Advertisement
Advertisement
May 2023
Sponsored by Shockwave Medical
The New Shockwave L6 Peripheral Intravascular Lithotripsy (IVL) Catheter
Larger sizes now available for large calcified vessels.


The Shockwave L6 Peripheral IVL Catheter is available in the United States only.
What are the specific challenges in treating heavily calcified iliac arteries?
Dr. Briggs: I am a big proponent of optimizing lower extremity arterial inflow by treating the aortoiliac and common femoral segment. Some of the happiest patients I see back in clinic after an intervention are typically those in whom I have restored flow through this segment. That said, up until a few months ago, there was a subset of these patients in whom I would alter my treatment paradigm. These were patients with severe calcification of the common iliac arteries (CIAs), regardless of TransAtlantic Inter-Society Consensus (TASC) II classification. Severe calcification presents several challenges to endovascular intervention. For one, severe calcification is largely unresponsive to balloon angioplasty.
Dr. Corl: We know that heavily calcified plaque negatively impacts procedural success and long-term durability. Large vessels such as the iliac arteries can be especially challenging. Calcified iliac arteries are at a higher risk of embolization, dissection, and perforation. These complications can be very unforgiving when they occur in the iliac arteries. Treatment options can be limited and often less effective in larger vessels due to equipment specifications such as actual sizes available as well as sheath and guidewire compatibility. Standard balloon angioplasty often requires high-pressure inflations to sufficiently dilate these calcified vessels. Higher-pressure inflations increase the risk of complications. Fibroelastic recoil is common after standard balloon angioplasty in calcified vessels. Atherectomy is typically not a useful or effective treatment option in larger vessels, and stent options are somewhat limited in larger vessels as well. Larger stents, such as covered stents and balloon-expandable stents, often require larger sheaths and are often only available with shorter shaft lengths. Due to these logistics, common femoral artery (CFA) access is typically required to deliver these stents.
How do you usually approach treating calcium in the iliac arteries?
Dr. Briggs: Efforts to have the calcification respond to balloon angioplasty may include inflating a semi-compliant balloon to extremely high pressures. Unfortunately, the balloon typically overinflates in the more compliant areas of the artery that are not severely calcified, which, in some circumstances, can lead to vessel dissection and rupture. In a high-flow vessel, like the CIA, this can be a life-threatening situation. As this area is also adjacent to the aortic bifurcation, management of unilateral iliac dissection or rupture may require aortic and contralateral iliac intervention with balloon occlusion, stent grafting, or even open conversion.
Dr. Corl: My algorithm starts with imaging with angiography from a radial access approach, followed by intravascular ultrasound (IVUS). IVUS provides an accurate reference vessel diameter and detailed plaque morphology. Before the Shockwave L6 balloon (Shockwave Medical) was available, I primarily used standard balloon angioplasty, often high pressure, followed by stent placement. Occasionally intravascular lithotripsy (IVL) with the Shockwave M5+ balloon (Shockwave Medical) was an option in patients with smaller than average iliac arteries.
Do you stent in the iliacs? If so, when and why?
Dr. Briggs: Primary stenting of the CIA, particularly with balloon-expandable covered stents, has been shown to be superior to bare-metal stents in complex iliac anatomy since the COBEST trial.1 In heavily calcified arteries, however, there is a risk of poor stent expansion of any stent. Further, as there is a balloon angioplasty component “baked into” the deployment of these stents, there remains a risk of vessel injury, dissection, and rupture. This risk is heightened by severe calcification. A conundrum is then created—does one advance and deploy a stent that may poorly expand due to severe calcification or predilate and risk vessel dissection and rupture?
Dr. Corl: I routinely use stents when treating the iliac arteries. I prefer balloon-expandable covered stents in the CIAs and self-expanding stents in the external iliac arteries (EIAs). Stents deployed in the CIA and EIA have excellent long-term patency when the stents are sized with IVUS guidance and full stent expansion is achieved. Moderate-to-severe vascular calcium can lead to stent underexpansion and malapposition. Effective plaque modification with IVL allows for full stent expansion without relying on high-pressure predilatation.
How is Shockwave L6 uniquely positioned to treat calcium in the iliac arteries?
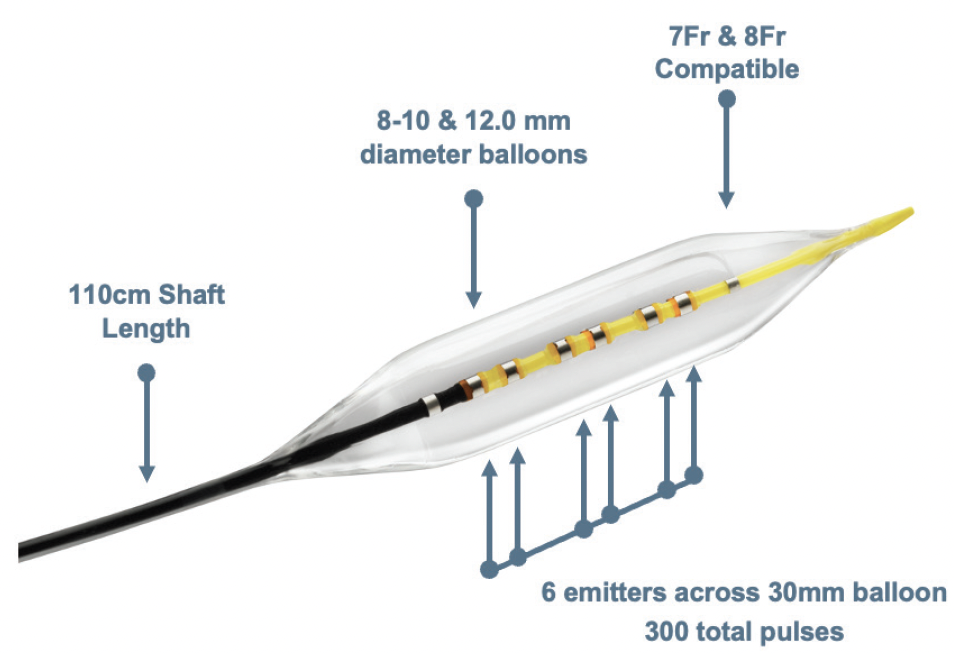
Dr. Briggs: IVL—and the Shockwave L6 specifically—has completely changed my management of heavily calcified CIAs. The Shockwave L6 catheter has uniformed sonic energy output across the entire length of the 30-mm balloon. This length is perfect, as the CIA is not much longer than 30 mm and its lesions are usually focal. The balloons also are at a larger diameter than the Shockwave M5+, from 8 to 12 mm. This is helpful for lesions in larger-diameter iliac vessels, as in men or in predilating focal iliac lesions for large-bore access for endovascular aneurysm repair, thoracic endovascular aortic repair, or transfemoral aortic valve repair.
Dr. Corl: The Shockwave L6 balloon has been a welcome addition to my iliac artery treatment algorithm. The balloon diameter range of 8 to 12 mm expands IVL to these large vessels. The 30-mm balloon length is ideal for the iliac arteries. This compact balloon length provides a focused, high-energy profile across the entire length of the balloon. This energy profile modifies both superficial and deep calcium, which improves transmural vessel compliance and decreases fibroelastic recoil. Stent expansion is optimized following effective IVL.
What do you find to be the most important feature(s) of Shockwave L6 and how would you describe it/their benefit(s)?
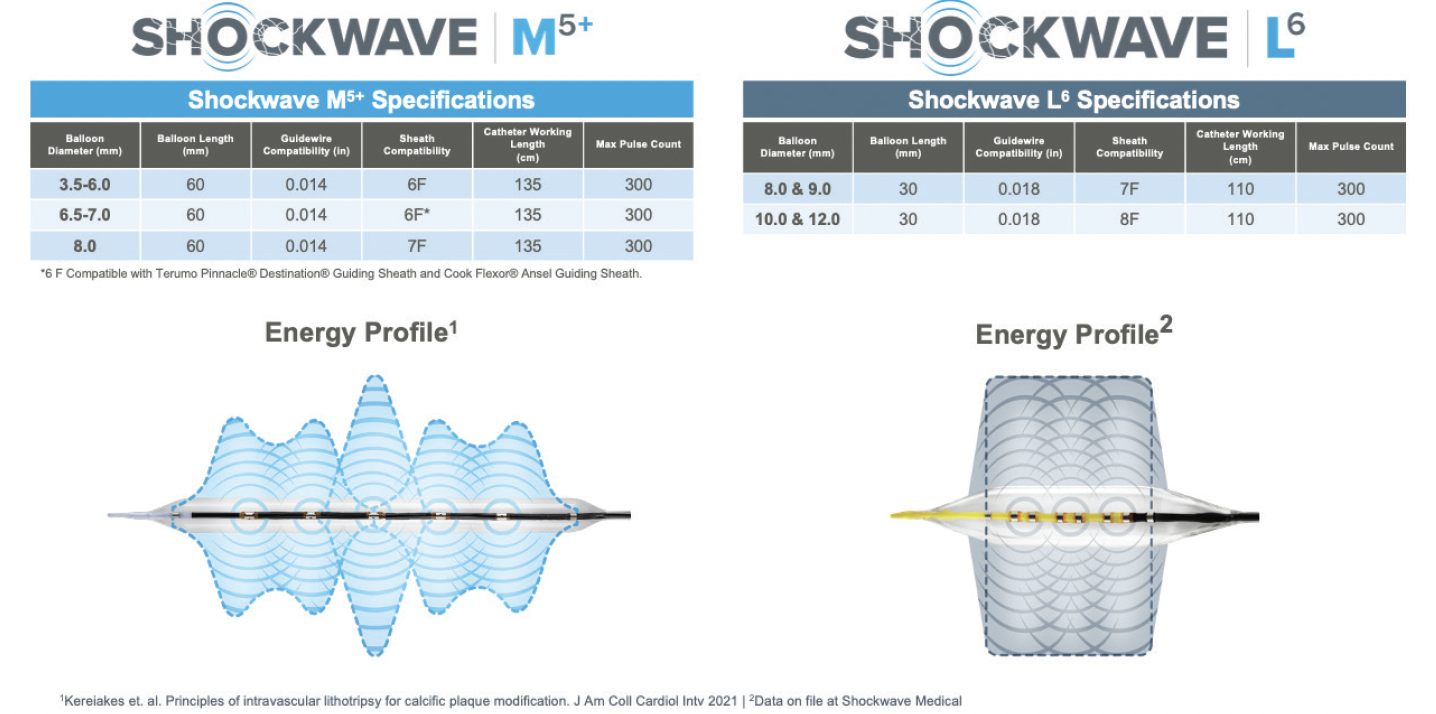
Dr. Briggs: As with most devices intended for CIA treatment, the Shockwave L6 utilizes a 7- or 8-F sheath. It has a 110-cm working length. The balloon catheter is on a 0.018-inch system, which provides moderate support for post-IVL stent deployment. The IVL therapy pressure is as low as 2 atm with a balloon burst pressure of 6 atm. These low pressures, combined with the uniform sonic energy output, provide impactful remodeling of tricky CIA calcified plaque with low risk of vessel injury.
Dr. Corl: The Shockwave L6 balloon diameter sizes are perfect for the iliac arteries. The larger balloon diameters attain appropriate vessel wall apposition to facilitate effective transmission of sonic energy. The compact emitter profile with six emitters positioned across the 30-mm balloon length creates a high sonic energy output across the length of the lithotripsy balloon. This energy profile reaches and fractures the deep calcium found in these large iliac arteries. The combination of excellent wall apposition with the larger balloons and the deep penetrating energy profile with the compact emitter design allows effective lesion preparation with an ultra-low-pressure inflation. With effective IVL, we can safely achieve full balloon expansion at 2 to 4 atm. The Shockwave L6 IVL catheters are 0.018-inch guidewire compatible, which provides adequate wire support for iliac artery interventions. The Shockwave L6 IVL balloon, like the other Shockwave balloons, is straightforward to use on an intuitively simple platform.
How do you generally size in the iliac arteries? What imaging modality do you use for sizing? Does the ultra-low pressure of Shockwave L6 impact your sizing strategy?
Dr. Briggs: I routinely obtain CTA in any patient with symptomatic aortoiliac artery inflow disease. CTA is helpful to size and strategize for any intervention and to allow for discussion of treatment plans with patients. My approach to treating severely calcified iliac vessels before IVL was either medical management alone, surgical bypass grafting, or performing a relatively risky endovascular intervention. I would primarily stent with a covered balloon-expandable stent and then postdilate, sometimes with high pressures, until there was < 30% residual stenosis and/or a lack of significant pressure gradient across the lesion. Although I have never ruptured a CIA myself, I would be prepared for this complication by utilizing the hybrid operating room with anesthesia as well as having available an aortic balloon occluder and an open instrument tray.
Currently, I cross my lesion with an 0.035-inch wire, upsize to a 7- or 8-F sheath, and exchange to a 0.018-inch wire. I then utilize 0.018-inch IVUS to confirm vessel sizing (from preintervention CTA) and location of landmarks such as the CIA ostia and bifurcation. I predilate a calcified CIA lesion with the Shockwave L6 IVL catheter at 2 atm and sometimes 4 atm until the balloon fully expands. IVL balloon sizing is at a 1.1 to 1 (balloon-to-artery) diameter ratio. Because of the low inflation pressures, I do not worry about dissection or rupture. I then deploy an appropriately sized balloon-expandable covered stent over the 0.018-inch wire to the CIA ostia or extend into the aorta by 1 to 2 mm. After intervention, I check my work with IVUS and antegrade digital subtraction angiography. I have not yet done IVL alone (or in conjunction with a drug-covered balloon) for aortoiliac TASC II A/B lesions but could imagine a scenario where that could be successful in select patients.2,3
Dr. Corl: I routinely perform IVUS for my peripheral interventions. IVUS provides accurate reference vessel measurements, which is key for sizing balloons and stents. Vessel diameter is often underestimated with angiography, which can lead to undersized balloons and stents. Undersized balloons and stents translate to poor long-term outcomes. IVL balloon sizing is very important. In the iliac arteries, I size the IVL balloon 1:1 (or greater) to vessel diameter based on IVUS measurements. The ability to modify calcified plaque with a low-pressure inflation permits safe IVL therapy with 1:1 (or greater) balloon sizing. Alternatively, high-pressure inflation with a standard balloon sized 1:1 in these densely calcified arteries carries a significant dissection and perforation risk.
Do you think the use of Shockwave L6 could impact your decision to stent?
Dr. Briggs: IVL using the Shockwave L6 balloon catheter has completely changed my treatment paradigm for severe CIA calcification. I have been able to treat more patients with lower morbidity endovascular intervention. The low-pressure, high-sonic energy output of the Shockwave L6 balloon remodels calcification in this anatomy in up to 12-mm-diameter vessels, which can then be stented or traversed with large-bore devices. This mechanism allows the provider to worry less about the catastrophic complications of stent compression, vessel dissection, or rupture. Finally, patients will be able to return to clinic happy that their lifestyle-limiting symptoms are relieved after an easily tolerated endovascular intervention.
Dr. Corl: I think I will continue to utilize stents in the CIA and EIA following IVL with the Shockwave L6 balloon. Vessel prep with IVL allows for optimal stent deployment in these heavily calcified vessels.
When do you see using Shockwave L6 versus Shockwave M5+ in the iliac arteries?
Dr. Briggs: The Shockwave M5+ balloon can also be useful in the iliacs. I find it to be better in long, calcified lesions where there is not much vessel caliber change like the EIA or femoropopliteal segment. The shorter length of Shockwave L6 is perfect for the CIA, as it is not much longer than 30 mm and lesions are usually focal. I also don’t need to worry about the shoulders of a longer balloon extending into a smaller-caliber EIA if the balloon is sized to the CIA, as vessel sizes can differ significantly.
Dr. Corl: Vessel diameter is the main consideration. The Shockwave L6 balloon is appropriate for iliac arteries > 8 mm in diameter, whereas the Shockwave M5+ is suited for vessels < 8 mm in diameter. The Shockwave L6 and Shockwave M5+ overlap at the 8-mm diameter, and either balloon can be used in an 8-mm-diameter iliac artery. In general, the Shockwave M5+ balloon may be better suited for more diffuse iliac disease, where the Shockwave L6 is more apt for a focal iliac stenosis. In most iliac arteries, I would typically select the Shockwave L6 IVL balloon to take advantage of the deep penetrating energy profile created by the compact layout of the six emitters. Another consideration is that the Shockwave M5+ IVL catheter has a 135-cm shaft compared to the 110-cm shaft on the Shockwave L6 catheter. The longer shaft on the Shockwave M5+ catheter can reach the iliac arteries from a radial access approach, which grants a radial-to-peripheral option for select patients.
There’s been significant improvement in Medicare hospital reimbursement for peripheral IVL. Does this impact your access to IVL at your institution?
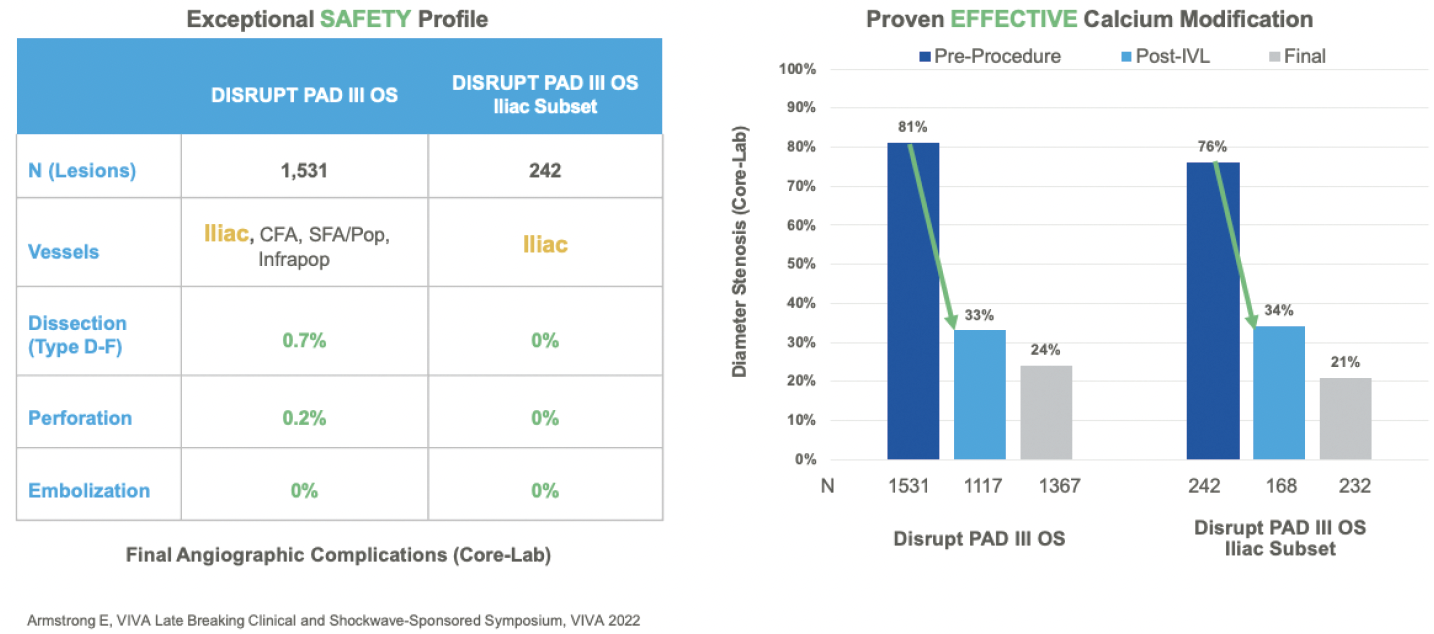
Dr. Briggs: IVL is now reimbursed at the level of other plaque-modifying devices, like atherectomy, but it is the only plaque-modifying device intended for use in the iliac arteries. At my institution, I had access to IVL for use in the iliac arteries before the new reimbursement paradigm. My partners and I used it for large-bore access to avoid iliac conduit and to increase calcified iliac arterial compliance before stent deployment. IVL has worked well for us for many years. Before IVL, when treating heavy iliac calcification, there seemed to be a much higher risk of vessel dissection, avulsion, rupture, and/or stent nonexpansion. These have a profound effect on cost, not to mention patient morbidity and mortality. Management of iliac complications, including intensive care unit care, hospital care, and reinterventions, are all quite expensive. So, while it is fantastic that IVL has seen significant improvement in hospital reimbursement, I would use it regardless. The data from Disrupt PAD support its use. At the end of the day, my job is to do what is best and safest for patients. I would use IVL in stenotic, calcified iliac arteries whether it was reimbursed or not.
Dr. Corl: Fortunately, at The Christ Hospital, access to IVL and other beneficial treatment technologies have always been based on patient need as opposed to relying on financial considerations. That being said, it is a huge plus that IVL reimbursement has improved to take some of the stress off the finances involved in these complex procedures.
1. Mwipatayi BP, Thomas S, Wong J, et al. A comparison of covered vs bare expandable stents for the treatment of aortoiliac occlusive disease. J Vasc Surg. 2011;54:1561-1570. doi: 10.1016/j.jvs.2011.06.097
2. Tetteroo E, van der Graaf Y, Bosch JL, et al. Randomised comparison of primary stent placement versus primary angioplasty followed by selective stent placement in patients with iliac-artery occlusive disease. Dutch Iliac Stent Trial Study Group. Lancet. 1998;351:1153-1159. doi: 10.1016/s0140-6736(97)09508-1
3. Tepe G, Brodmann M, Werner M, et al. Intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized Disrupt PAD III trial. JACC Cardiovasc Interv. 2021;14:1352-1361. doi: 10.1016/j.jcin.2021.04.010
Severe Aortic and CIA Calcifications; CIA Treated With Shockwave L6 IVL
By Charles Briggs, MD
CASE PRESENTATION
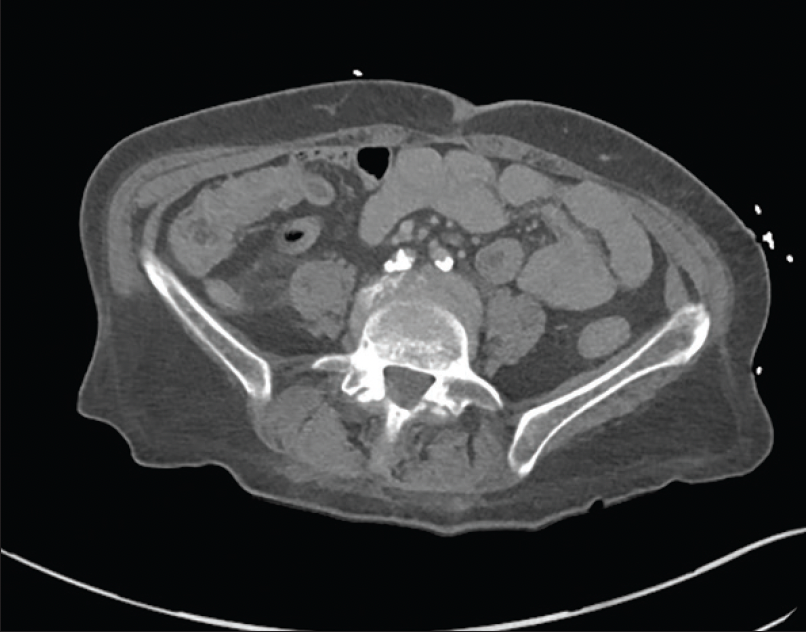
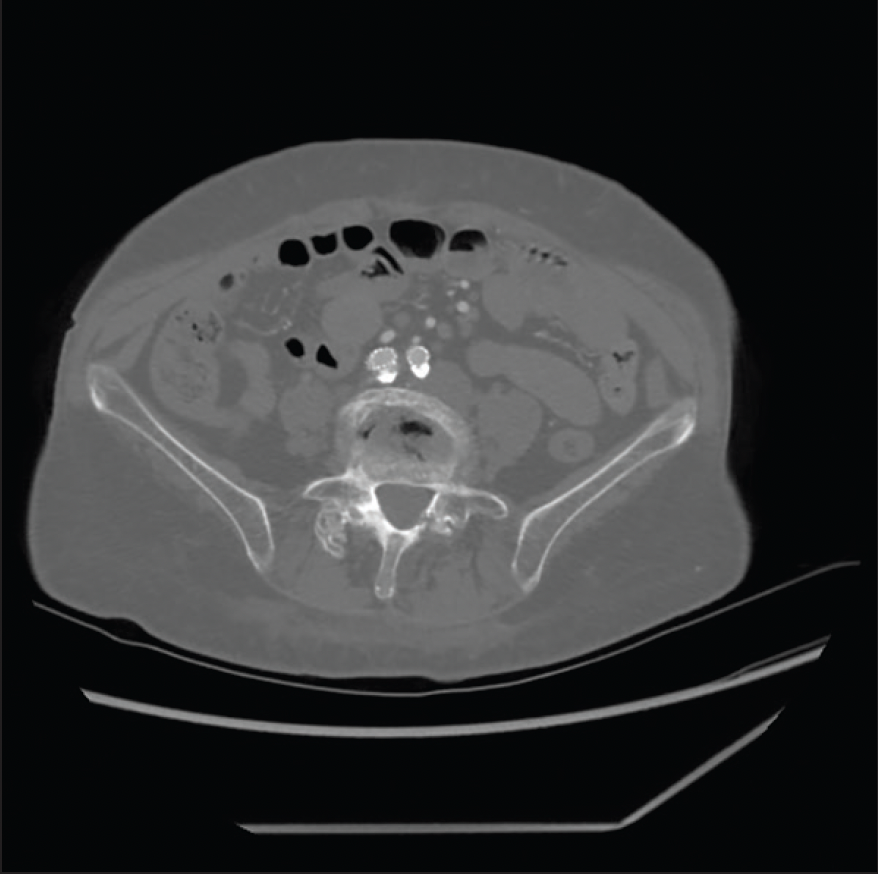
A woman in her early 60s was referred for right lower extremity lifestyle-limiting claudication and ischemic rest pain. She described lifestyle-limiting claudication of the right buttock, hip, thigh, and calf, as well as right foot numbness while in bed at night, which was relieved by getting up and “shaking it off.” Her comorbidities included coronary artery disease, heart failure with preserved ejection fraction, chronic obstructive pulmonary disease, atrial fibrillation, hypertension, hyperlipidemia, and type 2 diabetes mellitus. She was an active smoker. She was considered high risk for open surgery. Preoperative workup included an ankle-brachial index (ABI) of 0.42 on the right and 0.81 on the left. CTA of the abdomen and pelvis had been performed in 2019, which revealed severe calcification of the aortic bifurcation and CIAs with associated stenosis (Figure 1).
PROCEDURAL OVERVIEW
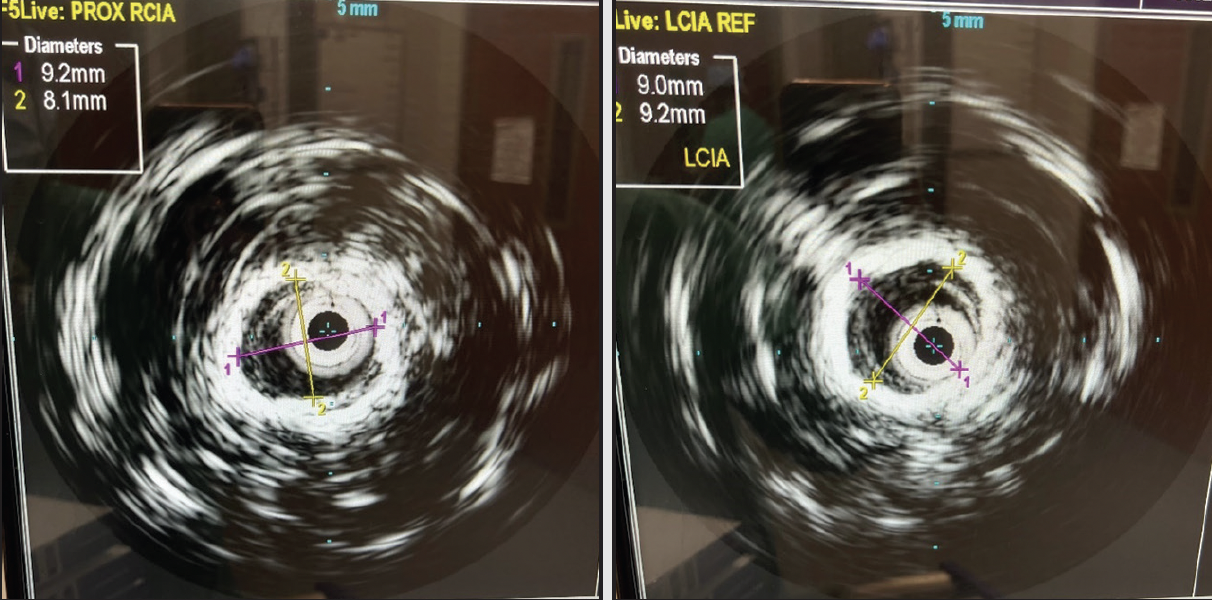
The patient was brought to the hybrid operating room, where she was anesthetized generally. Both groins were prepped, bilateral CFA access was achieved, and 5-F sheaths were placed bilaterally. From the left groin, a flush catheter was advanced into the abdominal aorta for aortography (Figure 2), given that there were no prior contrasted imaging results since 2019. From the right groin, I crossed the subtotally occluded CIA in a retrograde fashion with a 0.035-inch stiff straight Glidewire and Glidecath (Terumo Interventional Systems). The sheath was upsized bilaterally to 8 F. The wires were exchanged for 0.018-inch wires over which the IVUS catheter was advanced, which is helpful to confirm true luminal crossing, identify landmarks such as the CIA ostia, and obtain length and diameter measurements. The right CIA was predilated with a 4- X 40-mm balloon, as the IVUS catheter would not initially pass. After predilation, IVUS revealed a > 75% right CIA stenosis. CIA length was also shown to be 46 mm. IVUS also highlighted severe distal aortic stenosis and moderate left CIA stenosis. I elected to proceed with complete endovascular reconstruction of the aortic bifurcation.
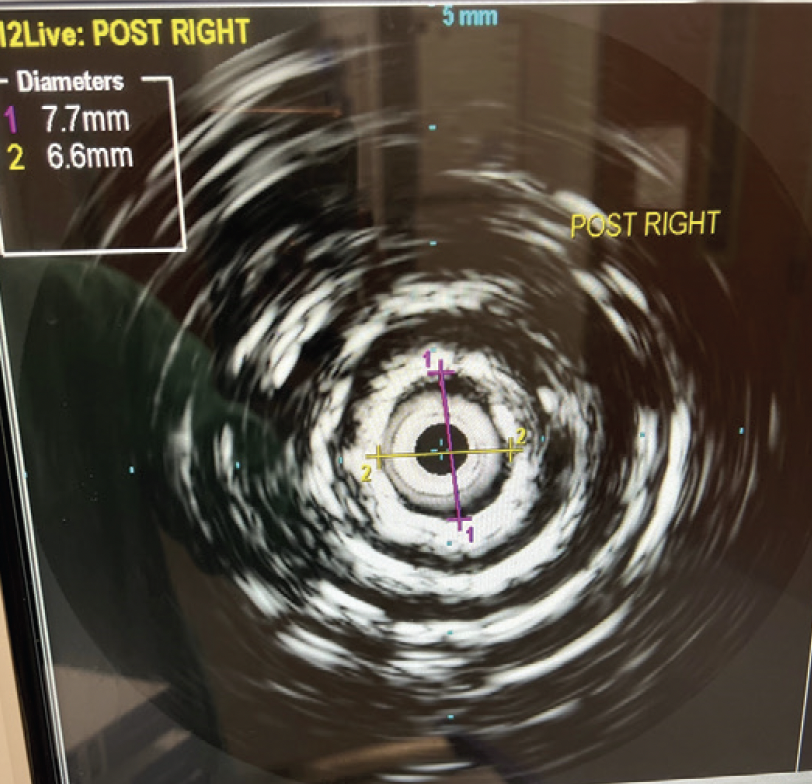
Over the 0.018-inch wires bilaterally, I advanced 8.0-mm Shockwave L6 IVL balloons to the proximal CIA, as maximal iliac diameter was 7.4 mm. IVL was done in a “kissing” fashion at 2 and then 4 atm for a total of two inflations. The lesions yielded quickly with the first low (2 atm) pressure inflation, but I elected to reinflate to 4 atm to be extra confident in my upcoming stent deployment. The Shockwave L6 design was helpful here, as I would otherwise have needed several wire exchanges between 0.035- and 0.014-inch to do IVL and stenting if I had used the Shockwave M5+ balloon. I also appreciated the 30-mm Shockwave L6 design in this case to avoid the shoulders of my balloon potentially causing a dissection in the relatively narrow, chronically underperfused EIAs. With its uniform sonic energy output, the Shockwave L6 IVL catheter also appeared more powerful, even at much lower pressures. I deployed an 8- X 59-mm Gore Viabahn VBX balloon-expandable covered stent (Gore & Associates) in the distal aorta over the 0.018-inch wire and postdilated it proximally with a 14- X 20-mm Atlas balloon (BD Interventional). The stent had been advanced and deployed through the left groin with the wire pulled back slightly from the right. The wire from the right was now advanced through the true lumen of the stent and confirmed with IVUS, which also showed the result of the right IVL treatment (Figure 3). Bilaterally, I then advanced and deployed 8- X 79-mm Viabahn VBX stents into the distal aortic stent, which also postdilated the distal aortic stent. IVUS was readvanced bilaterally, demonstrating an optimal result of the right CIA stent (Figure 4). Antegrade completion aortogram showed a satisfactory result (Figure 5). After sheath removal, the patient had palpable pedal pulses in both feet. Dual antiplatelet and maximal-dose statin therapy was prescribed.
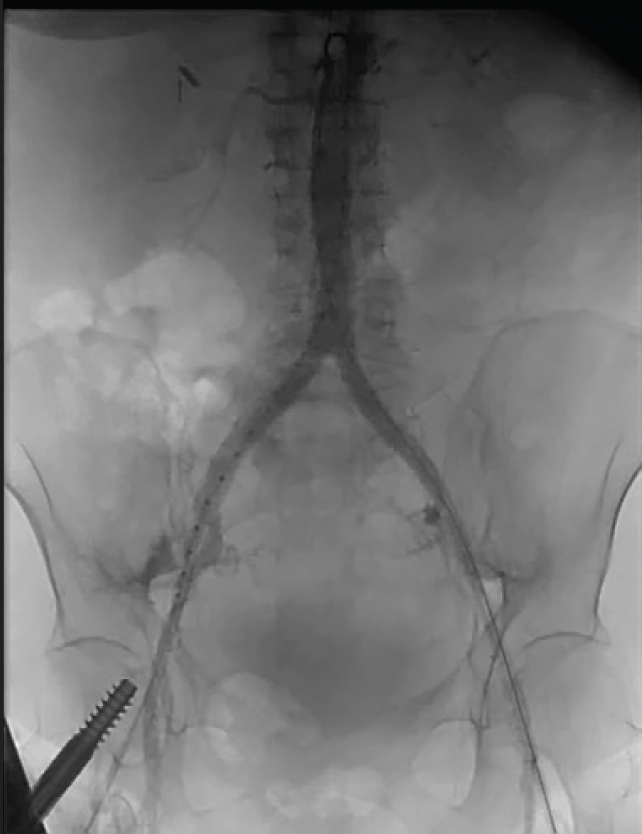
At follow-up, the patient was asymptomatic from a peripheral artery disease standpoint, with normal ABIs bilaterally. A postintervention CTA demonstrated widely patent aortic and bilateral CIA stents (Figure 6).
Use of Shockwave L6 IVL in Severely Calcified Iliac Arteries
By JD Corl, MD, FACC, FSCAI
CASE PRESENTATION
A woman in her early 70s was initially referred for evaluation and treatment of severe lifestyle-limiting claudication involving the bilateral lower extremities. Her claudication symptoms had worsened over the past 3 to 4 months. Her past medical history includes coronary artery disease, diabetes, hypertension, hyperlipidemia, tobacco abuse, obesity, and a history of a transient ischemic attack. A recent lower extremity duplex ultrasound study revealed monophasic waveforms in bilateral CFAs consistent with significant inflow disease.
PROCEDURAL OVERVIEW
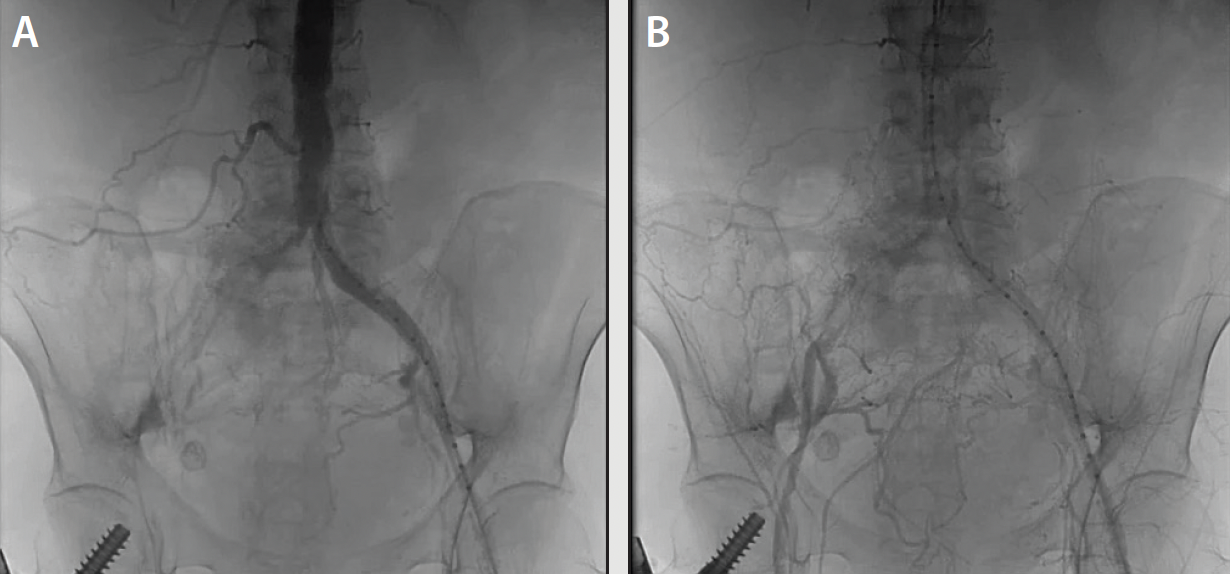
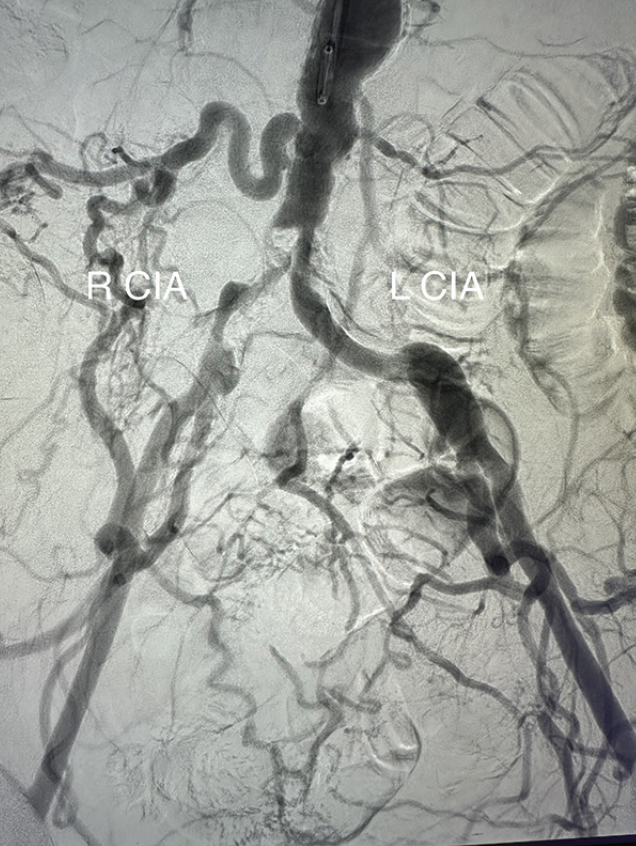
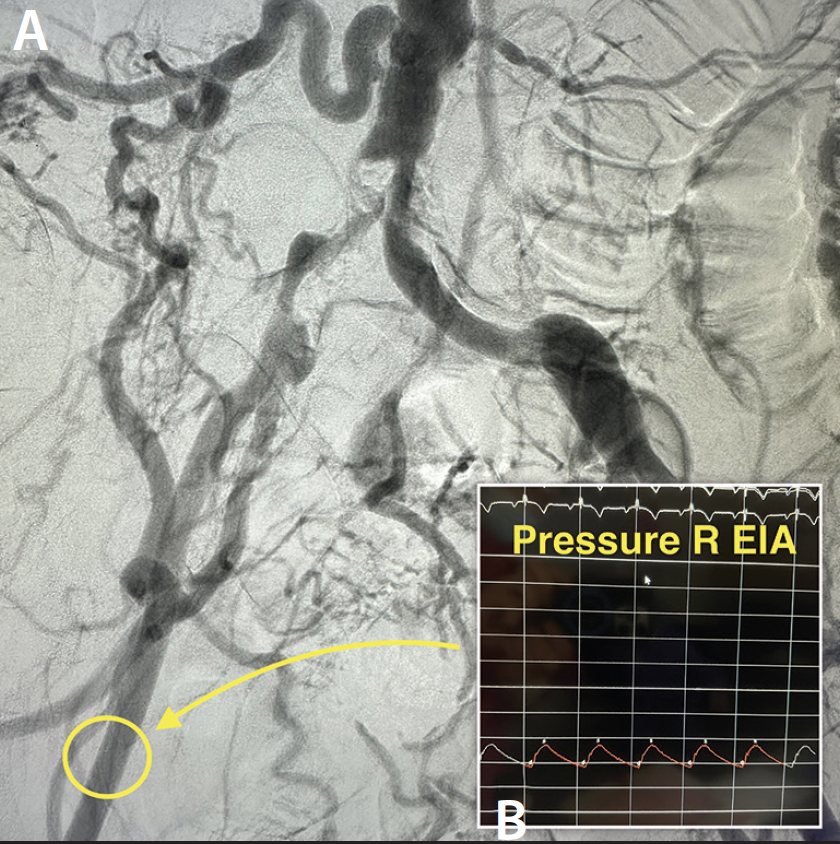
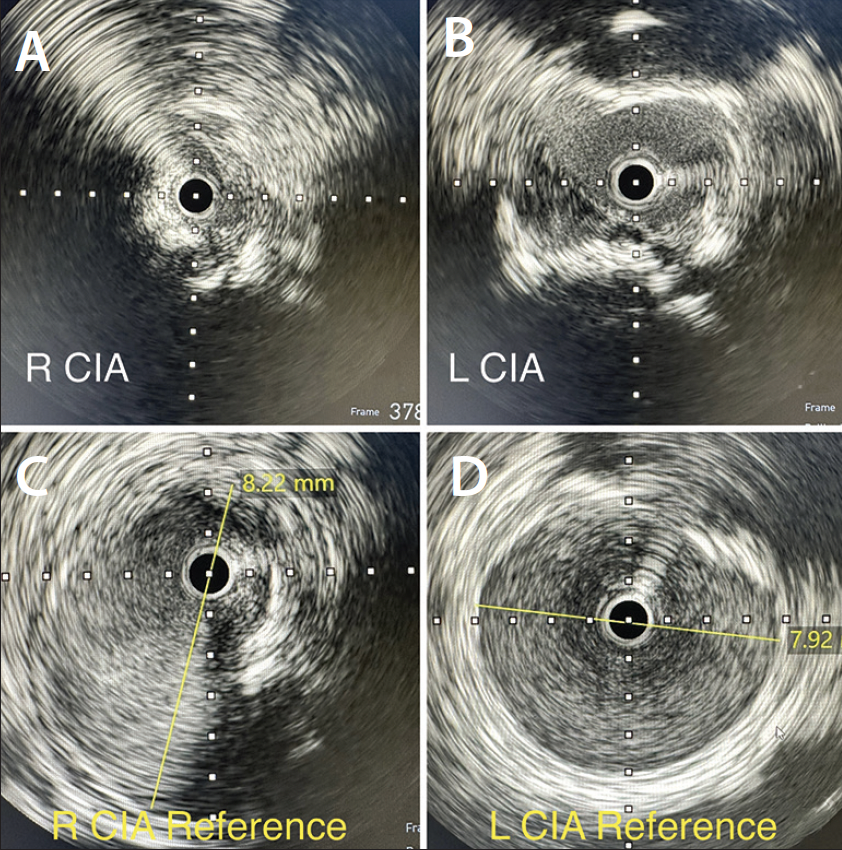
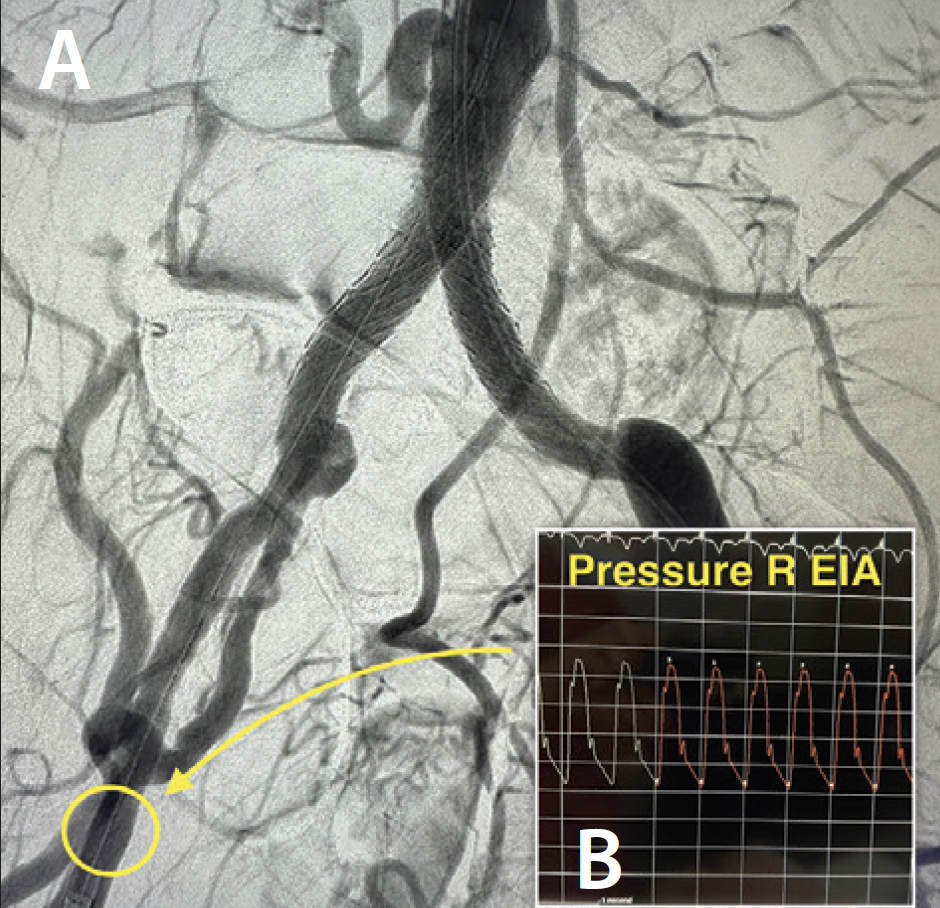
An abdominal aortogram from right radial access revealed a subtotal occlusion of the right CIA and a severe stenosis involving the left CIA (Figure 1). The pigtail catheter was removed, the radial sheath was exchanged for a 119-cm R2P sheath (Terumo Interventional Systems), and 8-F sheaths were placed in the bilateral CFAs using ultrasound-guided access. An invasive arterial blood pressure test obtained in the right EIA showed a severely dampened pressure waveform (Figure 2B). Both CIAs were successfully crossed with 0.018-inch guidewires via the CFA sheaths. IVUS showed severe calcification and heavy plaque burden with severe luminal narrowing in the right and left CIA (Figure 3A and 3B). The reference vessel diameters were 8.22 and 7.92 mm for the right and left CIA, respectively (Figure 3C and 3D).
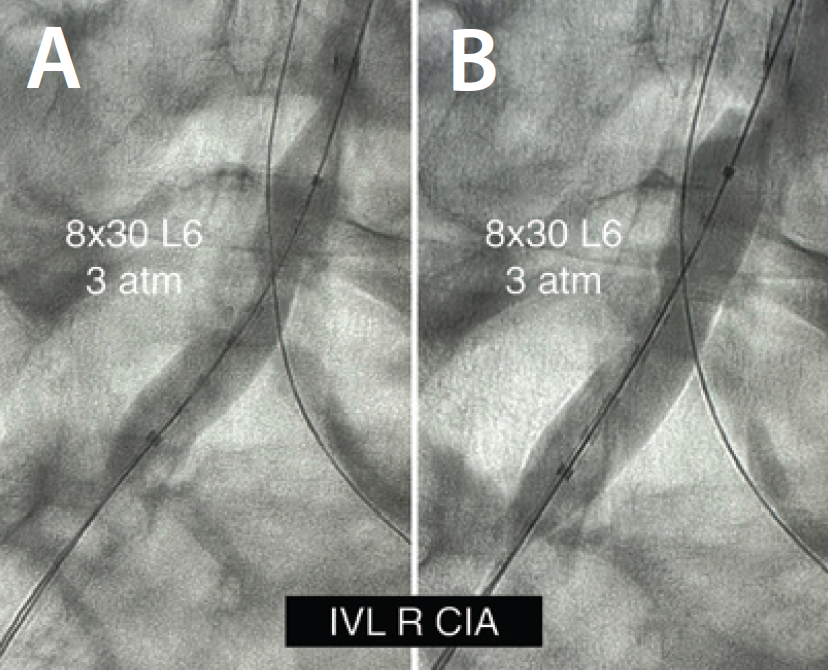
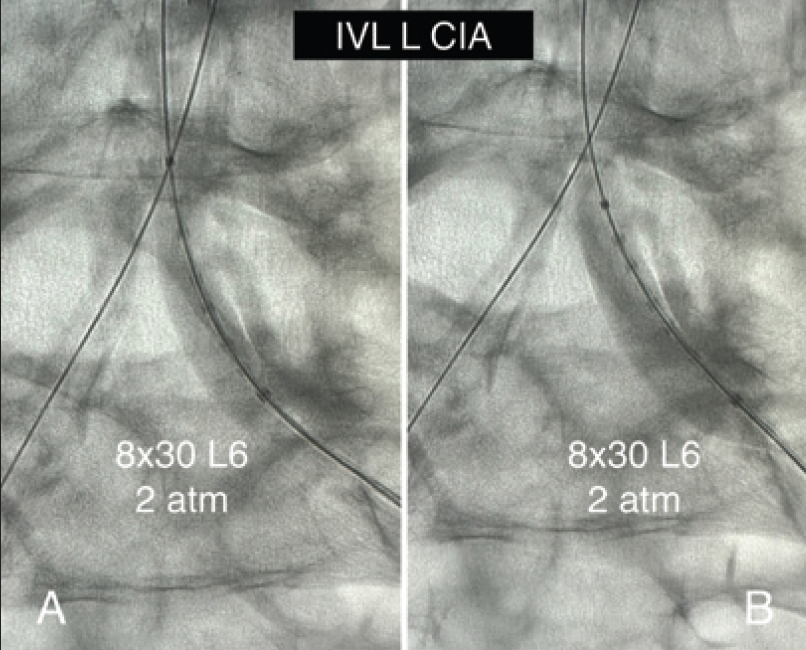
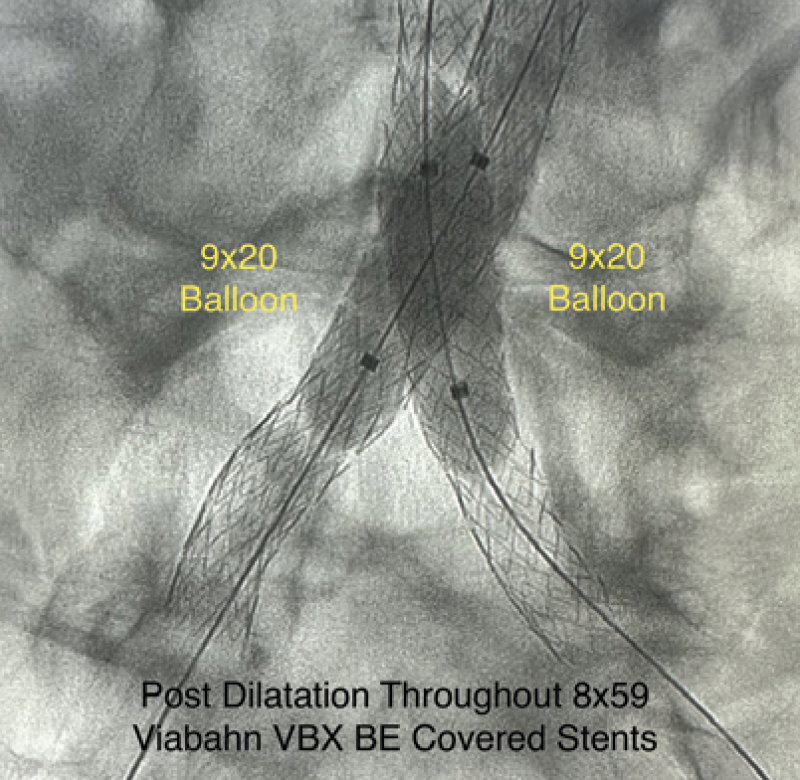
IVL was performed on the bilateral CIAs using an 8- X 30-mm Shockwave L6 catheter, delivering 180 pulses in the right CIA with six low-pressure inflations (Figure 4) and 120 pulses in the left CIA with four low-pressure inflations (Figure 5). All Shockwave L6 inflations ranged from 2 to 4 atm. A pair of 8- X 59-mm Viabahn VBX balloon-expandable covered stents were introduced and deployed simultaneously in bilateral CIAs extending up into the distal aorta using a kissing technique. Postdilatation was performed throughout both covered stents using two 9- X 20-mm balloons (Figure 6). The postintervention angiogram showed an excellent angiographic result (Figure 7A), with significant improvement and normalization of invasive blood pressure in the right EIA postintervention (Figure 7B).
DISCUSSION
Severely diseased, heavily calcified bilateral CIAs were safely treated with the ultra-low-pressure Shockwave L6 IVL catheter and Viabahn VBX balloon-expandable covered stents. Alternatively, these calcified stenoses could have been treated with high-pressure standard balloon angioplasty instead of IVL for vessel preparation. However, high-pressure balloon angioplasty in these vessels would carry significant risk of embolization, dissection, or perforation. IVL can effectively modify calcified plaque with low-pressure inflations (2-4 atm), minimizing the risk of complications related to barotrauma secondary to higher-pressure balloon inflations. IVL with the Shockwave L6 catheter is simple and intuitive. In this case, heavily calcified, severely stenosed CIAs (Figure 1) were successfully modified and prepped with an appropriately sized Shockwave L6 balloon. After the vessel was safely and effectively prepped with IVL, a pair of Viabahn VBX balloon-expandable covered stents were deployed with full expansion to achieve an excellent angiographic result (Figure 7A). This case further supports that severely calcified iliac arteries can be safely and effectively modified using the Shockwave L6 IVL catheter to facilitate optimal stent deployment.
Disclosures
Dr. Briggs: Paid consultant to Shockwave Medical.
Dr. Corl: Paid consultant to Shockwave Medical.
Views expressed are those of the authors and not necessarily those of Shockwave Medical.
Shockwave M5+, Shockwave M5, Shockwave S4 and Shockwave L6 Safety Information
In the United States: Rx only.
Indications for Use
The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary or cerebral vasculature.
Contraindications
Do not use if unable to pass 0.014″ (M5, M5+, S4) or 0.018″ (L6) guidewire across the lesion-Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings
Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device—Use the generator in accordance with recommended settings as stated in the Operator’s Manual.
Precautions
Use only the recommended balloon inflation medium—Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology.
Adverse effects
Possible adverse effects consistent with standard angioplasty include—Access site complications—Allergy to contrast or blood thinner—Arterial bypass surgery—Bleeding complications—Death—Fracture of guidewire or device—Hypertension/Hypotension—Infection/sepsis—Placement of a stent—Renal failure—Shock/pulmonary edema—Target vessel stenosis or occlusion—Vascular complications. Risks unique to the device and its use—Allergy to catheter material(s)—Device malfunction or failure—Excess heat at target site.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. www.shockwavemedical.com
Advertisement
Advertisement