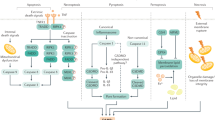
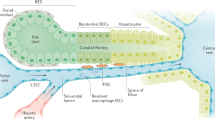
Abstract
Liver sinusoidal endothelial cells (LSECs) form the wall of the hepatic sinusoids. Unlike other capillaries, they lack an organized basement membrane and have cytoplasm that is penetrated by open fenestrae, making the hepatic microvascular endothelium discontinuous. LSECs have essential roles in the maintenance of hepatic homeostasis, including regulation of the vascular tone, inflammation and thrombosis, and they are essential for control of the hepatic immune response. On a background of acute or chronic liver injury, LSECs modify their phenotype and negatively affect neighbouring cells and liver disease pathophysiology. This Review describes the main functions and phenotypic dysregulations of LSECs in liver diseases, specifically in the context of acute injury (ischaemia–reperfusion injury, drug-induced liver injury and bacterial and viral infection), chronic liver disease (metabolism-associated liver disease, alcoholic steatohepatitis and chronic hepatotoxic injury) and hepatocellular carcinoma, and provides a comprehensive update of the role of LSECs as therapeutic targets for liver disease. Finally, we discuss the open questions in the field of LSEC pathobiology and future avenues of research.
Key points
-
Liver sinusoidal endothelial cells (LSECs) form the vascular wall of the hepatic microcirculatory system, the hepatic sinusoid, and exhibit unique phenotypic characteristics, including open fenestrae and lack of a basement membrane.
-
In health, LSECs have key roles maintaining hepatic homeostasis and are critical for several processes, including immune regulation, control of inflammation, modulation of vascular tone and regulation of the coagulation cascade.
-
LSECs become rapidly dedifferentiated during acute and chronic liver injuries, acquiring vasoconstrictor, proinflammatory and prothrombotic properties; this process, termed ‘capillarization’, contributes to the activation and dedifferentiation of other hepatic cells.
-
LSEC capillarization plays a key part in the pathophysiology of major liver diseases, including ischaemia–reperfusion injury, drug-induced liver injury, chronic liver disease and hepatocellular carcinoma; several LSEC molecular targets have been proposed as treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gracia-Sancho, J., Marrone, G. & Fernández-Iglesias, A. Hepatic microcirculation and mechanisms of portal hypertension. Nat. Rev. Gastroenterol. Hepatol. 16, 221–234 (2019).
Smedsrød, B. et al. Cell biology of liver endothelial and Kupffer cells. Gut 35, 1509–1516 (1994).
Marrone, G., Shah, V. H. & Gracia-Sancho, J. Sinusoidal communication in liver fibrosis and regeneration. J. Hepatol. 65, 608–617 (2016).
Gracia-Sancho, J. et al. Increased oxidative stress in cirrhotic rat livers: a potential mechanism contributing to reduced nitric oxide bioavailability. Hepatology 47, 1248–1256 (2008).
Wohlleber, D. & Knolle, P. A. The role of liver sinusoidal cells in local hepatic immune surveillance. Clin. Transl. Immunol. 5, e117 (2016).
Shetty, S., Lalor, P. F. & Adams, D. H. Liver sinusoidal endothelial cells — gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 15, 555–567 (2018).
Smedsrod, B., Pertoft, H., Gustafson, S. & Laurent, T. C. Scavenger functions of the liver endothelial cell. Biochem. J. 266, 313–327 (1990).
Elvevold, K. H., Nedredal, G. I., Revhaug, A. & Smedsrød, B. Scavenger properties of cultivated pig liver endothelial cells. Comp. Hepatol. 3, 4 (2004).
Sørensen, K. K. et al. Liver sinusoidal endothelial cells. Compr. Physiol. 5, 1751–1774 (2015).
Thomson, A. W. & Knolle, P. A. Antigen-presenting cell function in the tolerogenic liver environment. Nat. Rev. Immunol. 10, 753–766 (2010).
Crispe, I. N. Liver antigen-presenting cells. J. Hepatol. 54, 357–365 (2011).
Do, H., Healey, J. F., Waller, E. K. & Lollar, P. Expression of factor VIII by murine liver sinusoidal endothelial cells. J. Biol. Chem. 274, 19587–19592 (1999).
Kume, M. et al. Bacterial lipopolysaccharide decreases thrombomodulin expression in the sinusoidal endothelial cells of rats - a possible mechanism of intrasinusoidal microthrombus formation and liver dysfunction. J. Hepatol. 38, 9–17 (2003).
Yang, H. et al. Neutrophil adhesion and crawling dynamics on liver sinusoidal endothelial cells under shear flow. Exp. Cell Res. 351, 91–99 (2017).
Hilscher, M. B. et al. Mechanical stretch increases expression of CXCL1 in liver sinusoidal endothelial cells to recruit neutrophils, generate sinusoidal microthombi, and promote portal hypertension. Gastroenterology 157, 193–209.e9 (2019).
Meyer, J. et al. Platelet interactions with liver sinusoidal endothelial cells and hepatic stellate cells lead to hepatocyte proliferation. Cells 9, 1243 (2020).
Wisse, E. An electron microscopic study of the fenestrated endothelial lining of rat liver sinusoids. J. Ultrastruct. Res. 31, 125–150 (1970).
Widmann, J. J., Cotran, R. S. & Fahimi, H. D. Mononuclear phagocytes (Kupffer cells) and endothelial cells: Identification Of two functional cell types in rat liver sinusoids by endogenous peroxidase activity. J. Cell Biol. 52, 159–170 (1972).
Ogawa, K., Minase, T., Enomoto, K. & Onoé, T. Ultrastructure of fenestrated cells in the sinusoidal wall of rat liver after perfusion fixation. Tohoku J. Exp. Med. 110, 89–101 (1973).
Wisse, E., Jacobs, F., Topal, B., Frederik, P. & De Geest, B. The size of endothelial fenestrae in human liver sinusoids: Implications for hepatocyte-directed gene transfer. Gene Ther. 15, 1193–1199 (2008).
Wisse, E., De Zanger, R. B., Jacobs, R. & McCuskey, R. S. Scanning electron microscope observations on the structure of portal veins, sinusoids and central veins in rat liver. Scan. Electron. Microsc. 1441–1452 (1983).
Steffan, A.-M., Gendrault, J.-L., McCuskey, R. S., McCuskey, P. A. & Kirn, A. Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology 6, 830–836 (1986).
Eitzen, G. Actin remodeling to facilitate membrane fusion. Biochim. Biophys. Acta Mol. Cell Res. 1641, 175–181 (2003).
Yokomori, H. et al. Endothelin-1 suppresses plasma membrane Ca++-ATPase, concomitant with contraction of hepatic sinusoidal endothelial fenestrae. Am. J. Pathol. 162, 557–566 (2003).
Yokomori, H. et al. Rho modulates hepatic sinusoidal endothelial fenestrae via regulation of the actin cytoskeleton in rat endothelial cells. Lab. Invest. 84, 857–864 (2004).
Bingen, A., Gendrault, J. L. & Kim, A. in Cells of the Hepatic Sinusoid Vol. 2 (eds Wisse, E., Knook, D. L. & Decker, K.) 466–470 (Kupffer Cell Foundation, 1989).
Taira, K. Trabecular meshworks in the sinusoidal endothelial cells of the golden hamster liver: a freeze-fracture study. J. Submicrosc. Cytol. Pathol. 26, 271–277 (1994).
Guo, L., Zhang, H., Hou, Y., Wei, T. & Liu, J. Plasmalemma vesicle–associated protein: a crucial component of vascular homeostasis (review). Exp. Ther. Med. 12, 1639–1644 (2016).
Stan, R. V., Kubitza, M. & Palade, G. E. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc. Natl Acad. Sci. USA 96, 13203–13207 (1999).
Ioannidou, S. et al. An in vitro assay reveals a role for the diaphragm protein PV-1 in endothelial fenestra morphogenesis. Proc. Natl Acad. Sci. USA 103, 16770–16775 (2006).
Stan, R. V. et al. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev. Cell 23, 1203–1218 (2012).
Bankston, P. W. & Pino, R. M. The development of the sinusoids of fetal rat liver: morphology of endothelial cells, Kupffer cells, and the transmural migration of blood cells into the sinusoids. Am. J. Anat. 159, 1–15 (1980).
Herrnberger, L. et al. Formation of fenestrae in murine liver sinusoids depends on plasmalemma vesicle-associated protein and is required for lipoprotein passage. PLoS ONE 9, 1–26 (2014).
Braet, F., Spector, I., De Zanger, R. & Wisse, E. A novel structure involved in the formation of liver endothelial cell fenestrae revealed by using the actin inhibitor misakinolide. Proc. Natl Acad. Sci. USA 95, 13635–13640 (1998).
Tkachenko, E. et al. Caveolae, fenestrae and transendothelial channels retain PV1 on the surface of endothelial cells. PLoS ONE 7, e32655 (2012).
Auvinen, K. et al. Fenestral diaphragms and PLVAP associations in liver sinusoidal endothelial cells are developmentally regulated. Sci. Rep. 9, 1–16 (2019).
Cogger, V. C., O’Reilly, J. N., Warren, A. & Le Couteur, D. G. A standardized method for the analysis of liver sinusoidal endothelial cells and their fenestrations by scanning electron microscopy. J. Vis. Exp. https://doi.org/10.3791/52698 (2015).
Fernández-Iglesias, A., Ortega-Ribera, M., Guixé-Muntet, S. & Gracia-Sancho, J. 4 in 1: Antibody-free protocol for isolating the main hepatic cells from healthy and cirrhotic single rat livers. J. Cell. Mol. Med. 23, 877–886 (2018).
Maeso-Díaz, R. et al. Effects of aging on liver microcirculatory function and sinusoidal phenotype. Aging Cell 17, e12829 (2018).
Di Martino, J. et al. Actin depolymerization in dedifferentiated liver sinusoidal endothelial cells promotes fenestrae re-formation. Hepatol. Commun. 3, 213–219 (2019).
Mönkemöller, V., Øie, C., Hübner, W., Huser, T. & McCourt, P. Multimodal super-resolution optical microscopy visualizes the close connection between membrane and the cytoskeleton in liver sinusoidal endothelial cell fenestrations. Sci. Rep. 5, 1–10 (2015).
Zapotoczny, B., Szafranska, K., Kus, E., Chlopicki, S. & Szymonski, M. Quantification of fenestrations in liver sinusoidal endothelial cells by atomic force microscopy. Micron 101, 48–53 (2017).
Zapotoczny, B. et al. Tracking fenestrae dynamics in live murine liver sinusoidal endothelial cells. Hepatology 69, 876–888 (2019).
Halpern, K. B. et al. Single-cell spatial reconstruction reveals global division of labour in the mammalian liver. Nature 542, 1–5 (2017).
Halpern, K. B. et al. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat. Biotechnol. 36, 962 (2018).
MacParland, S. A. et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. Commun. 9, 1–21 (2018).
Aizarani, N. et al. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 572, 199–204 (2019).
Lemoinne, S. et al. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology 61, 1041–1055 (2015).
Carreira, C. M. et al. LYVE-1 is not restricted to the lymph vessels: Expression in normal liver blood sinusoids and down-regulation in human liver cancer and cirrhosis. Cancer Res. 61, 8079–8084 (2001).
DeLeve, L. D., Wang, X., McCuskey, M. K. & McCuskey, R. S. Rat liver endothelial cells isolated by anti-CD31 immunomagnetic separation lack fenestrae and sieve plates. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G1187–G1189 (2006).
Xie, G., Wang, L., Wang, X., Wang, L. & DeLeve, L. D. Isolation of periportal, midlobular, and centrilobular rat liver sinusoidal endothelial cells enables study of zonated drug toxicity. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1204–G1210 (2010).
Wree, A., Holtmann, T. M., Inzaugarat, M. E. & Feldstein, A. E. Novel drivers of the inflammatory response in liver injury and fibrosis. Semin. Liver Dis. 39, 275–282 (2019).
Ibrahim, S. H., Hirsova, P. & Gores, G. J. Non-alcoholic steatohepatitis pathogenesis: sublethal hepatocyte injury as a driver of liver inflammation. Gut 67, 963–972 (2018).
DeLeve, L. D., Wang, X. & Guo, Y. Sinusoidal endothelial cells prevent rat stellate cell activation and promote reversion to quiescence. Hepatology 48, 920–930 (2008).
Nieto, N. Oxidative-stress and IL-6 mediate the fibrogenic effects of rodent Kupffer cells on stellate cells. Hepatology 44, 1487–1501 (2006).
Wen, Y. Hepatic macrophages in liver homeostasis and diseases- diversity, plasticity and therapeutic opportunities. Cell. Mol. Immunol. 18, 45–56 (2021).
Warren, A. et al. Hepatic pseudocapillarization in aged mice. Exp. Gerontol. 40, 807–812 (2005).
Cogger, V. C. et al. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp. Gerontol. 38, 1101–1107 (2003).
Ito, Y. et al. Age-related changes in the hepatic microcirculation in mice. Exp. Gerontol. 42, 789–797 (2007).
Hide, D. et al. Ischemia/reperfusion injury in the aged liver: the importance of the sinusoidal endothelium in developing therapeutic strategies for the elderly. J. Gerontol. A Biol. Sci. Med. Sci. 75, 268–277 (2020).
Maeso-Díaz, R. et al. Aging influences hepatic microvascular biology and liver fibrosis in advanced chronic liver disease. Aging Dis. 10, 684–698 (2019).
Xie, G. et al. Role of differentiation of liver sinusoidal endothelial cells in progression and regression of hepatic fibrosis in rats. Gastroenterology 142, 918–927 (2012).
Xie, G. et al. Hedgehog signalling regulates liver sinusoidal endothelial cell capillarisation. Gut 62, 299–309 (2012).
Desroches-Castan, A. et al. Bone morphogenetic protein 9 is a paracrine factor controlling liver sinusoidal endothelial cell fenestration and protecting against hepatic fibrosis. Hepatology 70, 1392–1408 (2019).
Géraud, C. et al. Liver sinusoidal endothelium: a microenvironment-dependent differentiation program in rat including the novel junctional protein liver endothelial differentiation-associated protein-1. Hepatology 52, 313–326 (2010).
Géraud, C. et al. GATA4-dependent organ-specific endothelial differentiation controls liver development and embryonic hematopoiesis. J. Clin. Invest. 127, 1099–1114 (2017).
Winkler, M. et al. Endothelial GATA4 controls liver fibrosis and regeneration by preventing a pathogenic switch in angiocrine signaling. J. Hepatol. 74, 380–393 (2021).
Montalvo-Jave, E. E., Escalante-Tattersfield, T., Ortega-Salgado, J. A., Pina, E. & Geller, D. A. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J. Surg. Res. 147, 153–159 (2008).
Peralta, C., Jiménez-Castro, M. B. & Gracia-Sancho, J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J. Hepatol. 59, 1094–1106 (2013).
Dar, W. A., Sullivan, E., Bynon, J. S., Eltzschig, H. & Ju, C. Ischaemia reperfusion injury in liver transplantation: cellular and molecular mechanisms. Liver Int. 39, 788–801 (2019).
Caldwell-Kenkel, J. C., Thurman, R. G. & Lemasters, J. J. Selective loss of nonparenchymal cell viability after cold ischemic storage of rat livers. Transplantation 45, 834–837 (1988).
Jaeschke, H. Role of reactive oxygen species in hepatic ischemia-reperfusion injury and preconditioning. J. Invest. Surg. 16, 127–140 (2003).
Stewart, R. K. et al. A novel mouse model of depletion of stellate cells clarifies their role in ischemia/reperfusion- and endotoxin-induced acute liver injury. J. Hepatol. 60, 298–305 (2014).
Caldwell-Kenkel, J. C., Currin, R. T., Tanaka, Y., Thurman, R. G. & Lemasters, J. J. Reperfusion injury to endothelial cells following cold ischemic storage of rat livers. Hepatology 10, 292–299 (1989).
Selzner, N., Rudiger, H., Graf, R. & Clavien, P. A. Protective strategies against ischemic injury of the liver. Gastroenterology 125, 917–936 (2003).
Clemens, M. G. Nitric oxide in liver injury. Hepatology 30, 1–5 (1999).
Russo, L. et al. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology 55, 921–930 (2012).
Gracia-Sancho, J. et al. Flow cessation triggers endothelial dysfunction during organ cold storage conditions: strategies for pharmacologic intervention. Transplantation 90, 142–149 (2010).
Gracia-Sancho, J. et al. Simvastatin maintains function and viability of steatotic rat livers procured for transplantation. J. Hepatol. 58, 1140–1146 (2013).
DeLeve, L. D., Wang, X., Hu, L., Mccuskey, M. K. & Mccuskey, R. S. Rat liver sinusoidal endothelial cell phenotype is maintained by paracrine and autocrine regulation. Am. J. Physiol. Gastrointest. Liver Physiol. 287, G757–G763 (2004).
Lakshminarayanan, V., Drab-Weiss, E. A. & Roebuck, K. A. H2O2 and tumor necrosis factor-alpha induce differential binding of the redox-responsive transcription factors AP-1 and NF-kappaB to the interleukin-8 promoter in endothelial and epithelial cells. J. Biol. Chem. 273, 32670–32678 (1998).
Read, M. A. et al. The proteasome pathway is required for cytokine-induced endothelial-leukocyte adhesion molecule expression. Immunity 2, 493–506 (1995).
Perry, B. C., Soltys, D., Toledo, A. H. & Toledo-Pereyra, L. H. Tumor necrosis factor-alpha in liver ischemia/reperfusion injury. J. Invest. Surg. 24, 178–188 (2011).
Chen, G. Y. & Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Teoh, N. C. & Farrell, G. C. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J. Gastroenterol. Hepatol. 18, 891–902 (2003).
Casillas-Ramirez, A., Mosbah, I. B., Ramalho, F., Rosello-Catafau, J. & Peralta, C. Past and future approaches to ischemia-reperfusion lesion associated with liver transplantation. Life Sci. 79, 1881–1894 (2006).
Sindram, D., Porte, R. J., Hoffman, M. R., Bentley, R. C. & Clavien, P. A. Platelets induce sinusoidal endothelial cell apoptosis upon reperfusion of the cold ischemic rat liver. Gastroenterology 118, 183–191 (2000).
Lesurtel, M. et al. Platelet-derived serotonin mediates liver regeneration. Science 312, 104–107 (2006).
Miyashita, T. et al. Ischemia reperfusion-facilitated sinusoidal endothelial cell injury in liver transplantation and the resulting impact of extravasated platelet aggregation. Eur. Surg. 48, 92–98 (2016).
Go, K. L., Lee, S., Zendejas, I., Behrns, K. E. & Kim, J. S. Mitochondrial dysfunction and autophagy in hepatic ischemia/reperfusion injury. Biomed. Res. Int. 2015, 183469 (2015).
Hide, D. et al. Effects of warm ischemia and reperfusion on the liver microcirculatory phenotype of rats: underlying mechanisms and pharmacological therapy. Sci. Rep. 6, 22107 (2016).
Guixé-Muntet, S. et al. Cross-talk between autophagy and KLF2 determines endothelial cell phenotype and microvascular function in acute liver injury. J. Hepatol. 66, 86–94 (2017).
Qu, S. et al. Heme oxygenase 1 attenuates hypoxia-reoxygenation injury in mice liver sinusoidal endothelial cells. Transplantation 102, 426–432 (2018).
Greene, A. K. et al. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann. Surg. 237, 530–535 (2003).
Wang, L. et al. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J. Clin. Invest. 122, 1567–1573 (2012).
Batkai, S. et al. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J. 21, 1788–1800 (2007).
Pacher, P. & Hasko, G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 153, 252–262 (2008).
Marra, F. & Bertolani, C. Adipokines in liver diseases. Hepatology 50, 957–969 (2009).
Alvarez-Mercado, A. I., Bujaldon, E., Gracia-Sancho, J. & Peralta, C. The role of adipokines in surgical procedures requiring both liver regeneration and vascular occlusion. Int. J. Mol. Sci. 19, 3395 (2018).
Yokoyama, Y., Nimura, Y., Nagino, M., Bland, K. I. & Chaudry, I. H. Role of thromboxane in producing hepatic injury during hepatic stress. Arch. Surg. 140, 801–807 (2005).
Minamino, T. et al. Thromboxane A2 receptor signaling promotes liver tissue repair after toxic injury through the enhancement of macrophage recruitment. Toxicol. Appl. Pharmacol. 259, 104–114 (2012).
Isozaki, H., Okajima, K., Hara, H. & Kobayashi, M. The protective effect of thromboxane A2 synthetase inhibitor against ischemic liver injury. Surg. Today 24, 435–440 (1994).
Hide, D. et al. A novel form of the human manganese superoxide dismutase protects rat and human livers undergoing ischaemia and reperfusion injury. Clin. Sci. 127, 527–537 (2014).
Ito, T. et al. Sinusoidal protection by sphingosine-1-phosphate receptor 1 agonist in liver ischemia-reperfusion injury. J. Surg. Res. 222, 139–152 (2018).
Yadav, N. et al. Efficient reconstitution of hepatic microvasculature by endothelin receptor antagonism in liver sinusoidal endothelial cells. Hum. Gene Ther. 30, 365–377 (2019).
Wang, X., Maretti-Mira, A. C., Wang, L. & DeLeve, L. D. Liver-selective MMP-9 inhibition in the rat eliminates ischemia-reperfusion injury and accelerates liver regeneration. Hepatology 69, 314–328 (2019).
Wang, X. et al. Susceptibility of rat steatotic liver to ischemia-reperfusion is treatable with liver-selective matrix metalloproteinase inhibition. Hepatology 72, 1771–1785 (2020).
Andrade, R. J. et al. Drug-induced liver injury. Nat. Rev. Dis. Primers 5, 58 (2019).
Kaplowitz, N. Idiosyncratic drug hepatotoxicity. Nat. Rev. Drug Discov. 4, 489–499 (2005).
Chen, M., Suzuki, A., Borlak, J., Andrade, R. J. & Lucena, M. I. Drug-induced liver injury: interactions between drug properties and host factors. J. Hepatol. 63, 503–514 (2015).
Reuben, A. et al. Outcomes in adults with acute liver failure between 1998 and 2013: an observational cohort study. Ann. Intern. Med. 164, 724–732 (2016).
Donnelly, M. C. et al. Acute liver failure in Scotland: changes in aetiology and outcomes over time (the Scottish Look-Back Study). Aliment. Pharmacol. Ther. 45, 833–843 (2017).
Suzuki, H. & Sugiyama, Y. Transport of drugs across the hepatic sinusoidal membrane: Sinusoidal drug influx and efflux in the liver. Semin. Liver Dis. 20, 251–263 (2000).
Yuan, L. & Kaplowitz, N. Mechanisms of drug-induced liver injury. Clin. Liver Dis. 17, 507–518 (2013).
Hagenbuch, B. & Stieger, B. The SLCO (former SLC21) superfamily of transporters. Mol. Aspects Med. 34, 396–412 (2013).
Ito, Y., Bethea, N. W., Abril, E. R. & McCuskey, R. S. Early hepatic microvascular injury in response to acetaminophen toxicity. Microcirculation 10, 391–400 (2003).
McCuskey, R. S. Sinusoidal endothelial cells as an early target for hepatic toxicants. Clin. Hemorheol. Microcirc. 34, 5–10 (2006).
Teratani, T. et al. Free cholesterol accumulation in liver sinusoidal endothelial cells exacerbates acetaminophen hepatotoxicity via TLR9 signaling. J. Hepatol. 67, 780–790 (2017).
Ganey, P. E. et al. Role of the coagulation system in acetaminophen-induced hepatotoxicity in mice. Hepatology 46, 1177–1186 (2007).
Randle, L. E. et al. α1-Adrenoceptor antagonists prevent paracetamol-induced hepatotoxicity in mice. Br. J. Pharmacol. 153, 820–830 (2008).
Ito, Y., Abril, E. R., Bethea, N. W. & McCuskey, R. S. Inhibition of matrix metalloproteinases minimizes hepatic microvascular injury in response to acetaminophen in mice. Toxicol. Sci. 83, 190–196 (2005).
Liu, J. et al. The nitric oxide donor, V-PYRRO/NO, protects against acetaminophen-induced hepatotoxicity in mice. Hepatology 37, 324–333 (2003).
Deleve, L. D. Dacarbazine toxicity in murine liver cells: a model of hepatic endothelial injury and glutathione defense. J. Pharmacol. Exp. Ther. 268, 1261–1270 (1994).
DeLeve, L. D. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology 24, 830–837 (1996).
DeLeve, L. D., Wang, X., Kuhlenkamp, J. F. & Kaplowitz, N. Toxicity of azathioprine and monocrotaline in murine sinusoidal endothelial cells and hepatocytes: the role of glutathione and relevance to hepatic venoocclusive disease. Hepatology 23, 589–599 (1996).
Ito, Y. et al. Mechanisms and pathophysiological implications of sinusoidal endothelial cell gap formation following treatment with galactosamine/endotoxin in mice. Am. J. Physiol. Gastrointest. Liver Physiol 291, G211–G218 (2006).
DeLeve, L. D. et al. Sinusoidal endothelial cells as a target for acetaminophen toxicity. Direct action versus requirement for hepatocyte activation in different mouse strains. Biochem. Pharmacol. 53, 1339–1345 (1997).
McCuskey, R. S. et al. Ethanol binging exacerbates sinusoidal endothelial and parenchymal injury elicited by acetaminophen. J. Hepatol. 42, 371–377 (2005).
McCuskey, R. S. S. The hepatic microvascular system in health and its response to toxicants. Anat. Rec. 291, 661–671 (2008).
Garcia-Roman, R. & Frances, R. Acetaminophen-induced liver damage in hepatic steatosis. Clin. Pharmacol. Ther. 107, 1068–1081 (2020).
Zhang, Q. et al. Palmitate up-regulates laminin expression via ROS/integrin αvβ3 pathway in HLSECs. Oncotarget 10, 4083–4090 (2019).
Liu, J. et al. High glucose regulates LN expression in human liver sinusoidal endothelial cells through ROS/integrin αvβ3 pathway. Environ. Toxicol. Pharmacol. 42, 231–236 (2016).
Yang, R., Miki, K., He, X., Killeen, M. E. & Fink, M. P. Prolonged treatment with N-acetylcystine delays liver recovery from acetaminophen hepatotoxicity. Crit. Care 13, R55 (2009).
Sandilands, E. A. & Bateman, D. N. Adverse reactions associated with acetylcysteine. Clin. Toxicol. 47, 81–88 (2009).
Eugenio-Perez, D., Montes de Oca-Solano, H. A. & Pedraza-Chaverri, J. Role of food-derived antioxidant agents against acetaminophen-induced hepatotoxicity. Pharm. Biol. 54, 2340–2352 (2016).
Kigawa, G. et al. Improvement of portal flow and hepatic microcirculatory tissue flow with N-acetylcysteine in dogs with obstructive jaundice produced by bile duct ligation. Eur. J. Surg. 166, 77–84 (2000).
Yin, H. et al. Lactoferrin protects against acetaminophen-induced liver injury in mice. Hepatology 51, 1007–1016 (2010).
Coppell, J. A., Brown, S. A. & Perry, D. J. Veno-occlusive disease: cytokines, genetics, and haemostasis. Blood Rev. 17, 63–70 (2003).
Park, Y. D. et al. Impaired activity of plasma von Willebrand factor-cleaving protease may predict the occurrence of hepatic veno-occlusive disease after stem cell transplantation. Bone Marrow Transpl. 29, 789–794 (2002).
Fan, C. Q. & Crawford, J. M. Sinusoidal obstruction syndrome (hepatic veno-occlusive disease). J. Clin. Exp. Hepatol. 4, 332–346 (2014).
DeLeve, L. D. et al. Decreased hepatic nitric oxide production contributes to the development of rat sinusoidal obstruction syndrome. Hepatology 38, 900–908 (2003).
Nishigori, N. et al. Von Willebrand factor-rich platelet thrombi in the liver cause sinusoidal obstruction syndrome following oxaliplatin-based chemotherapy. PLoS ONE 10, 1–17 (2015).
Takada, S. et al. Soluble thrombomodulin attenuates endothelial cell damage in hepatic sinusoidal obstruction syndrome. In Vivo 32, 1409–1417 (2018).
Richardson, P. G. et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood 127, 1656–1665 (2016).
Otaka, F. et al. Thromboxane A2 receptor signaling in endothelial cells attenuates monocrotaline-induced liver injury. Toxicol. Appl. Pharmacol. 381, 114733 (2019).
Navarro, V. J. & Lucena, M. I. Hepatotoxicity induced by herbal and dietary supplements. Semin. Liver Dis. 34, 172–193 (2014).
Seeff, L. B., Bonkovsky, H. L., Navarro, V. J. & Wang, G. Herbal products and the liver: a review of adverse effects and mechanisms. Gastroenterology 148, 517–532.e3 (2015).
Andrade, R. J., Medina-Caliz, I., Gonzalez-Jimenez, A., Garcia-Cortes, M. & Lucena, M. I. Hepatic damage by natural remedies. Semin. Liver Dis. 38, 21–40 (2018).
European Medicines Agency. Committee on herbal medicinal products (HMPC) (EMA, 2017).
Kullak-Ublick, G. A. et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut 66, 1154–1164 (2017).
Kaplowitz, N., DeLeve, L., Kaplowitz, N. & DeLeve, L. Drug-Induced Liver Disease (Academic, 2013).
Xiong, A. et al. Metabolomic and genomic evidence for compromised bile acid homeostasis by senecionine, a hepatotoxic pyrrolizidine alkaloid. Chem. Res. Toxicol. 27, 775–786 (2014).
Ortega-Ribera, M. et al. Resemblance of the human liver sinusoid in a fluidic device with biomedical and pharmaceutical applications. Biotechnol. Bioeng. 115, 1–10 (2018).
Crispe, I. N. The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 (2009).
Limmer, A. et al. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat. Med. 6, 1348–1354 (2000).
Katz, S. C., Pillarisetty, V. G., Bleier, J. I., Shah, A. B. & DeMatteo, R. P. Liver sinusoidal endothelial cells are insufficient to activate T cells. J. Immunol. 173, 230–235 (2004).
Carambia, A. et al. TGF-β-dependent induction of CD4+CD25+Foxp3+ Tregs by liver sinusoidal endothelial cells. J. Hepatol. 61, 594–599 (2014).
Schurich, A. et al. Dynamic regulation of CD8 T cell tolerance induction by liver sinusoidal endothelial cells. J. Immunol. 184, 4107–4114 (2010).
Knolle, P. A., Böttcher, J. & Huang, L. R. The role of hepatic immune regulation in systemic immunity to viral infection. Med. Microbiol. Immunol. 204, 21–27 (2015).
Neumann, K. et al. Chemokine transfer by liver sinusoidal endothelial cells contributes to the recruitment of CD4+ T cells into the murine liver. PLoS ONE 10, e0123867 (2015).
Wittlich, M. et al. Liver sinusoidal endothelial cell cross-priming is supported by CD4 T cell-derived IL-2. J. Hepatol. 66, 978–986 (2017).
Caparrós, E. et al. Liver sinusoidal endothelial cells contribute to hepatic antigen-presenting cell function and Th17 expansion in cirrhosis. Cells 9, 1227 (2020).
Gola, A. et al. Commensal-driven immune zonation of the liver promotes host defence. Nature 589, 131–136 (2020).
Martin-Armas, M. et al. Toll-like receptor 9 (TLR9) is present in murine liver sinusoidal endothelial cells (LSECs) and mediates the effect of CpG-oligonucleotides. J. Hepatol. 44, 939–946 (2006).
Lalor, P. F. et al. Recruitment of lymphocytes to the human liver. Immunol. Cell Biol. 80, 52–64 (2002).
Cheluvappa, R. et al. Liver sinusoidal endothelial cells and acute non-oxidative hepatic injury induced by Pseudomonas aeruginosa pyocyanin. Int. J. Exp. Pathol. 89, 410–418 (2008).
Leong, S. S., Cazen, R. A., Yu, G. S., LeFevre, L. & Carson, J. W. Abdominal visceral peliosis associated with bacillary angiomatosis. Ultrastructural evidence of endothelial destruction by bacilli. Arch. Pathol. Lab. Med. 116, 866–871 (1992).
Cheluvappa, R. et al. Pathogenesis of the hyperlipidemia of Gram-negative bacterial sepsis may involve pathomorphological changes in liver sinusoidal endothelial cells. Int. J. Infect. Dis. 14, e857–e867 (2010).
Yao, Z. et al. Blood-borne lipopolysaccharide is rapidly eliminated by liver sinusoidal endothelial cells via high-density lipoprotein. J. Immunol. 197, 2390–2399 (2016).
Ganesan, L. P. et al. Scavenger receptor B1, the HDL receptor, is expressed abundantly in liver sinusoidal endothelial cells. Sci. Rep. 6, 20646 (2016).
Heesch, K. et al. The function of the chemokine receptor CXCR6 in the T cell response of mice against Listeria monocytogenes. PLoS ONE 9, e97701 (2014).
Oie, C. I. et al. Liver sinusoidal endothelial cells contribute to the uptake and degradation of entero bacterial viruses. Sci. Rep. 10, 898 (2020).
Liu, J. et al. TLR1/2 ligand-stimulated mouse liver endothelial cells secrete IL-12 and trigger CD8+ T cell immunity in vitro. J. Immunol. 191, 6178–6190 (2013).
Wu, J. et al. Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology 46, 1769–1778 (2007).
Huang, S. et al. LSECs express functional NOD1 receptors: a role for NOD1 in LSEC maturation-induced T cell immunity in vitro. Mol. Immunol. 101, 167–175 (2018).
Breiner, K. M. M., Schaller, H. & Knolle, P. A. A. Endothelial cell-mediated uptake of a hepatitis B virus: a new concept of liver targeting of hepatotropic microorganisms. Hepatology 34, 803–808 (2001).
Gripon, P. et al. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl Acad. Sci. USA 99, 15655–15660 (2002).
Schulze, A., Gripon, P. & Urban, S. Hepatitis B virus infection initiates with a large surface protein-dependent binding to heparan sulfate proteoglycans. Hepatology 46, 1759–1768 (2007).
Protzer, U., Maini, M. K. & Knolle, P. A. Living in the liver: hepatic infections. Nat. Rev. Immunol. 12, 201–213 (2012).
Baiocchini, A. et al. Liver sinusoidal endothelial cells (LSECs) modifications in patients with chronic hepatitis C. Sci. Rep. 9, 8760 (2019).
Bruns, T. et al. CMV infection of human sinusoidal endothelium regulates hepatic T cell recruitment and activation. J. Hepatol. 63, 38–49 (2015).
Frevert, U. et al. Intravital observation of plasmodium berghei sporozoite infection of the liver. PLoS Biol. 3, 1034–1046 (2005).
Tavares, J. et al. Role of host cell traversal by the malaria sporozoite during liver infection. J. Exp. Med. 210, 905–915 (2013).
Schuster, S., Cabrera, D., Arrese, M. & Feldstein, A. E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 15, 349–364 (2018).
Sanyal, A. J. et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the simtuzumab trials. Hepatology 70, 1913–1927 (2019).
Pasarín, M. et al. Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS ONE 7, e32785 (2012).
Francque, S. et al. Increased intrahepatic resistance in severe steatosis: endothelial dysfunction, vasoconstrictor overproduction and altered microvascular architecture. Lab. Invest. 92, 1428–1439 (2012).
Maeso-Díaz et al. New rat model of advanced NASH mimicking pathophysiological features and transcriptomic signature of the human disease. Cells 8, 1062 (2019).
Hammoutene, A. et al. A defect in endothelial autophagy occurs in patients with non-alcoholic steatohepatitis and promotes inflammation and fibrosis. J. Hepatol. 72, 528–538 (2020).
Pasarín, M. et al. Insulin resistance and liver microcirculation in a rat model of early NAFLD. J. Hepatol. 55, 1095–1102 (2011).
Sun, X. X. & Harris, E. N. New aspects of hepatic endothelial cells in physiology and nonalcoholic fatty liver disease. Am. J. Physiol. Cell Physiol. 318, 1200–1213 (2020).
Van der Graaff, D. et al. Severe steatosis induces portal hypertension by systemic arterial hyporeactivity and hepatic vasoconstrictor hyperreactivity in rats. Lab. Invest. 98, 1263–1275 (2018).
Semmler, G. et al. The impact of hepatic steatosis on portal hypertension. PLoS ONE 14, 1–14 (2019).
Zhou, L.-Y., Zeng, H., Wang, S. & Chen, J.-X. Regulatory role of endothelial PHD2 in the hepatic steatosis. Cell Physiol. Biochem. 48, 1003–1011 (2018).
Rogers, G. W. T., Dobbs, B. R. & Fraser, R. Decreased hepatic uptake of cholesterol and retinol in the dimethylnitrosamine rat model of cirrhosis. Liver 12, 326–329 (1992).
Fujita, K. et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology 50, 772–780 (2009).
Fraser, R., Dobbs, B. R. & Rogers, G. W. T. Lipoproteins and the liver sieve: the role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology 21, 863–874 (1995).
Simon, J. et al. Targeting hepatic glutaminase 1 ameliorates non- alcoholic steatohepatitis by restoring very-low- density lipoprotein triglyceride assembly article targeting hepatic glutaminase 1 ameliorates non-alcoholic steatohepatitis by restoring very-low-density lip. Cell Metab. 31, 605–622.e10 (2020).
Nedredal, G. I. et al. Porcine liver sinusoidal endothelial cells contribute significantly to intrahepatic ammonia metabolism. Hepatology 50, 900–908 (2009).
Miyao, M. et al. Pivotal role of liver sinusoidal endothelial cells in NAFLD/NASH progression. Lab. Invest. 95, 1130–1144 (2015).
Vassilopoulos, D. & Hadziyannis, S. J. in Practical Management of Liver Diseases (ed. Younossi, Z.) 26–38 (Cambridge Univ. Press, 2008).
Do, A. & Reau, N. S. Chronic viral hepatitis: current management and future directions. Hepatol. Commun. 4, 329–341 (2020).
Nguyen, V. T. T., Law, M. G. & Dore, G. J. Hepatitis B-related hepatocellular carcinoma: epidemiological characteristics and disease burden. J. Viral Hepat. 16, 453–463 (2009).
Attia, F., Megahed, K., Zhou, X. & Sun, P. The interactions between HBV and the innate immunity of hepatocytes. Viruses 12, 285 (2020).
Meng, Z., Chen, Y. & Lu, M. Advances in targeting the innate and adaptive immune systems to cure chronic hepatitis B virus infection. Front. Immunol. 10, 3127 (2020).
Yang, S. et al. MMP2/MMP9-mediated CD100 shedding is crucial for inducing intrahepatic anti-HBV CD8 T cell responses and HBV clearance. J. Hepatol. 71, 685–698 (2019).
Nahmias, Y., Casali, M., Barbe, L., Berthiaume, F. & Yarmush, M. L. Liver endothelial cells promote LDL-R expression and the uptake of HCV-like particles in primary rat and human hepatocytes. Hepatology 43, 257–265 (2006).
Abouelasrar Salama, S. et al. Induction of chemokines by hepatitis C virus proteins: synergy of the core protein with interleukin-1β and interferon-γ in liver bystander cells. J. Interf. Cytokine Res. 40, 195–206 (2020).
Rowe, I. A. et al. Paracrine signals from liver sinusoidal endothelium regulate hepatitis C virus replication. Hepatology 59, 375–384 (2013).
Brenndörfer, E. D. et al. Anti-tumor necrosis factor α treatment promotes apoptosis and prevents liver regeneration in a transgenic mouse model of chronic hepatitis C. Hepatology 52, 1553–1563 (2010).
Giugliano, S. et al. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology 148, 392–402.e13 (2015).
Schmidt, F. P. et al. Interferon- and ribavirin-free therapy with new direct acting antivirals (DAA) for chronic hepatitis C improves vascular endothelial function. Int. J. Cardiol. 271, 296–300 (2018).
Davis, J. S. et al. The effect of curing hepatitis C with direct-acting antiviral treatment on endothelial function. Antivir. Ther. 23, 687–694 (2018).
Wang, B.-Y., Ju, X.-H., Fu, B.-Y., Zhang, J. & Cao, Y.-X. Effects of ethanol on liver sinusoidal endothelial cells-fenestrae of rats. Hepatobiliary Pancreat. Dis. Int. 4, 422–426 (2005).
Nevzorova, Y. A., Boyer-Diaz, Z., Cubero, F. J. & Gracia-Sancho, J. Animal models for liver disease - a practical approach for translational research. J. Hepatol. 73, 423–440 (2020).
Gracia-Sancho, J. et al. Endothelial expression of transcription factor Kruppel-like factor 2 and its vasoprotective target genes in the normal and cirrhotic rat liver. Gut 60, 517–524 (2011).
Cogger, V. C., Hunt, N. J. & Le Couteur, D. G. in The Liver (eds Arias, I. M. et al.) 435–443 (Wiley, 2020).
Ruart, M. et al. Impaired endothelial autophagy promotes liver fibrosis by aggravating the oxidative stress response during acute liver injury. J. Hepatol. 70, 458–469 (2019).
Gracia-Sancho, J. et al. Enhanced vasoconstrictor prostanoid production by sinusoidal endothelial cells increases portal perfusion pressure in cirrhotic rat livers. J. Hepatol. 47, 220–227 (2007).
Rockey, D. C. & Weisiger, R. A. Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance. Hepatology 24, 233–240 (1996).
Graupera, M. et al. Cyclooxygenase-derived products modulate the increased intrahepatic resistance of cirrhotic rat livers. Hepatology 37, 172–181 (2003).
Planagumà, A. et al. The selective cyclooxygenase-2 inhibitor SC-236 reduces liver fibrosis by mechanisms involving non-parenchymal cell apoptosis and PPARγ activation. FASEB J. 19, 1120–1122 (2005).
Graupera, M. et al. 5-Lipoxygenase inhibition reduces intrahepatic vascular resistance of cirrhotic rat livers: a possible role of cysteinyl-leukotrienes. Gastroenterology 122, 387–393 (2002).
Rockey, D. C. & Chung, J. J. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology 114, 344–351 (1998).
Gracia-Sancho, J. et al. Evidence against a role for NADPH oxidase modulating hepatic vascular tone in cirrhosis. Gastroenterology 133, 959–966 (2007).
Rosado, E. et al. Interaction between NO and COX pathways modulating hepatic endothelial cells from control and cirrhotic rats. J. Cell. Mol. Med. 16, 2461–2470 (2012).
Lisman, T. & Luyendyk, J. P. Platelets as modulators of liver diseases. Semin. Thromb. Hemost. 44, 114–125 (2018).
Tripodi, A., Primignani, M., Mannucci, P. M. & Caldwell, S. H. Changing concepts of cirrhotic coagulopathy. Am. J. Gastroenterol. 112, 274–281 (2017).
Cerini, F. et al. Enoxaparin reduces hepatic vascular resistance and portal pressure in cirrhotic rats. J. Hepatol. 64, 834–842 (2016).
Bosch, J., Gracia-Sancho, J. & Abraldes, J. G. Cirrhosis as new indication for statins. Gut 69, 953–962 (2020).
Marrone, G. et al. The transcription factor KLF2 mediates hepatic endothelial protection and paracrine endothelial-stellate cell deactivation induced by statins. J. Hepatol. 58, 98–103 (2013).
Marrone, G. et al. KLF2 exerts antifibrotic and vasoprotective effects in cirrhotic rat livers: behind the molecular mechanisms of statins. Gut 64, 1434–1443 (2015).
Rodríguez, S. et al. A nitric oxide-donating statin decreases portal pressure with a better toxicity profile than conventional statins in cirrhotic rats. Sci. Rep. 7, 40461 (2017).
Hunt, N. J. et al. Manipulating fenestrations in young and old liver sinusoidal endothelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 316, G144–G154 (2019).
Zafra, C. et al. Simvastatin enhances hepatic nitric oxide production and decreases the hepatic vascular tone in patients with cirrhosis. Gastroenterology 126, 749–755 (2004).
Abraldes, J. G. et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology 136, 1651–1658 (2009).
Tripathi, D. M. et al. Simvastatin prevents progression of acute on chronic liver failure in rats with cirrhosis and portal hypertension. Gastroenterology 155, 1564–1577 (2018).
Pose, E. et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol. Hepatol. 5, 31–41 (2020).
Biecker, E. et al. Treatment of bile duct-ligated rats with the nitric oxide synthase transcription enhancer AVE 9488 ameliorates portal hypertension. Liver Int. 28, 331–338 (2008).
Matei, V. et al. The eNOS cofactor tetrahydrobiopterin improves endothelial dysfunction in livers of rats with CCl4 cirrhosis. Hepatology 44, 44–52 (2006).
Matei, V. et al. Three-day tetrahydrobiopterin therapy increases in vivo hepatic NOS activity and reduces portal pressure in CCl4 cirrhotic rats. J. Hepatol. 49, 192–197 (2008).
Yokoyama, Y. et al. Role of thromboxane A2 in early BDL-induced portal hypertension. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G453–G460 (2003).
Graupera, M. et al. Sinusoidal endothelial COX-1-derived prostanoids modulate the hepatic vascular tone of cirrhotic rat livers. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G763–G770 (2005).
Lin, L. et al. Amelioration of cirrhotic portal hypertension by targeted cyclooxygenase-1 siRNA delivery to liver sinusoidal endothelium with polyethylenimine grafted hyaluronic acid. Nanomed. Nanotechnol. Biol. Med. 13, 2329–2339 (2017).
Guillaume, M. et al. Recombinant human manganese superoxide dismutase reduces liver fibrosis and portal pressure in CCl4-cirrhotic rats. J. Hepatol. 58, 240–246 (2013).
Di Pascoli, M. et al. Resveratrol improves intrahepatic endothelial dysfunction and reduces hepatic fibrosis and portal pressure in cirrhotic rats. J. Hepatol. 58, 904–910 (2013).
Boyer-Diaz, Z. et al. A nutraceutical rich in docosahexaenoic acid improves portal hypertension in a preclinical model of advanced chronic liver disease. Nutrients 11, 1–14 (2019).
De Gottardi, A. et al. Postprandial effects of dark chocolate on portal hypertension in patients with cirrhosis: results of a phase 2, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 96, 584–590 (2012).
Loffredo, L. et al. Effects of dark chocolate on endothelial function in patients with non-alcoholic steatohepatitis. Nutr. Metab. Cardiovasc. Dis. 28, 143–149 (2018).
Gracia-Sancho, J., Villarreal, G., Zhang, Y. & García-Cardeña, G. Activation of SIRT1 by resveratrol induces KLF2 expression conferring an endothelial vasoprotective phenotype. Cardiovasc. Res. 85, 514–519 (2010).
Wu, W. et al. Flow-dependent regulation of Krüppel-like factor 2 is mediated by MicroRNA-92a. Circulation 124, 633–641 (2011).
Gongol, B. et al. Shear stress regulation of miR-93 and miR-484 maturation through nucleolin. Proc. Natl Acad. Sci. USA 116, 12974–12979 (2019).
Verbeke, L. et al. FXR agonist obeticholic acid reduces hepatic inflammation and fibrosis in a rat model of toxic cirrhosis. Sci. Rep. 6, 33453 (2016).
Schwabl, P. et al. The FXR agonist PX20606 ameliorates portal hypertension by targeting vascular remodelling and sinusoidal dysfunction. J. Hepatol. 66, 724–733 (2017).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
Rodríguez-Vilarrupla, A. et al. PPARα activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. J. Hepatol. 56, 1033–1039 (2012).
Tsai, H. C. et al. Beneficial effects of the peroxisome proliferator-activated receptor α/γ agonist aleglitazar on progressive hepatic and splanchnic abnormalities in cirrhotic rats with portal hypertension. Am. J. Pathol. 188, 1608–1624 (2018).
Boyer-Diaz, Z. et al. Pan-PPAR agonist lanifibranor improves portal hypertension and hepatic fibrosis in experimental advanced chronic liver disease. J. Hepatol. https://doi.org/10.1016/j.jhep.2020.11.045 (2020).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Matsuzaki, K. et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology 46, 48–57 (2007).
Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 380, 1450–1462 (2019).
Kin, M., Torimura, T., Ueno, T., Inuzuka, S. & Tanikawa, K. Sinusoidal capillarization in small hepatocellular carcinoma. Pathol. Int. 44, 771–778 (1994).
Wu, L. Q. et al. Phenotypic and functional differences between human liver cancer endothelial cells and liver sinusoidal endothelial cells. J. Vasc. Res. 45, 78–86 (2008).
Geraud, C. et al. Endothelial transdifferentiation in hepatocellular carcinoma: loss of stabilin-2 expression in peri-tumourous liver correlates with increased survival. Liver Int. 33, 1428–1440 (2013).
Thomann, S. et al. YAP orchestrates heterotypic endothelial cell communication via HGF/c-MET signaling in liver tumorigenesis. Cancer Res. 80, 5502–5514 (2020).
Pinato, D. J. et al. Immune-based therapies for hepatocellular carcinoma. Oncogene 39, 3620–3637 (2020).
Wadkin, J. C. R. et al. CD151 supports VCAM-1-mediated lymphocyte adhesion to liver endothelium and is upregulated in chronic liver disease and hepatocellular carcinoma. Am. J. Physiol. Gastrointest. Liver Physiol. 313, G138–G149 (2017).
Knolle, P. A. & Wohlleber, D. Immunological functions of liver sinusoidal endothelial cells. Cell Mol. Immunol. 13, 347–353 (2016).
Wu, K., Kryczek, I., Chen, L., Zou, W. & Welling, T. H. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 69, 8067–8075 (2009).
Matsuzaki, J. et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl Acad. Sci. USA 107, 7875–7880 (2010).
Benedicto, A. et al. Decreased expression of the β2 integrin on tumor cells is associated with a reduction in liver metastasis of colorectal cancer in mice. BMC Cancer 17, 827 (2017).
Benedicto, A. et al. Liver sinusoidal endothelial cell ICAM-1 mediated tumor/endothelial crosstalk drives the development of liver metastasis by initiating inflammatory and angiogenic responses. Sci. Rep. 9, 13111 (2019).
Yu, X. et al. Immune modulation of liver sinusoidal endothelial cells by melittin nanoparticles suppresses liver metastasis. Nat. Commun. 10, 574 (2019).
Sarcognato, S., Garcia-Lezana, T. & Villanueva, A. Mechanisms of action of drugs effective in hepatocellular carcinoma. Clin. Liver Dis. 14, 62–65 (2019).
Llovet, J. M., Montal, R., Sia, D. & Finn, R. S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 15, 599–616 (2018).
Li, W. et al. Regulation of tumorigenesis and metastasis of hepatocellular carcinoma tumor endothelial cells by microRNA-3178 and underlying mechanism. Biochem. Biophys. Res. Commun. 464, 881–887 (2015).
Xu, W. et al. Immunotherapy for hepatocellular carcinoma: recent advances and future perspectives. Ther. Adv. Med. Oncol. 11, 1758835919862692 (2019).
Guixé-Muntet, S. et al. Nuclear deformation mediates liver cell mechanosensing in cirrhosis. JHEP Rep. 2, 100145 (2020).
Scoazec, J. –Y. & Feldmann, G. Both macrophages and endothelial cells of the human hepatic sinusoid express the CD4 molecule, a receptor for the human immunodeficiency virus. Hepatology 12, 505–510 (1990).
Knolle, P. A. et al. Induction of cytokine production in naive CD4+ T cells by antigen- presenting murine liver sinusoidal endothelial cells but failure to induce differentiation toward T(h1) cells. Gastroenterology 116, 1428–1440 (1999).
March, S., Hui, E. E., Underhill, G. H., Khetani, S. & Bhatia, S. N. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology 50, 920–928 (2009).
Muro, H., Shirasawa, H., Kosugi, I. & Nakamura, S. Defect of Fc receptors and phenotypical changes in sinusoidal endothelial cells in human liver cirrhosis. Am. J. Pathol. 143, 105 (1993).
Harb, R. et al. Bone marrow progenitor cells repair rat hepatic sinusoidal endothelial cells after liver injury. Gastroenterology 137, 704–712 (2009).
Ohmori, S. et al. High expression of CD34-positive sinusoidal endothelial cells is a risk factor for hepatocellular carcinoma in patients with HCV-associated chronic liver diseases. Hum. Pathol. 32, 1363–1370 (2001).
Cui, S. et al. Enhanced CD34 expression of sinusoid-like vascular endothelial cells in hepatocellular carcinoma. Pathol. Int. 46, 751–756 (1996).
Zhao, S. et al. Tetramethylpyrazine attenuates sinusoidal angiogenesis via inhibition of hedgehog signaling in liver fibrosis. IUBMB Life 69, 115–127 (2017).
Couvelard, A. et al. Structural and functional differentiation of sinusoidal endothelial cells during liver organogenesis in humans. Blood 87, 4568–4580 (1996).
Volpes, R., van den Oord, J. J. & Desmet, V. J. Adhesive molecules in liver disease. Immunohistochemical distribution of thrombospondin receptors in chronic HBV infection. J. Hepatol. 10, 297–304 (1990).
Hollenbaugh, D. et al. Expression of functional CD40 by vascular endothelial cells. J. Exp. Med. 182, 33–40 (1995).
Knolle, P. A. & Gerken, G. Local control of the immune response in the liver. Immunol. Rev. 174, 21–34 (2000).
Leifeld, L. et al. Enhanced expression of CD80 (B7-1), CD86 (B7-2), and CD40 and their ligands CD28 and CD154 in fulminant hepatic failure. Am. J. Pathol. 154, 1711–1720 (1999).
Scoazec, J.-W. et al. Expression of complement-regulatory proteins in normal and UW-preserved human liver. Gastroenterology107, 505–516 (1994).
Oteiza, A., Li, R., McCuskey, R. S., Smedsrød, B. & Sørensen, K. K. Effects of oxidized low-density lipoproteins on the hepatic microvasculature. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G684–G693 (2011).
van Oosten, M., van de Bilt, E., de Vries, H. E., van Berkel, T. J. C. & Kuiper, J. Vascular adhesion molecule–1 and intercellular adhesion molecule–1 expression on rat liver cells after lipopolysaccharide administration in vivo. Hepatology 22, 1538–1546 (1995).
Volpes, R., van den Oord, J. J. & Desmet, V. J. Immunohistochemical study of adhesion molecules in liver inflammation. Hepatology 12, 59–65 (1990).
Volpes, R., van den Oord, J. J. & Desmet, V. J. Hepatic expression of intercellular adhesion molecule-1 (ICAM-1) in viral hepatitis B. Hepatology 12, 148–154 (1990).
Lohse, A. W. et al. Antigen-presenting function and B7 expression of murine sinusoidal endothelial cells and Kupffer cells. Gastroenterology 110, 1175–1181 (1996).
Øie, C. I. et al. Rat liver sinusoidal endothelial cells (LSECs) express functional low density lipoprotein receptor-related protein-1 (LRP-1). J. Hepatol. 55, 1346–1352 (2011).
Minhajat, R. et al. Organ-specific endoglin (CD105) expression in the angiogenesis of human cancers. Pathol. Int. 56, 717–723 (2006).
Adams, D. H., Burra, P., Hubscher, S. G., Elias, E. & Newman, W. Endothelial activation and circulating vascular adhesion molecules in alcoholic liver disease. Hepatology 19, 588–594 (1994).
Schrage, A. et al. Murine CD146 is widely expressed on endothelial cells and is recognized by the monoclonal antibody ME-9F1. Histochem. Cell Biol. 129, 441–451 (2008).
Connolly, M. K. et al. In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immunogenicity. J. Immunol. 185, 2200–2208 (2010).
Hansen, B., Arteta, B. & Smedsrød, B. The physiological scavenger receptor function of hepatic sinusoidal endothelial and Kupffer cells is independent of scavenger receptor class A type I and II. Mol. Cell. Biochem. 240, 1–8 (2002).
Malovic, I. et al. The mannose receptor on murine liver sinusoidal endothelial cells is the main denatured collagen clearance receptor. Hepatology 45, 1454–1461 (2007).
Asumendi, A., Alvarez, A., Martinez, I., Smedsrød, B. & Vidal-Vanaclocha, F. Hepatic sinusoidal endothelium heterogeneity with respect to mannose receptor activity is interleukin-1 dependent. Hepatology 23, 1521–1529 (1996).
Lai, W. K. et al. Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium: a role for capturing hepatitis C virus particles. Am. J. Pathol. 169, 200–208 (2006).
Bashirova, A. A. et al. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193, 671–678 (2001).
Na, H. et al. Novel roles of DC-SIGNR in colon cancer cell adhesion, migration, invasion, and liver metastasis. J. Hematol. Oncol. 10, 28 (2017).
Ramachandran, P. et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature 575, 512–518 (2019).
Zuo, Y. et al. Novel roles of liver sinusoidal endothelial cell lectin in colon carcinoma cell adhesion, migration and in-vivo metastasis to the liver. Gut 62, 1169–1178 (2013).
Liu, W. et al. Characterization of a novel C-type lectin-like gene, LSECtin: demonstration of carbohydrate binding and expression in sinusoidal endothelial cells of liver and lymph node. J. Biol. Chem. 279, 18748–18758 (2004).
Tang, L. et al. Liver sinusoidal endothelial cell lectin, LSECtin, negatively regulates hepatic T-cell immune response. Gastroenterology 137, 1498–1508.e5 (2009).
Arimoto, J. et al. Expression of LYVE-1 in sinusoidal endothelium is reduced in chronically inflamed human livers. J. Gastroenterol. 45, 317–325 (2010).
Politz, O. et al. Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem. J. 362, 155–164 (2002).
Lautenschlager, I. et al. Distribution of the major histocompatibility complex antigens on different cellular components of human liver. Cell. Immunol. 85, 191–200 (1984).
Uhrig, A. et al. Development and functional consequences of LPS tolerance in sinusoidal endothelial cells of the liver. J. Leukoc. Biol. 77, 626–633 (2005).
Kaipainen, A. et al. The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J. Exp. Med. 178, 2077–2088 (1993).
Ding, B. Sen et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468, 310–315 (2010).
Mandili, G. et al. Mouse hepatocytes and LSEC proteome reveal novel mechanisms of ischemia/reperfusion damage and protection by A2aR stimulation. J. Hepatol. 62, 573–580 (2015).
Ajamieh, H. et al. Acute atorvastatin is hepatoprotective against ischaemia-reperfusion injury in mice by modulating eNOS and microparticle formation. Liver Int. 35, 2174–2186 (2015).
Rabie, M. A., Zaki, H. F. & Sayed, H. M. Telluric acid ameliorates hepatic ischemia reperfusion-induced injury in rats: involvement of TLR4, Nrf2, and PI3K/Akt signaling pathways. Biochem. Pharmacol. 168, 404–411 (2019).
Sabry, M. M., Ramadan, N. M., Al Dreny, B. A., Rashed, L. A. & Abo El Enein, A. Protective effect of apelin preconditioning in a rat model of hepatic ischemia reperfusion injury; possible interaction between the apelin/APJ system, Ang II/AT1R system and eNOS. United European Gastroenterol. J. 7, 689–698 (2019).
Lassailly, G. et al. Nucleotide-binding oligomerization domain 1 (NOD1) modulates liver ischemia reperfusion through the expression adhesion molecules. J. Hepatol. 70, 1159–1169 (2019).
Deleve, L. D. et al. Sinusoidal obstruction syndrome (veno-occlusive disease) in the rat is prevented by matrix metalloproteinase inhibition. Gastroenterology 125, 882–890 (2003).
La Mura, V. et al. Effects of simvastatin administration on rodents with lipopolysaccharide-induced liver microvascular dysfunction. Hepatology 57, 1172–1181 (2013).
Welz, M. et al. Perforin inhibition protects from lethal endothelial damage during fulminant viral hepatitis. Nat. Commun. 9, 4805 (2018).
Abraldes, J. G. et al. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J. Hepatol. 46, 1040–1046 (2007).
Verbeke, L. et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology 59, 2286–2298 (2014).
Pietrosi, G. et al. Human amniotic stem cells improve hepatic microvascular dysfunction and portal hypertension in cirrhotic rats. Liver Int. 40, 2500–2514 (2020).
Hu, L. et al. AMPK agonist AICAR ameliorates portal hypertension and liver cirrhosis via NO pathway in the BDL rat model. J. Mol. Med. 97, 423–434 (2019).
Gracia–Sancho, J. et al. Emricasan ameliorates portal hypertension and liver fibrosis in cirrhotic rats through a hepatocyte–mediated paracrine mechanism. Hepatol. Commun. 3, 987–1000 (2019).
Zhang, R., Chen, J., Liu, D. & Wang, Y. Urotensin II receptor antagonist reduces hepatic resistance and portal pressure through enhanced eNOS-dependent HSC vasodilatation in CCl4-induced cirrhotic rats. Front. Med. 13, 398–408 (2019).
Bravo, M. et al. Restoration of liver sinusoidal cell phenotypes by statins improves portal hypertension and histology in rats with NASH. Sci. Rep. 9, 1–12 (2019).
Hide, D. et al. Simvastatin-loaded polymeric micelles are more effective and less toxic than conventional statins in a pre-clinical model of advanced chronic liver disease. Nanomedicine 29, 102267 (2020).
Meireles, C. Z. et al. Simvastatin attenuates liver injury in rodents with biliary cirrhosis submitted to hemorrhage/resuscitation. Shock 47, 370–377 (2017).
Acknowledgements
The authors acknowledge current and former members of the Liver Vascular Biology Research Group at IDIBAPS-Hospital Clínic de Barcelona and Inselspital-Bern and the Hepatic and Intestinal Immunobiology Group at Miguel Hernández University for their contributions and commitment towards basic and translational liver research; the funding agencies supporting the authors’ research, mainly the Spanish Ministry of Science and Innovation, the Instituto de Salud Carlos III, CIBEREHD, Generalitat de Catalunya, Generalitat Valenciana and the Swiss National Science Foundation (currently AES PI20/00220 and PI16/0967, PID2019-107036RB-I00, AGAUR-SGR2017-517, the CERCA Program, PROMETEO 2016/001 and SNF 320030_189252/1); and the International Society for Hepatic Sinusoidal Research (ISHSR) for its support of sinusoidal research and discussion.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
US National Library of Medicine ClinicalTrials.gov: https://clinicaltrials.gov/
Rights and permissions
About this article
Cite this article
Gracia-Sancho, J., Caparrós, E., Fernández-Iglesias, A. et al. Role of liver sinusoidal endothelial cells in liver diseases. Nat Rev Gastroenterol Hepatol 18, 411–431 (2021). https://doi.org/10.1038/s41575-020-00411-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-00411-3
This article is cited by
-
Construction of in vitro liver-on-a-chip models and application progress
BioMedical Engineering OnLine (2024)
-
Role of imbalanced gut microbiota in promoting CRC metastasis: from theory to clinical application
Cell Communication and Signaling (2024)
-
Cell senescence in liver diseases: pathological mechanism and theranostic opportunity
Nature Reviews Gastroenterology & Hepatology (2024)
-
Autophagic degradation of MVBs in LSECs promotes Aldosterone induced-HSCs activation
Hepatology International (2024)
-
Progress, application and challenges of liver organoids
Clinical Cancer Bulletin (2024)