Abstract
Purpose
The objective of this systematic review was to determine a minimum serum 25-hydroxyvitamin D (25OHD) threshold based on the risk of having rickets in young children. This work was commissioned by the WHO and FAO within the framework of the update of the vitamin D requirements for children 0–3 years old.
Methods
A systematic search of Embase was conducted to identify studies involving children below 4 years of age with serum 25OHD levels and radiologically confirmed rickets, without any restriction related to the geographical location or language. Study-level and individual participant data (IPD)-level random effects multi-level meta-analyses were conducted. The odds, sensitivity and specificity for rickets at different serum 25OHD thresholds were calculated for all children as well as for children with adequate calcium intakes only.
Results
A total of 120 studies with 5412 participants were included. At the study-level, children with rickets had a mean serum 25OHD of 23 nmol/L (95% CI 19–27). At the IPD level, children with rickets had a median and mean serum 25OHD of 23 and 29 nmol/L, respectively. More than half (55%) of the children with rickets had serum 25OHD below 25 nmol/L, 62% below 30 nmol/L, and 79% below 40 nmol/L. Analysis of odds, sensitivities and specificities for nutritional rickets at different serum 25OHD thresholds suggested a minimal risk threshold of around 28 nmol/L for children with adequate calcium intakes and 40 nmol/L for children with low calcium intakes.
Conclusion
This systematic review and IPD meta-analysis suggests that from a public health perspective and to inform the development of dietary requirements for vitamin D, a minimum serum 25OHD threshold of around 28 nmol/L and above would represent a low risk of nutritional rickets for the majority of children with an adequate calcium intake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Vitamin D is an essential nutrient for bone health [1] and possibly for other extra-skeletal health outcomes [1, 2]. The main sources of vitamin D are dietary intake and dermal synthesis during sunlight exposure [3]. Vitamin D (along with calcium and zinc) has been prioritized by the Food and Agriculture Organization (FAO) together with the World Health Organization (WHO), as part of the update of their 2004 nutrient requirements for children aged 0–3 years [4, 5]. Dietary Reference Values (DRV) for vitamin D, as estimates of the dietary requirements for the vitamin, are crucial from a public health perspective in providing a framework for the prevention of vitamin D deficiency and optimizing vitamin D status of individuals [6]. With the vitamin D DRV update in mind, a recent FAO-WHO-commissioned systematic review and meta-analysis evaluated circulating 25-hydroxyvitamin D (25OHD), parathyroid hormone and other newer potential biomarkers of vitamin D status (such as free and bioavailable 25OHD, 24,25-dihydroxyvitamin D, C3-epimer of 25OHD, and vitamin D3) in terms of their use in defining dietary requirements for vitamin D in young children [7]. The systematic review concluded that circulating 25OHD is a robust and reliable marker of vitamin D status in infants and children [7].
In setting DRVs for vitamin D, there is a need to clarify the relationship of serum 25OHD and the reference level of the critical indicator(s) of health outcomes for nutrient adequacy, taking into consideration sex, life-stage and vulnerable groups [8]. This serum 25OHD threshold, in turn, is used to establish the recommended vitamin D intake which maintains a stated percentage individuals above this threshold, and thus ensuring adequacy. For infants and children, the FAO-WHO prioritized the risk of rickets as the critical indicator amongst other skeletal and extra-skeletal health outcomes [9]. Rickets is a softening and weakening of bones at the growth plate, which can lead to painful and long-term health consequences [10], including potentially life-threatening complications [11]. It can be diagnosed based on clinical signs, biochemical tests and radiographies [10]. Several authorities and expert bodies have established vitamin D recommendations that indicate a minimum recommended serum 25OHD level, based on minimizing the risk of developing rickets in children, or osteomalacia in adults [3, 12]. However, there is a lack of consensus on this minimum 25OHD threshold, with values varying from 25 up to 50 nmol/L (see Table 1). Differences between these recommended serum 25OHD thresholds could be explained by differences in the body of evidence considered, variability in the vitamin D assays [13], and the characteristics of the populations, such as calcium intake [14] and sun exposure [15].
The present systematic review and individual participant data (IPD) meta-analysis was commissioned by the FAO-WHO with the key objective of determining a serum 25OHD threshold, based on the risk of rickets, to inform the setting of the vitamin D DRV for young children. In particular, emphasis was placed on the determination of a serum 25OHD threshold in the setting of adequate dietary calcium intake. This is important because of the DRV convention that setting a vitamin D intake requirement is based on the assumption that the intake of calcium and all other nutrients is adequate [3, 12, 16]. Of note, other authorities and expert bodies thus far were unable to include this aspect in their consideration of serum 25OHD thresholds.
Methods
The present systematic review and meta-analysis, including IPD analyses, follows the guidance provided as part of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-IPD statement [17]. Approval by a research ethics committee to conduct the IPD meta-analysis was not required because the aim of this secondary analysis was consistent with the ethical approval received for the individual studies. The current analysis was conducted on anonymized data.
Eligibility criteria
Studies involving generally healthy (apart from rickets) children below 4 years of age with total serum 25OHD levels (referred to as 25OHD henceforth) and radiologically confirmed active rickets were included. Studies in which the presence or absence of rickets were diagnosed only clinically or biochemically, but not radiologically, were excluded (to lower risk of misdiagnosis). Children 4 years and above or with conditions, such as low birth weight, prematurity, hereditary rickets, vitamin D resistant or dependent rickets, were excluded. If only serum 25OHD2 or 25OHD3 was measured, the study was excluded. The following study designs were included: cross-sectional, cohort, case–control, case report, case series, surveillance studies, before-after studies, and trials. Conference abstracts, systematic reviews, commentaries, and editorials were excluded. There were no restrictions related to the geographical location or the language.
Search strategy
A systematic search of Embase was conducted on 7 June 2022. The search strategy is shown in Appendix 1. The search was supplemented with a manual screening of the reference lists of included articles, reviews and key international vitamin D DRV reports from other authoritative bodies [3, 12, 18,19,20,21]. Study selection was conducted in duplicate by two reviewers.
Data collection processes, data items, IPD and data protection
Information on the characteristics of the study and their participants, the 25OHD measurement methods, as well as the method of estimation of calcium intake were extracted by one reviewer and verified by a second reviewer. Aggregate- and individual-level data (where available) for serum 25OHD and calcium intake were extracted. For before-after studies and trials with vitamin D supplementation, only the baseline data were extracted.
In the case of those identified priority studies that reported having measured calcium intake as well as serum 25OHD, collaboration, in the form of IPD sharing, was requested. The authors of each study were contacted by e-mail (up to a maximum of 3 times). For willing collaborators, data were initially de-identified at source before encryption and transfer by e-mail. In line with recently published principles and recommendations in relation to the sharing and reuse of IPD [22], data within the individual data files were used to establish an overall anonymized data file, as follows: only data on the prioritized IPD variables within the transferred files were included, there were no personal identifiers included. The anonymized data file was held in Excel® V15.30 (Microsoft Corporation, USA).
Data analysis
The statistical analyses were conducted in the graphical user interface RAnalyticFlow (version 3.1.8) with R (version 3.6.3). Serum 25OHD values were transformed into the common unit of nmol/L and calcium intake into mg/d, using the conversion factors 2.496 mol/g for 25OHD and 24.95 mmol/g for calcium. If means and standard deviations were not reported, they were estimated using medians, interquartile ranges, confidence intervals, standard errors, t values, P values, F values [23]. If data was only available in plots, it was extracted using PlotDigitizer [24]. Non-detectable levels of serum 25OHD were imputed using the midpoint between the detection level of the assay and zero.
The data distributions of the study-level estimates and individual-level data were plotted in histograms and outliers reviewed. Data were subjected to random effects multi-level meta-analyses, and ninety-five percent confidence intervals (95% CI) were computed. Studies that could not be meta-analyzed were summarized in a narrative manner.
The odds of having rickets at different serum 25OHD thresholds were calculated. The sensitivity (i.e. percentage of the population with disease correctly identified by the threshold) and specificity (i.e. percentage of the population without the disease correctly identified by the threshold) of different serum 25OHD thresholds to detect rickets were calculated and plotted as a receiver operating characteristic (ROC) curve. The maximal Youden index was calculated and used to determine at which serum threshold the sensitivity and specificity were maximized and thus represents the maximum potential effectiveness of a biomarker like serum 25OHD.
The sensitivity and specificity analyses were performed on the IPD subset of individuals with adequate calcium intake (as newly defined by FAO-WHO, i.e., Average Nutrient Requirement (ANR) values for 0–6 months-old, 210 mg/d, 7–11 months-old, 330 mg/d, and 1–3 year-olds, 490 mg/d) (Personal communication from Dr Jason Montez, WHO Scientist) as well as on the entire IPD dataset (irrespective of dietary calcium intake). To assess the robustness of the results, further sensitivity analyses were conducted. One sensitivity analysis was done including only IPD data with known adequate calcium intakes assessed by multiple 24h recalls. To be able to include IPD data where calcium intake was not reported, an additional sensitivity analysis was conducted with imputed missing calcium intake data. Where calcium intake was missing, it was assumed to be adequate in infants exclusively breastfed and in children with a diversified diet, including dairy products, and assumed to be insufficient in infants below 6 months of age with mixed feeding and in children above 5 months exclusively breastfed, with a low and null dairy intake, special unbalanced or vegan diet.
Results
Study characteristics
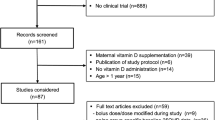
From a total of 1112 records identified within the search, a total of 120 studies with 5412 participants (mean age 17 months) were included (see Fig. 1). The majority of the studies were case reports (N = 39) and case series (N = 40), followed by case–control studies (N = 19) and trials (N = 19), cohort studies (N = 2) and a cross-sectional study (N = 1). The studies were conducted in all regions of the world, except Latin America. The countries in which most of the studies were conducted were the United States of America (N = 22), Nigeria (N = 14), India (N = 12), and Turkey (N = 11). The studies covered latitudes from 60.5°N to 40.9°S (mean 31.4° N). In the majority of the studies (79%), the skin pigmentation of the participants was dark. While most of the studies did not report which method was used to measure circulating 25OHD (N = 63), the remainder reported the use of competitive binding radioimmunoassay (N = 44), chemiluminescence immunoassay (N = 8), liquid chromatography-tandem mass spectrometry (LC–MS/MS) (N = 4), or high-performance liquid chromatography (HPLC) (N = 1). Only two studies reported participating in a vitamin D assay standardization program. The characteristics of the included studies are shown in Table 2.
Individual data on serum 25OHD was reported for 65 studies with 930 participants (mean age 31 months old, range 0–47 months old), of which 75% had radiologically confirmed rickets and 25% did not have rickets. Sixteen studies reported having measured calcium intake. Upon request for IPD on serum 25OHD and calcium intake, 11 studies agreed and provided the data (666 participants, meaning that 71.6% of the participants in the IPD dataset had a corresponding calcium intake measured) [25,26,27,28,29,30,31,32,33,34]. The remaining five studies did not respond or were not able to provide the data [35,36,37,38,39]. Among the 11 studies for which data was provided, calcium intake was estimated by multiple 24h recalls in 8 studies, by a single 24 h recall in one study, by a 3-day food record in one study, and a food frequency in one study. Using the FAO-WHO’s age-specific ANR values, 23% of participants had adequate calcium intake and 77% had insufficient calcium intake.
Study-level meta-analysis
The meta-analysis of all the studies (irrespective of study design and calcium intake) showed that children with rickets had a mean serum 25OHD of 23 nmol/L (N studies = 77, 95% CI 19–27), whereas children without rickets had a mean serum 25OHD of 62 nmol/L (N studies = 19, 95% CI 55–70). When restricting the meta-analysis to case–control studies (N studies = 16), the children with and without rickets had a mean serum 25OHD of 32 (95% CI 23–40) nmol/L and 64 nmol/L (95% CI 56–73) nmol/L, respectively. When restricting the meta-analysis to case reports, case series and trials, mean serum 25OHD in children with rickets was 17, 20, 26 nmol/L, respectively.
Eight studies were not meta-analyzable, because they did not report mean or median serum 25OHD. In one study [40], refugee children showing up at the hospital were screened for rickets. Of all the children screened, 28.5% had nutritional rickets, 40% had serum 25OHD < 30 nmol/L and 9% have serum 25OHD in the range 30–49 nmol/L. In another study [41], 47% of the infants had a serum 25OHD below 25 nmol/L and of those 72% had radiographic evidence of rickets. A case series [42] of children diagnosed with nutritional rickets found that 79% had serum 25OHD below 50 nmol/L. Another case series [43] of children diagnosed with nutritional rickets found that 62% had serum 25OHD below 25 nmol/L. A surveillance study [44] found that among children with serum 25OHD below 50 nmol/L, 77% had radiological changes associated with rickets.
Two trials [45, 46] compared 6-month-old exclusively breastfed infants of women who received vitamin D supplementation or no supplementation during postpartum. The first trial [45] found that 44% and 75% of children from non-supplemented mothers had serum 25OHD below 25 and 50 nmol/L, respectively; whereas 8% and 25% of children from vitamin D-supplemented mothers had serum 25OHD below 25 and 50 nmol/L, respectively. In the unsupplemented and supplemented groups, 3.4% and 3.6% of the children developed radiological rickets, respectively. In the second trial [46], the equivalent estimates for the development of radiological rickets in unsupplemented and supplemented children were 4% and 2%, respectively. One trial [47] found that at baseline, at 3–5 days of age, that 3% and 6% of infants with serum 25OHD < and > 27.5 nmol/L showed wrist ossification centers. At 6 months of age, after vitamin D supplementation with 100, 200 or 400 IU/d, none of the children showed any radiological signs of rickets [47].
IPD meta-analysis
Based on individual data and irrespective of dietary calcium intake (n = 930, mean 289 mg/d, median 230 mg/d), the serum 25OHD in 700 children with rickets ranged from non-detectable to 180 nmol/L, with a median of 23 nmol/L and mean of 29 nmol/L (95% CI 27–31). The distribution of the serum 25OHD in children with rickets is shown in Fig. 2. More than half (55%) of the children with rickets had serum 25OHD below 25 nmol/L, 62% below 30 nmol/L, 79% below 40 nmol/L, and 87% below 50 nmol/L. In 230 children without rickets, the median serum 25OHD was 57 nmol/L, with a mean of 62 nmol/L (95% CI 58–66).
The odds of having rickets increased exponentially as serum 25(OH)D concentrations decreased below 50 nmol/L, and dramatically so when concentrations fell below 30 nmol/L (see Fig. 3). The sensitivities and specificities of different serum 25OHD thresholds to detect rickets are shown in Fig. 4 and Supplemental Table 2. A sensitivity and specificity of 80% were reached at serum 25OHD concentrations of 42 and 38 nmol/L, respectively. The serum 25OHD threshold at which the sensitivity and specificity were maximized, i.e. the maximal Youden index, was at 40 nmol/L (sensitivity 79% and specificity 77%).
Including only children with adequate dietary calcium intake
When considering only the children with adequate calcium intakes (n = 640, mean 580 mg/d, median 522 mg/d), the odds of having rickets increased exponentially as serum 25(OH)D concentrations decreased below 60 nmol/L, and dramatically so when concentrations fell below ~ 25 nmol/L (see Fig. 5). The sensitivities and specificities of different serum 25OHD thresholds to detect rickets are shown in Fig. 6 and Supplemental Table 2. A sensitivity and specificity of 80% were reached at serum 25OHD concentrations of 32 and 28 nmol/L, respectively. The thresholds at which the sensitivity and specificity were maximized (i.e. Youden index) was 28 nmol/L. When including only studies with known adequate calcium intakes estimated from multiple 24 h recalls, the Youden index was at 33 nmol/L. When including studies with known adequate calcium intakes as well as assumed adequate calcium intakes (imputed), the Youden index was at 30 nmol/L.
Discussion
In terms of the establishment of a DRV for vitamin D for young children, identification and selection of an appropriate serum 25OHD threshold is critical. This serum 25OHD concentration should protect a majority of children against increased risk of nutritional rickets and thus form a basis for derivation of a recommended dietary intake which will allow young children to maintain serum 25OHD concentrations at or above this threshold. It is not intended as a clinical threshold diagnostic for rickets.
Expert authorities charged with the establishment of vitamin D recommendations have thus far generally relied on reported individual baseline serum 25OHD concentration data in case reports, and mean/median 25OHD concentration data from studies of other designs, without trying to meta-analyze data to set a serum 25OHD threshold [3, 12, 18]. For example, the Scientific Advisory Committee on Nutrition (SACN) in the UK concluded that individual and mean serum 25OHD concentrations of children with rickets were < 25 nmol/L in the majority of studies (44 included) in their DRV exercise in 2016 [18]. The US Institute of Medicine (IOM) in 2011 identified 13 studies in their DRV exercise, and while 6 studies reported mean or median serum 25OHD concentrations < 30 nmol/L in children with rickets, the remaining studies reported mean serum 25OHD concentrations > 30 nmol/L [range 36–50 nmol/L] [12]. The European Food Safety Authority (EFSA) expert panel concluded that there is no risk of vitamin D deficiency rickets with serum 25OHD concentrations at or above 50 nmol/L and adequate calcium intake [3]. In the present extensive systematic review, the meta-analysis of study-level data also showed that young children with radiologically confirmed rickets had a mean serum 25OHD concentration of 23 nmol/L (95% CI 19–27).
A key limitation in the interpretation of such study-level data is the fact that they could be confounded by dietary calcium, especially as many of the studies were from developing countries where calcium intakes may be low [12, 18]. Thus, whether the rickets in these studies was caused solely by vitamin D deficiency and/or by low calcium intake is not clear. The present work sought to address this key knowledge gap by obtaining IPD from those studies that measured calcium intake as well as serum 25OHD in children with rickets. This data enabled an analysis of sensitivities and specificities in relation to odds of rickets at different serum 25OHD thresholds, and consequently, the estimation of the maximal Youden index, which is a measure of the potential effectiveness of a biomarker and an index used for setting optimal thresholds on medical tests. The analysis suggested the serum 25OHD threshold at which the sensitivity and specificity were maximized, i.e. the maximal Youden index, was around 28 nmol/L in children with adequate calcium, whereas this increased to 40 nmol/L in the entire sample which included children with insufficient calcium intakes. The latter would be more reflective of the types of datasets that IOM, SACN and EFSA would have based their threshold decisions upon. If dietary calcium intake is low, and serum calcium concentrations decrease, the compensatory metabolic response is an accelerated conversion of 25OHD to 1,25-dihydroxyvitamin D (via parathyroid hormone), which normalizes serum calcium concentrations [18]. This increased 25OHD catabolism leads to an increased vitamin D requirement. The IOM have suggested that when calcium intakes are inadequate, vitamin D supplementation to the point of serum 25OHD concentration up to and beyond 75 nmol/L has no effect [12]. The present findings based on empirical data from young children with adequate calcium intakes are consistent with the suggestion by the IOM, as well as other agencies briefed with the development of vitamin D DRVs, that in the face of adequate calcium intake, the risk of nutritional rickets increases below a serum 25OHD concentration of 30 nmol/L and is minimal (although not absent) when serum 25(OH)D concentrations range between 30 and 50 nmol/L [3, 12, 18]. Another report that explored the interaction between 25OHD and calcium intake from a single study, also found that the risk of rickets increased below 40 nmol/L or even higher in children with the lowest calcium intakes [14].
Two major intertwined strengths of this review are the meta-analysis of IPD, to complement the study-level meta-analyses, and that differences in calcium intake could be accounted for and the analysis be restricted to children with adequate calcium intake. The comprehensive search of the literature for the relevant studies ensured that all those studies with measured calcium intakes were identified. IPD data for 25OHD was available for 65 studies (n = 930) out of 120 studies (n = 5412). IPD was requested from the 15 studies that reported having measured calcium intakes and was obtained from 10 of these studies. The comparison of sensitivities and specificities of different thresholds allowed the identification of an optimal minimum serum 25OHD threshold. The present work also emphasized rickets as confirmed radiologically, to reduce the risk of misdiagnosing children with or without rickets.
The limitations of this review were that, due to resource and time constraints, only one online database was searched systematically. However, to ensure no important and relevant studies were missed, the reference lists of other reviews were screened. Another limitation of this review, in common with all DRV exercises to-date, is the potential variability in the serum 25OHD measurement data amongst included studies that used different analytical methods. In fact, only two of the studies included reported participating in a vitamin D assay standardization program. The measurement of serum 25OHD can vary widely between assays and participation in a vitamin D assay standardization program is recommended [13]. In addition, it was not possible to assess whether there might be different serum 25OHD thresholds on the basis of ethnicity or ancestry because the majority of the studies were of dark-skinned participants. The available data was not reported in a sufficiently consistent matter to be able to take into account sun exposure and geographical location.
In conclusion, the present IPD-level meta-analyses suggest that a minimum serum 25OHD threshold of ~ 28 nmol/L and above would represent a low risk of nutritional rickets for the majority of children with an adequate calcium intake. However, a higher 25OHD threshold is likely necessary to prevent rickets in populations with low dietary calcium intakes, which includes the geographic areas of Africa and South Asia, where rickets remain widespread. This threshold while useful within a vitamin D DRV process, as indicative of the risk of disease, it is not intended as a clinical threshold diagnostic for rickets.
Data availability
The study-level data is available upon reasonable request to the corresponding author. Individual-level data can however not be shared.
References
Holick MF (2003) Vitamin D: a millenium perspective. J Cell Biochem 88:296–307. https://doi.org/10.1002/jcb.10338
Rosen CJ, Adams JS, Bikle DD et al (2012) The nonskeletal effects of vitamin D: an endocrine society scientific statement. Endocr Rev 33:456–492. https://doi.org/10.1210/er.2012-1000
EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) (2016) Dietary reference values for vitamin D. EFSA Journal 14:. https://doi.org/10.2903/j.efsa.2016.4547
Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements (2004) Vitamin and mineral requirements in human nutrition
. In: FAO/WHO nutrient requirements for children aged 0–36 months. https://www.who.int/groups/fao-who-nutrient-requirements-for-children-aged-0-36-months. Accessed 20 Sep 2022
Cashman KD (2015) Vitamin D: dietary requirements and food fortification as a means of helping achieve adequate vitamin D status. J Steroid Biochem Mol Biol 148:19–26. https://doi.org/10.1016/j.jsbmb.2015.01.023
Cashman KD, Ritz C, Carlin A, Kennedy M (2022) Vitamin D biomarkers for dietary reference intake development in children: a systematic review and meta-analysis. Am J Clin Nutr 115:544–558. https://doi.org/10.1093/ajcn/nqab357
Cashman KD, Kiely M (2011) Towards prevention of vitamin D deficiency and beyond: knowledge gaps and research needs in vitamin D nutrition and public health. Br J Nutr 106:1617–1627. https://doi.org/10.1017/S0007114511004995
Beauchesne AR, Cara KC, Krobath DM et al (2022) Vitamin D intakes and health outcomes in infants and preschool children: Summary of an evidence report. Ann Med 54:2278–2301. https://doi.org/10.1080/07853890.2022.2111602
World Health Organization (2019) Nutritional rickets: a review of disease burden, causes, diagnosis, prevention and treatment. World Health Organization, Geneva
Aul AJ, Fischer PR, O’Grady JS et al (2019) Population-based incidence of potentially life-threatening complications of hypocalcemia and the role of vitamin D deficiency. J Pediatr 211:98-104.e4. https://doi.org/10.1016/j.jpeds.2019.02.018
Ross A, Taylor C, Yaktine A (2011) Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. 5. Dietary Reference Intakes for Calcium and Vitamin D. Dietary Reference Intakes for Adequacy: Calcium and Vitamin D. National Academies Press (US), Washington (DC)
Sempos C, Binkley N (2020) 25-Hydroxyvitamin D assay standardisation and vitamin D guidelines paralysis. Public Health Nutr 23:1153–1164. https://doi.org/10.1017/S1368980019005251
Sempos CT, Durazo-Arvizu RA, Fischer PR et al (2021) Serum 25-hydroxyvitamin D requirements to prevent nutritional rickets in Nigerian children on a low-calcium diet—a multivariable reanalysis. Am J Clin Nutr 114:231–237. https://doi.org/10.1093/ajcn/nqab048
Bouillon R (2017) Comparative analysis of nutritional guidelines for vitamin D. Nat Rev Endocrinol 13:466–479. https://doi.org/10.1038/nrendo.2017.31
European Food Safety Authority (EFSA) (2017) Dietary Reference Values for nutrients Summary report. EFS3 14:. https://doi.org/10.2903/sp.efsa.2017.e15121
Stewart LA, Clarke M, Rovers M et al (2015) Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. JAMA 313:1657. https://doi.org/10.1001/jama.2015.3656
Scientific Advisory Committee on Nutrition (SACN) (2016) Vitamin D and Health
Munns CF, Shaw N, Kiely M et al (2016) Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab 101:394–415. https://doi.org/10.1210/jc.2015-2175
Cranney A, Horsley T, O’Donnell S, et al (2007) Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 1–235
Newberry SJ, Chung M, Shekelle PG, et al (2014) Vitamin D and Calcium: A Systematic Review of Health Outcomes (Update). Evid Rep Technol Assess (Full Rep) 1–929. https://doi.org/10.23970/AHRQEPCERTA217
Ohmann C, Banzi R, Canham S et al (2017) Sharing and reuse of individual participant data from clinical trials: principles and recommendations. BMJ Open 7:e018647. https://doi.org/10.1136/bmjopen-2017-018647
Higgins J, Green S (2019) Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration
PlotDigitizer software
Baroncelli GI, Bereket A, El Kholy M et al (2008) Rickets in the middle east: role of environment and genetic predisposition. J Clin Endocrinol Metab 93:1743–1750. https://doi.org/10.1210/jc.2007-1413
Dabas A, Dabas V, Dabla PK, et al (2022) Daily versus weekly oral vitamin D3 therapy for nutritional rickets in Indian children: A randomized controlled open-label trial. Br J Nutr 1–23. https://doi.org/10.1017/S0007114522001477
El Kholy M, Elsedfy H, Fernández-Cancio M et al (2017) Nutritional rickets: vitamin D, calcium, and the genetic make-up. Pediatr Res 81:356–363. https://doi.org/10.1038/pr.2016.222
Graff M, Thacher TD, Fischer PR et al (2004) Calcium absorption in Nigerian children with rickets. Am J Clin Nutr 80:1415–1421. https://doi.org/10.1093/ajcn/80.5.1415
Jones KDJ, Hachmeister CU, Khasira M et al (2018) Vitamin D deficiency causes rickets in an urban informal settlement in Kenya and is associated with malnutrition. Matern Child Nutr 14:e12452. https://doi.org/10.1111/mcn.12452
Oramasionwu GE, Thacher TD, Pam SD et al (2008) Adaptation of calcium absorption during treatment of nutritional rickets in Nigerian children. Br J Nutr 100:387–392. https://doi.org/10.1017/S0007114507901233
Thacher TD, Fischer PR, Pettifor JM et al (2000) Case-control study of factors associated with nutritional rickets in Nigerian children. J Pediatr 137:367–373. https://doi.org/10.1067/mpd.2000.107527
Thacher TD, Obadofin MO, O’Brien KO, Abrams SA (2009) The Effect of vitamin D2 and vitamin D3 on intestinal calcium absorption in nigerian children with rickets. J Clin Endocrinol Metab 94:3314–3321. https://doi.org/10.1210/jc.2009-0018
Thacher TD, Aliu O, Griffin IJ et al (2009) Meals and dephytinization affect calcium and zinc absorption in Nigerian children with rickets. J Nutr 139:926–932. https://doi.org/10.3945/jn.108.101030
Thacher TD, Fischer PR, Pettifor JM (2014) Vitamin D treatment in calcium-deficiency rickets: a randomised controlled trial. Arch Dis Child 99:807–811. https://doi.org/10.1136/archdischild-2013-305275
Aggarwal V, Seth A, Aneja S et al (2012) Role of calcium deficiency in development of nutritional rickets in indian children: a case control study. J Clin Endocrinol Metab 97:3461–3466. https://doi.org/10.1210/jc.2011-3120
Aggarwal V, Seth A, Marwaha RK et al (2013) Management of nutritional rickets in indian children: a randomized controlled trial. J Trop Pediatr 59:127–133. https://doi.org/10.1093/tropej/fms058
Ahmed S, Goldberg GR, Raqib R et al (2020) Aetiology of nutritional rickets in rural Bangladeshi children. Bone 136:115357. https://doi.org/10.1016/j.bone.2020.115357
Balasubramanian K, Rajeswari J, Gulab, et al (2003) Varying Role of vitamin D deficiency in the etiology of rickets in young children vs. adolescents in Northern India. J Trop Pediatr 49:201–206. https://doi.org/10.1093/tropej/49.4.201
Thacher TD, Ighogboja SI, Fischer PF (1997) Rickets without vitamin D deficiency in Nigerian children. Ambulatory Child Health 56–64
Acoglu EA, Yucel H, Polat E, Senel S (2020) Nutritional rickets with severe complications in syrian and iraqi refugee children. Indian Pediatr 57:64–66. https://doi.org/10.1007/s13312-020-1706-0
Jain V, Gupta N, Kalaivani M et al (2011) Vitamin D deficiency in healthy breastfed term infants at 3 months & their mothers in India: seasonal variation & determinants. Indian J Med Res 133:267–273
Lazol JP, Cakan N, Kamat D (2008) 10-year case review of nutritional rickets in children’s hospital of Michigan. Clin Pediatr (Phila) 47:379–384. https://doi.org/10.1177/0009922807311397
Shah BR, Finberg L (1994) Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr 125:487–490. https://doi.org/10.1016/S0022-3476(05)83303-7
Wheeler BJ, Dickson NP, Houghton LA et al (2015) Incidence and characteristics of vitamin D deficiency rickets in New Zealand children: a New Zealand paediatric surveillance unit study. Aust N Z J Public Health 39:380–383. https://doi.org/10.1111/1753-6405.12390
Naik P, Faridi MMA, Batra P, Madhu SV (2017) Oral Supplementation of parturient mothers with vitamin D and its effect on 25OHD status of exclusively breastfed infants at 6 months of age: a double-blind randomized placebo controlled trial. Breastfeed Med 12:621–628. https://doi.org/10.1089/bfm.2016.0164
Trivedi M, Faridi MMA, Aggarwal A et al (2020) Oral Vitamin D Supplementation to Mothers During Lactation—Effect of 25(OH)D Concentration on Exclusively Breastfed Infants at 6 Months of Age: A Randomized Double-Blind Placebo-Controlled Trial. Breastfeed Med 15:237–245. https://doi.org/10.1089/bfm.2019.0102
Specker BL, Ho ML, Oestreich A et al (1992) Prospective study of vitamin D supplementation and rickets in China. J Pediatr 120:733–739. https://doi.org/10.1016/s0022-3476(05)80236-7
National Health and Medical Research Council (Australia) NZ, Ministry of Health, Australia, Department of Health and Ageing (2006) Nutrient reference values for Australia and New Zealand. National Health and Medical Research Council, Canberra, A.C.T.
From Indian Academy of Pediatrics ‘Guideline for Vitamin D and Calcium in Children’ Committee., Khadilkar A, Khadilkar V, et al (2017) Prevention and Treatment of Vitamin D and Calcium Deficiency in Children and Adolescents: Indian Academy of Pediatrics (IAP) Guidelines. Indian Pediatr 54:567–573. https://doi.org/10.1007/s13312-017-1070-x
Nordic Council of Ministers NC of M (2008) Nordic Nutrition Recommendations 2012. Integrating nutrition and physical activity 5:1–3. https://doi.org/10.6027/Nord2014-002
Al-Atawi MS, Al-Alwan IA, Al-Mutair AN et al (2009) Epidemiology of nutritional rickets in children. Saudi J Kidney Dis Transpl 20:260–265
Alouf B, Grigalonis M (2005) Incidental finding of vitamin-D deficient rickets in an otherwise healthy infant–a reappraisal of current vitamin-D supplementation guidelines. J Natl Med Assoc 97:1170–1173
Amirlak I, Al Dhaheri W, Narchi H (2008) Dilated cardiomyopathy secondary to nutritional rickets. Ann Trop Paediatr 28:227–230. https://doi.org/10.1179/146532808X335688
Arnaud SB, Stickler GB, Haworth JC (1976) Serum 25-Hydroxyvitamin D in Infantile Rickets. Pediatrics 57:221
Ashraf S (2002) The prevalence of rickets among non-Caucasian children. Archives of Disease in Childhood 87:263-a-264. https://doi.org/10.1136/adc.87.3.263-a
Balkan C, Ersoy B, Nese N (2005) Myelofibrosis Associated with Severe Vitamin D Deficiency Rickets. J Int Med Res 33:356–359. https://doi.org/10.1177/147323000503300311
Beck-Nielsen SS, Jensen TK, Gram J et al (2009) Nutritional rickets in Denmark: a retrospective review of children’s medical records from 1985 to 2005. Eur J Pediatr 168:941–949. https://doi.org/10.1007/s00431-008-0864-1
Bereket A, Cesur Y, Ozkan B et al (2010) Circulating Insulin-like Growth Factor Binding Protein-4. JCRPE 2:17–20. https://doi.org/10.4274/jcrpe.v2i1.17
Bétend B, David L, Evrard A et al (1981) A patient with nutritional rickets stage 1 or partial hypoparathyroidism. Acta Paediatr 70:259–260. https://doi.org/10.1111/j.1651-2227.1981.tb05553.x
Bhimma R, Pettifor JM, Coovadia HM et al (1995) Rickets in black children beyond infancy in Natal. S Afr Med J 85:668–672
Blok BH, Grant CC, McNeil AR, Reid IR (2000) Characteristics of children with florid vitamin D deficient rickets in the Auckland region in 1998. N Z Med J 113:374–376
Blond MH, Gold F, Pierre F et al (1997) Nutritional fetal rickets. A case report. J Gynecol Obstet Biol Reprod (Paris) 26:834–836
Bloom E, Klein EJ, Shushan D, Feldman KW (2004) Variable Presentations of Rickets in Children in the Emergency Department. Pediatr Emerg Care 20:126–130. https://doi.org/10.1097/01.pec.0000113889.10140.7a
Brinsmead T, Frawley K, Conwell LS (2011) Images in pediatric endocrinology: vitamin D deficiency rickets and other nutritional deficiencies in a 12-month-old infant. Journal of Pediatric Endocrinology and Metabolism 24:. https://doi.org/10.1515/jpem.2011.085
Chatterjee D, S. Swamy MK, Gupta V, et al (2017) Safety and Efficacy of Stosstherapy in Nutritional Rickets. Jcrpe 9:63–69. https://doi.org/10.4274/jcrpe.3557
Chehade H, Girardin E, Rosato L et al (2011) Acute life-threatening presentation of vitamin D deficiency rickets. J Clin Endocrinol Metab 96:2681–2683. https://doi.org/10.1210/jc.2011-1112
Chuang L-H, Tung Y-C, Liu S-Y et al (2018) Nutritional rickets in Taiwanese children: experiences at a single center. J Formos Med Assoc 117:583–587. https://doi.org/10.1016/j.jfma.2017.08.013
Curtis JA, Kooh SW, Fraser D, Greenberg ML (1983) Nutritional rickets in vegetarian children. Can Med Assoc J 128:150–152
Dawodu A, Agarwal M, Sankarankutty M et al (2006) Higher prevalence of vitamin d deficiency in mothers of rachitic than nonrachitic children. J Pediatr 147:109–111. https://doi.org/10.1016/j.jpeds.2005.03.001
DeLucia MC, Mitnick ME, Carpenter TO (2003) Nutritional rickets with normal circulating 25-hydroxyvitamin D: a call for reexamining the role of dietary calcium intake in north american infants. J Clin Endocrinol Metab 88:3539–3545. https://doi.org/10.1210/jc.2002-021935
Duplechin RY, Nadkarni M, Schwartz RP (1999) Hypocalcemic tetany in a toddler with undiagnosed rickets. Ann Emerg Med 34:399–402. https://doi.org/10.1016/S0196-0644(99)70137-X
Elidrissy ATH, Sedrani SH, Lawson DEM (1984) Vitamin D deficiency in mothers of rachitic infants. Calcif Tissue Int 36:266–268. https://doi.org/10.1007/BF02405328
Elhassan Elidrissy ATH, Sandokji AM, Al-Magamsi MSF et al (2012) Nutritional rickets in Almadinah Almunawwarah: presentation and associated factors. J Taibah Univ Med Sci 7:35–40. https://doi.org/10.1016/j.jtumed.2012.07.002
Elzouki AY, Markestad T, Elgarrah M et al (1989) Serum concentrations of vitamin D metabolites in rachitic libyan children. J Pediatr Gastroenterol Nutr 9:507–512. https://doi.org/10.1097/00005176-198911000-00019
Eren E, Abuhandan M, Güzel B, et al (2015) A Treatable Cause of Cardiomyopathy: Vitamin D Deficiency. jcp 13:143–146. https://doi.org/10.4274/jcp.08370
Estrade S, Majorel C, Tahhan N et al (2017) Rachitisme carentiel sévère du nourrisson : de nouveau d’actualité. Arch Pediatr 24:737–742. https://doi.org/10.1016/j.arcped.2017.05.004
Eugster EA, Sane KS, Brown DM (1996) Minnesota rickets. Need for a policy change to support vitamin D supplementation. Minn Med 79:29–32
Fidan O, Alp H, Orbak Z, Karakelleoglu C (2015) Radiological score in malnourished and well-nourished children with active rickets. West Indian Med J. https://doi.org/10.7727/wimj.2015.008
Flot C, Porquet-Bordes V, Bacchetta J et al (2020) Demographic characteristics, risk factors, and presenting features of children with symptomatic nutritional rickets: a french series. Horm Res Paediatr 93:304–312. https://doi.org/10.1159/000511419
Gad K, Khan M, Mahmood K (2014) Afebrile seizures and electrocardiography abnormality: an unusual presentation of nutritional rickets. Scott Med J 59:e16–e19. https://doi.org/10.1177/0036933014547307
Garabédian M, Vainsel M, Mallet E et al (1983) Circulating vitamin D metabolite concentrations in children with nutritional rickets. J Pediatr 103:381–386. https://doi.org/10.1016/S0022-3476(83)80407-7
Ginat-Israeli T, Dranitzki Z, Straus U (2003) Nutritional rickets in infants immigrating to Israel from Ethiopia. Isr Med Assoc J 5:291–292
Hoecker CC, Kanegaye JT (2002) First place winner. J Emerg Med 23:367–370. https://doi.org/10.1016/S0736-4679(02)00570-X
Holick MF, Lim R, Dighe AS (2009) Case 3–2009: A 9-Month-Old Boy with Seizures. N Engl J Med 360:398–407. https://doi.org/10.1056/NEJMcpc0807821
Khan AK, Ali M, Anwar A et al (2020) Treatment Outcome of Oral Versus Injectable Vitamin D in Nutritional Rickets in Children. Pakistan Journal of Medical and Health Sciences 14:754–759
Kosecik M, Ertas T (2007) Dilated cardiomyopathy due to nutritional vitamin D deficiency rickets. Pediatr Int 49:397–399. https://doi.org/10.1111/j.1442-200X.2007.02367.x
Kreiter SR, Schwartz RP, Kirkman HN et al (2000) Nutritional rickets in African American breast-fed infants. J Pediatr 137:153–157. https://doi.org/10.1067/mpd.2000.109009
Kruse K (2000) Aktuelle Aspekte der Vitamin-D-Mangel-Rachitis. Monatsschrift Kinderheilkunde 148:588–595. https://doi.org/10.1007/s001120050600
Kubota T, Kotani T, Miyoshi Y et al (2006) A spectrum of clinical presentations in seven japanese patients with vitamin D deficiency. Clin Pediatr Endocrinol 15:23–28. https://doi.org/10.1297/cpe.15.23
Ladhani S, Srinivasan L, Buchanan C, Allgrove J (2004) Presentation of vitamin D deficiency. Arch Dis Child 89:781–784. https://doi.org/10.1136/adc.2003.031385
Lautatzis M-E, Sharma A, Rodd C (2019) A closer look at rickets and vitamin D deficiency in Manitoba: The tip of the iceberg. Paediatr Child Health 24:179–184. https://doi.org/10.1093/pch/pxy105
Lemoine A, Giabicani E, Lockhart V et al (2020) Case report of nutritional rickets in an infant following a vegan diet. Arch Pediatr 27:219–222. https://doi.org/10.1016/j.arcped.2020.03.008
Lin EL, Gottesman GS, McAlister WH et al (2020) Healing of vitamin D deficiency rickets complicating hypophosphatasia suggests a role beyond circulating mineral sufficiency for vitamin D in musculoskeletal health. Bone 136:115322. https://doi.org/10.1016/j.bone.2020.115322
Machiels F, De Maeseneer M, Van Snick A et al (1995) A rare cause of rickets in a young child. J Belge Radiol 78:276–277
Markestad T, Kolmannskog S, Arntzen E et al (1984) Serum concentrations of vitamin D metabolites in exclusively breast-fed infants at 70° North. Acta Paediatr 73:29–32. https://doi.org/10.1111/j.1651-2227.1984.tb09893.x
Meyer HE, Skram K, Berge IA et al (2017) Nutritional rickets in Norway: a nationwide register-based cohort study. BMJ Open 7:e015289. https://doi.org/10.1136/bmjopen-2016-015289
Mittal H, Rai S, Shah D et al (2014) 300,000 IU or 600,000 IU of oral vitamin D3 for treatment of nutritional rickets: A randomized controlled trial. Indian Pediatr 51:265–272. https://doi.org/10.1007/s13312-014-0399-7
Mittal M, Yadav V, Khadgawat R et al (2018) Efficacy and safety of 90,000 IU versus 300,000 IU single dose oral Vitamin D in nutritional rickets: A randomized controlled trial. Indian J Endocr Metab 22:760. https://doi.org/10.4103/ijem.IJEM_84_18
Molla AM, Badawi MH, Al-Yaish S et al (2000) Risk factors for nutritional rickets among children in Kuwait. Pediatr Int 42:280–284. https://doi.org/10.1046/j.1442-200x.2000.01230.x
Moncrieff M, Fadahunsi TO (1974) Congenital rickets due to maternal vitamin D deficiency. Arch Dis Child 49:810–811. https://doi.org/10.1136/adc.49.10.810
Mondal K, Seth A, Marwaha RK et al (2014) A randomized controlled trial on safety and efficacy of single intramuscular versus staggered oral dose of 600 000IU vitamin D in treatment of nutritional rickets. J Trop Pediatr 60:203–210. https://doi.org/10.1093/tropej/fmt105
Mughal MZ, Salama H, Greenaway T et al (1999) Lesson of the week: florid rickets associated with prolonged breast feeding without vitamin D supplementation. BMJ 318:39–40. https://doi.org/10.1136/bmj.318.7175.39
Abdullah M, Bigras JL, McCrindle BW, Mustafa A (1999) Dilated cardiomyopathy as a first sign of nutritional vitamin D deficiency rickets in infancy. Can J Cardiol 15:699–701
Oginni LM, Worsfold M, Oyelami OA et al (1996) Etiology of rickets in Nigerian children. J Pediatr 128:692–694. https://doi.org/10.1016/S0022-3476(96)80137-5
Oginni LM (2003) Radiological and biochemical resolution of nutritional rickets with calcium * COMMENTARY. Arch Dis Child 88:812–817. https://doi.org/10.1136/adc.88.9.812
Ojeda L, Ros MA, Tomás C et al (2010) Raquitismo carencial en un lactante de 5 meses. Patología poco común en nuestro medio. Anales de Pediatría 72:225–227. https://doi.org/10.1016/j.anpedi.2009.11.022
Olgun H, Ceviz N, Ozkan B (2003) A case of dilated cardiomyopathy due to nutritional vitamin D deficiency rickets. Turk J Pediatr 45:152–154
Orbak Z, Hatun S, Özkan B et al (2005) Rickets in early infancy: The characteristic features. Cocuk Sagligi ve Hastaliklari Dergisi 48:8–13
Ozkan B, Doneray H, Karacan M et al (2009) Prevalence of vitamin D deficiency rickets in the eastern part of Turkey. Eur J Pediatr 168:95–100. https://doi.org/10.1007/s00431-008-0821-z
Ozkan B, Doneray H, Keskin H (2009) The Effect of Vitamin D Treatment on Serum Adiponectin Levels in Children with Vitamin D Deficiency Rickets. JCRPE 1:262–265. https://doi.org/10.4274/jcrpe.v1i6.262
Pearson D, Barreto-Chang O, Shepard WE et al (2010) Vitamin D-deficient rickets in a child with cow’s milk allergy. Nutr Clin Pract 25:394–398. https://doi.org/10.1177/0884533610374199
Pedersen P, Michaelsen K, Mølgaard C (2007) Children with nutritional rickets referred to hospitals in Copenhagen during a 10-year period. Acta Paediatr 92:87–90. https://doi.org/10.1111/j.1651-2227.2003.tb00475.x
Pedrosa C, Ferraria N, Limbert C, Lopes L (2013) Hypovitaminosis D and severe hypocalcaemia: the rebirth of an old disease. Case Reports 2013:bcr2012007406–bcr2012007406. https://doi.org/10.1136/bcr-2012-007406
Perez-Rossello JM, Feldman HA, Kleinman PK et al (2012) Rachitic changes, demineralization, and fracture risk in healthy infants and toddlers with vitamin D deficiency. Radiology 262:234–241. https://doi.org/10.1148/radiol.11110358
Pietrek J, Otto-Buczkowska E, Kokot F et al (1980) Concentration of 25-hydroxyvitamin D in serum of infants under the intermittent high-dose vitamin D3 prophylactic treatment. Arch Immunol Ther Exp (Warsz) 28:805–814
Prentice A, Ceesay M, Nigdikar S et al (2008) FGF23 is elevated in Gambian children with rickets. Bone 42:788–797. https://doi.org/10.1016/j.bone.2007.11.014
Rajah J, Jubeh JA, Haq A et al (2008) Nutritional rickets and z scores for height in the United Arab Emirates: To D or not to D? Pediatr Int 50:424–428. https://doi.org/10.1111/j.1442-200X.2008.02700.x
Rajah J, Abdel-Wareth L, Haq A (2010) Failure of alphacalcidol (1α-hydroxyvitamin D3) in treating nutritional rickets and the biochemical response to ergocalciferol. J Steroid Biochem Mol Biol 121:273–276. https://doi.org/10.1016/j.jsbmb.2010.03.075
Ramavat LG (1999) Vitamin D deficiency rickets at birth in Kuwait. Indian J Pediatr 66:37–43. https://doi.org/10.1007/BF02752349
Robinson PD (2006) The re-emerging burden of rickets: a decade of experience from Sydney. Arch Dis Child 91:564–568. https://doi.org/10.1136/adc.2004.069575
Sakamoto Y, Ishijima M, Kinoshita M et al (2018) Association between leg bowing and serum alkaline phosphatase level regardless of the presence of a radiographic growth plate abnormality in pediatric patients with genu varum. J Bone Miner Metab 36:447–453. https://doi.org/10.1007/s00774-017-0851-6
Salama HM, El-Dayem SA, Yousef H et al (2010) The effects of L-thyroxin replacement therapy on bone minerals and body composition in hypothyroid children. Arch Med Sci 6:407–413. https://doi.org/10.5114/aoms.2010.14264
Saluja RK, Dewan P, Gomber S et al (2022) Low dose depot oral vitamin D 3 v. daily oral vitamin D 3 for treating nutritional rickets: a randomised clinical trial. Br J Nutr 127:1778–1783. https://doi.org/10.1017/S0007114521002713
Shah M, Salhab N, Patterson D, Seikaly MG (2000) Nutritional rickets still afflict children in north Texas. Tex Med 96:64–68
Shaikh U, Alpert PT (2006) Nutritional Rickets in Las Vegas, Nevada. Journal of Pediatric Endocrinology and Metabolism 19:. https://doi.org/10.1515/JPEM.2006.19.3.209
Sodri NI, Mohamed-Yassin M-S, Mohd Nor NS, Ismail IA (2021) Rickets Due to Severe Vitamin D and Calcium Deficiency During the COVID-19 Pandemic in Malaysia. Am J Case Rep 22:. https://doi.org/10.12659/AJCR.934216
Soliman AT, Al Khalaf F, Alhemaidi N et al (2008) Linear growth in relation to the circulating concentrations of insulin-like growth factor I, parathyroid hormone, and 25-hydroxy vitamin D in children with nutritional rickets before and after treatment: endocrine adaptation to vitamin D deficiency. Metabolism 57:95–102. https://doi.org/10.1016/j.metabol.2007.08.011
Soliman AT, El-Dabbagh M, Adel A et al (2010) Clinical responses to a mega-dose of vitamin D3 in infants and toddlers with vitamin D deficiency rickets. J Trop Pediatr 56:19–26. https://doi.org/10.1093/tropej/fmp040
Spence JT, Serwint JR (2004) Secondary Prevention of Vitamin D-Deficiency Rickets. Pediatrics 113:e70–e72. https://doi.org/10.1542/peds.113.1.e70
Stevens RL, Lyon C (2009) Nutritional vitamin D deficiency: a case report. Cases J 2:7000. https://doi.org/10.1186/1757-1626-2-7000
Thacher TD, Fischer PR, Pettifor JM et al (1999) A Comparison of Calcium, Vitamin D, or Both for Nutritional Rickets in Nigerian Children. N Engl J Med 341:563–568. https://doi.org/10.1056/NEJM199908193410803
Thacher TD, Fischer PR, Isichei CO, Pettifor JM (2006) Early response to vitamin D2 in children with calcium deficiency rickets. J Pediatr 149:840–844. https://doi.org/10.1016/j.jpeds.2006.08.070
Thacher TD, Fischer PR, Obadofin MO et al (2010) Comparison of metabolism of vitamins D2 and D3 in children with nutritional rickets. J Bone Miner Res 25:1988–1995. https://doi.org/10.1002/jbmr.99
Thacher TD, Fischer PR, Isichei CO et al (2012) Prevention of nutritional rickets in Nigerian children with dietary calcium supplementation. Bone 50:1074–1080. https://doi.org/10.1016/j.bone.2012.02.010
Thacher TD, Fischer PR, Tebben PJ et al (2013) Increasing incidence of nutritional rickets: a population-based study in olmsted county, Minnesota. Mayo Clin Proc 88:176–183. https://doi.org/10.1016/j.mayocp.2012.10.018
Thacher TD, Bommersbach TJ, Pettifor JM et al (2015) Comparison of limestone and ground fish for treatment of nutritional rickets in children in Nigeria. J Pediatr 167:148-154.e1. https://doi.org/10.1016/j.jpeds.2015.02.008
Train JJ, Yates RW, Sury MR (1995) Hypocalcaemic stridor and infantile nutritional rickets. BMJ 310:48–49. https://doi.org/10.1136/bmj.310.6971.48
Uday S, Fratzl-Zelman N, Roschger P et al (2018) Cardiac, bone and growth plate manifestations in hypocalcemic infants: revealing the hidden body of the vitamin D deficiency iceberg. BMC Pediatr 18:183. https://doi.org/10.1186/s12887-018-1159-y
Valério M, Pimentel Marcos S, Santos C, Leiria MJ (2015) Raquitismo: Ressurgimento do Passado. Acta Med Port 28:263. https://doi.org/10.20344/amp.5520
Vanstone MB, Oberfield SE, Shader L et al (2012) Hypercalcemia in Children Receiving Pharmacologic Doses of Vitamin D. Pediatrics 129:e1060–e1063. https://doi.org/10.1542/peds.2011-1663
Vierucci F, Del Pistoia M, Randazzo E et al (2017) The Spectrum of Vitamin D Deficiency: Description of a Family. Exp Clin Endocrinol Diabetes 125:478–484. https://doi.org/10.1055/s-0043-109699
Vuletic B, Markovic S, Igrutinovic Z et al (2016) Case report of an infant with severe vitamin D deficiency rickets manifested as hypocalcemic seizures. Srp Arh Celok Lek 144:90–93. https://doi.org/10.2298/SARH1602090V
Walter C, Muñoz-Santanach D, Marín del Barrio S et al (2010) Hipocalcemia sintomática secundaria a raquitismo carencial. Presentación de dos casos clínicos. Anales de Pediatría 72:343–346. https://doi.org/10.1016/j.anpedi.2009.12.014
Weinstein M (2003) A child with vitamin D deficiency rickets and suppurative arthritis. Pediatr Infect Dis J 22:290–291. https://doi.org/10.1097/00006454-200303000-00022
Williams AL, Cox J, Gordon CM (2008) Rickets in an Otherwise Healthy 11-Month-Old. Clin Pediatr (Phila) 47:409–412. https://doi.org/10.1177/0009922807310932
Yener E, Çoker C, Cura A et al (1995) Lymphocyte subpopulations in children with vitamin D deficient rickets. Pediatr Int 37:500–502. https://doi.org/10.1111/j.1442-200X.1995.tb03362.x
Yu JW, Pekeles G, Legault L, McCusker CT (2006) Milk allergy and vitamin D deficiency rickets: a common disorder associated with an uncommon disease. Ann Allergy Asthma Immunol 96:615–619. https://doi.org/10.1016/S1081-1206(10)63558-2
Acknowledgements
We would like to sincerely thank Dr Jason Montez for the database searches and the FAO/WHO expert group on nutrient requirements for children aged 0–36 months for their valuable input. We would like to thank the authors of the studies who provided individual data, more specifically (in alphabetical order), Giampiero Baroncelli (on behalf of the Research Unit on Rickets of the European Society for Pediatric Endocrinology, with Abdullah Bereket, Mohamed Salah El Kholy, Laura Audì Parera, Yasar Cesur, Yuzuncu Yil Behzat Ozkan, Mona Rashad, Monica Fernandez-Cancio, Yoseph Weisman, Giuseppe Saggese, Ze’ev Hochberg. Giampiero Baroncelli is the Representative of ERN-BOND. This work is generated within the European Reference Network for Rare Bone Diseases.), Aashima Dabas, Heba Hassan Elsedfy (with Laura Audí Parera and Mohamed Salah el Kholy), Kelsey Jones, and Tom Thacher.
Funding
Funding for this work was provided to MRL as an independent consultant by the World Health Organization (WHO). The other co-authors did not receive funding for this work.
Author information
Authors and Affiliations
Contributions
MRL designed the research protocol. MRL screened the studies, extracted the data, and conducted the analyses. KDC acted as the second reviewer. TDT, AD, GB and HHE provided individual participant data from studies. KDC provided inputs for the analyses. MRL wrote the manuscript. KDC, TDT, AD, GB, and HHE provided inputs on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rios-Leyvraz, M., Thacher, T.D., Dabas, A. et al. Serum 25-hydroxyvitamin D threshold and risk of rickets in young children: a systematic review and individual participant data meta-analysis to inform the development of dietary requirements for vitamin D. Eur J Nutr 63, 673–695 (2024). https://doi.org/10.1007/s00394-023-03299-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03299-2