

Volume 11 • 2022 www.AERjournal.com
Editor-in-Chief
Demosthenes G Katritsis, PhD, FRCP, FESC, FACC Hygeia Hospital, Athens, Greece
Section Editor – Clinical Electrophysiology and Ablation
Hugh Calkins, MD Johns Hopkins Medicine, Baltimore, MD, US
Section Editor – Implantable Devices
Ken Ellenbogen, MD, FHRS
Virginia Commonwealth University School of Medicine, Richmond, VA, US
Section Editor – Arrhythmia Mechanisms/Basic Science
Andrew Grace, MB, BS, PhD, FRCP Royal Papworth and Addenbrooke’s Hospitals, Cambridge, UK
Joseph G Akar, MD, PhD, FACC, FHRS
Yale University School of Medicine, New Haven, CT, US
Robert Anderson, MD, FRCPath
Newcastle University, Newcastle upon Tyne, UK
Charles Antzelevitch, PhD, FACC, FAHA, FHRS Lankenau Institute for Medical Research, Wynnewood, PA, US
Elena Arbelo, MD, PhD, MSc Hospital Clínic de Barcelona, Barcelona, Spain
Angelo Auricchio, MD, PhD
Fondazione Cardiocentro Ticino, Lugano, Switzerland
Adrian Baranchuk, MD, FACC, FRCPC, FCCS, FSIAC Queen’s University, Kingston, Canada
Carina Blomström-Lundqvist, MD, PhD
Uppsala University, Uppsala, Sweden
Johannes Brachmann, MD
Klinikum Coburg, II Med Klinik, Coburg, Germany
Josep Brugada, MD, PhD, FESC
Hospital Sant Joan de Déu, University of Barcelona, Barcelona, Spain
Pedro Brugada, MD, PhD
University of Brussels, UZ-BrusselVUB, Brussels, Belgium
Haran Burri, MD, MBBS, MBBCh, PhD, DNP University Hospital of Geneva, Geneva, Switzerland
Alfred Buxton, MD
Beth Israel Deaconess Medical Center, Boston, MA, US
David J Callans, MD University of Pennsylvania, Philadelphia, PA, US
A John Camm, PhD
St George’s University of London, London, UK
Section Editor – Arrhythmia Risk Stratification
Pier D Lambiase, BMBCh, PhD, FRCP Institute of Cardiovascular Science, University College London, and Barts Heart Centre, London, UK
Section Editor – Atrial Fibrillation
Gregory YH Lip, MD, FRCP, DFM, FACC, FESCH, FEHRA Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool, UK
Section Editor – Imaging in Electrophysiology
Sanjiv M Narayan, MD, PhD Stanford University Medical Center, CA, US
Editorial Board
Tze-Fan Chao, MD
Taipei Veterans General Hospital and National Yang Ming Chiao Tung University, Taipei, Taiwan
Shih-Ann Chen, MD National Yang Ming University School of Medicine, Taipei, Taiwan
Eue-Keun Choi, MD Seoul National University Hospital, Seoul, South Korea
KR Julian Chun, MD
CardioVascular Center Bethanien, Frankfurt, Germany
Harry Crijns, MD, PhD, FESC Maastricht University Medical Center, Maastricht, the Netherlands
Luigi Di Biase, MD, PhD Albert Einstein College of Medicine and Montefiore Medical Center, New York, NY, US
Sanjay Dixit, MD, PhD, FESC University of Pennsylvania
Perelman School of Medicine, Philadelphia, PA, US
Sabine Ernst, MD, PhD, FESC Royal Brompton & Harefield NHS Foundation Trust, London, UK
Yutao Guo, PhD, FESC
Chinese PLA General Hospital, Beijing, China
Dhiraj Gupta, MD, DM, FRCP, FESC Liverpool Heart and Chest NHS Foundation Trust, Liverpool, UK
Jeroen Hendriks, RN, MSc, PhD, FESC, FCSANZ Flinders University, Adelaide, Australia
Gerhard Hindricks, MD, PhD University of Leipzig, Leipzig, Germany
Carsten W Israel, MD
JW Goethe University, Frankfurt, Germany
Warren Jackman, MD, FHRS University of Oklahoma Health Sciences Center, Oklahoma City, OK, US
Pierre Jaïs, MD University of Bordeaux, CHU Bordeaux, France
Roy John, MBBB, PhD, FRCP Northshore University Hospital, New York, NY, US
Boyoung Joung, MD, PhD Yonsei University, Seoul, South Korea
Prapa Kanagaratnam, MB, BChir
Imperial College Healthcare NHS Trust, London, UK
Josef Kautzner, MD, PhD, FESC Institute for Clinical and Experimental Medicine, Prague, Czech Republic
Roberto Keegan, MD Hospital Privado del Sur, Bahia Blanca, Argentina
Karl-Heinz Kuck, MD, PhD
LANS Cardio, Kardiologie Hamburg, Hamburg, Germany
Francis E Marchlinski, MD University of Pennsylvania Health System, Philadelphia, PA, US
Joseph E Marine, MD, MBA, FACC, FHRS Johns Hopkins University School of Medicine, Baltimore, ML, US
John M Miller, MD, FACC, FAHA, FHRS Indiana University School of Medicine, Indianapolis, IN, US
Fred Morady, MD, FACC Cardiovascular Center, University of Michigan, MI, US
Andrea Natale, MD, FACC
Texas Cardiac Arrhythmia Institute, St David’s Medical Center, Austin, TX, US
Mark O’Neill, DPhil, FRCP, FHRS St Thomas’ Hospital and King’s College London, London, UK
Douglas Packer, MD
Mayo Clinic, St Mary’s Campus, Rochester, MN, US
Carlo Pappone, MD, PhD, FACC IRCCS Policlinico San Donato, Milan, Italy
Sunny S Po, MD University of Oklahoma Health Sciences Center, Oklahoma City, OK, US
Edward Rowland, MD, FRCP, FESC Barts Heart Centre, St Bartholomew’s Hospital, London, UK
Frédéric Sacher, MD, PhD Bordeaux University Hospital, Electrophysiology and Heart Modelling Institute, Bordeaux, France
Richard Schilling, MD, FESC Barts Health NHS Trust, London, UK
Afzal Sohaib, MBBS, MRCP, PhD, ECES Imperial College London and Barts Health NHS Trust, London, UK
Neil T Srinivasan, MBChB, PhD, MRCP, IBHRE-CEPS, IBHRE-CCDS Essex Cardiothoracic Centre, Basildon, Essex, UK
William G Stevenson, MD Vanderbilt School of Medicine, Nashville, TN, US
Richard Sutton, MB BS, DSc, FRCP, FACC, FESC, FAHA, FHRS, FEHRA, FBHRS
National Heart and Lung Institute, Imperial College London, London, UK
Marc A Vos, PhD, FAHA University Medical Center Utrecht, Utrecht, the Netherlands
Katja Zeppenfeld, MD, PhD, FESC, FEHRA
Leiden University Medical Center, Leiden, the Netherlands
Douglas P Zipes, MD Krannert Institute of Cardiology, Indiana University School of Medicine, Indianapolis, IN, US
© RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com
Volume 11 • 2022
1
www.AERjournal.com
www.AERjournal.com
Editorial
Publishing Director Leiah Norcott | Managing Editor Calum White
Production Editors Aashni Shah, Bettina Vine | Senior Graphic Designer Lewis Allen
Peer Review Editor Nicola Parsons | Editorial Coordinator Jemima Hegerty-Ward
Contact calum.white@radcliffe-group.com
Marketing
Marketing Director Lizzy Comber | Digital Marketing Manager Kati Marandi
Senior Marketing Coordinator Dawn von Bergen | Marketing Coordinator Calum Barlow
Marketing Executive | Bavneet Dhanjal
Contact lizzy.comber@radcliffe-group.com
Radcliffe Medical Media
Managing Director Jonathan McKenna
Sales Director David Bradbury | Agency Sales Director Gary Swanston
Senior Account Managers William Cadden, Brad Wilson
Account Manager Steven Cavanagh
Contact david.bradbury@radcliffe-group.com
Radcliffe Medical Education
Managing Director Rob Barclay
Sales Director Carrie Barclay
Education Account Manager Meadbh Metrustry
Contact carrie.barclay@radcliffe-group.com
Leadership
Chief Executive Officer David Ramsey
Chief Operations Officer Liam O’Neill
Official journal of
Published by Radcliffe Cardiology.


All information obtained by Radcliffe Cardiology and each of the contributors from various sources is as current and accurate as possible. However, due to human or mechanical errors, Radcliffe Cardiology, British Heart Rhythm Society and the contributors cannot guarantee the accuracy, adequacy or completeness of any information, and cannot be held responsible for any errors or omissions, or for the results obtained from the use thereof. Published content is for information purposes only and is not a substitute for professional medical advice. Where views and opinions are expressed, they are those of the author(s) and do not necessarily reflect or represent the views and opinions of Radcliffe Cardiology or British Heart Rhythm Society.
Radcliffe Cardiology, Unit F, First Floor, Bourne End Business Park, Cores End Road, Bourne End, Buckinghamshire SL8 5AS, UK © 2022 All rights reserved • ISSN: 2050-3369 • eISSN: 2050-3377
© RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Volume 11 • 2022
2
Aims and Scope
• Arrhythmia & Electrophysiology Review is an international, English language, peer-reviewed, open access journal that publishes articles continuously on www.AERjournal.com
• Arrhythmia & Electrophysiology Review aims to assist time-pressured physicians to stay abreast of key advances and opinion.
• Arrhythmia & Electrophysiology Review comprises balanced and comprehensive articles written by leading authorities.
• Arrhythmia & Electrophysiology Review provides comprehensive updates on salient issues to support physicians in developing their knowledge and effectiveness in day-to-day clinical practice.
Structure and Format
• Arrhythmia & Electrophysiology Review publishes review articles, expert opinion pieces, guest editorials and letters to the editor.
• The structure and degree of coverage assigned to each category of the journal is the decision of the Editor-in-Chief, with the support of the Editorial Board.
Abstracting and Indexing
Arrhythmia & Electrophysiology Review is abstracted, indexed and listed in PubMed, Crossref, Emerging Sources Citation Index, Scopus, Google Scholar and Directory of Open Access Journals. All articles are published in full on PubMed Central a month after publication. Radcliffe Group is an STM member publisher.
Editorial Expertise
Arrhythmia & Electrophysiology Review is supported by various levels of expertise:
• Overall direction from the Editor-in-Chief, supported by the Editorial Board comprising leading authorities from a variety of disciplines.
• Invited contributors who are recognised authorities in their fields.
• Peer review – conducted by experts appointed for their experience and knowledge of a specific topic.
• An experienced team of editors and technical editors.
Submissions and Instructions to Authors
• Contributors are identified by the Editor-in-Chief with the support of the Editorial Board and Managing Editor.
• Following acceptance of an invitation, the author(s) and Managing Editor, in conjunction with the Editor-in-Chief, formalise the working title and scope of the article.
• Instructions for authors and additional submission details are at www.radcliffecardiology.com/guideline/author-guidelines
• Authors wishing to discuss potential submissions should contact the Managing Editor, Calum White, calum.white@radcliffe-group.com
• Articles may be submitted directly at www.editorialmanager.com/aer
Ethics and Conflicts of Interest
The journal follows guidance from the International Committee of Medical Journal Editors and the Committee on Publication Ethics. We expect all parties involved in the journal’s publication to follow these guidelines. All authors must declare any conflicts of interest.
Open Access, Copyright and Permissions
Articles published in this journal are gold open access, which means the version of record is freely available, immediately upon publication, without charge. Articles may be published under a CC-BY-NC or CC-BY licence.
CC-BY-NC: Allows users to read, download, copy, redistribute and make derivative works for non-commercial purposes. The author retains all non-commercial rights for articles published herein under the CC-BY-NC 4.0 License (https://creativecommons.org/licenses/by-nc/4.0/legalcode). To support open access publication costs, Radcliffe charges an article publication charge upon acceptance of an unsolicited paper: £1,500 UK | €1,770 Eurozone | $1,970 all other countries. Waivers are available, as specified in the ‘For authors’ section on www.AERjournal.com. Permission to reproduce an article published under CC-BY-NC for commercial purposes, either in full or part, should be sought from the Managing Editor.
CC-BY: Allows users to read, download, copy, redistribute and make derivative works for any purpose, including commercially. Radcliffe offers publication under the CC-BY 4.0 License (https://creativecommons.org/ licenses/by/4.0/legalcode) to authors funded by UK Research Councils (UKRI) or The Wellcome Trust. The article publication charge is £1,750 | €2,069 Eurozone | $2,299 all other countries. The author retains all rights under this option.
Peer Review
• On submission, all articles are assessed by the Editor-in-Chief.
• Suitable manuscripts are sent for double-blind peer review.
• The Editor-in-Chief reserves the right to accept or reject any proposed amendments.
• Once a manuscript has been amended in accordance with the reviewers’ comments, it is assessed to ensure it meets expectations.
• The manuscript is sent to the Editor-in-Chief for final approval.
Distribution and Readership
Arrhythmia & Electrophysiology Review is an online publication, with articles published continuously on www.AERjournal.com. The journal is free to read online and PDF downloads are available for registered users. Print subscriptions are available upon request.
Online
All published manuscripts are free to read at www.AERjournal.com They are also available at www.radcliffecardiology.com, along with articles from the other journals in Radcliffe Cardiology’s cardiovascular portfolio – Cardiac Failure Review, European Cardiology Review, Interventional Cardiology, Journal of Asian Pacific Society of Cardiology and US Cardiology Review
Reprints
All articles included in Arrhythmia & Electrophysiology Review are available as reprints. Please contact the Sales Director, David Bradbury david.bradbury@radcliffe-group.com
© RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Volume 11 • 2022 www.AERjournal.com
Contents
What Cannot Be Missed: Important Publications on Electrophysiology in 2021
Sanjiv M Narayan, Hugh Calkins, Andrew Grace, Gregory YH Lip, Ken Ellenbogen, Pier D Lambiase and Demosthenes G Katritsis https://doi.org/10.15420/aer.2022.04
Ablation Lesion Assessment with MRI
Lluis Mont, Ivo Roca-Luque and Till F Althoff https://doi.org/10.15420/aer.2021.63
Bridging the Gap Between Artificial Intelligence Research and Clinical Practice in Cardiovascular Science: What the Clinician Needs to Know
Emily Shipley, Martha Joddrell, Gregory YH Lip and Yalin Zheng https://doi.org/10.15420/aer.2022.07
Contemporary Management of Complex Ventricular Arrhythmias
Benedict M Wiles, Anthony C Li, Michael C Waight and Magdi M Saba https://doi.org/10.15420/aer.2021.66
Arrhythmogenesis of Sports: Myth or Reality?
Saad Fyyaz and Michael Papadakis https://doi.org/10.15420/aer.2021.68
Global Substrate Mapping and Targeted Ablation with Novel Gold-tip Catheter in De Novo Persistent AF
Michael TB Pope and Timothy R Betts https://doi.org/10.15420/aer.2021.64
Preprocedural Discrimination of Posteroseptal Accessory Pathways Ablated from the Right Endocardium from Those Requiring a Left-sided or Epicardial Coronary Venous Approach
Mathieu Lebloa and Patrizio Pascale https://doi.org/10.15420/aer.2021.55
Future Directions for Mapping Atrial Fibrillation
Junaid AB Zaman, Andrew A Grace and Sanjiv M Narayan https://doi.org/10.15420/aer.2021.52
Association Between Left Atrial Appendage Morphology and Function and the Risk of Ischaemic Stroke in Patients with Atrial Fibrillation
Katarzyna Dudzińska-Szczerba, Piotr Kułakowski, Ilona Michałowska and Jakub Baran https://doi.org/10.15420/aer.2022.08
Prophylactic Cavotricuspid Isthmus Ablation in Atrial Fibrillation without Documented Typical Atrial Flutter: A Systematic Review and Meta-analysis
Yoga Waranugraha, Ardian Rizal, Mohammad Saifur Rohman, Chia-Ti Tsai and Fu-Chun Chiu https://doi.org/10.15420/aer.2021.37
Clinical Relevance of Sinus Rhythm Mapping to Quantify Electropathology Related to Atrial Fibrillation
Mathijs S van Schie and Natasja MS de Groot https://doi.org/10.15420/aer.2022.03
A Chronicle of Hybrid Atrial Fibrillation Ablation Therapy: From Cox Maze to Convergent
Riyaz A Kaba, Omar Ahmed, Elijah Behr and Aziz Momin https://doi.org/10.15420/aer.2022.05
Economic Evaluation of Catheter Ablation Versus Medical Therapy for the Treatment of Atrial Fibrillation from the Perspective of the UK
Lisa WM Leung, Zaki Akhtar, Christos Kontogiannis, Ryan J Imhoff, Hannah Taylor and Mark M Gallagher https://doi.org/10.15420/aer.2021.46
Mahaim Revisited
Eduardo Back Sternick, Damian Sanchez-Quintana, Hein JJ Wellens and Robert H Anderson https://doi.org/10.15420/aer.2022.12
Protecting Against Collateral Damage to Non-cardiac Structures During Endocardial Ablation for Persistent Atrial Fibrillation
Lisa WM Leung, Zaki Akhtar, Jamal Hayat and Mark M Gallagher https://doi.org/10.15420/aer.2021.67
e08
e09
e10
e11
e12
e13
e14
e15
© RADCLIFFE CARDIOLOGY 2022 Access at: www.AERjournal.com
e01
e02
e03
e04
e05
e06
e07
Contents
Arrhythmogenic Mitral Valve Prolapse
Theofanis George Korovesis, Paraskevi Koutrolou-Sotiropoulou and Demosthenes George Katritsis https://doi.org/10.15420/aer.2021.28
Ventricular Dyssynchrony and Pacing-induced Cardiomyopathy in Patients with Pacemakers, the Utility of Ultra-high-frequency ECG and Other Dyssynchrony Assessment Tools
Jan Mizner, Pavel Jurak, Hana Linkova, Radovan Smisek and Karol Curila https://doi.org/10.15420/aer.2022.01
Safety, Efficacy and Prognostic Benefit of Atrial Fibrillation Ablation in Heart Failure with Preserved Ejection Fraction
Nicolas Johner, Mehdi Namdar, Dipen Shah https://doi.org/10.15420/aer.2022.10
UK Expert Consensus Statement for the Optimal Use and Clinical Utility of Leadless Pacing Systems on Behalf of the British Heart Rhythm Society
Paul Roberts, Mohamed Hassan ElRefai, Paul Foley, Archana Rao, David Sharman, Riyaz Somani, Simon Sporton, Gary Wright, Amir Zaidi, Chris Pepper https://doi.org/10.15420/aer.2022.17
Catecholaminergic Polymorphic Ventricular Tachycardia
Mohamed Abbas, Chris Miles, Elijah R Behr https://doi.org/10.15420/aer.2022.09
e16
e17
e18
e19
© RADCLIFFE CARDIOLOGY 2022 Access at: www.AERjournal.com
e20
What Cannot Be Missed: Important Publications on Electrophysiology in 2021
Sanjiv M Narayan , 1 Hugh Calkins , 2 Andrew Grace , 3 Gregory YH Lip , 4 Ken Ellenbogen , 5 Pier D Lambiase 6,7 and Demosthenes G Katritsis8,9
1. Stanford University Medical Center, Palo Alto, CA, US; 2. Johns Hopkins Medical Institution, Baltimore, MD, US; 3. Royal Papworth and Addenbrooke’s Hospitals, Cambridge, UK; 4. Liverpool Centre for Cardiovascular Science, University of Liverpool, Liverpool, UK; 5. Virginia Commonwealth University School of Medicine, Richmond, VA, US; 6. UCL Institute of Cardiovascular Science, University College London, UK; 7. Barts Heart Centre, London, UK; 8. Hygeia Hospital, Athens, Greece; 9. Johns Hopkins University School of Medicine, Baltimore, MD, US
Disclosure: The authors are the editor-in-chief and section editors of Arrhythmia & Electrophysiology Review
Received: 19 January 2022 Accepted: 4 February 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e01. DOI: https://doi.org/10.15420/aer.2022.04
Correspondence: Demosthenes Katritsis, Hygeia Hospital, 4 Erythrou Stavrou St, Athens 15123, Greece. E: dkatrits@dgkatritsis.gr
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The editors are pleased to present the following important papers and brief summaries from 2021 for your attention.
Clinical Arrhythmias
Atrial Fibrillation
Lurie A, Wang J, Hinnegan KJ, et al. Prevalence of left atrial thrombus in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol 2021;77:2875–86. https://doi.org/10.1016/j.jacc.2021.04.036; PMID: 34112315.
• Left atrial thrombus prevalence is high in subgroups of anticoagulated patients with AF/atrial flutter, who may benefit from routine pre-procedural transoesophageal echocardiography before cardioversion or catheter ablation.
Gencer B, Djousse L, Al-Ramady OT, et al. Effect of long-term marine ω-3 fatty acids supplementation on the risk of atrial fibrillation in randomized controlled trials of cardiovascular outcomes: a systematic review and meta-analysis. Circulation 2021;144:1981–90. https://doi.org/10.1161/ CIRCULATIONAHA.121.055654; PMID: 34612056.
• In randomised controlled trials examining cardiovascular outcomes, marine ω-3 supplementation was associated with an increased risk of AF.
Schmidt AS, Lauridsen KG, Møller DS, et al. Anterior-lateral versus anteriorposterior electrode position for cardioverting atrial fibrillation. Circulation 2021;144:1995–2003. https://doi.org/10.1161/circulationaha.121.056301 PMID: 34814700.
• Anterior-lateral electrode positioning was more effective than anterior-posterior electrode positioning for biphasic cardioversion of AF.
Squara F, Elbaum C, Garret G, et al. Active compression versus standard anterior-posterior defibrillation for external cardioversion of atrial fibrillation: a prospective randomized study. Heart Rhythm 2021;18:360–5. https://doi.org/10.1016/j.hrthm.2020.11.005; PMID: 33181323.
• Active compression applied to the anterior defibrillation electrode is more effective for persistent AF cardioversion than standard
anterior-posterior cardioversion, with lower defibrillation threshold and higher success rate.
Lip GYH, Tran G, Genaidy A, et al. Improving dynamic stroke risk prediction in non-anticoagulated patients with and without atrial fibrillation: comparing common clinical risk scores and machine learning algorithms. Eur Heart J Qual Care Clin Outcomes 2021. https://doi.org/10.1093/ ehjqcco/qcab037; PMID: 33999139; online ahead of press.
• Large improvements in stroke risk prediction can be shown with a multimorbid index and a machine learning approach incorporating changes in risk related to ageing and incident comorbidities.
Chao TF, Joung B, Takahashi Y, et al. 2021 Focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost 2022;122:20–47. https://doi.org/10.1055/s-0041-1739411; PMID: 34773920.
• Guidelines on stroke prevention in AF from the Asia Pacific Heart Rhythm Society.
Ventricular Arrhythmias
Muser D, Nucifora G, Pieroni M, et al. Prognostic value of non-ischemic ring-like left ventricular scar in patients with apparently idiopathic nonsustained ventricular arrhythmias. Circulation 2021;143:1359–73. https:// doi.org/10.1161/CIRCULATIONAHA.120.047640; PMID: 33401956.
• In patients with apparently idiopathic non-sustained ventricular arrhythmias, non-ischaemic left ventricular scar with a ringlike pattern is associated with malignant arrhythmic events.
Cadrin-Tourigny J, Bosman LP, Wang W, et al. Sudden cardiac death prediction in arrhythmogenic right ventricular cardiomyopathy: a multinational collaboration. Circ Arrhythm Electrophysiol 2021;14:e008509. https://doi.org/10.1161/circep.120.008509; PMID: 33296238.
• Life-threatening ventricular arrhythmic events in patients with arrhythmogenic right ventricular cardiomyopathy can be predicted by a novel simple prediction model using only four clinical predictors.
EDITORIAL © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Foreword
Syncope
Sheldon R, Faris P, Tang A, et al. Midodrine for the prevention of vasovagal syncope: a randomized clinical trial. Ann Intern Med 2021;174:1349–56. https://doi.org/10.7326/M20-5415; PMID: 34339231.
• Midodrine can reduce the recurrence of syncope in healthy, younger patients with a high syncope burden.
Electrophysiology and Ablation
DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid Convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation: results of CONVERGE clinical trial. Circ Arrhythm Electrophysiol 2020;13:e009288. https://doi.org/10.1161/circep.120.009288; PMID: 33185144.
• The Hybrid Convergent procedure has superior effectiveness compared to catheter ablation for the treatment of persistent and long-standing persistent AF.
Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. https://doi.org/10.1056/nejmoa2029980; PMID: 33197159.
• Among patients receiving initial treatment for symptomatic, paroxysmal AF, there was a significantly lower rate of AF recurrence with catheter cryoballoon ablation than with antiarrhythmic drug therapy, as assessed by continuous cardiac rhythm monitoring.
Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. https://doi. org/10.1056/nejmoa2029554; PMID: 33197158.
• Cryoballoon ablation as initial therapy was superior to drug therapy for the prevention of atrial arrhythmia recurrence in patients with paroxysmal AF.
Kuniss M, Pavlovic N, Velagic V, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. Europace 2021;23:1033–41. https://doi.org/10.1093/europace/ euab029; PMID: 33728429.
• Cryoballoon catheter ablation was superior to anti-arrhythmic drug therapy, significantly reducing atrial arrhythmia recurrences in treatment naive patients with paroxysmal AF.
Heeger CH, Sohns C, Pott A, et al. Phrenic nerve injury during cryoballoonbased pulmonary vein isolation: results of the worldwide YETI registry. Circ Arrhythm Electrophysiol 2022;15:e010516. https://doi.org/10.1161/ CIRCEP.121.010516; PMID: 34962134.
• The incidence of phrenic nerve injury (PNI) during cryoballoon-based pulmonary vein isolation was 4.2%. Overall 97% of PNI cases recovered within 12 months.
Calkins H, Gache L, Frame D, et al. Predictive value of atrial fibrillation during the postradiofrequency ablation blanking period. Heart Rhythm 2021;18:366–73. https://doi.org/10.1016/j.hrthm.2020.11.020; PMID: 33242668.
• Freedom from AF recurrence during the blanking period is highly predictive of longer-term success in catheter ablation.
Katritsis DG, Marine JE, Katritsis G, et al. Spatial characterization of the tachycardia circuit of atrioventricular nodal re-entrant tachycardia. Europace 2021;23:1596–602. https://doi.org/10.1093/europace/euab130; PMID: 34240123.
• Successful ablation affects the tachycardia circuit without necessarily
abolishing slow conduction, probably by interrupting the circuit at the septal isthmus.
Reddy VY, Dukkipati SR, Neuzil P, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614–27. https://doi.org/10.1016/j. jacep.2021.02.014; PMID: 33933412.
• Pulmonary vein isolation (PVI) with a ‘single-shot’ pulse-field-ablation catheter results in excellent PVI durability and acceptable safety, with a low 1-year rate of atrial arrhythmia recurrence
Brignole M, Pentimalli F, Palmisano P, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J 2021;42:4731–9. https://doi.org/10.1093/eurheartj/ehab569; PMID: 34453840.
• Ablation plus cardiac resynchronisation was superior to pharmacological therapy in reducing mortality in patients with permanent AF and narrow QRS on ECG who had been hospitalised for heart failure, irrespective of their baseline left ventricular ejection fraction.
Katritsis G, Luther V, Jamil-Copley S, et al. Postinfarct ventricular tachycardia substrate: characterization and ablation of conduction channels using ripple mapping. Heart Rhythm 2021;18:1682–90. https:// doi.org/10.1016/j.hrthm.2021.05.016; PMID: 34004345.
• Conduction channels can be located using ripple mapping to analyse scar potentials. Ablation at channel entrances can eliminate scar-related potentials and is associated with good medium-term results.
Cardiac Implanted Electronic Devices
Gold MR, Lambiase PD, El-Chami MF, et al. Primary results from the understanding outcomes with the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation 2021;143:7–17. https://doi.org/10.1161/CIRCULATIONAHA.120.048728; PMID: 33073614.
• This study demonstrates high efficacy and safety with contemporary subcutaneous-ICD devices and programming despite the relatively high incidence of comorbidities in comparison with earlier subcutaneous-ICD trials.
Schaller RD, Brunker T, Riley MP, et al. Magnetic resonance imaging in patients with cardiac implantable electronic devices with abandoned leads. JAMA Cardiol 2021;6:549–56. https://doi.org/10.1001/ jamacardio.2020.7572; PMID: 33595595.
• The risk of MRI in patients with abandoned cardiac implanted electronic device leads was low in this large observational study, including patients who underwent examination of the thorax.
Vijayaraman P, Ponnusamy OC, Sharma PS, et al. Left bundle branch area pacing for cardiac resynchronization therapy: results from the International LBBASP Collaborative Study Group. JACC Clin Electrophysiol 2021;7:135–47. https://doi.org/10.1016/j.jacep.2020.08.015; PMID: 33602393.
• Left bundle area pacing is feasible and safe and provides an alternative option for cardiac resynchronisation therapy.
Vinther M, Risum N, Svendsen JH, et al. A randomized trial of His pacing versus biventricular pacing in symptomatic heart failure patients with left bundle branch block (His-Alternative). JACC Clin Electrophysiol 2021;7:1422–32. https://doi.org/10.1016/j.jacep.2021.04.003; PMID: 34167929.
Important Publications on Electrophysiology in 2021 ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
• In heart failure patients with left bundle branch block, His-based cardiac resynchronisation therapy provided similar clinical and physical improvement compared with biventricular cardiac resynchronisation therapy at the expense of higher pacing thresholds.
Basic Science and Future Directions
Ng FS, Toman O, Petru J, et al. Novel low-voltage multipulse therapy to terminate atrial fibrillation. JACC Clin Electrophysiol 2021;7:988–99. https://doi.org/10.1016/j.jacep.2020.12.014; PMID: 33812836.
• Low-voltage MultiPulse Therapy effectively terminated AF at voltages and energies known to be well tolerated or painless in some patients.
Choi YS, Yin RT, Pfenniger A, et al. Fully implantable and bioresorbable cardiac pacemakers without leads or batteries. Nat Biotechnol 2021;39:1228–38. https://doi.org/10.1038/s41587-021-00948-x; PMID: 34183859.
• A report of leadless, battery-free, fully implantable cardiac
pacemaker for postoperative control of cardiac rate and rhythm that undergoes complete dissolution and clearance by natural biological processes after a defined operating timeframe.
Rogers AJ, Selvalingam A, Alhusseini MI, et al. Machine learned cellular phenotypes in cardiomyopathy predict sudden death. Circ Res 2021;128:172–84. https://doi.org/10.1161/circresaha.120.317345 PMID: 33167779.
• Machine learning of action potential recordings in patients revealed novel phenotypes for long-term outcomes in ischaemic cardiomyopathy.
Giudicessi JR, Schram M, Bos JM, et al. Artificial intelligence-enabled assessment of the heart rate corrected QT Interval using a mobile electrocardiogram device. Circulation 2021;143:1274–86. https://doi. org/10.1161/circulationaha.120.050231; PMID: 33517677.
• Using smartphone-enabled electrodes, an artificial-intelligence deep neural network can predict accurately the QTc of a standard 12-lead ECG.
Important Publications on Electrophysiology in 2021 ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Ablation Lesion Assessment with MRI
Lluís Mont , 1,2,3 Ivo Roca-Luque 1,2,3 and Till F Althoff 1,2,4,5
1. Arrhythmia Section, Cardiovascular Institute, Clínic – University Hospital Barcelona, Barcelona, Catalonia, Spain; 2. Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Catalonia, Spain; 3. Centro de Investigación Biomédica en Red Cardiovascular (CIBERCV), Madrid, Spain; 4. Department of Cardiology and Angiology, Charité University Medicine Berlin, Berlin, Germany; 5. German Centre for Cardiovascular Research (DZHK), Berlin, Germany
Abstract
Late gadolinium enhancement (LGE) MRI is capable of detecting not only native cardiac fibrosis, but also ablation-induced scarring. Thus, it offers the unique opportunity to assess ablation lesions non-invasively. In the atrium, LGE-MRI has been shown to accurately detect and localise gaps in ablation lines. With a negative predictive value close to 100% it can reliably rule out pulmonary vein reconnection non-invasively and thus may avoid unnecessary invasive repeat procedures where a pulmonary vein isolation only approach is pursued. Even LGE-MRI-guided repeat pulmonary vein isolation has been demonstrated to be feasible as a standalone approach. LGE-MRI-based lesion assessment may also be of value to evaluate the efficacy of ventricular ablation. In this respect, the elimination of LGE-MRI-detected arrhythmogenic substrate may serve as a potential endpoint, but validation in clinical studies is lacking. Despite holding great promise, the widespread use of LGE-MRI is still limited by the absence of standardised protocols for image acquisition and post-processing. In particular, reproducibility across different centres is impeded by inconsistent thresholds and internal references to define fibrosis. Thus, uniform methodological and analytical standards are warranted to foster a broader implementation in clinical practice.
Keywords
Late gadolinium enhancement, MRI, ablation lesion, fibrosis
Disclosure: LM has received honoraria as a lecturer and consultant and research grants from Abbott Medical, Biosense Webster, Boston Scientific and Medtronic; and is a shareholder of Galgo Medical. All other authors have no conflicts of interest to declare.
Funding: The authors have received funding outside the present work from the Instituto de Salud Carlos III (FIS_PI16/00435 – FIS_CIBER16, CB16/11/00354) and Fundació la Marató de TV3 (20152730).
Received: 16 October 2021
Accepted: 11 December 2021
Citation: Arrhythmia & Electrophysiology Review 2022;11:e02. DOI: https://doi.org/10.15420/aer.2021.63
Correspondence: Till F Althoff, Arrhythmia Section, Cardiovascular Institute, Clínic – University Hospital Barcelona, C/ Villarroel 170, 08036 Barcelona, Spain. E: althoff@clinic.cat
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Late gadolinium enhancement (LGE) cardiac MRI is increasingly used to detect cardiac fibrosis in the context of arrhythmias.1–4 Fibrosis is a hallmark of arrhythmogenic cardiac remodelling and constitutes an important substrate in both atrial and ventricular arrhythmias.2 3
Of note, by exploiting the slow washout kinetics of gadolinium in extracellular space, LGE-MRI is not only capable of determining native fibrotic tissue, but also of detecting ablation-induced scarring.5–13 Several groups have reported LGE-MRI-based localisation of functional gaps in atrial ablation lesions with high accuracy, and even LGE-MRI-guided repeat pulmonary vein isolation (PV) has been demonstrated to be efficient and effective as a standalone approach.5 7 14 Although the use of LGE-MRI for ventricular ablation lesion assessment is lagging behind compared to the atrium, feasibility has been demonstrated in preclinical and early clinical studies.10 15 16 However, initial data on the accuracy of LGE-MRI-based lesion assessment were somewhat conflicting.5 6 17 18 This limited reproducibility of promising results across centres may have been because of differences in the methods of image acquisition, postprocessing and analysis.19 In addition, we have generated evidence that the timing of image acquisition with respect to different stages of lesion formation and scar remodelling also has to be considered.20
This review focuses on the assessment of chronic ablation lesions using LGE-MRI and its utility in clinical practice. The scope of this article does not include real-time monitoring of lesion formation through intraprocedural MRI, which – despite holding great promise – is still of limited clinical relevance because of current structural and technical limitations related to electromagnetic interference, as well as the relative absence of acute parameters that accurately predict definite lesion formation.21
Pathology of Lesion Formation
The pathology of radiofrequency (RF) ablation injury is well established in animal models and patients and is characterised by coagulation necrosis, haemorrhage and complete loss of cellular and vascular architecture, apart from a narrow peripheral transition zone.9 22–25 The response to ablation injury implies infiltration of immune cells and neovascularisation, with local inflammation and interstitial oedema being observed up to 8 weeks post-ablation.11 23,25 In parallel, activated fibroblasts proliferate and differentiate into myofibroblasts that generate fibrogenic signals, which perpetuate tissue repair and promote collagen deposition resulting in replacement of myocardium with fibrous scar tissue.22,24–26 While RF ablation and cryoablation fundamentally differ in the acute effect on the tissue and the mechanism of cell death, most of these basic principles of
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Clinical Electrophysiology and Ablation
Table 1: Image Post-processing (Normalisation and Thresholds to Define Lesions)
Oakes et al. 200939 Normal tissue (“lower region of the pixel intensity histogram between 2% and 40% of the maximum intensity within the region of interest [e.g. the left atrial wall]”)
Khurram et al. 201450
Mean LA blood pool signal intensity
Benito et al. 201738 Mean LA blood pool signal intensity
Harrison et al. 201517
Mean LA blood pool signal intensity
User-selected individual threshold (2–4 SD above the mean of ‘normal’†, based on the investigators’ discretion)
Universal threshold (upper limit of normal: IIR 0.97; dense scar: >1.6)
Universal threshold (upper limit of normal: IIR 1.2; dense scar: >1.32)
1.5 T, 15 min post gadolinium (0.1 mmol/kg Multihance [Braco Diagnostic],* 0.5 M)
1.5 T, 15–25 min post gadolinium (0.2 mmol/kg Magnevist [Bayer],* 0.5 M)
3 T, 20 min post gadolinium (0.2 mmol/kg Gadovist [Bayer], 1.0 M)
No fixed threshold, but visualisation of signal intensities in SD from reference 1.5 T, 20 min post gadolinium (0.2 mmol/kg Gadovist, 1.0 M)
Jefairi et al. 201951 Maximum signal intensity Universal threshold with possible individual adaptation (>50% maximum signal intensity)
Peters et al. 200740 LA blood pool signal intensity
1.5 T, 17 min post gadolinium (0.2 mmol/kg Dotarem [Guerbet], 0.5 M)
“Minimum threshold which eliminates most left atrial blood pool pixels” 1.5 T, 20–25 min post gadolinium (0.2 mmol/kg Magnevist,* 0.5 M)
Kurose et al. 202069 ‘Healthy’ LA wall >2 SDs above the mean of “healthy” LA wall 1.5 T, 15 min post gadolinium (0.1 mmol/kg Gadovist, 1.0 M)
Ventricular Lesion Assessment
Cochet et al. 201358 Maximal myocardial signal
Fernandez-Armenta et al. 201357
Maximal myocardial signal
Yan et al. 200653 Remote (healthy) myocardial segment
35–50% (BZ) or >50% (scar) of maximal signal intensity 1.5 T, 15 min post gadolinium (0.2 mmol/kg Dotarem, 0.5 M)
40–60% (BZ) or >60% (scar) of maximal signal intensity
2–3 SDs (BZ) or >3 SDs (scar) above remote myocardium
1.5 and 3 T, 7–10 min post gadolinium (0.2 mmol/kg Omniscan [GE Healthcare],* 0.5 M)
1.5 T, 10–15 min post gadolinium (0.15–0.2 mmol/kg Magnevist,* 0.5 M)
*Authorisation of these linear gadolinium-based contrast agents for cardiac MRI has been suspended in the EU. †Mean of ‘normal’ indicates the average signal intensity of this area. BZ = border zone; IIR = image intensity ratio (the ratio between the signal intensity of each single pixel and the mean LA blood pool intensity for each patient); LA = left atrium.
RF-ablation-induced scar formation appear to apply similarly to cryoablation, albeit less well established and with an arguably more preserved ultrastructural tissue integrity.27,28
It has been shown that scar formation and remodelling in response to MI is a dynamic and chronically sustained process that continues over years after the initial injury.29,30 Once recruited to injured myocardium, fibroblasts persist in the infarct scar for years where they continue to generate fibrogenic signals that perpetuate tissue repair and promote fibrosis.29,30 While data from long-term longitudinal studies on ablation-induced scarring are lacking, a recent analysis of post-mortem cardiac samples from patients with previous ventricular tachycardia (VT) ablation, indicates that we have to consider such long-term remodelling processes also in response to catheter ablation.25
LGE-MRI for the Detection of Ablation-induced Fibrosis Basic Principles
LGE-MRI for the detection of myocardial fibrosis was first used and histologically validated in a canine model of MI.31 Despite pathophysiological differences, both cardiac ablation and MI result in coagulation necrosis, loss of syncytial membrane integrity and eventually replacement fibrosis. LGE-MRI makes use of the expansion of extracellular space and thus increased volume of distribution for the contrast agent that is associated with replacement fibrosis, as well as the prolonged washout owing to decreased capillary density within the myocardial fibrotic tissue.32,33 Gadolinium-based contrast agents diffuse freely into the interstitium, but they cannot cross intact cell membranes and thus accumulate in the extracellular space. As gadolinium contrast agents
reduce the T1 relaxation time of adjacent tissue, LGE enhancement results in an increased signal intensity in T1-weighted MRI sequences. It is noteworthy though that LGE is not specific for fibrotic tissue, but can reflect other pathological processes associated with an expansion of the extracellular space such as inflammation and oedema formation, which impedes definite lesion assessment, particularly in the acute setting.34
Timing of Gadolinium Application
As indicated above, besides interstitial volume of distribution, LGE is determined by wash-in and washout kinetics of the contrast agent. Thus, the exact time delay between contrast administration and image acquisition is critical. While image acquisition is typically performed 7–15 minutes (ventricle) or 15–25 minutes (atrium) post contrast agent injection for differential spatial contrast between scar and normal tissue, there is no consensus among different centres. Moreover, in some cases the time delay may even be adapted, based on individual perfusion (cardiovascular function) and washout kinetics (renal function). At our centre, we acquire atrial images 20 minutes after an intravenous bolus of 0.2 mmol/kg of gadobutrol, whereas in the ventricle we acquire images 7–10 minutes after gadolinium injection.
Of note, the time to allow for gadolinium to enter lesions appears to be of particular relevance regarding the so-called dark core phenomenon. This phenomenon is characterised by a hypoenhanced region (dark core) within ablation lesions surrounded by a peripheral rim of LGE.11 15 16 35 The exact pathological correlate underlying this phenomenon remains unknown, but microvascular obstruction impeding gadolinium wash-in is likely to play a role (no reflow). Possibly owing to a lack of functional capillaries, gadolinium appears to enter ablation lesions via diffusion from
Ablation Lesion Assessment with MRI ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Thresholds
Atrial
Internal Reference for Normalisation
Defining Atrial Lesions Image Acquisition
Lesion Assessment
the lesion periphery.9 11 This hypothesis is based on the centripetal expansion of LGE towards the lesion centre resulting in a diminishment of the hypoenhanced dark core, which is observed with increasing time delays between gadolinium administration and image acquisition allowing for longer diffusion times.9,11
Contrast Agent and Dosage
The impact of different gadolinium-based contrast agents and doses on image quality are of particular interest in light of the recent safety concerns, not only regarding the induction of nephrogenic systemic fibrosis but also with respect to cerebral gadolinium deposits, the longterm clinical relevance of which is still unknown. Against this background, there may be a rationale to reduce doses of gadolinium-based contrast agents. While there has been no head-to-head-comparison of the different agents in the context of cardiac LGE imaging, recent data showed that Gadovist (Bayer) at 0.10 mmol/kg provides inferior LGE image quality than 0.15 and 0.20 mmol/kg with respect to ventricular scar assessment (image acquisition at a median of 9 minutes post gadolinium injection).36 Interestingly, there is evidence suggesting that scans with 3T provide a signal-to-noise ratio that allows for better delineation of scarred myocardium than 1.5 T scans, even with lower contrast concentration (0.10 versus 0.20 mmol/kg Dotarem [Guerbet]), but again, systematic comparative data are lacking.37
While the above-mentioned results obtained for Gadovist and Dotarem, respectively, may not be generalisable to other gadolinium-based contrast agents, it has to be noted that, of the contrast agents presented in this review, only Gadovist and Dotarem are authorised for cardiac MRI in the EU (Table 1).
Image Acquisition
While several protocols have been detailed and validated previously, to date no consensus has been reached regarding standardised image acquisition and magnetic resonance (MR) sequence.9 38–40 Typically 1.5 or 3 T scanners are used for post-contrast image acquisition employing fast 3D gradient echo sequences with ECG-gating and fat suppression. Low flip angles are applied to reduce saturation effects with short repetition times. To further optimise T1 contrast and signal intensities, inversion recovery sequences nullifying the signal of healthy ventricular myocardium are employed. Here, the optimum inversion time (TI) suppressing healthy myocardium (typically 250–300 ms) is determined empirically using a TI scout module prior to the acquisition of definite images. Healthy myocardium will thus appear hypoenhanced relative to scar tissue. TI values may have to be adapted during the scan to accommodate incremental T1 values of the normal myocardium owed to gadolinium washout.
To minimise cardiac motion artefacts, ECG gating is usually performed with the image acquisition window limited to <20% of the RR interval (typically 150–200 ms) and a trigger delay corresponding to atrial or ventricular mid-diastole, sparing atrial and ventricular contraction, respectively. In tachyarrhythmic patients, the trigger delay can be adapted according to the mean RR interval. However, we and others found signalto-noise ratios substantially reduced in patients with AF.39,41 Therefore, we strongly recommend cardioverting patients prior to the LGE-MRI study to avoid insufficient image quality. Long breath-holds required for image acquisition may be a limiting factor in some patients, a problem that is addressed by free-breathing 3D navigators that suppress respiratory motion artefacts through respiratory gating. Typical LGE-MRI sequences result in a voxel size of 1.25 x 1.25 x 2.5 mm with scan times of 10–15 minutes, depending on heart rate and breathing patterns.
The use of cardiac MRI in patients with implanted cardiac devices has been limited not only because of safety concerns, but also due to hyperintense image artefacts. While numerous studies and the advent of MR conditional cardiac pacemakers and ICDs have largely dispelled the safety concerns, image artefacts have remained a major limitation.42–44 The artefacts are the result of significant distortion of the MRI magnetic field induced by the metallic pacemaker or ICD components.45 46 They are typically located in the proximity (5–10 cm) of the device, with the distance being inversely associated with artefact size.45,46 Of note, the artefacts are particularly pronounced in LGE-MRI sequences as applied for ablation lesion assessment. The use of lower magnetic field strength and shorter echo times has been shown to reduce artefacts, but this may be at the cost of image signal intensity and contrast.46 Recently, based on the hypothesis that the device-related artefacts are caused by the limited spectral bandwidth of the inversion pulse that is typically applied in LGEMRI, specific wideband MR sequences have been established.47 48 We and other centres are now successfully employing these sequences, enabling high image quality without hyperintensity artefacts, even in the proximity of implanted devices.49
Image Post-processing
Several established open-source and commercial platforms for image post-processing are available. Most of these enable semiautomatic segmentation where manual tracings of the endocardial and/or epicardial borders are automatically adjusted to build a 3D anatomical shell. Relative signal intensities are then colour-coded and projected onto the 3D anatomical shell to create a relative LGE map discriminating healthy myocardium from scar tissue based on predefined thresholds. Some postprocessing software even allows for integration of LGE maps into common electroanatomical mapping (EAM) systems.
As mentioned above, to date there is no standardised method for LGE image acquisition, and the same applies to image post-processing and analysis, which may explain the limited reproducibility across different centres. Most importantly, as T1-weighted imaging is based on signal intensity contrast rather than directly measured absolute values, LGE quantification requires a consistent internal reference for normalisation as well as validated signal intensity thresholds discriminating healthy and scar tissue.
While methods using normalisation based on the somewhat arbitrary definition of healthy atrial myocardium have been described for atrial lesion assessment, we and others use the mean signal intensity of the blood pool as an internal reference. Our group has recently established a method quantifying signal intensity ratios using the mean signal intensity of the left atrial (LA) blood pool as a reference (signal intensity of each given voxel/mean signal intensity of the blood).38 Thresholds to define healthy myocardium (signal intensity ratio ≤1.2) and ablation-induced scarring (signal intensity ratio >1.32) in the atrium were derived from distinct cohorts of young healthy individuals as well as post-AF ablation patients, respectively, and subsequently validated in numerous clinical studies with respect to electroanatomical voltage mapping as well as clinical endpoints.5–7 However, it should be emphasised that various other methods using distinct internal references and thresholds have been validated (Table 1).17 39,50,51
For ventricular lesion assessment the ‘full width half maximum’ method is the most commonly used approach, although other methods defining remote ‘healthy’ myocardial segments as an internal reference for normalisation have been described.52–55 We found that the best agreement
Ablation Lesion Assessment with MRI ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 1: Ablation-induced Late Gadolinium Enhancement After Pulmonary Vein Isolation
with electroanatomical voltage mapping was achieved when applying thresholds of >60% (dense scar) and <40% (healthy tissue) of the maximum signal intensity, but again, other thresholds have been proposed too (Table 1).56–59
Timing of Lesion Assessment
As outlined above, we have to assume that ablation lesion formation is a dynamic process of sustained remodelling, which implies constant changes affecting cellular composition, extracellular space, water content and vascularisation, as well as small-vessel permeability and patency and intravascular pressure. These changes in turn, inevitably alter tissueinherent magnetic properties and wash-in/washout kinetics of gadolium.60,61 In fact, we found determination of definite atrial ablation lesions by LGE-MRI to be more accurate at 3 months post ablation than at later time points >12 months after ablation.20 Thus, it is evident that lesion assessment by LGE-MRI is dependent on the exact time point. Moreover, LGE is not specific for fibrosis and does not necessarily indicate formation of durable ablation lesions. In fact, particularly in the acute setting, LGE may represent oedema reflecting a transient inflammatory response, which usually resolves within the first month following ablation.11 62 In addition, the sensitivity of LGE to detect acute ablation lesions may be locally reduced by the above-mentioned dark core or no reflow phenomena, where limited diffusion times result in central hypoenhanced regions within ablation lesions. As diffusion distances are minimal in the thin-walled atrium, this phenomenon seems to be less relevant in the context of atrial ablation, where central hypoenhanced regions have only been observed in acute lesions.35 Even in the ventricle, these hypoenhanced regions are less frequently encountered in chronic lesions >1 month post-ablation, possibly because of on-going remodelling of scar tissue including neovascularisation, although data are somewhat conflicting in this regard (see the section Ventricular Ablation Lesions, below).11 15 16 63
Taken together, these time-dependent limitations may argue for a late timing of LGE-MRI-based lesion assessment. However, we have recently found a decreased detectability of atrial ablation lesions at very late timepoints, >12 months post-ablation, compared with an assessment at 3 months post ablation.20 The long-term decrease in LGE of pulmonary vein (PV)-encircling ablation lesions observed in this study could in theory also reflect true regression of ablation-induced fibrosis; in fact, such a phenomenon has been proposed previously as a possible explanation for non-durable ablation lesions and late AF recurrences. However, using invasive high-density mapping as a reference, we found the decrease in LGE over time to be because of reduced detectability of ablation-induced fibrosis by LGE-MRI at time-points >12 months post ablation. Again, the pathological correlate underlying this observation is unclear, but likely to involve on-going remodelling altering tissue-inherent magnetic properties and wash-in/washout kinetics of the gadolinium contrast agent.
Against this background, the time-point of 3 months post ablation that has become an established standard for lesion assessment with LGE-MRI in many labs, appears reasonable. This time-point has been shown to reliably indicate chronic lesion formation in the atrium and in the ventricle and has been rigorously validated with respect to functional gaps detected by EAM as well as clinical endpoints like AF recurrence.6 62 64 65
Atrial Ablation Lesions
LGE-MRI of the Atrium
While LGE-MRI is a well-established tool to aid or guide VT ablation, its usage for the assessment of fibrosis in the atrium is somewhat lagging behind. This is because of – in part – two reasons. Firstly, unlike the welldemarcated extensive post-MI scar, atrial fibrosis is typically less extensive and more diffuse. This renders detection difficult, as conventional T1weighted MRI relies on differential spatial contrast between normal tissue on one side and abnormal tissue on the other side. Secondly, differentiation
Ablation Lesion Assessment with MRI ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
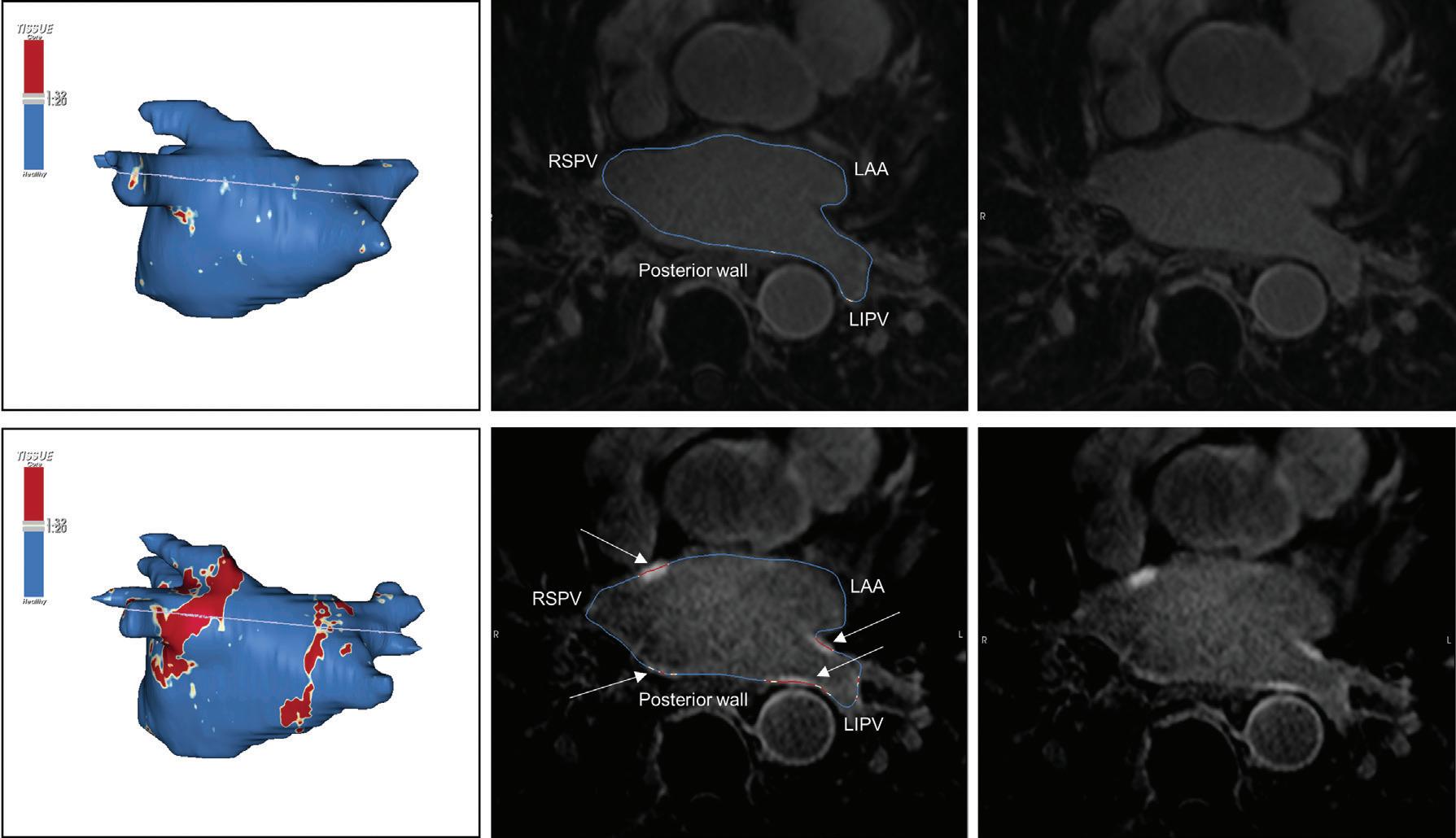
Preprocedural LGE-MRI (1 day before PVI) Post-ablation LGE-MRI (3 months after PVI) LIPV RIPV LSPV RSPV LIPV RIPV LSPV RSPV Postero-anterior view Postero-anterior view
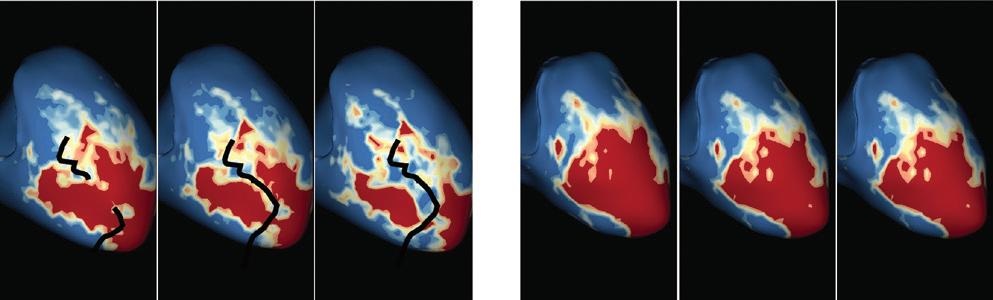
Left: 3D reconstruction of the LA with colour-coding based on image intensity ratios with thresholds for dense scar (red >1.32) and border zone (yellow 1.2–1.32), using ADAS 3D software (Adas3D Medical). Blue lines indicate the plane of the LA slices on the right. Middle: Overlay of the T1-weighted images with the LGE colour-coding described above. White arrows point to local ablation-induced LGE lesions. Right: T1-weighted LGE-MRI slice depicting the LA with evident LGE of PV ostial walls. LA = left atrium; LAA = left atrial appendage; LGE = late gadolinium enhancement; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; PV = pulmonary vein; PVI = pulmonary vein isolation; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
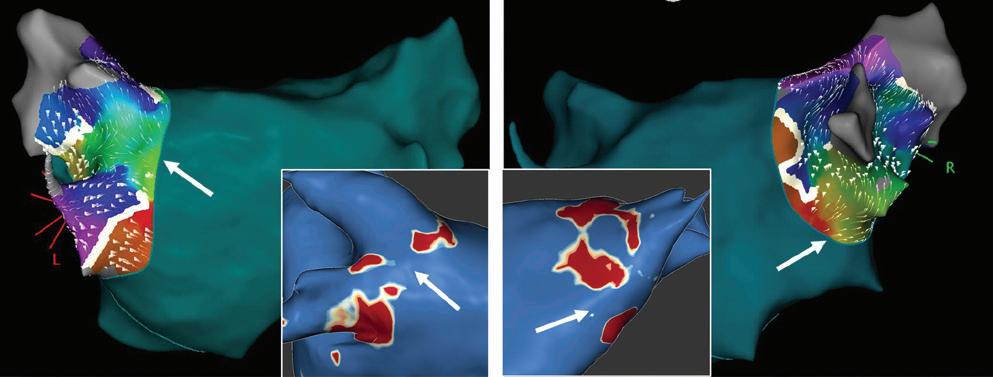
Figure 2: Gaps in Ablation Lesions After Pulmonary Vein Isolation
Examples of discontinuations of ablation-induced LGE lesions encircling the right (A) and left pulmonary veins (B), respectively, in a patient with AF recurrence after PVI. Left: 3D reconstruction of the LA with colour-coding based on image intensity ratios with thresholds for dense scar (red >1.32) and border zone (yellow 1.2–1.32), using ADAS 3D software). White arrows indicate local gaps. Pink lines indicate the plane of the left atrial LGE-MRI slices on the right; Middle: Overlay of the T1-weighted left atrial slices with the LGE colour-coding described above. White arrows indicate local gaps corresponding to the ones indicated in the 3D reconstructions on the left; Right: T1-weighted LGE-MRI slices without colour-coding. LA = left atrium; LAA = left atrial appendage; LGE = late gadolinium enhancement; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; PV = pulmonary vein; PVI = pulmonary vein isolation; RPV = right pulmonary vein; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
of spatial contrast is particularly difficult in the thin-walled atrium with wall thicknesses down to 1 mm, which approximates the limit of spatial resolution of MRI. However, recent advances in MR imaging techniques, such as 3D navigated inversion recovery sequences, have yielded improved resolution and signal-to-noise ratios enabling valid tissue characterisation also in the atrium.39,66 With respect to atrial ablation lesions it has to be considered that these constitute dense scar, which
facilitates discrimination from healthy tissue by LGE-MRI. Thus, even before validation of LGE-MRI for the detection of native atrial fibrosis, it was successfully employed for the assessment of atrial ablation-induced scarring (Figure 1).40,64,67
While initial studies evaluating the capability of LGE-MRI to accurately localise functional gaps within ablation lesions yielded conflicting results,
Ablation Lesion Assessment with MRI ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Preprocedural LGE-MRI (1 day before PVI) A B Post-ablation LGE-MRI (3 months after PVI) Preprocedural LGE-MRI (1 day before PVI) Post-ablation LGE-MRI (3 months after PVI) LIPV LAA RIPV LSPV RSPV LIPV LAA Superior view Superior view Superior view Superior view RIPV LSPV RSPV LIPV LAA RIPV LSPV RSPV LIPV LAA RIPV LSPV RSPV
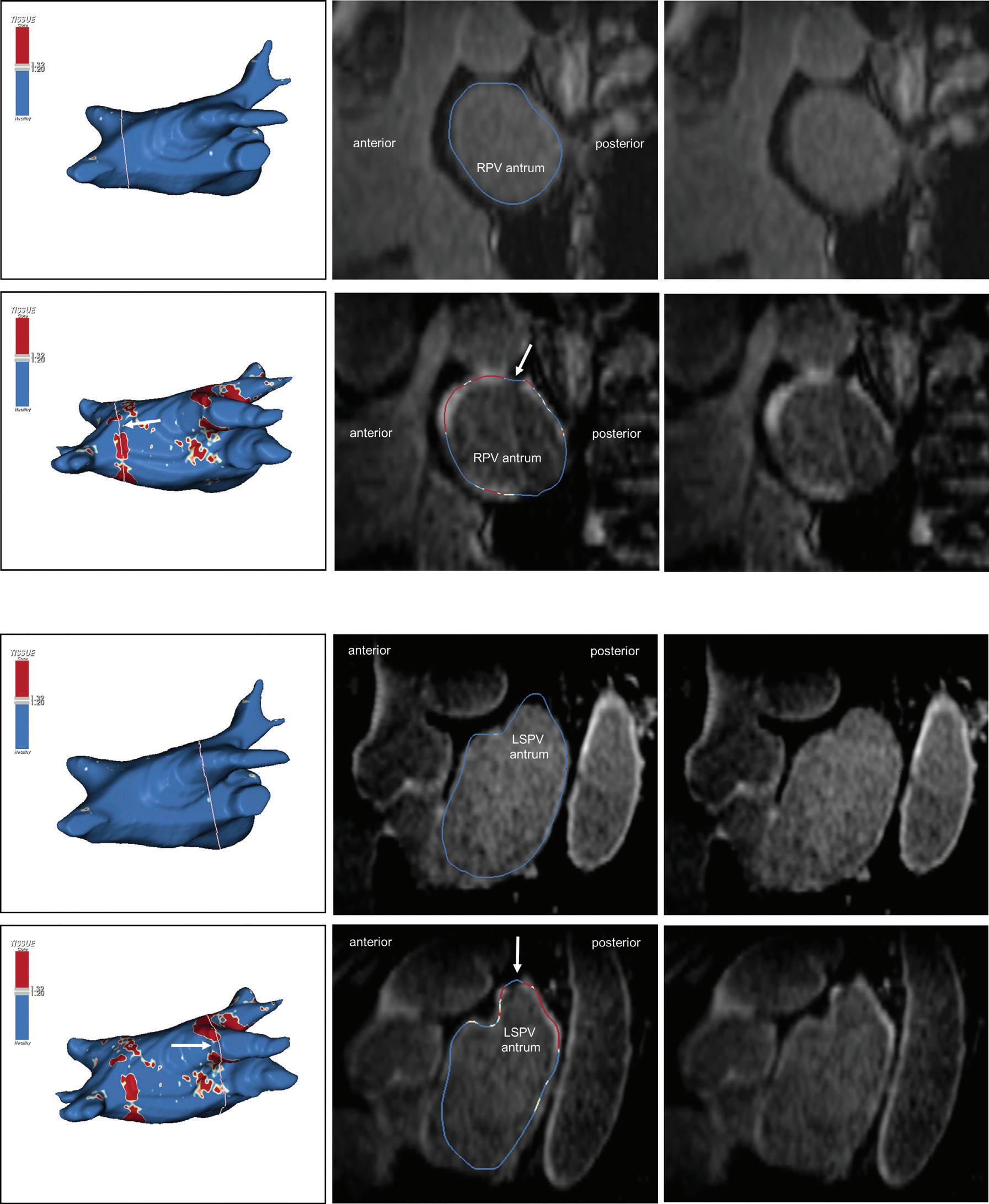
Figure 3: Agreement Between Electroanatomical Mapping and 3 Months Late Gadolinium Enhancement-MRI Regarding Gap Localisation
LGE-MRI-guided Repeat Ablation
Accumulating evidence indicates that LGE-MRI can detect and localise the gaps in ablation lesions with high accuracy (Figure 3).5–7 14 Overall, the accuracy and in particular the high sensitivity in the detection of gaps appears to be sufficient for LGE-MRI-guided repeat ablation – not only in the context of AF (Figure 4), but also with respect to post-ablation reentrant atrial arrhythmias (Figure 5).5 7 14
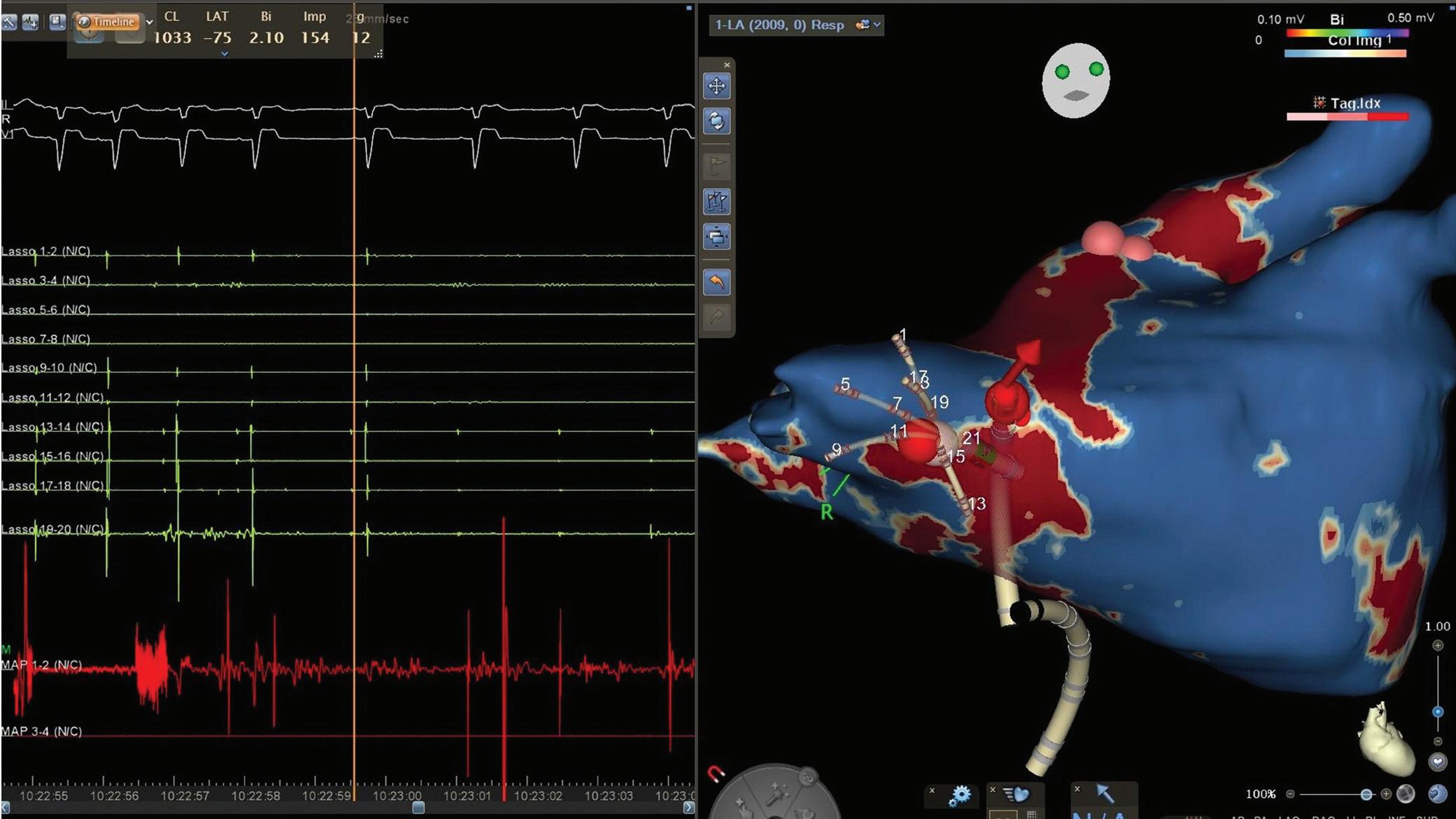
LPV entry
RPV entry
Activation maps of the LPVs and RPVs with conduction vectors (CARTO 3, coherent mapping with Pentaray catheter, Biosense Webster) indicating the entry site of the activation wave front (functional gaps) as detected during a repeat ablation procedure. Corresponding gaps detected by prior late gadolinium enhancement (LGE)-MRI (3 months post index ablation) are displayed in the small boxes. Colour-coding of the LGE maps (ADAS 3D software) is based on image intensity ratios with thresholds for dense scar (>1.32 red) and border zone (1.2–1.32 yellow), respectively. White arrows indicate localised functional gaps and LGE discontinuities, respectively. LPV = left pulmonary vein; RPV = right pulmonary vein.
depending on the performing centre, it has to be taken into account that time-points and protocols for image acquisition as well as post-processing methods varied substantially in these studies.5 6 17 18 As outlined above, this may account for the lack of reproducibility. Promoted by further technological and methodological advances in the last decade, LGE-MRI is now being established as a useful standard for risk stratification, patient selection and lesion assessment in the context of AF ablation in a growing number of specialised centres.2
Of note, the feasibility of lesion assessment with LGE-MRI has been demonstrated, both in the context of RF ablation and cryoablation. Interestingly, apart from wider lesions observed after ablation with the cryoballoon compared with point-by-point RF ablation, both techniques result in quite similar LGE lesion characteristics.68 69
Non-invasive Confirmation of Durable PVI
Most importantly, LGE-MRI can non-invasively evaluate and confirm durable PVI and may thus replace invasive repeat procedures confirming PVI. Today, it is a common practice that symptomatic recurrences beyond the 3 months post-ablation blanking period almost automatically trigger a repeat procedure. However, to an increasing degree, all four PVs are found isolated in those repeat procedures. As ablation of extra-PV targets has failed to show benefit in large randomised trials, more and more often we may end up performing these highly invasive procedures only to confirm durable PVI; or even worse, investigators might feel obliged to ablate extra-PV targets to justify the invasive procedure. Against this background it is noteworthy that LGE-MRI has been shown to be capable of reliably confirming durable PVI with positive predictive values approaching 100%.64 This is consistent with our experience where a complete circumferential LGE lesion set practically rules out PV reconnection. Thus, in patients with circumferential LGE lesions indicating durable PVI, there is no rationale for a repeat procedure, unless one is determined to target extra-PV structures (Figure 1).
However, it should be noted that in earlier studies, complete LGE lesions encircling all four PV were encountered only in around 7–28% of the repeat procedures.64,70 Although we have to assume that these numbers have increased with recent advances in ablation techniques, in our experience the majority of patients still display discontinuities in the LGE lesions (Figure 2).
Bisbal et al. were the first to demonstrate the feasibility of a merely LGEMRI-guided approach in repeat PVI procedures.5 They performed reablation based on a 3D reconstruction of the atrial LGE-MRI, which was integrated and merged into the EAM system, with the investigator blinded to any electrical information. A total of 15 patients underwent this LGEMRI-guided approach, with re-isolation being accomplished in 95.6% of the reconnected PVs. In a subsequent study, the same approach even proved superior to segmental PV re-isolation based on electrical signals.7 However, it has to be taken into account that this was not a randomised trial, but a case–control study with all its potential bias and limitations.
One possible explanation of the putative superiority of the LGE-MRIguided approach could be the higher sensitivity of LGE-MRI regarding the detection of gaps. As outlined above, the negative predictive value of LGE-MRI to rule out gaps is very high.20 However, gaps detected by LGEMRI do not always correspond to a functional gap based on electrical signals. While this may reflect a limited specificity of LGE-MRI and failure to detect local ablation-induced scarring, it may in part be explained by a limited sensitivity of catheter-based gap detection, particularly when conventional catheters are used instead of microelectrode catheters. Moreover, LGE-MRI-determined anatomical gaps might colocalise with non-conductive tissue or a site of dormant conduction, rendering catheterbased gap detection impossible.
Taken together, the higher sensitivity of the LGE-MRI regarding the detection of gaps may be at the cost of specificity, but it is less likely that gaps are omitted. Thus, while segmental electrogram-guided repeat PVI might occasionally result in undertreatment, LGE-MRI potentially leads to a more complete re-ablation.
Incomplete lesion sets can also constitute an arrhythmogenic substrate for reentrant atrial tachycardias, which is more likely in the case of extensive ablation strategies. Fochler et al. recently showed that incomplete lesions and resulting isthmi can be detected by LGE-MRI. They found that a dechannelling approach targeting LGE-MRI-detected isthmi, analogously to VT substrate ablation strategies, may be feasible and appropriate as a standalone approach in patients with recurrent atrial tachycardias after initial AF ablation.14
Lesion Assessment to Predict Ablation Outcome
Besides being a valuable tool for patient selection and guidance of repeat ablation procedures, LGE-MRI-based lesion assessment has yielded several predictors of AF recurrence, with the most obvious one being related to PV reconnection. Interestingly, Linhart et al. found LGE-MRIdetected gaps to predict AF recurrences, but it was not the presence of gaps per se or the number of gaps that predicted recurrences, but the cumulative length of all gaps added together relative to the circumference of ipsilateral PVs. In the 94 patients included in their study, the risk of recurrence increased by 16% with every 10% gap length relative to the PV-encircling ablation line. So, while a single small gap detected by LGEMRI may not be critical with respect to outcome, extensive or multiple gaps are critical.6
Ablation Lesion Assessment with MRI ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 4: Single-touch Late Gadolinium Enhancement-MRI-guided Repeat Pulmonary Vein Isolation
Late gadolinium enhancement (LGE) map (ADAS 3D software) integrated into the 3D mapping system (CARTO 3) for targeted ablation of a single LGE-discontinuity at the right superior pulmonary vein (PV) (right panel) resulting in immediate PV isolation upon radiofrequency application as reflected by the disappearance of the PV electrograms detected by the multipolar mapping catheter (Pentaray, Biosense Webster). Colour-coding of LGE map: Image intensity ratio thresholds for dense scar >1.32 (red) and border zone 1.2–1.32 (yellow).
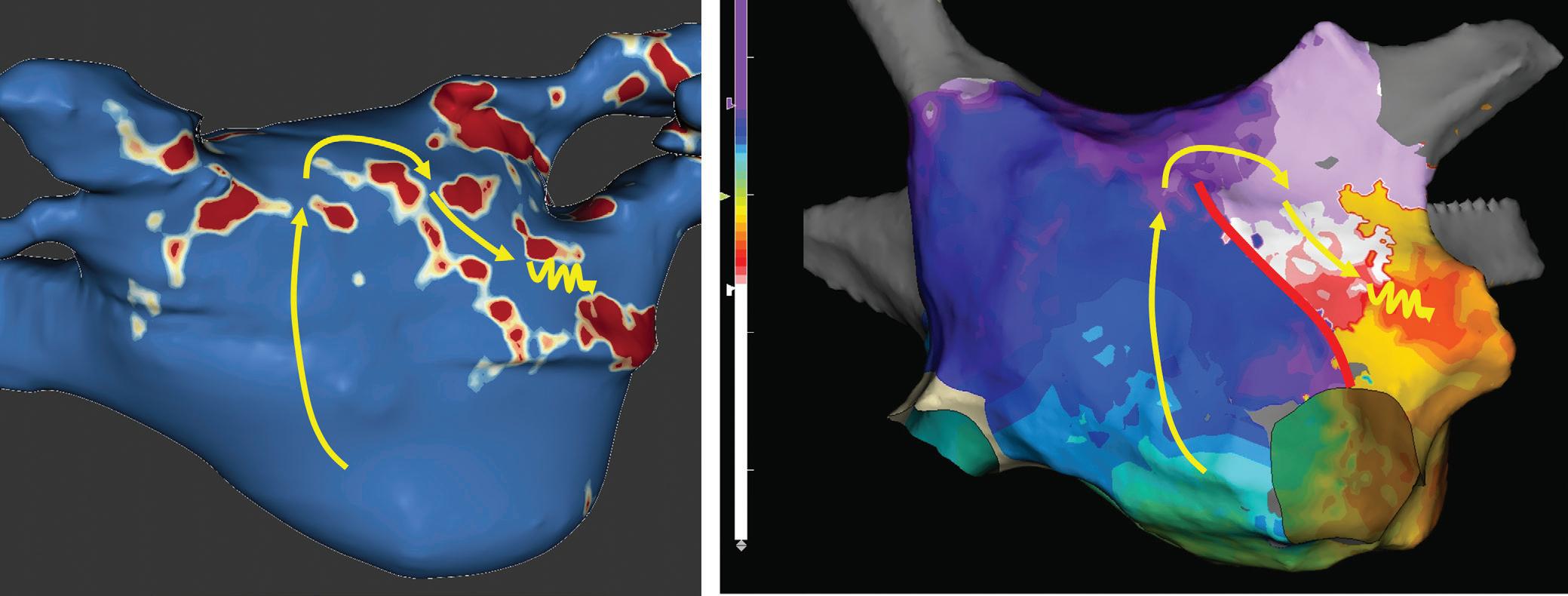
Figure 5: Recurrent Perimitral Flutter After Two Mitral Isthmus Ablations
Left: Late gadolinium enhancement map of the left atrium (LA) 3 months post mitral isthmus re-ablation (ADAS 3D software). The impulse propagation as determined by electroanatomical mapping (activation mapping with HD grid and EnSite Precision [Abbott Medical]) during the repeat procedure is indicated by yellow arrows. These illustrate how lesions from previous ablations force the wave front to go around the LA roof before meandering back to the mitral isthmus through gaps in the ablation line. Dechannelling by ablating the critical isthmus of slow conduction terminated the tachycardia and rendered it non-inducible. Colour-coding of late gadolinium enhancement map: image intensity ratio thresholds for dense scar >1.32 (red) and border zone 1.2–1.32 (yellow).
Right: LA activation during flutter (mapping with HD grid and EnSite Precision). Yellow arrows indicate impulse propagation. Line of conduction block illustrated by red line.
These data are in line with a study by Akoum et al. using LGE-MRI before and 3 months after AF ablation to evaluate ablation lesions and modification of potentially arrhythmogenic substrate.71 They also found the presence of LGE-MRI-detected gaps in PV encircling ablation lines per se not to be predictive of recurrences. However, besides LGE-MRIdetected baseline atrial fibrosis, they identified residual fibrosis, i.e.
fibrotic area not homogenised by ablation, as a predictor of AF recurrence.
Recent work by Kamali et al. assessed ablation lesions and their potential barrier function for electrical propagation in persistent AF.72 They identified the atrial area available for AF to propagate, as determined by LGE-MRI,
Ablation Lesion Assessment with MRI ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
200 ms 130 ms 0 ms -135 ms -200 ms -400 ms -500 ms
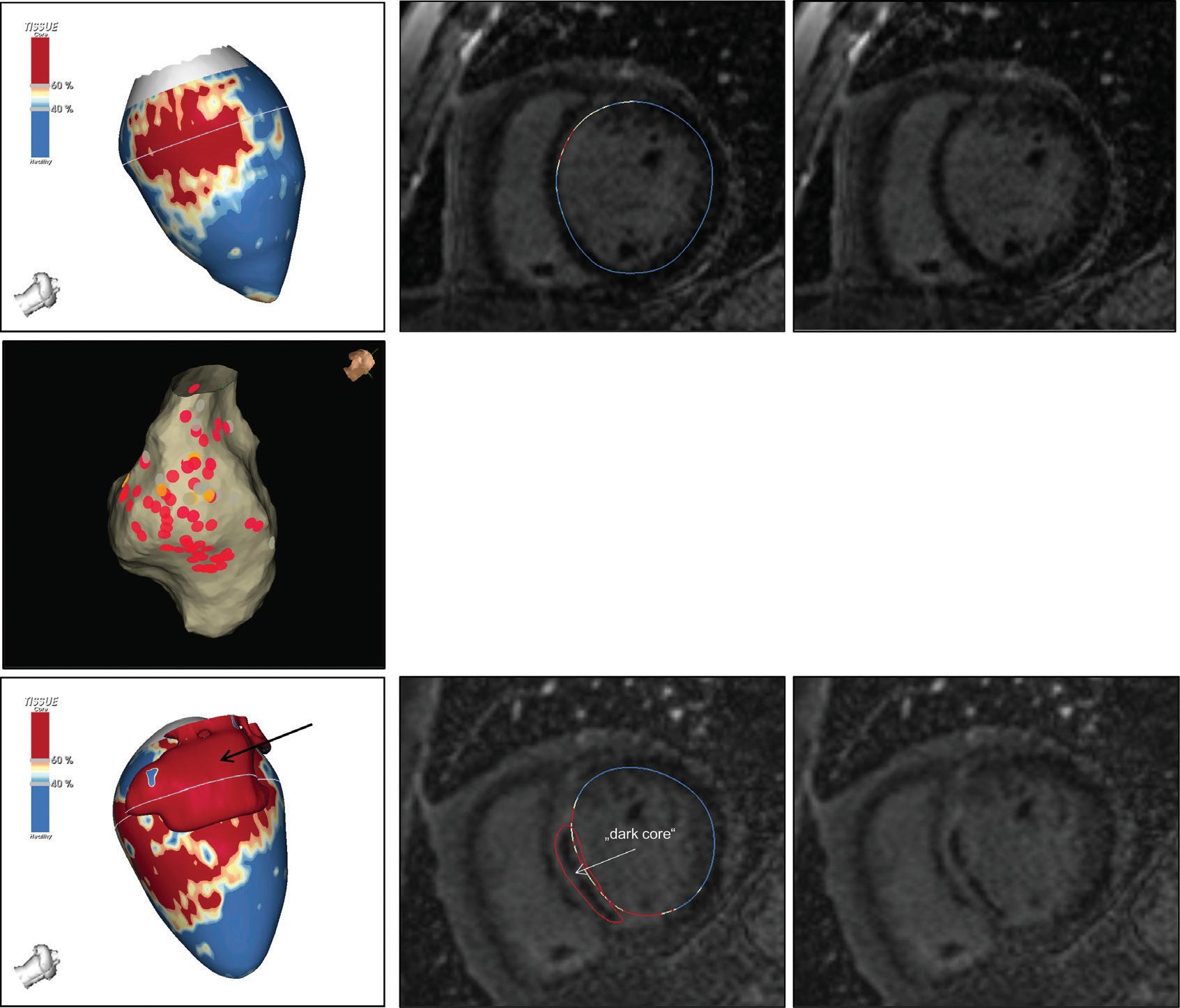
Figure 6: Ventricular Ablation Lesion Assessment
Preprocedural LGE-MRI (1 day before VT ablation)
Ablation points
Post-ablation LGE-MRI (3 months after VT ablation)
Left: 3D reconstruction of the left ventricle with LGE-based colour-coding based on thresholds for dense scar (red, >60% maximum of signal intensity) and border zone (yellow, 40–60% of maximum signal intensity), mapped using ADAS 3D. Shown are the layers at 30% of the transmurality (from endocardial to epicardial). For the post-ablation LGE-MRI (lower panel), an additional 3D reconstruction of the manually defined dark core in red (black arrow) is depicted. Blue lines indicate the plane of the short-axis slices on the right. The ablation points (TactiCath, Abbott Medical) are visualised using a 3D mapping system (EnSite Precision, Abbott Medical). Middle: Overlay of the T1-weighted short-axis slices with the colour-coding described above. The central hypoenhancement dark core of the ablation lesion is manually delineated (red border) to avoid misinterpretation as healthy tissue. Right: T1-weighted short-axis LGE-MRI slices without colour-coding. LGE = late gadolinium enhancement; VT = ventricular tachycardia.
as a predictor of recurrence after catheter ablation. Interestingly, this variable predicted recurrences better than did established predictors such as LA volume or total atrial fibrosis.
Ventricular Ablation Lesions Objective of Lesion Assessment in the Ventricle
Even though LGE-MRI was first established for ventricular tissue characterisation and is by now widely used as a clinical tool to guide VT ablation through detection of arrhythmogenic substrate, there has been less interest in ablation lesion assessment in the ventricle than the atrium. This may be because of the fact that ablation strategies, and thus requirements for non-invasive ablation lesion assessment, are fundamentally different in the ventricle compared to the atrium. While in the atrium continuity and transmurality of predefined lesion sets are assessed, in the ventricle the endpoint is rather elimination or modification of arrhythmogenic substrate. The capability of LGE-MRI to accurately localise arrhythmogenic substrate in terms of scar border zone and slow conduction channels in a 3D fashion is meanwhile well-established; this is also true for patients with implanted cardiac devices when employing specific wideband MRI sequences.4 49,56,57 73–75 However, the elimination of LGE-MRI-detected arrhythmogenic substrate as a potential endpoint of VT ablation has not been studied.
Feasibility of Ventricular Lesion Assessment
Several preclinical and few early observational clinical studies evaluated the feasibility of LGE-MRI for ventricular ablation lesion assessment. Most of these studies investigated lesion formation in the acute setting, at time points when reliable discrimination of irreversible lesions from transient oedema based on LGE is highly challenging, if not impossible.9 12 76–78 While oedema has been demonstrated to resolve within 1–2 weeks from ablation, formation of definite lesions appears to take up to 8 weeks.10 11 25
Of note, Yamashita et al. demonstrated strong correlation of depth and volume of LGE lesions with definite lesions as determined by gross pathology in a canine model 8 weeks after ablation.10
Studies in patients with idiopathic and ischaemic VT have demonstrated that these definite ablation lesions can be visualised by LGE-MRI even many months after the ablation.15,16
The Dark Core Phenomenon
For image post-processing and analyses it has to be taken into account that, in contrast to atrial ablation lesions, the above-mentioned dark core phenomenon, characterised by centrally hypoenhanced lesions, has been observed even in these chronic stages of ventricular lesion formation, thus complicating the assessment of ablation-induced scarring
Ablation Lesion Assessment with MRI ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 7: Assessing Elimination of Arrhythmogenic Fibrotic Substrate by Late Gadolinium Enhancement-MRI
Against this background, we have recently analysed the potential role of LGE-MRI to assess the long-term effect of VT ablation in terms of arrhythmogenic substrate elimination (unpublished data). Three to 6 months following the procedure, effective ablation was reflected by pronounced reduction of LGE-MRI-detected border zone scar volume and extent of slow conduction channels compared to the preprocedural LGEMRI (Figure 7). In patients undergoing repeat ablation procedures, this arrhythmogenic substrate elimination as determined by LGE-MRI correlated well with EAM. Thus, LGE-MRI-based lesion assessment may be of potential value to evaluate the efficacy of ventricular substrate ablation and to predict VT recurrences and clinical outcome. However, as mentioned above, clinical validation is warranted.
Left panel: LGE map of the left ventricle prior to substrate-based ventricular tachycardia ablation. LGE depicts an antero-apical scar. A 3D-analysis using the ADAS 3D software predicts a slow-conduction channel (black line) extending over 30 % of the transmurality that was confirmed by invasive electroanatomical mapping. Right panel: LGE map of the left ventricle 3 months post-ventricular tachycardia ablation. LGE indicates complete scar homogenisation and ‘dechannelling’ with ablation lesions covering the full substrate. Percentages indicate distinct layers of the transmurality from endocardial (0%) to epicardial (100%). LGE = late gadolinium enhancement.
in the ventricle.15 As current post-processing software algorithms are solely based on hyperenhancement, hypoenhanced lesion cores are not automatically identified and thus have to be delineated manually to avoid misinterpretation (Figure 6).
While Vunnam et al. have recently reported to have found ‘dark core’ lesions only up to 1 month after RF ablation but not at later stages, which is in line with previous preclinical and clinical data, Dabbagh et al. consistently observed lesions with hypoenhanced cores as late as 30 months post-ablation in all patients after repeat ablation of post-MI substrates.11 15 16 63
Of note, at our centre, we encounter centrally hypoenhanced lesions in around 60% of the patients at the systematic follow-up LGE-MRI 3–6 months post-VT ablation. Interestingly, in the study of Vunnam et al., comparison with pre-procedural LGE-MRI scans revealed that dark core lesions could only be observed in previously non-fibrotic myocardium without preexisting scar, suggesting that different wash-in/ washout kinetics in scarred versus non-scarred myocardium play a role in this context. This is in line with a study in patients devoid of structural heart disease in whom LGE-MRI was performed at a mean of 22 months after ablation of idiopathic VT, where no central hypoenhancement of lesions was encountered.16
Potential Clinical Value of Ventricular Lesion Assessment
While cumulative evidence is suggesting feasibility of LGE-MRI-based ventricular ablation lesion assessment, clinical validation is absent.
1. Pontecorboli G, Figueras I Ventura RM, Carlosena A, et al. Use of delayed-enhancement magnetic resonance imaging for fibrosis detection in the atria: a review. Europace 2017;19:180–9. https://doi.org/10.1093/europace/euw053;
PMID: 28172967
2. Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612;
PMID: 32860505
3. Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/ APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: executive summary. Europace 2020;22:450–95. https://doi.org/10.1093/europace/ euz332; PMID: 31995197
4. Berruezo A, Penela D, Jáuregui B, Soto-Iglesias D. The role of imaging in catheter ablation of ventricular arrhythmias.
Conclusion
LGE-MRI constitutes the gold standard for non-invasive ablation lesion assessment. In the context of atrial ablation, LGE-MRI-based lesion assessment is already employed in routine clinical settings for noninvasive confirmation of durable PVI and to guide repeat ablation procedures in selected centres. In contrast, ventricular lesion assessment by LGE-MRI is less well established. However, despite the lack of clinical validation, LGE-MRI-based evaluation of arrhythmogenic substrate elimination holds great promise as an efficacy endpoint for VT ablation and a potential predictor of recurrences and clinical outcome.
In light of the current limitations, there is clearly some work ahead of us. Most importantly, uniform methodological and analytical standards are warranted to increase reproducibility of results across centres. This in turn, will foster the acceptance of the method and a broader implementation into clinical practice.
Clinical Perspective
• Late-gadolinium-enhancement (LGE)-MRI offers the unique capability of non-invasive ablation lesion assessment.
• LGE-MRI can detect and localise functional gaps in atrial lesion sets with high accuracy, which also allows for MRI-guided repeat ablation.
• Because of a very high negative predictive value regarding the detection of functional gaps, LGE-MRI can reliably rule out pulmonary vein reconnection non-invasively and may thus avoid unnecessary invasive repeat procedures where a pulmonaryvein-isolation-only approach is pursued.
• Elimination of LGE-MRI-detected arrhythmogenic substrate may serve as a potential efficacy endpoint and predictor of clinical outcomes in ventricular tachycardia ablation.
Pacing Clin Electrophysiol 2021;44:1115–25. https://doi. org/10.1111/pace.14183; PMID: 33527461
5. Bisbal F, Guiu E, Cabanas-Grandio P, et al. CMR-guided approach to localize and ablate gaps in repeat AF ablation procedure. JACC Cardiovasc Imaging 2014;7:653–63. https:// doi.org/10.1016/j.jcmg.2014.01.014; PMID: 24813966
6. Linhart M, Alarcon F, Borras R, et al. Delayed gadolinium enhancement magnetic resonance imaging detected anatomic gap length in wide circumferential pulmonary vein ablation lesions is associated with recurrence of atrial fibrillation. Circ Arrhythm Electrophysiol 2018;11:e006659. https://doi.org/10.1161/CIRCEP.118.006659; PMID: 30562102
7. Quinto L, Cozzari J, Benito E, et al. Magnetic resonanceguided re-ablation for atrial fibrillation is associated with a lower recurrence rate: a case-control study. Europace 2020;22:1805–11. https://doi.org/10.1093/europace/euaa252; PMID: 33063124
8. Chubb H, Aziz S, Karim R, et al. Optimization of late
gadolinium enhancement cardiovascular magnetic resonance imaging of post-ablation atrial scar: a cross-over study. J Cardiovasc Magn Reson 2018;20:30. https://doi. org/10.1186/s12968-018-0449-8; PMID: 29720202
9. Dickfeld T, Kato R, Zviman M, et al. Characterization of radiofrequency ablation lesions with gadolinium-enhanced cardiovascular magnetic resonance imaging. J Am Coll Cardiol 2006;47:370–8. https://doi.org/10.1016/j. jacc.2005.07.070; PMID: 16412863
10. Yamashita K, Kholmovski E, Ghafoori E, et al. Characterization of edema after cryo and radiofrequency ablations based on serial magnetic resonance imaging. J Cardiovasc Electrophysiol 2019;30:255–62. https://doi. org/10.1111/jce.13785; PMID: 30375090
11. Ghafoori E, Kholmovski EG, Thomas S, et al. Characterization of gadolinium contrast enhancement of radiofrequency ablation lesions in predicting edema and chronic lesion size. Circ Arrhythm Electrophysiol
Ablation Lesion Assessment with MRI ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
LGE-MRI pre-ablation 60% 70% 80% 60% 70% 80% LGE-MRI 3 months post ablation
2017;10:e005599. https://doi.org/10.1161/CIRCEP.117.005599; PMID: 29079664
12. Nordbeck P, Hiller KH, Fidler F, et al. Feasibility of contrastenhanced and nonenhanced MRI for intraprocedural and postprocedural lesion visualization in interventional electrophysiology: animal studies and early delineation of isthmus ablation lesions in patients with typical atrial flutter. Circ Cardiovasc Imaging 2011;4:282–94. https://doi.org/10.1161/ CIRCIMAGING.110.957670; PMID: 21415125
13. Spragg DD, Khurram I, Nazarian S. Role of magnetic resonance imaging of atrial fibrosis in atrial fibrillation ablation. Arrhythm Electrophysiol Rev 2013;2:124–7. https://doi. org/10.15420/aer.2013.2.2.124; PMID: 26835053
14. Fochler F, Yamaguchi T, Kheirkahan M, et al. Late gadolinium enhancement magnetic resonance imaging guided treatment of post atrial fibrillation ablation recurrent arrhythmia. Circ Arrhythm Electrophysiol 2019;12:e007174. https://doi.org/10.1161/CIRCEP.119.007174; PMID: 31422685
15. Dabbagh GS, Ghannam M, Siontis KC, et al. Magnetic resonance mapping of catheter ablation lesions after postinfarction ventricular tachycardia ablation. JACC Cardiovasc Imaging 2021;14:588–98. https://doi.org/10.1016/j. jcmg.2020.08.041; PMID: 33248970
16. Ilg K, Baman TS, Gupta SK, et al. Assessment of radiofrequency ablation lesions by CMR imaging after ablation of idiopathic ventricular arrhythmias. JACC Cardiovasc Imaging 2010;3:278–85. https://doi.org/10.1016/j. jcmg.2009.09.028; PMID: 20223425
17. Harrison JL, Sohns C, Linton NW, et al. Repeat left atrial catheter ablation: cardiac magnetic resonance prediction of endocardial voltage and gaps in ablation lesion sets. Circ Arrhythm Electrophysiol 2015;8:270–8. https://doi.org/10.1161/
CIRCEP.114.002066; PMID: 25593109
18. Spragg DD, Khurram I, Zimmerman SL, et al. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm 2012;9:2003–9. https://doi.org/10.1016/j.hrthm.2012.08.039;
PMID: 23000671
19. Mărgulescu AD, Nuñez-Garcia M, Alarcón F, et al. Reproducibility and accuracy of late gadolinium enhancement cardiac magnetic resonance measurements for the detection of left atrial fibrosis in patients undergoing atrial fibrillation ablation procedures. Europace 2019;21:724–31. https://doi.org/10.1093/europace/euy314;
PMID: 30649273
20. Althoff TF, Garre P, Caixal G, et al. Late gadolinium enhancement-MRI determines definite lesion formation most accurately at three months post ablation compared to later time points. Pacing Clin Electrophysiol 2022;45:72–82. https://doi.org/10.1111/pace.14415; PMID: 34820857
21. Chubb H, Williams SE, Whitaker J, et al. Cardiac electrophysiology under MRI guidance: an emerging technology. Arrhythm Electrophysiol Rev 2017;6:85–93. https:// doi.org/10.15420/aer.2017.1.2; PMID: 28845235
22. Deneke T, Khargi K, Müller KM, et al. Histopathology of intraoperatively induced linear radiofrequency ablation lesions in patients with chronic atrial fibrillation. Eur Heart J 2005;26:1797–803. https://doi.org/10.1093/eurheartj/ehi255; PMID: 15855195
23. Gepstein L, Hayam G, Shpun S, et al. Atrial linear ablations in pigs. Chronic effects on atrial electrophysiology and pathology. Circulation 1999;100:419–26. https://doi. org/10.1161/01.CIR.100.4.419; PMID: 10421604
24. Harrison JL, Jensen HK, Peel SA, et al. Cardiac magnetic resonance and electroanatomical mapping of acute and chronic atrial ablation injury: a histological validation study. Eur Heart J 2014;35:1486–95. https://doi.org/10.1093/ eurheartj/eht560; PMID: 24419806
25. Glashan CA, Stevenson W, Zeppenfeld K. Lesion size and lesion maturation after radiofrequency catheter ablation for ventricular tachycardia in humans with nonischemic cardiomyopathy. Circ Arrhythm Electrophysiol 2021;14:e009808. https://doi.org/10.1161/CIRCEP.121.009808; PMID: 34397261
26. Althoff TF, Mont L. Novel concepts in atrial fibrillation ablation-breaking the trade-off between efficacy and safety. J Arrhythm 2021;37:904–11. https://doi.org/10.1002/
joa3.12592; PMID: 34386116
27. Khairy P, Dubuc M. Transcatheter cryoablation part I: preclinical experience. Pacing Clin Electrophysiol 2008;31:112–20. https://doi.org/10.1111/j.1540-8159.2007.00934.x;
PMID: 18181919
28. Manasse E, Colombo P, Roncalli M, Gallotti R. Myocardial acute and chronic histological modifications induced by cryoablation. Eur J Cardiothorac Surg 2000;17:339–40. https:// doi.org/10.1016/S1010-7940(99)00361-9; PMID: 10847689
29. Rog-Zielinska EA, Norris RA, Kohl P, Markwald R. The living scar – cardiac fibroblasts and the injured heart. Trends Mol
Med 2016;22:99–114. https://doi.org/10.1016/j. molmed.2015.12.006; PMID: 26776094
30. Sun Y, Weber KT. Infarct scar: a dynamic tissue. Cardiovasc Res 2000;46:250–6. https://doi.org/10.1016/S00086363(00)00032-8; PMID: 10773228
31. Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002. https://doi.org/10.1161/01.CIR.100.19.1992; PMID: 10556226
32. Bing R, Dweck MR. Myocardial fibrosis: why image, how to image and clinical implications. Heart 2019;105:1832–40. https://doi.org/10.1136/heartjnl-2019-315560; PMID: 31649047
33. Mewton N, Liu CY, Croisille P, et al. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011;57:891–903. https://doi.org/10.1016/j. jacc.2010.11.013; PMID: 21329834
34. Kiuchi K, Fukuzawa K, Nogami M, et al. Visualization of intensive atrial inflammation and fibrosis after cryoballoon ablation: PET/MRI and LGE-MRI analysis. J Arrhythm 2021;37:52–9. https://doi.org/10.1002/joa3.12454; PMID: 33664886
35. McGann C, Kholmovski E, Blauer J, et al. Dark regions of no-reflow on late gadolinium enhancement magnetic resonance imaging result in scar formation after atrial fibrillation ablation. J Am Coll Cardiol 2011;58:177–85. https:// doi.org/10.1016/j.jacc.2011.04.008; PMID: 21718914
36. Monti CB, Codari M, Cozzi A, et al. Image quality of late gadolinium enhancement in cardiac magnetic resonance with different doses of contrast material in patients with chronic myocardial infarction. Eur Radiol Exp 2020;4:21. https://doi.org/10.1186/s41747-020-00149-2; PMID: 32242266
37. Lim J, Park EA, Song YS, Lee W. Single-dose gadoterate meglumine for 3T late gadolinium enhancement MRI for the assessment of chronic myocardial infarction: intra-individual comparison with conventional double-dose 1.5T MRI. Korean J Radiol 2018;19:372–80. https://doi.org/10.3348/ kjr.2018.19.3.372; PMID: 29713214
38. Benito EM, Carlosena-Remirez A, Guasch E, et al. Left atrial fibrosis quantification by late gadolinium-enhanced magnetic resonance: a new method to standardize the thresholds for reproducibility. Europace 2017;19:1272–9. https://doi.org/10.1093/europace/euw219; PMID: 27940935
39. Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009;119:1758–67. https://doi.org/10.1161/CIRCULATIONAHA.108.811877; PMID: 19307477
40. Peters DC, Wylie JV, Hauser TH, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology 2007;243:690–5. https://doi.org/10.1148/radiol.2433060417; PMID: 17517928
41. Vijayakumar S, Kholmovski E, McGann C, Marrouche NF. Dependence of contrast to noise ratio between ablation scar and other tissues on patient heart rate and flip angle for late gadolinium enhancement imaging of the left atrium. J Cardiovasc Magn Reson 2012;14(Suppl 1):o107. https://doi. org/10.1186/1532-429X-14-S1-O107
42. Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–520. https://doi. org/10.1093/eurheartj/ehab364; PMID: 34455430
43. Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm 2017;14:e97–153. https://doi.org/10.1016/j.hrthm.2017.04.025; PMID: 28502708
44. Nazarian S, Hansford R, Rahsepar AA, et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med 2017;377:2555–64. https://doi. org/10.1056/NEJMoa1604267; PMID: 29281579
45. Dickfeld T, Tian J, Ahmad G, et al. MRI-guided ventricular tachycardia ablation: integration of late gadoliniumenhanced 3D scar in patients with implantable cardioverterdefibrillators. Circ Arrhythm Electrophysiol 2011;4:172–84. https://doi.org/10.1161/CIRCEP.110.958744; PMID: 21270103
46. Sasaki T, Hansford R, Zviman MM, et al. Quantitative assessment of artifacts on cardiac magnetic resonance imaging of patients with pacemakers and implantable cardioverter-defibrillators. Circ Cardiovasc Imaging 2011;4:662–70. https://doi.org/10.1161/ CIRCIMAGING.111.965764; PMID: 21946701
47. Rashid S, Rapacchi S, Shivkumar K, et al. Modified
wideband three-dimensional late gadolinium enhancement MRI for patients with implantable cardiac devices. Magn Reson Med 2016;75:572–84. https://doi.org/10.1002/ mrm.25601; PMID: 25772155
48. Rashid S, Rapacchi S, Vaseghi M, et al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology 2014;270:269–74. https://doi.org/10.1148/radiol.13130942; PMID: 24086074
49. Roca-Luque I, Van Breukelen A, Alarcon F, et al. Ventricular scar channel entrances identified by new wideband cardiac magnetic resonance sequence to guide ventricular tachycardia ablation in patients with cardiac defibrillators. Europace 2020;22:598–606. https://doi.org/10.1093/ europace/euaa021; PMID: 32101605
50. Khurram IM, Beinart R, Zipunnikov V, et al. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm 2014;11:85–92. https://doi.org/10.1016/j. hrthm.2013.10.007; PMID: 24096166
51. Jefairi NA, Camaioni C, Sridi S, et al. Relationship between atrial scar on cardiac magnetic resonance and pulmonary vein reconnection after catheter ablation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2019;30:727–40. https://doi.org/10.1111/jce.13908; PMID: 30847990
52. Amado LC, Gerber BL, Gupta SN, et al. Accurate and objective infarct sizing by contrast-enhanced magnetic resonance imaging in a canine myocardial infarction model. J Am Coll Cardiol 2004;44:2383–9. https://doi.org/10.1016/j. jacc.2004.09.020; PMID: 15607402
53. Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of postmyocardial infarction mortality. Circulation 2006;114:32–9. https://doi.org/10.1161/CIRCULATIONAHA.106.613414; PMID: 16801462
54. Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007;115:2006–14. https://doi.org/10.1161/CIRCULATIONAHA.106.653568;
PMID: 17389270
55. Peters DC, Appelbaum EA, Nezafat R, et al. Left ventricular infarct size, peri-infarct zone, and papillary scar measurements: a comparison of high-resolution 3D and conventional 2D late gadolinium enhancement cardiac MR. J Magn Reson Imaging 2009;30:794–800. https://doi. org/10.1002/jmri.21897; PMID: 19787731
56. Andreu D, Berruezo A, Ortiz-Pérez JT, et al. Integration of 3D electroanatomic maps and magnetic resonance scar characterization into the navigation system to guide ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2011;4:674–83. https://doi.org/10.1161/CIRCEP.111.961946; PMID: 21880674
57. Fernandez-Armenta J, Berruezo A, Andreu D, et al. Threedimensional architecture of scar and conducting channels based on high resolution ce-CMR: insights for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2013;6:528–37. https://doi.org/10.1161/CIRCEP.113.000264; PMID: 23685537
58. Cochet H, Komatsu Y, Sacher F, et al. Integration of merged delayed-enhanced magnetic resonance imaging and multidetector computed tomography for the guidance of ventricular tachycardia ablation: a pilot study. J Cardiovasc Electrophysiol 2013;24:419–26. https://doi.org/10.1111/ jce.12052; PMID: 23252727
59. Yamashita S, Sacher F, Mahida S, et al. Image integration to guide catheter ablation in scar-related ventricular tachycardia. J Cardiovasc Electrophysiol 2016;27:699–708. https://doi.org/10.1111/jce.12963; PMID: 26918883
60. Dall’Armellina E, Karia N, Lindsay AC, et al. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ Cardiovasc Imaging 2011;4:228–36. https://doi.org/10.1161/ CIRCIMAGING.111.963421; PMID: 21447711
61. Lima JA, Judd RM, Bazille A, et al. Regional heterogeneity of human myocardial infarcts demonstrated by contrastenhanced MRI. Potential mechanisms. Circulation 1995;92:1117–25. https://doi.org/10.1161/01.CIR.92.5.1117; PMID: 7648655
62. Badger TJ, Oakes RS, Daccarett M, et al. Temporal left atrial lesion formation after ablation of atrial fibrillation. Heart Rhythm 2009;6:161–8. https://doi.org/10.1016/j. hrthm.2008.10.042; PMID: 19187904
63. Vunnam R, Maheshwari V, Jeudy J, et al. Ventricular arrhythmia ablation lesions detectability and temporal changes on cardiac magnetic resonance. Pacing Clin Electrophysiol 2020;43:314–21. https://doi.org/10.1111/ pace.13886; PMID: 32052461
Ablation Lesion Assessment with MRI ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
64. Badger TJ, Daccarett M, Akoum NW, et al. Evaluation of left atrial lesions after initial and repeat atrial fibrillation ablation: lessons learned from delayed-enhancement MRI in repeat ablation procedures. Circ Arrhythm Electrophysiol 2010;3:249–59. https://doi.org/10.1161/CIRCEP.109.868356; PMID: 20335558
65. Kheirkhahan M, Baher A, Goldooz M, et al. Left atrial fibrosis progression detected by LGE-MRI after ablation of atrial fibrillation. Pacing Clin Electrophysiol 2019;43:402–11. https:// doi.org/10.1111/pace.13866; PMID: 31867751.
66. McGann C, Akoum N, Patel A, et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol 2014;7:23–30. https://doi.org/10.1161/ CIRCEP.113.000689; PMID: 24363354
67. McGann CJ, Kholmovski EG, Oakes RS, et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J Am Coll Cardiol 2008;52:1263–71. https://doi.org/10.1016/j. jacc.2008.05.062; PMID: 18926331
68. Alarcón F, Cabanelas N, Izquierdo M, et al. Cryoballoon vs. radiofrequency lesions as detected by late-enhancement cardiac magnetic resonance after ablation of paroxysmal atrial fibrillation: a case-control study. Europace 2020;22:382–7. https://doi.org/10.1093/europace/euz309;
PMID: 31821484
69. Kurose J, Kiuchi K, Fukuzawa K, et al. Lesion characteristics between cryoballoon ablation and radiofrequency ablation with a contact-force sensing catheter: late-gadolinium enhancement magnetic resonance imaging assessment. J Cardiovasc Electrophysiol 2020;31:2572–81. https://doi. org/10.1111/jce.14664; PMID: 32648326
70. Akoum N, Wilber D, Hindricks G, et al. MRI assessment of ablation-induced scarring in atrial fibrillation: analysis from the DECAAF study. J Cardiovasc Electrophysiol 2015;26:473–80. https://doi.org/10.1111/jce.12650; PMID: 25727106
71. Akoum N, Morris A, Perry D, et al. Substrate modification is a better predictor of catheter ablation success in atrial fibrillation than pulmonary vein isolation: an LGE-MRI study. Clin Med Insights Cardiol 2015;9:25–31. https://doi.org/10.4137/ CMC.S22100; PMID: 25983561
72. Kamali R, Kump J, Ghafoori E, et al. Area available for atrial fibrillation to propagate is an important determinant of recurrence after ablation. JACC Clin Electrophysiol 2021;7:896–908. https://doi.org/10.1016/j.jacep.2020.11.008; PMID: 33640348
73. Andreu D, Ortiz-Perez JT, Boussy T, et al. Usefulness of contrast-enhanced cardiac magnetic resonance in identifying the ventricular arrhythmia substrate and the
approach needed for ablation. Eur Heart J 2014;35:1316–26. https://doi.org/10.1093/eurheartj/eht510; PMID: 24394378
74. Andreu D, Penela D, Acosta J, et al. Cardiac magnetic resonance-aided scar dechanneling: influence on acute and long-term outcomes. Heart Rhythm 2017;14:1121–8. https:// doi.org/10.1016/j.hrthm.2017.05.018; PMID: 28760258
75. Berruezo A, Fernandez-Armenta J, Andreu D, et al. Scar dechanneling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythm Electrophysiol 2015;8:326–36. https://doi.org/10.1161/CIRCEP.114.002386; PMID: 25583983
76. Lardo AC, McVeigh ER, Jumrussirikul P, et al. Visualization and temporal/spatial characterization of cardiac radiofrequency ablation lesions using magnetic resonance imaging. Circulation 2000;102:698–705. https://doi. org/10.1161/01.CIR.102.6.698; PMID: 10931812
77. Celik H, Ramanan V, Barry J, et al. Intrinsic contrast for characterization of acute radiofrequency ablation lesions. Circ Arrhythm Electrophysiol 2014;7:718–27. https://doi. org/10.1161/CIRCEP.113.001163; PMID: 24988893
78. Tao S, Guttman MA, Fink S, et al. Ablation lesion characterization in scarred substrate assessed using cardiac magnetic resonance. JACC Clin Electrophysiol 2019;5:91–100. https://doi.org/10.1016/j.jacep.2018.11.001; PMID: 30678791
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Ablation Lesion Assessment with MRI
Bridging the Gap Between Artificial Intelligence Research and Clinical Practice in Cardiovascular Science: What the Clinician Needs to Know
Emily Shipley , 1,2 Martha Joddrell , 1,2 Gregory YH Lip 1,2 and Yalin Zheng
1. Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool Heart and Chest Hospital, Liverpool, UK;
2. Department of Cardiovascular and Metabolic Medicine, University of Liverpool, Liverpool, UK;
3. Department of Eye and Vision Science, University of Liverpool, Liverpool, UK
Keywords
Artificial intelligence, machine learning, cardiovascular, arrhythmia, AF, ECG, risk prediction
Disclosure: GYHL is a section editor on the Arrhythmia & Electrophysiology Review editorial board; this did not influence acceptance. All other authors have no conflicts of interest to declare.
Acknowledgement: The authors thank Dr Stephanie Harrison and Dr Deirdre Lane for their contributions to this work.
Received: 4 February 2022 Accepted: 4 February 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e03. DOI: https://doi.org/10.15420/aer.2022.07
Correspondence: Martha Joddrell, Department of Cardiovascular and Metabolic Medicine, University of Liverpool, William Henry Duncan Building, 6 West Derby St, Liverpool L7 8TX, UK. E: m.joddrell@liverpool.ac.uk
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Artificial intelligence (AI) and its applications in cardiovascular science have rapidly grown in recent years, with 77% more papers being published on the PubMed database in 2021 than in 2011 including the terms AI or machine learning (ML) and cardiovascular disease. An even steeper increase can be seen in papers including AI/ML and arrhythmias, with 89% more papers published in 2021 than in 2011.
Advancements have been aided by new ML techniques and increasing computing powers in the form of graphics processing units (GPUs), and availability of large databases, such as the UK Biobank.1
Increased implementation of these techniques in clinical practice would have the potential to significantly improve the management of arrhythmias for patients and clinicians alike, but is subject to significant obstacles. Figure 1 gives an overview of these benefits and concerns associated with AI in clinical practice.
Case for Artificial Intelligence in Arrhythmia Management
Patient Outcomes and Workload Reduction
Disease detection is a key area where AI could prove beneficial. The application of ML to ECG analysis provides a promising solution to lessen the demand placed on clinicians’ already-limited time. For AF, early detection and intervention are key to minimising the increasing risk of adverse outcomes and the healthcare costs associated with this arrhythmia.2
The use of AI can vastly reduce a medical practitioner’s workload as well as improve prognosis by early identification, diagnosis and appropriate management. For example, neural networks can be trained to analyse ECGs, and reinforcement learning can be used to help make dosage decisions.3 This is important given the move towards a more holistic or
integrated approach to AF care, for which studies have demonstrated an association with improved clinical outcomes.4
AI-based risk prediction models can identify and quantify risk factors with higher accuracy than traditional risk scores and enable the detection of factors that researchers are unaware correlate with an outcome. For example, logistic regression, gradient boosting, a decision tree and a neural network for stroke risk prediction obtained areas under the curve (AUCs) of 0.891, 0.881, 0.881 and 0.859, respectively, compared with a value of 0.780 obtained by the CHA2DS2VASc score.5
More widespread use has the potential to improve patient-centred care by further individualising a patient’s level of risk, thus enabling the management of modifiable risk factors. An added benefit would be the ability to account for the dynamic nature of risk in certain cardiovascular outcomes. For example, ML and the use of mobile health data could enable stroke risk prediction to adapt to treatment changes over time and incident risk factors, in contrast with the static nature of current standard risk scores.5
Data-driven Performance
AI applications are benefiting from the explosion of data creation happening currently. With new methods of collection, data are becoming more diverse, enabling improvement in performance of ML models.
Mobile health data applications have already aided prediction of arrhythmias including AF and VF, as well as supraventricular ectopic beat and ventricular ectopic beat.6
ML techniques such as natural language processing (NLP) have enabled researchers to make better use of the data in patients’ electronic health records. A study of >63 million individuals applied NLP to free-text data
EDITORIAL © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Artificial Intelligence
1,3
Figure 1: Benefits and Concerns of Artificial Intelligence in Cardiovascular Research
combined with structured electronic health record data, and correctly detected 3,976,056 further non-valvular AF cases, compared with using structured data alone.7
Evidently, introducing AI-based detection methods into clinical use could help clinicians screen a vast number of arrhythmia cases that may otherwise have gone undetected and, with appropriate treatment, reduce the likelihood of adverse outcomes in these patients.
Barriers to Clinical Use and Potential Solutions
Clinical Acceptance
As AI progresses, such tools are beginning to become accepted into clinical use. For example, in 2019, HeartFlow received approval from the Food and Drug Administration (FDA) to implement its non-invasive, realtime, virtual modelling tool for coronary artery disease intervention.8
Although the application of this tool has been a success, agencies such as the FDA can often limit the progress of AI systems by subjecting them to lengthy acceptance processes. The Artificial Intelligence/Machine Learning (AI/ML) Software as a Medical Device (SaMD) Action Plan proposes a regulatory framework for the use of AI and ML solutions in healthcare.9 Better, more explicit resources on approval processes, such as this regulatory framework, would enable researchers and developers to reduce delays and rejection by ensuring their applications meet approval requirements.
Another barrier to the real-world application of AI research is the standard of reporting. Reviews have observed that studies developing prediction models for clinical use are not providing transparent pictures of their methods and findings.10 As a result, these findings may not be trusted by patients or clinicians, used or replicated.
To address this, laws and protocols are being developed to advise AI researchers on how to thoroughly present their work, such as TRIPOD-AI and PROBAST-AI.11 They provide guidelines and tools that analysts should follow to prevent research waste and help readers identify key information to make a clear decision on the quality of the studies.
Research waste can also occur when applications are not designed with the needs of clinical purpose at the forefront. Regardless of outstanding performance in predicting an outcome, a model will not be deployed if a clinician requires the prediction of multiple outcomes simultaneously.
Shortcomings of Machine Learning
The complexity of the relationships modelled by deep learning, while a benefit of the technique, may also preclude its use in clinical practice. Applicability decreases if a model generalises too strongly to its training data, resulting in reduced performance with differing populations.
This may be overcome by creating training models using a variety of datasets, although problems arise when health datasets differ in features, even when the same variables are collected. For example, if a model is trained on a dataset that categorises alcohol consumption by <5 units/ week and ≥5 units/week, retraining with a dataset that categorises alcohol consumption by <2 units/week, 2–7 units/week, and ≥7 units/ week would prove difficult. Feature selection methods, as well as model calibration, provide a solution to this by preventing overfitting.
Similarly, any patterns, biases or outdated information in the data will influence a model’s robustness.
Incorrect and harmful applications could ensue if models are applied to unsuitable populations, which may be especially concerning in
Cardiovascular AI in Clinical Practice ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Accuracy Personalised risk prediction Benefits Concerns Cost-e ective Reduces clinical workload Clinical use Access to data Reporting of research Biased data Interpretability/explainability
Potential successes that artificial intelligence could have in cardiovascular research with corresponding concerns of use in clinical practice.
cardiovascular science given the life-and-death nature of many clinical decisions.
Gaining access to reflective, diverse data is another problem within AI because of the red tape of data privacy and protection legislation. Developing countries and isolated communities tend to have a lower rate of data collection hence are less likely to be incorporated into model training.12 As a result, individual personal complexities that could influence outcomes in these populations will not be reflected by models.
Additionally, algorithms built on a specific type of patient data gained from a congruent sample may not apply to those who differ in predictor values. This caveat is being slowly alleviated, with the growth of big data producing more representative and thorough datasets.
These issues, if not addressed, will present significant obstacles to the many potential benefits the clinical use of deep learning could bring.
Interpretability and Explainability
The increasing concern surrounding interpretability and explainability is potentially the most considerable barrier to acceptance of AI in healthcare.
Interpretability refers to the ability to observe the cause-and-effect relationships a model has learned and the outcomes that various factors will produce, e.g. a model predicts that a patient will develop lung cancer as the patient is a current smoker.
Explainability refers to how well the influence of a model’s parameters on its decision can be understood; a regression model’s coefficients explain that smoking results in a certain increase in the likelihood a patient has lung cancer, whereas a ‘black box’ deep neural network’s weights give a much less explicit insight of how the presence of smoking impacted the final prediction.
For risk prediction in particular, explainability can enable clinicians and patients to mitigate risk by identifying and managing the risk factors contributing most to the prediction.
Increased interpretability and explainability may also help highlight any
1. Elliott AD, Linz D, Mishima R, et al. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur Heart J 2020;41:1479–86. https://doi.org/10.1093/eurheartj/ehz897; PMID: 31951255.
2. Burdett P, Lip GYH. Atrial fibrillation in the United Kingdom: predicting costs of an emerging epidemic recognising and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J Qual Care Clin Outcomes 2022;8:187–94. https:// doi.org/10.1093/ehjqcco/qcaa093; PMID: 33346822.
3. Olier I, Ortega-Martorell S, Pieroni M, Lip GYH. How machine learning is impacting research in atrial fibrillation: implications for risk prediction and future management. Cardiovasc Res 2021;117:1700–17. https://doi.org/10.1093/cvr/ cvab169; PMID: 33982064.
4. Romiti GF, Pastori D, Rivera-Caravaca JM, et al. Adherence to the ‘atrial fibrillation better care’ pathway in patients with atrial fibrillation: impact on clinical outcomes – a systematic review and meta-analysis of 285,000 patients. Thromb Haemost 2021;122:406–14. https://doi. org/10.1055/a-1515-9630; PMID: 34020488.
5. Lip GYH, Tran G, Genaidy A, et al. Improving dynamic stroke risk prediction in non-anticoagulated patients with and without atrial fibrillation: comparing common clinical risk scores and machine learning algorithms. Eur Heart J Qual
biases embedded within the data by allowing the examination of a model’s choices. Various methods are being developed to help explain the decisions made by more complex models.
For risk prediction, Shapley values can be used to quantify each variable’s contribution.13 However, their calculation is computationally expensive, and the computation time required may be too expensive for a clinician to accept. For deep neural networks, saliency maps can be used to produce visualisations that highlight the patterns and areas of each beat that contribute to the model’s final outcome prediction.14
Conversely, some researchers question the necessity of interpretability, and argue that the pursuit of interpretable models is not necessary and holding back progression.15
There is a strong argument for interpretability for models making highstakes decisions such as treatment recommendations and dosage calculations, where the negative consequences of an incorrect decision may be substantial. However, for models designed to perform smaller tasks, such as the annotation of ECGs, interpretability may not be essential as long as the model demonstrates good performance.
Responsibility for decisions made by uninterpretable models is a discussion starting to arise now that system manufacturers are seeking approval for them to be deployed in a clinical setting. Since legislation on data and automatic systems are only a recent issue, there are no clear guidelines as to who would be at fault if a misdiagnosis were made by the machine. Although some believe this is a reason to delay use of algorithms in practice, a perquisite is that it takes blame away from overworked medical personnel who, by human nature, are bound to make mistakes occasionally.
Conclusion
Despite its barriers, one cannot deny the success, elevated accuracy and promise that AI is yielding in arrhythmia research. For this progress to be most useful in healthcare, it is imperative that the wall between AI research and clinical care be broken down, through both the implementation of solutions discussed here and the innovation of solutions.
Care Clin Outcomes 2021. https://doi.org/10.1093/ehjqcco/ qcab037; PMID: 33999139; epub ahead of press.
6. Sadrawi M, Lin CH, Lin YT, et al. Arrhythmia evaluation in wearable ECG devices. Sensors (Basel) 2017;17:2445. https:// doi.org/10.3390/s17112445; PMID: 29068369.
7. Elkin PL, Mullin S, Mardekian J, et al. Using artificial intelligence with natural language processing to combine electronic health record’s structured and free text data to identify nonvalvular atrial fibrillation to decrease strokes and death: evaluation and case-control study. J Med Internet Res 2021;23:e28946. https://doi.org/10.2196/28946; PMID: 34751659.
8. Qayyum S, Habib A, Kechyn S, et al. P01 Heartflow: experience of a high-volume district general hospital. Heart 2020;106(Suppl 3):A5.
9. Food and Drug Administration. Artificial Intelligence/Machine Learning (AI/ML) Software as a Medical Device (SaMD) Action Plan Silver Spring, MD: FDA, 2021. https://www.fda.gov/medicaldevices/software-medical-device-samd/artificial-intelligenceand-machine-learning-software-medical-device (accessed 15 March 2022).
10. Collins GS, Mallett S, Omar O, Yu LM. Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting. BMC Med 2011;9:103. https:// doi.org/10.1186/1741-7015-9-103; PMID: 21902820.
11. Collins GS, Dhiman P, Andaur Navarro CL, et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open 2021;11:e048008. https://doi.org/10.1136/ bmjopen-2020-048008; PMID: 34244270.
12. Yan Y, Zhang JW, Zang GY, Pu J. The primary use of artificial intelligence in cardiovascular diseases: what kind of potential role does artificial intelligence play in future medicine? J Geriatr Cardiol 2019;16:585–91. https://doi. org/10.11909/j.issn.1671-5411.2019.08.010; PMID: 31555325.
13. Wang S, Li J, Sun L, et al. Application of machine learning to predict the occurrence of arrhythmia after acute myocardial infarction. BMC Med Inform Decis Mak 2021;21:301. https://doi. org/10.1186/s12911-021-01667-8; PMID: 34724938.
14. Vijayarangan S, Murugesan B, Vignesh R, et al. Interpreting deep neural networks for single-lead ECG arrhythmia classification. Annu Int Conf IEEE Eng Med Biol Soc 2020:300–3. https://doi.org/10.1109/EMBC44109.2020.9176396; PMID: 33017988.
15. London AJ. Artificial intelligence and black-box medical decisions: accuracy versus explainability. Hastings Cent Rep 2019;49:15–21. https://doi.org/10.1002/hast.973; PMID: 30790315.
Cardiovascular AI in Clinical Practice ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Contemporary Management of Complex Ventricular Arrhythmias
Abstract
Percutaneous catheter ablation is an effective and safe therapy that can eliminate ventricular tachycardia, reducing the risks of both recurrent arrhythmia and shock therapies from a defibrillator. Successful ablation requires accurate identification of arrhythmic substrate and the effective delivery of energy to the targeted tissue. A thorough pre-procedural assessment is needed before considered 3D electroanatomical mapping can be performed. In contemporary practice, this must combine traditional electrophysiological techniques, such as activation and entrainment mapping, with more novel physiological mapping techniques for which there is an ever-increasing evidence base. Novel techniques to maximise energy delivery to the tissue must also be considered and balanced against their associated risks of complication. This review provides a comprehensive appraisal of contemporary practice and the evidence base that supports recent developments in mapping and ablation, while also considering potential future developments in the field.
Keywords
Ventricular arrhythmia, ventricular tachycardia, mapping, scar, ablation, endocardial, epicardial
Disclosure: BW has received unrestricted research fellowships and consultancy payments from Boston Scientific. MW and BW have received funding from the Advanced Ventricular Arrhythmia Training and Research Program administered by St George’s Hospital Charity. AL and MS have received unrestricted grants from Abbott Laboratories. Received: 29 November 2021
Accepted: 6 February 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e04. DOI: https://doi.org/10.15420/aer.2021.66
Correspondence: Benedict M Wiles, Cardiology Clinical Academic Group, St George’s University of London, Cramer Terrace, London SW17 0RE, UK. E: ben.wiles@doctors.org.uk
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Sudden cardiac death is a leading cause of global mortality, with 6 million deaths per year attributed to ventricular tachyarrhythmia.1 In at-risk groups, ICD improves survival, and is superior to medical therapy in both the primary and secondary prevention of sudden death.2–7 ICD therapy does not prevent arrhythmia, of course. Recurrence is common, even in the presence of anti-arrhythmic medications, which can themselves be associated with significant adverse effects.8–10 ICD shock therapies are also associated with increased mortality and reduced quality of life.11–14
Percutaneous catheter ablation is an effective and safe solution that can eliminate ventricular tachycardia (VT), reducing the risk of recurrent arrhythmia and future ICD therapies. In expert hands this can be achieved with low procedure-related complications and extremely low early mortality.15–20
Catheter Ablation: Indications
In patients with structural heart disease, expert consensus guidelines currently recommend catheter ablation for VT after failure of antiarrhythmic drug (AAD) therapy, or for patients in whom AAD therapy is either not tolerated or not desired (class I indication).21
The most robust evidence for catheter ablation is in patients with both VT and ischaemic heart disease (IHD). VANISH was a randomised controlled trial in which patients with IHD and an ICD, who experienced VT despite AAD therapy, were randomised to either catheter ablation or an escalation in AAD therapy. After a mean follow-up of >2 years, a significant reduction in the combined primary outcome of death, ICD shock or VT storm was
observed in the ablation cohort, with a subgroup analysis showing that patients who were already taking amiodarone obtained the greatest benefit.17 In the IHD population, catheter ablation is therefore recommended over escalation in AAD therapy for patients who experience VT despite amiodarone.
In patients with IHD, catheter ablation may also be considered after a first episode of monomorphic VT to reduce the future risk of shock therapies (class IIb indication). This recommendation is supported by the findings of SMASH-VT, a randomised controlled trial in which patients with a history of MI and a secondary prevention ICD indication were randomised to either ICD alone or ICD plus prophylactic catheter ablation. In this trial, appropriate ICD shocks were significantly reduced in the catheter ablation cohort with no increase in mortality.18
These findings were further supported by VTACH, a randomised controlled trial of ICD versus ICD and ablation, which recruited patients with prior stable VT, MI and reduced ejection fraction (<50%). Time to recurrence of VT or ventricular fibrillation (VF) was significantly longer in the ablation group (18.6 versus 5.9 months), while freedom from ventricular arrhythmia at 2 years was also significantly greater postablation (47% versus 29%).19
Ablation prior to ICD implantation is also a potentially promising strategy in patients with IHD, but this has not yet been shown to offer benefit over a deferred ablation strategy.20 However, we eagerly await the publication of on-going trials in this area, such as PARTITA (NCT01547208).16
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Clinical Electrophysiology and Ablation
1,2
, 1,2
1,2
1,2
Benedict M Wiles ,
Anthony C Li
Michael C Waight
and Magdi M Saba
1. Advanced Ventricular Arrhythmia Training and Research (AVATAR) Program, St George’s University Hospitals NHS Foundation Trust, London, UK; 2. Cardiology Clinical Academic Group, St George’s University of London, London, UK
In patients with non-ischaemic cardiomyopathy, catheter ablation is associated with lower rates of acute procedural success and higher rates of VT recurrence, when compared with ischaemic cardiomyopathy cohorts.22–24 In non-ischaemic cardiomyopathy, recurrence of VT postablation is dependent upon the underlying substrate, but may be reduced by early referral time after VT.25–27 Unfortunately, there remains an absence of randomised controlled data pertaining to VT management in non-ischaemic cardiomyopathy patients, although large case series of catheter ablation after failed medical therapy have shown that catheter ablation may reduce VT burden and result in greater discontinuation of AAD therapy.28 Catheter ablation of VT storm has also been shown to be effective, with recurrent storm rates as low as 5% after a median follow-up of 45 months.29
In idiopathic VT, catheter ablation is considered preferable to AAD therapy in symptomatic patients (class I indication) due to its high success and low recurrence rates.21 Catheter ablation may also be performed in patients who experience VT in the context of congenital heart disease, inherited arrhythmia syndromes or hypertrophic cardiomyopathy, and there is individualised guidance pertaining to each of these conditions within the guidelines.21
Approach to Catheter Ablation
There are two principal components to an effective catheter ablation procedure. The first is substrate identification, the process by which the critical substrate that is responsible for the initiation and/or maintenance of an arrhythmia is identified. Substrate identification requires an assimilation of all the available data, which might include pre-procedural investigations, the successful induction and mapping of a given VT circuit, and information obtained from invasive mapping during sinus rhythm. The second component is ablation, which requires sufficient energy to be delivered to the targeted tissue, ensuring permanent destruction and preventing further arrhythmia.
Substrate Identification Pre-procedure
Substrate identification begins with a comprehensive pre-procedural assessment that is critical to the creation of an appropriate ablation strategy. This should include a considered review of any documented tachycardia. The 12-lead ECG is the most useful diagnostic tool in localising the origin of a VT and always requires scrutiny.30–32 Intracardiac electrograms (EGMs), downloaded from implantable cardiac devices, are also worthy of attention. These can provide useful information on VT morphology, cycle length, the relative timings at each ventricular lead and, where anti-tachycardia pacing has been delivered, the results of entrainment from the right ventricle.33–36 Prior ambulatory monitoring should also be reviewed, especially in the treatment of ventricular ectopy, where variations in ectopic burden can accurately predict the site of origin in outflow tract arrhythmia.37
Where possible, patients should have cardiac imaging prior to ablation, to ensure that the operator has a comprehensive understanding of the arrhythmia substrate prior to invasive mapping. Echocardiography is readily available, non-ionising and inexpensive, but multimodality cross-sectional imaging is also increasingly available and used in most centres. MRI with late gadolinium enhancement and CT can be used to localise and quantify scar substrate. Their usage pre-procedure has been shown to reduce both total procedure time and volume of ablation, as well as improve acute and long-term outcomes.38 Where there is no contraindication, cardiac MRI is routinely performed in our centre for all patients who present with ventricular tachycardia. Cross-sectional imaging may also identify potential technical
challenges relating to either a proposed epicardial puncture or to obtaining retrograde ventricular access; for example, in the presence of significant peripheral vascular disease.
We believe that the need for epicardial access should be decided prior to ablation to ensure appropriate patient consent and to plan a suitable periprocedural anticoagulation strategy. In general, epicardial ablation should be considered where epicardial or mid-myocardial substrate is anticipated from either cross-sectional imaging or prior invasive mapping, or where the presenting ECG suggests an epicardial VT exit. Although, patientspecific factors, including body habitus and prior cardiac surgery, must also be considered.
In patients with coronary artery disease, an endocardial-only approach for first-line ablative therapy has been favoured in our centre, due to the absence of randomised controlled data to support a more invasive initial strategy. Retrospective observational data from a small study (n=15 in the combined endo-epicardial ablation group) did show that an initial combined procedure resulted in fewer readmissions for VT and fewer repeat ablations, but we have not routinely adopted this approach.39
Conversely, in patients with non-ischaemic cardiomyopathy, where the prevalence of epicardial substrate is much higher, we have adopted an endo-epicardial ablation approach as first line. In patients with arrhythmogenic cardiomyopathy, this strategy is supported by a recently published meta-analysis in which significantly higher freedom from recurrent arrhythmia or ICD therapies (84.6% versus 52.2%) and greater elimination of AADs (69.2% versus 21.7%) was observed in the combined ablation group through >3 years of follow-up.40
Invasive Voltage Mapping
In the catheter laboratory, substrate identification is achieved through careful three-dimensional electroanatomical mapping using a dedicated mapping system. This can be achieved with a point-by-point approach, using an ablation catheter with or without the addition of contact force sensing, or through the use of a multi-electrode catheter.
Multi-electrode catheter mapping produces higher-density maps, and has been shown to provide better discrimination of late potentials in randomised trials.41 A meta-analysis has also shown multi-electrode mapping to be associated with a reduction in mapping time, but does not reduce procedure or ablation time, nor confer improvements in acute procedural success or VT recurrence.42 Contact force sensing is also not associated with an improvement in hard endpoints, although retrospective trial data suggest a reduction in fluoroscopy time.43
The amplitude of local EGMs is used to differentiate normal tissue from scar tissue, with automated bipolar and unipolar voltage maps created simultaneously, and displayed on three-dimensional electroanatomical maps (EAM). Normal endocardial bipolar voltage, measured between two adjacent electrodes at the endocardial surface, is generally accepted as being >1.5 mV, with dense scar identified by bipolar voltages of <0.5 mV.44,45
However, voltage limit adjustment may be required to accurately identify VT channels with relatively higher voltages (within regions of low voltage scar).46 The direction of myocardial activation has also been shown to alter the voltage characteristics of myocardial scar.47 In this study by Tung et al., separate voltage maps were created using different wavefronts of activation (right ventricular pacing, left ventricular pacing, intrinsic conduction) and approximately 18% of critical sites for re-entrant VT were found in regions
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Management of Ventricular Arrhythmias
Figure 1: Endocardial and Epicardial Voltages
that displayed low voltages during one wavefront of activation, and normal voltage (>1.5 mV) during another wavefront of activation.47
A possible future solution might be the use of omnipolar EGMs in creating ventricular EAMs. These represent a new approach to voltage mapping, taking advantage of the directional properties of the electric field of a travelling wavefront on the surface of the myocardium. Preliminary studies have shown that omnipolar EGMs are orientation-independent, effectively virtual bipolar EGMs that are aligned along the direction of a wavefront.48 Ventricular mapping with omnipolar EGMs has subsequently been achieved in animal models, but the clinical application of this technology to ventricular tachycardia ablation remains unclear.49
Unipolar voltages are recorded between a single mapping electrode and a neutral electrode sited away from the myocardium. Unipolar maps, therefore, have a greater ‘field of view’, which can facilitate the identification of mid-myocardial and epicardial scar. Defining ‘normal’ references for unipolar voltage is challenging, and flexibility is required when interpreting unipolar maps (Figure 1). In our centre, we loosely use the cut-off values of >8.3 mV in the left ventricle and >5.5 mV on the right ventricular free wall to define normal unipolar amplitudes.50,51 However, in the presence of endocardial scar, a lower cut-off value is often required, while a higher upper limit of normal may be required in the presence of significant left ventricular hypertrophy.
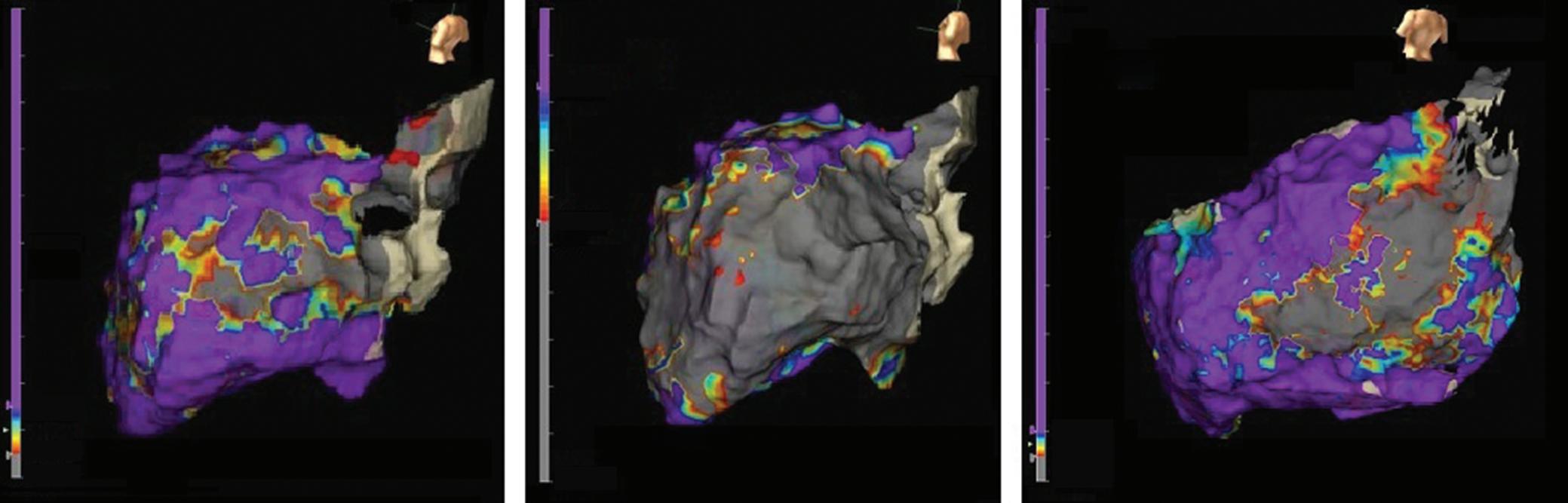
In the future, it may be possible to adopt more disease-specific unipolar reference ranges. Recent trials in both Chagas’ disease and remodelled post-infarct myocardium have suggested a shift away from standardised ranges to ensure accurate identification of substrate.52,53
Mapping During Ventricular Tachycardia
In the absence of spontaneous arrhythmia, programmed electrical stimulation is routinely used to induce VT. Where this is sustained – and occurs without haemodynamic collapse – activation and entrainment mapping can be performed. Unfortunately, approximately 60–70% of scar-related VT is associated with haemodynamic collapse.54,55 This has led to an increased interest in entirely substrate-based ablation, which has the potential benefit of identifying future substrate, at the expense of potentially unnecessary ablation.
The latest randomised controlled data support a substrate-based approach in patients with ischaemic heart disease and stable VT. When compared with a strategy in which only mappable VTs were targeted, the substrate-based group experienced less recurrent VT, required less AAD therapy and had fewer hospitalisations. There was also no difference in peri-procedural complications or 12-month mortality.56 A subsequent meta-analysis of six studies further supported the idea of complete substrate modification, with a significantly lower risk of the composite endpoint of arrhythmia recurrence and all-cause mortality, although more ablation was associated with a higher rate of complications.57
Mechanical circulatory support devices may be considered to maintain haemodynamic stability and preserve end organ perfusion while mapping sustained VT. Options include intra-aortic balloon pumps, atrial to femoral bypass systems, aorta-flow assist systems and extracorporeal membrane oxygenation.58 Observational data suggest that haemodynamic support can facilitate extended mapping, but at a cost of increased complications.59–61 To date, there are no randomised data to evaluate the efficacy of these strategies. Appropriate patient selection also remains challenging, although scoring systems have been developed to predict the risk of haemodynamic decompensation during VT ablation.62
Interestingly, general anaesthesia does not appear to reduce VT inducibility in the majority of patients, but it is associated with a greater need for haemodynamic support.63 Induction of VT under conscious sedation, rather than general anaesthesia, is therefore also worthy of consideration in cases where haemodynamic collapse is likely to prevent mapping in tachycardia.
In our centre, mapping of unstable tachycardias is frequently supported by pressor pre-loading and the strategic placement of multipolar catheters at the sites of interest prior to VT induction. This allows for a short period of mapping in tachycardia (~30 s), which can be sufficient to adequately identify critical substrate.
Activation Mapping
In activation mapping, the overall pattern of ventricular myocardial activation is determined from the timing of local EGMs and referenced to
Management of Ventricular Arrhythmias ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
A C B 1.5 mV 8.3 mV 5.5 mV 0.0 mV 1.0 mV 0.5 mV 0.0 mV 0.5 mV 0.0 mV
A–C: Three-dimensional electroanatomical maps collected from the same patient. A,B: Endocardial left ventricular maps; C: An epicardial left ventricular map. A: A bipolar amplitude map (scale 0.5–1.5 mV) on which only a small region of dense scar (grey) can be seen in the basal–lateral territory. B: The equivalent unipolar map (scale 5.5–8.3 mV) showing much more extensive scarring across this entire region. C: The epicardial bipolar map (scale 0.5–1.0 mV) delineates the epicardial scar. B,C: Extensive patchy or non-transmural mid-myocardial scarring would account for the discrepancy in scar territories observed.
Figure 2: Local Activation Time Mapping
a consistent point in time (e.g. the onset of the QRS complex). This process can be largely automated using EAM software.
In a focal ventricular arrythmia, the site of earliest activation is targeted for ablation, with local EGMs >24 ms prior to the earliest activation of the surface QRS a predictor of long-term success.64 Characteristically, the local EGM may also display a reverse polarity pattern on the distal and proximal bipoles of the ablation catheter, often with low amplitude initial components.65 However, this finding is not specific, and pseudo-focal patterns may also be present in deep substrate.
In re-entrant VT, activation maps require a different interpretation, as there is no focal activation point, but a continuous circuit of electrical activity (Figure 2). Local EGM signals that are immediately pre-QRS represent the point at which the wavefront exits the protected isthmus into the myocardium, while electrical activity through the critical isthmus is identified by the presence of mid-diastolic potentials. However, passive slow conduction is common within scar tissue, and mid-diastolic activation alone is not sufficient to determine active involvement in the re-entry circuit. The critical components of the re-entry circuit can therefore only be accurately distinguished by entrainment.
Entrainment Mapping
Entrainment is a manoeuvre in which pacing is delivered at a constant cycle length shorter than that of the tachycardia, but not excessively short, as this might alter the conduction properties of the critical substrate and lead to inaccurate information. The aim is to continuously reset the arrhythmia, but without termination, so that it continues, unchanged, on cessation of pacing. Observations made during and after entrainment are crucial to understanding the arrhythmia mechanism and in locating the critical substrate.
In scar VT, entrainment from the protected critical components of the circuit (critical isthmus and exit) results in a QRS morphology that is identical to that of the VT (entrainment with concealed fusion). By comparison, entrainment from elsewhere in the ventricle results in manifest fusion of the QRS complex. On cessation of entrainment, the postpacing interval minus the tachycardia cycle length can also be used to determine the distance between the pacing catheter tip and the protected circuit, with critical sites expected to have a postpacing interval minus the tachycardia cycle length of <30 ms (Figure 3).66
Additionally, the stimulus to QRS time that is observed during entrainment with concealed fusion can be used to further subclassify the circuit components. At the critical isthmus, a stimulus to QRS interval of 30–50% of the tachycardia cycle length is expected. Furthermore, the stimulus to QRS interval should be close in duration to the local EGM-QRS interval observed during activation mapping. The critical isthmus is the commonest target for ablation, and the point at which ablation is most likely to terminate the rhythm and prevent its recurrence.67
Substrate Mapping Without Sustained Ventricular Tachycardia Pace mapping
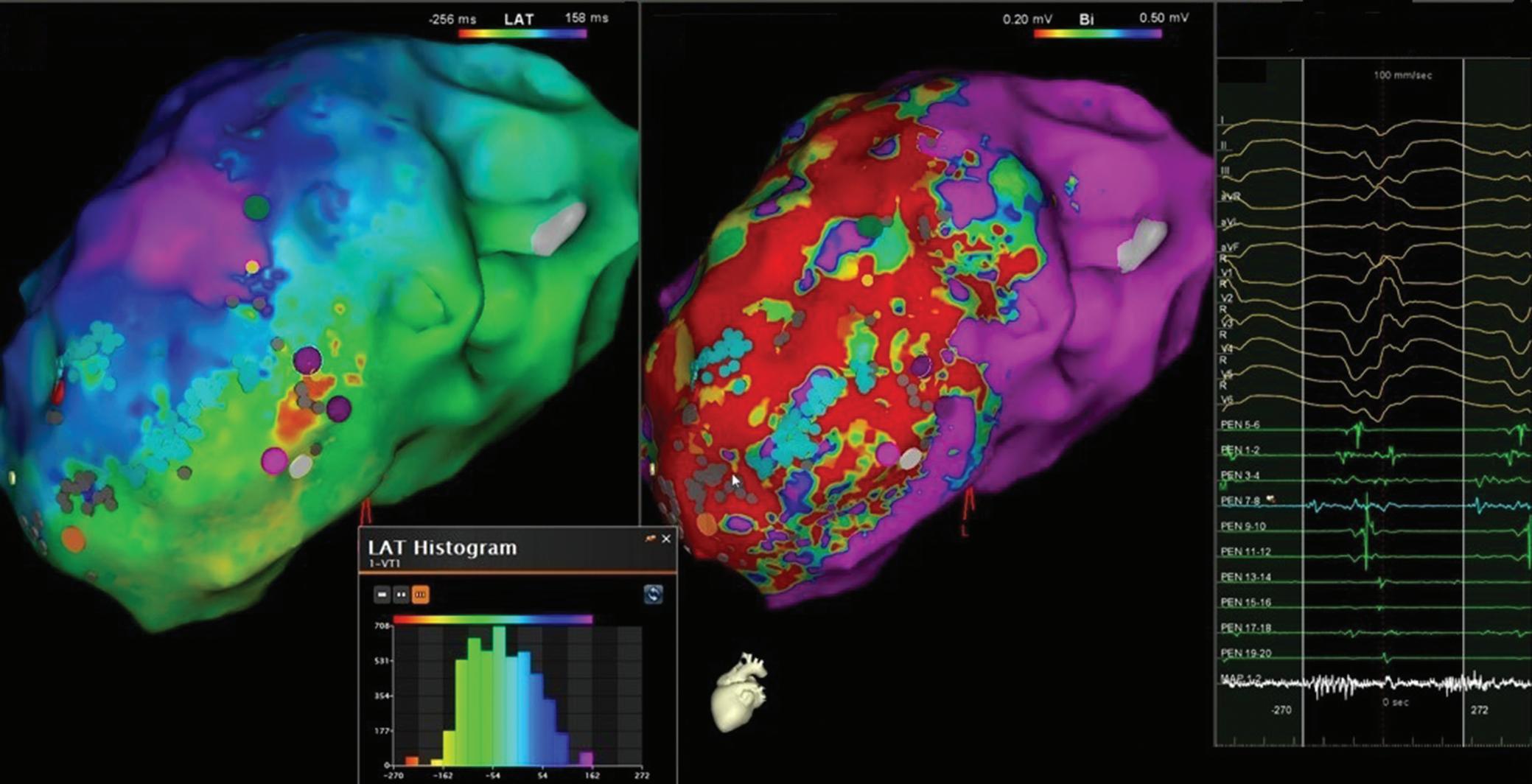
Pace mapping can be used to assist with identification of the site of origin of a focal arrhythmia or the exit site/distal protected isthmus of a reentrant circuit, but prior documentation of the clinical VT (or premature ventricular complexes) morphology is required. Pace mapping can also assist with the identification of substrate critical to the maintenance of re-entry (Figure 4).
The fundamental principle behind pace mapping is the positive correlation that exists, when healthy tissue is paced, between QRS morphological
Management of Ventricular Arrhythmias ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
A C B
A,B: 3D electroanatomical maps from the same patient. A: A local activation time map has been created during sustained ventricular tachycardia with the QRS complex placed in the middle of the ‘window of interest’ (C). B: A bipolar voltage map is shown (scale 0.2–0.5 mV) collected during right ventricular pacing. A large anterior wall scar is seen, with regions of heterogeneity. The local activation time map suggests a channel of conduction that occurs during mid-diastole, with the entrance shown in purple and the exit in red. This would be a good target for ablation, marked by the blue tags transecting this path. The absent part of the cycle length (missing bars on the histogram) could represent mid-myocardial conduction or voltage below the lower detection limit that has been applied on the mapping system. LAT = local activation time.
Figure 3: Entrainment
change and the distance the pacing catheter tip is moved. This allows a quantitative assessment of the pace mapping output, a process that can be automated by EAM software.68
However, adjacent tissue capture due to high pacing output in bipolar configuration, and possibly saline irrigation, may lead to alterations in QRS morphology, which might limit the accuracy of pace mapping. Consequently, some centres resort to unipolar pacing to minimise the degree of local tissue capture. Theoretically, wavefront activation during VT might also differ from a paced QRS from a perfect exit site, due to the absence of refractory myocardium that is only present during tachycardia, although this is not usually encountered clinically.
Recently, pace mapping has also been used to unmask VT isthmuses in patients with postinfarct re-entrant VT.69 In that study, the best pace map percentages were found, as expected, at the isthmus exit, while the poorest percentage pace maps were found adjacent to the scar border in the entrance zones and in the entrance part of the isthmus. On highdensity 3D pace maps, an abrupt change in pace map match percentage was therefore found to be associated with the central isthmus, matching the location of the wavefront identified on activation mapping.
Local Abnormal Ventricular Activities
Local abnormal ventricular activities are sharp, high-frequency ventricular potentials, which are usually low in amplitude and arise from pathological tissue.70 Importantly they are distinct from, and can therefore be uncoupled from, the larger far field ventricular EGM within which they reside or follow. They can be identified using a standard ablation catheter or more readily using a multi-electrode mapping catheter either during sinus rhythm or ventricular pacing. Elimination of all local abnormal ventricular activities provides a clear and reproducible end point for ablation and has been reported as both feasible and safe. Furthermore, where local abnormal ventricular activity can be identified, elimination is independently associated with superior survival from recurrent VT, although this has not been prospectively assessed in a randomised controlled trial.70
Late Potentials
Late potentials are local EGMs that occur after the terminal portion of the surface QRS, either entirely isolated from other local activity or due to continuous fractionation. They are present in the majority of patients who have had a MI, and can be easily identified when mapping during sinus rhythm (Figure 5).
The elimination of all late potentials is a potential endpoint for a substratebased ablation strategy. In a large study of ischaemic patients in whom the combined endpoint of non-inducibility and the elimination of all late potentials was employed, VT recurrence was reduced to low levels and a significant reduction in cardiac death was observed. However, this was not a randomised controlled trial.71
Scar Dechannelling
Scar dechannelling is a technique by which a scar is identified using standard bipolar voltage parameters, but then further EGM analysis is undertaken to identify the various entry points into this delineated scar. These channels can then be eliminated by ablation at the entry points, with a view to abolishing a potential isthmus from this territory.
This mapping technique requires software that can identify abnormal or delayed components on a local EGM (usually fractionated and low voltage) that are only marginally delayed with respect to the healthy far field component of the EGM (usually higher voltage and lower frequency). These signals are felt to represent scar channel entry points.
Scar dechannelling has been shown to result in low rates of VT recurrence and mortality, as well as limiting the extent of ablation required in more than half of patients. Incomplete channel elimination was also associated with higher VT recurrence rates.72 Cardiac MRIaided scar dechannelling, where MRI with late gadolinium enhancement tissue characterisation is integrated into the navigation system, has also been associated with a lower need for radiofrequency (RF) delivery, higher non-inducibility rates after substrate ablation and a higher VT recurrence-free survival.73
Management of Ventricular Arrhythmias ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
II I aVF V1 A1, A2 A2, A3 A3, A4 B1, B2 B2, B3 B3, B4 360 382 380 C1, C2 C2, C3 C3, C4 D1, D2 D2, D3 D3, D4 MAP V4 V5
ECG and electrogram data recorded during a ventricular tachycardia ablation. The patient is in sustained monomorphic ventricular tachycardia and the multi-electrode catheter is positioned in the epicardium at a region of interest. The ventricular tachycardia (cycle length 380 ms) has been successfully entrained (at 360 ms) from a multi-electrode catheter spline that had shown discrete diastolic potentials during tachycardia. Concealed fusion is evident (98% morphology match), and the post-pacing interval minus the tachycardia cycle length is 2 ms. The stimulus to QRS is also equal to the local electrogram-QRS (not annotated). Ablation here has a high likelihood of terminating the tachycardia.
ECG and electrogram data recorded during a ventricular tachycardia ablation. A single site within the left ventricular scar is being paced at a constant cycle length of 600 ms. Ventricular capture occurs, but the QRS morphology alternates between a septal exit site and a more lateral wall exit. The two exits have different stimulus to QRS intervals (68 ms versus 80 ms) and different stimulus to right ventricular apex intervals (162 ms versus 252 ms). Variation in RR interval, measured from the right ventricular apex catheter (514 ms versus 696 ms), is therefore also observed despite the ventricle being paced at a constant cycle length. This is an example of ‘multiple exit sites’, indicating that the catheter tip is capturing tissue that is likely to be involved in re-entry. This would be a good target for ablation.
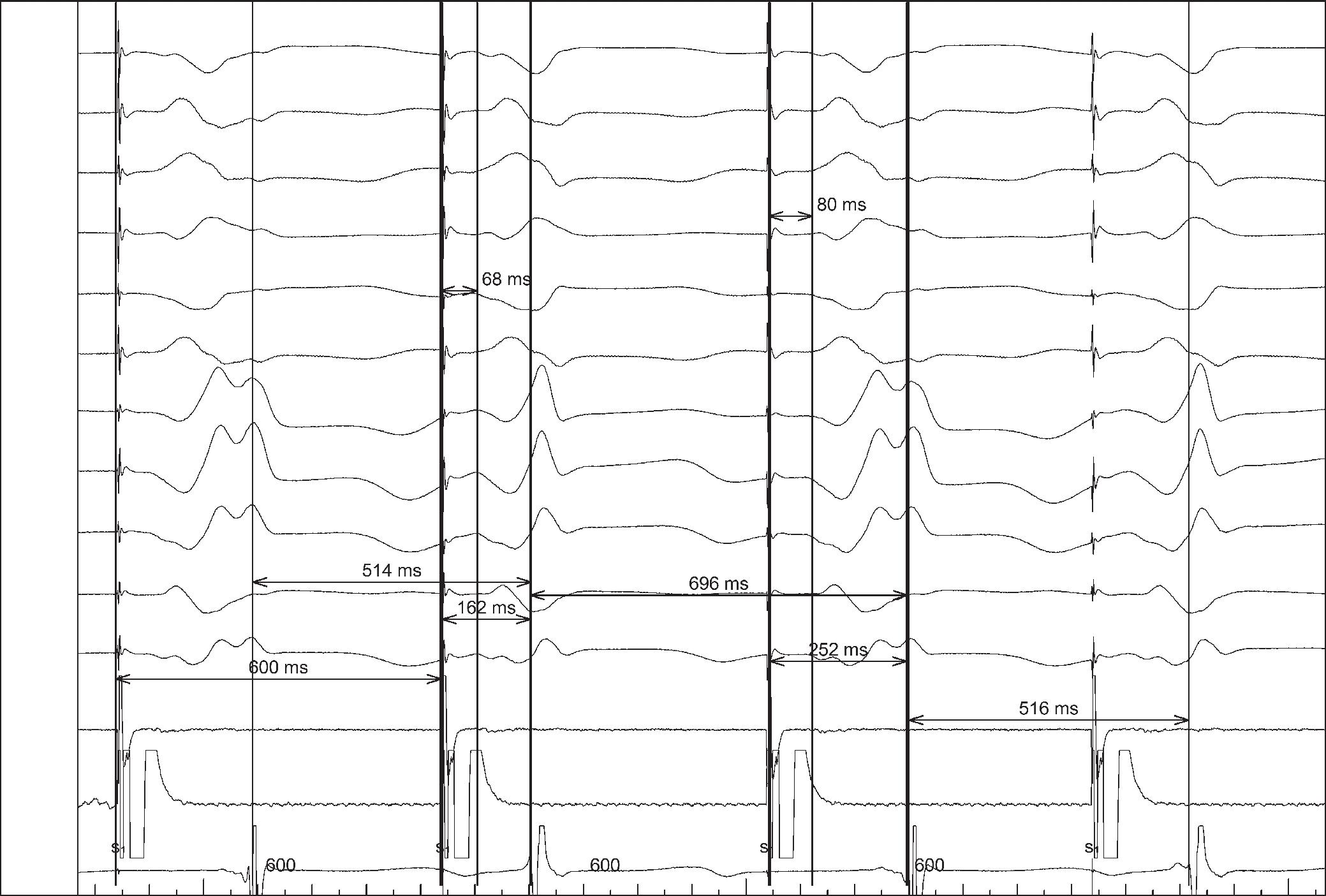
Functional Substate Mapping
Slow conduction and conduction block are pre-requisites for re-entry, and tissues that display these characteristics represent regions of interest in the hunt for critical VT substrate. Conduction velocity and conduction block are not fixed characteristics of a tissue, they depend upon the timing and direction of depolarising wavefronts. As such, functional tissue assessments can provide greater substrate characterisation than a purely voltage-based approach. In recent years, this has been the subject of significant research, and several advanced mapping techniques have emerged.
Isochronal Late Activation Maps
Isochronal late activation maps, produced in sinus rhythm or during constant ventricular pacing, have been proposed as a possible supplementary technique for identifying critical ablation targets. Isochronal late activation maps are readily created using EAM software. Local EGMs are timed at the latest bipolar component, signifying the completion of local activation, and displayed across equally distributed isochrones. Where conduction velocity slows, isochrones are observed to crowd together, and ‘deceleration zones’ can be identified. Sites critical to re-entry have been retrospectively shown to occur in these regions.74
VT ablation guided by the production of isochronal late activation maps has also been performed prospectively with successful termination sites found to colocalise to the deceleration zones in 95% of cases, with high rates of freedom from VT at 12 months.75
Decremental Evoked Potential Mapping
Decrement evoked potential (DEEP) mapping involves stressing possible substrate tissue to identify local conduction delay and unidirectional block, both of which are pre-requisites for re-entry. DEEP mapping was first described using a ventricular drive train followed by a single ventricular extrastimulus, delivered 20 ms above the refractory period of the ventricle.76 The response of local late potentials to this extrastimulus was mapped using EAM software. The potentials that were significantly delayed (>10 ms) in comparison with their relative timing during the drive chain were proposed as possible targets for ablation. However, an important limitation of DEEP mapping is the potential for proximal slowing of conduction to delay a downstream EGM, the critical site of delay thereby going undetected.
This DEEP mapping technique was assessed in a small, non-randomised study (n=20) in which all identified late potentials were interrogated by the introduction of an extrastimulus. Critical VT substrate was identified with high specificity, allowing for a more focused ablation strategy, and the majority of patients were rendered non-inducible for clinical VT.77
DEEP mapping has also been assessed using a sensed extrastimulus approach, removing the need for a drive train, facilitating a shorter mapping time.78 In this small (n=30), non-randomised study, ablation guided by a right ventricular sensed extrastimulus protocol rendered VT
Management of Ventricular Arrhythmias ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
aVR aVF V1 V2 V3 V4 V6 MAP P MAP D RVA D aVL I II III
Figure 4: Multiple Exit Site Pacing
non-inducible in 97% of patients, whereas 90% remained free from both symptomatic VT and ICD therapies at a median follow-up of 12 months.78
Substrate Mapping for VF
The ablation of VF most commonly involves the targeting of triggering premature ventricular complexes. These typically arise from the Purkinje system or scar border zone, and are characterised by short QRS duration and a short coupling interval. As with all ventricular ectopic ablation, they can be localised by a combination of activation mapping and pace mapping. In patients with a history of VF, the ablation of triggering ectopics has been shown to reduce recurrent VF in patients with ischaemic cardiomyopathy, Brugada syndrome, long-QT syndrome and early repolarisation syndrome.79–82
In the absence of triggering premature ventricular complexes, the ablation of low amplitude EGMs with fractionation or late potentials has been shown to reduce recurrent VF in patients with and without structural heart disease.83 In patients with idiopathic VF, empirical ablation of the left posterior fascicle, targeting the Purkinje potentials seen along the left ventricular inferoseptum, has also been shown to be of benefit in a small case series.84 An empiric strategy of interrogating the Purkinje’s network, papillary muscles and outflow tract regions by pace map matching against stored ICD template EGMs has also been shown to reduce arrhythmia burden and AAD usage.85
In patients with early repolarisation syndrome and recurrent VF, noninvasive electrocardiographic imaging has also been used in the identification of targets for ablation. Electrocardiographic imaging showed potential in identifying regions of abnormal conduction (delayed activation, conduction block, fractionation) and abnormal repolarisation.86 In a small, non-randomised trial, this technique, in combination with tradition electroanatomical mapping, has also been shown to result in effective ablation in symptomatic patients with recurrent VF.87
Recently, there has also been an increased interest in the identification of rotors and wavelets that might maintain ventricular fibrillation. A 64-electrode basket catheter has recently been used to obtain a substrate map during VF.88 In that small non-randomised study (n=6), patients with drug refractory VF had areas of localised re-entry identified during VF. Ablation of these targets resulted in a significant reduction in ICD shocks and all-cause mortality compared with a non-ablation control group.
Ablation Techniques
RF catheter ablation requires sufficient energy to be delivered to the targeted tissue to ensure permanent elimination of local conduction. In the early days of VT ablation, conventional electrode catheters were found to be ineffective in the ventricle, with the most likely explanation being the relatively small and shallow lesions they created.89,90 Larger irrigated-tip catheters have since become the routine standard of care.
Irrigation of room temperature saline at the catheter tip has been shown to reduce the electrode tip temperature, decrease the likelihood of thrombus and char formation, and result in larger and deeper lesions.91 Catheters with an 8 mm tip have been shown to have greater efficiency in the treatment of ventricular arrhythmias than 4 mm tipped catheters, but even with modern irrigated catheters, effective energy delivery can still be challenging.92 For example, when the arrhythmic substrate is located in areas that are difficult to access percutaneously (left ventricular summit or intra mural substrate), or where energy delivery is impeded by
Figure 5: Ablation of Late Potentials
A: A 3D electroanatomical map showing local activation time across the left ventricular epicardium collected during right ventricular pacing. The scale is set to show late potentials (grey) occurring >174 ms after QRS onset. A high-density multipolar mapping catheter is sited at one area of interest and the signals from that location are displayed in the panel on the right side, below a 12-lead ECG. Extremely late potentials can be seen across all of the bipolar electrodes. B: A 3D electroanatomical map from the same patient, now showing local bipolar voltage amplitudes prior to ablation. A: The regions of interest correspond to areas of dense scar (<0.5 mV). The maroon balls represent areas of subsequent ablation, the red tags delineate the course of an obtuse marginal branch of left circumflex coronary artery and the green tags mark the course of the left phrenic nerve. The multi-electrode mapping catheter is placed in a similar location to panel A. Successful ablation of all the late potentials can be observed in the right-hand panel.
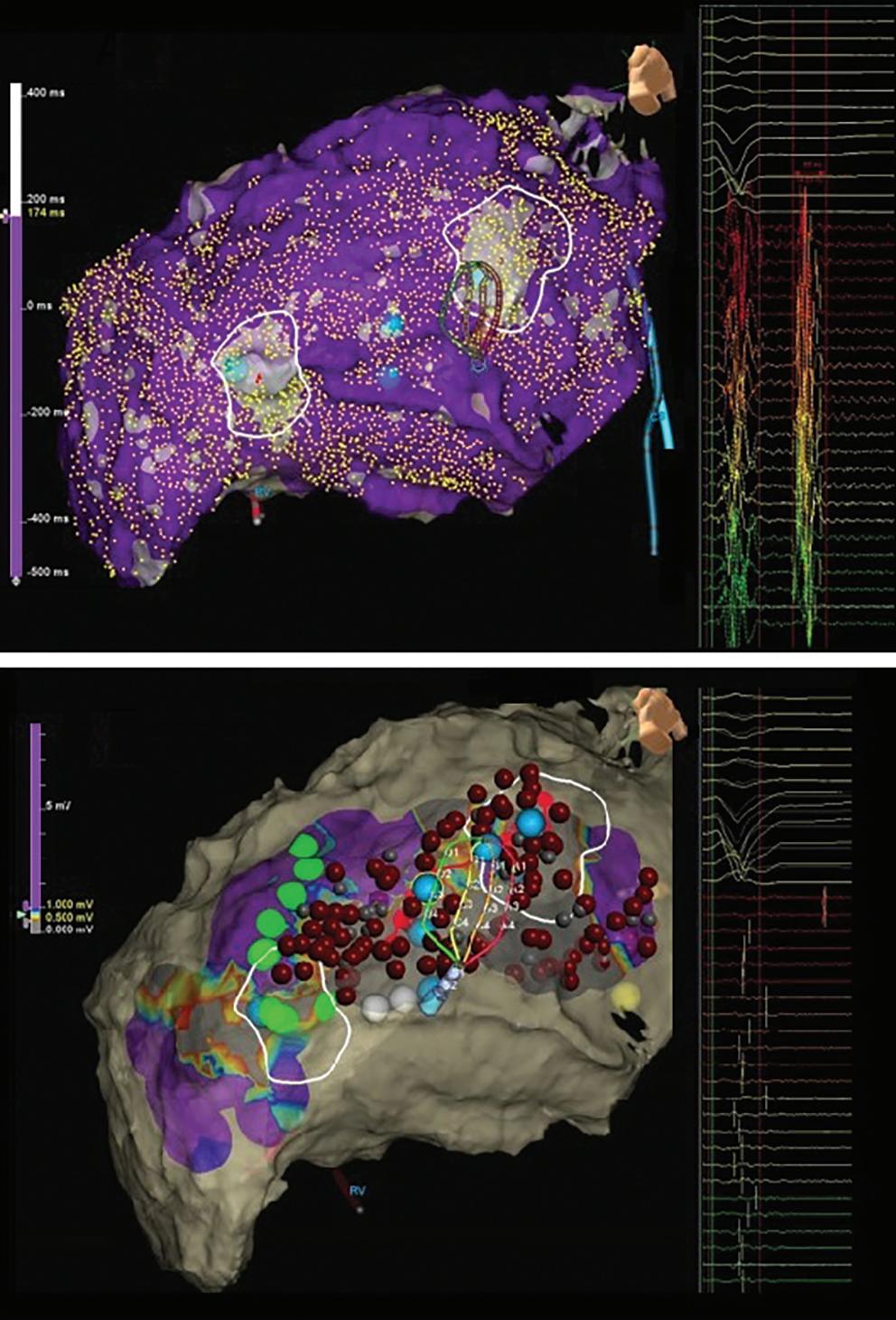
epicardial fat, coronary vessels or scar calcification. At all times, the potential benefit of delivering higher energy to the tissue must be balanced against the increased risk of complications.
Alternative Irrigation Solutions
In recent years, the use of alternative irrigants has provided a possible solution to these challenges. Solutions with lower ionic concentrations and charge densities have been shown to result in larger RF lesion volumes, as the increased impedance around the catheter tip decreases the dissipation of energy into the blood pool, maximising the energy conducted to the tissue.93
Half normal saline has been prospectively assessed as an alternative irrigant in a multicentre trial of 94 patients with ventricular arrhythmia refractory to standard ablation techniques. Half normal saline was found to be safe and effective, with acute rhythm suppression achieved in 83% of cases.94 Dextrose irrigation has also been trialled in an ovine ventricular model with lesion sizes comparable to those achieved with half normal
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Management of Ventricular Arrhythmias
A B
saline, both of which were larger in volume than normal saline irrigated lesions.95
As alternative irrigation solutions enhance energy transfer into the tissue, operators should be mindful of excessive tissue heating deep to the surface and the possibility of precipitating steam pops. In the aforementioned trials, steam pops were more frequently observed with both alternative irrigation solutions, more so with the use of dextrose, although without adverse consequence.
Bipolar Ablation
During RF ablation, the dispersion of energy is proportional to the distance from the catheter tip. Unipolar lesions are therefore limited to approximately 5–6 mm in depth, even with high power settings. This may be troublesome when the critical substrate is located deep in the tissue, rather than at the endocardial surface.
One possible solution is bipolar ablation, where current is applied between two ablation catheter tips that are positioned on either side of the targeted tissue. This approach has been prospectively tested in both non-ischaemic cardiomyopathy patients with an intraseptal substrate (n=21), and in patients with deep intramural substrate that could not be suppressed by unipolar RF (n=18). Non-inducibility was achieved in 89–95% of these cases with an excellent safety profile.96,97
We strongly consider this technique in our centre for patients requiring redo procedures and those with documented septal substrate on imaging. Direct loss of pacing capture from a diagnostic catheter strategically placed in an intramural septal vein, in close proximity to a scar, has also been used to facilitate – and acutely demonstrate the value of – bipolar ablation in targeting deep septal substrate.98
Retrograde Coronary Venous Ethanol
RF ablation of VT can fail because of inaccessibility of the critical substrate. In left ventricular summit VTs, for example, the critical substrate might only be identified on mapping of the coronary sinus and its tributaries. Retrograde coronary venous ethanol using an angioplasty balloon, to ensure retrograde flow of the ethanol, is a possible solution.99
In a prospective multicentre trial, retrograde coronary venous ethanol has been shown to be safe and effective, offering long-term control of drug and RF-refractory ventricular arrhythmia. Isolated or adjunctive retrograde coronary venous ethanol was successful in 98% of patients (n=56), 77% of whom remained free of recurrence at 12 months.100
Future Developments
There are several novel ideas in development that may significantly influence ventricular ablation in the coming years.
Needle Catheter Ablation
A novel RF catheter has been developed with an extendable and retractable 27-G needle that can be used to target deep intramural substrate. This technology is not presently available commercially, but a single study of 31 patients demonstrated control of otherwise refractory arrhythmias, with acceptable procedural risk.101 In a porcine model, this idea has been further developed to include an extendable infusion needle electrode capable of delivering warm saline directly to intramural tissue. This has been shown to cause near transmural lesions with no wall thinning or perforation, but has not yet been tested in humans.102
Pulsed Field Ablation
Pulsed field ablation (PFA) is a novel, non-thermal modality that selectively ablates myocardium with ultra-short electrical impulses that create microscopic pores in the cell membrane, known as electroporation. Unlike thermal ablation with RF, tissue-specific thresholds exist for electroporation, allowing for selective ablation of the cardiac myocytes. This was demonstrated in animal trials, where PFA created durable myocardial lesions with no discernible oesophageal damage.103–105
First-in-human trials of PFA have already been conducted in patients with AF. Ultra-rapid pulmonary vein and left atrial posterior wall isolation have been demonstrated using a single shot PFA catheter. Furthermore, combined RF and PFA ablation has also been performed using a novel lattice-tip catheter capable of delivering either technology.106–108 In each of these trials, excellent safety profiles and lesion durability were demonstrated.
A proof-of-concept study of endocardial ventricular PFA using porcine myocardium has also now been reported, with homogenous myocardiumspecific lesions successfully created.109
PFA appears to yield much promise, although the technology remains in its infancy and patient numbers remain very low. Long-term data on both safety and efficacy is also awaited, although further trials are anticipated for both atrial and ventricular ablation.
Stereotactic Body Radiation Therapy
Radiotherapy is a long-established therapeutic technique in which highenergy X-rays, gamma rays or a photon beam are targeted to abnormal tissue, most commonly cancer cells. In recent years, attention has turned to the use of radiotherapy as a possible treatment for ventricular arrhythmia, after myocardial irradiation was shown in animal models to create transmural fibrosis, similar to the results of catheter ablation.110,111
Both invasive and non-invasive techniques can be used to characterise the VT and localise the arrhythmic substrate as accurately as possible prior to radiotherapy. In a prospective case series of five patients, highdensity surface electrocardiographic data from a 252-electrode vest was used to characterise the VT, combined with a chest CT scan to delineate
Clinical Perspective
• Percutaneous catheter ablation is a safe and effective therapy that reduces recurrent ventricular tachycardia (VT) and shock therapies where anti-arrhythmic drugs have failed or not been tolerated.
• Prior to VT ablation, a thorough pre-procedural assessment is required, and should include a considered review of prior tachycardia and cross-sectional imaging.
• Haemodynamic stability during VT largely determines the methodology by which critical substrate is identified, with increasing evidence to support an entirely substrate-based approach where needed.
• Novel physiological mapping and advanced ablation techniques are increasingly used to supplement the traditional techniques of activation, entrainment and pace mapping.
• Ventricular ablation is a rapidly progressing field with several new technologies and ideas that may change the therapy landscape in the coming years.
Management of Ventricular
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Arrhythmias
the substrate using an entirely non-invasive approach.112 Post-therapy, a 99.9% reduction in overall VT burden was observed.
Longer-term follow-up data are now available from two prospective study cohorts. Safety and efficacy are demonstrated at >12 months, with reductions in VT burden and anti-arrhythmic drug therapy, as well as improvements in quality of life.113,114
These results are promising, but the evidence is restricted to case reports and small case series (both prospective and retrospective), in which the majority of recipients have failed standard ablation or been deemed unsuitable for invasive therapy. A randomised clinical trial is needed. It also remains to be seen what the potential application of this technology
1. Mehra R. Global public health problem of sudden cardiac death. J Electrocardiol 2007;40(Suppl):S118–22. https://doi. org/10.1016/j.jelectrocard.2007.06.023; PMID: 17993308.
2. Moss AJ, Hall WJ, Cannom DS, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 1996;335:1933–40. https://doi.org/10.1056/ NEJM199612263352601; PMID: 8960472.
3. Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. N Engl J Med 1999;341:1882–90. https://doi. org/10.1056/NEJM199912163412503; PMID: 10601507.
4. Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. Eur Heart J 2000;21:2071–8. https://doi. org/10.1053/euhj.2000.2476. PMID: 11102258.
5. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877–83. https://doi.org/10.1056/NEJMoa013474; PMID: 11907286.
6. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. https://doi. org/10.1056/NEJMoa043399; PMID: 15659722.
7. Luni FK, Singh H, Khan AR, et al. Mortality effect of ICD in primary prevention of nonischemic cardiomyopathy: a metaanalysis of randomized controlled trials. J Cardiovasc Electrophysiol 2017;28:538–43. https://doi.org/10.1111/ jce.13192; PMID: 28370885.
8. Holmberg M, Holmberg S, Herlitz J. Incidence, duration and survival of ventricular fibrillation in out-of-hospital cardiac arrest patients in Sweden. Resuscitation 2000;44:7–17. https://doi.org/10.1016/s0300-9572(99)00155-0; PMID: 10699695.
9. Larsen MP, Eisenberg MS, Cummins RO, Hallstrom AP. Predicting survival from out-of-hospital cardiac arrest: a graphic model. Ann Emerg Med 1993;22:1652–8. https://doi. org/10.1016/s0196-0644(05)81302-2; PMID: 8214853.
10. Valenzuela TD, Roe DJ, Cretin S, et al. Estimating effectiveness of cardiac arrest interventions: a logistic regression survival model. Circulation 1997;96:3308–13. https://doi.org/10.1161/01.cir.96.10.3308; PMID: 9396421.
11. Sears SF, Conti JB. Quality of life and psychological functioning of icd patients. Heart 2002;87:488–93. https:// doi.org/10.1136/heart.87.5.488; PMID: 11997430.
12. Irvine J, Dorian P, Baker B, et al. Quality of life in the Canadian Implantable Defibrillator Study (CIDS). Am Heart J 2002;144:282–9. https://doi.org/10.1067/mhj.2002.124049; PMID: 12177646.
13. Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med 2008;359:1009–17. https://doi. org/10.1056/NEJMoa071098; PMID: 18768944.
14. Proietti R, Labos C, Davis M, et al. A systematic review and meta-analysis of the association between implantable cardioverter-defibrillator shocks and long-term mortality. Can J Cardiol 2015;31:270–7. https://doi.org/10.1016/j. cjca.2014.11.023; PMID: 25746019.
15. Fernandez-Armenta J, Soto-Iglesias D, Silva E, et al. Safety and outcomes of ventricular tachycardia substrate ablation during sinus rhythm: a prospective multicenter registry. JACC Clin Electrophysiol 2020;6:1435–48. https://doi.org/10.1016/j. jacep.2020.07.028; PMID: 33121673.
16. Chen M, Wu S, Yao Y, et al. Pan-Asia United States Prevention of Sudden Cardiac Death Catheter Ablation trial (PAUSE-SCD): rationale and study design. J Interv Card Electrophysiol 2020;57:271–8. https://doi.org/10.1007/s10840-
could be in less frail patients, and whether it would supersede standard ablation techniques.
Conclusion
Catheter ablation is a safe and effective treatment strategy for VT, for which increasingly sophisticated techniques in both mapping and ablation have been recently developed. Contemporary VT ablation requires a comprehensive understanding of the principles of both patient preparation and invasive substrate mapping, to which additional and more novel techniques can then be appropriately applied.
Future advances, with the promise of reducing procedural risk and duration, and improving outcomes, are also eagerly anticipated.
019-00535-w; PMID: 30891654.
17. Sapp JL, Wells GA, Parkash R, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med 2016;375:111–21. https://doi.org/10.1056/NEJMoa1513614; PMID: 27149033.
18. Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic catheter ablation for the prevention of defibrillator therapy. N Engl J Med 2007;357:2657–65. https://doi.org/10.1056/ NEJMoa065457; PMID: 18160685.
19. Kuck KH, Schaumann A, Eckardt L, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010;375:31–40. https://doi.org/10.1016/S01406736(09)61755-4; PMID: 20109864.
20. Willems S, Tilz RR, Steven D, et al. Preventive or deferred ablation of ventricular tachycardia in patients with ischemic cardiomyopathy and implantable defibrillator (Berlin VT): A multicenter randomized trial. Circulation 2020;141:1057–67. https://doi.org/10.1161/CIRCULATIONAHA.119.043400; PMID: 32000514.
21. Cronin EM, Bogun FM, Maury P, et al. HRS/EHRA/APHRS/ LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. J Interv Card Electrophysiol 2019;21:1143–4. https://doi.org/10.1007/s10840-019-00663-3 PMID: 31984466.
22. Dinov B, Fiedler L, Schönbauer R, et al. Outcomes in catheter ablation of ventricular tachycardia in dilated nonischemic cardiomyopathy compared with ischemic cardiomyopathy: results from the prospective Heart Centre of Leipzig VT (HELP-VT) study. Circulation 2014;129:728–36. https://doi.org/10.1161/CIRCULATIONAHA.113.003063; PMID: 24211823.
23. Kumar S, Romero J, Mehta NK, et al. Long-term outcomes after catheter ablation of ventricular tachycardia in patients with and without structural heart disease. Heart Rhythm 2016;13:1957–63. https://doi.org/10.1016/j.hrthm.2016.07.001; PMID: 27392945.
24. Proietti R, Essebag V, Beardsall J, et al. Substrate-guided ablation of haemodynamically tolerated and untolerated ventricular tachycardia in patients with structural heart disease: effect of cardiomyopathy type and acute success on long-term outcome. Europace 2015;17:461–7. https://doi. org/10.1093/europace-euu326. PMID: 25488957.
25. Tokuda M, Tedrow UB, Kojodjojo P, et al. Catheter ablation of ventricular tachycardia in nonischemic heart disease. Circ Arrhythm Electrophysiol 2012;5:992–1000. https://doi. org/10.1161/CIRCEP.112.971341; PMID: 22942218.
26. Vaseghi M, Hu TY, Tung R, et al. Outcomes of catheter ablation of ventricular tachycardia based on etiology in nonischemic heart disease: an international ventricular tachycardia ablation center collaborative study. JACC Clin Electrophysiol 2018;4:1141–50. https://doi.org/10.1016/j. jacep.2018.05.007; PMID: 30236386.
27. Romero J, Di Biase L, Diaz JC, et al. Early versus Late referral for catheter ablation of ventricular tachycardia in patients with structural heart disease: a systematic review and meta-analysis of clinical outcomes. JACC Clin Electrophysiol 2018;4:374–82. https://doi.org/10.1016/j. jacep.2017.12.008; PMID: 30089564.
28. Muser D, Santangeli P, Castro SA, et al. Long-term outcome after catheter ablation of ventricular tachycardia in patients with nonischemic dilated cardiomyopathy. Circ Arrhythm Electrophysiol 2016;9:e004328. https://doi.org/10.1161/ CIRCEP.116.004328; PMID: 27733494.
29. Muser D, Liang JJ, Pathak RK, et al. Long-term outcomes of catheter ablation of electrical storm in nonischemic dilated
cardiomyopathy compared with ischemic cardiomyopathy. JACC Clin Electrophysiol 2017;3:767–78. https://doi. org/10.1016/j.jacep.2017.01.020; PMID: 29759543.
30. Miller JM, Marchlinski FE, Buxton AE, Josephson ME. Relationship between the 12-lead electrocardiogram during ventricular tachycardia and endocardial site of origin in patients with coronary artery disease. Circulation 1988;77:759–66. https://doi.org/10.1161/01.cir.77.4.759; PMID: 3349580.
31. Kuchar DL, Ruskin JN, Garan H. Electrocardiographic localization of the site of origin of ventricular tachycardia in patients with prior myocardial infarction. J Am Coll Cardiol 1989;13:893–903. https://doi.org/10.1016/07351097(89)90232-5; PMID: 2926041.
32. Segal OR, Chow AWC, Wong T, et al. A novel algorithm for determining endocardial VT exit site from 12-lead surface ECG characteristics in human, infarct-related ventricular tachycardia. J Cardiovasc Electrophysiol 2007;18:161–8. https:// doi.org/10.1111/j.1540-8167.2007.00721.x; PMID: 17338765.
33. Atienza F, Almendral J, Arenal Á, et al. Utilidad diagnóstica de los electrogramas almacenados por el desfibrilador automático implantable. Rev Esp Cardiol Supl 2008;8:76A–85. https://doi.org/10.1016/S1131-3587(08)73540-4
34. Almendral JM, Grogan EW, Cassidy DM, et al. Timing of the right ventricular apical electrogram during sustained ventricular tachycardia: relation to surface QRS morphology and potential clinical implications. Am J Cardiol 1984;54:1003–7. https://doi.org/10.1016/s00029149(84)80134-4; PMID: 6496320.
35. Yoshida K, Liu TY, Scott C, et al. The value of defibrillator electrograms for recognition of clinical ventricular tachycardias and for pace mapping of post-infarction ventricular tachycardia. J Am Coll Cardiol 2010;56:969–79. https://doi.org/10.1016/j.jacc.2010.04.043; PMID: 20828650.
36. Almendral J, Atienza F, Everss E, et al. Implantable defibrillator electrograms and origin of left ventricular impulses: an analysis of regionalization ability and visual spatial resolution. J Cardiovasc Electrophysiol 2012;23:506–14. https://doi.org/10.1111/j.1540-8167.2011.02233.x;
PMID: 22151407.
37. Waight MC, Li AC, Leung LW, et al. Hourly variability in outflow tract ectopy as a predictor of its site of origin. J Cardiovasc Electrophysiol 2022;33:7–16. https://doi.org/10.1111/ jce.15295; PMID: 34797600.
38. Kuo L, Liang JJ, Nazarian S, Marchlinski FE. Multimodality imaging to guide ventricular tachycardia ablation in patients with non-ischaemic cardiomyopathy. Arrhythm Electrophysiol Rev 2020;8:255–64. https://doi.org/10.15420/aer.2019.37.3;
PMID: 32685156.
39. Izquierdo M, Sánchez-Gómez JM, Ferrero de Loma-Osorio A, et al. Endo-epicardial versus only-endocardial ablation as a first line strategy for the treatment of ventricular tachycardia in patients with ischemic heart disease. Circ Arrhythm Electrophysiol 2015;8:882–9. https://doi.org/10.1161/ CIRCEP.115.002827; PMID: 26056239.
40. Bai R, Di Biase L, Shivkumar K, et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/ cardiomyopathy: arrhythmia-free survival after endoepicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol 2011;4:478–85. https://doi.org/10.1161/ CIRCEP.111.963066; PMID: 21665983.
41. Acosta J, Penela D, Andreu D, et al. Multielectrode vs. pointby-point mapping for ventricular tachycardia substrate ablation: a randomized study. Europace 2018;20:512–9. https://doi.org/10.1093/europace/euw406. PMID: 28069835.
42. De Silva K, Virk S, Nalliah CJ, et al. Multielectrode mapping versus point-by-point mapping for catheter ablation of
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Management of Ventricular Arrhythmias
Management of Ventricular Arrhythmias
ventricular tachycardia: a systematic review and metaanalysis. JACC Clin Electrophysiol 2020;6:876–8. https://doi. org/10.1016/j.jacep.2020.04.007; PMID: 32703573.
43. Elbatran AI, Li A, Gallagher MM, et al. Contact force sensing in ablation of ventricular arrhythmias using a 56-hole openirrigation catheter: a propensity-matched analysis. J Interv Card Electrophysiol 2021;60:543–53. https://doi.org/10.1007/ s10840-020-00756-4; PMID: 32440943.
44. Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation 2000;101:1288–96. https://doi. org/10.1161/01.cir.101.11.1288; PMID: 10725289.
45. Cassidy DM, Vassallo JA, Marchlinski FE, et al. Endocardial mapping in humans in sinus rhythm with normal left ventricles: activation patterns and characteristics of electrograms. Circulation 1984;70:37–42. https://doi. org/10.1161/01.cir.70.1.37; PMID: 6723010.
46. Mountantonakis SE, Park RE, Frankel DS, et al. Relationship between voltage map “channels” and the location of critical isthmus sites in patients with post-infarction cardiomyopathy and ventricular tachycardia. J Am Coll Cardiol 2013;61:2088–95. https://doi.org/10.1016/j.jacc.2013.02.031; PMID: 23524215.
47. Tung R, Josephson ME, Bradfield JS, Shivkumar K. Directional influences of ventricular activation on myocardial scar characterization: voltage mapping with multiple wavefronts during ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2016;9:e004155. https://doi. org/10.1161/CIRCEP.116.004155; PMID: 27516464.
48. Deno DC, Balachandran R, Morgan D, et al. Orientationindependent catheter-based characterization of myocardial activation. IEEE Trans Bio Med Eng 2017;64:1067–77. https:// doi.org/10.1109/TBME.2016.2589158; PMID: 27411215.
49. Porta-Sánchez A, Magtibay K, Nayyar S, et al. Omnipolarity applied to equi-spaced electrode array for ventricular tachycardia substrate mapping. Europace 2019;21:813–21. https://doi.org/10.1093/europace/euy304. PMID: 30726937.
50. Polin GM, Haqqani H, Tzou W, et al. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm 2011;8:76–83. https://doi.org/10.1016/j. hrthm.2010.09.088; PMID: 20933099.
51. Hutchinson MD, Gerstenfeld EP, Desjardins B, et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:49–55. https://doi.org/10.1161/ CIRCEP.110.959957; PMID: 21131557.
52. Soto-Becerra R, Bazan V, Bautista W, et al. Ventricular tachycardia in the setting of chagasic cardiomyopathy: use of voltage mapping to characterize endoepicardial nonischemic scar distribution. Circ Arrhythm Electrophysiol 2017;10:e004950. https://doi.org/10.1161/CIRCEP.116.004950;
PMID: 29133379.
53. Sramko M, Abdel-Kafi S, Geest RJ van der, et al. New adjusted cutoffs for “normal” endocardial voltages in patients with post-infarct LV remodeling. JACC Clin Electrophysiol 2019;5:1115–26. https://doi.org/10.1016/j. jacep.2019.07.007; PMID: 31648735.
54. Tung R, Vaseghi M, Frankel DS, et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: An International VT Ablation Center Collaborative Group study. Heart Rhythm Off J Heart Rhythm Soc 2015;12:1997–2007. https://doi.org/10.1016/j. hrthm.2015.05.036; PMID: 26031376.
55. Josephson ME. Electrophysiology of ventricular tachycardia: an historical perspective. J Cardiovasc Electrophysiol 2003;14:1134–48. https://doi. org/10.1046/j.1540-8167.2003.03322.x; PMID: 14521677.
56. Di Biase L, Burkhardt JD, Lakkireddy D, et al. Ablation of stable VTs versus substrate ablation in ischemic cardiomyopathy: the VISTA randomized multicenter trial. J Am Coll Cardiol 2015;66:2872–82. https://doi.org/10.1016/j. jacc.2015.10.026; PMID: 26718674.
57. Briceño DF, Romero J, Villablanca PA, et al. Long-term outcomes of different ablation strategies for ventricular tachycardia in patients with structural heart disease: systematic review and meta-analysis. Europace 2018;20:104–15. https://doi.org/10.1093/europace/eux109 PMID: 28575378.
58. Virk SA, Keren A, John RM, et al. Mechanical circulatory support during catheter ablation of ventricular tachycardia: indications and options. Heart Lung Circ 2019;28:134–45. https://doi.org/10.1016/j.hlc.2018.10.006; PMID: 30355468.
59. Vallabhajosyula S, Vallabhajosyula S, Vaidya VR, et al. Venoarterial extracorporeal membrane oxygenation support for ventricular tachycardia ablation: a systematic review.
ASAIO J 2020;66:980–5. https://doi.org/10.1097/ MAT.0000000000001125. PMID: 31977352.
60. Turagam MK, Vuddanda V, Atkins D, et al. Hemodynamic support in ventricular tachycardia ablation: an International VT Ablation Center Collaborative Group study. JACC Clin Electrophysiol 2017;3:1534–43. https://doi.org/10.1016/j. jacep.2017.07.005; PMID: 29759835.
61. Baratto F, Pappalardo F, Oloriz T, et al. Extracorporeal membrane oxygenation for hemodynamic support of ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2016;9:e004492. https://doi.org/10.1161/CIRCEP.116.004492; PMID: 27932426.
62. Muser D, Castro SA, Liang JJ, Santangeli P. Identifying risk and management of acute haemodynamic decompensation during catheter ablation of ventricular tachycardia. Arrhythm Electrophysiol Rev 2018;7:282–7. https://doi.org/10.15420/ aer.2018.36.3; PMID: 30588317.
63. Nof E, Reichlin T, Enriquez AD, et al. Impact of general anesthesia on initiation and stability of VT during catheter ablation. Heart Rhythm 2015;12:2213–20. https://doi. org/10.1016/j.hrthm.2015.06.018; PMID: 26072026.
64. Im SI, Voskoboinik A, Lee A, et al. Predictors of long-term success after catheter ablation of premature ventricular complexes. J Cardiovasc Electrophysiol 2021;32:2254–61. https://doi.org/10.1111/jce.15114; PMID: 34041816.
65. van Huls van Taxis CF, Wijnmaalen AP, den Uijl DW, et al. Reversed polarity of bipolar electrograms for predicting a successful ablation site in focal idiopathic right ventricular outflow tract arrhythmias. Heart Rhythm 2011;8:665–71. https://doi.org/10.1016/j.hrthm.2010.12.049; PMID: 21215326.
66. Stevenson WG, Sager PT, Friedman PL. Entrainment techniques for mapping atrial and ventricular tachycardias. J Cardiovasc Electrophysiol 1995;6:201–16. https://doi. org/10.1111/j.1540-8167.1995.tb00771.x; PMID: 7620645.
67. El-Shalakany A, Hadjis T, Papageorgiou P, et al. Entrainment/ mapping criteria for the prediction of termination of ventricular tachycardia by single radiofrequency lesion in patients with coronary artery disease. Circulation 1999;99:2283–9. https://doi.org/10.1161/01.cir.99.17.2283; PMID: 10226094.
68. Li A, Davis JS, Wierwille J, et al. Relationship between distance and change in surface ECG morphology during Pacemapping as a guide to ablation of ventricular arrhythmias: implications for the spatial resolution of Pacemapping. Circ Arrhythm Electrophysiol 2017;10:e004447. https://doi.org/10.1161/CIRCEP.116.004447; PMID: 28031213.
69. Chillou C de, Groben L, Magnin-Poull I, et al. Localizing the critical isthmus of postinfarct ventricular tachycardia: the value of pace-mapping during sinus rhythm. Heart Rhythm 2014;11:175–81. https://doi.org/10.1016/j.hrthm.2013.10.042; PMID: 24513915.
70. Jaïs P, Maury P, Khairy P, et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation 2012;125:2184–96. https://doi. org/10.1161/CIRCULATIONAHA.111.043216; PMID: 22492578.
71. Silberbauer J, Oloriz T, Maccabelli G, et al. Noninducibility and late potential abolition: a novel combined prognostic procedural end point for catheter ablation of postinfarction ventricular tachycardia. Circ Arrhythm Electrophysiol 2014;7:424–35. https://doi.org/10.1161/CIRCEP.113.001239; PMID: 24833642.
72. Berruezo A, Fernández-Armenta J, Andreu D, et al. Scar dechanneling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythm Electrophysiol 2015;8:326–36. https://doi.org/10.1161/CIRCEP.114.002386; PMID: 25583983.
73. Andreu D, Penela D, Acosta J, et al. Cardiac magnetic resonance-aided scar dechanneling: influence on acute and long-term outcomes. Heart Rhythm 2017;14:1121–8. https:// doi.org/10.1016/j.hrthm.2017.05.018; PMID: 28760258.
74. Irie T, Yu R, Bradfield JS, et al. Relationship between sinus rhythm late activation zones and critical sites for scarrelated ventricular tachycardia: a systematic analysis of isochronal late activation mapping. Circ Arrhythm Electrophysiol 2015;8:390–9. https://doi.org/10.1161/ CIRCEP.114.002637; PMID: 25740836.
75. Aziz Z, Shatz D, Raiman M, et al. Targeted ablation of ventricular tachycardia guided by wavefront discontinuities during sinus rhythm: a new functional substrate mapping strategy. Circulation 2019;140:1383–97. https://doi.org/10.1161/ CIRCULATIONAHA.119.042423; PMID: 31533463.
76. Jackson N, Gizurarson S, Viswanathan K, et al. Decrement evoked potential mapping: basis of a mechanistic strategy for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol 2015;8:1433–42. https://doi.org/10.1161/ CIRCEP.115.003083; PMID: 26480929.
77. Porta-Sánchez A, Jackson N, Lukac P, et al. Multicenter study of ischemic ventricular tachycardia ablation with
decrement-evoked potential (DEEP) mapping with extra stimulus. JACC Clin Electrophysiol 2018;4:307–15. https://doi. org/10.1016/j.jacep.2017.12.005; PMID: 30089555.
78. Srinivasan NT, Garcia J, Schilling RJ, et al. Multicenter study of dynamic high-density functional substrate mapping improves identification of substrate targets for ischemic ventricular tachycardia ablation. JACC Clin Electrophysiol 2020;6:1783–93. https://doi.org/10.1016/j.jacep.2020.06.037; PMID: 33357574.
79. Komatsu Y, Hocini M, Nogami A, et al. Catheter ablation of refractory ventricular fibrillation storm after myocardial infarction. Circulation 2019;139:2315–25. https://doi. org/10.1161/CIRCULATIONAHA.118.037997; PMID: 30929474.
80. Talib AK, Takagi M, Shimane A, et al. Efficacy of endocardial ablation of drug-resistant ventricular fibrillation in Brugada syndrome: long-term outcome. Circ Arrhythm Electrophysiol 2018;11:e005631. https://doi.org/10.1161/CIRCEP.117.005631; PMID: 30354308.
81. Haïssaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med 2008;358:2016–23. https://doi.org/10.1056/NEJMoa071968; PMID: 18463377.
82. Haïssaguerre M, Extramiana F, Hocini M, et al. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation 2003;108:925-8. https:// doi.org/10.1161/01.CIR.0000088781.99943.95; PMID: 12925452.
83. Nakamura T, Schaeffer B, Tanigawa S, et al. Catheter ablation of polymorphic ventricular tachycardia/fibrillation in patients with and without structural heart disease. Heart Rhythm 2019;16:1021–7. https://doi.org/10.1016/j. hrthm.2019.01.032; PMID: 30710740.
84. Rattanawong P, Ladia V, Minaskeian N, et al. Empirical ablation to prevent sequential Purkinje system recruitment: a novel therapy for idiopathic ventricular fibrillation. JACC Case Rep 2021;3:517–22. https://doi.org/10.1016/j. jaccas.2021.01.018; PMID: 34317571.
85. Salazar P, Beaser AD, Upadhyay GA, et al. EMPIRIC ablation OF POLYMORPHIC ventricular tachycardia/fibrillation in the absence of a mappable trigger: prospective feasibility and efficacy of pacemap matching to defibrillator electrograms. Heart Rhythm 2021;527102316-X:S1547. https://doi. org/10.1016/j.hrthm.2021.10.025; PMID: 34757186.
86. Zhang J, Hocini M, Strom M, et al. The electrophysiological substrate of early repolarization syndrome: noninvasive mapping in patients. JACC Clin Electrophysiol 2017;3:894–904. https://doi.org/10.1016/j.jacep.2016.12.017; PMID: 29130071.
87. Nademanee K, Haissaguerre M, Hocini M, et al. Mapping and ablation of ventricular fibrillation associated with early repolarization syndrome. Circulation 2019;140:1477–90. https://doi.org/10.1161/CIRCULATIONAHA.118.039022; PMID: 31542949.
88. Krummen DE, Ho G, Hoffmayer KS, et al. Electrical substrate ablation for refractory ventricular fibrillation: results of the AVATAR study. Circ Arrhythm Electrophysiol 2021;14:e008868. https://doi.org/10.1161/CIRCEP.120.008868; PMID: 33550811.
89. Morady F, Harvey M, Kalbfleisch SJ, et al. Radiofrequency catheter ablation of ventricular tachycardia in patients with coronary artery disease. Circulation 1993;87:363–72. https:// doi.org/10.1161/01.cir.87.2.363; PMID: 8425285.
90. Kim YH, Sosa-Suarez G, Trouton TG, et al. Treatment of ventricular tachycardia by transcatheter radiofrequency ablation in patients with ischemic heart disease. Circulation 1994;89:1094–102. https://doi.org/10.1161/01.cir.89.3.1094; PMID: 8124795.
91. Nakagawa H, Yamanashi WS, Pitha JV, et al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with a saline-irrigated electrode versus temperature control in a canine thigh muscle preparation. Circulation 1995;91:2264–73. https://doi. org/10.1161/01.cir.91.8.2264; PMID: 7697856.
92. Di Biase L, Burkhardt JD, Lakkireddy D, et al. Mapping and ablation of ventricular arrhythmias with magnetic navigation: comparison between 4- and 8-mm catheter tips. J Interv Card Electrophysiol 2009;26:133–7. https://doi.org/10.1007/s10840009-9416-5; PMID: 19639398.
93. Nguyen DT, Olson M, Zheng L, et al. Effect of irrigant characteristics on lesion formation after radiofrequency energy delivery using ablation catheters with actively cooled tips. J Cardiovasc Electrophysiol 2015;26:792–8. https://doi.org/10.1111/jce.12682; PMID: 25864402.
94. Nguyen DT, Gerstenfeld EP, Tzou WS, et al. Radiofrequency ablation using an open irrigated electrode cooled with halfnormal saline. JACC Clin Electrophysiol 2017;3:1103–10. https:// doi.org/10.1016/j.jacep.2017.03.006; PMID: 29759492.
95. Bennett R, Campbell T, Byth K, et al. Catheter ablation using half-normal saline and dextrose irrigation in an ovine ventricular model. JACC Clin Electrophysiol 2021;7:1229–39.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Management of Ventricular Arrhythmias
https://doi.org/10.1016/j.jacep.2021.05.002; PMID: 34217664.
96. Della Bella P, Peretto G, Paglino G, et al. Bipolar radiofrequency ablation for ventricular tachycardias originating from the interventricular septum: safety and efficacy in a pilot cohort study. Heart Rhythm 2020;17:2111–8. https://doi.org/10.1016/j.hrthm.2020.06.025; PMID: 32599177.
97. Igarashi M, Nogami A, Fukamizu S, et al. Acute and longterm results of bipolar radiofrequency catheter ablation of refractory ventricular arrhythmias of deep intramural origin. Heart Rhythm 2020;17:1500–7. https://doi.org/10.1016/j. hrthm.2020.04.028; PMID: 32353585.
98. Waight MC, Wiles BM, Li AC, Saba MM. Bipolar radiofrequency ablation of septal ventricular tachycardia facilitated by an intramural catheter. JACC Case Rep 2021;3:1119–24. https://doi.org/10.1016/j.jaccas.2021.05.010; PMID: 34471895.
99. Kreidieh B, Rodríguez-Mañero M, Schurmann P, et al. Retrograde coronary venous ethanol infusion for ablation of refractory ventricular tachycardia. Circ Arrhythm Electrophysiol 2016;9:10. https://doi.org/10.1161/CIRCEP.116.004352; PMID: 27406606.
100. Tavares L, Lador A, Fuentes S, et al. Intramural venous ethanol infusion for refractory ventricular arrhythmias: outcomes of a multicenter experience. JACC Clin Electrophysiol 2020;6:1420–31. https://doi.org/10.1016/j. jacep.2020.07.023; PMID: 33121671.
101. Stevenson WG, Tedrow UB, Reddy V, et al. Infusion needle radiofrequency ablation for treatment of refractory ventricular arrhythmias. J Am Coll Cardiol 2019;73:1413–25.
https://doi.org/10.1016/j.jacc.2018.12.070; PMID: 30922472.
102. John RM, Connell J, Termin P, et al. Characterization of warm saline-enhanced radiofrequency ablation lesions in the infarcted porcine ventricular myocardium. J Cardiovasc Electrophysiol 2014;25:309–16. https://doi.org/10.1111/ jce.12307; PMID: 24151953.
103. Stewart MT, Haines DE, Verma A, et al. Intracardiac pulsed field ablation: proof of feasibility in a chronic porcine model. Heart Rhythm 2019;16:754–64. https://doi.org/10.1016/j. hrthm.2018.10.030; PMID: 30385383.
104. Koruth JS, Kuroki K, Kawamura I, et al. Pulsed field ablation versus radiofrequency ablation: esophageal injury in a novel porcine model. Circ Arrhythm Electrophysiol 2020;13:e008303. https://doi.org/10.1161/CIRCEP.119.008303; PMID: 31977250.
105. Yavin H, Brem E, Zilberman I, et al. Circular multielectrode pulsed field ablation catheter lasso pulsed field ablation: lesion characteristics, durability, and effect on neighboring structures. Circ Arrhythm Electrophysiol 2021;14:e009229. https://doi.org/10.1161/CIRCEP.120.009229; PMID: 33417475.
106. Reddy VY, Neuzil P, Koruth JS, et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol 2019;74:315–26. https://doi.org/10.1016/j.jacc.2019.04.021; PMID: 31085321.
107. Reddy VY, Anic A, Koruth J, et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol 2020;76:1068–80. https://doi.org/10.1016/j.jacc.2020.07.007; PMID: 32854842.
108. Reddy VY, Anter E, Rackauskas G, et al. Lattice-tip focal ablation catheter that toggles between radiofrequency and
pulsed field energy to treat atrial fibrillation: A first-in-human trial. Circ Arrhythm Electrophysiol 2020;13:e008718. https://doi. org/10.1161/CIRCEP.120.008718; PMID: 32383391.
109. Koruth JS, Kuroki K, Iwasawa J, et al. Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation. Europace 2020;22:434–9. https://doi.org/10.1093/ europace/euz341; PMID: 31876913.
110. Sharma A, Wong D, Weidlich G, et al. Noninvasive stereotactic radiosurgery (CyberHeart) for creation of ablation lesions in the atrium. Heart Rhythm 2010;7:802–10. https://doi.org/10.1016/j.hrthm.2010.02.010; PMID: 20156591.
111. Blanck O, Bode F, Gebhard M, et al. Dose-escalation study for cardiac radiosurgery in a porcine model. Int J Radiat Oncol Biol Phys 2014;89:590–8. https://doi.org/10.1016/j. ijrobp.2014.02.036; PMID: 24751407.
112. Cuculich PS, Schill MR, Kashani R, et al. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med 2017;377:2325–36. https://doi.org/10.1056/NEJMoa1613773; PMID: 29236642.
113. Robinson CG, Samson PP, Moore KMS, et al. Phase I/II trial of electrophysiology-guided noninvasive cardiac radioablation for ventricular tachycardia. Circulation 2019;139:313–21. https://doi.org/10.1161/ CIRCULATIONAHA.118.038261; PMID: 30586734.
114. Neuwirth R, Cvek J, Knybel L, et al. Stereotactic radiosurgery for ablation of ventricular tachycardia. Europace 2019;21:1088–95. https://10.1093/europace/euz133 PMID: 31121018.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Arrhythmogenesis of Sports: Myth or Reality?
Saad Fyyaz and Michael Papadakis
Cardiovascular Clinical Academic Group, St George’s, University of London, St George’s University Hospitals NHS Foundation Trust, London, UK
Abstract
Regular exercise confers health benefits with cardiovascular mortality risk reduction through a variety of mechanisms. At a population level, evidence suggests that undertaking more exercise has greater benefits. In the modern era of sport, there has been an exponential rise in professional and amateur athletes participating in endurance events, with a progressively better understanding of the associated cardiac adaptations, collectively termed ‘athletes heart’. However, emerging data raise questions regarding the risk of potential harm from endurance exercise, with an increased risk of arrhythmia from adverse cardiac remodelling. Cross-sectional studies have demonstrated that athletes may exhibit a higher burden of AF, conduction tissue disease, ventricular arrhythmias, a cardiomyopathy-like phenotype and coronary artery disease. In an attempt to separate myth from reality, this review reports on the evidence supporting the notion of ‘too much exercise’, the purported mechanisms of exercise-induced cardiac arrhythmia and complex interplay with sporting discipline, demographics, genetics and acquired factors.
Keywords
Endurance athlete, masters athlete, AF, coronary artery disease, myocardial fibrosis, exercise dose
Disclosure: SF is funded by research grants from Cardiac Risk in the Young, which advocates for preparticipation cardiac screening of young athletes. MP has received research grants from Cardiac Risk in the Young.
Received: 14 December 2021 Accepted: 17 March 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e05. DOI: https://doi.org/10.15420/aer.2021.68
Correspondence: Michael Papadakis, Cardiovascular Clinical Academic Group, St George’s, University of London, Cranmer Terrace, London SW17 0RE, UK. E: mipapada@sgul.ac.uk
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The health benefits of exercise are well established and extend beyond the cardiovascular system.1 These benefits accrue from the modulation of traditional risk factors for atherosclerotic cardiovascular disease, as well as through an anti-inflammatory effect on the vascular endothelium and changes in autonomic regulation.2 A meta-analysis in almost 900,000 individuals demonstrated that the physically active group had a 35% reduction in the risk of cardiovascular death and 33% reduction in all-cause mortality.3
The WHO recommends a minimum of 150 minutes of moderate-intensity exercise or 75 minutes of vigorous-intensity exercise per week. 4 A cohort of nearly 650,000 individuals participating in physical activity at half these recommended levels, at the recommended levels and at three times the recommended levels, gained 1.8, 3.4 and 4.3 years of life, respectively.5 Higher cardiorespiratory fitness levels correlate with greater benefit, with a mortality risk reduction of 13% for each additional metabolic equivalent (MET) increase in exercise capacity.6,7 These data suggest that, at the population level, a greater volume of exercise results in greater cardiovascular benefit. A more cautious approach is necessary in individuals with established heart disease, where the volume and intensity of exercise may need to be moderated.8
Endurance athletes routinely exercise far beyond the WHO recommendations.9,10 The sustained elevation of cardiac pressure and volume loads associated with regular exercise promote a series of electrical, structural and functional adaptations, collectively termed ‘athlete’s heart’. The nature and magnitude of changes vary by sporting
discipline, ethnicity, age and sex, and can overlap with mild phenotypes of conditions associated with arrhythmias and sudden cardiac death (SCD).11 Extreme cavity dilatation, left ventricular (LV) hypertrophy, elevated coronary artery calcium (CAC) scores, acute cardiac biomarker release, myocardial fibrosis and cardiac arrhythmias have all been reported, raising concern of a reverse U-shaped relationship between the volume of exercise and cardiovascular health, with diminishing cardiovascular benefit and potential harm.12–15 Therefore, there is ongoing debate as to whether there is a threshold that constitutes ‘excess of exercise’, which may induce harm. To separate myth from reality, this review reports on the evidence supporting the notion of ‘too much exercise’ and the proposed mechanisms of exercise-induced cardiac arrhythmias in ostensibly healthy athletes.
AF
AF is the most common sustained arrhythmia in the general population; it is a major cause of ischaemic stroke, heart failure and impairment in cognition and quality of life, and increases the risk of death.16–18 The incidence of AF increases with age, given that age in itself is a determinant of AF. Moreover, advanced age is associated with cardiovascular risk factors, heart failure, structural heart disease, coronary artery disease and chronic kidney disease, all of which are linked with an increased risk of AF.19 It is well established that exercise mitigates such risk factors and, as such, regular exercise can prevent AF onset, as well as also improve symptoms, morbidity and mortality in those with established AF.20–22 A study of 6,000 veterans with a mean age of 56.8 years undergoing a symptom-limited exercise tolerance test found exercise capacity to be
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com
Arrhythmia Risk and Stratification
Athletes are at higher risk of developing AF compared with non-athletes
Adrenergic activation, vagal tone, inflammation, atrial dilatation and fibrosis are potential mechanisms of AF in atheletes
inversely related to the incidence of AF during a median follow-up of 8 years. The fittest individuals were found to have the lowest risk of developing AF, with a 21% decrease for each MET increase in exercise capacity.20 The Cardiovascular Health Study of 5,446 adults aged >65 years identified greater leisure time activity and walking as being associated with a lower incidence of AF, with progressively lower risk with greater activity levels, and a 44% risk reduction in those undertaking moderate physical activity.23 Importantly, however, risk reduction diminished in those undertaking high-intensity exercise (>6 METs).23
An emerging body of evidence has since supported a link between longterm intense endurance exercise and AF in an ‘exercise paradox’ (Figure 1). Larger epidemiological studies and several meta-analyses have demonstrated that the incidence of AF is two- to fivefold greater in endurance athletes than in non-athletes.24–29 The elevated risk in the athletic group dissipates with increasing age (>55 years) and the presence of cardiovascular risk factors. There is evidence to support the notion that exercise intensity, duration and type of sport affect the onset of AF. In a study of 52,755 cross-country skiers participating in a 90 km cross-country skiing race, the participants who completed more than five races were at highest risk of AF, and were more likely to develop AF than those who undertook one race (HR 1.29).24 Similar findings have been observed among healthy, middle-aged male physicians, with those participating in higher-intensity jogging having a 53% higher risk of AF compared with men who did not exercise.30 This would suggest that the association between exercise and AF is not restricted to elite athletes, and is also observed in the general population. However, the exact dose of exercise that confers risk of AF remains unclear, with high-quality prospective studies with well-defined study populations still lacking. A figure of around 1,500–2,000 lifetime exercise hours has been suggested as the threshold at which AF risk increases, with a peak age of onset at >40 years.31 AF in younger athletes is unusual and should prompt evaluation for underlying heart disease.32,33
Most studies investigating the relationship between AF and exercise have focused on male elite athletes, who historically dominated the landscape of elite sports. The link between exercise and AF in female athletes is less clear. In a large cohort of more than 140,000 male and 160,000 female athletes, increasing levels of physical activity were associated with AF in male, but not female, participants.34 A metaanalysis of 22 studies identified an increased risk of AF in men undertaking intense exercise but, conversely, intense exercise was protective in women.25 Similarly, a more recent meta-analysis also concluded that the general risk of AF is lower in female than male athletes.27 However, there remains a lack of data on high-level female
Younger male athletes (<55 years) at greater risk, with a lifetime of 1,500–2,000 training hours suggested as the risk threshold
Both mixed and endurance sports are associated with increased risk of AF
endurance athletes, who would surpass the level of exercise undertaken by the female participants of these studies.
The mechanism of AF in athletes is not well understood, with much of our knowledge based on animal models. Vagal tone, which is chronically elevated in athletes, is thought to be one of the most important contributors to the development of AF.35 In addition, atrial remodelling, in the form of atrial dilatation and fibrosis, is increasingly being recognised as an important factor. Atrial remodelling in athletes is considered to be a physiological response to exercise, because the overall reservoir function appears to be preserved with atrial dilatation; however, given that atrial dilatation in pathological conditions contributes to the development of AF, it remains to be seen how distinct atrial remodelling in athletes is from that seen in pathological states.36,37 AF episodes are most common during states of increased parasympathetic tone (rest, sleep), but sympathetic stimulation during exercise may also trigger AF, in association with atrial wall stretch and inflammatory cytokines.15 In a study of rat models of chronic endurance exercise, AF was induced after 16 weeks of training with identifiable atrial dilatation, fibrosis in the atria and right ventricle (RV) and autonomic changes, which did not fully resolve with detraining.14
Bradyarrhythmias
Sinus bradycardia and sinus pauses are common in endurance athletes. In a study of 62 former professional male cyclists, compared with 62 wellmatched controls (male golfers), the former endurance athletes demonstrated more frequent sinus bradycardia, sinus node dysfunction and pacemaker implantation for bradyarrhythmias relative to the control group.38 This is widely believed to be a consequence of high vagal tone, although, because these findings can persist despite detraining, adverse remodelling and fibrosis of the conduction system are also thought to be contributing factors.38 More recently, evidence suggests significant electrical remodelling within the sinus node, with downregulation of potassium/sodium hyperpolarisation activated cyclic nucleotide-gated channel 4 (HCN-4).39,40 A possible dose–response relationship has also been suggested, with a study of cross-country skiers demonstrating that those who participated in more races had a higher risk of sinus node disease or third-degree atrioventricular block.24
Ventricular Arrythmias Premature Ventricular Beats
Premature ventricular beats (PVB) are fairly common in athletes and are usually benign. However, they may be the only sign of heart disease, often leading to comprehensive evaluation. It is well established that PVBs may reflect the broader phenotype of cardiomyopathies and help
Arrhythmogenesis of Sports: Myth or Reality? ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 1: Characteristics of AF in Masters Athletes (Age >40 years)
Figure 2: Predictors of Malignant Ventricular Premature Beats in Athletes
differentiate pathology from physiological adaptation to exercise, particularly in athletes with mild phenotypic expression, often referred to as the ‘grey zone’.
Data supporting the notion that PVBs are more frequent in athletes and may represent a feature of athletic adaptation are contrasted by studies that show similar burden of ectopy in athletic and non-athletic individuals.41–43 Comparisons between studies are challenging due to differences in methodologies to record and report PVBs, as well as the absence of a standardised protocol guiding further investigation. PVBs have been reported in up to 14% of young athletes and 26% of veteran athletes, with no convincing association between sporting discipline, volume or intensity of exercise, years of sports participation and burden or complexity of PVBs.42–44 Furthermore, the overall burden of PVBs increases with age. These findings do not support the hypothesis that endurance sports activity increases the burden of ventricular arrhythmias.42–44
The PVB characteristics that imply association with disease are evolving (Figure 2). Traditionally, a frequency in excess of 2,000 PVBs/24 hours has been considered a red flag.41 Recently, however, evaluation of the morphology of PVBs, as a surrogate of ventricular origin, has emerged as the key factor in differentiating benign from potentially sinister PVBs.44–46 Frequent PVBs as a result of focal automaticity, emerging from the outflow tracts or from the fascicles of the left bundle branches, are relatively common and, in the absence of structural heart disease, should be considered benign.42,43 Other morphologies, such as PVBs with left or wide right bundle branch block or with intermediate or superior axis, are relatively uncommon and should be investigated further.46–48 Similarly, short coupling intervals, increasing PVB frequency during exercise and multifocal ectopy should prompt further evaluation. In particular, exerciseinduced PVBs with multiple and/or alternating morphologies (bidirectional) may raise suspicion of underlying catecholaminergic polymorphic ventricular tachycardia.49
Effects on Ion Channels
Regular exercise exerts a significant effect on the expression and function of cardiac ion channels. Athletes exhibit longer QT intervals than sedentary individuals, with corrected QT intervals of 470 ms in male athletes and 480 ms in female athletes accepted as the upper limits of normal.8 Exerciseinduced QT prolongation may confer an increased risk in individuals with underlying long QT syndrome (LQTS) because adrenergic surges and emotional stress may trigger arrhythmias in LQT1 and LQT2, respectively.50–53 Moreover, exercise-induced prolongation of the QT interval may pose
considerable challenges in differentiating physiological adaptation from congenital LQTS, and potentially offering false reassurance to athletes at risk. A recent study demonstrated an exercise-induced QT prolongation phenotype, mimicking congenital LQTS, which reverts back to normal after a period of detraining.54 Although no arrhythmic events were recorded, more data are needed to fully understand the arrhythmic risk in individuals with acquired QT prolongation.54
Similarly, repolarisation patterns on the athlete’s ECG may overlap with the Brugada phenotype, causing a diagnostic conundrum.55 Although there are no clear data supporting a relationship between exercise and SCD in patients with Brugada syndrome, enhanced vagal tone at rest and in early recovery following exercise has been postulated as a precipitant of arrhythmia in athletes with Brugada syndrome.56
The Left Ventricle
Elevations in cardiac preload and afterload with chronic exercise are associated with cardiac chamber enlargement, with a 10–20% increase in wall thickness and 10–15% increase in ventricular cavity dimensions. Consequently, differentiation between athletic adaptation to exercise and a mild phenotype of primary cardiomyopathies may be challenging even for the most experienced of sports cardiologists. Male endurance athletes are typically observed with the largest cavity dimensions, with up to 14% exceeding 60 mm, a threshold that typically raises suspicion of a primary dilated cardiomyopathy.57 Ethnicity is important to consider in the evaluation of LV wall thickness. For example, an LV wall thickness of >13 mm is rare among white athletes, whereas it is more prevalent in black athletes (2% versus 12%, respectively).55,58 Crucially, regardless of ethnicity, a maximum wall thickness exceeding 16 mm is uncommon and should prompt consideration and further evaluation for hypertrophic cardiomyopathy. In addition, LV cavity dilatation and hypertrophy may persist in up to 20% of athletes, despite detraining, suggesting that extremes of cardiovascular adaptation to exercise may be irreversible.59 In a study by Finocchiaro et al., none of the first-degree relatives of decedents with unexplained LV hypertrophy (30% competitive athletes) were diagnosed with hypertrophic cardiomyopathy, suggesting that extreme LV hypertrophy may be a source of arrhythmias.60
The Right Ventricle
At rest, the RV functions against a very low resistance and high compliance pulmonary circulation. However, during exercise, RV wall stress increases 30-fold, reflecting a minimal reduction in pulmonary vascular resistance and a significant rise in pulmonary artery systolic pressures. This raises the
Arrhythmogenesis of Sports: Myth or Reality? ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Structural heart disease Symptoms Family history of sudden cardiac death (<50 years) or inherited cardiac conditions Increased frequency of PVBs on exercise LBBB with intermediate/superior axis
RBBB with QRS >130 ms Complex PVBs
PVBs
Atypical
Short coupled
LBBB = left bundle branch block; PVBs = premature ventricular beats; RBBB = right bundle branch block.
in Endurance Athletes
possibility that repetitive intense exercise can induce structural changes and arrhythmias overlapping with arrhythmogenic right ventricular cardiomyopathy (ARVC), referred to as ‘exercise-induced ARVC’.61
Data from an animal model of endurance training demonstrated training-dependent RV fibrosis and tendency to arrhythmia following a 16-week exercise regime, which reversed after 8 weeks of exercise cessation.62 In a study of more than 300 athletes, RV enlargement meeting criteria for ARVC was seen in up to 45% of black athletes and 59% of white athletes, although none was diagnosed with ARVC.63 Studies have also reported transient RV dysfunction following endurance exercise, with greater dysfunction associated with more prolonged intense exercise, such as ultra-endurance events. In most studies there was no associated LV dysfunction, but there was correlation between the degree of RV dysfunction and elevation of troponin levels.64–66 Moreover, an evaluation of 46 endurance athletes presenting with arrhythmias by Heidbüchel et al. reported that 80% of arrhythmias were of RV origin and 89% of athletes fulfilled either definite (59%) or borderline/possible (30%) diagnostic criteria for ARVC.67 During a median follow-up of 5 years, 40% of athletes experienced major arrhythmic events defined as SCD, ICD shock or ventricular tachycardia.
Subsequent genetic analysis of genes associated with ARVC, identified pathogenic variants in only 12.8% of athletes, compared with 30–50% expected in ARVC.68 Although these studies support the notion of exercise-induced ARVC, it is important to note that they included a highly selected cohort of athletes presenting with ventricular arrhythmias, and the genetic yield in ARVC may be far lower than 50% in the context of sporadic rather than familial disease. Moreover, other studies in elite Olympic athletes competing over many years have failed to demonstrate significant pathological RV remodelling, suggesting that this may be applicable to the very extremes of endurance training in individuals with some genetic predisposition, although it may not represent the classic ARVC genotype.69
By the same token, repetitive exercise in those with an established diagnosis of ARVC is well recognised to increase the risk of SCD through the acceleration of RV dysfunction and induction of ventricular arrhythmias.70,71 A North American multidisciplinary study reported that patients engaging in competitive sports were at a twofold increased risk of ventricular tachyarrhythmias or death and earlier presentation of symptoms than patients who participated in recreational sports and sedentary individuals.72 Similar results have been confirmed in
Arrhythmogenesis of Sports: Myth or Reality? ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
AF
Figure 3: Proposed Mechanisms of Adverse Cardiac Remodelling that May Predispose to Arrhythmias
Myocyte necrosis (troponin release)
Sinus node disease AV block Adverse cardiac remodelling Arrhythmias Ventricular arrhythmias Strength of evidence
Sport, sex, ethnicity, age, genetics, acquired factors ARVC-like phenotype
↑ RV pressures Fibrosis Excessive LV remodelling DCM-like phenotype HCM-like phenotype
Autonomic dysfunction Ion channel remodelling Atrial dilatation Atherosclerosis
The figure underscores the complex interaction of demographics, sporting discipline, genetics and acquired factors. ARVC = arrhythmogenic right ventricular cardiomyopathy; AV = atrioventricular block; DCM = dilated cardiomyopathy; HCM = hypertrophic cardiomyopathy; LV = left ventricle; RV = right ventricle.
desmosomal mutation carriers with no phenotypic expression, underscoring the impact of exercise on the RV.73 Further studies and longitudinal data are required to better understand the interplay between exercise and the RV in health and disease states.
Myocardial Fibrosis
In patient populations, the presence of late gadolinium enhancement (LGE) is an established adverse risk factor for malignant arrhythmia, and in athletes has been associated with a risk of complex VA.47,48,74,75
A small number of studies have demonstrated myocardial fibrosis in ostensibly fit male masters athletes engaging in endurance exercise. In a study of 102 middle-aged marathon runners, 12% demonstrated myocardial fibrosis (compared with 4% of controls), of which 42% demonstrated a pattern consistent with MI predominantly in the territory of the left anterior descending artery.15 Furthermore, there was suggestion of a dose–response relationship because participation in a greater number of marathons was an independent predictor for the presence of LGE.76 Similarly, in a study of 106 male masters endurance athletes, 14% demonstrated myocardial fibrosis, with almost half demonstrating a pattern consistent with a previous MI.13 Of those with evidence of MI, only half demonstrated coronary stenosis in the relevant coronary artery, raising the possibility of subclinical infarction, due to demand ischaemia, coronary spasm or plaque rupture.13
In a study of 83 asymptomatic middle-aged triathletes, participation in longer swimming distances and cycling races was an independent predictor for the presence of non-ischaemic LGE, affecting 17% of male athletes but none of the female athletes.77 A recent meta-analysis concluded that the incidence of LGE was almost sevenfold higher in middle-aged endurance athletes compared with non-athletes, with most of this due to mid-myocardial or subepicardial LGE, with the next most common pattern being insertion point fibrosis.78 Further longitudinal studies are required to better understand the temporal association of non-ischaemic fibrosis with acquired risk factors, such as an episode of myocarditis, and its clinical relevance in masters athletes. This is relevant in the era of the COVID-19 pandemic, which has ignited interest about the prevalence and potential implications of asymptomatic (subclinical) myocardial inflammation in elite athletes. A recent registry of 1,597 competitive collegiate athletes infected with COVID-19 reported symptomatic (clinical) myocarditis in five athletes (0.3%).79 The routine use of cardiac MRI (CMR) in all athletes increased the diagnostic yield of myocarditis by 7.4-fold to 2.3%.79 Importantly, follow-up CMR in 27 of the 37 athletes diagnosed with myocarditis (73.0%) demonstrated resolution of myocardial oedema (T2 elevation) in all, and LGE indicative of myocardial fibrosis in 11 (41%).79 Similarly, in a cohort of more than 3,000 athletes with COVID-19 infection, myocarditis was identified in 0.5% of those who underwent clinically indicated CMR following clinical assessment, but in 3% of the cohort of 198 athletes who underwent screening CMR.80
Coronary Artery Disease
Exercise is well established to reduce traditional risk factors for coronary artery disease, although masters athletes have been demonstrated to show elevated CAC scores, which is a powerful adjunctive predictor of future cardiovascular events in non-athletes.13,76,81 In a study of 152 masters endurance athletes with low Framingham risk scores (mean age 54 years), 19% of male athletes had a CAC score ≥100 Agatston units, compared with 4% among the controls, and 11% of athletes had a CAC score >300 Agatston units, compared with none among the
controls.13 Furthermore, male athletes demonstrated twice as many atherosclerotic plaques (44% versus 22%), and 7.5% of male athletes demonstrated a luminal stenosis >50%, compared with none of the controls.13 Importantly, the significance of the elevated CAC scores may be mitigated by the plaque composition among athletes, which demonstrate a greater proportion of calcified plaques, which are considered more stable and less prone to rupture. In a study of 284 athletes, divided by lifelong exercise volume (<1,000, 1,000–2,000 and >2,000 MET-min/week), Aengevaeren et al. demonstrated that the most active athletes had a higher CAC score and more atherosclerotic plaque, but also a higher prevalence of calcified plaque.82 The longer-term longitudinal outcomes of endurance athletes remain unknown and further studies are warranted. In the Cooper Centre Longitudinal Study of more than 20,000 male participants, those performing >3,000 METmin of exercise per week were more likely to have CAC, without increased all-cause or cardiovascular mortality after a decade of followup.83 Another study reported on 8,425 men who underwent an assessment of cardiorespiratory fitness and CAC and, over a 8.4-year follow-up, identified that each additional MET of fitness corresponded to a 14% lower risk of cardiovascular death in an adjusted model and attenuated the risk associated with higher CAC levels.84
Conclusion
Exercise remains one of the most potent, cost-effective treatments against cardiovascular disease and cardiovascular mortality. Currently, evidence suggests that even high-intensity, high-volume exercise, and the associated lifestyle of elite endurance athletes, confers significant benefits, with athletes gaining an average of 5–7 years of life compared with sedentary individuals.85 Life-threatening arrhythmias remain overwhelmingly low, and mostly reflect underlying hereditary or congenital cardiac disease. Nevertheless, extremes of exercise may pose detrimental effects in an ‘exercise paradox’, with several routes of enquiry that require further study (Figure 3). Life-long endurance athletes seem to be at increased risk of AF in their 40s and a small number who participate in the most extreme of endurance sports may be predisposed to RV-related arrhythmias. More research is needed in better-defined cohorts with longterm follow-up.
Clinical Perspective
• The incidence of life-threatening arrhythmias in endurance athletes is low, and commonly reflects hereditary or congenital cardiac disease.
• Extremes of exercise may pose a detrimental effect; the proposed mechanisms are complex, with several routes of further enquiry ongoing.
• Athletes are at a higher risk of developing AF than non-athletes, particularly in their 40s, with both mixed and endurance sports conferring risk.
• Premature ventricular beats are common in athletes and are usually benign. Although ventricular arrhythmias have been associated with an exercise-induced arrhythmogenic phenotype, this seems to be applicable to the very extremes of endurance training in individuals with genetic predisposition.
• Further research is needed to ascertain the long-term significance of autonomic regulation and ion channel expression in endurance athletes, including extreme structural adaptations, coronary calcification, myocardial fibrosis and acute biomarker release.
Arrhythmogenesis of Sports: Myth or Reality? ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
1. McTiernan A, Kooperberg C, White E, et al. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative cohort study. JAMA 2003;290:1331–6. https://doi.org/10.1001/ jama.290.10.1331; PMID: 12966124.
2. Kasiakogias A, Sharma S. Exercise: the ultimate treatment to all ailments? Clin Cardio 2020;43:817–26. https://doi. org/10.1002/clc.23369; PMID: 32506511.
3. Nocon M, Hiemann T, Müller-Riemenschneider F, et al. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and metaanalysis. Eur J Cardiovasc Prev Rehabil 2008;15:239–46. https://doi.org/10.1097/HJR.0b013e3282f55e09; PMID: 18525377.
4. Bull FC, Al-Ansari SS, Biddle S, et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. https://doi.org/10.1136/bjsports-2020-102955; PMID: 33239350.
5. Moore SC, Patel AV, Matthews CE, et al. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med 2012;9:e1001335. https://doi.org/10.1371/journal. pmed.1001335; PMID: 23139642.
6. Kokkinos P, Myers J, Kokkinos JP, et al. Exercise capacity and mortality in black and white men. Circulation 2008;117:614–22. https://doi.org/10.1161/ CIRCULATIONAHA.107.734764; PMID: 18212278.
7. Mandsager K, Harb S, Cremer P, et al. Association of cardiorespiratory fitness with long-term mortality among adults undergoing exercise treadmill testing. JAMA Netw Open 2018;1:e183605. https://doi.org/10.1001/ jamanetworkopen.2018.3605; PMID: 30646252.
8. Pelliccia A, Sharma S, Gati S, et al. ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. https://doi.org/10.1093/ eurheartj/ehaa605; PMID: 32860412.
9. Lubbers M, Dedic A, Coenen A, et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J 2016;37:1232–43. https://doi.org/10.1093/eurheartj/ehv700; PMID: 26746631.
10. Marijon E, Uy-Evanado A, Reinier K, et al. Sudden cardiac arrest during sports activity in middle age. Circulation 2015;131:1384–91. https://doi.org/10.1161/ CIRCULATIONAHA.114.011988; PMID: 25847988.
11. Drezner JA, Malhotra A, Prutkin JM, et al. Return to play with hypertrophic cardiomyopathy: are we moving too fast? A critical review. Br J Sports Med 2021;55:1041–7. https://doi. org/10.1136/bjsports-2020-102921; PMID: 33472848.
12. Schnohr P, O’Keefe JH, Marott JL, et al. Dose of jogging and long-term mortality: the Copenhagen City Heart study. J Am Coll Cardiol 2015;65:411–9. https://doi.org/10.1016/j. jacc.2014.11.023; PMID: 25660917.
13. Merghani A, Maestrini V, Rosmini S, et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation 2017;136:126–37. https://doi.org/10.1161/ CIRCULATIONAHA.116.026964; PMID: 28465287.
14. Guasch E, Benito B, Qi X, et al. Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol 2013;62:68–77. https://doi.org/10.1016/j.jacc.2013.01.091; PMID: 23583240.
15. Breuckmann F, Möhlenkamp S, Nassenstein K, et al. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology 2009;251:50–7. https://doi.org/10.1148/ radiol.2511081118; PMID: 19332846.
16. Arbelo E, Aktaa S, Bollmann A, et al. Quality indicators for the care and outcomes of adults with atrial fibrillation. Europace 2021;23:494–5. https://doi.org/10.1093/europace/ euaa253; PMID: 32860039.
17. Wyndham CRC. Atrial fibrillation: the most common arrhythmia. Tex Heart Inst J 2000;27:257–67. PMID: 11093410.
18. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics – 2019 update: a report from the American Heart Association. Circulation 2019;139:e56–528. https://doi. org/10.1161/CIR.0000000000000659; PMID: 30700139.
19. Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612; PMID: 32860505.
20. Faselis C, Kokkinos P, Tsimploulis A, et al. Exercise capacity and atrial fibrillation risk in veterans: a cohort study. Mayo Clin Proc 2016;91:558–66. https://doi.org/10.1016/j. mayocp.2016.03.002; PMID: 27068670.
21. Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF Cohort study. J Am Coll Cardiol 2014;64:2222–31. https://doi.org/10.1016/j. jacc.2014.09.028; PMID: 25456757.
22. Pathak RK, Elliott A, Middeldorp ME, et al. Impact of CARDIOrespiratory FITness on arrhythmia recurrence in obese individuals with atrial fibrillation: the CARDIO-FIT study. J Am Coll Cardiol 2015;66:985–96. https://doi. org/10.1016/j.jacc.2015.06.488; PMID: 26113406.
23. Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults the cardiovascular health study. Circulation 2008;118:800–7. https://doi.org/10.1161/CIRCULATIONAHA.108.785626; PMID: 18678768.
24. Andersen K, Farahmand B, Ahlbom A, et al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J 2013;34:3624–31. https://doi. org/10.1093/eurheartj/eht188; PMID: 23756332.
25. Mohanty S, Mohanty P, Tamaki M, et al. Differential association of exercise intensity with risk of atrial fibrillation in men and women: evidence from a meta-analysis. J Cardiovasc Electrophysiol 2016;27:1021–9. https://doi. org/10.1111/jce.13023; PMID: 27245609.
26. Nielsen JR, Wachtell K, Abdulla J. The relationship between physical activity and risk of atrial fibrillation – a systematic review and meta-analysis. J Atr Fibrillation 2013;5:789. https:// doi.org/10.4022/jafib.789; PMID: 28496815.
27. Newman W, Parry-Williams G, Wiles J, et al. Risk of atrial fibrillation in athletes: a systematic review and metaanalysis. Br J Sports Med 2021;55:1233–8. https://doi. org/10.1136/bjsports-2021-103994; PMID: 34253538.
28. Gerche A La, Schmied CM. Atrial fibrillation in athletes and the interplay between exercise and health. Eur Heart J 2013;34:3599–602. https://doi.org/10.1093/eurheartj/eht265; PMID: 23884920.
29. Li X, Cui S, Xuan D, et al. Atrial fibrillation in athletes and general population: a systematic review and meta-analysis. Medicine 2018;97:e13405. https://doi.org/10.1097/ MD.0000000000013405; PMID: 30544416.
30. Aizer A, Gaziano JM, Cook NR, et al. Relation of vigorous exercise to risk of atrial fibrillation. Am J Cardiol 2009;103:1572–7. https://doi.org/10.1016/j. amjcard.2009.01.374; PMID: 19463518.
31. Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace 2009;11:1156–9. https://doi. org/10.1093/europace/eup197; PMID: 19633305.
32. Vlachos K, Mascia G, Martin CA, et al. Atrial fibrillation in Brugada syndrome: current perspectives. J Cardiovasc Electrophysiol 2020;31:975–84. https://doi.org/10.1111/ jce.14361; PMID: 31961030.
33. Pelliccia A, Maron BJ, Di Paolo FM, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol 2005;46:690–6. https://doi. org/10.1016/j.jacc.2005.04.052; PMID: 16098437.
34. Thelle DS, Selmer R, Gjesdal K, et al. Resting heart rate and physical activity as risk factors for lone atrial fibrillation: a prospective study of 309 540 men and women. Heart 2013;99:1755–60. https://doi.org/10.1136/ heartjnl-2013-303825; PMID: 23749790.
35. Guasch E, Mont L. Diagnosis, pathophysiology, and management of exercise-induced arrhythmias. Nat Rev Cardiol 2017;14:88–101. https://doi.org/10.1038/ nrcardio.2016.173; PMID: 27830772.
36. Seko Y, Kato T, Haruna T, et al. Association between atrial fibrillation, atrial enlargement, and left ventricular geometric remodeling. Sci Rep 2018;8:6366. https://doi.org/10.1038/ s41598-018-24875-1; PMID: 29686287.
37. D’Ascenzi F, Anselmi F, Focardi M, Mondillo S. Atrial enlargement in the athlete’s heart: assessment of atrial function may help distinguish adaptive from pathologic remodeling. J Am Soc Echocardiogr 2018;31:148–57. https:// doi.org/10.1016/j.echo.2017.11.009; PMID: 29246514.
38. Baldesberger S, Bauersfeld U, Candinas R, et al. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J 2008;29:71–8. https://doi.org/10.1093/eurheartj/ehm555; PMID: 18065754.
39. D’souza A, Bucchi A, Johnsen AB, et al. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat Commun 2014;5:3775. https://doi. org/10.1038/ncomms4775; PMID: 24825544.
40. Mesirca P, Nakao S, Nissen SD, et al. Intrinsic electrical remodeling underlies atrioventricular block in athletes. Circ Res 2021;129:e1–20. https://doi.org/10.1161/ CIRCRESAHA.119.316386; PMID: 33849278.
41. Biffi A, Pelliccia A, Verdile L, et al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol 2002;40:446–52. https://doi.org/10.1016/s0735-
1097(02)01977-0; PMID: 12142109.
42. Zorzi A, De Lazzari M, Mastella G, et al. Ventricular arrhythmias in young competitive athletes: prevalence, determinants, and underlying substrate. J Am Heart Assoc 2018;7:e009171. https://doi.org/10.1161/JAHA.118.009171; PMID: 29886418.
43. Zorzi A, Mastella G, Cipriani A, et al. Burden of ventricular arrhythmias at 12-lead 24-hour ambulatory ECG monitoring in middle-aged endurance athletes versus sedentary controls. Eur J Prev Cardiol 2018;25:2003–11. https://doi. org/10.1177/2047487318797396; PMID: 30160531.
44. Pelliccia A, De Martino L, Borrazzo C, et al. Clinical correlates and outcome of the patterns of premature ventricular beats in Olympic athletes: a long-term follow-up study. Eur J Prev Cardiol 2021;28:1038–47. https://doi. org/10.1177/2047487320928452; PMID: 32484042.
45. Corrado D, Drezner JA, D’Ascenzi F, Zorzi A. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. Br J Sports Med 2020;54:1142–8. https://doi.org/10.1136/ bjsports-2018-100529; PMID: 31481389.
46. Verdile L, Maron BJ, Pelliccia A, et al. Clinical significance of exercise-induced ventricular tachyarrhythmias in trained athletes without cardiovascular abnormalities. Heart Rhythm 2015;12:78–85. https://doi.org/10.1016/j.hrthm.2014.09.009; PMID: 25239428.
47. Zorzi A, Marra MP, Rigato I, et al. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive. Circ Arrhtyhm Electrophysiol 2016;9:e004229. https://doi. org/10.1161/CIRCEP.116.004229; PMID: 27390211.
48. Crescenzi C, Zorzi A, Vessella T, et al. Predictors of left ventricular scar using cardiac magnetic resonance in athletes with apparently idiopathic ventricular arrhythmias. J Am Heart Assoc 2021;10:e018206. https://doi.org/10.1161/ JAHA.120.018206; PMID: 33381977.
49. Haugaa KH, Leren IS, Berge KE, et al. High prevalence of exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia mutation-positive family members diagnosed by cascade genetic screening. Europace 2010;12:417–23. https://doi.org/10.1093/europace/ eup448; PMID: 20106799.
50. Schnell F, Behar N, Carré F. Long-QT syndrome and competitive sports. Arrhythm Electrophysiol Rev 2018;7:187–92. https://doi.org/10.15420/aer.2018.39.3; PMID: 30416732.
51. Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype–phenotype correlation in the long-QT syndrome. Circulation 2001;103:89–95. https://doi.org/10.1161/01.CIR.103.1.89; PMID: 11136691.
52. Mascia G, Arbelo E, Solimene F, et al. The long-QT syndrome and exercise practice: the never-ending debate. J Cardiovasc Electrophysiol 2018;29:489–96. https://doi. org/10.1111/jce.13410; PMID: 29292852.
53. Marrakchi S, Kammoun I, Bennour E, et al. Inherited primary arrhythmia disorders: cardiac channelopathies and sports activity. Herz 2020;45:142–57. https://doi.org/10.1007/ s00059-018-4706-2; PMID: 29744527.
54. Dagradi F, Spazzolini C, Castelletti S, et al. Exercise traininginduced repolarization abnormalities masquerading as congenital long QT syndrome. Circulation 2020;142:2405–15. https://doi.org/10.1161/CIRCULATIONAHA.120.048916; PMID: 33073610.
55. Papadakis M, Carre F, Kervio G, et al. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/AfroCaribbean origin. Eur Heart J 2011;32:2304–13. https://doi. org/10.1093/eurheartj/ehr140; PMID: 21613263.
56. Arai Y, Saul JP, Albrecht P, et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol Heart Circ Physiol 1989;256:h132–41. https://doi. org/10.1152/ajpheart.1989.256.1.h132; PMID: 2643348.
57. Pelliccia A, Culasso F, Di Paolo FM, Maron BJ. Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med 1999;130:23–31. https://doi.org/10.7326/0003-4819-1301-199901050-00005; PMID: 9890846.
58. Pelliccia A, Maron BJ, Spataro A, et al. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med 1991;324:295–301. https://doi. org/10.1056/NEJM199101313240504; PMID: 1824720.
59. Pelliccia A, Maron BJ, De Luca R, et al. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation 2002;105:944–9. https://doi. org/10.1161/hc0802.104534; PMID: 11864923.
60. Finocchiaro G, Dhutia H, Gray B, et al. Diagnostic yield of hypertrophic cardiomyopathy in first-degree relatives of decedents with idiopathic left ventricular hypertrophy. Europace 2020;22:632–42. https://doi.org/10.1093/europace/ euaa012; PMID: 32011662.
61. D’Ascenzi F, Pisicchio C, Caselli S, et al. RV remodeling in Olympic athletes. JACC Cardiovasc Imaging 2017;10:385–93.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Arrhythmogenesis of Sports: Myth or Reality?
Arrhythmogenesis of Sports: Myth or Reality?
https://doi.org/10.1016/j.jcmg.2016.03.017; PMID: 27544901.
62. Benito B, Gay-Jordi G, Serrano-Mollar A, et al. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011;123:13–22. https://doi.org/10.1161/CIRCULATIONAHA.110.938282; PMID: 21173356.
63. Zaidi A, Ghani S, Sharma R, et al. Physiological right ventricular adaptation in elite athletes of African and AfroCaribbean origin. Circulation 2013;127:1783–92. https://doi. org/10.1161/CIRCULATIONAHA.112.000270; PMID: 23538381.
64. Elliott AD, La Gerche A. The right ventricle following prolonged endurance exercise: are we overlooking the more important side of the heart? A meta-analysis. Br J Sports Med 2015;49:724–9. https://doi.org/10.1136/ bjsports-2014-093895; PMID: 25281542.
65. La Gerche A, Burns AT, Mooney DJ, et al. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J 2012;33:998–1006. https:// doi.org/10.1093/eurheartj/ehr397; PMID: 22160404.
66. La Gerche A, Claessen G, Dymarkowski S, et al. Exerciseinduced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J 2015;36:1998–2010. https://doi.org/10.1093/eurheartj/ ehv202; PMID: 26038590.
67. Heidbüchel H, Hoogsteen J, Fagard R, et al. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias: role of an electrophysiologic study in risk stratification. Eur Heart J 2003;24:1473–80. https://doi.org/10.1016/S0195668X(03)00282-3; PMID: 12919770.
68. La Gerche A, Robberecht C, Kuiperi C, et al. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart 2010;96:1268–74. https://doi. org/10.1136/hrt.2009.189621; PMID: 20525856.
69. Aengevaeren VL, Caselli S, Pisicchio C, et al. Right heart remodeling in Olympic athletes during 8 years of intensive exercise training. J Am Coll Cardiol 2018;72:815–7. https://doi. org/10.1016/j.jacc.2018.03.548; PMID: 30092959.
70. Finocchiaro G, Barra B, Molaro S, et al. Prevalence and clinical correlates of exercise-induced ventricular arrhythmias in arrhythmogenic right ventricular
cardiomyopathy. Int J Cardiovasc Imaging 2021 2022;38:389–96. https://doi.org/10.1007/s10554-021-02395-w; PMID: 34480708.
71. Saberniak J, Hasselberg NE, Borgquist R, et al. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail 2014;16:1337–44. https://doi.org/10.1002/ejhf.181; PMID: 25319773.
72. Ruwald AC, Marcus F, Estes NAM, et al. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J 2015;36:1735–43. https://doi. org/10.1093/eurheartj/ehv110; PMID: 25896080.
73. James CA, Bhonsale A, Tichnell C, et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathyassociated desmosomal mutation carriers. J Am Coll Cardiol 2013;62:1290–7. https://doi.org/10.1016/j.jacc.2013.06.033; PMID: 23871885.
74. Giusca S, Kelle S, Nagel E, et al. Differences in the prognostic relevance of myocardial ischaemia and scar by cardiac magnetic resonance in patients with and without diabetes mellitus. Eur Heart J Cardiovasc Imaging 2016;17:812–20. https://doi.org/10.1093/ehjci/jev220; PMID: 26358695.
75. Schnell F, Claessen G, La Gerche A, et al. Subepicardial delayed gadolinium enhancement in asymptomatic athletes: let sleeping dogs lie? Br J Sports Med 2016;50:111–7. https:// doi.org/10.1136/bjsports-2014-094546; PMID: 26224114.
76. Möhlenkamp S, Lehmann N, Breuckmann F, et al. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J 2008;29:1903–10. https://doi.org/10.1093/ eurheartj/ehn163; PMID: 18426850.
77. Tahir E, Starekova J, Muellerleile K, et al. Myocardial fibrosis in competitive triathletes detected by contrast-enhanced CMR correlates with exercise-induced hypertension and competition history. JACC Cardiovasc Imaging 2018;11:1260–70. https://doi.org/10.1016/j.jcmg.2017.09.016; PMID: 29248656.
78. Zhang CD, Xu SL, Wang XY, et al. Prevalence of myocardial fibrosis in intensive endurance training athletes: a systematic review and meta-analysis. Front Cardiovasc Med 2020;7:585692. https://doi.org/10.3389/fcvm.2020.585692; PMID: 33102537.
79. Daniels CJ, Rajpal S, Greenshields JT, et al. Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: results from the Big Ten COVID-19 cardiac registry. JAMA Cardiol 2021;6:1078–87. https://doi.org/10.1001/jamacardio.2021.2065; PMID: 34042947.
80. Moulson N, Petek BJ, Drezner JA, et al. SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation 2021;144:256–66. https://doi.org/10.1161/ CIRCULATIONAHA.121.054824; PMID: 33866822.
81. Budoff MJ, Shaw LJ, Liu ST, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007;49:1860–70. https://doi.org/10.1016/j.jacc.2006.10.079; PMID: 17481445.
82. Aengevaeren VL, Mosterd A, Braber TL, et al. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation 2017;136:138–48. https://doi.org/10.1161/CIRCULATIONAHA.117.027834; PMID: 28450347.
83. Defina LF, Radford NB, Barlow CE, et al. Association of allcause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol 2019;4:174–81. https://doi.org/10.1001/ jamacardio.2018.4628; PMID: 30698608.
84. Radford NB, DeFina LF, Leonard D, et al. Cardiorespiratory fitness, coronary artery calcium, and cardiovascular disease events in a cohort of generally healthy middle-age men: results from the Cooper Center longitudinal study. Circulation 2018;137:1888–95. https://doi.org/10.1161/ CIRCULATIONAHA.117.032708; PMID: 29343464.
85. Sanchis-Gomar F, Olaso-Gonzalez G, Corella D, et al. Increased average longevity among the Tour de France cyclists. Int J Sports Med 2011;32:644–7. https://doi. org/10.1055/s-0031-1271711; PMID: 21618162.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Global Substrate Mapping and Targeted Ablation with Novel Gold-tip Catheter in De Novo Persistent AF
Michael TB Pope 1,2 and Timothy R Betts 1,3
Abstract
Results from catheter ablation for persistent AF are suboptimal, with no strategy other than pulmonary vein isolation showing clear benefit. Recently employed empirical strategies beyond pulmonary vein isolation involve widespread atrial ablation in all patients and do not take into account patient-specific differences in AF mechanisms or phenotype. Charge density mapping using the non-contact AcQMap system (Acutus Medical) allows visualisation of whole-chamber activation during AF and reveals localised patterns of complex activation thought to represent important mechanisms for AF maintenance that can be targeted with focal ablation. In this review, the authors outline the fundamentals of this technology, the initial data exploring the mechanistic role of activation patterns seen and the application to ablation of persistent AF.
Keywords
Non-contact mapping, atrial fibrillation, charge density, core-to-boundary ablation
Disclosure: MTBP and TRB have received honoraria from Acutus Medical.
Received: 25 October 2021 Accepted: 14 February 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e06. DOI: https://doi.org/10.15420/aer.2021.64
Correspondence: Timothy Betts, Department of Cardiology, John Radcliffe Hospital, Headley Way, Oxford OX3 9DU, UK. E: tim.betts@ouh.nhs.uk
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The cornerstone of catheter ablation for AF was the discovery in the late 1990s of rapidly firing ectopic foci from the pulmonary veins (PV) initiating AF, and the observation that ablation of these ectopic foci could abolish the arrhythmia.1 Additional recognition of the complex tissue architecture of the PV-left atrial (LA) junction led to a progression in catheter ablation approaches from focal PV ablation to ostial segmental ablation and subsequently to wide area circumferential ablation incorporating part of the posterior LA wall and the complex PV-LA junction.2–4 Randomised controlled trials and meta-analyses have since demonstrated increased effectiveness of this antral approach, which is also thought to result in lower risk of pulmonary vein stenosis.3 5 6 In patients with paroxysmal AF, outcomes are good. At 12 months, success rates approach 65%, increasing to 80% following repeat procedures.7–11 However, the observation of late recurrences with long-term follow-up despite pulmonary vein isolation (PVI) and the significantly lower success rates in patients with persistent arrhythmia highlight the importance of additional non-pulmonary vein mechanisms in AF initiation and maintenance.7,8,12–14
Although several empirical ablation strategies including linear ablation of the LA roof and mitral isthmus or ablation of complex fractionated atrial electrograms have been employed, none have been shown demonstrably to result in incremental benefit beyond a strategy of PVI alone, which, in the STAR-AF 2 trial, resulted in a 59% rate of freedom from AF recurrence after 18 months of follow-up.15–19 Recent trials of PVI in combination with posterior wall isolation have been similarly disappointing.20,21 Improved outcomes have been identified in small trials of strategies employing extensive catheter and/or surgical ablation techniques targeting a large volume of atrial myocardium.22–24 However, the potential development of
stiff atrial syndrome resulting from such extensive iatrogenic scar formation risks counteracting the benefits of sinus rhythm restoration.25
Limitations of empirical strategies have subsequently led to attempts to develop individualised, patient-specific approaches targeting either localised areas of fibrosis or electrophysiological mechanisms.26 Results from animal models have suggested the existence of high-frequency localised and repetitive re-entrant circuits termed rotors, thought to represent ‘drivers’ responsible for maintenance of AF propagation.27–30 Early efforts to develop methods able to identify these mechanisms and thus guide ablation strategies showed promising results.31–35 However, recent randomised studies have been disappointing.21 36 37 These methods have largely relied on either dominant frequency analysis aimed at identifying zones of high-frequency activation, or phase mapping applied either to multi-electrode basket catheters or non-invasive body surface electrodes, which aim to identify phase singularities representing the pivot point of localised rotors.38 Further studies and evaluation of these techniques have revealed contrasting results and highlighted the limitations of these approaches.39–44
Charge density mapping (Acutus Medical) uses a non-contact approach to facilitate whole chamber mapping of atrial activation with the aim of identifying patient-specific electrophysiological mechanisms responsible for AF maintenance that can be targeted with ablation. In this review we aim to provide an overview of the background to this technology, the current clinical application and available evidence, as well as provide insights into the potential future benefits this technology may hold for patients.
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Clinical Electrophysiology and Ablation
1. Department of Cardiology, Oxford University Hospitals NHS Foundation Trust, Oxford, UK; 2. Department for Human Development and Health, University of Southampton, Southampton, UK; 3. University of Oxford Biomedical Research Centre, Oxford, UK
and Targeted Ablation with Novel Gold-tip Catheter
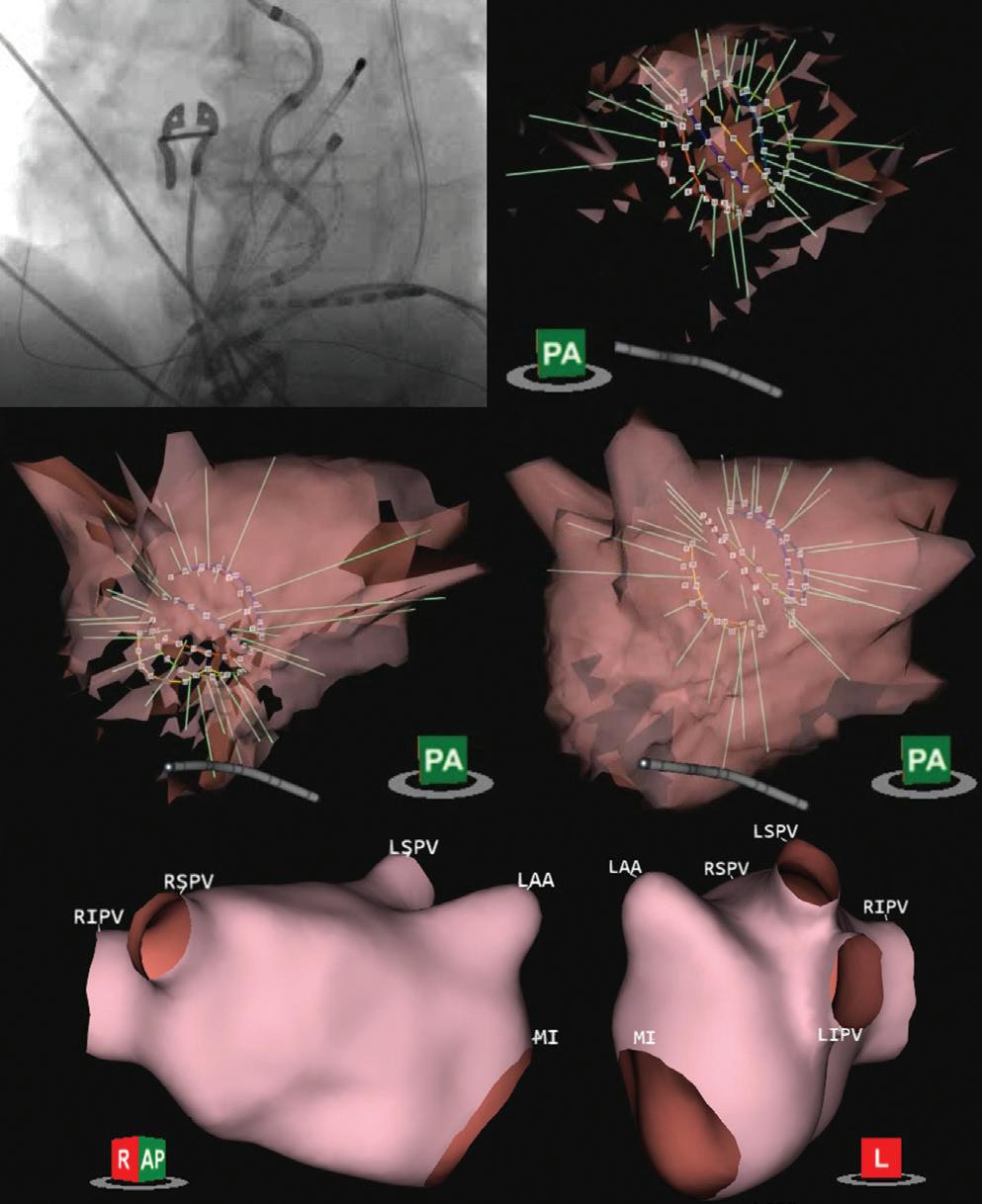
1: Ultrasound Generation of Atrial Anatomy
rather originates from the presence of charge. The summation of charge, in varying magnitudes and location, directly affects the structure and magnitude of the electric potential field, and thus the voltage measured with an electrode at a given location.
As an action potential is generated within the myocardium, facilitated by the movement of ions across the cell membrane, a small charge imbalance is created in the region of the adjacent extracellular space. Initial depolarisation is followed by recruitment of adjacent cells and spreads outwards, resulting in a macroscopic distribution of charge, a ‘density’ over space, with the largest gradient of charge density at the depolarising wavefront. This results in an ability to represent the cardiac signals in a more spatially compact and morphologically narrow waveform – the charge density electrogram.45
These local charges represent the sources of the potential field measured as an electrogram by conventional electrophysiological recording systems or as an electrocardiogram when measured on the body surface. The relationship between charge and potential (voltage signals) is fundamentally defined by Poisson’s equation, allowing direct computation of the potential field caused by a given electric charge. Although charge itself cannot be directly measured, a set of potential field measurements can be used to inversely solve Poisson’s equation to determine the originating charge distribution giving rise to them.46 Following subtraction of the far-field QRS-T wave signals, the AcQMap non-contact approach relies on this technique to calculate the continuous chamber-wide, distribution of charge that gives rise to any ongoing cardiac activity, critical to the visualisation of complex and temporally variable AF propagation.
Charge Density Non-contact Approach
The AcQMap system (Acutus Medical) is a combined imaging and electrophysiological mapping system that uses a diagnostic catheter comprising six splines, each of which incorporates eight ultrasound transducers interspersed with eight biopotential electrodes (Figure 1A). Through processing of up to 115,000 ultrasound points/minute, a highresolution 3D reconstruction of the atrial chamber anatomy is generated within a few minutes (Figures 1B–1E and Supplementary Material Video 1). This is based on a triangular mesh structure comprising constituent corners (termed vertices) of shared triangular faces, which is integrated into a registered coordinate system for spatial localisation based on tracking within an impedance field. The distance between vertices is approximately 1.5–2 mm, which represents the spatial resolution of the system. The 48 biopotential electrodes record raw intracardiac unipolar signals at a rate of 150,000 samples/second. An inverse solution is applied to reconstruct unipolar signals at each unique point (approximately 3,500 vertices) on the generated endocardial surface anatomy. The use of regularisation as well as the proximity of the electrodes to the potential source on the atrial endocardium serve to limit any errors introduced in the solution to the inverse problem.
Uniquely, this system uses charge density mapping to provide sharper, high-resolution visualisation of cardiac activation in comparison with voltage signals.45 Cardiac electrograms measured from either non-contact electrodes in the cardiac chamber, electrodes in contact with the cardiac tissue or from the body surface, are measures of electric potential in units of volts. However, this electric potential does not originate in isolation, but
Validation has been performed examining the reconstructed voltage signals against contact unipolar electrograms recorded by a multipolar circular mapping catheter during both sinus rhythm and AF.47 Validation measures assessed included both electrogram morphology and timing. During sinus rhythm, median morphology cross-correlation and timing difference was 0.85 (interquartile range [IQR] 0.71–0.94) and 6.4 ms (IQR 2.6–17.1), respectively, with values of 0.79 (IQR 0.69–0.88) and 14.4 ms (IQR 6.7–26.2), for morphology and timing difference during AF.47 The radial distance between the centre of the AcQMap catheter and the atrial surface significantly impacted results, particularly at distances ≥40 mm (Figure 2).47
Activation Pattern Characterisation
Annotation of the negative slope of the charge signal, which correlates with phase 0 of the action potential, identifies the zone of depolarisation at any instant in time and enables construction of a propagation history map without the need for a stable timing reference.45 This is visualised as a moving leading edge of a wavefront displayed as an isochronal map, with differing isochronal spacing representing changing conduction velocities. Visual interpretation of the propagation map is complemented by the integrated, automated AcQTrack platform, which tracks and analyses the spread of the activation wavefronts between all adjacent vertices on the chamber anatomy. While smooth planar wavefronts are discounted, directional changes in propagation are identified and characterised.
Three distinct patterns of propagation are identified including focal firing (FF), localised rotational activation (LRA) and localised irregular activation (LIA) (Figure 3 and Supplementary Material Videos 2–4). FF is identified if the activation at a vertex occurs at least 3 ms prior to all its immediate neighbours and is seen to propagate outwards centrifugally. LRA is identified by recording the degrees of conduction around a central point
Global Substrate Mapping
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
A B C D E
Figure
Fluoroscopic view of the AcQMap catheter (Acutus Medical) positioned within the left atrium (A). Ultrasound is used to generate the chamber anatomy (B–D) with the ultrasound beams illustrated by green lines emanating from the basket transducers. Following post-processing, the final anatomy is generated (E). AP = antero-posterior; L = left lateral; LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; MI = mitral isthmus; PA = postero-anterior; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein.
by calculating the cumulative angle differences of sequential conduction velocity vector directions around the central point and within a discrete area of approximately 300 mm2. If the rotational angle of conduction exceeds 270°, equivalent to a cumulative angle of wavefront activation change approaching 360°, rotation is detected at the central point. LIA is characterised by more complex directional changes in propagation but incorporates directional changes in activation differently from the criteria for LRA. Angle differences between the vector of a wavefront entering and leaving a confined region of approximately 200 mm2 are computed and if the difference exceeds 90°, LIA is detected.
Practical Considerations and Insights into AF Mechanisms
A major challenge in designing non-PV ablation strategies for persistent AF is the limitation in our understanding of AF mechanisms. Competing
theories of AF propagation revolve around questions of hierarchical versus anarchical mechanisms and the role of structural versus functional substrate properties.48 Similarly, identification of appropriate ablation targets guided by charge density mapping is influenced by a combination of practical aspects of how the technology is implemented into a procedure and understanding of the mechanistic significance of the patterns of AF activation identified.
Ablation, by definition, delivers a fixed therapy. This is an important consideration in designing strategies aimed at AF activation patterns. Targeting a fixed therapy to transient or migratory phenomena is unlikely to be effective, while sites of highly repetitive and spatially stable activation patterns represent more attractive ablation targets. An appropriate duration of mapping that reveals these stable phenomena, together with an understanding of their spatial stability over time, is
Global Substrate
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Mapping and Targeted Ablation with Novel Gold-tip Catheter
Xcorr = 0.87 Xcorr = 0.89 9 8 7 6 5 4 3 2 1 10 Xcorr = 0.97 Xcorr = 0.98 Xcorr = 0.90 Xcorr = 0.88 Xcorr = 0.71 Xcorr = 0.79 Xcorr = 0.89 Xcorr = 0.94 Noncontact Contact Sinus rhythm AF Correlation Timing di erence (ms) Correlation Timing di erence (ms) n=20 Radial distance (r) from centre of noncontact catheter 0.85 (0.71–0.94) 6.4 (2.6–17.1) 0.79 (0.69–0.88) 14.4 (6.7–26.2) ≤40 mm >40 mm p-value 0.87 (0.72–0.94) 5.7 (2.6–15.4) 0.81 (0.69–0.89) 12.3 (5.9–21.8) 0.73 (0.56–0.88) 15.1 (4.1–27.7) 0.67 (0.45–0.82) 28.3 (16.2–36.0) <0.01 <0.01 <0.01 <0.01
Figure 2: Validation Data for Non-contact Electrograms
Validation of inversely derived non-contact voltage electrograms against contact unipolar electrograms for both morphology and timing during sinus rhythm and AF. Accuracy diminishes significantly at radial distances ≥40 mm from the centre of the AcQMap catheter (Acutus Medical). In the table, values are median (interquartile range). Xcorr = cross-correlation. Source: Shi et al. 2020.47 Adapted with permission from Elsevier.
and Targeted Ablation with Novel Gold-tip Catheter
Figure 3: Examples of Complex Activation Patterns Identified Using Charge Density Propagation Mapping
The relative properties of LIA, LRA and FF may represent variation in their role in the maintenance of AF propagation and/or the progression from a trigger-dependent, to a substrate-dependent, trigger-independent mechanism. These differences may also reflect distinct phenotypes either reliant on focal ‘drivers’ to sustain downstream fibrillatory conduction, or able to self-sustain as a result of ‘maintainers’ that facilitate wave-break together with wavefront renewal processes perpetuating AF.52 These observations are important considerations in designing individualised ablation strategies and inform the hierarchy of ablation targets chosen. When present, high-frequency FF is targeted with the aim of eliminating AF ‘driver’ mechanisms, while more prevalent sites of LIA are ablated with a view to altering the substrate responsible for the maintenance of AF propagation.
Areas of localised complex conduction are identified including FF (A), which is characterised by centrifugal activation from a central point; LRA (B), which involves smooth conduction around a central zone of >270°; and LIA (C). In this example of LIA, a region of slow conduction (blue jagged line) results in isthmus-like conduction with directional change in propagation around this zone. Red represents the leading edge of the activation wavefront with purple (see inset) the trailing edge with a time difference of 80 ms. FF = focal firing; LIA = localised irregular activation; LRA = localised rotational activation.
therefore required. We have previously demonstrated that regions of high-frequency LIA show a high degree of spatiotemporal stability, in contrast to regions of LRA that show the least consistency over repeated mapping segments.49 Although isolated focal activations are frequently observed throughout the chamber, zones of high-frequency FF (occurring at a frequency of ≥1 every 3 seconds) demonstrate greater stability. Similarly, while mapping durations of 12 seconds are adequate to identify these stable zones of LIA, 20–25 seconds are required to reveal repetitive zones of either LRA or FF.
Lee et al. have recently published similar findings with high temporal stability of LIA and no meaningful relationship to low-voltage amplitude.50 Although bipolar voltage amplitude in regions of repetitive LIA appears preserved, these sites demonstrate an increase in conduction heterogeneity during short-coupled extra-stimulus pacing not observed in the remainder of the chamber.51 49 Considering the high degree of spatial stability, this suggests a relationship to underlying atrial structural properties with a potential role in AF maintenance, making sites of highfrequency LIA attractive targets for catheter ablation. From a practical perspective, analysis of a minimum of two to three mapping segments of 10 seconds duration is therefore required to accurately reveal the most stable phenomena and guide delivery of therapy.
In addition, there appears to be an inverse association between the frequency of focal and rotational activation patterns. Termination of AF during catheter ablation is associated with a higher frequency of FF, and patients with paroxysmal, compared to persistent AF demonstrate more FF with less LRA. Furthermore, we have shown that adenosine, which is known to shorten atrial refractoriness and provoke AF, promotes LRA alongside an acceleration in global AF cycle length. These findings suggest that LRA frequency may serve as a useful surrogate marker of atrial functional properties, but that transient and meandering LRA patterns represent unattractive targets for ablation.
These mechanisms described are distributed across both the LA and right atrium (RA). Much of the focus of work exploring AF mechanisms has been on the LA, without considering a bi-atrial perspective in AF maintenance. Simultaneous bi-atrial mapping using two linked AcQMap systems has helped to characterise the role of the RA. In most patients, AF propagation is maintained through balanced conduction between both chambers, while where one chamber dominates, this is as often the RA as the LA. In addition, electrophysiological substrate properties of the RA, including increasing frequency of LRA and shorter RA AFCL, but not the LA, appear to predict a failure of acute AF termination with ablation.
Ablation Strategy
Sites of repetitive LIA are most frequently distributed within the anterior and posterior LA and the septum and lateral walls of the RA, while zones of high-frequency FF are similarly distributed in addition to originating from the PVs.53 Importantly, these regions are commonly found in close proximity to the PV antra (particularly on the posterior wall), likely reflective of the complex structural properties of the PV-LA junction. Mapping prior to PVI allows incorporation of these sites within the wide antral lesion set. Repeat mapping both prior to and following PVI provides the additional benefit of revealing non-pulmonary vein sites with consistent patterns of activation across both timepoints that can help to determine the hierarchy of ablation targets.
We have described a ‘core-to-boundary’ approach that aims to deliver focused ablation to the core of sites of preferential conduction patterns while anchoring these regions to non-conducting boundaries to reduce the risk of generating substrate for organised macro-re-entrant atrial tachycardias (AT).54 This strategy compliments an approach of ‘map, ablate, re-map’ that takes advantage of the speed of map recording and generation to allow repeated evaluation of the impact of ablation delivered, alterations to the dynamic patterns of AF activation and identification of stable or emerging areas of interest.
The number of conduction pattern sites appears related to the individual AF phenotype and is greater in patients in AF at the start of the procedure, compared to patients in whom AF is induced, and is proportional to the duration of persistent AF.54 55 Although definitive evidence of the optimal endpoint for ablation is lacking, we propose a strategy focused on sequential ablation of sites of preferential conduction patterns across both chambers until either all sites are eliminated or AF terminates to either AT or sinus rhythm. This supports a low threshold for mapping and ablation of RA mechanisms following ablation in the LA but without incremental addition of empirical ablation with the aim of achieving sinus rhythm in all patients. In an unselected group of 135 patients undergoing AcQMap guided de novo or re-do ablation of persistent AF in our centre,
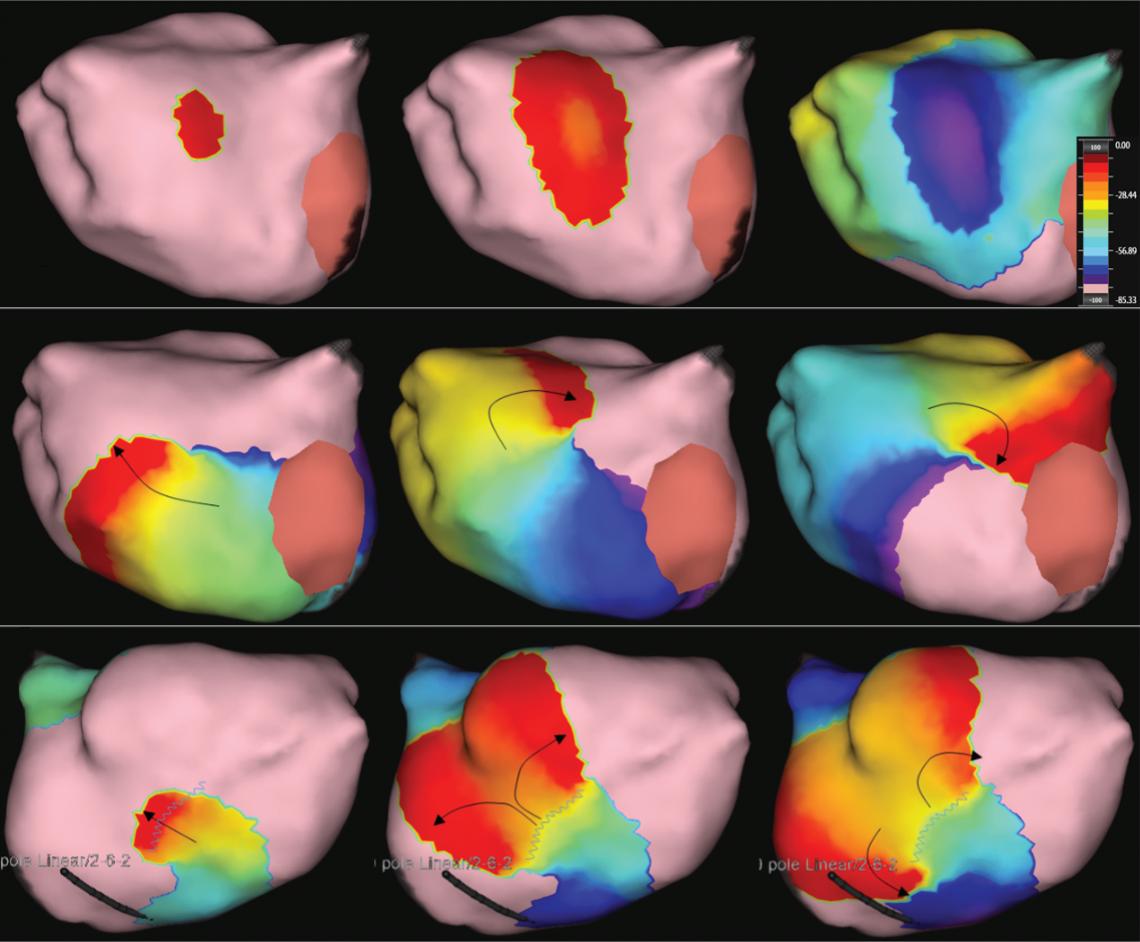
Global Substrate Mapping
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
0 ms A B C 9 ms 76 ms 0 ms 30 ms 57 ms 0 ms 17 ms 25 ms
acute termination with ablation is seen in 46%, reducing to 28% in patients attending the procedure in AF (as opposed to those in whom AF was induced). Evidence of correlation between acute AF termination and longterm procedural success is weak and therefore this should not be considered a definitive endpoint to be achieved in all patients after the elimination of targeted mechanisms.56
It should also be remembered that approximately 60% of patients undergoing first time procedures for persistent AF achieve good results from PVI alone.19 Where adoption of an individualised approach may prove particularly beneficial is in identifying those patients likely to do well with a strategy of PVI alone and limiting additional non-PV ablation only to those patients in whom this is likely to be required. As described above, the relative balance of FF and LRA appears related to acute AF termination. Different phenotypes can also be discerned in the pattern of whole chamber activation, with some patients demonstrating more ‘organised’ whole-chamber activation while others appear more chaotic and disorganised. Further work is needed to correlate these findings with long-term outcomes, but adapting the strategy to minimise additional ablation in patients with more organised whole chamber activation, while delivering more extensive ablation to bi-atrial AF mechanisms in others may prove most beneficial.
Mapping of Organised Atrial Tachycardias
ATs may arise either de novo or during AcQMap guided ablation of AF. The development of an algorithm for high-density mapping of stable atrial rhythms including both AT and paced rhythms provides more comprehensive utility of the AcQMap system beyond its application to mapping of AF and obviates the need for a second parallel mapping system. This algorithm, termed SuperMap accumulates multiple noncontact measurements acquired at different times and at different locations of the chamber by aligning beats according to timing and morphology of simultaneously recorded unipolar electrograms within the coronary sinus (CS). This allows separate grouping of beats with different cycle lengths or CS electrogram morphologies (for example, in the case of non-sustained tachycardias or unstable cycle lengths) that can be mapped concurrently and analysed separately. The atrial surface in proximity to the roving electrodes is highlighted during data collection to guide adequate spatial distribution of measurements. Charge densities are then computed by grouped beats, and the propagation history, an animated leading wavefront with color-coded activation histories, is generated for visualisation (Supplementary Material Video 5).
Ramak et al. have published the first description of the utility of this algorithm in a small series (n=7) of patients undergoing ablation of complex ATs following AF ablation.57 Shi et al. applied computational modelling to identify the optimum minimum number of catheter positions required to achieve a threshold of accuracy in local activation time annotation. A total of 60 separate locations was shown to meet this accuracy threshold which was achieved within a maximum of 3 minutes of continuous catheter roving. This formed the basis for subsequent clinical evaluation in a cohort of 20 patients undergoing ablation of ATs. Noncontact electrogram morphology and timing correlated well with contact electrograms obtained using the HD-grid (Abbot Medical).58
We have performed a systematic evaluation in 30 patients and a total of 60 separate maps including a comparison to contemporary contact mapping in 20 of these patients (42 individual maps). The SuperMap algorithm performed favourably in comparison to high-density contact mapping, accurately revealing the arrhythmia mechanism in 93% of
arrhythmias evaluated. This was achieved over a 37% shorter procedure time, requiring an average of 374 ± 214 seconds (6 minutes, 14 seconds) compared to 591 ± 332 seconds (9 minutes, 51 seconds) using contact mapping (p<0.0005). This was achieved alongside a high number of electrograms used for each map (7,201 ± 5,276 versus 3,380 ± 3,234; difference 3,821; 95% CI [2,207–5,435]; p<0.0005), therefore not compromising on mapping density.59
Gold-tip Ablation
The non-contact diagnostic capability of the AcQMap system is complemented by radiofrequency (RF) ablation therapy based around a 3.5 mm gold-tip contact-force sensing ablation catheter (Acutus Medical in partnership with Biotronik), with impedance-based catheter localisation. Gold is well known to exhibit a significantly higher thermal conductivity compared to platinum, which represents the most widely used material for catheter tip electrodes. Delivery of RF energy during ablation is limited in part by rising temperature at the catheter–tissue interface that risks excessive heating of the blood pool and formation of coagulum with associated risks of embolism.
The superior thermal conduction properties of gold have been found to result in deeper and broader ablation lesions when compared to platinum electrodes in vitro using both 4- and 8-mm electrode sizes.60 61 Although this difference is mitigated in part by use of irrigated tip catheters, in a randomised study of cavo-tricuspid isthmus ablation the use of gold-tip catheters resulted in a higher procedural success rate.62 In addition, gold allowed delivery of a higher mean power alongside lower catheter tip temperatures that resulted in a significantly lower rate of coagulum formation.62 Similar findings have been observed during irrigated RF ablation of the pulmonary veins with lower tip temperatures and higher energy delivery.63 These properties also allow RF delivery at significantly lower irrigation flow rates, with potential benefits particularly in patients with impaired left ventricular function, who form a significant proportion of patients now undergoing catheter ablation for AF.63
Contact force capability is integrated into the catheter thereby making this the only contact force sensing gold-tip ablation catheter commercially available, which can be used either in conjunction with the AcQMap mapping system or as a standalone ablation system. Lesion formation is informed by measurement of force-time integral during RF delivery. Contact force is measured using Fiber Bragg Gating (FBG) technology, which detects changes in the reflected wavelength of a light source that occur due to mechanical distortion (force). Changes in the reflected wavelength can also be caused by temperature changes thereby impacting on the accuracy of contact force measurements obtained.
Uniquely, the AcQBlate catheter incorporates an additional FBG system within a non-deformable section of the catheter in which measurements obtained reflect temperature changes and are used to provide constant correction to the contact force measurement obtained from the tip sensor. Acutus Medical commercial testing (unpublished data) when compared to the TactiCath SE (Abbott Medical) contact force ablation catheter suggests improved accuracy of contact force measurement both during and after RF ablation delivery (Figure 4). In this analysis conducted in vitro, a constant reference force of 1 g was applied and compared to the measured force detected by AcQBlate and TactiCath SE catheters during and immediately after RF ablation delivery. Wider variation was observed in force measured using TactiCath, which remained at 4 g after cessation of RF while the value returned to 1 g applied force after RF termination with the AcQBlate system (Figure 4).
Global Substrate Mapping and Targeted Ablation
Gold-tip Catheter ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
with Novel
Figure 4: Effect of Temperature on Measurement of Contact Force Using AcQBlate Catheter
become a widely adopted empirical approach. Patients treated using the AcQMap system had a 68% rate of freedom from recurrent AF or AT off antiarrhythmic drugs after a single procedure, which compared favourably with the 46% rate of freedom from arrhythmia seen in the PVI and PWI group (p=0.043).54
Although these data show significant promise in terms of clinical success using an AcQMap-guided approach, further evidence is eagerly awaited. The RECOVER trial (NCT03368781) is a single-arm study mirroring the design of UNCOVER-AF in patients undergoing re-do ablation procedures for AF, which has completed recruitment and is at the last stages of follow up, while the DISCOVER study (AcQMap Registry; NCT03893331) is a registry study aimed at delivering real-world data regarding utility and clinical outcomes in a target population of 500 patients undergoing treatment using the AcQMap system. However, the data available to date and on the horizon are poor substitutes for well-conducted randomised controlled trials and definitive evidence of benefit for patients undergoing ablation for AF will not be possible without this data.
Future Perspectives and Gaps in Evidence
Clinical Outcome Data
The UNCOVER-AF trial was the first outcome study performed using the AcQMap system and was a single arm prospective study including 129 patients who underwent first time ablation procedures for persistent AF across 13 centres in the UK, Europe and Canada.55 Patients included were aged 62.4 ± 8.6 years with 3.2 ± 3.9 years since AF diagnosis, including 1.9 ± 3.1 years since onset of persistent AF. The mean LA diameter was 43 ± 4 mm and 46% of patients had a CHA2DS2-VASc score of ≥2. Outcome assessment included both safety and effectiveness, with arrhythmia recurrence detected using a combination of 12-lead ECG and 24-hour continuous ECG monitoring at 3, 6, 9 and 12 months following the procedure.
Major adverse events within 24 hours of the procedure were observed in three patients and consisted of cardiac tamponade (n=2) and cerebral thromboembolism (n=1). In addition, one patient suffered an air embolism related to the steerable sheath within the LA and two patients suffered complications related to the femoral venous puncture site. Ablation resulted in termination of AF in 32% of patients with 68% undergoing direct current cardioversion (DCCV) and two patients remaining in AF at the end of the procedure. Of those in whom ablation resulted in AF termination, an average of 2.4 ± 1.5 non-PV sites were targeted, which increased to 3.4 ± 1.4 in those in whom DCCV restored sinus rhythm. The primary outcome of freedom from AF >30 seconds in duration at 12 months (on or off antiarrhythmic drugs) was achieved in 72.5% of patients, with a freedom from AF or AT in 69.2%. Although this compares favourably to results from STAR-AF2, the more recent VENUS randomised controlled trial, and the PRECEPT study, direct comparisons to results from alternative ablation strategies cannot accurately be drawn without a control arm and randomisation.19 24 64
We have recently published 2-year outcome data from a series of 40 patients treated across two UK tertiary centres in which we described our ‘core-to-boundary’ approach to the first-time ablation of persistent AF. In addition, we conducted a propensity matched comparison against a strategy of PVI combined with posterior wall isolation (PWI), which has
Although the AcQMap system contains the capability to collect contact mapping data, we have not discussed this as it is not part of the usual workflow in global substrate mapping and ablation of AF, although it is likely to be the subject of future technological development. We have demonstrated some of the insights that the AcQMap system has provided into mechanisms of AF and the potential benefit this may provide in terms of clinical outcomes.
There is much still to learn about the mechanisms of AF and how to optimally employ or interpret the mapping data generated. We have shown that regions of LIA are highly stable and likely to represent structural atrial properties, but it is unclear whether these sites are pathological, perhaps related to interstitial fibrosis or myofibre disarray, or are related to normal regions of changing fibre orientation and/or interatrial connections.
Detailed studies employing MRI and histological analysis are needed to explore this further. What is the optimal ablation approach? Should regions of FF be preferentially targeted, in keeping with the hypothesis of focal drivers, or should stable regions of LIA be treated as these are the regions of abnormal tissue responsible for fibrillatory conduction? What is the appropriate endpoint for ablation and how can the effect of ablation be accurately assessed and quantified in real-time to guide therapy? We have shown evidence highlighting the role of the RA but predicting which patients require RA ablation is difficult and not possible based on current mechanistic understanding.
In addition to furthering mechanistic understanding is the potential for technological developments that may yield further clinical application and advances. The ultrasound capability of the system is currently employed purely to generate endocardial surface anatomy. However, the ultrasound data generated can also be processed to yield data on atrial mechanical function, visualise contraction and potentially evaluate wall thickness and adjacent structures, which will greatly expand the overall system capability and application.
Use of the AcQMap catheter is also currently limited to the atria. A global mapping approach could be highly desirable within the ventricles both for mapping single or infrequent ectopy and for rapid assessment of ventricular tachycardia mechanisms that are haemodynamically poorly
Global Substrate Mapping and Targeted Ablation
Novel Gold-tip Catheter ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
with
Total force peak (g) Mean AlCath Force Verification results Mean TactiCath ± SD n=5 AiCath Force and n=5 TactiCath 16 14 12 10 8 6 4 2 0 Not to exceed 10 g during ablation After 5 s After 30 s After 60 s After ablation Force (g)
Effect of temperature on contact force measured during constant application of 1 g of force at different stages of ablation in vitro using the AcQBlate catheter (AlCath) and the TactiCath SE catheter (Abbott Medical). Bars represent mean and standard deviation. Source: Acutus Medical.
Mapping and Targeted Ablation with Novel Gold-tip Catheter
tolerated. Animal studies in this area are on-going and catheter adaptations to suit deployment within the ventricles are under investigation.
Conclusion
AcQMap is a non-contact charge density electrophysiological mapping system for the assessment of atrial arrhythmias that uses ultrasound reconstruction of atrial anatomy. This is coupled with a RF ablation delivery system based around a gold tip contact-force sensing ablation catheter. Visualisation of whole chamber activation and localised patterns of complex conduction during AF allows delivery of an individualised approach to catheter ablation incorporating non-pulmonary vein mechanisms of AF propagation.
A parallel algorithm for the synchronous non-contact mapping of ATs facilitates accurate and rapid high-density evaluation of complex AT mechanisms. Early evidence of clinical outcomes is promising but randomised clinical trials are needed to ultimately test the benefit of this approach to treating patients with AF.
1. Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. https:// doi.org/10.1056/NEJM199809033391003; PMID: 9725923.
2. Hocini M, Ho SY, Kawara T, et al. Electrical conduction in canine pulmonary veins: electrophysiological and anatomic correlation. Circulation 2002;105:2442–8. https://doi. org/10.1161/01.cir.0000016062.80020.11; PMID: 12021234.
3. Kanj M, Wazni O, Natale A. Pulmonary vein antrum isolation. Heart Rhythm 2007;4(3 Suppl):S73–9. https://doi.org/10.1016/j. hrthm.2006.12.036; PMID: 17336890.
4. Atienza F, Jalife J. Reentry and atrial fibrillation. Heart Rhythm 2007;4(3 Suppl):S13–6. https://doi.org/10.1016/j. hrthm.2006.12.004; PMID: 17336877.
5. Oral H, Scharf C, Chugh A, et al. Catheter ablation for paroxysmal atrial fibrillation: segmental pulmonary vein ostial ablation versus left atrial ablation. Circulation 2003;108:2355–60. https://doi.org/10.1161/01. CIR.0000095796.45180.88; PMID: 14557355.
6. Proietti R, Santangeli P, Di Biase L, et al. Comparative effectiveness of wide antral versus ostial pulmonary vein isolation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2014;7:39–45. https://doi.org/10.1161/ CIRCEP.113.000922; PMID: 24385448.
7. Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549. https://doi.org/10.1161/JAHA.112.004549; PMID: 23537812.
8. Knight BP, Novak PG, Sangrigoli R, et al. Long-term outcomes after ablation for paroxysmal atrial fibrillation using the second-generation cryoballoon: final results from STOP AF post-approval study. JACC Clin Electrophysiol 2019;5:306–14. https://doi.org/10.1016/j.jacep.2018.11.006; PMID: 30898232.
9. Andrade JG, Champagne J, Dubuc M, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019;140:1779–88. https://doi.org/10.1161/ CIRCULATIONAHA.119.042622; PMID: 31630538.
10. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. https://doi.org/10.1056/ NEJMoa2029980; PMID: 33197159.
11. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. https://doi.org/10.1056/NEJMoa2029554; PMID: 33197158.
12. Gökoğlan Y, Mohanty S, Güneş MF, et al. Pulmonary vein antrum isolation in patients with paroxysmal atrial fibrillation: more than a decade of follow-up. Circ Arrhythm Electrophysiol 2016;9:e003660. https://doi.org/10.1161/ CIRCEP.115.003660; PMID: 27162030.
13. Hoffmann E, Straube F, Wegscheider K, et al. Outcomes of cryoballoon or radiofrequency ablation in symptomatic paroxysmal or persistent atrial fibrillation. Europace 2019;21:1313–24. https://doi.org/10.1093/europace/euz155; PMID: 31199860.
14. Usui E, Miyazaki S, Taniguchi H, et al. Recurrence after
Clinical Perspective
• AF is a highly complex arrhythmia and significant challenges remain in developing successful strategies for catheter ablation, particularly beyond pulmonary vein isolation. The development of whole chamber non-contact charge density mapping provides a novel and unique approach to treatment.
• Detailed studies of the nature of complex activation patterns during AF have revealed important spatiotemporal properties of localised conduction that are important for the development of targeted ablation strategies.
• Emerging evidence supports the clinical benefit of an individualised ablation approach targeting complex patterns of local activation with anchoring of these sites to adjacent non-conducting structures.
• Non-contact mapping of organised atrial arrhythmias is a highly accurate and efficient technique for identification and ablation of atrial tachycardia.
“long-term success” in catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm 2015;12:893–8. https://doi. org/10.1016/j.hrthm.2015.01.043; PMID: 25640637.
15. Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044–53. https://doi.org/10.1016/j.jacc.2003.12.054; PMID: 15172410.
16. Yao Y, Zheng L, Zhang S, et al. Stepwise linear approach to catheter ablation of atrial fibrillation. Heart Rhythm 2007;4:1497–504. https://doi.org/10.1016/j. hrthm.2007.07.028; PMID: 17997359.
17. Zhang Z, Letsas KP, Zhang N, et al. Linear ablation following pulmonary vein isolation in patients with atrial fibrillation: a meta-analysis. Pacing Clin Electrophysiol 2016;39:623–30. https://doi.org/10.1111/pace.12841; PMID: 26970360.
18. Kim JS, Shin SY, Na JO, et al. Does isolation of the left atrial posterior wall improve clinical outcomes after radiofrequency catheter ablation for persistent atrial fibrillation? A prospective randomized clinical trial. Int J Cardiol 2015;181:277–83. https://doi.org/10.1016/j. ijcard.2014.12.035; PMID: 25535691.
19. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. https://doi.org/10.1056/NEJMoa1408288; PMID: 25946280.
20. Lee JM, Shim J, Park J, et al. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol 2019;5:1253–61. https://doi.org/10.1016/j.jacep.2019.08.021; PMID: 31753429.
21. Brachmann J, Hummel JD, Wilber DJ, et al. Prospective randomized comparison of rotor ablation vs conventional ablation for treatment of persistent atrial fibrillation – the REAFFIRM trial. Presented at 40th Annual Scientific Sessions, Heart Rhythm 2019, San Francisco, California, 9 May 2019. Presentation S-LBCT01-02.
22. Di Biase L, Burkhardt JD, Mohanty P, et al. Left atrial appendage isolation in patients with longstanding persistent AF undergoing catheter ablation: BELIEF trial. J Am Coll Cardiol 2016;68:1929–40. https://doi.org/10.1016/j. jacc.2016.07.770; PMID: 27788847.
23. DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation: results of CONVERGE clinical trial. Circ Arrhythm Electrophysiol 2020;13:e009288. https:// doi.org/10.1161/CIRCEP.120.009288; PMID: 33185144.
24. Valderrabano M, Peterson LE, Swarup V, et al. Effect of catheter ablation with vein of Marshall ethanol infusion vs catheter ablation alone on persistent atrial fibrillation: the Venus randomized clinical trial. JAMA 2020;324:1620–8. https://doi.org/10.1001/jama.2020.16195; PMID: 33107945.
25. Gibson DN, Di Biase L, Mohanty P, et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 2011;8:1364–71. https://doi.org/10.1016/j.hrthm.2011.02.026; PMID: 21354332.
26. Rivner H, Mitrani RD, Goldberger JJ. Atrial myopathy underlying atrial fibrillation. Arrhythm Electrophysiol Rev 2020;9:61–70. https://doi.org/10.15420/aer.2020.13;
PMID: 32983526.
27. Mandapati R, Skanes A, Chen J, et al. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation 2000;101:194–9. https://doi. org/10.1161/01.cir.101.2.194; PMID: 10637208.
28. Jalife J, Berenfeld O, Skanes A, Mandapati R. Mechanisms of atrial fibrillation: mother rotors or multiple daughter wavelets, or both? J Cardiovasc Electrophysiol 1998;9(8 Suppl):S2–12. PMID: 9727669.
29. Jalife J. Rotors and spiral waves in atrial fibrillation. J Cardiovasc Electrophysiol 2003;14:776–80. https://doi. org/10.1046/j.1540-8167.2003.03136.x; PMID: 12930260.
30. Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: a mechanism of atrial fibrillation. Cardiovasc Res 2002;54:204–16. https://doi.org/10.1016/ s0008-6363(02)00223-7; PMID: 12062327.
31. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation with or without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628–36. https://doi.org/10.1016/j. jacc.2012.05.022; PMID: 22818076.
32. Miller JM, Kalra V, Das MK, et al. Clinical benefit of ablating localized sources for human atrial fibrillation: the Indiana University FIRM registry. J Am Coll Cardiol 2017;69:1247–56. https://doi.org/10.1016/j.jacc.2016.11.079; PMID: 28279291.
33. Miller JM, Kowal RC, Swarup V, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 2014;25:921–9. https://doi.org/10.1111/jce.12474; PMID: 24948520.
34. Haissaguerre M, Hocini M, Denis A, et al. Driver domains in persistent atrial fibrillation. Circulation 2014;130:530–8. https://doi.org/10.1161/CIRCULATIONAHA.113.005421; PMID: 25028391.
35. Atienza F, Almendral J, Jalife J, et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009;6:33–40. https://doi.org/10.1016/j. hrthm.2008.10.024; PMID: 19121797.
36. Atienza F, Almendral J, Ormaetxe JM, et al. Comparison of radiofrequency catheter ablation of drivers and circumferential pulmonary vein isolation in atrial fibrillation: a noninferiority randomized multicenter RADAR-AF trial. J Am Coll Cardiol 2014;64:2455–67. https://doi.org/10.1016/j. jacc.2014.09.053; PMID: 25500229.
37. Buch E, Share M, Tung R, et al. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: a multicenter experience. Heart Rhythm 2016;13:636–41. https://doi.org/10.1016/j.hrthm.2015.10.031; PMID: 26498260.
38. Umapathy K, Nair K, Masse S, et al. Phase mapping of cardiac fibrillation. Circ Arrhythm Electrophysiol 2010;3:105–14. https://doi.org/10.1161/CIRCEP.110.853804; PMID: 20160178.
39. Child N, Clayton RH, Roney CH, et al. Unraveling the underlying arrhythmia mechanism in persistent atrial fibrillation: results from the STARLIGHT study. Circ Arrhythm Electrophysiol 2018;11:e005897. https://doi.org/10.1161/
Global Substrate
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Global Substrate Mapping and Targeted Ablation with Novel Gold-tip Catheter
CIRCEP.117.005897; PMID: 29858382.
40. Laughner J, Shome S, Child N, et al. Practical considerations of mapping persistent atrial fibrillation with whole-chamber basket catheters. JACC Clin Electrophysiol 2016;2:55–65. https://doi.org/10.1016/j.jacep.2015.09.017; PMID: 29766854.
41. Rodrigo M, Climent AM, Liberos A, et al. Technical considerations on phase mapping for identification of atrial reentrant activity in direct- and inverse-computed electrograms. Circ Arrhythm Electrophysiol 2017;10:e005008. https://doi.org/10.1161/CIRCEP.117.005008; PMID: 28887361.
42. Roney CH, Cantwell CD, Bayer JD, et al. Spatial resolution requirements for accurate identification of drivers of atrial fibrillation. Circ Arrhythm Electrophysiol 2017;10:e004899. https://doi.org/10.1161/CIRCEP.116.004899; PMID: 28500175.
43. Jarman JW, Wong T, Kojodjojo P, et al. Spatiotemporal behavior of high dominant frequency during paroxysmal and persistent atrial fibrillation in the human left atrium. Circ Arrhythm Electrophysiol 2012;5:650–8. https://doi.org/10.1161/ CIRCEP.111.967992; PMID: 22722660.
44. Salinet JL, Tuan JH, Sandilands AJ, et al. Distinctive patterns of dominant frequency trajectory behavior in drug-refractory persistent atrial fibrillation: preliminary characterization of spatiotemporal instability. J Cardiovasc Electrophysiol 2014;25:371–9. https://doi.org/10.1111/jce.12331; PMID: 24806529.
45. Grace A, Willems S, Meyer C, et al. High-resolution noncontact charge-density mapping of endocardial activation. JCI Insight 2019;4:e126422. https://doi.org/10.1172/ jci.insight.126422; PMID: 30895945.
46. Shi R, Norman M, Chen Z, Wong T. Individualized ablation strategy guided by live simultaneous global mapping to treat persistent atrial fibrillation. Future Cardiol 2018;14:237–49. https://doi.org/10.2217/fca-2017-0109; PMID: 29441808.
47. Shi R, Parikh P, Chen Z, et al. Validation of dipole density mapping during atrial fibrillation and sinus rhythm in human left atrium. JACC Clin Electrophysiol 2020;6:171–81. https://doi. org/10.1016/j.jacep.2019.09.012; PMID: 32081219.
48. Roney CH, Wit AL, Peters NS. Challenges associated with interpreting mechanisms of AF. Arrhythm Electrophysiol Rev 2020;8:273–84. https://doi.org/10.15420/aer.2019.08; PMID: 32685158.
49. Pope MT, Kuklik P, Briosa E Gala A, et al. Spatial and
temporal variability of rotational, focal, and irregular activity: practical implications for mapping of atrial fibrillation. J Cardiovasc Electrophysiol 2021;32:2393–403. https://doi. org/10.1111/jce.15170; PMID: 34260134.
50. Lee JMS, Nelson TA, Clayton RH, Kelland NF. Characterization of persistent atrial fibrillation with noncontact charge density mapping and relationship to voltage. J Arrhythmia 2021;38:77–85. https://doi.org/10.1002/ joa3.12661; PMID: 35222753.
51. Chierchia GB, Sieira J, Vanderper A, et al. Substrate mapping of the left atrium in persistent atrial fibrillation: spatial correlation of localized complex conduction patterns in global charge-density maps to low-voltage areas in 3D contact bipolar voltage maps. J Interv Card Electrophysiol 2021;62:539–47. https://doi.org/10.1007/s10840-020-009264; PMID: 33420713.
52. Dharmaprani D, Schopp M, Kuklik P, et al. Renewal theory as a universal quantitative framework to characterize phase singularity regeneration in mammalian cardiac fibrillation. Circ Arrhythm Electrophysiol 2019;12:e007569. https://doi. org/10.1161/CIRCEP.119.007569; PMID: 31813270.
53. Shi R, Chen Z, Butcher C, et al. Diverse activation patterns during persistent atrial fibrillation by noncontact chargedensity mapping of human atrium. J Arrhythm 2020;36:692–702. https://doi.org/10.1002/joa3.12361; PMID: 32782641.
54. Shi R, Chen Z, Pope MTB, et al. Individualized ablation strategy to treat persistent atrial fibrillation: core-toboundary approach guided by charge-density mapping. Heart Rhythm 2021;18:862–70. https://doi.org/10.1016/j. hrthm.2021.02.014; PMID: 33610744.
55. Willems S, Verma A, Betts TR, et al. Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping: UNCOVER AF trial. Circ Arrhythm Electrophysiol 2019;12:e007233. https://doi. org/10.1161/CIRCEP.119.007233; PMID: 31242746.
56. Kochhauser S, Jiang CY, Betts TR, et al. Impact of acute atrial fibrillation termination and prolongation of atrial fibrillation cycle length on the outcome of ablation of persistent atrial fibrillation: a substudy of the STAR AF II trial. Heart Rhythm 2017;14:476–83. https://doi.org/10.1016/j. hrthm.2016.12.033; PMID: 28011328.
57. Ramak R, Chierchia GB, Paparella G, et al. Novel noncontact
charge density map in the setting of post-atrial fibrillation atrial tachycardias: first experience with the Acutus SuperMap Algorithm. J Interv Card Electrophysiol 2021;61:187–95. https://doi.org/10.1007/s10840-020-00808-9; PMID: 32643104.
58. Shi R, Zaman JAB, Chen Z, et al. Novel aggregated multiposition noncontact mapping of atrial tachycardia in humans: from computational modeling to clinical validation. Heart Rhythm 2022;19:61–9. https://doi.org/10.1016/j. hrthm.2021.09.025; PMID: 34583060.
59. Pope MTB, Leo M, Briosa e Gala A, Betts TR. Clinical utility of non-contact charge density ‘SuperMap’ algorithm for the mapping and ablation of organized atrial arrhythmias. EP Europace 2022;24:747–54. https://doi.org/10.1093/europace/ euab271; PMID: 34871398.
60. Lewalter T, Bitzen A, Wurtz S, et al. Gold-tip electrodes – a new “deep lesion” technology for catheter ablation? In vitro comparison of a gold alloy versus platinum-iridium tip electrode ablation catheter. J Cardiovasc Electrophysiol 2005;16:770–2. https://doi. org/10.1111/j.1540-8167.2005.40832.x; PMID: 16050836.
61. Linhart M, Mollnau H, Bitzen A, et al. In vitro comparison of platinum-iridium and gold tip electrodes: lesion depth in 4 mm, 8 mm, and irrigated-tip radiofrequency ablation catheters. Europace 2009;11:565c70. https://doi.org/10.1093/ europace/eup040; PMID: 19251707.
62. Lewalter T, Weiss C, Spencker S, et al. Gold vs. platinumiridium tip catheter for cavotricuspid isthmus ablation: the AURUM 8 study. Europace 2011;13:102–8. https://doi. org/10.1093/europace/euq339; PMID: 20876601.
63. Linhart M, Liberman I, Schrickel JW, et al. Superiority of gold versus platinum irrigated tip catheter ablation of the pulmonary veins and the cavotricuspid isthmus: a randomized study comparing tip temperatures and cooling flow requirements. J Cardiovasc Electrophysiol 2012;23:717–21. https://doi.org/10.1111/j.1540-8167.2011.02267.x; PMID: 22429859.
64. Mansour M, Calkins H, Osorio J, et al. Persistent atrial fibrillation ablation with contact force-sensing catheter: the prospective multicenter PRECEPT trial. JACC Clin Electrophysiol 2020;6:958–69. https://doi.org/10.1016/j. jacep.2020.04.024; PMID: 32819531.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Preprocedural Discrimination of Posteroseptal Accessory Pathways Ablated from the Right Endocardium from Those Requiring a Left-sided or Epicardial Coronary Venous Approach
Mathieu Lebloa and Patrizio Pascale
Abstract
The success of radiofrequency catheter ablation of the accessory pathway (AP) depends on the accurate localisation of the bypass tract. In that respect, posteroseptal or inferior paraseptal APs often pose a diagnostic challenge because of the complex anatomy at the crux of the four cardiac chambers. Considering the differences in procedure risks and success rate depending on the need for a left-sided approach or a coronary sinus ablation, an accurate anticipation of the precise location of inferior paraseptal APs is critical to inform the consent process and guide the initial mapping strategy. Here, the preprocedural clues to discriminate APs that can be ablated from the right atrium, from those requiring a left-sided or epicardial coronary venous approach, are reviewed. Both manifest and concealed APs will be considered and, following the diagnostic process made by the operator before interpretation of the intra-cardiac signals, each of the following aspects will be addressed: clinical context and initial probability; and 12-lead ECG analysis during baseline ECG with manifest AP, maximal preexcitation, and orthodromic reciprocating tachycardia.
Keywords
Accessory pathway, ECG, posteroseptal, Wolff-Parkinson-White, delta wave
Disclosure: The authors have no conflicts of interest to declare.
Received: 14 September 2021 Accepted: 9 March 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e07. DOI: https://doi.org/10.15420/aer.2021.55
Correspondence: Patrizio Pascale, Arrhythmia Unit, Cardiovascular Department, Centre Hospitalier Universitaire Vaudois – BH 10-982, 1011 Lausanne, Switzerland.
E: patrizio.pascale@chuv.ch
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Radiofrequency catheter ablation has become the preferred treatment option for patients with symptomatic Wolff-Parkinson-White (WPW) syndrome or recurrent symptomatic orthodromic reciprocating tachycardia. The success of the procedure depends on the accurate localisation of the accessory pathway (AP). In that respect, posteroseptal or inferior paraseptal APs, which represent the second most common atrioventricular (AV) connection site after left free wall AP, often pose a diagnostic challenge. This reflects the complex anatomy at the crux of the four cardiac chambers, where a small area may encompass APs that may be approached from the right or left endocardium, or require an ablation performed epicardially inside the coronary sinus (CS).
APs located in the posteroseptal area can take a variety of courses. Four different course types may be distinguished (Figure 1):
• Endocardially between the inferior paraseptal right atrium and the right ventricle. This area includes the inferior part of the Koch’s triangle and the area surrounding the CS ostium.
• Endocardially between the inferior paraseptal left atrium and the left ventricle.
• Coursing between the inferior paraseptal right atrium and the left ventricle in the pyramidal space, given that the right atrium lies directly on the posterior superior process of the left ventricle. This anatomical conformation results from the fact that the interatrial
septum lies leftward to the interventricular septum and the tricuspid annulus is displaced 5–10 mm apically with respect to the mitral annulus.1 The right atrial endocardial aspect overlying the posterior superior process of the left ventricle lies between the most posterior aspect of the right fibrous trigone and the CS ostium, medial to the tricuspid valve. Because of its close proximity, ablation of these APs may be possible from the proximal CS. Based on past surgical experience, an important subgroup of inferior paraseptal APs is actually right atrial to left ventricular connections.2,3
• Epicardially, connecting the musculature overlying the CS to the ventricle. These connections are probably most often related to sleeve-like extensions of the CS musculature that cover the proximal portion of the middle cardiac vein or posterior coronary veins. In 70% of these APs the CS venous anatomy is normal, while in the remaining cases venous anomalies are identified, mostly in the form of CS diverticula.4 Most of these APs are ablated with a coronary venous approach but it is worth noting that a successful ablation within the CS does not imply a CS musculature-related connection but may merely reflect the proximity of the CS to atrioventricular muscle connections.5 Here, these APs are referred to as ‘epicardial CS’ APs.
Because of the anatomical complexity of the inferior paraseptal region and the fact that APs may overlap or extend in adjacent areas, these APs
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Clinical Electrophysiology and Ablation
Arrhythmia Unit, Cardiovascular Department, Centre Hospitalier Universitaire Vaudois and University of Lausanne, Lausanne, Switzerland
Figure 1: Schematic Diagram of the Four Subtypes of Posteroseptal AP in the AV Annuli (LAO View) Tricuspid
paraseptal region is 2.3 ± 0.5 cm distant from the CS orifice.15 Second, studies that have included the so-called epicardial CS APs in their analysis often considered together both left endocardial inferior paraseptal and left posterior APs.4 16 Finally, the proportion of APs requiring an epicardial coronary venous approach is dependent on the ablation strategy. In our practice, radiofrequency application within the CS is never performed before ruling out a possibly successful ablation on the left endocardial side in order to limit the risks of complications such as coronary artery damage.13,14 This strategy may potentially underrepresent the prevalence of epicardial CS APs compared with an ablation strategy performing CS ablation more liberally.
The four types of accessory pathway (AP) are as follows: 1, between the right atrium and the right ventricle fibre; 2, between the right atrium and the posterior superior process of the left ventricle; 3, between the left atrium and the left ventricle; and 4, between the ventricle and sleeve-like extensions of the coronary sinus (CS) musculature that cover either: 4a, the proximal portion of the middle cardiac vein (MCV) or posterior coronary veins, or 4b, venous anomalies mostly in the form of CS diverticula. AV = atrioventricular; IVC = inferior vena cava; LAO = left anterior oblique; LPS = left endocardial posteroseptal; PS-CS = posteroseptal epicardial CS; RPS = right endocardial posteroseptal; VS = venous structure such as diverticula.
often shared the lowest ablation success rate with right free wall APs in previous series.6 7 Similarly, a high recurrence rate has been reported, in the range of approximately 10%.6–8 In that respect, one must be aware that APs ablated within the coronary venous system present a much higher risk of developing slow conduction and decremental properties after an ablation attempt compared with other localisations.9 Finally, the need for a percutaneous subxiphoid approach in inferior paraseptal APs has more often been reported in case reports or series, possibly because of ablation limitations for CS musculature-related AP.10
The procedural risks of inferior paraseptal AP ablation will notably differ depending on whether a left-sided approach or a CS ablation is required, mainly as a consequence of the risk of embolisation or damage to coronary artery, respectively.4 11–14 Considering these differences, an accurate anticipation of the precise location of inferior paraseptal AP is critical to inform the discussion and consent process with the patient and to guide the mapping strategy. Here, we will review the clues to discriminate APs that can be ablated from the right atrium from those requiring a left-sided or epicardial coronary venous approach. Both manifest and concealed APs will be considered and, following the diagnostic process made by the operator before starting the interpretation of the intra-cardiac signals, each of the following aspects will be addressed:
• clinical context and initial probability; and
• 12-lead ECG analysis during baseline ECG with manifest AP, maximal pre-excitation and orthodromic reciprocating tachycardia.
Clinical Context and Initial Probability
The proportion of inferior paraseptal APs that can be ablated from the right atrium or that require a left-sided or epicardial CS approach has varied in previous reports for a number of reasons. First, the distinction between left inferior paraseptal and left posterior AP is ill-defined when considering ablation procedures performed under fluoroscopic guidance only. From an anatomical standpoint, the left boundary of the inferior
When left inferior paraseptal and epicardial CS APs are defined based on a successful ablation from 7 to 8 o’clock along the mitral annulus, and ≥1 cm within the CS (including its proximal branches), respectively, then the majority of inferior paraseptal APs (in the range of 50–60%) can be ablated from the right side.17–21 Epicardial APs requiring a coronary venous approach represent approximately 10–20% of inferior paraseptal APs, while the remaining APs can be successfully ablated on the septal mitral annulus.4 16 19–21
Two notable exceptions to this AP distribution are worth mentioning: the Ebstein’s anomaly and the permanent form of reciprocating tachycardia caused by slowly conducting bypass tracts. In Ebstein’s anomaly, the vast majority of inferior paraseptal APs are ablated on the anatomical tricuspid annulus.22–24 Regarding APs exhibiting the phenotype of permanent junctional reciprocating tachycardia, approximately three-quarters are located in the inferior paraseptal region (range, 50–88%).25–28 Of these, 80–100% can be successfully ablated with an exclusive right-sided approach, mostly around the CS ostium. The remaining cases are ablated within the CS or its proximal branches, or on the left septal mitral annulus.25 26 28 APs with Mahaim conduction characteristics will not be considered in this review because most of them originate at the lateral aspect of the tricuspid annulus. However, it is worth noting that inferior paraseptal locations may also be found.29
The 12-Lead ECG Analysis
The first step of the diagnostic process is based on the 12-lead ECG analysis of either the ventricular preexcitation pattern or, for concealed APs, the retrograde atrial activation during orthodromic reciprocating tachycardia. The preexcitation pattern may be analysed during baseline ECG or during maximal preexcitation, such as in antidromic reciprocating tachycardia, preexcited AF, adenosine infusion or during rapid atrial pacing manoeuvres performed during electrophysiological studies.
Baseline ECG Analysis in Manifest Accessory Pathway
Several algorithms based on the delta wave and/or QRS polarity have been previously proposed to characterise the AP localisation in WPW patients.17 20 21 30–36 However, their accuracy is limited mainly by the fact that the QRS complex is a fusion of varying degree with the activation from the His–Purkinje system. The interpretation is also flawed due to a number of other factors, such as the variations in body shape and/or size, the heart’s location and anatomical characteristics. Finally, up to 10% of patients may have more than one AP, which can make the ECG difficult to interpret. Therefore, these criteria provide only an approximate indication and attempts to distinguish localisations that are only 1–2 cm apart should be interpreted cautiously. Their accuracy is especially modest for certain locations, such as the inferior paraseptal region, considering the complex local anatomy as discussed previously.37
Localisation of Posteroseptal Accessory Pathways ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
His 1 2 3 4a 4b CS RPS LPS PS-CS MCV VS
Epicardial
annulus Mitral annulus
Endocardial pathway
pathway
Table 1: Polarity of the Delta Wave for Inferior Paraseptal Accessory Pathways According to ECG Lead and Ablation Site
Source: Pascale et al. 2020.19 Adapted with permission from Elsevier.
Regarding the specific characterisation of inferior paraseptal APs, the goal of most algorithms was to discriminate the laterality between right or left endocardial localisations. Only a limited number of studies have included in their analysis bypass tracts requiring ablation performed within the CS.4 17 19 20 27 38 These algorithms have considered either the QRS polarity or the delta wave polarity, the latter being generally measured from the onset of the earliest delta wave observed in any of the peripheral leads. Direct comparison between studies is limited by the fact that the analysis of the delta wave polarity substantially differed between studies (see below).
The most common characteristics that have been proposed by authors to distinguish inferior paraseptal APs from other localisations can be summarised as follows:
• Negative delta waves in at least two inferior leads, given that the delta wave in lead II less often displays a negative delta wave, and may be isoelectric or biphasic.17 19 21 27 31–33 The delta wave polarity was variably defined: some studies considered the initial 20 ms, and some the initial 40 ms.17 33 31 32 Isoelectric delta waves were also considered together with negative delta waves.17 31 33 In our practice, we rather consider both the first and the second half of the 40 ms period, and would suspect an inferior paraseptal AP when a negative component is observed in either period.19 We compared this method with the assessment of the initial 20 ms only.19 As illustrated in Table 1, considering only delta waves whose initial 20 ms are negative, the sensitivity to detect inferior paraseptal APs was notably reduced.
• A positive delta wave in leads I and aVL in order to distinguish them from left posterior/posterolateral APs (or ‘left free wall’ APs), which may display a negative delta wave.17,19,27,31,33 In our experience, a negative component of the delta wave (first 20 ms), or a fully isoelectric delta wave (40 ms), may sometimes be observed in left endocardial inferior paraseptal APs, but we did not observe this
pattern in right-sided or epicardial CS APs (Table 1).
• An early precordial lead transition. To distinguish inferior paraseptal APs from ‘right free wall’ APs, which generally display later precordial transitions. These right free wall APs comprise right posterior or posterolateral APs, but also right lateral bypass tracts given that the latter sometimes also display negative delta waves in inferior leads. This early transition was variably defined by authors as an R/S ratio >1 in V2;31 34 or an RS or Rs QRS pattern in V1–V3.33
Identification of Epicardial Coronary Sinus Accessory Pathway
Regarding the differential diagnosis of inferior paraseptal APs, there have been various attempts to specifically identify epicardial CS APs ablated within the CS based on a standard sinus rhythm ECG.17 19 20 The most widely reported pattern was first described by Arruda et al.17 They suggested that the identification of a negative delta wave in lead II was specifically associated with epicardial CS APs (100% sensitivity and specificity). Their stepwise ECG algorithm was based on the polarity of the initial 20 ms of the delta wave and the first step consisted in ruling out a left free wall AP based on the delta wave polarity in leads I and V1. However, this association between a negative delta wave in lead II and epicardial CS APs should by no means be regarded as definitive.
Indeed, in a later study involving almost 10-fold more epicardial CS APs, Arruda’s group reported a much lower sensitivity, with 70% of epicardial CS APs displaying a negative delta wave in lead II.4 Regarding the specificity of this association and its reproducibility in other study populations, we found that a negative delta wave in lead II was indeed significantly more often observed in epicardial CS APs compared with right or left inferior paraseptal APs.19 However, the specificity was limited considering that 35–40% of endocardial inferior paraseptal (left and right), left posterior and right free wall APs all displayed a negative delta wave in lead II. Accordingly, when applying the stepwise diagnostic algorithm proposed by Arruda et al. in our population, the specificity and positive predictive
Localisation of Posteroseptal Accessory Pathways ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Total n=273 Right Posteroseptal n=64, n (%) Left Posteroseptal n=33, n (%) Subepicardial Coronary Sinus n=13, n (%) Lead II • Negative δ wave (0–20 ms) • Negative component of the δ wave (0–20 and/or 20–40 ms) 64 104 26 (41) 41 (64) 13 (39) 19 (58) 10 (77) 12 (92) Lead III • Negative δ wave (0–20 ms) • Negative component of the δ wave (0–20 and/or 20–40 ms) 119 159 56 (88) 63 (98) 19 (58) 31 (94) 10 (77) 13 (100) Lead aVF • Negative δ wave (0–20 ms) • Negative component of the δ wave (0–20 and/or 20–40 ms) 96 143 46 (72) 59 (92) 16 (48) 30 (91) 10 (77) 12 (92) Lead aVL • Negative δ wave (0–20 ms) • Negative component of the δ wave (0–20 and/or 20–40 ms) 97 103 0 0 2 (6) 2 (6) 0 0 Lead aVR • Positive δ wave (0–20 and 20-40 ms) 6 2 (3) 2 (6) 2 (15) Lead V1 • Negative δ wave (0–20 ms) • Negative component of the δ wave (0–20 and/or 20–40 ms) • Positive δ wave (0–20 ms) • Positive component of the δ wave (0–20 and/or 20–40 ms) 70 106 150 190 45 (70) 54 (84) 9 (14) 14 (22) 3 (9) 6 (18) 20 (61) 24 (73) 3 (23) 3 (23) 8 (62) 11 (85)
value (PPV) of a negative delta wave in lead II to predict epicardial CS APs were only 68% and 18%, respectively.17,19 The sensitivity was 77%, which compares well with the aforementioned findings reported in the later study by Arruda’s group.4
Takahashi et al. also attempted to identify APs ablated within the CS in a selected population of 117 patients with manifest inferior paraseptal AP.20 They evaluated the initial 40 ms of the delta wave and also found that negative delta waves in lead II were more often observed in APs ablated within the CS compared with those ablated from the right or left endocardium (87% versus 21%, p<0.01). The sensitivity of that finding was high in their cohort (87%). However, similar to the results in the study by Pascale et al., the specificity and PPV of this association were low considering that the study population was a selected one, consisting of only inferior paraseptal APs (79% and 50%, respectively).19,20
Takahashi et al. also observed that a positive delta wave in lead aVR was more often observed in epicardial CS APs compared with right or left inferior paraseptal APs (57% versus 9%, p<0.01). In that selected population the specificity and PPV of this finding were 91% and 62%, respectively. The reproducibility of this result in the Pascale et al. study cannot be precisely assessed. The finding of a positive delta wave in lead aVR, both in the first and second half of the 40 ms period, was indeed specific for AP localised in the inferior paraseptal region, but it was rarely observed in the population from the Pascale et al. study: it was observed in only 15% of epicardial CS APs compared with 3% and 6% in right and left inferior paraseptal APs, respectively (p=0.07 and 0.31, respectively) (Table 1).19
In summary, there seems to be little evidence to support the fact that a specific ECG pattern enables selective discrimination of epicardial CS APs from other APs based on a standard sinus rhythm ECG. Considering the limitations in the ECG interpretation discussed above, the identification of such a specific ECG pattern seems unrealistic.
It seems therefore more realistic to aim for the identification of features that are specific to APs located at some distance from the overlapped endo- and epicardial components of the left atrium. As such, we think that an approach aiming to discriminate inferior paraseptal APs ablated from the right endocardium from left-sided APs (endocardial or epicardial CS) is most reasonable. This distinction is the most relevant in terms of procedural risk anticipation and procedural planning, given that a leftsided approach would be advised even if an epicardial CS AP is initially suspected.
Right Endocardial Posteroseptal versus Left-sided Posteroseptal Accessory Pathway
Based on our experience and previous data, there is no single ECG sign that allows to discriminate the laterality of most inferior paraseptal APs. Nevertheless, there are some ECG features based on the delta wave or QRS polarity that enable localisation of the subgroups of inferior paraseptal APs with a reasonable specificity. By summing the knowledge of these different criteria, the categorisation of a substantial proportion of APs may be achieved.
Delta Wave in the Frontal Plane
Regarding the delta wave polarity in the frontal plane, we were not able to find any specific pattern able to discriminate a significant number of right and left inferior paraseptal APs (Table 1).19 Similarly, Haghjoo et al. found that the delta wave polarity in the inferior leads could not distinguish right from left inferior paraseptal APs.21
In contrast, some authors suggested that the combination of a positive initial delta wave in lead II (0–20 ms) with a negative delta waves in leads III and aVF was specific for right inferior paraseptal AP given that it points towards a more right-sided location.33 In our experience, this pattern seems indeed specific for right endocardial septal AP but it is rarely observed, given that less than 10% of patients with inferior paraseptal AP had this pattern.19 Of note, as mentioned before, the delta wave, considered as a whole, is often ‘less negative’ in lead II in inferior paraseptal APs whether right- or left-sided.
The finding of a negative delta wave in all inferior leads has also been regarded as suggestive of a right endocardial AP, generally related to the CS orifice region.33 In our study population this finding provided the exact same information as a negative delta wave in lead II with respect to the AP localisation, given that all of those patients also had a negative initial delta wave in leads III and aVF.19 As such, this pattern instead suggested an epicardial CS AP and was also often observed in left endocardial APs (36%), as discussed before.
Finally, as mentioned above, when an inferior paraseptal AP is suspected, the finding of a negative component of the delta wave (first 20 ms) in leads I or aVL essentially rules out a right-sided, and possibly also an epicardial CS AP. Nevertheless here, again, this finding was rarely observed in left endocardial inferior paraseptal APs (Table 1).19
Accordingly, the delta wave polarity in the frontal leads seems to be of limited value to discriminate right versus left-sided APs.
Delta Wave in Precordial Leads
Regarding the delta wave polarity in the precordial leads, there are conflicting data concerning the assessment of V1 to distinguish between right- versus left-sided APs. As discussed before, these discrepancies may in part be related to the method used to define the delta wave polarity.
In their study assessing the initial 40 ms of the delta wave in patients with manifest inferior paraseptal AP, Takahashi et al. found that a negative delta wave in V1 was present only in right endocardial APs (28% versus 0%, p<0.01).20 A negative polarity was considered when the end of the delta wave was below the isoelectric line. In contrast, Haghjoo et al. evaluated the polarity of the initial 60 ms of the delta wave and found no statistically significant difference between the proportion of positive, negative and biphasic delta waves in V1 when comparing right endocardial, left endocardial and epicardial CS inferior paraseptal APs.21 In that study, polarity was considered negative or positive when the delta wave was entirely below or above the isoelectric line, respectively. In contrast to these findings, despite using the same delta wave polarity definition, Chiang et al. had previously reported that a positive delta wave in V1 could specifically differentiate left from right inferior paraseptal APs.31 In our previous study, we tested different ways of assessing the delta wave polarity including a separate assessment of the first and second half of the first 40 ms of the delta wave.19 As shown in Table 1, a negative initial delta wave (0–20 ms) pointed towards a right endocardial inferior paraseptal AP with a fair specificity. This pattern was observed in 70% of right inferior paraseptal APs, while only 9% and 23% of the left inferior paraseptal and epicardial CS APs had a negative initial delta wave, respectively (p<0.01 and p=0.001, respectively).
Similar findings were observed when the delta wave polarity defined by Takahashi et al. was applied to the population in the study by Pascale et al.: 69% of the right inferior paraseptal APs had a negative delta wave in V1
Localisation of Posteroseptal Accessory Pathways ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 2: The ‘Double Transition’ Pattern to Discriminate Right Endocardial Posterior/Posteroseptal Accessory Pathways
An abnormal transition pattern was observed in a subset of patients (blue arrows), with a proportion of the positive QRS component (displayed in per cent) in V1 < V2 > V3. The QRS polarity in lead V2 enabled further localisation of the AP along the tricuspid annulus: a positive QRS sum indicated an inferior paraseptal right endocardial AP (A–C), whereas a negative or isoelectric QRS sum in lead V2 predicted a more lateral localisation, as shown in a posterolateral right AP (D). AP = accessory pathway. Adapted from: Pascale et al. 2020.19 Used with permission from Elsevier.
while only 12% and 15% of the left inferior paraseptal and epicardial CS APs had a negative delta wave, respectively (p<0.01 for both comparisons).19
Arruda et al. showed that right inferior paraseptal APs could be more specifically identified by combining the finding of a negative, or isoelectric, initial delta wave in V1 with a negative delta wave in lead aVF.17
In the Pascale et al. study population, this pattern was fairly specific and provided 58% sensitivity, 93% specificity and 73% PPV.19 Its specificity could be further increased when only negative delta waves were considered in both V1 and aVF (45% sensitivity, 96% specificity and 78% PPV).19
In our experience, however, the most specific pattern is the finding of a negative delta wave in both the first and second half of the first 40 ms of the delta wave. This pattern was almost 100% specific for right endocardial AP and was observed in approximately half of the patients.19
Accordingly, lead V1 may provide some useful indication depending on how the delta wave polarity is defined.
Analysis of the QRS Polarity
Regarding the analysis of the QRS polarity, different authors have showed that an R/S ratio in V1 ≥1 is a sensitive and specific marker (up to 100%) to differentiate left from right inferior paraseptal endocardial APs (including the CS ostium).21 32 39 Of note, APs ablated from within the CS have not consistently been included in these analyses. However, it appears that even APs ablated from the most proximal part of the CS (<1–1.5 cm from the ostium) more often have an R/S ratio ≥1 in V1.1 18 21 39 40 Moreover, it may be anticipated that, the further from the CS ostium, the more likely it is that APs will have an R/S ratio in V1 ≥1. However, in a previous review, Haissaguerre et al. instead noted the value of an R/S ratio <1 in V1.40 In their experience, all inferior paraseptal APs with prominent negative QRS complexes in V1 were ablated from the right side (88% endocardially, 12% in the proximal CS). APs with prominent positive QRS complexes were ablated at the right endocardium, the proximal CS or left endocardium in 55%, 26% and 18% of cases, respectively.40
On the other hand, other authors did not find a significant yield of the R/S ratio to discriminate between right- and left-sided inferior paraseptal APs.31 33
We also evaluated the R/S ratio in V1 to discriminate right- from left-sided inferior paraseptal APs. We found that an R/S ratio ≥1 was significantly more frequent in left-sided APs: it was observed in 76% of left endocardial APs and in 69% of epicardial CS APs. Right endocardial APs had an R/S ratio ≥1 in 25% of cases (p<0.001 and p=0.002 compared to left endocardial and epicardial CS APs, respectively) (Pascale, 2021 unpublished data). What we observed on analysis of these data is that in the majority of cases of left-sided AP with a predominantly negative QRS complex in V1 there was in fact a weak degree of preexcitation with a relatively narrow QRS. Not surprisingly, this limitation is even more relevant when assessing the polarity of the QRS rather than that of the delta wave. This drawback may explain the differences between studies and must be kept in mind when assessing the R/S ratio in V1.
We recently reported a new ECG sign based on the QRS polarity that enables us to specifically identify inferior parasteptal and posterior APs that can be successfully ablated from the right endocardium from those needing another approach.19 Although a progressively increasing R wave proportion across the precordium is normally observed, we observed in a subset of patients an abnormal QRS transition pattern. The ECG sign consisted of the association of either a Q wave, or a predominantly negative wide QRS in V1 (defined as a QRS width >130 ms), with a proportion of the positive precordial QRS component in V2 greater than that in V1 and V3, which produced a ‘double transition’ pattern. This pattern is illustrated in Figure 2. Interestingly, this double transition had been observed by Xie et al., who noted that a little fewer than half of the patients with right inferior paraseptal AP had “a higher R wave in leads V2 and V4 than in V3”.35
In our study population of 273 patients, this pattern was 100% specific for an AP that could be ablated from the right endocardium and could be used to rule out the need for a left-sided approach or an ablation performed within the CS.19 Moreover, the AP localisation could be further
Localisation of Posteroseptal Accessory Pathways ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Right endocardial AP (100% specificity, 28% sensitivity) A I II III aVR aVL aVF ≈70% R ≈30% R ≈30% R ≈10% R ≈50% R 100% R 100% R 0% R 0% R – V1 + V2 – V3 ± V4 + V5 + V6 I II III aVR aVL aVF – V1 + V2 – V3 + V4 + V5 + V6 I II III aVR aVL aVF – V1 + V2 ± V3 + V4 + V5 + V6 I II III aVR aVL aVF – V1 ± V2 – V3 + V4 + V5 + V6 B C D 100% R 0% R 0% R 100% R 100% R 100% R 100% R 100% R 100% R 100% R 0% R ≈50% R 100% R 100% R 100% R • Q wave in V1 • Positive QRS component in V1 < V2 > V3
Table 2: Useful Baseline ECG Signs to Localise Posteroseptal Accessory Pathways
Negative δ wave in V1, defined as either:
• End of δ wave at 40 ms below the isoelectric line
• Negative δ wave (first 20 ms)
• Negative δ wave (first 20 ms) combined with a negative δ wave in lead aVF
• Negative δ wave in both the first and second half of the first 40 ms
QRS polarity: R/S ratio in V1 ≥1
The ‘double transition’ pattern
Q wave in V1 or predominantly negative wide QRS in V1 (QRS > 130 ms)
+ Proportion of the positive precordial QRS component in V1 < V2 > V3
Negative δ wave (first 20 ms) in lead I or aVLç
+ = mild association; ++ = strong association; +++ = highly specific association; AP = accessory pathway; CS = coronary sinus.
refined depending on the QRS polarity in V2. Namely, in the case of a positive QRS, the AP was localised on the right endocardial inferior paraseptal region, whereas in the case of a negative or isoelectric QRS in V2, the AP was localised more laterally on the tricuspid annulus. In that cohort, this double transition pattern helped to characterise the AP localisation of almost one out of seven APs referred for ablation, and almost half of the right endocardial inferior paraseptal APs.
In contrast, regarding the analysis of the QRS polarity in the frontal leads, the analysis of the R/S ratio does not seem to be of meaningful value. An R wave amplitude in lead I exceeding the S wave by ≥1.0 mV has been suggested as a feature that may help discriminate right- from left-sided inferior paraseptal AP.21,32 However, in our experience, most patients with left-sided inferior paraseptal APs indeed have a predominantly positive QRS complexes in lead I (Pascale 2021, unpublished data).
Useful baseline ECG signs to distinguish right endocardial inferior paraseptal APs from left-sided inferior paraseptal APs are summarised in Table 2
ECG Analysis During Maximal Preexcitation
As previously discussed, the analysis of the delta wave and, particularly, the QRS, during baseline ECG may be misleading as a result of the varying degree of fusion with the activation from the His–Purkinje system. Analysis during maximal preexcitation is therefore expected to notably increase the accuracy and reproducibility of the ECG to predict AP localisation. As such, this analysis should be the first diagnostic step in the electrophysiology lab in order to guide the initial mapping strategy. A stepwise algorithm during maximal preexcitation was recently developed by Pambrun et al.41 This four-step algorithm is based on the QRS amplitude and morphology in inferior leads, leads V1, V3 and lead I during rapid atrial pacing. Regarding specifically inferior paraseptal APs and the anticipation of the successful ablation approach, the algorithm can be adapted as illustrated in Figure 3
First, all inferior paraseptal AP had negative QRS in all three leads. Of note, in the case of isoelectric QRS, the polarity of the QRS was defined by its
More specific in the case of substantial degree of preexcitation (QRS > 130 ms?)
QRS polarity in V2 enables further refinement of AP localisation:
• If positive: right posteroseptal AP
• If negative or isoelectric: AP localised more laterally on the tricuspid annulus
Rarely observed (more frequent in left posterior or posterolateral APs)
initial deflection. Second, APs ablated from the right endocardium had a negative QRS in V1, while it was positive for epicardial CS and left endocardial AP. Right inferior paraseptal APs could be distinguished from posterior APs by a positive QRS in V3. Third, epicardial CS APs were identified when the QRS ratio V1/I was <1 (which located the AP left septally), and a notched QS was observed in lead II. The authors raise the hypothesis that this notching may reflect the inhomogeneous ventricular activation related to the various orientations of the sleeve-like CS extensions.
Regarding the algorithm accuracy, the identification was correct in 90% of the patients, as opposed to 63% with the Arruda et al. algorithm. For inferior paraseptal APs, the PPV was 97% and 77% for right and left inferior paraseptal APs, respectively. As expected, the lowest PPV was observed for epicardial CS APs.
Takahashi et al. specifically sought to identify ECG features to discriminate epicardial CS APs from right or left endocardial inferior paraseptal APs during maximal preexcitation.20 They found that a steep positive delta wave in lead aVR had the highest PPV (88%), which increased to 91% when combined with a deep S wave in V6 (R wave ≤ S wave). A positive delta wave in aVR was also reported as a specific finding of epicardial CS APs by Kobza et al.38
In our cohort of APs during maximal preexcitation, we could not reproduce this finding (n=242) (Pascale 2021, unpublished data). Of note, in the study by Takahashi et al. a negative delta wave in V1 was highly specific for right endocardial AP but, unexpectedly, a positive delta wave or a positive QRS were observed in approximately one-third of the patients with right endocardial AP, in contradiction to the above mentioned algorithm. In our cohort, a positive QRS in V1 (as defined in the Pambrun et al. algorithm) was observed in only approximately 10% of maximally preexcited right endocardial inferior paraseptal APs (Pascale 2021, unpublished data). Nevertheless, one must be aware that whenever a 3D system with precordial patches is used for mapping, the interpretation of V1 or V3 features may be flawed if such leads are recorded from a different position on the patient’s chest.
Localisation of Posteroseptal Accessory Pathways ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Right Endocardial Posteroseptal Favoured Left-sided Posteroseptal (Including Subepicardial CS) Favoured Caveats and Nuances
+ + ++ ++ ––More specific
More specific
+
+++
++
LPS = left posteroseptal; RP = right posterior; RPS = right posteroseptal. Adapted from: Pambrun et al. 2018.41 Used with permission from Oxford University Press.
The 12-Lead ECG Analysis During Orthodromic Reciprocating Tachycardia
Inferior paraseptal bypass tracts generally display negative P waves in all inferior leads and positive P waves in aVR, aVL and V1 during orthodromic reciprocating tachycardia. As can be expected considering the electrical ‘weight’ of the atria, and the difficulties in identifying the P wave morphology with the interference from the T wave, there are no criteria able to discriminate right and left inferior paraseptal APs with reasonable accuracy.42 43
Although present in a minority of patients, the analysis of the ventriculoatrial (VA) interval during functional bundle branch block (BBB) aberrancy may possibly provide some indication to localise the AP on the right or left endocardial side. Lengthening of the VA interval (or of the tachycardia cycle length provided that there are no changes in the atrioventricular interval) by 35 ms or more with the development of BBB indicates the involvement in the tachycardia of an ipsilateral free wall AP.44 Regarding inferior paraseptal pathways, the VA interval may prolong with left BBB albeit to a lesser extent, by 25 ms or less, but it does not vary with right BBB.44 45 Although the absence of VA changes during functional left BBB clearly points towards a successful right-sided approach, VA interval prolongation is often observed in inferior paraseptal APs ablated from the right atrium and CS ostium.18 46 This probably reflects the fact that some APs are actually connected to the posterior superior process of the left ventricle, as discussed previously (Figure 1).
Clinical Perspective
• The localisation of posteroseptal or inferior paraseptal accessory pathways (APs) poses a diagnostic challenge because of the complex anatomy of this region and the fact that a small area may encompass APs that may be approached from the right or left endocardium, or require an ablation performed epicardially inside the coronary sinus.
• There seems to be little evidence to support the fact that a specific ECG pattern enables selective discrimination of APs ablated within the coronary sinus from other APs based on a standard sinus rhythm ECG.
• Considering the limitations in the ECG interpretation, an approach aiming to discriminate subgroups of inferior paraseptal APs ablated from the right endocardium from left-sided APs (endocardial or ablated within the coronary sinus) seems more realistic.
• The baseline ECG signs that seem most useful and relevant to distinguish right endocardial inferior paraseptal APs from left-sided inferior paraseptal APs are based on the finding of a negative delta wave in V1 (depending, however, on how it is defined), the assessment of the R/S ratio in V1, and the finding of a Q wave or predominantly negative QRS in V1 with the ‘double precordial transition’.
Localisation of Posteroseptal Accessory Pathways ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Step 1 Step 2 Step 3 All negative inferior leads V1 polarity Non-posteroseptal AP Right endocardial AP (RP or RPS) Precordial polarity transition DCS LPS LPL Left endocardial AP RP RPS Notched QS in lead II ≥V3 <V3 Yes No <1 ≥1 V1/I ratio Yes No – +
Figure 3: Stepwise ECG Algorithm Based on Maximally Pre-excited QRS to Discriminate Posteroseptal Accessory Pathways
This
algorithm is based on maximally preexcited QRS to discriminate inferior paraseptal APs ablated from the right endocardium, from those requiring a left-sided or epicardially coronary venous approach. AP = accessory pathway; DCS = deep coronary sinus; LPL = left posterolateral;
Localisation of Posteroseptal Accessory Pathways
1. Jazayeri MR, Dhala A, Deshpande S, et al. Posteroseptal accessory pathways: an overview of anatomical characteristics, electrocardiographic patterns, electrophysiological features, and ablative therapy. J Interv Cardiol 1995;8:89–101. https://doi.org/10.1111/j.1540-8183.1995. tb00519.x; PMID: 10155221.
2. Sealy WC, Mikat EM. Anatomical problems with identification and interruption of posterior septal Kent bundles. Ann Thorac Surg 1983;36:584–95. https://doi.org/10.1016/S00034975(10)60690-X; PMID: 6639196.
3. Guiraudon GM, Klein GJ, Sharma AD, et al. Surgical ablation of posterior septal accessory pathways in the WolffParkinson-White syndrome by a closed heart technique. J Thorac Cardiovasc Surg 1986;92:406–13. https://doi. org/10.1016/S0022-5223(19)35794-0; PMID: 3528678.
4. Sun Y, Arruda M, Otomo K, et al. Coronary sinus-ventricular accessory connections producing posteroseptal and left posterior accessory pathways: incidence and electrophysiological identification. Circulation 2002;106:1362–7. https://doi.org/10.1161/01. CIR.0000028464.12047.A6; PMID: 12221053.
5. Liu E, Shehata M, Swerdlow C, et al. Approach to the difficult septal atrioventricular accessory pathway: the importance of regional anatomy. Circ Arrhythm Electrophysiol 2012;5:e63–6. https://doi.org/10.1161/CIRCEP.112.971135;
PMID: 22715241.
6. Calkins H, Yong P, Miller JM, et al. Catheter ablation of accessory pathways, atrioventricular nodal reentrant tachycardia, and the atrioventricular junction: final results of a prospective, multicenter clinical trial. Circulation 1999;99:262–70. https://doi.org/10.1161/01.CIR.99.2.262;
PMID: 9892593.
7. Dionne A, Gauvreau K, O’Leary E, et al. Risk factors for early recurrence following ablation for accessory pathways: the role of consolidation lesions. Circ Arrhythm Electrophysiol 2020;13:e008848. https://doi.org/10.1161/CIRCEP.120.008848;
PMID: 33017181.
8. Nakagawa H, Jackman WM. Catheter ablation of paroxysmal supraventricular tachycardia. Circulation 2007;116:2465–78. https://doi.org/10.1161/CIRCULATIONAHA.106.655746; PMID: 18025404.
9. Sternick EB, Correa FS, Rego S, et al. Postablation-acquired short atrioventricular Mahaim-type fibers: observations on their clinical, electrocardiographic, and electrophysiologic profile. Heart Rhythm 2012;9:850–8. https://doi.org/10.1016/j. hrthm.2012.02.011; PMID: 22338671.
10. Sternick EB, Faustino M, Correa FS, et al. Percutaneous catheter ablation of epicardial accessory pathways. Arrhythm Electrophysiol Rev 2017;6:80–4. https://doi.org/10.15420/ aer.2017.6.2; PMID: 28835839.
11. Belhassen B, Rogowski O, Glick A, et al. Radiofrequency ablation of accessory pathways: a 14 year experience at the Tel Aviv Medical Center in 508 patients. Isr Med Assoc J 2007;9:265–70. PMID: 17491219.
12. Sacher F, Wright M, Tedrow UB, et al. Wolff-Parkinson-White ablation after a prior failure: a 7-year multicentre experience. Europace 2010;12:835–41. https://doi. org/10.1093/europace/euq050; PMID: 20223787.
13. Solomon AJ, Tracy CM, Swartz JF, et al. Effect on coronary artery anatomy of radiofrequency catheter ablation of atrial insertion sites of accessory pathways. J Am Coll Cardiol 1993;21:1440–4. https://doi.org/10.1016/07351097(93)90321-Q; PMID: 8473653.
14. Stavrakis S, Jackman WM, Nakagawa H, et al. Risk of coronary artery injury with radiofrequency ablation and cryoablation of epicardial posteroseptal accessory pathways within the coronary venous system. Circ Arrhythm Electrophysiol 2014;7:113–9. https://doi.org/10.1161/ CIRCEP.113.000986; PMID: 24365648.
15. Davis LM, Byth K, Ellis P, et al. Dimensions of the human posterior septal space and coronary sinus. Am J Cardiol 1991;68:621–5. https://doi.org/10.1016/00029149(91)90354-N; PMID: 1877479.
16. Pap R, Traykov VB, Makai A, et al. Ablation of posteroseptal and left posterior accessory pathways guided by left atriumcoronary sinus musculature activation sequence. J Cardiovasc Electrophysiol 2008;19:653–8. https://doi.
org/10.1111/j.1540-8167.2008.01103.x; PMID: 18284500.
17. Arruda MS, McClelland JH, Wang X, et al. Development and validation of an ECG algorithm for identifying accessory pathway ablation site in Wolff-Parkinson-White syndrome. J Cardiovasc Electrophysiol 1998;9:2–12. https://doi. org/10.1111/j.1540-8167.1998.tb00861.x; PMID: 9475572.
18. Dhala AA, Deshpande SS, Bremner S, et al. Transcatheter ablation of posteroseptal accessory pathways using a venous approach and radiofrequency energy. Circulation 1994;90:1799–810. https://doi.org/10.1161/01.CIR.90.4.1799; PMID: 7923665.
19. Pascale P, Hunziker S, Denis A, et al. The “double transition”: a novel electrocardiogram sign to discriminate posteroseptal accessory pathways ablated from the right endocardium from those requiring a left-sided or epicardial coronary venous approach. Europace 2020;22:1703–11. https://doi.org/10.1093/europace/euaa200; PMID: 32984869.
20. Takahashi A, Shah DC, Jais P, et al. Specific electrocardiographic features of manifest coronary vein posteroseptal accessory pathways. J Cardiovasc Electrophysiol 1998;9:1015–25. https://doi.org/10.1111/j.1540-8167.1998. tb00879.x; PMID: 9817553.
21. Haghjoo M, Mahmoodi E, Fazelifar AF, et al. Electrocardiographic and electrophysiologic predictors of successful ablation site in patients with manifest posteroseptal accessory pathway. Pacing Clin Electrophysiol 2008;31:103–11. https://doi. org/10.1111/j.1540-8159.2007.00933.x; PMID: 18181918.
22. Iturralde P, Nava S, Salica G, et al. Electrocardiographic characteristics of patients with Ebstein’s anomaly before and after ablation of an accessory atrioventricular pathway. J Cardiovasc Electrophysiol 2006;17:1332–6. https://doi. org/10.1111/j.1540-8167.2006.00617.x; PMID: 17239096.
23. Reich JD, Auld D, Hulse E, et al. The Pediatric Radiofrequency Ablation Registry’s experience with Ebstein’s anomaly. J Cardiovasc Electrophysiol 1998;9:1370–7. https://doi.org/10.1111/j.1540-8167.1998.tb00113.x; PMID: 9869537.
24. Wei W, Zhan X, Xue Y, et al. Features of accessory pathways in adult Ebstein’s anomaly. Europace 2014;16:1619–25. https://doi.org/10.1093/europace/euu028; PMID: 24614573.
25. Aguinaga L, Primo J, Anguera I, et al. Long-term follow-up in patients with the permanent form of junctional reciprocating tachycardia treated with radiofrequency ablation. Pacing Clin Electrophysiol 1998;21:2073–8. https://doi. org/10.1111/j.1540-8159.1998.tb01126.x; PMID: 9826859.
26. Gaita F, Haissaguerre M, Giustetto C, et al. Catheter ablation of permanent junctional reciprocating tachycardia with radiofrequency current. J Am Coll Cardiol 1995;25:648–54. https://doi.org/10.1016/0735-1097(94)00455-Y; PMID: 7860909
27. Josephson ME. Clinical Cardiac Electrophysiology: Techniques and Interpretation. 2nd ed. Philadelphia, PA: Lea & Febiger, 1993.
28. Ticho BS, Saul JP, Hulse JE, et al. Variable location of accessory pathways associated with the permanent form of junctional reciprocating tachycardia and confirmation with radiofrequency ablation. Am J Cardiol 1992;70:1559–64. https://doi.org/10.1016/0002-9149(92)90457-A; PMID: 1466323.
29. Katritsis DG, Wellens HJ, Josephson ME. Mahaim accessory pathways. Arrhythm Electrophysiol Rev 2017;6:29–32. https:// doi.org/10.15420/aer.2016:35:1; PMID: 28507744.
30. Boersma L, Garcia-Moran E, Mont L, Brugada J. Accessory pathway localization by QRS polarity in children with WolffParkinson-White syndrome. J Cardiovasc Electrophysiol 2002;13:1222–6. https://doi. org/10.1046/j.1540-8167.2002.01222.x; PMID: 12521337.
31. Chiang CE, Chen SA, Teo WS, et al. An accurate stepwise electrocardiographic algorithm for localization of accessory pathways in patients with Wolff-Parkinson-White syndrome from a comprehensive analysis of delta waves and R/S ratio during sinus rhythm. Am J Cardiol 1995;76:40–6. https://doi. org/10.1016/S0002-9149(99)80798-x; PMID: 7793401.
32. Fitzpatrick AP, Gonzales RP, Lesh MD, et al. New algorithm for the localization of accessory atrioventricular connections using a baseline electrocardiogram. J Am Coll Cardiol
1994;23:107–16. https://doi.org/10.1016/0735-1097(94)905088; PMID: 8277067.
33. Fox DJ, Klein GJ, Skanes AC, et al. How to identify the location of an accessory pathway by the 12-lead ECG. Heart Rhythm 2008;5:1763–6. https://doi.org/10.1016/j. hrthm.2008.09.012; PMID: 18996058.
34. d’Avila A, Brugada J, Skeberis V, et al. A fast and reliable algorithm to localize accessory pathways based on the polarity of the QRS complex on the surface ECG during sinus rhythm. Pacing Clin Electrophysiol 1995;18:1615–27. https://doi.org/10.1111/j.1540-8159.1995.tb06983.x; PMID: 7491305.
35. Xie B, Heald SC, Bashir Y, et al. Localization of accessory pathways from the 12-lead electrocardiogram using a new algorithm. Am J Cardiol 1994;74:161–5. https://doi. org/10.1016/0002-9149(94)90090-6; PMID: 8023781.
36. Iturralde P, Araya-Gomez V, Colin L, et al. A new ECG algorithm for the localization of accessory pathways using only the polarity of the QRS complex. J Electrocardiol 1996;29:289–99. https://doi.org/10.1016/S00220736(96)80093-8; PMID: 8913903.
37. Katsouras CS, Greakas GF, Goudevenos JA, et al. Localization of accessory pathways by the electrocardiogram: which is the degree of accordance of three algorithms in use? Pacing Clin Electrophysiol 2004;27:189–93. https://doi. org/10.1111/j.1540-8159.2004.00409.x; PMID: 14764169.
38. Kobza R, Hindricks G, Tanner H, et al. Paraseptal accessory pathway in Wolff-Parkinson-White-syndrome: ablation from the right, from the left or within the coronary sinus/middle cardiac vein? J Interv Card Electrophysiol 2005;12:55–60. https://doi.org/10.1007/s10840-005-5841-2; PMID: 15717152.
39. Diker E, Ozdemir M, Tezcan UK, et al. QRS polarity on 12-lead surface ECG. A criterion for the differentiation of right and left posteroseptal accessory atrioventricular pathways. Cardiology 1997;88:328–32. https://doi. org/10.1159/000177354; PMID: 9197426.
40. Haissaguerre M, Gaita F, Marcus FI, Clementy J. Radiofrequency catheter ablation of accessory pathways: a contemporary review. J Cardiovasc Electrophysiol 1994;5:532–52. https://doi.org/10.1111/j.1540-8167.1994.tb01293.x; PMID: 8087297.
41. Pambrun T, El Bouazzaoui R, Combes N, et al. Maximal preexcitation based algorithm for localization of manifest accessory pathways in adults. JACC Clin Electrophysiol 2018;4:1052–61. https://doi.org/10.1016/j.jacep.2018.03.018; PMID: 30139487.
42. Chiang CE, Chen SA, Tai CT, et al. Prediction of successful ablation site of concealed posteroseptal accessory pathways by a novel algorithm using baseline electrophysiological parameters: implication for an abbreviated ablation procedure. Circulation 1996;93:982–91. https://doi.org/10.1161/01.CIR.93.5.982; PMID: 8598090.
43. Tai CT, Chen SA, Chiang CE, et al. A new electrocardiographic algorithm using retrograde P waves for differentiating atrioventricular node reentrant tachycardia from atrioventricular reciprocating tachycardia mediated by concealed accessory pathway. J Am Coll Cardiol 1997;29:394–402. https://doi.org/10.1016/S07351097(96)00490-1; PMID: 9014995.
44. Kerr CR, Gallagher JJ, German LD. Changes in ventriculoatrial intervals with bundle branch block aberration during reciprocating tachycardia in patients with accessory atrioventricular pathways. Circulation 1982;66:196–201. https://doi.org/10.1161/01.CIR.66.1.196; PMID: 7083507.
45. Jazayeri MR, Caceres J, Tchou P, et al. Electrophysiologic characteristics of sudden QRS axis deviation during orthodromic tachycardia. Role of functional fascicular block in localization of accessory pathway. J Clin Invest 1989;83:952–9. https://doi.org/10.1172/JCI113981; PMID: 2921328.
46. Extramiana F, Takatsuki S, Hayashi M, Leenhardt A. Functional bundle branch block and orthodromic reciprocating tachycardia cycle length: do not bet on accessory pathway location. Europace 2007;9:920–2. https://doi.org/10.1093/europace/eum183; PMID: 17804516.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Future Directions for Mapping Atrial Fibrillation
Abstract
Mapping for AF focuses on the identification of regions of interest that may guide management and – in particular – ablation therapy. Mapping may point to specific mechanisms associated with localised scar or fibrosis, or electrical features, such as localised repetitive, rotational or focal activation. In patients in whom AF is caused by disorganised waves with no spatial predilection, as proposed in the multiwavelet theory for AF, mapping would be of less benefit. The role of AF mapping is controversial at the current time in view of the debate over the underlying mechanisms. However, recent clinical expansions of mapping technologies confirm the importance of understanding the state of the art, including limitations of current approaches and potential areas of future development.
Keywords
AF, mapping, ablation, mechanisms, drivers
Disclosure: AAG and SMN are section editors on the Arrhythmia & Electrophysiology Review editorial board; this did not influence peer review. JABZ has no conflicts of interest to declare.
Funding: This work was supported in part by grants from the National Institutes of Health to SMN (HL83359, HL103800, HL149134).
Received: 30 August 2021 Accepted: 8 November 2021 Citation: Arrhythmia & Electrophysiology Review 2022;11:e08. DOI: https://doi.org/10.15420/aer.2021.52
Correspondence: Junaid AB Zaman, Keck School of Medicine, University of Southern California, 1510 San Pablo St, Ste 322E, Los Angeles, CA 90033, US.
E: Junaid.zaman@med.usc.edu
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Anatomically based pulmonary vein isolation (PVI) is the cornerstone of ablation, yet continues to achieve success rates of 55–75% at 12–18 months in randomised trials, depending on the population, which falls over time.1 Notably, there has been little benefit from adding several anatomical ablation strategies targeting the posterior wall, the mitral isthmus, left atrial roof or other linear lesions.2–5 Studies that add isolation of the left atrial appendage are ongoing.6 A dominant alternative viewpoint is that patients with AF likely differ in the anatomical locations for their mechanisms, underscoring the need to optimise AF mapping. This viewpoint is tempered by the reality that mapping systems to date have yielded divergent data, varying outcomes in different clinical series and complex mathematical approaches that are difficult to validate by clinical observation.7 In contrast, PVI and anatomical ablation lines are relatively straightforward to confirm clinically.
This review critically evaluates the bench-to-bedside evidence that localised regions of interest perpetuate AF, and describes clinical approaches used to identify these regions for ablation.8,9 We will attempt to address why the localised source hypothesis may be more important to some patients than others.10–15 We will intersperse our descriptions with thoughts on future directions to address challenges that we believe must be overcome to truly advance the field.
Mechanisms that Sustain Human AF
Once AF has been initiated by triggers from the pulmonary veins or other sites, its disorganised wavefronts must be continuously replenished for AF to sustain.16 Two mechanisms are proposed. In one model, disorganised
activity self-sustains because new wavelets are generated across atrial tissue over time. Computer simulations reveal that such wavelets can be generated by unstable spiral waves and wavebreak.17,18 Collision from repetitive foci in multiple spatial locations can also destabilise wave propagation, again producing new wavelets.19 This hypothesis does not require preferred regions of interest (Figure 1A), and effective therapy would require large-scale debulking of the atrium. In the second major model, preferred regions of interest are central to perpetuating AF. Such regions may represent localised rotational or focal sources, other electrical features such as repetitive activation patterns, or structural regions of fibrosis or tissue anisotropy that replenish disordered wavefronts and can thus be termed ‘drivers’ (Figures 1B, 1C and 1D).
Localised rotational or focal sources are a well-described mechanism for replenishing AF wavelets. Rotational circuits were first described in animal models of ventricular and AF in seminal work by Jalife and others in the 1990s.9 Classically, a rotor is defined as re-entry around an unexcited yet excitable core, activating too rapidly for surrounding tissue to keep up and resulting in disordered waves (‘fibrillatory conduction’). This has been shown in multiple animal studies using optical mapping and contact mapping and more recently in human atria during AF (Figures 1B and 1C).9 These features have been reported in patients by many techniques, and ablation at these localised sites alone may terminate AF (Figure 1D).8,20 The field has been confused by argument on the definitions of a rotor, yet this argument has little practical significance at the spatial resolution of clinical mapping. We use the terms rotational activation, localised re-entry and rotor interchangeably, leaving ultimate adjudication on this issue to
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Atrial Fibrillation
Junaid AB Zaman , 1 Andrew A Grace 2 and Sanjiv M Narayan 3
1. Keck School of Medicine, University of Southern California, Los Angeles, CA, US;
2. Department of Biochemistry, University of Cambridge, Cambridge, UK; 3. Cardiovascular Institute and Department of Medicine, Stanford University, CA, US
Figure 1: AF Shows Spatially Heterogeneous Disordered Activity
This may result from localised regional (hierarchical) or self-sustaining (non-hierarchical) mechanisms. A: Non-hierarchical mechanisms, which would require atrial debulking to eliminate; B: AF driver (a hierarchical mechanism) in ex-vivo human AF revealed by bi-atrial optical mapping, which could theoretically be eliminated by localised ablation driver near the RAA with action potentials spanning 100% of cycle length; C: Structural heterogeneities in human atria, with intramural fibrosis at the AF arrhythmogenic hub (red, driver) and surrounding intramural fibrosis (blue) on contrast-enhanced MRI; D: Re-entrant AF driver (hierarchical mechanism), ablation lesions (green dots) that terminated AF prior to PVI, and panoramic recording catheter (basket; left) and DE-CMR reconstruction (right).
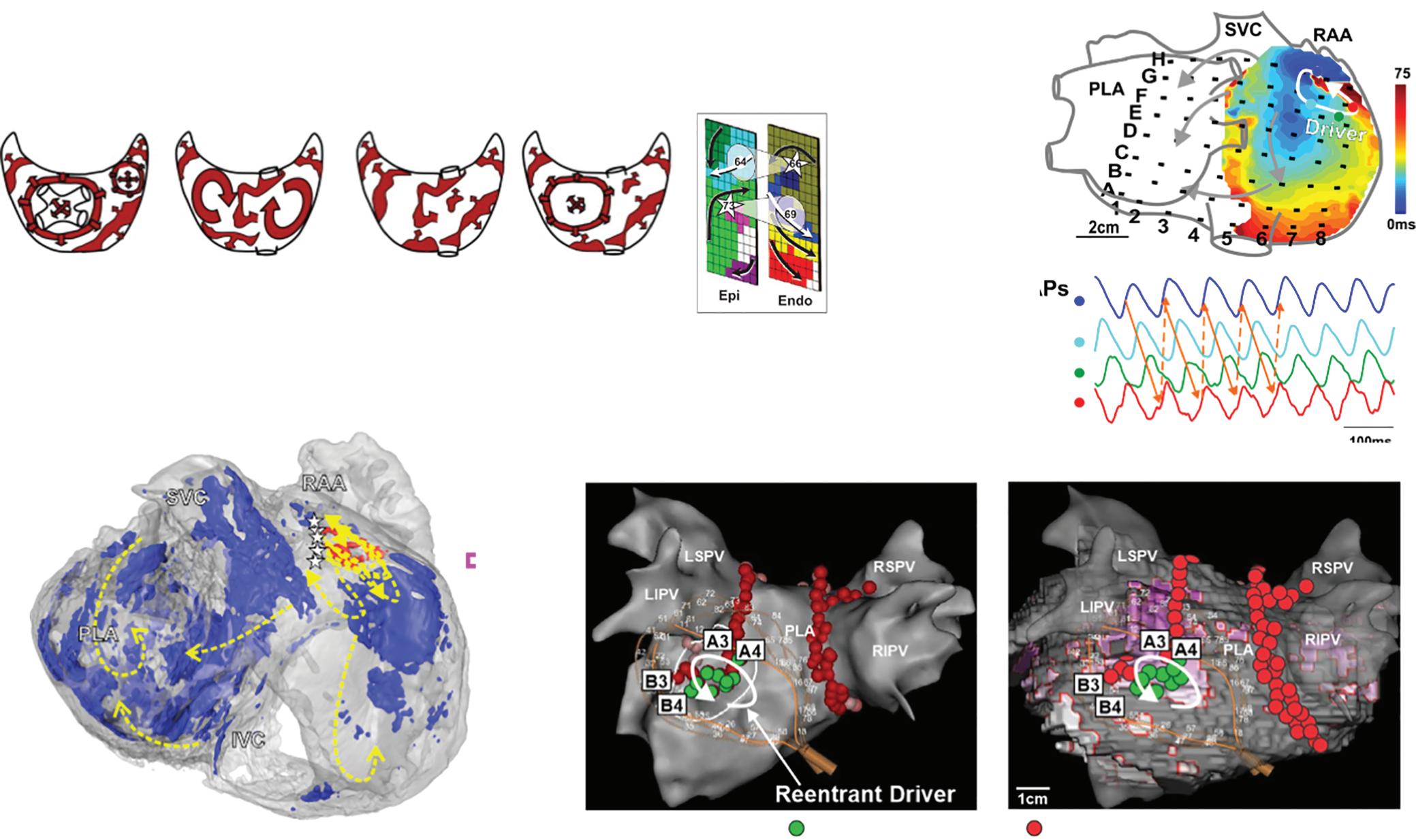
DE-CMRI = Delayed-enhanced cardiac MRI; IVC = inferior vena cava; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; PLA = posterior left atrium; PVI = pulmonary vein isolation; RAA = right atrial appendage; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava.
Sources: A: Eckstein et al. 2008.67 Reproduced with permission from Elsevier. de Groot et al. 2016.68 Reproduced with permission from Wolters Kluwer Health; B–D: Hansen et al. 2020.69 Reproduced from Wiley & Sons under a Creative Commons CC-BY-NC license.
future biophysical, tissue-level and cellular studies. Rapid focal activity can also produce fibrillatory activity and may be a source or driver for AF. The localised source hypothesis may explain how AF sustains in small volumes such as mouse hearts.21 This has always been difficult to explain by the multiwavelet hypothesis that requires large volumes, and how limited ablation alone may terminate persistent AF, which is also difficult to explain by the multiwavelet hypothesis.7,22
Clinical Mapping of AF in Patients
An increasing variety of approaches are available to map AF. Despite differences in how signals are recorded and analysed, most clinical systems reveal organised features in AF in both atria where ablation can acutely impact AF in patient subsets.
Table 1 summarises AF clinical mapping systems. Essentially all identify localised regions of interest in AF, such as focal impulses, rotational sources or other repetitive areas (including ‘localised disorder’). Spatially, three to five such sites are typically seen in each patient, approximately two-thirds of which are in the left atrium. Figures 2A, 2B and 2C show examples of mapped sites where ablation terminates AF. Multiple sites often co-exist and compete and, as shown in Figures 2D and 2E, when the re-entrant site (blue) dominates AF the focal site (pink) is quiescent and vice versa.23 The earliest clinical mapping system (focal impulse and rotor mapping [FIRM]), specifically designed to identify focal sources, provides ~80% concordance with simultaneous optical mapping of human AF, with the caveat that electromechanical decoupling agents were used to
eliminate atrial motion (a general requirement for optical mapping).24 Validation studies of other clinical mapping systems are pending.
We summarise reported AF mapping methods based on whether their primary recording approach is global (panoramic) or in a small field of view (and hence sequential) in the atria. Sequential approaches assume that AF sources, when identified, are relatively stationary during the timescale of mapping or return to preferred locations repetitively if they meander. We also compare systems based on whether they use contact electrodes, which have traditionally been the gold standard, or noncontact recordings such as body surface mapping, in which electrograms are computed mathematically. Randomised trial data do not yet support the benefits of ablating targets identified by any of these mapping systems, but early subset analyses of randomised trials are promising and several trials are ongoing (NCT04442113, NCT04473963, NCT02696265, NCT04428944, NCT04702451).25
Panoramic Contact Mapping
Focal Impulse and Rotor Mapping
FIRM was the first approach to systematically map localised putative drivers for AF and target them for ablation (Figure 2). FIRM analyses unipolar electrograms of AF from wide-area (panoramic) basket recordings, and applies physiological filters based on monophasic action potential dynamics and conduction velocity to remove noise (RhythmView, Abbott). Initial reports of FIRM in 2011 demonstrated localised rotors and focal sources in nearly all patients with AF, where ablation was able to
Challenges for AF Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
A C D B Multiple foci Unstable circuits Multiple wavelets Focus + multiple wavelets Endo and epicardial breakthrough
AF
Ablation through AF-driver fibrotic substrate terminates
AF terminating lesions Subsequent PVI ablation
Table 1: Clinical Mapping Approaches for AF
AT = atrial tachycardia; ECGI = electrocardiographic imaging; FIRM = focal impulse and rotor mapping; LA = left atrium; LAA = left atrial appendage; LPV = left pulmonary vein; PV = pulmonary vein; PVI = pulmonary vein isolation; RA = right atrium; RADAR = real-time electrogram analysis for drivers of AF; RCT = randomised controlled trial; STAR
terminate or slow AF and eliminate AF in 82.4% of patients. Results from this mapping approach are ~80% concordant with concurrent optical mapping of AF in explanted human atria, with promising results of ablation in meta-analyses.7,24,26,27 However, some clinical studies reported very poor results, for unclear reasons. Nevertheless, this has motivated newer techniques to overcome difficulties in reading maps, to provide better atrial coverage during AF, to better define ablation strategies once mapping is complete and also to identify patients in whom these mechanisms are more important than others.
AF sources from FIRM mapping arise in diverse locations; one-third in the right atrium.11,28,29 This intriguing figure may explain the 70–80% success ceiling of left atrial ablation for AF and the reported benefits of right atrial ablation in some patients.30,31 There were few complications during mapping.32 In a recent randomised clinical trial (REAFFIRM), intention-totreat analysis showed no difference in arrhythmia freedom between FIRM + PVI and PVI. However, in on-treatment analysis, the pre-specified PVI + FIRM group showed beneficial trends over the prespecified PVI alone group (77.7% versus 65.5% single procedure freedom from atrial arrhythmias at 1 year; p=0.09).25 These hypothesis-generating results are now being tested in on-going randomised trials of several AF map-guided ablation strategies.
stochastic trajectory analysis of ranked signals.
FIRM studies in 2011–2014 foreshadowed several current controversies in AF mapping. First, AF driver sites fluctuated over time, but some reappeared in conserved locations for very prolonged periods.33 While reappearing features were ideally prioritised for ablation, they may have been difficult to identify at some centres. A major focus is to reduce subjectivity in map reading, for which machine learning has had some success.34 AF sources may fluctuate if they compete with concurrent sites.23 From optical maps of human AF, fluctuations may also represent intramural migration of a driver.8 Conversely, body surface mapping (electrocardiographic imaging; ECGI) interprets these features as truly intermittent.13
Electrographic Flow Mapping
Electrographic flow (EGF) uses panoramic basket catheter recordings to compute the ‘average propagation of action potentials.’35 Unipolar electrogram data are used to reconstruct 64 discrete data streams with equal peak amplitudes. After filtering to reconstruct local extracellular voltage, EGF reconstructs an electrogram that is stated to be akin to an optical action potential at each electrode. One hundred consecutive voltage shapes covering 1.9 seconds are then fed into a Horn-Schunck optical flow algorithm to calculate average flow behaviour. Because of noise and imperfect mathematical modelling, flow vectors may scatter
Challenges for AF Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Mapping Technique AF Type Mapped Number of Ablation Targets Atrial Location Source Characterisation Acute Termination Percentage Freedom from AF at 12 Months, with/ without PVI Panoramic Contact Mapping FIRM (RhythmView)10,62,63 Paroxysmal, persistent and long standing persistent 3–5 LA 70% RA 30% PV 24% Stable rotations 76%, focal sources 24%10 56% (60% to sinus)10 RA in 22%62 In meta-analyses 27–53% (sinus or AT) Meta-analysis: 72.5%7 Persistent AF RCT: 77.7% (FIRM + PVI subgroup)25 Electrographic flow mapping (Ablacon)35,36 Persistent AF 4–6 LA 70% RA 30% PV 40% Rotational 51%, focal 49% 100% RA in 10% Pending Sequential Contact Mapping CARTOFINDER (Biosense Webster)37,38,42 Persistent and long standing persistent 1–3 LA 63% RA 27% Non-PV 79%64 Rotational activity 70%, focal activations 30–100%38,42 63% (58% to AT)42 15% (all sinus)64 71%38 70%64 Spatiotemporal dispersion (Volta Medical)43 Persistent AF 4–6 LA 80% RA 20% PV/LAA 80% Regions of microre-entry 95% (85% to AT) 85% without PVI (1.4 procedures, at 18 months) STAR45 Persistent AF 2–3 (post PVI) LA 95% RA 5% Early sites of activation 29% (75% to AT) 80% (AT/AF at 18 months) RADAR (CardioNXT) 46 Persistent AF Longstanding AF 3.9 ± 1.3 (LA) 2.5 ± 1.4 (RA) Inconsistent RA mapping Rotational (73%) and focal sites 55% 74% AF freedom at 13 months (on/off drugs) Non-contact Mapping Charge/dipole density (Acutus)48,65 Persistent AF 2–3 RA not mapped LA anterior 70% Localised irregular activity Localised rotational activity Focal activity 50–60% 73%65 Body surface, ECGI (CardioInsight, EP Solutions)13,51,66 Persistent and long standing persistent 3–6 LA 70% RA 30% LPV/LAA 82%13 LA 53% RA 27% Septum 20%51 Re-entries 80% Focal breakthrough 20%13 80% (66% to AT)13 64% (79% to AT) (PVs 37%, LA 35%, RA in 28%)51 85%13 78%51
=
Figure 2: Initial Clinical Reports of Localised Sources for Persistent AF by FIRM
(A) Rotational, termination to sinus
A Beginning of ablation at rotor #3 B After <1 min of ablation at rotor #3
C Electroanatomic map of right atrial sources
A: Rotational sources for persistent AF; B: Focal sources for persistent AF. Ablation at these sites alone (approximately two to four per patient) caused termination to sinus rhythm prior to PVI; C: Multiple overlapping positions of basket catheters in the atria ensure adequate atrial coverage and contact in contemporary practice. CS = coronary sinus; FIRM = focal impulse and rotor mapping; IVC = inferior vena cava; LA = left atrium LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; PVI = pulmonary vein isolation; RA = right atrium; SVC = superior vena cava. Sources: A: Miller et al. 2017.62 Reproduced with permission from Elsevier; B: Narayan et al. 2013.70 Reproduced with permission from Wolters Kluwer Health ; C: Zaman et al. 2017.71 Reproduced with permission from Elsevier.
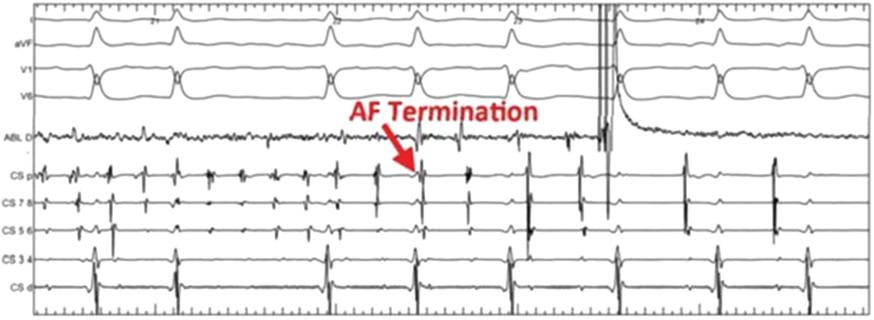
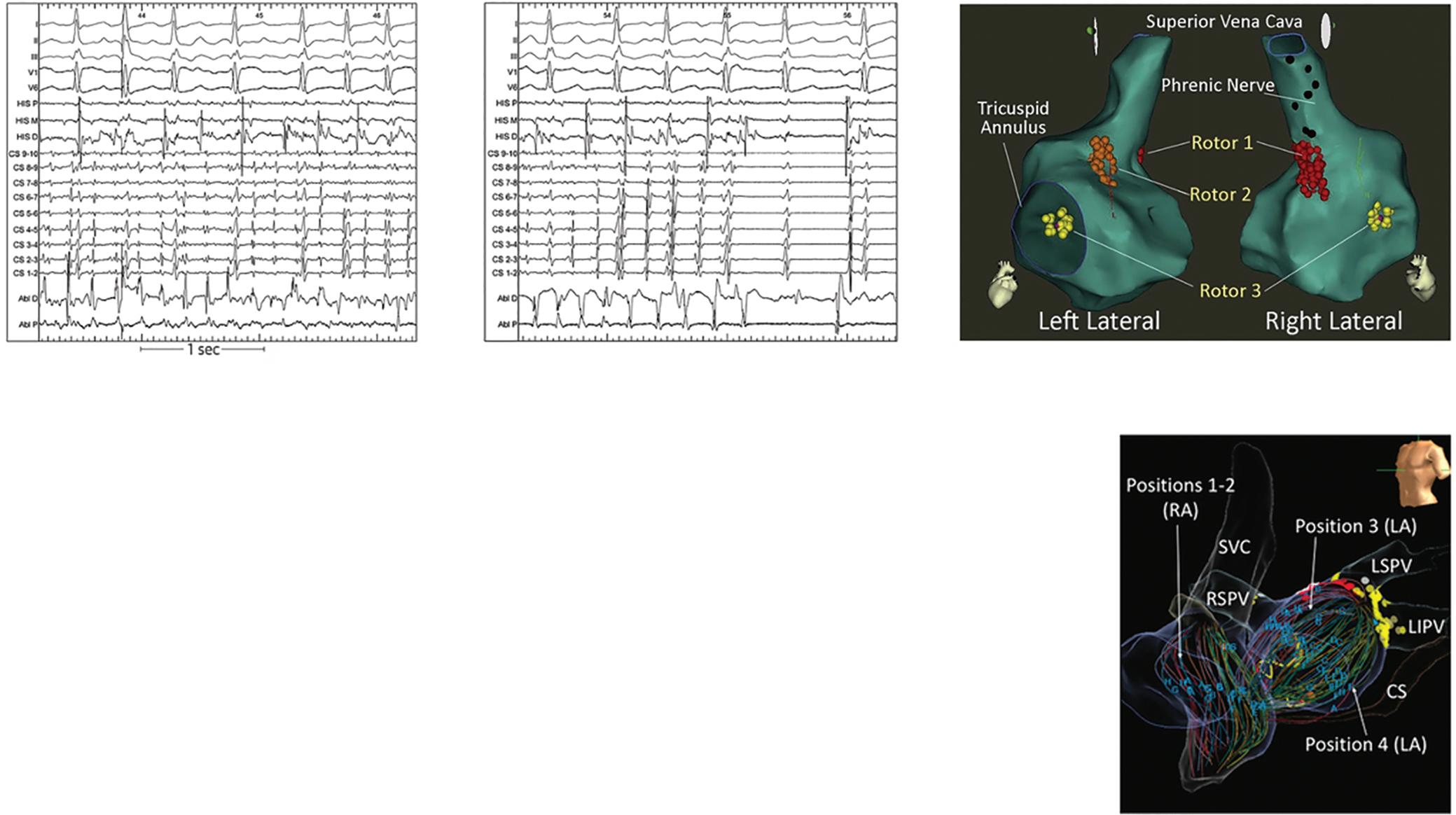
widely with random vector sizes and directions. Averaging cycles eliminates these random vectorial components, leaving consistent EGF vectors that dominate the final map.
EGF vectors may be consistent for several reasons. Flow vectors are constant at a given point if potentials originate from a local source, in which case they may travel in circular or spiral re-entry pathways, flow around a fixed anatomical obstacle, or follow a linear pathway. This principle of vector summation is a critical stated advantage over phase or activation mapping, where noise and artefacts cannot be easily eliminated by temporal averaging. The entirety of the resulting constant vector components is comprised in flow maps.
This approach has been proposed to distinguish primary (true) from secondary (passive) regions, although this has yet to be verified. Applied retrospectively to FIRM data, the approach identifies similar regions to FIRM including at sites where targeted ablation terminated AF.36 In that study, sites labelled as ‘active’ versus ‘passive’ did not identify sites of termination and non-termination. The commercially available system, Ablacon (Ablamap, Ablacon Inc.) is currently undergoing prospective evaluation to identify areas of importance for AF ablation.
Sequential High-density Contact Mapping CARTOFINDER
CARTOFINDER is the system from Biosense Webster integrated into current versions of the CARTO electroanatomic mapping system. CARTOFINDER uses combined unipolar and bipolar electrogram annotation to construct
high density activation maps using either a panoramic basket catheter (Constellation, Boston Scientific) in early studies, or a high-density PENTARAY (Biosense Webster) in more recent series.37–40
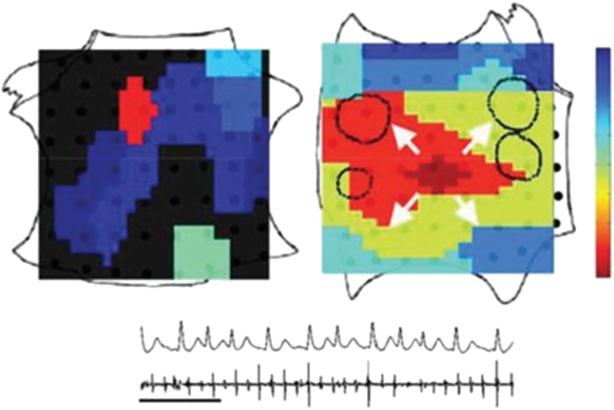
Mapping using CARTOFINDER can be integrated into routine clinical workflows, as it requires no additional equipment if one is already using CARTO for AF ablation if the software module is present.41 Mapping shows focal and rotational activation patterns as regions of interest (ROI).
Figure 3 shows a case of a 47-year-old man with new onset de novo persistent AF with extensive areas identified using CARTOFINDER pre-PVI, which were incorporated into the lesion set and ablated in addition after completion of PVI. Repeat mapping was performed to ensure modification of the ROI as an endpoint.
In recent series, there does not appear to be a relationship between these sites and low bipolar voltage as measured by the PENTARAY, in contrast to earlier work using basket catheters.41,42 Given the large number of institutions that use CARTO mapping as part of an AF ablation, more systematically collected data are emerging to guide mechanistic insight and tailored therapy approaches using this method.
Spatiotemporal Dispersion Mapping
In 2017 Seitz et al. described the use of spatiotemporal dispersion to identify areas of stable electrogram patterns across the splines of a high density PENTARAY catheter, which spanned the AF cycle length and on modelling were proposed to represent electrogram fingerprints of nearby
Challenges for AF Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
termination to sinus
(B) Focal,
FIRM ablation at LA focal source terminates AF RF O LA repetitive focal source in AF Right Atrium Left Atrium ECG CS 100 0 ms
(C)
Challenges for AF Mapping
AF Mapping Using PENTARAY Prior to Pulmonary Vein Isolation
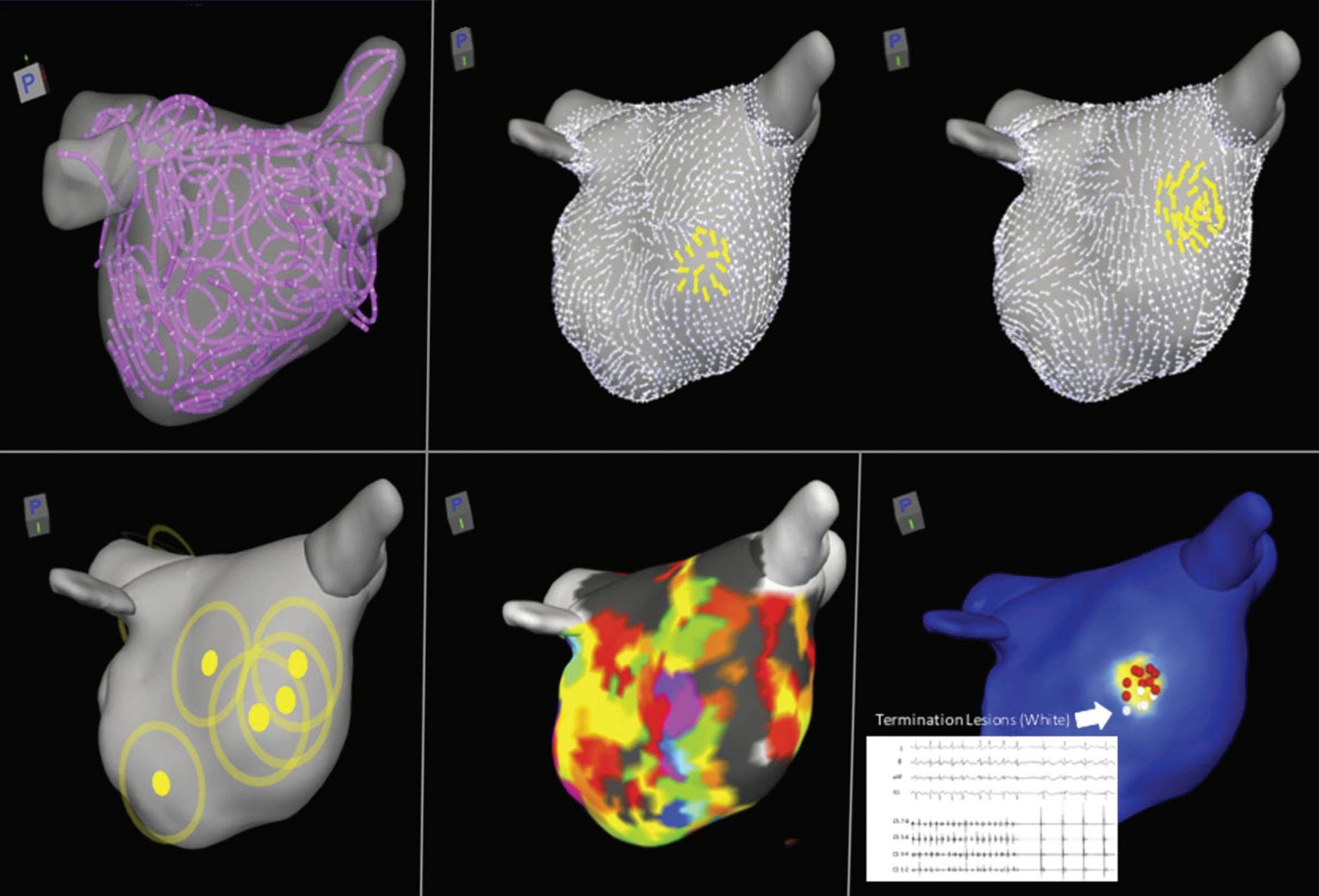
Left panel shows rotational activity (blue) spanning the junction of the right superior pulmonary vein and left atrial roof and focal activity (green) lies near the posterior antrum of the left pulmonary vein. Right panel shows the corresponding bipolar voltage map, with areas of heterogeneous voltage overlapping sites. White lines show planned modified pulmonary vein isolation lesion set to transect areas of driver and heterogeneous voltage, typically two to three targets per patient.
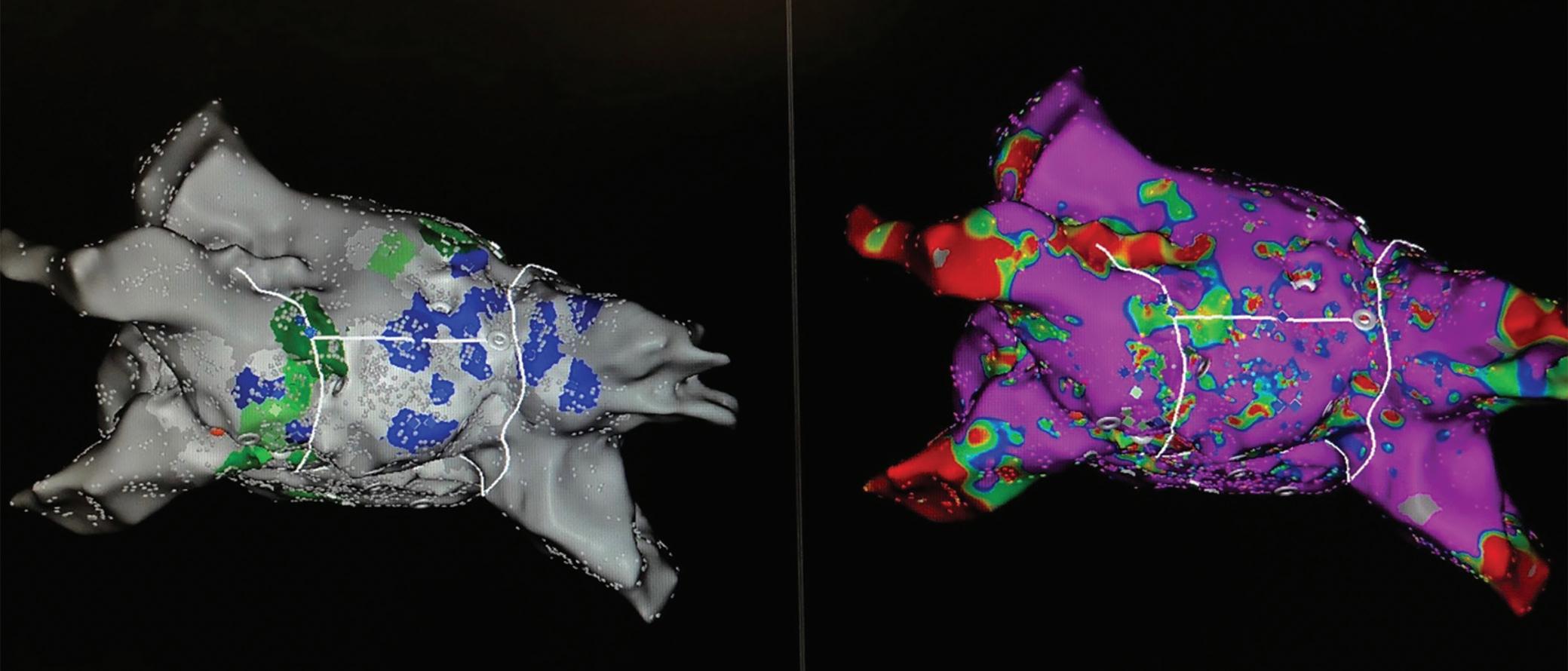
Using
A: Individual conduction vector maps for each phase; B: Combined to yield a driver density map; C: Voltage map collected during AF; D: Incorporated with this information to output the PADA map; E: AF successfully terminated with ablation (red and white dots) at the highlighted driver domain, typically approximately four per patient. LA = left atrium; PADA = probabilistic atrial driver assessment; RADAR = real-time electrogram analysis for drivers of AF. Source: Choudry et al. 2020.46 Reproduced with permission from Wolters Kluwer Health.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 3: CARTOFINDER
PADA
ablation lesions A B C D E High-density LA sampling Conduction vector phase 1
Conduction vector phase 2
Driver density map 7 rotors Voltage map
Figure 4: CardioNXT Mapping System
RADAR Mapping
map with
of 6
of 6
rotational drivers.43 Targeting these areas, without the need for activation assignment or annotation of electrograms, enabled high acute termination rates and high freedom from AF in a single-arm trial versus historical results from PVI only. Notably, the authors did not perform PVI in this series, with only spatiotemporal dispersion areas being targeted for ablation, but subsequent series have found it additive to PVI in terms of clinical efficacy.43,44
This approach has now been automated using artificial intelligence classification tools in a software system called Volta (Volta Medical), compatible with any multipolar catheter, currently under investigation in the Ev-AIFIB trial (NCT03434964) at eight sites in France.
Stochastic Trajectory Analysis of Ranked Signals
Stochastic trajectory analysis of ranked signals (STAR) mapping identifies atrial regions in AF that most often precede activation of neighbouring areas, calculated from multiple individual wavefront trajectories. By gathering data from hundreds of activations, a statistical model is formed, permitting regions of the atrium to be ranked by the amount of time that their activations precede those of adjacent regions.
The method imports electrogram signals, corresponding chamber geometry and catheter location data from electro-anatomical mapping systems. STAR maps allow the earliest sites of activity (ESA) to be identified on a digital replica of atrial geometry. For a site to be classified as an ESA it must lead for >75% of the time. STAR has been applied to panoramic contact baskets and as small multipolar mapping catheters. One
‘advantage’ of the approach is that it does not discriminate whether a site is rotational, focal or shows other activation patterns. The ablation strategy of targeting of areas of ESA is the same for each. In a recent single-centre study of 35 patients, after PVI an average of 2.6 ± 0.8 ESA were ablated. Out of the 86 STAR maps created post-PVI, the same ESA was identified on 73.8 ± 26.1% of maps. ESAs that resulted in AF termination were more likely to be identified on both pre- and post-PVI maps. During a follow-up of 18.5 ± 3.7 months, 28 (80%) patients were free from atrial tachycardia/AF.45 Additional data on this technique are pending.
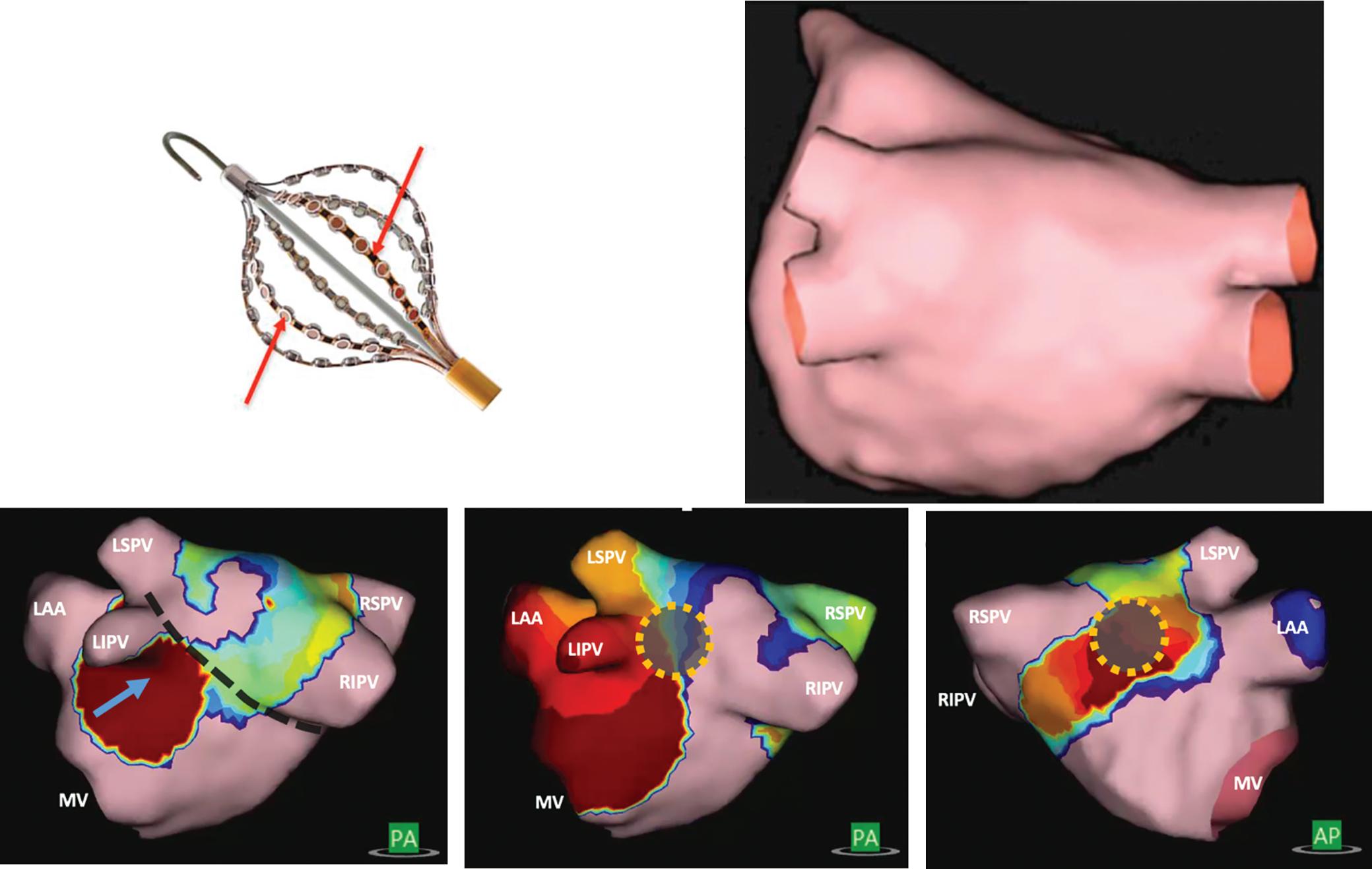
Real-time Electrogram Analysis for Drivers of AF
Another sequential high density mapping method for AF is termed realtime electrogram analysis for drivers of AF (RADAR). Using the coronary sinus as a reference, this system sorts and compiles electrograms from various anatomical locations to create a panoramic 3D conduction vector map that corresponds to each of a series of numerically calculated ‘phases’ or patterns of coronary sinus activation. Distance between electrodes is calculated using positions obtained from the electroanatomical mapping system. Using these distances and depolarisation timings at each electrode, conduction vector velocities are calculated between electrode pairs. An average of 587 electrode locations are used to map the chamber. Conduction vectors are computed using combinations of all of these electrode positions and depolarisation timings.
The commercial system (CardioNXT, CardioNXT.) computes sites of rotational activity and focal impulses from individual conduction vector maps, and combines them to create a driver density map (Figure 4). When
Challenges for AF Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
48 Low-impedance high-fidelity electrodes A C B 48 Ultrasound transducers 10 Fr shaft
Figure
5: Non-contact Mapping Using Charge Density
A: AcQMap catheter with 48 ultrasound transducers and electrodes; B: Ultrasound geometry created by rotation of the catheter in the centre of the chamber; C: Activation patterns projected on geometry by AcQMap are categorised as focal (left, with line of block in dash), localised rotational activation (middle) or localised irregular activity (right), typically two to three per patient. LAA = left atrial appendage; LIPV = left inferior pulmonary vein; LSPV = left superior pulmonary vein; MV = mitral valve; RIPV = right inferior pulmonary vein; RSPV = right superior pulmonary vein. Source: Upper panel: Willems et al. 2019.72 Reproduced with permission from Wolters Kluwer Health.
rotational or focal activity is detected in voltage border zones, the region is highlighted. These data are fused to create a single probabilistic atrial driver assessment map that incorporates repetition of drivers and areas of voltage transition to highlight putative AF driver domains, which can then be targeted for ablation.46
Initial results from ablation using this approach show 68% off-drug freedom from AF and 74% when on/off drug after a 13-month follow-up in 64 patients treated.
Non-contact Charge Density Mapping
Non-contact charge density mapping is an alternative approach to map non-stationary AF mechanisms, inspired by the non-contact technology used to map focal ventricular ectopics and appreciation of the membraneassociated charge origin of the cardiac electrical field.47
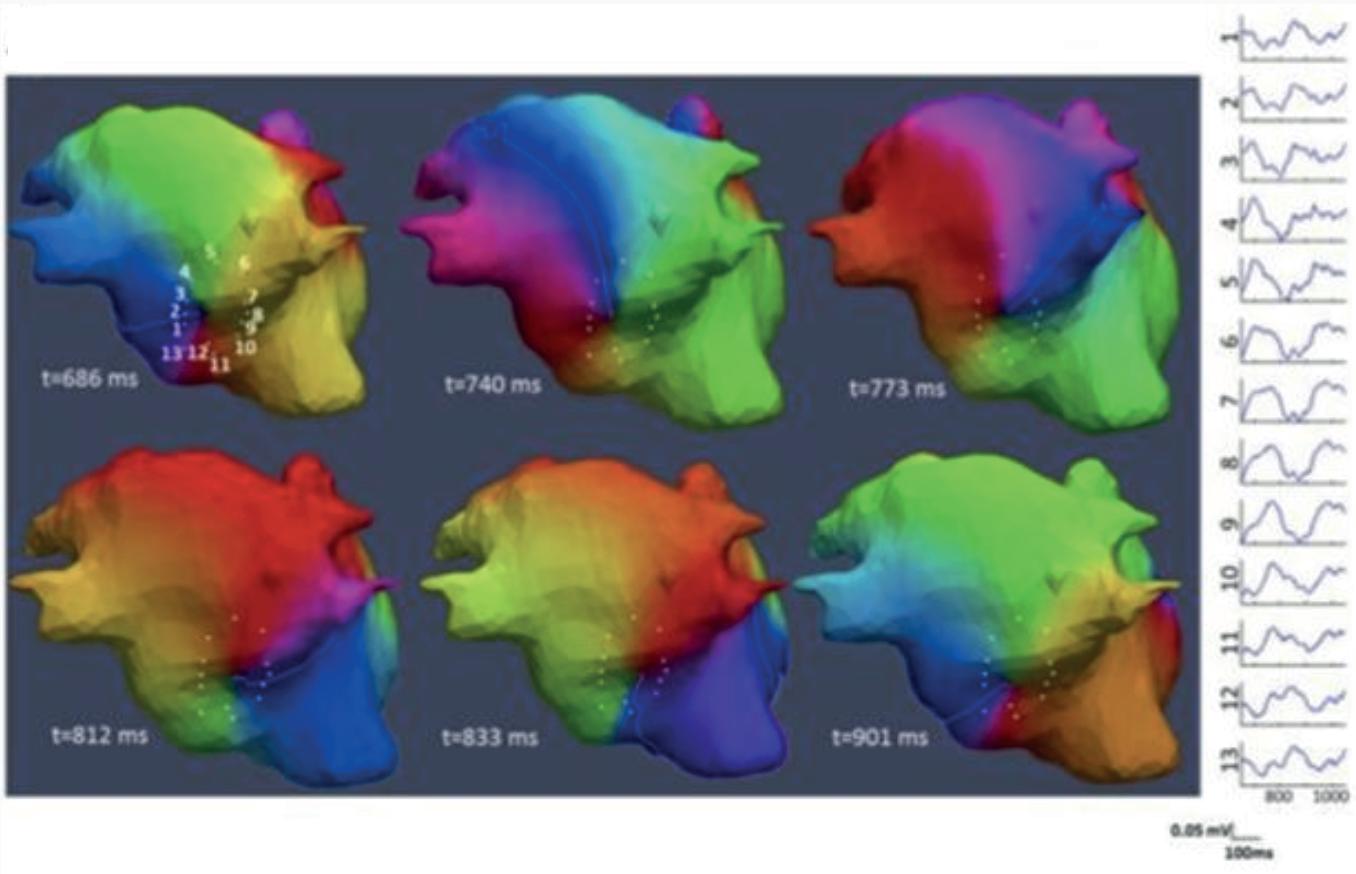
The AcQMap (Acutus Medical) catheter incorporates 48 ultrasound emitters used in real time to generate a 3D anatomy by rotation of the assembly in the centre of the atrial chamber (Figure 5). The physical principle that underpins this approach is that the charge-layer is the true source of the cardiac field, so that calculated charge density provides the most accurate source localisation. Unipolar electrograms (150 ks−1) are acquired and the charge density calculated at fixed times using a governing Poisson formulation and displayed as a movie on a dedicated console (Acutus).
for AF Mapping
The signals acquired provide acceptable correlations against contact electrograms (correlations >0.80).48,49 The system identifies areas in AF which exhibits localised rotational activity, focal beats and localised irregular activity, the most common patterns. Ablation at these areas has shown 72.3% freedom from AF at 12 months compared to PVI alone (Table 1
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Challenges for AF Mapping
) in an international multicentre series.50
Electrode vest measures ECG potentials A B Instrumentation setup Transverse CT images Heart–torso geometry CADIS (ECGI software) Body surface potentials 224-channel ECG Anterior Posterior Epicardial surface Potentials (mV) Electrograms (mV) Isochrones (ms) CT provides geometry
Figure 6: ECG Imaging Provides a Non-contact Workflow
A: Pre-procedure CT registers vest with surface torso anatomy. B: Aggregated density plots of driver activity, showing red areas of repetitive activity, typically four to six per patient. ECGI = electrocardiographic imaging. Source: A: Ramanathan et al. 2004.73 Reproduced with permission from Springer Nature. B: Haissaguerre et al. 2014.13 Reproduced with permission from Wolters Kluwer Health.
Phase maps of reconstructed (virtual) electrograms show singularity point on posterior left atrial shell, with reconstructed electrograms numbered 1–13; Source: Haïssaguerre et al. 2014.13 Reproduced with permission from Wolters Kluwer Health.
Figure 7: Phase Maps of Reconstructed Electrograms
Non-invasive Body Surface Mapping Electrocardiographic Imaging
The use of non-invasive body surface mapping using the inverse solution can resolve cardiac electrograms from multipolar ECGs acquired from the chest wall. The most studied approach clinically involves a 252-electrode surface vest (ECVUE, CardoInsight, Medtronic), which is placed on the patient before they undergo a non-contrast thoracic computed tomography scan to obtain high-resolution 3D images of the individual biatrial geometry and the relative electrode positions via segmentation.
The system reconstructs biatrial unipolar electrograms from torso potentials using mathematical computation. Activation maps are then computed by using the traditional unipolar electrogram intrinsic deflection-based (−dV/dTmax) method. Surrogates of the depolarisation and repolarisation wave fronts have also been computed from the isophase values equal, respectively, to π/2 and –π/2.13 Movies of activation and/or phase can then be used to guide ablation (Figures 6 and 7).
ECGI maps are reported to show ‘driver domains’ bi-atrially, which when targeted may reduce the complexity of AF leading ultimately to atrial tachycardias. This approach has been associated with higher freedom from AF compared to a historical stepwise ablation cohorts, although with up to a 50% recurrence rate with atrial tachycardia.13,51
Body Surface Mapping
A related technique uses body surface recording from a reduced 67 lead recording system without using CT scans to localise electrodes and has demonstrated singularity points on the body surface which become stable when filtered at the highest dominant frequency.52
Studies confirm the presence and detection of AF drivers using this approach when compared to panoramic basket catheters and ablation outcomes using this methodology either as a standalone or in combination with invasive mapping methods are awaited.53,54
Promising Novel Methods
The plethora of technologies seeking to identify AF mechanisms to guide ablation meet the unmet need to extend beyond PVI to treat patients with persistent AF. Another such system with similar early promising data is representation of electrical tracking or origin mapping, which uses a highdensity spiral catheter (AFOCUS II catheter, St Jude Medical) to identify spatiotemporal stability in persistent AF.55 Aside from novel mapping systems to visualise AF propagation, using an electrogram visualisation tool already available in CARTO (Ripple mapping), organised activation patterns detected by the Orion basket catheter in Rhythmia (Boston Scientific), consistent activation patterns detected on a PENTARAY and frequency analysis of electrograms have also been reported.56–60
1. Poole JE, Bahnson TD, Monahan KH, et al. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol 2020;75:3105–18. https://doi.org/10.1016/j.jacc.2020.04.065; PMID: 32586583.
2. Lee JM, Shim J, Park J, et al. The electrical isolation of the left atrial posterior wall in catheter ablation of persistent atrial fibrillation. JACC Clin Electrophysiol 2019;5:1253–61. https://doi.org/10.1016/j.jacep.2019.08.021; PMID: 31753429.
3. Verma A, Jiang C, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. https://doi.org/10.1056/NEJMoa1408288; PMID: 25946280.
4. Vogler J, Willems S, Sultan A, et al. Pulmonary vein isolation versus defragmentation: the CHASE-AF clinical trial. J Am Coll Cardiol 2015;66:2743–52. https://doi.org/10.1016/j. jacc.2015.09.088; PMID: 26700836.
Future Directions
Despite decades of research into fibrillatory propagation, clinical translation into therapy beyond PVI remains debated. Any AF mapping strategy must be explicable and confer additive value. Future AF mapping research must thus explain the interaction with pulmonary-vein-based mechanisms. Although AF termination is increasingly reported as an endpoint of these studies, it remains unclear whether this acute endpoint confers long term clinical benefit. How and why AF terminates in some patients but not others is an area we feel is vital towards developing a common vocabulary and framework to understand AF. Finally, as ablation technologies themselves are rapidly evolving to be more destructive yet safer, it is essential to develop robust endpoints and efficient approaches to map and ablate mechanistically important areas. We believe that better patient selection, by non-invasive imaging and body surface techniques, will help improve the impact of mapping studies in the future to those patients for whom an empirical lesion set, such as PVI is insufficient and better define substrate at an individual patient level.61
Conclusion
There is considerable interest in mapping AF to improve the results of anatomically based ablation in patients with persistent AF. A plethora of basic science as well as clinical studies support the role of spatial regions in sustaining AF. Studies are needed to clarify why ablating putative drivers produce widely differing results between centres, which has limited the ability of randomised trials to show benefit. The field could be advanced by identifying patients most likely to benefit from driver ablation, to standardise mapping and interpretation across centres, and to standardise strategies for ablation of potential drivers. Together, such studies may enable more precise map-based phenotyping of AF patients, and hence improved outcomes from ablation and pharmacological therapies.
Clinical Perspective
• Standard pulmonary vein isolation and empirical ablation strategies approach a ceiling of efficacy in persistent AF.
• AF mapping is increasingly used to identify putative areas of mechanistic importance that often reside beyond the pulmonary veins.
• Many mapping approaches exist, each with their own limitations and strengths.
• Similarities are emerging amongst the patterns observed between techniques and direct comparison to gold standard ex vivo techniques suggests patterns are real.
• Clinical outcome data are promising when compared to pulmonary vein isolation alone but yet to be confirmed in prospective randomised control trials.
5. Wong KCK, Paisey JR, Sopher M, et al. No benefit of complex fractionated atrial electrogram (CFAE) ablation in addition to circumferential pulmonary vein ablation and linear ablation: BOCA study. Circ Arrhythmia Electrophysiol 2015;8:1316–24. https://doi.org/10.1161/CIRCEP.114.002504; PMID: 26283145.
6. Heeger CH, Rillig A, Geisler D, et al. Left atrial appendage isolation in patients not responding to pulmonary vein isolation. Circulation 2019;139:712–5. https://doi.org/10.1161/ CIRCULATIONAHA.118.037451; PMID: 30689416.
7. Baykaner T, Rogers AJ, Meckler GL, et al. Clinical implications of ablation of drivers for atrial fibrillation. Circ Arrhythmia Electrophysiol 2018;11:e006119. https://doi. org/10.1161/CIRCEP.117.006119; PMID: 29743170.
8. Hansen BJ, Zhao J, Csepe TA, et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical
mapping in explanted human hearts. Eur Heart J 2015;36:2390–401. https://doi.org/10.1093/eurheartj/ehv233; PMID: 26059724.
9. Pandit SV, Jalife J. Rotors and the dynamics of cardiac fibrillation. Circ Res 2013;112:849–62. https://doi.org/10.1161/ CIRCRESAHA.111.300158; PMID: 23449547.
10. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628–36. https://doi.org/10.1016/j. jacc.2012.05.022; PMID: 22818076.
11. Miller JM, Kowal RC, Swarup V, et al. Initial independent outcomes from focal impulse and rotor modulation ablation for atrial fibrillation: multicenter FIRM registry. J Cardiovasc Electrophysiol 2014;25:921–9. https://doi.org/10.1111/jce.12474; PMID: 24948520.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Challenges for AF Mapping
12. Tomassoni G, Duggal S, Muir M, et al. Long-term follow-up of FIRM-guided ablation of atrial fibrillation: a single-center experience. J Innov Card Rhythm Manag 2015;6:2145–51. https://doi.org/10.19102/icrm.2015.061002
13. Haissaguerre M, Hocini M, Denis A, et al. Driver domains in persistent atrial fibrillation. Circulation 2014;130:530–8. https://doi.org/10.1161/CIRCULATIONAHA.113.005421; PMID: 25028391.
14. Buch E, Share M, Tung R, et al. Long-term clinical outcomes of focal impulse and rotor modulation for treatment of atrial fibrillation: a multicenter experience. Heart Rhythm 2016;13:636–41. https://doi.org/10.1016/j.hrthm.2015.10.031; PMID: 26498260.
15. Mohanty S, Gianni C, Trivedi C, et al. Impact of rotor ablation in non-paroxysmal AF patients: findings from the perprotocol population of the OASIS trial at long-term followup. Am Heart J 2018;205:145–8. https://doi.org/10.1016/j. ahj.2018.05.021; PMID: 30144981.
16. Elayi CS, Di Biase L, Bai R, et al. Identifying the relationship between the non-PV triggers and the critical CFAE sites post-PVAI to curtail the extent of atrial ablation in longstanding persistent AF. J Cardiovasc Electrophysiol 2011;22:119–205. https://doi.org/10.1111/j.1540-8167. 2011.02122.x; PMID: 21692897.
17. Moe GK, Abildskov JA. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J 1959;58:59–70. https://doi.org/10.1016/0002-870390274-1; PMID: 13661062.
18. Fenton FH, Cherry EM, Hastings HM, Evans SJ. Multiple mechanisms of spiral wave breakup in a model of cardiac electrical activity. Chaos 2002;12:852–92. https://doi. org/10.1063/1.1504242; PMID: 12779613.
19. Lee S, Sahadevan J, Khrestian CM, et al. Simultaneous bi-atrial high density (510–512 electrodes) epicardial mapping of persistent and long-standing persistent atrial fibrillation in patients: new insights into the mechanism of its maintenance. Circulation 2015;132:2108–17. https://doi. org/10.1161/CIRCULATIONAHA.115.017007; PMID: 26499963.
20. Hansen BJ, Csepe TA, Zhao J, et al. Maintenance of atrial fibrillation. Circ Arrhythmia Electrophysiol 2016;9:e004398. https://doi.org/10.1161/CIRCEP.116.004398; PMID: 27729340.
21. Vaidya D, Morley GE, Samie FH, Jalife J. Reentry and fibrillation in the mouse heart: a challenge to the critical mass hypothesis. Circ Res 1999;85:174–81. https://doi. org/10.1161/01.RES.85.2.174; PMID: 10417399.
22. Rappel -J, Zaman JABB, Narayan SM. Mechanisms for the termination of atrial fibrillation by localized ablation: Computational and clinical studies. Circ Arrhythmia Electrophysiol 2015;8:1325–33. https://doi.org/10.1161/ CIRCEP.115.002956; PMID: 26359479.
23. Kowalewski CAB, Shenasa F, Rodrigo M, et al. Interaction of localized drivers and disorganized activation in persistent atrial fibrillation. Circ Arrhythmia Electrophysiol 2018;11:e005846. https://doi.org/10.1161/CIRCEP.117.005846; PMID: 29884620.
24. Hansen BJ, Zhao J, Li N, et al. Human atrial fibrillation drivers resolved with integrated functional and structural imaging to benefit clinical mapping. JACC Clin Electrophysiol 2018;4:1501–15. https://doi.org/10.1016/j.jacep.2018.08.024; PMID: 30573112.
25. Brachmann J, Hummel JD, Wilber DJ, et al. Prospective randomized comparison of rotor ablation vs conventional ablation for treatment of persistent atrial fibrillation – the REAFFIRM trial. Presented at 40th Annual Scientific Sessions, Heart Rhythm 2019, San Francisco, CA, 9 May 2019. S-LBCT01-02. https://www.abstractsonline.com/ pp8/#!/5753/presentation/31210 (accessed 9 April 2022).
26. Ramirez FD, Birnie DH, Nair GM, et al. Efficacy and safety of driver-guided catheter ablation for atrial fibrillation: a systematic review and meta-analysis. J Cardiovasc Electrophysiol 2017;28:1371–8. https://doi.org/10.1111/jce.13313; PMID: 28800192.
27. Lin CY, Lin YJ, Narayan SM, et al. Comparison of phase mapping and electrogram-based driver mapping for catheter ablation in atrial fibrillation. Pacing Clin Electrophysiol 2019;42:216–23. https://onlinelibrary.wiley.com/doi/ abs/10.1111/pace.13573; PMID: 30536679.
28. Swarup V, Baykaner T, Rostamian A, et al. Stability of rotors and focal sources for human atrial fibrillation: focal impulse and rotor mapping (FIRM) of AF sources and fibrillatory conduction. J Cardiovasc Electrophysiol 2014;25;1284–92. https://doi.org/10.1111/jce.12559; PMID: 25263408.
29. Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:447–54. https://doi. org/10.1111/j.1540-8167.2012.02332.x; PMID: 22537106.
30. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart
Challenges for AF Mapping
Rhythm 2017;14:e275–444. https://doi.org/10.1016/j. hrthm.2017.05.012; PMID: 28506916.
31. Hocini M, Nault I, Wright M, et al. Disparate evolution of right and left atrial rate during ablation of long-lasting persistent atrial fibrillation. J Am Coll Cardiol 2010;55:1007–16. https://doi.org/10.1016/j.jacc.2009.09.060; PMID: 20202517.
32. Krummen DE, Baykaner T, Schricker AA, et al. Multicentre safety of adding focal impulse and rotor modulation (FIRM) to conventional ablation for atrial fibrillation. Europace 2017;19:769–74. https://doi.org/10.1093/europace/euw377; PMID: 28339546.
33. Narayan SM, Krummen DE, Enyeart MW, Rappel WJ. Computational mapping identifies localized mechanisms for ablation of atrial fibrillation. PLoS One 2012;7:e46034. https:// doi.org/10.1371/journal.pone.0046034; PMID: 23049929.
34. Alhusseini MI, Abuzaid F, Rogers AJ, et al. Machine learning to classify intracardiac electrical patterns during atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e008160. https://doi.org/10.1161/CIRCEP.119.008160; PMID: 32631100.
35. Bellmann B, Lin T, Ruppersberg P, et al. Identification of active atrial fibrillation sources and their discrimination from passive rotors using electrographical flow mapping. Clin Res Cardiol 2018;107:1021–32. https://doi.org/10.1007/s00392018-1274-7; PMID: 29744616.
36. Swerdlow M, Tamboli M, Alhusseini MI, et al. Comparing phase and electrographic flow mapping for persistent atrial fibrillation. Pacing Clin Electrophysiol 2019;42:499–507. https://doi.org/10.1111/pace.13649; PMID: 30882924.
37. Daoud EG, Zeidan Z, Hummel JD, et al. Identification of repetitive activation patterns using novel computational analysis of multielectrode recordings during atrial fibrillation and flutter in humans. JACC Clin Electrophysiol 2017 ;3:207–16. https://doi.org/10.1016/j.jacep.2016.08.001; PMID: 29759514.
38. Honarbakhsh S, Schilling RJ, Providencia R, et al. Automated detection of repetitive focal activations in persistent atrial fibrillation: validation of a novel detection algorithm and application through panoramic and sequential mapping. J Cardiovasc Electrophysiol 2019;30:58–66. https://doi. org/10.1111/jce.13752; PMID: 30255666.
39. Verma A, Sarkozy A, Skanes A, et al. Characterization and significance of localized sources identified by a novel automated algorithm during mapping of human persistent atrial fibrillation. J Cardiovasc Electrophysiol 2018;29:1480–8. https://doi.org/10.1111/jce.13742; PMID: 30230079.
40. Wolf M, Tavernier R, Zeidan Z, et al. Identification of repetitive atrial activation patterns in persistent atrial fibrillation by direct contact high-density electrogram mapping. J Cardiovasc Electrophysiol 2019;30:2704–12. https://doi.org/10.1111/jce.14214; PMID: 31588635.
41. Unland R, Bergau L, Hamriti M El, et al. Find me if you can: first clinical experience using the novel CARTOFINDER algorithm in a routine workflow for atrial fibrillation ablation. J Clin Med 2021;10:2979. https://doi.org/10.3390/ jcm10132979; PMID: 34279463.
42. Honarbakhsh S, Schilling RJ, Dhillon G, et al. A novel mapping system for panoramic mapping of the left atrium: application to detect and characterize localized sources maintaining atrial fibrillation. JACC Clin Electrophysiol 2017;4:124–34. https://doi.org/10.1016/j.jacep.2017.09.177; PMID: 29387810.
43. Seitz J, Bars C, Théodore G, et al. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: a wholly patient-tailored approach. J Am Coll Cardiol 2017;69:303–21. https://doi.org/10.1016/j. jacc.2016.10.065; PMID: 28104073.
44. Qin M, Jiang WF, Wu SH, et al. Electrogram dispersionguided driver ablation adjunctive to high-quality pulmonary vein isolation in atrial fibrillation of varying durations. J Cardiovasc Electrophysiol 2020;31:48–60. https://doi. org/10.1111/jce.14268; PMID: 31701626.
45. Honarbakhsh S, Hunter RJ, Ullah W, et al. ablation in persistent atrial fibrillation using stochastic trajectory analysis of ranked signals (STAR) mapping method. JACC Clin Electrophysiol 2019;5:817–29. https://doi.org/10.1016/j. jacep.2019.04.007; PMID: 31320010.
46. Choudry S, Mansour M, Sundaram S, et al. RADAR: a multicenter Food and Drug Administration investigational device exemption clinical trial of persistent atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e007825. https://doi. org/10.1161/CIRCEP.119.007825; PMID: 31944826.
47. Schilling RJ, Peters NS, Davies DW. Simultaneous endocardial mapping in the human left ventricle using a noncontact catheter: comparison of contact and reconstructed electrograms during sinus rhythm. Circulation 1998;98:887–98. https://doi.org/10.1161/01.CIR.98.9.887; PMID: 9738644.
48. Grace A, Willems S, Meyer C, et al. High-resolution noncontact charge-density mapping of endocardial
activation. JCI Insight 2019;4:e126422. https://doi.org/10.1172/ jci.insight.126422; PMID: 30895945.
49. Shi R, Parikh P, Chen Z, et al. Validation of dipole density mapping during atrial fibrillation and sinus rhythm in human left atrium. JACC Clin Electrophysiol 2020;6:171–81. https://doi. org/10.1016/j.jacep.2019.09.012; PMID: 32081219.
50. Willems S, Verma A, Betts TR, et al. Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping: UNCOVER AF trial. Circ Arrhythm Electrophysiol 2019;12:e007233. https://doi. org/10.1161/CIRCEP.119.007233; PMID: 31242746.
51. Knecht S, Sohal M, Deisenhofer I, et al. Multicentre evaluation of non-invasive biatrial mapping for persistent atrial fibrillation ablation: the AFACART study. Europace 2017;19:1302–9. https://doi.org/10.1093/europace/euw168; PMID: 28204452.
52. Rodrigo M, Guillem MS, Climent AM, et al. Body surface localization of left and right atrial high-frequency rotors in atrial fibrillation patients: a clinical-computational study. Heart Rhythm 2014;11:1584–91. https://doi.org/10.1016/j. hrthm.2014.05.013; PMID: 24846374.
53. Rodrigo M, Climent AM, Hernández-Romero I, et al. Noninvasive assessment of complexity of atrial fibrillation: correlation with contact mapping and impact of ablation. Circ Arrhythm Electrophysiol 2020;13:e007700. https://doi. org/10.1161/CIRCEP.119.007700; PMID: 32078374.
54. Yamashita S, Shah AJ, Mahida S, et al. Body surface mapping to guide atrial fibrillation ablation. Arrhythmia Electrophysiol Rev 2015;4:172–6. https://doi.org.10.154220/ aer.2015.4.3.172; PMID: 26835121.
55. Mann I, Linton NWF, Coyle C, et al. RETRO-MAPPING: a new approach to activation mapping in persistent atrial fibrillation reveals evidence of spatiotemporal stability. Circ Arrhythm Electrophysiol 2021;14:e009601. https://doi. org/10.1161/CIRCEP.121.009602; PMID: 33999670.
56. Zaman JAB, Kowalewski CAB, Narayan SM. Mapping ripples or waves in atrial fibrillation? J Cardiovasc Electrophysiol 2017;28:383–5. https://doi.org/10.1111/jce.13181; PMID: 28185356.
57. Takahashi Y, Iwai S, Yamashita S, et al. Novel mapping technique for localization of focal and reentrant activation during atrial fibrillation. J Cardiovasc Electrophysiol 2017;28:375–82. https://doi.org/10.1111/jce.13163; PMID: 28063269.
58. Lațcu DG, Enache B, Hasni K, et al. Sequential ultrahighdensity contact mapping of persistent atrial fibrillation: an efficient technique for driver identification. J Cardiovasc Electrophysiol 2021;32:29–40. https://doi.org/10.1111/jce.14803; PMID: 33155347.
59. Haïssaguerre M, Hocini M, Sanders P, et al. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation 2006;113:616–25. https://doi.org/10.1161/ CIRCULATIONAHA.105.546648; PMID: 16461833.
60. Lin YJ, Lo MT, Lin C, et al. Prevalence, characteristics, mapping, and catheter ablation of potential rotors in nonparoxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2013;6:851–8. https://doi.org/10.1161/CIRCEP.113.000318; PMID: 23983246.
61. Al-Kaisey AM, Parameswaran R, Kalman JM. Atrial fibrillation structural substrates: aetiology, identification and implications. Arrhythmia Electrophysiol Rev 2020;9:113–20. https://doi.org/10.15420/aer.2020.19; PMID: 33240506.
62. Miller JM, Kalra V, Das MK, et al. Clinical benefit of ablating localized sources for human atrial fibrillation: the Indiana University FIRM registry. J Am Coll Cardiol 2017;69:1247–56. https://doi.org/10.1016/j.jacc.2016.11.079; PMID: 28279291.
63. Spitzer SG, Károlyi L, Rämmler C, et al. treatment of recurrent non-paroxysmal atrial fibrillation using focal impulse and rotor mapping (FIRM)-guided rotor ablation: early recurrence and long-term outcomes. J Cardiovasc Electrophysiol 2017;28:31–8. https://doi.org/10.1111/jce.13110; PMID: 27766704.
64. Calvo D, Rubin J, Perez D, Morís C. Ablation of rotor domains effectively modulates dynamics of human longstanding persistent atrial fibrillation. Circ Arrhythmia Electrophysiol 2017;10:e005740. https://doi.org/10.1161/ CIRCEP.117.005740; PMID: 29254947.
65. NCT02825992. Utilizing novel dipole density capabilities to objectively visualize the etiology of rhythms in atrial fibrillation (UNCOVER-AF). ClinicalTrials.gov. 2020. https:// clinicaltrials.gov/ct2/show/NCT02825992 (accessed 14 January 2022).
66. Rodrigo M, Climent AM, Liberos A, et al. Technical considerations on phase mapping for identification of atrial reentrant activity in direct- and inverse-computed electrograms. Circ Arrhythm Electrophysiol 2017;10:e005008. https://doi.org/10.1161/CIRCEP.117.005008; PMID: 28887361.
67. Eckstein J, Verheule S, de Groot N, et al. Mechanisms of perpetuation of atrial fibrillation in chronically dilated atria. Prog Biophys Mol Biol 2008;97:435–51. https://doi.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
org/10.1016/j.pbiomolbio.2008.02.019; PMID: 18378284.
68. de Groot N, van der Does L, Yaksh A, et al. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circ Arrhythm Electrophysiol 2016;9:e003648. https://doi.org/10.1161/CIRCEP.115.003648; PMID: 27103089.
69. Hansen BJ, Zhao J, Helfrich KM, et al. Unmasking arrhythmogenic hubs of reentry driving persistent atrial fibrillation for patient-specific treatment. J Am Heart Assoc 2020;9:e017789. https://doi.org/10.1161/JAHA.120.017789; PMID: 33006292.
Challenges for AF Mapping
70. Narayan SN, Shivkumar K, Krummen DE, et al. Panoramic electrophysiology mapping but not electrogram morphology identifies stables sources for human atrial fibrillation: stable atrial fibrillation rotors and focal sources relate poorly to fractionated electrograms. Circ Arrhythm Electrophysiol 2013;6:58–67. https://doi.org/10.1161/CIRCEP.111.977264; PMID: 23392583.
71. Zaman JAB, Baykaner T, Clopton P, et al. Recurrent postablation paroxysmal atrial fibrillation shares substrates with persistent atrial fibrillation: an 11-center study. JACC Clin Electrophysiol 2017;3:393–402. https://doi.org/10.1016/j.
jacep.2016.10.006; PMID: 28596994.
72. Willems S, Verma A, Betts TR, et al. Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping. Circ Arrhythm Electrophysiol 2019;12:e007233. https://doi.org/10.1161/ CIRCEP.119.007233; PMID: 31242746.
73. Ramanathan C, Ghanem RN, Jia P, et al. Noninvasive electrocardiographic imaging for cardiac electrophysiology and arrhythmia. Nat Med 2004;10:422–8. https//:doi. org/10.1038/nm1011; PMID: 15034569.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Association Between Left Atrial Appendage Morphology and Function and the Risk of Ischaemic Stroke in Patients with Atrial Fibrillation
Katarzyna Dudzińska-Szczerba
Abstract
AF is the most common cardiac arrhythmia and has been identified as an independent risk factor for stroke. The European Society of Cardiology guidelines recommend a thromboembolic event risk assessment based on the CHA2DS2-VASc score. However, stroke also occurs in some patients with a low CHA2DS2-VASc score. Therefore, it is necessary to find new factors to improve thromboembolic risk stratification in AF patients. Over 90% of embolic strokes are caused by thrombi originating from the left atrial appendage (LAA). Thus, certain anatomical or functional parameters of the LAA could potentially be used to predict cardioembolic stroke. Studies have suggested that some of these factors, such as LAA morphology, number of LAA lobes, LAA dimensions, LAA volume, distance from the LAA ostium to the first bend of LAA, LAA orifice diameter, extent of LAA trabeculations, LAA takeoff, LAA flow velocity and LAA strain rate, are independently associated with a higher risk of stroke in a population of patients with AF and improve the performance of the CHA2DS2-VASc score. However, the results are conflicting and, so far, no new parameter has been added to the CHA2DS2-VASc score.
Keywords
Left atrium appendage, AF, ischaemic stroke
Disclosure: The authors have no conflicts of interest to declare.
Received: 5 February 2022
Accepted: 22 April 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e09. DOI: https://doi.org/10.15420/aer.2022.08
Correspondence: Katarzyna Dudzińska-Szczerba, Department of Cardiology, Centre of Postgraduate Medical Education, Grochowski Hospital, Grenadierów 51/59, 04-073 Warsaw, Poland. E: katarzyna.dudzinska27@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
AF is the most common cardiac arrhythmia.1 AF has been estimated to occur in 20.9 million men and 12.6 million women worldwide, with a higher incidence and prevalence rates in developed countries.2 Estimates suggest an AF prevalence of approximately 3% in adults, with greater prevalence in older persons and in patients with underlying conditions, such as hypertension, heart failure, coronary artery disease, valvular heart disease, obesity, diabetes or chronic kidney disease.1,2 AF is an increasingly serious health problem in the ageing population. Regardless of the presence of comorbidities, AF is associated with a twofold increased risk of all-cause mortality in women and a 1.5-fold increased risk in men.2 AF has been identified as an independent risk factor for stroke, increasing the incidence fivefold.3
Stroke is the leading cause of long-term disability and is associated with high healthcare costs worldwide.4–6 It has been estimated that 20% of ischaemic strokes are caused by cardiac embolism, most often in the course of AF.7 According to the guidelines, it is recommended that the risk of thromboembolic complications is assessed on the basis of the CHA2DS2VASc score.1,2,8 However, it is known that the CHA2DS2-VASc score identifies some, and not all, patients with an elevated risk of stroke or peripheral embolism.9,10 Despite a low CHA2DS2-VASc score, a patient may still experience stroke.11,12 In a meta-analysis from 2016, the summary
estimate for the annual risk of ischaemic stroke was 1.61% (95% CI [0–3.23%]) for a CHA2DS2-VASc score of 1 and 0.68% (95% CI [0.12–1.23%]) for a CHA 2 DS 2 -VASc score of 0.13
The temporal relationship between stroke and AF is also an interesting issue. The current guidelines combine all types of AF with respect to anticoagulation, the main determinants of which are comorbidities that translate into risk markers.1,2,8,14 In the ASSERT trial, the temporal relationship of an episode of AF with the onset of stroke was unclear; very few patients had subclinical AF in the month before their event.15 This raises the question whether AF, rather than being a contributory factor, causes changes in atrial structure and endothelial function that are associated with the risk of stroke.15,16 Among patients in clinical trials, those with non-paroxysmal AF appear to have a greater risk of stroke than those with paroxysmal AF.14 Moreover, continuous monitoring of AF with cardiac implantable devices has provided us with the concept of the ‘AF burden’. Typically, the greater the AF burden, the greater the risk of stroke; however, this relationship is not well characterised in relation to the threshold above which the risk increases.14 In large cohorts of patients with cardiac implantable electronic devices and continuous rhythm monitoring, stroke risk increased the most in days 1–5 following an AF of ≥5.5 hours in duration, and diminished rapidly thereafter.17 In addition, AF
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Atrial Fibrillation
, 1 Piotr Kułakowski , 2 Ilona Michałowska 3 and Jakub Baran 4
1. Department of Cardiology, Centre of Postgraduate Medical Education, Grochowski Hospital, Warsaw, Poland; 2. Division of Clinical Electrophysiology, Department of Cardiology, Centre of Postgraduate Medical Education, Grochowski Hospital, Warsaw, Poland;
3. Department of Radiology, Institute of Cardiology, Warsaw, Poland; 4. Division of Clinical Electrophysiology, Department of Cardiology, Centre of Postgraduate Medical Education, Grochowski Hospital, Warsaw, Poland
episodes lasting >23 hours on a given day were associated with the highest risk of stroke.17 This indicates the complexity of the variables that may be associated with the risk of AF-related stroke.
Imaging studies are revealing the more patients with no history of transient ischaemic attack (TIA) or stroke have experienced cerebral infarction, and this is associated with an improved availability and quality of imaging techniques. Silent cerebral ischaemia (SCIs) is fivefold more frequent than stroke in the general population.18 Despite the absence of clinically significant symptoms of stroke, SCIs are associated with the occurrence and progression of neurological and cognitive deficits that commonly go unnoticed. Furthermore, the presence of silent infarcts more than doubled the risk of later stroke and dementia.18 MRI of the brain has shown a high incidence of cerebral ischaemia in asymptomatic patients with AF. Based on brain MRI studies, SCI is found in 92% of patients with persistent AF, and in 89% of patients with paroxysmal AF.19 However, in patients without AF, SCI occurs almost half as often, only in 46% of patients.19
Over 90% of embolic strokes are caused by thrombi originating from the left atrial appendage (LAA).20 For this reason, certain anatomical or functional parameters of the LAA could potentially be used to predict cardioembolic strokes and improve the performance of the CHADS2 and CHA 2 DS 2 -VASc scores.
Anatomy of the Left Atrial Appendage
The LAA is a small, finger-like extension of the left atrium (LA). The LAA extends primarily between the anterior and lateral walls of the LA, parallel to the left pulmonary veins. The tip of the LAA is usually directed anterosuperiorly, overlapping the left border of the right ventricular outflow tract or the pulmonary trunk and the main stem of the left coronary or circumflex artery. However, it is not uncommon to find the tip of the LAA directed laterally and backwards (Figure 1).21,22 Despite a highly trabeculated endocardium, the LAA wall is very thin (~1 mm). The orifice of the LAA is usually oval. Round, triangular and water drop shapes are observed less frequently. A prominent ridge separates the orifice of the left pulmonary veins from the orifice of LAA, as well as the smooth
muscular wall of the LA from the mitral annulus. The orifice leads to the LAA neck, which opens into the lobulated body. The LAA varies in size, number of lobes, shape, ostium and dimensions.22 In a large study of postmortem hearts, Veinot et al. defined a lobe as a visible outpouching from the main tubular body of the LAA, usually demarcated by an external crease, with the tail itself also representing a lobe, although bends in the tail do not constitute new lobes.23 Veinot et al. found that two lobes were most common, with the number of lobes ranging from one to four, with the prevalence of one, two, three and four lobes being 20%, 54%, 23% and 3%, respectively.23 In 2010, Wang et al. classified the LAA morphology into four types based on CT and cardiac MRI – chicken wing, cauliflower, cactus and windsock (Figure 2).24
Function of the Left Atrial Appendage
The LAA has important neurohormonal and mechanical functions. The LAA has contractile properties, and its distensibility is larger than that of the LA. The endothelial cells of the LAA produce natriuretic peptides, namely atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP). Due to the greater extensibility and higher concentration of ANP in the LAA than in the LA, the LAA helps modulate LA pressure.25 Because natriuretic peptides play an important role in regulating fluid balance, filling the vascular bed and feeling thirsty, elimination of the LAA may impair this physiological regulation. Currently, there are limited data regarding the effect of LAA closure on plasma concentrations of natriuretic peptides.26–28 Experimental studies have shown that the mechanical activity of the LAA has no apparent effect on cardiac output, and therefore LAA occlusion or excision will not have any significant haemodynamic consequences.25
Undoubtedly, neurohormonal changes play a key role in the development of LA remodelling. Moreover, they also constitute the link between morphological changes and electrophysiological abnormalities that favour the triggering and maintenance of AF, creating a vicious circle between atrial cardiomyopathy and arrhythmias. Increased levels of a number of neurohormones, including ANP, BNP, angiotensin II and transforming growth factor-β1, are related to the relationship between atrial cardiomyopathy and arrhythmias.29 Mechanical stretching of the LA is the strongest factor stimulating increased ANP secretion.30 Two paracrine factors derived from endothelial cells play important roles in modulating ANP secretion, with endothelin enhancing ANP secretion and nitric oxide reducing it.30 ANP is a direct vasodilator that lowers blood pressure and inhibits the effect of renin, endothelin secretion, myocyte hypertrophy and collagen synthesis by fibroblasts.29 AF increases ANP concentrations, primarily as a result of haemodynamic effects, which indicates that the increase in ANP is an adaptive response to changing haemodynamic conditions in order to prevent remodelling.29,31
It has been reported that patients with a longer duration of AF have lower plasma ANP concentrations, suggesting that the reduction in ANP secretion in this population is most likely due to advanced atrial degenerative changes.32 BNP is an indicator of LA and left ventricular remodelling. Increased production of BNP occurs in response to increased stress on the heart wall as a result of volume and/or pressure overload. Such conditions are met in various phases of AF-related remodelling. In patients with AF, levels of BNP and N-terminal pro B-type natriuretic peptide are correlated with the type, and therefore duration, of arrhythmia.33 After electrical cardioversion of AF, a rapid decrease in BNP concentrations is observed, which may be the beginning, and also a marker, of reverse remodelling.29 It can be said that in the first phase of AF, biochemical remodelling takes place, which heralds structural remodelling.29
LAA Morphology and Function and Stroke Risk in AF Patients ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 1: CT of the Heart Showing the Anatomy of the Left Atrium Appendage and its Relationship with Adjacent Structures
ALMV = anterior leaflet of the mitral valve; LA = left atrium; LAA = left atrium appendage; LV = left ventricle; LVOT = left ventricular outflow tract; PLMV = posterior leaflet of the mitral valve.
Angiotensin II plays a key role in the pathogenesis of AF-related remodelling by stimulating interstitial fibrosis. Angiotensin II may also mediate thrombus formation by interacting with thromboxane receptors and via nitric oxide- and prostacyclin-dependent mechanisms.29
It has been shown that in patients with AF, the endocardium of the LAA exhibits an elevated expression profile of prothrombotic and proinflammatory proteins compared with the right atrial appendage (RAA), indicating increased thrombogenicity of the LAA compared with RAA.34 This may explain, at least in part, the observation that most clots are formed in the LAA in patients with AF.34
Virchow’s triad is a group of well-documented factors explaining the pathophysiological mechanism predisposing individuals to the development of venous thrombosis.35 LA thrombosis likely has a similar background, with slightly different factors leading to its occurrence. Structural remodelling of the LA goes hand-in-hand with functional remodelling of the LA, which is primarily manifested by deterioration of contractility of the LA and LAA. A major indicator of functional LA remodelling is a reduction in blood flow velocity within the LAA. The lack of LAA contractility associated with AF leads to blood stagnation, which promotes the formation of a thrombus. There are several mechanisms during AF that promote thrombogenesis: endocardial damage by atrial dilatation, endocardial denudation, fibroelastic infiltration of the extracellular matrix, haemostatic and platelet activation and growth factor changes during AF. All these factors complete the triad.25
Morphological and Haemodynamic Features of the Left Atrial Appendage and the Risk of a Thromboembolic Event
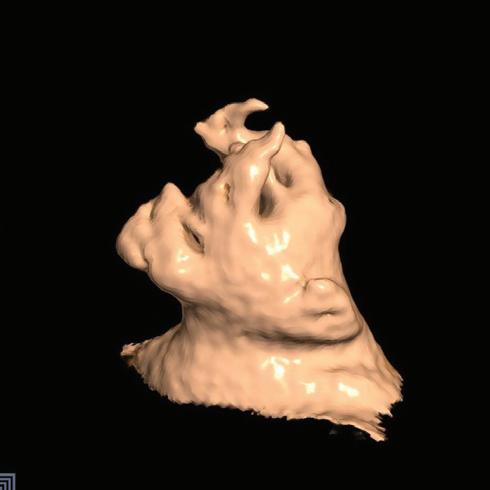
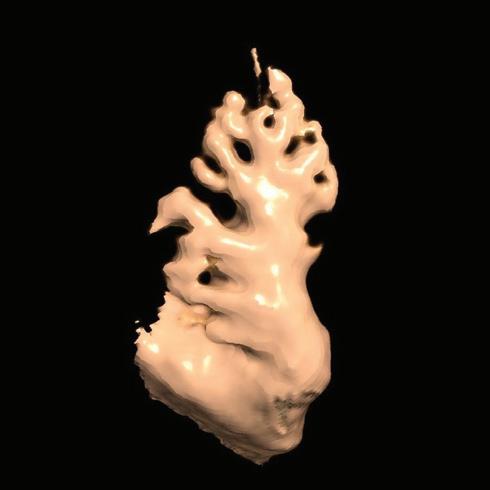
The LAA is a major thromboembolic source in patients with AF. Therefore, many studies have assessed the risk of stroke by analysing morphological and haemodynamic features of the LAA (Supplementary Material Table 1). There are three known morphological features of the LAA associated with ischaemic stroke: shape, size and fibrosis.36
A specific morphology of the LAA has been reported to be related to stroke in patients with AF. In 2012, Di Biase et al. studied the LAA by CT and MRI to classify different LAA morphologies and correlate the morphology with the history of embolic cerebrovascular events in patients with AF.37 The prevalence rate of stroke or TIA among the study population was 12% for those with a cactus morphology, 4% for those with a chicken wing morphology, 10% for those with a windsock morphology and 18% for those with a cauliflower morphology (p=0.003). The study showed that patients with a chicken wing morphology were less likely to have an embolic event after adjustment for comorbidities and CHADS2 score, whereas the cauliflower morphology appeared to be the most frequently associated with thromboembolism.37
Anselmino et al. investigated the correlation between LAA morphology studied by MRI or CT and the SCI detected by cerebral MRI in patients with AF undergoing transcatheter ablation (SCI was detected in 84.8% of patients).38 That study showed that LAA morphology was associated with SCI in AF patients. Patients with a chicken wing LAA morphology had a significantly lower risk of SCI, whereas those with windsock or cauliflower LAA morphology were at an increased risk of SCI.38 Lupercio et al. conducted the first meta-analysis, including eight studies and 2,596 patients (84% with a CHADS 2 score <2), to determine thromboembolic event risk for each LAA morphology in patients with AF with low to intermediate risk.39 The authors showed that chicken wing LAA morphology was associated with a lower risk of thromboembolic events than non-chicken wing morphology.39 This was
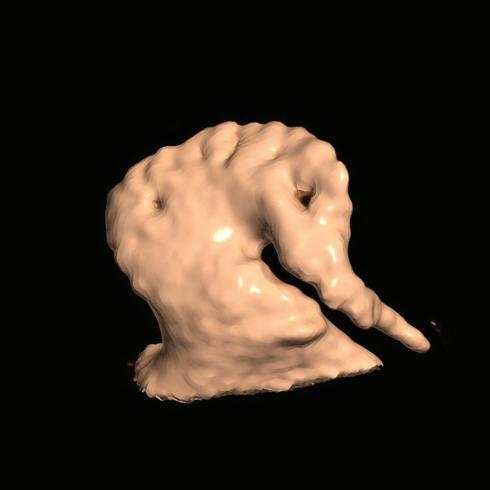
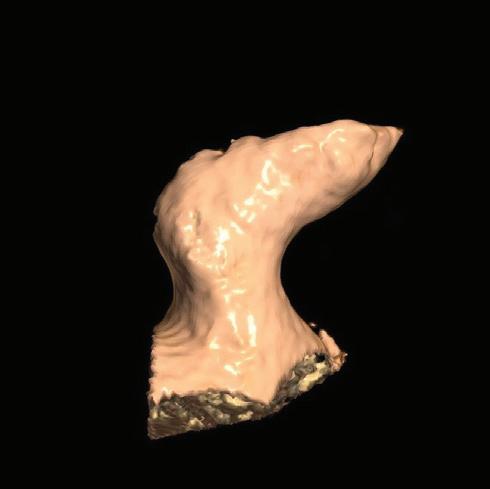
confirmed by other authors.40 In another study, Wang et al. showed that the number of LAA lobes is an independent risk factor and has a moderate predictive value for LA thrombus and LA spontaneous echo contrast among patients with AF factors.41
In addition to LAA shape, LAA size is also thought to play a role as a predictor of stroke. LAA dimensions assessed using multiple imaging modalities have been directly correlated with thromboembolic risk. For example, Beinart et al. showed that higher LAA volume (mean [± SD] 22.9 ± 9.6 cm3 versus 14.5 ± 7.1 cm3; p<0.001), larger LAA depth (3.76 ± 0.9 cm versus 3.21 ± 0.8 cm; p=0.006) and long (3.12 ± 0.7 cm versus 2.08 ± 0.7 cm; p<0.001) and short axes (2.06 ± 0.5 cm versus 1.37 ± 0.4 cm; p<0.001) of the LAA neck were associated with an increased risk of a history of stroke, and that LAA neck measurements (short axis × long axis) had the strongest correlation with stroke risk.42 Burrell et al. focused on the LAA volume, measured with MRI, in AF patients with or without a history of stroke.43 In that study, patients with a history of stroke had larger LAA mean volumes than control subjects (28.8 ± 13.5 cm3 versus 21.7 ± 8.27 cm3; p=0.002), and stroke risk was highest in patients with an LAA volume >34 cm3 43 In contrast, Di Biase et al. did not find the size of LAA to be a predictor of stroke.37
A larger LAA orifice area has also been shown to be associated with ischaemic stroke.44–46 Lee et al. found that patients with stroke had a larger LAA orifice area (mean ± SD 4.5 ± 1.5 cm2 versus 3.0 ± 1.1 cm2; p<0.001) and showed that LAA size was the most powerful predictor of stroke in patients with low CHA2DS2-VASc scores.44,45
Khurram et al. studied the association of LAA morphology and the extent of trabeculations, as well as orifice diameter and length, with prevalent stroke.47 The extent of LAA trabeculations (27.7% versus 14.4%; p=0.019) and a smaller LAA orifice diameter (mean [± SD] 2.26 ± 0.52 cm versus 2.78 ± 0.71 cm; p<0.001) were associated with a higher risk of stroke, and this may explain the association between cauliflower LAA morphology and stroke.47
LAA Morphology and Function and Stroke Risk in AF Patients ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
A B C D
Figure 2: Four Different Left Atrium Appendage Morphologies as Shown by CT
A: Chicken wing; B: Cauliflower; C: Cactus; D: Windsock.
The orientation of the LAA to adjacent cardiac structures has also been shown to be an additional risk factor for the presence of thrombus. Nedios et al. found an association between a higher LAA take-off (i.e. higher than the left superior pulmonary vein) with a tachycardia-mediated thrombogenic flow and an increased thromboembolic risk in patients with AF and a low CHA 2 DS 2 -VASc score.48 In another study, Dudzińska-Szczerba et al. showed that the distance from the LAA ostium to the first bend of the LAA was independently associated with stroke risk in patients with AF.49
AF is also known to be associated with structural remodelling of atrial tissue and fibrosis. This remodelling process is an integral part of the pathophysiology of arrhythmia and is necessary for a chronic arrhythmia to occur. Atrial fibrosis in patients with AF may play a significant role in the development of electromechanical dysfunction leading to stasis and localised contractile dysfunction that may form the focus of thrombus formation.50 Akoum et al. used late gadolinium enhancement cardiac MRI to detect structural LAA dysfunction in patients with AF, finding that LAA fibrosis was associated with reduced LAA flow velocities, and thus with blood stasis, thrombus formation and the risk of stroke.51,52
In addition to LAA morphology, LAA function has been associated with an increased risk of stroke in patients with AF. Lower LAA velocities have been shown to correlate with ischaemic stroke and thrombus formation. Post hoc analysis from the SPAF-III trial, which included 721 patients who underwent transoesophageal echocardiography (TOE), showed that peak LAA anterograde flow velocity <20 cm/s was independently associated with thrombus formation and the risk of cardioembolic stroke.53 Uretsky et al. found that mean LAA contraction velocity was lower in patients with LAA thrombus than in those without (10 ± 4 cm/s versus 22 ± 7 cm/s; p<0.001) and lower in patients with AF and a history of stroke/TIA than in those without (11 ± 3 cm/s versus 15 ± 6 cm/s; p=0.008).54 Moreover, Uretsky et al. noticed that nearly one-third of patients with LAA flow velocity (LAAFV) ≤11 cm/s had LAA thrombus.54 Several studies have reported complex interactions between LAA haemodynamics and geometry. For example, Lee et al. evaluated whether a specific LAA morphology was related to stroke and whether it was related to haemodynamic changes and the size of the LAA in AF patients.45 That study confirmed that a chicken wing LAA was associated with a decreased stroke risk. Moreover, it was shown that patients with a chicken wing LAA have a smaller LAA orifice area and higher LAA velocity than those with a non-chicken wing LAA. Thus, Lee et al. suggested that an increased LAA orifice and decreased LAAFV may be associated with increased stroke risk.45 In another study, Lee et al. observed that the presence of both increased LAA orifice area and decreased LAAFV were significant risk factors for stroke.44 Among patients with an LAAFV <37.0 cm/s, patients with a large LAA orifice (>3.5 cm2) had a greater incidence of stroke than those with an LAA orifice ≤3.5 cm2 (75% versus 23%; p<0.001).44
Others have come to similar conclusions. For example, one study showed that cauliflower LAA morphology was the most common morphology in patients with cardioembolic stroke (CES) and the least common in patients with AF and no history of cerebrovascular accident (AF/non-CVA); in contrast, chicken wing LAA morphology was most common in AF/non-CVA and the least prevalent in CES patients.46 Furthermore, LAA orifice diameters were larger in the cardioembolic TIA (CETIA) and CES groups than in the AF/non-CVA group. LAAFV was higher in the CES group than in the other groups. Multiple multinomial regression analyses showed that the cauliflower morphology was associated with CETIA and CES; however, after adjusting for LAA orifice diameters and LAAFV, LAA morphology was no longer associated with either CETIA or CES.46 Receiver operating
characteristic curve analysis showed that LAA orifice diameter and LAAFV accurately predicted CETIA and CES.46 In another study, significant differences were found in LAAFV between the specific LAA morphologies.55 LAAFV was higher in patients with chicken wing LAA than in those with cactus and cauliflower LAA. After adjusting for covariates, the study revealed that LAA morphology was a significant determinant of LAAFV and suggested that the relationship between a specific LAA morphology and stroke may also be explained, in part, by the change in LAAFV.55
There is continuous research for new markers of LAA dysfunction. In a recent study, Jankajova et al. analysed the significance of LAA deformation in relation to thromboembolic events using TOE.56 In that study, the velocity vector imaging-derived LAA strain rate was found to be a significant predictor of ischaemic stroke and systemic thromboembolism in patients with AF, with predictive power similar to the CHA2DS2-VASc score.56
Left Atrial Appendage Occlusion
Oral anticoagulation (OAC) is the standard therapy for stroke prevention in AF, but its use is associated with a risk of bleeding and its effect depends upon patients’ adherence to recommended treatment.
LAA occlusion (LAAO) provides an alternative to OAC for thromboembolic risk reduction in patients with non-valvular AF (NVAF). The most common rationale for LAA closure/exclusion in clinical practice is the observed high risk of bleeding or, less frequently, contraindications to the use of OAC, as well as the occurrence of ischaemic stroke during anticoagulant therapy.1
Evidence from three randomised control trials supports LAAO.57–59 Specifically, the WATCHMAN device (Boston Scientific) was compared to vitamin K antagonist therapy in randomised control trials (PROTECT AF and PREVAIL). These studies found that LAA closure was not inferior to stroke prevention treatment with vitamin K antagonist therapy in patients with AF with a moderate risk of stroke, with a possible reduction in bleeding during longer follow-up.57–59
A meta-analysis including patients from the PROTECT AF and PREVAIL trials and their respective registries reported that, in patients with NVAF who were at increased risk of stroke or bleeding, LAAO, compared with warfarin, resulted in improved rates of haemorrhagic stroke (0.15 versus 0.96 events/100 patient years; HR 0.22; p=0.004), cardiovascular/ unexplained death (1.1 versus 2.3 events/100 patient years; HR 0.48; p=0.006) and non-procedural bleeding (6.0% versus 11.3%; HR 0.51; p=0.006).60 Although there was a greater incidence of ischaemic stroke in the device group (1.6 versus 0.9 events/100 patient years; HR 1.95; p=0.05), the rates of ischaemic stroke were no longer significantly different between the device and warfarin groups after exclusion of procedure-related strokes.60
As direct oral anticoagulants (DOACs) also provide a significant reduction in haemorrhagic stroke and mortality compared with warfarin, the question may be raised as to whether LAAO or DOAC therapy may be more appropriate for a given patient. This issue was recently raised in the non-inferiority study PRAGUE 17. Among high-risk patients with NVAF, LAAO use was not less effective in preventing cardiovascular or neurological events than DOAC.61
A recent meta-analysis compared the results of the three currently available randomised control trials assessing LAAO versus OAC, namely the PROTECT AF, PREVAIL and PRAGUE 17 trials.62 That analysis found no significant differences in ischaemic stroke (RR 1.48; p=0.13) or overall major bleeding
LAA Morphology and Function and Stroke Risk in AF Patients ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
(RR 0.89; p=0.46) between groups. Compared with OAC, LAAO provided a significant reduction in haemorrhagic stroke (RR 0.22; p=0.002), nonprocedural major bleeding (RR 0.53; p<0.001), cardiovascular death (RR 0.65; p=0.03) and all-cause death (RR 0.78; p=0.04).62
In addition, multiple registries have reported favourable outcomes for the WATCHMAN, Amplatzer Cardiac Plug and Amulet devices (Abbott) in patients with a higher bleeding risk and contraindications to short-term OAC for whom less intensive post-LAAO anticoagulation regimens were required.63 There is also growing evidence of the efficacy and safety of newer devices with distinct characteristics that may be of value to specific patient subgroups.63
Numerous observational studies have demonstrated the feasibility and safety of LAA surgical closure/shutdown. Residual LAA flow or incomplete LAA closure may be associated with an increased risk of stroke. In a multicentre randomised trial involving participants with AF and a CHA2DS2VASc score of at least 2.0, who had undergone cardiac surgery and most of whom continued to receive ongoing antithrombotic therapy, stroke or systemic embolism occurred in 4.8% of participants in the occlusion group, compared with 7.0% in the non-occlusion group (HR 0.67; 95% CI [0.53–0.85]; p=0.001). The incidence of perioperative bleeding, heart failure or death did not differ significantly between the trial groups.64
There is a need for studies of adequate power to determine the best indications for LAAO or LAA exclusion versus DOAC therapy in patients with relative or absolute contraindications to anticoagulant therapy and in patients with ischaemic stroke during anticoagulant treatment, as well as to evaluate appropriate anticoagulant treatment after LAA closure.
Right Atrial Appendage and the Risk of Embolism
Although thrombi migrating from the LAA are usually blamed for peripheral embolisation of cardiac origin, the RAA may occasionally be the source of embolism. Currently, the literature lacks detailed data on the anatomy, function and incidence of thrombus in the RAA. Pulmonary embolism (PE) and paradoxical migration through the patent foramen ovale with a risk of systemic embolism, including stroke, are potential complications of thrombus in the RAA.65 However, the clinical relevance and the incidence of thrombus in the RAA are not well understood.66
The RAA is a triangular extension of the right atrium (RA) with a mean area of 3 cm2 that wraps around the aortic root and is composed of a trabecular network of pectinate muscles.67,68 The sagittal bundle connects the terminal crest with the apex of the RAA.68 The following appendage shapes have been identified: horse head, parrot beak, anvil, sailboat and indeterminate. The number of lobes ranges from one to six.69
The risk of thromboembolic events has been shown to increase with morphological complexity, larger surface area and dysfunction of the RAA. The presence of spontaneous echo contrast in the RA is also an independent predictor of thrombus formation in the RAA.70
Based on data from TOE, thrombi are much less common in the RAA than in the LAA, with an incidence, according to various reports, that ranges from 0.5% to 0.8% in AF/atrial flutter patients (versus 5.9–10.3% in the LAA).71,72 It seems that the lower incidence of thrombus formation in the RAA compared to LAA may be due to differences in the morphology and anatomical location of the two structures. The wide RAA ostium may facilitate thrombus migration. Conversely, the location of the RAA adjacent to the inlet of the superior vena cava may reduce the coagulation capacity.
The lower reported number of RAA thrombi in TOE may also be secondary to problems with the visualisation of this structure.73
One study, which included a total of 23,796 autopsies, suggested that the incidence of thrombus in the RA was the same as in the LA, reaching 3.1%. However, there were no data as to the proportion of thrombi located in the appendages.74
Another controversial and often overlooked issue in clinical practice is PE in patients with AF.66 In the previously mentioned study, it was shown that 7% of patients who died of PE had a right-sided intracardiac thrombus and 62% had no other potential source of embolisation.74 The incidence of AF in PE patients ranges from 15% to 21% and is much higher than in the general population.73 It has been shown that the occurrence of AF is associated with a significantly higher risk of another episode of venous thromboembolism compared with patients with sinus rhythm.73,75 Several studies have shown that patients with PE without concomitant deep vein thrombosis more often have a history of AF than patients with PE associated with deep vein thrombosis.76,77 The relationship between AF and PE has a pathophysiological background. The stagnation of blood in the atria, along with the pectin muscles, is a substrate for thrombus formation in AF. However, there are insufficient clinical data on the relationship between these two conditions. This could be for a variety of reasons, including the inability to visualise the RAA using TOE, which is routinely performed after a diagnosis of PE, and the lack of recommendations for TOE examination in this patient group. Therefore, it can assumed that the actual incidence of thrombus in the RAA in PE patients has not been established. Another reason may be that episodes of PE often remain clinically silent in patients with AF. In addition, most studies of right heart thrombosis did not analyse the incidence of AF in this population.73
Paradoxical embolism in the presence of patent foramen ovale may be another manifestation of the RAA emboli. Data in the literature are scarce, but some authors have reported that RAA function is altered in relatively young patients with patent foramen ovale and cryptogenic stroke.78 Thus, searching for RAA thrombi in patients with patent foramen ovale and cryptogenic stroke or peripheral arterial embolism may be advocated in selected patients.
In summary, the treatment of patients with RAA thrombi is currently unclear. Some authors suggest that the management of thrombus in the RAA should be the same as for LAA thrombus. It has been proposed that in all patients undergoing TOE for LAA thrombus evaluation prior to cardioversion, thrombus in the RAA should also be excluded.66,71,72 If PE occurs in a patient with AF and without deep vein thrombosis, screening for RAA thrombus by TOE should also be performed.66 However, the optimal management of right-sided thrombi remains unclear.
Conclusion
Numerous studies have shown that some anatomical and functional LAA features are independently associated with stroke prevalence in a population of patients with AF and could improve the performance of the CHAD 2 and CHA 2 DS2-VASc scores. However, the results of studies are often contradictory (e.g. LAA shape and stroke risk). None of the LAA parameters has been universally accepted as a strong risk factor or included in the currently used stroke risk scales. Thus, more research is needed to establish the role of LAA parameters in stroke risk assessment in patients with AF. Percutaneous LAAO can be a safe and effective nonpharmacological treatment option for patients with NVAF.
LAA Morphology and Function and Stroke Risk in AF Patients ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Clinical Perspective
• The European Society of Cardiology guidelines recommend a thromboembolic event risk assessment based on the CHA2DS2-VASc score in patients with non-valvular AF; however, stroke occurs also in some patients with low CHA2DS2-VASc scores.
• The leading cause of embolic strokes is thrombi originating from the left atrial appendage (LAA); therefore, some anatomical or functional parameters of the LAA could potentially be used to predict cardioembolic stroke and improve the performance of CHA2DS2-VASc scores.
• New factors need to be found to improve thromboembolism risk stratification in the AF patient population.
• LAA occlusion provides an alternative to oral anticoagulation for thromboembolic risk reduction in patients with non-valvular AF.
1. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612; PMID: 32860505.
2. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. https://doi.org/10.1093/eurheartj/ehw210; PMID: 27567408.
3. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke 1991;22:983–8. https://doi.org/10.1161/01. STR.22.8.983; PMID: 1866765.
4. Writing Group Members, Mozaffarian D, Benjamin EJ, et al. Executive summary: heart disease and stroke statistics –2016 update: a report from the American Heart Association. Circulation 2016;133:447–54. https://doi.org/10.1161/ CIR.0000000000000366; PMID: 26811276.
5. GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1545–602. https://doi.org/10.1016/S0140-6736(16)31678-6; PMID: 27733282.
6. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544. https://doi.org/10.1016/S0140-6736(16)31012-1; PMID: 27733281.
7. Członkowska A, Niewada M. Selected diseases of the nervous system: stroke. In: Gajewski P, ed. Interna Szczeklika 2019. Kraków: Medycyna Praktyczna, 2019; 2259–60 [in Polish].
8. January CT, Wann LS, Calkins H et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation 2019;140:e125–51. https:// doi.org/10.1161/CIR.0000000000000665; PMID: 30686041.
9. Van Staa TP, Setakis E, Di Tanna GL, et al. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost 2011;9:39–48. https://doi. org/10.1111/j.1538-7836.2010.04085.x; PMID: 21029359.
10. Fang MC, Go AS, Chang Y, et al. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J Am Coll Cardiol 2008;51:810–5. https://doi.org/10.1016/j.jacc.2007.09.065; PMID: 18294564.
11. Potpara TS, Polovina MM, Licina MM, et al. Reliable identification of ‘truly low’ thromboembolic risk in patients initially diagnosed with ‘lone’ atrial fibrillation: the Belgrade atrial fibrillation study. Circ Arrhythm Electrophysiol 2012;5:319–26. https://doi.org/10.1161/CIRCEP.111.966713; PMID: 22319004.
12. Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124. https://doi. org/10.1136/bmj.d124; PMID: 21282258.
13. Joundi RA, Cipriano LE, Sposato LA, et al. Ischemic stroke risk in patients with atrial fibrillation and CHA2DS2-VASc score of 1: systematic review and meta-analysis. Stroke 2016;47:1364–7. https://doi.org/10.1161/ STROKEAHA.115.012609; PMID: 27026630.
14. Botto GL, Tortora G, Casale MC, et al. Impact of the pattern of atrial fibrillation on stroke risk and mortality. Arrhythm Electrophysiol Rev 2021;10:68–76. https://doi.org/10.15420/ aer.2021.01; PMID: 34401178.
15. Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation 2014;129:2094–9. https://doi. org/10.1161/CIRCULATIONAHA.113.007825; PMID: 24633881.
16. Freestone B, Lip GY. The endothelium and atrial fibrillation. The prothrombotic state revisited. Hamostaseologie 2008;28:207–12. https://doi.org/10.1055/s-0037-1617102; PMID: 18836646.
17. Singer DE, Ziegler PD, Koehler JL, et al. Temporal association between episodes of atrial fibrillation and risk of ischemic stroke. JAMA Cardiol 2021;6:1364–9. https://doi. org/10.1001/jamacardio.2021.3702; PMID: 34586356.
18. Vermeer SE, Longstreth WT Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007;6:611–9. https://doi.org/10.1016/S1474-4422(07)70170-9; PMID: 17582361.
19. Gaita F, Corsinovi L, Anselmino M, et al. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. J Am Coll Cardiol 2013;62:1990–7. https://doi.org/10.1016/j. jacc.2013.05.074; PMID: 23850917.
20. Johnson WD, Ganjoo AK, Stone CD, et al. The left atrial appendage: our most lethal human attachment! Surgical implications. Eur J Cardiothorac Surg 2000;17:718–22. https:// doi.org/10.1016/S1010-7940(00)00419-X; PMID: 10856866.
21. Naksuk N, Padmanabhan D, Yogeswaran V, et al. Left atrial appendage: embryology, anatomy, physiology, arrhythmia and therapeutic intervention. JACC Clin Electrophysiol 2016;2:403–12. https://doi.org/10.1016/j.jacep.2016.06.006; PMID: 29759858.
22. Beigel R, Wunderlich NC, Ho SY, et al. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging 2014;7:1251–65. https://doi. org/10.1016/j.jcmg.2014.08.009; PMID: 25496544.
23. Veinot JP, Harrity PJ, Gentile F, et al. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation 1997;96:3112–5. https://doi.org/10.1161/01.CIR.96.9.3112; PMID: 9386182.
24. Wang Y, Di Biase L, Horton RP, et al. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. J Cardiovasc Electrophysiol 2010;21:973–82. https://doi. org/10.1111/j.1540-8167.2010.01814.x; PMID: 20550614.
25. Collado FMS, Lama von Buchwald CM, Anderson CK, et al. Left atrial appendage occlusion for stroke prevention in nonvalvular atrial fibrillation. J Am Heart Assoc 2021;10:e022274. https://doi.org/10.1161/JAHA.121.022274; PMID: 34668395.
26. Majunke N, Sandri M, Adams V, et al. Atrial and brain natriuretic peptide secretion after percutaneous closure of the left atrial appendage with the watchman device. J Invasive Cardiol 2015;27:448–52. PMID: 25999139.
27. Cruz-Gonzalez I, Palazuelos Molinero J, Valenzuela M, et al. Brain natriuretic peptide levels variation after left atrial appendage occlusion. Catheter Cardiovasc Interv 2016;87:e39–43. https://doi.org/10.1002/ccd.25985; PMID: 26033157.
28. Bartus K, Podolec J, Lee RJ, et al. Atrial natriuretic peptide and brain natriuretic peptide changes after epicardial percutaneous left atrial appendage suture ligation using LARIAT device. J Physiol Pharmacol 2017;68:117–23. PMID: 28456775.
29. Zapolski T, Kamińska A, Wysokiński A. Atrial fibrillation and left atrium remodelling – pathophysiology and prognostic implications. Post Nauk Med 2015;27:8B.
30. Dietz JR. Mechanisms of atrial natriuretic peptide secretion from the atrium. Cardiovasc Res 2005;68:8–17. https://doi. org/10.1016/j.cardiores.2005.06.008; PMID: 15993390.
31. Yoshihara F, Nishikimi T, Sasako Y, et al. Plasma atrial
natriuretic peptide concentration inversely correlates with left atrial collagen volume fraction in patients with atrial fibrillation: plasma ANP as a possible biochemical marker to predict the outcome of the maze procedure. J Am Coll Cardiol 2002;39:288–94. https://doi.org/10.1016/S07351097(01)01719-3; PMID: 11788221.
32. Van Den Berg MP, Crijns HJ, Van Veldhuisen DJ, et al. Atrial natriuretic peptide in patients with heart failure and chronic atrial fibrillation: role of duration of atrial fibrillation. Am Heart J 1998;135:242–4. https://doi.org/10.1016/S00028703(98)70088-2; PMID: 9489971.
33. Meune C, Wahbi K, Vermillet A, et al. N-Terminal-proBrain natriuretic peptide measurement at presentation to identify patients with recent onset of atrial fibrillation. Int J Cardiol 2012;154:208–9. https://doi.org/10.1016/j.ijcard.2011.10.060; PMID: 22075419.
34. Breitenstein A, Glanzmann M, Falk V, et al. Increased prothrombotic profile in the left atrial appendage of atrial fibrillation patients. Int J Cardiol 2015;185:250–5. https://doi. org/10.1016/j.ijcard.2015.03.092; PMID: 25814212.
35. Chung I, Lip GY. Virchow’s triad revisited: blood constituents. Pathophysiol Haemost Thromb 2003;33:449–54. https://doi.org/10.1159/000083844; PMID: 15692259.
36. Yaghi S, Song C, Gray WA, et al. Left atrial appendage function and stroke risk. Stroke 2015;46:3554–9. https://doi. org/10.1161/STROKEAHA.115.011273; PMID: 26508750.
37. Di Biase L, Santangeli P, Anselmino M, et al. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012;60:531–8. https:// doi.org/10.1016/j.jacc.2012.04.032; PMID: 22858289.
38. Anselmino M, Scaglione M, Di Biase L, et al. Left atrial appendage morphology and silent cerebral ischemia in patients with atrial fibrillation. Heart Rhythm 2014;11:2–7. https://doi.org/10.1016/j.hrthm.2013.10.020; PMID: 24120872.
39. Lupercio F, Carlos Ruiz J, Briceno DF, et al. Left atrial appendage morphology assessment for risk stratification of embolic stroke in patients with atrial fibrillation: a metaanalysis. Heart Rhythm 2016;13:1402–9. https://doi. org/10.1016/j.hrthm.2016.03.042; PMID: 27016474.
40. Anan AR, Fareed J, Suhaib J, et al. Left atrial appendage morphology as a determinant for stroke risk assessment in atrial fibrillation patients: systematic review and metaanalysis. J Atr Fibrillation 2019;12:2183. https://doi. org/10.4022/jafib.2183; PMID: 32002111.
41. Wang F, Zhu M, Wang X, et al. Predictive value of left atrial appendage lobes on left atrial thrombus or spontaneous echo contrast in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord 2018;18:153. https://doi.org/10.1186/ s12872-018-0889-y; PMID: 30064363.
42. Beinart R, Heist EK, Newell JB, et al. Left atrial appendage dimensions predict the risk of stroke/TIA in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:10–5. https://doi.org/10.1111/j.1540-8167.2010.01854.x;
PMID: 20662984.
43. Burrell LD, Horne BD, Anderson JL, et al. Usefulness of left atrial appendage volume as a predictor of embolic stroke in patients with atrial fibrillation. Am J Cardiol 2013;112:1148–52. https://doi.org/10.1016/j.amjcard.2013.05.062;
PMID: 23827402.
44. Lee JM, Shim J, Uhm JS, et al. Impact of increased orifice size and decreased flow velocity of left atrial appendage on stroke in nonvalvular atrial fibrillation. Am J Cardiol 2014;113:963–9. https://doi.org/10.1016/j.amjcard.2013.11.058; PMID: 24462064.
45. Lee JM, Seo J, Uhm JS, et al. Why is left atrial appendage morphology related to strokes? An analysis of the flow velocity and orifice size of the left atrial appendage. J Cardiovasc Electrophysiol 2015;26:922–7. https://doi. org/10.1111/jce.12710; PMID: 25959871.
46. Lee Y, Park HC, Lee Y, et al. Comparison of morphologic features and flow velocity of the left atrial appendage among patients with atrial fibrillation alone, transient ischemic attack, and cardioembolic stroke. Am J Cardiol
LAA Morphology and Function and Stroke Risk in AF Patients ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
2017;119:1596–604. https://doi.org/10.1016/j. amjcard.2017.02.016; PMID: 28364953.
47. Khurram IM, Dewire J, Mager M, et al. Relationship between left atrial appendage morphology and stroke in patients with atrial fibrillation. Heart Rhythm 2013;10:1843–9. https:// doi.org/10.1016/j.hrthm.2013.09.065; PMID: 24076444.
48. Nedios S, Koutalas E, Kornej J, et al. Cardiogenic stroke despite low CHA2DS2-VASc score: Assessing Stroke Risk by Left Atrial Appendage Anatomy (ASK LAA). J Cardiovasc Electrophysiol 2015;26:915–21. https://doi.org/10.1111/ jce.12749; PMID: 26178767.
49. Dudzińska-Szczerba K, Michałowska I, Piotrowski R, et al. Assessment of the left atrial appendage morphology in patients after ischemic stroke – the Assam study. Int J Cardiol 2021;330:65–72. https://doi.org/10.1016/j. ijcard.2021.01.001; PMID: 33524464.
50. Mahnkopf C, Kwon Y, Akoum N. Atrial fibrosis, ischaemic stroke and atrial fibrillation. Arrhythm Electrophysiol Rev 2021;10:225–9. https://doi.org/10.15420/aer.2021.51; PMID: 35106172.
51. Akoum N, Marrouche N. Assessment and impact of cardiac fibrosis on atrial fibrillation. Curr Cardiol Rep 2014;16:518. https://doi.org/10.1007/s11886-014-0518-z; PMID: 24950676.
52. Akoum N, Fernandez G, Wilson B, et al. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2013;24:1104–9. https://doi. org/10.1111/jce.12199; PMID: 23844972.
53. Goldman ME, Pearce LA, Hart RG, et al. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF-III] study). J Am Soc Echocardiogr 1999;12:1080–7. https://doi.org/10.1016/S0894-7317(99)70105-7; PMID: 10588784.
54. Uretsky S, Shah A, Bangalore S, et al. Assessment of left atrial appendage function with transthoracic tissue Doppler echocardiography. Eur J Echocardiogr 2009;10:363–71. https://doi.org/10.1093/ejechocard/jen339; PMID: 19193710.
55. Fukushima K, Fukushima N, Kato K, et al. Correlation between left atrial appendage morphology and flow velocity in patients with paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging 2016;17:59–66. https://doi.org/10.1093/ ehjci/jev117; PMID: 25944049.
56. Jankajova M, Kubikova L, Valocik G, et al. Left atrial appendage strain rate is associated with documented thromboembolism in nonvalvular atrial fibrillation. Wien Klin Wochenschr 2019;131:156–64. https://doi.org/10.1007/s00508019-1469-6; PMID: 30824998.
57. Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for
prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009;374:534-542. https://doi.org/10.1016/S0140-6736(09)61343-X; PMID: 19683639.
58. Reddy VY, Doshi SK, Sievert H, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) trial. Circulation 2013;127:720–9. https://doi.org/10.1161/ CIRCULATIONAHA.112.114389; PMID: 23325525.
59. Holmes DR Jr, Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. Am Coll Cardiol 2014;64:1–12. https://doi.org/10.1016/j. jacc.2014.04.029; PMID: 24998121.
60. Holmes DR Jr, Doshi SK, Kar S, et al. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: a patient-level meta-analysis. J Am Coll Cardiol 2015;65:2614–23. https://doi.org/10.1016/j. jacc.2015.04.025; PMID: 26088300.
61. Osmancik P, Herman D, Neuzil P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–35. https://doi.org/10.1016/j.jacc.2020.04.067; PMID: 32586585.
62. Turagam MK, Osmancik P, Neuzil P, et al. Left atrial appendage closure versus oral anticoagulants in atrial fibrillation: a meta-analysis of randomized trials. J Am Coll Cardiol 2020;76:2795–7. https://doi.org/10.1016/j. jacc.2020.08.089; PMID: 33272374.
63. Cruz-González I, Trejo-Velasco B. Percutaneous left atrial appendage occlusion in the current practice. Kardiol Pol 2021;79:255–68. https://doi.org/10.33963/KP.15864; PMID: 33687872.
64. Whitlock RP, Belley-Cote EP, Paparella D, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. https://doi. org/10.1056/NEJMoa2101897; PMID: 33999547.
65. Omran H, Jung W, MacCarter D, et al. Right atrial thrombi and depressed right atrial appendage function after cardioversion of atrial fibrillation. Echocardiography 1999;16:245–51. https://doi.org/10.1111/j.1540-8175.1999. tb00809.x; PMID: 11175145.
66. Ozer O, Sari I, Davutoglu V. Right atrial appendage: forgotten part of the heart in atrial fibrillation. Clin Appl Thromb Hemost 2010;16:218–20. https://doi. org/10.1177/1076029608323088; PMID: 18840628.
67. Malik SB, Kwan D, Shah AB, et al. The right atrium: gateway to the heart – anatomic and pathologic imaging findings. Radiographics 2015;35:14–31. https://doi.org/10.1148/
rg.351130010; PMID: 25590385.
68. Kamiński R, Grzybiak M, Nowicka E, et al. Macroscopic morphology of right atrial appendage in humans. Kardiol Pol 2015;73:183–7. https://doi.org/10.5603/KP.a2014.0170; PMID: 25179484.
69. Rissi R, Marques MJ, Neto HS. Checking the shape and lobation of the right atrial appendage in view of their clinical relevance. Anat Sci Int 2019;94:324–9. https://doi. org/10.1007/s12565-019-00489-z; PMID: 31073851.
70. de Divitiis M, Omran H, Rabahieh R, et al. Right atrial appendage thrombosis in atrial fibrillation: its frequency and its clinical predictors. Am J Cardiol 1999;84:1023–8. https:// doi.org/10.1016/s0002-9149(99)00492-0; PMID: 10569657.
71. Cresti A, García-Fernández MA, Miracapillo G, et al. Frequency and significance of right atrial appendage thrombi in patients with persistent atrial fibrillation or atrial flutter. J Am Soc Echocardiogr 2014;27:1200–7. https://doi. org/10.1016/j.echo.2014.08.008; PMID: 25240491.
72. Cresti A, García-Fernández MA, De Sensi F, et al. Prevalence of auricular thrombosis before atrial flutter cardioversion: a 17-year transoesophageal echocardiographic study. Europace 2016;18:450–6. https://doi.org/10.1093/europace/ euv128; PMID: 26017468.
73. Ptaszynska-Kopczynska K, Kiluk I, Sobkowicz B. Atrial fibrillation in patients with acute pulmonary embolism: clinical significance and impact on prognosis. BioMed Res Int 2019;2019:7846291. https://doi.org/10.1155/2019/7846291; PMID: 31531368.
74. Ogren M, Bergqvist D, Eriksson H, et al. Prevalence and risk of pulmonary embolism in patients with intracardiac thrombosis: a population-based study of 23 796 consecutive autopsies. Eur Heart J 2005;26:1108–14. https://doi. org/10.1093/eurheartj/ehi130; PMID: 15695529.
75. Lutsey PL, Norby FL, Alonso A, et al. Atrial fibrillation and venous thromboembolism: evidence of bidirectionality in the Atherosclerosis Risk in Communities Study. J Thromb Haemost 2018;16:670–9. https://doi.org/10.1111/jth.13974; PMID: 29431904.
76. Keller K, Prochaska JH, Coldewey M, et al. History of deep vein thrombosis is a discriminator for concomitant atrial fibrillation in pulmonary embolism. Thromb Res 2015;136:899–906. https://doi.org/10.1016/j. thromres.2015.08.024; PMID: 26376038.
77. van Langevelde K, Srámek A, Vincken PW, et al. Finding the origin of pulmonary emboli with a total-body magnetic resonance direct thrombus imaging technique. Haematologica 2013;98:309–15. https://doi.org/10.3324/ haematol.2012.069195; PMID: 22801962.
78. Pöyhönen P, Kuusisto J, Pirinen J, et al. Right atrium and cryptogenic ischaemic stroke in the young: a case-control study. Open Heart 2021;8:e001596. https://doi.org/10.1136/ openhrt-2021-001596; PMID: 34006504.
LAA Morphology and Function and Stroke Risk in AF Patients ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Prophylactic Cavotricuspid Isthmus Ablation in Atrial Fibrillation without Documented Typical Atrial Flutter: A Systematic Review and Meta-analysis
Yoga Waranugraha , 1 Ardian Rizal , 1 Mohammad Saifur Rohman , 1 Chia-Ti Tsai 2,3 and Fu-Chun Chiu 4
1. Department of Cardiology and Vascular Medicine, Faculty of Medicine, University of Brawijaya, Malang, Indonesia; 2. Division of Cardiology, Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan; 3. Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan; 4. Division of Cardiology, Department of Internal Medicine, National Taiwan University Hospital, Yun-Lin Branch, Yun-Lin, Taiwan
Abstract
Background: The advantage of prophylactic cavotricuspid isthmus (CTI) ablation for AF patients without documented atrial flutter is still unclear. The present study aimed to evaluate the role of prophylactic CTI ablation in this population. Methods: A systematic review and meta-analysis study was conducted. The overall effects estimation was conducted using random effects models. The pooled effects were presented as the risk difference and standardised mean difference for dichotomous and continuous outcomes, respectively. Results: A total of 1,476 patients from four studies were included. The risk of atrial tachyarrhythmias following a successful catheter ablation procedure was greater in the pulmonary vein isolation + CTI ablation group than pulmonary vein isolation alone group (34.8% versus 28.2%; risk difference 0.08; 95% CI [0.00–0.17]; p=0.04). Prophylactic CTI ablation was associated with a higher recurrent AF rate (33.8% versus 27.1%; risk difference 0.07; 95% CI [0.01–0.13]; p=0.02). Additional prophylactic CTI ablation to pulmonary vein isolation significantly increased the radio frequency application time (standardised mean difference 0.52; 95% CI [0.04–1.01]; p=0.03). Conclusion: This study suggested that prophylactic CTI ablation was an ineffective and inefficient approach in AF without documented typical atrial flutter patients.
Keywords
AF, atrial flutter, atrial tachyarrhythmias, cavotricuspid isthmus ablation, pulmonary vein isolation
Disclosure: The authors have no conflicts of interest to declare.
Data Availability: The data that support the findings of this study are available in the main article and in the supplementary material.
Received: 26 June 2021
Accepted: 28 December 2021
Citation: Arrhythmia & Electrophysiology Review 2022;11:e10. DOI: https://doi.org/10.15420/aer.2021.37
Correspondence: Yoga Waranugraha, Department of Cardiology and Vascular Medicine, Faculty of Medicine, University of Brawijaya, Veteran St, Malang 65145, Indonesia. E: mr.waranugraha@ub.ac.id
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
AF and atrial flutter (AFL) commonly coexist, and reveal a strong clinical interrelationship.1 The presence of AFL is documented in 20.6% of AF patients.2 Pulmonary vein (PV) firing during AF episodes makes an essential contribution to initiating typical AFL.3 In contrast, AF induced by pacing protocol during typical AFL ablation is a strong predictor for AF.4
Complete PV isolation (PVI) by single-shot or point-by-point catheter ablation approach is the cornerstone of catheter ablation in AF patients.5 Compared with antiarrhythmic drugs (AADs), catheter ablation significantly reduces the AF recurrence rate, provides better symptom control and improves the quality of life in AF patients.6 7
In AF patients with left ventricular ejection fraction (LVEF) ≤35%, catheter ablation gives additional benefit in reducing hospitalisation due to worsening heart failure and all-cause mortality.8 However, the main issue is the high rate of atrial tachyarrhythmias (ATAs) following the catheter ablation procedure.9 10 Substrate modification, linear ablation and stepwise catheter ablation approaches have been conducted with a view to improving clinical outcomes.11–19
Ablation of the cavotricuspid isthmus (CTI) is well known as the therapeutic strategy for typical AFL.20 This approach has also been proposed as an additional ablation procedure to PVI for AF patients in improving ATAsfree survival. A prior meta-analysis of randomised controlled trials (RCTs) revealed that prophylactic PVI during CTI ablation successfully improved the 1-year ATAs-free survival rate.21 However, several previous studies about prophylaxis CTI ablation during PVI in AF patients demonstrated conflicting results.14,22–24 This systematic review and meta-analysis study aimed to evaluate the advantage of prophylactic CTI ablation in AF patients without documented AFL.
Methods
Literature Search
According to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidance, we conducted a systematic review and meta-analysis study.25 Until January 2021, articles comparing PVI + prophylactic CTI ablation versus PVI alone in AF patients without documented AFL were identified from the electronic scientific databases including ClinicalTrials.gov, ScienceDirect, PubMed, Cochrane and
SYSTEMATIC REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Clinical Electrophysiology and Ablation
Figure 1: Flow Diagram of the Study Selection Process
Records identified through database searching (ClinicalTrials.gov, ScienceDirect, PubMed, Cochrane, ProQuest) (n=141)
Records after duplicates removed (n=89)
Screening
Records screened (n=89)
Full-text articles assessed for eligibility (n=15)
Studies included in the qualitative synthesis (n=4)
Studies included in the quantitative synthesis (meta-analysis; n=4)
Additional records identified through other sources (n=12)
The quality assessment of RCTs was performed using version 2 of the Cochrane risk of bias tool in randomised trials.27 Two investigators performed the study quality assessment. The disagreement between both investigators was resolved through discussion and the third investigator’s second opinion.
Data Extraction
Records excluded due to unrelated with our research (n=74)
Full-text articles excluded due to: Included AFL patients (n=3) Meta-analysis (n=1) Review articles (n=3) Unavailability of full text (n=4)
ProQuest. We used the following keywords: ‘atrial fibrillation’ OR ‘AF’ OR ‘AFib’ AND ‘catheter ablation’ OR ‘ablation’ AND ‘pulmonary vein isolation’ OR ‘PVI’ AND ‘cavotricuspid isthmus’ OR ‘CTI’. We also manually looked for potentially relevant articles from other sources, such as the reference lists of all eligible articles or Google Scholar. The literature search was conducted by two investigators.
Eligibility Criteria
We used the following inclusion criteria: original research articles comparing PVI + prophylactic CTI ablation versus PVI alone in AF patients without documented AFL; catheter ablation purposed for rhythm control; written in English; availability of the data about ATAs, AF and AFL during the follow-up period; and availability of the procedural aspect data, including all-procedural complications, fluoroscopy time, procedure time or radiofrequency (RF) application time. We also excluded articles with the following criteria: duplications; treatment group and control group were incomparable; documented AFL prior to catheter ablation; outcomes of interest were not reported; using the data from similar studies; unavailability of full text; cross-sectional study; meta-analysis; review article; editorial; or case reports.
Endpoints
In this meta-analysis, the study endpoints were divided into clinical and procedural endpoints. Clinical endpoints involved ATAs, recurrent AF and new-onset AFL during the follow-up period. The atrial tachyarrhythmias were defined as documented AF, AFL and/or atrial tachycardia episodes by ECG or Holter monitor with duration ≥30 seconds after a 3-month blanking period. The procedural time, fluoroscopy time, RF application time and all-procedural complications were the procedural endpoints in this study.
Study Quality Assessment
For cohort studies, the study quality was evaluated using the Newcastle–Ottawa scale. It consisted of three domains with the highest score of 9. A cohort study was categorised as a good-quality study if it had: three or four stars in the selection domain; one or two stars in the comparability domain; and two or three stars in the outcome domain.26
Two investigators conducted the data extraction process. Essential information about the following was extracted from each study: the name of the first author; year of publication; study design; centres involved; country; number of patients; type of arrhythmia; blanking period duration; follow-up period duration; and arrhythmia detection methods. We also extracted data about patient baseline characteristics from each study, including: demographic (sex and age); AF (paroxysmal AF proportion, AF duration and CHA 2 DS2-VASc score); comorbidities (heart failure, hypertension, stroke, coronary artery disease and diabetes); and echocardiographic parameters (LVEF, left atrial diameter and left atrial volume index).
We also identified important information about: the incidence of ATAs, AF or AFL following the catheter ablation procedure; the procedural endpoints involved in procedural time, fluoroscopy time or RF application time; and all-procedural complications. We demonstrated the numerical data as the mean and SD. The mean and SD could also be estimated from the median and interquartile range using the Tiejun Tong group formula.28–30 The number and percentage represented categorical data.
Statistical Analysis
The statistical analysis was performed by two investigators using Review Manager (RevMan) version 5.3 (Cochrane). This process was conducted based on the standard guideline direction.31 We used Q-statistics to investigate heterogeneity among studies. The heterogeneity was identified if the p-value for heterogeneity was <0.1 or the I2 statistic was >50%.32–35 Meta-analyses were conducted using random effects models to anticipate clinical and methodological diversity among the included studies.34 The pooled effects were presented as a risk difference (RD), and standardised mean difference (SMD) for dichotomous and continuous outcomes. We also estimated the 95% CI of each pooled effect. A p-value <0.05 was considered statistically significant. The funnel plot was used to estimate the presence of the publication bias.33
Results Study Selection Process
In the initial search phase, we successfully identified a total of 141 articles from electronic scientific databases (ClinicalTrials.gov [n=6], ScienceDirect [n=66], PubMed [n=21], Cochrane [n=5] and ProQuest [n=43]), and 12 articles from the reference lists of eligible articles. After removal of duplicates, we had 89 articles. In the beginning, 74 articles were excluded due to being unrelated to our systematic review and meta-analysis. In the next phase, we also excluded 10 articles because of the following: included AFL patients (n=3), meta-analysis (n=1), review (n=3) and unavailability of full text (n=4). Finally, four good-quality studies, including two cohort studies and two RCTs, were involved in this systematic review and meta-analysis.14 22–24 The flow diagram of the study selection process is shown in Figure 1
Baseline Characteristics
We summarised the characteristics of the studies in Supplementary Material Table 1. The study from Kim et al. only involved paroxysmal AF patients.22 However, other studies also included another type of AF, such
Prophylactic CTI Ablation in AF without Documented Typical AFL ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Identification Included
Eligibility
AFL = atrial flutter.
Figure 2: Study Quality Assessment
Randomised controlled trials quality assessment using risk of bias 2
Randomisation process
Deviations from the intended interventions
Missing outcome data
Measurement of the outcome
Selection of the reported result
Cohort studies quality assessment using the Newcastle–Ottawa scale
Mesquita et al. 201824
A: Risk of bias 2 for randomised controlled trials; B: Newcastle–Ottawa scale for cohort studies. NOS = Newcastle–Ottawa scale.
as persistent AF or long-standing persistent AF.14 23 24 3D mapping systems, such as CARTO or EnSite NavX, were used in four studies.14,22–24 The blanking period duration in all studies was 3 months, and the shortest follow-up period was 12 months. AADs were given to some of the patients during the follow-up period.14 22–24 However, the data about the usage rates of AADs during follow-up were available in only two studies. The usage rates of AADs were 18.9% and 47.8% in the studies by Pontoppidan et al. and Mesquita et al., respectively. No significant difference was found in the AADs usage rate between both groups.14 24 Besides standard 12-lead ECG recording, all studies used ambulatory monitor systems, such as the Holter monitor or event recorder, to detect arrhythmia.14 22–24
A total of 1,476 AF patients from four studies were included in this systematic review and meta-analysis (724 patients in the PVI + prophylactic CTI ablation group and 752 patients in the PVI alone group).14 22–24 The mean age of all study participants was 58.5 ± 10.8 years, and 68.7% of them were men. The CHA 2 DS2-VASc scores were available in three studies, with an overall mean value of 1.7 ± 2.9.22–24 Comorbid conditions, including heart failure, hypertension, stroke, coronary artery disease and diabetes, were found in 7.3%, 45.8%, 4.7%, 4.7% and 10% of study participants, respectively. The data about LVEF and left atrial diameter were reported in three studies.14,22,23 The overall mean value of the LVEF and left atrial diameter were 64.0% ± 20.7% and 41.3 mm ± 39.1 mm, respectively.
Only a study by Mesquita et al. provided left atrial volume index data. The mean left atrial volume index in the PVI + prophylactic CTI ablation group and PVI alone group were 57.3 ± 3.5 ml/m2 and 55.3 ± 3.0 ml/m2, respectively.24 Patient characteristics in each study are summarised in Supplementary Material Table 2
Study Quality, Heterogeneity and Publication Bias
Based on version 2 of the Cochrane risk of bias tool in randomised trials assessment, two RCTs involved in this meta-analysis were not at high risk of bias. According to the Newcastle–Ottawa scale, two cohort studies were classified as good-quality studies (Figure 2). Heterogeneity was present in the meta-analysis of atrial tachyarrhythmias (I2=57%, p-value for heterogeneity=0.07), new-onset atrial flutter (I2=61%, p-value for heterogeneity=0.11), procedure time (I2=97%, p-value for heterogeneity <0.01), fluoroscopy time (I2=99%, p-value for heterogeneity <0.01) and radiofrequency application time (I2=79%, p-value for heterogeneity=0.03). This heterogeneity could be caused by the various AF types, comorbid conditions, atrial size, operator experience and follow-up duration. However, we did not find the heterogeneity in the meta-analysis of recurrent atrial fibrillation (I2=31%, p-value for heterogeneity=0.23) and procedural complications (I2=42%, p-value for heterogeneity=0.19). Because two cohort studies and two RCTs were involved in this systematic review and meta-analysis study, the pooled effects were conducted using random effects models to anticipate clinical and methodological diversity among included studies (Figures 3 and 4).14 22–24 As shown in the funnel plot (Figure 5), publication biases were not found in the analysis of atrial tachyarrhythmias, recurrent atrial fibrillation, new-onset atrial flutter, procedural complications and RF application time.
Clinical Endpoints
In the analysis of clinical endpoints, the risk of ATAs following a successful catheter ablation procedure was higher in the PVI + CTI ablation group than PVI alone group (34.8% versus 28.2%; RD 0.08; 95% CI [0.00–0.17]; p=0.04). We also conducted subgroup analysis for recurrent AF and newonset AFL following the catheter ablation procedure. Additional CTI
Prophylactic CTI Ablation in AF without Documented Typical AFL ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
A B Study Study Kim et al. 202122 D1 + + + ! ! ! + + + + + + + +D1 D2 D3 D4 D5 D2 D3 D4 D5 Overall Pontoppidan
Some concerns High risk Low risk
et al. 200914
Selection Comparability Outcome Representative of the exposed cohort Selection of the nonexposed cohort Ascertainment of exposure Outcome of interest was not present at the start of the study Main factor Additional factor Assessment of outcome Su cient follow-up time Adequacy of follow-up NOS 8 9 Lee
et al. 201923
Figure 3: Forest Plot of the Clinical Endpoints
Τ2=0.00; χ2=4.34; d.f.=3 (p=0.23); I2=31%
Τ2=0.00; χ2=2.54; d.f.=1 (p=0.11); I2=61%
A: Atrial tachyarrhythmias; B: Recurrent AF; C: New-onset atrial flutter following single catheter ablation. abl = ablation; CTI = cavotricuspid isthmus; M-H = Mantel–Haenszel; PVI = pulmonary vein isolation.
ablation was associated with a greater recurrent AF rate (33.8% versus 27.1%; RD 0.07; 95% CI [0.01–0.13]; p=0.02). However, both groups revealed a non-significant difference in new-onset AFL (2.1% versus 2.3%; RD 0.00; 95% CI [−0.05, 0.05]; p=0.87) during the follow-up period (Figure 3).
Procedural Endpoints
The procedure time (SMD 0.55; 95% CI [–0.16, 1.26]; p=0.13) and fluoroscopy time (SMD –0.20; 95% CI [–1.72, 1.32 min]; p=0.79) between both groups were not significantly different. However, additional prophylactic CTI ablation to PVI significantly increased RF application time (SMD 0.52; 95% [CI 0.04–1.01]; p=0.03). Both groups had a similar rate of all-procedural complications (2.3% versus 2.3%; RD 0.00; 95% CI [−0.04, 0.04]; p=0.97; Figure 3). We did not perform a subgroup analysis of the specific procedural complication, because only two studies reported it.14,22 Moreover, the number of specific complications in each study was too low. Therefore, the subgroup analysis was not possible (Figure 4).
Discussion
Main Results and the Current Recommendation
We tried to assess whether prophylactic CTI ablation could improve outcomes in AF patients without documented AFL. Our main result was
that PVI + prophylactic CTI ablation was not superior to PVI alone in reducing ATAs, recurrent AF and new-onset AFL following a successful catheter ablation procedure. Moreover, prophylactic CTI ablation was associated with a higher risk of ATAs and recurrent AF. Prophylactic CTI ablation also did not increase all-procedural complications. However, it was associated with longer RF application time.
The current guideline strongly recommends PVI as a rhythm control strategy for paroxysmal AF and persistent AF.5 On the other side, CTI ablation is recommended for recurrent and symptomatic CTI-dependent AFL.20 The rates of ATAs following those procedures are still high. In AF patients undergoing PVI ablation, 29.4–42% of patients developed ATAs during the follow-up period.36–38 However, in AFL patients, the ATAs can be found in 16.7–50% of patients after CTI ablation.39,40
The Link Between Atrial Flutter and AF
A long time ago, AFL and AF were thought to be different arrhythmias, driven by a single macroreentrant arrhythmia circuit and multiple reentrant wave fronts in both atria. However, in the real clinical setting, approximately 50% of AFL patients suffer from AF during long-term follow-up.41 The studies from Watson et al. and Waldo et al. provided new
Prophylactic CTI Ablation in AF without Documented Typical AFL ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Atrial tachyarrhythmias Kin et al. 202122 Lee et al. 201923 Mesquita et al. 201824 Pontoppidan et al. 200914 A Study or subgroup Total events Heterogeneity: Tau2=0.00; χ2=7.04; d.f.=3 (p=0.07); I2=57% Test for overall e ect: Z=2.04 (p=0.04) Total (95% CI) 724 252 212 752 100.0% 0.08 [0.00–0.17] PVI + CTI abl Events 50 22 149 31 Total 183 57 411 73 PVI 47 12 129 24 Total 183 82 411 76 29.2% 18.2% 35.4% 17.2% Weight Risk di erence M-H, random, 95% CI Risk di erence M-H, random, 95% CI 0.02 [ 0.07, 0.11] 0.24 [0.09, 0.39] 0.05 [ 0.02, 0.11] 0.11 [ 0.05, 0.26] Favours [PVI + CTI abl] Favours [PVI] 1 0.5 0 0.5 1 Recurrent AF Kin et al. 202122 Lee et al. 201923 Mesquita et al. 201824 Pontoppidan et al. 200914 B Study or subgroup Total (95% CI) 724 245 204 752 100.0% 0.07 [0.01–0.13] PVI + CTI abl Events 47 18 149 31 Total 183 57 411 73 41 10 129 24 Total 183 82 411 76 30.0% 15.2% 41.9% 12.9% Weight Risk di erence M-H, random, 95% CI Risk di erence M-H, random, 95% CI 0.03 [ 0.05, 0.12] 0.19 [0.05–0.33] 0.05 [ 0.02, 0.11] 0.11 [ 0.05, 0.26] Favours [PVI + CTI abl] Favours [PVI] 1 0.5 0 0.5 1 Events PVI Events Total events Heterogeneity:
Test for overall e ect:
New-onset atrial flutter Kin et al. 202122 Lee et al. 201923 C Study or subgroup Total (95% CI) PVI + CTI abl Events 3 2 5 6 Total 183 57 6 0 Total 183 82 59.8% 40.2% Weight Risk di erence M-H, random, 95% CI Risk di erence M-H, random, 95% CI 0.02 [ 0.05, 0.02] 0.04 [ 0.02, 0.09] 0.00 [ 0.05, 0.05] Favours [PVI + CTI abl] Favours [PVI] 1 0.5 0 0.5 1 PVI Events Total events Heterogeneity:
overall
240 265 100.0%
Z=2.39 (p=0.02)
Test for
e ect: Z=0.17 (p=0.87)
Figure 4: Forest Plot of the Procedural Endpoints
A: Procedure times; B: Fluoroscopy times; C: Radiofrequency application time; D: All-procedural complications. Abl = ablation; CTI = cavotricuspid isthmus; IV = inverse variance; M-H = Mantel–Haenszel; PVI = pulmonary vein isolation; Std. = standard.
insight into understanding the mechanism of AF and AFL. Those studies observed that a transitional period of AF typically preceded the AFL onset.42 43 The transition from an AF to AFL mechanism was described in an animal study by Ortiz et al. That study revealed that the conversion from AF to AFL was due to: the formation of a long line of the functional block; the presence of stable re-entry circuits; and the re-emergence of the slow conduction area. In contrast, the conversion from AFL to AF following these several mechanisms caused the following: a decrease in the cycle length; disappearance of the slow conducting area; a decrease in the length of the functional block line; and an unstable re-entry circuit with the very short cycle length with various appearance, shape and location.44
Three years later, a human study by Roithinger et al. documented the existence of the stereotypical pattern of the subendocardial organisation during the transformation from AF to AFL.45 In 2001, Hsieh et al. reported that several ectopic beats initiated the spontaneous transformation from typical AFL to AF. It could be eliminated by performing catheter ablation to those ectopic foci.46 Therefore, it is thought that a better outcome will be
achieved by performing prophylactic PVI in AFL patients undergoing CTI ablation or conducting prophylactic CTI ablation in AF patients undergoing PVI.
Prophylactic Cavotricuspid Isthmus Ablation During Pulmonary Vein Isolation
Several studies were conducted to assess the advantage of prophylactic CTI ablation in AF patients without documented AFL.14,22–24 An RCT by Pontoppidan et al. revealed that prophylactic CTI ablation failed to reduce the recurrent AF or new-onset AFL.14 An RCT with a longer follow-up duration by Kim et al. also showed similar results.22 A cohort study with a large number of patients by Mesquita et al. also demonstrated that prophylactic CTI ablation failed to increase AF-free survival.24 The high rate of post-catheter ablation atypical AFL in the PVI alone group could be due to a macroreentrant circuit resulting from atrial remodelling. A retrospective study from Lee et al. showed that additional CTI ablation was associated with higher ATAs after the catheter ablation procedure. However, in that study, the higher ATAs in the prophylactic CTI ablation group could be caused by left atrial remodelling. In that group, patients
Prophylactic CTI Ablation in AF without Documented Typical AFL ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Procedure time A Study or subgroup Kin et al. 202122 Mesquita et al. 201824 Pontoppidan et al. 200914 Mean PVI + CTI abl 174.2 188.9 117.4 76.5 18.4 53.7 183 411 73 176.8 171.1 87.5 72.6 16.9 32.9 183 411 76 SD Total Total PVI Mean SD 33.7% 34.1% 32.2% Weight −0.03 [−0.24, 0.17] 1.01 [0.086–1.15] 0.67 [0.34–1.00] 667 670 Total (95% CI) 100.0% 0.55 [−0.16, 1.26] Heterogeneity: Τ2=0.38; χ2=66.08; d.f.=3 (p=0.00001); I2=97% Test for overall e ect: Z=2.04 (p=0.13) −10 −5 0 5 10 Std. mean di erence IV, random, 95% CI Favours [PVI + CTI abl] Favours [PVI] Fluoroscopy time B Study or subgroup Mesquita et al. 201824 Pontoppidan et al. 200914 Mean PVI + CTI abl 10.1 15.9 1.9 13.6 411 73 484 487 12.1 9.8 2.2 6 411 76 SD Total Total PVI Mean SD 50.5% 49.5% Weight Std. mean di erence IV, random, 95% CI Std. mean di erence IV, random, 95% CI −0.97 [−1.12, −0.83] 0.58 [0.25–0.91] Total (95% CI) 100.0% −0.20 [−1.72, 1.32] Heterogeneity: Τ2=1.19; χ2=72.12; d.f.=1 (p=0.00001); I2=99% Test for overall e ect: Z=0.26 (p=0.79) −10 −5 0 5 10 Std. mean di erence IV, random, 95% CI Favours [PVI + CTI abl] Favours [PVI] Radiofrequency application time C Study or subgroup Mesquita et al. 201824 Pontoppidan et al. 200914 Mean PVI + CTI abl 48 32.6 17 10.4 75 73 148 189 43 25.2 18 8.5 113 76 SD Total Total PVI Mean SD 51.3% 48.7% Weight Std. mean di erence IV, random, 95% CI 0.28 [−0.01, 0.58] 0.78 [0.44–1.11] Total (95% CI) 100.0% 0.52 [0.04–1.01] Heterogeneity: Τ2=0.10; χ2=4.75; d.f.=1 (p=0.03); I2=79% Test for overall e ect: Z=2.12 (p=0.03) −10 −5 0 5 10 Std. mean di erence IV, random, 95% CI Favours [PVI + CTI abl] Favours [PVI] All-procedural complications D Study or subgroup Kim et al. 202122 Pontoppidan et al. 200914 PVI + CTI abl 1 5 6 6 183 73 256 259 3 3 183 76 Total Total PVI 74.5% 25.5% Weight 0.01 [−0.03, 0.01] 0.03 [−0.04, 0.04] Total (95% CI) 100.0% −0.00 [−0.04, 0.04] Heterogeneity: Τ2=0.00; χ2=1.72; d.f.=1 (p=0.19); I2=42% Test for overall e ect: Z=0.03 (p=0.97) −10 −5 0 5 10 Favours [PVI + CTI abl] Favours [PVI] Events Events Risk di erence M-H, random, 95% CI Risk di erence M-H, random, 95% CI Total events
had a larger left atrial size (39.67 ± 5.07 mm versus 35.95 ± 5.56 mm; p<0.01). A study from Lee et al. gave us the important lesson that left atrial remodelling or left atrial size plays a major role in the development of ATAs.23
To the best of our knowledge, our study is the first systematic review and meta-analysis study that quantified the efficacy and safety profile of prophylactic CTI ablation in AF patients without documented AFL. A previous meta-analysis study by Romero et al. compared PVI + CTI ablation with PVI alone in AF patients.47 However, that meta-analysis included two RCTs that involved AF patients with documented coexisting AFL.48 49 In our meta-analysis, we did not only exclude the studies from
Wazni et al. and Mohanty et al., but we also added a study from Kim et al.48 49 22 We provided data about procedural aspects, including procedure time, fluoroscopy time, RF application time and all-procedural complications, that were not provided by the prior meta-analysis study by Romero et al.47
Our study revealed that prophylactic CTI ablation could not reduce the risk of ATAs during the follow-up period. Our result supported the prior study by Romero et al. (RR 1.29; 95% CI [0.93–1.79]; p=0.13).47 In our metaanalysis, we also found that prophylactic CTI ablation failed to reduce recurrent AF. This finding could be influenced by the results of the study by Lee et al.23 In that study, the left atrial size in both groups was
Prophylactic CTI Ablation in AF without Documented Typical AFL ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
SE(SMD) SMD RD RD RD SE(RD) SE(RD) RD SE(RD) A B C D E SE(RD) 0 −1 −2 0.1 0.08 0.04 0.06 0.02 0 0.1 0.08 0.04 0.06 0.02 0 0.1 0.08 0.04 0.06 0.02 0 0.1 0.08 0.04 0.06 0.02 0 0.2 0.15 0.1 0.05 0 1 2 0 −0.5 −1 0.5 1 0 −0.5 −1 0.5 1 0 −0.5 −1 0.5 1 0 −0.5 −1 0.5 1
Figure 5: Funnel Plots
A: Atrial tachyarrhythmias; B: Recurrent AF; C: New-onset atrial flutter; D: All procedural complications; and E: Radiofrequency application time show no publication bias.
significantly different. That could be a significant confounder. A larger atrial size was associated with a higher AF recurrence rate following PVI.50 51 Our meta-analysis demonstrated that prophylactic CTI ablation did not reduce new-onset AFL in AF without documented typical AFL patients. In both groups, the most AF ablation was PVI.14,22–24 Additional ablation strategies, such as linear ablation, complex fractionated atrial electrogram ablation and superior vena cava isolation, were conducted if PVI was not effective.14 23 A prior study from Ipek et al. showed that linear ablation for AF was correlated with a higher left AFL incidence following AF ablation.52 In contrast, CTI ablation is the well-known treatment of choice for CTIdependent AFL, and it is crucial to differentiate between CTI-dependent and non-CTI-dependent AFL.20 In this meta-analysis, data about the specific types of AFL were only available in a study by Lee et al.23 Therefore, we could not conduct a subgroup analysis.
As expected, prophylactic CTI ablation required a longer RF application time. However, the procedure time, fluoroscopy time and procedural complications between both groups were not significantly different. Our results suggested that conducting prophylactic CTI ablation in AF patients without documented AFL was ineffective and inefficient. Our results also supported the AF catheter ablation strategy in most of the centres. The first-time AF catheter ablation strategy is high-power, short-duration PVI only.53 54 If any atrial tachyarrhythmia is documented during the follow-up period, additional linear ablation can be considered.
Limitations
Special attention is required in interpreting the results of our study due to the presence of several limitations. First, even though only studies that assessed prophylactic CTI ablation in AF patients without documented AF were included, most of those studies were small studies with a limited number of participants. Second, almost all studies in this meta-analysis involved paroxysmal AF and persistent AF patients. We did not conduct the subgroup analysis based on AF subtypes because: only four studies were included in this meta-analysis; the study from Kim et al. only involved
1. Waldo AL, Feld GK. Inter-relationships of atrial fibrillation and atrial flutter: mechanisms and clinical implications. J Am Coll Cardiol 2008;51:779–86. https://doi.org/10.1016/j. jacc.2007.08.066; PMID: 18294560.
2. Stempfel S, Aeschbacher S, Blum S, et al. Symptoms and quality of life in patients with coexistent atrial fibrillation and atrial flutter. Int J Cardiol Heart Vasc 2020;29:100556. https:// doi.org/10.1016/j.ijcha.2020.100556; PMID: 32577496.
3. Kaneshiro T, Yoshida K, Sekiguchi Y, et al. Crucial role of pulmonary vein firing as an initiator of typical atrial flutter: evidence of a close relationship between atrial fibrillation and typical atrial flutter. J Arrhythm 2017;33:86–91. https:// doi.org/10.1016/j.joa.2016.07.013; PMID: 28416972.
4. Romero J, Diaz JC, Di Biase L, et al. Atrial fibrillation inducibility during cavotricuspid isthmus-dependent atrial flutter ablation as a predictor of clinical atrial fibrillation. A meta-analysis. J Interv Card Electrophysiol 2017;48:307–15. https://doi.org/10.1007/s10840-016-0211-9; PMID: 28070875.
5. Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612; PMID: 32860505.
6. Mark DB, Anstrom KJ, Sheng S, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–85. https://doi.org/10.1001/ jama.2019.0692; PMID: 30874716.
7. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. https://doi.org/10.1001/jama.2019.0693; PMID: 30874766.
8. Marrouche NF, Brachmann J, Andresen D, et al. Catheter
paroxysmal AF patients; other studies included paroxysmal AF and persistent AF, and/or long-standing persistent AF; and we could not access the patients’ individual-level data.14 22–24 Third, the unequal baseline characteristics, especially the left atrial size, could be a confounder, because atrial remodelling plays an essential role as the substrate for ATAs. Fourth, the differences in the mapping system, catheter ablation technology, ablation strategy, operator experience, follow-up period duration, the physicians’ preference-directed AADs usage during the follow-up period and arrhythmia detection method were other issues that could affect the outcomes.
Conclusion
In this meta-analysis, conducting prophylactic CTI ablation during PVI in AF patients without documented AFL did not reduce the risk of ATAs, recurrent AF and new-onset AFL in AF patients. Moreover, prophylactic CTI ablation was associated with a higher risk of ATAs and recurrent AF. In this population, conducting prophylactic CTI ablation during PVI prolonged RF application time. Our study suggested that prophylactic CTI ablation was an ineffective and inefficient approach for AF patients without documented AFL. RCTs with better methods, larger scale and longer follow-up durations are required to obtain better evidence.
Clinical Perspective
• Pulmonary vein isolation (PVI) is the cornerstone of catheter ablation in AF patients.
• Prophylactic cavotricuspid isthmus (CTI) ablation during PVI in AF patients without documented atrial flutter (AFL) fails to reduce the risk of ATAs, recurrent AF and new-onset AFL.
• Prophylactic CTI ablation during PVI in AF patients without documented AFL prolongs radiofrequency application time.
• Prophylactic CTI ablation during PVI in AF patients without documented AFL does not increase complication rates.
ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. https://doi.org/10.1056/NEJMoa1707855; PMID: 29385358.
9. Balk EM, Garlitski AC, Alsheikh-Ali AA, et al. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol 2010;21:1208–16. https://doi.org/10.1111/j.1540-8167.2010. 01798.x; PMID: 20487117.
10. Ganesan AN, Shipp NJ, Brooks AG, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549. https://doi.org/10.1161/JAHA.112.004549; PMID: 23537812.
11. Jaïs P, Hocini M, Hsu LF, et al. Technique and results of linear ablation at the mitral isthmus. Circulation 2004;110:2996–3002. https://doi.org/10.1161/01. CIR.0000146917.75041.58; PMID: 15520313.
12. Di Biase L, Elayi CS, Fahmy TS, et al. Atrial fibrillation ablation strategies for paroxysmal patients: randomized comparison between different techniques. Circ Arrhythm Electrophysiol 2009;2:113–9. https://doi.org/10.1161/ CIRCEP.108.798447; PMID: 19808455.
13. Oral H, Chugh A, Yoshida K, et al. A randomized assessment of the incremental role of ablation of complex fractionated atrial electrograms after antral pulmonary vein isolation for long-lasting persistent atrial fibrillation. J Am Coll Cardiol 2009;53:782–9. https://doi.org/10.1016/j.jacc.2008.10.054; PMID: 19245970.
14. Pontoppidan J, Nielsen JC, Poulsen SH, et al. Prophylactic cavotricuspid isthmus block during atrial fibrillation ablation in patients without atrial flutter: a randomised controlled trial. Heart 2009;95:994–9. https://doi.org/10.1136/ hrt.2008.153965; PMID: 19261602.
15. Rolf S, Kircher S, Arya A, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol
2014;7:825–33. https://doi.org/10.1161/CIRCEP.113.001251; PMID: 25151631.
16. Dong JZ, Sang CH, Yu RH, et al. Prospective randomized comparison between a fixed ‘2C3L’ approach vs. stepwise approach for catheter ablation of persistent atrial fibrillation. Europace 2015;17:1798–806. https://doi.org/10.1093/ europace/euv067; PMID: 25957039.
17. Schreiber D, Rostock T, Fröhlich M, et al. Five-year follow-up after catheter ablation of persistent atrial fibrillation using the stepwise approach and prognostic factors for success. Circ Arrhythm Electrophysiol 2015;8:308–17. https://doi. org/10.1161/CIRCEP.114.001672; PMID: 25744570.
18. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. https://doi.org/10.1056/NEJMoa1408288; PMID: 25946280.
19. Waranugraha Y, Rizal A, Setiawan D, Aziz IJ. Additional complex fractionated atrial electrogram ablation does not improve the outcomes of non-paroxysmal atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Indian Heart J 2021;73:63–73. https://doi. org/10.1016/j.ihj.2020.11.004; PMID: 33714411.
20. Brugada J, Katritsis DG, Arbelo E, et al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia: the Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC). Eur Heart J 2020;41:655–720. https://doi. org/10.1093/eurheartj/ehz467; PMID: 31504425.
21. Koerber SM, Turagam MK, Gautam S, et al. Prophylactic pulmonary vein isolation during cavotricuspid isthmus ablation for atrial flutter: a meta-analysis. Pacing Clin Electrophysiol 2019;42:493–8. https://doi.org/10.1111/ pace.13637; PMID: 30779174.
22. Kim SH, Oh YS, Choi Y, et al. Long-term efficacy of prophylactic cavotricuspid isthmus ablation during atrial fibrillation ablation in patients without typical atrial flutter: a
Prophylactic CTI Ablation in AF without Documented Typical AFL ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
prospective, multicentre, randomized trial. Korean Circ J 2021;51:58–64. https://doi.org/10.4070/kcj.2020.0174; PMID: 33150753.
23. Lee WC, Fang HY, Chen HC, et al. Additional cavotricuspid isthmus block ablation may not improve the outcome of atrial fibrillation ablation. Pacing Clin Electrophysiol 2019;42:1421–8. https://doi.org/10.1111/pace.13799; PMID: 31482578.
24. Mesquita J, Ferreira AM, Cavaco D, et al. Impact of prophylactic cavotricuspid isthmus ablation in atrial fibrillation recurrence after a first pulmonary vein isolation procedure. Int J Cardiol 2018;259:82–7. https://doi. org/10.1016/j.ijcard.2018.01.025; PMID: 29579616.
25. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and metaanalyses of studies that evaluate health care interventions: explanation and elaboration. PLOS Med 2009;6:e1000100. https://doi.org/10.1371/journal.pmed.1000100; PMID: 19621070.
26. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. http://www.ohri.ca/ programs/clinical_epidemiology/oxford.asp (accessed 22 February 2021).
27. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. https://doi.org/10.1136/bmj.l4898; PMID: 31462531.
28. Tiejun Tong group. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. http://www.math.hkbu.edu. hk/~tongt/papers/median2mean.html (accessed 26 February 2021).
29. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or midquartile range. Stat Methods Med Res 2018;27:1785–805. https://doi.org/10.1177/0962280216669183; PMID: 27683581.
30. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. https://doi.org/10.1186/1471-2288-14-135; PMID: 25524443.
31. Cleophas TJ, Zwinderman AH. Modern meta-analysis: review and update of methodologies. Cham, Switzerland: Springer International Publishing, 2017. https://doi.org/10.1007/978-3319-55895-0.
32. Fletcher J. What is heterogeneity and is it important? BMJ 2007;334:94–6. https://doi.org/10.1136/ bmj.39057.406644.68; PMID: 17218716.
33. Waranugraha Y, Rizal A, Setiawan D, Aziz IJ. The benefit of atrioventricular junction ablation for permanent atrial fibrillation and heart failure patients receiving cardiac resynchronization therapy: an updated systematic review and meta-analysis. Indian Pacing Electrophysiol J 2021;21:101–
11. https://doi.org/10.1016/j.ipej.2020.12.005; PMID: 33548449.
34. Anderson RD, Ariyarathna N, Lee G, et al. Catheter ablation versus medical therapy for treatment of ventricular tachycardia associated with structural heart disease: systematic review and meta-analysis of randomized controlled trials and comparison with observational studies. Heart Rhythm 2019;16:1484–91. https://doi.org/10.1016/j. hrthm.2019.05.026; PMID: 31150816.
35. Waranugraha Y, Rizal A, Syaban MFR, et al. Direct comparison of non-vitamin K antagonist oral anticoagulant versus warfarin for stroke prevention in non-valvular atrial fibrillation: a systematic review and meta-analysis of realworld evidences. Egypt Heart J 2021;73:70. https://doi. org/10.1186/s43044-021-00194-1; PMID: 34379219.
36. Voskoboinik A, Moskovitch JT, Harel N, et al. Revisiting pulmonary vein isolation alone for persistent atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm 2017;14:661–7. https://doi.org/10.1016/j. hrthm.2017.01.003; PMID: 28434446.
37. Cheng WH, Lo LW, Lin YJ, et al. Ten-year ablation outcomes of patients with paroxysmal atrial fibrillation undergoing pulmonary vein isolation. Heart Rhythm 2019;16:1327–33. https://doi.org/10.1016/j.hrthm.2019.03.028; PMID: 30946970.
38. Sau A, Howard JP, Al-Aidarous S, et al. Meta-analysis of randomized controlled trials of atrial fibrillation ablation with pulmonary vein isolation versus without. JACC Clin Electrophysiol 2019;5:968–76. https://doi.org/10.1016/j. jacep.2019.05.012; PMID: 31439299.
39. Celikyurt U, Knecht S, Kuehne M, et al. Incidence of newonset atrial fibrillation after cavotricuspid isthmus ablation for atrial flutter. Europace 2017;19:1776–80. https://doi. org/10.1093/europace/euw343; PMID: 28069839.
40. Kwon HJ, Lee SS, Park YJ, et al. Effectiveness and safety of high-power and short-duration ablation for cavotricuspid isthmus ablation in atrial flutter. Pacing Clin Electrophysiol 2020;43:941–6. https://doi.org/10.1111/pace.14019; PMID: 28069839.
41. Cosío FG. Atrial flutter, typical and atypical: a review. Arrhythm Electrophysiol Rev 2017;6:55–62. https://doi. org/10.15420/aer.2017.5.2; PMID: 28835836.
42. Watson RM, Josephson ME. Atrial flutter. I. Electrophysiologic substrates and modes of initiation and termination. Am J Cardiol 1980;45:732–41. https://doi. org/10.1016/0002-9149(80)90115-0; PMID: 7361663.
43. Waldo AL, Cooper TB. Spontaneous onset of type I atrial flutter in patients. J Am Coll Cardiol 1996;28:707–12. https:// doi.org/10.1016/0735-1097(96)00223-9; PMID: 8772760.
44. Ortiz J, Niwano S, Abe H, et al. Mapping the conversion of atrial flutter to atrial fibrillation and atrial fibrillation to atrial flutter. Insights into mechanisms. Circ Res 1994;74:882–94. https://doi.org/10.1161/01.RES.74.5.882; PMID: 8156635.
45. Roithinger FX, Karch MR, Steiner PR, et al. Relationship
between atrial fibrillation and typical atrial flutter in humans: activation sequence changes during spontaneous conversion. Circulation 1997;96:3484–91. https://doi. org/10.1161/01.CIR.96.10.3484; PMID: 9396445.
46. Hsieh MH, Tai CT, Tsai CF, et al. Mechanism of spontaneous transition from typical atrial flutter to atrial fibrillation: role of ectopic atrial fibrillation foci. Pacing Clin Electrophysiol 2001;24:46–52. https://doi.org/10.1046/j.1460-9592.2001. 00046.x; PMID: 11227968.
47. Romero J, Patel K, Briceno D, et al. Cavotricuspid isthmus line in patients undergoing catheter ablation of atrial fibrillation with or without history of typical atrial flutter: a meta-analysis. J Cardiovasc Electrophysiol 2020;31:1987–95. https://doi.org/10.1111/jce.14614; PMID: 32530541.
48. Wazni O, Marrouche NF, Martin DO, et al. Randomized study comparing combined pulmonary vein-left atrial junction disconnection and cavotricuspid isthmus ablation versus pulmonary vein-left atrial junction disconnection alone in patients presenting with typical atrial flutter and atrial fibrillation. Circulation 2003;108:2479–83. https://doi. org/10.1161/01.CIR.0000101684.88679.AB; PMID: 14610012.
49. Mohanty S, Mohanty P, Di Biase L, et al. Results from a single-blind, randomized study comparing the impact of different ablation approaches on long-term procedure outcome in coexistent atrial fibrillation and flutter (APPROVAL). Circulation 2013;127:1853–60. https://doi. org/10.1161/CIRCULATIONAHA.113.001855; PMID: 23572499.
50. Akutsu Y, Kaneko K, Kodama Y, et al. Association between left and right atrial remodeling with atrial fibrillation recurrence after pulmonary vein catheter ablation in patients with paroxysmal atrial fibrillation: a pilot study. Circ Cardiovasc Imaging 2011;4:524–31. https://doi.org/10.1161/ CIRCIMAGING.110.962761; PMID: 21778328.
51. Zhuang J, Wang Y, Tang K, et al. Association between left atrial size and atrial fibrillation recurrence after single circumferential pulmonary vein isolation: a systematic review and meta-analysis of observational studies. Europace 2012;14:638–45. https://doi.org/10.1093/europace/eur364; PMID: 22117033.
52. Ipek GE, Marine JE, Habibi M, et al. Association of left atrial function with incident atypical atrial flutter after atrial fibrillation ablation. Heart Rhythm 2016;13:391–8. https://doi. org/10.1016/j.hrthm.2015.09.028; PMID: 26416618.
53. Kottmaier M, Popa M, Bourier F, et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace 2020;22:388–93. https://doi. org/10.1093/europace/euz342; PMID: 31872249.
54. Waranugraha Y, Rizal A, Firdaus AJ, et al. The superiority of high-power short-duration radiofrequency catheter ablation strategy for atrial fibrillation treatment: a systematic review and meta-analysis study. J Arrhythm 2021;37:975–89. https:// doi.org/10.1002/joa3.12590; PMID: 34386124.
Ablation in AF without Documented Typical AFL ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Prophylactic CTI
Clinical Relevance of Sinus Rhythm Mapping to Quantify Electropathology Related to Atrial Fibrillation
Mathijs S van Schie and Natasja MS de Groot
Department of Cardiology, Erasmus Medical Center, Rotterdam, the Netherlands
Abstract
Progression of AF is accompanied by structural and electrical remodelling, resulting in complex electrical conduction disorders. This is defined as electropathology and it increases with the progression of AF. The severity of electropathology, thus, defines the stage of AF and is a major determinant of effectiveness of AF therapy. As specific features of AF-related electropathology are still unknown, it is essential to first quantify the electrophysiological properties of atrial tissue and then to examine the inter- and intra-individual variation during normal sinus rhythm. Comparison of these parameters between patients with and without a history of AF unravels quantified electrophysiological features that are specific to AF patients. This can help to identify patients at risk for early onset or progression of AF. This review summarises current knowledge on quantified features of atrial electrophysiological properties during sinus rhythm and discusses its relevance in identifying AF-related electropathology.
Keywords
Sinus rhythm mapping, AF, electrophysiology, electropathology
Disclosures: The authors have no conflicts of interest to declare.
Funding: NMSdG is supported by funding grants from CVON-AFFIP (grant number 914728), NWO-Vidi (grant number 91717339), Biosense Webster USA (ICD 783454) and Medical Delta.
Received: 16 January 2022 Accepted: 25 May 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e11. DOI: https://doi.org/10.15420/aer.2022.03
Correspondence: Natasja MS de Groot, Unit Translational Electrophysiology, Department of Cardiology, Erasmus Medical Center, Dr Molewaterplein 40, 3015GD Rotterdam, the Netherlands. E: n.m.s.degroot@erasmusmc.nl
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Electrophysiological mapping strategies targeting atrial tachyarrhythmia, such as AF, have improved considerably over the past few years. However, designing effective ablation strategies for persistent AF remains a major challenge.1 Since the development of the pulmonary vein isolation procedure in the late 1990s, several additional ablation strategies have been proposed, including linear lesions, complex fractionated electrogram (EGM) ablation, low-voltage area (LVA) ablation, and identification and ablation of rotational activity and presumed trigger sites.2–5 Although these techniques showed promising results in single-centre studies, they did not lead to widespread improvement in procedural outcome in multicentre studies.6 This can only partly be due to the fact that there is still an inadequate understanding of the mechanisms and electropathological substrate underlying AF.
Arrhythmogenesis depends on the presence of a substrate and a trigger. AF initially starts in a paroxysmal form, which is mainly based on the presence of triggers.2 Progression of AF is accompanied by structural and electrical remodelling, which can typically be described as a progressive change in electrophysiological properties of the myocardium caused by cardiovascular comorbidities and AF itself.7 This results in complex electrical conduction disorders, which is defined as electropathology.8 With the progression of AF, there is an increase in electropathology. The severity of electropathology thus defines the stage of AF and is a major determinant of the effectiveness of AF therapy.
In an attempt to find the substrate underlying AF, the vast majority of studies directly focus on electrophysiological properties measured during AF or during atrial pacing. However, what the specific features of AFrelated electropathology are, is at present unknown. These features may include conduction delay and block, signal morphology, such as potential voltages and fractionation, and electrical asynchrony between the endoand epicardium, as illustrated in Figure 1. It is essential to first quantify electrophysiological properties of atrial tissue and then to examine the inter- and intra-individual variation in these quantified parameters during normal sinus rhythm (SR). Next, comparison of these parameters between patients with and without a history of AF reveals quantified electrophysiological features that are specific to AF patients. The most suitable objective parameter can then help identify patients at risk for early onset or progression of AF. This review summarises current knowledge on quantified features of atrial electrophysiological properties during SR and explores its relevance in identifying AF-related electropathology.
Excitation of the Atria
It is common knowledge that during SR, electrical activity originates from the sino-atrial node (SAN) area, from where the activation first spreads over the right atrium (RA) and towards the left atrium (LA) via Bachmann’s bundle (BB), coronary sinus (CS) musculature or interatrial septum. BB is considered to be the preferential route of interatrial conduction as it is a highly organised bundle of muscular fibres arranged in parallel fashion
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Atrial Fibrillation
Figure 1: Excitation and Electrophysiological Properties of the Atria
A: Smooth excitation of the right and left atrium via Bachmann’s bundle. B: Impaired conduction across Bachmann’s bundle in a patient with AF results in alternative interatrial routes. C: Colour-coded total excitation maps of the right and left atrium on a schematic posterior view of the atria derived from epicardial mapping. Arrows indicate main trajectories of sinus rhythm wavefront at different atrial regions. D: Quantification of conduction disorders. Left: colour-coded activation map based on local activation times. Thick black lines represent conduction block according to a time difference between adjacent electrodes of ≥12 ms. Middle: local activation pattern with isochronal map. Isochrones are drawn at 5 ms. Right: conduction velocity map of the corresponding local activation maps. E: typical examples of different types of unipolar (upper) and bipolar (lower) electrograms. Morphology of unipolar single potentials is classified according to the relative R to S wave amplitude. CFAE = complex fractionated atrial electrograms; CFP = complex fractionated potential; IVC = inferior vena cava; LAT = local activation time; LDP = long double potential; PV = pulmonary vein; SDP = short double potential; SP = single potential; SVC = superior vena cava.
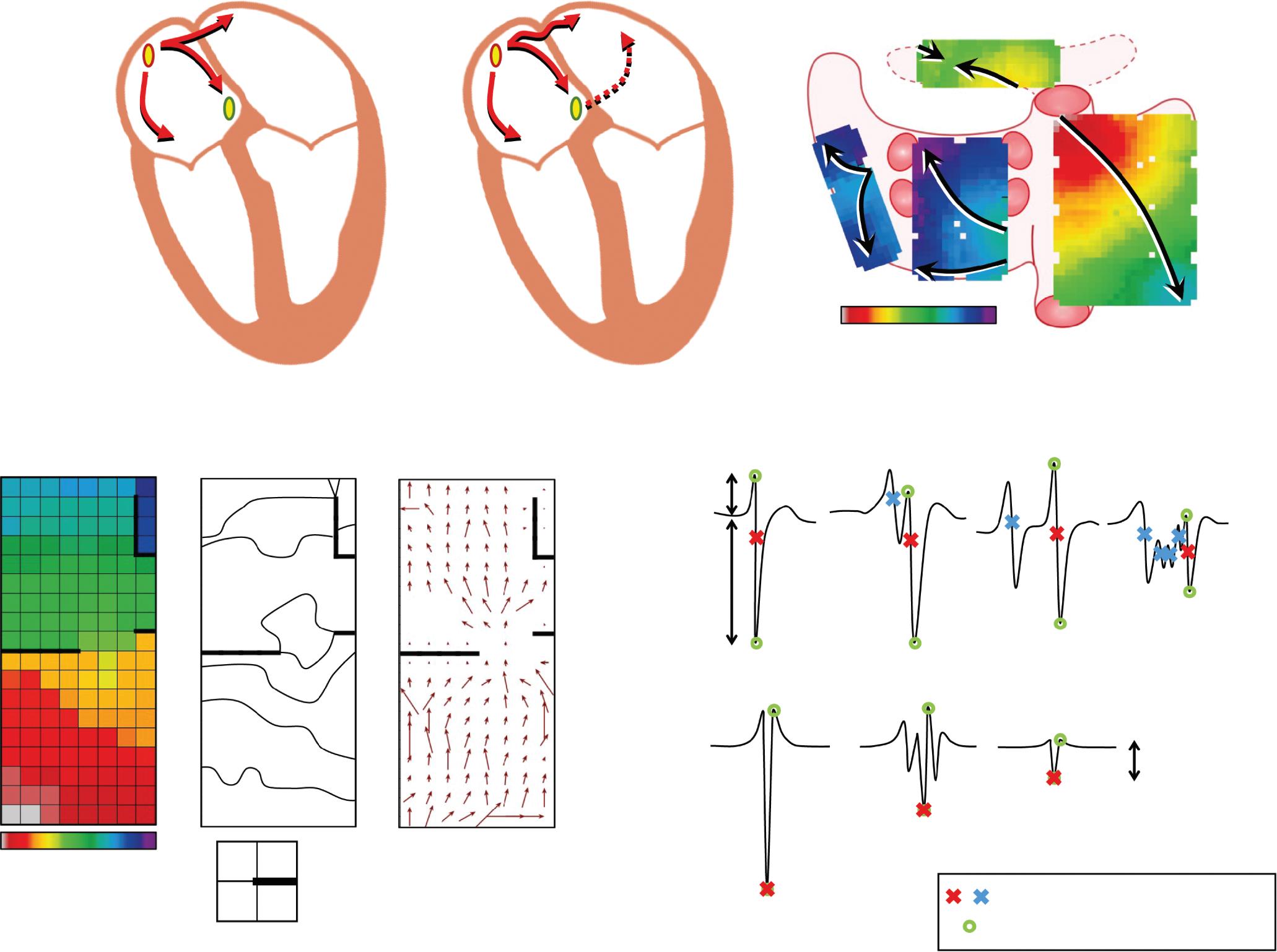
and it is by far the largest of the anatomical interatrial connections.9 Detailed analysis of SR activation was first performed by Boineau et al., who performed epicardial mapping in dogs.10 Later, Cox et al. were the first to create an epicardial isochronal map of one SR beat activating both the right and left atrium in a human.11 Since the 1990s, the advent of electroanatomical mapping systems allowed detailed measurement of impulse propagation inside the human atria. Knowledge of atrial excitation patterns during SR and its variations enabled detection of propagation abnormalities associated with arrhythmia, such as AF.
Spread of the SR wavefront is influenced by membrane properties, tissue structure and wavefront geometry.12,13 Conduction disorders are caused by structural atrial remodelling due to, e.g. (long-standing) pressure and/or volume overload, inflammation, atherosclerosis, myocardial ischaemia/ infarction, interstitial fibrosis or an abnormal anatomy.14–18 Structural remodelling may lead to non-uniform tissue anisotropy and local disorders in conduction, such as slowing of conduction or conduction block (CB). Areas of CB (frequently defined as large activation time differences between two adjacent electrodes or the presence of double potentials)
are thought to play a crucial role in the genesis and perpetuation of AF.12 19 The presence of lines of CB makes it more likely for reentrant circuits to develop, which may increase the likelihood of AF.20 Lines of CB affect propagation of the expanding SR wavefront and could be either structural or functional in nature.
Spach et al. demonstrated that ageing leads to greater changes in conduction when wavefront directions differ, leading to low-voltage, fractionated potentials.21 Wong et al. used CS pacing to assess electrophysiological features during wavefronts propagating in different directions.22 They observed that there was direction-dependent slowing of conduction, prolongation of total atrial excitation times and an increase in number and length of CB lines. These differences were more pronounced in patients with chronic atrial stretch and were associated with a greater susceptibility to develop AF. On the other hand, conduction abnormalities could also be rate-dependent. Huang et al. used CS pacing with cycle lengths varying between 1,000 and 250 ms and demonstrated that rate-dependent CB was present in 94% of patients with AF induction.23 However, by examining 339 atrial extrasystoles during epicardial mapping,
Value of Sinus Rhythm Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Conduction properties Unipolar Bipolar Conduction block ∆LAT ³12 ms 31 0 60 5 5 10 10 15 15 30 35 50 40 45 ms 31 24 15 R S SP SDP CFAE Normal Low voltage Primary/secondary LAT Peak-to-peak amplitude LDP CFP <0.5 mV Non-remodelled atria A B C D E
with AF Total excitation map 0 150 ms Electrogram morphology PV PV PV PV SVC IVC
Patient
Teuwen et al. showed that the incidence of conduction disorders was mainly associated with the degree of aberrancy but not with prematurity.24 Conduction abnormalities could therefore be hidden during normal SR, resulting in a possible underestimation of conduction disorders in studies that only focus on SR.
Conduction Abnormalities in Electrically Non-remodelled Atria
In patients with Wolff-Parkinson-White syndrome with non-dilated atria, Konings et al. were the first to demonstrate that the RA free wall of these near healthy hearts was activated uniformly by a single broad activation wave without any conduction disorders.25 However, Hansson et al. demonstrated in three out of 12 patients undergoing coronary artery bypass grafting (CABG; 63 ± 7.6 years) or surgical transection of an accessory pathway (55 ± 9.9 years) that epicardial mapping of the RA free wall during SR revealed small areas of conduction disorders, covering 2–12% of the total recording area.26 Moreover, the presence of areas of CB was also described at the junction of the right superior pulmonary vein (PV) and the LA in 18 patients (67 ± 11 years) without AF.27 In contrast, Lanters et al. demonstrated in 209 CABG patients (66 ± 9.6 years) with electrically non-remodelled atria that some degree of CB (median 1.3%, range 0.1–4.3%) was present in all patients.28 There was a considerable intra-atrial, but also inter-individual variation in prevalence of CB. However, a predilection site was present at the superior intercaval RA although it did not reflect CB elsewhere in the atria and had no correlation with the development of postoperative AF.
Conduction Abnormalities Predisposing to Postoperative AF
Various studies report on an association between CB during SR and development of AF. Sakamoto et al. performed intra-operative mapping during SR of the RA free wall in 52 patients with a variety of structural heart diseases.29 The presence of non-uniform activation patterns (defined as areas of CB or fusion of multiple wavefronts) was observed in 15 patients (29%) and was associated with development of postoperative AF. Kharbanda et al. examined the RA by simultaneous endo-epicardial mapping in 80 patients and found a relationship between transmural CB at the inferior RA and postoperative AF.30 Other atrial sites were also related to development of postoperative AF. By performing epicardial mapping at BB, Teuwen et al. found that a high amount (defined as >4% CB) and long lines of CB (defined as ≥12 mm) predisposed for early postoperative AF in CABG patients (Table 1).9
Two studies described the association between areas of CB during SR for intraoperative AF inducibility in patients without a history of AF. RobertsThomson et al. compared patients with atrial septal defects with control subjects and demonstrated the presence of conduction disorders in the LA and increased inducibility of AF in the study group.31 In 54 patients with structural heart disease without a history of AF, Van Staveren et al. found that longer lines of CB at BB were related to AF inducibility.32 These observations further support a relationship between the presence of conduction abnormalities and the development of AF.
Conduction Abnormalities Associated with AF Episodes
During SR, electrical activity originates from the SAN area and conducts to the atrial myocardium via several exit pathways.33 The presence of areas of CB around the SAN causes a blockage of these exit pathways leading to shifts of SAN exit sites. Kharbanda et al. demonstrated that the SAN exit pathway in patients with AF was located more caudally compared to
patients without AF.34 By using simultaneous endo-epicardial mapping, they also demonstrated that conduction disorders at the RA were more pronounced in patients with a history of AF.30 In contrast, in an epicardial mapping study involving 253 patients with various underlying heart diseases, Mouws et al. demonstrated that although RA excitation during SR was prolonged in patients with a history of AF, there was no relation between the SAN exit site and total atrial excitation time.35
Previous studies have also demonstrated that patients with a history of AF have more conduction disorders in the RA and LA compared to patients without AF. In 268 patients with and without a history of AF, Mouws et al. showed that patients with AF more often present with continuous lines of CB located at the PV area.36 Heida et al. have demonstrated that patients with various underlying heart diseases and a history of AF had more conduction disorders than patients without AF throughout both atria.37 Van der Does et al. identified the lateral LA as a location with more conduction disorders in patients with valvular heart disease.38 In all aforementioned studies, disturbed conduction at BB during SR was most strongly correlated with the presence of atrial remodelling related to AF episodes (Supplementary Material Table 1).
Bachmann’s Bundle: A Key Player in Arrhythmogenesis?
BB is the preferential route of interatrial conduction. Lemery et al. performed endocardial mapping in 20 patients with either paroxysmal or persistent AF and demonstrated that LA activation occurred via BB in all patients.39 However, Tapanainen et al. demonstrated that although BB is the most common interatrial route, the LA was activated primarily via the rim of the oval fossa region at the interatrial septum or via the CS ostial connections in 15 out of 50 patients with paroxysmal AF.40 A limitation of these studies was that mapping was performed with a low spatial resolution in a small number of patients. Most importantly, BB was not included in these studies as mapping was performed only at the endocardial side.
In several studies it was demonstrated that BB is a predilection site for conduction disorders to occur.35,37,41 In an intraoperative epicardial mapping study, Mouws et al. demonstrated that total atrial excitation times were longer in patients with a history of AF compared to those without AF (136 ± 20 ms versus 114 ± 17 ms, p<0.001).35 This was mainly caused by longer total activation time of the RA and BB (RA: 73 ± 13 ms versus 67 ± 14 ms, p=0.018; BB: 106 ± 20 ms versus 87 ± 16 ms, p<0.001). This resulted in alternative routes for BB and left atrioventricular groove (LAVG) activation, as BB was activated either via one wavefront from right to left, from the central part or via multiple wavefronts. The LAVG was then activated via either BB, the PV area or via both routes, depending on which route had the shortest interatrial excitation time. An increased total activation time of BB was caused by the presence of extensive conduction disorders. Teuwen et al. indeed demonstrated that particularly long lines of longitudinal CB are more pronounced in patients with AF episodes undergoing CABG.9 The resulting delayed right-to-left excitation favoured conduction via other interatrial routes, such as the limbus of the oval fossa, the CS and interatrial bundles both superior and inferior along BB. As demonstrated by Mouws et al., a combination of conduction disorders and distinct parts of the SR wavefront entering asynchronously, the posterior LA can lead to an increased risk of AF.42 Conduction disorders giving rise to alternative propagation routes may be the result of damage to the thick and thin septa surrounding BB myocytes, considerably affecting interatrial conduction.43 This was further supported by the observation of Mouws et al. that LAVG excitation via only the PV area was
Value of Sinus Rhythm Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Table 1: Atrial Excitation in Patients with AF
is the most common route between the atria, the LA was activated primarily via:
• Rim of the oval fossa
• CS ostial connections
delay right-to-left excitation, thereby favouring conduction via other
AVD = aortic valve disease; BB = Bachmann’s bundle; CABG = coronary artery bypass graft; CAD = coronary artery disease; DN PoAF= de novo postoperative AF; CS = coronary sinus; endo = endocardial; epi = epicardial; LA = left atrium; LAVG = left atrioventricular groove; LPsAF = longstanding persistent AF; MVD = mitral valve disease; PAF = paroxysmal AF; PsAF = persistent AF; PVA = pulmonary vein area; RA = right atrium; SAN = sino-atrial node; TAT = total activation time.
considerably slower than via BB (90 ± 18 ms versus 101 ± 20 ms, p<0.001).35 Patients with either AF, LA dilation or mitral valve disease (MVD) had particularly longer total excitation times of the atria, which was mainly determined by impaired conduction along BB. Based on these observations it was suggested that total atrial excitation times are particularly affected by conduction disorders at BB and RA, which are likely related to the presence of AF. This was also demonstrated by Heida et al., who recently showed in a large cohort of 447 patients that a history of AF was associated with the slowing of conduction; patients with AF had more conduction times (CT) ≥4 ms (≈50 cm/s), especially at the BB, LA and PV areas.37 In addition, maximum CT for AF patients was larger than in non-AF patients. In the majority of studies, it was consistently demonstrated that an increased amount of conduction disorders at BB is present in AF patients. The highly organised architecture of BB could make this structure more vulnerable to structural remodelling and consequently disturbances in conduction that can even be identified during SR.
Pro-arrhythmic Features of Ablation-created Conduction Block
There appears to be a clear link between the presence of conduction disorders and the development of AF. However, CB can also be introduced during extensive catheter ablation procedures targeting complex fractionated EGMs or LVAs, for example. From a theoretical point of view, the resulting ablation lesions causing local CB may be involved in the initiation and perpetuation of AF. This is also the case if linear lesions were incomplete. Postoperative arrhythmias are not uncommon after AF surgery and are typically reentrant and related to the surgically created lesions.44 A combination of pre-existing conduction disorders and manually created lesions can therefore also provide additional substrate underlying AF recurrence.
Assessment of Cardiac Conduction Velocity
Cardiac conduction velocity (CV) provides important information on the properties of the underlying myocardium and is therefore widely used in electrophysiological studies. Changes in intercellular electrical coupling
and tissue structure contribute to conduction heterogeneity and CV reduction, which in turn play a major role in the initiation and perpetuation of AF.21 However, there is no agreement over which is the best technique to compute CV.45 Calculation of CV is frequently based on a certain distance travelled by a propagating wavefront in a unit of time. This results in an accurate CV estimate if the propagation is uniform and the direction is known. A straightforward method to estimate CV is by using isochronal maps in which isopotential lines are drawn over a fixed time interval. The CV is then estimated by examining the distance travelled over a fixed time window. To also automatically include an estimation of the local propagation direction, a minimum of three electrodes is typically required to establish a velocity vector. Also, an adequate spatial resolution is required to minimise CV estimation errors, especially when working with complex and heterogeneous activation wavefronts. During standard electrophysiology studies, multi-electrode catheters enable CV estimation by techniques such as triangulation.45 Using regularly spaced high-density electrode arrays, simultaneous recordings on a high spatial resolution scale enable analysis of complex and heterogeneous patterns of activation by using techniques such as finite differences, polynomial surface fitting, discrete velocity vectors or omnipolar EGMs (O-EGMs).46,47 A disadvantage of the finite differences, polynomial surface fitting and O-EGM techniques is that they require data interpolation and smoothening, thereby masking local conduction heterogeneity.
Local CV can be estimated by triangulation using 3D electroanatomical activation maps. Using this methodology, Stiles et al. demonstrated that CV in patients with paroxysmal AF was especially lower at the RA and LA septal parts compared to controls with a left-sided accessory pathway (RA: 210 ± 50 cm/s versus 130 ± 30 cm/s, p<0.001; LA: 220 ± 40 cm/s versus 120 ± 20 cm/s, p<0.001).48 Prabhu et al. demonstrated that during CS pacing in patients with (long-standing) persistent AF, CV in the RA was lower compared to the LA (93 ± 15 cm/s versus 101 ± 19 cm/s, p=0.02), also particularly at the septal parts of the atria.49 Another study by Zheng et al. reported that during SR, the average CVs of the RA were lower in paroxysmal AF patients compared to controls with atrioventricular nodal
Value of Sinus Rhythm Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Authors Study Population Site of Interest Mapping Sites Recording Site Patients (n) AF Patients (n) AF Type Outcomes Kharbanda et al. 202134 CAD MVD AVD SAN exit pathways RA Endo-epi 20 8 PAF AF patients: caudal sinus node activity
Lemery et al. 200439 AF Interatrial conduction RA, LA Endo 20 15 5 PAF PsAF LA activation mainly via BB Tapanainen et al. 200940 AF Interatrial conduction RA, LA Endo 50 50 PAF Although
caused by changes in preferential SAN exit pathways
BB
Teuwen et al. 20169 CABG Interatrial conduction BB Epi 185 13 56 PAF DN PoAF Conduction disorders
interatrial routes Mouws et al. 201835 CAD MVD AVD Interatrial conduction RA, BB, PVA, LA Epi 253 33 9 1 PAF PsAF LPsAF AF patients found to have: • Alternative routes for BB and LAVG activation • Prolongation of TAT Mouws et al. 201842 CAD MVD AVD LA PVA Epi 327 47 14 1 PAF PsAF LPsAF AF patients found to have: • Complex patterns with multiple entry sites
reentrant tachycardia (60 ± 12 cm/s versus 83 ± 13 cm/s, p<0.05).50 Remarkably, CV estimates in this control group were much lower than CVs assessed in other studies.9 26 51 In addition, no differences were found between the septum and other parts of the atria. Although the triangulation methodology has been automated to generate high-density CV maps of clinically acquired data, studies focusing on CV comparison in AF patients are lacking.52
Teuwen et al. reported that CV (measured by using isochronal maps) across BB is about 90 cm/s in patients undergoing CABG, which was comparable with an average CV of 88 cm/s at the RA free wall in patients with Wolff-Parkinson-White syndrome and patients with ischaemic and/or valvular heart disease.9,26,46,53 However, in the case of local conduction heterogeneities, a more sophisticated method is required. Van Schie et al. Have developed a discrete velocity vectors methodology to estimate local CVs.46 It was demonstrated that patients with paroxysmal AF have slower conduction across BB (± 10 cm/s) during SR compared to patients without AF. In addition, more areas of slow CV (<30 cm/s) were found at the BB and PV area. Heida et al. recently reported similar observations in a casecontrol study of 34 patients with and without a history of AF.53 In a specific population of MVD patients, a history of AF was characterised by decreased CV and unipolar single potential amplitudes at BB due to loss of S wave amplitudes.54 Areas of slowed conduction have frequently been linked to low-voltage potentials, which formed the basis for current ablation strategies targeting LVAs for AF.12,55–57
Voltage Mapping Techniques
Structurally remodelled tissue gives rise to slowing of conduction or conduction disorders. These areas are frequently identified using the spatial distribution of EGM amplitudes, commonly known as voltage mapping. However, there is still a lack of consensus on how to accurately use voltage mapping to target the AF substrate and how to define abnormal voltage.58 Also, there are several voltage modalities which can be used such as unipolar, bipolar and multipolar (omnipolar/Laplacian) voltage mapping.59 In short, a unipolar EGM (U-EGM) is recorded as an extracellular potential difference between one single electrode on the tissue relative to an indifferent electrode, while a bipolar EGM (Bi-EGM) is simply the subtraction of two U-EGMs. Therefore, a U-EGM can be regarded as the sum of instantaneous current dipoles of a wavefront, reflecting cardiac electrical activity of the tissue surrounding the recording electrode. As the amplitude depends on the volume of simultaneously activated cardiac tissue, synchronous activation of the myocardium results in relatively large amplitude U-EGMs, whereas areas of asynchronously activated myocardium cause a decrease in U-EGM amplitudes.19
Although U-EGMs are more sensitive to far-field and remote activations, U-EGM potential morphology contains additional information on the progression of the wavefront.54 Nevertheless, Bi-EGMs are still predominantly used in clinical practice as Bi-EGMs are less sensitive to noise and represent more local information from the tissue between two electrodes. However, Bi-EGMs have high directional sensitivity, which is particularly important when targeting voltage abnormalities during stable SR.60 A so-called omnipolar mapping technique has been proposed to overcome this directional sensitivity. Using multiple neighbouring electrodes, O-EGMs are mathematically constructed to represent maximal bipolar voltage along the direction of a propagating wavefront.47 However, a major disadvantage of all voltage mapping techniques is their dependency on the electrode size, as larger electrodes result in lower voltages and consequently more LVAs in the tissue.61 It therefore remains difficult to directly compare study outcomes.
Bipolar Voltage Mapping
Several studies have shown local variation in bipolar voltages during SR, in which the majority of the studies focused on the LA only (Supplementary Material Table 2). In addition, all studies described regional voltage analyses as part of ablation therapy targeting LVAs. These areas are commonly defined as bipolar voltages ≤0.5 mV.62 Marcus et al. were the first to characterise regional differences in bipolar voltage during SR in 22 patients with paroxysmal AF.63 They demonstrated that AF patients exhibited significantly more LVAs in the septum and posterior LA walls compared to patients with focal atrial tachycardia. However, a major disadvantage of this study was that the AF patients were mapped with an 8 mm catheter, whereas all control patients were mapped with a 4 mm catheter. During pacing from the CS, Teh et al. demonstrated that patients with AF have circumscriptive areas of lower voltages, more LVAs, slowing of conduction and fractionated EGMs compared to age-matched control patients with a left-sided accessory pathway.64 These changes were more pronounced in persistent AF patients who were first cardioverted when they did not present in SR.
Two years later, Rolf et al. were then the first to describe the use of SR voltage mapping guiding AF substrate modification after circumferential PV isolation and demonstrated that LVAs occur more frequently in patients with persistent AF compared to patients with paroxysmal AF.4 Lin et al. also demonstrated a decrease in mean LA voltage and a higher incidence of LVAs during SR with progression of AF.65 Regional LA differences were studied in more detail by Kapa et al., who demonstrated that there was a heterogeneity in voltage distribution in all patients regardless of prior LA ablation.66 The highest voltage was found in the LA floor and lowest in the posterior LA wall adjoining the PVs, while Rodríguez-Mañero et al. found the highest values in the LA appendage.67 Kogawa et al. compared these regional differences between patients with paroxysmal or persistent AF during SR.68 They demonstrated that bipolar voltages were specifically lower at the LA septum, roof and posterior wall, right superior PV and its antrum, right superior PV carina and right inferior PV antrum in patients with persistent AF.
As AF therapy commonly targets in the LA, data on regional differences in bipolar biatrial voltage during SR is lacking. However, Stiles et al. showed that mean RA and LA bipolar voltage was reduced in 25 patients with paroxysmal AF compared with 25 patients with left-sided accessory pathways.48 More specifically, areas at the high-lateral RA, posterior LA and LA roof in AF patients were more likely to be LVAs. Although during CS pacing, Prabhu et al. demonstrated that global bipolar voltage did not differ between the RA and LA, but voltages were larger at the posterior LA compared to posterior RA in patients with (long-standing) persistent AF.49 Although specific regional differences in bipolar voltages are more recognised, cut-off values to identify LVAs are currently not customised to the different atrial voltage distributions. Despite ablation therapy based on bipolar LVAs possibly being beneficial in certain patient populations, the efficacy and long-term outcome remains controversial.56 It is for these reasons that U-EGMs are regaining popularity.
Unipolar Voltage Mapping
Unlike Bi-EGMs, U-EGMs are directionally independent and provide additional information on wavefront progression. Remarkably, studies focusing on unipolar voltage distribution during SR are still lacking, although the use of U-EGMs has regained interest in clinical practice. Using biatrial electroanatomical mapping, Prabhu et al. demonstrated that unipolar voltages were higher in the LA compared to the RA in patients with (long-standing) persistent AF during CS pacing (2.95 ± 1.14
Value of Sinus Rhythm Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 2: Endo-epicardial Asynchrony and Focal Activation Pattern
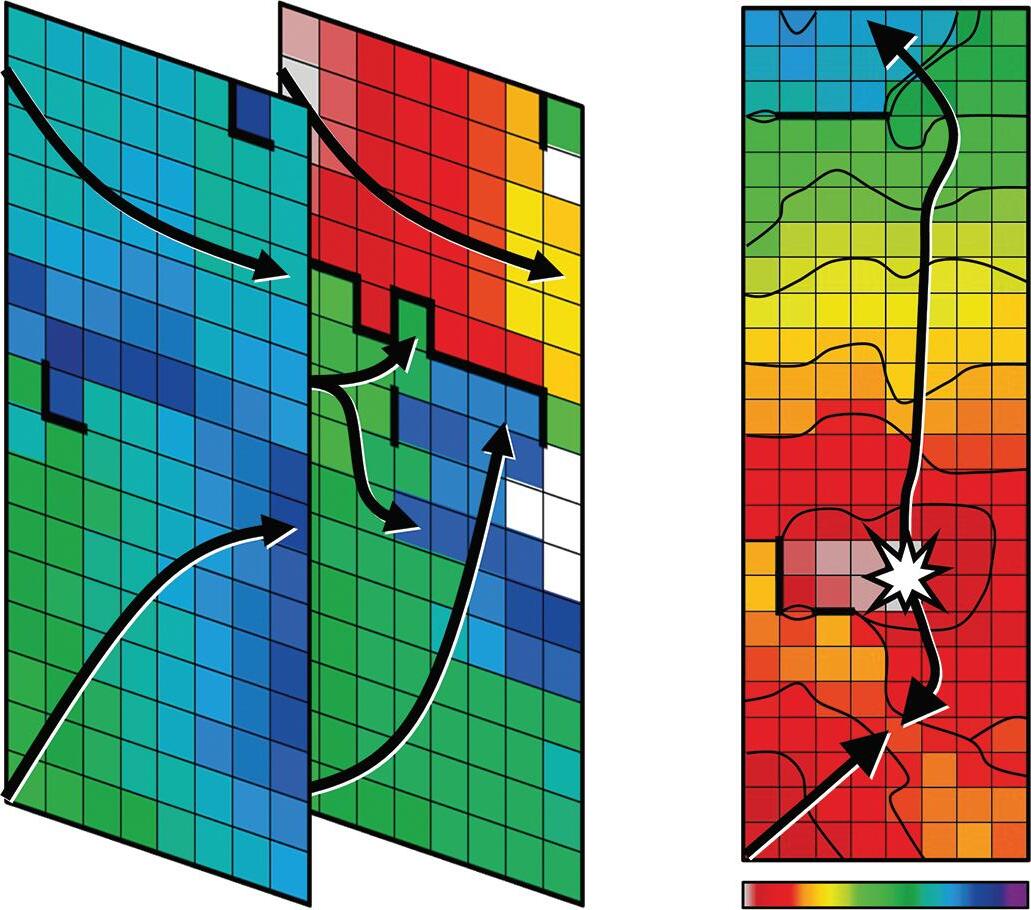
demonstrated that fractionation during SR is also found in areas characterised by normal voltages and CV.72 Nevertheless, fractionation is still commonly used to identify arrhythmogenic substrate with either U-EGMs or Bi-EGMs. However, fractionation can also occur during SR in patients without electrically remodelled atria, although it is more frequent in patients with AF.73
While potential annotation in U-EGMs is quite straightforward, Bi-EGM annotation is more complex, particularly when identifying complex fractionated potentials. It is therefore not surprising that no predilection sites for fractionation or differences in the degree and spatial distribution of fractionation between patients with paroxysmal or persistent AF could be found in a large review of 84 studies targeting mostly Bi-EGM, complex fractionated EGMs.74
Epicardial Endocardial
A: Endo-epicardial asynchrony recorded during sinus rhythm at the left atrium. Arrows indicate the main direction of the propagating wavefront and thick black lines represent conduction block. The activation wavefront enters the recording area at the upper part of the endocardial side only and is blocked in the middle of the area. The middle and lower parts of the recording area are activated 30 ms later and within the following 30 ms the whole recorded epicardial area was also activated.80 B: Focal activation pattern recorded during sinus rhythm at Bachmann’s bundle. Isochrones are drawn at 5 ms intervals and the origin of the focal activation pattern is demonstrated by an asterisk.
versus 2.28 ± 0.65 mV, p=0.002).49 More specifically, unipolar voltages were particularly higher in the posterior, lateral and septal parts of the LA.
Another U-EGM voltage parameter was introduced by Lin et al., who used peak negative voltages to study the voltage distribution in patients with paroxysmal AF compared to other supraventricular tachycardia.69 These peak negative voltages are defined as the negative portions of U-EGMs. They demonstrated that global RA peak negative voltages were reduced and more heterogeneously distributed in AF patients compared to patients with either atrioventricular nodal reentrant tachycardia, focal atrial tachycardia or atrial flutter. Van Schie et al. have demonstrated that unipolar voltage is lower in areas of slowed conduction or CB, or areas containing fractionated potentials, which might indicate areas of arrhythmogenic tissue.70 In this study, unipolar LVAs were found in all 67 patients with MVD with and without a history of paroxysmal AF, although lower voltages and more LVAs were found at BB in paroxysmal AF patients. There were no predilection sites for low voltages to occur. Patients with paroxysmal AF were also characterised by decreased single potential amplitudes at BB due to loss of S-wave amplitudes together with a decreased CV.54 Remarkably, no further studies are available focusing on unipolar voltages during SR. Recently, Van Schie et al. demonstrated that bipolar LVAs can still contain large unipolar voltages and high CVs.60 Future studies could therefore focus on the combination of unipolar and bipolar voltages to improve voltage-guided ablation therapy.
Use of Electrogram Morphology and Fractionation
Another parameter of potential morphology includes potential fractionation. However, it remains unclear how fractionated potentials need to be defined as many different definitions and recording methodologies have been introduced.71 Although fractionated EGMs are frequently linked to LVAs and slowed conduction, Viles-Gonzalez et al.
Fractionation of U-EGMs, on the other hand, has been studied less extensively. In the late 1990s, Konings et al. described a classification of U-EGMs based on the number of negative deflections, which is easy to measure, especially during SR.75 Van Schie et al. have demonstrated that MVD patients with paroxysmal AF had more unipolar fractionated potentials at the PV area compared to those without a history of AF.70 However, fractionation can also be a consequence of variation in the anatomy of the atrial wall and therefore functional anisotropy. U-EGM and Bi-EGM fractionation has also been linked to asynchronous activation of the endo- and epicardium, which may be a significant mechanism for the persistence of AF.76–78 Van der Does et al. demonstrated a moderate-high sensitivity (65–78%) for areas of electrical asynchrony between endo- and epicardial layer for U-EGM and Bi-EGM fractionation.77 However, fractionation could not only be explained by local endo-epicardial differences in fractionation, but also by inhomogeneous conduction patterns in solely the endo- or epicardial plane. However, whether EGM fractionation therefore represents a proper target for ablative therapy remains questionable.
Endo-epicardial Asynchrony and Breakthrough Waves
Endo-epicardial asynchrony (EEA) and breakthrough waves do not only exist during AF but also during SR, particularly in areas with a thicker atrial wall.79 The presence of intramural conduction disorders may enhance EEA (Figure 2). Kharbanda et al. were the first to demonstrate that patients with (long-standing) persistent AF already have more EEA during SR in the inferior RA compared to those without AF.30 This was also linked to a higher amount of conduction disorders in that area. Kharbanda et al. also demonstrated in a case report that extensive EEA was present in the LA in three patients with paroxysmal AF.80 Although this study included only three patients, the highest degree of EEA was found in the patient with the longest history of AF. This indicates that even during SR the degree of EEA could indeed be related to AF duration and that early intervention may prevent progression of AF.
Enhanced EEA may result in transmural propagation of waves which breakthrough in the opposite layer. These sites can be identified as focal activation patterns (FAPs). These FAPs occur frequently during AF and they are the key elements of AF-related electropathology.81 82 FAPs are not only present during AF, but also during SR. Mouws et al. demonstrated that epicardial FAPs are present in over a third of patients at various sites in the RA, LA and BB, particularly in thicker parts of the atrial wall.83 There was also a clear difference in R/S ratio between unipolar potentials recorded at SAN-FAP sites and other epicardial FAPs; SAN-FAPs were more often characterised by a full S wave
Value of Sinus Rhythm Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
35 30 25 20 15 10 10 10 5 30 25 20 15 10 10 10 0 60 ms
Endo-epicardial synchrony A B Focal activation pattern
morphology, while other FAPs consisted more often of an RS or rS wave morphology. This could indicate that muscular connections between the endo- and epicardium underlie FAPs and that a slight degree of EEA is already enough for FAPs to occur in some areas during SR. As the incidences and spatial distribution of these FAPs during SR were similar between patients with and without history of AF, most of these FAPs could be based on anatomical substrate and therefore be physiological. It is likely that further aggravation of structural remodelling enhances local conduction disorders and EEA, facilitating transmural propagation of wavefronts and hence the development of AF. In addition, AF-induced remodelling may further facilitate EEA and enhance the occurrence of FAPs during AF, thereby promoting AF persistence. When a more extensive arrhythmogenic substrate is present in the atria, ablative therapy is more likely to fail.
Clinical Implications and Future Directions
During SR, several electrophysiological parameters have been identified as indicators of AF-related electropathology. These indicators were not only found at the LA, but also at the RA and even more frequently at BB. This clearly indicates the presence of AF-related electropathology outside the LA, confirming the hypothesis that AF is not just a solely left-sided disease. Even during SR, electropathology may be missed when mapping is performed only at the endocardium or epicardium. Hence, conduction is 3D and complex even during SR. Remarkably, patients with AF already
1. Roten L, Derval N, Jais P. Catheter ablation for persistent atrial fibrillation: elimination of triggers is not sufficient. Circ Arrhythm Electrophysiol 2012;5:1224–32. https://doi. org/10.1161/CIRCEP.112.974873; PMID: 23250552.
2. Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. https:// doi.org/10.1056/NEJM199809033391003; PMID: 9725923.
3. Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044–53. https://doi.org/10.1016/j.jacc.2003.12.054; PMID: 15172410.
4. Rolf S, Kircher S, Arya A, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:825–33. https://doi.org/10.1161/CIRCEP.113.001251; PMID: 25151631.
5. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (Conventional Ablation for Atrial Fibrillation with or without Focal Impulse and Rotor Modulation) trial. J Am Coll Cardiol 2012;60:628–36. https://doi.org/10.1016/j. jacc.2012.05.022; PMID: 22818076.
6. Schmidt B, Brugada J, Arbelo E, et al. Ablation strategies for different types of atrial fibrillation in Europe: results of the ESC-EORP EHRA Atrial Fibrillation Ablation Long-Term registry. Europace 2020;22:558–66. https://doi.org/10.1093/ europace/euz318; PMID: 31821488.
7. Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995;92:1954–68. https://doi.org/10.1161/01.cir.92.7.1954; PMID: 7671380.
8. Allessie MA, de Groot NM, Houben RP, et al. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol 2010;3:606–15. https://doi.org/10.1161/CIRCEP.109.910125; PMID: 20719881.
9. Teuwen CP, Yaksh A, Lanters EA, et al. Relevance of conduction disorders in Bachmann’s bundle during sinus rhythm in humans. Circ Arrhythm Electrophysiol 2016;9:e003972. https://doi.org/10.1161/CIRCEP.115.003972; PMID: 27153879.
10. Boineau JP, Schuessler RB, Mooney CR, et al. Multicentric origin of the atrial depolarization wave: the pacemaker complex. Relation to dynamics of atrial conduction, P-wave changes and heart rate control. Circulation 1978;58:1036–48. https://doi.org/10.1161/01.cir.58.6.1036; PMID: 709760.
11. Cox JL, Canavan TE, Schuessler RB, et al. The surgical treatment of atrial fibrillation II. Intraoperative
have more electropathology detectable during SR compared to patients without AF, particularly at BB. However, which parameters are most suitable as indicators for this purpose remains unknown. It also remains unclear whether electropathology is a cause or consequence of AF. Certainly, AF itself can also contribute to a certain amount of electropathology. On the other hand, electropathology can be manually introduced during catheter ablation therapy. To investigate whether electropathology during SR is associated with AF, the next step in mapping is to correlate quantified electrophysiological parameters during SR with parameters measured during AF at same site. Once AF-related electropathology is identified, it can be used as an electrical marker to guide ablative therapy of AF.
Clinical Perspective
• Progression of AF is accompanied by structural and electrical remodelling, which results in complex electrical conduction disorders defined as electropathology.
• AF-related electropathology is not only confined to the left atrium, but it is also present in the right atrium and at Bachmann’s bundle.
• Patients with AF already have more electropathology detectable during sinus rhythm compared to patients without AF.
electrophysiologic mapping and description of the electrophysiologic basis of atrial flutter and atrial fibrillation. J Thorac Cardiovasc Surg 1991;101:406–26. https://doi. org/10.1016/S0022-5223(19)36723-6; PMID: 1999934.
12. Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 2004;84:431–88. https://doi.org/10.1152/ physrev.00025.2003; PMID: 15044680.
13. Spach MS, Dolber PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res 1988;62:811–32. https://doi.org/10.1161/01.res.62.4.811; PMID: 2450697.
14. Darby AE, Dimarco JP. Management of atrial fibrillation in patients with structural heart disease. Circulation 2012;125:945–57. https://doi.org/10.1161/ CIRCULATIONAHA.111.019935; PMID: 22354975.
15. Alasady M, Abhayaratna WP, Leong DP, et al. Coronary artery disease affecting the atrial branches is an independent determinant of atrial fibrillation after myocardial infarction. Heart Rhythm 2011;8:955–60. https:// doi.org/10.1016/j.hrthm.2011.02.016; PMID: 21338715.
16. Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J 2015;79:495–502. https://doi.org/10.1253/circj.CJ-15-0138; PMID: 25746525.
17. Heeringa J, van der Kuip DA, Hofman A, et al. Subclinical atherosclerosis and risk of atrial fibrillation: the Rotterdam study. Arch Intern Med 2007;167:382–7. https://doi. org/10.1001/archinte.167.4.382; PMID: 17325300.
18. Hernandez-Madrid A, Paul T, Abrams D, et al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown-up Congenital Heart Disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace 2018;20:1719–53. https://doi.org/10.1093/europace/eux380; PMID: 29579186.
19. Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: a major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol 1997;20:397–413. https://doi. org/10.1111/j.1540-8159.1997.tb06199.x; PMID: 9058844.
20. Ortiz J, Niwano S, Abe H, et al. Mapping the conversion of atrial flutter to atrial fibrillation and atrial fibrillation to atrial flutter. Insights into mechanisms. Circ Res 1994;74:882–94. https://doi.org/10.1161/01.res.74.5.882; PMID: 8156635.
21. Spach MS, Dolber PC. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic
level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res 1986;58:356–71. https://doi.org/10.1161/01. res.58.3.356; PMID: 3719925.
22. Wong CX, John B, Brooks AG, et al. Direction-dependent conduction abnormalities in the chronically stretched atria. Europace 2012;14:954–61. https://doi.org/10.1093/europace/ eur428; PMID: 22308090.
23. Huang D, Marine JE, Li JB, et al. Association of ratedependent conduction block between eccentric coronary sinus to left atrial connections with inducible atrial fibrillation and flutter. Circ Arrhythm Electrophysiol 2017;10:e004637. https://doi.org/10.1161/CIRCEP.116.004637; PMID: 28039281.
24. Teuwen CP, Kik C, van der Does LJME, et al. Quantification of the arrhythmogenic effects of spontaneous atrial extrasystole using high-resolution epicardial mapping. Circ Arrhythm Electrophysiol 2018;11:e005745. https://doi. org/10.1161/CIRCEP.117.005745; PMID: 29269560.
25. Konings KT, Kirchhof CJ, Smeets JR, et al. High-density mapping of electrically induced atrial fibrillation in humans. Circulation 1994;89:1665–80. https://doi.org/10.1161/01. cir.89.4.1665; PMID: 8149534.
26. Hansson A, Holm M, Blomstrom P, et al. Right atrial free wall conduction velocity and degree of anisotropy in patients with stable sinus rhythm studied during open heart surgery. Eur Heart J 1998;19:293–300. https://doi.org/10.1053/ euhj.1997.0742; PMID: 9519324.
27. Lee G, Spence S, Teh A, et al. High-density epicardial mapping of the pulmonary vein-left atrial junction in humans: insights into mechanisms of pulmonary vein arrhythmogenesis. Heart Rhythm 2012;9:258–64. https://doi. org/10.1016/j.hrthm.2011.09.010; PMID: 21907170.
28. Lanters EAH, Yaksh A, Teuwen CP, et al. Spatial distribution of conduction disorders during sinus rhythm. Int J Cardiol 2017;249:220–5. https://doi.org/10.1016/j.ijcard.2017.08.067; PMID: 28888481.
29. Sakamoto S, Yamauchi S, Yamashita H, et al. Intraoperative mapping of the right atrial free wall during sinus rhythm: variety of activation patterns and incidence of postoperative atrial fibrillation. Eur J Cardiothorac Surg 2006;30:132–9. https://doi.org/10.1016/j.ejcts.2006.03.060; PMID: 16730998.
30. Kharbanda RK, Knops P, van der Does LJME, et al. Simultaneous endo-epicardial mapping of the human right atrium: unraveling atrial excitation. J Am Heart Assoc 2020;9:e017069. https://doi.org/10.1161/JAHA.120.017069; PMID: 32808551.
31. Roberts-Thomson KC, John B, Worthley SG, et al. Left atrial remodeling in patients with atrial septal defects. Heart Rhythm 2009;6:1000–6. https://doi.org/10.1016/j. hrthm.2009.03.050; PMID: 19493703.
Value of Sinus Rhythm Mapping ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
32. van Staveren LN, van der Does WFB, Heida A, et al. AF inducibility is related to conduction abnormalities at Bachmann’s bundle. J Clin Med 2021;10:5536. https://doi. org/10.3390/jcm10235536; PMID: 34884237.
33. Stiles MK, Brooks AG, Roberts-Thomson KC, et al. Highdensity mapping of the sinus node in humans: role of preferential pathways and the effect of remodeling. J Cardiovasc Electrophysiol 2010;21:532–9. https://doi. org/10.1111/j.1540-8167.2009.01644.x; PMID: 19912447.
34. Kharbanda RK, Wesselius FJ, van Schie MS, et al. Endoepicardial mapping of in vivo human sinoatrial node activity. JACC Clin Electrophysiol 2021;7:693–702. https://doi. org/10.1016/j.jacep.2020.11.017; PMID: 33640354.
35. Mouws EMJP, Lanters EAH, Teuwen CP, et al. Impact of ischemic and valvular heart disease on atrial excitation: a high-resolution epicardial mapping study. J Am Heart Assoc 2018;7:e008331. https://doi.org/10.1161/JAHA.117.008331; PMID: 29519812.
36. Mouws EMJP, van der Does LJME, Kik C, et al. Impact of the arrhythmogenic potential of long lines of conduction slowing at the pulmonary vein area. Heart Rhythm 2019;16:511–9. https://doi.org/10.1016/j.hrthm.2018.10.027; PMID: 30744910.
37. Heida A, van der Does WFB, van Staveren LN, et al. Conduction heterogeneity: impact of underlying heart disease and atrial fibrillation. JACC Clin Electrophysiol 2020;6:1844–54. https://doi.org/10.1016/j. jacep.2020.09.030; PMID: 33357582.
38. van der Does LJME, Lanters EAH, Teuwen CP, et al. The effects of valvular heart disease on atrial conduction during sinus rhythm. J Cardiovasc Transl Res 2020;13:632–9. https:// doi.org/10.1007/s12265-019-09936-8; PMID: 31773460.
39. Lemery R, Soucie L, Martin B, et al. Human study of biatrial electrical coupling: determinants of endocardial septal activation and conduction over interatrial connections. Circulation 2004;110:2083–9. https://doi.org/10.1161/01. CIR.0000144461.83835.A1; PMID: 15466628.
40. Tapanainen JM, Jurkko R, Holmqvist F, et al. Interatrial rightto-left conduction in patients with paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2009;25:117–22. https://doi.org/10.1007/s10840-008-9359-2; PMID: 19283459.
41. Houck CA, Lanters EAH, Heida A, et al. Distribution of conduction disorders in patients with congenital heart disease and right atrial volume overload. JACC Clin Electrophysiol 2020;6:537–48. https://doi.org/10.1016/j. jacep.2019.12.009; PMID: 32439038.
42. Mouws EMJP, Kik C, van der Does LJME, et al. Novel insights in the activation patterns at the pulmonary vein area. Circ Arrhythm Electrophysiol 2018;11:e006720. https://doi. org/10.1161/CIRCEP.118.006720; PMID: 30520348.
43. van Campenhout MJ, Yaksh A, Kik C, et al. Bachmann’s bundle: a key player in the development of atrial fibrillation? Circ Arrhythm Electrophysiol 2013;6:1041–6. https://doi. org/10.1161/CIRCEP.113.000758; PMID: 24129206.
44. McElderry HT, McGiffin DC, Plumb VJ, et al. Proarrhythmic aspects of atrial fibrillation surgery: mechanisms of postoperative macroreentrant tachycardias. Circulation 2008;117:155–62. https://doi.org/10.1161/ CIRCULATIONAHA.107.688358; PMID: 18158363.
45. Cantwell CD, Roney CH, Ng FS, et al. Techniques for automated local activation time annotation and conduction velocity estimation in cardiac mapping. Comput Biol Med 2015;65:229–42. https://doi.org/10.1016/j. compbiomed.2015.04.027; PMID: 25978869.
46. van Schie MS, Heida A, Taverne YJHJ, et al. Identification of local atrial conduction heterogeneities using high-density conduction velocity estimation. Europace 2021;23:1815–25. https://doi.org/10.1093/europace/euab088; PMID: 33970234.
47. Deno DC, Balachandran R, Morgan D, et al. Orientationindependent catheter-based characterization of myocardial activation. IEEE Trans Bio Med Eng 2017;64:1067–77. https:// doi.org/10.1109/TBME.2016.2589158; PMID: 27411215.
48. Stiles MK, John B, Wong CX, et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the ‘second factor’. J Am Coll Cardiol 2009;53:1182–91. https://doi.org/10.1016/j.jacc.2008.11.054; PMID: 19341858.
49. Prabhu S, Voskoboinik A, McLellan AJA, et al. A comparison of the electrophysiologic and electroanatomic characteristics between the right and left atrium in persistent atrial fibrillation: is the right atrium a window into the left? J Cardiovasc Electrophysiol 2017;28:1109–16. https:// doi.org/10.1111/jce.13297; PMID: 28730651.
50. Zheng Y, Xia Y, Carlson J, et al. Atrial average conduction velocity in patients with and without paroxysmal atrial fibrillation. Clin Physiol Funct Imaging 2017;37:596–601. https://doi.org/10.1111/cpf.12342; PMID: 26762841.
51. Kojodjojo P, Kanagaratnam P, Markides V, et al. Age-related changes in human left and right atrial conduction. J Cardiovasc Electrophysiol 2006;17:120–7. https://doi.org/10.1111/ j.1540-8167.2005.00293.x; PMID: 16533247.
52. Cantwell CD, Roney CH, Ali RL, et al. A software platform for the comparative analysis of electroanatomic and imaging data including conduction velocity mapping. Annu Int Conf IEEE Eng Med Biol Soc 2014;2014:1591–4. https://doi. org/10.1109/EMBC.2014.6943908; PMID: 25570276.
53. Heida A, van Schie MS, van der Does WFB, et al. Reduction of conduction velocity in patients with atrial fibrillation. J Clin Med 2021;10:2614. https://doi.org/10.3390/jcm10122614; PMID: 34198544.
54. van Schie MS, Starreveld R, Roos-Serote MC, et al. Classification of sinus rhythm single potential morphology in patients with mitral valve disease. Europace 2020;22:1509–19. https://doi.org/10.1093/europace/euaa130; PMID: 33033830.
55. Miyamoto K, Tsuchiya T, Narita S, et al. Bipolar electrogram amplitudes in the left atrium are related to local conduction velocity in patients with atrial fibrillation. Europace 2009;11:1597–605. https://doi.org/10.1093/europace/eup352; PMID: 19910315.
56. Yagishita A, Gimbel JR, Olivera SDE, et al. Long-term outcome of left atrial voltage-guided substrate ablation during atrial fibrillation: a novel adjunctive ablation strategy. J Cardiovasc Electrophysiol 2017;28:147–55. https://doi. org/10.1111/jce.13122; PMID: 27862561.
57. Roberts-Thomson KC, Kistler PM, Sanders P, et al. Fractionated atrial electrograms during sinus rhythm: relationship to age, voltage, and conduction velocity. Heart Rhythm 2009;6:587–91. https://doi.org/10.1016/j. hrthm.2009.02.023; PMID: 19329365.
58. Sim I, Bishop M, O’Neill M, Williams SE. Left atrial voltage mapping: defining and targeting the atrial fibrillation substrate. J Interv Card Electrophysiol 2019;56:213–27. https:// doi.org/10.1007/s10840-019-00537-8; PMID: 31076965.
59. de Groot NMS, Shah D, Boyle PM, et al. Critical appraisal of technologies to assess electrical activity during atrial fibrillation: a position paper from the European Heart Rhythm Association and European Society of Cardiology Working Group on eCardiology in collaboration with the Heart Rhythm Society, Asia Pacific Heart Rhythm Society, Latin American Heart Rhythm Society and Computing in Cardiology. Europace 2022;24:313–30. https://doi. org/10.1093/europace/euab254; PMID: 34878119.
60. van Schie MS, Kharbanda RK, Houck CA, et al. Identification of low-voltage areas: a unipolar, bipolar, and omnipolar perspective. Circ Arrhythm Electrophysiol 2021;14:e009912. https://doi.org/10.1161/CIRCEP.121.009912; PMID: 34143644.
61. Abdi B, van Schie MS, Groot NMS, Hendriks RC. Analyzing the effect of electrode size on electrogram and activation map properties. Comput Biol Med 2021;134:104467. https:// doi.org/10.1016/j.compbiomed.2021.104467; PMID: 34044208.
62. Kottkamp H, Berg J, Bender R, et al. Box isolation of fibrotic areas (BIFA): a patient-tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol 2016;27:22–30. https://doi.org/10.1111/jce.12870; PMID: 26511713.
63. Marcus GM, Yang Y, Varosy PD, et al. Regional left atrial voltage in patients with atrial fibrillation. Heart Rhythm 2007;4:138–44. https://doi.org/10.1016/j.hrthm.2006.10.017; PMID: 17275746.
64. Teh AW, Kistler PM, Lee G, et al. Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. J Cardiovasc Electrophysiol 2012;23:232–8. https://doi. org/10.1111/j.1540-8167.2011.02178.x; PMID: 21955090.
65. Lin Y, Yang B, Garcia FC, et al. Comparison of left atrial electrophysiologic abnormalities during sinus rhythm in patients with different type of atrial fibrillation. J Interv Card Electrophysiol 2014;39:57–67. https://doi.org/10.1007/s10840013-9838-y; PMID: 24113851.
66. Kapa S, Desjardins B, Callans DJ, et al. Contact electroanatomic mapping derived voltage criteria for characterizing left atrial scar in patients undergoing ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2014;25:1044–52. https://doi.org/10.1111/jce.12452; PMID: 24832482.
67. Rodriguez-Manero M, Valderrabano M, Baluja A, et al. Validating left atrial low voltage areas during atrial fibrillation and atrial flutter using multielectrode automated electroanatomic mapping. JACC Clin Electrophysiol 2018;4:1541–52. https://doi.org/10.1016/j.jacep.2018.08.015; PMID: 30573117.
68. Kogawa R, Okumura Y, Watanabe I, et al. Left atrial remodeling: regional differences between paroxysmal and persistent atrial fibrillation. J Arrhythm 2017;33:483–7. https:// doi.org/10.1016/j.joa.2017.06.001; PMID: 29021854.
69. Lin YJ, Tai CT, Huang JL, et al. Characterization of right atrial substrate in patients with supraventricular tachyarrhythmias. J Cardiovasc Electrophysiol 2005;16:173–80. https://doi. org/10.1046/j.1540-8167.2005.40513.x; PMID: 15720456.
70. van Schie MS, Starreveld R, Bogers AJJC, de Groot NMS. Sinus rhythm voltage fingerprinting in patients with mitral valve disease using a high-density epicardial mapping approach. Europace 2021;23:469–78. https://doi.org/10.1093/ europace/euaa336; PMID: 33432326.
71. van der Does LJ, de Groot NM. Inhomogeneity and complexity in defining fractionated electrograms. Heart Rhythm 2017;14:616–24. https://doi.org/10.1016/j. hrthm.2017.01.021; PMID: 28104483.
72. Viles-Gonzalez JF, Gomes JA, Miller MA, et al. Areas with complex fractionated atrial electrograms recorded after pulmonary vein isolation represent normal voltage and conduction velocity in sinus rhythm. Europace 2013;15:339–46. https://doi.org/10.1093/europace/eus321; PMID: 23148118.
73. Tanigawa M, Fukatani M, Konoe A, et al. Prolonged and fractionated right atrial electrograms during sinus rhythm in patients with paroxysmal atrial fibrillation and sick sinus node syndrome. J Am Coll Cardiol 1991;17:403–8. https://doi. org/10.1016/s0735-1097(10)80106-8; PMID: 1991897.
74. Starreveld R, van der Does LJME, de Groot NMS. Anatomical hotspots of fractionated electrograms in the left and right atrium: do they exist? Europace 2019;21:60–72. https://doi. org/10.1093/europace/euy059; PMID: 29688325.
75. Konings KT, Smeets JL, Penn OC, et al. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation 1997;95:1231–41. https://doi. org/10.1161/01.cir.95.5.1231; PMID: 9054854.
76. de Groot N, van der Does L, Yaksh A, et al. Direct proof of endo-epicardial asynchrony of the atrial wall during atrial fibrillation in humans. Circ Arrhythm Electrophysiol 2016;9:e003648. https://doi.org/10.1161/CIRCEP.115.003648; PMID: 27103089.
77. van der Does LJME, Starreveld R, Kharbanda RK, et al. Detection of endo-epicardial asynchrony in the atrial wall using one-sided unipolar and bipolar electrograms. J Cardiovasc Transl Res 2021;14:902–11. https://doi.org/10.1007/ s12265-021-10111-1; PMID: 33782858.
78. van der Does LJME, Knops P, Teuwen CP, et al. Unipolar atrial electrogram morphology from an epicardial and endocardial perspective. Heart Rhythm 2018;15:879–87. https://doi.org/10.1016/j.hrthm.2018.02.020; PMID: 29476825.
79. Schuessler RB, Kawamoto T, Hand DE, et al. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation 1993;88:250–63. https://doi.org/10.1161/01.cir.88.1.250; PMID: 8319340.
80. Kharbanda RK, Kik C, Knops P, et al. First evidence of endoepicardial asynchrony of the left atrial wall in humans. JACC Case Rep 2020;2:745–9. https://doi.org/10.1016/j. jaccas.2020.02.027; PMID: 34317340.
81. de Groot NM, Houben RP, Smeets JL, et al. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation 2010;122:1674–82. https://doi.org/10.1161/CIRCULATIONAHA.109.910901; PMID: 20937979.
82. Eckstein J, Zeemering S, Linz D, et al. Transmural conduction is the predominant mechanism of breakthrough during atrial fibrillation: evidence from simultaneous endoepicardial high-density activation mapping. Circ Arrhythm Electrophysiol 2013;6:334–41. https://doi.org/10.1161/ CIRCEP.113.000342; PMID: 23512204.
83. Mouws EMJP, Lanters EAH, Teuwen CP, et al. Epicardial breakthrough waves during sinus rhythm: depiction of the arrhythmogenic substrate? Circ Arrhythm Electrophysiol 2017;10:e005145. https://doi.org/10.1161/CIRCEP.117.005145; PMID: 28912205.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Value of Sinus Rhythm Mapping
A Chronicle of Hybrid Atrial Fibrillation Ablation Therapy: From
Cox Maze to Convergent
Riyaz A Kaba , 1,2 Omar Ahmed , 1 Elijah Behr 1 and Aziz Momin1,2
Abstract
The burden of AF is increasing in prevalence and healthcare resource usage in the UK and worldwide. It can result in impaired quality of life for affected patients, as well as increased risk of stroke, heart failure and mortality. A holistic, integrated approach to AF management is recommended, which may include a focus on reducing risk factors and on medical management with anticoagulation and anti-arrhythmic drugs. There are also various ablation strategies that may be considered when anti-arrhythmic drugs fail to alleviate symptoms and reduce AF burden. These ablation techniques range from standalone percutaneous endocardial catheter ablation to open surgical ablation procedures concomitant with cardiac surgery. More recently, hybrid ablation that combines aspects of both surgical and electrophysiologically targeted ablation has been described. This article reviews the evolution of ablation strategies, beginning with the origin of the Cox maze IV procedure and continuing to the recent hybrid convergent approach, and provides a summary of the associated outcomes.
Keywords
Atrial fibrillation, hybrid ablation, history
Disclosure: RAK and AM conduct educational sessions for AtriCure. All other authors have no conflicts of interest to declare.
Acknowledgements: The authors thank Ms Yashasvi Awasthi and Dr Kristen Plasseraud for their kind assistance in the preparation of this article.
Received: 24 January 2022 Accepted: 6 May 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e12. DOI: https://doi.org/10.15420/aer.2022.05
Correspondence: Riyaz A. Kaba, Cardiovascular Clinical Academic Group, Molecular and Clinical Sciences Institute, St George’s, University of London and St George’s University Hospitals NHS Foundation Trust, London SW17 0QT, UK. E: rkaba@sgul.ac.uk
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
AF Burden and Multi-pronged Approaches to Management
AF is the most common cardiac arrhythmia and is estimated to affect more than 46 million people worldwide, with a prevalence that is expected to continue to increase in the next 30–40 years.1 AF can have substantial impacts on quality of life and carries an increased risk of ischaemic stroke, heart failure and mortality.2 A recent study in the UK reported a 20% mortality rate within the first year of AF diagnosis, with excess deaths due to AF primarily attributable to cardiovascular disease, infection and metabolic disorders.3 Additionally, AF is economically burdensome to the healthcare system. In the UK, it has been estimated that AF may have resulted in direct healthcare costs of £1,435 million–£2,548 million in 2020, primarily from hospital admissions.4 5
Current European Society of Cardiology (ESC) guidelines recommend a holistic approach to AF management, using the ABC framework to streamline patient care, focused on stroke risk assessment, symptoms and concomitant conditions and risk factors.6 An integrated approach to patient care in AF is aimed at reducing adverse events and mortality through consideration of optimal treatment pathways. Risk factor reduction, medical management with anticoagulation and rate- or rhythmcontrolling drugs, and ablation are all potential aspects of an AF treatment paradigm, with careful patient selection being essential when considering
the various treatment pathways. With respect to medical management, recent evidence has suggested that early rhythm control through antiarrhythmic drugs (AADs) or ablation may result in better cardiovascular outcomes than traditional care focused on rate control.7 Overall, AADs are given a higher level of recommendation as first-line AF therapy compared with catheter ablation.6 8 However, recent randomised clinical trials have suggested that endocardial cryoballoon ablation may achieve better clinical outcomes as a first approach compared with AADs in paroxysmal AF.9–11 A meta-analysis of randomised clinical trials also suggested that endocardial radiofrequency (RF) ablation is associated with a reduced rate of AF recurrence, albeit a similar rate of adverse events, compared with AADs.12
Traditionally, if AADs are used as first-line therapy and subsequently fail, ablative therapies are then typically recommended. The appropriate type of ablation in part depends on the type and duration of AF, as well as on the risk factors for AF recurrence and anatomical factors.
Favourable success rates have been achieved with endocardial catheter ablation for drug-refractory paroxysmal AF, which is defined as terminating spontaneously or with intervention within 7 days of onset.13 In paroxysmal AF, endocardial catheter ablation with RF or cryothermal (cryoballoon) energy is primarily focused on isolation of the pulmonary veins (PVs), which
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Atrial Fibrillation
1. Cardiovascular Clinical Academic Group, Molecular and Clinical Sciences Institute, St George’s, University of London and St George’s University Hospitals NHS Foundation Trust, London, UK; 2. Department of Cardiology, Ashford and St Peter’s NHS Foundation Trust, Chertsey, Surrey, UK
were identified in 1998 as being the primary source of AF triggers.14 Pulmonary vein isolation (PVI) is considered the cornerstone of AF treatment. However, persistent AF (continuous AF for more than 7 days and up to 12 months) and longstanding persistent AF (continuous AF for more than 12 months) are more difficult to treat with conventional PVI-focused endocardial ablation (Supplementary Material Figure 1). Better outcomes for these patients are typically reported with surgical ablation; however, it is limited in scope because a more invasive procedure is usually performed concomitantly with planned cardiac surgical procedures. Hybrid AF ablation aims to leverage the advantages of endocardial and surgical ablation and offset their limitations in a standalone, minimally invasive procedure. In this review we examine the evolution of hybrid epicardial–endocardial ablation from the spectrum of percutaneous endocardial and surgical epicardial ablation techniques and provide a perspective on potential advances to hybrid ablation procedures.
Endocardial Ablation
Two recent clinical trials suggested that newer endocardial catheters may achieve favourable results in persistent AF occurring for up to 6–12 months in duration. Freedom from atrial arrhythmias without initiating new or higher dose AADs was 54.8% at 12 months following cryoballoon ablation in patients with a mean persistent AF duration of 7.2 months.15 One repeat ablation was permitted in the 3-month blanking period. In another study, patients with a mean persistent AF duration of 15.9 months had a freedom from atrial arrhythmias off new or higher dose AADs of 61.7% at 15 months with RF catheter ablation (including up to two repeat ablations in the 6-month period following the index procedure).16 Even with these advances, there are some limitations to endocardial ablation. One confounder is the phenomenon of endocardial–epicardial dissociation wherein the endocardial and epicardial surfaces have offset phase and activation patterns.17 In effect, endocardial-only (or epicardial-only) ablation may not be sufficient. Additionally, endocardial ablation focused on PV isolation does not address extra-PV triggers and substrates that become more prevalent in persistent and longstanding persistent AF, such as the left atrial posterior wall and left atrial appendage (LAA).18–21 Although meta-analyses have reported a potential benefit of endocardial posterior wall ablation in PVI, and there is a growing trend to undertake such an approach, there is no absolute consensus on posterior wall ablation that is used routinely.18 22 Measures to reduce thermal injury risk may in turn limit the ability to create durable, transmural lesions. Endocardial ablation focused on PVI has particularly poor results in longstanding persistent AF, with variable results reported for adjunctive substrate ablation.23,24
Cox Maze Surgical Ablation
Concomitant Surgical Ablation
Surgical ablation, notably the Cox maze procedure, is associated with better outcomes in persistent and longstanding persistent AF. The Cox
maze procedure was originally a cut-and-sew technique, using a series of incisions and sewing, to create a maze of transmural conduction blocks to prevent macro re-entry circuits. The first Cox maze procedure was performed in 1987, and subsequently 32 patients received the original Cox maze I procedure.25,26 The technique was then modified due to the observation that some patients could not generate an appropriate sinus tachycardia during exercise and had occasional left atrial dysfunction. Incisions around the sinoatrial node were eliminated and replaced with a right atrial counterincision, with additional modification to move the left atrial dome incision posteriorly. This became the Cox maze II, which was performed in 15 patients.26 However, the changes in the Cox maze II technique required complex surgery to the superior vena cava (SVC), including pericardial patching of one incision into the SVC orifice, and did not completely resolve issues with Cox maze I. Interatrial conduction was found to be delayed using both techniques. Thus, the Cox maze III lesion pattern was created, in which again the left atrial dome lesion was moved posteriorly to the extent that it was posterior to the SVC, enabling easier exposure of the left atrium. The evolution from Cox maze I to Cox maze III both protected the sinoatrial node and improved interatrial conduction compared with the previous iterations. In the first 123 patients treated with these cut-and-sew techniques, three early deaths occurred and the most common complications were fluid accumulation (resolved with spironolactone) and perioperative atrial arrhythmias. At a single centre, long-term rhythm outcomes of the cut-and-sew Cox maze procedure in 198 patients were highly favourable, with an estimated 92% freedom from AF at 14 years in patients who had Cox maze as a standalone procedure, and 97% freedom from AF in patients who had a concomitant procedure at 10 years.27 However, overall, the cut-and-sew technique was not widely adopted due to its technical difficulty and substantial morbidity, primarily left atrial dysfunction and pacemaker implantation.
Linear ablation using various energy sources was evaluated. Ablation with RF and cryothermal energy devices offered an alternative means of creating transmural lesions in order to block abnormal conduction as an alternative to cutting and sewing. Gaynor et al. reported the first prospective outcomes of the Cox maze IV procedure, which replaced most of the incisions with RF and cryothermal ablations, leaving only one left atrial and two right atrial incisions.28 Early results suggested a high level of clinical success, with 6-month freedom from AF of 91% with no operative mortality. Outcomes of the cut-and-sew maze technique have been compared with those of Cox maze IV ablation with RF energy augmented by cryoablation, and were found to have similar long-term rhythm outcomes, mortality rates and need for pacemaker intervention.29 The distinction is that the use of surgical ablation devices substantially decreased the technical complexity and length of the procedure, permitting more widespread adoption. Experimental data in explanted human hearts have demonstrated that two applications of bipolar RF energy can achieve 100% transmurality.30
Since the initial report, an investigational device exemption clinical trial and post-approval study reported the safety and efficacy of Cox maze IV procedures using bipolar RF (Figure 1) and cryoablation, and long-term success with surgical ablation concomitant with cardiac surgery procedures.31–34 An important consideration to reported outcomes after surgical ablation is the lesion set used. A true Cox maze IV lesion set is biatrial (Figure 2). Left atrial lesions are focused on right and left PV antrum isolation with roof and floor connecting lines, a connecting lesion to the LAA, and the mitral valve annulus.31 Right atrial lesions include the SVC and inferior vena cava, right atrial appendage lines to the freewall and tricuspid valve annulus, and tricuspid valve annulus to atriotomy. The
Chronicle of Hybrid AF Ablation Therapy ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 1: Bipolar Radiofrequency Clamp Used for Cardiac Ablation
Source: Reproduced with permission from AtriCure, Inc.
LAA is also closed. To complete the Cox maze IV lesion set, RF ablation is used for most lesions, with cryoablation applied to the tricuspid and mitral valves to avoid valvular stenosis. Surgical ablation can also be performed with cryoablation only, however, the Cox maze III lesion set is performed in this case. Observational studies have compared cryothermal surgical ablation with RF ablation alone and RF with adjunctive cryoablation. Vural et al. reported that there was no significant difference in sinus rhythm restoration at 12 months between surgical ablation performed with cryothermal energy only compared with RF energy.35 The authors created identical lesion sets with cryothermal and RF energy, however, the lesions shown are not the full Cox maze lesion set and do not show a coronary sinus lesion. Additionally, a limitation of RF-only ablation is that the mitral and tricuspid annular pathways cannot be fully addressed. As previously mentioned, cryothermal energy is preferred because it preserves the collagen matrix and avoids valvular stenosis. Ad et al. compared the outcomes of concomitant Cox maze III with cryothermal energy with those of Cox maze IV with RF and adjunctive cryothermal energy.36 Sinus rhythm restoration regardless of AADs was statistically similar between the cryothermal and RF–cryothermal groups. However, off AADs, sinus rhythm restoration was higher with cryothermal ablation at 6, 36 and 60 months after the procedure, with fewer cardioversions and catheter ablations during 60-month follow-up. Embolic stroke rate was significantly decreased with cryothermal-only ablation.36
Based on its efficacy without the addition of increased operative mortality or morbidity, concomitant surgical ablation with mitral valve surgeries has been given a class 1A recommendation and a class 1B recommendation in non-mitral valve surgeries by the Society of Thoracic Surgeons (STS).37
The ESC/European Association of Cardio-Thoracic Surgery (EACTS) guidelines assign a class 2a (level A) recommendation to concomitant surgical ablation with cardiac surgery.6
Despite the reported positive long-term outcomes and consensus recommendations, concomitant Cox maze IV procedures are still not
performed across the board in patients with preoperative AF who are undergoing planned cardiac surgery. In an analysis of the STS database between 2011 and 2014, 48% of patients with AF who underwent nonemergency cardiac operations had concomitant AF ablation.37 After propensity matching, patients who had surgical ablation had improved 30-day mortality and stroke rates. Lower rates of concomitant surgical ablation have been reported with non-mitral valve surgeries, despite published evidence suggesting that they have similar safety and effectiveness to concomitant ablation with mitral valve surgeries.38,39
Standalone Surgical Ablation
Not all patients who have AF are candidates for or are in need of a primary cardiac surgery procedure, such as coronary artery bypass grafting or valve repair. Long-term outcomes of standalone Cox maze procedures performed via sternotomy or right thoracotomy have been reported.40,41 Procedures were performed with cardiopulmonary bypass support. Ad et al. analysed the STS database from 2012 to 2016 and found that standalone AF ablation procedures increased by 7% during that time period.41 However, based on the database, most standalone surgical ablation procedures between 2014 and 2017 were performed off-pump. Off-pump surgical ablation is performed on the beating heart and therefore atriotomies and endocardial lesions are not made.
Thoracoscopic Ablation
Video-assisted and totally thoracoscopic approaches to surgical ablation have been described.42 43 Epicardial RF ablation is restricted to the left atrium and is used to isolate the PVs and make other lesions such as roof line, floor line and ganglionated plexi, and the LAA is closed. The totally thoracoscopic maze (TT maze) procedure uses bilateral port access to isolate the PVs, create a box lesion and trigonum line, and close the LAA.44 The Wolf mini-maze procedure includes video-assisted thoracoscopic PV isolation, ganglionated plexi ablation and LAA closure.45 The two procedural terms are sometimes used interchangeably, however, one difference in the approaches is that the mini-maze procedure uses
Chronicle of Hybrid AF Ablation Therapy ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 2: Cox Maze IV Lesion Set Performed with Bipolar Radiofrequency and Cryothermal Ablation
Left atrial appendage
Coronary sinus
Mitral annulus
Circumflex coronary artery IVC
SVC
Right atrial appendage
Right coronary artery
Tricuspid annulus
1 1 2 2 3 3 4 4 5 5 6a 6b
Bipolar RF clamp Surgical incision Cryothermal: endocardial Cryothermal: epicardial
IVC = inferior vena cava; RF = radiofrequency; SVC = superior vena cava. Source: Reproduced with permission from AtriCure, Inc.
5–6 cm bilateral incisions for working ports whereas the TT maze procedure uses smaller bilateral working ports.45 Tricuspid and mitral valve annulus lesions that are usually made with cryoablation in the Cox maze IV procedure are not created with these epicardial approaches.
The effectiveness of epicardial surgical video-assisted thoracoscopic ablation has been shown to be superior to that of endocardial catheter ablation in a randomised trial, albeit with higher procedural complications.46 However, success rates reported in the literature are generally higher with full, on-pump Cox maze IV surgical ablation than epicardial surgical ablation (93% versus 80% with anti-arrhythmic agents, respectively, and 87% versus 72% without anti-arrhythmic agents, respectively).47 Potential reasons for this are that epicardial-only lesions are restricted to the left atrium, and it is difficult to ensure transmural ablation when ablating only from the epicardial surface.
Hybrid Epicardial–Endocardial Ablation Strategies
The concept of a hybrid epicardial–endocardial ablation procedure was developed in part to combine advantageous aspects of existing surgical and electrophysiology approaches into a minimally invasive technique that could be performed on the beating heart by a multidisciplinary team. In particular, key criteria to be met were the creation of transmural, contiguous lesions through both epicardial and endocardial ablation, the use of intraoperative, anatomical lesion visualisation (by the surgeon) and subsequent electro-topographical mapping (by the electrophysiologist) to verify procedural success, and the promotion of multidisciplinary patient care.48 There are two main approaches to hybrid ablation, distinguished by the means of pericardial access and epicardial ablation technique. One hybrid ablation approach accesses the posterior left atrium thoracoscopically and the other approach achieves left atrial access endoscopically through a subxiphoid (or, less commonly, transdiaphragmatic) incision. Overall, the goals of each approach are similar: to overcome the limitations of epicardial-only surgical ablation and endocardial-only catheter ablation by combining techniques to isolate the posterior left atrium and PVs.

Hybrid Thoracoscopic Ablation
In 2011 a thoracoscopic hybrid epicardial–endocardial technique was described by Mahapatra et al. (Figure 3).49 This procedure differs by approach, which is totally thoracoscopic, and ablation requires different
RF devices. The epicardial lesion set is aimed at isolating the posterior wall and PVs, with endocardial ablation used to address gaps identified by electrophysiological mapping. The LAA can be addressed thoracoscopically as concomitant with the ablation procedure. A meta-analysis of hybrid thoracoscopic ablation compared with endocardial catheter ablation found higher freedom from atrial arrhythmia recurrence with the hybrid technique, although complications were increased with hybrid ablation.50 The authors noted that six of the 13 studies on hybrid ablation included early procedural experience or complications in some of the first patients treated. Seventy-one per cent of patients were in sinus rhythm off AADs at least 12 months after the hybrid procedure, compared with 50% with catheter ablation.50 Individual complications that were higher with hybrid thoracoscopic ablation included bleeding requiring transfusion, conversion to sternotomy, cardiac tamponade, hospital mortality, pacemaker implantation, phrenic nerve injury, pneumothorax, and insignificant PV stenosis. Rates of other complications such as bleeding requiring reoperation, groin haematoma requiring therapy, PV stenosis requiring stenting, and stroke/transient ischaemic attack were statistically similar between the groups. In general, there are some safety considerations for unilateral thoracoscopic hybrid ablation with respect to the potential for prolonged unilateral lung ventilation, duration of hospital stay, and occurrence of common complications such as pleuropericarditis.51
Preliminary outcomes from a randomised trial comparing thoracoscopic hybrid ablation with catheter ablation were recently reported.52 In 41 patients, 83% of patients who received hybrid ablation were free from atrial arrhythmias without AADs at 12 months compared with 45% who received endocardial catheter ablation (p=0.015), with a similar quality of life and no reported increase in major adverse events in the hybrid group. An additional randomised IDE (investigational device exemption) trial and a single-arm trial are pending.
Hybrid Convergent Ablation
Kiser et al. first described the hybrid convergent procedure, in which epicardial lesions are created by the cardiothoracic surgeon under endoscopic visualisation, followed by electroanatomical mapping and catheter ablation by the electrophysiologist to complete PVI and address gaps left by the epicardial lesion set.48 Unlike traditional epicardial surgical ablation using bipolar clamps, a unipolar, irrigated RF catheter was used through a pericardioscopic cannula with a guidewire. In this respect, the hybrid convergent procedure is the least invasive hybrid procedure currently available. As with thoracoscopic hybrid ablation, preexisting pericardial adhesions may be prohibitive for the use of the hybrid convergent procedure in patients who have had prior cardiac surgery. The initial epicardial lesion set was extensive and closely resembled the extra-cardiac maze lesion set, which was originally performed concomitant with cardiac surgery as described by Kiser et al. in 2007 (Figure 4), and later as a paracardioscopic minimally invasive procedure.53,54 This included linear ablation of the posterior PV antrum, as well as ablation on the anterior aspect of the PVs, along the coronary sinus, ligament of Marshall, and SVC. Following surgical closure, electrophysiological mapping was performed to guide endocardial catheter ablation, which was focused on PVI, coronary sinus isolation and cavotricuspid isthmus (CTI).48 The goal of the original combined procedure was to achieve isolation of the posterior left atrium and PVs, isolation or conduction block of the coronary sinus, and CTI conduction block.
Several studies were published that used this early lesion set and early generations of the unipolar RF device.55–57 Overall, the rhythm outcomes achieved were favourable for a population of primarily persistent and
Chronicle of Hybrid AF Ablation Therapy ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 3: Hybrid Thoracoscopic Epicardial–Endocardial Lesion Set Described by Mahapatra et al.
Coronary sinus line
Cavotricuspid line
Source: Mahapatra et al. 2011.49 Reproduced with permission from Elsevier.
longstanding persistent AF patients, with only one study reporting unfavourable outcomes.57 Early experience with the procedure indicated some safety concerns, including a few reports of atrio-oesophageal fistula.55 57 In part this risk was mitigated through oesophageal temperature monitoring and saline irrigation of the pericardial space. The lesion set also evolved over time, which may have also helped avoid collateral damage. As illustrated in a recent review by Wats et al., epicardial lesions that required manipulation and curving of the distal end of the catheter were eliminated, and epicardial ablation was focused exclusively on the left atrial posterior wall.58 Posterior wall isolation through a box lesion set was originally reported as having additive benefit to surgical ablation clinical outcomes.59 The left atrial posterior wall has a propensity to harbour AF triggers and substrate in persistent and longstanding persistent AF. A substantial posterior wall reconnection rate has been reported following endocardial catheter ablation only, therefore a hybrid epicardial–endocardial ablation strategy may be better to support durable posterior wall isolation.20 Several studies reported the use of an epicardial posterior wall box with hybrid convergent ablation.60–62 Then, the CONVERGE trial introduced the contemporary epicardial lesion pattern, which consists of posterior wall homogenisation through the creation of parallel, overlapping rows of contiguous lesions using the most recent generation of unipolar RF catheter (EPi-Sense, AtriCure, Inc.) in a linear configuration (Figure 5).63 During the time of the trial the primary access technique to reach the left atrium also changed, from dividing the central tendon of the diaphragm to using a subxiphoid incision for cannula insertion (Supplementary Material Figure 2). This change avoided the potential for postoperative development of transdiaphragmatic hernias. Two studies have reported significantly fewer complications in cases in which the subxiphoid approach was used compared with the transdiaphragmatic approach.64 65 Wats et al. published a comprehensive review of clinical outcomes following hybrid convergent ablation that spanned this evolution of the procedure, from its early inception to the lead-up to the CONVERGE clinical trial.58
The CONVERGE trial randomised patients with symptomatic, drugrefractory, persistent and longstanding persistent AF to hybrid epicardial–endocardial convergent ablation (PVI, posterior wall isolation and CTI) or endocardial RF catheter ablation (PVI, roof line, CTI and complex fractionated atrial electrograms at the discretion of the operator).66 Patients in the trial had a mean AF duration of 4.4 years. The trial met its primary effectiveness endpoint, demonstrating significantly higher freedom from atrial arrhythmias off new or a higher dose of AADs of 67.7% through 12 months compared with 50.0% for catheter ablation. It also met the primary safety endpoint, with a 7.8% major adverse event rate within 30 days, below the pre-specified safety goal. Several recent observational studies have reported clinical performance and safety results using the same lesion strategy and unipolar device as CONVERGE, including one propensity score-matched study in which the lesion strategy and device were compared with endocardial catheter ablation.64,65,67,68 A recent review by DeLurgio et al. discussed the results of CONVERGE with regard to the published observational studies that compared hybrid convergent ablation with endocardial catheter ablation, the observed reduction in AF burden that has been reported during follow-up after the procedure, and outcomes in the long-standing persistent AF population.69
As hybrid convergent ablation evolved, physicians performing the procedure gained experience to develop best practices with respect to institutional set up, multidisciplinary heart team collaboration, patient selection, medication strategies, procedural execution and follow-up care.70 Overall, there is recognition that the set up of the procedure does
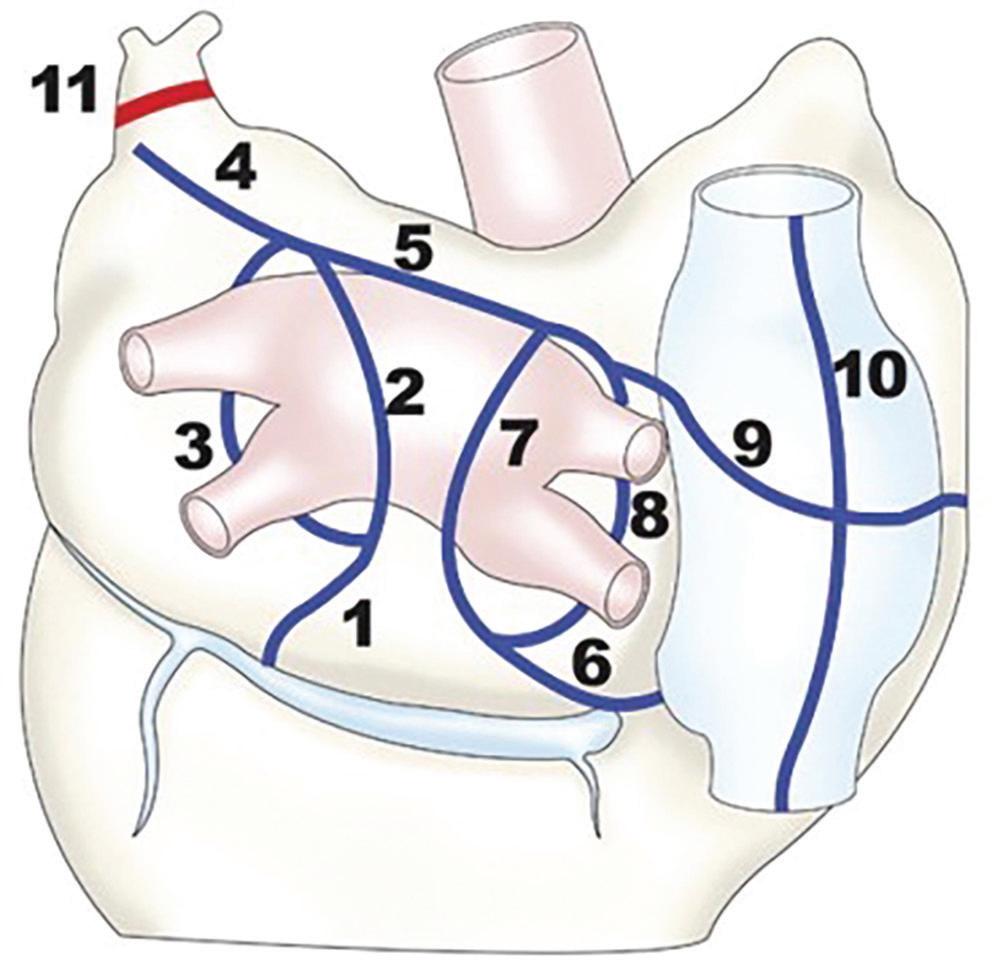
Source:
Source: Reproduced with permission from AtriCure, Inc.
not consist simply of a sequential performance of the surgical and electrophysiological ablation procedures, but instead requires extensive planning and collaboration often coordinated by the electrophysiology team.70 71
Future Perspectives for Hybrid Convergent Ablation
Some outstanding questions remain for hybrid convergent procedures that could be the focus of future studies. The epicardial and endocardial
Chronicle of Hybrid AF Ablation Therapy ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 4: Extra-cardiac Maze Lesion Set
Figure 5: Posterior Wall Ablation in the Contemporary Hybrid Convergent Procedure
Kiser et al. 2007.53 Reproduced with permission from Elsevier.
portions of the procedure can be performed on the same day of hospitalisation or alternatively can be staged separately by several weeks, which can be influenced by institutional logistics or reimbursement for healthcare providers. Outcomes for both strategies have been reported but not statistically compared in a case-controlled study. The role that prior catheter ablation plays in the outcome of hybrid ablation is also important for further investigation. CONVERGE included only ablation-naïve patients, but several observational studies have included patients who have received prior ablation. A recent single-centre study compared the recurrence of atrial arrhythmias and AF between patients who had a history of prior ablation before hybrid ablation and those who received de novo hybrid ablation and found no significant differences in these outcomes.72 However, patients who had a history of prior catheter ablation less frequently needed endocardial PVI and cardioversion (to restore sinus rhythm) during the hybrid convergent procedure. Larger, multicentre analyses may be warranted.
CONVERGE and other published studies used irrigated RF catheters for endocardial ablation, however, some investigators have incorporated the use of endocardial cryoballoon in hybrid convergent procedures.65,72 Endocardial cryoballoon was reported to be non-inferior to RF ablation for paroxysmal AF treatment, and the STOP PERSISTENT AF trial demonstrated safety and effectiveness of endocardial cryoballoon for the treatment of early (duration less than 6 months) persistent AF.73,15 Outcomes of hybrid convergent ablation using endocardial cryoballoon have not been formally compared with endocardial RF ablation and may be relevant for future evaluation.
The LAA is routinely closed in Cox maze surgical ablation procedures and can also be managed thoracoscopically. The LAA itself harbours electrical activity and is also the predominant site of thrombus formation in AF.21 Some investigators have reported preliminary data on the addition of thoracoscopic LAA exclusion as part of hybrid convergent procedures, and this is an emerging area for further study.74–76
Conclusion
AF is burdensome to both patients and the healthcare system, with an increasing prevalence worldwide. The genesis of AF is complex and still not entirely clear. AF can be present prior to the observation of structural heart disease, wherein it can contribute to atrial pathologies, such as mitral and tricuspid valve regurgitation. Conversely, the presence and progression of structural heart disease can also create the milieu for AF to initiate and progress through pathological substrate. In most cases, AF is present in the absence of structural heart disease, potentially due to poorly controlled risk factors, comorbidities and/or genetics. In effect, the
1. Kornej J, Borschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res 2020;127:4–20. https:// doi.org/10.1161/CIRCRESAHA.120.316340; PMID: 32716709.
2. Dorian P, Jung W, Newman D, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36:1303–9. https://doi.org/10.1016/s0735-1097(00)00886-x; PMID: 11028487.
3. Chung SC, Sofat R, Acosta-Mena D, et al. Atrial fibrillation epidemiology, disparity and healthcare contacts: a population-wide study of 5.6 million individuals. Lancet Reg Health Eur 2021;7:100157. https://doi.org/10.1016/j. lanepe.2021.100157; PMID: 34405204.
4. Burdett P, Lip GYH. Atrial fibrillation in the UK: predicting costs of an emerging epidemic recognising and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J
specific role for and extent of ablation depend on the context of the patient’s clinical condition.
Studies have identified the predominant sites harbouring arrhythmogenic triggers, which include first and foremost the PVs, and then the left atrial posterior wall and LAA, among others. In addition, as AF progresses, triggers outside the PVs become more prevalent, which is a consideration when choosing the ablation strategy appropriate for the patient. The LAAOS III trial demonstrated that closing the LAA in patients with AF undergoing cardiac surgery reduced the rate of stroke compared with leaving the LAA open.77 Therefore, closing the LAA, when accessible, is also a consideration during surgical ablation for AF. It has been nearly 25 years since the first cut-and-sew Cox maze procedure. Since then, the field of AF treatment has seen numerous innovations and procedural advancements with respect to cardiac ablation in both cardiothoracic surgery and electrophysiology. Hybrid convergent ablation is a newer approach that combines aspects of both disciplines to achieve PV and posterior wall isolation. Procedures from across the spectrum, from biatrial Cox maze to hybrid epicardial–endocardial ablation to endocardial catheter ablation alone, have been developed to address the primary sites of AF triggers and substrates across a range of clinical scenarios, taking into consideration the relative invasiveness of the ablation procedure and the minimal ablation targets, to effectively and safely restore normal sinus rhythm (Supplementary Material Figure 3). Careful patient selection when evaluating the available approaches for AF treatment is critical in the context of multidisciplinary, holistic AF care.
Clinical Perspective
• Outcomes from endocardial ablation therapy for persistent AF remain suboptimal compared with the treatment for paroxysmal AF, particularly in patients with the long-standing form and those with significantly dilated atria.
• Hybrid ablation is a well-established form of therapy for persistent AF and has better outcomes than endocardial ablation alone, given that the lesions sets are more reliably transmural. However, compared with endocardial ablations, the complication rates from traditional (thoracoscopic) hybrid ablations are greater, owing to multi-port thoracoscopic access to the pericardial space in order to deliver the epicardial ablations.
• Convergent hybrid ablation therapy is relatively new and is the least invasive of all of the hybrid approaches; a single port of entry through a small subxiphoid incision provides direct access to the pericardial space, which circumvents the more traumatic and prolonged transthoracic approach.
Qual Care Clin Outcomes 2022;8:187–94. https://doi. org/10.1093/ehjqcco/qcaa093; PMID: 33346822.
5. Kotalczyk A, Lip GYH. Disparities in atrial fibrillation: a call for holistic care. Lancet Reg Health Eur 2021;7:100160. https:// doi.org/10.1016/j.lanepe.2021.100160; PMID: 34557846.
6. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. https://doi.org/10.1093/eurheartj/ehaa612; PMID: 32860505.
7. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. https://doi.org/10.1056/NEJMoa2019422; PMID: 32865375.
8. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. https://doi.org/10.1016/j. hrthm.2017.05.012; PMID: 28506916.
9. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. https://doi.org/10.1056/NEJMoa2029554; PMID: 33197158.
10. Kuniss M, Pavlovic N, Velagic V, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. Europace 2021;23:1033–41. https://doi.org/10.1093/europace/euab029; PMID: 33728429.
11. Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. https://doi.org/10.1056/ NEJMoa2029980; PMID: 33197159.
Chronicle of Hybrid AF Ablation Therapy ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
12. Turagam MK, Musikantow D, Whang W, et al. Assessment of catheter ablation or antiarrhythmic drugs for first-line therapy of atrial fibrillation: a meta-analysis of randomized clinical trials. JAMA Cardiol 2021;6:697–705. https://doi. org/10.1001/jamacardio.2021.0852; PMID: 33909022.
13. Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. https:// doi.org/10.1001/jama.2009.2029; PMID: 20103757.
14. Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. https:// doi.org/10.1056/NEJM199809033391003; PMID: 9725923.
15. Su WW, Reddy VY, Bhasin K, et al. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the multicenter STOP Persistent AF trial. Heart Rhythm 2020;17:1841–7. https://doi.org/10.1016/j.hrthm.2020.06.020; PMID: 32590151.
16. Mansour M, Calkins H, Osorio J, et al. Persistent atrial fibrillation ablation with contact force-sensing catheter: the prospective multicenter PRECEPT trial. JACC Clin Electrophysiol 2020;6:958–69. https://doi.org/10.1016/j. jacep.2020.04.024; PMID: 32819531.
17. Parameswaran R, Kalman JM, Royse A, et al. Endocardial–epicardial phase mapping of prolonged persistent atrial fibrillation recordings: high prevalence of dissociated activation patterns. Circ Arrhythm Electrophysiol 2020;13:e008512. https://doi.org/10.1161/CIRCEP.120.008512; PMID: 32634027.
18. Della Rocca DG, Tarantino N, Trivedi C, et al. Non-pulmonary vein triggers in nonparoxysmal atrial fibrillation: implications of pathophysiology for catheter ablation. J Cardiovasc Electrophysiol 2020;31:2154–67. https://doi.org/10.1111/ jce.14638; PMID: 32583488.
19. Lim HS, Hocini M, Dubois R, et al. Complexity and distribution of drivers in relation to duration of persistent atrial fibrillation. J Am Coll Cardiol 2017;69:1257–69. https:// doi.org/10.1016/j.jacc.2017.01.014; PMID: 28279292.
20. Kaba RA, Momin A, Camm J. Persistent atrial fibrillation: the role of left atrial posterior wall isolation and ablation strategies. J Clin Med 2021;10:3129. https://doi.org/10.3390/ jcm10143129; PMID: 34300301.
21. Kaba RA, Ahmed O, Momin A. Electrical isolation of the left atrial appendage: a new frontier in the treatment for atrial fibrillation. J Cardiovasc Dis Diagn 2020;8:1–6. https://doi. org/10.37421/jcdd.2020.8.411
22. Thiyagarajah A, Kadhim K, Lau DH, et al. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2019;12:e007005. https://doi. org/10.1161/CIRCEP.118.007005; PMID: 31401853.
23. Tilz RR, Rillig A, Thum AM, et al. Catheter ablation of longstanding persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J Am Coll Cardiol 2012;60:1921–9. https://doi.org/10.1016/j.jacc.2012.04.060; PMID: 23062545.
24. Brooks AG, Stiles MK, Laborderie J, et al. Outcomes of longstanding persistent atrial fibrillation ablation: a systematic review. Heart Rhythm 2010;7:835–46. https://doi.org/10.1016/j. hrthm.2010.01.017; PMID: 20206320.
25. Cox JL, Schuessler RB, D’Agostino HJ Jr, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569–83. https://doi.org/10.1016/S0022-5223(19)36684-X; PMID: 2008095.
26. Cox JL, Boineau JP, Schuessler RB, et al. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. J Thorac Cardiovasc Surg 1995;110:473–84. https://doi.org/10.1016/S00225223(95)70244-X; PMID: 7637365.
27. Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg 2003;126:1822–8. https://doi.org/10.1016/ s0022-5223(03)01287-x; PMID: 14688693.
28. Gaynor SL, Diodato MD, Prasad SM, et al. A prospective, single-center clinical trial of a modified Cox maze procedure with bipolar radiofrequency ablation. J Thorac Cardiovasc Surg 2004;128:535–42. https://doi.org/10.1016/j. jtcvs.2004.02.044; PMID: 15457154.
29. Aranda-Michel E, Serna-Gallegos D, Kilic A, et al. The impact of the Cox-maze technique on freedom from atrial fibrillation. Ann Thorac Surg 2021;112:1417–23. https://doi. org/10.1016/j.athoracsur.2020.11.027; PMID: 33345780.
30. Khiabani AJ, MacGregor RM, Manghelli JL, et al. Bipolar radiofrequency ablation on explanted human hearts: how to ensure transmural lesions. Ann Thorac Surg 2020;110:1933–9. https://doi.org/10.1016/j.athoracsur.2020.04.079; PMID: 32522634.
31. McCarthy PM, Gerdisch M, Philpott J, et al. Three-year outcomes of the postapproval study of the AtriCure Bipolar Radiofrequency Ablation of Permanent Atrial Fibrillation Trial. J Thorac Cardiovasc Surg 2020. https://doi.org/10.1016/j. jtcvs.2020.09.099; PMID: 33129501; epub ahead of press.
32. Philpott JM, Zemlin CW, Cox JL, et al. The ABLATE trial: safety and efficacy of Cox Maze-IV using a bipolar radiofrequency ablation system. Ann Thorac Surg 2015;100:1541–8. https://doi.org/10.1016/j. athoracsur.2015.07.006; PMID: 26387721.
33. Khiabani AJ, MacGregor RM, Bakir NH, et al. The longterm outcomes and durability of the Cox-Maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg 2022;163:629–41. https://doi.org/10.1016/j.jtcvs.2020.04.100; PMID: 32563577.
34. Ad N, Holmes SD, Stone LE, et al. Rhythm course over 5 years following surgical ablation for atrial fibrillation. Eur J Cardiothorac Surg 2015;47:52–8. https://doi.org/10.1093/ejcts/ ezu059; PMID: 24585551.
35. Vural Ü, Balci AY, Ağlar AA, Kızılay M. Which method to use for surgical ablation of atrial fibrillation performed concomitantly with mitral valve surgery: radiofrequency ablation versus cryoablation. Braz J Cardiovasc Surg 2018;33:542–52. https://doi.org/10.21470/1678-9741-20180130; PMID: 30652742.
36. Ad N, Holmes SD, Rongione AJ, et al. Does surgical ablation energy source affect long-term success of the concomitant Cox maze procedure? Ann Thorac Surg 2017;104:29–35. https://doi.org/10.1016/j.athoracsur.2017.04.004; PMID: 28577848.
37. Badhwar V, Rankin JS, Damiano RJ Jr, et al. The Society of Thoracic Surgeons 2017 clinical practice guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg 2017;103:329–41. https://doi.org/10.1016/j. athoracsur.2016.10.076; PMID: 28007240.
38. Ad N, Holmes SD, Rongione AJ, et al. The long-term safety and efficacy of concomitant Cox maze procedures for atrial fibrillation in patients without mitral valve disease. J Thorac Cardiovasc Surg 2019;157:1505–14. https://doi.org/10.1016/j. jtcvs.2018.09.131; PMID: 30578060.
39. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm 2017;33:369–409. https://doi. org/10.1016/j.joa.2017.08.001; PMID: 29021841.
40. Lapenna E, De Bonis M, Giambuzzi I, et al. Long-term outcomes of stand-alone maze IV for persistent or longstanding persistent atrial fibrillation. Ann Thorac Surg 2020;109:124–31. https://doi.org/10.1016/j. athoracsur.2019.05.061; PMID: 31325420.
41. Ad N, Holmes SD, Friehling T. Minimally invasive stand-alone Cox maze procedure for persistent and long-standing persistent atrial fibrillation: perioperative safety and 5-year outcomes. Circ Arrhythm Electrophysiol 2017;10:e005352. https://doi.org/10.1161/CIRCEP.117.005352; PMID: 29138143.
42. Wolf RK, Schneeberger EW, Osterday R, et al. Videoassisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130:797–802. https://doi.org/10.1016/j. jtcvs.2005.03.041; PMID: 16153931.
43. Yilmaz A, Van Putte BP, Van Boven WJ. Completely thoracoscopic bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2008;136:521–2. https://doi.org/10.1016/j. jtcvs.2008.01.035; PMID: 18692667.
44. van Laar C, Geuzebroek GS, Hofman FN, Van Putte BP. The totally thoracoscopic left atrial maze procedure for the treatment of atrial fibrillation. Multimed Man Cardiothorac Surg 2016;2016:mmv043. https://doi.org/10.1093/mmcts/mmv043; PMID: 26993056.
45. Wolf RK. Surgical treatment of atrial fibrillation. Methodist Debakey Cardiovasc J 2021;17:56–64. https://doi.org/10.14797/ VNDG5944; PMID: 34104322.
46. Boersma LV, Castella M, van Boven W, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation 2012;125:23–30. https://doi.org/10.1161/CIRCULATIONAHA.111.074047; PMID: 22082673.
47. Je HG, Shuman DJ, Ad N. A systematic review of minimally invasive surgical treatment for atrial fibrillation: a comparison of the Cox-Maze procedure, beating-heart epicardial ablation, and the hybrid procedure on safety and efficacy. Eur J Cardiothorac Surg 2015;48:531–40. https://doi. org/10.1093/ejcts/ezu536; PMID: 25567961.
48. Kiser AC, Landers M, Horton R, et al. The convergent procedure: a multidisciplinary atrial fibrillation treatment. Heart Surg Forum 2010;13:e317–21. https://doi.org/10.1532/ HSF98.20091112; PMID: 20961832.
49. Mahapatra S, LaPar DJ, Kamath S, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation
for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg 2011;91:1890–8. https://doi.org/10.1016/j.athoracsur.2011.02.045; PMID: 21619988.
50. van der Heijden CAJ, Vroomen M, Luermans JG, et al. Hybrid versus catheter ablation in patients with persistent and longstanding persistent atrial fibrillation: a systematic review and meta-analysis. Eur J Cardiothorac Surg 2019;56:433–43. https://doi.org/10.1093/ejcts/ezy475; PMID: 30698685.
51. de Asmundis C, Varnavas V, Sieira J, et al. Two-year followup of one-stage left unilateral thoracoscopic epicardial and transcatheter endocardial ablation for persistent and longstanding persistent atrial fibrillation. J Interv Card Electrophysiol 2020;58:333–43. https://doi.org/10.1007/ s10840-019-00616-w; PMID: 31520292.
52. Maesen B, Weberndörfer V, Vroomen M, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation versus transvenous catheter ablation in persistent and longstanding persistent atrial fibrillation: 1-year results of the HARTCAP-AF randomized controlled trial. Presented at the 26th Annual International Atrial Fibrillation Symposium. Abstract AFS 2021-04. J Cardiovasc Electrophysiol 2021;32:1467–1515. https://doi.org/10.1111/jce.14975
53. Kiser AC, Wimmer-Greinecker G, Chitwood WR. Totally extracardiac maze procedure performed on the beating heart. Ann Thorac Surg 2007;84:1783–5. https://doi. org/10.1016/j.athoracsur.2007.08.027; PMID: 17954121.
54. Kiser AC, Cockfield W. Paracardioscopic ex-maze procedure for atrial fibrillation. Multimed Man Cardiothorac Surg 2010;2010:mmcts.2008.003863. https://doi.org/10.1510/ mmcts.2008.003863; PMID: 24412848.
55. Gehi AK, Mounsey JP, Pursell I, et al. Hybrid epicardial–endocardial ablation using a pericardioscopic technique for the treatment of atrial fibrillation. Heart Rhythm 2013;10:22–8. https://doi.org/10.1016/j.hrthm.2012.08.044; PMID: 23064043.
56. Kiser AC, Landers MD, Boyce K, et al. Simultaneous catheter and epicardial ablations enable a comprehensive atrial fibrillation procedure. Innovations (Phila) 2011;6:243–7. https://doi.org/10.1097/IMI.0b013e31822ca15c; PMID: 22437982.
57. Edgerton Z, Perini AP, Horton R, et al. Hybrid procedure (endo/epicardial) versus standard manual ablation in patients undergoing ablation of longstanding persistent atrial fibrillation: results from a single center. J Cardiovasc Electrophysiol 2016;27:524–30. https://doi.org/10.1111/ jce.12926; PMID: 26766149.
58. Wats K, Kiser A, Makati K, et al. The convergent atrial fibrillation ablation procedure: evolution of a multidisciplinary approach to atrial fibrillation management. Arrhythm Electrophysiol Rev 2020;9:88–96. https://doi. org/10.15420/aer.2019.20; PMID: 32983530.
59. Voeller RK, Bailey MS, Zierer A, et al. Isolating the entire posterior left atrium improves surgical outcomes after the Cox maze procedure. J Thorac Cardiovasc Surg 2008;135:870–7. https://doi.org/10.1016/j.jtcvs.2007.10.063; PMID: 18374771.
60. Gersak B, Zembala MO, Muller D, et al. European experience of the convergent atrial fibrillation procedure: multicenter outcomes in consecutive patients. J Thorac Cardiovasc Surg 2014;147:1411–6. https://doi.org/10.1016/j. jtcvs.2013.06.057; PMID: 23988287.
61. Civello K, Smith C, Boedefeld W. Combined endocardial and epicardial ablation for symptomatic atrial fibrillation: single center experience in 100+ consecutive patients. J Innovations Cardiac Rhythm Manag 2013;4:1–7. https://doi.org/10.19102/ icrm.2013.040906
62. Zembala M, Filipiak K, Kowalski O, et al. Staged hybrid ablation for persistent and longstanding persistent atrial fibrillation effectively restores sinus rhythm in long-term observation. Arch Med Sci 2017;13:109–17. https://doi. org/10.5114/aoms.2015.53960; PMID: 28144262.
63. DeLurgio DB, Ferguson E, Gill J, et al. Convergence of epicardial and endocardial RF ablation for the treatment of symptomatic persistent AF (CONVERGE trial): rationale and design. Am Heart J 2020;224:182–91. https://doi. org/10.1016/j.ahj.2020.02.016; PMID: 32416333.
64. Larson J, Merchant FM, Patel A, et al. Outcomes of convergent atrial fibrillation ablation with continuous rhythm monitoring. J Cardiovasc Electrophysiol 2020;31:1270–6. https://doi.org/10.1111/jce.14454; PMID: 32219901.
65. Makati KJ, Sherman AJ, Gerogiannis I, Sood N. Safety and efficacy of convergent hybrid procedure using cryo as endocardial energy source for the treatment of atrial fibrillation. Circ Arrhythm Electrophysiol 2020;13:e008556. https://doi.org/10.1161/CIRCEP.120.008556; PMID: 33003965.
66. DeLurgio DB, Crossen KJ, Gill J, et al. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation: results of
Chronicle of Hybrid AF Ablation Therapy ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
CONVERGE clinical trial. Circ Arrhythm Electrophysiol 2020;13:e009288. https://doi.org/10.1161/ CIRCEP.120.009288; PMID: 33185144.
67. Gulkarov I, Wong B, Kowalski M, et al. Convergent ablation for persistent atrial fibrillation: single center experience. J Card Surg 2019;34:1037–43. https://doi.org/10.1111/jocs.14204; PMID: 31374587.
68. Maclean E, Yap J, Saberwal B, et al. The convergent procedure versus catheter ablation alone in longstanding persistent atrial fibrillation: a single centre, propensitymatched cohort study. Int J Cardiol 2020;303:49–53. https:// doi.org/10.1016/j.ijcard.2019.10.053; PMID: 32063280.
69. DeLurgio DB, Gill JS, Ahsan S, et al. Hybrid convergent procedure for the treatment of persistent and long-standing persistent atrial fibrillation. Arrhythm Electrophysiol Rev 2021;10:198–204. https://doi.org/10.15420/aer.2021.24; PMID: 34777825.
70. Makati KJ, Sood N, Lee LS, et al. Combined epicardial and endocardial ablation for atrial fibrillation: best practices and
guide to hybrid convergent procedures. Heart Rhythm 2021;18:303–12. https://doi.org/10.1016/j.hrthm.2020.10.004; PMID: 33045430.
71. Wijesuriya N, Papageorgiou N, Maclean E, et al. The role of the electrophysiologist in convergent ablation. Arrhythm Electrophysiol Rev 2020;9:8–14. https://doi.org/10.15420/ aer.2019.06; PMID: 32637114.
72. Kress DC, Erickson L, Mengesha TW, et al. Characterizing recurrence following hybrid ablation in patients with persistent atrial fibrillation. J Patient Cent Res Rev 2020;7:227–38. https://doi.org/10.17294/2330-0698.1744; PMID: 32760754.
73. Kuck KH, Brugada J, Furnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med 2016;374:2235–45. https://doi.org/10.1056/ NEJMoa1602014; PMID: 27042964.
74. Kress DC, Erickson L, Perez Moreno AC, et al. Abstract 14352: staged hybrid atrial fibrillation ablation with addition of endoscopic left atrial appendage closure reduces
arrhythmia recurrence between 3 and 12 months compared to non-staged hybrid without closure. Circulation 2020;142:14352. https://doi.org/10.1161/circ.142. suppl_3.14352
75. Tonks R, Lantz G, Mahlow J, et al. Short and intermediate term outcomes of the convergent procedure: initial experience in a tertiary referral center. Ann Thorac Cardiovasc Surg 2020;26:13–21. https://doi.org/10.5761/atcs.oa.19-00164; PMID: 31495813.
76. Gegechkori N, Yang F, Miller A, et al. Comparison of hybrid ablation for persistent atrial fibrillation with and without left atrial appendage closure. Report of one-year follow-up. Presented at Venice Arrhythmias, Venice, Italy, 4 October 2019. J Interv Card Electrophysiol 2020;57:164. https://doi. org/10.1007/s10840-019-00665-1
77. Whitlock RP, Belley-Cote EP, Paparella D, et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N Engl J Med 2021;384:2081–91. https://doi. org/10.1056/NEJMoa2101897; PMID: 33999547.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Chronicle of Hybrid AF Ablation Therapy
Economic Evaluation of Catheter Ablation Versus Medical Therapy for the Treatment of Atrial Fibrillation from the Perspective of the UK
Abstract
Randomised evidence supports an early rhythm control strategy as treatment for AF, and catheter ablation outperforms medical therapy in terms of effectiveness when studied as first- and second-line treatment. Despite evidence consistently showing that catheter ablation treatment is superior to medical therapy in most AF patients, only a small proportion receive ablation, in some cases after a prolonged trial of ineffective medical therapy. Health economics research in electrophysiology remains limited but is recognised as being important in influencing positive change to ensure early access to ablation services for all eligible patients. Such information has informed the updated recommendations from the recently published National Institute for Health and Care Excellence clinical guideline on the diagnosis and management of AF, but increased awareness is needed to drive real-world adoption and to ensure patients are quickly referred to specialists. In this article, economic evaluations of catheter ablation versus medical therapy are reviewed.
Keywords
Economic evaluation, cost-effectiveness, catheter ablation, medical therapy, atrial fibrillation
Disclosure: LWML has previously received research support from Attune Medical. RJI is an employee of CTI Clinical Trial and Consulting Services, which is a consultant to Biosense Webster. HT is an employee of Johnson & Johnson. MMG has received funding from Attune Medical and was a paid speaker for Cook Medical and Boston Scientific. All other authors have no conflicts of interest to declare.
Received: 1 August 2021 Accepted: 14 March 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e13. DOI: https://doi.org/10.15420/aer.2021.46
Correspondence: Lisa WM Leung, Department of Cardiology, St George’s Hospital, Blackshaw Rd, London SW17 0QT, UK. E: lleung@sgul.ac.uk
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
AF is an increasingly common condition worldwide, with over one million people affected in the UK alone.1 AF is important due to its associated comorbidities, including risk of stroke and hospitalisations from heart failure.2 Therefore, AF has a considerable impact on both patients and healthcare providers. The treatment of AF is much more than just establishing the need for long-term anticoagulation; research has progressively shown that early rhythm control can improve patient outcomes and that this may be prognostically relevant.3 In addition to older studies on the effectiveness of catheter ablation, there is increasing evidence demonstrating that, as a first-line treatment, it is more effective at achieving rhythm control than medical therapy, without significantly adding to the risk of adverse events.4–10 In line with this evidence, the European Society of Cardiology 2020 AF guidelines recommended that “AF catheter ablation for [pulmonary vein isolation (PVI)] should/may be considered as first-line rhythm control therapy to improve symptoms in selected patients with symptomatic paroxysmal AF episodes and symptomatic persistent AF (without major risk factors for AF recurrence) as an alternative to [antiarrhythmic drug (AAD)] class I or III, considering patient choice, benefit, and risk.”11
Although there is an evidence-based shift to support catheter ablation as early treatment for AF in some international guidelines, changes in realworld practice are often slower due to decisions regarding restructuring of healthcare resource allocation, which cannot be achieved without
health economics research to help identify the most appropriate solution. Another challenge is awareness of the updated treatment guidelines by clinicians involved earlier in the AF treatment pathway, including general practitioners and general cardiologists.
In this review article, the importance of health economic data on the treatment of AF is discussed with the aim of creating awareness about the latest evidence and recommendations that will help inform changes in real-world practice.
Definitions
Most economic evaluations in healthcare are cost-effectiveness studies. The unit of cost-effectiveness is measured in cost per quality-adjusted life years (QALYs) gained, where QALY is a general unit to express 1 year of good health (i.e. time and quality of life benefit). Cost utility analysis guides procurement decisions and involves calculation of incremental costs and effects of a certain treatment, combining the unit measure as the incremental cost-effectiveness ratio (ICER; i.e. cost per QALY). Modelling can use data derived from randomised controlled trials or from a combination of data pulled from health and economic data sources, which then go through a decision–analytical process on probability. To manage potential bias, sensitivity analysis can explore potential sources and uncertainty. Probabilistic sensitivity analysis is preferred to assess any uncertainty or unknowns in the model because it allows potentially
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Clinical Electrophysiology and Ablation
Lisa WM Leung , 1 Zaki Akhtar , 1 Christos Kontogiannis , 1 Ryan J Imhoff , 2 Hannah Taylor 3 and Mark M Gallagher 1
1. Department of Cardiology, St George’s Hospital NHS Foundation Trust, London, UK; 2. Real-World Evidence and Late Phase Research, CTI Clinical Trial and Consulting Services Inc, Covington, KY, US; 3. Johnson & Johnson Medical Ltd, Berkshire, UK
multiple aspects of uncertainty to be reflected at the same time for a better overall picture. In addition, this type of analysis provides the best estimate for Markov models with multiple non-linear inputs. Markov models are useful in health economics and for a condition such as AF because they evaluate risk over time and have the capacity to allow events to occur more than once. There are assumptions that a patient is in one of a set number of defined health states in the model.
Economic Model Data and Clinicians
Clinicians are familiar with interpreting data relating to the clinical effectiveness of treatments, but understandably less familiar identifying and interpreting economic data. However, economic evaluation of treatment options has always been an important factor in decision making in healthcare systems. For the UK, the National Institute for Health and Care Excellence (NICE) uses clinical and cost-effectiveness data to determine whether certain treatment options should be recommended. In turn, the National Health Service (NHS) uses NICE guidance to inform service or treatment availability and their associated costs. NICE assesses cost-effectiveness to maximise health gain from available resources; this acknowledges that budgets are limited, and bridging scientific data with economic detail can bring best possible care into effect.12 Finding the most appropriate methodology for costeffectiveness analysis should consider the nature of the condition in question. It is important for a common condition like AF, but the same analysis cannot be applied for much rarer diseases, such as spinal muscular atrophy.13 Since the 1970s, demand for all sectors of health care has increased rapidly and has been met by increasing growth of technology and pharmacological therapy. However, there has not been the same degree of growth in resources to match the demand. Kernick’s review on health economics for the medical physician sums up the importance of economic evaluation and the different types of studies used to match the appropriate scenario.14 Cost-effectiveness is the most common type of study used to compare interventions or treatments for the same condition for similar clinical outcomes. Results are presented in the form of a ratio (e.g. ICER). This may be supported by a cost utility analysis, which offers insights into the quality and quantity of life, with the unit of measure being the QALY.
Cost-effectiveness of Catheter Ablation Versus Medical Therapy
Previous studies on the cost-effectiveness of catheter ablation versus medical therapy used trial data to provide clinical inputs into the model, with short time horizons and a narrow focus on the outcomes measured. This provided the incentive to conduct a new evaluation that may be more applicable to real-world patients. A 2014 study by Reynolds et al. compared the cost-effectiveness of cryoballoon ablation with that of AADs in the UK, finding an ICER of £21,957, which was above the £20,000 willingness to pay (WTP) threshold.15 That study only looked at a time horizon of 5 years and did not consider events such as hospitalisations from heart failure, which may explain the higher ICER for cryoballoon ablation treatment. Hospitalisations or the utilisation of healthcare facilities are important variables to consider in a long-term condition like AF. In addition, the study considered the costs relating to older-generation cryoballoon catheters at that time, which may affect components in the overall costs to the healthcare provider. The improvements in methodology and operator effectiveness within the past decade should have seen improved outcomes, measured by overall freedom from AF, shorter inpatient stays and fewer complications (i.e. reduced healthcare provider costs), which, overall, would make catheter ablation more cost-effective (lower ICER).
A 2019 study performed in Australia by Gao and Moodie looked at the cost-effectiveness of catheter ablation versus medical therapy in patients with both AF and heart failure, yielding an ICER that was above the WTP threshold.16 However, that study only evaluated the impact of reduced mortality. Therefore, again, healthcare facility use and other clinical events were not accounted for, which are important variables with significant effects on overall cost and quality of life.
Aronsson et al. published a cost-effectiveness substudy in 2014 of their randomised controlled trial on first-line treatment of paroxysmal AF using radiofrequency compared with AADs.17 In their substudy of the same population, Aronsson et al. found that ablation was more cost-effective for younger patients (age <50 versus >50 years), but their analysis was concentrated on order of treatment as opposed to defining what treatment should be offered based on age. That study was interesting because the ICER calculated by the authors was different to the costeffectiveness analysis from a separate study, namely RAAFT, with that the ablation procedure was significantly more expensive in the study by Aronsson et al.18 These clinical randomised studies offered invaluable insights into ablation treatment compared with medical therapy but, when it comes to cost-analysis based on the same data, there are limitations on local cost analysis and its application towards long-term benefit.
In summary, these main studies offer some insights into the economics of ablation with some variation in study design and focus. More of these studies are required to follow on from clinical trials to allow research findings to be interpreted within this context and whether that enhances the study findings. However, it is important to recognise that this approach is still imperfect, because these short- to medium-term results are used to provide economic evaluation over a longer period of time or even lifetime horizon analysis. One way to improve on these analyses is to incorporate real-world data whenever possible.
Use of Real-world Evidence Data
Clinical trials often have protocols that influence usage, are performed at top-performing, high-volume clinical sites and generally have relatively small sample sizes. By using large real-world populations to derive many of the estimates used in a health economics model, the results become more generalisable and can capture benefits that may not be seen in smaller, randomised trials. This approach may become more widespread since NICE announced more routine use of real-world data as part of their 5-year strategy launched in April 2021.19
We proposed an economic model analysis of ablation versus medical therapy from the perspective of the UK NHS, with the data summary presented at the European Heart Rhythm Association 2021 and full results now widely available.20,21 In this study, data were extracted from real-world studies and supplemented the systematic literature review and metaanalysis from established clinical trials. Compared with existing model data, the uniqueness of this study rested with the real-world data input. A patientlevel Markov health-state transition model was used to conduct a cost–utility analysis. The population included patients previously treated for AF with medical therapy, including those with heart failure, simulated over a lifetime horizon. Figure 1 illustrates AF treatment in the model. Patients entered the model from the age of 64 years. Data sources included published literature on healthcare resource utilisation and cardiovascular event rates in real-world patients, a systematic literature review and metaanalysis of randomised controlled trials for AF recurrence (Figure 2A,B) and publicly available government data/reports on costs relevant to the NHS. From this unique perspective, catheter ablation (by any modality) resulted in
Cost-effectiveness of Catheter Ablation Versus Medical Therapy for AF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
a favourable ICER per additional QALY gained compared with medical therapy. Figure 3 illustrates that catheter ablation is a cost-effective treatment at various WTP thresholds, including £20,000. Rhythm control was significantly more successful in the catheter ablation group and sustained this superiority over the years that the patients remained in the model. The cost-effectiveness was evident in time horizons tested beyond the 5-year time constraint of older studies. This study concluded that catheter ablation is a cost-effective second-line treatment for AF compared with medical therapy from the perspective of the UK NHS.20,21
As with other studies, our study has limitations. Although the cost input into the model included state-of-the-art radiofrequency catheters, the clinical evidence evaluated the effectiveness of ablation procedures using older technology. Therefore, the clinical benefit is underestimated. In addition, real-world data were primarily extracted from a US-based population study by Noseworthy et al., which included a large wellmatched patient population.22 Although the clinical endpoints should not vary dramatically, there may be differences in patient assessment and clinical practice that would not be translatable to care in the UK. In addition, this model did not account for inevitable crossover from medical therapy to catheter ablation, which is common in clinical practice (e.g. 27.5% of patients crossed over from medical therapy to ablation in the CABANA study), but this was done to ensure a clear comparison of the ablation and medical therapy treatment strategies to assess costeffectiveness.23 It is important to note that in the current environment, worsened by the coronavirus pandemic, it is not unusual to find patients deemed suitable for catheter ablation remaining on a waiting list for the procedure over a prolonged period, over three cycles (9 months) duration as per the model. In this period, AAD therapy may be used as a bridging measure. This period is akin to a treatment crossover despite original intentions by both patient and specialist opinion, and only adds further to healthcare provider costs, in addition to reducing patient quality of life.
Finally, this model only considers direct costs to the health providers: the
NHS and prescribed specialised services. The model does not capture expenses for patients, nor does it consider burdens such as missed time from work, reduced productivity or the burden on caregivers, particularly for those suffering a disabling adverse event.
Recent NICE Guidelines
The latest NICE clinical guideline on the diagnosis and management of AF was published in April 2021.19 This was an update on the 2014 guideline, using recently published clinical and health economic data on the treatment options for AF.
The cost-effectiveness analysis of catheter ablation treatment as secondline treatment (after failure of at least one AAD) found that radiofrequency point-by-point ablation was more cost-effective over a lifetime than cryoballoon or laser balloon ablation.19 The NICE network meta-analysis calculated a 1% difference in AF recurrence between cryoballoon ablation (32%) and radiofrequency point-by-point ablation (31%); however, radiofrequency point-by-point ablation remained the most cost-effective treatment option after scenario analyses adjusting for cost and healthcare resource use.19 This demonstrates the importance of considering 12-month outcomes as part of routine clinical decision making: even a 1% difference in AF recurrence has an impact due to the costs of additional redo procedures. Redo procedures may also add burden to waiting lists, which could limit access to ablation for new patients. Due to the difference in cost-effectiveness, the updated guidelines recommend that patients should be offered radiofrequency point-by-point ablation unless there are specific clinical reasons why an alternative should be used instead (e.g. patient factors or wishes).19 The analysis itself was based on patients with paroxysmal AF and this recommendation was for patients with paroxysmal AF and applied to those with symptomatic persistent AF who cannot have or are unsuitable for long-term AAD therapy.
The NICE analysis used data from randomised controlled trials and health economic studies to populate usage parameters over a lifetime period
Cost-effectiveness of Catheter Ablation Versus Medical Therapy for AF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Death Rhythm control achieved AF recurrence Treatment: ablation or medical therapy Stop rhythm control: futility Cardiovascular adverse events: • MI • Stroke • Bleeding event • Cardiac arrest 1. Catheter ablation or 2. Medical therapy Attempt AAD treatment AF controlled? Suitable for AF ablation Not evaluated Yes No Patient presents with AF Continue rhythm control Yes AAD = antiarrhythmic drug.
Figure 1: Model Structure for the Treatment of AF and AF Recurrence in the 2021 Study
Source: Leung et al. 2022.21 Adapted from Wiley & Sons under a Creative Commons CC BY-NC 4.0 licence.
Figure 2: Arrhythmia Recurrence Data
and it was calculated that radiofrequency using a point-by-point method was cost effective with an ICER well below the current WTP threshold (£9,764 with a WTP threshold of £20,000).
Studies published from 2003 onwards were included by NICE; therefore, some technology may no longer be used. For example, of the 16 studies reporting AF recurrence for radiofrequency point-by-point ablation, only four used contact force (CF)-sensing catheters.24–27 We know from a previous meta-analysis of CF-sensing catheters versus conventional catheters that CF-sensing catheters are more effective at treating AF, with lower rates of AF recurrence at the 12-month follow up.28 And so, although the analysis did not find a significant difference in AF recurrence rates across all modalities, we can extrapolate from this that, with CF-sensing catheters only, radiofrequency may show a superior edge in efficacy.
Applicability of Economic Models
Despite economic models relying on some data inputs specific to the authors’ healthcare systems, the analysis can still be highly useful for
the international community as a rough guide: often the clinical and population data are applicable, but there may be differences in regional costs and healthcare resource use. The UK model used clinical data already established in the medical literature and included patients outside of the UK. To our knowledge, it is the first comprehensive economic evaluation of ablation treatment compared with medical therapy that uses real-world data. It is also novel to use cost data for state-of-the-art radiofrequency ablation technology. Thus, the most recent analysis should also be meaningful to international clinicians and healthcare providers.
The latest economic evaluation analysis and the older analyses imply that there should be prioritisation of arrhythmia services where AF ablation treatment is available and an extension of these services to improve accessibility to the population in need by ensuring patients are referred to specialists quickly if their first treatment fails. Regular review of the clinical and economic data is necessary to keep up with incremental changes in technology, first-line approaches and in different AF subtypes. For all
Cost-effectiveness of Catheter Ablation Versus Medical Therapy for AF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Study A B Events Total Estimate 95% CI Blomstrom-Lundqvist et al. 201929 Forleo et al. 200930 Jais et al. 200831 Krittayaphong
al. 200332 Mont et al. 201433 Oral et al. 200634 Packer et al. 201923 Pappone et al. 200635 Stabile et al. 200636 Wilber et al. 201037 16 74 20 35 42 55 9 15 34 48 59 69 360 629 75 99 63 69 51 61 Random e ects model 729 1,154 Heterogeneity: I2=94%, χ2=145.7 (p<0.01) 20 40 60 80 Event percentage 69.2 [53.5–82.9] 27.1 [18.7–36.5] 21.6 [13.1–31.7] 57.1 [40.7–72.9] 76.4 [64.4–86.5] 60.0 [35.0–82.5] 70.8 [57.3–82.7] 85.5 [76.3–92.8] 57.2 [53.3–61.1] 75.8 [66.9–83.6] 91.3 [83.6–96.7] 83.6 [73.4–91.7] Study Events Total Estimate 95% CI Blomstrom-Lundqvist et al. 201929 Forleo et al. 200930 Jais et al. 200831 Krittayaphong et al. 200332 Mont et al. 201433 Oral et al. 200634 Packer et al. 201923 Pappone et al. 200635 Stabile et al. 200636 Wilber et al. 201037 11 73 7 35 6 52 3 14 39 98 26 77 217 611 14 99 30 68 39 106 Random e ects model Heterogeneity: I2=85%, χ2=58.5 (p<0.01) 10 20 30 40 50 Event percentage 15.1 [7.8–24.1] 20.0 [8.6–34.6] 11.5 [4.4–21.5] 21.4 [4.7–45.8] 39.8 [30.4–49.6] 33.8 [23.7–44.6] 35.5 [31.8–39.4] 14.1 [8.0–21.7] 44.1 [32.6–56.0] 36.8 [27.9–46.2]
et
AF recurrence data with (A) medical therapy and (B) catheter ablation treatment at 12 months. Source: Leung et al. 2022.21 Reproduced from Wiley & Sons under a Creative Commons CC BY-NC 4.0 licence.
clinicians, the updated data should make us question our local and wider arrhythmia service provision and how it meets the current data and guidelines.
AF Subtypes and First-line Treatment
There is public interest and debate over the potential benefits and costeffectiveness of catheter ablation versus medical therapy, particularly in patients with persistent AF. The recent UK cost-effectiveness study examined patients with all types of AF and did not specifically break out or model patients with paroxysmal versus persistent AF, using populationlevel treatment effects that were applicable to all AF subtypes.21 This was done for two reasons. First, by evaluating all AF patients, it gives a more comprehensive view of the real-world cost-effectiveness of ablation to inform policy and reimbursement decisions. Second, there is a lack of direct published evidence, particularly in real-world studies, comparing catheter ablation to medical therapy in persistent AF. As more evidence becomes available, it will be important to conduct future health economic research on the subtypes of AF. Although results on AF were not split into subtype in this study, a subgroup of those with heart failure and AF were analysed and, in fact, ablation treatment was most cost-effective in this scenario, which matches the known clinical benefit of ablation for those with persistent AF and related cardiomyopathy.21
The same limitations apply to questions surrounding the health and economic benefits associated with first-line treatment of AF; as clinicians adopt the European Society of Cardiology recommendations on secondline catheter ablation treatment, this should generate real-world data to inform future analyses.
Conclusion
Economic model analyses have shown that catheter ablation is a highly cost-effective treatment for AF compared with medical therapy from the perspective of the UK. Alongside increasing evidence of the clinical effectiveness of catheter ablation, AF ablation services should be prioritised with clear referral pathways to make ablation more accessible to the population in need.
Further Work
Economic modelling has informed and influenced the latest NICE guidelines on the management of AF and, in general, this data analysis has the power to change healthcare resource allocation. Further work is recommended to improve the use of these data to guide future guidelines and treatment recommendations: real-world data inputs into economic models can help create a more accurate analysis of treatment
1. Public Health England. Atrial fibrillation prevalence estimates. 2020. https://www.gov.uk/government/ publications/atrial-fibrillation-prevalence-estimates-for-localpopulations (accessed 27 January 2022).
2. Zoni-Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol 2014;6:213–20. https://doi.org/10.2147/CLEP. S47385; PMID: 24966695.
3. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. https://doi.org/10.1056/NEJMoa2019422; PMID: 32865375.
4. Hakalahti A, Biancari F, Cosedis Nielsen JC, Raatikainen MJ. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: systematic review and meta-analysis. Europace 2015;17:370–8. https://doi.org/10.1093/europace/euu376; PMID: 25643988.
5. Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med 2021;384:316–24. https://doi.org/10.1056/NEJMoa2029554;
3: Trade-off Between Cost and Probability of Cost-effectiveness Favours Catheter Ablation Over Medical Therapy
costs for the same clinical condition, and therefore a more effective use of resources. In addition, previously published randomised trials focused on ablation treatment as a second-line treatment but, with the emerging data from recent randomised clinical trials investigating the effectiveness of ablation versus medical therapy as first-line treatment, economic model analysis in this context would provide further supportive insights into the benefits of early rhythm control and how this can be put into effect within the constraints of our healthcare systems.
Clinical Perspective
• Economic evaluation is essential to bridge advances in scientific evidence towards implementation of updated best-practice guidelines and improve healthcare services to the public.
• Economic evaluation can be improved by the utilisation of real-world data in addition to clinical trial data inputs because real-world data are more reflective of the current clinical situation and therefore more applicable in real-world care.
• For the treatment of AF, further work on economic evaluation is required, particularly to look at AF subsets separately and the use of ablation as first-line treatment.
PMID: 33197158.
6. Andrade JG, Wells GA, Deyell MW, et al. Cryoballoon or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. https://doi.org/10.1056/NEJMoa2029980; PMID: 33197159.
7. Kuniss M, Pavlovic N, Velagic V, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. EP Europace 2021;23:1033–41. https://doi.org/10.1093/europace/euab029; PMID: 33728429.
8. Leung LW, Akhtar Z, Seshasai SRK, Gallagher MM. First-line management of paroxysmal atrial fibrillation: is it time for a ‘pill in the bin’ approach? A discussion on the STOP AF First, EARLY AF, Cryo-FIRST, and EAST-AF NET 4 clincal trials. EP Europace 2022;24:533–7. https://doi.org/10.1093/europace/ euab259; PMID: 34850953.
9. Reynolds MR, Walczak J, White SA, et al. Improvements in symptoms and quality of life in patients with paroxysmal atrial fibrillation treated with radiofrequency catheter ablation versus antiarrhythmic drugs. Circ Cardiovasc Qual Outcomes 2010;3:615–23. https://doi.org/10.1161/ CIRCOUTCOMES.110.957563; PMID: 20940250.
10. Reynolds MR, Zimetbaum P, Josephson ME, et al. Costeffectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ Arrhythm Electrophysiol 2009;2:362–9. https:// doi.org/10.1161/CIRCEP.108.837294; PMID: 19808491.
11. Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;1:373–498. https://doi.org/10.1093/eurheartj/ehaa612; PMID: 32860505.
12. National Institute for Health and Care Excellence. Developing NICE guidelines: the manual. London: NICE, 2014. https://www. nice.org.uk/process/pmg20/chapter/introduction (accessed 28 January 2022).
13. National Institute for Health and Care Excellence. Onasemnogene abeparvovec for treating spinal muscular atrophy London: NICE, 2021. https://www.nice.org.uk/guidance/
Cost-effectiveness of Catheter Ablation Versus Medical Therapy for AF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
0.25 £20,000 Willingness to pay £15,000 £10,000 £5,000 £20,000 £0 £25,000 £30,000 £35,000 £40,000 0 0.50 AAD Catheter ablation Probability of being cost-e ective 0.75 1
Figure
AAD = antiarrhythmic drug. Source: Leung et al. 2022.21 Reproduced from Wiley & Sons under a Creative Commons CC BY-NC 4.0 licence.
Cost-effectiveness of Catheter Ablation Versus Medical Therapy for AF
hst15/chapter/1-Recommendations (accessed 27 January 2022).
14. Kernick DP. Introduction to health economics for the medical practitioner. Postgrad Med J 2003;79:147–50. https://doi. org/10.1136/pmj.79.929.147; PMID: 12697913.
15. Reynolds MR, Lamotte M, Todd D, et al. Cost-effectiveness of cryoballoon ablation for the management of paroxysmal atrial fibrillation. Europace 2014;16:652–9. https://doi. org/10.1093/europace/eut380; PMID: 24390386.
16. Gao L, Moodie M. Modelling the lifetime cost-effectiveness of catheter ablation for atrial fibrillation with heart failure. BMJ Open 2019;9:e031033. https://doi.org/10.1136/ bmjopen-2019-031033; PMID: 31492790.
17. Aronsson M, Walfridsson H, Janzon M, et al. The costeffectiveness of radiofrequency catheter ablation as firstline treatment for paroxysmal atrial fibrillation: results from a MANTRA-PAF substudy. Europace 2015;17:48–55. https://doi. org/10.1093/europace/euu188; PMID: 25341739.
18. Khaykin Y, Wang X, Natale A, et al. Cost comparison of ablation versus antiarrhythmic drugs as first-line therapy for atrial fibrillation: an economic evaluation of the RAAFT pilot study. J Cardiovasc Electrophysiol 2009;20:7–12. https://doi. org/10.1111/j.1540-8167.2008.01303.x; PMID: 18803564.
19. National Institute for Health and Care Excellence. Atrial fibrillation: diagnosis and management. London: NICE, 2021. https://www.nice.org.uk/guidance/ng196 (accessed 27 January 2022).
20. Leung L, Imhoff RJ, Frame D, et al. Cost-effectiveness of catheter ablation versus medical therapy for the treatment of atrial fibrillation in the United Kingdom. EP Europace 2021;23(Suppl 3):euab116.531. https://doi.org/10.1093/ europace/euab116.531
21. Leung LWM, Imhoff RJ, Marshall HJ, et al. Cost-effectiveness of catheter ablation versus medical therapy for the treatment of atrial fibrillation in the United Kingdom. J Cardiovasc Electrophysiol 2022;33:164–75. https://doi. org/10.1111/jce.15317; PMID: 34897897.
22. Noseworthy PA, Gersh BJ, Kent DM, et al. Atrial fibrillation ablation in practice: assessing CABANA generalizability. Eur Heart J 2019;40:1257–64. https://doi.org/10.1093/eurheartj/
ehz085; PMID: 30875424.
23. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. https://doi.org/10.1001/jama.2019.0693; PMID: 30874766.
24. Andrade JG, Champagne J, Dubuc M, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation 2019;140:1779–88. https://doi.org/10.1161/ CIRCULATIONAHA.119.042622; PMID: 31630538.
25. Bin Waleed K, Yin X, Yang X, et al. Short and long-term changes in platelet and inflammatory biomarkers after cryoballoon and radiofrequency ablation. Int J Cardiol 2019;285:128–32. https://doi.org/10.1016/j. ijcard.2019.02.054; PMID: 30857843.
26. Gunawardene MA, Hoffmann BA, Schaeffer B, et al. Influence of energy source on early atrial fibrillation recurrences: a comparison of cryoballoon vs. radiofrequency current energy ablation with the endpoint of unexcitability in pulmonary vein isolation. Europace 2018;20:43–9. https://doi.org/10.1093/europace/euw307; PMID: 27742775.
27. Kuck KH, Furnkranz A, Chun KR, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: reintervention, rehospitalization, and quality-oflife outcomes in the FIRE AND ICE trial. Eur Heart J 2016;37:2858–65. https://doi.org/10.1093/eurheartj/ehw285; PMID: 27381589.
28. Afzal MR, Chatta J, Samanta A, et al. Use of contact force sensing technology during radiofrequency ablation reduces recurrence of atrial fibrillation: a systematic review and meta-analysis. Heart Rhythm 2015;12:1990–6. https://doi. org/10.1016/j.hrthm.2015.06.026; PMID: 26091856.
29. Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–68. https:// doi.org/10.1001/jama.2019.0335; PMID: 30874754.
30. Forleo GB, Mantica M, De Luca L, et al. Catheter ablation of atrial fibrillation in patients with diabetes mellitus type 2: results from a randomized study comparing pulmonary vein isolation versus antiarrhythmic drug therapy. J Cardiovasc Electrophysiol 2009;20:22–8. https://doi. org/10.1111/j.1540-8167.2008.01275.x; PMID: 18775050.
31. Jais P, Cauchemez B, Macle L, et al. Catheter ablation versus antiarrhythmic drugs for atrial fibrillation: the A4 study. Circulation 2008;118:2498–505. https://doi.org/10.1161/ CIRCULATIONAHA.108.772582; PMID: 19029470.
32. Krittayaphong R, Raungrattanaamporn O, Bhuripanyo K, et al. A randomized clinical trial of the efficacy of radiofrequency catheter ablation and amiodarone in the treatment of symptomatic atrial fibrillation. J Med Assoc Thai 2003;86(Suppl 1):S8–16. PMID: 12866763.
33. Mont L, Bisbal F, Hernandez-Madrid A, et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: a multicentre, randomized, controlled trial (SARA study). Eur Heart J 2014;35:501–7. https://doi. org/10.1093/eurheartj/eht457; PMID: 24135832.
34. Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med 2006;354:934–41. https://doi.org/10.1056/ NEJMoa050955; PMID: 16510747.
35. Pappone C, Augello G, Sala S, et al. A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF study. J Am Coll Cardiol 2006;48:2340–7. https:// doi.org/10.1016/j.jacc.2006.08.037; PMID: 17161267.
36. Stabile G, Bertaglia E, Senatore G, et al. Catheter ablation treatment in patients with drug-refractory atrial fibrillation: a prospective, multi-centre, randomized, controlled study (Catheter Ablation for the Cure of Atrial Fibrillation Study). Eur Heart J 2006;27:216–21. https://doi.org/10.1093/eurheartj/ ehi583; PMID: 16214831.
37. Wilber DJ, Pappone C, Neuzil P, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 2010;303:333–40. https:// doi.org/10.1001/jama.2009.2029; PMID: 20103757.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Mahaim Revisited
Eduardo Back Sternick , 1 Damian Sanchez-Quintana , 2 Hein JJ Wellens3 and Robert H Anderson 4
Abstract
The name Ivan Mahaim is well-known to electrophysiologists. However, alternative anatomical substrates can produce the abnormal rhythms initially interpreted on the basis of the pathways he first described. These facts have prompted suggestions that Mahaim should be deprived of his eponym. It is agreed that specificity is required when describing the pathways that produce the disordered cardiac conduction, and that the identified pathways should now be described in an attitudinally appropriate fashion. The authors remain to be convinced that understanding will be enhanced simply by discarding the term ‘Mahaim physiology’ from the lexicon. It is fascinating to look back at the history of accessory atrioventricular junctional conduction pathways outside the normal accessory atrioventricular conduction system, and their possible role in rhythm disturbances. It took both the anatomist and the clinical arrhythmologist quite some time to understand the complex anatomical architecture and the ensuing electrophysiological properties. Over the years, the name Mahaim was often mentioned in those discussions, although these pathways were not the ones that produced the eponym. The reason for this review, therefore, is to present relevant information about the person and what followed thereafter.
Keywords
Ventricular pre-excitation, atrioventricular node, nodoventricular pathways, fasciculoventricular pathways, atriofascicular tract
Disclosure: RHA is on the Arrhythmia & Electrophysiology Review editorial board; this did not influence peer review. All other authors have no conflicts of interest to declare.
Acknowledgement: Part of this review was produced in the final months of the life of HW. We dedicate it to his eternal memory.
Received: 25 February 2022
Accepted: 24 March 2022
Citation: Arrhythmia & Electrophysiology Review 2022;11:e14. DOI: https://doi.org/10.15420/aer.2022.12
Correspondence: Eduardo Sternick, Arrhythmia and Electrophysiology Department, Biocor Institute, Alameda do Morro 85, Olympus, T-4, Suite 1900, Nova Lima, Minas Gerais, 34083006, Brazil. E: eduardosternick@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Of late, there has been a significant resurgence of interest in the writings of Ivan Mahaim. We have ourselves recently produced a series of reviews in which we discussed the features of the unusual arrhythmias that are now usually explained on the basis of anatomical substrates bearing his name.1–3 It has recently been suggested, however, that the eponym ‘Mahaim’ should be discontinued.4 We have sympathy for this viewpoint. We accept that anatomical substrates of arrhythmias are better described in descriptive fashion, not least because one of us reported the first clinical case of a ‘Mahaim fibre’ in 1971, while another of us was involved in an attempt to establish the concept of descriptive nomenclature as long ago as 1976.5,6 We are also strong supporters of the need for descriptive nomenclature to be attitudinally correct.7 We wonder, however, whether discontinuation of the eponym, as suggested by those writing recently in Heart Rhythm, will produce the suggested clarification of the underlying substrates for the abnormal rhythms?4
Despite the very best efforts of those who have sought to avoid eponyms in general, it is unlikely that the name will disappear. It is also the case that a detailed reading of the writings of Mahaim reveals much to be admired.8–10 Mahaim himself cannot be blamed for the misinterpretations of these writings by subsequent generations of electrophysiologists. In this review, in defence of Mahaim, we summarise the initial findings of this
Belgian, who practised throughout his career in Switzerland, discussing their relevance to modern-day electrophysiologists. It will then remain to be seen whether the eponym disappears from the electrophysiological lexicon.
The Career of Ivan Mahaim
Mahaim was born in Liege, Belgium, on 25 June 1897.11 He was educated in Switzerland, where he entered the University of Lausanne in 1918 to study medicine, receiving his degree in 1925. Having undertaken postgraduate training with Wenckebach in Vienna, and LeClerc in Paris, he returned to Lausanne in 1929, where he was appointed associate professor at the university. During his career, he published 100 manuscripts, mostly in his native French, but with some significant contributions in the English literature. His magnum opus was published early in his career, and was entitled Les Maladies Organiques du Faisceau de His-Tawara. In this book, he described detailed findings of his study of over 400 individuals with problems of rhythm originating from the atrioventricular conduction axis.
Many of Mahaim’s investigations were supplemented by equally detailed histological studies. As we will describe, he revisited these early experiences later in his career, when summarising his findings in the descriptions of the pathways that earned him the eponym. As we will also
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Clinical Electrophysiology and Ablation
1. Arrhythmia and Electrophysiology Department, Biocor Institute, Nova Lima, Brazil; 2. Department of Anatomy and Cell Biology, Universidad de Extremadura, Badajoz, Spain; 3. Cardiovascular Research Centre Maastricht, the Netherlands; 4. Biosciences Institute, Newcastle University, Newcastle upon Tyne, UK
Figure 1: Paraspecific Connections
discuss, and as was pointed out by Lüderitz when summarising his overall achievements, he showed great prescience.11 Lüderitz points to his speculations with regard to the potential of cardiac surgery.
Mahaim was also remarkably prescient in contemplating the role of the alternative structures that are now interpreted in his name as the substrates for pre-excitation, even though the concept on which he based his interpretations proved to be spurious.11 He was also very much a ‘renaissance man’. In the latter part of his life, he established a reputation as a world-class scholar in the achievements of Ludwig van Beethoven.11 It is, nonetheless, his significant achievements in describing the detailed anatomy of the atrioventricular conduction axis, and its direct connections with the ventricular septal myocardium, on which we will concentrate.
The ‘Paraspecific’ Myocardial Connections
Upper panel: The drawing in the illustration was made by Mahaim to support his notion that, when the ‘specific’ connection provided by the atrioventricular conduction axis was diseased, the ‘paraspecific’ connections, provided either by the connections he described between the axis and the crest of the ventricular septum, labelled ‘cx’, or the pathways allegedly described by Kent, labelled ‘Kt’, could continue to permit atrioventricular conduction. Mahaim had conducted animal experiments, severing the normal pathways, as shown by the level ‘s. Kt’, to substantiate his concepts. Lower panel: The drawing shown was made by Mahaim to illustrate the ‘paraspecific’ pathways he had identified between the base of the atrioventricular conduction axis and the crest of the muscular septum. Mahaim believed that these pathways, labelled 1 through 5, could provide the substrate for normal atrioventricular conduction when the axis itself was diseased and incapable of conducting to the ventricles. Pathway 5 passes from the base of the atrioventricular node (Taw.), whereas pathways 1 through 3 take their origin from the non-branching bundle (tr. c). Pathway 4 runs from the left bundle branch (Br. g). Br. d. = right branch; Br. g. = left branch; cx. = upper connections (fasciculoventricular connections); fibr. = fibrosis; Kt = Kent’s fibers; m. aur. fibr. = retro-tawarian fibro-adipose plaque of the interatrial septum; mitr. = mitral valve; m. v. = partially fibrotic ventricular myocardium; m. v. sp. = a portion of ventricular myocardium included in the membranous septum; o.d. = right atrium; o.g. = left atrium; Sin. = sinus node; S. Kt = represents the experimental section performed by Kent; Taw. = tawara knot; Tr. c = main trunk; tric. = tricuspid; v. a.d. r. = right aortic valve. Upper panel: Source: Mahaim et al. 1947.10 Reproduced with permission from Elsevier. Lower panel: Source: Mahaim et al. 1941.9 Reproduced with permission from Karger.
Figure 2: Nodoventricular Connections
Mahaim began his research into the diseases that might afflict the myocardial connection between the atrial and ventricular myocardial masses at a time when the substrate for atrioventricular conduction remained controversial. This was because, although Wilhelm His Junior had provided an excellent account of the bundle that now bears his name, the English physiologist, Arthur Stanley Kent, had continued to maintain in the second decade of the 20th century that multiple myocardial pathways crossed the insulating planes of the atrioventricular junctions.12,13 In making these claims regarding multiple myocardial connections across the atrioventricular junctions in the normal heart, Kent was unequivocally wrong. He was partially correct in so far as the structures he illustrated do, indeed, exist. We now know that they are remnants of a ring of histologically specialised tissue that, in the developing heart, surrounds the embryonic interventricular communication. With ongoing normal development, the ring becomes remodelled as the direct connection is established between the cavities of the right atrium and right ventricle. The specialised myocardial tissues normally become sequestrated within the vestibule of the tricuspid valve. They persist as the entities discovered and illustrated by Kent.
As is recognised in the title of Mahaim’s book, it was Tawara who clarified the arrangement of the solitary pathway that, in the normal heart, provides the substrate for atrioventricular conduction. This is the pathway described by Mahaim as being ‘specific’ for atrioventricular conduction. On the basis of his research, specifically on the interpretation of the electrocardiographic changes, he inferred that other connections must exist between the atrial and ventricular myocardial masses to permit conduction when the major pathway was itself diseased. It was these additional pathways that he named the ‘paraspecific’ connections. He summarised his findings in this regard in the review published in 1947.9 In this work, he speculated that the pathways may not be confined to the connections that he had previously observed extending between the major pathway itself, but could also involve the structures illustrated by Kent. Mahaim, therefore, was well aware that pathways other than those we now name in his memory could provide alternative substrates for atrioventricular conduction.
The Pathways Described by Mahaim
It was direct connections between the base of the atrioventricular conduction axis and the crest of the ventricular septum that had been the focus of Mahaim’s histological studies. In his animal experiments, he had made cuts, as shown in the upper panel of Figure 1, observing that atrioventricular conduction persisted despite the severing of the midseptal components of the ventricular bundle branches. These findings, along with the interpretations of the electrocardiograms obtained from patients known to have acquired diseases of the conduction axis, underscored his proposal that the histological connections he identified
Mahaim Revisited ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Sin. Kt o.d. o.g. Taw Tr. c cx. S. Kt m. aur. fibr Taw tric. m. v. sp Br. d. m. v fibr mitr Tr. c. Br. g. v. a d r m. v Tendon
Compact atrioventricular node Nodoventricular connection
of Todaro
The section shows an obvious nodoventricular connection from a neonatal heart. Such connections were not found in 21 datasets obtained from adult hearts.
(Figure 1, lower panel) could provide the alternative pathways necessary to provide the observed atrioventricular conduction.
We recently had the opportunity to assess the findings of Mahaim through our own access to a series of data sets prepared so as to provide serial sections through a series of atrioventricular conduction axes obtained from adults.14 Our initial assessment of these data sets was confined to examination of the atrioventricular node and the penetrating atrioventricular bundle. Within this analysis, we did not encounter any nodoventricular connections, although we did find one connection between the origin of the penetrating bundle and the crest of the ventricular septum. Such a pathway was shown by Mahaim as #5 in the illustration we have now reproduced (Figure 1, lower panel).
In our previous analysis of hearts obtained from neonates and infants, it had been usual to find extensive remnants of the atrioventricular node dispersed within the insulating fibrous tissues of the atrioventricular junctions.15 These arrangements have previously been described as ‘foetal dispersion’. They were implicated by some investigators as a potential anatomical substrate for sudden infant death, although that hypothesis was never proven.16 In some neonatal hearts, the remnants can be sufficiently extensive to produce obvious nodoventricular connections (Figure 2). We were unable to identify such connections in our adult data sets.14 We have now extended our analysis of these data sets to include the non-branching and branching components of the conduction axis. This has permitted us to identify with certainty the origin of the right bundle branch.
We were surprised, therefore, to find, in addition to the pathway described at the origin of the penetrating atrioventricular bundle, several further examples of fasciculoventricular connections (Figure 3).
As is well-known to electrophysiologists, and now responsible for much of the confusion regarding the use of ‘Mahaim’ as an eponym, the arrhythmias described in his name can also be produced by a quite different anatomical substrate. This is the so-called ‘atriofascicular pathway’. The use of this term is not without its own problems. When the morphological members of the European Study Group on Pre-excitation suggested names for the anatomical substrates producing pre-excitation, they proposed that Mahaim’s ‘paraspecific’ pathways should be subcategorised as either nodoventricular or fasciculoventricular connections.6 This suggestion has been well received. At the same time, the study group proposed that the so-called ‘atrio-Hisian’ connections should be dubbed atriofascicular pathways. Nowadays, of course, the pathways considered to be ‘atriofascicular’ are far removed from the para-Hisian area. These pathways, nonetheless, can produce electrophysiological findings remarkably similar to those believed to produce ‘Mahaim’ pre-excitation through the paraspecific pathways. The substrate now believed to produce the alternative ‘Mahaim pre-excitation’ was first identified by ourselves, although we were unaware, at the time, of the significance of our finding. In our analysis of hearts obtained from patients dying with known preexcitation, we discovered an accessory insulated atrioventricular pathway in a patient with Ebstein’s malformation, but also known to have regular WolffParkinson-White syndrome
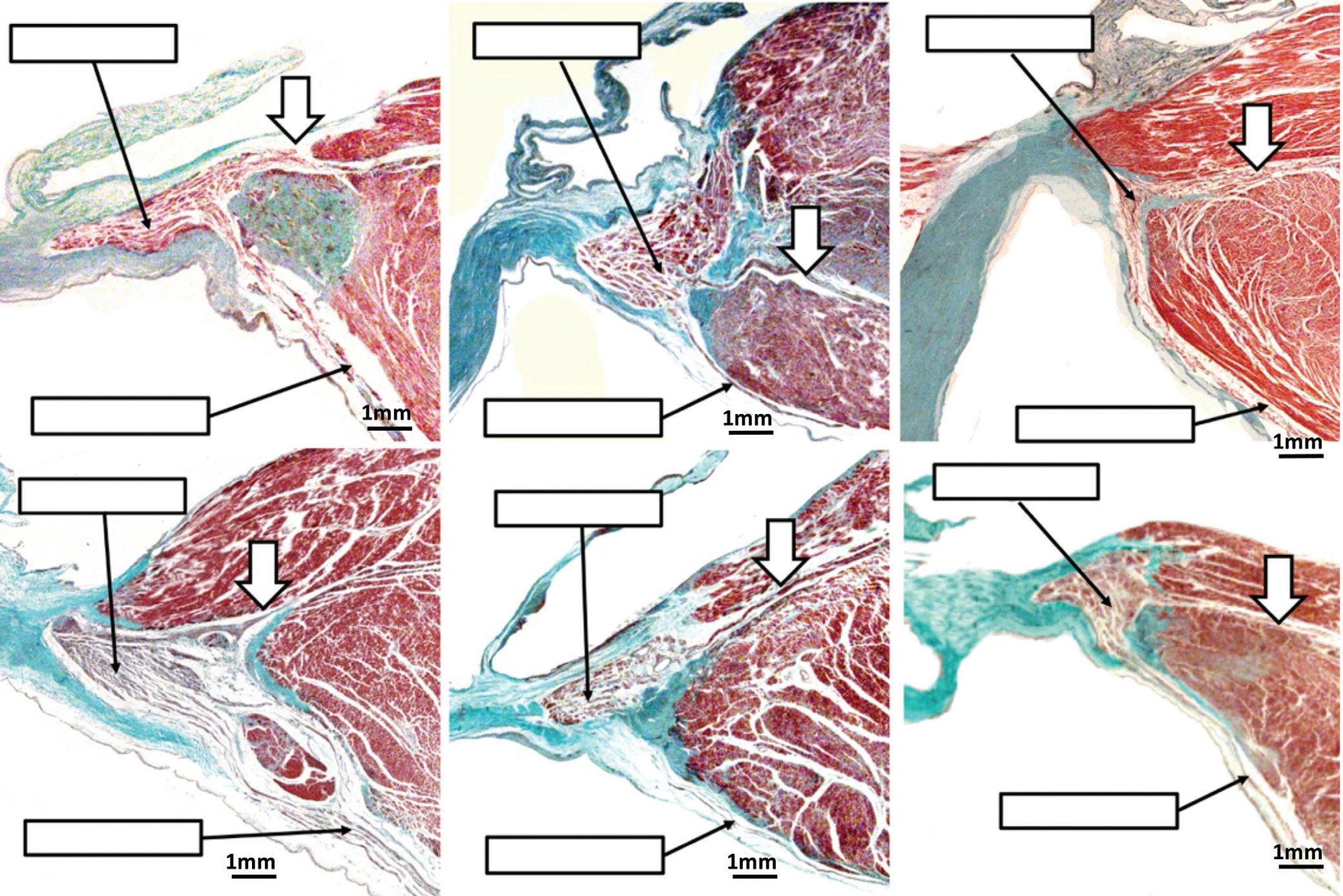
Mahaim Revisited ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
(Figure 4, upper right panel).17
Branching
Branching
Branching
Left bundle branch Left bundle branch Left bundle branch Left bundle branch Left bundle branch Left bundle branch
Figure 3: Fasciculoventricular Connection
Branching bundle Branching bundle
bundle
bundle
bundle Branching bundle
The sections show the six examples of connections between the ventricular components of the atrioventricular conduction axis and the crest of the ventricular septum (arrows). In all, we studied 21 datasets obtained from adult hearts. In each data set shown, the right bundle branch was found to arise separately from the more distal part of the branching atrioventricular bundle.
The upper right hand panel shows the accessory atrioventricular node we identified in a patient known to have had Wolff-Parkinson-White syndrome. We were able to trace an insulated tract from the node. We now know, as shown in the upper left hand panel, that the accessory node and tract can be part of a circuit involving the right bundle branch and the regular atrioventricular node. Hence, the description of the tract as being ‘atriofascicular’. The middle and lower panels show ‘Mahaim’ physiology. The middle panel shows the response to intravenous adenosine during accessory atrioventricular node antidromic tachycardia, with progressive increase of the atrioventricular interval (320–370 ms) until termination. The lower panel shows decremental atrioventricular conduction during atrial with increasing rates, in a left-sided short atrioventricular ‘Mahaim-type’ pathway. There is decremental conduction through both the atrioventricular node and the ‘Mahaim’ pathway (atrioventricular interval increased from 65 to 170 ms and 114 to 170 ms, respectively), until conduction through the atrioventricular conduction axis reverses at the onset of antidromic tachycardia. AV = atrioventricular.
This patient was also shown to have well-formed, left-sided, muscular atrioventricular connections. The right-sided connection we found was unusual, as it took its origin from an accessory atrioventricular node of the type initially illustrated by Kent. We were able to trace an insulated tract arising from the node, but we could not identify the ventricular insertion of the tract. It was the experience of Klein et al. that revealed how, in certain circumstances, the tract could pass into the moderator band and unite with the right bundle branch.18 It was this experience that prompted description of the pathways as being ‘atriofascicular’. The findings validate the existence of additional ‘paraspecific’ pathways
involving the structures illustrated by Kent, thus pointing again to the prescience of Ivan Mahaim.
As we now know that the so-called atrio-Hisian pathways almost certainly represent the final connection between the atrial myocardium and the atrioventricular conduction axis prior to its insulation as the His bundle, there is no longer a need to employ ‘atriofascicular’, as initially suggested by the European Study Group.14 The way is clear, therefore, to stratify the anatomical substrates for so-called ‘Mahaim pre-excitation’ using the descriptive terms of nodoventricular, fasciculoventricular and atriofascicular pathways. However, does this mean we then need to strip Mahaim of his eponym?
The Electrophysiological Significance of Mahaim Arrhythmias
It is certainly true that all accessory myocardial pathways having slow and decremental properties of conduction, regardless of their specific morphology, share a common electrophysiological characteristic. It is this property that has widely become known as Mahaim physiology (Figure 4, middle and lower panels). In keeping with this approach, the different variants of pre-excitation produced by the pathways are now commonly lumped together as ‘Mahaim fibres’. Already in 1971, one of us had published a case of a young boy suffering from tachycardias.5 In Figure 5, we show details from this first patient with Mahaim tachycardia who underwent an electrophysiological study. At that time, only the presence of an anatomical nodoventricular connection, as described by Mahaim, could explain the unusual findings. In retrospect, the findings of the gradual decremental increase in ventricular pre-excitation, the development of a QRS configuration with the features of left bundle branch block during atrial single test stimulation, and the reproducible initiation and termination of the tachycardia by appropriately timed atrial and ventricular premature beats, could also have been produced by an accessory atrioventricular pathway having nodal-like properties. This, of course, is the substrate described by Klein et al. 17 years later, and currently known as the atriofascicular pathway.18
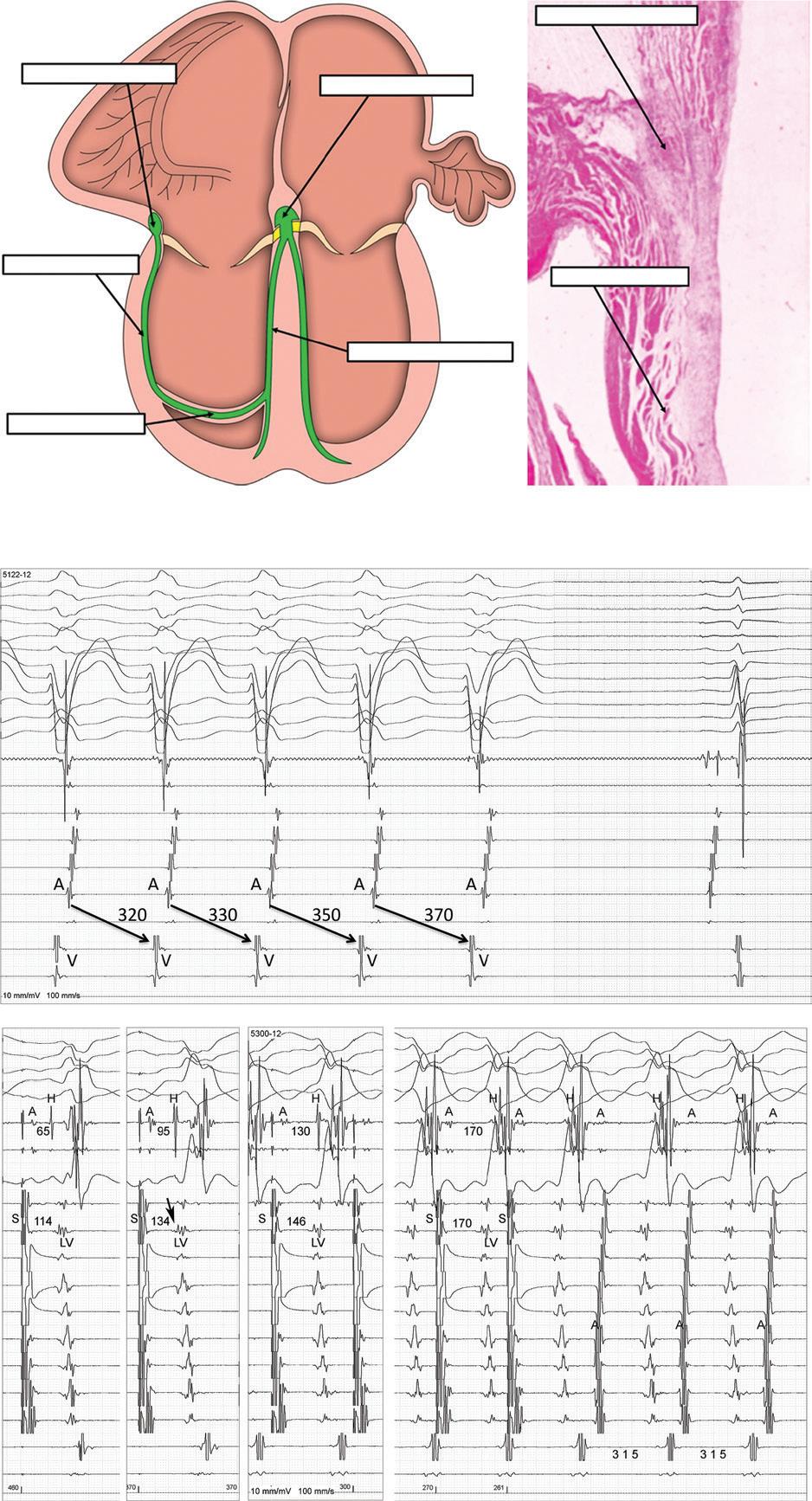
Among the connections identified by Mahaim himself, it was only the fasciculoventricular variety that had not been implicated in re-entrant circuits.19 Recently, however, cases have been reported involving a circus movement tachycardia, supposedly incorporating a fasciculoventricular connection.20 The fasciculoventricular pathways, more usually, function as bystander conduits in other tachycardias, such as atrioventricular re-entry or atrioventricular nodal re-entrant tachycardia. We know that anterograde conduction through such bystander pathways needs to be identified correctly during an electrophysiologic study, so as to avoid harm to the atrioventricular node should the fasciculoventricular pathway be mistakenly targeted for ablation. Fasciculoventricular pathways seem to be more frequent than atrioventricular bypass tracts, and probably ubiquitous structures.21 Thus, in our ongoing studies, we found fasciculoventricular pathways in 14 of 21 data sets prepared from normal hearts.22
In the other variants of ventricular pre-excitation involving a ‘Mahaim’ pathway, macro-reentry in the circuits can lead to antidromic tachycardia.23 This is because the circuits involve anterograde conduction over the pathway with decremental properties, and retrograde conduction through the normal atrioventricular conduction axis. The retrograde conduction may occur via the right bundle branch or through the branches of the left bundle. These variations can change the ventricular-His timing, as well as the tachycardia cycle length, giving rise to complex tachycardias.24,25 The atriofascicular pathway involving an accessory atrioventricular node, and
Mahaim Revisited ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Accessory AV node Regular AV node Insulated bundle Right bundle branch Moderator band Accessory AV node Insulated bundle Laboratório de Eletrofisiologia Cardíaca – Biocor Instituto Nova Lima Bras l Version WINDOWS XP : EPTRACER V1.072 I II III AVR AVL AVF V1 V2 V3 V4 V5 V6 His d His p SC d 2 3 4 5 VD d VD p STIM I II III V1 V6 His d His p UNIP CS d 2 3 4 5 6 7 8 9 RV d RV p STIM
Figure 4: Accessory Atrioventricular Node (Atriofascicular Pathway)
Figure 5: Unrecognised Electrophysiological Evidence for an Atriofascicular Pathway
The tracings were obtained during a study in an 8-year-old boy made in 1971. To the best of our knowledge, this was the first patient with a Mahaim tachycardia who underwent an electrophysiological study. The left upper hand panel shows the electrocardiogram during sinus rhythm, with a PR interval of 120 ms and minimal ventricular pre-excitation. The right hand panel shows a tachycardia with left bundle branch block QRS configuration, and advancement of the next tachycardia cycle by an atrial premature beat. This manoeuvre proves an extra-nodal proximal atrial end and accessory pathway participating in the tachycardia circuit. At that time, and until the 1980s, the Mahaim pathway was interpreted as a nodoventricular pathway (lower left panel). AV = atrioventricular. Source: Wellens 1971.5 Reproduced from University Park Press under a Creative Commons CC0 1.0 licence.
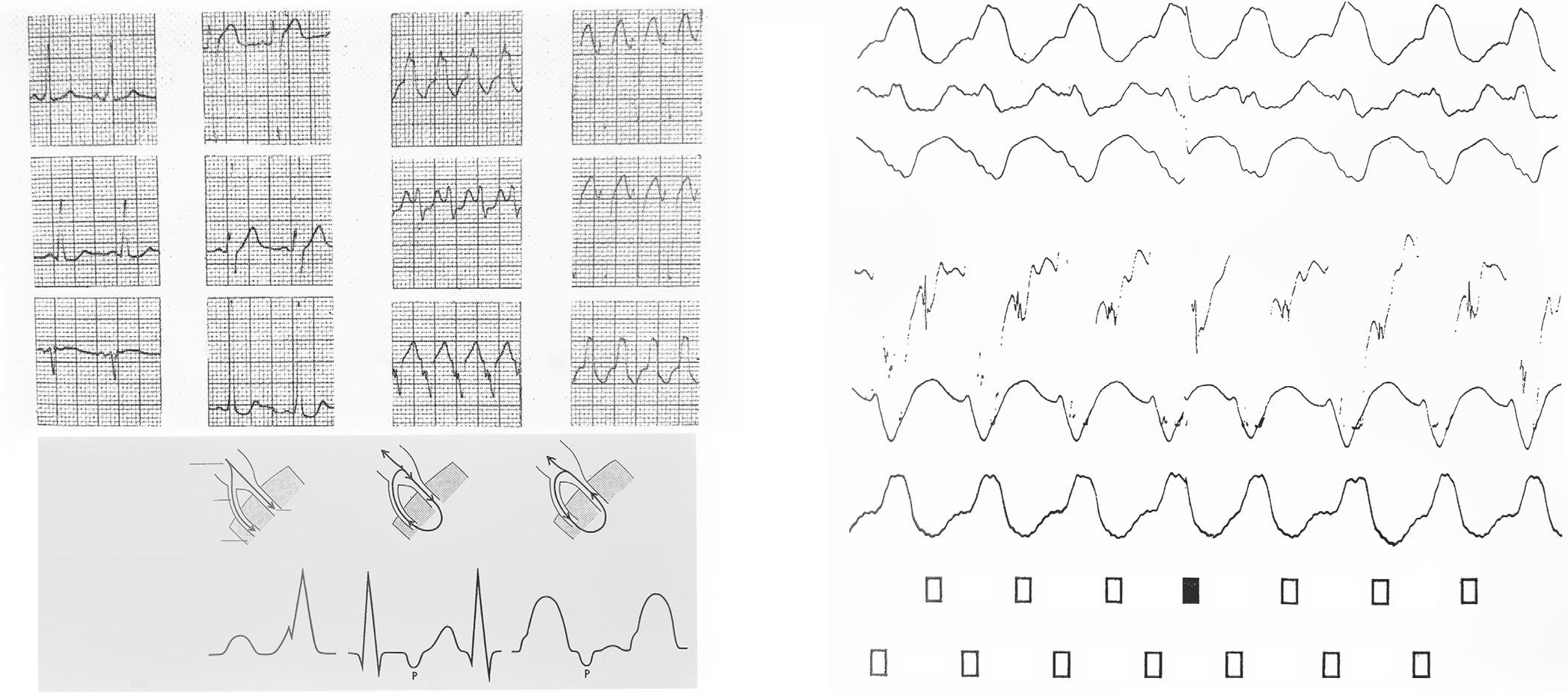
usually located at the acute margin of the right atrioventricular junction, is the most frequent structure involved. The macro-reentrant tachycardia using such a circuit would better be described as an antidromic accessory atrioventricular nodal re-entrant tachycardia.
Short decrementally conducting accessory pathways originating from the base of the compact atrioventricular node, which may occur at both the right and the left atrioventricular junctions, could produce comparable circuits.2 These nodoventricular pathways can be used anterogradely in a circus movement tachycardia, with ventriculo-atrial dissociation, as the atrial myocardium is not part of the circuit. They can also be used retrogradely, giving rise to narrow complex tachycardias with atrioventricular dissociation.3 These pathways, which are those described by Mahaim, can also be involved in other uncommon arrhythmic mechanisms, such as spontaneous automatic rhythms, or non-paroxysmal tachycardia due to double atrioventricular conduction. More recently, they have been implicated in nodoventricular-atrial re-entry, which may cause pseudo-atrioventricular block.26–28
Conclusion
As parts of groups, we have been involved for several decades in this long process of unravelling the historical anatomical and electrophysiological characteristics of the variants of ventricular pre-excitation. We endorse
1. Anderson RH, Sanchez-Quintana D, Mori S, et al. Unusual variants of pre-excitation: from anatomy to ablation: part I –understanding the anatomy of the variants of ventricular pre-excitation. J Cardiovasc Electrophysiol 2019;30:2170–80. https://doi.org/10.1111/jce.14106; PMID: 31397515.
2. Correa FS, Lokhandwala Y, Filho FC, et al. Part II—Clinical presentation, electrophysiologic characteristics, and when and how to ablate atriofascicular pathways and long and short decrementally conducting accessory pathways. J Cardiovasc Electrophysiol 2019;30:3079–96. https://doi. org/10.1111/jce 14203; PMID: 31588593.
3. Soares Correa F, Lokhandwala Y, Sánchez-Quintana D, et al.
the notion that cardiac structures are best described according not only to their structure-function relations, but also according to their correct attitudinal position inside the thorax of the living person.1 We also take the stance, nonetheless, that some eponyms, providing they have withstood the test of time for the medical community at large, need not be discarded simply for the sake of the ‘political correctness’. Terms, such as His, Purkinje and Wolff-Parkinson-White, are not only wellknown, but are part of our past. The same goes for the name of Ivan Mahaim. These eponyms are an integral part of the heritage that defines us. We are unaware of any evidence showing that use of the terms, ‘Mahaim pathway’ or ‘Mahaim physiology’, negatively impacts the message to be conveyed as any patient undergoes an electrophysiological study, in which the object is to establish the specific mechanism of the arrhythmia, and the location and structure of its anatomical substrate. Only when this object is achieved can the proper treatment be determined.
It remains the fact that recognition of the potential presence of a so-called ‘Mahaim pathway’ means that there will be fun at the electrophysiology lab! Eponyms have not disappeared, nor are they likely to go away. The main issue with their use is to be sure that the honouree is worthy of the structure thus named. Ivan Mahaim, in our opinion, remains more than worthy of the reference, as long as it is properly used.
Unusual variants of pre-excitation: from anatomy to ablation: part III – clinical presentation, electrophysiologic characteristics, when and how to ablate nodoventricular, nodofascicular, fasciculoventricular pathways, along with considerations of permanent junctional reciprocating tachycardia. J Cardiovasc Electrophysiol 2019;30:3097–115. https://doi.org/10.1111/jce.14247; PMID: 31646696.
4. Balaji S, Tchou P, Kanter R. Mahaim fibers: should they be renamed? Heart Rhythm 2020;17:161–2. https://doi. org/10.1016/j.hrthm.2019.07.025; PMID: 31351139.
5. Wellens HJJ. Normal PR interval-small delta wave-normal QRS complex. In: Wellens HJJ. Electrical Stimulation of the
Heart in the Study and Treatment of Tachycardias Baltimore: University Park Press, 1971;97–109.
6. Anderson RH, Becker AE, Brechenmacher C, et al. Ventricular preexcitation. A proposed nomenclature for its substrates. Eur J Cardiol 1975;3:27–36. PMID: 1132407.
7. Cosio FC, Anderson RH, Kuck K, et al. Living anatomy of the atrioventricular junctions. A guide to electrophysiological mapping. A consensus statement from the Cardiac Nomenclature Study Group, Working Group of Arrhythmias, European Society of Cardiology, and the Task Force on Cardiac Nomenclature from NASPE. Circulation 1999;100:e31–7. https://doi.org/10.1161/01.cir.100.5.e31;
Mahaim Revisited ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
I II III V1 V3 V6 I II III V1 V3 V6 I II III His V1 V6 AV node Mahaim bundle His bundle Ventricular septum Atrium 280 280 240 290 280 280 Ventricle 280 280 280 250 290 280
PMID: 10430823.
8. Mahaim I, Benatt A. New research on the superior connections of the left branch of the bundle of His-Tawara with the interventricular septum. Cardiologia 1937;1:61–73 [in French]. https://doi.org/10.1159/000164567
9. Mahaim I, Winston MR. Comparative anatomy and experimental pathology research on high connections of the bundle of His-Tawara. Cardiologia 1941;5:189–260 [in French]. https://doi.org/10.1159/000164707
10. Mahaim I. Kent’s fibers and the AV paraspecific conduction through the upper connection of the bundle of His-Tawara. Am Heart J 1947;33:651–3. https://doi.org/10.1016/00028703(47)90080-x; PMID: 20238575.
11. Lüderitz B. Ivan Mahaim (1897-1965). J Interv Card Electrophysiol 2003;8:155. https://doi. org/10.1023/a:1023617119093; PMID: 12766508.
12. Anderson RH, Mori S. Wilhelm His Junior and his bundle. J Electrocardiol 2016;49:637–43. https://doi.org/10.1016/j. jelectrocard.2016.06.003; PMID: 27324867.
13. Kent AFS. The structure and function of the mammalian heart. In: Report of the British Association for the Advancement of Science. London: John Murray, 1915;226–9.
14. Anderson RH, Sanchez-Quintana D, Mori S, et al. Re-evaluation of the structure of the atrioventricular node and its connections with the atrium. Europace 2020;22:821–30. https://doi.org/10.1093/europace/euaa031: PMID: 32304217.
15. Anderson RH, Ho SY, Gillette PC, Becker AE. Mahaim, Kent and abnormal atrioventricular conduction. Cardiovasc Res 1996;31:480–91. https://doi.org/10.1016/S0008-
6363(96)00011-9; PMID: 8689639.
16. Anderson RH, Bouton J, Burrow CT, Smith A. Sudden death in infancy: a study of the cardiac specialized tissue. Br Med J 1974;2:135–9. https://doi.org/10.1136/bmj.2.5911.135; PMID: 4825111.
17. Becker AE, Anderson RH, Durrer D, Wellens HJJ. The anatomical substrates of Wolff-Parkinson-White syndrome. A clinicopathologic correlation in seven patients. Circulation 1978;57:870–9. https://doi.org/10.1161/01.cir.57.5.870; PMID: 639209.
18. Klein GJ, Guiraudon GM, Kerr CR, et al. ‘Nodoventricular’ accessory pathway: evidence for a distinct accessory atrioventricular pathway with atrioventricular node-like properties. J Am Coll Cardiol 1988;11:1035–40. https://doi. org/10.1016/s0735-1097(98)90063-8; PMID: 3128586.
19. Sternick EB, Gerken LM, Vrandecic MO, Wellens HJJ. Fasciculoventricular pathways: clinical and electrophysiologic characteristics of a variant of preexcitation. J Cardiovasc Electrophysiol 2003;14:1057–63. https://doi.org/10.1046/j.1540-8167.2003.03206.x; PMID: 14521658.
20. Chung R, Wazni O, Dresing T, et al. Clinical presentation of ventricular-Hisian and ventricular-nodal accessory pathways. Heart Rhythm 2019;16:369–77. https://doi.org/10.1016/j. hrthm.2018.08.006; PMID: 30103070.
21. Suzuki T, Nakamura Y, Yoshida S, et al. Differentiating fasciculoventricular pathway from Wolff-Parkinson-White syndrome by electrocardiography. Heart Rhythm 2014;11:686–90. https://doi.org/10.1016/j.hrthm.2013.11.018; PMID: 24252285.
22. Macías Y, Tretter JT, Anderson RH, et al. Miniseries 1 – part IV: how frequent are fasciculo-ventricular connections in the normal heart? Europace 2022;24:464–72. https://doi. org/10.1093/europace/euab286; PMID: 34999781.
23. Katritsis DG, Wellens HJJ, Josephson ME. Mahaim accessory pathways. Arrhythm Electrophysiol Rev 2017;6:29–32. https:// doi.org/10.15420/aer.2016:35:1; PMID: 28507744.
24. Sternick EB, Scarpelli RB, Gerken LM, Wellens HJJ. Wide QRS tachycardia with sudden rate acceleration. What is the mechanism? Heart Rhythm 2009;6:1670–3. https://doi. org/10.1016/j.hrthm.2008.12.007; PMID: 19879548.
25. Gandhavadi M, Sternick EB, Jackman WM, et al. Characterization of the distal insertion of atriofascicular accessory pathways and mechanisms of QRS patterns in atriofascicular antidromic tachycardia. Heart Rhythm 2013;10:1385–92. https://doi.org/10.1016/j. hrthm.2013.07.009; PMID: 23851064.
26. Sternick EB, Sosa EA, Scanavacca MI, Wellens HJJ. Dual conduction in a Mahaim fiber. J Cardiovasc Electrophysiol 2004;15:1212–5. https://doi. org/10.1046/j.1540-8167.2004.04036.x; PMID: 15485450.
27. Sternick EB, Sosa EA, Timmermans C, et al. Automaticity in Mahaim fibers. J Cardiovasc Electrophysiol 2004;15:738–44. https://doi.org/10.1046/j.1540-8167.2004.03615.x; PMID: 15250854.
28. Tuohy S, Saliba W, Pai M, Tchou P. Catheter ablation as a treatment for atrioventricular block. Heart Rhythm 2018;15:90–6. https://doi.org/10.1016/j.hrthm.2017.08.015; PMID: 28823599.
Mahaim Revisited ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Protecting Against Collateral Damage to Non-cardiac Structures During Endocardial Ablation for Persistent Atrial Fibrillation
Lisa WM Leung , 1 Zaki Akhtar , 1 Jamal Hayat 2 and Mark M Gallagher 1
1. Department of Cardiology, St George’s Hospital NHS Foundation Trust, London, UK;
2. Department of Gastroenterology, St George’s Hospital NHS Foundation Trust, London, UK
Abstract
Injury to structures adjacent to the heart, particularly oesophageal injury, accounts for a large proportion of fatal and life-altering complications of ablation for persistent AF. Avoiding these complications dictates many aspects of the way ablation is performed. Because avoidance involves limiting energy delivery in areas of interest, fear of extracardiac injury can impede the ability of the operator to perform an effective procedure. New techniques are becoming available that may permit the operator to circumvent this dilemma and deliver effective ablation with less risk to adjacent structures. The authors review all methods available to avoid injury to extracardiac structures to put these developments in context.
Keywords
Ablation, AF, oesophagus, oesophageal injury, atrio-oesophageal fistula, oesophageal temperature management
Disclosure: LWML previously received research support from Attune Medical. MMG received research funding from Attune Medical and acted as a consultant and paid speaker for Boston Scientific and Cook Medical. All other authors have no conflicts of interest to declare.
Received: 30 November 2021 Accepted: 13 April 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e15. DOI: https://doi.org/10.15420/aer.2021.67
Correspondence: Mark M Gallagher, Department of Cardiology, St George’s Hospital, Blackshaw Rd, London SW17 0QT, UK. E: mark_m_gallagher@hotmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Normalisation of heart rhythm in patients vulnerable to atrial arrhythmia is now largely procedural rather than pharmacological.1 It is based on ablation of the myocardium in lines to obstruct arrhythmia circuits, or on destroying or encircling zones of the myocardium that trigger arrhythmia. In AF, ablation necessarily includes isolation of the pulmonary veins; for persistent AF, more extensive lesion sets are often used, particularly isolation of the posterior wall.2,3 Unlike paroxysmal AF, right atrial lesions are also often required. Lesion sets in persistent AF are often directed at targets, such as sites of automaticity, fractionation or low amplitude that may be found in a variety of locations, including the left atrial appendage, the crista terminalis and the superior vena cava, locations seldom targeted in other conditions.
Transmurality and contiguity are prerequisites for an effective lesion set. For an endocardial delivery of thermal energy to create tissue necrosis to the epicardial surface, some tissue injury must occur beyond that limit. To achieve consistent transmurality across a large lesion set, the operator must accept that damage will extend into adjacent structures. A skilled and experienced operator can do this in a controlled manner, but the risk is not eliminated.
Most of the structures that abut the human atrium (Figure 1) tolerate such injury without threat to life; small amounts of lung tissue and the diaphragmatic muscle can be considered dispensable, although symptomatic injury to the lung and airways does occur in cryoballoon ablation.4 Injury to the oesophagus is different: atrio-oesophageal fistula (AEF), as well as fistulae from the oesophageal lumen to the mediastinum or the pericardial space, have been reported in approximately 0.1% of ablations for atrial arrhythmias and most cases are fatal.5–8 Fistulae from
the atrium to a bronchus have also been reported; like AEF, they are often lethal.9 The true incidence of atriobronchial fistulas is not known, but radiological studies have highlighted the close proximity of the lung to the left atrium (Figure 2), in particular along the course where ablation of the pulmonary veins would be performed.10 Nerves are also vulnerable, particularly the perioesophageal neural plexus and the phrenic nerves.
Importance of the Oesophagus
In its infancy, ablation therapy for AF was dogged by a risk of complications related to the practicalities of catheter placement and energy delivery. Vascular injuries at the access site, cardiac injuries, thromboembolism and pulmonary vein stenosis were all common. The incidence of all of these injuries has declined steadily, progress that is attributable, in part, to technical refinements, such as the use of ultrasound to guide vascular puncture. It may also be related to accumulated experience among the community of physicians performing ablation. AEF has been stubbornly resistant to this progress, with an incidence remaining at approximately 0.01–0.3% over the past two decades.5,9 Evidence of milder thermal oesophageal injury (Figure 3) can be found in as many as 47% of cases.11 As other complications decline, and as procedure numbers increase, AEF becomes ever more important.
Perioesophageal Neural Injury
The perioesophageal vagal plexus influences the function of the gastrointestinal tract, including the gallbladder; clinical issues faced by those who experience vagal plexus injury as a result of catheter ablation include gastroparesis, bowel hypomotility (Figure 4) and acalculous cholecystitis.12,13 In a study involving 535 patients, 13 (2.4%) patients
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Clinical Electrophysiology and Ablation
Figure 1: Posterior Relationships of the Heart
Superior vena cava
Pulmonar y veins
Right lung
Vagal plexus
Oesophagus
Inferior vena cava
Ascending aor ta
Pulmonar y trunk
Pulmonar y veins
Descending aor ta
Left lung
Diaphragm
From an anterior viewpoint, the thoracic cavity is seen after removal of the heart and part of the pericardium. Any of the structures depicted can be involved in the extracardiac extension of thermal lesions created during catheter ablation, as can the phrenic nerves and the bronchi, which are not shown. Most clinically significant extracardiac lesions occur in the phrenic nerves, the perioesophageal plexus or the oesophagus.
Figure 2: CT of the Left Atrium and Adjacent Structures
imaging evidence) were more likely to have a ‘middle-positioned’ oesophagus (located in line with the spinal vertebrae) and additional posteriorly directed lesions, suggesting that the factors that promote injury to the plexus parallel those responsible for injury to the oesophagus.15 It may be hoped, although should not be assumed, that the same protective strategies may be applied to both sets of factors.
Other Neural Injury
Autonomic responses, including profound changes in the sinus rate or the occurrence of atrioventricular block, are commonly seen during AF ablation, particularly when delivering therapy around the superior pulmonary veins. The sites where such responses are elicited correspond to the known location of autonomic ganglia, so these events are often termed ‘ganglionic responses’ and are believed to result from damage to the ganglia. This is unique among the extracardiac effects of ablation in being regarded as beneficial: the occurrence of ganglionic responses is correlated with a successful ablation, and no downside to the effect has been reported.16 Evidence suggests that this represents a direct benefit of ganglionic injury, rather than ganglionic responses being just an indicator of lesion transmurality.16
The right phrenic nerve lies close to the right superior pulmonary vein and is vulnerable to ablation at its ostium. This is particularly a feature of cryoballoon ablation, where some degree of transient phrenic nerve dysfunction occurs in as many as 25% of cases, but clinically important injury occurs in fewer than 1% of cases.17
The left phrenic nerve is vulnerable to injury during ablation in the left atrial appendage. Ablation in this area is not a routine part of AF ablation in most centres, so is not seen as a major issue. When it does occur, it can be permanently disabling.
Strategies for Avoidance of Injury
The process of thermal injury progressing to a serious complication could be interrupted by: avoiding the delivery of thermal insult to the myocardium; delivering energy only at sites well removed from vulnerable structures; creating smaller lesions; moving vulnerable structures away from the site of delivery; intervening to control the temperature in the vicinity of vulnerable structures; or interrupting the sequence of events that progresses from initial injury to serious consequences. Some of these strategies can be facilitated by precise localisation of the structure at risk. All these strategies have been explored and all are in routine use in some form.
Avoiding Lesions in Areas of Danger:
Anatomical Considerations
Three slices are represented, taken at 1-cm intervals, and showing the level of the upper part of the superior pulmonary veins (A, B), the level of the carina between the veins (C, D) and the inferior margin of the pulmonary veins (E, F). The left-hand panels (A, C, E) are not annotated; in the right-hand panels (B, D, F) the oesophagus is outlined in yellow, the SVC is outlined in blue, the Ao is outlined in red and lung tissue is outlined in green. Ao = aorta; L = left; LA = left atrium; LVOT = left ventricular outflow tract; R = right; RA = right atrium; RIFV = right inferior pulmonary vein; RSPV = right superior pulmonary vein; SVC = superior vena cava.
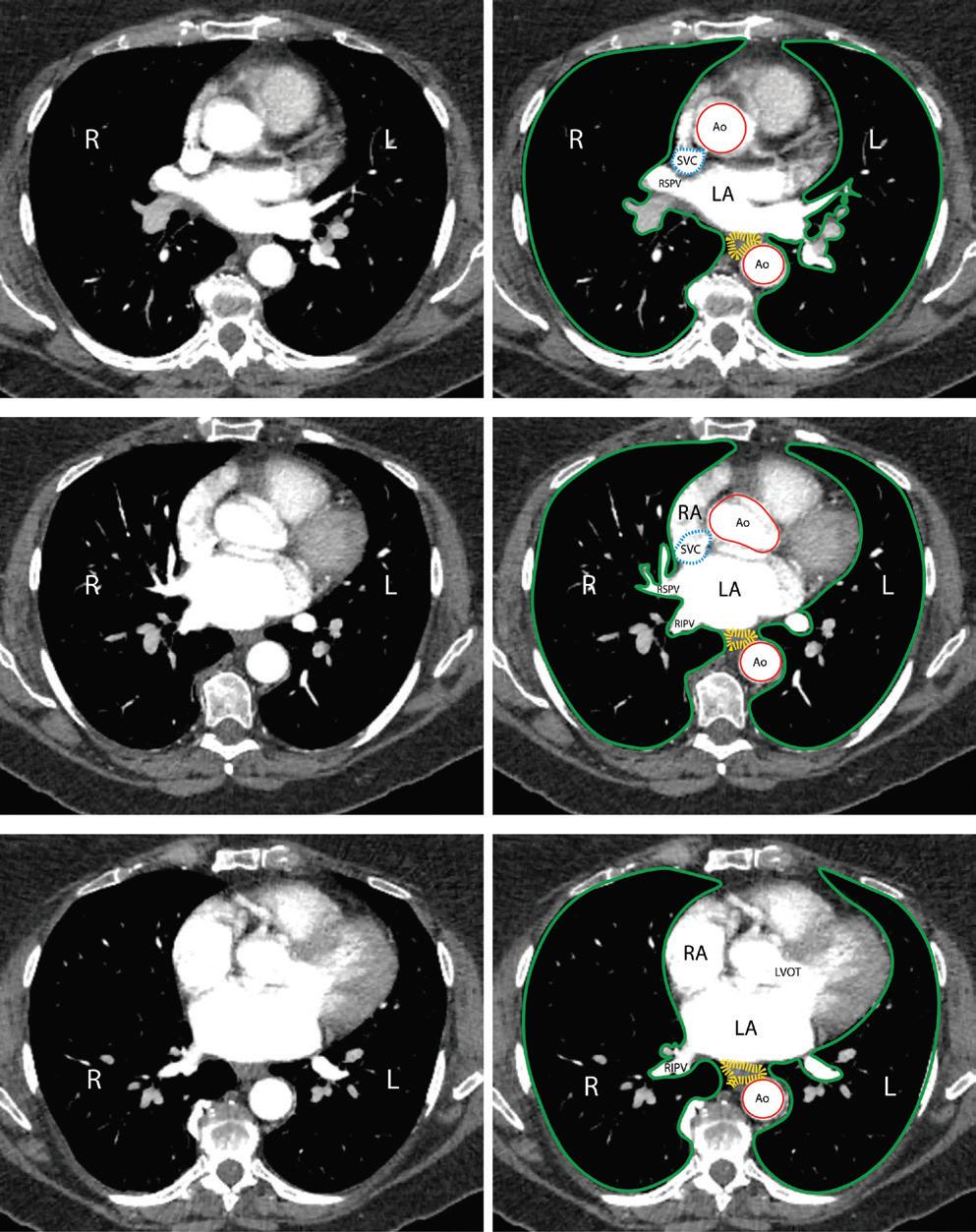
experienced severe gastric hypomotility, which, for the majority, gradually resolved over a period of 4 months.14
Yakabe et al. conducted a study of the utility of preprocedural CT to predict acute gastroparesis after AF ablation.15 They found that cases of confirmed gastroparesis (symptom profile, X-ray radiography or other
Ablation lesions can extend to approximately 7–9 mm from the tip of a catheter delivering radiofrequency (RF) energy, but slightly less for current cryotherapy catheters.18 The distance from the atrial chamber to the oesophageal lumen is shortest in the posterior wall; it is more than 15 mm at the anterior margin of the pulmonary veins.6,7 In principle, a lesion set composed of the deepest lesions possible could encircle the veins and the posterior wall without ever coming in reach of the oesophagus (Figure 5). In practice, this line is difficult to accomplish: it would involve a line across the roof of the left atrium more anterior than is usual, and a line across the floor that is much more inferior and anterior than usual. The more convenient alternatives that are in common use approach within 5–10 mm of an oesophagus lying in its typical position.7
The position of the oesophagus is neither predictable nor constant. In some cases, it is closer to the right pulmonary veins; in others, it is closer
Preventing Collateral Injury in AF
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Ablation
A B C D E F
3: Oesophageal Mucosal Lesions Detected on Endoscopy After Ablation
Disorders of the Upper Gastrointestinal Tract After Ablation
A patient who experienced severe eructation and dyspepsia after ablation underwent endoscopy 10 days after the procedure and was found to have gastric dilatation with retained gastric contents.
A: Abdominal radiography confirmed the presence of gastric bubble (arrow), with both fluid and gas (arrows) in the lower gastrointestinal tract (B, C). The dilatation resolved progressively (arrow), as shown on a repeat MRI bowel scan at 4 months (D); the bowel was no longer distended. By 1 year, the patient’s symptoms had resolved completely.
to the left. Even within the course of a single procedure, the oesophagus may move from one side to the other.19 Precise identification of the position of the oesophagus in real time is a logical part of any strategy of avoidance. Because procedures are typically guided by fluoroscopy, it makes sense to place an object or substance in the oesophageal lumen that makes it visible: contrast agents, nasogastric tubes and temperature probes are commonly used. A simple tubular design in the oesophageal marker weakens the strategy because the wall of the oesophagus most at risk of injury may still lie at a distance from that marker. None of these anatomical avoidance strategies has been evaluated in isolation. In real practice, because the risk of AEFs may occur despite a minimum lesion set created for pulmonary vein isolation, the strategy of the operators falls back on limitation of ablation power and time, for any posteriorly directed lesion.
The distance from the atrial cavity to any large airway is longer than the distance to the oesophagus. The left and right main bronchi are visible to the operator on the fluoroscopic imaging used for most ablation procedures. Because of the rarity of serious injury to the bronchi, operators do not usually take specific measures to avoid them.
Establishing the position of the phrenic nerve and minimising ablation in its vicinity is vital when using RF energy in the superior vena cava, in the left atrial appendage or inside the right superior pulmonary vein (as opposed to its antrum). Intraprocedure imaging cannot detect the nerve,
Lesion Sets
The lesion set represented in red is commonly used in treating persistent AF; separate loops are used to encircle the pulmonary veins in pairs, then lines are created across the roof of the atrium and across the lower part of the posterior wall to isolate the zone between the veins. This traditional lesion set involves redundancy because it creates three separate but contiguous areas that are isolated from each other and isolated from the remainder of the atrial myocardium. An alternative lesion set, shown in green, would create a single zone of isolation, eliminating the lesions on the posterior wall between the veins and using a much lower line across the inferior wall of the left atrium to close the inferior margin. Although it would involve fewer lesions, this inferior line is technically difficult.
so it is localised by stimulating electrically at high amplitude and looking for phrenic contraction. In cryoballoon ablation, phrenic nerve injury is avoided by eschewing deep engagement of the right pulmonary veins,
Preventing Collateral Injury in AF Ablation ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure
Figure 4: Motility
A B C A B C D Superior Right superior vein Right inferior vein Left superior vein Left inferior vein Inferior Coronary sinus
Figure 5: Left Atrial
Severe lesions (A, B; circled) are rare, but mild lesions (C; circled) involving erythema or superficial ulceration are commonly found on endoscopy performed in the early days after ablation for AF.
and the severity of injury is minimised by stimulating the nerve proximally throughout the procedure and terminating therapy promptly at the earliest sign of weakening. A similar approach can be used in treating the superior vena cava.
Minimising Lesion Depth Near Sensitive Structures
Lesion depth in RF ablation is a function of the power and duration of delivery and the force of catheter contact with the myocardium. Lesion size and shape are also influenced by features of catheter design, such as the mode of irrigation and the size of the tip electrode, as well as by the orientation of the catheter with respect to the endocardial surface. Confounding factors, such as local blood flow, make it difficult to predict the exact size of any lesion produced by any one delivery of energy, and the accumulation of heat in the tissue further complicates matters when successive lesions are delivered close together and in quick succession.
There is clear evidence that delivering more energy increases the risk of serious oesophageal injury; protocols that are designed to produce shallower lesions have been shown to protect against endoscopically detected oesophageal thermal lesions, including limitation of contact force.20,21 The success of this strategy requires that the depth of the lesion is accurately predicted: to this end, algorithms that incorporate all important determinants are commonly used. Combined with a disciplined consistency in interlesion distance and timing, most variables contributing to lesion depth are controlled.
Moving the Target
Most of the structures that are at risk during ablation are relatively fixed in location, but the oesophagus is mobile with respect to the heart. This movement can be inconvenient for an operator trying to avoid it, but the phenomenon can also be harnessed. A transoesophageal echo probe can be used to move the oesophagus to the left when ablating near the right veins and to the right when ablating near the left veins.22 The range of movement achievable is such that even lesions in the middle of the left atrial posterior wall can be kept at more than 1 cm from the most lateral part of the oesophageal lumen. However, this method cannot be standardised for every patient, and moving the oesophagus often leaves a trailing edge, which gives the false impression of adequate displacement. There are also dedicated devices designed for the purpose of oesophageal deviation in left atrial ablations with varying results from experimental use.23–27 As yet there are no randomised trial data to validate this method of oesophageal protection.
Altering the Ablation Modality
Myocardial ablation is currently most often performed by heating the tissue using RF energy. Cryotherapy is also commonly used, and pulsed field energy is an emerging modality. Ablation using laser or ultrasound energy has been described, but neither is currently in routine use.28 Because most ablations historically have been done with RF energy, and because most reported cases of oesophageal injury are from RF cases, RF ablations serve as a reference against which other modalities can be judged.
RF energy is an ancient technique by the standards of interventional electrophysiology, but it can be applied in new ways. High-power, shortduration ablation was originally applied to deliveries of 40–50 W, typically for a period of 10–20 s; more recently, a power of 90 W for just 4 s has entered routine use.29–31
Pulsed field ablation shows promise as a method for ablating the myocardium without collateral damage to adjacent structures.32,33
Because the injury produced by pulsed field energy is proportional to cell size, the myocardium is disproportionately affected and the tissues of the oesophagus are relatively spared. This promised theoretical advantage awaits rigorous testing in clinical practice. Preliminary results are encouraging: in a series of 121 consecutive cases of ablation for AF performed with pulsed field energy, no instance of clinically significant oesophageal injury was reported.33
Cryotherapy has been linked to cases of life-threatening oesophageal injury. The prevalence of injuries appears to be less than that associated with RF energy, but no randomised comparison has been performed of sufficient size to determine the relative risk.34 Most cryoablation for atrial fibrillation has been done using a single brand of balloon-tipped catheter, but other products are now available. No systematic evaluation of the risk of oesophageal injury has been conducted with any of these products.
Oesophageal Luminal Temperature Monitoring
Clinical studies have shown that an increase in oesophageal luminal temperature is linked to oesophageal injury and so, in standard practice, oesophageal temperature probes are widely used to guide the procedure, particularly during the creation of ablation lesions on the posterior aspect of the left atrium.35–52 Various different models of temperature probe have been used in conjunction with different ablation protocols. This form of oesophageal protection is the most common in routine practice, apart from limitation of ablation power, time and contact force. Despite its longterm use in clinical practice, the method was not evaluated scientifically until recently. Trial evidence is discouraging: the OPERA study, a recent landmark randomised trial, showed no evidence of benefit.35 On balance, the evidence suggests that the use of a temperature probe has the opposite effect to that intended, increasing the risk of oesophageal injury.48,49 This effect could relate to the physical presence of a foreign object in the oesophagus, or just reflect a less cautious approach when operators perceive that they are protected by a device.
Ex vivo studies also highlight the limitations of temperature monitoring; each model of temperature probe has its own physical profile and response time, which differ widely.53 The extent of protection, if any, is not standardised between probes and opens up the question as to the usefulness of this strategy at all when other studies show that use of a probe did not offer additional protection compared with controls.35,36
Controlling the Luminal Temperature
The lumen of the oesophagus can be cooled or warmed by fluid infused into the lumen or by a device placed in the lumen. Direct injection of water has been used in three controlled trials, each individually inconclusive but significant in concert.54 Building on this success, a cooling device that offers thermostat-controlled adjustment of the luminal temperature in the range 4–42°C (EnsoETM; Attune Medical) has been found to offer significant protection in a randomised trial of 120 patients evaluated by endoscopy.55
The EnsoETM device offers features that cannot be achieved by the infusion of saline: first, the protection is controlled in the sense that the temperature is set at a level chosen by the operator and maintained constant by a thermostat; and, second, the device is highly effective because the flow rate through the tube is 2.4 l/min, sufficient to convey a far larger amount of thermal energy into or away from the oesophageal lumen than can be achieved by any other available method. Direct infusion of a cooling fluid via an open-ended catheter is limited by the ability of the body to absorb that fluid. In a 90-min procedure, the EnsoETM
Preventing Collateral Injury in AF Ablation ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Table 1: Methods and Evidence in Preventing Serious Oesophageal Injury
Method Advantages Disadvantages
Minimise lesion depth Familiar; may reduce other complications
Avoid general area of oesophagus Familiar
Identify and avoid oesophagus Familiar
Move oesophagus away from danger None identified
Titrate energy to temperature Familiar; may reduce other complications
Control the temperature Intuitive; proven effectiveness and efficiency
Avoid thermal methods
Not yet established
Suppress gastric acid production Easy
Possibility of reducing procedure efficacy and efficiency
Possibility of reducing procedure efficacy and efficiency
Possibility of reducing procedure efficacy and efficiency
Demonstrated risk of physical injury to oesophagus
Possibility of reducing procedure efficacy and efficiency
Expense
Expense
Expense; drug adverse effects and interactions
circulates >200 l water, more than an order of magnitude greater than can be achieved without recirculation.
Control of the oesophageal temperature has been applied to cryoablation as well as to RF methods, and is undergoing clinical evaluation in an ongoing multicentre randomised trial (NCT04079634). The warming balloon marketed in parallel with the Adagio Ultralow cryoablation system is irrigated, but the extent and physical dimensions have not yet been studied in detail. The whole equipment is therefore quite new compared with other ablation modalities and unknown with regards to comparative safety, efficacy and efficiency parameters.
Secondary Prevention of Collateral Injury
Most injuries to extracardiac structures do not require specific treatment. Phrenic nerve palsy either resolves spontaneously or remains permanently. Injuries to the perioesophageal plexus resulting in gastroparetic symptoms may be confirmed by imaging, but the treatment is conservative.
Again, the oesophagus is different. Injuries progress from local tissue injury through necrosis to fistula formation, a process that may be due to luminal bacteria and acid. The slow progression of this process presents an opportunity for early intervention, but only for the vigilant. The assessment of those who may have sustained oesophageal injury requires knowledge of the condition by the initial medical responder. Often the clinical presentation is with fevers, and blood cultures and blood tests may reveal bacteraemia.56,57 Symptoms may include chest pain, and clinical examination may reveal acute neurological deficits. Any of this array of symptoms after ablation should alert the clinician. Early surgery is usually required.58–60 The placement of oesophageal stents, which involves a less invasive procedure, has a role, at least when fistulation is localised to the pericardium.
Summary of Preventative Strategies
Among the preventative strategies described, most are unsupported by randomised trial data (Table 1). Although it is widely accepted that reductions in contact force and power can reduce significant thermal
Level of Evidence
Support from retrospective analysis of real-world data
Equivocal evidence from retrospective analysis of real-world data
Equivocal evidence from retrospective analysis of real-world data
Balance of evidence suggests that available devices increase risk
Balance of evidence suggests that this strategy, in combination with current devices, increases risk
Support from large randomised trial and meta-analysis of smaller randomised trials
Support from retrospective analysis of real-world data
None
injury in RF ablation, this and oesophageal temperature monitoring are not backed up by trial data. Active oesophageal temperature control has shown clear benefit in randomised trials, and oesophageal deviation devices are undergoing evaluation.54,55 Oesophageal protection in cryotherapy has received less attention, but is important, especially now that ultra-low cryotherapy has emerged.
No single strategy has emerged to prevent all extracardiac injury. In practice, many strategies are used in every case, and it is appropriate that a mix of strategies should continue to be used. In a matter of patient safety, every plausible protection should be employed.
Conclusion
The extension of ablation-related injury into extracardiac structures is an important cause of complications of AF ablation. Structures at risk include the phrenic nerves and the bronchi, which are routinely protected by minimising lesion creation in their vicinity. Protection of the oesophagus is the highest priority due to the lethality of oesophageal injury. The oesophagus and the nerve plexus surrounding it have traditionally been protected by monitoring of the intraluminal temperature, but the evidence does not support this approach. Oesophageal cooling does provide effective protection during RF ablation. Pulsed field ablation as an alternative to thermal methods offers protection against any extracardiac injury in theory due to its specificity for the myocardium, but real-world data are needed.
Clinical Perspective
• Effective ablation requires transmural lesions, meaning that some injury must extend beyond the heart.
• Injury to the phrenic nerves and the perioesophageal neural plexus is an important complication of ablation for AF.
• Injury to the oesophagus is common but usually mild.
• Severe oesophageal injury is an important cause of ablationrelated death.
2022;24:533–7. https://doi.org/10.1093/europace/euab259; PMID: 34850953.
propensity-matched analysis. Pacing Clin Electrophysiol 2020;43:68–77. https://doi.org/10.1111/pace.13852; PMID: 31808165.
Preventing Collateral Injury in AF Ablation ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
1. Leung LWM, Akhtar Z, Seshasai SRK, Gallagher MM. First-line management of paroxysmal atrial fibrillation: is it time for a ‘pill in the bin’ approach? A discussion on the STOP AF First, EARLY AF, Cryo-FIRST, and EAST-AF NET 4 clinical trials. Europace
2. Elbatran AI, Gallagher MM, Li A, et al. Isolating the entire pulmonary venous component versus isolating the pulmonary veins for persistent atrial fibrillation: a
3. Loring Z, Holmes DN, Matsouaka RA, et al. Procedural patterns and safety of atrial fibrillation ablation: findings
Preventing Collateral Injury in AF Ablation
from Get With The Guidelines–Atrial Fibrillation. Circ Arrhythm Electrophysiol 2020;13:e007944. https://doi.org/10.1161/ CIRCEP.119.007944; PMID: 32703018.
4. Verma N, Gillespie CT, Argento AC, et al. Bronchial effects of cryoballoon ablation for atrial fibrillation. Heart Rhythm 2017;14:12–6. https://doi.org/10.1016/j.hrthm.2016.10.012; PMID: 28007093.
5. Kapur S, Barbhaiya C, Deneke T, Michaud GF. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation 2017;136:1247–55. https://doi. org/10.1161/CIRCULATIONAHA.117.025827; PMID: 28947480.
6. Lemola K, Sneider M, Desjardins B, et al. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation 2004;110:3655–60. https://doi.org/10.1161/01. CIR.0000149714.31471.FD; PMID: 15569839.
7. Sánchez-Quintana D, Cabrera JA, Climent V, et al. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation 2005;112:1400–5. https://doi.org/10.1161/ CIRCULATIONAHA.105.551291; PMID: 16129790.
8. Gillinov AM, Pettersson G, Rice TW. Esophageal injury during radiofrequency ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2001;122:1239–40. https://doi.org/10.1067/ mtc.2001.118041; PMID: 11726904.
9. Velghe D, Apers T, Devriendt S, et al. Atriobronchial fistula complicated by septic cerebral air emboli after pulmonary vein ablation. Crit Care Med 2017;45:e867–71. https://doi. org/10.1097/CCM.0000000000002438; PMID: 28441232.
10. Wu MH, Wongcharoen W, Tsao HM, et al. Close relationship between the bronchi and pulmonary veins: implications for the prevention of atriobronchial fistula after atrial fibrillation ablation. J Cardiovasc Electrophysiol 2007;18:1056–9. https://doi.org/10.1111/j.1540-8167.2007.00915.x; PMID: 17666059.
11. Schmidt M, Nolker G, Marschang H, et al. Incidence of oesophageal wall injury post pulmonary vein antrum isolation for treatment of patients with atrial fibrillation. Europace 2008;10:205–9. https://doi.org/10.1093/europace/ eun001; PMID: 18256125.
12. Tusboi I, Hayashi M, Miyauchi Y, et al. Anatomical factors associated with perioesophageal vagus nerve injury after catheter ablation of atrial fibrillation. J Nippon Med Sch 2014;81:248–57. https://doi.org/10.1272/jnms.81.248; PMID: 25186578.
13. Miyazaki S, Taniguchi H, Kusa S, et al. Factors associated with periesophageal vagal nerve injury after pulmonary vein antrum isolation. J Am Heart Assoc 2014;3:e001209. https:// doi.org/10.1161/JAHA.114.001209; PMID: 25249299.
14. Shah D, Dumonceau JM, Burri H, et al. Acute pyloric spasm and gastric hypomotility: an extracardiac adverse effect of percutaneous radiofrequency ablation for atrial fibrillation. J Am Coll Cardiol 2005;46:327–30. https://doi.org/10.1016/j. jacc.2005.04.030; PMID: 16022963.
15. Yakabe D, Fukuyama Y, Araki M, Nakamura T. Anatomical evaluation of the esophagus using computed tomography to predict acute gastroparesis following atrial fibrillation ablation. J Arrhythm 2021;37:1330–6. https://doi.org/10.1002/ joa3.12625; PMID: 34621432.
16. Lemery R, Birnie D, Tang ASL, et al. Feasibility study of endocardial mapping of ganglionated plexuses during catheter ablation of atrial fibrillation. Heart Rhythm 2006;3:387–96. https://doi.org/10.1016/j.hrthm.2006.01.009; PMID: 16567283.
17. Gang Y, Gonna H, Domenichini G, et al. Evaluation of the Achieve mapping catheter in cryoablation for atrial fibrillation: a prospective randomized trial. J Interv Card Electrophysiol 2016;45:179–87. https://doi.org/10.1007/s10840015-0092-3; PMID: 26698158.
18. Nakagawa H, Ikeda A, Sharma T, et al. Comparison of in vivo tissue temperature profile and lesion geometry for radiofrequency ablation with high power–short duration and moderate power–moderate duration: effects of thermal latency and contact force on lesion formation. Circ Arrhythm Electrophysiol 2021;14:e009899. https://doi.org/10.1161/ CIRCEP.121.009899; PMID: 34138641.
19. Good E, Oral H, Lemola K, et al. Movement of the esophagus during left atrial catheter ablation for atrial fibrillation. J Am Coll Cardiol 2005;46:2107–10. https://doi. org/10.1016/j.jacc.2005.08.042; PMID: 16325049.
20. Bahnson TD. Strategies to minimize the risk of esophageal injury during catheter ablation for atrial fibrillation. Pacing Clin Electrophysiol 2009;32:248–60. https://doi. org/10.1111/j.1540-8159.2008.02210.x; PMID: 19170916.
21. Zhang X, Kuang X, Gao X, et al. RESCUE-AF in patients undergoing atrial fibrillation ablation: the RESCUE-AF trial. Circ Arrhythm Electrophysiol 2019;12:e007044. https://doi. org/10.1161/CIRCEP.118.007044; PMID: 32125792.
22. Herweg B, Johnson N, Postler G, et al. Mechanical esophageal deflection during ablation of atrial fibrillation.
Pacing Clin Electrophysiol 2006;29:957–61. https://doi. org/10.1111/j.1540-8159.2006.00470.x; PMID: 16981919.
23. Koruth JS, Reddy VY, Miller MA, et al. Mechanical esophageal displacement during catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2012;23:147–54. https://doi.org/10.1111/j.1540-8167.2011.02162.x; PMID: 21914018.
24. Palaniswamy C, Koruth JS, Mittnacht AJ, et al. The extent of mechanical esophageal deviation to avoid esophageal heating during catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2017;3:1146–54. https://doi.org/10.1016/j. jacep.2017.03.017; PMID: 29759498.
25. Bhardwaj R, Naniwadekar A, Whang W, et al. Esophageal deviation during atrial fibrillation ablation: clinical experience with a dedicated esophageal balloon retractor. JACC Clin Electrophysiol 2018;4:1020–30. https://doi. org/10.1016/j.jacep.2018.04.001; PMID: 30139483.
26. Parikh V, Swarup V, Hantla J, et al. Feasibility, safety, and efficacy of a novel preshaped nitinol esophageal deviator to successfully deflect the esophagus and ablate left atrium without esophageal temperature rise during atrial fibrillation ablation: the DEFLECT GUT study. Heart Rhythm 2018;15:1321–7. https://doi.org/10.1016/j.hrthm.2018.04.017; PMID: 29678784.
27. Aguinaga L, Palazzo A, Bravo A, et al. Esophageal deviation with vacuum suction and mechanical deflection during ablation of atrial fibrillation: first in man evaluation. J Cardiovasc Electrophysiol 2021;32:67–70. https://doi. org/10.1111/jce.14801; PMID: 33155334.
28. Reyes G, Ruyra X, Valderrama F, et al. High intensity focused ultrasound ablation for atrial fibrillation: results from the National Spanish Registry. Minerva Cardioangiol 2016;64:501–6. PMID: 26006216.
29. Ravi V, Poudyal A, Abid QU, et al. High-power short duration vs. conventional radiofrequency ablation of atrial fibrillation: a systematic review and meta-analysis. Europace 2021;23:710–21. https://doi.org/10.1093/europace/euaa327; PMID: 33523184.
30. Shin DG, Lim HE. Esophageal endoscopy after high-power and short-duration ablation in atrial fibrillation patients. Korean Circ J 2021;51:154–6. https://doi.org/10.4070/ kcj.2020.0488; PMID: 33525070.
31. Kaneshiro T, Kamioka M, Hijioka N, et al. Characteristics of esophageal injury in ablation of atrial fibrillation using a high-power short-duration setting. Circ Arrhythm Electrophysiol 2020;13:e008602. https://doi.org/10.1161/ CIRCEP.120.008602; PMID: 32915644.
32. Wittkampf FHM, van Es R, Neven K. Electroporation and its relevance for cardiac catheter ablation. JACC Clin Electrophysiol 2018;4:977–86. https://doi.org/10.1016/j. jacep.2018.06.005; PMID: 30139498.
33. Reddy VY, Dukkipati SR, Neuzil P, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol 2021;7:614–27. https://doi.org/10.1016/j.jacep.2021.02.014; PMID: 33933412.
34. Piccini JP, Braegelmann KM, Simma S, et al. Risk of atrioesophageal fistula with cryoballoon ablation of atrial fibrillation. Heart Rhythm 2020;1:173–9. https://doi. org/10.1016/j.hroo.2020.05.007; PMID: 34113871.
35. Schoene K, Arya A, Grashoff F, et al. Oesophageal Probe Evaluation in Radiofrequency Ablation of atrial fibrillation (OPERA): results from a prospective randomized trial. Europace 2020;22:1487–94. https://doi.org/10.1093/ europace/euaa209; PMID: 32820324.
36. Meininghaus DG, Blembel K, Waniek C, et al. Temperature monitoring and temperature-driven irrigated radiofrequency energy titration do not prevent thermally-induced esophageal lesions in pulmonary vein isolation: a randomized study controlled by esophagoscopy before and after catheter ablation. Heart Rhythm 2021;18:928-34. https:// doi.org/10.1016/j.hrthm.2021.02.003; PMID: 33561587.
37. Di Biase L, Saenz LC, Burkhardt DJ, et al. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol 2009;2:108–12. https://doi.org/10.1161/CIRCEP.108.815266; PMID: 19808454.
38. Ahmed H, Neuzil P, d’Avila A, et al. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm 2009;6:962–9. https://doi.org/10.1016/j. hrthm.2009.03.051; PMID: 19560085.
39. Di Biase L, Dodig M, Saliba W, et al. Capsule endoscopy in examination of esophagus for lesions after radiofrequency catheter ablation: a potential tool to select patients with increased risk of complications. J Cardiovasc Electrophysiol 2010;21:839–44. https://doi. org/10.1111/j.1540-8167.2010.01732.x; PMID: 20163496.
40. Sause A, Tutdibi O, Pomsel K, et al. Limiting esophageal
temperature in radiofrequency ablation of left atrial tachyarrhythmias results in low incidence of thermal esophageal lesions. BMC Cardiovasc Disord 2010;10:52. https://doi.org/10.1186/1471-2261-10-52; PMID: 20977747.
41. Halm U, Gaspar T, Zachäus M, et al. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol 2010;105:551–6. https://doi. org/10.1038/ajg.2009.625; PMID: 19888201.
42. Leite LR, Santos SN, Maia H, et al. Luminal esophageal temperature monitoring with a deflectable esophageal temperature probe and intracardiac echocardiography may reduce esophageal injury during atrial fibrillation ablation procedures: results of a pilot study. Circ Arrhythm Electrophysiol 2011;4:149–56. https://doi.org/10.1161/ CIRCEP.110.960328; PMID: 21325208.
43. Contreras-Valdes FM, Heist EK, Danik SB, et al. Severity of esophageal injury predicts time to healing after radiofrequency catheter ablation for atrial fibrillation. Heart Rhythm 2011;8:1862–8. https://doi.org/10.1016/j. hrthm.2011.07.022; PMID: 21782773.
44. Fürnkranz A, Bordignon S, Schmidt B, et al. Luminal esophageal temperature predicts esophageal lesions after second-generation cryoballoon pulmonary vein isolation. Heart Rhythm 2013;10:789–93. https://doi.org/10.1016/j. hrthm.2013.02.021; PMID: 23428962.
45. Knopp H, Halm U, Lamberts R, et al. Incidental and ablationmediated findings during upper gastrointestinal endoscopy in patients after ablation of atrial fibrillation: a retrospective study of 425 patients. Heart Rhythm 2014;11:574–8. https:// doi.org/10.1016/j.hrthm.2014.01.010; PMID: 24418167.
46. Fürnkranz A, Bordignon S, Bohmig M, et al. Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm 2015;12:268–74. https://doi. org/10.1016/j.hrthm.2014.10.033; PMID: 25446159.
47. Metzner A, Burchard A, Wohlmuth P, et al. Increased incidence of esophageal thermal lesions using the secondgeneration 28-mm cryoballoon. Circ Arrhythm Electrophysiol 2013;6:769–75. https://doi.org/10.1161/CIRCEP.113.000228; PMID: 23748208.
48. Muller P, Dietrich JW, Halbfass P, et al. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm 2015;12:1464–9. https://doi.org/10.1016/j.hrthm.2015.04.005; PMID: 25847474.
49. Halbfass P, Muller P, Nentwich K, et al. Incidence of asymptomatic oesophageal lesions after atrial fibrillation ablation using an oesophageal temperature probe with insulated thermocouples: a comparative controlled study. Europace 2017;19:385–91. https://doi.org/10.1093/europace/ euw070; PMID: 27540039.
50. Deneke T, Nentwich K, Berkovitz A, et al. High-resolution infrared thermal imaging of the esophagus during atrial fibrillation ablation as a predictor of endoscopically detected thermal lesions: results from the HEAT-AF study. Circ Arrhythm Electrophysiol 2018;11:e006681. https://doi. org/10.1161/CIRCEP.118.006681; PMID: 30376732.
51. Daly MG, Melton I, Roper G, et al. High resolution infrared thermography of esophageal temperature during radiofrequency ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2018;11:e005667. https://doi.org/10.1161/ CIRCEP.117.005667; PMID: 29449354.
52. Chen S, Chun KRJ, Tohoku S, et al. Esophageal endoscopy after catheter ablation of atrial fibrillation using ablationindex guided high-power: Frankfurt AI-HP ESO I. JACC Clin Electrophysiol 2020;6:1253–61. https://doi.org/10.1016/j. jacep.2020.05.022; PMID: 33092751.
53. Turagam MK, Miller S, Sharma SP, et al. Differences in transient thermal response of commerical esophageal temperature probes. JACC Clin Electrophysiol 2019;5:1280–8. https://doi.org/10.1016/j.jacep.2019.07.013; PMID: 31753433.
54. Leung LW, Gallagher MM, Santangeli P, et al. Esophageal cooling for protection during left atrial ablation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2020;59:347–55. https://doi.org/10.1007/s10840-019-006615; PMID: 31758504.
55. Leung LWM, Bajpai A, Zuberi Z, et al. Randomized comparison of oesophageal protection with a temperature control device: results of the IMPACT study. Europace 2021;23:205–15. https://doi.org/10.1093/europace/euaa276; PMID: 33205201.
56. Pappone C, Vicedomini G, Santinelli V. Atrio-esophageal fistula after AF ablation: pathophysiology, prevention and treatment. J Atr Fibrillation 2013;6:860. https://doi. org/10.4022/jafib.860; PMID: 28496888.
57. Gandjbakhch E, Mandel F, Dagher Y, et al. Incidence, epidemiology, diagnosis and prognosis of atriooesophageal fistula following percutaneous catheter ablation: a French nationwide survey. Europace 2021;23:557–64. https://doi.org/10.1093/europace/euaa278;
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
PMID: 33221901.
Preventing Collateral Injury in AF Ablation
58. Mohanty S, Santangeli P, Mohanty P, et al. Outcomes of atrioesophageal fistula following catheter ablation of atrial fibrillation treated with surgical repair versus esophageal stenting. J Cardiovasc Electrophysiol 2014;25:579–84. https:// doi.org/10.1111/jce.12386; PMID: 25013875.
59. Back Sternick E, Soares Correa F, Ferber Drumond L, et al. Esophago-pericardial fistula after catheter ablation of atrial fibrillation: a review. J Cardiovasc Electrophysiol 2020;31:2600–6. https://doi.org/10.1111/jce.14723; PMID: 32829527.
60. Han HC, Ha FJ, Sanders P, et al. Atrioesophageal fistula.
Clinical presentation, procedural characteristics, diagnostic investigations, and treatment outcomes. Circ Arrhythm Electrophysiol 2017;10:e005579. https://doi.org/10.1161/ CIRCEP.117.005579; PMID: 29109075.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Arrhythmogenic Mitral Valve Prolapse
Theofanis George Korovesis , Paraskevi Koutrolou-Sotiropoulou and Demosthenes George Katritsis
Department of Cardiology, Hygeia Hospital, Athens, Greece
Abstract
Mitral valve prolapse (MVP) is a common condition present in 1–3% of the population. There has been evidence that a subset of MVP patients is at higher risk of sudden cardiac death. The arrhythmogenic mechanism is related to fibrotic changes in the papillary muscles caused by the prolapsing valve. ECG features include ST-segment depression, T wave inversion or biphasic T waves in inferior leads, and premature ventricular contractions arising from the papillary muscles and the fascicular system. Echocardiography can identify MVP and mitral annular disjunction, a feature that has significant negative prognostic value in MVP. Cardiac MRI is indicated for identifying fibrosis. Patients with high-risk features should be referred for further evaluation. Catheter ablation and mitral valve repair might reduce the risk of malignant arrhythmia. MVP patients with high-risk features and clinically documented ventricular arrhythmia may also be considered for an ICD.
Keywords
Arrhythmia, mitral valve prolapse, sudden cardiac death, mitral annular disjunction, premature ventricular contractions, papillary muscle fibrosis, echocardiography
Disclosure: DGK is editor-in-chief of Arrhythmia & Electrophysiology Review; this did not influence peer review. All other authors have no conflicts of interest to declare.
Received: 4 June 2021
Accepted: 2 February 2022
Citation: Arrhythmia & Electrophysiology Review 2022;11:e16. DOI: https://doi.org/10.15420/aer.2021.28
Correspondence: Theofanis George Korovesis, Department of Cardiology, Hygeia Hospital, Erythrou Stavrou 5 str, Marousi, 151 23, Greece. E: faniskorovesis@gmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Definitions
Mitral valve prolapse (MVP) has been conventionally defined as a superior displacement of the mitral leaflets of more than 2 mm above the annular plane in the long axis view into the left atrium during systole. This can affect one or both leaflets and can be due to disruption or elongation of the leaflets, chordae and papillary muscles. It is also characterised by fibromyxomatous degeneration of the leaflets with a maximal thickness of more than 5 mm during diastole (Barlow’s valve). However, MVP without mitral valve thickening (<5 mm) has also been recognised.1 A prodromal morphology of a familial form of MVP has also been described, characterised by an elongated posterior leaflet and an anteriorly displaced coaptation zone (towards the aorta) with no displacement of the leaflets in the left atrium.2 This morphology shares, either completely or partially, the haplotype of complete MVP, thereby suggesting that it might be either a precursor or the result of incomplete penetrance of the MVP phenotype.3
Epidemiology
MVP is a common valve condition in developed countries, affecting 1–3% of the population.1,4 MVP may lead to significant mitral regurgitation (MR), left ventricular dysfunction, infective endocarditis, thromboembolism and arrhythmias, such as AF and ventricular arrhythmias, even leading to sudden cardiac death (SCD).5 Patients without significant MR have been shown to enjoy excellent survival rates.6 However, there is a subset of patients with MVP that may incur a higher risk of ventricular tachycardia (VT) and mortality, regardless of the presence of MR.7 In this study however, patients with coronary artery disease and severe MR were not excluded, which might confound VT and mortality outcomes.8,9 The true incidence of SCD due to MVP varies depending on the methods used to evaluate the event (autopsy versus survivors) and the available clinical
data (ECG, echocardiogram, cardiac MRI [CMR]) and therefore may be underestimated. In autopsy-based studies there is a wide variability in the incidence of MVP-related SCD because of the uncertain causative relationship between the finding of MVP at autopsy and arrhythmic SCD.10 In a series of 339 autopsy-defined sudden arrhythmic deaths, MVP was identified in seven, while six more were identified by reviewing echocardiograms, indicating that MVP-related SCD may be underestimated even in post-mortem studies.11
Pathophysiological Mechanism
On the microscopic level, MVP is characterised by marked proliferation of the spongiosa, a glycosaminoglycan and proteoglycan-rich middle layer contained within a spongy elastin network. This causes interruption of the fibrosa, the underlying, collagen-rich lower layer located towards the ventricular side of the valve. Secondary effects include fibrotic changes on the mitral valve leaflets, thinning and elongation of the chordae tendinae, which is also caused by accumulation of glycosaminoglycans and ventricular friction lesions.12 There is some evidence the MVP itself may be the cause of the papillary and peripapillary fibrotic changes.13 The pathological systolic shortening and deformation may exert excessive traction on the papillary muscle, therefore inducing fibrotic change.14,15 These fibrotic changes, friction lesions and myocardial stretching may give rise to ventricular arrhythmias either through re-entry or triggered activity.16 Furthermore, there is evidence of increased sympathetic activity, coupled with decreased vagal activity and elevated catecholamine levels in MVP patients with a high ventricular arrhythmic load.17 This autonomic dysfunction not only increases the frequency of ectopic activity but also predisposes the ventricular myocardium to ectopic activity.
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com
Risk and
Arrhythmia
Stratification
To summarise, the pathogenesis of arrhythmia in MVP is best explained when we consider the myocardial hypertrophy and fibrosis as the substrate with the mechanical stretch being the trigger of the arrhythmia.18
Clinical and ECG Features of Mitral Valve Prolapse
MVP-related VT seems to occur more commonly in women – being as high as 70–90% in some studies.19 There is no clear reason for this discrepancy and is probably multifactorial. A majority of patients with MVP-related SCD demonstrate T wave inversions, ST segment depressions, or biphasic T waves on the inferior leads (II, III, aVF) on ECG.7,20 However, this finding alone is not specific enough to classify a patient’s risk by itself. Premature ventricular contractions (PVC) are another common finding in MVP patients. Although MR patients exhibit more PVCs compared with non-MR patients, even non-MR patients exhibit pleomorphic PVCs more commonly than the general population.7,21 These PVCs seem to arise from the papillary muscle, the fascicular system and the outflow tract, areas which are often found to be under
mechanical stress and undergoing fibrosis in MVP (Figure 1 and Supplementary Material Figure 1).22
In a retrospective study that involved 25 MVP patients who underwent catheter ablation of PVCs, 27 PVCs originating from the papillary muscles were identified and they all demonstrated a right bundle branch block pattern. Of these, 17 arose from the posteromedial papillary muscle and demonstrated a superior axis with negative forces in leads II and III, while 10 originated from the anterolateral papillary muscle and demonstrated an inferiorly directed axis (n=5) or inferior lead discordance (n=5), with negative QRS in lead II and positive QRS in lead III. Furthermore, four patients presented with PVC-associated VT and a fifth patient, who died during follow-up, presented with PVC-mediated cardiomyopathy. ST-T wave changes were present in 15 patients, affecting the inferior leads in five patients, the lateral leads in four and both in six patients. In a series of 14 patients with MVP and ventricular ectopy, at least one of the ectopic foci originated from the papillary muscle or the Purkynje system and ablation at these foci resulted in symptom relief and a reduction in defibrillator shocks in 12 of these patients.23 A cross-sectional study that examined 100 MVP patients and 100 healthy subjects detected early repolarisation phenomena (a notch in the descending arm of the QRS complex and ST elevation) in 74% of the patients (51% in inferior leads, 23% in I, aVL, and 8% in all three leads) while these phenomena were only observed in six subjects in the control group.24
Echocardiography
Echocardiography can easily recognise MVP in the parasternal long axis view and is defined as a superior displacement of the mitral leaflets of more than 2 mm into the left atrium during systole. Also leaflet length, redundancy and thickness are evaluated during diastole, and 3D echocardiography can also estimate the extent of the prolapsed segments (Figure 2).
The literature suggests that mitral annular disjunction (MAD) is a significant negative prognostic factor in MVP, characterised by a detachment of the ‘roots’ of the annulus from the ventricular myocardium. The posterior leaflet under the P1 and P2 segments of the valve is most commonly affected because the anterior leaflet is supported by the dense fibrous trigones of the cardiac skeleton. A frame-by-frame analysis of a 2D echocardiography in the parasternal long axis view usually confirms the diagnosis (Figure 3 and Supplementary Material Figures 2 and 3). MAD can also be recognised in the apical four-chamber view (Figure 4) and 3D echocardiography has shown that the disjunctive annulus exhibits paradoxical expansion and flattening during systole. MAD is not always present in patients who have MVP and it can be found in patients without MVP. In a Norwegian study which enrolled 116 patients with MAD, 34% of them presented with severe VT.25 This study supported that papillary muscle fibrosis may be linked to severe arrhythmic events and showed an association among PVCs, arrhythmic events and MAD independently of the presence of MVP, indicating that MAD itself may be an arrhythmogenic entity. However, more studies are needed to prove and better define this.
MR frequently develops in patients with MVP. It is quantified by echocardiography based on the recommendations for the non-invasive evaluation of MR more frequently uses the proximal isovelocity surface area and vena contracta method. One study showed that severe MR is an independent predictor of ventricular arrhythmias.26 However, in a recent systematic review of MVP and SCD most of the patients experienced SCD in the setting of non-severe MR. Even patients with mild or moderate MR are at risk of SCD.27
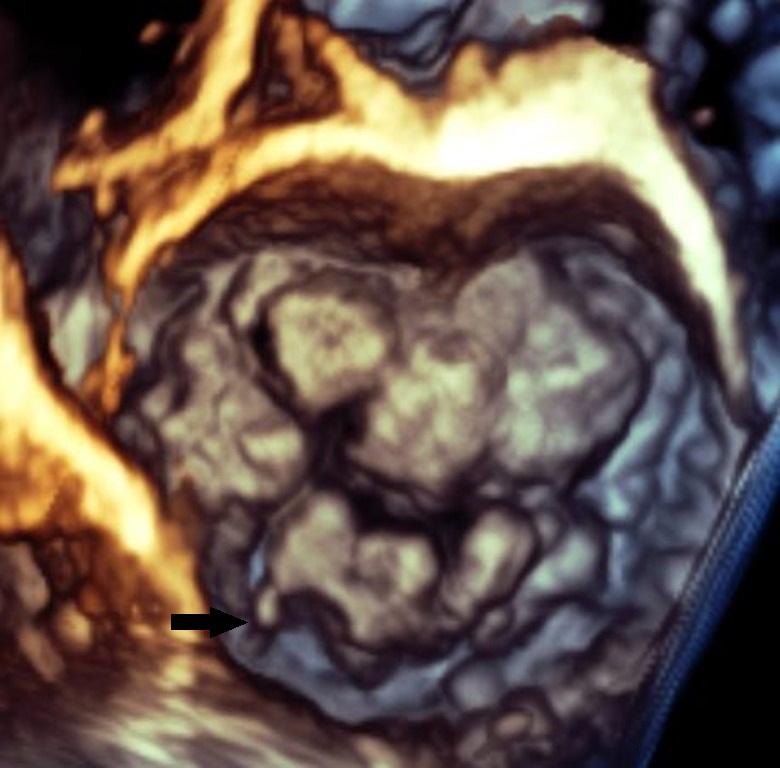
Arrhythmogenic Mitral Valve Prolapse ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 1: 12-Lead ECG with Premature Ventricular Contractions Arising Either from the Mitral Annulus or from the Posterior Papillary Muscle
I V1 V2 V3 V4 V5 V6 II III aVR aVL aVF V1
Figure 2: Bileaflet Mitral Valve Prolapse with Flail (Black Arrow) Posterior Lateral Scallop Imaged with 3D Echocardiography
Finally, in a study of 21 patients with bileaflet MVP and MR, patients at a high risk (67% versus 22%; p<0.08) to develop a ventricular arrhythmia were identified by increased peak systolic lateral velocity of the lateral mitral annulus >1.6 m/s.28 This so called Pickelhaube sign was not present in the low-risk group. However larger studies are required to confirm the validity of this sign.
Cardiac MRI
After identifying fibrotic changes at post-mortem, Basso et al. theorised that these changes can be identified in vivo by means of CMR. CMR is the ideal diagnostic test to identify specific arrhythmogenic fibrotic regions and also provide evidence of MAD (Figure 5).29 In a series of 43 cases of SCD in young patients with MVP papillary muscle fibrosis (88%) or inferobasal fibrosis (93%) was identified and late gadolinium enhancement (LGE) distribution on CMR correlated with histopathological findings.20 In another series of 62 cases, fibrosis of at least one papillary muscle or the adjacent left ventricular (LV) wall was identified.30 In a study comparing the prevalence of LV fibrosis in MR patients with and without MVP, higher prevalence was identified in the MVP group (36.7% versus 6.7%; p<0.001).31 The fibrotic pattern was most commonly found in the basal inferolateral (31.1%) and basal inferior (10.7%) wall. During follow-up, an arrhythmic event occurred in 4.5% (n=8) of MVP patients, with the highest event rate being recorded in MVP patients with replacement fibrosis. This study also suggests that MVP may promote fibrotic changes beyond those that follow the MR-associated volume overload.
A forensic study of 17 MVP-SCD patients and 17 matched controls, showed that LV fibrosis was significantly higher in the posterior and lateral walls compared with the anterior wall and interventricular septum with a significant endocardial-to-epicardial fibrotic gradient, while the right ventricular fibrotic changes were comparable between the two groups, thereby suggesting that both localised and generalised fibrosis contributes to the pathogenesis of SCD.32 However, the posteromedial and anterolateral papillary muscles, the inferobasal portion as well as leaflet thickness and annular disjunction were not evaluated, as these are areas of interest relating to localised fibrotic change. Also, inability to assess atrial enlargement does not exclude the suspicion of subclinical MR, especially in the context of increased cardiac mass.33
A prospective study of 400 MVP patients detected replacement myocardial fibrosis by LGE CMR in 110 patients, most commonly localised in the basal inferolateral wall and the papillary muscle. Fibrosis was detected in mild MR (13%), 28% in moderate and 37% in severe MR and was associated with a more dilated LV and more frequent ventricular arrhythmias (VAs) (45% versus 26%, p<0.0001).34 In the subgroup of patients with mild MR, abnormal LV dilatation in the absence of volume overload was detected in 16% of patients, furthering the evidence of MVP-related fibrosis, and VAs in 25%. Replacement fibrosis was associated with worse 4-year cardiovascular event-free survival.
Another retrospective study of 41 patients with MVP indicates that even the presence of diffuse subclinical ventricular fibrosis was associated with a higher ventricular arrhythmic load and might be a precursor of focal papillary fibrosis.35
Finally, severe curling, defined as an unusual systolic motion of the posterior mitral ring >3.5 mm, has been associated with myocardial fibrosis and has been identified with LGE in a study of 54 MVP patients.36 A 3.5 mm cut-off was the median in this study.
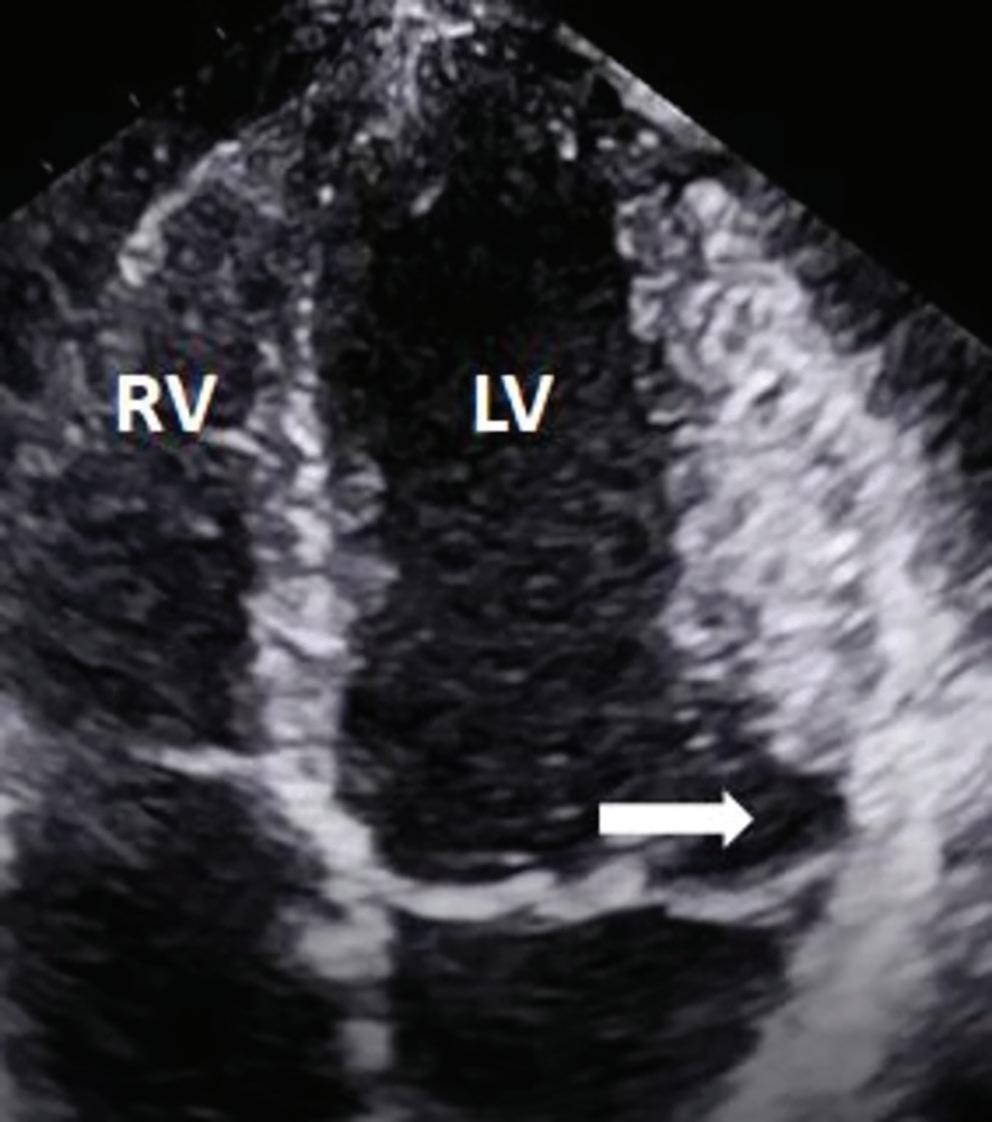
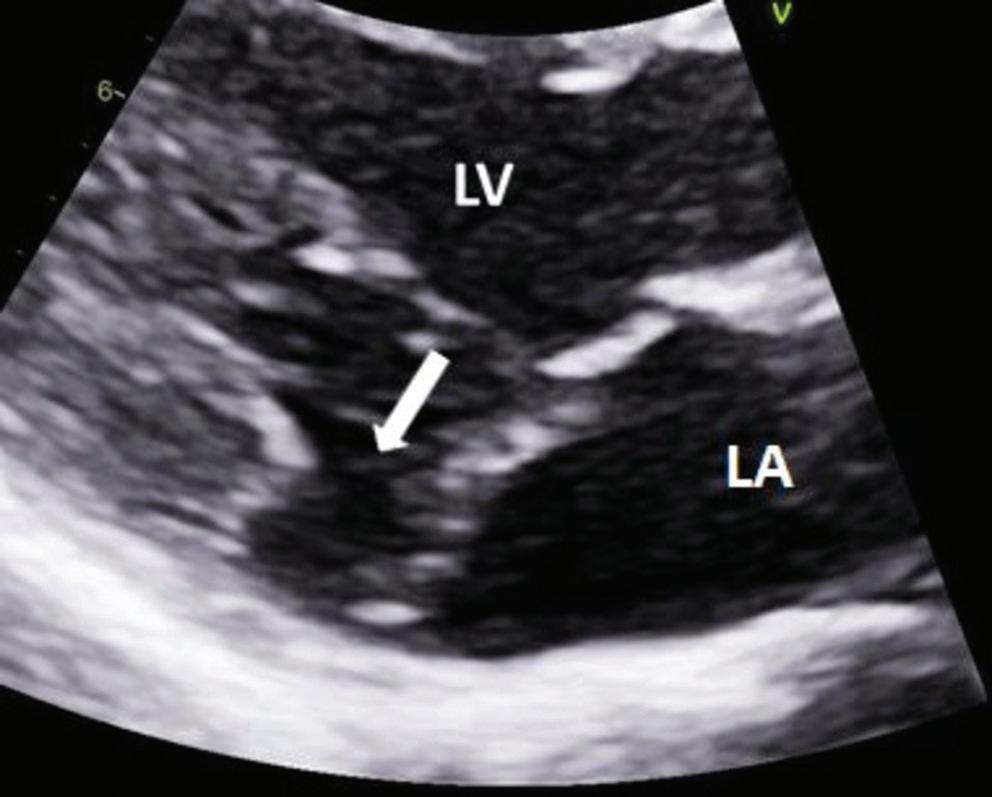
Risk Stratification
Risk stratification of MVP is not clear despite the plethora of risk factors associated with it because there is still no strong predictor of malignant arrhythmias. Additionally, it is not clear which combination of risk factors incurs the highest risk. Another challenge is that people with high risk exist among a much larger population who are traditionally perceived as
Arrhythmogenic Mitral Valve Prolapse ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 3: Mitral Annular Disjunction Characterised by the Detachment of the Roots of the Posterior Part of the Mitral Annulus Under P1 and P2 Segments
Figure 4: Presence of Mitral Annular Disjunction at the Four-chamber View on Transthoracic Echocardiography
The white arrow shows detachment of the ‘roots’ of the annulus from ventricular myocardium at the parasternal long axis view transthoracic echocardiography. LA = left atrium; LV = left ventricle.
The white arrow shows detachment of the ‘roots’ of the annulus from ventricular myocardium. LV = left ventricle; RV = right ventricle.
low-risk patients. However, identification of features, such as complex ventricular ectopy, echocardiographic features of leaflet redundancy or MAD and repolarisation abnormalities on ECG should lead to further evaluation with a 24-hours, 7-day rhythm Holter monitoring of the VA load and also CMR evaluation of the LV regardless of the LV ejection fraction, the degree of MR and the LV end systolic diameter. Bileaflet MVP and leaflet thickening have been considered as high-risk features of SCD but prognostic significance of these features is unclear.20,22,37 While it seems that there is a 42% prevalence of bileaflet MVP in young idiopathic out-ofhospital cardiac arrest survivors, bileaflet MVP without any additional risk factors did not significantly increase the risk of SCD or defibrillator implantation.22,37
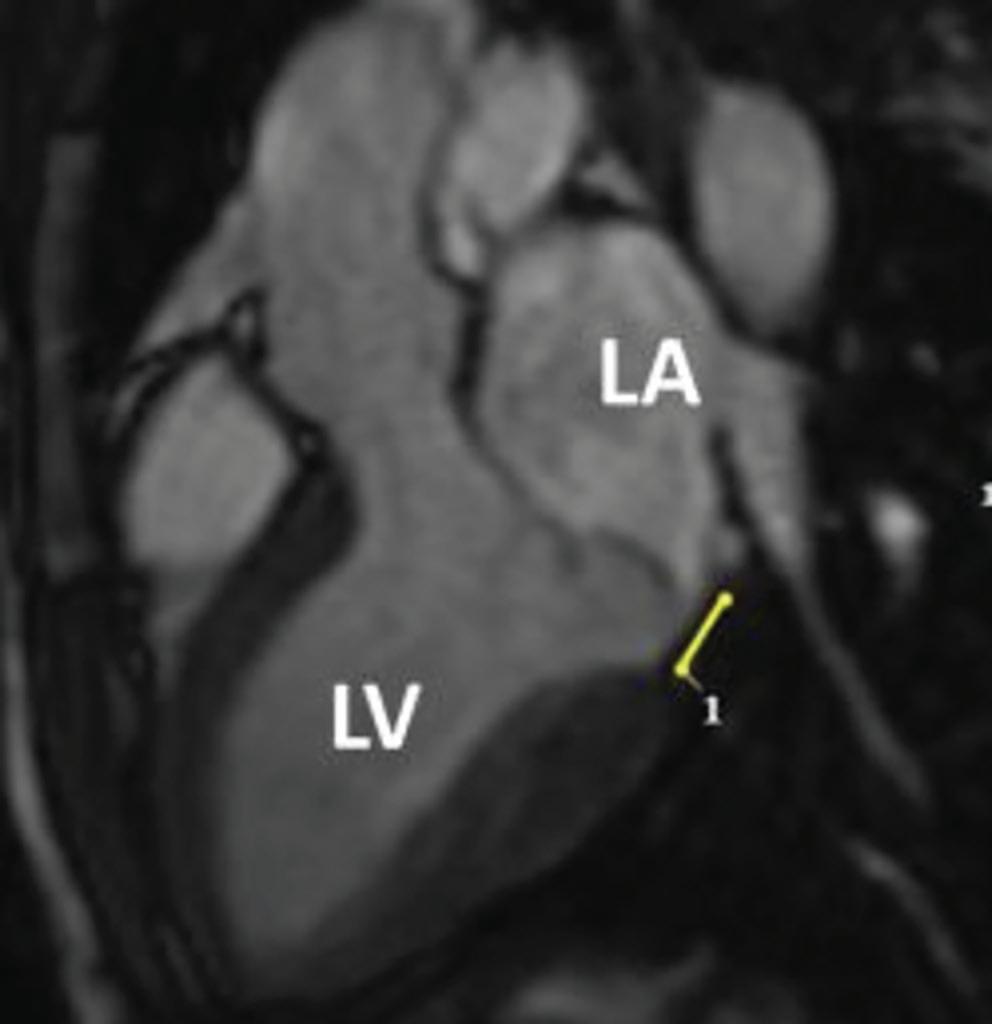
The literature suggests that SCD more commonly occurs in younger women with MVP, however this has not been verified in all studies.38,7 In patients with MVP and syncope it is imperative to determine the nature of the syncope. Arrhythmic syncope is suspected if it occurs either during strenuous exercise or during rest without any previous warning symptoms. For patients with MVP, suspected arrhythmic syncope and evidence of scarring, it is acceptable to consider an electrophysiology study. If it is unremarkable or not feasible, implantation of a loop recorder may be considered dependent on the amount of high-risk features present.39 However, it should be mentioned that programmed electrical ventricular stimulation as a means to stratify the VA risk is of limited significance as the induction of polymorphic VT is a non-specific finding.40 Patients with a truly positive study should undergo implantation of an ICD.
The American Heart Association and the European Society of Cardiology guidelines for VA and SCD prevention have no specific recommendation for MVP risk stratification.5,41 The management of low-risk MVP patients with polymorphic VA consists of lifestyle modifications such as avoiding stimulants such as caffeine, tobacco, alcohol and recreational drugs, and the agents of choice are β-blockers in both symptomatic and asymptomatic, non-sustained or sustained VA. An ICD would be indicated
for the secondary prevention of out-of-hospital cardiac arrest. There are no validated scores to help stratify risk and decisions should be shared with the patient after a frank, transparent and honest discussion. Role of family history of MVP, MAD and SCD can also help identify risk, but specificity is unknown. Family members of SCD patients who are identified as having MVP should undergo evaluation, but there is a lack of evidence to support this.
Catheter Ablation
Catheter ablation of scar-related malignant arrhythmias has been reported as an effective treatment.23,42 PVC triggers, which are often located on the papillary muscles, can be mapped and ablation can be performed successfully, which also leads to a reduction in the need for ICD therapies.23 Papillary muscle ablation can be technically demanding because of catheter instability, intramural origin and the need to ablate at the base of the papillary muscle.43 However, in a study of 15 MVP patients that underwent ablation of high-burden PVCs, ventricular bigeminy, nonsustained or sustained VT, five patients developed haemodynamically significant VT or VF and underwent ICD implantation over 9 years of follow-up. In three of these five patients there was also appropriate ICD therapy.44 This late recurrence can arise from arrhythmic foci not present at the time of ablation, highlighting the need for long-term follow-up of these patients.
Mitral Valve Repair and Exercise Recommendations
Mitral valve repair should reverse the deformed structure and relieve the papillary muscles, promoting positive remodeling and reducing the arrhythmic load. There is evidence that mitral valve surgery can be beneficial in younger patients (<42 years).45–49 However, data are limited and surgery may not effectively reduce the rate of malignant arrhythmias.50
Due to the benign clinical course of the condition in most people, asymptomatic patients with mild or moderate MR can participate in competitive and informal sports. Exercise restrictions should only be recommended in athletes with MVP that present the following features: prior arrhythmogenic syncope, sustained or repetititve non-sustained VT, or complex VAs recorded on Holter monitoring, T wave inversions on inferior leads, long QT, basal inferoseptal wall fibrosis, bileaflet mitral valve prolapse, severe MR, LV systolic dysfunction (LV ejection fraction <50%), prior embolic event, or family history of MVP-related SCD. Athletes presenting with any of these features should participate only in lowintensity competitive sports such as golf, alternatively low-intensity aerobic exercise should be encouraged on the basis of maintaining general well-being.51,52 The incidence of adverse cardiovascular events in patients without any high-risk features is 0.5%, according to one study.53
ICD
There are limited data supporting prophylactic ICD implantation in patients with MVP and high-risk features. In a study of 13 MVP patients who underwent ICD implantation as secondary prevention, nine had documented non-sustained VT, two had received anti-tachycardia pacing and two had received defibrillation.54 Therefore, MVP patients with highrisk features should be closely monitored for malignant arrhythmias and may be considered for ICD implantation.
Lack of Solid Evidence
Due to the nature of the disease, most of the data regarding the incidence, diagnosis and possible treatment options come from
Arrhythmogenic Mitral Valve Prolapse ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 5: Cardiac MRI with Evidence of Mitral Annular Disjunction (Yellow Line)
LA = left atrium; LV = left ventricle.
Arrhythmogenic Mitral Valve Prolapse
inadequately powered studies either due to confounding factors or low sample number.7–9,22–25,27,32,43–50 The scarcity of large-scale, randomised, double-blind studies on the subject should also be noted. Further studies will help clear doubts by adding solid data to the current knowledge on MVP.
Conclusion
There is an association between MVP and SCD. Identifying the most
Clinical Perspective
at-risk individuals is a demanding task which requires careful evaluation of patients with clinical and echocardiographic features. The presence and history of PVCs play a prominent role and further evaluation with CMR, 24-hour ambulatory monitoring or invasive procedures may be indicated in selected patients. Further large-scale studies are required to clearly identify the risk factors that could lead to an accurate identification of high-risk individuals, paving the way for appropriate and successful primary prevention intervention.
• Mitral valve prolapse, albeit a common condition, is associated with ventricular arrhythmias and sudden cardiac death.
• Electrocardiographic features include ST-segment depression, T wave inversion or biphasic T waves in the inferior leads, QT prolongation and premature ventricular complexes.
• Echocardiography can identify mitral valve prolapse as well as mitral annular disjunction, a high-risk feature for ventricular arrhythmias, while late gadolinium enhancement cardiac MRI can detect the fibrotic arrhythmogenic regions in the papillary muscles and ventricular myocardium.
• Patients with high-risk features should be referred for further evaluation, but risk stratification is challenging.
• An ICD and/or mitral valve repair should be considered in truly high-risk patients.
1. Freed LA, Benjamin EJ, Levy D, et al. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol 2002;40:1298–304. https://doi.org/10.1016/ s0735-1097(02)02161-7; PMID: 12383578.
2. Delling FN, Rong J, Larson MG, et al. Evolution of mitral valve prolapse: insights from the Framingham Heart Study. Circulation 2016;133:1688–95. https://doi.org/10.1161/ CIRCULATIONAHA.115.020621; PMID: 27006478.
3. Nesta F, Leyne M, Yosefy C, et al. New locus for autosomal dominant mitral valve prolapse on chromosome 13: clinical insights from genetic studies. Circulation 2005;112:2022–30. https://doi.org/10.1161/CIRCULATIONAHA.104.516930; PMID: 16172273.
4. Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005–11. https://doi.org/10.1016/S01406736(06)69208-8; PMID: 16980116.
5. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. Circulation 2021;143:e35–71. https://doi.org/10.1161/ CIR.0000000000000932; PMID: 33332149.
6. Avierinos JF, Gersh BJ, Melton LJ 3rd, et al. Natural history of asymptomatic mitral valve prolapse in the community. Circulation 2002;106:1355–61. https://doi.org/10.1161/01. cir.0000028933.34260.09; PMID: 12221052.
7. Essayagh B, Sabbag A, Antoine C, et al. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol 2020;76:637–49. https://doi.org/10.1016/j. jacc.2020.06.029; PMID: 32762897.
8. Alqarawi W, Cheung CC, Davies B, Krahn AD. Arrhythmic mitral valve prolapse: looking for a needle in a haystack. J Am Coll Cardiol 2020;76:2688–9. https://doi.org/10.1016/j. jacc.2020.08.085; PMID: 33243388.
9. Han HC, Teh AW, Hare DL, et al. The clinical demographics of arrhythmic mitral valve prolapse. J Am Coll Cardiol 2020;76:2689–90. https://doi.org/10.1016/j. jacc.2020.09.591; PMID: 33243389.
10. Oliva A, Brugada R, D’Aloja E, et al. State of the art in forensic investigation of sudden cardiac death. Am J Forensic Med Pathol 2011;32:1–16. https://doi.org/10.1097/ PAF.0b013e3181c2dc96; PMID: 20083991.
11. Delling FN, Aung S, Vittinghoff E, et al. Antemortem and post-mortem characteristics of lethal mitral valve prolapse among all countrywide sudden deaths. JACC Clin Electrophysiol 2021;7:1025–34. https://doi.org/10.1016/j. jacep.2021.01.007; PMID: 33640349.
12. Delling FN, Vasan RS. Epidemiology and pathophysiology of mitral valve prolapse: new insights into disease progression, genetics, and molecular basis. Circulation 2014;129:2158–70. https://doi.org/10.1161/CIRCULATIONAHA.113.006702; PMID: 24867995.
13. Huttin O, Pierre S, Venner C, et al. Interactions between mitral valve and left ventricle analysed by 2D speckle
tracking in patients with mitral valve prolapse: one more piece to the puzzle. Eur Heart J Cardiovasc Imaging 2017;18:323–31. https://doi.org/10.1093/ehjci/jew075; PMID: 27099279.
14. Basso C, Calabrese F, Corrado D, Thiene G. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc Res 2001;50:290–300. https://doi.org/10.1016/s00086363(01)00261-9; PMID: 11334833.
15. Basso C, Perazzolo Marra M. Mitral annulus disjunction: emerging role of myocardial mechanical stretch in arrhythmogenesis. J Am Coll Cardiol 2018;72:1610–2. https:// doi.org/10.1016/j.jacc.2018.07.069; PMID: 30261962.
16. Maruyama T, Fukata M. Increased coupling interval variability – mechanistic, diagnostic and prognostic implication of premature ventricular contractions and underlying heart diseases. Circ J 2015;79:2317–9. https://doi. org/10.1253/circj.CJ-15-0963; PMID: 26376670.
17. Sniezek-Maciejewska M, Dubiel JP, Piwowarska W, et al. Ventricular arrhythmias and the autonomic tone in patients with mitral valve prolapse. Clin Cardiol 1992;15:720–4. https://doi.org/10.1002/clc.4960151029; PMID: 1395181.
18. Basso C, Iliceto S, Thiene G, Perazzolo Marra M. Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation 2019;140:952–64. https://doi.org/10.1161/ CIRCULATIONAHA.118.034075; PMID: 31498700.
19. Zuppiroli A, Mori F, Favili S, et al. Arrhythmias in mitral valve prolapse: relation to anterior mitral leaflet thickening, clinical variables, and color Doppler echocardiographic parameters. Am Heart J 1994;128:919–27. https://doi. org/10.1016/0002-8703(94)90590-8; PMID: 7942485.
20. Basso C, Perazzolo Marra M, Rizzo S, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015;132:556–66. https://doi.org/10.1161/ CIRCULATIONAHA.115.016291; PMID: 26160859.
21. Kligfield P, Hochreiter C, Kramer H, et al. Complex arrhythmias in mitral regurgitation with and without mitral valve prolapse: contrast to arrhythmias in mitral valve prolapse without mitral regurgitation. Am J Cardiol 1985;55:1545–9. https://doi.org/10.1016/00029149(85)90970-1; PMID: 4003297.
22. Sriram CS, Syed FF, Eric Ferguson ME, et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol 2013;62:222–30. https://doi.org/10.1016/j. jacc.2013.02.060; PMID: 23563135.
23. Syed FF, Ackerman MJ, McLeod CJ, et al. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol 2016;9:e004005. https://doi.org/10.1161/CIRCEP.116.004005; PMID: 27103091.
24. Peighambari MM, Alizadehasl A, Totonchi Z. Electrocardiographic changes in mitral valve prolapse syndrome. J Cardiovasc Thorac Res 2014;6:21–3. https://doi. org/10.5681/jcvtr.2014.004; PMID: 24753827.
25. Dejgaard LA, Skjølsvik ET, Lie ØH, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol
2018;72:1600–9. https://doi.org/10.1016/j.jacc.2018.07.070; PMID: 30261961.
26. Turker Y, Ozaydin M, Acar G, et al. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. Int J Cardiovasc Imaging 2010;26:139–45. https://doi.org/10.1007/ s10554-009-9514-6; PMID: 19847667.
27. Han HC, Ha FJ, Teh AW, et al. Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc 2018;7:e010584. https://doi.org/10.1161/JAHA.118.010584; PMID: 30486705.
28. Muthukumar L, Rahman F, Jan MF, et al. The Pickelhaube sign: novel echocardiographic risk marker for malignant mitral valve prolapse syndrome. JACC Cardiovasc Imaging 2017;10:1078–80. https://doi.org/10.1016/j.jcmg.2016.09.016; PMID: 28017396.
29. Fulton BL, Liang JJ, Enriquez A, et al. Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol 2018;29:146–53. https:// doi.org/10.1111/jce.13374; PMID: 29059484.
30. Sheppard MN, Steriotis AK, Sharma S. Letter by Sheppard et al regarding article ‘Arrhythmic mitral valve prolapse and sudden cardiac death’. Circulation 2016;133:e458. https://doi. org/10.1161/CIRCULATIONAHA.115.018775; PMID: 27022045.
31. Kitkungvan D, Nabi F, Kim RJ, et al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol 2018;72:823–34. https://doi. org/10.1016/j.jacc.2018.06.048; PMID: 30115220.
32. Han HC, Parsons SA, Curl CL, et al. Systematic quantification of histologic ventricular fibrosis in isolated mitral valve prolapse and sudden cardiac death. Heart Rhythm 2021;18:570–6. https://doi.org/10.1016/j.hrthm.2020.12.021; PMID: 33359875.
33. Rizzo S, Perazzolo Marra M, De Gaspari M, Basso C. The missing pieces in the puzzle of arrhythmic mitral valve prolapse: papillary muscles, mitral annulus dysjunction, and myocardial scarring. Heart Rhythm 2021;18:577–8. https://doi. org/10.1016/j.hrthm.2021.01.004; PMID: 33429105.
34. Constant Dit Beaufils AL, Huttin O, Jobbe-Duval A, et al. Replacement myocardial fibrosis in patients with mitral valve prolapse: relation to mitral regurgitation, ventricular remodeling, and arrhythmia. Circulation 2021;143:1763–74. https://doi.org/10.1161/CIRCULATIONAHA.120.050214; PMID: 33706538.
35. Bui AH, Roujol S, Foppa M, et al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017;103:204–9. https://doi.org/10.1136/ heartjnl-2016-309303; PMID: 27515954.
36. Perazzolo Marra M, Basso C, De Lazzari M, et al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging 2016;9:e005030. https://doi.org/10.1161/ CIRCIMAGING.116.005030; PMID: 27516479.
37. Nordhues BD, Siontis KC, Scott CG, et al. Bileaflet mitral valve prolapse and risk of ventricular dysrhythmias and death. J Cardiovasc Electrophysiol 2016;27:463–8. https://doi. org/10.1111/jce.12914; PMID: 26749260.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
38. Enriquez-Sarano M. Mitral annular disjunction: the forgotten component of myxomatous mitral valve disease. JACC Cardiovasc Imaging 2017;10:1434–36. https://doi.org/10.1016/j. jcmg.2017.03.001; PMID: 28528160.
39. Brignole M, Moya A, de Lange FJ, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–948. https://doi.org/10.1093/eurheartj/ehy037; PMID: 29562304.
40. Morady F, Shen E, Bhandari A, et al. Programmed ventricular stimulation in mitral valve prolapse: analysis of 36 patients. Am J Cardiol 1984;53:135–8. https://doi. org/10.1016/0002-9149(84)90697-0; PMID: 6691249.
41. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–867. https://doi.org/10.1093/eurheartj/ehv316; PMID: 26320108.
42. Hong-Tao Y, Yang M, Zhong L, et al. Ventricular premature contraction associated with mitral valve prolapse. Int J Cardiol 2016;221:1144–9. https://doi.org/10.1016/j. ijcard.2016.06.252; PMID: 27522301.
43. Van Herendael H, Zado ES, Haqqani H, et al. Catheter ablation of ventricular fibrillation: importance of left
Arrhythmogenic Mitral Valve Prolapse
ventricular outflow tract and papillary muscle triggers. Heart Rhythm 2014;11:566–73. https://doi.org/10.1016/j. hrthm.2013.12.030; PMID: 24398086.
44. Marano PJ, Lim LJ, Sanchez JM, et al. Long-term outcomes of ablation for ventricular arrhythmias in mitral valve prolapse. J Interv Card Electrophysiol 2021;61:145–54. https:// doi.org/10.1007/s10840-020-00775-1; PMID: 32506159.
45. Naksuk N, Syed FF, Krittanawong C, et al. The effect of mitral valve surgery on ventricular arrhythmia in patients with bileaflet mitral valve prolapse. Indian Pacing Electrophysiol J 2016;16:187–91. https://doi.org/10.1016/j. ipej.2016.10.009; PMID: 28401865.
46. Pocock WA, Barlow JB, Marcus RH, Barlow CW. Mitral valvuloplasty for life-threatening ventricular arrhythmias in mitral valve prolapse. Am Heart J 1991;121:199–202. https:// doi.org/10.1016/0002-8703(91)90976-o; PMID: 1985363.
47. Vaidya VR, DeSimone CV, Damle N, et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol 2016;46:137–43. https:// doi.org/10.1007/s10840-015-0090-5; PMID: 26768434.
48. Eriksson MJ, Bitkover CY, Omran AS, et al. Mitral annular disjunction in advanced myxomatous mitral valve disease: echocardiographic detection and surgical correction. J Am Soc Echocardiogr 2005;18:1014–22. https://doi.org/10.1016/j. echo.2005.06.013; PMID: 16198877.
49. Newcomb AE, David TE, Lad VS, et al. Mitral valve repair for advanced myxomatous degeneration with posterior
displacement of the mitral annulus. J Thorac Cardiovasc Surg 2008;136:1503–9. https://doi.org/10.1016/j. jtcvs.2008.05.059; PMID: 19114198.
50. Vohra J, Sathe S, Warren R, et al. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin Electrophysiol 1993;16:387–93. https://doi.org/10.1111/j.1540-8159.1993.tb01599.x; PMID: 7681188.
51. Pelliccia A, Sharma S, Gati S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Rev Esp Cardiol (Engl Ed) 2021;74:545. https://doi.org/10.1016/j.rec.2021.05.003; PMID: 34020769.
52. Maron BJ, Ackerman MJ, Nishimura RA, et al. Task Force 4: HCM and other cardiomyopathies, mitral valve prolapse, myocarditis, and Marfan syndrome. J Am Coll Cardiol 2005;45:1340–5. https://doi.org/10.1016/j.jacc.2005.02.011; PMID: 15837284.
53. Caselli S, Mango F, Clark J, et al. Prevalence and clinical outcome of athletes with mitral valve prolapse. Circulation 2018;137:2080–2. https://doi.org/10.1161/ CIRCULATIONAHA.117.033395; PMID: 29735594.
54. Bumgarner JM, Patel D, Kumar A, et al. Management and outcomes in mitral valve prolapse with ventricular arrhythmias undergoing ablation and/or implantation of ICDs. Pacing Clin Electrophysiol 2019;42:447–52. https://doi. org/10.1111/pace.13613; PMID: 30680747.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Ventricular Dyssynchrony and Pacing-induced Cardiomyopathy in Patients with Pacemakers, the Utility of Ultra-high-frequency ECG and Other Dyssynchrony Assessment Tools
Jan Mizner , 1 Pavel Jurak , 2 Hana Linkova , 1 Radovan Smisek 2 and Karol Curila 1
Abstract
The majority of patients tolerate right ventricular pacing well; however, some patients manifest signs of heart failure after pacemaker implantation and develop pacing-induced cardiomyopathy. This is a consequence of non-physiological ventricular activation bypassing the conduction system. Ventricular dyssynchrony was identified as one of the main factors responsible for pacing-induced cardiomyopathy development. Currently, methods that would allow rapid and reliable ventricular dyssynchrony assessment, ideally during the implant procedure, are lacking. Paced QRS duration is an imperfect marker of dyssynchrony, and methods based on body surface mapping, electrocardiographic imaging or echocardiography are laborious and time-consuming, and can be difficult to use during the implantation procedure. However, the ventricular activation sequence can be readily displayed from the chest leads using an ultra-high-frequency ECG. It can be performed during the implantation procedure to visualise ventricular depolarisation and resultant ventricular dyssynchrony during pacing. This information can assist the electrophysiologist in selecting a pacing location that avoids dyssynchronous ventricular activation.
Keywords
Pacing-induced cardiomyopathy, cardiac pacing, ventricular dyssynchrony assessment, ultra-high-frequency ECG
Disclosure: JM, PJ, HL, RS and KC have received funding from Charles University Research Centre and the Ministry of Health of the Czech Republic. Participating research institutions have filed a European patent application EP 19212534.2: Method of electrocardiographic signal processing and apparatus for performing the method.
Funding: This paper was supported by the Charles University Research Centre program number UNCE/MED/002, 260530/SVV/2020 and the Ministry of Health of the Czech Republic grant number NU21-02-00584.
Received: 1 January 2022 Accepted: 9 April 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e17. DOI: https://doi.org/10.15420/aer.2022.01
Correspondence: Karol Curila, Cardiocenter, Third Faculty of Medicine, Charles University, Srobarova 50, 100 34 Prague, Czech Republic. E: karol.curila@fnkv.cz
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Permanent cardiac pacing is a standard, reliable and widely accessible method of bradycardia treatment. Since the first implantable pacemaker was developed in 1959 by Rune Elmqvist, cardiac pacing has undergone a dynamic technological revolution.1
Pacemaker and lead technology have developed rapidly, and modern pacemakers are now automatic and more reliable. Epicardial leads have been replaced by transvenous leads and pacemakers are programmed to sense underlying cardiac activity and provide pacing only when needed.
The increased life expectancies of the steadily growing elderly population have led to increased permanent pacemaker (PPM) implantation rates and new challenges in treating bradycardia.2
Even though PPMs brought indisputable benefits to patients with bradycardia, the constantly rising standards for patient wellbeing and better patient follow-up have revealed patients who fail to tolerate conventional right ventricular (RV) pacing.3–10
In some of them, left ventricular ejection fraction (LVEF) can decline after pacing. This condition is known as pacing-induced cardiomyopathy
(PICM). The current literature has several working sets of diagnostic criteria for identifying PICM, primarily based on changes in the LVEF (Table 1). The authors’ review of published research found these were the four most frequent PICM definitions:
• Decreased LVEF by 10% or more or below 50% regardless of patient symptoms;8,11
• Decreased LVEF below 45% or a decline in LVEF that is greater than 10% after PPM implantation;12
• Decreased LVEF below 40% or an indication to CRT upgrade;7
• Decreased LVEF by 5% or more with heart failure (HF) symptoms with no other aetiology of HF.13
The literature estimates that 6–22% of all patients undergoing PPM implantation fulfil the criteria for PICM within 3–16 years.7,8,11 This wide range of prevalence is associated with differences in PICM definitions, the variability of the studied populations and variable lengths of follow-up.14
Moreover, there is a rising awareness that, at least in some patients, permanent RV pacing can lead to symptoms of HF without significant changes in LVEF, a condition called PICM syndrome.15
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com
Implantable devices
1. Department of Cardiology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic; 2. Institute of Scientific Instruments of the Czech Academy of Sciences, Brno, Czech Republic
Table 1: Pacing-induced Cardiomyopathy Definitions and Incidences
1: resultant LVEF ≤40% if baseline LVEF was ≥50% or an absolute reduction of the LVEF ≥5% if baseline LVEF was <50%
2: resultant LVEF ≤40% if baseline LVEF was ≥50%, or an absolute reduction of the LVEF ≥10% if baseline LVEF was ≤50%
CRT = cardiac resynchronisation therapy; HF = heart failure; LV = left ventricular; PICM = pacing-induced cardiomyopathy; QRSd = QRS duration.
As shown recently, HF can often appear in a timescale of months, not years, after PPM implantation. A Danish national registry-based study including almost 28,000 patients undergoing PPM implantation found that almost 11% of them manifested with HF. This was significantly more than in the control group of patients without PPM, and most of these events occurred within 6 months of PPM implantation.10
Pathophysiology of Pacinginduced Cardiomyopathy
Physiological heart activation preserves atrioventricular (AV) and intraventricular conduction via the heart’s conduction system. This mechanism preserves AV synchrony and synchronous ventricular contraction.
The velocity of electrical signal transmission in Purkinje fibres is in a range of 2–4 m/s, as opposed to 0.4–0.8 m/s in ventricular muscle cells.16 RV pacing bypasses the physiological pathway, leading to slow myocyte-tomyocyte signal transmission, with a single electrical signal breakthrough in the RV apex or septum (depending on the stimulation site).
This results in disproportional RV and, more importantly, left ventricular (LV) mechanical and electrical activation, with the initial depolarisation occurring at the pacing site followed by delayed depolarisation of remote LV segments.17 These consequences of RV pacing are generally regarded as electro-mechanical ventricular dyssynchrony.
Different types of ventricular dyssynchrony are recognisable, i.e. interventricular (between the right and left ventricles) and intraventricular (within the right and left ventricles) electro-mechanical dyssynchrony.
Interventricular dyssynchrony can be measured using conventional Doppler echocardiographic imaging as the difference between the opening times of the pulmonary and aortic valves, i.e., aortopulmonary ejection delay.18,19 Intraventricular LV dyssynchrony is understood as a delay of mechanical activation between the various LV segments. It can be assessed using tissue Doppler imaging (TDI), or 2D speckle-tracking strain analysis, and real-time 3D echocardiography.20
The relationship between LV dyssynchrony and RV apical pacing was first shown in 2006 by Tops et al. in patients with AF treated with PPM implantation and subsequent AV nodal ablation. Here, LV dyssynchrony, measured using TDI, developed in almost 50% of patients after a mean follow-up of 3.8 years. Patients with LV dyssynchrony had a significant decline in LVEF and worsened New York Heart Association (NYHA) scores; in patients without LV dyssynchrony, the LVEF remained unchanged, and the NYHA score improved.21
As was shown soon after, RV apical pacing results in dyssynchronous LV contractions immediately after the start of pacing, even in patients with structurally normal hearts, and the presence of mechanical ventricular dyssynchrony, caused by RV pacing, was identified as the critical determinant of the detrimental effect of RV pacing on LV function.22–26
The time difference between the activation of individual LV segments leads to structural alteration and asymmetrical remodelling. This results from asymmetrical workloads between early activated septal and late activated LV lateral wall segments, reducing the workload for the septum and increasing the workload for the LV lateral wall. This is followed by thinning of the septum and hypertrophy of late activated LV lateral wall segments.27 It has been reported that the efficiency of cardiac pump function (the amount of stroke work generated by a unit of oxygen consumed) is approximately 30% lower in dyssynchronous than in synchronous hearts.28
As a result of non-physiological RV pacing, changes in ventricular blood perfusion, neurohumoral innervation and fatty acid metabolism have also been observed. Moreover, dyssynchrony results in changes in local myocardium oxygen demand. Different effective workloads of particular ventricular segments also cause changes in segmental myocardial perfusion and regional myocardial perfusion defects, even in the absence of coronary artery disease (CAD).29 30
Moreover, cardiac pacing has been associated with increased noradrenaline levels in myocardial tissue; in clinical research, early activated LV segments have been associated with a redistribution of sympathetic activity that resulted in regional LV defects of 123I-MIBG uptake.31,32
Pacing-induced Cardiomyopathy and Ventricular Dyssynchrony Assessment ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Study Patients (n) PICM Definition Average Follow-up (Years) PICM Incidence Risk Factors for PICM Development Khurshid et al.8 257 Decrease in LVEF ≥10% resulting in LVEF <50% 3.3 20% Male sex, prolonged spontaneous QRSd, prolonged paced QRSd Kim et al.11 130 Decrease in LVEF ≥10%, with a resultant LVEF<50% 4.7 16% Paced QRSd Kiehl et al.7 823 Resultant LVEF ≤40% or CRT upgrade 4.3 12% Baseline LV dysfunction and ≥20% ventricular pacing burden Lee et al.13 234 LVEF decrease >5% with symptoms of HF without other aetiology for HF 15.6 21% Higher ventricular pacing burden Old age Prolonged paced QRSd Higher myocardial scar score Kaye
118 Definition
Definition
Definition
baseline LVEF 3.5 Definition 1: 9% Definition 2: 6% Definition 3: 39% Higher ventricular pacing burden
et al.14
3: an absolute reduction of LVEF ≥10% irrespective of
Altered myocardial metabolism can contribute to fibrosis, myofibrillar disarray and changes in cardiac extracellular matrix (ECM) metabolism. These changes are not detectable using conventional imaging methods, but they can be assessed in tissues and possibly also in the blood circulation.
RV apical pacing in dogs has been associated with asymmetrical hypertrophy of the late-activated lateral wall segments and led to ECM remodelling and overexpression of the collagen type II gene. Additionally, the lateral wall exhibited increased amounts of matrix-metalloproteinases (MMP), MMP-2, MMP-9, TIMP-1 and TIMP-3 expression.33
Adomian et al. identified myofibrillar disarray in nine out of 12 canine hearts after 3 months of RV apical pacing.34 Similar observations were confirmed in one of only a few clinical studies on histological changes following RV pacing in humans. In the study, chronic RV pacing led to myofibrillar hypertrophy, fatty depositions and the development of cardiac interstitial fibrosis.35
Risk Factors for Pacing-induced Cardiomyopathy and Dyssynchrony Assessment
There are known risk factors for PICM development in patients with frequent RV pacing, i.e., decreased pre-implant LVEF, older age, coronary artery disease (CAD) and wide spontaneous or paced QRS durations (QRSd).
According to recent research, the potentially harmful burden of RV pacing is lower (around 20%) than previously suggested by results from the MOST trial.5,7,13,14 The major problem in relying on these risk factors is their limited predictive value, so new and better methods for PICM risk assessment are needed.
As mentioned above, dyssynchronous LV ventricular contraction can be assessed using echocardiography, which has been shown to better identify patients at the highest risk of developing PICM.
Interventricular dyssynchrony, as a risk factor for PICM in patients with RV pacing, was assessed in the study by Bansal et al.23 This group demonstrated that patients with a significant aortopulmonary ejection delay (>40 ms) were more prone to developing a decrease in LVEF than patients with lower values. Multivariate analysis showed that significant interventricular dyssynchrony and a high burden of RV pacing were the only predictors of an LVEF decrease of >10%.23
RV pacing not only results in various forms of dyssynchrony but also affects LV function sooner than can be detected by LVEF measurements. As shown by Ahmad et al., LV function measured using global longitudinal strain (GLS) deteriorates much sooner compared to when it is measured using LVEF. Furthermore, the study showed that GLS values decline as soon as 1 month after the start of RV septal pacing, and the same patients had a decline in LVEF of ≥5% over 12 months of follow-up. In this study, lower values of GLS were an independent predictor of a decline in the LVEF during follow-up.36
Thus, the data show that echocardiography can be a valuable tool for risk stratification of PICM development. It can identify patients with dyssynchronous ventricular contractions due to RV pacing, which appear to be at the highest risk of a further decline in the LVEF. However, its use during the implant procedure is limited and, for that reason, new methods of dyssynchrony assessment are needed.
The traditional tool for non-invasive dyssynchrony assessment has been the surface 12-lead ECG. The most often used parameter of synchronous ventricular activation is the QRSd. This can be easily measured during implantation procedures and, for this reason, appears to be an ideal parameter for ventricular dyssynchrony assessment.
Although it has been shown in some studies that a wider paced QRSd is an independent multivariable predictor for PICM development, this has not been confirmed in other research.8,11,37,7,23 Its major limitation is that a conventional ECG visualises only the combined depolarisation of both ventricles and does not have the ability to assess their separate activation.38 QRS morphology offers more insight into ventricular activation patterns; however, this assessment is subject to significant error. Additionally, there are several definitions of left bundle branch block.39
Another ECG-derived parameter of dyssynchrony is the QRS area (QRSa).40 It is derived from orthogonal chest leads or calculated from a standard surface 12-lead ECG and converted to 3D vectorcardiography (Figure 1).41 The QRSa is an easily obtainable, reproducible parameter, which can be automatically calculated.42
Large QRS areas have been positively associated with volumetric responses to cardiac resynchronisation therapy (CRT) and are superior in predicting CRT responses over QRSd or QRS morphologies.43
In CRT patients, a decrease in the QRSa was an independent predictor of survival and reverse cardiac remodelling, especially in patients with larger baseline QRSa.44 Also in CRT patients, the QRSa has been shown to correlate better with LV lateral wall activation delay, measured by invasive electroanatomical mapping, than with QRSd or QRS morphology.45 In patients with bradycardia, QRSa has been studied and compared during RV septal, deep septal and left bundle branch area pacing.45 Unfortunately, it has never been studied and compared in patients with various types of RV pacing.
ECG imaging (ECGi) is a complex, non-invasive imaging tool based on body surface potential mapping (BSPM). It reconstructs electroanatomical epicardial activation from a combination of approximately 240 surface electrodes and computed tomography (CT) acquired hearttorso geometry (Figure 1). It creates over 2,500 epicardial unipolar electrocardiograms.
From these, a variety of interventricular as well as LV or RV dyssynchrony parameters can be calculated.46 These include: ventricular electrical uncoupling (VEU), which is the difference between mean LV and RV activation times and is thus considered to be an interventricular dyssynchrony parameter; LV total activation time (LVTAT); and the difference between the maximum and minimum activation times – the total activation time (TAT).
ECGi has been used primarily in patients with heart failure and various types of ventricular conduction defects or RV apical pacing.47 These studies show that the method provides detailed information about ventricular depolarisation patterns and predicts the response of these patients to biventricular resynchronisation therapy.48,49 No study has used ECGi to show the differences between various types of pacing in bradycardia patients or PICM prediction.
The ECG belt (Medtronic) is a simplified BSPM system consisting of 40 body surface electrodes, which do not require a CT or MRI scan for dyssynchrony assessment. The data are processed offline and generate colour-coded isochronal maps from the anterior and posterior chest view
Pacing-induced Cardiomyopathy
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
and Ventricular Dyssynchrony Assessment
Figure 1: Schematic Demonstration of Different Tools for Non-invasive Assessment of Ventricular Electrical Dyssynchrony
A: Schematic demonstration of QRS area calculation from orthogonal ECG leads. B: Visualisation of ventricular dyssynchrony using an ECG belt in patient with left bundle branch block (LBBB). C: Visualisation of ventricular depolarisation using an ECG imaging in patients with LBBB. D: Visualisation of ulta-high-frequency ECG ventricular depolarisation using V1–V8 chest leads in patient with LBBB. F = foot; G = ground; L = Left; N = neutral; R = right.
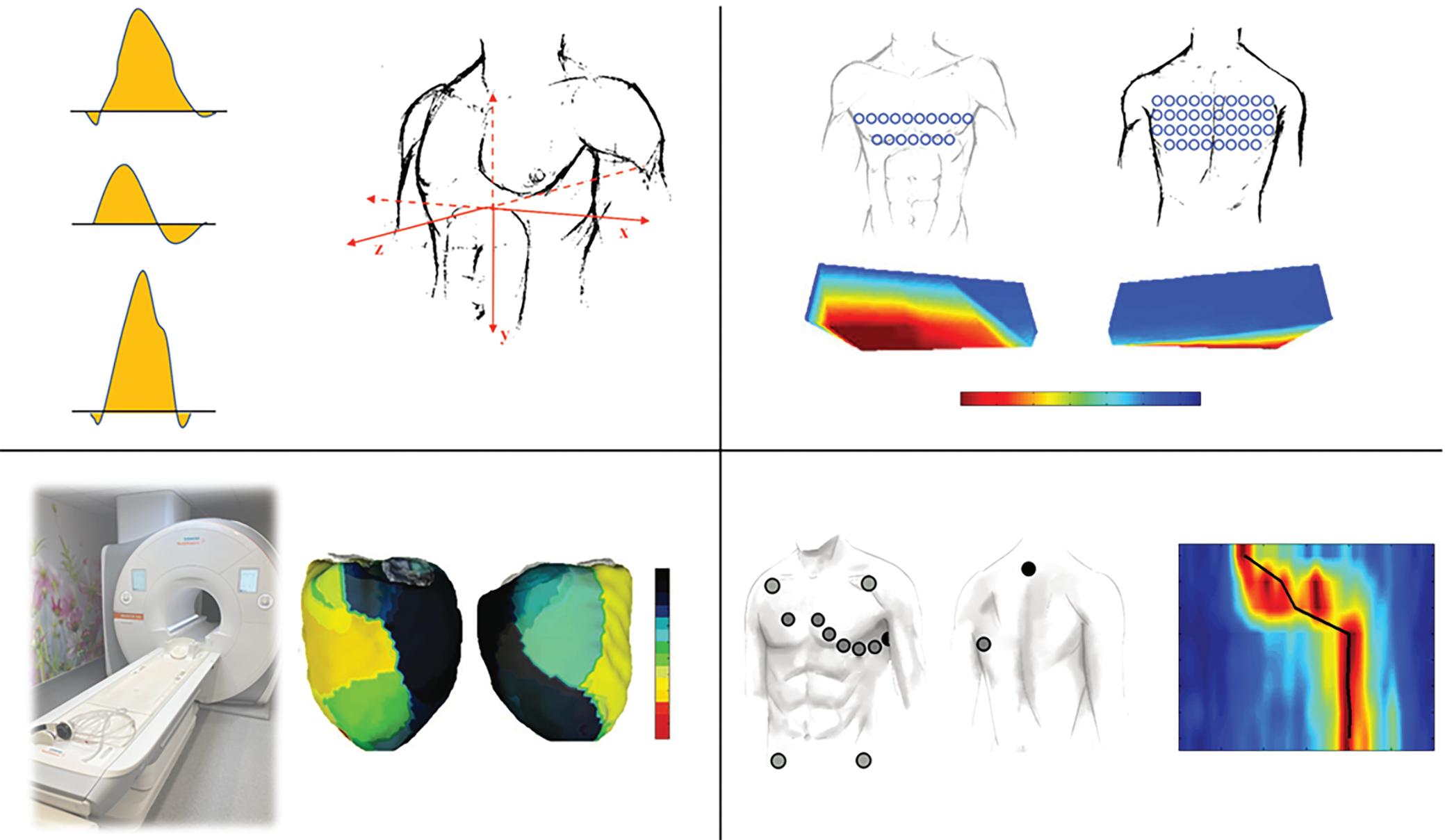
Figure 2: Ultra-high-frequency ECG Map in Right Ventricular Septal Pacing
Ventricular activation patterns and dyssynchrony parameters can also be measured using ultra-high-frequency ECG (UHF-ECG), which is currently available for non-commercial, research purposes in a limited number of clinical centres. UHF-ECG displays the sequence of ventricular activation using an analysis of the ultra-high frequency components of ventricular myocyte action potentials in perimyocardial tissue.50,51
The ventricular activation sequence under standard chest leads (V1–V6 or V1–V8 configuration) is displayed in depolarisation maps, usually in 1–3 minutes, making the method suitable for clinical practice. The broad-band QRS complex is constructed as the average of the 16 normalised median amplitude envelopes of the 16 frequency bands (150–1000 Hz) and displayed as a coloured map for chest leads.
The calculation of e-DYS (delay from first activated lead to the latest), RVLVd (delay from the first activated lead to V1), and LVLWd (delay from the first activated lead to V8) are shown. e-Dys = parameter of ventricular electrical dyssynchrony; LVLWd = left ventricular lateral wall delay; RWLVd = right ventricular lateral wall delay.
(Figure 1). The most often used dyssynchrony parameters derived from the ECG belt are the standard deviation of activation times (SDAT) and left thorax activation times (LTAT). These parameters have shown to be predictive of CRT response and useful for optimising CRT therapy.48,49
Compared to ECGi, the method is less expensive, less time consuming and easier to operate, which enables its use during implant procedures. However, the need for additional chest leads and the complexity of visualisation of ventricular depolarisation patterns make it less applicable in standard clinical care.
Local activation times are calculated as the centre of mass of the UHFQRS above the 50% threshold of the baseline-to-peak amplitude for each chest lead. The parameter of ventricular electrical dyssynchrony – e-DYS – is calculated using the time difference between the first and last activated centre of mass.
Additional and more specific parameters, such as RV or LV lateral wall activation delay (RVLWd or LVLWd) as a distance from the first activated centre of mass to V1 and V8, respectively, can be calculated in milliseconds (ms) (Figure 2). A comparison of advantages, disadvantages, and possible clinical utility of non-invasive dyssynchrony assessment tools is summarised in Table 2
Recently, using a UHF-ECG, Curila et al. showed differences in ventricular activation patterns during pacing.52 In their study, they paced various RV
Pacing-induced Cardiomyopathy and Ventricular Dyssynchrony Assessment ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
A C Anterior Posterior ms 200 0 D B X Y Z QRSarea = QRS2area x + QRS2area y + QRS2area z 0 20 40 60 80 100 120 ms R N F C1 C2 C3 C4 C5 C6 C7 C8 G L V1 V2 V3 V4 V5 V6 V7 V8 80 0 40 80 40 ms
RVLWd V1 V2 V3 V4 V5 V6 V7 V8 0 50 Right ventricle lateral wall activation 100 ms 150 200 Left ventricle lateral wall activation First activation 19 ms 61 ms LVLWd 72 ms e-Dys
Table 2: Comparison of the Non-invasive Dyssynchrony Assessment Tools
Method and Measures of Dyssynchrony
Vectorcardiography (QRS area) Feasible during the implantation, low cost, fully automatic algorithm available, reproducible42
ECG belt (SDAT, LTAT) Feasible during the implant procedure, without need for CT examination, less time consuming compared to ECGi
ECGi (VEU, LVTAT, RVTAT, TAT) Provides most detailed non-invasive electro-anatomical activation mapping of both LV and RV
UHF-ECG (e-DYS, RVLWd, LVLWd) Feasible during implantation, fully automatic. Provides qualitative and quantitative information about LV and RV depolarisation
Provides quantitative but not qualitative measurements. Does not offer a way to assess LV and RV activation separately
Multiple leads still make the system too complicated for everyday clinical use
CT or MRI scan required Time-consuming, expensive and non-feasible in daily clinical praxis
No validation study available until now; signal averaging is needed due to low amplitudes of analysed signals; UHF-ECG is not commercially available until now
• CRT response prediction43
• CRT optimisation65
• CRT response prediction48
• CRT optimisation49 66
• CRT response prediction67
• CRT optimisation68
• Ventricular depolarisation visualisation in LBBB and IVCD patients47
• Describing the differences between various types of physiological or RV pacing52 58 59
CRT = cardiac resynchronisation therapy; e-DYS = parameter of ventricular electrical dyssynchrony; ECGi = ECG imaging; IVCD = intraventricular conduction delay; LBBB = left bundle branch block; LTAT = left thorax activation times; LV = left ventricle; LVLWd = left ventricular lateral wall delay; LVTAT = left ventricular total activation time; RV = right ventricle; RVLWd = right ventricular lateral wall delay; RVTAT = right ventricular total activation time; SDAT = standard deviation of activation times; TAT = total activation time; UHF-ECG = ultra-high-frequency ECG; VEU = ventricular electrical uncoupling.
Figure 3: Pacing Locations and Representative Ultra-high-frequency ECG Maps for Pacing Sites
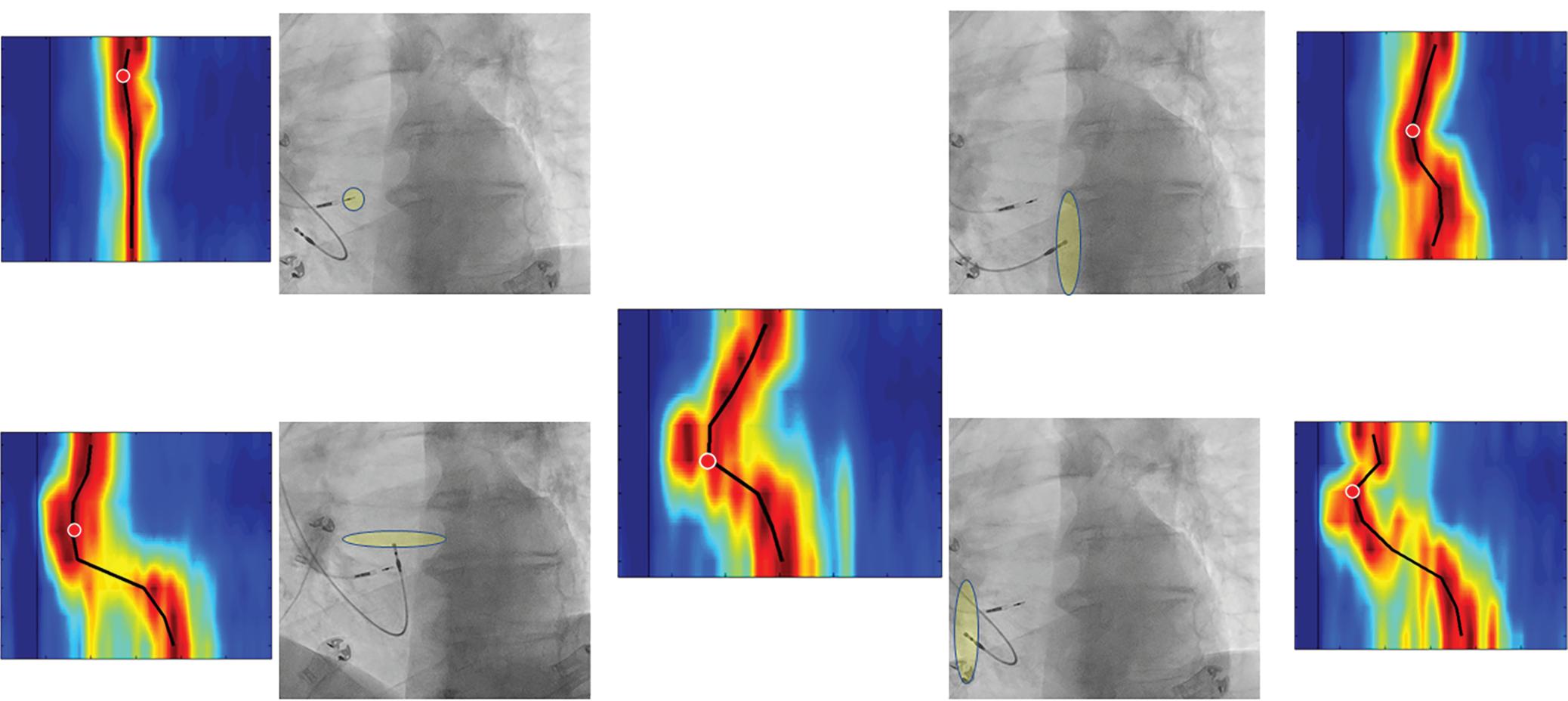
Pacing locations and representative ultra-high-frequency ECG maps for nsHB or RBBp; right ventricular septal pacing, right ventricular apical pacing, right ventricular anterior wall pacing, and right ventricular lateral wall pacing. All myocardial captures of the right ventricle produced more significant LVLWd and RVLWd than nsHB or RBBp. LVLWd = left ventricular lateral wall delay; nsHB = nonselective His bundle; RBBp = right bundle branch pacing; RVLWd = right ventricular lateral wall delay.
locations during the implant procedure in patients with bradycardia. They showed that significant differences in RV and LV activation delays were present during pacing from the basal septum with myocardial and His bundle or right bundle branch engagement (nonselective His bundle or right bundle branch pacing), the pacing of the RV septum with pure myocardial capture, the pacing of the RV apex, and pacing of the RV anterior or RV lateral wall (Figure 3). The shortest LVLWd was observed during nonselective His bundle or right bundle branch pacing, while the longest LVLWd was observed during RV anterior and lateral wall pacing. A slight difference in LVLWd between RV septal and apical pacing was observed, although the latter caused a much longer QRSd.
Curila et al. also showed that significant differences exist between various pacing locations on the RV septum.52 Pacing the RV inflow tract caused a
significantly shorter LVLWd than pacing septal myocytes in the RV outflow tract, during which LVLWd values were very similar to values seen during RV apical pacing. RV apical capture was the only studied capture type that caused significant RV activation delays. Variations in the RV and LV activation delay could be explained by differences between pacing locations and the character of the electrical wave-front propagation in both ventricles.
When the velocity of depolarisation wave-front propagation was measured in the leads placed above the LV lateral wall, it was found to be similar during RV apical, anterior and lateral wall pacing, and all were significantly longer compared to RV septal pacing. This is likely to be a result of different types of electrical wave-front propagation. During RV septal pacing, the LV Purkinje system is used for activation; however,
Pacing-induced Cardiomyopathy and Ventricular Dyssynchrony
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Assessment
Clinical Utility in Published Literature
Advantages Disadvantages
V1 nsHB or RBBp ms ms ms Left Anterior Oblique Projection Left Anterior Oblique Projection Left Anterior Oblique Projection Left Anterior Oblique Projection Right Ventricular Anterior Wall Pacing Right Ventricular Apical Pacing LVLWd 54 ms, RVLWd 42 ms LVLWd 90 ms, RVLWd 16 ms LVLWd 7 ms, RVLWd 4 ms LVLWd 96 ms, RWLVd 18 ms LVLWd 20 ms, RVLWd 22 ms V2 V3 V4 V5 V6 V7 V8 80 40 0 40 80 V1 Right Ventricular Septal Pacing ms ms V2 V3 V4 V5 V6 V7 V8 80 40 0 40 80 V1 Right Ventricular Lateral Wall Pacing V2 V3 V4 V5 V6 V7 V8 80 40 0 40 80 V1 V2 V3 V4 V5 V6 V7 V8 80 40 0 40 80 V1 V2 V3 V4 V5 V6 V7 V8 80 40 0 40 80
Pacing-induced Cardiomyopathy and Ventricular Dyssynchrony Assessment
Figure 4: Ultra-high-frequency ECG Maps and Left Ventricular Lateral Wall Delay with Right Ventricular Apical and Right Ventricular Septal Pacing
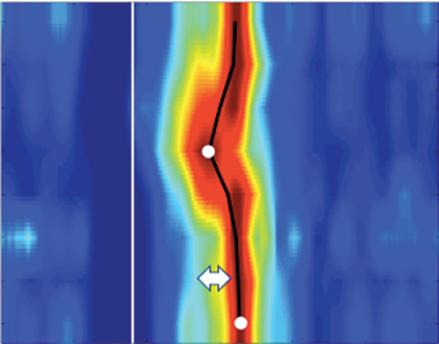
unreliable ECG or X-ray criteria, which led to incorrect lead fixations towards the anterior wall in a substantial percentage of patients, i.e. the pacing location, which, based on UHF-ECG data, can lead to more delayed LV lateral wall depolarisation than RV apical pacing.56 57
Recently, His-Purkinje conductive system pacing techniques were introduced. These include His bundle pacing (HBP), left bundle branch pacing (LBBP) and left ventricular septal pacing (LVSP). As shown recently, these techniques better preserve physiological ventricular activation than RV pacing.52,58,59 This favourable effect on ventricular activation during HBP was shown to reduce HF hospitalisations in patients requiring more than 20% ventricular pacing compared to RV apical or septal pacing.60
However, as shown subsequently, HBP has some limitations, such as higher pacing thresholds, which can lead to premature battery depletion, lower sensing values and lower success rates in patients with bundle branch blocks; these limitations have restricted its use in all patients.61,62
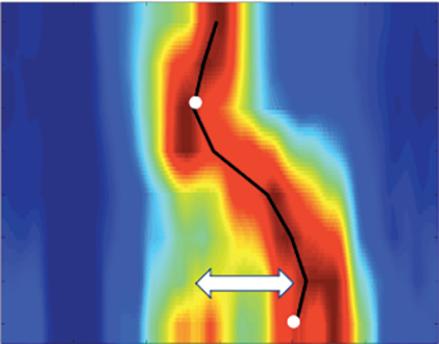
Moreover, in some studies, the risk of reintervention on pacing lead repositioning was unacceptably high.63 For that reason, more distal pacing lead placement (i.e., LBBP or LVSP) is now preferred by many specialists. Although these methods are less physiological than HBP, a recent multicentre, observational study showed they reduce the incidence of death and HF hospitalisations compared to RV apical or septal pacing.64
during RV apical, anterior, and lateral wall pacing, slow myocardial cell-tocell propagation plays a more significant role.
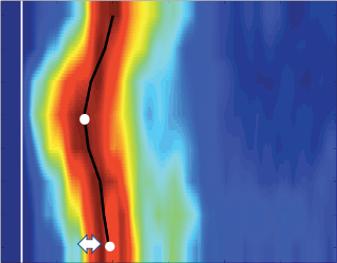
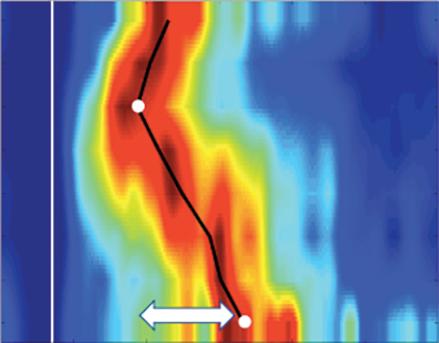
Although the averaged values showed significant differences between RV pacing sites, a closer review of the data revealed significant individual variability between the patients included in the study (data not published). There were patients with minimal LV and RV lateral wall delays during RV apical or RV septal pacing, but there were others in whom pacing the same locations resulted in much greater ventricular dyssynchrony (Figure 4).
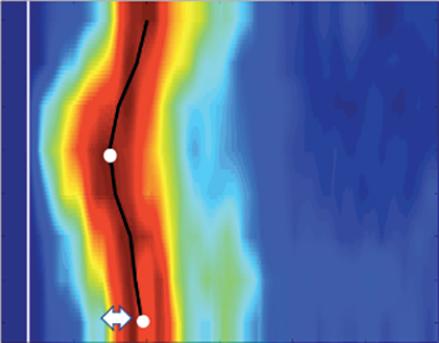
In summary, UHF-ECG can visualise ventricular depolarisation patterns in various types of ventricular pacing during the implant procedure. Significant differences have been found between the pacing locations studied and individual patients using the same pacing locations. A multicentre clinical trial to determine if UHF-ECG dyssynchrony can serve as an additional tool for predicting patients with the highest risk of PICM has been started (NCT04908033). Unfortunately, the availability of UHFECG is limited. Until now, the hardware has been available only in nine centres and shortly it is going to be installed in another five centres.
Possible Prevention and Treatment of Pacing-induced Cardiomyopathy
Initially, PICM development was thought to result from RV apical pacing, which produced wide QRS complexes. It was hypothesised that narrowing the paced QRS duration during RV septal pacing would reduce PICM development. Unfortunately, no clinical trial comparing RV septal to apical pacing showed any clinical benefit of RV septal pacing.53 54 Additionally, no benefits in mortality or HF hospitalisations were observed in trials comparing biventricular pacing with RV pacing in patients with bradycardia.55 56
However, some of these studies had important shortcomings, which limited the potential benefit of reduced ventricular dyssynchrony during RV septal over RV apical pacing. RV septal lead placement was based on
Whether these approaches will lead to a better clinical outcome than RV pacing needs to be demonstrated in prospective, randomised clinical trials. Even if these promising pacing methods prove effective, they are still more complex and require dedicated implant tools and advanced equipment in the operating room.
For that reason, at least for now, these methods are probably best suited for patients at the highest risk of pacing-induced cardiomyopathy.
Conclusion
Declining LVEF and the development of HF, as the main signs of pacinginduced cardiomyopathy, are not uncommon complications of permanent RV pacing.
These complications occur because of non-physiological ventricular activation, with resultant asynchronous ventricular contractions, which are detectable soon after the start of RV pacing.
Several methods based on the processing of signals generated by ventricular depolarisation or echocardiography can be used to assess ventricular dyssynchrony. While these methods provide the electrophysiologist with exact information about the resultant pattern of ventricular depolarisation associated with a specific pacing location, they are complex, time consuming and cannot be readily performed during standard implant procedures.
However, this information can be obtained using UHF-ECG, which can be used to visualise ventricular depolarisation patterns. This method analyses high-frequency ECG signals in a 12- or 14-lead ECG. It uses standard chest leads, and information about ventricular activation is available in less than 3 minutes. UHF-ECG is a promising method for real-time feedback during implant procedures since it visualises and quantifies the activation delay of specific ventricular segments. This additional information helps the electrophysiologist avoid pacing locations that produce dyssynchronous ventricular activation.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
V1 ms Patient 1 Patient 2 Patient 3 Patient 4 V2 V3 V4 V5 V6 V7 V8 V1 V2 V3 V4 V5 V6 V7 V8 80 40 0 40 80 ms 80 40 0 40 80 V1 ms V2 V3 V4 V5 V6 V7 V8 V1 V2 V3 V4 V5 V6 V7 V8 80 40 0 40 80 ms 80 40 0 40 80 LVLWd 14 ms LVLWd 58 ms LVLWd 16 ms LVLWd 62 ms
Comparison of ultra-high-frequency ECG maps and LVLWd in four patients with right ventricular apical (patients 1 and 2) and right ventricular septal pacing (patients 3 and 4). A significant difference in LVLWd is seen between patients with pacing leads at similar locations.
LVLWd = left ventricular lateral wall delay.
Pacing-induced Cardiomyopathy and Ventricular Dyssynchrony Assessment
Clinical Perspective
• Dyssynchronous ventricular activation is one of the most important factors responsible for right ventricular pacing having a deleterious effect, and occurs in a subset of patients with pacemakers.
• Identifying patients with ventricular dyssynchrony during pacing would allow the most appropriate pacing method to be selected.
• Ventricular dyssynchrony can be measured using echocardiography or complex methods of ventricular depolarisation visualisation, but these are not well suited for use during standard implant procedures.
• Ultra-high-frequency ECGs can visualise the sequence of ventricular activation and identify patients with dyssynchronous ventricular depolarisation during pacing.
• If ultra-high-frequency ECG demonstrates an ability to predict patients at risk of decreased left ventricular ejection fraction due to pacing, then it may help to individualise treatment in patients with bradycardia.
1. Furman S, Schwedel JB. An intracardiac pacemaker for Stokes-Adams seizures. N Engl J Med 1959;261:943–8. https://doi.org/10.1056/NEJM195911052611904; PMID: 13825713.
2. Bradshaw PJ, Stobie P, Knuiman MW, et al. Trends in the incidence and prevalence of cardiac pacemaker insertions in an ageing population. Open Heart 2014;1:e000177. https://doi.org/10.1136/openhrt-2014-000177; PMID: 25512875.
3. Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular with an implantable defibrillator. JAMA 2002;288:3115–23. https://doi.org/10.1001/jama.288.24.3115; PMID: 12495391.
4. Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013;368:1585–93. https://doi.org/10.1056/ NEJMoa1210356; PMID: 23614585.
5. Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. https://doi. org/10.1161/01.CIR.0000072769.17295.B1; PMID: 12782566.
6. Chan JYS, Fang F, Zhang Q, et al. Biventricular pacing is superior to right ventricular pacing in bradycardia patients with preserved systolic function: 2-year results of the PACE trial. Eur Heart J 2011;32:2533–40. https://doi.org/10.1093/ eurheartj/ehr336; PMID: 21875860.
7. Kiehl EL, Makki T, Kumar R, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm 2016;13:2272–8. https://doi.org/10.1016/j.hrthm.2016.09.027;
PMID: 27855853.
8. Khurshid S, Epstein AE, Verdino RJ, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm 2014;11:1619–25. https://doi. org/10.1016/j.hrthm.2014.05.040; PMID: 24893122.
9. Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation 2006;113:2082–8. https://doi.org/10.1161/ CIRCULATIONAHA.105.608356; PMID: 16636167.
10. Tayal B, Fruelund P, Sogaard P, et al. Incidence of heart failure after pacemaker implantation: a nationwide Danish Registry-based follow-up study. Eur Heart J 2019;40:3641–8. https://doi.org/10.1093/eurheartj/ehz584; PMID: 31504437.
11. Kim JH, Kang KW, Chin JY, et al. Major determinant of the occurrence of pacing-induced cardiomyopathy in complete atrioventricular block: a multicentre, retrospective analysis over a 15-year period in South Korea. BMJ Open 2018;8:e019048. https://doi.org/10.1136/ bmjopen-2017-019048; PMID: 29439074.
12. Zhang H, Zhou YJ, Zeng YJ. Prognostic factors of pacinginduced cardiomyopathy. Chin Med J (Engl) 2020;133:1533–9. https://doi.org/10.1097/CM9.0000000000000856; PMID: 32568868.
13. Lee SA, Cha MJ, Cho Y, et al. Paced QRS duration and myocardial scar amount: predictors of long-term outcome of right ventricular apical pacing. Heart Vessels 2016;31:1131–9. https://doi.org/10.1007/s00380-015-0707-8; PMID: 26142378.
14. Kaye G, Ng JY, Ahmed S, et al. The prevalence of pacinginduced cardiomyopathy (PICM) in patients with long term right ventricular pacing − is it a matter of definition? Heart Lung Circ 2019;28:1027–33. https://doi.org/10.1016/j. hlc.2018.05.196; PMID: 30017634.
15. Merchant FM, Mittal S. Pacing induced cardiomyopathy. J Cardiovasc Electrophysiol 2020;31:286–92. https://doi. org/10.1111/jce.14277; PMID: 31724791.
16. Draper MH and Mya-Tu M. A comparison of the conduction velocity in cardiac tissues of various mammals. Q J Exp
Physiol Cogn Med Sci 1959;44:91–109. https://doi.org/10.1113/ expphysiol.1959.sp001379; PMID: 13624016.
17. Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony: implications for therapy. J Am Coll Cardiol 2009;54:764–76. https://doi.org/10.1016/j.jacc.2009.06.006; PMID: 19695453.
18. Rouleau F, Merheb M, Geffroy S, et al. Echocardiographic assessment of the interventricular delay of activation and correlation to the QRS width in dilated cardiomyopathy. Pacing Clin Electrophysiol 2001;24:1500–6. https://doi. org/10.1046/j.1460-9592.2001.01500.x; PMID: 11707043.
19. Ghio S, Constantin C, Klersy C, et al. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J 2004;25:571–8. https://doi.org/10.1016/j.ehj.2003.09.030; PMID: 15120054.
20. Marsan NA, Breithardt OA, Delgado V, et al. Predicting response to CRT. The value of two- and three-dimensional echocardiography. Europace 2008;10:73–9. https://doi. org/10.1093/europace/eun219; PMID: 18955403.
21. Tops LF, Schalij MJ, Holman ER, et al. Right ventricular pacing can induce ventricular dyssynchrony in patients with atrial fibrillation after atrioventricular node ablation. J Am Coll Cardiol 2006;48:1642–8. https://doi.org/10.1016/j. jacc.2006.05.072; PMID: 17045901.
22. Delgado V, Tops LF, Trines SA, et al. Acute effects of right ventricular apical pacing on left ventricular synchrony and mechanics. Circ Arrhythm Electrophysiol 2009;2:135–45. https://doi.org/10.1161/CIRCEP.108.814608; PMID: 19808458.
23. Bansal R, Parakh N, Gupta A, et al. Incidence and predictors of pacemaker-induced cardiomyopathy with comparison between apical and non-apical right ventricular pacing sites. J Interv Card Electrophysiol 2019;56:63–70. https://doi. org/10.1007/s10840-019-00602-2; PMID: 31363943.
24. Fang F, Luo XX, Zhang Q, et al. Deterioration of left ventricular systolic function in extended Pacing to Avoid Cardiac Enlargement (PACE) trial: the predictive value of early systolic dyssynchrony. Europace 2015;17:ii47–53. https://doi.org/10.1093/europace/euv130; PMID: 26842115.
25. Schmidt M, Rittger H, Marschang H, et al. Left ventricular dyssynchrony from right ventricular pacing depends on intraventricular conduction pattern in intrinsic rhythm. Eur J Echocardiogr 2009;10:776–83. https://doi.org/10.1093/ ejechocard/jep069; PMID: 19515706.
26. Pastore G, Noventa F, Piovesana P, et al. Left ventricular dyssynchrony resulting from right ventricular apical pacing: relevance of baseline assessment. Pacing Clin Electrophysiol 2008;31:1456–62. https://doi. org/10.1111/j.1540-8159.2008.01209.x; PMID: 18950303.
27. Van Oosterhout MFM, Prinzen FW, Arts T, et al. Asynchronous electrical activation induces asymmetrical hypertrophy of the left ventricular wall. Circulation 1998;98:588–95. https://doi.org/10.1161/01.CIR.98.6.588; PMID: 9714117.
28. Prinzen FW, Lumens J, Duchenn J, Vernooy K. Electroenergetics of biventricular, septal and conduction system pacing. Arrhythm Electrophysiol Rev 2021;10:250–7. https://doi. org/10.15420/aer.2021.30; PMID: 35106177.
29. Tse HF, Lau CP. Long-term effect of right ventricular pacing on myocardial perfusion and function. J Am Coll Cardiol 1997;29:744–9. https://doi.org/10.1016/S07351097(96)00586-4; PMID: 9091519.
30. Skalidis EI, Kochiadakis GE, Koukouraki SI, et al. Myocardial perfusion in patients with permanent ventricular pacing and normal coronary arteries. J Am Coll Cardiol 2001;37:124–9. https://doi.org/10.1016/S0735-1097(00)01096-2; PMID: 11153726.
31. Lee MA, Dae MW, Langberg JJ, et al. Effects of long-term right ventricular apical pacing on left ventricular perfusion,
innervation, function and histology. J Am Coll Cardiol 1994;24:225–32. https://doi.org/10.1016/07351097(94)90567-3; PMID: 8006270.
32. Marketou ME, Simantirakis EN, Prassopoulos VK, et al. Assessment of myocardial adrenergic innervation in patients with sick sinus syndrome: effect of asynchronous ventricular activation from ventricular apical stimulation. Heart 2002;88:255–9. https://doi.org/10.1136/heart.88.3.255; PMID: 12181217.
33. Lin JM, Lai LP, Lin CS, et al. Left ventricular extracellular matrix remodeling in dogs with right ventricular apical pacing. J Cardiovasc Electrophysiol 2010;21:1142–9. https://doi. org/10.1111/j.1540-8167.2010.01765.x; PMID: 20384649.
34. Adomian GE, Beazell J. Myofibrillar disarray produced in normal hearts by chronic electrical pacing. Am Heart J 1986;112:79–83. https://doi.org/10.1016/00028703(86)90682-4; PMID: 3728292.
35. Karpawich PP, Rabah R, Haas JE. Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol 1999;22:1372–7. https://doi.org/10.1111/j.1540-8159.1999. tb00631.x; PMID: 10527019.
36. Ahmed FZ, Motwani M, Cunnington C, et al. One-month global longitudinal strain identifies patients who will develop pacing-induced left ventricular dysfunction over time: the pacing and ventricular dysfunction (PAVD) study. PLoS One 2017;12:e0162072. https://doi.org/10.1371/journal. pone.0162072; PMID: 28095413.
37. Lee WC, Fang HY, Chen HC, et al. Post-pacemaker implant QRS duration and heart failure admission in patients with sick sinus syndrome and complete atrioventricular block. ESC Heart Fail 2019;6:686–93. https://doi.org/10.1002/ ehf2.12445; PMID: 31111655.
38. Poole JE, Singh JP, Birgersdotter-Green U. QRS duration or QRS morphology; what really matters in cardiac resynchronization therapy? J Am Coll Cardiol 2016;67:1104–17. https://doi.org/10.1016/j.jacc.2015.12.039; PMID: 26940932.
39. van Deursen CJM, Blaauw Y, Witjens MI, et al. The value of the 12-lead ECG for evaluation and optimization of cardiac resynchronization therapy in daily clinical practice. J Electrocardiol 2014;47:202–11. https://doi.org/10.1016/j. jelectrocard.2014.01.007; PMID: 24444866.
40. Heckman LIB, Luermans JGLM, Curila K, et al. Comparing ventricular synchrony in left bundle branch and left ventricular septal pacing in pacemaker patients. J Clin Med 2021;10:822. https://doi.org/10.3390/jcm10040822; PMID: 33671420.
41. Kors JA, van Herpen G, Sittig AC, van Bemmel JH. Reconstruction of the Frank vectorcardiogram from standard electrocardiographic leads: diagnostic comparison of different methods. Eur Heart J 1990;11:1083–92. https://doi. org/10.1093/oxfordjournals.eurheartj.a059647; PMID: 2292255.
42. Plesinger F, van Stipdonk AMW, Smisek R, et al. Fully automated QRS area measurement for predicting response to cardiac resynchronization therapy. J Electrocardiol 2020;63:159–63. https://doi.org/10.1016/j. jelectrocard.2019.07.003; PMID: 31324399.
43. van Stipdonk AMW, ter Horst I, Kloosterman M, et al. QRS area is a strong determinant of outcome in cardiac resynchronization therapy. Circ Arrhythm Electrophysiol 2018;11:e006497. https://doi.org/10.1161/CIRCEP.118.006497; PMID: 30541356.
44. Ghossein MA, van Stipdonk AMW, Plesinger F, et al. Reduction in the QRS area after cardiac resynchronization therapy is associated with survival and echocardiographic response. J Cardiovasc Electrophysiol 2021;32:813–22. https:// doi.org/10.1111/jce.14910; PMID: 33476467.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Pacing-induced Cardiomyopathy and Ventricular Dyssynchrony Assessment
45. Mafi Rad M, Wijntjens GWM, Engels EB, et al. Vectorcardiographic QRS area identifies delayed left ventricular lateral wall activation determined by electroanatomic mapping in candidates for cardiac resynchronization therapy. Heart Rhythm 2016;13:217–25. https://doi.org/10.1016/j.hrthm.2015.07.033; PMID: 26232766.
46. Sedova K, Repin K, Donin G, et al. Clinical utility of body surface potential mapping in CRT patients. Arrhythm Electrophysiol Rev 2021;10:113–9. https://doi.org/10.15420/ aer.2021.14; PMID: 34401184.
47. Eschalier R, Ploux S, Lumens J, et al. Detailed analysis of ventricular activation sequences during right ventricular apical pacing and left bundle branch block and the potential implications for cardiac resynchronization therapy. Heart Rhythm 2015;12:137–43. https://doi.org/10.1016/j. hrthm.2014.09.059; PMID: 25285646.
48. Gage RM, Curtin AE, Burns KV, et al. Changes in electrical dyssynchrony by body surface mapping predict left ventricular remodeling in patients with cardiac resynchronization therapy. Heart Rhythm 2017;14:392–9. https://doi.org/10.1016/j.hrthm.2016.11.019; PMID: 27867072.
49. Ben Johnson WB, Vatterott PJ, Peterson MA, et al. Body surface mapping using an ECG belt to characterize electrical heterogeneity for different left ventricular pacing sites during cardiac resynchronization: relationship with acute hemodynamic improvement. Heart Rhythm 2017;14:385–91. https://doi.org/10.1016/j.hrthm.2016.11.017; PMID: 27871987.
50. Jurak P, Curila K, Leinveber P, et al. Novel ultra-highfrequency electrocardiogram tool for the description of the ventricular depolarization pattern before and during cardiac resynchronization. J Cardiovasc Electrophysiol 2020;31:300–7. https://doi.org/10.1111/jce.14299; PMID: 31788894.
51. Jurak P, Bear LR, Nguyên UC, et al. 3-dimensional ventricular electrical activation pattern assessed from a novel high-frequency electrocardiographic imaging technique: principles and clinical importance. Sci Rep 2021;11:11469. https://doi.org/10.1038/s41598-021-90963-4; PMID: 34075135.
52. Curila K, Jurak P, Halamek J, et al. Ventricular activation pattern assessment during right ventricular pacing: ultra-
high-frequency ECG study. J Cardiovasc Electrophysiol 2021;32:1385–94. https://doi.org/10.1111/jce.14985; PMID: 33682277.
53. Zografos TA, Siontis KC, Jastrzebski M, et al. Apical vs. nonapical right ventricular pacing in cardiac resynchronization therapy: a meta-analysis. EP Europace 2015;17:1259–66. https://doi.org/10.1093/europace/euv048; PMID: 25829472.
54. Da Costa A, Gabriel L, Romeyer-Bouchard C, et al. Focus on right ventricular outflow tract septal pacing. Arch Cardiovasc Dis 2013;106:394–403. https://doi.org/10.1016/j. acvd.2012.08.005; PMID: 23850059.
55. Funck RC, Mueller HH, Lunati M, et al. Characteristics of a large sample of candidates for permanent ventricular pacing included in the biventricular pacing for atrioventricular block to prevent cardiac desynchronization study (BioPace). Europace 2014;16:354–62. https://doi.org/10.1093/ europace/eut343; PMID: 24200715.
56. Curtis AB, Worley SJ, Chung ES, et al. Improvement in clinical outcomes with biventricular versus right ventricular pacing: the BLOCK HF study. J Am Coll Cardiol 2016;67:2148–57. https://doi.org/10.1016/j.jacc.2016.02.051; PMID: 27151347.
57. Domenichini G, Sunthorn H, Fleury E, et al. Pacing of the interventricular septum versus the right ventricular apex: a prospective, randomized study. Eur J Intern Med 2012;23:621–7. https://doi.org/10.1016/j.ejim.2012.03.012; PMID: 22939807.
58. Curila K, Prochazkova R, Jurak P, et al. Both selective and nonselective His bundle, but not myocardial, pacing preserve ventricular electrical synchrony assessed by ultrahigh-frequency ECG. Heart Rhythm 2020;17:607–14. https:// doi.org/10.1016/j.hrthm.2019.11.016; PMID: 31805370.
59. Curila K, Jurak P, Jastrzebski M, et al. Left bundle branch pacing compared to left ventricular septal myocardial pacing increases interventricular dyssynchrony but accelerates left ventricular lateral wall depolarization. Heart Rhythm 2021;18:1281–9. https://doi.org/10.1016/j. hrthm.2021.04.025; PMID: 33930549.
60. Abdelrahman M, Subzposh FA, Beer D, et al. Clinical outcomes of His bundle pacing compared to right
ventricular pacing. J Am Coll Cardiol 2018;71:2319–30. https:// doi.org/10.1016/j.jacc.2018.02.048; PMID: 29535066.
61. Upadhyay GA, Vijayaraman P, Nayak HM, et al. His corrective pacing or biventricular pacing for cardiac resynchronization in heart failure. J Am Coll Cardiol 2019;74:157–9. https://doi.org/10.1016/j.jacc.2019.04.026; PMID: 31078637.
62. Sharma PS, Ellenbogen KA, Trohman RG. Permanent His bundle pacing: the past, present, and future. J Cardiovasc Electrophysiol 2017;28:458–65. https://doi.org/10.1111/ jce.13154; PMID: 28032941.
63. Teigeler T, Kolominsky J, Vo C, et al. Intermediate-term performance and safety of His-bundle pacing leads: a single-center experience. Heart Rhythm 2021;18:743–9. https://doi.org/10.1016/j.hrthm.2020.12.031; PMID: 33418127.
64. Sharma PS, Patel NR, Ravi V, et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: results from the Geisinger-Rush Conduction System Pacing registry. Heart Rhythm 2022;19:3–11. https://doi. org/10.1016/j.hrthm.2021.08.033; PMID: 34481985.
65. Ghossein MA, Zanon F, Salden F, et al. Left ventricular lead placement guided by reduction in QRS area. J Clin Med 2021;10:5935. https://doi.org/10.3390/jcm10245935; PMID: 34945236.
66. Bank AJ, Gage RM, Curtin AE, et al. Body surface activation mapping of electrical dyssynchrony in cardiac resynchronization therapy patients: potential for optimization. J Electrocardiol 2018;51:534–41. https://doi. org/10.1016/j.jelectrocard.2017.12.004; PMID: 29273234.
67. Ploux S, Eschalier R, Whinnett ZI, et al. Electrical dyssynchrony induced by biventricular pacing: implications for patient selection and therapy improvement. Heart Rhythm 2015;12:782–91. https://doi.org/10.1016/j.hrthm.2014.12.031; PMID: 25546811.
68. Pereira H, Jackson TA, Sieniewicz B, et al. Non-invasive electrophysiological assessment of the optimal configuration of quadripolar lead vectors on ventricular activation times. J Electrocardiol 2018;51:714–9. https://doi. org/10.1016/j.jelectrocard.2018.05.006; PMID: 29997019.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Safety, Efficacy and Prognostic Benefit of Atrial Fibrillation Ablation in Heart Failure with Preserved Ejection Fraction
Nicolas Johner , Mehdi Namdar and Dipen C Shah Cardiology Division, Geneva University Hospital, Geneva, Switzerland
Abstract
Up to 65% of patients with heart failure with preserved ejection fraction (HFpEF) develop AF during the course of the disease. This occurrence is associated with adverse outcomes, including pump failure death. Because AF and HFpEF are mutually reinforcing risk factors, sinus rhythm restoration may represent a disease-modifying intervention. While catheter ablation exhibits acceptable safety and efficacy profiles, no randomised trials have compared AF ablation with medical management in HFpEF. However, catheter ablation has been reported to result in lower natriuretic peptides, lower filling pressures, greater peak cardiac output and improved functional capacity in HFpEF. There is growing evidence that catheter ablation may reduce HFpEF severity, hospitalisation and mortality compared to medical management. Based on indirect evidence, early catheter ablation and minimally extensive atrial injury should be favoured. Hence, individualised ablation strategies stratified by stepwise substrate inducibility provide a logical basis for catheter-based rhythm control in this heterogenous population. Randomised trials are needed for definitive evidence-based guidelines.
Keywords
AF, catheter ablation, pulmonary vein isolation, heart failure, heart failure with preserved ejection fraction, rhythm control.
Disclosure: DCS has received consultant-speaker board fees from Biosense Webster, Biotronik, St. Jude Medical and Boston Scientific. He is a consultant and member of the scientific advisory board of ArgaTech and SentiAR. All other authors have no conflicts of interest to declare.
Funding: DCS was a recipient of a Swiss National Science Foundation grant during this project.
Received: 12 February 2022
Accepted: 23 May 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e18. DOI: https://doi.org/10.15420/aer.2022.10
Correspondence: Dipen C Shah, Cardiology Division, University Hospital of Geneva, Rue Gabrielle-Perret-Gentil 4, 1205 Geneva, Switzerland.
E: dipen.shah@hcuge.ch
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Heart failure (HF) and AF are major causes of morbidity, hospitalisation and mortality. AF and HF with preserved ejection fraction (HFpEF) or reduced ejection fraction (HFrEF) frequently coexist due to complex pathophysiological interactions.1 More than half of HF patients exhibit AF during the course of their disease and more than one-third of AF patients exhibit incident HF.2 The association is even stronger with HFpEF, and AFHFpEF patients exhibit a poorer prognosis compared to either condition alone.2–4 The management of AF in HFpEF is therefore a crucial issue for which the specificities of the AF-HFpEF population need to be taken into account.
Catheter ablation is a first-line treatment option for AF in selected patients. Randomised controlled trials (RCTs) have demonstrated its superiority to pharmacological treatment in terms of symptom and rhythm control, as well as prognostic outcomes including mortality, including in HFrEF.5 6 Nevertheless, there is a relative scarcity of data regarding the management of AF in HFpEF and the role of catheter ablation.
In the present non-systematic review, we discuss the role of catheter ablation in AF-HFpEF and the current evidence regarding its safety, efficacy and prognostic benefit.
Heart Failure with Preserved Ejection Fraction
HFpEF and HFrEF contribute equally to the burden of HF in the community, with HFpEF accounting for the majority of HF hospitalisations.7 HFpEF is a clinical syndrome that results from an inability of the heart to meet adequate cardiac output at normal filling pressures, in the absence of overt left ventricular (LV) systolic dysfunction at rest. The HFpEF population is phenotypically diverse because of substantial pathophysiological heterogeneity. Filling pressures are infrequently measured directly in clinical practice and the diagnosis of HFpEF is based on multimodal clinical workup showing signs and symptoms of HF, echocardiographic evidence of diastolic dysfunction and/or increased filling pressures and increased natriuretic peptide levels.8 In addition, isolated mimics of HFpEF, such as valvular heart disease, pericardial constriction and arrhythmias (including AF), need to be differentiated.
There is currently no established prognosis-modifying treatment in HFpEF, and evidence-based management is limited to diuretic drugs to alleviate signs and symptoms of congestion and screening of treatable comorbid conditions, therefore emphasising a pressing need to identify diseasemodifying treatments.9
Current guidelines define HFpEF as LV ejection fraction (LVEF) ≥50%, HF
REVIEW © RADCLIFFE CARDIOLOGY 2022 www.AERjournal.com Atrial Fibrillation
with mid-range ejection fraction (HFmrEF) as LVEF 40–49% and HFrEF as LVEF <40%.8,9 Consistent with most AF-HFpEF literature, in the present review a preserved ejection fraction refers to an LVEF ≥50% unless otherwise specified; likewise, HFmrEF is grouped with HFrEF if not separately reported.
Epidemiology and Pathophysiology of AF-HFpEF
The prevalence of AF in the general adult population is approximately 3%, ranging from 0.1% among adults younger than 55 years to 9% among those older than 80 years.10–12 Similarly, HF exhibits an estimated 2% prevalence in the general adult population, rising to 8–10% in adults older than 75 years.1 13–15 Both AF and HF have an approximate lifetime risk of one in four to one in three.15–18 The incidence and prevalence of AF are rising in parallel to the growing burden of AF risk factors and population ageing. While the overall incidence of HF in high-income countries appears to have started to decline with an estimated peak in the mid1990s, the prevalence seems stable or increasing, possibly related to improved HF survival up to recent years and population ageing.1,3,19–23 Importantly, the incidence of HFpEF has increased, in divergence from a decreasing incidence of HFrEF: an analysis on the Framingham Heart Study and Cardiovascular Health Study cohorts found that the age- and sex-standardised incidence of HFpEF rose from 4.7 to 6.8 per 1,000 person-years from the 1990–1999 decade to 2000–2009, while the standardised incidence of HFrEF declined from 6.6 to 6.2 per 1,000 person-years.23 This epidemiological shift may be related to a growing prevalence of HFpEF risk factors – including ageing – in parallel with improvements in the prevention and treatment of coronary artery disease, including a decline in ST-elevation MI.
AF and HFpEF or HFrEF are strongly associated, with AF occurring in more than half of HF subjects, and HF occurring in more than one-third of AF subjects.2 The bidirectional temporal relationship between AF and HF is apparent in the Framingham Heart Study. Among 382 individuals who were diagnosed with both AF and HF between 1948 and 1995, 38% developed AF first, 41% developed HF first, and 21% were diagnosed with both conditions on the same day.24 AF subjects exhibited an incidence of HF of 33 per 1,000 person-years, and HF subjects exhibited an incidence of AF of 54 per 1,000 person-years. The association is even stronger between AF and HFpEF. Data from major HF registries consistently show a higher prevalence of AF in HFpEF compared to HFrEF, ranging from 32 to 65% in HFpEF and 23 to 53% in HFrEF.25 In a more recent analysis from the Framingham Heart Study, among individuals with new-onset HF, the prevalence of pre-existing AF was higher in HFpEF than in HFrEF (32% versus 23%, respectively; p=0.002), and HFpEF subjects were more likely to exhibit AF at any time compared to HFrEF subjects (62% versus 55%; p=0.02).2 Interestingly, HFpEF and HFrEF were associated with similar risks of incident (future) AF. On the other hand, among individuals with new-onset AF, the rates of pre-existing HFpEF and HFrEF were similar. Among AF subjects who subsequently developed HF, 50% developed HFpEF, 40% developed HFrEF and 10% were unclassified. Interestingly, while AF individuals exhibited higher incidence rates of both HFpEF and HFrEF compared to non-AF individuals, multivariable adjustment for shared risk factors showed prevalent AF to be an independent predictor of incident HFpEF, but not HFrEF.
The association between AF and HF is related to complex and reciprocal pathophysiological mechanisms, the details of which are beyond the scope of this review. Briefly, AF and HF have a propensity to cause each other, in addition to sharing common risk factors that frequently contribute to the clinical picture; both conditions may also perpetuate or worsen
each other over time in a mutually reinforcing fashion.
Shared risk factors include age, hypertension, diabetes, obesity, obstructive sleep apnoea (OSA), alcohol intake, chronic kidney disease (CKD), smoking and coronary artery disease (CAD).1,3,25 The high prevalence of comorbid conditions in AF-HFpEF can be appreciated in a large 2016–2017 real-word database in which, among 56,395 AF-HFpEF patients, 87.0% had hypertension, 41.7% had CAD, 33.8% had diabetes, 29.0% had stage ≥3 CKD, 28.6% had chronic obstructive pulmonary disease (COPD), 25.3% were obese and 15.8% had OSA.26 These risk factors cause alterations to atrial and ventricular function through inflammation, fibrosis, haemodynamic stress and ischaemia, resulting in structural, mechanical and electrophysiological remodelling.27,28 AF may cause or worsen HF through the loss of atrial systole and atrioventricular synchrony, rapid ventricular rates (including tachycardia-mediated cardiomyopathy per se), and irregular ventricular rhythm, leading in turn to reduced cardiac output, increased filling pressures and neurohormonal activation. Conversely, HF may cause AF through atrial mechanical stress and remodelling secondary to increased filling pressures, altered electrophysiological properties of the atrial tissue, abnormal calcium handling and neurohormonal and adrenergic activation.29
The epidemiological data from the Framingham Heart Study described above are compatible with a partially causal relationship between AF and HFpEF (prevalent AF independently predicts incident HFpEF), while shared risk factors appear to be a major driver for the association between prevalent AF and incident HFrEF (the association is lost after multivariable adjustment). Conversely, the similar risk of incident AF in HFpEF and HFrEF individuals could reflect that, when AF does not predate HF, the mechanisms that promote the development of AF in HF are partly shared between HFpEF and HFrEF.
While observational, these data provide a basic rationale for catheter ablation of AF as a disease-modifying intervention in HFpEF.
AF Leads to Adverse Outcomes in HFpEF
AF is an established predictor of mortality in HFrEF, as confirmed by several meta-analyses, and there is compelling evidence to support a similar effect in HFpEF.30–32
In the 1980–2012 Framingham Heart Study analysis, multivariableadjusted analysis found that new-onset AF predicted higher all-cause mortality in both pre-existing HFrEF (HR 2.72; 95% CI [2.12–3.48]; p<0.0001) and HFpEF (HR 1.83; 95% CI [1.41–2.37]; p<0.0001), with a worse prognosis in pre-existing HFrEF compared to HFpEF (p for comparison=0.02).2 Similar results have been reported in several, but not all, HFpEF RCTs and registries and confirmed by meta-analysis.31 For example, in the Swedish Heart Failure Registry (SwedeHF), from 2000 to 2012, 9,595 patients had HFpEF (LVEF ≥50%), including 6,250 (65%) with AF at any point during follow-up and 3,345 (35%) without AF.33 Compared to sinus rhythm (SR)-HFpEF, and after multivariable adjustment, AF-HFpEF was associated with higher rates of all-cause mortality, HF hospitalisation, and stroke or transient ischaemic attack. Also notably, in the Americas component (n=1,765) of the TOPCAT trial, an RCT that randomised 3,445 HFpEF patients (LVEF ≥45%) older than 50 years to receive either spironolactone or placebo, both prevalent AF at enrolment (adjusted HR 1.34; 95% CI [1.09–1.65]) and incident AF post-randomisation (adjusted HR 2.53; 95% CI [1.80–3.55]) were associated with higher all-cause mortality over a mean follow-up of 2.9 years.4 34 Prevalent AF and incident AF were also associated with higher rates of the trial’s primary outcome (a
AF Ablation in HFpEF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
composite of cardiovascular mortality, aborted cardiac arrest and hospitalisation for HF). The cause of excess death in AF-HFpEF was further assessed in another TOPCAT Americas post hoc analysis by Saksena et al., who compared 446 patients with AF on baseline ECG at inclusion with 1,319 patients with sinus rhythm (SR) at inclusion.35 Cardiovascular mortality at 5 years was much higher in AF-HFpEF patients compared to SR-HFpEF (30% versus 18%; p=0.014). Interestingly, pump failure death was more frequent in AF-HFpEF compared to SR-HFpEF (13% versus 5%; p=0.007), while the rate of sudden cardiac death was similar in both groups (10% versus 7%; p=not significant). These results may reflect the clinical impact of the adverse haemodynamic effects of AF, leading to worsening HF in the setting of HFpEF. Consistent with this hypothesis, Lam et al. reported that, compared to SR-HFpEF, AF-HFpEF patients exhibited lower peak oxygen consumption, higher N-terminal brain natriuretic peptide and higher left atrial (LA) volume index.36
Consistent with the findings from the TOPCAT trial, in which excess mortality associated with AF was greater for incident AF than prevalent AF, observational data on the temporal relationship between AF, HF and all-cause mortality have shown that new-onset AF carries a worse prognosis than prevalent AF, including in HFpEF patients.2,30
Considering this likely causal relationship between AF and HFpEF, the vicious circle of mutually reinforcing perpetuation and worsening that occurs when AF and HFpEF co-exist, and the adverse outcomes associated with AF-HFpEF, it can be reasonably hypothesised that efficacious – and preferably early – intervention to restore and maintain SR is likely to improve outcomes. The issue is particularly relevant given the very high prevalence of AF in HFpEF and the absence of established prognosismodifying treatment in HFpEF.
Rhythm Control Versus Rate Control in AF-HFpEF
The current management of AF-HFpEF follows the principles of AF management in the general population and is based on the prevention of thromboembolism (anticoagulation), symptom management (rhythm control versus rate control) and risk factor management. The choice between rhythm control and rate control has been guided by patient symptoms and shared decision making, as RCTs comparing medical rhythm control to medical rate control have shown no prognostic benefit of rhythm control over rate control.37 However, contemporary RCTs that included catheter ablation as a rhythm control intervention showed improved cardiovascular outcomes in selected patients, including HFrEF5 and early AF (≤1 year), suggesting that rhythm control – and in particular by means of catheter ablation – may have prognostic benefit beyond symptom management.38,39 A meta-analysis that pooled data from 11 contemporary RCTs (3,598 patients) confirmed that catheter-based rhythm control resulted in lower all-cause mortality (OR 0.51; p=0.0003) and fewer hospital readmissions (OR 0.44; p=0.003) compared to medical rate control in HF (mostly HFrEF) patients.40 Consistent with historical data, medical rhythm control did not improve outcomes compared to medical rate control, and in fact was associated with higher hospital readmission rate (OR 1.25; p=0.01).
In contrast, there are no RCTs comparing rhythm to rate control in HFpEF. However, while conflicting data exist, observational evidence suggests prognostic benefit of rhythm control over rate control. In an analysis of the Get With The Guidelines-Heart Failure registry, Kelly et al. identified 15,682 patients aged ≥65 years who were discharged from hospital with a diagnosis of AF and HFpEF. At the time of discharge, 1,857 were treated with rhythm control and 13,825 received rate
control.41 Patients receiving rhythm control were younger (median age 81 versus 83 years), but other baseline characteristics were similar. At 1 year, patients receiving rhythm control exhibited lower rates of allcause mortality (30.8% versus 37.5%; p<0.01), all-cause readmissions (62.0% versus 64.6%; p=0.02), ischaemic stroke readmissions (1.56% versus 2.3%; p=0.02) and HF readmissions (26.3% versus 27.7%; p=0.05), compared to those receiving rate control. The association between rhythm control and survival was preserved after multivariable adjustment (adjusted HR 0.86; 95% CI [0.75–0.98]; p=0.02), while other outcomes were not independently associated with treatment strategy. In contrast, in a smaller observational cohort study that included 447 AF-HFpEF subjects, of whom 40 were treated with rhythm control, allcause mortality over an average 4.1-year follow-up was not significantly reduced in the rhythm control group after propensity-score adjustment (adjusted HR 0.70; 95% CI [0.42–1.16]; p=0.16).42
In a retrospective observational multicentre study, Machino-Ohtsuka et al. compared 79 AF-HFpEF patients receiving rhythm control with 79 propensity-score-matched AF-HFpEF patients treated with rate control.43 Rhythm control was associated with a lower rate of a composite endpoint of cardiovascular death or HF hospitalisation (adjusted HR 0.30; 95% CI [0.18–0.98]; p=0.04) over a 24-month median follow-up. However, there were no significant differences in all-cause mortality and cardiovascular mortality between the two groups.
Catheter Ablation of AF in HFpEF
HF has been reported as a risk factor for arrhythmia recurrence after AF ablation.44 45 However, RCTs and subsequent meta-analyses of RCTs have demonstrated the superiority of catheter ablation compared to pharmacological rhythm and rate control in HFrEF for the reduction of allcause mortality and hospital readmission over follow-ups of up to 60 months. 5 6 40 46 47 RCTs have also shown an improvement in LVEF following catheter ablation compared to medical management.5,46
In contrast, there are no dedicated RCTs assessing the safety, efficacy and prognostic benefit of AF ablation in HFpEF. However, substantial data are available from registries, observational studies, and post-hoc analyses of RCTs, from which meaningful insight can be derived for clinical practice and future research directions.
Safety and Efficacy
A growing body of observational data comparing HFpEF to HFrEF and/or to no HF indicates acceptable safety and efficacy profiles of catheter ablation of AF in HFpEF, that seem to mirror findings from HFrEF cohorts.48–57 For example, in a single-centre observational retrospective cohort study, Aldaas et al. analysed 547 patients who underwent de novo catheter ablation of AF, of whom 51 had HFpEF, 40 had HFrEF and 456 had no HF.50 HFpEF patients were more often female compared to HFrEF, exhibited a higher prevalence of hypertension and end-stage renal disease compared to no HF and a higher prevalence of OSA and COPD compared to both HFrEF and no HF. LA diameter, the prevalence of persistent AF and the prevalence of CAD were greater in HFpEF and HFrEF compared to no HF. Periprocedural complication rates did not differ significantly between groups and the rate of atrial arrhythmia recurrence at 5 years was similar. All-cause hospitalisation was more frequent in HF compared to no HF but did not differ significantly between HFpEF and HFrEF. All-cause mortality did not differ between groups. Importantly, these results were observed despite a higher prevalence of comorbid conditions and risk factors for post-ablation AF recurrence in HF participants.
AF Ablation in HFpEF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Similar findings were reported by Yamauchi et al. in another single-centre observational retrospective study that included 502 consecutive patients who underwent non-paroxysmal AF ablation, of whom 293 had HFpEF, 84 had HFrEF and 125 had no HF.49 Compared to no HF and HFrEF, patients with HFpEF were older, more frequently female and had a higher prevalence of arterial hypertension. LA diameter and CHA2DS2-VASc score were higher in HFpEF and HFrEF compared to no HF. Of note, the proportion of patients with long-standing persistent AF was lower in HFpEF and HFrEF compared to no HF in this cohort. All-cause mortality at 1 year did not differ significantly between groups and no deaths occurred in HFpEF. There were no significant differences between groups in the rates of AF recurrence, repeat ablation, presence of SR at 1 year, and HF hospitalisation, with the exception of a higher rate of HF hospitalisation in HFrEF compared to no HF. Of note, the rate of 1-year AF-free survival in HFpEF was 83.6%, and 94.8% were in SR at 1 year.
A recent meta-analysis of six observational studies (three prospective, three retrospective) comparing 1-year outcomes of catheter ablation of AF in HFpEF versus HFrEF included a total of 1,505 patients (51% HFpEF, 49% HFrEF).58 There were no significant differences in the rates of periprocedural complications and hospitalisations. Mortality was lower in HFpEF compared to HFrEF (RR 0.41; 95% CI [0.18–0.94]; p=0.04). The risk of AF recurrence at 1 year did not differ between the two groups.
Prognostic Benefit of Catheter Ablation of AF in HFpEF
Despite the lack of dedicated RCTs, available evidence suggests that catheter ablation of AF in HFpEF individuals is not only safe and effective but also has prognostic benefit in terms of mortality and hospital readmission, as well as other markers of disease severity. Table 1 summarises the relevant studies.
Firstly, uncontrolled before/after data provides evidence that SR restoration by catheter ablation may modify the course of the disease in AF-HFpEF, likely by altering the vicious circle of mutually reinforcing perpetuation of AF and HFpEF. Compared to baseline, catheter ablation was shown to result in lower natriuretic peptides levels, smaller LA diameter and area, lower rest and exercise pulmonary capillary wedge pressure, greater peak cardiac output, and improved New York Heart Association (NYHA) functional capacity at 6–12 months post-ablation.49 59 60 In addition, all-cause hospital admission rate has been reported to decrease by 28.5% (p<0.001) following catheter ablation, as compared to before ablation.57 In one study, assessment at 1 year post-ablation showed resolution of HFpEF (as per European Society of Cardiology diagnostic criteria) in 42.9% of participants after the first ablation procedure and in 51% after multiple procedures.9,59 Consistent with a causal effect, HFpEF resolution was strongly correlated with freedom from arrhythmia recurrence after ablation. In addition, atrial functional mitral regurgitation, a frequent occurrence in AF-HFpEF, has been found to improve significantly following AF ablation, likely as a result of reverse remodelling of the LA and of the mitral valve apparatus.61 Such haemodynamic improvements associated with favourable post-ablation reverse remodelling likely play a role in the clinical benefit observed following catheter ablation in AF-HFpEF.
Secondly, observational comparative data are consistent with a prognostic benefit of catheter ablation over medical therapy in terms of hospital readmission. In a single-centre retrospective comparative study, Fukui et al. analysed 85 consecutive patients who were diagnosed with HFpEF (LVEF ≥50%, clinical HF, and LV diastolic dysfunction) and AF.56 Fifty patients received medical therapy alone, and 35 patients with drug-
refractory AF received catheter ablation. No major complications occurred. Freedom from arrhythmia recurrence did not differ significantly between the ablation group and the pharmacological rhythm control group (n=24). Nevertheless, over a mean follow-up of 792 days, catheter ablation was associated with a substantially lower rate of HF rehospitalisation (9% versus 48%, log-rank p=0.0039), as well as all-cause rehospitalisation (log-rank p=0.0284). Multivariable analysis showed catheter ablation to be the only independent predictor of freedom from HF rehospitalisation (OR 0.15; 95% CI [0.04–0.46]; p<0.001). Importantly, freedom from HF rehospitalisation was associated with SR maintenance (regardless of treatment arm) as compared to patients who exhibited arrythmia recurrence or who were in AF throughout the study (log-rank p=0.0185). Because catheter ablation was performed based on the presence of drug-refractory AF, likely selecting patients with a higher propensity to maintain AF, this finding supports a causal relationship between catheter ablation and freedom from HF rehospitalisation and renders confounding by indication unlikely.
Thirdly, randomised data have provided evidence for a reduction in mortality following catheter ablation compared to medical treatment. In a recently reported post-hoc analysis from the CABANA trial, a multicentre RCT which randomised 2,204 AF patients to catheter ablation or drug therapy, Packer et al. identified 778 patients with HF and functional capacity NYHA class II or greater, of whom 79% had HFpEF, 11.7% HFmrEF and 9.3% HFrEF.62 Subgroup intention-to-treat (ITT) analysis showed that, in HFpEF, catheter ablation resulted in markedly lower all-cause mortality at 4 years compared to drug therapy (3.3% versus 8.6%; HR 0.40; 95% CI [0.18–0.88]). It should be noted that this analysis was performed using imputation of missing baseline LVEF values. Subgroup analysis restricted to complete LVEF data (73% of the sample) showed no significant reduction in all-cause mortality in HFpEF (4.2% versus 8.3%; HR 0.51; 95% CI [0.23–1.12]). Nevertheless, full sample ITT analysis regardless of LVEF subgroup (but with an estimated 79% HFpEF) showed that, compared to drug therapy, catheter ablation resulted in a 44% relative reduction in first AF recurrence (HR 0.56; 95% CI [0.42–0.74]), as well as lower AF burden at all follow-up time points, sustained improvement in quality of life, lower rate of CABANA primary outcome (a composite of all-cause mortality, disabling stroke, serious bleeding or cardiac arrest; HR 0.64; 95% CI [0.41–0.99]) and lower all-cause mortality (6.1% versus 9.3%; HR 0.57; 95% CI [0.33–0.96]).
It should be noted that studies based on real-world data have reported conflicting findings. An analysis based on the Nationwide Readmissions Database from 2016–2017 failed to show prognostic benefit.26 Among 16,848 AF-HFpEF patients, of whom 1,053 underwent catheter ablation and 15,795 propensity-matched (1:15) controls did not, there was no significant difference in 1-year all-cause mortality, HF rehospitalisation and all-cause rehospitalisation between the two groups. However, AF rehospitalisation at 1 year occurred in substantially fewer patients in the ablation group compared to no ablation (4.9% versus 10.5%; HR 0.44; 95% CI [0.33–0.57]; p<0.001), which is compatible with clinically meaningful anti-AF efficacy.
It should also be noted that there are scarce data regarding the effect of AF ablation at different stages of HFpEF. Based on exercise right heart catheterisation, early HFpEF (i.e. exercise-induced only) has been defined as elevated LV filling pressures during exercise with normal LV filling pressures at rest (peak exercise pulmonary capillary wedge pressure ≥25 mmHg with resting pulmonary capillary wedge pressure <15 mmHg), while overt HFpEF – presumably representing more advanced disease –
AF Ablation in HFpEF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Table 1: Summary of Relevant Studies Assessing the Prognostic Benefit of AF Ablation in AF-HFpEF
Rattka et al. 202059
Sugumar et al. 202160
with AF-HFpEF undergoing cryoballoon AF ablation (77% paroxysmal)
with AF-HFpEF undergoing AF ablation (34% paroxysmal)
Yamauchi et al. 202149 Patients with persistent AF and HFpEF undergoing AF ablation
293
of HFpEF ESC criteria at ≥6 months Remission of HFpEF in 45% after single procedure Decrease in resting and peak exercise PCWP
12 months Remission of HFpEF (BNP <100 pg/ml) at 12 months NYHA functional class
Observational controlled data (catheter ablation versus medical therapy)
Fukui et al. 202056 Consecutive patients managed for AF-HFpEF (39% paroxysmal)
Arora et al. 202026 Patients with AF-HFpEF who underwent AF ablation and propensity-matched (1:15) AF-HFpEF patients without catheter ablation
85 (35 ablation, 50 usual medical therapy)
16,848 (1,053 ablation, 15,795 no ablation)
22 ± 16 months
Hospital readmission (all-cause and HF related)
184 days HF hospital readmission, mortality, AF readmission, all-cause readmission, at 1 year
Post-hoc analysis from a randomised controlled trial (catheter ablation versus medical therapy)
Packer et al. 202162 Subgroup of AF-HFpEF patients enrolled in the CABANA83 randomised trial (AF ablation versus drug therapy)
610 (295 ablation, 315 medical therapy)†
48.5 months
All-cause mortality, disabling stroke, serious bleeding, cardiac arrest
Decrease in LA area
Remission of HFpEF in 77% after multiple procedures
Decrease in LA diameter
Improved NYHA functional class
Lower all-cause hospital readmission and lower HF hospital readmission in the ablation group (9% versus 48%)
No significant difference in all-cause and HF hospital readmission
No significant difference in mortality
Lower AF hospital readmission in the ablation group (4.9% versus 10.5%)
A 60% reduction in all-cause mortality in the ablation group (4-year mortality of 3.3% versus 8.6%)
No significant difference in the trial primary composite endpoint
*Study/subgroup population, participant number and main outcomes refer to the subgroup data relevant to catheter ablation of AF in HFpEF and do not necessarily reflect the overall study population of the referenced study. †After imputation for missing left ventricular ejection fraction data. BNP = brain natriuretic peptide; ESC = European Society of Cardiology; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; LA = left atrium; NYHA = New York Heart Association; PCWP = pulmonary capillary wedge pressure.
has been defined as elevated LV filling pressures at rest (resting pulmonary capillary wedge pressure ≥15 mmHg).63 When systematic HFpEF screening is performed among AF patients regardless of symptoms, the majority of AF-HFpEF patients have been found to exhibit early HFpEF (64–74%).60 64 In one study in which 74% of AF-HFpEF patients had early HFpEF, catheter ablation of AF resulted in a significant decrease in peak exercise pulmonary capillary wedge pressure, as well as lower natriuretic peptide levels and greater peak cardiac output.60 The available data therefore suggest prognostic benefit of AF ablation in early HFpEF. However, to our knowledge, there are no reports of ablation outcomes stratified by HFpEF stage, which is an issue that requires further study.
AF Catheter Ablation Strategies with Potential Benefit in HFpEF
Based on indirect evidence, AF ablation strategies that are likely to provide the most benefit in HFpEF should favour AF ablation early in the course of the disease and minimise the extent of LA lesions through individualised substrate modification.
The timing of catheter ablation in AF-HFpEF merits attention, as the prognostic benefit may be greater with early intervention. From a
pathophysiological perspective, given the progressive nature of the AFHFpEF vicious circle with progressively worsening LA/LV haemodynamics, AF burden and LA/LV remodelling, early disease-modifying intervention is likely to provide additional benefit. Moreover, as mentioned above, data from the randomised TOPCAT trial and observational data from large registries show that new-onset AF is associated with a worse prognosis than prevalent AF in HFpEF, emphasising this potential window of opportunity for early intervention.4 2 30 Finally, RCTs have shown additive prognostic benefit from early catheter ablation in a general AF population and in HFrEF.38,65 It is therefore likely that catheter ablation early after AF diagnosis in AF-HFpEF would provide greater prognostic benefit.
Likewise, the timing of catheter ablation with respect to the progression of HF may affect outcomes. The severity of HF, which may be assessed by the NYHA functional classification, is known to influence the efficacy, prognostic benefit, and risk/benefit ratio of several therapeutic interventions in HFrEF.9 NYHA functional class is also correlated with the prevalence of AF in HFrEF.66 Currently available data on catheter ablation in AF-HFpEF provide little insight into the subject and future studies are needed to assess possibly differential outcomes of AF ablation in different NYHA HFpEF subgroups. Furthermore, given the considerable
AF Ablation in HFpEF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Study Study/Subgroup Population* Participants (n)* Follow-up Main Outcomes* Results Uncontrolled before and after data Elkaryoni et al. 202057 Patients with AF-HFpEF undergoing AF ablation (AF type not documented) 506 120 days All-cause hospital admission in the 120 days before versus after AF ablation A 28.5% relative reduction in
hospital
rate
all-cause
admission
(HFpEF subgroup)
Patients
35 29 ± 20 months Remission of HFpEF
criteria
12 months Remission
ESC
at
of HFpEF in 43% after single procedure and 51% after multiple procedures
20 12
months Remission
Patients
± 6
pathophysiological heterogeneity of HFpEF, and heterogeneity in underlying electrophysiological AF substrate, management tailored not only to disease severity but also to specific aetiological subgroups is likely to improve outcomes.67
Regarding the benefit of minimising the extent of LA injury, there is consistent evidence showing that LA lesions from catheter ablation and the resulting scar formation may adversely affect LA reservoir, conduit, booster pump and neurohormonal functions.68 LA electromechanical synchrony may be impaired by iatrogenic conduction disturbances, and LA ejection fraction has been described to decrease proportionally to post-ablation scar volume.69 70 Importantly, LA compliance may be further reduced following catheter ablation, leading in extreme cases to increased filling pressures with pulmonary hypertension, an entity that has been referred to as stiff LA syndrome. In a retrospective study of 499 unselected patients who underwent AF ablation, 8.2% exhibited an increase in right ventricular systolic pressure (RVSP) >10 mmHg on echocardiogram with an RVSP >35 mmHg post-ablation.71 This outcome was associated with echocardiographic features of LV diastolic dysfunction. In contrast, in another study where 1,380 consecutive patients were prospectively assessed before and after catheter ablation for the occurrence of stiff LA syndrome, pulmonary hypertension with LA diastolic dysfunction confirmed by right heart catheterisation or direct LA pressure measurement was found in only 1.4% of patients and was not associated with LV diastolic dysfunction.72 It can be hypothesised that differing mechanisms may lead to increased pulmonary artery pressure following catheter ablation, namely the stiff LA syndrome proper (due to LA diastolic dysfunction) versus the development/unmasking of LV diastolic dysfunction. Both entities are of particular relevance to catheter ablation in AF-HFpEF. While the available data seem to indicate improvements in haemodynamic parameters, including a decrease in filling pressures and resolution of LV diastolic dysfunction following catheter ablation in AF-HFpEF, this issue merits continued assessment in future AF-HFpEF trials.59 60 In the meantime, careful attention should be given to minimise atrial injury when catheter ablation is performed.
Given the interindividual variability in non-pulmonary vein (PV) AF substrate, and suboptimal outcomes of PV isolation alone, a rational strategy to achieve minimal atrial injury while optimising rhythm outcomes should involve individualised non-PV substrate modification. Briefly, nonPV substrate modification strategies have used differing methods in attempts to identify and localise AF substrate, including low-voltage area ablation, complex fractionated atrial electrogram ablation, focal impulse and rotor modulation, dominant frequency mapping, AF ‘nest’ ablation and anatomically guided linear ablations.73–78 In addition to the identification of electroanatomical AF substrate per se, strategies to minimise the extent of atrial injury have used functional ablation endpoints, including AF termination and AF non-inducibility, to guide the extent of substrate modification.45 By analogy with other tachyarrhythmias for which persistent arrhythmia/inducibility at procedure end is associated with poor rhythm outcomes, AF ablation strategies guided by functional endpoints seek to demonstrate efficient elimination of the atrial substrate necessary to sustain AF. Crucially, such strategies may allow more selective substrate modification compared to systematic elimination of all identified ablation targets. Recent work from our group showed that inducibility of sustained AF by burst-pacing immediately after PV isolation (PVI) was associated with a higher rate of AF recurrence at 24 months.79 In a subsequent study based on sequential non-PV substrate modification (fractionated electrogram ablation) guided by stepwise AF inducibility testing, we showed that achievement of AF non-inducibility or termination
during stepwise persistent AF ablation was associated with fewer AF recurrences at 24 months (HR 0.31; 95% CI [0.12–0.84]; p=0.021).80
Likewise, AF recurrence after catheter ablation was associated with progression in AF inducibility at repeat catheter ablation defined as persistently inducible AF at further steps of the redo procedure compared to the index procedure.81 These findings suggest that individualised and selective substrate modification guided by repeated AF inducibility testing may result in favourable rhythm outcomes while minimising the extent of LA injury. Consistent with this inference, our AF inducibility-guided stepwise catheter ablation approach resulted in 68% of paroxysmal AF patients and 29% of persistent AF patients to be treated by PVI alone, with an overall 82% and 76% rate of 24-month AF-free survival, respectively. In the absence of randomised trials, a rational approach to catheter ablation in AF-HFpEF should involve similarly individualised substrate modification, given the adverse effects of LA scarring in HFpEF. 79 80
Interestingly, novel non-thermal ablation technology such as pulsed field ablation may have less impact on LA compliance by triggering different tissue repair mechanisms. Nakatani et al. recently showed that, compared to thermal ablation (radiofrequency or cryoablation), pulsed field ablation was associated with similar decreases in LA reservoir and booster pump functions and larger late gadolinium enhancement volume at cardiac magnetic resonance in the acute phase (<3 hours after ablation), while recovery of LA mechanical function and disappearance of the majority of late gadolinium enhancement in the chronic stage (3 months after ablation) was observed.82 Of note, both alterations persisted in the chronic stage following thermal ablation. These findings suggest that tissue repair following pulsed field ablation involves less chronic fibrosis compared to thermal ablation, therefore resulting in better preservation of LA mechanical function.
Limitations
In the absence of dedicated RCTs, the prognostic benefit of AF ablation in HFpEF cannot be inferred with certainty. Data from observational studies, registries and post-hoc analyses should be interpreted with the usual caution. In particular, given the overlap between AF haemodynamic features and HFpEF diagnostic criteria, there is a risk of HFpEF overdiagnosis in AF patients, which may lead to biased data. RCTs comparing the outcomes of catheter ablation with medical management in HFpEF are therefore needed.36
Conclusion
Figure 1 summarises the pathophysiology of AF-HFpEF and the current evidence indicating a prognostic benefit of catheter ablation of AF in HFpEF.
The majority of HFpEF patients develop AF during the course of the disease and this occurrence is associated with adverse outcomes. Rhythm control may represent a disease-modifying treatment opportunity in AF-HFpEF, and observational data are indicative of prognostic benefit compared to rate control. While there are no RCTs comparing catheter ablation to medical management of AF in HFpEF, catheter ablation has been widely reported to have an acceptable safety and efficacy profile in HFpEF patients. In addition, data from observational studies, large registries and post-hoc analysis from RCTs suggest that catheter ablation reduces HFpEF severity, hospitalisation rates and all-cause mortality. Prospective RCTs are needed to confirm these results. Stratification by HFpEF aetiology, severity and timing of AF ablation is likely to provide insight towards the tailored management of the heterogeneous HFpEF population. Based on indirect evidence, it seems reasonable to currently
AF Ablation in HFpEF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 1: AF-HFpEF Pathophysiology and Current Evidence Indicating Prognostic Benefit of Catheter Ablation
favour early catheter ablation and to minimise the extent of atrial injury during ablation.
Clinical Perspective
• Up to 65% of heart failure with preserved ejection fraction patients develop AF during the course of the disease, and the occurrence of AF is predictive of worse outcomes, including pump failure death.
• Catheter ablation of AF in heart failure with preserved ejection fraction has an acceptable safety and efficacy profile that seems to mirror findings from HFrEF cohorts.
• Growing evidence suggests that catheter ablation of AF may lead to a decrease in heart failure with preserved ejection fraction severity, lower hospitalisation rate and lower all-cause mortality, but randomised trials are needed.
• Early catheter ablation may confer additive benefit, given the role of AF in disease progression.
*From uncontrolled before and after data. †From observational data. ‡From post-hoc analysis of a multicenter randomised control trial.62 HFpEF = heart failure with preserved ejection fraction; LA = left atrium; NT-proBNP = N-terminal brain natriuretic peptide; NYHA = New York Heart Association; peak VO2 = peak oxygen consumption.
1. Ling LH, Kistler PM, Kalman JM, et al. Comorbidity of atrial fibrillation and heart failure. Nat Rev Cardiol 2016;13:131–47. https://doi.org/10.1038/nrcardio.2015.191; PMID: 26658575
2. Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved Versus reduced ejection fraction. Circulation 2016;133:484–92. https://doi. org/10.1161/CIRCULATIONAHA.115.018614; PMID: 26746177
3. Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–33. https://doi.org/10.1016/j. jacc.2013.11.053; PMID: 24491689
4. Cikes M, Claggett B, Shah AM, et al. Atrial fibrillation in heart failure with preserved ejection fraction: the TOPCAT trial. JACC Heart Fail 2018;6:689–97. https://doi.org/10.1016/j. jchf.2018.05.005; PMID: 30007557
5. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. https://doi.org/10.1056/NEJMoa1707855; PMID: 29385358
6. Di Biase L, Mohanty P, Mohanty S, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC Multicenter Randomized Trial. Circulation 2016;133:1637–44. https://doi.org/10.1161/ CIRCULATIONAHA.115.019406; PMID: 27029350
7. van Riet EES, Hoes AW, Wagenaar KP, et al. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 2016;18:242–52. https://doi.org/10.1002/ ejhf.483; PMID: 26727047
8. Pieske B, Tschöpe C, Boer RA de, et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:391–412. https://doi.org/10.1002/ejhf.1741; PMID: 32133741
9. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. https://doi.org/10.1002/ ejhf.592; PMID: 27207191
10. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–5. https://doi.org/10.1001/jama.285.18.2370; PMID: 11343485
• The extent of atrial injury during ablation should be minimised to avoid further reduction in left atrial compliance, which might worsen heart failure with preserved ejection fraction through increased filling pressures.
11. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. https://doi. org/10.1161/CIRCULATIONAHA.113.005119; PMID: 24345399
12. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation 2021;143:e254–743. https://doi. org/10.1161/CIR.0000000000000950; PMID: 33501848
13. Mosterd A, Hoes AW, de Bruyne MC, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; the Rotterdam Study. Eur Heart J 1999;20:447–55. https://doi.org/10.1053/euhj.1998.1239; PMID: 10213348
14. Redfield MM, Jacobsen SJ, Burnett JC, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202. https://doi.org/10.1001/ jama.289.2.194; PMID: 12517230
15. Bleumink GS, Knetsch AM, Sturkenboom MCJM, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure the Rotterdam Study. Eur Heart J 2004;25:1614–9. https://doi. org/10.1016/j.ehj.2004.06.038; PMID: 15351160
16. Staerk L, Wang B, Preis SR, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ 2018;361:k1453. https://doi.org/10.1136/bmj.k1453; PMID: 29699974
17. Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042–6. https://doi.org/10.1161/01. CIR.0000140263.20897.42; PMID: 15313941
18. Magnussen C, Niiranen TJ, Ojeda FM, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE consortium (biomarker for cardiovascular risk assessment in Europe). Circulation 2017;136:1588–97. https:// doi.org/10.1161/CIRCULATIONAHA.117.028981; PMID: 29038167
19. Gerber Y, Weston SA, Redfield MM, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000–2010. JAMA Intern Med 2015;175:996–1004. https://doi.org/10.1001/jamainternmed.2015.0924; PMID: 25895156
20. Vasan RS, Zuo Y, Kalesan B. Divergent temporal trends in morbidity and mortality related to heart failure and atrial fibrillation: age, sex, race, and geographic differences in the United States, 1991–2015. J Am Heart Assoc 2019;8:e010756. https://doi.org/10.1161/JAHA.118.010756; PMID: 30955391
21. Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep 2014;11:404–15. https://doi.org/10.1007/s11897-014-0220-x; PMID: 25182014
22. Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet 2018;391:572–80. https://doi. org/10.1016/S0140-6736(17)32520-5; PMID: 29174292
23. Tsao CW, Lyass A, Enserro D, et al. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail 2018;6:678–85. https://doi.org/10.1016/j.jchf.2018.03.006; PMID: 30007560
24. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5. https://doi.org/10.1161/01. CIR.0000072767.89944.6E; PMID: 12771006
25. Ariyaratnam JP, Lau DH, Sanders P, Kalman JM. Atrial fibrillation and heart failure: epidemiology, pathophysiology, prognosis, and management. Card Electrophysiol Clin 2021;13:47–62. https://doi.org/10.1016/j.ccep.2020.11.004; PMID: 33516407
26. Arora S, Jaswaney R, Jani C, et al. Catheter ablation for atrial fibrillation in patients with concurrent heart failure. Am J Cardiol 2020;137:45–54. https://doi.org/10.1016/j. amjcard.2020.09.035; PMID: 33002464
27. Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol 2014;63:2335–45. https://doi.org/10.1016/j. jacc.2014.02.555; PMID: 24613319
28. Normand C, Kaye DM, Povsic TJ, Dickstein K. Beyond pharmacological treatment: an insight into therapies that target specific aspects of heart failure pathophysiology. Lancet 2019;393:1045–55. https://doi.org/10.1016/S01406736(18)32216-5; PMID: 30860030
29. Prabhu S, Voskoboinik A, Kaye DM, Kistler PM. Atrial fibrillation and heart failure — cause or effect? Heart Lung Circ 2017;26:967–74. https://doi.org/10.1016/j.hlc.2017.05.117; PMID: 28684095
30. Odutayo A, Wong CX, Williams R, et al. Prognostic importance of atrial fibrillation timing and pattern in adults with congestive heart failure: a systematic review and metaanalysis. J Card Fail 2017;23:56–62. https://doi.org/10.1016/j. cardfail.2016.08.005; PMID: 27565044
31. Cheng M, Lu X, Huang J, et al. The prognostic significance of atrial fibrillation in heart failure with a preserved and reduced left ventricular function: insights from a metaanalysis. Eur J Heart Fail 2014;16:1317–22. https://doi. org/10.1002/ejhf.187; PMID: 25371247
32. Mamas MA, Caldwell JC, Chacko S, et al. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail 2009;11:676–83. https://doi. org/10.1093/eurjhf/hfp085; PMID: 19553398
33. Sartipy U, Dahlström U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid-range, and reduced
AF Ablation in HFpEF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Shared pathophysiology of AF and HFpEF: Catheter ablation of AF in HFpEF: A B C 1 Shared risk factors A AF causes HF B HF causes AF C 2 AF HFpEF Reciprocal perpetuation AF-HFpEF ↓ Peak VO2 ↑ NT-proBNP ↑ LA dilation ↑ Hospitalisation ↑ Mortality 3 Resolves HFpEF*/prevents reinforcement 1 Improves haemodynamics, NT-proBNP and NYHA functional class* 2 Reduces hospitalisations,† all-cause mortality‡ 3
ejection fraction. JACC Heart Fail 2017;5:565–74. https://doi. org/10.1016/j.jchf.2017.05.001; PMID: 28711451
34. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383–92. https://doi.org/10.1056/NEJMoa1313731; PMID: 24716680
35. Saksena S, Slee A, Lakkireddy D, et al. Abstract 15198: Predictors of adverse cardiovascular outcomes in patients with atrial fibrillation and heart failure With preserved systolic function: A Topcat Americas post hoc analysis. Circulation 2020;142(Suppl 3):A15198. https://doi.org/10.1161/ circ.142.suppl_3.15198
36. Lam CSP, Rienstra M, Tay WT, et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail 2017;5:92–8. https://doi.org/10.1016/j.jchf.2016.10.005; PMID: 28017355
37. Caldeira D, David C, Sampaio C. Rate versus rhythm control in atrial fibrillation and clinical outcomes: updated systematic review and meta-analysis of randomized controlled trials. Arch Cardiovasc Dis 2012;105:226–38. https://doi.org/10.1016/j.acvd.2011.11.005; PMID: 22633297
38. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. https://doi.org/10.1056/NEJMoa2019422; PMID: 32865375
39. Eysenck W, Saba M. Rhythm control in heart failure patients with atrial fibrillation. Arrhythm Electrophysiol Rev 2020;9:161–6. https://doi.org/10.15420/aer.2020.23; PMID: 33240512
40. Chen S, Pürerfellner H, Meyer C, et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J 2020;41:2863–73. https://doi.org/10.1093/eurheartj/ehz443; PMID: 31298266
41. Kelly JP, DeVore AD, Wu J, et al. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: insights from get with the guidelines—heart failure. J Am Heart Assoc 2019;8:e011560. https://doi.org/10.1161/JAHA.118.011560; PMID: 31818219
42. Zhang P, Chamberlain AM, Hodge DO, et al. Outcomes of incident atrial fibrillation in heart failure with preserved or reduced ejection fraction: a community-based study. J Cardiovasc Electrophysiol 2020;31:2275–83. https://doi. org/10.1111/jce.14632; PMID: 32584498
43. Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Relationships between maintenance of sinus rhythm and clinical outcomes in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Cardiol 2019;74:235–44. https://doi.org/10.1016/j.jjcc.2019.02.014; PMID: 30910388
44. Rostock T, Salukhe TV, Steven D, et al. Long-term singleand multiple-procedure outcome and predictors of success after catheter ablation for persistent atrial fibrillation. Heart Rhythm 2011;8:1391–7. https://doi.org/10.1016/j. hrthm.2011.04.012; PMID: 21699825
45. Johner N, Namdar M, Shah DC. Individualised approaches for catheter ablation of AF: patient selection and procedural endpoints. Arrhythm Electrophysiol Rev 2019;8:184–90. https:// doi.org/10.15420/aer.2019.33.2; PMID: 31463056
46. Turagam MK, Garg J, Whang W, et al. Catheter ablation of atrial fibrillation in patients with heart failure: a metaanalysis of randomized controlled trials. Ann Intern Med 2019;170:41–50. https://doi.org/10.7326/M18-0992; PMID: 30583296
47. Virk SA, Bennett RG, Chow C, et al. Catheter ablation versus medical therapy for atrial fibrillation in patients with heart failure: a meta-analysis of randomised controlled trials. Heart Lung Circ 2019;28:707–18. https://doi.org/10.1016/j. hlc.2018.10.022; PMID: 30509786
48. Black-Maier E, Ren X, Steinberg BA, et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm 2018;15:651–7. https://doi.org/10.1016/j.hrthm.2017.12.001; PMID: 29222043
49. Yamauchi R, Morishima I, Okumura K, et al. Catheter ablation for non-paroxysmal atrial fibrillation accompanied by heart failure with preserved ejection fraction: feasibility and benefits in functions and B-type natriuretic peptide. Europace 2021;23:1252–61. https://doi.org/10.1093/europace/ euaa420; PMID: 33693617
50. Aldaas OM, Malladi CL, Mylavarapu PS, et al. Comparison of outcomes after ablation of atrial fibrillation in patients with heart failure with preserved versus reduced ejection fraction. Am J Cardiol 2020;136:62–70. https://doi. org/10.1016/j.amjcard.2020.09.018; PMID: 32941815
51. Cha YM, Wokhlu A, Asirvatham SJ, et al. Success of ablation for atrial fibrillation in isolated left ventricular diastolic dysfunction: a comparison to systolic dysfunction and normal ventricular function. Circ Arrhythm Electrophysiol 2011;4:724–32. https://doi.org/10.1161/CIRCEP.110.960690; PMID: 21747059
52. Ichijo S, Miyazaki S, Kusa S, et al. Impact of catheter ablation of atrial fibrillation on long-term clinical outcomes in patients with heart failure. J Cardiol 2018;72:240–6. https://doi.org/10.1016/j.jjcc.2018.02.012; PMID: 29609877
53. Vecchio N, Ripa L, Orosco A, et al. Atrial fibrillation in heart failure patients with preserved or reduced ejection fraction. Prognostic significance of rhythm control strategy with catheter ablation. J Atr Fibrillation 2019;11:2128. https://doi. org/10.4022/jafib.2128; PMID: 31139301
54. Eitel C, Ince H, Brachmann J, et al. Atrial fibrillation ablation strategies and outcome in patients with heart failure: insights from the German ablation registry. Clin Res Cardiol 2019;108:815–23. https://doi.org/10.1007/s00392-019-014113; PMID: 30788620
55. Jayanna MB, Mohsen A, Inampudi C, et al. Procedural outcomes of patients with heart failure undergoing catheter ablation of atrial fibrillation. Am J Ther 2019;26:e333–8. https://doi.org/10.1097/MJT.0000000000000931; PMID: 30893071
56. Fukui A, Tanino T, Yamaguchi T, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol 2020;31:682–8. https://doi. org/10.1111/jce.14369; PMID: 31985099
57. Elkaryoni A, Al Badarin F, Spertus JA, et al. Comparison of the effect of catheter ablation for atrial fibrillation on allcause hospitalization in patients with versus without heart failure (from the Nationwide Readmission Database). Am J Cardiol 2020;125:392–8. https://doi.org/10.1016/j. amjcard.2019.10.048; PMID: 31780075
58. Aldaas OM, Lupercio F, Darden D, et al. Meta-analysis of the usefulness of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. Am J Cardiol 2021;142:66–73. https://doi.org/10.1016/j. amjcard.2020.11.039; PMID: 33290688
59. Rattka M, Pott A, Kühberger A, et al. Restoration of sinus rhythm by pulmonary vein isolation improves heart failure with preserved ejection fraction in atrial fibrillation patients. Europace 2020;22:1328–36. https://doi.org/10.1093/ europace/euaa101; PMID: 32449907
60. Sugumar H, Nanayakkara S, Vizi D, et al. A prospective STudy using invAsive haemodynamic measurements foLLowing catheter ablation for AF and early HFpEF: STALL AF-HFpEF. Eur J Heart Fail 2021;23:785–96. https://doi. org/10.1002/ejhf.2122; PMID: 33565197
61. Nishino S, Watanabe N, Ashikaga K, et al. Reverse remodeling of the mitral valve complex after radiofrequency catheter ablation for atrial fibrillation: a serial 3-dimensional echocardiographic study. Circ Cardiovasc Imaging 2019;12:e009317. https://doi.org/10.1161/ CIRCIMAGING.119.009317; PMID: 31594407
62. Packer DL, Piccini JP, Monahan KH, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation 2021;143:1377–90. https:// doi.org/10.1161/CIRCULATIONAHA.120.050991; PMID: 33554614
63. Borlaug BA, Nishimura RA, Sorajja P, et al. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–95. https://doi.org/10.1161/CIRCHEARTFAILURE.109.930701; PMID: 20543134
64. Meluzin J, Starek Z, Kulik T, et al. Prevalence and predictors of early heart failure with preserved ejection fraction in patients with paroxysmal atrial fibrillation. J Card Fail 2017;23:558–62. https://doi.org/10.1016/j.cardfail.2017 .04.002; PMID: 28408305
65. Anselmino M, Matta M, D’Ascenzo F, et al. Catheter ablation of atrial fibrillation in patients with left ventricular systolic dysfunction: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol 2014;7:1011–8. https://doi.org/10.1161/ CIRCEP.114.001938; PMID: 25262686
66. Dries DL, Exner DV, Gersh BJ, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: a retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J Am Coll Cardiol 1998;32:695–703. https://doi.org/10.1016/s0735-1097(98)00297-6; PMID: 9741514
67. Mukherjee RK, Williams SE, O’Neill MD, O’Neill MD. Atrial fibrillation ablation in patients with heart failure: one size does not fit all. Arrhythm Electrophysiol Rev 2018;7:84–90. https://doi.org/10.15420/aer.2018.11.3
68. Packer M. Effect of catheter ablation on pre-existing abnormalities of left atrial systolic, diastolic, and neurohormonal functions in patients with chronic heart failure and atrial fibrillation. Eur Heart J 2019;40:1873–9. https://doi.org/10.1093/eurheartj/ehz284; PMID: 31081029
69. Johner N, Namdar M, Shah DC. Intra- and interatrial conduction abnormalities: hemodynamic and arrhythmic significance. J Interv Card Electrophysiol 2018;52:293–302. https://doi.org/10.1007/s10840-018-0413-4; PMID: 30128800
70. Wylie JV, Peters DC, Essebag V, et al. Left atrial function and scar after catheter ablation of atrial fibrillation. Heart Rhythm 2008;5:656–62. https://doi.org/10.1016/j. hrthm.2008.02.008; PMID: 18452866
71. Witt CM, Fenstad ER, Cha YM, et al. Increase in pulmonary arterial pressure after atrial fibrillation ablation: incidence and associated findings. J Interv Card Electrophysiol 2014;40:47–52. https://doi.org/10.1007/s10840-014-9875-1; PMID: 24532114
72. Gibson DN, Di Biase L, Mohanty P, et al. Stiff left atrial syndrome after catheter ablation for atrial fibrillation: clinical characterization, prevalence, and predictors. Heart Rhythm 2011;8:1364–71. https://doi.org/10.1016/j.hrthm.2011.02.026; PMID: 21354332
73. Rolf S, Kircher S, Arya A, et al. Tailored atrial substrate modification based on low-voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2014;7:825–33. https://doi.org/10.1161/CIRCEP.113.001251; PMID: 25151631
74. Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol 2004;43:2044–53. https://doi.org/10.1016/j.jacc.2003.12.054; PMID: 15172410
75. Verma A, Jiang CY, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. https://doi.org/10.1056/NEJMoa1408288; PMID: 25946280
76. Narayan SM, Krummen DE, Shivkumar K, et al. Treatment of atrial fibrillation by the ablation of localized sources: CONFIRM (conventional ablation for atrial fibrillation with or without focal impulse and rotor modulation) trial. J Am Coll Cardiol 2012;60:628–36. https://doi.org/10.1016/j. jacc.2012.05.022; PMID: 22818076
77. Atienza F, Almendral J, Jalife J, et al. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm 2009;6:33–40. https://doi.org/10.1016/j. hrthm.2008.10.024; PMID: 19121797
78. Pachon M JC, Pachon M EI, Pachon M JC, et al. A new treatment for atrial fibrillation based on spectral analysis to guide the catheter RF-ablation. Europace 2004;6:590–601. https://doi.org/10.1016/j.eupc.2004.08.005; PMID: 15519263
79. Johner N, Namdar M, Shah DC. Sustained and selfterminating atrial fibrillation induced immediately after pulmonary vein isolation exhibit differences in coronary sinus electrical activity from onset. J Cardiovasc Electrophysiol 2020;31:150–9. https://doi.org/10.1111/jce.14296; PMID: 31778260
80. Johner N, Namdar M, Shah DC. Right atrial complexity evolves with stepwise left-sided persistent atrial fibrillation substrate ablation and predicts outcomes. JACC Clin Electrophysiol 2020;6:1619–30. https://doi.org/10.1016/j. jacep.2020.06.021; PMID: 33334439
81. Johner N, Shah DC, Giannakopoulos G, et al. Evolution of post-pulmonary vein isolation atrial fibrillation inducibility at redo ablation: electrophysiological evidence of extrapulmonary vein substrate progression. Heart Rhythm 2019;16:1160–6. https://doi.org/10.1016/j.hrthm.2019.02.026;
PMID: 30818093
82. Nakatani Y, Sridi-Cheniti S, Cheniti G, et al. Pulsed field ablation prevents chronic atrial fibrotic changes and restrictive mechanics after catheter ablation for atrial fibrillation. Europace 2021;23:1767–76. https://doi. org/10.1093/europace/euab155; PMID: 34240134
83. Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. https://doi.org/10.1001/jama.2019.0693;
PMID: 30874766
AF Ablation in HFpEF ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
UK Expert Consensus Statement for the Optimal Use and Clinical Utility of Leadless Pacing Systems on Behalf of the British Heart Rhythm Society
Paul R Roberts,1 Mohamed ElRefai,1 Paul Foley,2 Archana Rao,3 David Sharman,4 Riyaz Somani,5 Simon Sporton,6 Gary Wright,7 Amir Zaidi,8 Chris Pepper9
1. Wessex Cardiothoracic Centre, University Hospital Southampton NHS Trust, Southampton, UK; 2. Department of Cardiology, Great Western Hospital NHS Foundation Trust, Swindon, UK; 3. Department of Cardiology, Liverpool Heart and Chest Hospital NHS Foundation Trust, Liverpool, UK; 4. Department of Cardiology, Northampton General Hospital NHS Trust, Northampton, UK; 5. Department of Cardiology, Glenfield Hospital, University Hospitals of Leicester NHS Trust, Leicester, UK; 6. Department of Cardiology, Barts Health NHS Trust, London, UK; 7. Department of Cardiology, NHS Golden Jubilee, Glasgow, UK; 8. Department of Cardiology, Manchester University NHS Trust, Manchester, UK; 9. Department of Cardiology, Leeds Teaching Hospitals NHS Trust, Leeds, UK
Abstract
Pacemakers are a key technology in the treatment of bradyarrhythmias. Leadless pacemakers (LP) were introduced to address limitations of transvenous devices. However, guidelines and other restrictions have led to LPs becoming niche products. The aim of this consensus statement was to determine the strength of opinion of UK implantation experts as to how LPs can be more optimally used. Using a modified Delphi approach, a panel of LP experts developed 36 statements that were used to form a survey that was distributed to LP implanters in the UK. Stopping criteria included a 3-month window for response, a minimum 25% response rate and at least 75% of statements achieving the threshold for consensus (agreed at 66%). In all, 31 of 36 statements reached consensus, and 23 of these achieved ≥90% agreement. Five statements did not achieve consensus. On the basis of these results, seven recommendations were proposed. The implementation of these recommendations may increase the use of LPs, with the aim of improving patient outcomes.
Keywords
Leadless pacing, cardiac pacing, consensus, expert opinion
Disclosure: PRR has received honoraria from Medtronic, Boston Scientific and EBR Systems. ME has received research funding from Boston Scientific. AR has received honoraria from Medtronic, Boston Scientific and Phillips. SS has received honoraria from Medtronic, Boston Scientific and Biotronik. PF, DS, RS, GW, AZ, CP have received honoraria from Medtronic.
Funding: This study was managed and facilitated on behalf of the authors by Triducive Partners Limited, who received funding from Medtronic. The authors did not receive any funding from Medtronic for their involvement in this study.
Acknowledgements: The authors thank Tim Warren and Thomas Scoble of Triducive Partners Limited for their support in facilitating the project, analysing the data, contributing to writing the manuscript and reviewing the final draft.
Received: 24 June 2022
Accepted: 8 July 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e19. DOI: https://doi.org/10.15420/aer.2022.17
Correspondence: Paul Roberts, University Hospital Southampton NHS Foundation Trust, Tremona Road, Southampton SO16 6DY, UK. E: Paul.Roberts@uhs.nhs.uk
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
The implantation of permanent transvenous pacemakers has long been established as the first line treatment for patients with bradyarrhythmias. Continuous device improvements and an ageing population have led to a corresponding increase in implantations, with approximately 1,000 units per million people implanted annually in Europe.1
However, transvenous pacing still has several limitations, leading to significant complications in 9–12% of patients.2,3 Complications may be acute (<30 days after implantation) and can include bleeding/haematoma, pneumothorax, pericardial effusion/perforation, infection and lead displacement. Chronic complications include lead fractures and infections, with rates particularly high at the time of generator change.
The development of leadless pacemakers was intended to address some of the limitations seen with transvenous pacemakers. The first leadless pacemaker was implanted in 2012. In all, 1,423 Nanostim devices
(Nanostim Inc./St Jude Medical/Abbott Medical) were implanted before the device was withdrawn due to several cases of premature battery depletion.4
The first Micra transcatheter pacing system was implanted in 2013 (Micra transcatheter pacing system; Medtronic) and, to date, almost 150,000 devices have been implanted worldwide. The safety and efficacy of this device have been studied extensively. During trials, the utility of this device was demonstrated, with a 99% successful implantation rate (719 patients of 725 recruited) and a 96% primary safety end point (patients should be free of system- or procedure-related major complications).5 Registry data following the investigational device exemption study continue to demonstrate 99% procedural success rates and low complication rates (2.7% at 12 months).6 The second-generation Micra transcatheter pacing system uses the signal generated by the device’s accelerometer to sense atrial activity and then sequentially pace the right ventricle, providing a
EXPERT OPINION © 2022 The Author(s). Published by Radcliffe Group Ltd. www.AERjournal.com
Implantable Devices
VDD pacing mode. Initial studies demonstrated a mean atrioventricular rate of synchrony of 87%.7
A key advantage of using leadless pacemakers over transvenous devices is the marked reduction in pacemaker-related infection. Pacemaker-related infections occur in 7–12% of cases of transvenous pacemakers, and the risk triples in replacement procedures.3,8 During clinical trials of leadless pacemakers, there was an absence of pacemaker-related infections, even in bacteraemia settings.3,2 It is likely that this is the result of encapsulation of the device within the right ventricle and the absence of leads in the vasculature and generator on the chest wall.
Although there is currently no head-to-head randomised controlled trial for leadless devices again transvenous pacemakers, the currently available evidence base suggests that leadless pacemakers have favourable complication rates, with a 63% lower rate of complications than transvenous devices.6 As the number of devices implanted increases, the literature identifies certain patient populations where leadless pacing is considered advantageous. This includes patients with prior cardiac device infection, patients on haemodialysis and patients in whom there is an expectation of low levels of pacing in a young population (e.g. cardioinhibitory vasovagal syncope).8–11
Despite these advantages, current guidance within the UK limits the use of leadless devices only for the purposes of research or when conventional pacemakers are contraindicated.12 Although the 2021 guidelines from the European Society of Cardiology (ESC) state that leadless devices can be used when the risk of infection is high, incorporating shared decisionmaking and taking into account life expectancy considerations, leadless pacing remains a relatively niche procedure.13
Recent efforts have been made by groups of Austrian and Polish healthcare professionals (HCPs) to identify the indications and contraindications for the wider use of leadless pacemakers, developing a set of criteria through which this could be achieved within their healthcare settings.3,14 Given the state of leadless pacemaker implantation and the positions taken by the Austrian and Polish researchers, the intent of this study was to determine how leadless pacing could be more optimally used within the UK NHS.
A comprehensive literature review on leadless pacemakers was compiled and presented to a panel of experts in leadless pacing device implantation from across the UK. The panel convened in January 2022 to discuss current challenges around the optimal clinical use of leadless pacing. Using a modified Delphi methodology guided by an independent facilitator, the panellists identified five main topics of focus:
• problems that are experienced with transvenous pacing and need to be appreciated/acknowledged;
• the relative risk of leadless systems;
• patient types suitable for leadless pacemakers who may be at risk from transvenous devices;
• the role of a national register; and
• logistical requirements for the safe delivery of leadless pacemakers in the UK.
These topics were discussed further, with 36 statements developed and used to create an online questionnaire using Microsoft Forms. The questionnaire was distributed to 72 leadless implanters identified as
working within the UK by PRR. Stopping criteria were agreed as a 3-month time period to collect responses (February–April 2022), a minimum 25% response rate, and at least 75% of statements achieving the agreement threshold for consensus. These criteria were set to allow for the greatest number of HCPs to respond given the pressures currently being experienced by the health service in relation to the COVID-19 pandemic. Given the speciality of the field, the threshold for consensus agreement was set at 66%. Consensus agreement was further defined as ‘high’ at ≥66% and ‘very high’ at ≥90%.
Respondents used a 4-point Likert scale (strongly disagree, tend to disagree, tend to agree and strongly agree) to indicate their corresponding level of agreement with each statement. The questionnaire also captured some demographic data for further analysis, including years of experience in implanting cardiac pacing devices, years of experience in implanting leadless devices and the number of leadless devices implanted per year.
Completed anonymised surveys were collated and analysed by an independent facilitator to produce an arithmetic agreement score for each statement. This information was then reviewed by the panel of experts to determine what recommendations could be made based on the responses received.
Because this study only sought the anonymous opinions of healthcare professionals, ethics approval was not sought. However, a statement of consent was provided at the start of the survey, and all completing participants provided consent in line with this statement.
Outcome of the Delphi Process
Of the 72 implanters identified, four could not be contacted for inclusion in the study; thus, 68 invitations sent out. Of these, 27 responses were received (40% response rate) and analysed.
From the first round of consensus, 23 of 36 statements attained very high (≥90%) agreement, eight attained high (<90% and ≥66%) agreement and five did not reach the threshold for consensus (<66%; Figure 1; Table 1). Given the high level of agreement attained for the statements and that the stopping criteria had been met, it was decided not to undertake a second round of testing.
The results demonstrate a strong degree of support for most statements, with more experienced clinicians showing a lower degree of support overall than more junior colleagues (Supplementary Figure 1). However, this association was less clear when examining the experience of respondents with implanting leadless devices (Supplementary Figure 2).
Discussion
Perception of the Safety of Leadless Pacemakers
It is clear from the level of agreement with Statements 6 and 7 (Table 1; 56% and 44%, respectively) that respondents are unclear as to the perceptions of the wider healthcare community around the safety of leadless pacemakers.
During discussion of the results, the panellists agreed that it is a challenge to know what other HCPs, especially those who refer patients on for pacemaker implantation, think about the safety and use of a leadless device over a more traditional transvenous pacemaker. It was also noted that, to date, patients offered leadless devices are those who are at greater risk of a complication to begin with, which therefore may inversely affect the perception of the safety of the device.
UK Expert Consensus Statement for the Clinical Utility of Leadless Pacing ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
The panellists suggested that this is an area where improvements could be made by expanding the education around leadless pacemakers so that clinicians and referring colleagues are more aware of the advantages of the systems and how they can be used to improve patient outcomes.
Which Patients Benefit Most From a Leadless Pacemaker
Part of the intent of this study was to define suitable patient types who would benefit from leadless pacemaker implantation. This would build on the findings of previous studies to help establish the position of UK implanters. Based on the agreement from Statements 15–19 and 21–26 (Table 1), the panellists offered patient criteria for considering leadless pacemaker implantation, as presented in Table 2
It is possible that the sub-threshold agreement level for Statement 19 (48%) indicates that the responders considered that single-chamber transvenous pacemakers were entirely reasonable in an uncomplicated population with AF and bradycardia. AF with bradycardia is supported as a basic criterion for leadless pacemaker implantation in both the ESC 2021 guidelines and within the study examining the position of Austrian HCPs conducted by Steinwender et al.3,13
Most of the recommended patient populations relate specifically to complications associated with transvenous systems that are mitigated by a leadless pacemaker. Infection has been recognised as a very remote complication of leadless pacemakers, with no devices having to be removed as a consequence of infection in either the investigational device exemption study or the postapproval registry.1,6 Consequently, this device is attractive for patients who are at a high risk of infection, including those on haemodialysis, those with a previous cardiac device infection, those who are immunocompromised, those undergoing steroid therapy or receiving biological drugs and those with indwelling vascular catheters. Other recommendations are largely justified by the anatomical advantage of not having leads in blood vessels or a generator (i.e. patients undergoing thoracic radiotherapy, younger patients and patients with congenital heart disease who may be younger and not have appropriate venous access for transvenous pacing).
Further to this list, the cost of the device should be taken into consideration because there is variation across the UK. Therefore, the panellists recommended that leadless devices should be used in a targeted approach that takes into account patient experience and quality of life factors.
National Register Needs
The strength of the response to Statements 27–29 suggests that implanters recognise the need for a specific register to capture information around the use of leadless devices, including their risks and complication rates. The panellists suggested that these data should be input by implanters to ensure accuracy. Furthermore, the panellists agreed that the National Institute for Cardiovascular Outcomes Research (NICOR) database is not currently able to manage the information needs of leadless pacemakers, but that it could be expanded to provide the appropriate fields. However, it is beyond the scope of the present study to provide recommendations as to how this should be achieved.
Logistical Requirements for Delivering Leadless Systems
There was consensus that ultrasound should be used when implanting leadless pacemakers. It has been demonstrated that complication rates
for femoral access for electrophysiology procedures are lower if ultrasound is used.15 In that meta-analysis of 7,858 patients, the incidence of vascular complications in the ultrasound group was 1.2%, compared with 3.2% in the anatomic landmark guided group (p<0.00001).15 Because the introducer sheaths for leadless devices are large (e.g. 23 Fr), it would seem logical that safety would be enhanced if ultrasound was used. The low complication rate and high success rates associated with leadless pacemaker implantation may be attributed, in part, to the extensive training available for this procedure and the experience of operators. Consequently, maintaining this high level of training and ensuring ongoing experience with recommended minimal annual numbers would seem appropriate, and was reflected by consensus on these points.
The evidence base on the Micra device indicates that the incidence of pericardial perforation requiring surgical intervention is low. In the postapproval registry, two of the 1,817 patients recruited (0.1%) required surgical intervention6. Despite this low number, there was no consensus about undertaking leadless pacing in non-cardiac surgical centres. However, there was consensus that centres should have a defined pathway in place to access cardiac surgical support. This would include procedures performed in a cardiac surgical centre and a non-cardiac surgical centre. In the latter situation, the process would be similar to that for the rare occasions when percutaneous coronary intervention or AF ablation require surgical input. This would need to be a predefined process of urgent transfer, recognising that any delay may adversely affect outcome. Similarly, it was recognised that centres implanting leadless pacemakers should have robust pathways in place to address any complications associated with the device or the procedure.
The use of shared decision-making is widely acknowledged as an important part of patient care and features highly within the NHS Long Term Plan, as well as General Medical Council guidance on consent.16,17 Not surprisingly, the use of shared decision-making in deciding on leadless pacing reached 100% consensus.
Recommendations
Based on the levels of agreement from 27 responses, the authors offer the following set of recommendations:
• Education for implanters and referrers regarding the benefits and safety of leadless pacing systems should be improved.
• Awareness and training on the use of leadless devices should be improved for non-leadless implanters.
• A registry should be developed to track the complications and risks associated with the use of leadless devices.
UK Expert Consensus Statement for the Clinical Utility of Leadless Pacing ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure 1: Combined Consensus Agreement Scores
The dark green line represents the consensus threshold of 66% and the light green line represents the threshold for very high consensus (90%).
for the Clinical Utility of Leadless Pacing
• Leadless devices should be more widely used so that implanters can better understand and mitigate the risks involved with the device.
• Leadless pacemakers should be considered in certain patient populations (Table 2).
• The choice to use a leadless pacemaker should be clinically driven to ensure the best outcome for the patient.
• A robust and defined pathway for timely cardiac surgical support for leadless pacing should be developed.
Table 1: Defined Consensus Statements and Corresponding Levels of Agreement from 27 Responses
UK Expert Consensus
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Statement
No. Statement Score (%) Topic A: Problems that are experienced with transvenous pacing and need to be appreciated/acknowledged 1 There is a clear need for leadless pacing in NHS clinical practice 100 2 There is a perception that leadless pacing is underutilised in NHS clinical practice 85 3 There is an existing evidence base that demonstrates clinical limitations with transvenous pacing 96 4 Leadless pacing has a lower rate of infection compared with transvenous pacing 96 5 Leadless pacing has lower rates of complications versus transvenous pacing 81 6 Leadless pacing is perceived as a safer alternative by NHS implanters than transvenous pacing 56 7 Leadless pacing is perceived as a safer alternative by NHS referrers than transvenous pacing 44 8 It is acceptable to implant more than one leadless pacemaker over the patient’s lifetime 100 9 Leadless pacing should be considered in order to preserve vascular access 81 10 It is reasonable to consider leadless pacing in order to reduce lead-related complications 96 Topic B: Relative risk of leadless systems 11 The consequence of a complication with a leadless pacemaker is no more severe than with a transvenous pacer 56 12 The relative risk of a leadless pacemaker is dependent on the profile of the patient 93 13 An evidence base exists for patients at greater risk of cardiac perforation 81 Topic C: Suitable patient types for leadless pacemakers that may be at risk from transvenous devices 14 Patient choice should always be considered when selecting a pacing option 100 15 Patients requiring a pacemaker who are considered to be at high risk of infection should be eligible for leadless pacing 100 16 Patients requiring a pacemaker who have end-stage renal disease should be eligible for leadless pacing 100 17 Patients requiring a pacemaker who have experienced previous device infections should be eligible for leadless pacing 100 18 Patients requiring a pacemaker who have anatomical constraints complicating or precluding a transvenous pacemaker should be eligible for leadless pacing 100 19 Any patient with AF and bradycardia should be eligible for leadless pacing 48 20 Patients requiring a pacemaker who are unwilling to consider a conventional transvenous device should be eligible for leadless pacing 85 21 Patients eligible for a pacemaker that should be considered for leadless pacing include those who are immunocompromised 96 22 Patients eligible for a pacemaker that should be considered for leadless pacing include those taking biological medicines 78 23 Patients eligible for a pacemaker that should be considered for leadless pacing include those undergoing radiotherapy 70 24 Leadless pacing should be an option for selected appropriate patients with congenital heart disease 100 25 Patients under the age of 40 years can be considered for leadless pacing 78 26 Patients who have, or are at, a high probability of needing indwelling catheters as part of the disease management plan should be considered for leadless pacing 100 Topic D: The role for a national register 27 The usage and outcomes of leadless pacing should be measured in a national registry 100 28 A national registry would help appropriate patient access to leadless pacing 96 29 A national registry would help appropriate NHS funding decisions for leadless pacing 96 Topic E: Logistical requirements for safe delivery of leadless pacemakers in the UK 30 Ultrasound should be used to guide vascular access for leadless pacing 93 31 Formal training and proctoring help improve the outcome of leadless pacing 100 32 Implanters should perform a requisite annual number of leadless pacing implants to maintain competence 100 33 Leadless pacing should not be limited to cardiac surgical support centres only 59 34 There should be a robust and defined pathway to access timely cardiac surgical support support when leadless pacing is used 93 35 There should be a robust pathway to deal with potential complications where leadless pacing is used 100 36 Shared decision-making with the patient is always required when deciding the appropriate pacing option 100
The results of this study are a representative sample of the opinions of implanters currently operating within the field. This provides a useful basis for the panel to propose recommendations to improve the use of leadless devices on a patient-centred basis.
As with all consensus studies, the wording of statements may have affected the levels of agreement attained. Future work could refine the statements found less agreeable in the present study to determine what elements are driving the agreement shown.
Conclusion
This consensus document is based on the expert opinion of 27 leadless pacemaker implanters currently operating within the UK, representing a response rate of 40%. The results provide a strong indication of the opinions of these specialists.
This study highlights that there are elements within the current approach to the use of leadless pacemakers that should be modified to improve the clinical utility of the device with a patient-centric focus, including patient types suitable for implantation, the role of a national register and the logistical requirements for delivering the system.
The implementation of the seven recommendations listed above may increase the use of leadless pacemakers, with the aim of improving patient outcomes.
Table 2: Recommended Patient Criteria for Considering Leadless Pacemaker Implantation
• High risk of infection
• End-stage renal disease
• Previous device infection
• Anatomical constraints complicating/precluding transvenous pacing
• Immunocompromised
• Biological medicines (including immunosuppressants and steroids)
• Undergoing radiotherapy
• Congenital heart disease
• Under 40 years of age
• Have, or at high probability of needing, indwelling vascular catheters
Clinical Perspective
• Leadless pacing appears to be a safe and effective alternative to conventional transvenous pacing.
• A Delphi model was used to evaluate opinions on aspects of leadless pacing in the UK, including problems associated with transvenous pacing, risks of leadless pacing, patient types for leadless pacing, the role of a national register and the logistics of delivering leadless pacing.
• The results of the Delphi process and expert opinion resulted in seven recommendations, including the need for a national register.
1. Sideris S, Archontakis S, Dilaveris P, et al. Leadless cardiac pacemakers: current status of a modern approach in pacing. Hellenic J Cardiol 2017;58:403–10. https://doi.org/10.1016/j. hjc.2017.05.004; PMID: 28529181.
2. El-Chami MF, Bonner M, Holbrook R, et al. Leadless pacemakers reduce risk of device-related infection: review of the potential mechanisms. Heart Rhythm 2020;17:1393–7. https://doi.org/10.1016/j.hrthm.2020.03.019; PMID: 32247833.
3. Steinwender C, Lercher P, Schukro C, et al. State of the art: leadless ventricular pacing: a national expert consensus of the Austrian Society of Cardiology. J Interv Card Electrophysiol 2020;57:27–37. https://doi.org/10.1007/s10840-019-00680-2; PMID: 31863250.
4. Sperzel J, Hamm C, Hain A. Nanostim-leadless pacemaker. Herzschr Elektophys 2018;29:327–33. https://doi.org/10.1007/ s00399-018-0598-3; PMID: 30341551.
5. Reynolds D, Duray GZ, Omar R, et al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016;374:533–41. https://doi.org/10.1056/NEJMoa1511643; PMID: 26551877.
6. El-Chami MF, Al-Samadi F, Clementy N, et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018;15:1800–7. https://doi.org/10.1016/j.hrthm.2018.08.005; PMID: 30103071.
7. Chintz L, Ritter P, Khelae SK, et al. Accelerometer-based atrioventricular synchronous pacing with a ventricular leadless pacemaker: results from the Micra atrioventricular
feasibility studies. Heart Rhythm 2018;15:1363–71. https://doi. org/10.1016/j.hrthm.2018.05.004; PMID: 29758405.
8. El-Chami MF, Clementy N, Garweg C, et al. Leadless pacemaker implantation in haemodialysis patients: experience with the Micra transcatheter pacemaker. JACC Clin Electrophysiol 2019;5:162–70. https://doi.org/10.1016/j. jacep.2018.12.008; PMID: 30784685.
9. El-Chami MF, Johanse JB, Zaidi A, et al. Leadless pacemaker implants in patients with pre-existing infections: results from the Mircra postapproval registry. J Cardiovasc Electrophysiol 2019;30:569–74. https://doi.org/10.1111/ jce.13851; PMID: 30661279.
10. Roberts PR, Pepper C, Rinaldi CA, et al. The use of a single chamber leadless pacemaker for the treatment of cardioinhibitory vasovagal syncope. Int J Cardiol Heart Vasc 2019;23:100349. https://doi.org/10.1016/j.ijcha.2019.100349; PMID: 30976654.
11. Turagam MK, Gopinathannair R, Park PH, et al. Safety and efficacy of leadless pacemaker for cardioinhibitory vasovagal syncope. Heart Rhythm 2020;17:1575–81. https:// doi.org/10.1016/j.hrthm.2020.05.006; PMID: 32389681.
12. Nice Institute for Health and Care Excellence (NICE). Leadless Cardiac Pacemaker Implantation for Bradyarrhythmias. London: NICE, 2018. https://www.nice.org.uk/guidance/ ipg626/resources/leadless-cardiac-pacemaker-implantationfor-bradyarrhythmias-pdf-1899873986002117 (accessed 1 December 2021).
13. Glikson M, Nielsen JC, Kronborg MB, et al. 2021 ESC
guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–520. https://doi. org/10.1093/eurheartj/ehab364; PMID: 34455430.
14. Kempa M, Mitkowski P, Kowalski O, et al. Expert opinion of a Working Group on Leadless Pacing appointed by the National Consultant in Cardiology and the Board of the Heart Rhythm Section of the Polish Cardiac Society. Kardiol Pol 2021;79:604–8. https://doi.org/10.33963/KP.15982; PMID: 34125944.
15. Triantafyllou K, Karkos CD, Fragakis N, et al. Ultrasoundguided versus anatomic landmark-guided vascular access in cardiac electrophysiology procedures: a systemic review and meta-analysis. Indian Pacing Electrophysiol J 2022;22:145–53. https://doi.org/10.1016/j.ipej.2022.01.005; PMID: 35143989.
16. National Health Service (NHS). NHS Long Term Plan. NHS, 2019. www.longtermplan.nhs.uk (accessed 1 December 2021).
17. General Medical Council (GMC). Guidance on Professional Standards and Ethics for Doctors: Decision Making and Consent Manchester: GMC, 2020. https://www.gmc-uk.org/-/media/ documents/gmc-guidance-for-doctors---decision-makingand-consent-english_pdf-84191055.pdf (accessed 1 December 2021).
UK Expert Consensus Statement for the Clinical Utility of Leadless Pacing ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Catecholaminergic Polymorphic Ventricular Tachycardia
Mohamed Abbas , 1 Chris Miles 2 and Elijah Behr 2
1. Department of Cardiology, Royal Stoke University Hospital, Stoke-on-Trent, UK;
2. Cardiovascular Clinical Academic Group, Molecular and Clinical Sciences Institute, St George’s, University of London and St George’s University Hospitals NHS Foundation Trust, London, UK
Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmia syndrome characterised by adenergically mediated bidirectional and/or polymorphic ventricular tachycardia. CPVT is a significant cause of autopsy-negative sudden death in children and adolescents, although it can also affect adults. It is often caused by pathogenic variants in the cardiac ryanodine receptor gene as well as other rarer genes. Early identification and risk stratification is of major importance. β-blockers are the cornerstone of therapy. Sodium channel blockers, specifically flecainide, have an additive role. Left cardiac sympathetic denervation is playing an increasing role in suppression of arrhythmia and symptoms. Concerns have been raised, however, about the efficacy of implantable cardioverter defibrillator therapy and the risk of catecholamine driven proarrhythmic storms. In this review, we summarise the clinical characteristics, genetics, and diagnostic and therapeutic strategies for CPVT and describe recent advances and challenges.
Keywords
β-blockers, catecholaminergic polymorphic ventricular tachycardia, flecainide, left cardiac sympathetic denervation, ryanodine receptor mutation, sudden cardiac death
Disclosure: The authors have no conflicts of interest to declare.
Received: 11 January 2022
Accepted: 2 July 2022 Citation: Arrhythmia & Electrophysiology Review 2022;11:e20. DOI: https://doi.org/10.15420/aer.2022.09
Correspondence: Elijah Behr, Cardiovascular Clinical Academic Group, Molecular and Clinical Sciences Institute, St George’s University Hospitals NHS Foundation Trust, Blackshaw Road, London SW17 0QT, UK. E: ebehr@sgul.ac.uk
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare inherited arrhythmia syndrome or channelopathy characterised by exercise- or emotional stress-induced bidirectional or polymorphic ventricular tachycardia in the setting of a structurally normal heart and a normal ECG.1 It is estimated to affect 1 in 10,000 people with reported mortality rates as high as 30–50% by the age of 35 years.2–4 This review presents an update on our current understanding of the condition and its clinical implications.
Clinical Presentation
CPVT is a heterogeneous disease that manifests primarily in children, with a mean age of 7–9 years at presentation. It is recognised as an important cause of unheralded sudden cardiac death (SCD) in young people.3 5 6 Although most patients will present before the age of 10; there are some reported cases where the patient has first presented in their 20s and 30s.7 Many people with CPVT will have a family history of SCD at a young age and/or stress-induced syncope or presyncope.8 9
Patients with CPVT can be asymptomatic and only diagnosed as part of family screening. Symptomatic patients usually present with exerciseinduced or emotional stress-related syncope accounting for up to 80% of cases.5 A significant subset of patients present for the first time with cardiac arrest due to ventricular tachycardia (VT) or VF (Figure 1).7,10–12 There are few differences based on sex with a tendency towards earlier manifestation in boys compared to girls.7
Patients with CPVT have a structurally normal heart and a normal 12-lead ECG at rest. The characteristic arrhythmias are bidirectional VT or polymorphic VT in the context of a catecholaminergic surge (Figure 1). This may cause haemodynamic compromise that results in syncope. Faster or sustained episodes of VT may degenerate into VF, resulting in sudden arrhythmic death or aborted cardiac arrest. Some episodes may be shortlived and terminate spontaneously, often causing palpitations and/or presyncope.7,13,14
Physical examination of patients with CPVT is often unremarkable. The syncopal episodes at presentation are often considered vasovagal events or caused by neurological triggers. A considerable proportion will be incorrectly diagnosed with epilepsy due to their presentation with seizure-like activity during syncope.5,2 The typical delay between the first syncope and the establishment of the diagnosis has been reported as 2 years.7
Genetic Basis
CPVT principally arises from pathogenic variants in two genes: ryanodine receptor 2 (RYR2) and calsequestrin 2 (CASQ2). RYR2 is inherited in an autosomal dominant manner and accounts for most cases (55–65%).7 Conversely, CASQ2 demonstrates autosomal recessive inheritance and accounts for approximately 5% of cases. RYR2 is a sarcoplasmic reticulum membrane channel protein expressed in cardiac tissues, and CASQ2 is a calcium storage protein in the sarcoplasmic reticulum. Both
REVIEW © 2022 The Author(s). Published by Radcliffe Group Ltd. www.AERjournal.com
Electrophysiology and Ablation
Clinical
1: Bidirectional Ventricular Tachycardia Degenerating to VF
before considering genetic testing.4 One study suggested the potential use of phenotype-enhanced variant classification in CPVT using a newly developed diagnostic scorecard to reduce the burden of RYR2 variants of unknown significance (VUS). The study was retrospectively conducted and revalidated in two big centres. The readjudication using amended standards dropped the VUS rate significantly from 48% to 7% in the primary centre with similar results seen in the validation centre. These results illustrate the potential value of incorporating a standardised assessment of phenotypic strength into the variant classification and reporting process.22
Criteria, Diagnostic Studies and Differential Diagnosis
Clinical Criteria
proteins play a critical role in regulating intracellular calcium. Pathogenic variants in these genes may result in excessive calcium release during diastole that result in intracellular calcium overload and subsequently delayed afterdepolarisation and triggered activity that could induce VT and VF. Other CPVT-associated genes have been reported with variable frequency of disease causation.15 Using the ClinGen gene curation framework, all CPVT reported genes had been reviewed by an expert panel who classified five genes in addition to RYR2 and CAQ2 as having definitive to moderate evidence for disease causation in CPVT (three calmodulin genes – CALM1, CALM2, CALM3, triadin [TRDN] and trans2,3-enoyl-CoA reductase-like [TECRL]). Three disputed genes (potassium inwardly rectifying channel subfamily j member 2 [KCNJ2], plakophilin 2 [PKP2], sodium voltage-gated channel alpha subunit 5 [SCN5A]) were deemed unrepresentative of CPVT. The remaining reported gene (ankyrin 2 [ANK2]) was too common in the population to be the cause of disease.16
Patients with RYR2 pathogenic variants have similar clinical courses when compared to patients without RYR2 7 CASQ2-CPVT may be more severe and more resistant to β-blockers, although this observation lacks comparison with a large series of individuals.5 In patients with no family history of CPVT, sporadic mutations are likely to be the cause. Of note, missense mutations in RYR2 have also been linked to arrhythmogenic right ventricular cardiomyopathy (ARVC-2) characterised by exerciseinduced polymorphic VT that does not appear to have a reentrant mechanism, occurring in the absence of significant structural abnormalities.17 This probably represents an early phenotypic misclassification.
Consensus guidelines recommend genetic testing for patients with clinically suspected CPVT identifying the index case in a family (the proband), and provide subsequent cascade screening of family members to detect relatives harbouring the causative variant.18 In a large retrospective study of autopsy-negative sudden death, RYR2 pathogenic variants were the most common molecular diagnosis, and when a rare variant burden analysis (a statistical test of association) was undertaken, RYR2 was the only gene associated significantly with autopsy-negative sudden death.19
Nevertheless, results from genetic testing should be interpreted with caution and require careful adjudication of variant pathogenicity.20 Data from exome sequence collection, such as that by the Exome Aggregation Consortium, showed that approximately 3% of healthy individuals harbour a rare protein-altering variant in the RYR2 gene.21 The lower the clinical pre-test probability for CPVT, the higher the rate of false positive results, emphasising the importance of comprehensive clinical phenotyping
CPVT should be suspected in patients with exertional syncope or syncope occurring during acute emotion. It should also be suspected in patients presenting with aborted SCD triggered by acute emotional stress or exercise. An exercise test may show ventricular arrhythmia in the form of polymorphic or bidirectional VT. According to the most recent international guidelines from the Heart Rhythm Society (HRS), the European Heart Rhythm Association (EHRA) and the Asia Pacific Heart Rhythm Society, CPVT is diagnosed in the presence of a structurally normal heart, normal ECG and unexplained exercise- or catecholamine-induced bidirectional VT or polymorphic ventricular premature beats or VT in people aged under 40 years. The diagnosis can be made in patients (who can be the index case or family member) who have a pathogenic mutation and in family members of a CPVT index case with a normal heart who have exercise-induced premature ventricular contractions or bidirectional/ polymorphic VT. Although very rare, the diagnosis can be made in people over 40 years in which case coronary disease should be excluded.23
Diagnostic Studies
The resting ECG is normal. Thus, the primary role of the resting ECG in patients with CPVT is to exclude other possibilities of arrhythmic cardiac syncope in young patients, such as long QT syndrome (LQTS) and Brugada syndrome.24 Sinus bradycardia has been reported in about 20% of CPVT patients.25 Prominent U waves are also observed in a subset and may also be related to altered intracellular calcium handling.26 Supraventricular arrhythmias, which might be catecholamine related, have been reported and may accompany sinus node dysfunction.8
The exercise ECG test is the most useful diagnostic tool in patients with suspected CPVT and it has a primary role in guiding therapy in confirmed cases. During exercise, isolated premature ventricular complexes (PVCs) often present first. As the exercise continues, they usually develop into ventricular bigeminy followed by polymorphic complexes. If the exercise is stopped at this stage, the ventricular complexes are likely to disappear. These exercise-induced ventricular complexes might be the only abnormality observed in some patients with CPVT who are mildly affected by the disease. Characteristically, the heart rate during which the ventricular dysrhythmias occur is between 100 and 130 BPM, which is typically reproducible. The complexity of the ventricular dysrhythmias is likely to worsen as the exercise workload increases. The occurrence of exercise-induced bidirectional VT and 180° rotation of the QRS axis from beat to beat is highly characteristic of CPVT. Some patients, however, will only develop polymorphic VT in the absence of a stable QRS vector alternans.8,27
Although premature ventricular complexes can also be observed in healthy subjects in response to exercise testing, certain features of PVCs
Catecholaminergic Polymorphic Ventricular Tachycardia ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Figure
Credit to Dr Andrew Martin. Source: The Cardiac Society of Australia and New Zealand.71 Reproduced with permission from The Cardiac Society of Australia and New Zealand.
during an exercise test can potentially assist in distinguishing CPVT from healthy controls. These features include: a larger number of PVCs, first appearance at higher workload, a left bundle branch block and an inferior axis, bigeminy or trigeminy at peak stress, QRS duration of more than 120 ms, a coupling interval of more than 400 ms, and disappearance in the first minute of the recovery. Of note, a positive exercise test for bidirectional or polymorphic VT is highly predictive of CPVT, however, a negative test is unreliable for ruling out the condition.28 29
Continuous ambulatory monitoring can also reveal arrhythmias typical for CPVT if the sinus rate of the patient exceeds the arrhythmia-inducing threshold during monitoring. Ambulatory monitoring can be useful in young children, who may find that performing a maximal exercise stress test is difficult. Implantation of a loop recorder can also be valuable in selected patients. On the other hand, provocation testing by IV infusion of isoproterenol or epinephrine can help diagnose patients with concealed CPVT who are unable to exercise.30 The test is considered positive for CPVT if three or more beats of polymorphic VT or bidirectional VT are provoked and borderline if polymorphic couplets, PVCs or non-sustained monomorphic VT are induced.31
Family Screening
CPVT has unfavourable clinical manifestations and prognosis. It is therefore essential to expand the evaluation to first- and second-degree relatives to find other potential CPVT cases in a family. Clinically, exercise testing and Holter monitoring are used for screening. Of note, some CPVT patients may have a negative exercise test in early childhood and become positive later in life. Therefore, regular follow-up with repeated exercise stress tests is indicated.
About 50% of the relatives identified by cascade screening as carrying the RYR2 mutation have a CPVT phenotype. Screening of family members by genetic testing is therefore recommended when a pathogenic or likely pathogenic variant has been identified in the proband. Genetic evaluation facilitates diagnosis in silent carriers and allows implementation of preventive pharmacological therapy and a reproductive risk assessment.23,32
Differential Diagnosis
When evaluating a patient with exertional symptoms attributed to ventricular arrhythmias, one should rule out other conditions that might present similarly. Arrhythmogenic right ventricular cardiomyopathy (ARVC) predisposes to VT and SCD in young people; however, it typically presents with an abnormal ECG, monomorphic VT and evidence of structural changes on cardiac imaging.33 In one study, some patients with a clinical diagnosis of CPVT exhibited PKP2 pathogenic mutation which suggests that the progression of the PKP2-dependent electropathy can be independent of structural disturbance and can precipitate exerciseassociated presentations prior to the development of an overt cardiomyopathy, clinically mimicking CPVT.34 This phenotypic overlap calls for careful imaging assessment (echocardiogram/cardiac magnetic resonance) in all patients with suspected CPVT. LQTS may present with exercise-related syncope, typically in the LQT1 variant.35 Occasionally, some individuals with Andersen Tawil syndrome, a variant of LQTS, may develop bidirectional VT similar to that of CPVT; however, the presence of QTU prolongation on ECG, syndromic features, the lower risk of sudden death and inconsistency of the relationship of arrhythmia to adrenergic activity distinguish this condition from CPVT.7, 36 Short-coupled torsades de pointes (SC-TdP) may also present similarly. It occurs in the setting of structurally normal heart and normal ECG. However, the onset of the SC-
TdP is not related to adrenergic stimuli and is not associated with the typical bidirectional pattern of CPVT tachycardia.32 Another heritable cause of polymorphic VT is familial ST depression syndrome, although its presentation is not related to exercise and some patients will have left ventricular systolic dysfunction.37–39
Risk Stratification and Treatment
The occurrence of cardiac arrest is associated with a higher risk of future arrhythmic episodes. Symptomatic patients with arrhythmic syncope are more likely to develop a cardiac event compared to those who are asymptomatic. Similarly, diagnosis at a young age is linked to adverse outcomes.3 Persistence of complex ectopy during exercise tests may be a marker of adverse outcomes as well.3 Ventricular arrhythmias are usually not inducible at programmed electrical stimulation, making it inadequate for management and risk stratification. Furthermore, the predictive value of inducibility of ventricular arrhythmias by catecholamine infusion or exercise for risk stratification has not been demonstrated.
The role of genetic testing in risk stratification is evolving. The attribution of pathogenicity to a rare variant in the RYR2 gene is challenging, given the high rate of rare, yet benign variants. CPVT-causing variants tend to cluster in functionally relevant domains. Patients with RYR2 variants affecting the C-terminus (CTD amino acid) are at higher risk of lifethreatening arrhythmic events independent of clinical presentation and the type of β-blocker used.40 In a retrospective observational study, de novo RYR2 variants were more likely to be located in the C-terminus domain and less likely in the N-terminus domain than in the familial group. In this cohort, the de novo cases presented with an arrhythmic event (syncope or cardiac arrest) at a younger age.41
Lifestyle changes and supportive care are crucial for all patients with CPVT. Furthermore, all patients with CPVT should be treated with pharmacological therapies to reduce the incidence of the primary manifestations of the disease. Non-pharmacological treatments should be considered in selected patients with persistent symptoms and/or exercise-induced ventricular arrhythmias despite optimal medical therapy, and device therapy is recommended for secondary prevention.
Sport and Lifestyle Changes
Due to the nature of the condition, long-term management strategies in CPVT have the objective of reducing adrenergic stimulation. Lifestyle modification, including the avoidance of emotional distress, is advised. A position statement by the European Association of Preventive Cardiology (EAPC) and the European Heart Rhythm Association (EHRA) suggests the following for patients with CPVT:42
• Competitive and intensive leisure time sports are not recommended.
• Low-to-moderate-intensity leisure-time sports may be considered if stress tests show the absence of any ventricular ectopy or arrhythmia when using appropriate treatment, and if the patient is asymptomatic for a minimum of 3 months, including patients with an implantable cardioverter defibrillator (ICD).
• Restrict sports activity to a moderate intensity of 30–60 minutes a day on 3–7 days a week, similar to the general population.
• Carriers of a pathogenic CPVT mutation without an overt phenotype should be managed as patients with manifest CPVT and be restricted to low-intensity sports.
• Follow-up should include stress test and/or continuous ECG monitoring during low-intensity sports to ensure control of exerciseinduced ventricular arrhythmias.
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Catecholaminergic Polymorphic Ventricular Tachycardia
• Avoid stressful and emotional situations, dehydration, electrolyte disturbance and hyperthermia.36,42,43
Despite these recommendations, preliminary retrospective analysis suggests that with appropriate treatment, sports practice may be possible if sudden bursts of exercise are avoided.44 Based on previous exercise testing, individualised maximum heart rate limits may be specified. Of paramount importance is that a diagnosis of CPVT can be a burden for patients as well as their families and appropriate psychological support should be considered early on.45
Pharmacological Therapy
β-blockers are the first-line therapy. Current guidelines recommend treating symptomatic patients with β-blockers.20 High-dose nadolol 1–2 mg/kg is preferred to other types of β-blockers as patients on nadolol have lower event rates.46 47 In countries where nadolol is not available, another non-selective β-blocker, such as propranolol, could be used as an alternative. It is important to emphasise that β-blockers should be administered throughout pregnancy in affected women. Asymptomatic patients with positive pathogenic mutations for CPVT and negative exercise tests have lower arrhythmic event rates on long-term follow-up (4.4 per 1,000 person-years), nevertheless, they may be considered for prophylactic β-blocker therapy.32,23
Although β-blockers are the mainstay treatment for CPVT, they are not fully effective in all patients. Van der Werf et al. reported 37% of patients on β-blocker will continue to develop ventricular arrhythmias at 8 years follow-up. Furthermore, the fatality rate on β-blocker has been reported as being between 2.6–9.2%.20,48 Therefore, regular evaluation with exercise tests is mandatory to up-titrate the β-blocker to the maximum tolerated doses, emphasise the importance of strict compliance with therapy and identify those who may benefit from treatment escalation.7
Flecainide effectively reduces ventricular arrhythmias in symptomatic patients with CPVT already on a maximum tolerated dose of β-blocker. The current guidelines recommend the consideration of flecainide in addition to β-blocker in patients with recurrent syncope, ventricular arrhythmias on exercise stress test, or to reduce appropriate ICD therapy.23 The usual daily dose of flecainide ranges between 100 and 300 mg.49,8 A recent randomised crossover trial has shown flecainide to be very effective when taken in addition to β-blocker in suppressing ventricular arrhythmias in patients with CPVT. The authors suggested that titrating flecainide dose depending on plasma trough levels achieved better outcomes.50 In another study, the antiarrhythmic effect appeared to be independent of the specific CPVT genetic subtype.51 Flecainide monotherapy has been reported in some cases as well as a small observational study; however, there are no randomised trials to evaluate its effect on patients who are not also taking β-blockers.52,53 As it stands, flecainide monotherapy is not recommended in patients who can tolerate β-blockers.23
At present, the only measure of the efficacy of flecainide is the burden of ventricular arrhythmia using the exercise test. It is, however, essential to recognise that this was not a reliable predictor of cardiac events in some reports. Therefore, Lieve et al. suggest that the optimal therapeutic response is evaluated by comparing ventricular arrhythmia burden on exercise before and after the initiation of flecainide.54 Flecainide reduced ventricular arrhythmias effectively in patients on maximally tolerated β-blocker therapy with symptoms or ventricular arrhythmias during their exercise test. These results were similar in patients with pathogenic
variants in RYR2, CASQ2 and genotypically negative cases. Nevertheless, large studies are needed to fully evaluate the efficacy of flecainide in prevention of cardiac events in the long term.
Data that support the use of calcium channel blockers are relatively scanty. Studies by Swan et al. in 2005, Rosso et al. in 2007 and Katz et al. in 2010 have investigated the efficacy of verapamil in addition to β-blocker in suppressing ventricular arrhythmias in CPVT patients.55–57 The burden of ventricular arrhythmias was significantly reduced in all three studies in the short term; however, verapamil did not prevent events in these groups in the long term.58 Moreover, in all three studies, verapamil did not add any negative chronotropic effect as the mean heart rate was similar before and after verapamil. Therefore, the current guidelines do not recommend verapamil as first- or second-line therapy. Yet, it could be considered in some patients on an individual basis, particularly in carriers of a specific CASQ2 mutation.53 59
Non-pharmacological Therapy
The European Society of Cardiology (ESC) guidelines and the HRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes recommend ICD implantation in CPVT patients who experienced cardiac arrest, recurrent syncope, or polymorphic/bidirectional VT despite optimal medical therapy and/or left cardiac sympathetic denervation (LCSD).23 60 ICD as a stand-alone therapy in an asymptomatic patient with CPVT is contraindicated due to the risk of adrenergic storms.23 Despite guideline recommendations, real-world data suggests a trend towards a more liberal use of ICDs in CPVT patients.12 61 Given the nature of CPVT and its potentially malignant course even with optimal medical therapy, implanting an ICD seems reasonable. However, ICDs are not without their problems.
Early reports suggested that ICDs might be proarrhythmic in CPVT.62 63 Roston et al. concluded in a systematic review and meta-analysis that ICDs effectively terminate VF but are not as effective in terminating VT.61 Recently, Van der Werf et al. studied 136 patients who presented with SCD in whom CPVT was diagnosed, subsequently leading to the initiation of guideline-directed therapy with β-blocker and flecainide with or without LCSD. 58% of the patients had an ICD implanted. After a median follow-up of 4.8 years, a composite outcome of SCD, sudden cardiac arrest, syncope or appropriate ICD shocks occurred in 47% of the patients with ICD and only 15.8% of the patients without ICD. Moreover, inappropriate ICD shocks occurred in 24% and other device-related complications in 29% of the patients. Although limited by being observational data, this study suggests a lack of survival benefit in patients with ICDs; instead, ICDs are associated with a high rate of appropriate and inappropriate shocks along with other device-related complications.64
Another prospective cohort study followed up 216 patients with CPVT on β-blockers only for a mean duration of 7.5 years. Twenty-eight patients (13%) had a life-threatening arrhythmic event; 18 had an ICD already implanted prior to the event, with subsequent zero mortality; and four of the other ten patients (40%) died. Based on these findings, the authors argued that ICD implantation offers important protection in CPVT. However, relevant clinical information was not presented on whether the patients who died had symptoms while on β-blocker or had a normal exercise test on follow-up before they had cardiac arrest. Notably, data on long-term arrhythmic events in CPVT patients on dual therapy (β-blocker and flecainide) with or without LCSD are still lacking.41
Based on the data available and in the absence of randomised trials, it
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Catecholaminergic Polymorphic Ventricular Tachycardia
Catecholaminergic Polymorphic Ventricular Tachycardia
seems reasonable to recommend a conservative approach towards using ICDs in patients with CPVT in line with the current guidelines. Indeed, when an ICD is implanted following a shared decision-making process, the treating physician should seek to minimise the risk of appropriate and inappropriate therapy by optimising medical treatment with or without LCSD. Supraventricular tachycardias are common in CPVT patients and they should be treated promptly as they facilitate ventricular arrhythmias as well as possibly resulting in inappropriate ICD therapies. ICD programming with an extended monitoring period could also help to minimise these risks, aiming to treat only high-rate arrhythmias, such as VF.
Left cardiac sympathetic denervation is a surgical procedure in which the lower two-thirds of the left thoracic stellate ganglion along with T2–T4 ganglia are ablated, resulting in an interruption of the major source of norepinephrine release in the heart.65 LCSD is at least moderately effective in reducing arrhythmic events in CPVT patients according to multiple reports, although long-term data are still needed.6,43,49,66,67 LCSD is not curative and is not recommended as monotherapy for CPVT patients. There are some limitations related to procedural risks depending on the chosen surgical approach whether that is video-assisted thoracoscopic surgery or open thoracotomy. Possible outcomes including failure of the procedure should always be discussed before surgery to manage the patient and the family’s expectations.68 As it stands, LCSD may be considered in patients with CPVT who are symptomatic with recurrent syncope, polymorphic/bidirectional VT, or appropriate ICD shocks despite maximal medical therapy and/or intolerant of or with contraindication to β-blocker.23
Using a Stepwise Approach
All CPVT patients should be on a β-blocker and be given advice regarding lifestyle changes and avoiding strenuous exercise. Follow-up with exercise testing is mandatory to evaluate the clinical response to therapy. Patients with exercise-induced ventricular arrhythmias in the form of ventricular couplets, non-sustained VT, polymorphic or bidirectional VT should be considered for flecainide add-on therapy. If they continue to have exercise-induced ventricular arrhythmia and/or symptoms, they may be offered an ICD with or without LCSD, although earlier use of the LCSD and avoidance of an ICD is advocated by some. All CPVT cases should be managed by experts in the field in a referral centre.7 Figure 2 is a flowchart summarising a suggested care pathway for patients with CPVT.
Knowledge Gap and Future Directions
Despite significant progress in understanding CPVT, there are still uncertainties and gaps in our knowledge. In day-to-day practice, clinicians may make difficult diagnostic and therapeutic decisions without solid evidence. Such difficulties contribute to most clinical dilemmas and must be shared with the patients and their families. Examples include the definition of optimal exercise stress test protocol and the accepted findings on stress tests on follow-up. Furthermore, the background rate of RYR2 rare variants is reported as 3% in a general white population, and functional evidence to support causality is only present in a minority. Interpretation of these rare variants in daily practice remains a significant source of uncertainty and discomfort, particularly when discussing test results with bereaved families.6 Indeed, many patients with CPVT remain variant-negative; therefore, future discovery of genes and gene modifiers will continue with a particular focus on genome-wide association studies.
Figure 2: Suggested Care Pathway for Catecholaminergic Polymorphic Ventricular Tachycardia Index Cases
Presentation
Referral point
Investigations
Phenotype
Genetic test
Suspected index case with exercise-induced syncope or palpitations
Family history of CPVT or sudden arrhythmic death
Specialist centre for inherited cardiac conditions
ECG, exercise test, Holter and echocardiogram
Consider cardiac MRI, genetic testing and catecholamine provocation test
Probable or definite CPVT diagnosis
Yes
Advice on lifestyle and supportive care
Management
Low likelihood of CPVT Consider other causes of syncope and palpitations
No
As appropriate for any underlying condition
Follow-up
Commence β-blockers, consider flecainide with or without left cardiac sympathetic denervation if still symptomatic
Consider implantable cardioverter defibrillator with optimal programming if previous ventricular fibrillation arrest or recurrent syncope while on optimal therapy
Yes
Regular evaluation with exercise testing and Holter ECG
Cascade screening for first degree relatives
No, unless a diagnosis of another inherited cardiac condition was made
Discharge from centre for inherited cardiac conditions
Lifestyle changes and the ‘acceptable’ level of exercise in probands and asymptomatic affected relatives is another clinical dilemma frequently faced by clinicians. On the management aspect, although nadolol is not available in many countries, there is a lack of data on the use of other non-selective β-blockers in symptomatic patients. On the other hand, in asymptomatic patients with RYR2 pathogenic variants identified on family screening, the safety of not treating with β-blockers has yet to be studied fully. Moreover, studies are required to evaluate the role of more proactive treatment in some high-risk groups (for example, patients with RYR2 variant affecting the C-terminus and those with homozygous variants in the CASQ2 gene). More work is needed to examine the role of electrophysiological testing and catheter ablation in atrial and ventricular arrhythmias in refractory CPVT cases. Finally, gene therapy is a promising new therapeutic option for CPVT.69,70
There is a need for more comprehensive risk stratification tools to aid the management of CPVT patients coupled with interventional randomised trials to evaluate existing and novel therapeutic options. However, CPVT has a low prevalence, and more referral centres need to collaborate to address these priorities. Great examples of the outcomes that can be achieved in this type of collaboration have been demonstrated by the multicentre observational registry that was established in 2014 with future findings expected.47 64
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
CPVT = catecholaminergic polymorphic ventricular tachycardia.
Catecholaminergic Polymorphic Ventricular Tachycardia
Clinical Perspective
• Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited arrhythmia syndrome that is characterised by stress-induced bidirectional and/or polymorphic VT.
• Exercise tests play an important role in the diagnosis of CPVT as well as monitoring the response to medical therapy in affected patients.
• β-blockers remain the cornerstone therapy for CPVT. The addition of flecainide often further reduces the incidence of arrhythmic events in symptomatic patients.
• Lifestyle changes including avoidance of emotional stress as well as avoidance of competitive and intensive leisure-time sport are recommended.
1. Nguyen PT, Scheinman MM, Seger J. Polymorphous ventricular tachycardia: clinical characterization, therapy, and the QT interval. Circulation 1986;74:340–9. https://doi.org/10.1161/01.cir.74.2.340; PMID: 3731424
2. Tester DJ, Medeiros-Domingo A, Will ML, et al. Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clin Proc 2012;87:524–39. https://doi.org/10.1016/ jmayocp.2012.02.017; PMID: 22677073
3. Hayashi M, Denjoy I, Extramiana F, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation 2009;119:2426–34. https://doi.org/10.1161/ CIRCULATIONAHA.108.829267; PMID: 19398665
4. Kapplinger JD, Pundi KN, Larson NB, et al. Yield of the RYR2 genetic test in suspected catecholaminergic polymorphic ventricular tachycardia and implications for test interpretation. Circ Genom Precis Med 2018;11:e001424. https://doi.org/10.1161/CIRCGEN.116.001424; PMID: 29453246
5. Napolitano C, Bloise R, Monteforte N, Priori SG. Sudden cardiac death and genetic ion channelopathies: long QT, Brugada, short QT, catecholaminergic polymorphic ventricular tachycardia, and idiopathic ventricular fibrillation. Circulation 2012;125:2027–34. https://doi.org/10.1161/ CIRCULATIONAHA.111.055947; PMID: 22529064
6. Pflaumer A, Wilde AAM, Charafeddine F, Davis AM. 50 years of catecholaminergic polymorphic ventricular tachycardia (CPVT) – time to explore the dark side of the moon. Heart Lung Circ 2020;29:520–8. https://doi.org/10.1016/j. hlc.2019.10.013; PMID: 31859141
7. Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation 2002;106:69–74. https://doi.org/10.1161/01. cir.0000020013.73106.d8; PMID: 12093772
8. Napolitano C, Mazzanti A, Bloise R, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. In: GeneReviews [Internet]. Seattle, US: University of Washington, 2004. PMID: 20301466.
9. van der Werf C, Nederend I, Hofman N, et al. Familial evaluation in catecholaminergic polymorphic ventricular tachycardia: disease penetrance and expression in cardiac ryanodine receptor mutation-carrying relatives. Circ Arrhythm Electrophysiol 2012;5:748–56. https://doi.org/10.1161/ CIRCEP.112.970517; PMID: 22787013
10. Choi G, Kopplin LJ, Tester DJ, et al. Spectrum and frequency of cardiac channel defects in swimming-triggered arrhythmia syndromes. Circulation 2004;110:2119–24. https:// doi.org/10.1161/01.CIR.0000144471.98080.CA; PMID: 15466642
11. Tester DJ, Kopplin LJ, Will ML, Ackerman MJ. Spectrum and prevalence of cardiac ryanodine receptor (RyR2) mutations in a cohort of unrelated patients referred explicitly for long QT syndrome genetic testing. Heart Rhythm 2005;2:1099–105. https://doi.org/10.1016/j.hrthm.2005.07.012;
PMID: 16188589
12. Roston TM, Vinocur JM, Maginot KR, et al. Catecholaminergic polymorphic ventricular tachycardia in children: analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ Arrhythm Electrophysiol 2015;8:633–42. https://doi.org/10.1161/
CIRCEP.114.002217; PMID: 25713214
13. Baltogiannis GG, Lysitsas DN, di Giovanni G, et al. CPVT: arrhythmogenesis, therapeutic management, and future perspectives. A brief review of the literature. Front Cardiovasc Med 2019;6:92. https://doi.org/10.3389/ fcvm.2019.00092; PMID: 31380394
14. Pérez-Riera AR, Barbosa-Barros R, de Rezende Barbosa MPC, et al. Catecholaminergic polymorphic ventricular tachycardia, an update. Ann Noninvasive Electrocardiol 2018;23:e12512. https://doi.org/10.1111/anec.12512;
PMID: 29048771
15. Gray B, Behr ER. New insights into the genetic basis of inherited arrhythmia syndromes. Circ Cardiovasc Genet 2016;9:569–77.https://doi.org/10.1161/ CIRCGENETICS.116.001571; PMID: 27998945
16. Walsh R, Adler A, Amin AS, et al. Evaluation of gene validity for CPVT and short QT syndrome in sudden arrhythmic death. Eur Heart J 2022;43:1500–10. https://doi.org/10.1093/ eurheartj/ehab687; PMID: 34557911
17. Tiso N, Stephan DA, Nava A, et al. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2). Hum Mol Genet 2001;10:189–94. https://doi. org/10.1093/hmg/10.3.189; PMID: 11159936
18. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–867. https://doi.org/10.1093/eurheartj/ehv316; PMID: 26320108
19. Lahrouchi N, Raju H, Lodder EM, et al. Utility of post-mortem genetic testing in cases of sudden arrhythmic death syndrome. J Am Coll Cardiol 2017;69:2134–45. https://doi. org/10.1016/j.jacc.2017.02.046; PMID: 28449774
20. Lek M, Karczewski KJ, Minikel EV, et al. Analysis of proteincoding genetic variation in 60,706 humans. Nature 2016;536:285–91. https://doi.org10.1038/nature19057; PMID: 27535533.
21. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–24. https://doi. org/10.1038/gim.2015.30; PMID: 25741868
22. Giudicessi JR, Lieve KVV, Rohatgi RK, et al. Assessment and validation of a phenotype-enhanced variant classification framework to promote or demote RYR2 missense variants of uncertain significance. Circ Genom Precis Med 2019;12:e002510. https://doi.org/10.1161/ CIRCGEN.119.002510; PMID: 31112425
23. Imberti JF, Underwood K, Mazzanti A, Priori SG. Clinical challenges in catecholaminergic polymorphic ventricular tachycardia. Heart Lung Circ 2016;25:777–83. https://doi. org/10.1016/j.hlc.2016.01.012; PMID: 26948768
24. Liu N, Ruan Y, Priori SG. Catecholaminergic polymorphic ventricular tachycardia. Prog Cardiovasc Dis 2008;51:23–30. https://doi.org/10.1016/j.pcad.2007.10.005; PMID: 18634915
25. Postma AV, Denjoy I, Kamblock J, et al. Catecholaminergic polymorphic ventricular tachycardia: RYR2 mutations, bradycardia, and follow up of the patients. J Med Genet 2005;42:863–70. https://doi.org/10.1136/jmg.2004.028993; PMID: 16272262
26. Leenhardt A, Lucet V, Denjoy I, et al. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation 1995;91:1512–9. https://doi.org/10.1161/01.cir.91.5.1512; PMID: 7867192
27. Sumitomo N, Harada K, Nagashima M, et al. Catecholaminergic polymorphic ventricular tachycardia: electrocardiographic characteristics and optimal therapeutic strategies to prevent sudden death. Heart 2003;89:66–70. https://doi.org/10.1136/heart.89.1.66; PMID: 12482795
28. Blich M, Marai I, Suleiman M, et al. Electrocardiographic comparison of ventricular premature complexes during exercise test in patients with CPVT and healthy subjects. Pacing Clin Electrophysiol 2015;38:398–402. https://doi. org/10.1111/pace.12574; PMID: 25627675
29. Steriotis AK, Nava A, Rampazzo A, et al. Follow-up with exercise test of effort-induced ventricular arrhythmias linked
to ryanodine receptor type 2 gene mutations. Am J Cardiol 2012;109:1015–9. https://doi.org/10.1016/j. amjcard.2011.11.033; PMID: 22221940
30. Stiles MK, Wilde AAM, Abrams DJ, et al. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. Heart Rhythm 2021;18:e1–50. https://doi.org/10.1016/j. hrthm.2020.10.010; PMID: 33091602
31. Obeyesekere MN, Klein GJ, Modi S, et al. How to perform and interpret provocative testing for the diagnosis of Brugada syndrome, long-QT syndrome, and catecholaminergic polymorphic ventricular tachycardia. Circ Arrhythm Electrophysiol 2011;4:958–64. https://doi.org/10.1161/ CIRCEP.111.965947; PMID: 22203660
32. Chokr MO, Darrieux FC, Hardy CA, et al. Short-coupled variant of ‘torsades de pointes’ and polymorphic ventricular tachycardia. Arq Bras Cardiol 2014;102:e60–4. https://doi. org/10.5935/abc.20140075; PMID: 25004426
33. Ackerman MJ, Zipes DP, Kovacs RJ, Maron BJ. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 10: the cardiac channelopathies: a scientific statement From the American Heart Association and American College of Cardiology. J Am Coll Cardiol 2015;66:2424–8. https://doi.org/10.1016/j. jacc.2015.09.042; PMID: 26542662
34. Tester DJ, Ackerman JP, Giudicessi JR, et al. Plakophilin-2 truncation variants in patients clinically diagnosed with catecholaminergic polymorphic ventricular tachycardia and decedents with exercise-associated autopsy negative sudden unexplained death in the young. JACC Clin Electrophysiol 2019;5:120–7. https://doi.org/10.1016/j. jacep.2018.09.010; PMID: 30678776
35. Ozawa J, Ohno S, Fujii Y, et al. Differential diagnosis between catecholaminergic polymorphic ventricular tachycardia and long QT syndrome type 1 – modified Schwartz score. Circ J 2018;82:2269–76. https://doi. org/10.1253/circj.CJ-17-1032; PMID: 29925740
36. Inoue YY, Aiba T, Kawata H, et al. Different responses to exercise between Andersen-Tawil syndrome and catecholaminergic polymorphic ventricular tachycardia. Europace 2018;20:1675–82. https://doi.org/10.1093/europace/ eux351; PMID: 29309601
37. Christensen AH, Nyholm BC, Vissing CR, et al. Natural history and clinical characteristics of the first 10 Danish families with familial ST-depression syndrome. J Am Coll Cardiol 2021;77:2617–9. https://doi.org/10.1016/j. jacc.2021.03.313; PMID: 34016271
38. Bundgaard H, Jøns C, Lodder EM, et al. A novel familial cardiac arrhythmia syndrome with widespread ST-segment depression. N Engl J Med 2018;379:1780–1. https://doi. org/10.1056/NEJMc1807668; PMID: 30380381
39. Christensen AH, Vissing CR, Pietersen A, et al. Electrocardiographic findings, arrhythmias, and left ventricular involvement in familial ST-depression syndrome. Circ Arrhythm Electrophysiol 2022;15:e010688. https://doi. org/10.1161/CIRCEP.121.010688; PMID: 35357203.
40. Shimamoto K, Ohno S, Kato K, et al. Impact of cascade screening for catecholaminergic polymorphic ventricular tachycardia type 1. Heart 2022;108:840–7. https://doi. org/10.1136/heartjnl-2021-320220; PMID: 35135837
41. Mazzanti A, Kukavica D, Trancuccio A, et al. Outcomes of patients with catecholaminergic polymorphic ventricular tachycardia treated with β-blockers. JAMA Cardiol 2022;7:504–12. https://doi.org/10.1001/ jamacardio.2022.0219; PMID: 35353122
42. Heidbuchel H, Arbelo E, D’Ascenzi F, et al. Recommendations for participation in leisure-time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions. Part 2: ventricular arrhythmias, channelopathies, and implantable defibrillators: a position statement of the section of sports
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com
Catecholaminergic Polymorphic Ventricular Tachycardia
cardiology and exercise from the European Association of Preventive Cardiology (EAPC) and the European Heart Rhythm Association (EHRA), both associations of the European Society of Cardiology. Europace 2021;23:147–8. https://doi.org/10.1093/europace/euaa106; PMID: 32596731.
43. Wilde AA, Bhuiyan ZA, Crotti L, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med 2008;358:2024–9. https://doi.org/10.1056/NEJMoa0708006; PMID: 18463378
44. Ostby SA, Bos JM, Owen HJ, et al. Competitive sports participation in patients with catecholaminergic polymorphic ventricular tachycardia: a single center’s early experience. JACC Clin Electrophysiol 2016;2:253–62. https://doi. org/10.1016/j.jacep.2016.01.020; PMID: 29766881
45. Richardson E, Spinks C, Davis A, et al. Psychosocial implications of living with catecholaminergic polymorphic ventricular tachycardia in adulthood. J Genet Couns 2018;27:549–57. https://doi.org/10.1007/s10897-017-0152-1; PMID: 28940060
46. Leren IS, Saberniak J, Majid E, et al. Nadolol decreases the incidence and severity of ventricular arrhythmias during exercise stress testing compared with β1-selective β-blockers in patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2016;13:433–40. https://doi.org/10.1016/j.hrthm.2015.09.029; PMID: 26432584
47. Peltenburg PJ, Kallas D, Bos JM, et al. An international multicenter cohort study on β-blockers for the treatment of symptomatic children with catecholaminergic polymorphic ventricular tachycardia. Circulation 2022;145:333–44. https:// doi.org/10.1161/CIRCULATIONAHA.121.056018; PMID: 34874747
48. van der Werf C, Zwinderman AH, Wilde AA. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace 2012;14:175–83. https://doi. org/10.1093/europace/eur277; PMID: 21893508
49. Dusi V, De Ferrari GM, Pugliese L, Schwartz PJ. Cardiac sympathetic denervation in channelopathies. Front Cardiovasc Med 2019;6:27. https://doi.org/10.3389/ fcvm.2019.00027; PMID: 30972341
50. Kannankeril PJ, Moore JP, Cerrone M, et al. Efficacy of flecainide in the treatment of catecholaminergic polymorphic ventricular tachycardia: a randomized clinical trial. JAMA Cardiol 2017;2:759–66. https://doi.org/10.1001/jamacardio.2017.1320;
PMID: 28492868
51. Watanabe H, van der Werf C, Roses-Noguer F, et al. Effects of flecainide on exercise-induced ventricular arrhythmias and recurrences in genotype-negative patients with catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2013;10:542–7. https://doi.org/10.1016/j.
hrthm.2012.12.035; PMID: 23286974
52. Pott C, Dechering DG, Reinke F, et al. Successful treatment of catecholaminergic polymorphic ventricular tachycardia with flecainide: a case report and review of the current literature. Europace 2011;13:897–901. https://doi.org/10.1093/ europace/euq517; PMID: 21292648
53. Padfield GJ, AlAhmari L, Lieve KV, et al. Flecainide monotherapy is an option for selected patients with catecholaminergic polymorphic ventricular tachycardia intolerant of β-blockade. Heart Rhythm 2016;13:609–13. https://doi.org/10.1016/j.hrthm.2015.09.027; PMID: 26416620
54. Lieve KV, Wilde AA, van der Werf C. The role of flecainide in the management of catecholaminergic polymorphic ventricular tachycardia. Arrhythm Electrophysiol Rev 2016;5:45–9. https://doi.org/10.15420/aer.2016.3.3; PMID: 27403293
55. Katz G, Khoury A, Kurtzwald E, et al. Optimizing catecholaminergic polymorphic ventricular tachycardia therapy in calsequestrin-mutant mice. Heart Rhythm 2010;7:1676–82. https://doi.org/10.1016/j.hrthm.2010.07.004; PMID: 20620233
56. Rosso R, Kalman JM, Rogowski O, et al. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2007;4:1149–54. https://doi.org/10.1016/j. hrthm.2007.05.017; PMID: 17765612
57. Swan H, Laitinen P, Kontula K, Toivonen L. Calcium channel antagonism reduces exercise-induced ventricular arrhythmias in catecholaminergic polymorphic ventricular tachycardia patients with RyR2 mutations. J Cardiovasc Electrophysiol 2005;16:162–6. https://doi. org/10.1046/j.1540-8167.2005.40516.x; PMID: 15720454
58. Rosso R, Kalman J, Rogowsky R. Long-term effectiveness of beta blocker and calcium blocker combination therapy in patients with CPVT. Heart Rhythm 2010;7:S382–445. https:// doi.org/10.1016/j.hrthm.2010.03.034
59. Alcalai R, Wakimoto H, Arad M, et al. Prevention of ventricular arrhythmia and calcium dysregulation in a catecholaminergic polymorphic ventricular tachycardia mouse model carrying calsequestrin-2 mutation. J Cardiovasc Electrophysiol 2011;22:316–24. https://doi. org/10.1111/j.1540-8167.2010.01877.x; PMID: 20807279
60. Priori SG, Wilde AA, Horie M, et al. Executive summary: HRS/ EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace 2013;15:1389–406. https:// doi.org/10.1093/europace/eut272; PMID: 23994779.
61. Roston TM, Jones K, Hawkins NM, et al. Implantable cardioverter-defibrillator use in catecholaminergic polymorphic ventricular tachycardia: a systematic review. Heart Rhythm 2018;15:1791–9. https://doi.org/10.1016/j.
hrthm.2018.06.046; PMID: 30063211
62. Miyake CY, Webster G, Czosek RJ, et al. Efficacy of implantable cardioverter defibrillators in young patients with catecholaminergic polymorphic ventricular tachycardia: success depends on substrate. Circ Arrhythm Electrophysiol 2013;6:579–87. https://doi.org/10.1161/CIRCEP.113.000170; PMID: 23667268
63. Roses-Noguer F, Jarman JW, Clague JR, Till J. Outcomes of defibrillator therapy in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 2014;11:58–66. https:// doi.org/10.1016/j.hrthm.2013.10.027; PMID: 24120999
64. van der Werf C, Lieve KV, Bos JM, et al. Implantable cardioverter-defibrillators in previously undiagnosed patients with catecholaminergic polymorphic ventricular tachycardia resuscitated from sudden cardiac arrest. Eur Heart J 2019;40:2953–61. https://doi.org/10.1093/eurheartj/ ehz309; PMID: 31145795
65. Hofferberth SC, Cecchin F, Loberman D, Fynn-Thompson F. Left thoracoscopic sympathectomy for cardiac denervation in patients with life-threatening ventricular arrhythmias. J Thorac Cardiovasc Surg 2014;147:404–9. https://doi. org/10.1016/j.jtcvs.2013.07.064; PMID: 24268954
66. Schwartz PJ, Ackerman MJ, Antzelevitch C, et al. Inherited cardiac arrhythmias. Nat Rev Dis Primers 2020;6:58. https:// doi.org/10.1038/s41572-020-0188-7; PMID: 32678103
67. Sgrò A, Drake TM, Lopez-Ayala P, Phan K. Left cardiac sympathetic denervation in the management of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia: a meta-regression. Congenit Heart Dis 2019;14:1102–12. https://doi.org/10.1111/chd.12855; PMID: 31621201
68. Niaz T, Bos JM, Sorensen KB, et al. Left cardiac sympathetic denervation monotherapy in patients with congenital long QT syndrome. Circ Arrhythm Electrophysiol 2020;13:e008830. https://doi.org/10.1161/CIRCEP.120.008830; PMID: 33198487
69. Bezzerides VJ, Caballero A, Wang S, et al. Gene therapy for catecholaminergic polymorphic ventricular tachycardia by inhibition of Ca2+/calmodulin-dependent kinase II. Circulation 2019;140:405–19. https://doi.org/10.1161/ CIRCULATIONAHA.118.038514; PMID: 31155924
70. Hajjar RJ, Lyon AR. Gene therapy for the treatment of catecholaminergic polymorphic ventricular tachycardia. Circulation 2014;129:2633–5. https://doi.org/10.1161/ CIRCULATIONAHA.114.010586; PMID: 24958749
71. The Cardiac Society of Australia and New Zealand (CSANZ). Guidelines for the diagnosis and management of Catecholaminergic Polymorphic Ventricular Tachycardia. 2011; Sydney: CSANZ. https://www.csanz.edu.au/wp-content/ uploads/2013/11/Diagnosis-and-Management-of-CPVT.pdf (accessed 7 July 2022).
ARRHYTHMIA & ELECTROPHYSIOLOGY REVIEW www.AERjournal.com



































































