT can potentially occur in every section of the cerebral venous system [7]. A relatively uncommon and often overlooked subset of CVT is the deep cerebral veins thrombosis (DCVT) involving the vein of Rosenthal, internal cerebral veins, and vein of Galen [8]. DCVT is estimated in about 10% of patients with CVT, and it occurs as an isolated event in approximately 1% or accompanies superficial cerebral vein and sinus thrombosis in 15% of the cases [9], but only a few studies explored clinical features of this subgroup of patients [10].
The clinical presentation of DCVT may be multifaced and puzzling. DCVT may cause coma with signs of decerebration and extrapyramidal hypertonia, resulting in death or resolve in several weeks but with disabling neurologic sequelae [11]. Occasionally, it may show a more benign presentation with mild symptoms of increased intracranial pressure (ICP) and confusion with only minor neurologic sequelae [12, 13].
Due to variable clinical and neuroradiological presentation, DCVT may be overlooked on initial brain non-contrast-enhanced CT (NCCT) examination in patients with CVT and diagnosis is even more difficult in patients with isolated DCVT making the diagnosis challenging and delaying treatment [8]. Because prompt anticoagulant therapy has a great impact on CVT outcome, early diagnosis is essential, especially in patients with involvement of the deep cerebral veins [11].
Recently, thrombectomy and thrombolysis have been proposed for the treatment of dural sinus thrombosis; however, the evidence is still low and current guidelines do not recommend them [14].
Neuroimaging examinations play a key role in the initial diagnosis of CVT [15]. Although brain MRI shows better diagnostic accuracy and may have a role in monitoring thrombus dissolution during anticoagulant therapy, brain CT is usually the first examination performed in an emergency setting [16]. Several studies on dural sinuses thrombosis (DST) demonstrated that the sensitivity and specificity for the direct visualization of the spontaneous hyperdense thrombus on NCCT are low [17]. Only in 25% of patients DST can be visualized with NCCT as an increased density within the thrombosed dural sinus [18]. In DCVT, the spontaneous thrombus hyperdensity is more easily observed in internal cerebral veins than the vein of Galen because the latter one commonly appears hyperdense in normal subjects, and clot identification may be challenging [11, 19].
Aside from venous thrombus hyperdensity, brain CT may also provide information about brain parenchymal involvement. In DCVT patients, a “butterfly-shaped” low density in the basal ganglia and thalamus, indicating venous edema, has frequently been described in the emergency setting [13, 20]. Bilateral involvement is common, but unilateral involvement can also be seen [21], and probably venous collaterals drainage plays a role in this variable presentation [22].
However, NCCT may be normal in about 10% of patients [23] and the direct detection of DCVT thrombosis by contrast-enhanced CT (CeCT) remains the gold standard for the diagnosis [24].
Neuroradiological features of DST have been extensively studied, but studies on DCVT are scarce and a recent meta-analysis reported available clinical data in only 120 patients with DCVT worldwide [10]. Quantitative studies about density measurements on involved dural venous sinuses may improve NCCT accuracy for CVT diagnosis in an emergency setting [25,26,27], while only a few studies have quantified the veins’ density in DCVT [28]. Moreover, score systems, aimed to assess the extension of CVT, have been developed only in cerebral venous dural sinuses [29]; while in patients with DCVT associated or not to DST, a comprehensive score system capable of assessing parenchymal changes along with venous thrombosis extension or quantitative venous density measurements is lacking.
The purposes of this study, in patients with DCVT, were to (1) describe CT findings at the diagnosis based on both parenchymal changes and vessel occlusion extension in order to develop qualitative neuroradiological severity scores, (2) assess quantitative measurements of density in thrombosed deep brain veins and venous dural sinuses in order to improve the diagnostic accuracy of NCCT, and (3) evaluate neuroradiological and clinical features at diagnosis and follow-up in order to predict the outcome.
Material and methods
Patients
Patients were identified by reviewing the hospital database between 2008 and 2021, and 14 consecutive patients with DCVT were collected.
The following items were recorded: age, gender, risk factors for venous thrombosis, clinical symptoms at the onset, the interval from symptoms’ onset to diagnosis, D-dimer values on admission, imaging modalities, neuroradiological findings including the extent of thrombosis, parenchymal edema and hemorrhage, treatment, in-hospital mortality, and outcome (Table 1).
Patients were enrolled if the following inclusion criteria were met: the patients received brain NCCT when admitted to the emergency department, and DCVT was confirmed by CeCT within the following 6 h. In studies where intravenous iodinated contrast was administered 6 h after NCCT, patients with unsatisfactory image quality due to motion artifacts and patients with coexistence of other acute intracranial brain diseases or recent brain surgery were excluded.
Moreover, we selected asymptomatic patients without CVT (“healthy controls”), paired up with the previous ones according to gender and age, who underwent NCCT and CeCT for the routine follow-up of brain aneurysms; none of them showed intracranial hemorrhage at NCCT.
The optimal treatment protocol for DCVT patients was made individually according to current guidelines [14]. All enrolled patients had received subcutaneous injections of low molecular weight heparin (LMWH) according to the patient’s weight or unfractionated heparin (UFH) during hospitalization. Thereafter, oral anticoagulant therapy was continued for at least 6 months. The dose of warfarin was adjusted to achieve a target international normalized ratio (INR) between 2 and 3.
None of the patients underwent a thrombectomy. Neurological sequelae at follow-up visits were also recorded. The clinical outcome at 3 months was evaluated by a stroke neurologist according to the modified Rankin Scale (mRS) [30]. The long-term clinical outcome (6 months) was documented by mRS using a structured telephone interview.
Imaging technique
All patients underwent multimodal brain CT imaging, including NCCT and multiphase computed tomography angiography (mCTA).
Briefly, CT imaging was performed using a 64-multislice CT (GE MEDICAL SYSTEM Optima CT660 645, GE Healthcare, Milwaukee, Wisconsin).
Acquisition parameters for NCCT were 120 kv and 44 mAs, and the acquisition duration was 9 s. Acquisition parameters for mCTA were 100 kV and 4 mAs, and acquisition duration was 21 s; after administration of 80 mL of contrast media at 4 mL/s flow, the acquisition was composed of three subsequent phases (separated by an interval of 8 s): The first phase (acquired in the arterial phase) extends from the aortic arch to the vertex, and the next two phases (acquired in the early and late venous phases) from the occipital foramen to the vertex.
Imaging analysis
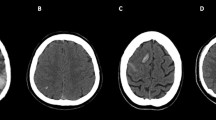
On the first available acute NCCT performed at symptoms’ onset, image-based classification systems were designed considering the topographic distribution of intracranial lesions (unilateral vs. bilateral thalami; edema in basal ganglia and other brain regions; parenchymal and ventricular hemorrhage), mass effect (midline shift), and hydrocephalus development. The Intracranial Imaging Score (IIS) was developed, assigning 1 point for each item, and the Venous Occlusion Imaging Score (VOIS) was developed, considering major venous dural sinuses, deep veins system, and cortical veins involvement, assigning either 1 or 2 points for unilateral or bilateral absence of contrast opacification on CeCT (Table 2; Fig. 1). Higher scores indicated a severer neuroradiological involvement.
Brain CT features in patients with venous system thrombosis. Axial unenhanced CT (a–j); enhanced CT (k–n). Intracranial changes. (1) Topographic distribution of parenchymal density changes: unilateral thalamus edema (left-side hypodensity, arrow in a); thalami edema (bilateral hypodensity, arrows in b); unilateral thalamus and basal ganglia edema (left-side hypodensity, arrows in c); thalami and basal ganglia edema (bilateral hypodensity, arrows in d); other parenchymal venous strokes (left temporal edema, arrow in e). (2) Hemorrhage: unilateral parenchymal hemorrhage (arrow in f); bilateral parenchymal hemorrhage (arrows in g); intraventricular hemorrhage (arrowhead in g). (3) Mass effect: midline shift (h); hydrocephalus (asterisks in i, j). Venous system occlusion. (1) Dural sinuses thrombosis (DST): unilateral (arrow in k) or bilateral (arrows in l) transverse and sigmoid sinuses involvement. (2) Deep cerebral veins thrombosis (DCVT): unilateral (arrow in m) or bilateral (arrows in n) cerebral internal veins involvement
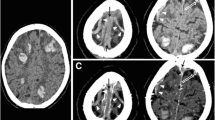
Two independent neuroradiologists with 14-year experience performed quantitative image analysis both in the DCVT group and in the control group. They were asked to measure (1) dural sinuses attenuation by tracking a round large ROI in standardized regions of the cerebral dural venous system such as posterior superior sagittal sinus, the confluence of the venous sinuses (Torcular Herophili), left and right transverse-sigmoid sinus passage, without including the adjacent bone, CSF, or dura; (2) deep venous system attenuation by drawing a round small ROI within internal cerebral veins and vein of Galen without including adjacent structures (Fig. 2).
Mean attenuation values in the different sinuses segments and deep veins in patients with dural sinuses and deep cerebral veins thrombosis. Axial unenhanced CT (a–h). (1) Dural sinuses attenuation by tracking a round ROI in standardized regions of the cerebral dural venous system: posterior superior sagittal sinus (red round ROI in c), Torcular Herophili (red round ROI in d), transverse-sigmoid sinus passage (red round ROI in d). (2) Deep venous system attenuation by drawing a round small ROI within internal cerebral veins (red round ROI in g), vein of Galen (red round ROI in h)
Average Hounsfield Unit (HU) was defined as the mean density of all above dural sinus segments and structures of the deep venous system in the control group and the mean density of dural sinus segments and structures of the deep venous system involved by thrombosis in the DCVT group.
An identical method of measurement in the lumen of the intracranial non-calcified portion of the internal carotid artery (ICA) was recorded in order to compare arterial values with venous measurements.
Because normalized ratios of HU to hematocrit have been proposed to improve the accuracy in detecting CVT on an NCCT [27], subjects were required to have a hematocrit drawn within 24 h of NCCT scan performance for calculation of an HU/hematocrit (HU/H) ratio. If more than one hematocrit was available, the lab value most closely approximating the time of CT imaging was considered.
Veno-arterial (VA) difference was calculated by subtracting the internal carotid artery attenuation from the mean sinus density or mean deep veins density.
Follow-up CT or MRI at 1–3 months was evaluated for all alive patients and was compared directly with initial imaging studies to categorize the recanalization status into three types: complete, partial, or no recanalization.
Statistical analysis
Descriptive statistics were expressed as the median and interquartile range [IQR] for continuous variables if not otherwise specified.
Pearson correlation was used to compare the neuroradiological scores and the time window between symptoms’ onset and radiological confirmation on CT, the IIS and VOIS, the neuroradiological severity scores and the mRS, the neuroradiological severity scores and the vessels recanalization status, and the vessels recanalization status and the mRS. The rank correlation was used to compare parenchymal stroke extension (unilateral versus bilateral) and VOIS.
Mann–Whitney test was used to assess the difference between patients and controls about average HU, HU/H, and VA both in the dural sinuses and deep venous system. Pearson correlation was also used to compare quantitative HU measurements (average HU, HU/H, VA difference) both in dural sinuses and deep venous system and the neuroradiological severity scores, quantitative HU measurements and the mRS, and quantitative HU measurements and the vessels recanalization status.
About the average density of the vein system, receiver operating characteristic (ROC) curves analysis and area under the curves (AUC) were used to determine optimal cut-off values capable of differentiating patients with thrombosis from patients without thrombosis at early CT.
Statistical significance was set at P < 0.05. Statistical analyses were conducted using Statistical Package for the Social Sciences (SPSS) for Windows version 25.0 (SPSS Inc., Chicago, IL, USA).
Results
The clinical, neuroimaging, and treatment data are summarized in Tables 1, 2, and 3.
Clinical data
A total of 14 adults were included in the study. The median age was 51 years [IQR 43–58]. The mean time between symptoms’ onset and diagnostic confirmation on CT was 3,1 days (range: 0–7 days).
At diagnosis, 12 out of 14 patients showed headache and change of consciousness progressing to coma and 5 patients died during the in-hospital stay. They were frequently accompanied by focal neurological deficits such as motor deficits and language disorders (Table 1).
CT imaging score in the acute setting
These data demonstrate that, in patients with DCVT, the overall clinical prognosis is influenced mainly by the severity of intracranial involvement.
The first NCCT available at the symptoms’ onset revealed intracranial changes with a variable severity spectrum, while CeCT revealed a variable unilateral or bilateral venous occlusion involving both dural sinuses and the deep venous system.
Higher scores in neuroradiological scales (IIS, VOIS) were related to severer intracranial changes and bilateral venous occlusion (Table 3); a positive correlation between IIS and VOIS was found (p 0,02, r: 0,60), but no correlation between parenchymal involvement (unilateral vs. bilateral) and venous occlusion extension and between neuroradiological severity scores and symptoms’ onset time was found (p > 0,05) (Table 4).
Vessel HU analysis
Mean density, mean HU/H ratio, and VA differences, measured on initial NCCT, were significantly higher in sinus segments and deep veins involved by CVT than in patent ones of the control group (dural sinuses: 70,03 ± 6,63 vs. 56,17 ± 4,21 HU, p < 0.0001; 1,76 ± 0,29 vs. 1,38 ± 0,11, H/H, p < 0.0001; 24,25 ± 4,17 vs. 15,17 ± 5,79 VA differences, p < 0.0001. Deep venous system: 56,89 ± 3,13 vs. 38,76 ± 3,41 HU, p < 0.001; 1,44 ± 0,28 vs. 0,95 ± 0,06 H/H, p < 0.001; 11,10 ± 6,99 vs. − 2,23 ± 4,29 VA differences, p < 0.0001) (Table 5). The distribution of hematocrit values was normal both in patients and the control group. The median hematocrit of the study population was 40,4% (range: 37–45), while in the control group, it was 40,7% (range: 37–45).
In order to determine optimal threshold criteria for absolute attenuation values and standardized HU values, receiver operator curves (ROC) were derived both for dural sinuses and deep venous systems. The AUC for HU values related to DVCT and DST demonstrated an excellent diagnostic value. In the identification of DST, HU values greater than 61 showed AUC 0,98 (95% CI 0,95–1) with a sensitivity of 93% and a specificity of 82%, a HU/H ratio greater than 1,5 as a stand-alone value showed AUC 0,95 (95% CI 0,88–1) with a sensitivity of 93% and a specificity of 75%; VA difference greater than 20 HU showed AUC 0,89 (95% CI 0,79–0,99) with a sensitivity of 93% and a specificity of 79%.
In the identification of DCVT, HU values greater than 48,5 were pathognomonic with AUC 1 (95% CI 1–1); also, HU/H ratio greater than 1,13 showed AUC 1 (95% CI 1–1); VA difference greater than 6,5 HU showed AUC 0,96 (0,92–1) with a sensitivity of 86% and specificity of 99% (Table 6; Fig. 3). No correlation between quantitative venous HU measurements and neuroradiological scores was found (p > 0,05).
Receiver operating characteristic curves for quantitative parameters in dural sinuses and deep cerebral veins thrombosis. Differences between average HU, HU/H ratio, and VA difference measurement in evaluating dural sinuses thrombosis (a) and deep cerebral veins thrombosis (b). Dural sinuses thrombosis (DST): average HU values > 61: AUC 0,98 (95% CI 0,95–1,01); HU/H ratio > 1,5: AUC 0,95 (95% CI 0,88–1); VA HU values difference > 20: AUC 0,89 (95% CI 0,79–0,99). Deep cerebral veins thrombosis (DCVT): average HU values > 48,5: AUC 1 (95% CI 1–1), HU/H > 1,13: AUC 1 (95% CI 1–1); VA HU values difference > 6,5: AUC 0,96 (0,92–1). AUC: area under the curve; HU: Hounsfield unit; Hounsfield unit/hematocrit ratio: HU/H ratio; veno-arterial (VA) difference
Imaging findings and neurological outcome at the follow-up
Follow-up CT and/or magnetic resonance imaging (MRI) scans, whenever available (9 out of 14 patients), ranged from 15 days to 3 months (Table 1). By 3 months, 8 patients showed a complete recanalization and 1 patient showed partial recanalization with complete or almost complete regression of the parenchymal lesions. Among patients who died by the first week of in-hospital stay, short-term follow-up CeCTs were performed in 3 patients, and they did not show recanalization.
Neurological examination at 3 months and 6 months follow-up visit is shown in Table 1.
A positive correlation between the IIS at the diagnosis and mRS (p 0,003, r = 0,73), between the IIS and vessels recanalization status (p 0,002, r = 0,75), and between the vessels recanalization status and mRS (p < 0,001, r = 0,98) was found; on the other hand, no correlation was found between quantitative venous HU measurements and mRS, between the VOIS and mRS, and between quantitative HU measurements and vessels recanalization status (p > 0,05) (Table 4).
Discussion
The clinical presentation of CVT is multifaced [31], and whether the diagnostic suspect may be based on clinical symptoms, diagnostic confirmation requires neuroimaging studies [32,33,34]. Clinical presentation of CVT may vary depending on brain parenchymal involvement, the site extension of the thrombosis, and symptom duration [35], making the outcome hardly predictable [13, 20]. The diagnosis of CVT may be difficult, and the diagnostic delay may cause an extension of thrombosis across multiple venous sinuses or veins and worse venous infarcts leading to major long-term sequelae [36, 37]. Delay may be even longer in patients with DCVT [8].
The first goal of our study was to assess, in patients with DCVT, at the diagnosis, both intracranial changes and venous occlusion extension in order to develop a qualitative venous system neuroradiological severity score. NCCT revealed thalami and basal ganglia venous strokes with or without hemorrhagic infarction, effect mass, and hydrocephalus, although the interval between the symptoms’ onset and confirmatory CT was variable. The cumulative effect of these lesions predisposes patients to a different neuroradiological severity spectrum.
We found unilateral involvement of the thalamus in 7 patients, and according to previous reports, the left thalamus stroke was more frequent (6 out of 7) than the contralateral one, probably because the venous nondominant anatomic left side predisposes the deep left-sided system to an early insufficient collateral venous drainage [38].
All patients with DCVT also showed involvement of dural venous sinuses, probably because DCVT was superimposed on a preexisting DST at a variable time from the symptom onset.
We assessed both intracranial involvement and venous occlusion extension by means of two different score systems (IIS and VOIS). We found that, although the bilateral thrombotic involvement was related to a higher IIS, venous strokes were not always related to bilateral venous occlusion. Moreover, IIS and VOIS scores were not related to the timing of symptoms’ onset. Altogether, these findings suggest that the variable picture of intracranial changes may be influenced by individual anatomical factors, and venous system drainage status has been suggested as possible co-factor influencing, in some cases, hydrocephalus development and midline shift [22].
The second aim was to assess quantitative measurements of density within thrombosed deep brain veins and venous sinuses in order to evaluate the accuracy of NCCT in identifying patients with DCVT.
Previous studies reported that, in the dural sinuses system, qualitative NCCT evaluation is not accurate to detect DST because the hyperdense appearance of sinus may also be appreciated in the non-thrombotic dural venous system due to partial volume effects caused by adjacent bony structures [39].
On the other hand, some quantitative studies reported that HU values on NCCT as single values or along with hematocrit (HU/H ratio) can improve diagnostic accuracy in detecting sinus dural thrombosis than visual assessment alone; however, the sensitivity and specificity of HU values varied among studies mainly due to heterogeneity in study design, patients features, imaging methods, and standard reference [15, 25, 28, 40]. Accordingly, our data demonstrated a significant difference in venous sinuses densities and standardized HU measurements between groups of patients with DST and controls [16, 40], confirming that quantitative values may improve the diagnostic accuracy of NCCT in detecting venous thrombosis. Venous sinuses’ HU attenuation threshold values greater than 61 HU, a HU/H greater than 1.5, or a difference greater than 20 HU between venous and arterial HU were excellent values, capable of identifying thrombosed dural sinuses with the best combination of sensitivity and specificity. In thrombosed dural sinuses, we found HU cut-off values slightly lower than previously reported [16, 40], and it could be due to the subacute stage of clot at the time of CT examination. Conversely, only few quantitative studies evaluated the veins of the deep venous system; it is probably due to the high diagnostic confidence of attenuated deep vein sign in the correct detection of the thrombosed deep cerebral veins on qualitative NCCT evaluation because these veins are much less prone to partial volume artifacts due to their localization [39].
In agreement with previous studies, we found that the diagnostic accuracy of the quantitative NCCT evaluation was excellent [28] in patients with DCVT, and deep veins HU attenuation threshold values greater than 48,5 and HU/H greater than 1,13 were pathognomonic of thrombosis in cerebral deep veins.
No correlation was found between quantitative HU measurements of the venous system and the neuroradiological severity scores. This finding suggests that the age of the clot is not associated with the severity of intracranial changes or thrombotic extension, probably because other causes may play a role in this process [11].
The last goal was to compare both neuroradiological severity scores and CT quantitative density measurements with neurological sequalae and vessel recanalization status in order to predict the clinical outcome.
The overall prognosis was good in 70% of patients, while in 30%, it worsened to death. We found a positive correlation between IIS and mRS scores, between the venous recanalization status and mRS score, and between IIS and the venous recanalization status, while we found no correlation between the symptoms’ onset time and mRS score, between VOIS and mRS scores, between quantitative HU measurements and the mRS score, and between quantitative HU measurements and the venous recanalization status. These data demonstrate that, in patients with DCVT, the overall clinical prognosis is influenced mainly by the severity of parenchymal changes and marginally by symptoms’ duration or the venous occlusion extension. Edema, hemorrhage, and mass effect represent indirect imaging signs of venous stroke, and altogether, these findings may be linked to a more severe clinical outcome, while uni- or bilateral involvement was unremarkable. Recanalization of thrombosed veins frequently occurs in patients in whom the parenchymal damage was less severe. This finding may have several explanations, but probably a key role may be played by draining collateral veins [11] which allows a longer time for an anticoagulant to act, increasing the effectiveness of anticoagulant action on thrombosed veins.
We are aware that the retrospective design of the study and the small sample size may be considered a study limitation, and multicenter studies are warranted to confirm these results.
Further studies should clarify whether either anticoagulant therapy or draining collaterals’ status is an independent contributor to prognosis or whether better-draining collaterals status may improve prognosis by enhancing the effectiveness of anticoagulant therapy. This topic might have a possible therapeutic relevance because it might explain the variable effectiveness of anticoagulant therapy in patients with CVT.
Conclusion
Quantitative and qualitative CT assessment in patients with DVCT has a diagnostic relevance. Although the severity of intracranial involvement was not influenced by the time of symptoms’ onset or extension of venous occlusion, clinical outcome was related to intracranial involvement and venous recanalization. The role of draining collateral veins should be studied because it may determine the severity of parenchymal changes and because it may have therapeutic relevance.
Data Availability
The data that support the findings of this study are available upon reasonable request.
References
Bousser M-G, Ferro JM (2007) Cerebral venous thrombosis: an update. Lancet Neurol 6:162–170. https://doi.org/10.1016/S1474-4422(07)70029-7
Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Cantú C, Bousser M-G, Stam J (2009) Cerebral venous and sinus thrombosis in women. Stroke 40:2356–2361. https://doi.org/10.1161/STROKEAHA.108.543884
Connors JM, Levy JH (2020) Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost JTH 18:1559–1561. https://doi.org/10.1111/jth.14849
Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 382:e38. https://doi.org/10.1056/NEJMc2007575
Siegler JE, Klein P, Yaghi S, Vigilante N, Abdalkader M, Coutinho JM, Abdul Khalek F, Nguyen TN (2021) Cerebral vein thrombosis with vaccine-induced immune thrombotic thrombocytopenia. Stroke 52:3045–3053. https://doi.org/10.1161/STROKEAHA.121.035613
Hoelscher C, Sweid A, Ghosh R, Al Saiegh F, Keppetipola KM, Farrell CJ, Jallo J, Jabbour P, Tjoumakaris S, Gooch MR, Rosenwasser RH, Shah SO (2020) Cerebral deep venous thrombosis and COVID-19: case report. J Neurosurg 1–4. https://doi.org/10.3171/2020.5.JNS201542
Bousser MG, Chiras J, Bories J, Castaigne P (1985) Cerebral venous thrombosi – a review of 38 cases. Stroke 16:199–213. https://doi.org/10.1161/01.str.16.2.199
Pfefferkorn T, Crassard I, Linn J, Dichgans M, Boukobza M, Bousser M-G (2009) Clinical features, course and outcome in deep cerebral venous system thrombosis: an analysis of 32 cases. J Neurol 256:1839–1845. https://doi.org/10.1007/s00415-009-5206-3
Heckmann JG, Richter G, Struffert T, Schwab S (2008) Deep cerebral vein thrombosis misdiagnosed as tumour. Emerg Med J EMJ 25:861–862. https://doi.org/10.1136/emj.2008.058057
Yeo LL, Lye PP, Yee KW, Cunli Y, Ming TT, Ho AF, Sharma VK, Chan BP, Tan BY, Gopinathan A (2020) Deep cerebral venous thrombosis treatment : endovascular case using aspiration and review of the various treatment modalities. Clin Neuroradiol 30:661–670. https://doi.org/10.1007/s00062-020-00920-3
Crawford SC, Digre KB, Palmer CA, Bell DA, Osborn AG (1995) Thrombosis of the deep venous drainage of the brain in adults. Analysis of seven cases with review of the literature. Arch Neurol 52:1101–1108. https://doi.org/10.1001/archneur.1995.00540350095021
Bousser MG, Chiras J, Bories J, Castaigne P (1985) Cerebral venous thrombosis – a review of 38 cases. Stroke 16:199–213. https://doi.org/10.1161/01.str.16.2.199
Haley EC, Brashear HR, Barth JT, Cail WS, Kassell NF (1989) Deep cerebral venous thrombosis. Clinical, neuroradiological, and neuropsychological correlates. Arch Neurol 46:337–340. https://doi.org/10.1001/archneur.1989.00520390103026
Ferro JM, Bousser M-G, Canhão P, Coutinho JM, Crassard I, Dentali F, di Minno M, Maino A, Martinelli I, Masuhr F, Aguiar de Sousa D, Stam J, Organization ES (2017) European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis – endorsed by the European Academy of Neurology. Eur J Neurol 24:1203–1213. https://doi.org/10.1111/ene.13381
van Dam LF, van Walderveen MAA, Kroft LJM, Kruyt ND, Wermer MJH, van Osch MJP, Huisman MV, Klok FA (2020) Current imaging modalities for diagnosing cerebral vein thrombosis – a critical review. Thromb Res 189:132–139. https://doi.org/10.1016/j.thromres.2020.03.011
Bonatti M, Valletta R, Lombardo F, Zamboni GA, Turri E, Avesani G, Mansueto G, Manfredi R, Schifferle G (2021) Accuracy of unenhanced CT in the diagnosis of cerebral venous sinus thrombosis. Radiol Med (Torino) 126:399–404. https://doi.org/10.1007/s11547-020-01263-2
Garetier M, Rousset J, Pearson E, Tissot V, Gentric J-C, Nowak E, Ferre J-C, Timsit S (1987) Ben Salem D (2014) Value of spontaneous hyperdensity of cerebral venous thrombosis on helical CT. Acta Radiol Stockh Swed 55:1245–1252. https://doi.org/10.1177/0284185113513977
Provenzale JM, Kranz PG (2011) Dural sinus thrombosis: sources of error in image interpretation. AJR Am J Roentgenol 196:23–31. https://doi.org/10.2214/AJR.10.5323
Segall HD, Ahmadi J, McComb JG, Zee CS, Becker TS, Han JS (1982) Computed tomographic observations pertinent to intracranial venous thrombotic and occlusive disease in childhood. State of the art, some new data, and hypotheses. Radiology 143:441–449. https://doi.org/10.1148/radiology.143.2.7071346
Averback P (1978) Primary cerebral venous thrombosis in young adults: the diverse manifestations of an underrecognized disease. Ann Neurol 3:81–86. https://doi.org/10.1002/ana.410030112
Küker W, Schmidt F, Friese S, Block F, Weller M (2001) Unilateral thalamic edema in internal cerebral venous thrombosis: is it mostly left? Cerebrovasc Dis Basel Switz 12:341–345. https://doi.org/10.1159/000047732
Rahman NU, al-Tahan AR (1993) Computed tomographic evidence of an extensive thrombosis and infarction of the deep venous system. Stroke 24:744–746. https://doi.org/10.1161/01.str.24.5.744
Rao KC, Knipp HC, Wagner EJ (1981) Computed tomographic findings in cerebral sinus and venous thrombosis. Radiology 140:391–398. https://doi.org/10.1148/radiology.140.2.7255715
Rodallec MH, Krainik A, Feydy A, Hélias A, Colombani J-M, Jullès M-C, Marteau V, Zins M (2006) Cerebral venous thrombosis and multidetector CT angiography: tips and tricks. Radiogr Rev Publ Radiol Soc N Am Inc 26 Suppl 1:S5–18; discussion S42–43. https://doi.org/10.1148/rg.26si065505
Besachio DA, Quigley EP, Shah LM, Salzman KL (2013) Noncontrast computed tomographic Hounsfield unit evaluation of cerebral venous thrombosis: a quantitative evaluation. Neuroradiology 55:941–945. https://doi.org/10.1007/s00234-013-1194-1
Buyck P-J, De Keyzer F, Vanneste D, Wilms G, Thijs V, Demaerel P (2013) CT density measurement and H: H ratio are useful in diagnosing acute cerebral venous sinus thrombosis. AJNR Am J Neuroradiol 34:1568–1572. https://doi.org/10.3174/ajnr.A3469
Black DF, Rad AE, Gray LA, Campeau NG, Kallmes DF (2011) Cerebral venous sinus density on noncontrast CT correlates with hematocrit. AJNR Am J Neuroradiol 32:1354–1357. https://doi.org/10.3174/ajnr.A2504
Linn J, Pfefferkorn T, Ivanicova K, Müller-Schunk S, Hartz S, Wiesmann M, Dichgans M, Brückmann H (2009) Noncontrast CT in deep cerebral venous thrombosis and sinus thrombosis: comparison of its diagnostic value for both entities. AJNR Am J Neuroradiol 30:728–735. https://doi.org/10.3174/ajnr.A1451
Wu Z, Xie Y, Xiong S, Li J, Sun X, Shi Q, Dan W, Jiang L (2021) The Venous Occlusion Image Score: a novel quantitative scoring instrument for cerebral venous sinus thrombosis. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 30:105845. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105845
Banks JL, Marotta CA (2007) Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke 38:1091–1096. https://doi.org/10.1161/01.STR.0000258355.23810.c6
Shimizu Y, Tsuchiya K, Fujisawa H (2019) Deep venous thrombosis with decreased cerebral blood flow to the thalamus was completely restored by factor Xa inhibitor. J Stroke Cerebrovasc Dis Off J Natl Stroke Assoc 28:e39–e43. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.01.023
Crassard I, Bousser M-G (2004) Cerebral venous thrombosis. J Neuro-Ophthalmol Off J North Am Neuro-Ophthalmol Soc 24:156–163. https://doi.org/10.1097/00041327-200406000-00011
Ferro JM, Canhão P, Stam J, Bousser M-G, Barinagarrementeria F, Investigators ISCVT (2004) Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke 35:664–670. https://doi.org/10.1161/01.STR.0000117571.76197.26
Roland T, Jacobs J, Rappaport A, Vanheste R, Wilms G, Demaerel P (2010) Unenhanced brain CT is useful to decide on further imaging in suspected venous sinus thrombosis. Clin Radiol 65:34–39. https://doi.org/10.1016/j.crad.2009.09.008
Palena LM, Toni F, Piscitelli V, Maffei M, Leonardi M (2009) CT diagnosis of cerebral venous thrombosis: importance of the first examination for fast treatment. Neuroradiol J 22:137–149. https://doi.org/10.1177/197140090902200201
Rizzo L, Crasto SG, Rudà R, Gallo G, Tola E, Garabello D, De Lucchi R (2010) Cerebral venous thrombosis: role of CT, MRI and MRA in the emergency setting. Radiol Med (Torino) 115:313–325. https://doi.org/10.1007/s11547-010-0493-4
Digge P, Prakashini K, Bharath KV (2018) Plain CT vs MR venography in acute cerebral venous sinus thrombosis: triumphant dark horse. Indian J Radiol Imaging 28:280–284. https://doi.org/10.4103/ijri.IJRI_328_17
Menon D, Sarojam MK, Gopal R (2019) Unilateral thalamic infarct: a rare presentation of deep cerebral venous thrombosis. Ann Indian Acad Neurol 22:221–224. https://doi.org/10.4103/aian.AIAN_488_17
Linn J, Brückmann H (2010) Cerebral venous and dural sinus thrombosis* : state-of-the-art imaging. Clin Neuroradiol 20:25–37. https://doi.org/10.1007/s00062-010-9035-7
Alsafi A, Lakhani A, Carlton Jones L, Lobotesis K (2015) Cerebral venous sinus thrombosis, a nonenhanced ct diagnosis? Radiol Res Pract 2015:581437. https://doi.org/10.1155/2015/581437
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
We declare that all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
Rosalinda Calandrelli declares that she has no conflict of interest.
Francesca Colò declares that she has no conflict of interest.
Aldo Broccolini declares that he has no conflict of interest.
Giacomo Della Marca declares that he has no conflict of interest.
Giovanni Frisullo declares that he has no conflict of interest.
Cesare Colosimo declares that he is a scientific consultant for Bracco Diagnostics Inc. and Bayer HealthCare.
Fabio Pilato declares that he has no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Calandrelli, R., Colò, F., Broccolini, A. et al. Deep cerebral venous system involvement in patients with cerebral sinus thrombosis. A proposal of neuroradiological score systems useful for clinical assessment. Neurol Sci 44, 2049–2060 (2023). https://doi.org/10.1007/s10072-023-06656-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06656-5