Abstract
The flux balances of carbon and chlorine between subduction into the deep mantle and volcanic emissions into the atmosphere are crucial for the habitability of our planet1,2. However, pervasive loss of fluids from subducting slabs has been thought to cut off the delivery of both carbon and chlorine to the deep mantle owing to their high mobility under hydrous conditions3,4. Our new high-pressure experiments show that most carbonates (>75 wt%) in carbonate-rich crustal rocks—one of the main subducting carbon reservoirs—survive devolatilization and hydrous melting in cold and warm subduction zones, indicating that their subduction has driven the deep carbon cycle since the Mesoproterozoic. We found that KCl and NaCl, respectively, become stable phases crystallizing from hydrous carbonatite melts with low chlorine solubility in warm and hot subduction zones, resulting in the sequestration of chlorine in the solid residue in downwelling slabs. Accordingly, the subduction of carbonate-rich rocks facilitated highly effective recycling of both chlorine and carbon into the deep mantle at intermediate stages of Earth’s history and led to declining atmospheric pCO2 and the formation of carbon-rich and chlorine-rich mantle reservoirs since the Mesoproterozoic. This period of optimal carbon and chlorine subduction may explain the ages of eclogitic diamonds and the formation of the HIMU mantle source.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the paper or in the supplementary files and are also available at https://doi.org/10.6084/m9.figshare.22698292.v1. Source data are provided with this paper.
References
Broadley, M. W., Barry, P. H., Ballentine, C. J., Taylor, L. A. & Burgess, R. End-Permian extinction amplified by plume-induced release of recycled lithospheric volatiles. Nat. Geosci. 11, 682–687 (2018).
Dasgupta, R. & Hirschmann, M. M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 298, 1–13 (2010).
Kelemen, P. B. & Manning, C. E. Reevaluating carbon fluxes in subduction zones, what goes down, mostly comes up. Proc. Natl Acad. Sci. 112, E3997–E4006 (2015).
Barnes, J. D., Manning, C. E., Scambelluri, M. & Selverstone, J. in The Role of Halogens in Terrestrial and Extraterrestrial Geochemical Processes: Surface, Crust, and Mantle (eds Harlov, D. E. & Aranovich, L.) 545–590 (Springer, 2018).
Foley, S. F. & Fische, T. P. An essential role for continental rifts and lithosphere in the deep carbon cycle. Nat. Geosci. 10, 897–902 (2017).
Hirschmann, M. M. Comparative deep Earth volatile cycles: the case for C recycling from exosphere/mantle fractionation of major (H2O, C, N) volatiles and from H2O/Ce, CO2/Ba, and CO2/Nb exosphere ratios. Earth Planet. Sci. Lett. 502, 262–273 (2018).
Stewart, E. M. & Ague, J. J. Pervasive subduction zone devolatilization recycles CO2 into the forearc. Nat. Commun. 11, 6220 (2020).
Hanyu, T. et al. Tiny droplets of ocean island basalts unveil Earth’s deep chlorine cycle. Nat. Commun. 10, 60 (2019).
Plank, T. & Manning, C. E. Subducting carbon. Nature 574, 343–352 (2019).
Ague, J. J. & Nicolescu, S. Carbon dioxide released from subduction zones by fluid-mediated reactions. Nat. Geosci. 7, 355–360 (2014).
Farsang, S. et al. Deep carbon cycle constrained by carbonate solubility. Nat. Commun. 12, 4311 (2021).
Poli, S. Carbon mobilized at shallow depths in subduction zones by carbonatitic liquids. Nat. Geosci. 8, 633–636 (2015).
Schettino, E. & Poli, S. in Carbon in Earth’s Interior (eds Manning, C. E., Lin, J.-F. & Mao, W. L.) 209–221 (American Geophysical Union, 2020).
Martin, L. A. J. & Hermann, J. Experimental phase relations in altered oceanic crust: implications for carbon recycling at subduction zones. J. Petrol. 59, 299–320 (2018).
Grassi, D. & Schmidt, M. W. The melting of carbonated pelites from 70 to 700 km depth. J. Petrol. 52, 765–789 (2011).
Chen, X. et al. Melting of carbonated pelite at 5.5–15.5 GPa: implications for the origin of alkali-rich carbonatites and the deep water and carbon cycles. Contrib. Mineral. Petrol. 177, 2 (2021).
Plank, T. in Treatise on Geochemistry 2nd edn (eds Holland, H. D. & Turekian, K. K.) 607–629 (Elsevier, 2014).
Staudigel, H., Plank. T., White, B. & Schmincke, H.-U. in Subduction: Top to Bottom (eds Bebout, G. E., Scholl, D. W., Kirby, S. H. & Platt, J. P.) 19–38 (American Geophysical Union, 2013).
Van den Bleeken, G. & Koga, K. T. Experimentally determined distribution of fluorine and chlorine upon hydrous slab melting, and implications for F–Cl cycling through subduction zones. Geochim. Cosmochim. Acta 171, 353–373 (2015).
Li, H. & Hermann, J. Apatite as an indicator of fluid salinity: an experimental study of chlorine and fluorine partitioning in subducted sediments. Geochim. Cosmochim. Acta 166, 267–297 (2015).
Kerrick, D. M. & Connolly, J. A. D. Metamorphic devolatilization of subducted marine sediments and the transport of volatiles into the Earth’s mantle. Nature 411, 293–296 (2001).
Kendrick, M. A., Scambelluri, M., Honda, M. & Phillips, D. High abundances of noble gas and chlorine delivered to the mantle by serpentinite subduction. Nat. Geosci. 4, 807–812 (2011).
Philippot, P., Agrinier, P. & Scambelluri, M. Chlorine cycling during subduction of altered oceanic crust. Earth Planet. Sci. Lett. 161, 33–44 (1998).
Scambelluri, M. & Philippot, P. Deep fluids in subduction zones. Lithos 55, 213–227 (2001).
Rüpke, L. H., Morgan, J. P., Hort, M. & Connolly, J. A. D. Serpentine and the subduction zone water cycle. Earth Planet. Sci. Lett. 223, 17–34 (2004).
Tsuno, K., Dasgupta, R., Danielson, L. & Righter, K. Flux of carbonate melt from deeply subducted pelitic sediments: geophysical and geochemical implications for the source of Central American volcanic arc. Geophys. Res. Lett. 39, L16307 (2012).
Thomsen, T. B. & Schmidt, M. W. Melting of carbonated pelites at 2.5–5.0 GPa, silicate–carbonatite liquid immiscibility, and potassium–carbon metasomatism of the mantle. Earth Planet. Sci. Lett. 267, 17–31 (2008).
Litasov, K. D. & Ohtani, E. Phase relations in the peridotite–carbonate–chloride system at 7.0–16.5 GPa and the role of chlorides in the origin of kimberlite and diamond. Chem. Geol. 262, 29–41 (2009).
Safonov, O. G., Kamenetsky, V. S. & Perchuk, L. L. Links between carbonatite and kimberlite melts in chloride–carbonate–silicate systems: experiments and application to natural assemblages. J. Petrol. 52, 1307–1331 (2010).
Safonov, O. G., Perchuk, L. L. & Litvin, Y. A. Melting relations in the chloride–carbonate–silicate systems at high-pressure and the model for formation of alkalic diamond–forming liquids in the upper mantle. Earth Planet. Sci. Lett. 253, 112–128 (2007).
Syracuse, E. M., van Keken, P. E. & Abers, G. A. The global range of subduction zone thermal models. Phys. Earth Planet. Inter. 183, 73–90 (2010).
Hawkesworth, C. J., Cawood, P. A., Dhuime, B. & Kemp, T. I. S. Earth’s continental lithosphere through time. Annu. Rev. Earth Planet. Sci. 45, 169–198 (2017).
Dhuime, B., Wuestefeld, A. & Hawkesworth, C. J. Emergence of modern continental crust about 3 billion years ago. Nat. Geosci. 8, 552–555 (2015).
Ronov, A. B. Common tendencies in the chemical evolution of the earth’s crust, ocean and atmosphere. Geokhiniiya 8, 715–743 (1964).
Palin, R. M. et al. Secular change and the onset of plate tectonics on Earth. Earth Sci. Rev. 207, 103172 (2020).
Martin, H. Effect of steeper Archean geothermal gradient on geochemistry of subduction-zone magmas. Geology 14, 753–756 (1986).
Komiya, T., Hayashi, M., Maruyama, S. & Yurimoto, H. Intermediate-P/T type Archean metamorphism of the Isua supracrustal belt: implications for secular change of geothermal gradients at subduction zones and for Archean plate tectonics. Am. J. Sci. 302, 806–826 (2002).
Tsujimori, T. & Ernst, W. G. Lawsonite blueschists and lawsonite eclogites as proxies for palaeo-subduction zone processes: a review. J. Metamorph. Geol. 32, 437–454 (2014).
Sheldon, N. D. Precambrian paleosols and atmospheric CO2 levels. Precambrian Res. 147, 148–155 (2006).
Kah, L. C. & Riding, R. Mesoproterozoic carbon dioxide levels inferred from calcified cyanobacteria. Geology 35, 799–802 (2007).
Kanzaki, Y. & Murakami, T. Estimates of atmospheric CO2 in the Neoarchean–Paleoproterozoic from paleosols. Geochim. Cosmochim. Acta 159, 190–219 (2015).
Berner, R. A. The Phanerozoic Carbon Cycle: CO2 and O2 (Oxford Univ. Press, 2004).
Klein-BenDavid, O., Wirth, R. & Navon, O. TEM imaging and analysis of microinclusions in diamonds: a close look at diamond-growing fluids. Am. Mineral. 91, 353–365 (2006).
Kamenetsky, M. B. et al. Kimberlite melts rich in alkali chlorides and carbonates: a potent metasomatic agent in the mantle. Geology 32, 845–848 (2004).
Maas, R., Kamenetsky, M. B., Sobolev, A. V., Kamenetsky, V. S. & Sobolev, N. V. Sr, Nd, and Pb isotope evidence for a mantle origin of alkali chlorides and carbonates in the Udachnaya kimberlite, Siberia. Geology 33, 549–552 (2005).
Palyanov, Y. N., Shatsky, V. S., Sobolev, N. V. & Sokol, A. G. The role of mantle ultrapotassic fluids in diamond formation. Proc. Natl Acad. Sci. 104, 9122–9127 (2007).
Cabral, R. A. et al. Volatile cycling of H2O, CO2, F, and Cl in the HIMU mantle: a new window provided by melt inclusions from oceanic hot spot lavas at Mangaia, Cook Islands. Geochem. Geophys. Geosyst. 15, 4445–4467 (2014).
Herzberg, C., Condie, K. & Korenaga, J. Thermal history of the Earth and its petrological expression. Earth Planet. Sci. Lett. 292, 79–88 (2010).
Dasgupta, R. Ingassing, storage, and outgassing of terrestrial carbon through geologic time. Rev. Mineral. Geochem. 75, 183–229 (2013).
Skora, S. et al. Hydrous phase relations and trace element partitioning behaviour in calcareous sediments at subduction-zone conditions. J. Petrol. 56, 953–980 (2015).
Veizer, J. & Mackenzie, F. T. in Treatise on Geochemistry (eds Holland, H. D. & Turekian, K. K.) 369–407 (Elsevier, 2003).
Shirey, S. B. & Richardson, S. H. Start of the Wilson cycle at 3 Ga shown by diamonds from subcontinental mantle. Science 333, 434–436 (2011).
Driese, S. G. et al. Neoarchean paleoweathering of tonalite and metabasalt: implications for reconstructions of 2.69 Ga early terrestrial ecosystems and paleoatmospheric chemistry. Precambrian Res. 189, 1–17 (2011).
Kasting, J. F. Theoretical constraints on oxygen and carbon dioxide concentrations in the Precambrian atmosphere. Precambrian Res. 34, 205–229 (1987).
Krissansen-Totton, J., Arney, G. N. & Catling, D. C. Constraining the climate and ocean pH of the early Earth with a geological carbon cycle model. Proc. Natl Acad. Sci. 115, 4105–4110 (2018).
Alt, J. C. & Teagle, D. A. H. The uptake of carbon during alteration of ocean crust. Geochim. Cosmochim. Acta 63, 1527–1535 (1999).
Coogan, L. A. & Gillis, K. M. Evidence that low-temperature oceanic hydrothermal systems play an important role in the silicate-carbonate weathering cycle and long-term climate regulation. Geochem. Geophys. Geosyst. 14, 1771–1786 (2013).
Shilobreeva, S., Martinez, I., Busigny, V., Agrinier, P. & Laverne, C. Insights into C and H storage in the altered oceanic crust: results from ODP/IODP Hole 1256D. Geochim. Cosmochim. Acta 75, 2237–2255 (2011).
Dick, H. J. B., MacLeod, C. J., Blum, P. & the Expedition 360 Scientists. Expedition 360 Preliminary Report: Southwest Indian Ridge Lower Crust and Moho. International Ocean Discovery Program. https://doi.org/10.14379/iodp.pr.360.2016 (2016).
Tsuno, K. & Dasgupta, R. The effect of carbonates on near-solidus melting of pelite at 3 GPa: relative efficiency of H2O and CO2 subduction. Earth Planet. Sci. Lett. 319, 185–196 (2012).
Mann, U. & Schmidt, M. W. Melting of pelitic sediments at subarc depths: 1. Flux vs. fluid-absent melting and a parameterization of melt productivity. Chem. Geol. 404, 150–167 (2015).
Dasgupta, R., Hirschmann, M. M. & Dellas, N. The effect of bulk composition on the solidus of carbonated eclogite from partial melting experiments at 3 GPa. Contrib. Mineral. Petrol. 149, 288–305 (2005).
Hammouda, T. High-pressure melting of carbonated eclogite and experimental constraints on carbon recycling and storage in the mantle. Earth Planet. Sci. Lett. 214, 357–368 (2003).
Kiseeva, E. S. et al. An experimental study of carbonated eclogite at 3·5–5·5 GPa—implications for silicate and carbonate metasomatism in the cratonic mantle. J. Petrol. 53, 727–759 (2012).
Thomson, A. R., Walter, M. J., Kohn, S. C. & Brooker, R. A. Slab melting as a barrier to deep carbon subduction. Nature 529, 76–79 (2016).
Chen, C. F., Förster, M. W., Foley, S. F. & Liu, Y. S. Massive carbon storage in convergent margins initiated by subduction of limestone. Nat. Commun. 12, 4463 (2021).
Förster, M. W., Foley, S. F., Marschall, H. R., Alard, O. & Buhre, S. Melting of sediments in the deep mantle produces saline fluid inclusions in diamonds. Sci. Adv. 5, eaau2620 (2019).
Clift, P. D. A revised budget for Cenozoic sedimentary carbon subduction. Rev. Geophys. 55, 97–125 (2017).
Li, K., Li, L., Pearson, D. G. & Stachel, T. Diamond isotope compositions indicate altered igneous oceanic crust dominates deep carbon recycling. Earth Planet. Sci. Lett. 516, 190–201 (2019).
Staudigel, H., Hart, S. R., Schmincke, H.-U. & Smith, B. M. Cretaceous ocean crust at DSDP Sites 417 and 418: carbon uptake from weathering versus loss by magmatic outgassing. Geochim. Cosmochim. Acta 53, 3091–3094 (1989).
Jarrard, R. D. Subduction fluxes of water, carbon dioxide, chlorine, and potassium. Geochem. Geophys. Geosyst. 4, 8905 (2003).
He, T. et al. Determination of Cl, Br, and I in geological materials by sector field inductively coupled plasma mass spectrometry. Anal. Chem. 91, 8109–8114 (2019).
Yaxley, G. M. & Brey, G. P. Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: implications for petrogenesis of carbonatites. Contrib. Mineral. Petrol. 146, 606–619 (2004).
Franzolin, E., Schmidt, M. W. & Poli, S. Ternary Ca–Fe–Mg carbonates: subsolidus phase relations at 3.5 GPa and a thermodynamic solid solution model including order/disorder. Contrib. Mineral. Petrol. 161, 213–227 (2011).
Hermann, J. & Spandler, C. J. Sediment melts at sub-arc depths: an experimental study. J. Petrol. 49, 717–740 (2008).
Saha, S. & Dasgupta, R. Phase relations of a depleted peridotite fluxed by a CO2-H2O fluid—implications for the stability of partial melts versus volatile-bearing mineral phases in the cratonic mantle. J. Geophys. Res. Solid Earth 124, 10089–10106 (2019).
Carpentier, M., Chauvel, C., Maury, R. C. & Mattielli, N. The “zircon effect” as recorded by the chemical and Hf isotopic compositions of Lesser Antilles forearc sediments. Earth Planet. Sci. Lett. 287, 86–99 (2009).
Poli, S. Melting carbonated epidote eclogites: carbonatites from subducting slabs. Prog. Earth Planet. Sci. 3, 27 (2016).
Acknowledgements
S.F.F. and C.C. are funded by ARC grant FL180100134 and S.S.S. by Macquarie University support funds for the FL project. M.W.F. is funded by Macquarie University grant MQRF0001074-2020. The Ocean Discovery Project provided the marine limestone and sediment samples. We acknowledge the facilities of the Centre for Advanced Microscopy at the Australian National University, Canberra. We thank Z. Hu and T. He for analysis of Cl contents of the starting materials. We acknowledge I. Ezad for proofreading this manuscript.
Author information
Authors and Affiliations
Contributions
C.C., M.W.F. and S.F.F. designed the study. C.C. and S.S.S. carried out the experiments. C.C. and M.W.F. performed analytical measurements. C.C. wrote the manuscript and all authors contributed to interpreting data and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Ananya Mallik and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 The compositions of starting materials in this study compared with previous high-pressure experiments and natural sediments and altered basalts.
a, CO2 and H2O contents of starting materials from this study (CS2, CS1 and CS5) and from previous high-pressure experiments on carbonated sediments26,27,50,60,61 and oceanic crust12,14,62,63,64. The model CaO–Al2O3–SiO2–CO2–H2O system is shown for comparison13. b–f, Chemical compositions of sedimentary columns subducting at global trenches17, the Lesser Antilles sediments from DODP Site 144 (ref. 77), altered oceanic crust18 and starting materials in this study. The global weighted average composition of subducted sediments (GLOSS-II)17 is shown for comparison.
Extended Data Fig. 2 Representative backscattered electron images of run products at 3 and 4 GPa.
a–c, 3 GPa. d–i, 4 GPa. a,d–e, Subsolidus experiments. b,c,f–i, Above-solidus experiments. Silicate melt at 3 GPa (b,c) and 4 GPa (g). Carbonatite melt at T = 900 °C and 4 GPa (f). KCl at T = 850 °C (h) and both KCl and NaCl at T = 900 °C (i).
Extended Data Fig. 3 Representative backscattered electron images of experimental charges at 5 GPa.
a–c, Subsolidus experiments. d–i, Above-solidus experiments. KCl in carbonatite melt (f) and in the solid residues (g). NaCl in carbonatite melts (h) and in the solid residues (i).
Extended Data Fig. 6 The chemical compositions of carbonate-rich phases in the experiments.
The chemical compositions of carbonate precipitated from fluid and of calcite (a) and compositions of silicate and carbonatite melts (b) in this study. Carbonate precipitates and melts from previous experiments on hydrated carbonated gabbro12 and carbonated sediments26,27,50 are shown for comparison. The stability regions of (Mg, Fe)-calcite and siderite–magnesite solid solution are from ref. 78.
Extended Data Fig. 7 Subduction of carbonate-rich crustal rocks through time.
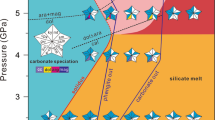
a, Schematic illustration showing subduction of carbonate-rich crustal rocks and replenishment of carbon and/or chlorine to cratonic mantle roots and deep sources of HIMU-type ocean island basalts (OIBs). Stability of carbonate and chloride during subduction of carbonate-rich crustal rocks influenced by the infiltration of Cl-rich fluid in the cold (b), warm (c) and hot (d) subduction regimes.
Extended Data Fig. 8 The chemical compositions of minerals in the experiments.
Major element chemistry of garnet, jadeite and carbonates showing systematic changes with temperature. Their compositions are independent of the bulk compositions of the starting materials.
Extended Data Fig. 9 Representative backscattered electron images of the experimental charges in the unpolished halves of capsules.
KCl coexists with other residual minerals as inclusions (a) and coexists with carbonatite melts (b) in the experiment at 950 °C. c, NaCl coexists with carbonatite melts in the experiment at 1,100 °C.
Supplementary information
Supplementary Data 1
Compilation of age of eclogitic diamonds constrained by Sm–Nd and Re–Os isochrons for inclusions (n > 2) in diamonds. n = the number of inclusions for the isochron. Note that we compile the isochron ages only when n > 2.
Supplementary Data 2
Major element compositions of phengite, garnet, jadeite, aragonite, (Mg, Fe) calcite, epidote and calcite in the experiments.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, C., Förster, M.W., Foley, S.F. et al. Carbonate-rich crust subduction drives the deep carbon and chlorine cycles. Nature 620, 576–581 (2023). https://doi.org/10.1038/s41586-023-06211-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06211-4
This article is cited by
-
Crystal structure of calcite-type Ca1–xMnxCO3 solid solution by X-ray diffraction and Raman spectroscopy
Physics and Chemistry of Minerals (2024)
-
Fertile upper mantle peridotite xenoliths indicate no wholesale destruction of cratonic root in East Asia
Communications Earth & Environment (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.