Abstract
Immunotherapy based on immunecheckpoint blockade (ICB) using antibodies induces rejection of tumours and brings clinical benefit in patients with various cancer types1. However, tumours often resist immune rejection. Ongoing efforts trying to increase tumour response rates are based on combinations of ICB with compounds that aim to reduce immunosuppression in the tumour microenvironment but usually have little effect when used as monotherapies2,3. Here we show that agonists of α2-adrenergic receptors (α2-AR) have very strong anti-tumour activity when used as monotherapies in multiple immunocompetent tumour models, including ICB-resistant models, but not in immunodeficient models. We also observed marked effects in human tumour xenografts implanted in mice reconstituted with human lymphocytes. The anti-tumour effects of α2-AR agonists were reverted by α2-AR antagonists, and were absent in Adra2a-knockout (encoding α2a-AR) mice, demonstrating on-target action exerted on host cells, not tumour cells. Tumours from treated mice contained increased infiltrating T lymphocytes and reduced myeloid suppressor cells, which were more apoptotic. Single-cell RNA-sequencing analysis revealed upregulation of innate and adaptive immune response pathways in macrophages and T cells. To exert their anti-tumour effects, α2-AR agonists required CD4+ T lymphocytes, CD8+ T lymphocytes and macrophages. Reconstitution studies in Adra2a-knockout mice indicated that the agonists acted directly on macrophages, increasing their ability to stimulate T lymphocytes. Our results indicate that α2-AR agonists, some of which are available clinically, could substantially improve the clinical efficacy of cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information. scRNA-seq and RNA-seq data were obtained from in-house experiments. Raw and processed data are available from the Gene Expression Omnibus under accession codes GSE227601 and GSE226290, respectively. Intermediate processed results have been archived at Zenodo DOIs https://doi.org/10.5281/zenodo.7751788 and https://doi.org/10.5281/zenodo.7789646. Source data are provided with this paper.
Code availability
Supporting R and Python scripts, with installation instructions and version numbers, are publicly available at GitHub (https://github.com/BVDElab/Tumour_immune_rejection) and archived at Zenodo. Code to generate the corresponding analyses or figures is available at https://doi.org/10.5281/zenodo.7751788 for Figs. 3i–j, 4a–d and Extended Data Fig. 5f; and at https://doi.org/10.5281/zenodo.7789646 for Extended Data Figs. 4h and 9c.
References
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Marin-Acevedo, J. A., Kimbrough, E. O. & Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol. 14, 45 (2021).
Rotte, A., Jin, J. Y. & Lemaire, V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann. Oncol. 29, 71–83 (2018).
Giovannitti, J. A. Jr., Thoms, S. M. & Crawford, J. J. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth. Prog. 62, 31–39 (2015).
Kim, T. J., Sun, J., Lu, S., Zhang, J. & Wang, Y. The regulation of beta-adrenergic receptor-mediated PKA activation by substrate stiffness via microtubule dynamics in human MSCs. Biomaterials 35, 8348–8356 (2014).
Sucharov, C. C., Dockstader, K., Nunley, K., McKinsey, T. A. & Bristow, M. β-Adrenergic receptor stimulation and activation of protein kinase A protect against α1-adrenergic-mediated phosphorylation of protein kinase D and histone deacetylase 5. J. Card. Fail. 17, 592–600 (2011).
Wang, W. & Cao, X. β-Adrenergic signaling in tumor immunology and immunotherapy. Crit. Rev. Immunol. 39, 93–103 (2019).
Allard, B., Allard, D., Buisseret, L. & Stagg, J. The adenosine pathway in immuno-oncology. Nat. Rev. Clin. Oncol. 17, 611–629 (2020).
Ching, M. M., Reader, J. & Fulton, A. M. Eicosanoids in cancer: prostaglandin E2 receptor 4 in cancer therapeutics and immunotherapy. Front. Pharmacol. 11, 819 (2020).
Huijbers, I. J. et al. An inducible mouse model of melanoma expressing a defined tumor antigen. Cancer Res. 66, 3278–3286 (2006).
Zhu, J. et al. Resistance to cancer immunotherapy mediated by apoptosis of tumor-infiltrating lymphocytes. Nat. Commun. 8, 1404 (2017).
Hennequart, M. et al. Constitutive IDO1 expression in human tumors is driven by cyclooxygenase-2 and mediates intrinsic immune resistance. Cancer Immunol. Res. 5, 695–709 (2017).
Hedner, T. et al. Yohimbine pharmacokinetics and interaction with the sympathetic nervous system in normal volunteers. Eur. J. Clin. Pharmacol. 43, 651–656 (1992).
Altman, J. D. et al. Abnormal regulation of the sympathetic nervous system in α2A-adrenergic receptor knockout mice. Mol. Pharmacol. 56, 154–161 (1999).
Godinho-Silva, C., Cardoso, F. & Veiga-Fernandes, H. Neuro-immune cell units: a new paradigm in physiology. Ann. Rev. Immunol. 37, 19–46 (2019).
Starke, K., Endo, T. & Taube, H. D. Relative pre- and postsynaptic potencies of alpha-adrenoceptor agonists in the rabbit pulmonary artery. Naunyn Schmiedebergs Arch. Pharmacol. 291, 55–78 (1975).
MacMillan, L. B., Hein, L., Smith, M. S., Piascik, M. T. & Limbird, L. E. Central hypotensive effects of the alpha2a-adrenergic receptor subtype. Science 273, 801–803 (1996).
Zahalka, A. H. & Frenette, P. S. Nerves in cancer. Nat. Rev. Cancer 20, 143–157 (2020).
Yang, J. H., Lee, E. O., Kim, S. E., Suh, Y. H. & Chong, Y. H. Norepinephrine differentially modulates the innate inflammatory response provoked by amyloid-β peptide via action at β-adrenoceptors and activation of cAMP/PKA pathway in human THP-1 macrophages. Exp. Neurol. 236, 199–206 (2012).
Wu, J. J. et al. G protein-coupled receptor kinase 2 regulating β2-adrenergic receptor signaling in M2-polarized macrophages contributes to hepatocellular carcinoma progression. Onco Targets Ther. 12, 5499–5513 (2019).
Qiao, G. et al. β-Adrenergic signaling blocks murine CD8+ T-cell metabolic reprogramming during activation: a mechanism for immunosuppression by adrenergic stress. Cancer Immunol. Immunother. 68, 11–22 (2019).
Mohammadpour, H. et al. β2 adrenergic receptor-mediated signaling regulates the immunosuppressive potential of myeloid-derived suppressor cells. J. Clin. Invest. 129, 5537–5552 (2019).
Gandhi, S. et al. Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin. Cancer Res. 27, 87–95 (2021).
Rohrer, D. K., Chruscinski, A., Schauble, E. H., Bernstein, D. & Kobilka, B. K. Cardiovascular and metabolic alterations in mice lacking both β1- and β2-adrenergic receptors. J. Biol. Chem. 274, 16701–16708 (1999).
Kokolus, K. M. et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 7, e1405205 (2018).
Kim, R. et al. Ferroptosis of tumour neutrophils causes immune suppression in cancer. Nature 612, 338–346 (2022).
Fukata, M. et al. Innate immune signaling by Toll-like receptor-4 (TLR4) shapes the inflammatory microenvironment in colitis-associated tumors. Inflamm. Bowel Dis. 15, 997–1006 (2009).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Park, L. et al. Innate immunity receptor CD36 promotes cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA 110, 3089–3094 (2013).
McArdel, S. L., Terhorst, C. & Sharpe, A. H. Roles of CD48 in regulating immunity and tolerance. Clin. Immunol. 164, 10–20 (2016).
Readinger, J. A., Mueller, K. L., Venegas, A. M., Horai, R. & Schwartzberg, P. L. Tec kinases regulate T-lymphocyte development and function: new insights into the roles of Itk and Rlk/Txk. Immunol. Rev. 228, 93–114 (2009).
Ushio, A. et al. CCL22-producing resident macrophages enhance T cell response in Sjogren’s syndrome. Front. Immunol. 9, 2594 (2018).
Peng, S. L., Gerth, A. J., Ranger, A. M. & Glimcher, L. H. NFATc1 and NFATc2 together control both T and B cell activation and differentiation. Immunity 14, 13–20 (2001).
Tsaytler, P., Harding, H. P., Ron, D. & Bertolotti, A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332, 91–94 (2011).
Zhang, L. et al. Single-cell analyses inform mechanisms of myeloid-targeted therapies in colon cancer. Cell 181, 442–459 (2020).
Voon, D. C., Hor, Y. T. & Ito, Y. The RUNX complex: reaching beyond haematopoiesis into immunity. Immunology 146, 523–536 (2015).
Mariathasan, S. et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554, 544–548 (2018).
Gide, T. N. et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell 35, 238–255 (2019).
Castillo, L. F., Rivero, E. M., Goffin, V. & Luthy, I. A. Alpha2-adrenoceptor agonists trigger prolactin signaling in breast cancer cells. Cell. Signal. 34, 76–85 (2017).
Christensen, L. A., Finch, R. A., Booker, A. J. & Vasquez, K. M. Targeting oncogenes to improve breast cancer chemotherapy. Cancer Res. 66, 4089–4094 (2006).
Kanno, N. et al. Stimulation of alpha2-adrenergic receptor inhibits cholangiocarcinoma growth through modulation of Raf-1 and B-Raf activities. Hepatology 35, 1329–1340 (2002).
Maccari, S. et al. α-Adrenoceptor stimulation attenuates melanoma growth in mice. Br. J. Pharmacol. 179, 1371–1383 (2022).
Szpunar, M. J., Burke, K. A., Dawes, R. P., Brown, E. B. & Madden, K. S. The antidepressant desipramine and α2-adrenergic receptor activation promote breast tumor progression in association with altered collagen structure. Cancer Prev. Res. 6, 1262–1272 (2013).
Lavon, H. et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br. J. Anaesth. 120, 188–196 (2018).
Grebe, K. M. et al. Sympathetic nervous system control of anti-influenza CD8+ T cell responses. Proc. Natl Acad. Sci. USA 106, 5300–5305 (2009).
Eng, J. W. et al. A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol. Immunother. 63, 1115–1128 (2014).
Nevin, J. T., Moussa, M., Corwin, W. L., Mandoiu, I. I. & Srivastava, P. K. Sympathetic nervous tone limits the development of myeloid-derived suppressor cells. Sci. Immunol. https://doi.org/10.1126/sciimmunol.aay9368 (2020).
Qiao, G. et al. Chronic adrenergic stress contributes to metabolic dysfunction and an exhausted phenotype in T cells in the tumor microenvironment. Cancer Immunol. Res. 9, 651–664 (2021).
Shanker, A. et al. Thymocyte-intrinsic genetic factors influence CD8 T cell lineage commitment and affect selection of a tumor-reactive TCR. J. Immunol. 172, 5069–5077 (2004).
Ohshima, M., Itami, C. & Kimura, F. The α2A-adrenoceptor suppresses excitatory synaptic transmission to both excitatory and inhibitory neurons in layer 4 barrel cortex. J. Physiol. 595, 6923–6937 (2017).
Gajewski, T. F., Renauld, J. C., Van Pel, A. & Boon, T. Costimulation with B7-1, IL-6, and IL-12 is sufficient for primary generation of murine antitumor cytolytic T lymphocytes in vitro. J. Immunol. 154, 5637–5648 (1995).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Soneson, C., Love, M. I. & Robinson, M. D. Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Res 4, 1521 (2015).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Pedregosa, F. et al. Scikit-learn: Machine Learning in Python. Journal of Machine Learning Research 12; 2825-2830 (2011).
Sainburg, T., McInnes, L. & Gentner, T. Q. Parametric UMAP embeddings for representation and semisupervised learning. Neural Comput. 33, 2881–2907 (2021).
Thorsson, V. et al. The immune landscape of cancer. Immunity 48, 812–830 (2018).
Saltz, J. et al. Spatial organization and molecular correlation of tumor-infiltrating lymphocytes using deep learning on pathology images. Cell Rep. 23, 181–193 (2018).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007). (2007).
Waskom, M. L. seaborn: statistical data visualization. J. Open Source Softw. 6, 3021 (2021).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558 (2018).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Wolf, F. A., Angerer, P. & Theis, F. J. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 19, 15 (2018).
Levine, J. H. et al. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell 162, 184–197 (2015).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
Griffiths, J. A., Richard, A. C., Bach, K., Lun, A. T. L. & Marioni, J. C. Detection and removal of barcode swapping in single-cell RNA-seq data. Nat. Commun. 9, 2667 (2018).
McCarthy, D. J., Campbell, K. R., Lun, A. T. & Wills, Q. F. Scater: pre-processing, quality control, normalization and visualization of single-cell RNA-seq data in R. Bioinformatics 33, 1179–1186 (2017).
Lun, A. T., McCarthy, D. J. & Marioni, J. C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res. 5, 2122 (2016).
(2023) (2023) (2022) GSEApy: a comprehensive package for performing gene set enrichment analysis in Python Abstract Bioinformatics 39(1) 10.1093/bioinformatics/btac757.
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Poon, C. C. et al. Differential microglia and macrophage profiles in human IDH-mutant and -wild type glioblastoma. Oncotarget 10, 3129–3143 (2019).
Ochocka, N. et al. Single-cell RNA sequencing reveals functional heterogeneity of glioma-associated brain macrophages. Nat. Commun. 12, 1151 (2021).
Zhang, Q. et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 179, 829–845 (2019).
Acknowledgements
We thank I. Grisse for editorial assistance; P. Gomez for large-scale production of different mouse strains; P. Coulie for comments; and M.-P. Le and M. Formenti for technical assistance. J.Z. was supported by Fondation Contre le Cancer (grant no. 2019-094). This work was supported by WELBIO (Walloon Excellence in Life Sciences and Biotechnology), Belgium (grant no. WELBIO-CR-2019C-05); Fonds pour la Recherche Scientifique (FNRS), Belgium (grant nos EOS O000518F and PDR T.0091.18); Fondation contre le Cancer, Belgium (grant no. 2018-090); Ludwig Cancer Research; and de Duve Institute. The results shown here are in whole or part based on data generated by the TCGA Research Network (https://www.cancer.gov/tcga).
Author information
Authors and Affiliations
Contributions
J.Z. conceived the project and analysed the data. J.Z. and L.B. performed all of the in vivo experiments. L.B. performed the in vitro experiments. S.N. analysed the single-cell sequencing and bulk RNA-seq data for the MC38-OVA tumour model; L.G. and M.M. analysed the single-cell sequencing data for the TC-1 tumour model. B.J.V.d.E. helped to conceive and design experiments, analyse data and interpret results. J.Z. and B.J.V.d.E. wrote the manuscript. All of the authors were involved in the data interpretation and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
J.Z. and B.J.V.d.E. are listed as inventors on patent applications (WO 2020/083982, WO 2021/214129, Université Catholique de Louvain) related to using α2-AR agonists in cancer immunotherapy. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Roberta Zappasodi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
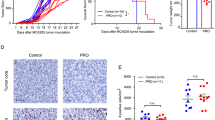
Extended Data Fig. 1 Anti-tumour effects of clonidine and guanabenz in multiple tumour models.
a, Survival curves of mice bearing MC38 colon carcinomas, B16F1 or B16F10 melanomas treated with CLD, GBZ, or vehicle. b, Progression of colon carcinomas MC38-OVA in mice treated with different doses of CLD. c, Anti-tumour effects of α2-AR agonists as monotherapy in multiple tumour models. GBZ or CLD was given daily (a, c: 5 mg/kg i.p.) starting at day 0 when tumours reached the indicated size. d, Progression of melanomas B16F10-OVA in C57BL/6J (immunocompetent) or NSG mice (immunodeficient) treated with vehicle (Con), GBZ or GFC. Data from one representative experiment out of two performed. GBZ (5 mg/kg) or GFC (2 mg/kg) was given daily (i.p.) starting 7 days after tumour implantation (day 0 = start of treatment). e, Progression of induced TiRP melanomas in mice treated with vehicle or GBZ. Left: mean growth curve; Right: individual growth curves. GBZ (5 mg/kg) was given daily (i.p.) starting at day 0 when tumours reached around 100 mm3. The number of mice per group is indicated in brackets. Data (mean ± s.e.m) from one representative experiment out of three (e). P values determined by: ordinary two-way ANOVA (b-e), Log-rank (Mantel-Cox) test was used for the statistical evaluation of survival (a), p < 0.0001.
Extended Data Fig. 2 Adra2a, Adra2b and Adra2c expression in murine tumour cell lines (a), effect of agonists on tumour cell lines in vitro (b,c), and gating strategy for flow cytometry analysis of TME cells (d).
a, Expression of Adra2a, Adra2b and Adra2c in murine tumour cell lines was measured by RT-qPCR. b, Effect of α2-AR agonists on cell viability of different tumour cell lines. Cells were treated with different concentrations of GBZ, CLD or GFC for 48 h and cell viability was measured by MTT assay. c, Effect of α2-AR agonists on cell proliferation of different tumour cell lines. CFSE assay was used to measure proliferation. During each round of cell division, intracellular CFSE becomes diluted and is quantified (Median fluorescence intensity (MFI) of CFSE) to determine proliferation rate. d, Gating strategy on tumour samples for tumour-infiltrating CD8+ T cells (Figs. 1b,d, 2b, 3e,g, 4f,g, Extended Data Figs. 4b,c, 7c), MDSCs (PMN-MDSC: CD11b+ Gr-1high, Ly6Clow, Ly6Ghigh, M-MDSC:CD11b+ Gr-1low, Ly6Chigh, Ly6Glow) (Fig. 3g, Extended Data Figs. 4c,f, 6f) and macrophages (CD11b+, F4/80+) (Fig. 4e, Extended Data Figs. 6b,c,d,e, 8a,c, 10a). e, Gating strategy for Extended Data Fig. 6g. f, Gating strategy for Fig. 3g. g, Gating strategy for Fig. 3d, Extended Data Fig. 6j. h, Gating strategy for Extended Data Fig. 6i. Data (mean ± s.e.m, technical duplicates for a and c, technical sextuplicate for b) from one representative out of two independent experiments.
Extended Data Fig. 3 Effect of α2-AR agonists on human tumour xenografts in humanized models (a-c), combination of α2-AR agonists with ICB (d-h), and genotyping of Adra2a-KO mice (ij).
a-b, Progression of human xenografts of colorectal carcinomas LS411N (a) or prostate carcinomas PC-3 (b) in NSG mice (immunodeficient) treated with vehicle, GBZ or CLD after adoptive transfer of allogeneic human PBMC. Human PBMC were injected (i.v.) on day 0 when tumours reached the indicated size. Treatments (5 mg/kg daily i.p.) started on the same day. Mean ± s.e.m from one representative out of two independent experiments. c, Expression of ADRA2A and ADRA2B in human tumour cell lines measured by RT-qPCR. Data from one representative out of two independent experiments. d, Survival of MC38-OVA tumour-bearing mice from Fig. 1f treated with GBZ, CLD, and/or anti-PD-1, as indicated. e, Individual tumour growth curves for mice from (d) (CR: complete tumour rejection). f, Survival of TC-1 tumour-bearing mice treated with CLD, anti-PD-1 or both. g, Progression of colon carcinomas CT26 in mice treated with GBZ, anti-PD-1, anti-CTLA4, or combinations of these. Treatments started on day 0 (7 days after tumour implantation). h, Progression of melanomas B16F1 in mice treated with CLD, anti-PD-1, or both. CLD and GBZ were given daily (5 mg/kg i.p.) starting at day 0 when tumours reached the indicated size. Anti-CTLA4 (40 µg) and anti-PD-1 (200 µg) antibodies (i.p.) were given 1 day before the first injection of α2-AR agonists, then twice a week. i, Expression of Adra2a, Adra2b and Adra2c measured by RT-qPCR in bulk tissue from MC38, B16F10-OVA and CT26 tumours. Each dot represents an individual mouse. j, Genotyping of Adra2a-KO mice. Genotyping was performed by PCR on genomic DNA from mouse tails, using primers described in the materials and methods. Mice with only the mutant band detected are identified as Adra2a-KO mice. Shown is the genotyping result for one batch of mice. Genotyping was repeated for all mice used. For gel source data, see Supplementary Fig. 1a. Mean ± s.e.m. P values determined by: ordinary two-way ANOVA (a,b,g,h), Log-rank (Mantel-Cox) test was used for the statistical evaluation of mouse survival (d and f), **** p < 0.0001.
Extended Data Fig. 4 Effect of clonidine on T cells and myeloid cells (a-f), and transciptomic analysis of treated tumours (g-h).
a, Effect of CLD on TCRP1A CD8+ T-cell proliferation in vitro (left panel) and activity as measured by T-cell degranulation (middle panel) and Ifn-γ ELISA (right panel). b, CD4+ and CD8+ T-cell infiltration in tumours, lymph nodes and spleens of MC38-OVA tumour-bearing mice analysed by flow cytometry after 7 days of treatment with vehicle (n = 14) or CLD (n = 15). CLD was given daily by i.p. injections (5 mg/kg) starting when tumours reached 50 mm3, and T-cell analysis was performed 7 days later. c, Progression, CD8+ T-cell infiltration and PMN-MDSC infiltration/apoptosis, of induced TiRP melanomas in TiRP mice treated with CLD or GFC after ACT of 10 million activated TCRP1A CD8+ T cells. d, GBZ and CLD induce apoptosis of PMN-MDSC in vitro. PMN-MDSCs were isolated from induced TiRP melanomas using MDSC isolation kit (Miltenyi Biotec) and were cultured and treated with GBZ or CLD for 24 h. Apoptosis was analysed by flow cytometry using Annexin V. Left: technical duplicate from one representative experiment out of two independent experiments; Right: Data pooled from technical duplicates/triplicates of MDSCs isolated from 4 different batches of mice (mean ± s.e.m.). e, T-cell suppressive activity of Ly6G+ myeloid cells (PMN-MDSC) isolated from induced TiRP melanomas, as measured by T-cell apoptosis (left) and T-cell number after co-culture (right). Technical duplicates from one representative experiment out of two. f, PMN-MDSC infiltration and CD8+ T-cell apoptosis in MC38 tumours from wild-type or Adra2a-KO mice treated with vehicle, GBZ or CLD. CLD, GBZ (5 mg/kg) and GFC (2 mg/kg) were given daily (i.p.), starting on day 0 when tumours reached a size between 50 and 100 mm3. Tumours were analysed ex vivo on day 6. g, RT-qPCR confirmation of mRNA expression of selected genes identified in the RNA-seq analysis. Mean fold change relative to untreated wild-type mice ± s.e.m. for MC38-OVA tumours from wild-type or Adra2a-KO mice treated with CLD and GBZ, as explained in f. Mean ± s.e.m, n = 4 independent biological samples. h, UMAP projection of L1-normalised gene expression using the genes of the upregulated (Post_Up) and the downregulated (Post_Down) signatures as defined in Supplementary Table 2, highlighting differences between MC38-OVA tumours from wild-type and Adra2a-KO mice treated with CLD or GBZ, as compared to untreated tumours (6 conditions, n = 3 per group). Clusters were identified with hierarchical clustering, using Spearman correlations between the samples as distance measure. Data (mean ± s.e.m) from one representative experiment out of two independent experiments (a–f). P values determined by: ordinary two-way ANOVA (c), t-test (Unpaired, two-tailed) (columns, a,b,c,d,f), **** p < 0.0001.
Extended Data Fig. 5 Single-cell RNAseq analysis of tumours from mice treated or not with clonidine (CLD).
a, Schematic representation of the scRNAseq workflow. b, UMAP representation of the merged dataset for treated and untreated MC38-OVA tumours. See Supplementary Table 1 for the markers used for cluster identification. c, Dotplot representing highly variable genes with relevance for macrophage activation. See main text for a description of the markers defining clusters 1 and 3. Cluster 2 is characterized by high expression of Fcer1g, Tyrobp, Cd7, C1qa, C1qb, Cd72, Trem2, and is similar to the anti-inflammatory macrophages as described before72. Cluster 4 is characterized by high expression of Thbs1, Lgals3, Fn1, Arg1, Ly6c2 and Tgfbi and is hence similar to the described population of intermediate monocyte-macrophage73. In another paper, these cells were identified as MDSC-like macrophages74. Cluster 5 is characterized by a high expression of genes encoding proliferation-related proteins (Tubb5, Stmn1, Hmgb1, H2afz and Top2a). d, Violin plots (Scanpy) showing the effect of CLD treatment on expression of 10 differentially expressed genes associated with H2 (two-sided Wilcoxon rank sum test with Benjamini-Hochberg correction for multiple testing, p.adj < 10-5) in each of the macrophage subpopulations versus the dataset mean. e, Enrichment plots (GSEA) in CD8+ T cells indicate an increase in CD8+ T-cell activation, which coincides with an increased CD8+ T cell infiltration shown in Fig. 4b. f, Single cell RNAseq analysis of TC-1 lung carcinomas treated or not with CLD. Relative proportions of immune cells in each cluster based on the unsupervised shared nearest-neighbour graph clustering of TC-1 tumours (pools of 10 tumours per group). Class identities were assigned using reference-based cell type annotations using the MC38-OVA tumours (see Fig. 4) as reference. The apparent reduction of macrophages in treated samples is only relative, and results from the increased proportion of CD4+ T cells.
Extended Data Fig. 6 Role of macrophages in the anti-tumour effect of α2-AR agonists.
a, Expression of Adra2a, Adra2b and Adra2c in macrophages (CD11b+, F4/80+, MHC II+), PMN-MDSCs (CD11b+, Gr1+ Ly6Ghigh Ly6Clow) and CD8+ T cells (CD45+ CD8+) sorted from MC38-OVA tumours (a pool of 8 tumours) by BD FACSAria™ III Cell Sorter. Technical duplicates from one representative experiment out of two. b, Surface MHC II expression and phagocytic activity of TME macrophages from MC38-OVA tumours in mice treated with vehicle or GBZ. c, Surface MHC II expression of TME macrophages from CT26 tumours in mice treated with vehicle, CLD or GBZ. d, Surface MHC II expression (left) and phagocytic activity (right) of TME macrophages from TC-1 tumours in wild-type or Adra2a-KO mice treated with vehicle or CLD (n = 5 per group). e, Efficiency of macrophage depletion by liposomal clodronate. Representative flow cytometry dot plots (left) and statistic bar graph (right) showing the efficacy of macrophage depletion following in vivo administration of liposomal clodronate to MC38-OVA tumour-bearing mice, as indicated in Fig. 4f. f, Depletion of PMN-MDSCs by liposomal clodronate. The abundance of PMN-MDSCs in tumours was evaluated by flow cytometry following CLD treatment and in vivo administration of liposomal clodronate to MC38-OVA tumour-bearing mice. Mean ± s.e.m. n = 7, 7, 8, 8 biologically independent samples. g, Migration of adoptively transferred CD45.1+ macrophages into lymph nodes and tumours of MC38-OVA tumour-bearing mice (CD45.2). Drugs were administered daily by i.p. injection: GBZ, CLD, 5 mg/kg. Treatments started when tumours reach around 100 mm3. Mice were euthanized after 4 days of treatment. h, Effect of CLD on the proliferation of murine allogeneic CD4+ and CD8+ T cells co-cultured with WT or Adra2a-KO macrophages. T cells were counted by flow cytometry.Technical duplicates from one representative experiment out of three . i, Effect of CLD (2.5 μM) on the proliferation of murine OT-II CD4+ and OT-I CD8+ T cells estimated by CFSE dilution after co-culture with WT or Adra2a-KO syngeneic macrophages pulsed with ovalbumin protein. An anti-MHC II monoclonal antibody (50 µg ml−1) was added as indicated. Technical triplicates from one representative experiment out of three. j, Ifn-γ production by OT-I CD8+ T cells co-cultured as in (i) and stimulated with E.G7-OVA tumour cells for 16 h. Intracellular Ifn-γ was measured by flow cytometry. Each dot represents the mean of technical duplicates. n = 4 independent biological replicates. Data (mean ± s.e.m) from one representative experiment out of three (b–f,i) except for g (all mice shown). P values determined by t-test (Unpaired, two-tailed) (b,d,f,i,j). **** p < 0.0001.
Extended Data Fig. 7 Role of CD4+ T cells in the effects of α2-AR agonists.
a, Representative flow cytometry dot plots showing the efficacy of CD4+ T-cell depletion 7 days after in vivo administration of the anti-CD4 mAb in tumour (left) and spleen (right). b, Progression of TC-1 tumours in mice treated with CLD, anti-CD4, or both. c, Infiltration of CD4+ and CD8+ T cells in the tumour, lymph node and spleen of TC-1 tumour-bearing mice 7 days after treatment with CLD, anti-CD4, or both. The apparently increased CD8+ T cell proportion in CD4-depleted samples is only relative, and so appears because of the disappearance of CD4+ T cells in those samples. d and e, Progression of TC-1 (d) or MC38-OVA (e) tumours in mice treated with CLD, anti-MHC II, or both. CLD was given daily (5 mg/kg i.p.) starting at day 0 when tumours reached the indicated size. Anti-CD4 antibody (500 μg) was given 1 day before starting CLD treatment, then 250 μg twice per week. Anti-MHC II (500 µg) antibody (i.p.) was given 1 day before the first injection of CLD, then twice a week. Data (mean ± s.e.m) from one representative out of three independent experiments (c) except for b,d and e (all mice shown). P values determined by: ordinary two-way ANOVA (b,d,e), t-test (Unpaired, two-tailed) (c). **** p < 0.0001.
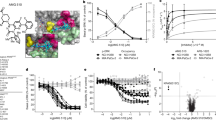
Extended Data Fig. 8 Effects of α2-AR agonists on the cAMP-PKA signalling pathway.
a, MC38-tumour bearing mice (10 mice per group) were treated with CLD or vehicle for 7 days. Tumours and lymph nodes were collected and pooled for isolation of CD11b+ cells. Left panels: Cell lysates were analysed for phospho-CREB and total CREB by western blotting. Beta-actin was used as loading control. The phospho-CREB to total CREB ratio is indicated below the blots. Right panels: MHC II expression of CD11b+ cells in the lymph nodes and tumours from the same mice used in the left panels. b, Quantification of cAMP levels in in vitro differentiated M1 macrophages treated with different concentrations of CLD for 15 min. Mean ± s.e.m of one experiment (technical triplicates). c, M1 macrophages differentiated in vitro from WT or Adra2a-KO bone marrow cells were treated with CLD (2.5 µM) for 16 h. Cell lysates were then analysed for phospho-CREB and total CREB by western blotting (left panels). Macrophages treated with PKA inhibitor (H-89 dihydrochloride hydrate, 1 µM) were used as controls. Beta-actin was used as loading control. The phospho-CREB to total CREB ratio is indicated below the blots. MHC II expression by the same cells is shown on the right panel. For gel source data, see Supplementary Fig. 1b and c. Data (mean ± s.e.m) from one representative out of two independent experiments (a,c). P values determined by t-test (Unpaired, two-tailed). **** p < 0.0001.
Extended Data Fig. 9 Association of α2-AR tumour signalling with survival of patients with cancer.
a, Kaplan–Meier curves for Overall Survival (OS) and Progression-free Survival (PFS) related to ADRA2 metagene (sum of ADRA2A, ADRA2B, ADRA2C) expression in human lung adenocarcinoma (LUAD) analysed using clinical data from TCGA (n = 515). b, Heatmap showing correlation of the expression of immune modulators genes with ADRA2 metagene expression across the TCGA cancer types (30 cancer types; ACC: n = 77, BLCA: n = 413, BRCA: n = 1130, CESC: n = 304, COAD: n = 331, ESCA: n = 181, GBM: n = 158, HNSC: n = 518, KICH: n = 66, KIRC: n = 542, KIRP: n = 288, LGG: n = 508, LIHC: n = 369, LUAD: n = 537, LUSC: n = 503, MESO: n = 87, OV: n = 423, PAAD: n = 178, PCPG: n = 176, PRAD: n = 503, READ: n = 91, SARC: n = 258, SKCM: n = 102, STAD: n = 414, TGCT: n = 132, THCA: n = 504, THYM: n = 119, UCEC: n = 189, UCS: n = 57, UVM: n = 79). c, Heatmaps showing the expression fold changes of genes downregulated (Post_Down) or upregulated (Post_Up) by CLD and GBZ in MC38-OVA tumours from WT or Adra2a-KO mice, as compared to untreated tumours (6 conditions, n = 3 per group). d, Cox Proportional Hazards (CoxPH) regression analysis across clinical trials in TIDE37,38 (BLCA: n = 348, SKCM: n = 41), using as input the human ortholog genes of the up- and downregulated gene signatures post treatment with CLD or GBZ (Post_Up and Post_Down). Negative Z-scores indicate better survival in the high expression group per signature. e, Boxplots highlighting statistically significant differences between high and low expression of the Post_Up and Post_Down gene signatures across a set of immune parameters calculated for TCGA (n = 9320, 30 cancer types). Groups were selected based on median expression of Post_Up or Post_Down signatures. Boxes represent the median, 1st and 3rd quartiles; outliers were determined based on the interquartile range and ignored for visualization. P values are determined by Log-rank-test (a), CoxPh regression-test (d), Two-sidedWelch t-tests (e). p < 0.05 is considered significant.
Extended Data Fig. 10 Anti-tumour effect of clonidine administered via the oral route (a,b), and schematic model of the anti-tumour activity of α2-AR agonists (c).
a, Progression of MC38-OVA colon carcinomas in mice treated with drinking water supplemented with clonidine (CLD) (10 μg ml−1). b, Phagocytic activity of TME macrophages from mice after 7 days of treatment as in (a) (n = 8 per group). Data (mean ± s.e.m) from one representative out of three independent experiments. P values determined by ordinary two-way ANOVA (left) and t-test (Unpaired, two-tailed, right). **** p < 0.0001. c, Model of the identified mechanisms underlying the anti-tumour activity of α2-AR agonists in the context of immunotherapy. Tumour-associated macrophages and myeloid-derived suppressor cells (MDSCs) contribute to immunosuppression in the tumour microenvironment, partly in response to factors such as adenosine and prostaglandins, which trigger the cAMP/pCREB signalling pathway. Agonists of the α2-adrenergic receptor (blue) inhibit adenylate cyclase (AC), thereby blocking the immunosuppressive cAMP/pCREB signalling. Macrophages now better stimulate anti-tumour CD4 and CD8 T cells, while MDSCs die by apoptosis. The schematic in c was created using BioRender.
Supplementary information
Supplementary Information
Uncropped gels (Supplementary Fig. 1) and Supplementary Tables 1 and 2.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, J., Naulaerts, S., Boudhan, L. et al. Tumour immune rejection triggered by activation of α2-adrenergic receptors. Nature 618, 607–615 (2023). https://doi.org/10.1038/s41586-023-06110-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06110-8
This article is cited by
-
Exercise accelerates recruitment of CD8+ T cell to promotes anti-tumor immunity in lung cancer via epinephrine
BMC Cancer (2024)
-
Non–small cell lung cancer and immune checkpoint inhibitor therapy: does non-alcoholic fatty liver disease have an effect?
BMC Cancer (2024)
-
Construction of an ER stress-related prognostic signature for predicting prognosis and screening the effective anti-tumor drug in osteosarcoma
Journal of Translational Medicine (2024)
-
Dexmedetomidine promotes colorectal cancer progression via Piwil2 signaling
Cellular Oncology (2024)
-
The effects of dexmedetomidine on postoperative tumor recurrence and patient survival after breast cancer surgery: a feasibility study
Anesthesiology and Perioperative Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.