Abstract
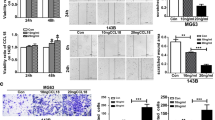
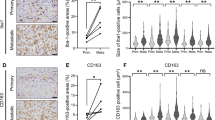
Tumor-associated macrophages (TAMs) play an important role in tumor growth and metastasis. However, the involvement of TAMs infiltration in pulmonary osteosarcoma (OS) metastasis remains poorly understood. Therefore, the effect of OS cells on macrophages migration was investigated by in vivo and in vitro experiments to evaluate the infiltration and mechanism of TAMs in pulmonary OS metastases. The results showed that the zinc finger protein ZIM3 was upregulated in OS cells than in osteoblasts and activated the expression of CCL25, which subsequently promoted the migration of M2 macrophages. CCL25 or ZIM3 silencing in OS cells inhibited the infiltration of M2 macrophages and the formation of pulmonary metastatic nodules in a mouse model of pulmonary OS metastasis and prolonged the survival of the mice. Furthermore, bioinformatics analyses revealed that CCL25 and ZIM3 expressions are negatively correlated with the prognosis of OS patients. In conclusion, this study found that a large number of M2 TAMs were recruited into pulmonary metastatic nodules of OS through the activation of the ZIM3-CCL25 axis in OS cells, thereby facilitating OS metastasis. Therefore, the suppression of ZIM3-CCL25-induced recruitment of M2 TAMs to the metastatic sites might be considered as a therapeutic approach to inhibit the growth of pulmonary OS metastases.






Similar content being viewed by others
References
Casali PG, Bielack S, Abecassis N et al (2018) Bone sarcomas: ESMO-PaedCan-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 29:iv79–iv95. https://doi.org/10.1093/annonc/mdy310
Alexander JH, Binitie OT, Letson GD, Joyce DM (2021) Osteosarcoma: an evolving understanding of a complex disease. J Am Acad Orthop Surg 29:e993–e1004. https://doi.org/10.5435/jaaos-d-20-00838
Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R (2018) Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther 18:39–50. https://doi.org/10.1080/14737140.2018.1413939
Meazza C, Scanagatta P (2016) Metastatic osteosarcoma: a challenging multidisciplinary treatment. Expert Rev Anticancer Ther 16:543–556. https://doi.org/10.1586/14737140.2016.1168697
Huang J, Li J, Zheng S et al (2020) Tumor microenvironment characterization identifies two lung adenocarcinoma subtypes with specific immune and metabolic state. Cancer Sci 111:1876–1886. https://doi.org/10.1111/cas.14390
Zhang Y, Zhao Y, Li Q, Wang Y (2021) Macrophages, as a promising strategy to targeted treatment for colorectal cancer metastasis in tumor immune microenvironment. Front Immunol 12:685978. https://doi.org/10.3389/fimmu.2021.685978
Mills CD, Lenz LL, Harris RA (2016) A breakthrough: macrophage-directed cancer immunotherapy. Cancer Res 76:513–516. https://doi.org/10.1158/0008-5472.Can-15-1737
Li X, Liu R, Su X, Pan Y, Han X, Shao C, Shi Y (2019) Harnessing tumor-associated macrophages as aids for cancer immunotherapy. Mol Cancer 18:177. https://doi.org/10.1186/s12943-019-1102-3
Bao X, Shi R, Zhao T, Wang Y, Anastasov N, Rosemann M, Fang W (2021) Integrated analysis of single-cell RNA-seq and bulk RNA-seq unravels tumour heterogeneity plus M2-like tumour-associated macrophage infiltration and aggressiveness in TNBC. Cancer Immunol Immunother 70:189–202. https://doi.org/10.1007/s00262-020-02669-7
Nam SH, Kim D, Lee D et al (2018) Lysyl-tRNA synthetase-expressing colon spheroids induce M2 macrophage polarization to promote metastasis. J Clin Invest 128:5034–5055. https://doi.org/10.1172/jci99806
Lin Y, Xu J, Lan H (2019) Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol 12:76. https://doi.org/10.1186/s13045-019-0760-3
Wu JY, Huang TW, Hsieh YT et al (2020) Cancer-Derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell 77:213–27.e5. https://doi.org/10.1016/j.molcel.2019.10.023
Schmall A, Al-Tamari HM, Herold S et al (2015) Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J Respir Crit Care Med 191:437–447. https://doi.org/10.1164/rccm.201406-1137OC
Huang YK, Wang M, Sun Y, Di Costanzo N, Mitchell C, Achuthan A, Hamilton JA, Busuttil RA, Boussioutas A (2019) Macrophage spatial heterogeneity in gastric cancer defined by multiplex immunohistochemistry. Nat Commun 10:3928. https://doi.org/10.1038/s41467-019-11788-4
Loyher PL, Hamon P, Laviron M et al (2018) Macrophages of distinct origins contribute to tumor development in the lung. J Exp Med 215:2536–2553. https://doi.org/10.1084/jem.20180534
Steenbrugge J, Breyne K, Demeyere K et al (2018) Anti-inflammatory signaling by mammary tumor cells mediates prometastatic macrophage polarization in an innovative intraductal mouse model for triple-negative breast cancer. J Exp Clin Cancer Res 37:191. https://doi.org/10.1186/s13046-018-0860-x
Chen Y, Zhang S, Wang Q, Zhang X (2017) Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol 10:36. https://doi.org/10.1186/s13045-017-0408-0
Huang R, Wang S, Wang N et al (2020) CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis 11:234. https://doi.org/10.1038/s41419-020-2435-y
Liu C, Yao Z, Wang J et al (2020) Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ 27:1765–1781. https://doi.org/10.1038/s41418-019-0460-0
Wang N, Liu W, Zheng Y et al (2018) CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis 9:880. https://doi.org/10.1038/s41419-018-0876-3
Wang D, Sun H, Wei J, Cen B, DuBois RN (2017) CXCL1 Is Critical for Premetastatic niche formation and metastasis in colorectal cancer. Cancer Res 77:3655–3665. https://doi.org/10.1158/0008-5472.Can-16-3199
Li R, Zhou R, Wang H et al (2019) Gut microbiota-stimulated cathepsin K secretion mediates TLR4-dependent M2 macrophage polarization and promotes tumor metastasis in colorectal cancer. Cell Death Differ 26:2447–2463. https://doi.org/10.1038/s41418-019-0312-y
Trikha P, Sharma N, Pena C et al (2016) E2f3 in tumor macrophages promotes lung metastasis. Oncogene 35:3636–3646. https://doi.org/10.1038/onc.2015.429
Mi X, Xu R, Hong S, Xu T, Zhang W, Liu M (2020) M2 Macrophage-derived exosomal lncRNA AFAP1-AS1 and MicroRNA-26a affect cell migration and metastasis in esophageal cancer. Mol Therapy Nucleic acids 22:779–790. https://doi.org/10.1016/j.omtn.2020.09.035
Wu J, Gao W, Tang Q et al (2021) M2 macrophage-derived exosomes facilitate HCC metastasis by transferring αM β2 integrin to tumor cells. Hepatology (Baltimore, Md.) 73:1365–80. https://doi.org/10.1002/hep.31432
Lan J, Sun L, Xu F et al (2019) M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res 79:146–158. https://doi.org/10.1158/0008-5472.Can-18-0014
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B (2019) Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer 18:64. https://doi.org/10.1186/s12943-019-0976-4
Geng Y, Fan J, Chen L et al (2021) A notch-dependent inflammatory feedback circuit between macrophages and cancer cells regulates pancreatic cancer metastasis. Cancer Res 81:64–76. https://doi.org/10.1158/0008-5472.Can-20-0256
Walens A, DiMarco AV, Lupo R, Kroger BR, Damrauer JS, Alvarez JV (2019) CCL5 promotes breast cancer recurrence through macrophage recruitment in residual tumors. eLife. https://doi.org/10.7554/eLife.43653
Seoane S, Martinez-Ordoñez A, Eiro N et al (2019) POU1F1 transcription factor promotes breast cancer metastasis via recruitment and polarization of macrophages. J Pathol 249:381–394. https://doi.org/10.1002/path.5324
Li J, Zhao C, Li Y et al (2022) Osteosarcoma exocytosis of soluble LGALS3BP mediates macrophages toward a tumoricidal phenotype. Cancer Lett 528:1–15. https://doi.org/10.1016/j.canlet.2021.12.023
Verrier S, Peroglio M, Voisard C, Lechmann B, Alini M (2011) The osteogenic differentiation of human osteoprogenitor cells on Anodic-Plasma-Chemical treated Ti6Al7Nb. Biomaterials 32:672–680. https://doi.org/10.1016/j.biomaterials.2010.09.028
Zhang P, Li J (2021) Down-regulation of circular RNA hsa_circ_0007534 suppresses cell growth by regulating miR-219a-5p/SOX5 axis in osteosarcoma. J Bone Oncol 27:100349. https://doi.org/10.1016/j.jbo.2021.100349
Liang JQ, Zhou ZT, Bo L, Tan HN, Hu JH, Tan MS (2021) Phosphoglycerate kinase 1 silencing by a novel microRNA microRNA-4523 protects human osteoblasts from dexamethasone through activation of Nrf2 signaling cascade. Cell Death Dis 12:964. https://doi.org/10.1038/s41419-021-04250-1
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141:52–67. https://doi.org/10.1016/j.cell.2010.03.015
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW (2006) Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 66:11238–11246. https://doi.org/10.1158/0008-5472.Can-06-1278
Qian B, Deng Y, Im JH, Muschel RJ, Zou Y, Li J, Lang RA, Pollard JW (2009) A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PLoS ONE 4:e6562. https://doi.org/10.1371/journal.pone.0006562
Maimon A, Levi-Yahid V, Ben-Meir K et al (2021) Myeloid cell-derived PROS1 inhibits tumor metastasis by regulating inflammatory and immune responses via IL-10. J Clin Invest. https://doi.org/10.1172/jci126089
Chen YQ, Li PC, Pan N et al (2019) Tumor-released autophagosomes induces CD4+ T cell-mediated immunosuppression via a TLR2-IL-6 cascade. J Immunother Cancer 7:178. https://doi.org/10.1186/s40425-019-0646-5
Lee CC, Lin JC, Hwang WL, Kuo YJ, Chen HK, Tai SK, Lin CC, Yang MH (2018) Macrophage-secreted interleukin-35 regulates cancer cell plasticity to facilitate metastatic colonization. Nat Commun 9:3763. https://doi.org/10.1038/s41467-018-06268-0
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545. https://doi.org/10.1016/s0140-6736(00)04046-0
Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW (2011) CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475:222–225. https://doi.org/10.1038/nature10138
Uehara S, Song K, Farber JM, Love PE (2002) Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3(high)CD69+ thymocytes and gammadeltaTCR+ thymocytes preferentially respond to CCL25. J Immunol 168:134–142. https://doi.org/10.4049/jimmunol.168.1.134
Spinnen J, Fröhlich K, Sinner N, Stolk M, Ringe J, Shopperly L, Sittinger M, Dehne T, Seifert M (2021) Therapies with CCL25 require controlled release via microparticles to avoid strong inflammatory reactions. J Nanobiotechnol 19:83. https://doi.org/10.1186/s12951-021-00830-7
Johnson-Holiday C, Singh R, Johnson E, Singh S, Stockard CR, Grizzle WE, Lillard JW (2011) CCL25 mediates migration, invasion and matrix metalloproteinase expression by breast cancer cells in a CCR9-dependent fashion. Int J Oncol 38:1279–1285. https://doi.org/10.3892/ijo.2011.953
Niu Y, Tang D, Fan L, Gao W, Lin H (2020) CCL25 promotes the migration and invasion of non-small cell lung cancer cells by regulating VEGF and MMPs in a CCR9-dependent manner. Exp Ther Med 19:3571–3580. https://doi.org/10.3892/etm.2020.8635
Morikawa R, Nakamoto N, Amiya T et al (2021) Role of CC chemokine receptor 9 in the progression of murine and human non-alcoholic steatohepatitis. J Hepatol 74:511–521. https://doi.org/10.1016/j.jhep.2020.09.033
Wurbel MA, McIntire MG, Dwyer P, Fiebiger E (2011) CCL25/CCR9 interactions regulate large intestinal inflammation in a murine model of acute colitis. PLoS One 6:e16442. https://doi.org/10.1371/journal.pone.0016442
Umar S, Palasiewicz K, Van Raemdonck K et al (2021) CCL25 and CCR9 is a unique pathway that potentiates pannus formation by remodeling RA macrophages into mature osteoclasts. Eur J Immunol 51:903–914. https://doi.org/10.1002/eji.202048681
Jacquelot N, Enot DP, Flament C et al (2016) Chemokine receptor patterns in lymphocytes mirror metastatic spreading in melanoma. J Clin Invest 126:921–937. https://doi.org/10.1172/jci80071
Cortés M, Sanchez-Moral L, de Barrios O et al (2017) Tumor-associated macrophages (TAMs) depend on ZEB1 for their cancer-promoting roles. EMBO J 36:3336–3355. https://doi.org/10.15252/embj.201797345
Chen XJ, Deng YR, Wang ZC et al (2019) Hypoxia-induced ZEB1 promotes cervical cancer progression via CCL8-dependent tumour-associated macrophage recruitment. Cell Death Dis 10:508. https://doi.org/10.1038/s41419-019-1748-1
Ma L, Yu L, Jiang BC et al (2021) ZNF382 controls mouse neuropathic pain via silencer-based epigenetic inhibition of Cxcl13 in DRG neurons. J Exp Med. https://doi.org/10.1084/jem.20210920
Jen J, Wang YC (2016) Zinc finger proteins in cancer progression. J Biomed Sci 23:53. https://doi.org/10.1186/s12929-016-0269-9
Cheng Y, Geng H, Cheng SH et al (2010) KRAB zinc finger protein ZNF382 is a proapoptotic tumor suppressor that represses multiple oncogenes and is commonly silenced in multiple carcinomas. Cancer Res 70:6516–6526. https://doi.org/10.1158/0008-5472.Can-09-4566
Kusic DM, Roberts WN, Jarvis JP, Zhang P, Scheinfeldt LB, Rajula KD, Brenner R, Dempsey MP, Zajic SC (2020) rs11670527 Upstream of ZNF264 Associated with Body Mass Index in the Coriell Personalized Medicine Collaborative. Mil Med 185:649–655. https://doi.org/10.1093/milmed/usz216
Kaplan RN, Riba RD, Zacharoulis S et al (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438:820–827. https://doi.org/10.1038/nature04186
Zhang M, Song C, Li G, Chen L, Ma R, Yu X, Gong P, Wang X (2020) Transplantation of umbilical cord blood mononuclear cells attenuates the expression of IL-1β via the TLR4/NF-κB pathway in hypoxic-ischemic neonatal rats. J Neurorestoratology 8:122–130. https://doi.org/10.26599/jnr.2020.9040015
Helm O, Held-Feindt J, Grage-Griebenow E et al (2014) Tumor-associated macrophages exhibit pro- and anti-inflammatory properties by which they impact on pancreatic tumorigenesis. Int J Cancer 135:843–861. https://doi.org/10.1002/ijc.28736
Lee S, Heinrich EL, Li L et al (2015) CCR9-mediated signaling through β-catenin and identification of a novel CCR9 antagonist. Mol Oncol 9:1599–1611. https://doi.org/10.1016/j.molonc.2015.04.012
Funding
This work was supported by the Shaanxi Province Innovation Talent Promotion Program, Youth Science and Technology Star Project (grant number: 2020KJXX-028) and the National Program on Key Research and Development Project of China (grant number: 2018YFE0114200).
Author information
Authors and Affiliations
Contributions
JL contributed to conceptualization, methodology, software, validation, formal analysis, investigation, writing the original draft, writing, reviewing and editing; CZ contributed to conceptualization, methodology, validation, formal analysis, writing, reviewing and editing; DW performed data curation; SW carried out formal analysis; DW contributed to investigation and resources; FC provided software; YY contributed to methodology and validation; JL contributed to formal analysis and resources; XH was involved in conceptualization, project administration, and funding acquisition; JQ contributed to conceptualization, methodology, writing, reviewing and editing, project administration, and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Zhao, C., Wang, D. et al. ZIM3 activation of CCL25 expression in pulmonary metastatic nodules of osteosarcoma recruits M2 macrophages to promote metastatic growth. Cancer Immunol Immunother 72, 903–916 (2023). https://doi.org/10.1007/s00262-022-03300-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-022-03300-7