Abstract
Forests in the northeastern US are experiencing shifts in community composition due to the northward migration of warm-adapted tree species and certain species’ declines (for example, white ash and eastern hemlock) due to invasive insects. Changes in belowground fungal communities and associated functions will inevitably follow. Therefore, we sought to investigate the relative importance of two important tree characteristics—mycorrhizal type [ectomycorrhizal (EcM) or arbuscular mycorrhizal (AM)] and leaf habit (deciduous or evergreen) on soil fungal community composition and organic matter cycling. We sampled soil in the organic and mineral horizons beneath two AM-associated (Fraxinus americana and Thuja occidentalis) and two ECM-associated tree species (Betula alleghaniensis and Tsuga canadensis), with an evergreen and deciduous species in each mycorrhizal group. To characterize fungal communities and organic matter decomposition beneath each tree species, we sequenced the ITS1 region of fungal DNA and measured the potential activity of carbon- and nitrogen-targeting extracellular enzymes. Each tree species harbored distinct fungal communities, supporting the need to consider both mycorrhizal type and leaf habit. However, between tree characteristics, mycorrhizal type better predicted fungal communities. Across fungal guilds, saprotrophic fungi were the most important group in shaping fungal community differences in soils beneath all tree species. The effect of leaf habit on carbon- and nitrogen-targeting hydrolytic enzymes depended on tree mycorrhizal association in the organic horizon, while oxidative enzyme activities were higher beneath EcM-associated trees across both soil horizons and leaf habits.
Similar content being viewed by others
Highlights
-
Tree species harbored distinct soil fungal communities, with mycorrhizal type explaining more variation than leaf habit.
-
Among guilds, saprotrophic fungi contributed most to differences in community composition.
-
Oxidative enzyme activities were higher beneath ectomycorrhizal-associated trees.
Introduction
Climate change and invasive insects are shifting the distribution of tree species and altering the composition of forest communities. As some tree species migrate northward with changing temperature and precipitation regimes (Jo and others 2019; Sharma and others 2022), forests of the northeastern US are also experiencing tree species declines in the Fraxinus and Tsuga genera due to the invasions of the emerald ash borer and hemlock woolly adelgid, respectively (Horsley and others 2002; Morin and others 2017). Changes in forest tree communities will have a profound effect on the soils they inhabit. The species composition of tree communities has bearing on the belowground communities of soil organisms that live on and around their roots, including symbiotic mycorrhizal fungi (Otsing and others 2021), and free-living saprotrophic and pathotrophic fungi (Tedersoo and others 2016). Soil carbon (C) storage and nitrogen (N) cycling are also known to vary below different tree species (Hicks Pries and others 2022; Crowley and Lovett 2017; Knops and others 2002). Northeastern forests at higher latitude and elevation have lower tree species diversity relative to forests further south in New England (Thompson and others 2013). Consequently, northern New England forests should be more susceptible to pests and species loss, given the relatively low functional redundancy among tree species (Ellison and others 2005). Understanding the relationships between tree traits and belowground fungal communities and soil decomposition processes will allow us to better predict the effects of forest community change (Reich 2014).
Forest community change will shift the relative abundances of trees with differing mycorrhizal associations, either arbuscular mycorrhizal (AM) or ectomycorrhizal (EcM) fungi (Jo and others 2019), an important tree trait that influences soil C and N cycling. Mycorrhizal fungi play an important role in soil N cycling by taking up soil N, which they give to trees in exchange for photosynthetically-derived sugars. The carbon-to-nitrogen (C:N) ratio of soil connects to how organisms such as mycorrhizal fungi search for and extract nutrients (Phillips and others 2013). Soils under EcM-associated trees generally have higher soil C:N ratios relative to AM-associating trees, with a larger proportion of soil N in less-available organic forms (Zhu and others 2018). EcM fungi have evolved over sixty times from saprotrophic lineages, and some of these species have retained the genetic capacity to mine N from organic matter by producing N- and C-targeting enzymes or via fenton chemistry (Pellitier and Zak 2018), where production of hydrogen peroxide in concert with soil ferrous iron creates oxidizing hydroxyl radicals (Buée and others 2007; Orwin and others 2011). In contrast, AM fungi are limited to scavenging inorganic N released from organic matter by saprotrophic microbes, creating an inorganic N economy (Lambers and others 2008; Phillips and others 2013). When soil N is limiting, EcM competes with saprotrophic microbes for organic N, contributing to N limitation (Frey 2019). In a process known as the Gadgil effect, these interactions sometimes slow saprotrophic litter decomposition in EcM-dominated soils, as enzymes are N-rich and energetically expensive (Fernandez and Kennedy 2016; Gadgil and Gadgil 1974). Nitrogen limitation can alter microbial community composition to favor saprotrophic species that are better equipped to survive in a low N environment (Cheeke and others 2017; Güsewell and Gessner 2009).
Though there is a rich literature on the effects of mycorrhizal fungi on soil microbial community composition (Fitch and others 2020; Liang and others 2016) and associated decomposition processes (Cheeke and others 2017; Wurzburger and Brookshire 2017), the effect of leaf habit (evergreen vs. deciduous) on soil fungal community composition and function is not often accounted for (Cornelissen and others 2001; Sun and others 2018). This oversight may conflate mycorrhizal effects with leaf habit effects since there is a wide range of representation between mycorrhizal types and evergreen or deciduous leaf habits, though most temperate and boreal evergreen trees are associated with EcM fungi (Averill and others 2019). Trees can broadly be classified as deciduous or evergreen, where each group employs different strategies to grow and recycle N from leaf litter (Loehle 1988). In contrast with the high C:N ratios and multiyear lifespan of evergreen leaves, deciduous leaves generally have lower C:N ratios and are shed annually. These strategies, or leaf habits, have different effects on soil litter decomposition and N availability, with subsequent effects on soil microbial community composition (Chen and others 2016; Dijkstra and Cheng 2007; Osborne and others 2021). Whereas deciduous litter generally undergoes rapid decomposition (Prescott and others 2000), leading to soils with shallower organic horizons and fast N cycling (Ollinger and others 2002), the high C:N and lignin-rich evergreen litter decays more slowly and promotes deeper organic horizons (Lu and others 2021; Silver and Miya 2001). The relatively slow turnover of N from evergreen-derived soil organic matter may lead to N limitation, constraining enzyme production and nutrient release (Rowe and others 2006). This N-limited environment in soils below evergreen trees tends to promote higher fungal biomass relative to deciduous soils (Awad and others 2019) and supports a different fungal community, including species of saprotrophic fungi that can produce oxidative enzymes necessary to break down lignin in evergreen tree leaves (Sinsabaugh 2010). In addition, differences in soil fungal community composition between deciduous and evergreen forests may be driven in part by the saprotrophic fungi who experience seasonal pulses of leaf litter inputs, with subsequent variation in nutrient availability (Jacob and others 2009; Nottingham and others 2009).
Given that most AM-associated trees have lower leaf tissue C:N and lignin:N compared to most EcM-associated trees (Averill and others 2019), we have yet to disentangle the effects of leaf habit and mycorrhizal type on observed patterns of decomposition and fungal community composition across AM- and EcM-associated forests. In the northeastern US, mixed evergreen–deciduous forests are common, and AM and EcM associations are observed for both evergreen and deciduous trees. Although most evergreen trees associate with EcM fungi in this region, trees in the Cupressaceae family associate with AM fungi (Brundrett and Tedersoo 2020), creating an opportunity to analyze the effects of mycorrhizal fungi and leaf habit in a factorial design. To investigate the relative importance of tree species, mycorrhizal association, and leaf habit on soil fungal communities and their associated functions, we sampled soil under four tree species, each representing a unique leaf habit and mycorrhizal type combination: Fraxinus americana (deciduous, AM-associated), Thuja occidentalis (evergreen, AM-associated), Betula alleghaniensis (deciduous, EcM-associated), and Tsuga canadensis (evergreen, EcM-associated). Due to the effects of mycorrhizal fungi on soil nutrient economies (Phillips and others 2013), our first hypothesis was that trees of each mycorrhizal type (AM vs. EcM) would foster distinct mycorrhizal and free-living (that is, saprotroph and pathotroph) soil fungal communities regardless of differences in leaf habit (evergreen vs. deciduous). However, due to the need to decompose lignin-rich leaf litter, saprotrophic fungal communities would be affected by leaf habit, resulting in more similar saprotrophic communities beneath AM- and EcM-associated evergreens. Further, because both leaf habit and tree mycorrhizal association have been shown to affect fungal communities, these traits may interact to shape distinct communities beneath each tree species. Both saprotrophic and some ectomycorrhizal fungi influence decomposition through extracellular enzyme production, targeting organic compounds for C, N, and phosphorus (P). Consequently, we hypothesized that extracellular enzyme activities would align with fungal community structure.
Methods
Site Selection
This study was conducted at eight forest sites in Vermont and New Hampshire, USA (Table S1). We sampled soils from 24 plots (10 m in diameter) that were dominated (> 50% basal area) by one of four tree species. Each tree species was either AM- or EcM-associated and had either an evergreen or deciduous leaf habit: F. americana L. (AM deciduous), T. occidentalis L. (AM evergreen), B. alleghaniensis Britton (EcM deciduous), and T. canadensis L. Carrière (EcM evergreen), hereafter referred to as ash, cedar, birch, and hemlock, respectively. We sampled soil under each species at multiple sites and under multiple species within sites when possible (Table S1). To address the potential confounding effects of tree species and site characteristics, we extracted information about annual precipitation, mean annual temperature (PRISM Climate Group 2014), soil series, parent material, and slope range for each site using publicly available data (Vermont Center for Geographic Information 2022; Soil Survey Geographic (SSURGO) database New Hampshire, 2021). For plot and species-level characteristics, we measured organic horizon depth, soil texture, and soil pH.
Soil Collection and Processing
We collected soil from the organic horizon and from the top 10 cm of the uppermost mineral soil horizon at the center of each plot, each of which was dominated (> 80%) by one focal tree species, where the focal species was always close to the plot center. All soil was sampled during the snow-free season in 2017–19. At the center of each plot, we removed the Oi layer and collected soil from the Oe + Oa horizon and from the top 10 cm of the mineral B horizon, as the A horizon was often found in a mixed OA horizon. When an E horizon was present, we collected from 10 cm of mineral soil from just below the E horizon. Soil samples were brought back to the laboratory and stored at 4°C. Within a week of collection, mineral soil samples were sieved (< 2 mm), and organic horizon soils were root-picked and homogenized. A subset of all samples was dried at 60°C for 48 h, and ground, while the rest was stored at − 20°C for downstream DNA extraction. Ground soils were analyzed for C and N concentrations on an elemental analyzer (Carlo Erba Instruments, Wigan, UK). Percent clay at each sample site was analyzed by hydrometer grain size analysis. pH was measured on a Mettler Toledo S220 pH/ion meter, using a 1:1 ratio of soil to water.
DNA Extraction and Functional Classification
Fungal DNA was extracted from organic horizon and mineral soils using the Qiagen PowerSoil Kit (Qiagen Science Inc., Germantown, MD). Samples were prepared at the University of Wisconsin-Madison Biotechnology Center similarly to the process detailed in Illumina’s 16S Metagenomic Sequencing Library Preparation Protocol, Part # 15044223 Rev. B (Illumina Inc., San Diego, CA), where the ITS1 region was amplified using the forward primers ITS1-f (CTTGGTCATTTAGAGGAAGTAA) and reverse primer ITS4 (TCCTCCGCTTATTGATATGC) (Gardes and Bruns 1993). Duplicate PCR reactions were conducted under the conditions outlined in Caporaso and others (2011). PCR products were cleaned using an AxyPrep Mag PCR clean-up kit (Axygen Biosciences, Union City, CA). Quality of the cleaned products was assessed using an Agilent DNA 1000 kit (Agilent Technologies, Santa Cara, CA), and concentrations were quantified using Qubit dsDNA HS Assay Kit (Thermofisher Scientific). Libraries were pooled in an equimolar fashion prior to sequencing. Paired-end, 300-bp sequencing was performed by Illumina MiSeq (v3). Sequences were processed using R software (R Core Team 2021, version 3.6.1) following the DADA2 ITS-specific pipeline ("DADA2" package; Callahan and others 2016). The ITS1 region was extracted using itsxpress for downstream community analysis (Rivers and others 2018). Unique amplicon sequence variants (ASVs) were identified to the lowest confident taxonomic level using a Naïve Bayesian Classifier from the UNITE ITS database (Wang and others 2007). Singletons and non-fungal ASVs were removed, and samples were rarefied to a sequencing depth of 16,803 using the "rrarefy" function in R ("vegan" package; Oksanen and others 2020). Thereafter, amplicon sequence variant (ASV) relative abundance was calculated for each sample. ASVs were assigned functional guild classifications (EcM, saprotroph, and pathotroph) using FUNGuild (Nguyen and others 2016). ASVs not belonging to these three functional guilds were classified as “other” where functional information was available, and as “unknown ecology” when taxonomic and/or functional information was not available. Because ITS sequencing may be biased against AM fungi (Lekberg and others 2018), we did not include those ASVs in this analysis. While all DNA barcodes used for fungal sequencing have been shown to over- or under-represent certain taxa, the ITS1 region has been shown to produce similar representations of fungal community composition as compared to ITS2 and SSU (O’Brien and others 2005). We acknowledge that our fungal community data are relative and not absolute abundances because they are not accompanied by soil microbial biomass or another indication of abundance (Gloor and others 2017).
Extracellular Enzyme Assays
We performed fluorometric and colorimetric assays of extracellular enzyme activity (EEA) using methods modified from German and others (2011) and Saiya-Cork and others (2002). We quantified potential activity of the following hydrolases, where the enzyme abbreviation is followed by the enzyme’s product and target substrate: β-glucosidase (BG; glucose from cellulose), cellobiohydrolase (CB; disaccharides from cellulose), N-acetyl-glucosaminidase (NAG; monosaccharides from chitin), leucine-aminopeptidase (LAP; amino acids from proteins), and acid phosphatase (AP; phosphate ions from organic compounds); and two oxidases: phenol oxidase (PO) and peroxidase (PER). In brief, soil subsamples (1 g) were homogenized using an immersion blender with 125 ml of Milli-Q water (pH ~ 7), to ensure capturing in situ soil pH. For the hydrolases, 200 ul of sample homogenate was pipetted into 96-well plates with 50 μl of each 4-methylumbelliferone-labeled fluorogenic substrate in replicates of four and incubated in the dark at 12°C (reflecting average in situ temperature) (Koch and others 2007). Fluorescence was measured on a microplate spectrophotometer (365-nm excitation and 450-nm emission) for hydrolases after the addition of 15 ul of 1 M NaOH. For phenol oxidase and peroxidase, the samples were mixed with 50 ul l-3,4-dihydroxyphenylalanine (L-DOPA) or 50-ul L-DOPA and 20-ul hydrogen peroxide, respectively, incubated in the dark at field temperature, and then, their absorbance (460 nm) was measured on the microplate reader (Tecan Spark).
Statistical Analysis
We characterized fungal communities through several steps: First, we evaluated multivariate homogeneity of group dispersions with a Bray–Curtis similarity matrix (functions “betadisper” and “permutest,” vegan package 2.5–7 in R v. 4.0.5; Oksanen and others 2020). The effects of tree species (the interaction between leaf habit and mycorrhizal type), horizon, and soil pH on fungal community structure were first evaluated by performing a PERMANOVA (“adonis” function) on all ASVs, and differences were visualized using non-metric multidimensional scaling (NMDS). The relative abundances of fungal functional groups (saprotrophs, pathotrophs, and EcM) were fitted to the NMDS using the “envfit” function. Given a significant interaction between leaf habit and mycorrhizal type from the initial PERMANOVA result, we then performed a pairwise comparison using the “adonis.pair” function to compare dissimilarities among tree species (that is, the mycorrhizal type by leaf habit interaction). To complement the NMDS, we evaluated the effects of tree species (the interaction between leaf habit and mycorrhizal type), horizon, and pH on these functional guilds using a linear model followed by Tukey’s HSD post hoc comparisons. We evaluated the contribution of individual fungal species to community dissimilarity using similarity percentages (SIMPER) analysis on the Bray–Curtis distance matrix with the “simper” function (vegan package 2.5–7). We evaluated relative contributions to community dissimilarity by performing comparisons across tree species in one SIMPER analysis and two separate comparisons for each mycorrhizal type and leaf habit. Given the results from the SIMPER analyses, which revealed that ASVs within the genus Mortierella made the largest contribution to community dissimilarity, we evaluated differences in Mortierella relative abundance across tree species using one-way ANOVA.
We used linear mixed modeling (“lmer” function, lme4 package 1.1–26; Bates and others 2015) to predict singular or PCA grouped potential enzyme activities in response to leaf habit (evergreen and deciduous) and mycorrhizal type groups (AM and EcM) (Bates and others 2015). We used a principal component analysis to group enzymes for subsequent statistical analyses based on relatedness across samples and functional categorization. BG, CB, and NAG were grouped on principal component axis 1, which was extracted for downstream analysis (Figure S1). Oxidative enzymes (PO and PER) were summed to represent overall potential oxidative activity. In all models, we included the soil C:N ratio, pH, and interactions with horizon as predictors that would likely affect potential enzyme activities. Lastly, we included “site” and “substrate batch” as random effects to account for variance that may have been associated across sites and weekly batches of enzyme assay substrates. We performed model selection by sequentially removing interactions and then predictors with the least significant p-value and verifying that the AIC value for the newest model did not increase relative to the previous model (Bolker and others 2009). We verified model variance homogeneity by plotting model residuals by each of the model predictors at each step of the model selection process and model normality by plotting model residuals in a histogram. If there was a significant interaction, we used the “contrast” function in the emmeans package (Lenth 2021) to perform pairwise comparisons to estimate marginal means. We used the “ggpredict” function to visualize the estimated marginal effects, which measure the association between a change in the predictor with a change in the response variable, from the model output. We also analyzed the effects of tree species, leaf habit, and mycorrhizal type on soil C:N ratio using linear mixed modeling and AIC sequential model selection, including sampling site as a random effect.
Results
Site Soil Characteristics
Organic horizon depth varied across sites (Table S1) and was highest beneath cedar and lowest beneath ash trees (Table S2). Soil C:N was higher in soils under EcM-associated relative to AM-associated trees (ANOVA, t75 = 3.53, p < 0.001; Table S2) and in soils with evergreen relative to deciduous trees (t75 = 3.57, p < 0.001). Soil pH was lower in soils under EcM-associated trees (t37 = − 2.61, p = 0.013; Figure S2) and did not differ by leaf habit (t3.3 = 1.49, p = 0.226). Across sampling sites, soil pH was highest in soils from cedar plots (Table S2 and Figure S3).
Fungal Community Composition
Fungal community composition was distinct beneath each tree species (two-way PERMANOVA interaction, F1,45 = 2.28, p < 0.001). Differences in fungal community composition were largest between the AM-associated evergreen species (T. occidentalis) and the ECM-associated evergreen species (T. canadensis), with less distinction between the AM- and ECM-associated deciduous species (pairwise PERMANOVA, p = 0.002 and 0.033, respectively; Figure 1A). As expected, EcM relative abundance was higher in soil beneath EcM-associated trees (linear model (lm), t1,42 = 3.32, p = 0.002; Figure 2). The differences in fungal community composition between AM and EcM soils were not driven exclusively by differences in the relative abundance of mycorrhizal fungi; we observed similar results by analyzing the free-living fungal community separately (two-way PERMANOVA interaction, F1,45 = 1.16, p = 0.046; Figure 1B). Similar to the full community analysis, the free-living fungal communities in soils under evergreen hemlock and cedar trees were more different than in soils under deciduous ash and birch trees (pairwise PERMANOVA, p = 0.005 and 0.029, respectively). Saprotroph relative abundance was higher in soils under hemlock (EcM evergreen) relative to cedar (AM evergreen) trees, but was relatively similar in soils under birch and ash deciduous trees (lm interaction, t1,42 = 3.0, Tukey HSD, p = 0.063 and p = 0.35, respectively). Pathotroph relative abundance was higher in the mineral relative to the organic horizon soils (lm, t1,43 = 2.23, p = 0.031) and higher in evergreen relative to deciduous soils (t1,43 = 2.12, p = 0.039), regardless of dominant tree mycorrhizal type. Soil pH also significantly affected fungal community composition (PERMANOVA; F1,45 = 2.54, p < 0.001; Figure S3), but did not affect the relative abundance of fungal guilds.
Non-metric multidimensional scaled representation of fungal community composition with A all ASVs and B free-living taxa (mycorrhizal taxa removed). Each color represents plots from a tree species of one mycorrhizal type (blue for EcM and yellow for AM), and the shade of the color represents leaf habit (darker for evergreen and lighter for deciduous). Circles represent the mineral soil, while triangles represent the organic horizon. Ellipses show the standard error of plots within one species and horizon, where dashed lines are the mineral soil and solid lines are the organic horizon. Arrows indicate significant correlations (p < 0.05) between the relative abundances of fungal guilds (saprotroph, EcM, and pathotroph) and differences in fungal community composition. The overall stress for these ordinations is 0.156 for A and 0.157 for B.
Functional guild relative abundance proportions in each tree species: hemlock (EcM evergreen), birch (EcM deciduous), ash (AM deciduous), and cedar (AM evergreen). Each color represents a functional guild. ASVs that did not fall into the main functional guilds were labeled as “Unidentified ecology.” ASVs that were not identified to any taxonomic level were defined as “Other.”
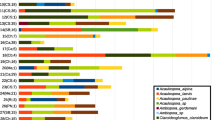
Given that each tree species fostered distinct soil fungal communities, we analyzed the contributions of individual fungal taxa to community dissimilarity (beta diversity) between tree species. To understand how the effect of tree species relates to the effects of mycorrhizal type and leaf habit, we then analyzed the contributions of both mycorrhizal type and leaf habit to overall community dissimilarity. We found that the effects of mycorrhizal type and leaf habit were both important factors contributing to fungal community dissimilarity, but mycorrhizal type explained twice the variability (10%) relative to leaf habit (5%) for a total of 15% (SIMPER analysis, p < 0.05; Figure 3A). When fungal community dissimilarity was compared among tree species, significantly contributing taxa explained a total of 85% of community dissimilarity, and three tree species comparisons explained over 20% each (Figure 4A). Of the 30 taxa that contributed the most to community dissimilarity in mycorrhizal type and leaf habit comparisons, 14 were saprotrophic, and saprotrophs as a functional group had the greatest contribution to dissimilarity (Figure 3B). Similarly, 15 out of 27 taxa in the tree species comparisons were saprotrophs, contributing a total of 73% to community dissimilarity (Figure 4B). The genus Mortierella is highly ranked in both analyses and contributed significantly to differences in fungal communities between cedar (AM evergreen) and hemlock (EcM evergreen) and between cedar and ash (AM deciduous) soils. Differences in the relative abundance of Mortierella across tree species supports this finding, where soils under cedar trees harbored the lowest relative abundance in comparison with all other tree species (Tukey’s HSD, p < 0.001), while ash, birch, and hemlock soils contained similar Mortierella relative abundances. For comparative reference, the relative abundance of ASVs across tree species is shown in Figure S4.
Average contribution of individual fungal taxa to overall community dissimilarity (beta diversity, p < 0.05) between mycorrhizal types (dark gray) or leaf habits (light gray), based on SIMPER analysis: A Each bar represents an ASV identified to the lowest level, and stacked boxes within a bar represent individual ASVs identified to the same level (that is, three to “Unassigned” or two to “Mortierella”). Associated fungal guilds are represented by colored boxes. B Each stacked bar represents one of the four fungal guilds, and outlined boxes within each bar represent an individual ASV’s contribution.
Average contribution of individual fungal ASVs to overall community dissimilarity between tree species, based on SIMPER analysis: A Each bar represents an ASV identified to the lowest level and stacked boxes within a bar represent individual ASVs identified to the same level (that is, three to “Unassigned” or two to “Mortierella”). Associated fungal guilds are represented by colored boxes. B Each stacked bar represents one of the four fungal guilds, and outlined boxes within each bar represent an individual ASV’s contribution. Only significantly contributing (p < 0.05) taxa were included from each tree species comparison.
Potential Enzyme Activities
As with the fungal communities, potential enzyme activities differed across tree species, mycorrhizal type, and leaf habit. In organic horizons, there was a species effect (interaction between the effect of mycorrhizal association and leaf habit) on C-targeting enzyme activities, represented by the combined activities of B-glucosidase, cellobiohydrase, and N-acetyl glucosaminidase as principal component one (PC1) (t33.7 = 2.72, p = 0.010). Specifically, C-targeting enzymes had higher potential activity in soils below hemlock (EcM evergreen) relative to birch deciduous trees (EcM evergreen) (pairwise comparisons from LMER, p < 0.001) and in soils below ash (AM deciduous) relative to birch (EcM deciduous) trees (p = 0.006, Figure 5A). C-targeting enzyme activities did not differ among species in the mineral soil. Across soil horizons, leucine aminopeptidase (LAP) had higher potential activity in soils below ash (AM deciduous) relative to birch (EcM deciduous) trees, but was similar in soils below hemlock and cedar (LMER, p = 0.026, Figure 5C). Potential LAP activity was also higher in the organic relative to mineral horizons (LMER, t66.5 = 3.53, p < 0.001) and marginally increased with soil C:N (t36.6 = 3.53, p = 0.075). The potential activity of oxidative enzymes was higher in EcM relative to AM soils across leaf habits and soil horizons (t71 = 2.91, p = 0.005; Figure 5B). The only enzyme whose activity was affected by pH was AP, and its potential activity decreased with pH (t36.7 = − 2.0, p = 0.054).
Potential enzyme activities across mycorrhizal type, leaf habit, and soil horizon: A Principal component 1 representing B-glucosidase, cellobiosidase, and N acetylglucosaminidase, where significant effects occurred only in the organic horizon, B combined oxidase, and C leucine aminopeptidase potential activities. Error bars represent standard error of marginal effects averages. For all panels, response variables show the marginal effects (that is, the isolated effects of the explanatory, x-axis variable).
Discussion
Effects of Mycorrhizal Type on Fungal Community Composition
We add to the small, but growing literature evaluating the effects of tree species on soil fungal community composition and function representing the two major leaf habits and mycorrhizal types (see Midgley and Sims 2020; Heděnec and others 2023). Comparing the relative differences between mycorrhizal type and leaf habit showed that fungal communities were distinct between tree species, and more affected by mycorrhizal type than leaf habit. These findings support the hypothesis that belowground traits (for example, roots and associated mycorrhizal fungi) have a greater effect on the free-living fungal community than aboveground traits such as leaf litter (See and others 2019; Whalen and others 2021). These effects remained even when mycorrhizal taxa were removed from the analysis. Thus, our first hypothesis that fungal community composition would differ between mycorrhizal types was supported.
Several fundamental differences between AM and EcM fungal function likely contributed to these distinct communities, including the effects on saprotrophic fungi, how mycorrhiza may alter exudation with soil inorganic N availability, and their association with soil pH. First, the process of exuding organic molecules to saprotrophic microbes to prime further decomposition and nutrient release is likely different between AM- and EcM-associated roots and fungi (Keller and others 2021). Saprotrophic microbes use exudates from roots and associated mycorrhizal fungi as an energy source to manufacture extracellular enzymes (de Vries and Caruso 2016; Schneider and others 2012). Second, these differences in photosynthate exudation relate to the strategies EcM and AM fungi use to obtain N, where AM-dominated soils generally have higher levels of inorganic N (Lin and others 2022). Under increasing available soil N, AM-associated roots and fungi have been shown to increase exudation, while EcM fungi decreased exudation to the rhizosphere, coinciding with reduced rhizosphere bacterial biomass (Keller and Phillips 2019; Gorka and others 2019). Differences in N availability have also been shown to affect fungal community composition across natural N gradients (Cox and others 2010; Pellitier and others 2021). This effect of N variability on fungal communities may be linked to root exudation rates, as there is field evidence that variation in seasonal exudation rates affects free-living microbial community composition and potential enzyme activities (Kaiser and others 2010; Voříšková and others 2014). Lastly, the effect of soil pH on microbial communities is well documented (Lauber and others 2009; Carrino-Kyker and others 2016), and we found that pH affected microbial community composition. Across horizons, soil pH was more strongly associated with tree mycorrhizal type than with leaf habit (Figure S2). Soils beneath EcM-associated trees were more acidic than AM soils, supporting findings from other studies (Midgley and Sims 2020; Heděnec and others 2023). Whether these pH differences are due to plant habitat preference (Curtis 1946; Pellitier and others 2021), or differences caused by the mycorrhiza themselves (Lin and others 2022) are still up for debate. However, taking pH into account in our models, we still found a significant effect of mycorrhizal type on fungal communities. The mycorrhizal type effect on fungal community structure is thus not solely due to differences in soil pH.
Effects of Leaf Habit on Soil Fungal Community Composition
Our second hypothesis, that evergreen litter would promote similar saprotroph communities, was not supported; instead, saprotrophic communities beneath deciduous tree species were more similar than those beneath evergreen trees. We hypothesize that EcM fungi affected saprotrophic fungi via N limitation in evergreen but not in deciduous soils, leading to more similar saprotrophic communities in soils beneath deciduous trees than evergreen soils (Fernandez and others 2020). A potential explanation for this pattern may be that EcM fungi compete with saprotrophs for N only when N is limiting (Smith and Wan 2019), which would be more likely under evergreen relative to deciduous trees. Increased competition from EcM could select for saprotrophic communities dominated by competitive taxa adept at resource acquisition, which may have fostered highly distinct fungal communities in the hemlock soils compared with birch soils. Indeed, we found that the highest soil C:N (and lowest soil %N) was under evergreen EcM trees relative to both deciduous EcM- and AM-associated trees in this study. We suggest that faster leaf litter decomposition under birch relative to hemlock trees (Finzi and others 2014) likely elevated N availability (Mueller and others 2012), reducing N competition between EcM and saprotrophic fungi. This fast-decaying deciduous litter may have produced similar nutrient conditions to those observed in soils under AM trees, leading to more similar fungal community compositions (Chen and others 2019).
Connecting Fungal Community Composition to Extracellular Enzyme Activities
We expected leaf habit to be more closely related to oxidative enzyme activities than tree mycorrhizal association because evergreen litter generally has higher lignin:N ratios than deciduous litter (Northeastern Ecosystem Research Cooperative 2010; Taylor and others 1989), and oxidative activity is key to decomposing lignin-rich litter (Ollinger and others 2002; Sinsabaugh 2010). However, we found that mycorrhizal type better explained oxidative enzyme activity, with higher oxidative activity in EcM-dominated soils versus AM-dominated soils (Figure 5B). Two complementary mechanisms can help explain these results. First, though saprotrophs are often thought of as the major wood and lignin decomposers (Janusz and others 2017; Lindahl and Tunlid 2015), some species of EcM fungi have retained the ability to produce oxidative enzymes (Kohler and others 2015), and many species are capable of oxidizing substrates via fenton chemistry (Op De Beeck and others 2018, 2020). Oxidation via fenton chemistry can be mistaken for extracellular oxidative enzyme activity in enzyme assays like those used in our study (Jones and others 2020). Consequently, a combination of these mechanisms, either direct enzyme production or fenton chemistry, could explain results from recent studies showing evidence that EcM fungi are associated with higher levels of peroxidase activity (Talbot and others 2015; Bödeker and others 2014), and support our findings where higher oxidase activity was found in soils beneath EcM-associated trees regardless of leaf habit.
Second, higher potential oxidative enzyme activity observed in soils under EcM-associated trees may also be related to relatively greater melanized fungal necromass as a C source for saprotrophic microbes (Drigo and others 2012). EcM fungal necromass has been shown to have a higher average melanin concentration compared to AM fungi (Siletti and others 2017), and melanin is composed of a group of complex phenolic polymers that require oxidative breakdown. The genus Mortierella has been shown to produce oxidative enzymes (for example, Lisov and others 2021) and is broadly associated with fungal necromass decomposition (Fernandez and Kennedy 2018; Siletti and others 2017). In our study, Mortierella was the most important taxa explaining differences in fungal community composition across tree species and exhibited the lowest relative abundance in soils beneath AM cedar trees (Figure S4). Greater fungal necromass in soils beneath EcM-associated trees may promote a higher relative abundance of Mortierella, which may help to explain the elevated oxidative activity observed in these plots. Further, melanized fungal necromass is an important source of SOM in EcM relative to AM systems, since melanin slows the decomposition of EcM tissues and increases its incorporation into SOM (Schweigert and others 2015; Fernandez and others 2019). Decomposition of melanized fungal necromass has also been shown to significantly influence decomposer community composition (Fernandez and Kennedy 2018; Fernandez and Koide 2014). These differences in Mortierella relative abundance are an example of how mycorrhizal type can affect fungal community composition and associated enzyme activities.
The potential activities of NAG and C-targeting enzymes (that is, PC1) were higher and more variable in the organic horizon and differed by tree species (Figure 5a), implying the effect of leaf habit depended on mycorrhizal type. In organic horizon soils, potential activity was higher below evergreen relative to deciduous leaf habit, but only when the trees were EcM-associated. These results indicate either there may be a need for more saprotrophic enzyme production beneath EcM-associated evergreen trees, or that the mycorrhizal fungi present are contributing directly to the overall enzyme activity (Kohler and others 2015). Because enzymes are energetically expensive to produce (Wallenstein and Burns 2011), these differing responses suggest that there must be a need for additional enzymatic decomposition in soils beneath EcM-associated evergreen trees, such as mining N from organic compounds into plant-available forms. A related, underlying mechanism could be connected to how tree roots of different mycorrhizal associations alter exudation with increasing N availability. EcM-associated deciduous trees may have had lower exudate transfer through their associated mycorrhizal fungi, relative to EcM evergreen trees in response to lower C:N ratios (Table S2) and decreased C-targeting enzyme production and activity (Kotroczó and others 2014; Gorka and others 2019). In soils beneath AM-associated trees, the similar C- and N-targeting enzyme activity suggests that roots and associated AM fungi behaved similarly, with the potential explanation that there was no difference in root rhizodeposition between leaf habits. Higher C-targeting and peptide-targeting enzyme activity in soils under ash (AM deciduous) relative to birch (EcM deciduous) (Figure 5a, c) indicates that AM-associated plants may have increased exudation to the rhizosphere under increasing soil N availability while EcM-associated roots decreased exudation (Keller and others 2021; Keller and Phillips 2019). Lower C:N ratios in soils under ash relative to birch trees support this hypothesis, as does their relatively higher rate of leaf litter decomposition (Sun and others 2018) and accompanied N release.
Another potential mechanism contributing to these tree species-specific results is the prevalence of chitin, an important C-rich and N-containing component of fungal tissue targeted by chitinases, one of the three enzyme activities measured and represented by PC1 (Figure S1). Chitin should be found more prevalently in EcM-dominated soils as EcM fungi produce significantly more biomass than AM fungi (Frey 2019) and because ECM-dominated forest soils harbor more fungal biomass in general (Cheeke and others 2017). Furthermore, the presence of chitin in SOM has been shown to regulate chitinase production across multiple lineages of EcM fungi (Maillard and others 2018). In this study, we also observed higher chitinase activity in soils under hemlock (EcM evergreen) compared with birch (EcM deciduous) trees (Figure 5a). Soils beneath hemlock trees are often characterized by high fungal necromass and chitin contents, which could stimulate chitinase activity (Maillard and others 2018), although the mechanism is not well-established. Soils with higher N availability have been shown to promote EcM species with shorter exploration types (Hobbie and Agerer 2010; Lilleskov and others 2011); in contrast, soils with lower N availability (for example, hemlock) may support medium- to long-distance exploration types. Although the relationship between exploration type and total fungal necromass production is not yet resolved, these longer distance explorers could conceivably produce greater necromass and chitin inputs to soils, elevating chitinase activity. Differences in the composition (and associated exploration types) of EcM in hemlock versus birch forest soils could thus help to explain the observed differences in chitinase activities between these two leaf habits. In summary, these divergent potential enzyme activities across tree species indicate that mycorrhizal associations and leaf habits are both important tree traits that affect C- and N-targeting enzyme production.
Conclusion
Considering both tree mycorrhizal type and leaf habit are necessary for predicting tree species effects on soil fungal communities and organic matter cycling (Hicks Pries and others 2022). However, mycorrhizal association better explained fungal community composition relative to leaf habit. Mycorrhiza can affect saprotrophic community composition via exudation of photosynthate and competition from EcM fungi, depending on N availability. Because leaf habit affects soil N availability, this tree characteristic is inextricably linked to the effects of root and mycorrhizal fungal exudation. The effects of leaf habit on C- and N-targeting hydrolytic enzyme activities depended on mycorrhizal type, and oxidative enzyme activities were explained by tree mycorrhizal association. This study is limited by the representation of each leaf habit and mycorrhizal type by only one species, but overall demonstrates the importance of considering leaf habit when trying to understand the effects of tree mycorrhizal associations on soil fungal communities and functions. To support and strengthen these findings, future studies on the relative importance of these two characteristics should include other AM-associated trees with lower quality litter such as Magnolia sp. (Serna-González and others 2019) and EcM-associated trees with higher quality litter such as Tilia sp. or Carya sp. (DeForest and Snell 2020; Northeastern Ecosystem Research Cooperative 2010). Climate change and invasive pests will increase forest disturbances and shift forest communities. Our findings indicate that removal of even a single-tree species could have significant effects on soil fungal communities and decomposition-related functions in northeastern forests.
Data Availability
The URL to access our data and code is https://data.ess-dive.lbl.gov/datasets/doi:10.15485/1962817.
References
Averill C, Bhatnagar JM, Dietze MC, Pearse WD, Kivlin SN. 2019. Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proc Natl Acad Sci 116(46):23163–23168. https://doi.org/10.1073/pnas.1906655116.
Awad A, Majcherczyk A, Schall P, Schröter K, Schöning I, Schrumpf M, Ehbrecht M, Boch S, Kahl T, Bauhus J, Seidel D, Ammer C, Fischer M, Kües U, Pena R. 2019. Ectomycorrhizal and saprotrophic soil fungal biomass are driven by different factors and vary among broadleaf and coniferous temperate forests. Soil Biol Biochem 131:9–18. https://doi.org/10.1016/j.soilbio.2018.12.014.
Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01.
Bödeker ITM, Clemmensen KE, de Boer W, Martin F, Olson Å, Lindahl BD. 2014. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol 203(1):245–256. https://doi.org/10.1111/nph.12791.
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evolut 24(3):127–135. https://doi.org/10.1016/j.tree.2008.10.008.
Brundrett MC, Tedersoo L. 2020. Resolving the mycorrhizal status of important northern hemisphere trees. Plant Soil 454(1):3–34. https://doi.org/10.1007/s11104-020-04627-9.
Buée M, Courty PE, Mignot D, Garbaye J. 2007. Soil niche effect on species diversity and catabolic activities in an ectomycorrhizal fungal community. Soil Biol Biochem 39(8):1947–1955. https://doi.org/10.1016/j.soilbio.2007.02.016.
Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13(7):581–583. https://doi.org/10.1038/nmeth.3869.
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 108(Supplement 1):4516–4522. https://doi.org/10.1073/pnas.1000080107.
Carrino-Kyker SR, Kluber LA, Petersen SM, Coyle KP, Hewins CR, DeForest JL, Smemo KA, Burke DJ. 2016. Mycorrhizal fungal communities respond to experimental elevation of soil pH and P availability in temperate hardwood forests. FEMS Microbiol Ecol 92(3):fiw024. https://doi.org/10.1093/femsec/fiw02.
Cheeke TE, Phillips RP, Brzostek ER, Rosling A, Bever JD, Fransson P. 2017. Dominant mycorrhizal association of trees alters carbon and nutrient cycling by selecting for microbial groups with distinct enzyme function. New Phytol 214(1):432–442. https://doi.org/10.1111/nph.14343.
Chen W, Koide RT, Adams TS, DeForest JL, Cheng L, Eissenstat DM. 2016. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc Natl Acad Sci 113(31):8741–8746. https://doi.org/10.1073/pnas.1601006113.
Chen L, Xiang W, Wu H, Ouyang S, Lei P, Hu Y, Ge T, Ye J, Kuzyakov Y. 2019. Contrasting patterns and drivers of soil fungal communities in subtropical deciduous and evergreen broadleaved forests. Appl Microbiol Biotechnol 103(13):5421–5433. https://doi.org/10.1007/s00253-019-09867-z.
Cornelissen J, Aerts R, Cerabolini B, Werger M, van der Heijden M. 2001. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia 129(4):611–619. https://doi.org/10.1007/s004420100752.
Cox F, Barsoum N, Lilleskov EA, Bidartondo MI. 2010. Nitrogen availability is a primary determinant of conifer mycorrhizas across complex environmental gradients. Ecol Lett 13(9):1103–1113. https://doi.org/10.1111/j.1461-0248.2010.01494.x.
Crowley KF, Lovett GM. 2017. Effects of nitrogen deposition on nitrate leaching from forests of the northeastern United States will change with tree species composition. Can J For Res. https://doi.org/10.1139/cjfr-2016-0529.
Curtis JD. 1946. Preliminary observations on Northern White Cedar in Maine. Ecology 27(1):23–36. https://doi.org/10.2307/1931014.
de Vries FT, Caruso T. 2016. Eating from the same plate? Revisiting the role of labile carbon inputs in the soil food web. Soil Biol Biochem 102:4–9. https://doi.org/10.1016/j.soilbio.2016.06.023.
DeForest JL, Snell RS. 2020. Tree growth response to shifting soil nutrient economy depends on mycorrhizal associations. New Phytol 225(6):2557–2566. https://doi.org/10.1111/nph.16299.
Dijkstra FA, Cheng W. 2007. Interactions between soil and tree roots accelerate long-term soil carbon decomposition. Ecol Lett 10(11):1046–1053. https://doi.org/10.1111/j.1461-0248.2007.01095.x.
Drigo B, Anderson IC, Kannangara GSK, Cairney JWG, Johnson D. 2012. Rapid incorporation of carbon from ectomycorrhizal mycelial necromass into soil fungal communities. Soil Biol Biochem 49:4–10. https://doi.org/10.1016/j.soilbio.2012.02.003.
Ellison AM, Bank MS, Clinton BD, Colburn EA, Elliott K, Ford CR, Foster DR, Kloeppel BD, Knoepp JD, Lovett GM, Mohan J, Orwig DA, Rodenhouse NL, Sobczak WV, Stinson KA, Stone JK, Swan CM, Thompson J, Von Holle B, Webster JR. 2005. Loss of foundation species: consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3(9):479–486. https://doi.org/10.1890/1540-9295(2005)003[0479:LOFSCF]2.0.CO;2.
Fernandez CW, Heckman K, Kolka R, Kennedy PG. 2019. Melanin mitigates the accelerated decay of mycorrhizal necromass with peatland warming. Ecol Lett 22(3):498–505. https://doi.org/10.1111/ele.13209.
Fernandez CW, Kennedy PG. 2016. Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? New Phytol 209(4):1382–1394. https://doi.org/10.1111/nph.13648.
Fernandez CW, Kennedy PG. 2018. Melanization of mycorrhizal fungal necromass structures microbial decomposer communities. J Ecol 106(2):468–479. https://doi.org/10.1111/1365-2745.12920.
Fernandez CW, Koide RT. 2014. Initial melanin and nitrogen concentrations control the decomposition of ectomycorrhizal fungal litter. Soil Biol Biochem 77:150–157. https://doi.org/10.1016/j.soilbio.2014.06.026.
Fernandez CW, See CR, Kennedy PG. 2020. Decelerated carbon cycling by ectomycorrhizal fungi is controlled by substrate quality and community composition. New Phytol 226(2):569–582. https://doi.org/10.1111/nph.16269.
Finzi AC, Raymer PCL, Giasson M-A, Orwig DA. 2014. Net primary production and soil respiration in New England hemlock forests affected by the hemlock woolly adelgid. Ecosphere 5(8):art98. https://doi.org/10.1890/ES14-00102.1.
Fitch AA, Lang AK, Whalen ED, Geyer K, Hicks Pries C. 2020. Fungal community, not substrate quality, drives soil microbial function in Northeastern U.S. Temperate Forests. Front For Glob Change. https://doi.org/10.3389/ffgc.2020.569945.
Frey SD. 2019. Mycorrhizal fungi as mediators of soil organic matter dynamics. Ann Rev Ecol Evolut Syst 50(1):237–259. https://doi.org/10.1146/annurev-ecolsys-110617-062331.
Gadgil RL, Gadgil PD. 1974. Suppression of litter decomposition by mycorrhizal roots of Pinus radiata. N Z J For Sci 5:33–41.
Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol Ecol 2(2):113–118. https://doi.org/10.1111/j.1365-294x.1993.tb00005.x.
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD. 2011. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43(7):1387–1397. https://doi.org/10.1016/j.soilbio.2011.03.017.
Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. 2017. Microbiome datasets are compositional: and this is not optional. Front Microbiol. https://doi.org/10.3389/fmicb.2017.02224.
Gorka S, Dietrich M, Mayerhofer W, Gabriel R, Wiesenbauer J, Martin V, Zheng Q, Imai B, Prommer J, Weidinger M, Schweiger P, Eichorst SA, Wagner M, Richter A, Schintlmeister A, Woebken D, Kaiser C. 2019. Rapid transfer of plant photosynthates to soil bacteria via ectomycorrhizal hyphae and its interaction with nitrogen availability. Front Microbiol. https://doi.org/10.3389/fmicb.2019.00168.
Güsewell S, Gessner MO. 2009. N:P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct Ecol 23(1):211–219. https://doi.org/10.1111/j.1365-2435.2008.01478.x.
Heděnec P, Zheng H, Pessanha Siqueira D, Lin Q, Peng Y, Kappel Schmidt I, Guldberg Frøslev T, Kjøller R, Rousk J, Vesterdal L. 2023. Tree species traits and mycorrhizal association shape soil microbial communities via litter quality and species mediated soil properties. For Ecol Manag 527:120608. https://doi.org/10.1016/j.foreco.2022.120608.
Hicks Pries CE, Lankau R, Ingham GA, Legge E, Krol O, Forrester J, Fitch A, Wurzburger N. 2022. Differences in soil organic matter between EcM- and AM-dominated forests depend on tree and fungal identity. Ecology n/a(n/a):e3929. https://doi.org/10.1002/ecy.3929
Hobbie EA, Agerer R. 2010. Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 327(1):71–83. https://doi.org/10.1007/s11104-009-0032-z.
Horsley SB, Long RP, Bailey SW, Hallett RA, Wargo PM. 2002. Health of Eastern North American sugar maple forests and factors affecting decline. North J Appl For 19(1):34–44. https://doi.org/10.1093/njaf/19.1.34.
Jacob M, Weland N, Platner C, Schaefer M, Leuschner C, Thomas FM. 2009. Nutrient release from decomposing leaf litter of temperate deciduous forest trees along a gradient of increasing tree species diversity. Soil Biol Biochem 41(10):2122–2130. https://doi.org/10.1016/j.soilbio.2009.07.024.
Janusz G, Pawlik A, Sulej J, Świderska-Burek U, Jarosz-Wilkołazka A, Paszczyński A. 2017. Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev 41(6):941–962. https://doi.org/10.1093/femsre/fux049.
Jo I, Fei S, Oswalt CM, Domke GM, Phillips RP. 2019. Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Sci Adv 5(4):eaav6358. https://doi.org/10.1126/sciadv.aav6358.
Jones ME, LaCroix RE, Zeigler J, Ying SC, Nico PS, Keiluweit M. 2020. Enzymes, manganese, or iron? Drivers of oxidative organic matter decomposition in soils. Environ Sci Technol 54(21):14114–14123. https://doi.org/10.1021/acs.est.0c04212.
Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Rasche F, Zechmeister-Boltenstern S, Sessitsch A, Richter A. 2010. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol 187(3):843–858. https://doi.org/10.1111/j.1469-8137.2010.03321.x.
Keller AB, Brzostek ER, Craig ME, Fisher JB, Phillips RP. 2021. Root-derived inputs are major contributors to soil carbon in temperate forests, but vary by mycorrhizal type. Ecol Lett 24(4):626–635. https://doi.org/10.1111/ele.13651.
Keller AB, Phillips RP. 2019. Relationship between belowground carbon allocation and nitrogen uptake in saplings varies by plant mycorrhizal type. Front For Glob Change. https://doi.org/10.3389/ffgc.2019.00081.
Knops JMH, Bradley KL, Wedin DA. 2002. Mechanisms of plant species impacts on ecosystem nitrogen cycling. Ecol Lett 5(3):454–466. https://doi.org/10.1046/j.1461-0248.2002.00332.x.
Koch O, Tscherko D, Kandeler E. 2007. Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Glob Biogeochem Cycles. https://doi.org/10.1029/2007GB002983.
Kohler A, Kuo A, Nagy LG, Morin E, Barry KW, Buscot F, Canbäck B, Choi C, Cichocki N, Clum A, Colpaert J, Copeland A, Costa MD, Doré J, Floudas D, Gay G, Girlanda M, Henrissat B, Herrmann S, Martin F. 2015. Convergent losses of decay mechanisms and rapid turnover of symbiosis genes in mycorrhizal mutualists. Nat Genet 47(4):410–415. https://doi.org/10.1038/ng.3223.
Kotroczó Z, Veres Z, Fekete I, Krakomperger Z, Tóth JA, Lajtha K, Tóthmérész B. 2014. Soil enzyme activity in response to long-term organic matter manipulation. Soil Biol Biochem 70:237–243. https://doi.org/10.1016/j.soilbio.2013.12.028.
Lambers H, Raven J, Shaver G, Smith S. 2008. Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evolut 23:95–103. https://doi.org/10.1016/j.tree.2007.10.008.
Lauber CL, Hamady M, Knight R, Fierer N. 2009. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75(15):5111–5120. https://doi.org/10.1128/AEM.00335-09.
Lekberg Y, Vasar M, Bullington LS, Sepp S-K, Antunes PM, Bunn R, Larkin BG, Öpik M. 2018. More bang for the buck? Can arbuscular mycorrhizal fungal communities be characterized adequately alongside other fungi using general fungal primers? New Phytol 220(4):971–976.
Lenth RV. 2021. emmeans: Estimated Marginal Means, aka Least-Squares Means (R package version 1.7.3). https://CRAN.R-project.org/package=emmeans
Liang M, Liu X, Gilbert GS, Zheng Y, Luo S, Huang F, Yu S. 2016. Adult trees cause density-dependent mortality in conspecific seedlings by regulating the frequency of pathogenic soil fungi. Ecol Lett 19(12):1448–1456. https://doi.org/10.1111/ele.12694.
Lilleskov EA, Hobbie EA, Horton TR. 2011. Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition. Fungal Ecol 4(2):174–183. https://doi.org/10.1016/j.funeco.2010.09.008.
Lin G, Craig ME, Jo I, Wang X, Zeng D-H, Phillips RP. 2022. Mycorrhizal associations of tree species influence soil nitrogen dynamics via effects on soil acid–base chemistry. Glob Ecol Biogeogr 31(1):168–182. https://doi.org/10.1111/geb.13418.
Lindahl BD, Tunlid A. 2015. Ectomycorrhizal fungi—potential organic matter decomposers, yet not saprotrophs. New Phytol 205(4):1443–1447. https://doi.org/10.1111/nph.13201.
Lisov A, Belova O, Zavarzina A, Konstantinov A, Leontievsky A. 2021. The role of laccase from zygomycetous fungus mortierella elasson in humic acids degradation. Agronomy 11(11):11. https://doi.org/10.3390/agronomy11112169.
Loehle C. 1988. Tree life history strategies: the role of defenses. Can J For Res 18(2):209–222. https://doi.org/10.1139/x88-032.
Lu C, Kotze DJ, Setälä HM. 2021. Evergreen trees stimulate carbon accumulation in urban soils via high root production and slow litter decomposition. Sci Total Environ 774:145129. https://doi.org/10.1016/j.scitotenv.2021.145129.
Maillard F, Didion M, Fauchery L, Bach C, Buée M. 2018. N-Acetylglucosaminidase activity, a functional trait of chitin degradation, is regulated differentially within two orders of ectomycorrhizal fungi: Boletales and Agaricales. Mycorrhiza 28(4):391–397. https://doi.org/10.1007/s00572-018-0833-0.
Midgley MG, Sims RS. 2020. Mycorrhizal association better predicts tree effects on soil than leaf habit. Front For Glob Change 3:74. https://doi.org/10.3389/ffgc.2020.00074.
Morin RS, Liebhold AM, Pugh SA, Crocker SJ. 2017. Regional assessment of emerald ash borer, Agrilus planipennis, impacts in forests of the Eastern United States. Biol Invasions 19(2):703–711. https://doi.org/10.1007/s10530-016-1296-x.
Mueller KE, Hobbie SE, Oleksyn J, Reich PB, Eissenstat DM. 2012. Do evergreen and deciduous trees have different effects on net N mineralization in soil? Ecology 93(6):1463–1472. https://doi.org/10.1890/11-1906.1.
Nguyen NH, Song Z, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG. 2016. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248. https://doi.org/10.1016/j.funeco.2015.06.006.
Northeastern Ecosystem Research Cooperative. 2010. Compilation of foliar chemistry data for the northeastern United States and southeastern Canada [Text/xml]. KNB Data Repository. https://doi.org/10.5063/AA/NERC.12.6.
Nottingham AT, Griffiths H, Chamberlain PM, Stott AW, Tanner EVJ. 2009. Soil priming by sugar and leaf-litter substrates: a link to microbial groups. Appl Soil Ecol 42(3):183–190. https://doi.org/10.1016/j.apsoil.2009.03.003.
O’Brien HE, Parrent JL, Jackson JA, Moncalvo J-M, Vilgalys R. 2005. Fungal community analysis by large-scale sequencing of environmental samples. Appl Environ Microbiol 71(9):5544–5550. https://doi.org/10.1128/AEM.71.9.5544-5550.2005.
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. 2020. vegan: Community Ecology Package (2.5–7). https://CRAN.R-project.org/package=vegan.
Ollinger SV, Smith ML, Martin ME, Hallett RA, Goodale CL, Aber JD. 2002. Regional variation in foliar chemistry and N cycling among forests of diverse history and composition. Ecology 83(2):339–355. https://doi.org/10.1890/0012-9658(2002)083[0339:RVIFCA]2.0.CO;2.
Op De Beeck M, Troein C, Peterson C, Persson P, Tunlid A. 2018. Fenton reaction facilitates organic nitrogen acquisition by an ectomycorrhizal fungus. New Phytol 218(1):335–343. https://doi.org/10.1111/nph.14971.
Op De Beeck M, Troein C, Siregar S, Gentile L, Abbondanza G, Peterson C, Persson P, Tunlid A. 2020. Regulation of fungal decomposition at single-cell level. ISME J 14(4):4. https://doi.org/10.1038/s41396-019-0583-9.
Orwin KH, Kirschbaum MUF, John MGS, Dickie IA. 2011. Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecol Lett 14(5):493–502. https://doi.org/10.1111/j.1461-0248.2011.01611.x.
Osborne BB, Soper FM, Nasto MK, Bru D, Hwang S, Machmuller MB, Morales ML, Philippot L, Sullivan BW, Asner GP, Cleveland CC, Townsend AR, Porder S. 2021. Litter inputs drive patterns of soil nitrogen heterogeneity in a diverse tropical forest: results from a litter manipulation experiment. Soil Biol Biochem 158:108247. https://doi.org/10.1016/j.soilbio.2021.108247.
Otsing E, Anslan S, Ambrosio E, Koricheva J, Tedersoo L. 2021. Tree species richness and neighborhood effects on ectomycorrhizal fungal richness and community structure in boreal forest. Front Microbiol. https://doi.org/10.3389/fmicb.2021.567961.
Pellitier PT, Zak DR. 2018. Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: why evolutionary history matters. New Phytol 217(1):68–73. https://doi.org/10.1111/nph.14598.
Pellitier PT, Zak DR, Argiroff WA, Upchurch RA. 2021. Coupled shifts in ectomycorrhizal communities and plant uptake of organic nitrogen along a soil gradient: an isotopic perspective. Ecosystems 24(8):1976–1990. https://doi.org/10.1007/s10021-021-00628-6.
Phillips RP, Brzostek E, Midgley MG. 2013. The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199(1):41–51. https://doi.org/10.1111/nph.12221.
Prescott CE, Zabek LM, Staley CL, Kabzems R. 2000. Decomposition of broadleaf and needle litter in forests of British Columbia: influences of litter type, forest type, and litter mixtures. Can J For Res 30(11):1742–1750. https://doi.org/10.1139/x00-097.
PRISM Climate Group. 2014. Oregon State University. https://prism.oregonstate.edu
R Core Team. 2021. A language and environment for statistical computing. R Foundation for Statistical Computing, Version 3.2.1. https://www.R-project.org/
Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J Ecol 102(2):275–301. https://doi.org/10.1111/1365-2745.12211.
Rivers AR, Weber KC, Gardner TG, Liu S, Armstrong SD. 2018. ITSxpress: software to rapidly trim internally transcribed spacer sequences with quality scores for marker gene analysis. F1000Research 7:1418. https://doi.org/10.12688/f1000research.15704.1.
Rowe EC, Evans CD, Emmett BA, Reynolds B, Helliwell RC, Coull MC, Curtis CJ. 2006. Vegetation type affects the relationship between soil carbon to nitrogen ratio and nitrogen leaching. Water Air Soil Pollut 177(1):335–347. https://doi.org/10.1007/s11270-006-9177-z.
Saiya-Cork KR, Sinsabaugh RL, Zak DR. 2002. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34(9):1309–1315. https://doi.org/10.1016/S0038-0717(02)00074-3.
Schneider T, Keiblinger KM, Schmid E, Sterflinger-Gleixner K, Ellersdorfer G, Roschitzki B, Richter A, Eberl L, Zechmeister-Boltenstern S, Riedel K. 2012. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J 6(9):1749–1762. https://doi.org/10.1038/ismej.2012.11.
Schweigert M, Herrmann S, Miltner A, Fester T, Kästner M. 2015. Fate of ectomycorrhizal fungal biomass in a soil bioreactor system and its contribution to soil organic matter formation. Soil Biol Biochem 88:120–127. https://doi.org/10.1016/j.soilbio.2015.05.012.
See CR, Luke McCormack M, Hobbie SE, Flores-Moreno H, Silver WL, Kennedy PG. 2019. Global patterns in fine root decomposition: climate, chemistry, mycorrhizal association and woodiness. Ecol Lett 22(6):946–953. https://doi.org/10.1111/ele.13248.
Serna-González M, Urrego-Giraldo LE, Osorio NW, Valencia-Ríos D. 2019. Mycorrhizae: a key interaction for conservation of two endangered Magnolias from Andean forests. Plant Ecol Evolut 152(1):30–40. https://doi.org/10.5091/plecevo.2019.1398.
Sharma S, Andrus R, Bergeron Y, Bogdziewicz M, Bragg DC, Brockway D, Cleavitt NL, Courbaud B, Das AJ, Dietze M, Fahey TJ, Franklin JF, Gilbert GS, Greenberg CH, Guo Q, Lambers JHR, Ibanez I, Johnstone JF, Kilner CL, Clark JS. 2022. North American tree migration paced by climate in the West, lagging in the East. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2116691118.
Siletti CE, Zeiner CA, Bhatnagar JM. 2017. Distributions of fungal melanin across species and soils. Soil Biol Biochem 113:285–293. https://doi.org/10.1016/j.soilbio.2017.05.030.
Silver WL, Miya RK. 2001. Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129(3):407–419. https://doi.org/10.1007/s004420100740.
Sinsabaugh RL. 2010. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol Biochem 42(3):391–404. https://doi.org/10.1016/j.soilbio.2009.10.014.
Smith GR, Wan J. 2019. Resource-ratio theory predicts mycorrhizal control of litter decomposition. New Phytol 223(3):1595–1606. https://doi.org/10.1111/nph.15884.
Soil Survey Geographic (SSURGO) database for New Hampshire. 2021. Retrieved December 30, 2022, from https://www.nhgeodata.unh.edu/datasets/NHGRANIT::soil-survey-geographic-ssurgo-database-for-new-hampshire/about
Sun T, Hobbie SE, Berg B, Zhang H, Wang Q, Wang Z, Hättenschwiler S. 2018. Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc Natl Acad Sci 115(41):10392–10397. https://doi.org/10.1073/pnas.1716595115.
Talbot JM, Martin F, Kohler A, Henrissat B, Peay KG. 2015. Functional guild classification predicts the enzymatic role of fungi in litter and soil biogeochemistry. Soil Biol Biochem 88:441–456. https://doi.org/10.1016/j.soilbio.2015.05.006.
Taylor BR, Parkinson D, Parsons WFJ. 1989. Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70(1):97–104. JSTOR. https://doi.org/10.2307/1938416
Tedersoo L, Bahram M, Cajthaml T, Põlme S, Hiiesalu I, Anslan S, Harend H, Buegger F, Pritsch K, Koricheva J, Abarenkov K. 2016. Tree diversity and species identity effects on soil fungi, protists and animals are context dependent. ISME J 10(2):2. https://doi.org/10.1038/ismej.2015.116.
Thompson JR, Carpenter DN, Cogbill CV, Foster DR. 2013. Four centuries of change in northeastern United States forests. PLoS ONE 8(9):e72540. https://doi.org/10.1371/journal.pone.0072540.
Vermont Center for Geographic Information. 2022. https://geodata.vermont.gov/
Voříšková J, Brabcová V, Cajthaml T, Baldrian P. 2014. Seasonal dynamics of fungal communities in a temperate oak forest soil. New Phytol 201(1):269–278. https://doi.org/10.1111/nph.12481.
Wallenstein MD, Burns RG. 2011. Ecology of extracellular enzyme activities and organic matter degradation in soil: a complex community-driven process. In: Methods of soil enzymology. Wiley. pp 35–55. https://doi.org/10.2136/sssabookser9.c2
Wang Q, Garrity GM, Tiedje JM, Cole JR. 2007. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73(16):5261–5267. https://doi.org/10.1128/AEM.00062-07.
Whalen ED, Lounsbury N, Geyer K, Anthony M, Morrison E, van Diepen LTA, Le Moine J, Nadelhoffer K, van den Enden L, Simpson MJ, Frey SD. 2021. Root control of fungal communities and soil carbon stocks in a temperate forest. Soil Biol Biochem 161:108390. https://doi.org/10.1016/j.soilbio.2021.108390.
Wurzburger N, Brookshire ENJ. 2017. Experimental evidence that mycorrhizal nitrogen strategies affect soil carbon. Ecology 98(6):1491–1497. https://doi.org/10.1002/ecy.1827.
Zhu K, McCormack ML, Lankau RA, Egan JF, Wurzburger N. 2018. Association of ectomycorrhizal trees with high carbon-to-nitrogen ratio soils across temperate forests is driven by smaller nitrogen not larger carbon stocks. J Ecol 106(2):524–535. https://doi.org/10.1111/1365-2745.12918.
Acknowledgements
We would like to thank Owen Krol for help with sample processing and enzyme assay method optimization, the UW Madison Biotechnology Center for providing ITS sequencing, Craig Layne, and Brad and Ann Caswell for help locating sampling sites, and the Vermont Nature Conservancy for access to Long Pond sites. This work was supported as part of the Terrestrial Ecosystem Science Program by the Office of Science, Office of Biological and Environmental Research, of the U.S. Department of Energy under contract DE-SC0020228.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions: AAF prepared the original draft, contributed to data collection, methodology development, data analysis, and visualization. AKL conceived the study and contributed to data collection, data analysis, and reviewing and editing. EDW contributed to bioinformatics, data analysis, visualization, and reviewing and editing. EMH contributed to data collection and reviewing and editing. SBG contributed to data collection and reviewing and editing. CHP provided resources and contributed to data analysis, visualization, and reviewing and editing.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fitch, A.A., Lang, A.K., Whalen, E.D. et al. Mycorrhiza Better Predict Soil Fungal Community Composition and Function than Aboveground Traits in Temperate Forest Ecosystems. Ecosystems 26, 1411–1427 (2023). https://doi.org/10.1007/s10021-023-00840-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-023-00840-6