Abstract
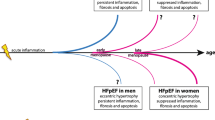
Heart failure (HF) is growing in prevalence, due to an increase in aging and comorbidities. Heart failure with reduced ejection fraction (HFrEF) is more common in men, whereas heart failure with preserved ejection fraction (HFpEF) has a higher prevalence in women. However, the reasons for these epidemiological trends are not clear yet. Since HFpEF affects mostly postmenopausal women, sex hormones should play a pivotal role in HFpEF development. Furthermore, for HFpEF, contrary to HFrEF, effective therapeutic approaches are missing. Interestingly, studies evidenced that some therapies can have better results in women than in HFpEF men, emphasizing the necessity of understanding these observations at a molecular level. Thus, herein, we review the molecular mechanisms of estrogen and androgen actions in the heart in physiological conditions and explain how its dysregulation can lead to disease development. This clarification is essential in the road for an effective personalized management of HF, particularly HFpEF, towards the development of sex-specific therapeutic approaches.




Similar content being viewed by others
Availability of data and material
This article reviews literature and therefore does not contain any associated data and materials.
Code availability
Not applicable.
References
Upadhya B, Kitzman DW (2020) Heart failure with preserved ejection fraction: New approaches to diagnosis and management. Clin Cardiol 43:145–155. https://doi.org/10.1002/clc.23321
Shim CY (2020) Heart failure with preserved ejection fraction: the major unmet need in cardiology. Korean Circ J 50:1051–1061. https://doi.org/10.4070/kcj.2020.0338
Wintrich J, Kindermann I, Ukena C, Selejan S, Werner C, Maack C, Laufs U, Tschöpe C, Anker SD, Lam CSP et al (2020) Therapeutic approaches in heart failure with preserved ejection fraction: past, present, and future. Clin Res Cardiol 109:1079–1098. https://doi.org/10.1007/s00392-020-01633-w
Meyer P, Beale AL (2020) Sex differences in heart failure with preserved ejection fraction therapy: potential mechanisms and clinical implications. EMJ Cardiol 8(1):82–91
Dworatzek E, Mahmoodzadeh S (2017) Targeted basic research to highlight the role of estrogen and estrogen receptors in the cardiovascular system. Pharmacol Res 119:27–35. https://doi.org/10.1016/j.phrs.2017.01.019
Regitz-Zagrosek V, Kararigas G (2017) Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol Rev 97:1–37. https://doi.org/10.1152/physrev.00021.2015
Li J, Chen X, McClusky R, Ruiz-Sundstrom M, Itoh Y, Umar S, Arnold AP, Eghbali M (2014) The number of X chromosomes influences protection from cardiac ischaemia/reperfusion injury in mice: one X is better than two. Cardiovasc Res 102:375–384. https://doi.org/10.1093/cvr/cvu064
Ventura-Clapier R, Dworatzek E, Seeland U, Kararigas G, Arnal J-F, Brunelleschi S, Carpenter TC, Erdmann J, Franconi F, Giannetta E et al (2017) Sex in basic research: concepts in the cardiovascular field. Cardiovasc Res 113:711–724. https://doi.org/10.1093/cvr/cvx066
Queirós AM, Eschen C, Fliegner D, Kararigas G, Dworatzek E, Westphal C, Sanchez Ruderisch H, Regitz-Zagrosek V (2013) Sex- and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. Int J Cardiol 169:331–338. https://doi.org/10.1016/j.ijcard.2013.09.002
Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM (2018) Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 138:198–205. https://doi.org/10.1161/CIRCULATIONAHA.118.034271
Levinsson A, Dube MP, Tardif JC, de Denus S (2018) Sex, drugs, and heart failure: a sex-sensitive review of the evidence base behind current heart failure clinical guidelines. ESC Heart Fail 5:745–754. https://doi.org/10.1002/ehf2.12307
Merrill M, Sweitzer NK, Lindenfeld J, Kao DP (2019) Sex differences in outcomes and responses to spironolactone in heart failure with preserved ejection fraction: a secondary analysis of TOPCAT trial. JACC Heart Fail 7:228–238. https://doi.org/10.1016/j.jchf.2019.01.003
McMurray JJV, Jackson AM, Lam CSP, Redfield MM, Anand IS, Ge J, Lefkowitz MP, Maggioni AP, Martinez F, Packer M et al (2020) Effects of sacubitril-valsartan versus valsartan in women compared with men with heart failure and preserved ejection fraction. Circulation 141:338–351. https://doi.org/10.1161/CIRCULATIONAHA.119.044491
Litvinukova M, Talavera-Lopez C, Maatz H, Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Heinig M, Lee M et al (2020) Cells of the adult human heart. Nature 588:466–472. https://doi.org/10.1038/s41586-020-2797-4
Walker CJ, Schroeder ME, Aguado BA, Anseth KS, Leinwand LA (2021) Matters of the heart: Cellular sex differences. J Mol Cell Cardiol 160:42–55. https://doi.org/10.1016/j.yjmcc.2021.04.010
Hu Z, Liu W, Hua X, Chen X, Chang Y, Hu Y, Xu Z, Song J (2021) Single-cell transcriptomic atlas of different human cardiac arteries identifies cell types associated with vascular physiology. Arterioscler Thromb Vasc Biol 41:1408–1427. https://doi.org/10.1161/ATVBAHA.120.315373
Paulus WJ, Tschope C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–271. https://doi.org/10.1016/j.jacc.2013.02.092
Regitz-Zagrosek V (2020) Sex and Gender Differences in heart failure. Int J Heart Fail 2:157–181. https://doi.org/10.36628/ijhf.2020.0004
Sasaki H, Nagayama T, Blanton RM, Seo K, Zhang M, Zhu G, Lee DI, Bedja D, Hsu S, Tsukamoto O et al (2014) PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Invest 124:2464–2471. https://doi.org/10.1172/jci70731
Kanashiro-Takeuchi RM, Heidecker B, Lamirault G, Dharamsi JW, Hare JM (2009) Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin Transl Sci 2:134–142. https://doi.org/10.1111/j.1752-8062.2009.00094.x
Witvrouwen I, Van Craenenbroeck EM, Abreu A, Moholdt T, Kränkel N (2019) Exercise training in women with cardiovascular disease: differential response and barriers – review and perspective. Eur J Prev Cardiol 28:779–790. https://doi.org/10.1177/2047487319838221
Bahls M, Friedrich N, Pietzner M, Wachter R, Budde K, Hasenfuss G, Nauck M, Pressler A, Felix SB, Edelmann F et al (2019) Heterogeneous metabolic response to exercise training in heart failure with preserved ejection fraction. J Clin Med 8:591. https://doi.org/10.3390/jcm8050591
Dunlay SM, Roger VL, Redfield MM (2017) Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 14:591–602. https://doi.org/10.1038/nrcardio.2017.65
Lei L, Mao Y (2018) Hormone treatments in congestive heart failure. J Int Med Res 46:2063–2081. https://doi.org/10.1177/0300060518761262
Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M (2017) The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol Sex Differ 8:33. https://doi.org/10.1186/s13293-017-0152-8
Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E, Heart ft, Group EpRSR (1998) Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 280:605–613. https://doi.org/10.1001/jama.280.7.605
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC et al (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333. https://doi.org/10.1001/jama.288.3.321
Hodis HN, Mack WJ, Henderson VW, Shoupe D, Budoff MJ, Hwang-Levine J, Li Y, Feng M, Dustin L, Kono N et al (2016) Vascular effects of early versus late postmenopausal treatment with estradiol. N Engl J Med 374:1221–1231. https://doi.org/10.1056/NEJMoa1505241
Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JE, Merriam GR et al (2014) Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women. Ann Intern Med 161:249–260. https://doi.org/10.7326/m14-0353
Maki PM (2013) Critical window hypothesis of hormone therapy and cognition: a scientific update on clinical studies. Menopause 20:695–709. https://doi.org/10.1097/GME.0b013e3182960cf8
Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, Køber L, Jensen J-EB (2012) Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ Brit Med J 345:e6409. https://doi.org/10.1136/bmj.e6409
Su JJ, Park SK, Hsieh TM (2014) The effect of testosterone on cardiovascular disease: a critical review of the literature. Am J Mens Health 8:470–491. https://doi.org/10.1177/1557988314522642
Elagizi A, Köhler TS, Lavie CJ (2018) Testosterone and cardiovascular health. Mayo Clin Proc 93:83–100. https://doi.org/10.1016/j.mayocp.2017.11.006
Tao J, Liu X, Bai W (2020) Testosterone supplementation in patients with chronic heart failure: a meta-analysis of randomized controlled trials. Front Endocrinol 11:110. https://doi.org/10.3389/fendo.2020.00110
Barrientos G, Llanos P, Basualto-Alarcón C, Estrada M (2020) Androgen-regulated cardiac metabolism in aging men. Front Endocrinol 11:316. https://doi.org/10.3389/fendo.2020.00316
Armeni E, Lambrinoudaki I (2017) Androgens and cardiovascular disease in women and men. Maturitas 104:54–72. https://doi.org/10.1016/j.maturitas.2017.07.010
Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A et al (2010) Adverse events associated with testosterone administration. N Engl J Med 363:109–122. https://doi.org/10.1056/NEJMoa1000485
Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER, Wenger NK, Bhasin S, Barrett-Connor E, Swerdloff RS, Stephens-Shields A, Cauley JA et al (2017) Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA 317:708–716. https://doi.org/10.1001/jama.2016.21043
Hutson DD, Gurrala R, Ogola BO, Zimmerman MA, Mostany R, Satou R, Lindsey SH (2019) Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol Sex Differ 10:4. https://doi.org/10.1186/s13293-019-0219-9
Gurrala R, Kilanowski-Doroh IM, Hutson DD, Ogola BO, Zimmerman MA, Katakam PVG, Satou R, Mostany R, Lindsey SH (2021) Alterations in the estrogen receptor profile of cardiovascular tissues during aging. Geroscience 43:433–442. https://doi.org/10.1007/s11357-021-00331-3
Zhang Y, Liu B, Zhao R, Zhang S, Yu XY, Li Y (2020) The influence of sex on cardiac physiology and cardiovascular diseases. J Cardiovasc Transl Res 13:3–13. https://doi.org/10.1007/s12265-019-09898-x
Kerkhof PLM, Peace RA, Heyndrickx GR, Meijboom LJ, Sprengers RW, Handly N (2018) Heart function analysis in cardiac patients with focus on sex-specific aspects. Adv Exp Med Biol 1065:361–377. https://doi.org/10.1007/978-3-319-77932-4_23
Merz AA, Cheng S (2016) Sex differences in cardiovascular ageing. Heart 102:825–831. https://doi.org/10.1136/heartjnl-2015-308769
Aryan L, Younessi D, Zargari M, Banerjee S, Agopian J, Rahman S, Borna R, Ruffenach G, Umar S, Eghbali M (2020) The role of estrogen receptors in cardiovascular disease. Int J Mol Sci 21:4314. https://doi.org/10.3390/ijms21124314
Menazza S, Murphy E (2016) The Expanding Complexity of Estrogen Receptor Signaling in the Cardiovascular System. Circ Res 118:994–1007. https://doi.org/10.1161/CIRCRESAHA.115.305376
Boese AC, Kim SC, Yin K-J, Lee J-P, Hamblin MH (2017) Sex differences in vascular physiology and pathophysiology: estrogen and androgen signaling in health and disease. Am J Physiol Heart Circ Physiol 313:H524–H545. https://doi.org/10.1152/ajpheart.00217.2016
Grohé C, Kahlert S, Löbbert K, Stimpel M, Karas RH, Vetter H, Neyses L (1997) Cardiac myocytes and fibroblasts contain functional estrogen receptors 1. FEBS Lett 416:107–112. https://doi.org/10.1016/s0014-5793(97)01179-4
Grohe C, Kahlert S, Lobbert K, Vetter H (1998) Expression of oestrogen receptor alpha and beta in rat heart: role of local oestrogen synthesis. J Endocrinol 156:R1. https://doi.org/10.1677/joe.0.156r001
Ropero AB, Eghbali M, Minosyan TY, Tang G, Toro L, Stefani E (2006) Heart estrogen receptor alpha: distinct membrane and nuclear distribution patterns and regulation by estrogen. J Mol Cell Cardiol 41:496–510. https://doi.org/10.1016/j.yjmcc.2006.05.022
Chung T-H, Wang S-M, Liang J-Y, Yang S-H, Wu J-C (2009) The interaction of estrogen receptor α and caveolin-3 regulates connexin43 phosphorylation in metabolic inhibition-treated rat cardiomyocytes. Int J Biochem Cell Biol 41:2323–2333. https://doi.org/10.1016/j.biocel.2009.06.001
Lizotte E, Grandy SA, Tremblay A, Allen BG, Fiset C (2009) Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem 23:75–86. https://doi.org/10.1159/000204096
Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, Weiske J, Pregla R, Hetzer R, Regitz-Zagrosek V (2006) Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB J 20:926–934. https://doi.org/10.1096/fj.05-5148com
Nordmeyer J, Eder S, Mahmoodzadeh S, Martus P, Fielitz J, Bass J, Bethke N, Zurbrugg HR, Pregla R, Hetzer R et al (2004) Upregulation of myocardial estrogen receptors in human aortic stenosis. Circulation 110:3270–3275. https://doi.org/10.1161/01.CIR.0000147610.41984.E8
Deschamps AM, Murphy E (2009) Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol 297:H1806–H1813. https://doi.org/10.1152/ajpheart.00283.2009
Filice E, Recchia AG, Pellegrino D, Angelone T, Maggiolini M, Cerra MC (2009) A new membrane G protein-coupled receptor (GPR30) is involved in the cardiac effects of 17beta-estradiol in the male rat. J Physiol Pharmacol 60:3–10
Patel VH, Chen J, Ramanjaneya M, Karteris E, Zachariades E, Thomas P, Been M, Randeva HS (2010) G-protein coupled estrogen receptor 1 expression in rat and human heart: Protective role during ischaemic stress. Int J Mol Med 26:193–199. https://doi.org/10.3892/ijmm_00000452
Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J et al (2004) Mitochondrial localization of estrogen receptor beta. Proc Natl Acad Sci USA 101:4130–4135. https://doi.org/10.1073/pnas.0306948101
Jazbutyte V, Kehl F, Neyses L, Pelzer T (2009) Estrogen receptor alpha interacts with 17β-hydroxysteroid dehydrogenase type 10 in mitochondria. Biochem Biophys Res Commun 384:450–454. https://doi.org/10.1016/j.bbrc.2009.04.139
Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E (2005) Estrogen receptor-beta mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol 288:H469–H476. https://doi.org/10.1152/ajpheart.00723.2004
Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, van Eys G, Grohe C, Doevendans PA (2006) Estrogen receptor beta protects the murine heart against left ventricular hypertrophy. Arterioscler Thromb Vasc Biol 26:1524–1530. https://doi.org/10.1161/01.ATV.0000223344.11128.23
Pedram A, Razandi M, Aitkenhead M, Levin ER (2005) Estrogen inhibits cardiomyocyte hypertrophy in vitro. Antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 280:26339–26348. https://doi.org/10.1074/jbc.M414409200
Levin ER, Hammes SR (2016) Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol 17:783–797. https://doi.org/10.1038/nrm.2016.122
Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER (2008) Estrogen Inhibits Cardiac Hypertrophy: Role of Estrogen Receptor-β to Inhibit Calcineurin. Endocrinology 149:3361–3369. https://doi.org/10.1210/en.2008-0133
Pedram A, Razandi M, Narayanan R, Dalton JT, McKinsey TA, Levin ER (2013) Estrogen regulates histone deacetylases to prevent cardiac hypertrophy. Mol Biol Cell 24:3805–3818. https://doi.org/10.1091/mbc.E13-08-0444
Eom GH, Cho YK, Ko J-H, Shin S, Choe N, Kim Y, Joung H, Kim H-S, Nam K-I, Kee HJ et al (2011) Casein kinase-2α1 induces hypertrophic response by phosphorylation of histone deacetylase 2 S394 and its activation in the heart. Circulation 123:2392–2403. https://doi.org/10.1161/CIRCULATIONAHA.110.003665
Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C et al (2004) 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ Res 95:692–699. https://doi.org/10.1161/01.RES.0000144126.57786.89
Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KDR, Schaefer E, Kajstura J, Anversa P, Sussman MA (2001) Myocardial Akt activation and gender. Circ Res 88:1020–1027. https://doi.org/10.1161/hh1001.090858
Morselli E, Santos RS, Criollo A, Nelson MD, Palmer BF, Clegg DJ (2017) The effects of oestrogens and their receptors on cardiometabolic health. Nat Rev Endocrinol 13:352–364. https://doi.org/10.1038/nrendo.2017.12
Shen T, Ding L, Ruan Y, Qin W, Lin Y, Xi C, Lu Y, Dou L, Zhu Y, Cao Y et al (2014) SIRT1 functions as an important regulator of estrogen-mediated cardiomyocyte protection in angiotensin II-induced heart hypertrophy. Oxid Med Cell Longev 2014:713894. https://doi.org/10.1155/2014/713894
Wang L, Tang ZP, Zhao W, Cong BH, Lu JQ, Tang XL, Li XH, Zhu XY, Ni X (2015) MiR-22/Sp-1 links estrogens with the up-regulation of cystathionine gamma-lyase in myocardium, which contributes to estrogenic cardioprotection against oxidative stress. Endocrinology 156:2124–2137. https://doi.org/10.1210/en.2014-1362
Sbert-Roig M, Bauzá-Thorbrügge M, Galmés-Pascual BM, Capllonch-Amer G, García-Palmer FJ, Lladó I, Proenza AM, Gianotti M (2016) GPER mediates the effects of 17β-estradiol in cardiac mitochondrial biogenesis and function. Mol Cell Endocrinol 420:116–124. https://doi.org/10.1016/j.mce.2015.11.027
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38. https://doi.org/10.1016/S0009-8981(03)00003-2
Pei H, Wang W, Zhao D, Su H, Su G, Zhao Z (2019) G protein-coupled estrogen receptor 1 inhibits angiotensin II-induced cardiomyocyte hypertrophy via the regulation of PI3K-Akt-mTOR signalling and autophagy. Int J Biol Sci 15:81–92. https://doi.org/10.7150/ijbs.28304
Wang H, Sun X, Lin MS, Ferrario CM, Van Remmen H, Groban L (2018) G protein-coupled estrogen receptor (GPER) deficiency induces cardiac remodeling through oxidative stress. Transl Res 199:39–51. https://doi.org/10.1016/j.trsl.2018.04.005
Mahmoodzadeh S, Pham TH, Kuehne A, Fielitz B, Dworatzek E, Kararigas G, Petrov G, Davidson MM, Regitz-Zagrosek V (2012) 17β-Estradiol-induced interaction of ERα with NPPA regulates gene expression in cardiomyocytes. Cardiovasc Res 96:411–421. https://doi.org/10.1093/cvr/cvs281
Luo T, Kim JK (2016) The role of estrogen and estrogen receptors on cardiomyocytes: an overview. Can J Cardiol 32:1017–1025. https://doi.org/10.1016/j.cjca.2015.10.021
Saito T, Ciobotaru A, Bopassa JC, Toro L, Stefani E, Eghbali M (2009) Estrogen contributes to gender differences in mouse ventricular repolarization. Circ Res 105:343–352. https://doi.org/10.1161/CIRCRESAHA.108.190041
Ribeiro R Jr, Pavan BMM, Potratz FF, Fiorim J, Simoes MR, Vargas Dias FM, Moulin Lima FL, Fernandes AA, Vassallo DV, Stefanon I (2012) Myocardial contractile dysfunction induced by ovariectomy requires AT1 receptor activation in female rats. Cell Physiol Biochem 30:1–12. https://doi.org/10.1159/000339041
Johnson BD, Zheng W, Korach KS, Scheuer T, Catterall WA, Rubanyi GM (1997) Increased expression of the cardiac L-type calcium channel in estrogen receptor-deficient mice. J Gen Physiol 110:135–140. https://doi.org/10.1085/jgp.110.2.135
Marni F, Wang Y, Morishima M, Shimaoka T, Uchino T, Zheng M, Kaku T, Ono K (2009) 17β-Estradiol modulates expression of low-voltage-activated CaV3.2 T-type calcium channel via extracellularly regulated kinase pathway in cardiomyocytes. Endocrinology 150:879–888. https://doi.org/10.1210/en.2008-0645
Rattanasopa C, Phungphong S, Wattanapermpool J, Bupha-Intr T (2015) Significant role of estrogen in maintaining cardiac mitochondrial functions. J Steroid Biochem Mol Biol 147:1–9. https://doi.org/10.1016/j.jsbmb.2014.11.009
Chen Y, Zhang Z, Hu F, Yang W, Yuan J, Cui J, Hao S, Hu J, Zhou Y, Qiao S (2015) 17β-estradiol prevents cardiac diastolic dysfunction by stimulating mitochondrial function: A preclinical study in a mouse model of a human hypertrophic cardiomyopathy mutation. J Steroid Biochem Mol Biol 147:92–102. https://doi.org/10.1016/j.jsbmb.2014.12.011
Rowe GC, Jiang A, Arany Z (2010) PGC-1 coactivators in cardiac development and disease. Circ Res 107:825–838. https://doi.org/10.1161/circresaha.110.223818
Ventura-Clapier R, Garnier A, Veksler V (2008) Transcriptional control of mitochondrial biogenesis: the central role of PGC-1α. Cardiovasc Res 79:208–217. https://doi.org/10.1093/cvr/cvn098
Rhoden EL, Ribeiro EP, Teloken C, Souto CAV (2005) Diabetes mellitus is associated with subnormal serum levels of free testosterone in men. BJU Int 96:867–870. https://doi.org/10.1111/j.1464-410X.2005.05728.x
Laughlin GA, Barrett-Connor E, Bergstrom J (2008) Low serum testosterone and mortality in older men. J Clin Endocrinol Metab 93:68–75. https://doi.org/10.1210/jc.2007-1792
Malkin CJ, Pugh PJ, Morris PD, Asif S, Jones TH, Channer KS (2010) Low serum testosterone and increased mortality in men with coronary heart disease. Heart 96:1821–1825. https://doi.org/10.1136/hrt.2010.195412
Gururani K, Jose J, George PV (2016) Testosterone as a marker of coronary artery disease severity in middle aged males. Indian Heart J 68:S16–S20. https://doi.org/10.1016/j.ihj.2016.07.002
Ayaz O, Howlett SE (2015) Testosterone modulates cardiac contraction and calcium homeostasis: cellular and molecular mechanisms. Biol Sex Differ 6:9. https://doi.org/10.1186/s13293-015-0027-9
Imperlini E, Mancini A, Alfieri A, Martone D, Caterino M, Orru S, Buono P (2015) Molecular effects of supraphysiological doses of doping agents on health. Mol Biosyst 11:1494–1506. https://doi.org/10.1039/c5mb00030k
Heywood LH (1980) Testosterone levels in the male laboratory rat: variation under experimental conditions. Int J Androl 3:519–529. https://doi.org/10.1111/j.1365-2605.1980.tb00140.x
Altamirano F, Oyarce C, Silva P, Toyos M, Wilson C, Lavandero S, Uhlén P, Estrada M (2009) Testosterone induces cardiomyocyte hypertrophy through mammalian target of rapamycin complex 1 pathway. J Endocrinol 202:299. https://doi.org/10.1677/joe-09-0044
Duran J, Oyarce C, Pavez M, Valladares D, Basualto-Alarcon C, Lagos D, Barrientos G, Troncoso MF, Ibarra C, Estrada M (2016) GSK-3β/NFAT signaling is involved in testosterone-induced cardiac myocyte hypertrophy. PLoS ONE 11:e0168255. https://doi.org/10.1371/journal.pone.0168255
Golden KL, Marsh JD, Jiang Y (2004) Testosterone regulates mRNA levels of calcium regulatory proteins in cardiac myocytes. Horm Metab Res 36:197–202. https://doi.org/10.1055/s-2004-814445
Parks RJ, Howlett SE (2013) Sex differences in mechanisms of cardiac excitation-contraction coupling. Pflugers Arch 465:747–763. https://doi.org/10.1007/s00424-013-1233-0
Zhang L, Wu S, Ruan Y, Hong L, Xing X, Lai W (2011) Testosterone suppresses oxidative stress via androgen receptor-independent pathway in murine cardiomyocytes. Mol Med Rep 4:1183–1188. https://doi.org/10.3892/mmr.2011.539
Bell JR, Mellor KM, Wollermann AC, Ip WT, Reichelt ME, Meachem SJ, Simpson ER, Delbridge LM (2011) Aromatase deficiency confers paradoxical postischemic cardioprotection. Endocrinology 152:4937–4947. https://doi.org/10.1210/en.2011-1212
Price T, Aitken J, Simpson ER (1992) Relative expression of aromatase cytochrome P450 in human fetal tissues as determined by competitive polymerase chain reaction amplification. J Clin Endocrinol Metab 74:879–883. https://doi.org/10.1210/jcem.74.4.1548354
Wilson C, Contreras-Ferrat A, Venegas N, Osorio-Fuentealba C, Pávez M, Montoya K, Durán J, Maass R, Lavandero S, Estrada M (2013) Testosterone increases GLUT4-dependent glucose uptake in cardiomyocytes. J Cell Physiol 228:2399–2407. https://doi.org/10.1002/jcp.24413
Mallat Z, Fornes P, Costagliola R, Esposito B, Belmin J, Lecomte D, Tedgui A (2001) Age and gender effects on cardiomyocyte apoptosis in the normal human heart. J Gerontol A Biol Sci Med Sci 56:M719–M723. https://doi.org/10.1093/gerona/56.11.m719
Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P (1995) Gender differences and aging: effects on the human heart. J Am Coll Cardiol 26:1068–1079. https://doi.org/10.1016/0735-1097(95)00282-8
Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Dworatzek E, Staub E, Martus P, Noppinger PR, Kintscher U et al (2010) Female sex and estrogen receptor-β attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 298:R1597–R1606. https://doi.org/10.1152/ajpregu.00825.2009
Mahmoodzadeh S, Dworatzek E (2019) The role of 17β-estradiol and estrogen receptors in regulation of Ca2+ channels and mitochondrial function in cardiomyocytes. Front Endocrinol 10:310. https://doi.org/10.3389/fendo.2019.00310
Curl CL, Wendt IR, Kotsanas G (2001) Effects of gender on intracellular [Ca2+] in rat cardiac myocytes. Pflugers Arch 441:709–716. https://doi.org/10.1007/s004240000473
Farrell SR, Ross JL, Howlett SE (2010) Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 299:H36–H45. https://doi.org/10.1152/ajpheart.00299.2010
Parks RJ, Ray G, Bienvenu LA, Rose RA, Howlett SE (2014) Sex differences in SR Ca2+ release in murine ventricular myocytes are regulated by the cAMP/PKA pathway. J Mol Cell Cardiol 75:162–173. https://doi.org/10.1016/j.yjmcc.2014.07.006
Vizgirda VM, Wahler GM, Sondgeroth KL, Ziolo MT, Schwertz DW (2002) Mechanisms of sex differences in rat cardiac myocyte response to beta-adrenergic stimulation. Am J Physiol Heart Circ Physiol 282:H256–H263. https://doi.org/10.1152/ajpheart.2002.282.1.H256
Chu SH, Sutherland K, Beck J, Kowalski J, Goldspink P, Schwertz D (2005) Sex differences in expression of calcium-handling proteins and beta-adrenergic receptors in rat heart ventricle. Life Sci 76:2735–2749. https://doi.org/10.1016/j.lfs.2004.12.013
Arieli Y, Gursahani H, Eaton MM, Hernandez LA, Schaefer S (2004) Gender modulation of Ca(2+) uptake in cardiac mitochondria. J Mol Cell Cardiol 37:507–513. https://doi.org/10.1016/j.yjmcc.2004.04.023
Ostadal B, Netuka I, Maly J, Besik J, Ostadalova I (2009) Gender differences in cardiac ischemic injury and protection–experimental aspects. Exp Biol Med (Maywood) 234:1011–1019. https://doi.org/10.3181/0812-MR-362
Milerova M, Drahota Z, Chytilova A, Tauchmannova K, Houstek J, Ostadal B (2016) Sex difference in the sensitivity of cardiac mitochondrial permeability transition pore to calcium load. Mol Cell Biochem 412:147–154. https://doi.org/10.1007/s11010-015-2619-4
Rosenkranz-Weiss P, Tomek RJ, Mathew J, Eghbali M (1994) Gender-specific differences in expression of mRNAs for functional and structural proteins in rat ventricular myocardium. J Mol Cell Cardiol 26:261–270. https://doi.org/10.1006/jmcc.1994.1029
Kararigas G, Bito V, Tinel H, Becher E, Baczko I, Knosalla C, Albrecht-Küpper B, Sipido KR, Regitz-Zagrosek V (2012) Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J Am Coll Cardiol 59:410–417. https://doi.org/10.1016/j.jacc.2011.09.054
Colom B, Oliver J, Roca P, Garcia-Palmer FJ (2007) Caloric restriction and gender modulate cardiac muscle mitochondrial H2O2 production and oxidative damage. Cardiovasc Res 74:456–465. https://doi.org/10.1016/j.cardiores.2007.02.001
Colom B, Oliver J, Garcia-Palmer FJ (2014) Sexual dimorphism in the alterations of cardiac muscle mitochondrial bioenergetics associated to the ageing process. J Gerontol A Biol Sci Med Sci 70:1360–1369. https://doi.org/10.1093/gerona/glu014
Ribeiro RF, Ronconi KS, Morra EA, Do Val Lima PR, Porto ML, Vassallo DV, Figueiredo SG, Stefanon I (2016) Sex differences in the regulation of spatially distinct cardiac mitochondrial subpopulations. Mol Cell Biochem 419:41–51. https://doi.org/10.1007/s11010-016-2748-4
Vijay V, Han T, Moland CL, Kwekel JC, Fuscoe JC, Desai VG (2015) Sexual dimorphism in the expression of mitochondria-related genes in rat heart at different ages. PLoS ONE 10:e0117047. https://doi.org/10.1371/journal.pone.0117047
Barba I, Miro-Casas E, Torrecilla JL, Pladevall E, Tejedor S, Sebastian-Perez R, Ruiz-Meana M, Berrendero JR, Cuevas A, Garcia-Dorado D (2017) High-fat diet induces metabolic changes and reduces oxidative stress in female mouse hearts. J Nutr Biochem 40:187–193. https://doi.org/10.1016/j.jnutbio.2016.11.004
Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E (2010) Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res 106:1681–1691. https://doi.org/10.1161/circresaha.109.213645
Sanchez-Ruderisch H, Queirós AM, Fliegner D, Eschen C, Kararigas G, Regitz-Zagrosek V (2019) Sex-specific regulation of cardiac microRNAs targeting mitochondrial proteins in pressure overload. Biol Sex Differ 10:8. https://doi.org/10.1186/s13293-019-0222-1
Saad NS, Elnakish MT, Ahmed AAE, Janssen PML (2018) Protein kinase A as a promising target for heart failure drug development. Arch Med Res 49:530–537. https://doi.org/10.1016/j.arcmed.2018.12.008
Wang F, Yang J, Sun J, Dong Y, Zhao H, Shi H, Fu L (2015) Testosterone replacement attenuates mitochondrial damage in a rat model of myocardial infarction. J Endocrinol 225:101–111. https://doi.org/10.1530/joe-14-0638
Pedram A, Razandi M, O’Mahony F, Lubahn D, Levin ER (2010) Estrogen receptor-β prevents cardiac fibrosis. Mol Endocrinol 24:2152–2165. https://doi.org/10.1210/me.2010-0154
Wang H, Zhao Z, Lin M, Groban L (2015) Activation of GPR30 inhibits cardiac fibroblast proliferation. Mol Cell Biochem 405:135–148. https://doi.org/10.1007/s11010-015-2405-3
Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V (2010) 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res 85:719–728. https://doi.org/10.1093/cvr/cvp350
Chung CC, Hsu RC, Kao YH, Liou JP, Lu YY, Chen YJ (2014) Androgen attenuates cardiac fibroblasts activations through modulations of transforming growth factor-beta and angiotensin II signaling. Int J Cardiol 176:386–393. https://doi.org/10.1016/j.ijcard.2014.07.077
Iwasaki T, Mukasa K, Yoneda M, Ito S, Yamada Y, Mori Y, Fujisawa N, Fujisawa T, Wada K, Sekihara H et al (2005) Marked attenuation of production of collagen type I from cardiac fibroblasts by dehydroepiandrosterone. Am J Physiol Endocrinol Metab 288:E1222-1228. https://doi.org/10.1152/ajpendo.00370.2004
Ikeda Y, Aihara K, Sato T, Akaike M, Yoshizumi M, Suzaki Y, Izawa Y, Fujimura M, Hashizume S, Kato M et al (2005) Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis. J Biol Chem 280:29661–29666. https://doi.org/10.1074/jbc.M411694200
Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, Knosalla C, Golz S, Hetzer R, Regitz-Zagrosek V (2014) Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail 16:1160–1167. https://doi.org/10.1002/ejhf.171
Petrov G, Regitz-Zagrosek V, Lehmkuhl E, Krabatsch T, Dunkel A, Dandel M, Dworatzek E, Mahmoodzadeh S, Schubert C, Becher E et al (2010) Regression of myocardial hypertrophy after aortic valve replacement. Circulation 122:S23–S28. https://doi.org/10.1161/CIRCULATIONAHA.109.927764
Petrov G, Dworatzek E, Schulze TM, Dandel M, Kararigas G, Mahmoodzadeh S, Knosalla C, Hetzer R, Regitz-Zagrosek V (2014) Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc Imaging 7:1073–1080. https://doi.org/10.1016/j.jcmg.2014.06.017
Dworatzek E, Mahmoodzadeh S, Schriever C, Kusumoto K, Kramer L, Santos G, Fliegner D, Leung Y-K, Ho S-M, Zimmermann W-H et al (2019) Sex-specific regulation of collagen I and III expression by 17β-Estradiol in cardiac fibroblasts: role of estrogen receptors. Cardiovasc Res 115:315–327. https://doi.org/10.1093/cvr/cvy185
Montalvo C, Villar AV, Merino D, García R, Ares M, Llano M, Cobo M, Hurlé MA, Nistal JF (2012) Androgens contribute to sex differences in myocardial remodeling under pressure overload by a mechanism involving TGF-β. PLoS ONE 7:e35635. https://doi.org/10.1371/journal.pone.0035635
Michel FS, Magubane M, Mokotedi L, Norton GR, Woodiwiss AJ (2017) Sex-specific effects of adrenergic-induced left ventricular remodeling in spontaneously hypertensive rats. J Card Fail 23:161–168. https://doi.org/10.1016/j.cardfail.2016.09.017
Dworatzek E, Baczko I, Kararigas G (2016) Effects of aging on cardiac extracellular matrix in men and women. Proteom Clin Appl 10:84–91. https://doi.org/10.1002/prca.201500031
Wang X, Tan Y, Xu B, Lu L, Zhao M, Ma J, Liang H, Liu J, Yu S (2018) GPR30 attenuates myocardial fibrosis in diabetic ovariectomized female rats: role of iNOS signaling. DNA Cell Biol 37:821–830. https://doi.org/10.1089/dna.2018.4208
Li L, Haynes MP, Bender JR (2003) Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA 100:4807–4812. https://doi.org/10.1073/pnas.0831079100
Ueda K, Karas RH (2013) Emerging evidence of the importance of rapid, non-nuclear estrogen receptor signaling in the cardiovascular system. Steroids 78:589–596. https://doi.org/10.1016/j.steroids.2012.12.006
Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR (2003) Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem 278:2118–2123. https://doi.org/10.1074/jbc.M210828200
Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR (2000) Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci USA 97:5930–5935. https://doi.org/10.1073/pnas.97.11.5930
Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW (2007) Direct interactions with Gαi and Gβγ mediate nongenomic signaling by estrogen receptor α. Mol Endocrinol 21:1370–1380. https://doi.org/10.1210/me.2006-0360
dos Santos RL, da Silva FB, Ribeiro RF, Stefanon I (2014) Sex hormones in the cardiovascular system. Horm Mol Biol Clin Investig 18:89. https://doi.org/10.1515/hmbci-2013-0048
Sobrino A, Oviedo PJ, Novella S, Laguna-Fernandez A, Bueno C, García-Pérez MA, Tarín JJ, Cano A, Hermenegildo C (2010) Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-α. J Mol Endocrinol 44:237. https://doi.org/10.1677/jme-09-0112
Villar IC, Hobbs AJ, Ahluwalia A (2008) Sex differences in vascular function: implication of endothelium-derived hyperpolarizing factor. J Endocrinol 197:447. https://doi.org/10.1677/joe-08-0070
Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, Hobbs AJ, Ahluwalia A (2005) Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice. Circulation 111:796–803. https://doi.org/10.1161/01.CIR.0000155238.70797.4E
Sickinghe AA, Korporaal SJA, den Ruijter HM, Kessler EL (2019) Estrogen contributions to microvascular dysfunction evolving to heart failure with preserved ejection fraction. Front Endocrinol (Lausanne) 10:442. https://doi.org/10.3389/fendo.2019.00442
Jesmin S, Mowa CN, Sultana SN, Shimojo N, Togashi H, Iwashima Y, Kato N, Sato A, Sakuma I, Hiroe M et al (2010) VEGF signaling is disrupted in the hearts of mice lacking estrogen receptor alpha. Eur J Pharmacol 641:168–178. https://doi.org/10.1016/j.ejphar.2010.05.020
Torres-Estay V, Carreño DV, Francisco IFS, Sotomayor P, Godoy AS, Smith GJ (2015) Androgen receptor in human endothelial cells. J Endocrinol 224:R131. https://doi.org/10.1530/joe-14-0611
Mukherjee TK, Dinh H, Chaudhuri G, Nathan L (2002) Testosterone attenuates expression of vascular cell adhesion molecule-1 by conversion to estradiol by aromatase in endothelial cells: implications in atherosclerosis. Proc Natl Acad Sci USA 99:4055–4060. https://doi.org/10.1073/pnas.052703199
Simoncini T, Mannella P, Fornari L, Varone G, Caruso A, Genazzani AR (2003) Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology 144:3449–3455. https://doi.org/10.1210/en.2003-0044
Goglia L, Tosi V, Sanchez AM, Flamini MI, Fu X-D, Zullino S, Genazzani AR, Simoncini T (2010) Endothelial regulation of eNOS, PAI-1 and t-PA by testosterone and dihydrotestosterone in vitro and in vivo. Mol Hum Reprod 16:761–769. https://doi.org/10.1093/molehr/gaq049
Yu J, Akishita M, Eto M, Ogawa S, Son B-K, Kato S, Ouchi Y, Okabe T (2010) Androgen receptor-dependent activation of endothelial nitric oxide synthase in vascular endothelial cells: role of phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 151:1822–1828. https://doi.org/10.1210/en.2009-1048
Yu J, Akishita M, Eto M, Koizumi H, Hashimoto R, Ogawa S, Tanaka K, Ouchi Y, Okabe T (2012) Src kinase-mediates androgen receptor-dependent non-genomic activation of signaling cascade leading to endothelial nitric oxide synthase. Biochem Biophys Res Commun 424:538–543. https://doi.org/10.1016/j.bbrc.2012.06.151
Kumanov P, Tomova A, Kirilov G (2007) Testosterone replacement therapy in male hypogonadism is not associated with increase of endothelin-1 levels. Int J Androl 30:41–47. https://doi.org/10.1111/j.1365-2605.2006.00706.x
Kumanov P, Tomova A, Kirilov G, Dakovska L, Schinkov A (2002) Increased plasma endothelin levels in patients with male hypogonadism. Andrologia 34:29–33. https://doi.org/10.1046/j.1439-0272.2002.00468.x
Liu R, Ding L, Yu M-H, Wang H-Q, Li W-C, Cao Z, Zhang P, Yao B-C, Tang J, Ke Q et al (2014) Effects of dihydrotestosterone on adhesion and proliferation via PI3-K/Akt signaling in endothelial progenitor cells. Endocrine 46:634–643. https://doi.org/10.1007/s12020-013-0081-1
Zhang H, Shi L, Ren G-Q, Sun W-W, Wang Y-B, Chen Y-K, Yin J-N, Wan B (2016) Dihydrotestosterone modulates endothelial progenitor cell function via RhoA/ROCK pathway. Am J Transl Res 8:4300–4309
Foresta C, Caretta N, Lana A, De Toni L, Biagioli A, Ferlin A, Garolla A (2006) Reduced number of circulating endothelial progenitor cells in hypogonadal men. J Clin Endocrinol Metab 91:4599–4602. https://doi.org/10.1210/jc.2006-0763
Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW (2003) Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation 108:3115–3121. https://doi.org/10.1161/01.CIR.0000106906.56972.83
Annibalini G, Agostini D, Calcabrini C, Martinelli C, Colombo E, Guescini M, Tibollo P, Stocchi V, Sestili P (2014) Effects of sex hormones on inflammatory response in male and female vascular endothelial cells. J Endocrinol Invest 37:861–869. https://doi.org/10.1007/s40618-014-0118-1
Death AK, McGrath KCY, Sader MA, Nakhla S, Jessup W, Handelsman DJ, Celermajer DS (2004) Dihydrotestosterone promotes vascular cell adhesion molecule-1 expression in male human endothelial cells via a nuclear factor-κB-dependent pathway. Endocrinology 145:1889–1897. https://doi.org/10.1210/en.2003-0789
Zhang X, Wang L-Y, Jiang T-Y, Zhang H-P, Dou Y, Zhao J-H, Zhao H, Qiao Z-D, Qiao J-T (2002) Effects of testosterone and 17-β-estradiol on TNF-α-induced E-selectin and VCAM-1 expression in endothelial cells: Analysis of the underlying receptor pathways. Life Sci 71:15–29. https://doi.org/10.1016/S0024-3205(02)01567-9
Hatakeyama H, Nishizawa M, Nakagawa A, Nakano S, Kigoshi T, Uchida K (2002) Testosterone inhibits tumor necrosis factor-α-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett 530:129–132. https://doi.org/10.1016/s0014-5793(02)03440-3
Nheu L, Nazareth L, Xu G-Y, Xiao F-Y, Luo R-Z, Komesaroff P, Ling S (2011) Physiological effects of androgens on human vascular endothelial and smooth muscle cells in culture. Steroids 76:1590–1596. https://doi.org/10.1016/j.steroids.2011.09.015
Hoetzer GL, MacEneaney OJ, Irmiger HM, Keith R, Van Guilder GP, Stauffer BL, DeSouza CA (2007) Gender Differences in circulating endothelial progenitor cell colony-forming capacity and migratory activity in middle-aged adults. Am J Cardiol 99:46–48. https://doi.org/10.1016/j.amjcard.2006.07.061
Rousseau A, Ayoubi F, Deveaux C, Charbit B, Delmau C, Christin-Maitre S, Jaillon P, Uzan G, Simon T (2010) Impact of age and gender interaction on circulating endothelial progenitor cells in healthy subjects. Fertil Steril 93:843–846. https://doi.org/10.1016/j.fertnstert.2008.10.062
Tan Z, Wang T-H, Yang D, Fu X-D, Pan J-Y (2003) Mechanisms of 17β-estradiol on the production of ET-1 in ovariectomized rats. Life Sci 73:2665–2674. https://doi.org/10.1016/S0024-3205(03)00605-2
Bilsel AS, Moini H, Tetik E, Aksungar F, Kaynak B, Özer A (2000) 17β-Estradiol modulates endothelin-1 expression and release in human endothelial cells. Cardiovasc Res 46:579–584. https://doi.org/10.1016/s0008-6363(00)00046-8
Pearson LJ, Yandle TG, Nicholls MG, Evans JJ (2008) Regulation of endothelin-1 release from human endothelial cells by sex steroids and angiotensin-II. Peptides 29:1057–1061. https://doi.org/10.1016/j.peptides.2008.02.003
Stanhewicz AE, Wenner MM, Stachenfeld NS (2018) Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circ Physiol 315:H1569–H1588. https://doi.org/10.1152/ajpheart.00396.2018
Mishra JS, Hankins GD, Kumar S (2016) Testosterone downregulates angiotensin II type-2 receptor via androgen receptor-mediated ERK1/2 MAP kinase pathway in rat aorta. J Renin Angiotensin Aldosterone Syst 17:1470320316674875. https://doi.org/10.1177/1470320316674875
Tanaka M, Nakaya S, Watanabe M, Kumai T, Tateishi T, Kobayashi S (1997) Effects of ovariectomy and estrogen replacement on aorta angiotensin-converting enzyme activity in rats. Jpn J Pharmacol 73:361–363. https://doi.org/10.1254/jjp.73.361
Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB (1999) Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension 33:323–328. https://doi.org/10.1161/01.HYP.33.1.323
Bukowska A, Spiller L, Wolke C, Lendeckel U, Weinert S, Hoffmann J, Bornfleth P, Kutschka I, Gardemann A, Isermann B et al (2017) Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med (Maywood) 242:1412–1423. https://doi.org/10.1177/1535370217718808
Debortoli AR, do Nascimento Rouver W, Delgado NTB, Mengal V, Claudio ERG, Pernomian L, Bendhack LM, Moysés MR, dos Santos RL (2017) GPER modulates tone and coronary vascular reactivity in male and female rats. J Mol Endocrinol 59:171. https://doi.org/10.1530/jme-16-0117
Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER (2002) ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol 16:100–115. https://doi.org/10.1210/mend.16.1.0757
Ueda K, Lu Q, Baur W, Aronovitz MJ, Karas RH (2013) Rapid estrogen receptor signaling mediates estrogen-induced inhibition of vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol 33:1837–1843. https://doi.org/10.1161/atvbaha.112.300752
Orshal JM, Khalil RA (2004) Gender, sex hormones, and vascular tone. Am J Physiol Regul Integr Comp Physiol 286:R233–R249. https://doi.org/10.1152/ajpregu.00338.2003
Sivritas D, Becher MU, Ebrahimian T, Arfa O, Rapp S, Bohner A, Mueller CF, Umemura T, Wassmann S, Nickenig G et al (2011) Antiproliferative effect of estrogen in vascular smooth muscle cells is mediated by Kruppel-like factor-4 and manganese superoxide dismutase. Basic Res Cardiol 106:563–575. https://doi.org/10.1007/s00395-011-0174-z
Lopes RAM, Neves KB, Pestana CR, Queiroz AL, Zanotto CZ, Chignalia AZ, Valim YM, Silveira LR, Curti C, Tostes RC (2014) Testosterone induces apoptosis in vascular smooth muscle cells via extrinsic apoptotic pathway with mitochondria-generated reactive oxygen species involvement. Am J Physiol Heart Circ Physiol 306:H1485–H1494. https://doi.org/10.1152/ajpheart.00809.2013
Kelly DM, Jones TH (2013) Testosterone: a vascular hormone in health and disease. J Endocrinol 217:R47–R71. https://doi.org/10.1530/joe-12-0582
Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE (2012) Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol 302:H115–H123. https://doi.org/10.1152/ajpheart.00046.2011
Hogg ME, Vavra AK, Banerjee MN, Martinez J, Jiang Q, Keefer LK, Chambon P, Kibbe MR (2012) The role of estrogen receptor α and β in regulating vascular smooth muscle cell proliferation is based on sex. J Surg Res 173:e1–e10. https://doi.org/10.1016/j.jss.2011.09.021
Straface E, Vona R, Gambardella L, Ascione B, Marino M, Bulzomi P, Canu S, Coinu R, Rosano G, Malorni W et al (2009) Cell sex determines anoikis resistance in vascular smooth muscle cells. FEBS Lett 583:3448–3454. https://doi.org/10.1016/j.febslet.2009.09.052
Dias FMV, Ribeiro RF Jr, Fernandes AA, Fiorim J, Travaglia TCF, Vassallo DV, Stefanon I (2014) Na+K+-ATPase activity and K+ channels differently contribute to vascular relaxation in male and female rats. PLoS ONE 9:e106345. https://doi.org/10.1371/journal.pone.0106345
Lyle MA, Alabdaljabar MS, Han YS, Brozovich FV (2020) The vasculature in HFpEF vs HFrEF: differences in contractile protein expression produce distinct phenotypes. Heliyon 6:e03129. https://doi.org/10.1016/j.heliyon.2019.e03129
Lontay B, Bodoor K, Weitzel DH, Loiselle D, Fortner C, Lengyel S, Zheng D, Devente J, Hickner R, Haystead TJ (2010) Smoothelin-like 1 protein regulates myosin phosphatase-targeting subunit 1 expression during sexual development and pregnancy. J Biol Chem 285:29357–29366. https://doi.org/10.1074/jbc.M110.143966
Mishra S, Kass DA (2021) Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 18:400–423. https://doi.org/10.1038/s41569-020-00480-6
Funding
This work was supported by Portuguese Foundation for Science and Technology (FCT), European Union, QREN, FEDER and COMPETE for funding the LAQV-REQUIMTE (UIDB/50006/2020), UnIC (UIDB/IC/00051/2020 and UIDP/00051/2020), and UMIB (UIDB/00215/2020 and UIDP/00215/2020) research units and the research projects DOCnet (NORTE-01–0145-FEDER-000003), NETDIAMOND (POCI-01–0145-FEDER-016385), and EXIT-HF (POCI-01–0145-FEDER-030011).
Author information
Authors and Affiliations
Contributions
R.N.F., M.S., F.A., R.F., and C.F. had the idea for the article; R.N.F., F.T., J.S.N., and C.F. performed the literature search; R.N.F., F.T., and C.F. drafted the manuscript; and M.S., F.A., A.L.M., and R.F. critically revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article reviews literature and therefore does not require ethics approval.
Consent to participate
This article reviews literature and therefore does not require consent to participate.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferreira, C., Trindade, F., Ferreira, R. et al. Sexual dimorphism in cardiac remodeling: the molecular mechanisms ruled by sex hormones in the heart. J Mol Med 100, 245–267 (2022). https://doi.org/10.1007/s00109-021-02169-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-021-02169-w