Structures of a Bifunctional Cell Wall Hydrolase CwlT Containing a Novel Bacterial Lysozyme and an NlpC/P60 dl-Endopeptidase.
Xu, Q., Chiu, H.J., Farr, C.L., Jaroszewski, L., Knuth, M.W., Miller, M.D., Lesley, S.A., Godzik, A., Elsliger, M.A., Deacon, A.M., Wilson, I.A.(2014) J Mol Biol 426: 169-184
- PubMed: 24051416
- DOI: https://doi.org/10.1016/j.jmb.2013.09.011
- Primary Citation of Related Structures:
4FDY - PubMed Abstract:
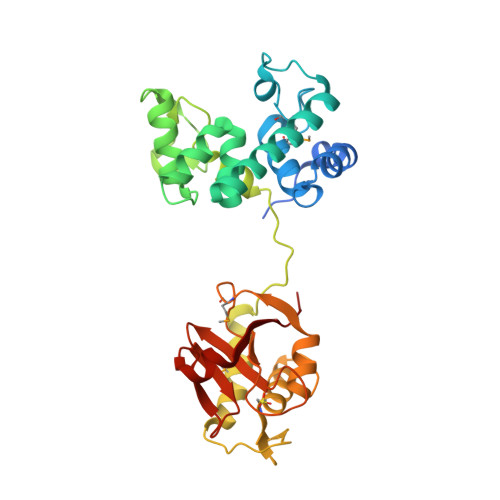
Tn916-like conjugative transposons carrying antibiotic resistance genes are found in a diverse range of bacteria. Orf14 within the conjugation module encodes a bifunctional cell wall hydrolase CwlT that consists of an N-terminal bacterial lysozyme domain (N-acetylmuramidase, bLysG) and a C-terminal NlpC/P60 domain (γ-d-glutamyl-l-diamino acid endopeptidase) and is expected to play an important role in the spread of the transposons. We determined the crystal structures of CwlT from two pathogens, Staphylococcus aureus Mu50 (SaCwlT) and Clostridium difficile 630 (CdCwlT). These structures reveal that NlpC/P60 and LysG domains are compact and conserved modules, connected by a short flexible linker. The LysG domain represents a novel family of widely distributed bacterial lysozymes. The overall structure and the active site of bLysG bear significant similarity to other members of the glycoside hydrolase family 23 (GH23), such as the g-type lysozyme (LysG) and Escherichia coli lytic transglycosylase MltE. The active site of bLysG contains a unique structural and sequence signature (DxxQSSES+S) that is important for coordinating a catalytic water. Molecular modeling suggests that the bLysG domain may recognize glycan in a similar manner to MltE. The C-terminal NlpC/P60 domain contains a conserved active site (Cys-His-His-Tyr) that appears to be specific to murein tetrapeptide. Access to the active site is likely regulated by isomerism of a side chain atop the catalytic cysteine, allowing substrate entry or product release (open state), or catalysis (closed state).
Organizational Affiliation:
Joint Center for Structural Genomics (http://www.jcsg.org); Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, 2575 Sand Hill Road, Menlo Park, CA 94025, USA.