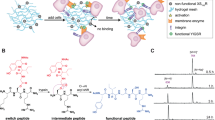
Abstract
The communication of cells with their surroundings is mostly encoded in the epitopes of structural and signalling proteins present in the extracellular matrix (ECM). These peptide epitopes can be incorporated in biomaterials to serve as function-encoding molecules to modulate cell–cell and cell–ECM interactions. In this Review, we discuss natural and synthetic peptide epitopes as molecular tools to bioengineer bioactive hydrogel materials. We present a library of functional peptide sequences that selectively communicate with cells and the ECM to coordinate biological processes, including epitopes that directly signal to cells, that bind ECM components that subsequently signal to cells, and that regulate ECM turnover. We highlight how these epitopes can be incorporated in different biomaterials as individual or multiple signals, working synergistically or additively. This molecular toolbox can be applied in the design of biomaterials aimed at regulating or controlling cellular and tissue function, repair and regeneration.
Key points
-
The communication between cells and their extracellular matrix (ECM) is encoded in peptide epitopes of structural and signalling ECM proteins, acting as molecular ‘orchestrators’ of multiple cell functions.
-
Peptide epitopes can be incorporated in biomaterials to achieve selective and specific communication with cells and tissues.
-
Biomaterials designed with specific peptide epitopes can control cell adhesion, differentiation, immunomodulation and extracellular matrix turnover.
-
A peptide epitope molecular toolbox can be applied for the design of functional synthetic ECMs in tissue engineering and bioengineering applications.
Similar content being viewed by others
Introduction
Molecular and cellular building blocks communicate with each other in a coordinated manner to define the structure and function of tissues. In particular, the extracellular matrix (ECM) is a key regulator of cell communication and regulation. The ECM is a meshwork composed of water, proteins (including collagens, fibronectin, elastin and laminins), proteoglycans, signalling cues (such as growth factors and cytokines), minerals and ions1. ECM composition varies among tissues and provides an adaptive microenvironment2, exhibiting precise, often co-existing, mechanisms, including feedback loops, to control molecule concentration3, protein disorder–order transitions to temporally control signalling4, and enzymatic degradation5 to allow turnover. For example, in bone healing, evolving and coordinated signalling events finely encode and regulate inflammation, immunomodulation, recruitment of cells, osteogenesis, angiogenesis and ultimately tissue repair6. Therefore, the ECM not only provides a rich resource of signals to interact with cells, but can also serve as inspiration for the design of biomaterials aimed at communicating with cells.
Biomaterials are used in vaccines, drug delivery systems, imaging tools, prostheses, biosensors, bioelectrodes, tissue engineering scaffolds and surgical equipment7. Most biomaterials rely on physiochemical properties, such as surface charge, chemical composition, topography and stiffness, to interact with biomolecules and cells, resulting in a limited capacity to guide biological responses. To design biomaterials that precisely communicate with biological structures, selectivity, specificity, dynamicity, hierarchy and multifunctionality can be implemented; however, to achieve clinical translation, regulatory and financial constraints should be considered in biomaterial design8.
In particular, protein epitopes can be used as building blocks to engineer cell-instructive biomaterials9. Proteins are composed of amino acid chains called peptides, which are referred to as epitopes if they contain a bioactive sequence capable of interacting with specific cell receptors or antibodies. These short amino acid sequences are the main ‘function-encoding language’ of proteins and can be used as building blocks of biomaterials. In particular, self-assembling peptides are molecular building blocks with high propensity to self-assemble into ECM-like nanofibres and hydrogels in aqueous environments10. This material platform enables the presentation of peptide epitopes on the surface of nanofibres to actively interact with cells, initiate specific cell signalling pathways, serve as therapeutic agents, or target cells and tissues in vivo11. The versatile molecular design of self-assembling peptides also allows the engineering of 3D nanofibrillar hydrogels with a range of mechanical, chemical and biological properties for applications in cell culture, cell delivery and biofabrication12,13.
Compared with full-length proteins, peptides show less degradation, more long-term functionality, higher purity, and less sensitivity to pH and temperature, and, in some cases, they can achieve more precise cell signalling14. In addition to ECM epitopes, synthetic amino acid sequences can be designed by artificial intelligence (AI) and by taking information from phage display15, complementing natural peptides as structural and signalling building blocks of biomaterials that can interact, communicate with and respond to their cellular surroundings.
In this Review, we discuss the design of biomaterials based on peptides as signalling building blocks. We provide a library of peptide epitopes that can serve as a molecular toolbox for the bioengineering of synthetic hydrogels with biological functions such as structural support, signalling and dynamicity (Fig. 1). Based on the function encoded in the hydrogel, we discuss peptides exploited for direct signalling, which foster cell adhesion, differentiation and immunomodulatory effects; peptides binding signalling or structural cues, such as growth factors, proteins and minerals; and enzymatically cleavable peptides to create degradable hydrogels. Finally, we describe the combination of the aforementioned peptides to induce synergistic or additive effects.
a, Classification and examples of peptide epitopes. b, Synthetic hydrogels endowed with function-encoding epitopes can induce multiple biological effects, such as adhesion, differentiation, immunomodulation and matrix turnover in encapsulated cells. ECM, extracellular matrix; MMP, matrix metalloproteinase.
Peptide sequences for direct signalling
Cell adhesion receptors, in particular, integrins and cadherins16, are pivotal for cell–cell and cell–ECM communication, transferring environmental information into intracellular pathways. An integrin receptor is a heterodimer structure containing an α and a β subunit, whose pairings are specific for ligand-binding interactions. For example, RGD peptides, which are present in many ECM proteins, including vitronectin, fibrinogen, osteopontin, bone sialoprotein and fibronectin17, are recognized and bound by multiple integrin subunits18, such as αvβ3, αvβ5, αvβ1, αvβ6, αvβ8, α5β1, α8β1 and αIIbβ319. Integrins can engage with elements of the cytoskeleton, such as actin, vinculin and talin molecules, transducing external information into changes of cell shape, cell architecture, motility and differentiation20. Above a certain threshold adhesion area, integrin adhesion strength increases exponentially with the number of bound integrins, and with recruited vinculin and talin21. Moreover, adhesion strength is modulated by integrin bonds, bond force and distribution along the cell contact area22.
Cadherins display extracellular tandem repeats in cell-to-cell junctions to create linkages with actin-containing cytoskeletons, while they interact intracellularly with catenins to modulate differentiation and morphogenesis23,24,25. Alongside cell adhesion, different transmembrane receptors are exploited by cells to bind specific growth factors and cytokines. Here, ‘direct signalling’ refers to the direct interaction of cell surface receptors with peptide epitopes to trigger intracellular signalling. Peptides bound by cell receptors include peptides promoting cell adhesion, mimicking growth factor epitopes, or regulating immunomodulatory effects (Fig. 2, Table 1 and Supplementary Table 1).
a,b, Peptide epitopes inducing direct signalling include: (a) adhesion peptides operating, for example, through integrin binding, and (b) growth factor-mimetic peptides mimicking, for example, transforming growth factor-β (TGFβ) molecules that bind to TGFβ receptors (TGFβR). c,d, Other peptides have been explored for immunomodulatory effects, including (c) antimicrobial peptides able to disrupt bacteria cell membranes and (d) immunomodulatory peptides that elicit immune-cell responses. MHC, major histocompatibility complex; Smad, small mothers against decapentaplegic protein.
Peptide sequences promoting cell adhesion and growth
Cell adhesion epitopes derived from fibronectin
The RGD peptide, present in the fibronectin domain FNIII9-10, is one of the most studied peptide sequences for cell adhesion. Synthetic polymers, such as polyethylene glycol (PEG) diacrylate (PEGDA), polyvinyl alcohol and poly-2-hydroxyethylmethacrylate, can be functionalized with RGD-containing motifs to control the adhesion and spreading of mammalian cells26. RGD-containing peptides can also be implemented in self-assembling peptide systems as structural and signalling building blocks, for example, in peptide amphiphile (PA) hydrogels to induce cell adhesion27, enamelogenesis28 and bone formation29; in RADA16-I hydrogels to differentiate human tendon stem and progenitor cells30; and in FEFEFKFK hydrogels for delivery and recovery of cardiac progenitor cells31. In these supramolecular hydrogels, RGD display can be controlled through co-assembly to modulate RGD density32, by host–guest moieties for dynamic display33, or to integrate surface topographies34. Thus, incorporation of RGD endows synthetic hydrogels with cell adhesion features.
Cell adhesion epitopes derived from laminins
Laminin-derived peptides, including IKVAV from the laminin α-chain, YIGSR, PDSGR, RYVVLPR from the laminin β1-chain, and RNIAEIIKDI from the laminin γ-chain, can be applied to induce cell aggregation and cluster formation in angiogenesis and neurogenesis35. For example, peptide-decorated synthetic polymers, such as poly(HEMA-co-AEMA) with YIGSR/IKVAV, and poly(tetrafluoroethylene-co-hexafluoropropylene) with YIGSR/IKVAV/RGD, have been explored in neural tissue engineering36,37. PEGDA with IKVAV and poly(urethane urea)/PEG with YIGSR can promote in vitro endothelialization38 and in vivo angiogenesis in a mouse model39. Self-assembling RADA16 hydrogels containing IKVAV, YIGSR and PDSGR promote cell attachment, and increase the expression of the neural marker βIII-tubulin in vitro and neurite network formation of mouse embryonic stem cells40; for example, IKVAV epitopes incorporated within PA hydrogels promote in vitro differentiation of neural progenitor cells and prevent differentiation of astrocytes, which are involved in scar formation after injury41. Thus, IKVAV-displaying PAs have been explored to reduce astrocyte-derived scars and aid regeneration of descending motor fibres and ascending sensory fibres in a mouse model42,43. Laminin is also an important component of the liver, and therefore, RADA16-I hydrogels comprising YIGSR can be used to culture hepatocyte for drug screening44. In addition, laminins are present in the pericellular matrix of nucleus pulposus cells, regulating nucleus pulposus cell attachment. PEG-based hydrogels containing IKVAV and AG73 peptides (full sequences are CSRARKQAASIKVAVSADR and CGGRKRLQVQLSIRT, respectively, from laminin-111 globular domains) can induce a juvenile phenotype in human degenerate nucleus pulposus cells through modulation of hydrogel stiffness and epitope density45. Therefore, laminin-mimetic peptides can serve as building blocks to enhance cell attachment and improve tissue regeneration in endothelial and neural tissues.
Cell adhesion epitopes derived from tenascins
Peptides derived from tenascins, a class of glycoproteins involved in neurogenesis, skeletogenesis and vasculogenesis, can be used as function-encoding tools to promote cell adhesion; for example, tenascin C is involved in spinal-cord regeneration, axon ingrowth and brain development46, and thus, hydrogels containing peptides from tenascin C have been explored for neural tissue engineering. In particular, binding of the tenascin-derived octapeptide VFDNFVLK to α7β1 integrins increases neurite length and promotes cell attachment in cerebral granule and cortical neurons47. Cell migration, differentiation and neurite formation can be promoted in neurospheres cultured on tenascin-C-mimicking PAs48. Similarly, a tenascin-C-derived peptide can direct mesenchymal stem cell (MSC) differentiation towards the osteogenic lineage, promoting cell viability, cell differentiation and mineralization in vitro49. Moreover, acetyl-VFDNFVLK, which is the shortest bioactive sequence derived from tenascin C, can form biocompatible and injectable hydrogels at physiological pH50. This hydrogel was tested in vitro as cell substrate for various neural and non-neural-derived cell lines (L929, C6, NIH3T3 and LN18), exhibiting low cytotoxicity and promoting cell adhesion and migration50.
Cell adhesion epitopes derived from collagens
Collagens provide structural support to soft and hard tissues, binding to integrins to support cell attachment. The collagen superfamily is made of 46 different polypeptide chains that supramolecularly assemble into 28 collagen types, depending on their hierarchical organization and function. As such, collagen types can be found as fibrillar components, such as type I, II, III and V, in bone and cartilage; as organized meshwork, including type IV, VI, VIII and X, in the cornea and dermis; and as anchoring fibrils, such as type VII, in the bladder51. The collagen-mimetic peptide KOD ((PKG)4-(POG)4-(DOG)4) establishes strong lysine–aspartate salt-bridged hydrogen bonds and can hierarchically assemble into stable triple helices52. In a thrombosis in vitro model using whole human blood, KOD-based hydrogels promote platelet adhesion, with blood clotting and degradation rates similar to those triggered by animal-derived collagen53. Furthermore, injection of POG-containing PA hydrogels into a degenerate rabbit intervertebral-disc (IVD) model leads to increased deposition of ECM components of the IVD, including glycosaminoglycans (GAGs) and collagen type II54, promoting recovery of the intervertebral disc within 2 weeks. The collagen-type-I-derived peptides DGEA and GFOGER have been investigated in bone tissue engineering. DGEA-displaying PA hydrogels increase the expression of the osteogenic markers alkaline phosphatase, RUNX2 and osteocalcin (OCN) in vitro55, and GFOGER-presenting peptides stimulate osteoblast adhesion and differentiation, and bone formation when used as coatings of polymeric plugs press-fitted into rat femoral defects56.
In addition, RADA16 hydrogels comprising the collagen type I epitope FPGER as part of FPGERGVEGPGP peptides can modulate keratinocyte and dermal fibroblast migration and proliferation in vitro57. Collagen mimicry can involve peptides that self-assemble into collagen-like bundles or collagen-mimetic motifs, to recreate signalling and structural functionalities of collagens.
Cell adhesion epitopes derived from cadherins
Cadherins are transmembrane cell receptors responsible for the transmission of signals between cells, cell migration and maintenance of tissue structure. For example, N-cadherins are essential for MSC condensation, which is a crucial step in cartilage morphogenesis. Cadherins contain the evolutionary-conserved tripeptide HAV, which is an important cell-to-cell adhesion mediator. This motif mediates both MSC–MSC (‘homotypic’) and MSCs–osteoblast (‘orthotypic’) interactions; for example, methacrylated hyaluronic acid hydrogels with HAV sequences promote early chondrogenesis of MSCs in vitro and neocartilage deposition in vivo when implanted in subchondral pockets of nude mice58. Similarly, HAVDI, a pentapeptide located in the extracellular domain-1 of N-cadherins, attached to the N-terminus of KLD12 peptide hydrogels triggers chondrogenesis of human MSCs in vitro by suppressing canonical Wnt/β-catenin signalling, a master regulator of osteogenesis and chondrogenesis59. Methacrylated gelatin (GelMA) conjugated to N-cadherin-mimetic peptides promote the differentiation of induced pluripotent stem cells into neurons, functionally better than Matrigel60.
Other cell adhesion epitopes targeting integrins
Given the pivotal role of integrin binding in mediating angiogenesis, osteogenesis and wound healing, integrin targeting is a key design consideration for many biomaterials61. For example, hydrogels based on streptococcal collagen-like proteins containing the sequence GFPGER, derived from collagens interacting with α1β1 and α2β1 integrins, promote in vitro endothelial cell adhesion on tissue-engineered vascular grafts62 and in vitro osteogenesis in 3D hydrogels63. Osteogenic differentiation can also be promoted with the phage-display-derived sequence RRETAWA that specifically interacts with α5β1 integrin64. Wound healing of a splinted excisional wound model in type II diabetic mice can be accelerated using PEG that contains the laminin-derived dodecapeptide A5G81 (AGQWHRVSVRWG), which targets α3β1 and α6β1 integrins65. Derived from the fibrinogen γC and βC chains, the peptides P1 (GWTVFQKRLDGS), P2 (YSMKKTTMKIIPFNRLTIG) and GWTVIQNRQ can also target integrins (αMβ2 binding sites) to support cell adhesion and migration66. Integrin-targeting peptides have also been derived from netrin-1 (QWRDTWARRLRKFQREKKGKCRKA), targeting α6β4 and α3β1 integrins67, from fibronectin and vascular cell adhesion molecule (CAM)-1 (DELPQLVTLPHPNLHGPEILDVPST) targeting α4β1 integrin68, and from thrombospondin (for example, the domain CSVTCG) binding β1 integrins69,70. However, within the majority of published studies, RGD remains the best studied integrin-binding peptide (89%), followed by IKVAV (6%), YIGSR (4%) and other sequences (1%)71. In addition, laminin-derived IKLLI, LRGDN and SINNNR, fibronectin-derived KQAGDV and PRARI, collagen-type-IV-derived NYYSNS, nidogen-1-derived FRGDGQ71, and CAMs-derived QIDS and LDT are being explored72.
Peptide sequences mimicking growth factors
Cells sense their microenvironment through cell receptors that sequester and bind growth factors from this microenvironment. Growth factor ligands bind to serine/threonine kinase receptor complexes, which in turn become phosphorylated and initiate a downstream intracellular cascade that reaches the nucleus and regulates transcription. Growth factor receptors can also move along the plasma membrane and cluster into small domains to enhance signalling73. Moreover, growth factors can cross-talk with integrins73. Growth factor-binding and unbinding are regulated by feedback controls; growth factor ligands can be sequestered in their latent form to prevent growth factor signalling. Growth factors are involved in numerous pivotal cellular processes, such as cell proliferation, migration and differentiation, and they orchestrate embryonic development, tissue homeostasis and repair by influencing and directing stem cell phenotype; for example, bone morphogenic proteins (BMPs) have a key role in osteogenesis; vascular endothelial growth factors (VEGFs) enable angiogenesis; and transforming growth factors (TGFβs) are important regulators of chondrogenesis74. Growth factor-mimetic peptides can be incorporated in 3D synthetic hydrogels to avoid rapid denaturation and clearance of full-length growth factors, yet ensuring reactivity, specificity and efficiency.
Growth factor-mimetic peptides for osteogenesis, chondrogenesis and discogenesis
Peptides derived from BMPs can be used in osteogenesis applications. BMPs are multifunctional growth factors from the TGFβ superfamily, which play a crucial role in heart, brain, cartilage and bone development75. BMPs have been investigated for multiple therapeutic interventions; for example, BMP-7 has shown great regenerative chondrogenic potential and anti-apoptotic effects in vitro and in vivo76. The BMP-7-derived peptide epitope KPSSAPTQLN can be conjugated to the C-terminus of a RADA16-I hydrogel to create hydrogels (RADA-KPSS) with anti-inflammatory and anti-apoptosis effects77; for example, this system reduces the expression of catabolic enzymes and increases secretion of GAGs in intervertebral-disc nucleus pulposus cells77, and induces migration, proliferation and ECM deposition of human nucleus-pulposus-derived stem cells in vitro78. The BMP-2-derived peptides DWIVA and ‘knuckle epitope’ KIPKASSVPTELSAISTLYL79 can be implemented in SSVPT-containing hydrogels, which show high osteogenic potential by increasing alkaline phosphatase, osteopontin production, and in vitro mineral deposition when cultured with murine osteoblasts and MSCs79. Furthermore, SSVPT-displaying PA hydrogels can induce osteogenic MSC differentiation in vitro and promote rat skull defect repair in vivo80. LIANAK, a TGFβ1-simulating peptide, can be conjugated to a self-assembling RADA16 hydrogel to increase the expression of chondrogenic genes and ECM deposition81. Combined with decellularized cartilage matrix, the hydrogel promotes neocartilage formation and restoration of the subchondral unit in a rabbit model of full-thickness knee cartilage defect81.
Growth factor-mimetic peptides for angiogenesis
Biomaterials that promote angiogenesis are of great interest for various applications, including tissue engineering to promote vascularization of tissue-engineered constructs and to mimic native levels of oxygenation, nutrients and waste removal, as well as in vitro models to perform drug screening studies. In particular, VEGF has a key role in angiogenesis and endothelial cell proliferation. The VEGF-mimicking peptide KLTWQELYQLKYKGI (KLT) from the VEGF helix region 17–35 binds to VEGF receptors, activates cell signalling and promotes capillary formation82. A KLT-decorated RADA16-I hydrogel promotes endothelial cell growth and induces capillary-like tubule formation in 2D and 3D, as well as neovascularization in a chicken embryo chorioallantoic membrane assay83. Moreover, an injectable KLT-containing self-assembling peptide hydrogel promotes angiogenesis, infiltration of haematopoietic stem cells and MSCs, and rapid host–gel integration in a rat model84. Basic fibroblast growth factor (bFGF), also known as FGF-2, also has pleiotropic effects, including angiogenesis and wound healing. In particular, the peptide domain 106–120 in FGF-2 is a partial agonist of FGF receptors85. The FGF-2-mimicking peptide YRSRKYSSWYVALKR can be applied to formulate angiogenic peptide nanoribbon hydrogels, which can promote the proliferation and migration of human umbilical vein endothelial cells in vitro, with similar bioactivity as native FGF-286. The osteonectin-derived GHK tripeptide87 is also pro-angiogenic, and can be implemented in a hyaluronic acid tyramine (HA-Tyr)/Laponite/PA) nanocomposite hydrogel to stimulate osteoblastic differentiation of human MSCs and neovessel formation in vitro, as well as bone formation in a rabbit maxillary sinus floor bone model88. Derived from osteopontin, the heptapeptide SVVYGLR coupled to RADA16 hydrogels can stimulate in vitro angiogenesis of rat lung endothelial cells with a comparable level to VEGF89, which may serve as treatment for myocardial infarction90. Similarly, MSC-laden RADA16-I hydrogels grafted with QHREDG, derived from an integrin-binding motif of angiopoietin-1 (Ang-1), reduces infarct size in a rat model owing to a paracrine effect that results in downregulation of the pro-inflammatory cytokines interleukin (IL)-6 and IL-191.
Growth factor-mimetic peptides for neurogenesis
Synthetic hydrogels can be designed to promote nerve regeneration. Nerve growth factor (NGF) promotes neurite outgrowth and regulates peripheral sensory and sympathetic cell function, and brain-derived growth factor (BDNF) provides neuroprotection and regeneration of injured neurons. The peptides CTDIKGKCTGACDGKQC and RGIDKRHWNSQ, derived from NGF and BDNF, respectively, can promote neurite proliferation and neuroprotection similar to the full-length growth factors92,93. In a RADA16-RGIDKRHWNSQ hydrogel, MSCs can be differentiated into neuron-like cells, with neurites and dendrites extending in 3D both in vitro and in vivo92. The cyclic tetrapeptide RKKADP is derived from loop 4 of native BDNF, and can activate BDNF-associated tyrosine kinase B (TrkB) signalling, thereby promoting primary cortical neuron assembly into an electrically conductive network94. Similarly, RADA16-NGF hydrogels can be used as bioactive ‘fillers’ of nerve conduits to bridge sciatic nerve defects, triggering nerve-conduction and re-myelinization in rats95. In addition, the fibroblast growth factor ligand motif EVYVVAENQQGKSKA, derived from the neural cell adhesion molecule (NCAM), promotes in vitro neurite sprouting andspinal-cord-derived neural stem cell adhesion and migration96.
Peptide sequences inducing immunomodulatory effects
Peptide hydrogels with naturally derived antimicrobial effects
Cationic amphipathic peptides possess intrinsic antimicrobial properties owing to the presence of positive charges that promote interactions and interdigitation within negatively charged bacteria membranes, causing bacterial cell death97. Taking advantage of their inherent antimicrobial properties, antimicrobial self-assembling hydrogels can be designed using the antimicrobial peptide (AMP) WGIRRILKYGKRS (WMR), which is derived from the native sequence of the marine peptide myxinidin present in the epidermal mucus of hagfishes (Myxine glutinosa)98. Compared with the myxinidin sequence, WMR peptides possess an extra tryptophan amino acid at the N terminus and a higher number of positively charged arginine residues, which confers strong antimicrobial activity on the sequence99. WMR-grafted PAs self-assembled into nanofibres can be used as substrates to inhibit biofilm formation of the Gram-negative bacteria Pseudomonas aeruginosa and the pathogenic fungus Candida albicans100, which are often involved in clinical infections. Antimicrobial activity and high cytocompatibility can also be achieved by presenting melittin101, a natural honeybee-venom-derived AMP, on the surface of synthetic multidomain peptides. In this design, upon assembly on peptides, melittin conformation is altered to selectively inhibit bacterial growth (Escherichia coli), while preserving biocompatibility towards mammalian cell membranes (mouse fibroblasts)102. Melittin can also be applied as antimicrobial coating; for example, a melittin-derived peptide (GIGAVLKVLTTGLPALISWIKRKRQQ) film layered on chitosan-antibiotic coatings of titanium bone implants results in the rapid killing and prevention of biofilm formation of methicillin- and vancomycin-resistant Staphylococcus aureus103. Melittin can further be used in combination with a cecropin peptide, derived from the haemolymph of Hyalophora cecropia moth, to create a hybrid melittin–cecropin epitope (KWKLFKKIGIGAVLKVLTTGLPALISC) with high stability and antimicrobial effect against E. coli when immobilized on polymeric films104. A biocompatible bacterial-membrane-disrupting self-assembled peptide system (CASP-K6), containing multiple lysine residues, shows strong bactericidal effect against P. aeruginosa105. Similarly, the presence of lysines in β-sheet-rich hexapeptides linked to lysine tripeptides (NAVSIQKKK) leads to antimicrobial activity against E. coli and S. aureus. Peptide hydrogels with designed antimicrobial effects are biocompatible in human lung fibroblasts and red blood cells106.
Peptide hydrogels with inherent antimicrobial effects
Injectable hydrogels can be made antimicrobial by incorporating peptides with antimicrobial properties. For example, β-hairpin self-assembling AMPs, such as PEP6R107, MAX1108 and MARG1109, inhibit growth of P. aeruginosa, Gram-positive and Gram-negative pathogens (for example, S. epidermidis, Streptococcus pyogenes, Klebsiella pneumoniae and E. coli)108, and methicillin-resistant S. aureus109. Low-molecular-weight peptide hydrogels based on Fmoc-FFE and Nap-FFE peptides also have bactericidal effects against S. aureus and S. epidermidis, when functionalized with the cationic group spermine110. Furthermore, NapFFKK and NapFFFKK show anti-biofilm activity against Gram-positive and Gram-negative strains, and co-assembly of FFKK with the drug naproxen leads to anti-inflammatory properties against cyclooxygenase enzymes, which are associated with chronic wound scar-tissue formation111,112. PA nanofibres, synthesized through mineralization of Fmoc-FFECG with silver nanoparticles, harness the antimicrobial properties of silver113 and elicit long-term in vitro antimicrobial activity against E. coli and Bacillus subtilis114. Such antimicrobial peptide-based biomaterials may be applied to treat bacterial infections after surgery or to overcome antibiotic resistance.
Peptide hydrogels that enhance T cell and B cell immune responses
Peptides can also serve as chemically defined immune adjuvants; for example, a peptide system (O-Q11) based on the Q11 self-assembling domain in tandem with an ovalbumin region (that is, OVA323–339) that contains antigenic epitopes recognized by T and B cells self-assembles into nanofibres in physiological conditions. Intranasally or subcutaneously administered nanofibres elicit a strong immune response in mice without adjuvants, causing high levels of immunoglobulins and promoting T cell activation and an antigenic response if conjugated to an H1N1 influenza epitope115. Furthermore, displaying Q11 on a virulence factor from tuberculosis116 or conjugating Q11 to (NANP)3, a malaria peptide epitope117, also results in an immune response in mice. In general, OVA sequences offer useful immunogenic epitopes; for example, peptide amphiphilic nanofibres biotinylated to OVA proteins cause antigen-specific splenocyte proliferation and CD8+ T cell responses when administered subcutaneously in mice118. OVA323–339 can also be conjugated to β-sheet-rich FKFEFKFE hydrogels; the chirality of specific amino acids in these hydrogels has a crucial role in the stability of T cell immune responses in mice119. Moreover, the OVA epitope SIINFEKL has self-assembling capabilities and may thus be used as hydrogel immune adjuvant system120.
Peptide hydrogels eliciting immunity against viruses
Peptide vaccines are based on peptide epitopes that elicit immune responses against specific viruses; for example, a self-assembled multimeric peptide amphiphilic system enhances immunity against enterovirus 71 (EV71), a Picornaviridae virus that causes hand, foot and mouth diseases121. Here, the peptide amphiphilic nanofibres display the two antigenic epitopes, virus particles 1 (YPTFGEHKQEKDLEY) and 3 (HYRAHARDGVFDYYT) from the enterovirus 71, leading to humoral and cell immune responses in mice. The serum from these immunized mice can neutralize EV71 and protect host cells121. CD8+ T cell epitopes and toll-like receptor 7/8 agonists R848 and R837 can be co-assembled in EAK16-II peptide hydrogels to elicit a cytotoxic T cell response against human immunodeficiency virus-1 (HIV-1) when they are administered subcutaneously in mice122. KFE8 peptide hydrogels can be used as adjuvants in combination with envelope protein domain III (EIII) and injected subcutanously, conferring immune protection against the West Nile virus in mice, similar to gold-standard adjuvants123. A shear-thinning peptide (FLIVIGSIIGPGGDGPGGD, known as h9e) can induce immunity against porcine reproductive and respiratory syndrome virus124 and H1N1 influenza125. Finally, the conserved peptide sequence RSAIEDLLFDKV, derived from coronavirus spike proteins, assembles and disassembles into β-sheet amyloid tapes depending on the pH, which is being explored for peptide vaccine design126.
Peptide sequences for binding ECM components
Peptide motifs can also bind and sequester ECM components, in particular, ECM molecules, and ions (Fig. 3a–c; Table 2; Supplementary Table 2).
Peptide sequences binding growth factors
Peptides directly binding growth factors
Soluble growth factors are commonly used in vitro and in the clinic to direct cell fate. However, they are often supplied at supraphysiological doses to ensure high concentration and efficacy over a long time period, which causes off-target effects, such as ectopic bone, neurological morbidities and cancer127. Alternatively, to avoid side effects, rapid clearance and degradation of exogenous growth factors in vivo, biomaterials can deliver growth factors to maximize growth factor retention, delivery and signalling. Growth factors bound to a solid phase closely recapitulate how growth factors are presented in native ECMs, mimicking sequestering and release dynamics, and improving their efficacy as therapeutics. In particular, growth factor-binding peptides can be implemented in 3D scaffolds to sequester and create growth factor reservoirs and gradients.
Growth factor-binding peptides are typically derived from cell receptors that bind growth factors; for example, a hydrogel based on a thermosensitive copolymer, consisting of poly(N-isopropylacrylamide)-grafted hyaluronic acid (Hyal-pN) functionalized with functional dendrimers, can bind BMP-2 (YPVHST) and TGFβ1 (HSNGLPL). The copolymer undergoes sol–gel transitions, which allows injection and progressive release of loaded growth factors128. Similarly, TGFβ1-binding PAs (HSNGLPL) induce chondrogenic differentiation of MSCs in vitro and cartilage regeneration in a rabbit model129. The same heptapeptide grafted on photo-crosslinkable GelMA also promotes chondrogenic differentiation of MSCs in vitro and accelerates cartilage tissue repair by recruiting TGFβ1 in situ in a full-thickness cartilage defect rabbit model130. A fully deamidated version of HSNGLPL retains 25% more TGFβ1 molecules than the amidated version, with a significant increase in chondrogenic markers and matrix deposition by ATDC5 chondrogenic cells encapsulated in the TGFβ-binding peptide hydrogels131. Similar peptide amphiphilic hydrogels based on the peptide motif TSPHVPYGGGS can retain endogenous and exogenous BMP-2132, increasing the expression of osteogenic markers by C2C12 pre-myoblast cells in vitro and achieving complete spinal fusion in a rat model. Remarkably, this effect is achieved with low doses (1 μg BMP-2), compared with collagen controls (10 μg BMP-2)133.
High growth factor retention and prolonged delivery are also important in cell therapies of myocardial infarction. Biotinylated nanofibres based on a ‘biotin sandwich’ of biotinylated insulin-like growth factor-1 (IGF-1) complexed with streptavidin bound to biotinylated peptides decreases caspase-3 cleavage, increases myocyte cross-sectional area, and improves systolic function after injection into the infarct zone of rat myocardium134. Biotin–streptavidin bonds can also be exploited to tether IGF-1 and TGFβ1 to the surface of self-assembling (KLDL)3 nanofibres to controllably deliver these growth factors in vitro for potential applications in cartilage tissue regeneration135. Through non-specific peptide–growth factor interactions, a RADA16-II hydrogel can sustainably deliver platelet-derived growth factor (PDGF) into the myocardium of rats, decreasing infarct size after ischaemia and reperfusion136.
Peptides binding growth factors through heparin-mimetic and heparin-binding domains
Glycan-mimetic peptides provide a versatile toolbox to bind multiple growth factors. Glycans bind to more than 300 secreted or membrane-bound proteins, such as growth factors, enzymes and chemokines, thereby regulating various biological processes. For example, the GAGs heparan sulfate and heparin promiscuously bind several growth factors. PAs can be designed to display dominant motifs of sulfated polysaccharides that bind and activate multiple growth factors137; here, the PA nanofibres are glycoconjugated with N-acetylglucosamine (GlcNAc) monosaccharides to interact with heparan-sulfate-binding growth factors, such as BMP-2, BMP-4, FGF-1, FGF-2, VEGF, Shh, and the growth factor inhibitor Noggin, to promote full spinal fusion in a rat model with low sub-therapeutic doses of BMP-2 (100 ng)137. GAG-mimetic peptide amphiphilic systems, based on the peptide epitope VVAGEGDK(p-sulfobenzoyl)S can also sequester BMP-2 and promote in vitro osteogenesis of Saos-2 osteosarcoma cells138. Similarly, heparin-mimetic peptide nanofibres bind VEGF and induce angiogenesis in vitro and in vivo139. Other similar systems show moderate-to-high binding affinity to growth factors, including hepatocyte growth factor (HGF), FGF-2, BMP-2 and NGF140.
Heparin, in particular, has high binding affinity to growth factors, and thus, peptides can be explored that bind heparin to subsequently bind growth factors. For example, the sequence LRKKLGKA, derived from the Cardin–Weintraub heparin-binding consensus sequence XBBBXXBX (where X and B are hydrophobic and basic amino acids, respectively), can be applied to design heparin-binding peptide amphiphilics. Owing to its anionic nature, heparin co-assembles with positively charged peptides, generating hydrogels that bind VEGF and FGF-2 to promote vascularization in a rat corneal assay141. These heparin-binding peptide amphiphilics also promote angiogenesis in the dorsal skinfold chamber142 and infarction143 models, and they can infilitrate the interior of islet cells in vitro, which may be explored for the treatment of diabetes type 1144. Retention and delivery of VEGF can also be achieved in a RADA16-I hydrogel145 that contains GAG-mimetic and GAG-binding peptides.
Peptide sequences binding other ECM components
Peptides binding ECM molecules through selective binding
The capacity to bind multiple molecular and biological components is key to emulate the compositional complexity of native tissues. In particular, selective binding between peptides and ECM components may enable the design of hydrogels that can controllably present or release these macromolecules. For example, a poly(allyl amine hydrochloride) hydrogel selectively binds a carcinoembryonic antigen, a glycosylated surface protein overexpressed by several carcinomas146. Similarly, poly(methacrylic acid) hydrogels decorated with hepsin-targeting peptides (IPLVVPL) selectively bind hepsin-overexpressing tumour cells147. Nap-FFG peptides are low-molecular-weight gelators that selectively bind and self-assemble into coatings at the surface of platelets, preventing human platelet aggregation in vitro148. Tunable assembly of Fmoc-dipeptides (that is, Fmoc-YN, Fmoc-YS, Fmoc-YL and Fmoc-VL) with β-lactoglobulin and bovine serum albumin (BSA) can be achieved by varying amino acid polarity149. FF-based hydrogels can also be designed to self-assemble in the presence of haemoglobin, myoglobin, serum albumin and fibrinogen, by exploiting covalent Schiff base bonding150. In addition, supramolecular systems can be engineered based on tyrosine and pyridine-containing gelators to selectively bind nickel (Ni2+)151 and chloride ions (Cl−)152.
Peptides binding ECM molecules through non-selective binding
Peptides that enable non-selective binding of ECM components based on complementary charges and hydrophobic forces allow the incorporation of multiple macromolecules and the assembly of materials with a high level of structural hierarchy and functionality. For example, non-specific interactions at liquid–liquid interfaces between a positively charged PA (V3A3K3) and negatively charged hyaluronic acid results in the formation of a diffusion barrier and subsequent co-assembly of the peptide and hyaluronic acid into structures of different sizes153. Similar co-assembling approaches by non-specific interactions can be applied to organize proteins into hierarchical ensembles; for example, by tuning the charge of PAs (K2, K3, K4), the conformation of disordered elastin-like polypeptides (ELPs) can be modulated to trigger co-assembly mechanisms that result in different hierarchical material structures and properties, including the capacity to grow and heal154. This approach enables the use of hydrodynamic forces to hierarchically guide assembly155, organize exogenous components, such as graphene oxide156, or tune peptide structures (Fmoc-FFK) to increase material stability157. Further modulation of the charge density and epitope presentation of PAs allows co-assembly with multiple ECM components to generate complex, yet controlled, cell-instructive matrices; for example, to model the tumour microenvironment (TME) of ovarian cancer, keratin can be co-assembled with multiple peptides (VPGIGH2K for hydrogel stability, RGDS for cell adhesion, GHK for angiogenesis)158, and to recreate the TME of pancreatic cancer, fibronectin, collagen, laminin and hyaluronan can be integrated in an EEE-containing PA159. Tough hydrogels can be created by merging PAs with polypeptides mimicking resilin160, a tough and extensible rubber-like protein found in insects, or Laponite161.
Peptides binding ions to tailor organic–inorganic interactions
ECMs of some tissues, such as bone and enamel, are nanocomposites of organic and inorganic phases, which are hierarchically organized to provide high strength and fracture toughness. In these tissues, the local distribution and concentration of ions and minerals, such as calcium and phosphate deposits, are essential to ensure structure and function. For example, in bone, the organic phase is mainly collagen type I, whereas the inorganic phase is hydroxyapatite, a naturally occurring mineral form of calcium apatite. Peptides have an important role in biomineralization, guiding organic–inorganic interactions. In particular, hydroxyapatite aggregates and its crystal formation can be tuned by interaction between negatively and positively charged amino acids (for example, glutamate and arginine) with Ca2+ and PO43− ions. Fmoc-FF, Fmoc-R and hydroxyapatite phases can be combined to create a multicomponent peptide-based scaffold for bone regeneration. In this system, Fmoc-FF provides structural rigidity, and Fmoc-R serves as template for biomineralization owing to the presence of arginine amino acids162. These hydroxyapatite-containing hydrogels are biocompatible and mechanically robust (stiffness up to 30 kPa) and can thus be applied in cell culture as biomimetic scaffolds for bone regeneration studies162. Fmoc-FF/S can also be combined with Fmoc-RGD and hydroxyapatite nanocrystals to deposit hydroxyapatite on RGD motifs, from R and D amino acids, and stimulate the differentiation of Raw 264.7 macrophages into osteoclasts163. In addition, peptide sequences can be rationally designed to direct biomineralization; for example, the dodecapeptide NPYHPTIPQSVH binds hydroxyapatite crystal surfaces164, and the heptapeptide MLPHHGA, conjugated to a β-hairpin-forming sequence (MDG1), directs hydroxyapatite formation in the presence of cementoblast cells165.
In dental enamel, the organic matrix plays a crucial role in the development of its hierarchical inorganic structure and mechanical properties. Negatively charged macromolecules of the enamel ECM, including glycoproteins and proteins, such as amelogenin, enamelin and amelotin166, bind Ca2+ ions and initiate apatite crystal nucleation and growth. To mimic this process, a low-viscosity solution of an anionic P11-4 self-assembling peptide can be designed that infiltrates and polymerizes into caries lesions, providing a biomimetic scaffold for hydroxyapatite nucleation. This peptide increases enamel mineralization and de novo crystalline hydroxyapatite depositions in vitro, and achieved mineralization of enamel in a clinical safety trial (UK National Research Ethics System; project number [10/H1207/75]) conducted in healthy adults with class V ‘white spot’ lesions167. Mimicking enamel glycoproteins, 26 and 32 amino acid amelogenin-inspired peptides (P26 and P32) can be engineered as hydrogels to promote the bottom-up formation of multilayered hydroxyapatite crystals on sectioned human molar teeth168. Similarly, enamel remineralization can be achieved with shorter amelogenin mimics, such as LEAWPATDKTKREEVD169 and TKREEVD170, and with the tripeptide motif (DSS) derived from dentin phosphoprotein171. Moreover, the peptide DDDEEKFLRRIGRFG, derived from statherin, a protein present in saliva, can control fluorapatite formation172.
Peptide sequences to create dynamic matrices
Peptides cleaved by matrix metalloproteinases
In healthy tissues, ECM deposition and degradation are tightly regulated to ensure homeostasis and matrix turnover. Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that are often overexpressed during tissue remodelling and in pathological conditions such as cancer173. The MMP-sensitive sequence GPX1G*LX2G (in which * denotes the cleavage site, X1 is A or L, and X2 is G) can be incorporated into hydrogels to control their enzymatic degradation and to create cleavable synthetic matrices; for example, the MMP-2-sensitive sequence GTAGLIGQ174 can be implemented as cleavable backbone of PA hydrogels to deliver cisplatin and test cancer treatment in vitro. Similarly, the MMP-9-sensitive sequence GL within the peptide FFAGLDD allows the in vitro release of doxorubicin from phenylacetyl-PAs, able to switch from micellar aggregates to fibres upon degradation of the MMP-sensitive site175. A diphenylalanine-based peptide hydrogel, comprising an MMP-sensitive tetrapeptide (PLGL) linked to a neuroprotective hexapeptide (NAVSIQ), can be injected to the brain of mice and release the hexapeptide upon cleavage by MMP-9176. Self-assembling PA–hyaluronic acid membranes that contain an MMP-1-cleavable epitope (GPQGIWGQ) allow the study of cell–ECM remodelling interactions177.
Many MMP-sensitive peptides have been optimized to ensure high enzymatic sensitivity and specificity, including sequences derived from phage-display libraries, such as SGESPAYYTA (optimized for MMP-2)178, GAPFALRLV (optimized for MMP-3 and MMP-7)179, GGYAELRMGG (optimized for MMP-11)180 and GPLGLWAR (optimized for MMP-13)181, GPQGIAGQ (sensitive to MMP-1, -2, -3, -7, -8 and -9182), GPLGMRGL (cleavable by MMP-13181), and the sequences VPMSMRGG and IPESLRAG, which have high degradation rates with MMP-1 and MMP-2183. MMP-sensitive epitopes have also been applied for the design of dynamic matrices, induction of morphogenesis, and enhanced cell invasion; for example, implementing the MMP-sensitive GPQGIWGQ epitope in PEG hydrogels allows proteolytic remodelling and organogenesis of intestinal stem cells in vitro184. Similarly, renal tubulogenesis can be achieved in vitro by tuning the mechanics and degradability of heparin-based hydrogels containing the MMP-sensitive GPQGIWGQ sequence185. Alginate gels modified with the MMP-sensitive PVGLIG epitope can deliver human MSCs and ensure cell invasion upon subcutaneous implantation in a mouse model and subsequent degradation186.
Peptides cleaved by other enzymes
In addition to MMPs, other enzymes have been explored to create degradable peptide-based hydrogels; for example, PAs that display the consensus sequence RRX1SX2, where X1 can be any residue and X2 must be a hydrophobic residue, are sensitive to cleavage by protein kinase A (PKA)187. Accordingly, PA nanofibres displaying RRASV assemble and disassemble in response to a specific enzyme. When exposed to PKA, the nanofibres are phosphorylated and disassemble, whereas in the presence of ALP, the phosphate groups are cleaved, restoring the assembly capabilities of the system187. The serine protease α-chymotrypsin cleaves the sequence KKFFVLK next to the F residues, resulting in products with different tendencies to form nanotubes or spherical micelles188. Similarly, cathepsin-sensitive sequences can be exploited to create degradable matrices; for example, PEGDA hydrogels grafted with the cathepsin-K-sensitive peptide GGGMGPSGPWGGK specifically degrade in response to osteoclasts, but not in response to osteoblasts, in bone resorption studies using porcine cortical bone slices as substrates189. The cathepsin-B-cleavable peptide AVPIAQFRRG can be conjugated to a pro-apoptotic peptide drug and doxorubicin to achieve high cancer cell specificity in tumour-bearing mice190. In addition, by incorporating ester backbones in β-sheet nanofibres and gels, they can be made degradable by hydrolysis191. Therefore, degradation can be tailored using specific peptides and by varying their secondary structure192 or amino acids composition193 to create cell-responsive, dynamic biomaterials conducive to cell remodelling and matrix turnover (Fig. 3d, Table 3 and Supplementary Table 3).
Hydrogels with synergistic peptide motifs
To recapitulate the dynamic complexity of the ECM, biomaterial designs should allow spatiotemporal control, avoid epitope redundancies and consider feedback loops. The incorporation of cleavable peptides enables matrix turnover, spatiotemporal changes and feedback controls driven by specific conditions (for example, pH changes, enzymes). Furthermore, peptides can be designed to target specific cell receptors and biological effects to avoid redundancies. In addition, self-assembling peptides allow the implementation of multiple signals within the same biomaterial through interpenetration of different epitope-displaying peptide nanofibres. Such 3D synthetic hydrogels containing multiple peptide epitopes can achieve synergistic and additive effects (Supplementary Fig. 1, Table 4 and Supplementary Table 4).
Mixture of cell adhesion epitopes
ECM components often work synergistically; for example, fibronectin contains an RGDS loop and the pentapeptide PHSRN, which are located close to each other on the folded protein194 and synergistically bind to α5β1 integrin if their distance is 3.2 nm (ref. 195). Accordingly, a multidomain peptide system that displays RGDS and PHSRN at a distance between the epitopes of approximately 3.2 nm allows control over endothelial cell adhesion and integrin upregulation in vitro196. By co-assembling different epitope-displaying chains, multiple peptide epitopes can be incorporated within the same hydrogel; for example, Q11 bearing RGDS, IKVAV, REDV and YIGSR can be co-assembled in various combinations to formulate homogeneous gels with different mechanical and biological properties, depending on the concentrations at which the peptide epitopes are mixed197. Interestingly, RGDS and YIGSR exhibit an antagonist effect, whereas RGDS and IKVAV show an additive, synergistic effect197. In addition, RGD and IKVAV can be mixed for cardiac tissue engineering198, IKVAV and YIGSR for neurogenesis199, and RGDS with phosphoserine for bone regeneration studies29.
Mixture of cell adhesion and growth factor-binding epitopes
Cell adhesion peptides can also be combined with growth factor-binding epitopes; for example, polyethylene acrylate can be used to unfold fibronectin, by adsorption and assembly of the protein on the methyl groups of polyethylene acrylate monomers, which cause conformational changes in fibronectin and expose its epitopes (FNIII9-10 and FNIII12-14) to enable synergistic adhesion and growth factor signalling73. 3D fibrin hydrogels functionalized with recombinant fibronectin that contains two bioactive fragments (FNIII9-10 and FNIII12-14) can be loaded with VEGF to promote wound healing in diabetic mice. The same platform can be loaded with BMP-2 for bone regeneration in non-healing bone defects200. A PEG hydrogel that incorporates full-length fibronectin and has tunable physical properties can retain BMP-2 and VEGF to aid in bone regeneration and vascularization in vivo201. RAD16-I can be combined with fibronectin- and laminin-derived motifs, and the heparin-binding sequence TAGSCLRKFSTM, to culture hepatocytes44. Moreover, synthetic stem cell niches can be designed using RADA16-I functionalized with SKPPGTSS, a bone-marrow homing motif, FHRRIKA, a heparin-binding motif, and PRGDSGYRGDS, a two-unit RGDS cell adhesion motif202. In addition, RADA16 hydrogels can be decorated with RGD- and VEGF-derived epitopes to promote dentin-pulp regeneration203, and EAK16-II hydrogels can be functionalized with RGD and (GRGDSP)4K from fibronectin, FRHRNRKGY from vitronectin, IKVAV from laminin, and IGF-1 for nervous tissue engineering204. Similarly, a hydrogel containing a BDNF-mimicking motif and the IKVAV sequence may achieve peripheral nerve regeneration when used as filler of hollow chitosan tubes to bridge 10-mm-long sciatic nerve defects in rats205.
A multi-epitope approach can also be pursued to stimulate osteogenesis and bone formation; for example, ELP-based membranes containing REDV, RGDS and the statherin-derived peptide DDDEEKFLRRIGRFG improve endothelialization206, cell adhesion and calcium phosphate binding, respectively, to increase osteoblastic differentiation in vitro and bone deposition in a critical-size rat calvarial defect model207. Similarly, a co-assembling PA hydrogel comprising RGDS for cell adhesion, SVVYGLR for angiogenesis, and DGEA for osteoblastic differentiation promotes growth of bone spheroids in a co-culture with endothelial cells and MSCs208.
Mixture of multiple growth factor-mimicking epitopes
Epitopes that signal multiple growth factors can be integrated in the same hydrogel; for example, a combination of the peptides CTDIKGKCTGACDGKQC (mimicking NGF) and RGIDKRHWNSQ (mimicking BDNF) can be used as filler of nerve conduits to synergistically promote peripheral nerve regeneration of 10-mm-long sciatic nerve defects in rats95. Similarly, a BDNF-mimicking peptide can be combined with a VEGF-mimicking motif in a RADA16-based hydrogel to promote peripheral nerve regeneration and bridge a critical-size sciatic nerve gap in rats209. In addition to its angiogenic properties, VEGF has neurotrophic and neuroprotective effects. Therefore, rat Schwann cells cultured on the BDNF/VEGF-functionalized hydrogels show adhesion, spreading and markers of myelinization209. Furthermore, the functionalized hydrogel induces sprouting of myelinated fibres, regeneration of axons and functional motor recovery in vivo209.
Mixture of cell adhesion, growth factor-mimicking and enzyme-cleavable epitopes
Matrix dynamicity, cell adhesion and growth factor signalling can also be achieved within the same system. For example, the multidomain peptide system ‘SLanc’ incorporates the cell adhesion sequence RGDS, an MMP-2 sensitive peptide, and a VEGF-mimicking peptide. Subcutaneous injection of SLanc hydrogels in rats promotes the formation of a large number of blood vessels surrounding the implant in 7 days, with rapid host cell infiltration and lack of fibrous encapsulation84. RGD can also be combined with an MMP-2-sensitive peptide sequence within a hydrogel carrier for the delivery of MSCs to increase MSC attachment and cell remodelling of the scaffold210. A PA system containing RGD and a MMP-2-sensitive cleavage site (TPGPQGIAGQ) enables the biofabrication of tissue templates that ‘self-detach’ during cell culture for downstream applications211. A 3D PEG ECM-inspired hydrogel that contains 13 cell adhesion integrin-binding and 7 MMP-degradable peptides has been designed to mimic the diversity of proteins in human bone marrow. Here, the peptides were selected from native tissue through a proteomic-based and biomechanics method212. The specific combination of bone-marrow-derived adhesion cues and cleavable sites recreates the bulk modulus of native bone marrow and provides a biomimetic niche for MSC growth and differentiation212. Similarly, a PEG-based hydrogel containing nine integrin-binding and five MMP-degradable peptides present in native brain enables in vitro control and maintenance of astrocyte quiescence with minimal activation213.
Peptide hydrogels on the market
Some peptide-based or peptide-displaying hydrogels have started to reach commercial and medical applications (Supplementary Table 5). For example, as reagents for biomedical research, PuraMatrix from 3-D Matrix (based on the RADARADARADARADARADA (RADA16) sequence), elastin-like recombinamers from Technical Proteins Nanobiotechnology S.L., and HydroMatrix from Sigma-Aldrich provide ECM-mimicking, biocompatible, reproducible and chemically defined platforms with tunable properties. Commercially available peptide hydrogels are also often used in tissue engineering for multiple cell types, including primary cells, cell lines and stem cells214. In addition, peptide-based hydrogels are gaining interest as medical products; for example, RADA16 hydrogels are sold as injectable haemostatic agents, known as PuraStat, PuraBond and PuraSinus, for topical haemostatic control of postoperative bleeding in vascular anamostoses and endoscopic operations215. Similarly, a chiral version of EAK16 (Sciobio) is used to treat severe bedsore and chronic diabetic ulcers, and to promote wound healing216. Other peptide-based systems that have been tested in clinical trials are available on the market, including the mineralizing peptide P11-4 (Curolox) for the treatment of dental caries217, and T45K (AC5)218 for haemostatic control in patients undergoing antiplatelet therapy. Of note, clinical trials investigating peptide hydrogels are currently mainly focused on the treatment of dental caries, tooth diseases, haemorrhages and skin diseases.
Outlook
In the ECM, integrated biomolecules work together to communicate with cells, and regulate cell and tissue function. Bioengineered platforms aiming to recreate complex biological scenarios or develop efficient regenerative therapies must communicate with biological systems in a similar manner. Despite regulatory challenges (Box 1), mimicry of ECM functionalities in biomaterial design would enable the generation of biomaterials that closely recreate in vivo mechanisms8. Peptide epitopes can be used as active molecular building blocks of hydrogel materials, operating as cell-instructive supramolecular ensembles both in vitro and in vivo. Peptide epitopes can be designed to increase cell adhesion and migration, promote cell differentiation, trigger immune response, or regulate ECM turnover. Furthermore, multiple epitopes can be combined to trigger functional effects in a synergistic or additive manner, closely resembling how living systems operate219,220. Moreover, peptides can serve as modular molecular building blocks capable of binding or co-assembling with ECM or synthetic macromolecules to improve the compositional diversity, structural complexity and functionality of biomaterials.
New functional peptide sequences can be discovered by phage display, in which a range of peptide-displaying phage particles are screened to identify peptides that bind to a specific target15. This approach does not require knowledge of full-length proteins15 and allows testing of 109 peptide combinations, in addition to revealing the encoding genes a posteriori. Moreover, artificial intelligence and machine learning may offer an inverse (but complementary) approach, through which full-length protein structures can be analysed to identify new peptides. For example, secondary structures of nearly 2 million protein helices have been compared by a pattern-based search engine to identify and locate antimicrobial motifs and to provide design rules to control protein-protein interactions221. Similarly, a combination of machine learning and multivariate outlier detection models has helped to discover new non-haemolytic peptides and provide de novo design guidelines222. The list of functional peptides yet to be discovered is expected to grow rapidly. Indeed, in 2022, the artificial intelligence network AlphaFold predicted the structure of >200 million proteins from ~1 million species, covering almost every known protein223. From these proteins, peptide epitopes can be identified, in particular from disordered regions of proteins, which are thought to play a key role in protein structure and signalling224. These peptide discovery tools could provide new sequences that can be implemented in biomaterials to investigate disease mechanisms and cell–biomaterial interactions, and to design therapies225.
Furthermore, co-assembling peptides with structural proteins or inorganic fillers may enable the engineering of hydrogel nanocomposites; for example, co-assembly of short self-assembling peptides with macromolecules, such as resilin-like polypeptides160, elastin-like proteins154,156, or hyaluronic acid88,153,177, increases the mechanical properties and bioactivity of the peptides. Similarly, peptides can be co-assembled with rigid 2D nanomaterials, such as graphene oxide226 and the synthetic clay Laponite88, to formulate peptide-based constructs for load-bearing applications. Moreover, manufacturing strategies, such as inkjet printing, electrospinning and bioprinting, can be applied to create peptide hydrogel nanostructures with defined hierarchical organization and high geometrical complexity across multiple scales, mimicking the structural and functional hierarchy of biological systems12.
References
Karamanos, N. K. et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 288, 6850–6912 (2021).
Hynes, R. O. Extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Hannezo, E. & Heisenberg, C.-P. Mechanochemical feedback loops in development and disease. Cell 178, 12–25 (2019).
Peysselon, F., Xue, B., Uversky, V. N. & Ricard-Blum, S. Intrinsic disorder of the extracellular matrix. Mol. Biosyst. 7, 3353–3365 (2011).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Schell, H. et al. The haematoma and its role in bone healing. J. Exp. Orthop. 4, 5 (2017).
Ratner, B. D. & Zhang, G. in Biomaterials Science 4th edn (eds Wagner, W. R. et al.) 21–34 (Academic, 2020).
Azevedo, H. S. & Mata, A. Embracing complexity in biomaterials design. Biomater. Biosyst. 6, 100039 (2022).
Oliver-Cervelló, L., Martin-Gómez, H. & Mas-Moruno, C. New trends in the development of multifunctional peptides to functionalize biomaterials. J. Pept. Sci. 28, e3335 (2021).
Hartgerink, J. D., Beniash, E. & Stupp, S. I. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc. Natl Acad. Sci. USA 99, 5133–5138 (2002).
Boekhoven, J. & Stupp, S. I. 25th anniversary article: Supramolecular materials for regenerative medicine. Adv. Mater. 26, 1642–1659 (2014).
Hedegaard, C. L. & Mata, A. Integrating self-assembly and biofabrication for the development of structures with enhanced complexity and hierarchical control. Biofabrication 12, 32002 (2020).
Ligorio, C., Hoyland, J. A. & Saiani, A. Self-assembling peptide hydrogels as functional tools to tackle intervertebral disc degeneration. Gels 8, 211 (2022).
Rodda, A. E., Meagher, L., Nisbet, D. R. & Forsythe, J. S. Specific control of cell–material interactions: targeting cell receptors using ligand-functionalized polymer substrates. Prog. Polym. Sci. 39, 1312–1347 (2014).
Martins, I. M., Reis, R. L. & Azevedo, H. S. Phage display technology in biomaterials engineering: progress and opportunities for applications in regenerative medicine. ACS Chem. Biol. 11, 2962–2980 (2016). This Review highlights the potential of phage display technologies for the discovery of biologically functional peptide sequences.
Hynes, R. O. The emergence of integrins: a personal and historical perspective. Matrix Biol. 23, 333–340 (2004).
Arnaout, M. A., Mahalingam, B. & Xiong, J.-P. Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 21, 381–410 (2005).
Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 12, 697–715 (1996).
Kapp, T. G. et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep. 7, 39805 (2017).
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Gallant, N. D., Michael, K. E. & García, A. J. Cell adhesion strengthening: contributions of adhesive area, integrin binding, and focal adhesion assembly. Mol. Biol. Cell 16, 4329–4340 (2005).
Gallant, N. D. & García, A. J. Model of integrin-mediated cell adhesion strengthening. J. Biomech. 40, 1301–1309 (2007).
Garcia, M. A., Nelson, W. J. & Chavez, N. Cell–cell junctions organize structural and signaling networks. Cold Spring Harb. Perspect. Biol. 10, a029181 (2018).
Gumbiner, B. M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 6, 622–634 (2005).
Shapiro, L. & Weis, W. I. Structure and biochemistry of cadherins and catenins. Cold Spring Harb. Perspect. Biol. 1, a003053 (2009).
Hersel, U., Dahmen, C. & Kessler, H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385–4415 (2003).
Webber, M. J. et al. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater. 6, 3–11 (2010).
Huang, Z. et al. Bioactive nanofibers instruct cells to proliferate and differentiate during enamel regeneration. J. Bone Miner. Res. 23, 1995–2006 (2008).
Mata, A. et al. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials 31, 6004–6012 (2010).
Yin, H. et al. Three-dimensional self-assembling nanofiber matrix rejuvenates aged/degenerative human tendon stem/progenitor cells. Biomaterials 236, 119802 (2020).
Burgess, K. A. et al. Functionalised peptide hydrogel for the delivery of cardiac progenitor cells. Mater. Sci. Eng. C 119, 111539 (2021).
Zhou, M. et al. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 30, 2523–2530 (2009).
Redondo-Gómez, C., Padilla-Lopategui, S., Azevedo, H. S. & Mata, A. Host–guest-mediated epitope presentation on self-assembled peptide amphiphile hydrogels. ACS Biomater. Sci. Eng. 6, 4870–4880 (2020).
Mata, A. et al. Micropatterning of bioactive self-assembling gels. Soft Matter 5, 1228–1236 (2009).
Genové, E., Shen, C., Zhang, S. & Semino, C. E. The effect of functionalized self-assembling peptide scaffolds on human aortic endothelial cell function. Biomaterials 26, 3341–3351 (2005).
Yu, T. T. & Shoichet, M. S. Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials 26, 1507–1514 (2005).
Tong, Y. W. & Shoichet, M. S. Peptide surface modification of poly(tetrafluoroethylene-co-hexafluoropropylene) enhances its interaction with central nervous system neurons. J. Biomed. Mater. Res. 42, 85–95 (1998).
Jun, H.-W. & West, J. L. Endothelialization of microporous YIGSR/PEG-modified polyurethaneurea. Tissue Eng. 11, 1133–1140 (2005).
Ali, S., Saik, J. E., Gould, D. J., Dickinson, M. E. & West, J. L. Immobilization of cell-adhesive laminin peptides in degradable PEGDA hydrogels influences endothelial cell tubulogenesis. Bioresearch Open Access 2, 241–249 (2013).
Li, Q., Chow, K. L. & Chau, Y. Three-dimensional self-assembling peptide matrix enhances the formation of embryoid bodies and their neuronal differentiation. J. Biomed. Mater. Res. A 102, 1991–2000 (2014).
Silva, G. A. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 303, 1352–1355 (2004).
Tysseling-Mattiace, V. M. et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 28, 3814–3823 (2008).
Álvarez Z. et al. Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury. Science 374, 848–856 (2021). This article describes how the motion of molecules within peptide nanofibres can affect regeneration in a mouse model of severe spinal cord injury.
Genové, E. et al. Functionalized self-assembling peptide hydrogel enhance maintenance of hepatocyte activity in vitro. J. Cell. Mol. Med. 13, 3387–3397 (2009).
Barcellona, M. N. et al. Control of adhesive ligand density for modulation of nucleus pulposus cell phenotype. Biomaterials 250, 120057 (2020).
Yu, Y.-M. et al. The extracellular matrix glycoprotein tenascin-C promotes locomotor recovery after spinal cord injury in adult zebrafish. Neuroscience 183, 238–250 (2011).
Meiners, S., Nur-e-Kamal, M. S. & Mercado, M. L. Identification of a neurite outgrowth-promoting motif within the alternatively spliced region of human tenascin-C. J. Neurosci. 21, 7215–7225 (2001).
Sever, M., Gunay, G., Guler, M. O. & Tekinay, A. B. Tenascin-C derived signaling induces neuronal differentiation in a three-dimensional peptide nanofiber gel. Biomater. Sci. 6, 1859–1868 (2018).
Sever, M., Mammadov, B., Guler, M. O. & Tekinay, A. B. Tenascin-C mimetic peptide nanofibers direct stem cell differentiation to osteogenic lineage. Biomacromolecules 15, 4480–4487 (2014).
Sharma, P., Kaur, H. & Roy, S. Designing a tenascin-C-inspired short bioactive peptide scaffold to direct and control cellular behavior. ACS Biomater. Sci. Eng. 5, 6497–6510 (2019).
Rezvani Ghomi, E., Nourbakhsh, N., Akbari Kenari, M., Zare, M. & Ramakrishna, S. Collagen-based biomaterials for biomedical applications. J. Biomed. Mater. Res. B 109, 1986–1999 (2021).
O’Leary, L. E. R., Fallas, J. A., Bakota, E. L., Kang, M. K. & Hartgerink, J. D. Multi-hierarchical self-assembly of a collagen mimetic peptide from triple helix to nanofibre and hydrogel. Nat. Chem. 3, 821–828 (2011). This article describes the design and synthesis of a peptide that replicates the multi-hierarchical self-assembly of natural collagen into native triple helix conformation.
Kumar, V. A. et al. A nanostructured synthetic collagen mimic for hemostasis. Biomacromolecules 15, 1484–1490 (2014).
Uysal, O. et al. Collagen peptide presenting nanofibrous scaffold for intervertebral disc regeneration. ACS Appl. Bio Mater. 2, 1686–1695 (2019).
Anderson, J. M. et al. Osteogenic differentiation of human mesenchymal stem cells synergistically enhanced by biomimetic peptide amphiphiles combined with conditioned medium. Acta Biomater. 7, 675–682 (2011).
Wojtowicz, A. M. et al. Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 31, 2574–2582 (2010).
Bradshaw, M. et al. Designer self-assembling hydrogel scaffolds can impact skin cell proliferation and migration. Sci. Rep. 4, 6903 (2014).
Liming, B., Murat, G., Robert, L. M. & Jason, A. B. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl Acad. Sci. USA 110, 10117–10122 (2013).
Li, R. et al. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials 145, 33–43 (2017).
O’Grady, B. J. et al. Development of an N-cadherin biofunctionalized hydrogel to support the formation of synaptically connected neural networks. ACS Biomater. Sci. Eng. 6, 5811–5822 (2020).
Dhavalikar, P. et al. Review of integrin-targeting biomaterials in tissue engineering. Adv. Healthc. Mater. 9, 2000795 (2020).
Cosgriff-Hernandez, E. et al. Bioactive hydrogels based on designer collagens. Acta Biomater. 6, 3969–3977 (2010).
Becerra-Bayona, S. M., Guiza-Arguello, V. R., Russell, B., Höök, M. & Hahn, M. S. Influence of collagen-based integrin α(1) and α(2) mediated signaling on human mesenchymal stem cell osteogenesis in three dimensional contexts. J. Biomed. Mater. Res. A 106, 2594–2604 (2018).
Gandavarapu, N. R., Alge, D. L. & Anseth, K. S. Osteogenic differentiation of human mesenchymal stem cells on α5 integrin binding peptide hydrogels is dependent on substrate elasticity. Biomater. Sci. 2, 352–361 (2014).
Zhu, Y. et al. Potent laminin-inspired antioxidant regenerative dressing accelerates wound healing in diabetes. Proc. Natl Acad. Sci. USA 115, 6816–6821 (2018).
Lishko, V. K. et al. Multiple binding sites in fibrinogen for integrin alphaMbeta2 (Mac-1). J. Biol. Chem. 279, 44897–44906 (2004).
Yebra, M. et al. Recognition of the neural chemoattractant Netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev. Cell 5, 695–707 (2003).
Makarem, R. et al. Competitive binding of vascular cell adhesion molecule-1 and the HepII/IIICS domain of fibronectin to the integrin alpha 4 beta 1. J. Biol. Chem. 269, 4005–4011 (1994).
Calzada, M. J. et al. Identification of novel β1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J. Biol. Chem. 279, 41734–41743 (2004).
Prater, C. A., Plotkin, J., Jaye, D. & Frazier, W. A. The properdin-like type I repeats of human thrombospondin contain a cell attachment site. J. Cell Biol. 112, 1031–1040 (1991).
Huettner, N., Dargaville, T. R. & Forget, A. Discovering cell-adhesion peptides in tissue engineering: beyond RGD. Trends Biotechnol. 36, 372–383 (2018).
Plow, E. F., Haas, T. A., Zhang, L., Loftus, J. & Smith, J. W. Ligand binding to integrins. J. Biol. Chem. 275, 21785–21788 (2000).
Salmerón-Sánchez, M. & Dalby, M. J. Synergistic growth factor microenvironments. Chem. Commun. 52, 13327–13336 (2016).
Mitchell, A. C., Briquez, P. S., Hubbell, J. A. & Cochran, J. R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 30, 1–12 (2016).
Chen, D., Zhao, M. & Mundy, G. R. Bone morphogenetic proteins. Growth Factors 22, 233–241 (2004).
Gu, T. et al. Human bone morphogenetic protein 7 transfected nucleus pulposus cells delay the degeneration of intervertebral disc in dogs. J. Orthop. Res. 35, 1311–1322 (2017).
Li, X. et al. Functional self-assembled peptide scaffold inhibits tumor necrosis factor-alpha-induced inflammation and apoptosis in nucleus pulposus cells by suppressing nuclear factor-κB signaling. J. Biomed. Mater. Res. A 106, 1082–1091 (2018).
Li, X.-C. et al. BMP7-based functionalized self-assembling peptides protect nucleus pulposus-derived stem cells from apoptosis in vitro. Tissue Eng. Part. A 22, 1218–1228 (2016).
Madl, C. M., Mehta, M., Duda, G. N., Heilshorn, S. C. & Mooney, D. J. Presentation of BMP-2 mimicking peptides in 3D hydrogels directs cell fate commitment in osteoblasts and mesenchymal stem cells. Biomacromolecules 15, 445–455 (2014).
Liang, P. et al. Bioactive 3D scaffolds self-assembled from phosphorylated mimicking peptide amphiphiles to enhance osteogenesis. J. Biomater. Sci. Polym. Ed. 30, 34–48 (2019).
Ye, W. et al. Articular cartilage reconstruction with TGF-β1-simulating self-assembling peptide hydrogel-based composite scaffold. Acta Biomater. https://doi.org/10.1016/j.actbio.2022.05.012 (2022).
D’Andrea, L. D. et al. Targeting angiogenesis: structural characterization and biological properties of a de novo engineered VEGF mimicking peptide. Proc. Natl Acad. Sci. USA 102, 14215–14220 (2005).
Liu, X. et al. In vivo studies on angiogenic activity of two designer self-assembling peptide scaffold hydrogels in the chicken embryo chorioallantoic membrane. Nanoscale 4, 2720–2727 (2012).
Kumar, V. A. et al. Highly angiogenic peptide nanofibers. ACS Nano 9, 860–868 (2015).
Baird, A., Schubert, D., Ling, N. & Guillemin, R. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proc. Natl Acad. Sci. USA 85, 2324–2328 (1988).
Rubert Pérez, C. M., Álvarez, Z., Chen, F., Aytun, T. & Stupp, S. I. Mimicking the bioactivity of fibroblast growth factor-2 using supramolecular nanoribbons. ACS Biomater. Sci. Eng. 3, 2166–2175 (2017).
Pickart, L. & Margolina, A. Regenerative and protective actions of the GHK-Cu peptide in the light of the new gene data. Int. J. Mol. Sci. 19, 1987 (2018).
Okesola, B. O. et al. Growth-factor free multicomponent nanocomposite hydrogels that stimulate bone formation. Adv. Funct. Mater. 30, 1906205 (2020).
Hamada, Y. et al. Angiogenic activity of osteopontin-derived peptide SVVYGLR. Biochem. Biophys. Res. Commun. 310, 153–157 (2003).
Gao, X.-R., Xu, H.-J., Wang, L.-F., Liu, C.-B. & Yu, F. Mesenchymal stem cell transplantation carried in SVVYGLR modified self-assembling peptide promoted cardiac repair and angiogenesis after myocardial infarction. Biochem. Biophys. Res. Commun. 491, 112–118 (2017).
Cai, H. et al. Self-assembling peptide modified with QHREDGS as a novel delivery system for mesenchymal stem cell transplantation after myocardial infarction. FASEB J. 33, 8306–8320 (2019).
Shi, W. et al. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 45, 247–261 (2016).
Colangelo, A. M. et al. A new nerve growth factor-mimetic peptide active on neuropathic pain in rats. J. Neurosci. 28, 2698–2709 (2008).
Edelbrock, A. N. et al. Supramolecular nanostructure activates TrkB receptor signaling of neuronal cells by mimicking brain-derived neurotrophic factor. Nano Lett. 18, 6237–6247 (2018).
Lu, C. et al. Bioactive self-assembling peptide hydrogels functionalized with brain-derived neurotrophic factor and nerve growth factor mimicking peptides synergistically promote peripheral nerve regeneration. ACS Biomater. Sci. Eng. 4, 2994–3005 (2018).
Zou, Z. et al. Biocompatibility and bioactivity of designer self-assembling nanofiber scaffold containing FGL motif for rat dorsal root ganglion neurons. J. Biomed. Mater. Res. A 95A, 1125–1131 (2010).
Li, L.-L., An, H.-W., Peng, B., Zheng, R. & Wang, H. Self-assembled nanomaterials: design principles, the nanostructural effect, and their functional mechanisms as antimicrobial or detection agents. Mater. Horiz. 6, 1794–1811 (2019).
Subramanian, S., Ross, N. W. & MacKinnon, S. L. Myxinidin, a novel antimicrobial peptide from the epidermal mucus of hagfish, Myxine glutinosa L. Mar. Biotechnol. 11, 748–757 (2009).
Lombardi, L. et al. Antimicrobial peptides at work: interaction of myxinidin and its mutant WMR with lipid bilayers mimicking the P. aeruginosa and E. coli membranes. Sci. Rep. 7, 44425 (2017).
Lombardi, L. et al. Enhancing the potency of antimicrobial peptides through molecular engineering and self-assembly. Biomacromolecules 20, 1362–1374 (2019).
Guha, S. et al. Applications and evolution of melittin, the quintessential membrane active peptide. Biochem. Pharmacol. 193, 114769 (2021).
Chen, W. et al. Self-assembled peptide nanofibers display natural antimicrobial peptides to selectively kill bacteria without compromising cytocompatibility. ACS Appl. Mater. Interfaces 11, 28681–28689 (2019).
Zarghami, V., Ghorbani, M., Pooshang Bagheri, K. & Shokrgozar, M. A. Melittin antimicrobial peptide thin layer on bone implant chitosan-antibiotic coatings and their bactericidal properties. Mater. Chem. Phys. 263, 124432 (2021).
Xiao, M. et al. Chemically immobilized antimicrobial peptide on polymer and self-assembled monolayer substrates. Langmuir 34, 12889–12896 (2018).
Sarkar, B. et al. Membrane-disrupting nanofibrous peptide hydrogels. ACS Biomater. Sci. Eng. 5, 4657–4670 (2019).
Adak, A., Ghosh, S., Gupta, V. & Ghosh, S. Biocompatible lipopeptide-based antibacterial hydrogel. Biomacromolecules 20, 1889–1898 (2019).
Veiga, A. S. et al. Arginine-rich self-assembling peptides as potent antibacterial gels. Biomaterials 33, 8907–8916 (2012).
Salick, D. A., Kretsinger, J. K., Pochan, D. J. & Schneider, J. P. Inherent antibacterial activity of a peptide-based beta-hairpin hydrogel. J. Am. Chem. Soc. 129, 14793–14799 (2007).
Salick, D. A., Pochan, D. J. & Schneider, J. P. Design of an injectable β-hairpin peptide hydrogel that kills methicillin-resistant Staphylococcus aureus. Adv. Mater. 21, 4120–4123 (2009).
Dewangan, R. P., Jain, A., Tanwar, S., Yar, M. S. & Pasha, S. Self assembly and hydrogelation of spermine functionalized aromatic peptidomimetics against planktonic and sessile methicillin resistant S. aureus. RSC Adv. 6, 112656–112666 (2016).
Laverty, G. et al. Ultrashort cationic naphthalene-derived self-assembled peptides as antimicrobial nanomaterials. Biomacromolecules 15, 3429–3439 (2014).
McCloskey, A. P. et al. Self-assembling ultrashort NSAID-peptide nanosponges: multifunctional antimicrobial and anti-inflammatory materials. RSC Adv. 6, 114738–114749 (2016).
Pazos, E. et al. Nucleation and growth of ordered arrays of silver nanoparticles on peptide nanofibers: hybrid nanostructures with antimicrobial properties. J. Am. Chem. Soc. 138, 5507–5510 (2016).
Wang, Y. et al. Silver mineralization on self-assembled peptide nanofibers for long term antimicrobial effect. J. Mater. Chem. 22, 2575–2581 (2012).
Si, Y., Wen, Y., Kelly, S. H., Chong, A. S. & Collier, J. H. Intranasal delivery of adjuvant-free peptide nanofibers elicits resident CD8+ T cell responses. J. Control. Rel. 282, 120–130 (2018).
Kelly, S. H. et al. Enabling sublingual peptide immunization with molecular self-assemblies. Biomaterials 241, 119903 (2020). This article reports short self-assembling peptides as nanofibre-based sublingual vaccines with low immunogenicity and durable systemic antibody responses.
Rudra, J. S. et al. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials 33, 6476–6484 (2012).
Demircan, M. B. et al. Biotin functionalized self-assembled peptide nanofiber as an adjuvant for immunomodulatory response. Biotechnol. J. 15, 2000100 (2020).
Appavu, R. et al. Enhancing the magnitude of antibody responses through biomaterial stereochemistry. ACS Biomater. Sci. Eng. 1, 601–609 (2015).
Kamalov, M. et al. Ovalbumin epitope SIINFEKL self-assembles into a supramolecular hydrogel. Sci. Rep. 9, 2696 (2019).
Kim, Y.-G. et al. Self-assembled multi-epitope peptide amphiphiles enhance the immune response against enterovirus 71. Nanomaterials 10, 2342 (2020).
Ding, Y. et al. Self-assembling peptide for co-delivery of HIV-1 CD8+ T cells epitope and Toll-like receptor 7/8 agonists R848 to induce maturation of monocyte derived dendritic cell and augment polyfunctional cytotoxic T lymphocyte (CTL) response. J. Control. Rel. 236, 22–30 (2016).
Friedrich, B. M., Beasley, D. W. C. & Rudra, J. S. Supramolecular peptide hydrogel adjuvanted subunit vaccine elicits protective antibody responses against West Nile virus. Vaccine 34, 5479–5482 (2016).
Li, X., Galliher-Beckley, A., Huang, H., Sun, X. & Shi, J. Peptide nanofiber hydrogel adjuvanted live virus vaccine enhances cross-protective immunity to porcine reproductive and respiratory syndrome virus. Vaccine 31, 4508–4515 (2013).
Huang, H. et al. Design of a shear-thinning recoverable peptide hydrogel from native sequences and application for influenza H1N1 vaccine adjuvant. Soft Matter 7, 8905–8912 (2011).
Castelletto, V. & Hamley, I. W. Amyloid and hydrogel formation of a peptide sequence from a coronavirus spike protein. ACS Nano https://doi.org/10.1021/acsnano.1c10658 (2022). This article reports the formation of amyloid structures and self-supporting hydrogels starting from a conserved coronavirus spike protein peptide.
Carragee, E. J., Hurwitz, E. L. & Weiner, B. K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 11, 471–491 (2011).
Seelbach, R. J. et al. Injectable hyaluronan hydrogels with peptide-binding dendrimers modulate the controlled release of BMP-2 and TGF-β1. Macromol. Biosci. 15, 1035–1044 (2015).
Shah, R. N. et al. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc. Natl Acad. Sci. USA 107, 3293–3298 (2010).
Ju, X. et al. A photo-crosslinked proteinogenic hydrogel enabling self-recruitment of endogenous TGF-β1 for cartilage regeneration. Smart Mater. Med. 3, 85–93 (2022).
Lewis, J. A. et al. Transforming growth factor β-1 binding by peptide amphiphile hydrogels. ACS Biomater. Sci. Eng. https://doi.org/10.1021/acsbiomaterials.0c00679 (2020).
Behanna, H. A., Donners, J. J. J. M., Gordon, A. C. & Stupp, S. I. Coassembly of amphiphiles with opposite peptide polarities into nanofibers. J. Am. Chem. Soc. 127, 1193–1200 (2005).
Lee, S. S. et al. Gel scaffolds of BMP-2-binding peptide amphiphile nanofibers for spinal arthrodesis. Adv. Healthc. Mater. 4, 131–141 (2015).
Davis, M. E. et al. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl Acad. Sci. USA 103, 8155–8160 (2006).
Miller, R. E., Kopesky, P. W. & Grodzinsky, A. J. Growth factor delivery through self-assembling peptide scaffolds. Clin. Orthop. Relat. Res. 469, 2716–2724 (2011).
Hsieh, P. C. H., Davis, M. E., Gannon, J., MacGillivray, C. & Lee, R. T. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J. Clin. Invest. 116, 237–248 (2006).
Lee, S. S. et al. Sulfated glycopeptide nanostructures for multipotent protein activation. Nat. Nanotechnol. 12, 821–829 (2017). This article reports the design and synthesis of highly versatile sulfated glycopeptides able to bind five critical proteins displaying different binding domains.
Kocabey, S., Ceylan, H., Tekinay, A. B. & Guler, M. O. Glycosaminoglycan mimetic peptide nanofibers promote mineralization by osteogenic cells. Acta Biomater. 9, 9075–9085 (2013).
Mammadov, R. et al. Heparin mimetic peptide nanofibers promote angiogenesis. Biomacromolecules 12, 3508–3519 (2011).
Mammadov, R., Mammadov, B., Guler, M. O. & Tekinay, A. B. Growth factor binding on heparin mimetic peptide nanofibers. Biomacromolecules 13, 3311–3319 (2012).
Rajangam, K. et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 6, 2086–2090 (2006).
Ghanaati, S. et al. Dynamic in vivo biocompatibility of angiogenic peptide amphiphile nanofibers. Biomaterials 30, 6202–6212 (2009).
Webber, M. J. et al. Capturing the stem cell paracrine effect using heparin-presenting nanofibers to treat cardiovascular diseases. J. Tissue Eng. Regen. Med. 4, 600–610 (2010).
Chow, L. W., Wang, L., Kaufman, D. B. & Stupp, S. I. Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials 31, 6154–6161 (2010).
Guo, H. et al. Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochem. Biophys. Res. Commun. 424, 105–111 (2012).
Casey, B. J. & Kofinas, P. Selective binding of carcinoembryonic antigen using imprinted polymeric hydrogels. J. Biomed. Mater. Res. A 87A, 359–363 (2008).
Xue, B. et al. Peptide-functionalized hydrogel cubes for active tumor cell targeting. Biomacromolecules 19, 4084–4097 (2018).
Zheng, W. et al. Surface-induced hydrogelation inhibits platelet aggregation. J. Am. Chem. Soc. 135, 266–271 (2013).
Javid, N. et al. Cooperative self-assembly of peptide gelators and proteins. Biomacromolecules 14, 4368–4376 (2013).
Yuan, T., Fei, J., Xu, Y., Yang, X. & Li, J. Stimuli-responsive dipeptide-protein hydrogels through schiff base coassembly. Macromol. Rapid Commun. https://doi.org/10.1002/marc.201700408 (2017).
Basak, S., Nanda, J. & Banerjee, A. Multi-stimuli responsive self-healing metallo-hydrogels: tuning of the gel recovery property. Chem. Commun. 50, 2356–2359 (2014).
Basak, S., Nandi, N. & Banerjee, A. Selective binding of hydrogen chloride and its trapping through supramolecular gelation. Chem. Commun. 50, 6917–6919 (2014).
Capito, R. M., Azevedo, H. S., Velichko, Y. S., Mata, A. & Stupp, S. I. Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science 319, 1812–1816 (2008).
Inostroza-Brito, K. E. et al. Co-assembly, spatiotemporal control and morphogenesis of a hybrid protein–peptide system. Nat. Chem. 7, 897–904 (2015). This article describes the use of peptide amphiphiles to guide the design of peptide–protein structures with hierarchical organization and spatiotemporal control.
Hedegaard, C. L. et al. Hydrodynamically guided hierarchical self-assembly of peptide–protein bioinks. Adv. Funct. Mater. 28, 1703716 (2018).
Majkowska, A. et al. Interfacial self-assembly to spatially organize graphene oxide into hierarchical and bioactive structures. Front. Mater. 7, 167 (2020).
Ghosh, M. et al. Disordered protein stabilization by co-assembly of short peptides enables formation of robust membranes. ACS Appl. Mater. Interfaces 14, 464–473 (2022).
Hedegaard, C. L. et al. Peptide-protein coassembling matrices as a biomimetic 3D model of ovarian cancer. Sci. Adv. 6, eabb3298 (2020).
Osuna de la Peña, D. et al. Bioengineered 3D models of human pancreatic cancer recapitulate in vivo tumour biology. Nat. Commun. 12, 5623 (2021).
Okesola, B. O. et al. Covalent co-assembly between resilin-like polypeptide and peptide amphiphile into hydrogels with controlled nanostructure and improved mechanical properties. Biomater. Sci. 8, 846–857 (2020).
Okesola, B. O. et al. De novo design of functional coassembling organic–inorganic hydrogels for hierarchical mineralization and neovascularization. ACS Nano 15, 11202–11217 (2021).
Ghosh, M. et al. Arginine-presenting peptide hydrogels decorated with hydroxyapatite as biomimetic scaffolds for bone regeneration. Biomacromolecules 18, 3541–3550 (2017).
Vitale, M. et al. Hydroxyapatite-decorated Fmoc-hydrogel as a bone-mimicking substrate for osteoclast differentiation and culture. Acta Biomater. 138, 144–154 (2022).
Chung, W.-J., Kwon, K.-Y., Song, J. & Lee, S.-W. Evolutionary screening of collagen-like peptides that nucleate hydroxyapatite crystals. Langmuir 27, 7620–7628 (2011).
Gungormus, M. et al. Self assembled bi-functional peptide hydrogels with biomineralization-directing peptides. Biomaterials 31, 7266–7274 (2010).
Abbarin, N., San Miguel, S., Holcroft, J., Iwasaki, K. & Ganss, B. The enamel protein amelotin is a promoter of hydroxyapatite mineralization. J. Bone Miner. Res. 30, 775–785 (2015).
Brunton, P. A. et al. Treatment of early caries lesions using biomimetic self-assembling peptides — a clinical safety trial. Br. Dent. J. 215, E6–E6 (2013).
Mukherjee, K. et al. Amelogenin peptide-chitosan hydrogel for biomimetic enamel regrowth. Front. Dent. Med. 2, 697544 (2021).
Wang, D. et al. Controlling enamel remineralization by amyloid-like amelogenin mimics. Adv. Mater. 32, 2002080 (2020).
Li, Q.-L. et al. A novel self-assembled oligopeptide amphiphile for biomimetic mineralization of enamel. BMC Biotechnol. 14, 32 (2014).
Yang, Y. et al. 8DSS-promoted remineralization of initial enamel caries in vitro. J. Dent. Res. 93, 520–524 (2014).
Shuturminska, K. et al. Elastin-like protein, with statherin derived peptide, controls fluorapatite formation and morphology. Front. Physiol. 8, 368 (2017).
Niland, S., Riscanevo, A. X. & Eble, J. A. Matrix metalloproteinases shape the tumor microenvironment in cancer progression. Int. J. Mol. Sci. 23, 146 (2022).
Kim, J.-K., Anderson, J., Jun, H.-W., Repka, M. A. & Jo, S. Self-assembling peptide amphiphile-based nanofiber gel for bioresponsive cisplatin delivery. Mol. Pharm. 6, 978–985 (2009).
Kalafatovic, D. et al. MMP-9 triggered micelle-to-fibre transitions for slow release of doxorubicin. Biomater. Sci. 3, 246–249 (2015).
Adak, A. et al. Extracellular matrix (ECM)-mimicking neuroprotective injectable sulfo-functionalized peptide hydrogel for repairing brain injury. ACS Biomater. Sci. Eng. 6, 2287–2296 (2020).
Ferreira, D. S. et al. Molecularly engineered self-assembling membranes for cell-mediated degradation. Adv. Healthc. Mater. 4, 602–612 (2015).
Chen, E. I. et al. A unique substrate recognition profile for matrix metalloproteinase-2. J. Biol. Chem. 277, 4485–4491 (2002).
Smith, M. M., Shi, L. & Navre, M. Rapid identification of highly active and selective substrates for stromelysin and matrilysin using bacteriophage peptide display libraries. J. Biol. Chem. 270, 6440–6449 (1995).
Pan, W. et al. Identification of peptide substrates for human MMP-11 (stromelysin-3) using phage display. J. Biol. Chem. 278, 27820–27827 (2003).
Deng, S. J. et al. Substrate specificity of human collagenase 3 assessed using a phage-displayed peptide library. J. Biol. Chem. 275, 31422–31427 (2000).
Nagase, H. & Fields, G. B. Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Biopolymers 40, 399–416 (1996).
Patterson, J. & Hubbell, J. A. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 31, 7836–7845 (2010).
Gjorevski, N. et al. Designer matrices for intestinal stem cell and organoid culture. Nature 539, 560–564 (2016).
Weber, H. M., Tsurkan, M. V., Magno, V., Freudenberg, U. & Werner, C. Heparin-based hydrogels induce human renal tubulogenesis in vitro. Acta Biomater. 57, 59–69 (2017).
Fonseca, K. B. et al. Injectable MMP-sensitive alginate hydrogels as hMSC delivery systems. Biomacromolecules 15, 380–390 (2014).
Webber, M. J., Newcomb, C. J., Bitton, R. & Stupp, S. I. Switching of self-assembly in a peptide nanostructure with a specific enzyme. Soft Matter 7, 9665–9672 (2011).
Dehsorkhi, A., Hamley, I. W., Seitsonen, J. & Ruokolainen, J. Tuning self-assembled nanostructures through enzymatic degradation of a peptide amphiphile. Langmuir 29, 6665–6672 (2013).
Hsu, C.-W., Olabisi, R. M., Olmsted-Davis, E. A., Davis, A. R. & West, J. L. Cathepsin K-sensitive poly(ethylene glycol) hydrogels for degradation in response to bone resorption. J. Biomed. Mater. Res. Part. A 98A, 53–62 (2011).
Shim, M. K. et al. Cancer-specific drug–drug nanoparticles of pro-apoptotic and cathepsin B-cleavable peptide-conjugated doxorubicin for drug-resistant cancer therapy. Biomaterials 261, 120347 (2020).
Tian, Y. F., Hudalla, G. A., Han, H. & Collier, J. H. Controllably degradable β-sheet nanofibers and gels from self-assembling depsipeptides. Biomater. Sci. 1, 1037–1045 (2013).
Shi, Y., Ferreira, D. S., Banerjee, J., Pickford, A. R. & Azevedo, H. S. Tuning the matrix metalloproteinase-1 degradability of peptide amphiphile nanofibers through supramolecular engineering. Biomater. Sci. 7, 5132–5142 (2019).
Giano, M. C., Pochan, D. J. & Schneider, J. P. Controlled biodegradation of self-assembling β-hairpin peptide hydrogels by proteolysis with matrix metalloproteinase-13. Biomaterials 32, 6471–6477 (2011).
Pankov, R. & Yamada, K. M. Fibronectin at a glance. J. Cell Sci. 115, 3861–3863 (2002).
Krammer, A., Craig, D., Thomas, W. E., Schulten, K. & Vogel, V. A structural model for force regulated integrin binding to fibronectin’s RGD-synergy site. Matrix Biol. 21, 139–147 (2002).
Pashuck, E. T. et al. Controlled sub-nanometer epitope spacing in a three-dimensional self-assembled peptide hydrogel. ACS Nano 10, 11096–11104 (2016).
Jung, J. P., Moyano, J. V. & Collier, J. H. Multifactorial optimization of endothelial cell growth using modular synthetic extracellular matrices. Integr. Biol. 3, 185–196 (2011).
Horgan, C. C. et al. Characterisation of minimalist co-assembled fluorenylmethyloxycarbonyl self-assembling peptide systems for presentation of multiple bioactive peptides. Acta Biomater. 38, 11–22 (2016).
Sur, S. et al. A hybrid nanofiber matrix to control the survival and maturation of brain neurons. Biomaterials 33, 545–555 (2012).
Martino, M. M. et al. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci. Transl. Med. 3, 100ra89 (2011).
Trujillo, S. et al. Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials 252, 120104 (2020).
Liu, X. et al. Functionalized self-assembling peptide nanofiber hydrogels mimic stem cell niche to control human adipose stem cell behavior in vitro. Acta Biomater. 9, 6798–6805 (2013).
Xia, K. et al. RGD- and VEGF-mimetic peptide epitope-functionalized self-assembling peptide hydrogels promote dentin-pulp complex regeneration. Int. J. Nanomed. 15, 6631–6647 (2020).
Brun, P. et al. 3D Synthetic peptide-based architectures for the engineering of the enteric nervous system. Sci. Rep. 9, 5583 (2019).
Yang, S. et al. Self-assembling peptide hydrogels functionalized with LN- and BDNF- mimicking epitopes synergistically enhance peripheral nerve regeneration. Theranostics 10, 8227–8249 (2020).
Tejeda-Montes, E. et al. Bioactive membranes for bone regeneration applications: effect of physical and biomolecular signals on mesenchymal stem cell behavior. Acta Biomater. 10, 134–141 (2014).
Tejeda-Montes, E. et al. Mineralization and bone regeneration using a bioactive elastin-like recombinamer membrane. Biomaterials 35, 8339–8347 (2014).
Derkus, B. et al. Multicomponent hydrogels for the formation of vascularized bone-like constructs in vitro. Acta Biomater. 109, 82–94 (2020). This article reports multicomponent peptide hydrogels containing key epitopes of the native bone matrix to promote both osteogenesis and angiogenesis in vitro.
Lu, J. et al. Synergistic effects of dual-presenting VEGF- and BDNF-mimetic peptide epitopes from self-assembling peptide hydrogels on peripheral nerve regeneration. Nanoscale 11, 19943–19958 (2019).
Galler, K. M., Aulisa, L., Regan, K. R., D’Souza, R. N. & Hartgerink, J. D. Self-assembling multidomain peptide hydrogels: designed susceptibility to enzymatic cleavage allows enhanced cell migration and spreading. J. Am. Chem. Soc. 132, 3217–3223 (2010).
Gouveia, R. M., Castelletto, V., Hamley, I. W. & Connon, C. J. New self-assembling multifunctional templates for the biofabrication and controlled self-release of cultured tissue. Tissue Eng. A 21, 1772–1784 (2015).
Jansen, L. E. et al. A poly(ethylene glycol) three-dimensional bone marrow hydrogel. Biomaterials 280, 121270 (2022). This articles describes the synthesis of a synthetic hydrogel capable of incorporating the complex signals of native bone marrow through a mixture of multiple peptide epitopes.
Galarza, S., Crosby, A. J., Pak, C. & Peyton, S. R. Control of astrocyte quiescence and activation in a synthetic brain hydrogel. Adv. Healthc. Mater. 9, 1901419 (2020).
Zhang, S. Discovery and design of self-assembling peptides. Interface Focus. 7, 20170028 (2017).
Sankar, S. et al. Clinical use of the self-assembling peptide RADA16: a review of current and future trends in biomedicine. Front. Bioeng. Biotechnol. 9, 679525 (2021).
Luo, Z., Wang, S. & Zhang, S. Fabrication of self-assembling D-form peptide nanofiber scaffold d-EAK16 for rapid hemostasis. Biomaterials 32, 2013–2020 (2011).
Bröseler, F. et al. Randomised clinical trial investigating self-assembling peptide P(11)-4 in the treatment of early caries. Clin. Oral. Investig. 24, 123–132 (2020).
Rahmani, G. et al. First safety and performance evaluation of T45K, a self-assembling peptide barrier hemostatic device, after skin lesion excision. Dermatol. Surg. 44, 939–948 (2018).
Natarajan, M., Lin, K.-M., Hsueh, R. C., Sternweis, P. C. & Ranganathan, R. A global analysis of cross-talk in a mammalian cellular signalling network. Nat. Cell Biol. 8, 571–580 (2006).
Asahara, T. et al. Synergistic effect of vascular endothelial growth factor and basic fibroblast growth factor on angiogenesis in vivo. Circulation 92, 365–371 (1995).
Tsai, C.-Y. et al. Helical structure motifs made searchable for functional peptide design. Nat. Commun. 13, 102 (2022).
Plisson, F., Ramírez-Sánchez, O. & Martínez-Hernández, C. Machine learning-guided discovery and design of non-hemolytic peptides. Sci. Rep. 10, 16581 (2020).
Callaway, E. ‘The entire protein universe’: AI predicts shape of nearly every known protein. Nature 608, 15–16 (2022).
van der Lee, R. et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 114, 6589–6631 (2014).
Cao, B. et al. Bacteriophage-based biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 145, 73–95 (2019).
Ligorio, C. et al. TGF-β3-loaded graphene oxide — self-assembling peptide hybrid hydrogels as functional 3D scaffolds for the regeneration of the nucleus pulposus. Acta Biomater. 127, 116–130 (2021).
Tysseling, V. M. et al. Self-assembling peptide amphiphile promotes plasticity of serotonergic fibers following spinal cord injury. J. Neurosci. Res. 88, 3161–3170 (2010).
Gelain, F., Bottai, D., Vescovi, A. & Zhang, S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS ONE 1, e119 (2006).
Balaoing, L. R. et al. Laminin peptide-immobilized hydrogels modulate valve endothelial cell hemostatic regulation. PLoS ONE 10, e0130749 (2015).
Stauffer, W. R. & Cui, X. T. Polypyrrole doped with 2 peptide sequences from laminin. Biomaterials 27, 2405–2413 (2006).
Eren Cimenci, C., Kurtulus, G. U., Caliskan, O. S., Guler, M. O. & Tekinay, A. B. N-cadherin mimetic peptide nanofiber system induces chondrogenic differentiation of mesenchymal stem cells. Bioconjug. Chem. 30, 2417–2426 (2019).
Pan, H. et al. Bone induction by biomimetic PLGA copolymer loaded with a novel synthetic RADA16-P24 peptide in vivo. Mater. Sci. Eng. C 33, 3336–3345 (2013).
Lee, J.-Y. et al. Osteoblastic differentiation of human bone marrow stromal cells in self-assembled BMP-2 receptor-binding peptide-amphiphiles. Biomaterials 30, 3532–3541 (2009).
Kumar, V. A. et al. Treatment of hind limb ischemia using angiogenic peptide nanofibers. Biomaterials 98, 113–119 (2016).
Caprini, A. et al. A novel bioactive peptide: assessing its activity over murine neural stem cells and its potential for neural tissue engineering. N. Biotechnol. 30, 552–562 (2013).
Amanda, M. et al. Multifunctionalized hydrogels foster hNSC maturation in 3D cultures and neural regeneration in spinal cord injuries. Proc. Natl Acad. Sci. USA 116, 7483–7492 (2019).
Gelain, F. et al. New bioactive motifs and their use in functionalized self-assembling peptides for NSC differentiation and neural tissue engineering. Nanoscale 4, 2946–2957 (2012).
Rudra, J. S., Tian, Y. F., Jung, J. P. & Collier, J. H. A self-assembling peptide acting as an immune adjuvant. Proc. Natl Acad. Sci. USA 107, 622 LP–622627 (2010).
Pompano, R. R. et al. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv. Healthc. Mater. 3, 1898–1908 (2014).
Anderson, C. F. et al. Collagen-binding peptide-enabled supramolecular hydrogel design for improved organ adhesion and sprayable therapeutic delivery. Nano Lett. https://doi.org/10.1021/acs.nanolett.2c00967 (2022).
Amosi, N. et al. Acidic peptide hydrogel scaffolds enhance calcium phosphate mineral turnover into bone tissue. Acta Biomater. 8, 2466–2475 (2012).
Jin, H.-E. et al. Biomimetic self-templated hierarchical structures of collagen-like peptide amphiphiles. Nano Lett. 15, 7138–7145 (2015).
Jun, H.-W., Yuwono, V., Paramonov, S. E. & Hartgerink, J. D. Enzyme-mediated degradation of peptide-amphiphile nanofiber networks. Adv. Mater. 17, 2612–2617 (2005).
Chau, Y. et al. Incorporation of a matrix metalloproteinase-sensitive substrate into self-assembling peptides — a model for biofunctional scaffolds. Biomaterials 29, 1713–1719 (2008).
Bacinello, D., Garanger, E., Taton, D., Tam, K. C. & Lecommandoux, S. Enzyme-degradable self-assembled nanostructures from polymer–peptide hybrids. Biomacromolecules 15, 1882–1888 (2014).
Chen, C. et al. Rational design of short peptide-based hydrogels with MMP-2 responsiveness for controlled anticancer peptide delivery. Biomacromolecules 18, 3563–3571 (2017).
Zhou, Y. et al. Injectable extracellular vesicle-released self-assembling peptide nanofiber hydrogel as an enhanced cell-free therapy for tissue regeneration. J. Control. Rel. 316, 93–104 (2019).
Lin, Y.-A., Ou, Y.-C., Cheetham, A. G. & Cui, H. Rational design of MMP degradable peptide-based supramolecular filaments. Biomacromolecules 15, 1419–1427 (2014).
Li, N. et al. Amphiphilic peptide dendritic copolymer-doxorubicin nanoscale conjugate self-assembled to enzyme-responsive anti-cancer agent. Biomaterials 35, 9529–9545 (2014).
Toledano, S., Williams, R. J., Jayawarna, V. & Ulijn, R. V. Enzyme-triggered self-assembly of peptide hydrogels via reversed hydrolysis. J. Am. Chem. Soc. 128, 1070–1071 (2006).
Guilbaud, J.-B. et al. Enzymatic catalyzed synthesis and triggered gelation of ionic peptides. Langmuir 26, 11297–11303 (2010).
Swanekamp, R. J., Welch, J. J. & Nilsson, B. L. Proteolytic stability of amphipathic peptide hydrogels composed of self-assembled pleated β-sheet or coassembled rippled β-sheet fibrils. Chem. Commun. 50, 10133–10136 (2014).
Xie, Y. et al. Enzyme–substrate interactions promote the self-assembly of amino acid derivatives into supramolecular hydrogels. J. Mater. Chem. B 4, 844–851 (2016).
Benoit, D. S. W. & Anseth, K. S. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials 26, 5209–5220 (2005).
Niece, K. L., Hartgerink, J. D., Donners, J. J. J. M. & Stupp, S. I. Self-assembly combining two bioactive peptide-amphiphile molecules into nanofibers by electrostatic attraction. J. Am. Chem. Soc. 125, 7146–7147 (2003).
Rong, Y., Zhang, Z., He, C. & Chen, X. Bioactive polypeptide hydrogels modified with RGD and N-cadherin mimetic peptide promote chondrogenic differentiation of bone marrow mesenchymal stem cells. Sci. China Chem. 63, 1100–1111 (2020).
Naghdi, P. et al. Survival, proliferation and differentiation enhancement of neural stem cells cultured in three-dimensional polyethylene glycol–RGD hydrogel with tenascin. J. Tissue Eng. Regen. Med. 10, 199–208 (2016).
He, X., Ma, J. & Jabbari, E. Effect of grafting RGD and BMP-2 protein-derived peptides to a hydrogel substrate on osteogenic differentiation of marrow stromal cells. Langmuir 24, 12508–12516 (2008).
Øvrebø, Ø. et al. Design and clinical application of injectable hydrogels for musculoskeletal therapy. Bioeng. Transl. Med. 7, e10295 (2022). This article highlights hydrogel design strategies to meet the current requirements of the European Medical Device Regulation for clinical translation.
Acknowledgements
The work was financially supported by the Medical Research Council (UK Regenerative Medicine Platform Hub Acellular Smart Materials 3D Architecture, MR/R015651/1), the ERC PoC Grant NOVACHIP, and project number AOCMF-21-04S from the AO Foundation, AO CMF. AO CMF is a clinical division of the AO Foundation — an independent medically guided not-for-profit organization.
Author information
Authors and Affiliations
Contributions
C.L. and A.M. developed the manuscript outline. C.L. researched data for the article, made substantial contributions to discussions of the content and wrote the manuscript. C.L. and A.M. drafted, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Seung-Woo Cho and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
International Council for Harmonization (ICH): https://www.ich.org
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ligorio, C., Mata, A. Synthetic extracellular matrices with function-encoding peptides. Nat Rev Bioeng 1, 518–536 (2023). https://doi.org/10.1038/s44222-023-00055-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00055-3
This article is cited by
-
Exploring the interaction between extracellular matrix components in a 3D organoid disease model to replicate the pathophysiology of breast cancer
Journal of Experimental & Clinical Cancer Research (2023)