Abstract
SNAIL is a key transcriptional regulator in embryonic development and cancer. Its effects in physiology and disease are believed to be linked to its role as a master regulator of epithelial-to-mesenchymal transition (EMT). Here, we report EMT-independent oncogenic SNAIL functions in cancer. Using genetic models, we systematically interrogated SNAIL effects in various oncogenic backgrounds and tissue types. SNAIL-related phenotypes displayed remarkable tissue- and genetic context-dependencies, ranging from protective effects as observed in KRAS- or WNT-driven intestinal cancers, to dramatic acceleration of tumorigenesis, as shown in KRAS-induced pancreatic cancer. Unexpectedly, SNAIL-driven oncogenesis was not associated with E-cadherin downregulation or induction of an overt EMT program. Instead, we show that SNAIL induces bypass of senescence and cell cycle progression through p16INK4A-independent inactivation of the Retinoblastoma (RB)-restriction checkpoint. Collectively, our work identifies non-canonical EMT-independent functions of SNAIL and unravel its complex context-dependent role in cancer.
Similar content being viewed by others
Introduction
SNAIL is overexpressed in > 70% of human pancreatic ductal adenocarcinomas (PDAC)1 and a wide number of other tumour entities, such as intestinal, breast, lung and liver cancer2,3,4,5,6. SNAIL expression is believed to be a key driver of tumour aggressiveness and metastasis formation via the induction of an epithelial-to-mesenchymal transition (EMT) program and the subsequent acquisition of stem cell-like features5,7,8,9,10,11,12. Accordingly, it is often correlated with poor prognosis and shortened survival of cancer patients. However, the specific in vivo functions of SNAIL and the role of EMT during tumour progression in different tumour types remain largely unexplored10,13,14,15,16. In an in vivo selection model of highly metastatic PDAC cells, we have demonstrated previously that SNAIL drives EMT and subsequently metastasis formation17. This runs counter to recent findings in an autochthonous mouse model of PDAC showing that Snail deletion does not influence the metastatic phenotype, but sensitizes tumours to chemotherapy13. This prompted us to re-investigate and mechanistically probe the function of SNAIL in vivo by systematic and comprehensive genetic gain- and loss-of-function approaches using a variety of disease-relevant genetically engineered autochthonous mouse models as well as human cancers.
Here, we show context-dependent oncogenic functions of the transcriptional regulator SNAIL in cancer, which are independent of its role as a regulator of the EMT process. SNAIL-induced phenotypes depend on both, the genetic context of the tumour and its tissue of origin, ranging from protective effects that delay tumour onset in intestinal cancer models to a dramatic acceleration of pancreatic cancer development and aggressiveness. Mechanistically, we demonstrate that SNAIL acts as transcriptional activator that bypasses oncogenic KRAS-induced senescence and drives the cell cycle by p16INK4A-independent inactivation of the Retinoblastoma (RB)-restriction checkpoint of senescence, thereby inducing context-dependent cancer progression. This knowledge provides opportunities to target SNAIL-driven cancers, which are a major clinical problem due to their high aggressiveness and lethality.
Results
SNAIL-driven cancer progression is highly context-specific
To investigate the function of SNAIL in different cancer types in vivo, we created a latent Snail allele silenced by a lox-stop-lox (LSL) cassette as a knock-in (KI) at the mouse Rosa26 locus (LSL-R26Snail/+ mouse line, termed SnailKI/+; Supplementary Fig. S1a–c). SNAIL expression was then activated in several genetically engineered murine autochthonous cancer models: i) a PDAC model that depends on Cre-induced expression of oncogenic KRASG12D in the Ptf1a lineage of the pancreas (Ptf1aCre/+;LSL-KrasG12D/+, termed PKrasG12D/+); ii) a classical WNT-driven intestinal cancer model, induced by loss of the tumour suppressor adenomatosis polyposis coli (Apc) due to Cre-mediated deletion of a floxed Apc allele in intestinal epithelial cells (Villin-Cre;Apclox/+, termed VApcΔInt); and iii) two different models of serrated intestinal cancer driven by either oncogenic KRASG12D or BRAFV637E, based on Villin-Cre-induced activation of latent oncogenic KrasG12D (Villin-Cre;LSL-KrasG12D/+ termed VKrasG12D/+) or BrafV637E (Villin-Cre;LSL-BrafV637E/+, termed VBrafV637E/+). In this way we mimicked the acquisition of SNAIL expression in different tumour types and subtypes driven by distinct oncogenes and signalling pathways.
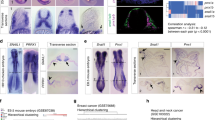
Concomitant transgenic expression of SNAIL and activation of oncogenic KRASG12D in the pancreas of Ptf1aCre/+;LSL-KrasG12D/+;LSL-R26Snail/+ mice (termed PKrasG12D/+;SnailKI/+) accelerated the formation of acinar to ductal metaplasia (ADM) and PDAC precursor lesions (pancreatic intraepithelial neoplasia (PanIN)), and substantially increased cancer development (Fig. 1a–h and Supplementary Fig. S1d–f). Consistent with this, PDAC gene set enrichment was already apparent in one-month old mice with aberrant SNAIL expression (Fig. 1f). All animals in the tumour watch cohort developed PDAC with a median survival of 190 days, compared to 465 days in PKrasG12D/+ animals. Biallelic Snail expression in Ptf1aCre/+;LSL-KrasG12D/+,LSL-R26Snail/Snail mice (termed PKrasG12D/+;SnailKI/KI) drastically reduced survival further to a median of only 64 days (Fig. 1g, h).
a Genetic strategy to activate SNAIL and KRASG12D expression in the pancreas. b Immunoblot analysis of SNAIL protein expression in pancreas of 1-month-old Ptf1aCre/+;LSL-KrasG12D/+ (PKrasG12D/+) and Ptf1aCre/+;LSL-KrasG12D/+;LSL-R26Snail/+ (PKrasG12D/+;SnailKI/+) compound mutant mice. c Representative hematoxylin and eosin (H&E), Alcian blue (AB), Muc5a, CK19 and BrdU stains of acinar to ductal metaplasia (ADM) and pancreatic intraepithelial neoplasia (PanIN) in 1-month-old PKrasG12D/+ and PKrasG12D/+;SnailKI/+ mice. Note the almost complete remodelling of pancreatic tissue in PKrasG12D/+;SnailKI/+ animals. Scale bars, 50 μm. d Quantification of ADM and PanIN progression in % of total lesions at age of one-month (error bars, mean ± SEM; n = 4 per genotype; 3 representative slides per mouse; *p = 0.037, Mann-Whitney two-tailed test). e Quantification of ductal and acinar structures after in vitro culture of acinar explants for 5 days (n = 3 control/PKrasG12D/+ mice, n = 5 PKrasG12D/+;SnailKI/+ mice; mean ± SEM; *p = 0.036, Mann-Whitney two-tailed test). f Gene-set enrichment analysis (GSEA) using mRNA expression profiles of PKrasG12D/+;SnailKI/+ (red) and PKrasG12D/+ (blue) pancreata of 1-month-old mice (n = 2 per genotype) computed and corrected for multiple testing using the Benjamini–Hochberg procedure (for statistical details, please see methods section). Genes were ranked using Signal-to-Noise ratio statistics according to their correlation. Vertical black lines mark the position of each gene in the data set. Normalized Enrichment Score: 3.15; Nominal p-value < 0.0001; False Discovery Rate (FDR) q-value < 0.0001. g Kaplan-Meier survival curves of PKrasG12D/+ (n = 125; 465 days median survival), PKrasG12D/+;SnailKI/+ (n = 42; 190 days median survival) and PKrasG12D/+;SnailKI/KI (n = 28; 64 days median survival) mice (***p < 0.0001, log-rank test, Bonferroni correction). h Macroscopic view and representative H&E and BrdU staining of pancreata with PDAC of three endpoint mice per genotype (tu tumour; st stomach; sp spleen). Scale bars, 50 µm. Note: The PKrasG12D/+ cohort in (g) is the same shown in Figs. 4b, 6e, f and the PKrasG12D/+;SnailKI/+ cohort is the same shown in Figs. 6e, l. Source data of Fig. 1 are provided in the Source Data file.
To assess the impact of SNAIL on tumour development, progression and survival of intestinal cancer, where SNAIL is aberrantly expressed in 78% of cases18, we used three different genetically engineered models that mirror major histopathological and molecular disease subtypes19,20,21 (Fig. 2 and Supplementary Fig. S2). Surprisingly, in the classical Apc loss-of-function model (VApcΔInt) that is driven by activation of canonical WNT signalling20, there was a trend towards prolonged survival (median survival of 419 vs. 355 days; p = 0.12) and reduced number of adenomas and carcinomas per animal upon aberrant SNAIL expression (Fig. 2a–e; p = 0.08 and 0.14, respectively). SNAIL had no major effect on tumour morphology and histopathology (Fig. 2f). This stands in sharp contrast to the dramatic pro-tumorigenic effects observed in the KRAS-driven PDAC model and suggests that SNAIL has context-specific functions in different tumour entities and/or oncogenic backgrounds. To test the hypothesis that SNAIL cooperates specifically with KRAS, but not WNT pathway activation, to induce cancer progression across tumour entities, we activated SNAIL in vivo in a KRASG12D-driven model of serrated intestinal cancer (Fig. 2g). This specific CRC subtype is characterized by a serrated histopathological morphology and progresses through a hyperplasia - serrated adenoma - serrated carcinoma sequence distinct from the classical WNT-driven CRC progression model described by Vogelstein and colleagues22, which is characterized by an adenoma-carcinoma sequence without hyperplasia and serrated morphology23,24. Unexpectedly, aberrant SNAIL expression failed to accelerate oncogenesis. Instead, we observed a weak trend towards prolonged survival in the KRAS-driven intestinal cancer model (median survival of 502 days (VKrasG12D/+;SnailKI/+) vs 354 days (VKrasG12D/+); p = 0.29; Fig. 2h, i). Accordingly, we detected no obvious change in the number of adenomas and carcinomas per animal, as well as in the grading of the tumours (Fig. 2j–n). These data indicate that the tissue of origin and not the oncogenic driver dictates the functional role of SNAIL in tumour progression in vivo.
a Strategy to activate Snail expression in intestinal epithelium in the Villin-Cre;Apclox/+ (termed VApcΔInt) model of intestinal cancer. b Kaplan-Meier survival curves of Villin-Cre;Apclox/+;LSL-R26Snail/+ (termed VApcΔInt;SnailKI/+; n = 10, median survival 419 days) and VApcΔInt mice (n = 8, median survival 355 days); p = 0.12, log-rank test. c qRT-PCR of Snail mRNA expression normalized to Cyclophilin A in intestinal tumours of VApcΔInt (n = 3) and VApcΔInt;SnailKI/+ (n = 4) endpoint mice (Mean ± SEM; **p = 0.0035, unpaired two-tailed t-test with We’ch’s correction). d Number of adenomas in VApcΔInt (n = 8) and VApcΔInt;SnailKI/+ (n = 9) endpoint mice. Mean ± SEM, p = 0.08, Mann-Whitney two-tailed test. e Percentage of carcinoma-bearing mice (left) and carcinoma number (right) in VApcΔInt (n = 8) and VApcΔInt;SnailKI/+ (n = 9) endpoint mice. Left, two-tailed Fisher’s exact test, p > 0.9999; right, Mann-Whitney two-tailed test, mean ± SEM, p = 0.14. f Representative H&E and Ki67 staining of invasive intestinal carcinoma of three VApcΔInt and VApcΔInt;SnailKI/+ mice. g Strategy to express Snail in intestinal epithelium in the Villin-Cre;LSL-KrasG12D/+ model (termed VKrasG12D/+) of intestinal cancer. h Kaplan-Meier survival curves of VKrasG12D/+ (n = 5, median survival 354 days) and Villin-Cre;KrasG12D/+;LSL-R26Snail/+ (termed VKrasG12D/+;SnailKI/+) mice (n = 5, median survival 502 days); p = 0.29, log-rank test. i qRT-PCR of Snail mRNA expression in the intestine of VKrasG12D/+ (n = 5) and VKrasG12D/+;SnailKI/+ endpoint mice (n = 3). Mean ± SEM, **p = 0.005, unpaired two-tailed t-test with Welch’s correction. j Number of adenomas in VKrasG12D/+ (n = 5) and VKrasG12D/+;SnailKI/+ (n = 5) endpoint mice. Mean ± SEM, Mann-Whitney two-tailed test; p = 0.795 (ns, not significant). k Representative H&E and Ki67 staining of intestinal adenoma in three VKrasG12D/+ and VkrasG12D/+;SnailKI/+ mice. l Percentage of carcinoma-bearing mice (left) and carcinoma number (right) in VKrasG12D/+ (n = 5) and VKrasG12D/+;SnailKI/+ (n = 5) endpoint mice. Left, two-tailed Fisher’s exact test (p > 0.9999); right, two-tailed Student’s t-test (p = 0.72), mean ± SEM. m Representative H&E staining of intestinal carcinoma in two VKrasG12D/+ and three VKrasG12D/+;SnailKI/+ mice. n Pathological grading of intestinal carcinomas from VApcΔInt (n = 44), VApcΔInt;SnailKI/+ (n = 21), VKrasG12D/+ (n = 2), VKrasG12D/+;SnailKI/+ (n = 4), VBrafV637E/+ (n = 8) and VBrafV637E/+;SnailKI/+ (n = 6) mice; two-tailed Fisher’s exact test. FC, fold change; ns, not significant; scale bars, 50μm for all images. Source data are provided in the Source Data file.
In the BrafV637E-driven model of serrated intestinal cancer, we observed more invasive carcinomas in animals with aberrant SNAIL expression (Villin-Cre;LSL-BrafV637E/+;SnailKI/+; termed VBrafV637E/+;SnailKI/+) than in VBrafV637E/+ animals, and also more adenomas per animal (Supplementary Fig. S2a–f). Median survival was reduced to 392 days in the VBrafV637E/+;SnailKI/+ cohort vs. 481 days in VBrafV637E/+; mice (Supplementary Fig. S2b; p = 0.0321). However, there was no overt consistent change in the grade of carcinomas observed towards more undifferentiated tumours in any of the three intestinal cancer models (Fig. 2f, m, n and Supplementary Fig. S2f).
These observations support the notion that SNAIL acts as a classical cancer-promoting oncogene particularly in KRAS-driven pancreatic and to a far lesser extent in BRAF-driven intestinal cancer, demonstrating its context-specific functions in cancer.
Snail activation fails to repress E-cadherin and does not induce an EMT program in PDAC
SNAIL is a master regulator of EMT that is associated with cancer aggressiveness, metastasis and decreased patient survival in various cancer types, such as PDAC5,7,14,25,26. We therefore analysed the effect of Snail activation on morphological and transcriptional EMT readouts in our autochthonous cancer models in vivo.
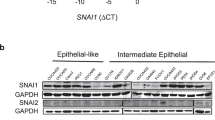
H&E, E-cadherin (Cdh1) and CK19 stainings revealed differentiated and undifferentiated cancers regardless of genotype in our three different PDAC models (Fig. 3a, b and Supplementary Fig. 3a–c). However, histopathological grading indicated a trend towards less undifferentiated/sarcomatoid cancers (Grade 4) in the PKrasG12D/+;SnailKI model in a Snail gene dose-dependent fashion (Fig. 3a and Supplementary Fig. S3a–c). Morphologically, PKrasG12D/+;SnailKI/+ and PKrasG12D/+;SnailKI/KI mice displayed a phenotype characterized by budding of epithelial tumour cells with the formation of small solid tumour cell nests (Supplementary Fig. S3b, c). These areas retained epithelial differentiation, visible as CK19 and E-cadherin positivity, adjacent to tubular ductal structures (Supplementary Fig. S3b). Aberrant SNAIL expression therefore did not drive PDAC development to a more undifferentiated or sarcomatoid phenotype.
a Grading of PKrasG12D/+ (n = 32), PKrasG12D/+;SnailKI/+ (n = 19) and PKrasG12D/+;SnailKI/KI PDAC mice (n = 17). Grade 4=undifferentiated/sarcomatoid86. b Representative staining of SNAIL and E-cadherin in PDAC sections of endpoint mice (n = 3 per genotype). c E-cadherin western blot of pancreas of 1-month-old mice (n = 2 per genotype). d qRT-PCR of Cdh1 mRNA expression normalized to Cyclophilin A (CypA) in pancreas of 1-month-old mice (Control, n = 3; PKrasG12D/+ n = 5; PKrasG12D/+;SnailKI/+ n = 6; PKrasG12D/+;SnailKI/KI n = 4). Mean ± SEM, unpaired two-tailed t-test with Welch’s and Bonferroni correction. e qRT-PCR of Snail (left) and Cdh1 (right) mRNA expression of PDAC cells with or without transgenic Snail expression (epi, epithelial (n = 27); mes, mesenchymal (n = 11)). Each dot represents one PDAC cell line. Mean ± SEM. ***p = 0.0005, unpaired two-tailed t-test with Welch’s correction. f Percentage of PKrasG12D/+ (n = 20), PKrasG12D/+;SnailKI/+ (n = 12) and PKrasG12D/+;SnailKI/KI (n = 8) PDAC cell lines with indicated morphology. g E-cadherin immunocytochemistry (green) of PDAC cells with or without transgenic Snail expression (n = 3 independent experiments). DAPI counterstain (blue). h SNAIL and E-cadherin western blot of PKrasG12D/+;SnailKI/+ (n = 2), PKrasG12D/+;SnailKI/KI (n = 1) (left) and Snail-transduced (RCAS-TVA system) PDAC cells (right) (n = 1). i PDAC cells from PKrasG12D/+, PKrasG12D/+;SnailKI/+ and floxed PKrasG12D/+;SnailKO/KO knock-out (KO) mice (n = 2 per genotype) treated with TGFβ for 72 h. j Total liver (left) and lung (right) metastasis rate of endpoint PKrasG12D/+ (n = 16), PKrasG12D/+;SnailKI/+ (n = 17) and PKrasG12D/+;SnailKI/KI (n = 17) PDAC mice. **p = 0.01 (liver) and p = 0.005 (lung), two-tailed Fisher’s exact test with Bonferroni correction. Nd. Not detected. k Representative H&E, CK19 and Ki67 staining of liver and lung metastases of three PKrasG12D/+;SnailKI/+ mice. l Representative E-cadherin staining of intestinal tumours of VApcΔInt and VApcΔInt;SnailKI/+ endpoint mice (n = 3 per genotype). m qRT-PCR of Cdh1 mRNA expression in intestinal tumours of VApcΔInt (n = 3) and VApcΔInt;SnailKI/+ (n = 4) endpoint mice. Mean ± SEM, unpaired two-tailed t-test with Welch’s correction. n–o qRT-PCR of Cdh1 mRNA expression in colon samples of (n) VKrasG12D/+ (n = 5) and VKrasG12D/+;SnailKI/+ (n = 3), and (o) VBrafV637E/+ (n = 5) and VBrafV637E/+;SnailKI/+ (n = 7) mice. Mean ±SEM, unpaired two-tailed t-test with Welch’s correction. FC Fold change, ns not significant; scale bars 50 μm. Source data are provided in the Source Data file.
SNAIL is known to repress the transmembrane glycoprotein CDH1 (E-cadherin) in several contexts, thereby inducing EMT, migration, invasion and metastasis7,27. Surprisingly, expression of SNAIL had no effect on CDH1 expression in vivo, nor in cultured primary PDAC cell isolates in vitro (Fig. 3b–e). E-cadherin protein and mRNA expression levels were comparable in cells with and without transgenic Snail expression (Fig. 3b–e). There was a marked decrease in the proportion of mesenchymal, and an increase in the epithelial phenotype in tumour cell lines from the PKrasG12D/+;SnailKI/+ model compared to PKrasG12D/+ controls (Fig. 3f and Supplementary Fig. S3d). Thus, there was no shift to a mesenchymal phenotype in the Snail-transgenic cell lines, not even with biallelic Snail expression, although we observed a > 5-fold higher expression of Snail in mesenchymal vs. epithelial PDAC cells without transgene (Fig. 3e, f and Supplementary Fig. S3d). Furthermore, global mRNA expression profiles revealed no EMT-related signatures and epithelial cancer cell isolates from PKrasG12D/+;SnailKI transgenic mice clustered exclusively with epithelial PDAC cells from PKrasG12D/+-driven models, irrespectively of the Trp53 mutational status (Supplementary Fig. S3e). To activate SNAIL expression in established epithelial PDAC cells, we transduced them with a retroviral Snail expression cassette. E-cadherin localization, expression levels, and cell morphology remained unchanged (Fig. 3g, h, Supplementary Fig. S3f), indicating that SNAIL expression alone is not sufficient to induce an overt EMT program in PDAC cells. To investigate whether SNAIL expression influences the ability of PDAC cells to undergo EMT, epithelial PDAC cells isolated from PKrasG12D/+, PKrasG12D/+;SnailKI/+ and from conditional pancreas-specific Snail knock-out (KO) mice (PKrasG12D/+;SnailKO/KO; see also Fig. 4a–d) were treated with the strong EMT-inducer TGFβ. All TGFβ-treated cells, regardless of genotype, underwent rapid EMT and displayed mesenchymal morphology (Fig. 3i), providing genetic evidence that SNAIL is dispensable for EMT-induction.
a Upper panel: Genetic strategy to conditionally delete a floxed Snail allele (Snaillox) in the pancreas of KrasG12D expressing mice. Lower panel: Genotyping PCR to test Snail-deletion (Snail-KO) using DNA from PDAC cells (cells) and tumour tissue with non-recombined stroma (tu) of PKrasG12D/+ and PKrasG12D/+;SnailKO/KO mice (n = 2 per genotype). Lower left panel: Floxed Snail allele (fl): 480 bp, Snail WT allele (WT): 395 bp, deleted Snail allele: no band. Lower right panel: deleted Snail allele (del): 492 bp; floxed Snail and WT allele: no band. b Kaplan-Meier survival curves of indicated genotypes of PKrasG12D/+;SnailKO/KO mice (n = 8; median survival 380 days), compared to PKrasG12D/+ (n = 125; median survival 465 days). ns, not significant, log-rank test. c Representative H&E-stained PDAC tissue sections of PKrasG12D/+;SnailKO/KO mice with undifferentiated (upper panel) and differentiated (lower panel) morphology (n = 6). d Pathological grading of PdACs of PKrasG12D/+ (n = 32) and PKrasG12D/+;SnailKO/KO mice (n = 6). e Genetic strategy to conditionally delete Cdh1 and express Snail in the pancreas of PKrasG12D/+ mice. f Kaplan-Meier survival curves of indicated genotypes of Pdx1-Cre;KrasG12D/+;SnailKI/+;Cdh1KO/+ (n = 8; median survival 78 days), compared to Pdx1-Cre;KrasG12D/+;SnailKI/+ mice (n = 8; median survival 166 days). ***p = 0.0008, log rank test. g qPCR analysis of Cdh1 mRNA expression in PDACs of Pdx1-Cre;KrasG12D/+;SnailKI/+;Cdh1KO/+ (n = 5) and Pdx1-Cre;KrasG12D/+;SnailKI/+ (n = 3) endpoint mice. Cdh1 mRNA levels were normalized to Cyclophilin A. Mean ± SEM, *p = 0.036, Mann-Whitney two-tailed test. FC, fold change. h Representative H&E-stained PDAC tissue sections of indicated Pdx1-Cre;KrasG12D/+;SnailKI/+ and Pdx1-Cre;KrasG12D/+;SnailKI/+;Cdh1KO/+ mice with differentiated and undifferentiated morphology (n = 8 per genotype). i Pathological grading of PDACs in Pdx1-Cre;KrasG12D/+;SnailKI/+ (n = 8) and Pdx1-Cre;KrasG12D/+;SnailKI/+;Cdh1KO/+ mice (n = 8). Note: The PKrasG12D/+ cohort in (b) is the same shown in Figs. 1g, 6e, f. Source data of Fig. 4 are provided in the Source Data file.
Beside the unchanged tumour differentiation status, what is also surprising is that aberrant SNAIL expression did not increase metastasis into liver and lung. Indeed, heterozygous transgenic PKras;SnailKI/+ mice had a trend towards reduced metastases, and biallellic PKras;SnailKI/KI mice significantly fewer (Fig. 3j, k). The capacity of autochthonous tumours to metastasize to liver and lung is thus independent of SNAIL expression, as demonstrated earlier13.
Results from the three different models of intestinal cancer were notably similar. Adenomas and carcinomas were mainly well differentiated and showed no histopathological signs or features of EMT induction regardless of SNAIL expression. Consistent with this, E-cadherin expression was retained in SNAIL-expressing adenomas and carcinomas (Fig. 3l–o), indicating that aberrant SNAIL expression is insufficient to drive a full EMT program in the intestinal epithelium.
To validate our findings in genetic loss-of-function models in vivo, we deleted Snail in KRAS-driven PDAC using the Cre/loxP system (Fig. 4a). As previously demonstrated13, this did not significantly alter PDAC development. Snail knock-out animals displayed similar tumour burden and overall survival to control mice (Fig. 4b–d). Importantly, and in line with our previous findings, loss of Snail did not drive PDAC to a well-differentiated epithelial phenotype nor block development of undifferentiated cancers that had already undergone an EMT program (Fig. 4c, d). Deletion of Snail does not therefore block EMT in vitro or in vivo.
Using a genetic approach to assess E-cadherin/Cdh1 function in the aberrant Snail expression model, we knocked out one floxed allele of the Cdh1 tumour suppressor in Pdx1-Cre;KrasG12D/+;SnailKI/+ mice (Fig. 4e). E-cadherin mRNA levels were reduced in Pdx1-Cre;KrasG12D/+;SnailKI/+;Cdh1lox/+ animals, which showed dramatically shortened median survival (78 days) compared to Pdx1-Cre; KrasG12D/+;SnailKI/+ (166 days) mice (Fig. 4f, g) and a clear shift of the tumours towards an undifferentiated mesenchymal phenotype (Fig. 4h, i). These data reveal that E-cadherin expression and function is independent of SNAIL in PDAC, and suppresses tumour progression and mesenchymal transition in vivo.
SNAIL bypasses senescence during pancreatic carcinogenesis
To discern how aberrant SNAIL expression promotes rapid tumour progression in the PKrasG12D/+;SnailKI/+ PDAC model, independent of overt EMT induction, we investigated early tumour barriers and events in tumour formation. Oncogene-induced senescence (OIS), a feature of KRAS-driven premalignant PanIN lesions of the pancreas28,29, is a cellular stress response, which blocks proliferation so protecting cells from neoplastic transformation30. As reported previously, nearly all PanINs of the PKrasG12D/+ model displayed positive senescence-associated β-galactosidase (SA-β-gal) staining, the most reliable marker of OIS in the pancreas31. In contrast, we observed a dramatically reduced rate of OIS in PKrasG12D/+;SnailKI/+ mice (Fig. 5a, b), which displayed an almost complete loss of OIS in premalignant PanIN lesions (Fig. 5b). In addition, we observed loss of senescence-associated gene sets controlled by the retinoblastoma (RB) tumour suppressor gene in expression profiles of PanIN bearing PKrasG12D/+;SnailKI/+ pancreata (Fig. 5c). In contrast, deletion of Snail in the PKrasG12D/+ model (PKrasG12D/+;SnailKO/KO) induced a strong senescence phenotype as evidenced by SA-β-gal staining of PanIN bearing pancreatic tissue sections (Fig. 5d), indicating that SNAIL is indeed capable of impacting OIS.
a Representative images of senescence-associated β-galactosidase (SA-β-gal) staining of pancreata with PanIN lesions of PKrasG12D/+ (n = 13) and PKrasG12D/+;SnailKI/+ (n = 12) mice. Scale bars, 50 μm. LG, low grade; HG, high grade. b Quantification of SA-β-gal-stained PanIN lesions from PKrasG12D/+ (n = 13) and PKrasG12D/+;SnailKI/+ (n = 12) mice. Mean ± SEM, *p < 0.0001, Mann-Whitney two-tailed test. LG, low grade; HG, high grade. c Gene set enrichment analysis (GSEA) of mRNA expression profiling of 1-month-old mice (n = 2 per genotype) computed and corrected for multiple testing using the Benjamini–Hochberg procedure (for statistical details, please see methods section) shows significant enrichment of Rb1 targets senescent genes (CHICAS_RB1_TARGETS_SENESCENT) in PKrasG12D/+;SnailKI/+ (red) vs. PKrasG12D/+ (blue) pancreata. Normalized Enrichment Score: 2.68; Nominal p-value < 0.001; False Discovery Rate (FDR) q-value < 0.001. d Representative images of SA-β-gal staining of pancreata with PanIN lesions of PKrasG12D/+;SnailKO/KO mice (n = 2). Scale bars, 50 μm. LG Low grade, HG High grade. e Viability of Human Pancreatic Duct Epithelial (HPDE) cells after activation of KRASG12D alone or in combination with SNAIL. HPDE cells transduced with lentiviral constructs for doxycycline-inducible expression of EGFP, KRASG12D ( + mock vector) or KRASG12D + SNAIL were treated with 100 ng ml-1 doxycycline. Viability was assessed by CellTiter-Glo assay after 72 h and is displayed as % of the respective untreated controls. Mean ± SEM. n = 3 independent experiments; *p = 0.033, unpaired two-tailed t-test with Welch’s correction. f Representative images of SA-β-Gal staining of HPDE cells treated for 3 days with doxycycline (100 ng ml-1) to induce activation of EGFP, KRASG12D + mock or KRASG12D + SNAIL. n = 3 independent experiments. The percentage of SA-β-gal+ cells is indicated in the upper right corner. Scale bar, 10 µm. Source data of Fig. 5 are provided in the Source Data file.
To probe SNAIL function in a human model and validate the relevance of our findings for human PDAC development, we employed immortalized human pancreatic duct epithelial (HPDE) lineage cells and engineered them with doxycycline-inducible human KRASG12D and SNAIL expression vectors (Fig. 5e, f and Supplementary Fig. S4a). Activation of oncogenic KRASG12D in HPDE cells induced strong morphological changes as well as OIS, as demonstrated by SA-β-gal staining, accompanied by a decrease in cell viability compared to EGFP transduced controls. In contrast, co-expression of KRASG12D and SNAIL reverted this phenotype almost completely, resulting in a loss of OIS and increased HPDE proliferation (Fig. 5e, f, Supplementary Fig. S4a). PDAC cells isolated from full blown tumours displayed no SA-β-gal staining and senescence phenotype, indicating that OIS is bypassed during early steps of pancreatic carcinogenesis as described previously28 (Supplementary Fig. S4b). Thus, we demonstrate in genetic mouse and engineered human pancreatic epithelial lineage cells that SNAIL expression bypasses OIS, thereby driving pancreatic cancer initiation.
SNAIL overcomes senescence without inactivating the TRP53/p21CIP1 axis
Oncogene-induced senescence is associated with induction of the tumour suppressor TRP53 and/or p16INK4A depending on cellular context and their loss facilitates tumor formation (Supplementary Fig. S4c)30,32. As shown by immunohistochemistry, TRP53 and its functional downstream target p21CIP1 are expressed in premalignant PanIN lesions and full-blown PDAC cells of PKrasG12D/+;SnailKI/+ mice in vivo (Fig. 6a, b). Because mutant gain- or loss-of-function TRP53 is unable to transcriptionally activate p21CIP1 28, these data support the notion that i) expression and function of the TRP53/p21CIP1 axis is intact in our model, and ii) the observed bypass of senescence in PKrasG12D/+;SnailKI/+ pancreata is independent of the TRP53 pathway. To further substantiate these findings, we show upregulation of TRP53 and p21CIP1 protein abundance in full-blown PKrasG12D/+;SnailKI/+ PDAC cells upon treatment with the topoisomerase inhibitor etoposide in vitro (Fig. 6c) and enrichment of TRP53 downstream targets in pancreatic gene expression profiles of PanIN-bearing PKrasG12D/+;SnailKI/+ mice (Fig. 6d).
a, b Representative Trp53 and p21CIP1 stainings of PanINs (a) and PDAC (b) of PKrasG12D/+;SnailKI/+ animals (n = 3 each). c Western blot of TRP53 and p21CIP1 expression in PKrasG12D/+;SnailKi/+ (n = 3) and PKrasG12D/+ (n = 1) PDAC cell lines after 6 h 20 μM etoposide (Eto) or vehicle (DMSO) treatment. d GSEA of mRNA expression of 1-month-old mice (n = 2 per genotype) computed and corrected for multiple testing using the Benjamini–Hochberg procedure (for statistical details, see methods section) shows significant enrichment of KEGG p53 signalling pathway genes in PKrasG12D/+;SnailKI/+ (red) vs. PKrasG12D/+ (blue). Normalized Enrichment Score: 1.98; Nominal p-value < 0.001; False Discovery Rate (FDR) q-value < 0.001. e, f Kaplan-Meier survival curves of PKrasG12D/+ (n = 125; 465 days), PKrasG12D/+;SnailKI/+ (n = 42; 190 days), PKrasG12D/+;Trp53R172H/+ (n = 28; 117 days) and PKrasG12D/+;SnailKI/+;Trp53R172H/+ (n = 22; 90 days) animals. ***p < 0.0001, log-rank test with Bonferroni correction. g Representative p16INK4A staining of PanINs (left) and PDAC (right) of PKrasG12D/+;SnailKI/+ mice (n = 3 each). h qRT-PCR analysis of p16Ink4a (left) and p19Arf (right) mRNA expression in PDAC of endpoint mice (PKrasG12D/+ n = 5; PKrasG12D/+;SnailKI/+ n = 12; PKrasG12D/+;SnailKI/KI n = 12). Mean ± SEM, **p = 0.0094, *p = 0.0365, Mann-Whitney two-tailed test. i Scheme of Cdkn2a gene locus and p16Ink4a genotyping strategy. The non-related proteins p16INK4A and p19ARF are encoded both by the Cdkn2a locus. Red arrows, primer positions. UTR, untranslated region. Scheme according to35. j PCR of p16Ink4a genomic sequence integrity in PDAC cell lines of PKrasG12D/+ (n = 30), PKrasG12D/+;SnailKI/+ (n = 13); PKrasG12D/+;SnailKI/KI (n = 9) endpoint mice. Gabra, internal positive control. k Quantification of PCR analysis of p16Ink4a genomic sequence integrity of data in panel (j). **p = 0.0016, two-tailed Fisher’s exact test. l Kaplan-Meier survival curves of PKrasG12D/+;SnailKI/+ (n = 42; 190 days), PKrasG12D/+;SnailKI/+;p16Ink4a*/+ (n = 22; 156 days) and PKrasG12D/+;SnailKI/+;Cdkn2alox/+ with loss of p16INK4A and p19ARF (n = 17; 108 days). ***p < 0.0001, log-rank test with Bonferroni correction. Note: PKrasG12D/+ cohort in panel (e, f) is the same shown in Figs. 1g, 4b, and PKrasG12D/+;SnailKI/+ cohort of panel e and l is the same shown in Fig. 1g. Source data of Fig. 6 are provided in the Source Data file. Scale bars, 50 μm. ns, not significant.
To test the functional relevance of the TRP53 pathway in an in vivo model, we used a genetically engineered p53 mutant allele, which lacks canonical TRP53 function and p21CIP1 induction33. These animals harbour the murine orthologue of the Li-Fraumeni hotspot mutation R175H at the endogenous murine Trp53 locus (Trp53R172H). In this p53 mutant background, PKrasG12D/+;SnailKI/+;Trp53R172H/+ mice displayed a dramatically accelerated PDAC formation and showed in vivo evidence for synergy between aberrant SNAIL expression and functional p53 inactivation. All animals in the tumour watch cohort developed invasive PDAC. Median survival of PKrasG12D/+;SnailKI/+;Trp53R172H/+ mice (90 days) was significantly less than PKrasG12D/+;SnailKI/+ (190 days) and PKrasG12D/+;Trp53R172H/+ (117 days) mice (Fig. 6e, f). These data demonstrate that TRP53 and SNAIL function, at least in part, via non-overlapping and non-redundant pathways and tumour barriers.
The cell cycle regulator p16INK4A, which blocks G1 to S phase progression of the cell cycle, is implicated in OIS and tumour suppression and is frequently lost during KRAS-driven pancreatic carcinogenesis (Fig. 6g–k and Supplementary Fig. S4c)32. It is encoded by the Cdkn2a locus together with p19ARF, which is involved in TRP53 activation by inhibiting Mdm234. Immunohistochemistry revealed strong expression of p16INK4A in PanIN lesions and full-blown PDAC of PKrasG12D/+;SnailKI/+ mice (Fig. 6g), indicating intact p16INK4A function. Furthermore, p16Ink4a/p19Arf mRNA expression in PDAC tissue was greater in PKrasG12D/+;SnailKI/+ than PKrasG12D/+ animals, and increased further in PKrasG12D/+;SnailKI/KI animals (Fig. 6h). We therefore tested the integrity of the genomic sequence of the p16Ink4a locus in cell lines isolated from the three different models (Fig. 6i, j). We observed loss of p16Ink4a in 60% of PKrasG12D/+ PDAC cell lines tested. In contrast, only 38,5% of PKrasG12D/+;SnailKI/+ and none of the PKrasG12D/+;SnailKI/KI cell lines were deficient for p16Ink4a (Fig. 6j, k). These findings suggest that Snail might inactivate the p16-RB controlled cell cycle/OIS restriction checkpoint downstream of p16Ink4a, to block OIS and drive PDAC progression.
To test this hypothesis, we again used genetic in vivo models of pancreatic tumour evolution. We crossed PKrasG12D/+ and PKrasG12D/+;SnailKI/+ animals with either p16Ink4a* mutant loss of function mice35, or Cdkn2alox mice with conditional knock-out of both p16Ink4a and p19Arf gene products36. All animals of the tumour watch cohort developed invasive PDAC. However and as hypothesized, PKrasG12D/+;SnailKI/+;p16Ink4a*/+ mice showed no statistically significant difference in median survival compared to PKrasG12D/+;SnailKI/+ mice (Fig. 6l). These data demonstrate at the level of genetics that p16Ink4a and SNAIL function via overlapping pathways/shared tumour barriers. In line, PKrasG12D/+;SnailKI/+;Cdkn2alox/+ mice with loss of both, the p16Ink4a and the p19ARF tumour suppressor barriers, had dramatically reduced median survival (Fig. 6l), confirming our data obtained with the Trp53R172H mutant in vivo model (Fig. 6e, f).
Taken together, our mechanistic dissection of three important tumor suppressor genes of KRAS-driven carcinogenesis provides strong in vivo genetic evidence that SNAIL bypasses senescence and drives tumour development independent of TRP53 inactivation and downstream of the p16Ink4a cell cycle restriction check-point, e.g., via blocking the RB-controlled senescence-pathway.
Consistent with this hypothesis, we observed increased proliferation of premalignant PanIN lesions, as evidenced by BrdU labelling and Ki67 staining (Fig. 7a, b and Supplementary Fig. S4d). In addition, we demonstrate the enrichment of genes that promote cell cycle progression by gene expression profiling of PanIN bearing PKrasG12D/+;SnailKI/+ vs. PKrasG12D/+ pancreata of one-month-old mice (Fig. 7c), as well as increased DNA damage and apoptosis induction by aberrant SNAIL expression (Supplementary Fig. S4e–i). It has been previously shown that E2F activation in response to RB inactivation leads to p53-dependent apoptosis37,38,39,40,41. Thus, combining p53 inactivation with aberrant SNAIL expression accelerates tumorigenesis most likely due to the prevention of p53-dependent apoptosis.
a Representative BrdU stainings of ADMs and PanINs of PKrasG12D/+ (n = 11) and PKrasG12D/+;SnailKI/+ (n = 5) mice. b Percentage of BrdU positive cells in ADMs/PanINs of PKrasG12D/+;SnailKI/+ (n = 5) and PKrasG12D/+ (n = 11) mice. Mean ± SEM, **p = 0.006, unpaired two-tailed t-test with Welch’s correction. c GSEA 1-month-old mice (n = 2 per genotype) computed and corrected for multiple testing using Benjamini–Hochberg procedure (statistical details see methods) shows significant enrichment of KEGG cell-cycle genes in PKrasG12D/+;SnailKi/+ (red) vs. PKrasG12D/+ (blue) pancreata. Normalized Enrichment Score (NES): 2.38; Nominal p-value < 0.001; False Discovery Rate (FDR) q-value < 0.001. d, e Representative stainings (d) and quantification of pRb-S807/811 positive PanINs (e) of PKrasG12D/+;SnailKI/+ and PKrasG12D/+ animals (n = 4 per genotype). Mean ± SEM, *p = 0.029, Mann-Whitney two-tailed test. f GSEA corrected for multiple testing using Benjamini–Hochberg procedure shows significant enrichment of hallmark E2F target genes in PKrasG12D/+;SnailKI/+ (red) vs. PKrasG12D/+ (blue) in 1-month-old mice (n = 2 per genotype). NES: 2.44; Nominal p-value < 0.001; FDRq-value < 0.001. g Chromatin-immunoprecipitation (ChIP) of SNAIL binding to E-boxes of indicated promoters in PKrasG12D/+ (n = 3) and PKrasG12D/+;SnailKI/+ (n = 3) PDAC cell lines ± Trp53 mutation as indicated. %input calculation; IgG, negative control. Mean ± SEM. *p = 0.05, Mann-Whitney one-tailed test. h Ccnb1 and E2f3 promoter activity in PKrasG12D/+ (n = 3) and PKrasG12D/+;SnailKI/KI (n = 3) PDAC cells (three independent experiments). Mean ± SEM, *p = 0.026, unpaired one-tailed Student’s t-test. i Volcano-plot representing enriched proteins in PKrasG12D/+;SnailKI/+ PDAC cells upon Snail or IgG ChIP, respectively, followed by mass-spectrometry based quantification of co-precipitated proteins (two independent experiments in triplicate for each condition). x-axis, log2-fold change; y-axis, adjusted p-value of the two-sample t-test (two-tailed, FDR < 0.05, s0 = 1). 141 of 1039 proteins were significant vs. IgG control. Pathway-enrichment analysis of significant proteins with MSigDB Hallmarks (upper right panel) and Reactome (lower panel) databases. j Scheme of genome-scale CRISPR/Cas9 negative-selection screen (PKrasG12D/+;SnailKO/KO, PKrasG12D/+;SnailKI/+, PKrasG12D/+;SnailKI/KI cells; n = 4). k Differences in β-scores (SnailKI overexpression (OE) - SnailKO knock-out (KO) cells) were used for Reactome database enrichment analysis (FDR ≤ 0.05; difference in β score < −1). Scale bars, 50 μm. ns not significant. LG low grade; HG high grade. Source data of Fig. 7 provided in Source Data file.
Phosphorylation and thereby inactivation of the tumour suppressor RB by cyclin dependent kinase (CDK)/cyclin complexes, which is negatively regulated by p16Ink4a, is an essential step to bypass the firm G1-phase arrest of senescent cells and initiate cell cycle activation and proliferation42. RB inactivation leads to dissociation of the E2F complex thereby activating the expression of E2F target genes, which can then drive cell cycle progression as well as apoptosis, as observed in our model40,41,43. In line, we detected phosphorylation of RB and thus inactivation of the RB-controlled cell cycle/senescence checkpoint (Fig. 7d, e) in PanIN lesions together with concomitant enrichment of E2F target genes (Fig. 7f) in the PKrasG12D/+;SnailKI/+ model. Although gene expression profiling of bulk tissues is confounded by the increased number of PanIN lesions in PKrasG12D/+;SnailKI/+ mice at an age of one month, and gene sets that promote cell cycle progression overlap substantially with E2F target genes, our various different in vivo models and datasets provide strong evidence for the hypothesis that SNAIL might bypass senescence downstream of p16Ink4a via interference with the RB-controlled cell cycle/senescence restriction check-point.
SNAIL is a transcriptional regulator of the cell cycle
Cyclins, such as cyclin A1 (CCNA1) or cyclin B1 (CCNB1) interact with cyclin-dependent kinases (CDK) to phosphorylate and thereby inactivate RB, which releases E2F transcription factors to enter the nucleus and activate transcription of target genes essential for the transition from G1 to S phase and progression of the cell cycle. Because our data provide genetic evidence that SNAIL bypasses senescence and drives the cell cycle in vivo, we validated the upregulation of important cell cycle regulators and downstream effectors. Transcriptomic profiling and qRT-PCR analysis revealed a marked increase in mRNA expression of several cell cycle-related genes, including cyclins and cyclin-dependent kinases, in pancreata with aberrant SNAIL expression compared with PKrasG12D/+ controls (Supplementary Fig. S5a, b), in line with the proliferation and E2F signature shown in Fig. 7c, f.
SNAIL, as a transcription factor, functions primarily via binding to promoter and enhancer regions of the target genes. To test whether SNAIL binds to promoter regions of genes that positively regulate the cell cycle, we analysed publicly available chromatin immunoprecipitation (ChIP)-seq cancer cell line datasets11 and compared them to significantly enriched genes in pancreas of one-month-old PKrasG12D/+;SnailKI/+ mice. Of 69 enriched genes implicated in proliferation and cell cycle progression, 62 were bound by SNAIL in their promoter region (Supplementary Fig. S5c). Calculation of the odds ratio for this enrichment (8.53; Supplementary Fig. S5c) strongly suggested that the presence of the vast majority (89.9%) of genes in the SNAIL-bound fraction was not due to chance. Thus, SNAIL has a clear preference for binding to genes that promote cell cycle progression. To validate these findings functionally in the PKrasG12D/+;SnailKI/+ PDAC model, we performed ChIP experiments using cell lines isolated from PKrasG12D/+ and PKrasG12D/+;SnailKI/+ mice with and without Trp53 mutation, and selected SNAIL targets from the ChIP-seq study. This revealed binding of SNAIL to E-boxes in promoter regions of multiple genes, such as Ccnb1, Ccnb2, Ccnd1, E2f2 and E2f3 (Fig. 7g and Supplementary Fig. S5d), all known to drive the cell cycle and bound by SNAIL in the published ChIP-seq data (Supplementary Fig. S5c). In line with the ChIP-seq data set, we did not observe binding of SNAIL to the Ccna1 promoter region, even though this cyclin mRNA is overexpressed in the PKrasG12D/+;SnailKI/+ model (Supplementary Fig. S5a). To test whether SNAIL is indeed capable of activating the expression of the identified cell cycle regulators, we performed promoter reporter assays using again our primary PDAC cell cultures. While aberrant SNAIL expression did not increase reporter gene activity of cyclin D1 (Ccnd1) and E2f2 promoter reporter constructs, cyclin B1 (Ccnb1; p = 0.026), cyclin B2 (Ccnb2; p = 0.0423) and E2f3 (p = 0.065) demonstrated evidence of gene activation (Fig. 7h and Supplementary Fig. S5e).
These findings support a context-specific function of SNAIL in vivo, which bypasses senescence by direct binding and activating important positive regulators of the cell cycle. To gain more insights into SNAIL-mediated gene activation, we studied potential co-regulators from chromatin cross-linked to SNAIL by proteomics and performed chromatin immunoprecipitation coupled to mass spectrometry (ChIP-MS). This allowed us to identify 141 significantly enriched putative chromatin-bound partners of SNAIL (Fig. 7i and Supplementary Data 1). Activating transcription factors, such as NFKB2 and SMAD2, nuclear receptors and coactivators, chromatin remodelers and histone modifiers, such as KDM1A were enriched together with SNAIL (Fig. 7i and Supplementary Data 1). Subsequent pathway analysis of significantly enriched genes revealed regulators of the cell cycle, such as MYC and E2F targets, and genes involved in progression through the G2M checkpoint (Fig. 7i). Further, several genes, e.g., RNA-binding proteins, involved in RNA pol II transcription and transcription termination, RNA metabolism, processing, splicing and transport were significantly enriched together with SNAIL, indicating a role of SNAIL in alternative splicing and RNA biology, which might contribute to its function in regulating cell cycle progression. Of note, NFKB/E2F interactions have previously been shown to control the timing of cell proliferation44 and SMAD2 silencing decreased PDAC cell division45. In addition, KDM1A has been recently linked to gene activation46 and PDAC cell cycle progression47. These data suggest that multiple interactions of SNAIL might contribute to its context-dependent role as a transcriptional activator and regulator of the cell cycle.
To identify functional relevant targets of SNAIL driving PDAC progression and maintenance, we performed pooled genome-wide CRISPR/Cas9 loss-of-function (viability) screens with cell lines isolated from PKrasG12D/+;SnailKI mice with aberrant SNAIL expression compared to PDAC cells from SNAIL-deficient PKrasG12D/+;SnailKO/KO animals (Fig. 7j, k, Supplementary Fig. S6 and Supplementary Data 2). We determined differential sensitivity scores48 by calculating the difference in β-score between SNAIL overexpressing and deficient cells and further analysed genes displaying a negative differential sensitivity score, pointing to enhanced depletion in PKrasG12D/+;SnailKI cells. This allowed us to identify 238 statistically significant genes, whose depletion led to the specific drop-out of cells with aberrant SNAIL expression.
Pathway analysis of these hits enabled us to uncover the specific genetic dependencies and vulnerabilities of cells with aberrant SNAIL expression, such as cell cycle and checkpoint regulation, E2F targets, NF-kB signaling, RNA pol II transcription and chromatin modifications (Fig. 7k and Supplementary Fig. S6b, c). Importantly, we observed that these pathways and processes correlated to a high degree with the ChIP-MS analysis of Fig. 7i, thereby cross-validating our findings by functional genetic screens. To probe the contribution of the differentially expressed cell cycle regulators for cell viability of SNAIL-driven PDAC, we correlated the β-scores of the PKrasG12D/+;SnailKI cells with their gene expression levels. As shown in Supplementary Fig. S6d, 32 out of a total of the 49 differentially expressed cell cycle-related genes of Supplementary Fig. S5 displayed a significant differential β-score indicating selective depletion in cells with aberrant SNAIL expression.
Aberrant SNAIL expression is prognostic in human PDAC
To assess a potential EMT-independent link of aberrant SNAIL expression with cell cycle progression in human PDAC, we analysed SNAIL and CDH1 abundance in differentiated and undifferentiated human PDAC specimens and cell lines, and analysed data of resected primary tumours49. We observed high levels of SNAIL expression in both differentiated and undifferentiated human PDAC specimens and cell lines in accordance with our findings in genetic mouse models corroborating EMT-independent functions of SNAIL also in human PDAC (Fig. 8a, b). In addition, we discerned undifferentiated specimens, which lack both, SNAIL and CDH1 expression (Fig. 8a). Gene expression profiling of primary resected differentiated human PDAC specimen with high CDH1 expression revealed no correlation with SNAIL abundance (p = 0.77; Fig. 8c). However, we observed a strong positive correlation between SNAIL and the expression of important cell cycle regulators, such as CDK4, CCNA1, CCND1, CCND2, CCNE1 and E2F3 (Fig. 8d). Strikingly, many of these genes have been identified as direct targets of SNAIL in murine PDAC (Fig. 7 and Supplementary Fig. S5) and functionally validated by genome wide CRISPR/Cas9-based negative selection screens (Fig. 8d right panel). In addition, tumours with high SNAIL expression were strongly associated with a poorer disease-free survival (DFS) and overall survival (OS) after surgical resection (Fig. 8e, f). Further, we observed a trend towards resistance against chemotherapy with gemcitabine in a small cohort with available clinical data of the resected PDAC patients (6 gemcitabine sensitive and 24 resistant PDAC cases; p = 0.065) (Fig. 8g). While intriguing and consistent with published experimental studies in mice, reporting that Snail knockout sensitizes PDAC tumours to gemcitabine treatment13, these human studies will require larger sample sets and prospective analyses in future.
a Left: Representative staining of SNAIL in human PDAC sections of differentiated (G1/2) and undifferentiated tumours (G3/4) of the Human Protein Atlas version 20.1 (http://www.proteinatlas.org)85. Images and clinical data are available from https://www.proteinatlas.org/ENSG00000124216-SNAI1/pathology/pancreatic+cancer#ihc. Right: Representative SNAIL and CDH1 staining in serial sections of an independent PDAC patient cohort with G1/2 and G3/4 tumours (n = 11). Scale bars, 50 µm. b qRT-PCR of SNAIL (SNAI1; left) and E-cadherin (CDH1; right) mRNA expression of human PDAC cell lines (epithelial, n = 9; mesenchymal, n = 16). Mean ± SEM. Left: ns, not significant (p = 0.835) unpaired two-tailed t-test; Right: p < 0.0001, Mann-Whitney two-tailed test. c SNAI1 (left) and CDH1 (middle) expression across SNAI1 quartile group of resected primary human PDAC samples (Q1 to Q4, n = 88). Mean ± SEM. p = 1.7e-26 (SNAI1) and 0.37 (CDH1), one-way ANOVA-test. Right: Pearson correlation of SNAIL and CDH1 expression across all PDAC samples (n = 88). Two-tailed Pearson correlation coefficient r = 0.031, r2 = 0.0009949, p = 0.7705 (not significant), 95% confidence interval −0.1791 to 0.2395. d Left: Heatmap of top 20 significant cell cycle related genes with the highest variance across SNAI1 quartile groups. Colour code, row-wise scaling of RNA expression. Row clustered using hierarchical clustering on Euclidean distance. Note: 11 out of the 20 human genes overlap with the cell cycle related genes of Supplementary Fig. S5b identified in the murine model (depicted in red). Right: Cross-species validation of cell cycle regulators. β-scores from genome-wide CRISPR/Cas9 negative-selection screen of PKrasG12D/+;SnailKI cell lines are indicated. Genes with FDR-q value > 0.05 are marked with an X on the bar. e, f Kaplan-Meier analysis of PDAC patients (n = 111). e Disease-Free Survival (DFS) p = 0.0178 log-rank test and (f) Overall Survival (p = 0.0094 log-rank test), in samples with aberrant high SNAI1 expression (Q4) compared to the rest (Q1-3). g Correlation of SNAIL expression with gemcitabine treatment resistance of human PDAC patients. Density distribution of SNAI1 mRNA expression across gemcitabine resistant (n = 6) or sensitive (n = 24) samples, p = 0.065, two-tailed Wilcoxon rank test. Source data of Fig. 8 are provided in the Source Data file.
Discussion
Understanding the specific in vivo functions of SNAIL, which is aberrantly expressed in a wide variety of epithelial cancers and often correlated with poor patient outcome, is crucial for improving patient stratification and clinical interventions5,14. SNAIL has been extensively and convincingly characterized as a master regulator of the embryonic EMT program, which triggers cancer cell plasticity, migration and metastatic spread in various tumour types5,8,11. In contrast, little is known about EMT-independent oncogenic functions of SNAIL in cancer initiation and progression. Specifically, non-redundant functions of this EMT transcription factor (TF) in autochthonous tumours remain elusive26. We employed complex genetic in vivo modelling to address this important question in a comprehensive and systematic manner across different cancer types, oncogenic drivers and pathways. This enabled us to discover a complex non-redundant context-specific EMT-independent framework of SNAIL function in epithelial PDAC that bypasses oncogenic KRAS-induced senescence and drives the cell cycle by p16INK4A-independent inactivation of the RB-restriction checkpoint of senescence and the cell cycle. Importantly, our data demonstrate that SNAIL acts in this context as a transcriptional activator, rather than via its canonical function as a transcriptional repressor50; it binds directly to the canonical E-boxes of a variety of important cell cycle regulators, such as cyclins, CDKs and E2F TFs to drive the cell cycle as evidenced by ChIP experiments and reporter gene assays. This allows sustained proliferation of epithelial PDAC cells and thus tumour progression independent of overt EMT induction and contrasts with WNT- and KRAS-driven intestinal cancer subtypes, which are refractory towards aberrantly expressed SNAIL. In line with our findings, SNAIL has recently been shown to be dispensable for the EMT process in PDAC, which is controlled by the EMT transcription factor Zeb151.
Collectively, our studies constitute a comprehensive analysis of SNAIL function in cancer. SNAIL has been identified and validated as an intrinsic cancer driver, and there are strong indications that both, the cell and tissue of origin as well as the genetic context dictates the function of SNAIL as a cancer driver. This improves our understanding of the diverse in vivo functions of SNAIL and will enable SNAIL downstream targets to be defined within the cell cycle machinery in epithelial PDAC. Our discovery has potentially important clinical implications, since it provides a framework for patient stratification and opens avenues for therapeutic interventions. Therapeutics targeting the cell cycle have been developed in recent years, which provide efficient opportunities to block cell cycle progression, e.g., via blockade of CDK4/6 activity52. Furthermore, considering the association of SNAIL with Gemcitabine resistance, it would seem worthwhile evaluating whether targeting SNAIL downstream effectors can improve the efficacy of current therapies for PDAC. Such treatment options are urgently needed. PDAC is a highly lethal and refractive disease with overall 5-year survival rates below 9%53.
Methods
Mouse strains and tumour models
LSL-KrasG12D/+ 54,55, Pdx1-Cre54, Ptf1aCre/+ 56,57, LSL-Trp53R172H/+ 33,58, LSL-R26TvailacZ/+ 59, Cdkn2alox/+ 36, p16Ink4a*/+ 35, Villin-Cre60, LSL-BrafV637E/+ 21, Apclox/+ 20, Cdh1lox/+ 61, Snaillox/+ 62 mice have been previously reported. All strains were on a mixed C57Bl/6 J;129S6/SvEv genetic background and interbred to obtain compound mutant mice of both sexes that develop autochthonous tumours in the pancreas and intestine. The sex, substrain, age and number of all animals analysed in this study in every experiment is provided in the “Source Data” Excel file. All animal studies were conducted in compliance with European guidelines for the care and use of laboratory animals and were approved by the Institutional Animal Care and Use Committees (IACUC) of the local authorities of Technische Universität München and the Regierung von Oberbayern (animal protocol number: ROB-55.2-2532.Vet_02-17-79 and 55.2-1-54-2532-31-11). The maximal tumour size/burden permitted by the IACUC and the local authorities (Regierung von Oberbayern) is 1.5 cm in diameter, which was not exceeded in our study. Euthanasia was performed by cervical dislocation. Animals were housed under specific pathogen- free conditions (SPF) in a dedicated facility, with a light-dark cycle of 12:12 hours, a relative air humidity between 45 and 65% and a temperature between 20 and 24 °C.
Construction of the targeting vector and generation of the LSL-Rosa26 Snail mouse line
Rosa26 targeting by a knock-in strategy was performed based on the pROSA26–1 plasmid59. Murine Snail cDNA (Snai1 cDNA; Library: IRAV MGC Mouse verified full length amplified cDNA; Clone: IRAVp968A0443D6, German Science Centre for Genome Research) was cloned into the targeting vector 3´ of a loxP-flanked transcriptional and translational stop element (loxP-stop-loxP, LSL) with a neomycin resistance cassette (Supplementary Fig. S1a). The targeting vector was linearized, electroporated into W4/129S6 embryonic stem cells, selection with 250 µg/ml geneticin imposed, and correctly targeted cell clones identified by PCR59. Gene targeting was verified by Southern blot with an external 32P-labeled 5´ probe and EcoRV digested genomic DNA (Supplementary Fig. S1b). The Southern blot images were processed with an Amersham automatic Hyperprocessor (Amersham Biosciences). Two verified cell clones were injected into C57BL/6 J blastocysts (Polygene). Germ-line transmission was achieved in 2/2 clones harbouring the targeted allele. The mice were genotyped using a 3-primer PCR strategy (ref. 59, Table 1 and Supplementary Fig. S1c).
Transduction of tumour cells using the RCAS-TVA system
To overexpress SNAIL in cell lines via the RCAS-TVA system59,63, the murine Snail cDNA was amplified and cloned into the pCR-Blunt II-TOPO vector (Invitrogen). After AatII/NdeI digestion, Snail cDNA was ligated to a modified pENTR/D-TOPO (Invitrogen) vector carrying dsRed under the control of the EF1α promoter 3’ to the Snail insertion side. Further cloning into RCASBP(A)-Att-CCDB-Att (modified from RCASBP(A), kindly provided by Stephen H. Hughes) was performed using the GatewayR LR Clonase (Invitrogen) mix to generate the final retroviral construct.
To generate RCAS vectors, the chicken fibroblast cell line DF-1 (American Type Culture Collection # CRL-12203 (RRID:CVCL_0570) was transfected using Superfect (Qiagen) with 2.5 µg purified RCAS plasmid. Fresh virus-containing supernatant was filtered through 0.45 μm pores and added to the medium of murine tumour cells carrying the TVA receptor59. Transduction with fresh supernatant was repeated daily until 80% cells showed expression of the dsRed reporter gene (Supplementary Fig. S3f).
Histology and immunohistochemistry
Murine tissue specimens were fixed overnight in 4% buffered formalin, dehydrated, embedded in paraffin and sectioned (2.5 µm thick). ADM, PanIN lesions and intestinal adenomas and carcinomas were quantified using haematoxylin and eosin (H&E)-stained sections64. Quantification was carried out blinded to the genotype.
TUNEL staining was conducted using the In Situ Cell Death Detection Kit, POD (Roche). Alcian blue staining and immunohistochemistry were performed using standard procedures64. If not stated otherwise, antigen retrieval was performed in citrate buffer, pH 6.0 in a microwave oven. The following primary antibodies were used: Muc5a (antigen retrieval Tris/EDTA pH 9.0, 1:200, 45M1 #MS-145-P1, Neomarkers), Cytokeratin 19 (1:300, TROMA 3 Developmental Studies Hybridoma Bank), E-cadherin (1:100, #610181, BD Biosciences), Rabbit anti-Ki67 (1:50, #MA5-14520, SP6, ThermoFischer), p-γ-H2AX (1:500, #05-636, Millipore), Cleaved Caspase 3 (1:250, #9664, Cell Signaling Technology), BrdU (1:500, #MCA2060, AbD Serotec), pRB (1:100, #8516, Cell Signaling Technology), p16INK4A (1:50, #sc-1661, Santa Cruz Biotechnology), TRP53 (1:400, #NCL-p53-CM5p, Novocastra/Leica Microsystems), p21CIP1 (1:50, #sc-397, Santa Cruz Biotechnology), SNAIL (1:50, #3879, Cell Signaling Technology).
For BrdU assay, 5 mg/kg 5-bromo-2’-deoxyuridine (BrdU), dissolved in sterile PBS, was injected intraperitoneally into animals 2 h before sacrifice.
Images were acquired with AxioVision Rel 4.8 and Aperio ImageScope v12.3.3. For counting of BrdU-, pRB-, and p-γ-H2AX-positive cells in ADMs and PanINs, one- to three-months old PKrasG12D/+;SnailKI/+ mice and one-month to two-year old PKrasG12D/+ animals were used. Quantification was carried out blinded to the genotype.
Metastasis quantification
At sacrifice, abdominal organs and lungs were investigated macroscopically for metastases17,65. Macroscopic pictures were taken using a Stemi SV 11 stereomicroscope (Zeiss) and processed with AxioVision Rel 4.8 software. For microscopic quantification, at least ten series of sections (100 µm between each series) of paraffin-embedded lungs and livers were prepared, H&E stained and investigated for the presence of metastases. Quantification was carried out blinded to the genotype.
Senescence-associated β-galactosidase (SA-β-gal) analysis
To obtain cryosections, tissue was fixed in 4% buffered formalin for 2 h, dehydrated in a sucrose series (15% sucrose for 4 h and 30% sucrose overnight), embedded in Tissue-Tek (Sakura Finetek), snap-frozen and sectioned (6 µm). Sections were dried overnight, and staining performed using the Senescence β-Galactosidase Staining Kit (Cell Signaling Technology)66. ADMs and PanINs from three different slides per pancreas were assessed for SA-β-gal quantification. The number of cells displaying positive SA-β-gal staining was counted and divided by the total number of cells per PanIN lesion, and expressed as % positive cells per lesion in the respective graphs. Quantification was done blinded to the genotype. SA-β-gal staining of cells in culture was performed as recommended by the manufacturer of the the Senescence β-Galactosidase Staining Kit and quantified blinded to the genotype as % positive cells.
Immunocytochemistry
Cells were washed 3 times in cold PBS and fixed 10 min in cold methanol. Washing was repeated following permeabilisation in 0.3% Triton X-100 in PBS for 10 min. Blocking was done for 30 min at 37 °C with 5% donkey serum before incubation with the primary E-cadherin antibody (1:80, #AF748, R&D Systems) for 2 h at 37 °C. After washing 3 times with PBS, cells were incubated with secondary antibody (Alexa Fluor® 488 donkey anti-goat, 1:100, # A-11055, Invitrogen) for 30 min at 37 °C. Washing was repeated and cells were covered with a cover glass using Vectashield mounting medium with DAPI. Images of the slides were acquired with AxioVision Rel 4.8 and Aperio ImageScope v12.3.3.
Cell lines and cell culture
Primary PDAC cell cultures were isolated from autochthonous mouse PDAC tumours and cultured in DMEM medium with 10% Fetal Bovine Serum17,65. The following human PDAC cell lines from from the American Type Culture Collection (ATCC), German Collection of Microorganisms and Cell Cultures (DSMZ) or Cell bank were used: AsPC-1 (CVCL_0152) ATCC# CRL-1682; Capan-2 (CVCL_0026) ATCC# HTB-80; CFPAC-1 (CVCL_1119) ATCC# CRL-1918; DAN-G (CVCL_0243) DSMZ# ACC 249; HPAC (CVCL_3517) ATCC# CRL-2119; HPAF-II (CVCL_0313) ATCC# CRL-1997; Hs 766 T (CVCL_0334) ATCC# HTB-134; HuP-T4 (CVCL_1300) DSMZ# ACC 223; IMIM-PC1 (CVCL_4061) https://www.cellosaurus.org/CVCL_4061; KP-4 (CVCL_1338) Cell bank# RCB1005; MIA PaCa-2 (CVCL_0428) ATCC# CRL-1420; Panc 02.03 (CVCL_1633) ATCC# CRL-2553; Panc 03.27 (CVCL_1635) ATCC# CRL-2549; Panc 04.03 (CVCL_1636) ATCC# CRL-2555; Panc 05.04 (CVCL_1637) ATCC# CRL-2557; Panc 08.13 (CVCL_1638) ATCC# CRL-2551; PANC-1 (CVCL_0480) ATCC# CRL-1469; Panc 10.05 (CVCL_1639) ATCC# CRL-2547; PaTu 8902 (CVCL_1845) DSMZ# ACC 179; PaTu 8988 s (CVCL_1846) DSMZ# ACC 204; PL45 (CVCL_3567) ATCC# CRL-2558; PSN1 (CVCL_1644) ATCC# CRL-3211; SU.86.86 (CVCL_3881) ATCC# CRL-1837; SW1990 (CVCL_1723) ATCC# CRL-2172; YAPC (CVCL_1794) DSMZ# ACC 38267. The Human Pancreatic Duct Epithelial (HPDE) cells (H6c7; RRID: CVCL_0P38) were obtained from Kerafast (#ECA001-FP), and the avian TVA receptor positive chicken embryonic fibroblast cell line DF-1 (RRID:CVCL_0570) from ATCC# CRL-12203.
All human cell lines were authenticated through STR or SNP profiling (last correct authentication in 2022). All murine cell lines were re-genotyped and tested for correct recombination of the respective alleles (last re-genotyping in 2022). The chicken fibroblast cell line DF-1 was authenticated by genotyping PCR for presence of the avian TVA receptor. All cells used were cultivated for less than 30 passages and tested negative for mycoplasma contamination by PCR. The electrophoresis DNA gel pictures were acquired with the Gel Doc™ XR + system (Biorad).
PDAC cell doubling time calculation
For PDAC cell doubling time calculation, 1000–2000 cells per well were seeded out in triplicates in 96 Well plates. Cell viability was determined on the following day (Day 0) and again 72 hours after the initial measurement (Day 3) by CellTiter-Glo assay (Promega). Doubling times were calculated blinded to the genotype by the formula given in equation number 1:
Equation Number 1:
Stimulation of PDAC cells with TGFβ
Cells at 50% confluence were cultured for 24 h in FCS-free DMEM before treatment with 10 ng/ml TGFβ or vehicle (10 nM citric acid, 2 mg/ml BSA). Cell morphology was documented after 72 h.
Acinar explants and acinar-ductal metaplasia (ADM) assay
Directly after sacrifice, pancreata of one-month old mice were injected with 2 ml Collagenase P solution (1.33 mg/ml Collagenase P (Roche) in HBSS (Gibco)), cut out, minced with a scalpel and gently shaken for 30 min at 37 °C in 5 ml Collagenase P solution. All subsequent steps were performed at 4 °C in a laminar flow cabinet and all centrifugation steps were carried out for 3 min at 180 x g. Cells were resuspended in 10 ml 5% FBS in HBSS and incubated 10 min for sedimentation of the cellular fraction. Supernatant was aspirated carefully, and the pellet was washed 3 times with 5% FBS in HBSS. Cells in 10 ml 5% FBS in HBSS were transferred into a new tube through a 100 µm cell strainer, slowly laid over 20 ml 30% FBS in HBSS and centrifuged. Cells were resuspended in 2 ml recovery medium (acinar cell medium, see below, with 30% FCS), incubated at 37 °C for 1 h, centrifuged and resuspended in a 1:1 mixture of acinar cell medium (containing 0.1% bovine serum albumin, 0.2 mg/ml soybean trypsin inhibitor (Sigma), 1% ITS premix (Corning), 50 µg/ml bovine pituitary extract (ThermoFisher), 0.1% FBS, 0.5% penicillin/streptomycin, 0.25 µg/ml Fungizone antimycotic (ThermoFisher) in Waymouth’s medium (Gibco) and rat tail collagen type I (Corning). Per pancreas, cells were seeded into 16 wells of a 48-well plate on a previously prepared collagen layer (final collagen concentration 2.5 mg/ml) and covered with another collagen layer before adding acinar cell medium. Medium was changed every 24 h.
Five days after seeding, images were acquired with AxioVision Rel 4.8 software and the percentage of ductal structures of the total amount of acinar explants was determined by counting 5 microscopic fields of view at 100x magnification for each pancreas. Quantification was blinded to the genotype.
Whole cell lysates and western blot
Whole cell lysates and proteins from tissue were harvested and subjected to western blotting using the following primary antibodies: SNAIL (1:500, #3895, Cell Signaling Technology), E-cadherin (1:2000, #610181, BD Biosciences), HSP90 (1:250, #sc-13119, Santa Cruz Biotechnology), TRP53 (1:1000, #NCL-p53-CM5p, Novocastra/Leica Microsystems), p21CIP1 (1:200, #sc-397, Santa Cruz Biotechnology), β-Actin (1:4000, #A5316, Sigma-Aldrich) and α-Tubulin (1:5000, #T9026, Sigma-Aldrich). The western blot images were collected using the Odyssey infrared imaging system with the Odyssey Software V1.2 (Li-Cor Biosciences).
Quantitative real-time PCR (qPCR)
Total RNA was isolated from tissues and cell lines with the RNeasy Kit (Qiagen) following reverse transcription (Applied Biosciences). 1 µg RNA was used for generation of 50 µl cDNA. qPCR was performed with the StepOnePlus real time PCR system (Applied Biosystems) by using the StepOne Software v2.3. Power SYBR Green PCR Master Mix was used in a 25 µl mixture containing 100 nM of each primer. Only primers with an amplification efficiency between 1.8 and 2.2 were applied. qPCR primers are given in Table 2. mRNA expression was analysed on 5 µl of 1:5 diluted cDNA in either duplicate or triplicate. All expression values were normalized to the housekeeping gene Cyclophilin A (CypA) or GAPDH. A melt curve was performed after each run to check for unwanted primer dimerization. Data analysis was carried out using Excel version 16.65 (Microsoft Corporation) according to 2-ΔΔCt method.
Analysis of p16Ink4a genomic sequence integrity
Genomic DNA was isolated from PDAC cell lines using the DNeasy Blood & Tissue Kit (Qiagen). The integrity of the p16Ink4a locus was tested by PDAC amplification and gel electrophoresis using 10 ng DNA and the primers given in Table 3. GABRA was used as positive control.
Quantitative chromatin immunoprecipitation (ChIP)
ChIP was performed using SimpleChIP Enzymatic Chromatin IP Kit (#9003, Cell Signaling Technology) according to the manufacturer’s protocol using SNAIL antibody (1:50, #3879, Cell Signaling Technology) and rabbit IgG (1:50, #2729, C15D3, Cell Signaling Technology) as negative control and H3 (1:50, #2650, Cell Signaling Technology) as positive control. Binding of SNAIL to the DNA regions of interest was determined by qPCR using the primers listed in Table 4 and analysed by the percent input method68.
Chromatin immunoprecipitation coupled to mass spectrometry (ChIP-MS)
ChIP was performed using freshly prepared cell lysates of murine primary PDAC cells (P144) isolated from the PKrasG12D/+;SnailKI/+ model. For each condition, three biological replicates were used. Briefly, 107 cells were fixed in 1% v/v formaldehyde (FA) in Phosphate buffered saline (PBS) at room temperature (RT) for 10 min. After incubating with 1.25 M glycine and washing twice with PBS, the samples were resuspended in IP buffer (50 mM Tris-HCl pH 8.0, 100 mM NaCl, 5 mM EDTA pH 8.0, 1.7% v/v Triton X-100, 0.3% v/v SDS, protease and phosphatase inhibitors). Subsequently, chromatin was sonicated (4 × 10 cycles at 4 °C; 30 s ON, 30 seconds OFF each cycle) using a Bioruptor Plus (Diagenode, Denville, NJ, United States) to an average size of 500 bp. The samples were then centrifuged at 3500 x g for 20 min at 4 °C, and the supernatant was used for estimation of protein concentration with the bicinchoninic acid (BCA) assay based on the manufacturer’s instructions (Pierce™ BCA Protein Assay Kit, Thermo Fisher Scientific). 1 mg of extract from each sample was used for immunoprecipitation with anti-Snail Rabbit mAb (1:50, #3879, C15D3, Cell Signaling) or anti-IgG Rabbit Ab (#2729, Cell Signaling, 5 µg) by incubating overnight at 4 °C on a rotating wheel. The next day, the antibody-bound complexes were precipitated with protein A + G-coupled magnetic beads (Millipore, Sigma) washed three times with low salt buffer (50 mM HEPES pH 7.5, 140 mM NaCl, 1% v/v Triton X-100), once with high salt buffer (50 mM HEPES pH 7.5, 500 mM NaCl, 1% v/v Triton X-100) and once with TBS. Immunoprecipitates were eluted from the beads and digested after incubating with the freshly prepared elution buffers I (2 M Urea, 50 mM Tris-HCl pH 7.5, 2 mM Dithiothreitol, 20 µg/mL Trypsin) and II (2 M Urea, 50 mM Tris-HCl pH 7.5, 10 mM Chloroacetamide) for 30 min and 5 min at 37 °C, respectively. Both eluates were combined and further incubated overnight at 25 °C. Subsequently, the tryptic peptides were acidified with 1% v/v Trifluoroacetic acid (TFA) solution, and transferred on top of styrene-divinylbenzene reverse-phase sulfonate (SDB-RPS; three layers) in-house made StageTips for desalting. Finally, they were concentrated (45 °C, 20 min) using a centrifugal evaporator (Eppendorf) until dryness, and analszed by liquid chromatography-coupled to mass spectrometry (LC-MS/MS) after reconstitution in MS compatible buffer [2% acetonitrile (ACN) v/v, 0.1 % v/v TFA]69.
LC-MS/MS analysis and data processing
All peptide samples were measured in a single-shot manner in a Q-Exactive HF-X hybrid quadrupole-orbitrap mass spectrometer (Thermo Fisher Scientific) after peptide separation by high-performance liquid chromatography (nanoLC 1200, Thermo Fisher Scientific) using a 50 cm reversed-phase column (made in house, packed with 1.9 µm C18 ReproSil particles). Peptides were eluted over a 90-minute-gradient from 0% to 95% buffer B (0.1% formic acid and 80% ACN) with a flow rate of 300 nL/minute.
Full scans were obtained from 300 to 1650 m/z with a target value of 3 × 106 ions at a resolution of 60,000 at 200 m/z. The fifteen most intense ions (Top15) of each full scan were fragmented with higher-energy collisional dissociation (HCD) (target value 1 × 105 ions, maximum injection time 120 ms, isolation window 1.4 m/z, underfill ratio 1%), and fragments were detected in the Orbitrap mass analyzer at a resolution of 15,000 at 200 m/z.
Mass spectrometry data analysis
Raw MS data files were processed using MaxQuant (version 1.6.1.0) to calculate peptide intensities with the integrated Andromeda search engine with FDR < 0.01 both at the protein and peptide levels. Oxidized methionine (M) and acetylation (protein N-terminus) were set as variable modifications, and carbamidomethyl (C) as fixed modification. Only peptides with a minimal length of seven amino acids were considered and the “match between runs” option was enabled for the biological replicates within each condition with a matching time window of 0.7 min. For protein and peptide identification, the UniProt database from mouse (September 2014) including 51,210 entries were used. Each raw file and biological replicate was treated as one independent experiment.
For bioinformatics analyses, the Perseus platform70 (version 1.6.7.0) was used. The R environment (version 3.6.2) was used for data visualization. Pre-processing of the label-free proteomics data included: (a) exclusion of reverse, potential contaminants and peptide identified only by site, (b) log2 transformation of peptide intensities, and (c) peptides without intensity values in less than 67% of the values in at least one bait group were filtered out. Missing values were replaced from a normal distribution window (width 0.3, downshift 1.8 standard deviations).
For statistical analysis, the two-sample t-test was implemented (FDR < 0.05, s0 = 1) and identified 141 proteins as significant (out of 1039 quantified proteins) between the two conditions (bait vs. negative control).
Lentivirus production and transduction
For lentivirus production, HEK293FT cells were transfected using TransIT-LT1 (Mirus Bio LLC) transfection reagent according to the manufacturer’s instructions with lentiviral packaging plasmids psPAX2 and pMD2.G and the respective lentiviral transfer plasmids. Virus-containing supernatant was collected 48 and 72 h after transfection, filtered through a 0.45 µm filter and stored at −80 °C. Cell lines were transduced in the presence of 8 μg ml−1 polybrene and selected with the respective selection antibiotic (Puromycin or Blasticidin).
Inducible activation of KRASG12D and Snail in HPDE Cells
Inducible expression vectors for GFP and mutant KrasG12D based on the pInducer20 vector system have been used in HPDE cells32. To generate an inducible expression system for Snail, cDNA of human SNAIL was cloned into the pInducer20-Blast (RRID:Addgene 109334)71 and verified by sequencing.
HPDE cells were cultivated in Keratinocyte-SFM medium (ThermoFisher). To induce expression of the respective target genes, cells were treated for the indicated time points with 100 ng ml−1 doxycycline.
Promoter reporter assays
The promoter reporter constructs for E2F2 (#MPRM38445-LvPG04-GC), E2F3 (MPRM40957-LvPG04-GC), CCNB1 (MPRM49947-LvPG04-GC) and CCNB2 (MPRM39222-LvPG04-GC) were purchased from BioCat GmbH (Heidelberg, Germany) and transduced into PKrasG12D/+ and PKrasG12D/+;SnailKI/KI PDAC cell lines.
The Secrete-Pair Dual Luminescence Assay Kit (#LF032-GC, BioCat GmbH, Heidelberg, Germany) was used according to the manufacturer’s instructions to analyse reporter activity blinded to the genotype. In brief, cell lines transduced with the reporter constructs were seeded in 6-well plates and medium was collected after 24 h. For measurement of Gaussia Luciferase (GLuc), 10 µL of the collected culture medium were pipetted in duplicates into a white opaque 96-well plate. GLuc Assay Working Solution was prepared with Buffer GL-S according to the manufacturer’s instructions and 100 µL was added per well. After incubation for 1 min at room temperature, luminescence was measured using a CLARIOstar microplate reader (BMG Labtech GmbH). For transduction normalization, Secreted Alkaline Phosphatase (SEAP) was measured. Therefore, medium was heated at 65 °C for 15 min and SEAP Assay Working Solution prepared according to the manufacturer’s protocol. 100 µL SEAP Assay Working Solution were added to 10 µL medium per sample in duplicates in a white opaque 96-well plate. Luminescence was measured after 5 min incubation at room temperature in a CLARIOstar microplate reader (BMG Labtech GmbH). Ratios of the mean Gaussia Luciferase (GLuc) to the mean Secreted Alkaline Phosphatase (SEAP) were calculated to determine reporter activity.
pGL3 Basic luciferase reporter plasmids containing Cyclin D1 (Ccnd1) promoter fragments (RRID:Addgene_32727 and RRID:Addgene_32726)72 were used to determine Cyclin D1 promoter activity blinded to the genotype. PKrasG12D/+ and PKrasG12D/+;SnailKI/KI PDAC cell cultures were transfected with the reporter constructs using Effectene Transfection Reagent (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. In each sample, 40 ng phRL-TK (Promega) Renilla Luciferase control reporter vector was co-transfected as an internal control for transfection efficiency. The medium was changed on the next day and the Dual-luciferase reporter assay system (Promega) was used according to the manufacturer’s instructions to determine luciferase activity 48 h post transfection.
Genome-wide CRISPR/Cas9 negative selection screens
PKrasG12D/+;SnailKI and PKrasG12D/+;SnailKO/KO Cas9-expressing cell lines were used to perform the genome-wide CRISPR/Cas9 loss-of-function screens at 500x coverage, as in48. Briefly, cells were transduced with the Brie library (Addgene #73633) and the screens were performed in side-by-side duplicates. At the end of the experiment, cells were harvested, genomic DNA was isolated using the Blood & Cell Culture DNA Maxi Kit (Qiagen), and sgRNA libraries were generated. The pooled sgRNA libraries were sequenced using an Illumina NextSeq 500 (custom read and indexing primers spiked in) with a depth of 35 Mio reads48. MAGeCK v0.5.9.473 was used for downstream analysis and β-scores calculated with the maximum likelihood estimation (mle) method by employing data of non-targeting control sgRNAs. The obtained β-score depicts enrichment (positive score) or depletion (negative score) of the sgRNAs compared to their initial abundance. To calculate selectively depleted genes in the PKrasG12D/+;SnailKI model, the difference in the β-score between PKrasG12D/+;SnailKI and PKrasG12D/+;SnailKO/KO PDAC cells was determined. Enrichments were performed on the genes annotated as non-essential, presenting an FDR ≤ 0.05 in both PKrasG12D/+;SnailKI and PKrasG12D/+;SnailKO/KO cells, and showing a difference in beta score ≤ −1 by using the MSigDB v7.1 gene sets provided by Broad Institute, Massachusetts Institute of Technology and Harvard University as in48.
Gene expression profiling and gene set enrichment analysis (GSEA)
mRNA was extracted using the RNeasy Kit (Qiagen). Quality was checked using the Experion RNA StdSens analysis Kit (Bio-Rad). For mRNA analysis of PDAC cell lines, 250 ng of each sample were processed with the GeneChip 3’ IVT express Kit (Affymetrix). Fragmentation and hybridization to GeneChip mouse genome 430 2.0 array chips (Affymetrix) was performed by the Institute for Medical Microbiology, Immunology and Hygiene, Technische Universität München. For mRNA analysis of pancreatic and PDAC tissues, 500 ng isolated mRNA was processed with the Ambion WT expression Kit (Applied Biosystems). Fragmentation and labelling were performed using the GeneChip WT terminal labelling Kit (Affymetrix). Hybridization to GeneChip mouse gene 1.0 ST array chips (Affymetrix) was carried out by the Institute for Medical Microbiology, Immunology and Hygiene, Technische Universität München. Data were collected with an Affymetrix Scanner 3000 7 G and the Affymetrix GeneChip Command Console Software (AGCC). The expression intensity of each gene was determined by using the Affymetrix Microarray Analysis Suite (MAS) 5.0 software.
All analyses were carried out using R version 3.1.274 and Bioconductor version 3.075. Microarray data were processed with the RMA method76, following quantile normalization77. For initial correlation analysis, pairwise Pearson correlation was computed on the normalized intensity values. Differential gene expression between mesenchymal and epithelial cell lines was analysed with Limma version 3.22.078. A probe set was considered to be differentially expressed with a Benjamini-Hochberg adjusted79 p-value of 0.05 and an absolute fold change >2. Annotations were downloaded from ENSEMBL (GRCm38.p3)80. The top 50 upregulated or downregulated genes were hierarchically clustered with Ward’s minimum variance method81. The dissimilarities between samples were squared before cluster updating as implemented in R.
We performed gene set enrichment analysis (GSEA)82 using GSEA v3.0 jar package and MSigDB v6.2 gene sets provided by Broad Institute of Massachusetts Institute of Technology and Harvard University. GSEA was conducted with RMA normalized microarray data. Parameters were set as follows: phenotype was defined as “PKrasG12D/+;SnailKI/+“ versus “PKrasG12D/+“; gene sets were permuted for 1000 times; enrichment statistic for scoring was set as “weighted” and genes were ranked based on “t-Test” metric; other parameters were set as default. The cut-off for a significant FDR q-value as well as NOM p-value was set at 0.05.
Human primary PDAC cohort
RNAseq data from resected primary PDAC tumours are accessible via International Cancer Genome Consortium (ICGC), as reported in Connor et al.49 were analysed using R version 4.2.12. Adapters and bad quality reads were trimmed with Trimmomatic version 2.3883. Filtered reads were aligned to human genome (hg19) and quantified using STAR version 2.6.084. Raw read count per gene was normalized to the library size using Counts Per Million (CPM). The resected cohort (n = 177) was divided into epithelial-like (n = 88) and mesenchymal-like samples (n = 89) based on CDH1 expression. Samples with high CDH1 expression, i.e., above CDH1 median expression, were classified as epithelial subtype, whereas low CDH1 expression samples were considered as mesenchymal subtype. Epithelial samples were further divided according to the expression of SNAI1 mRNA using quartile distribution were Q1 and Q4 contain the samples with the lowest and highest SNAI1 expression respectively. Differential expression of cell cycle-related genes across Q1 to Q4 subgroups was determined using an ANOVA test. Top 20 significantly altered cell-cycle genes across SNAI1 quartiles are depicted on the heatmap (see Fig. 8d).
Among the epithelial-like PDAC cohort, 32 patients received adjuvant chemotherapy with Gemcitabine. 24 were sensitive and 6 resistant. The response of 2 patients is unknown. The differential expression of SNAI1 mRNA between Gemcitabine resistant (n = 6) and sensitive (n = 24) samples was assessed using Wilcoxon rank test.
Survival analysis was performed on the complete resected dataset (111/177 samples with follow-up annotation). Samples with aberrant SNAI1 expression (Quantile 4, Q4) were compared to the other samples (Q1 to Q3). Difference of survival was determined with a Cox proportional hazards regression model. P-value below 0.05 was considered as significant.
To assess the expression of SNAIL in human PDAC sections of differentiated (G1/2) and undifferentiated tumours (G3/4), we used publicly available immunohistochemical stainings of the Human Protein Atlas version 20.185, which are available from https://www.proteinatlas.org/ENSG00000124216-SNAI1/pathology/pancreatic+cancer#ihc, as well as a cohort of PDAC tissue samples purchased from Biomax.us (PA961a Pancreatic cancer tissue array with normal pancreatic tissue, https://www.biomax.us/PA961a) and stained for SNAIL and CDH1.
Additional statistical methods and data analysis
No statistical method was used to determine sample size a priory. In Supplementary Fig. 1e, one outlier in the PKrasG12D/+;SnailKI/+ cohort that differed significantly from the other observations, has been removed from the analysis (please see Source Data file, Supplementary Fig. 1e, for detailed information on outlier definition). No other data were excluded from other datasets. Randomization was not appropriate for experiments described in this study. The investigators were blinded to allocation during experiments and outcome assessment. Graphical depiction and statistical analysis were performed with GraphPad Prism v5 and v8. Unless otherwise indicated, all data were determined from at least 3 independent experiments and expressed as mean values ± SEM. For comparisons between data sets, log-rank test, Fisher’s exact test, one- or two-tailed t-test with or without Welch’s correction or Mann-Whitney test were employed and resulting p-values are indicated in the respective figures. The significance level was set to 0.05. If more than one statistical test was performed simultaneously on a single data set, a Bonferroni-adjusted significance level was calculated to account for the increased possibility of false-positive results. Percentage of mice with intestinal carcinoma, cell morphology and p16Ink4a genomic sequence integrity were compared by Fisher’s exact test. Metastasis rates were compared by Fisher’s exact test followed by multiple testing correction with Benjamini Hochberg procedure. Survival analysis of the mouse models was carried out by the log-rank test.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All microarray data generated in this study have been deposited in the ArrayExpress database (https://www.ebi.ac.uk/arrayexpress/) with accession # E-MTAB-8173 and # E-MTAB-8174. Protein data generated by mass spectrometry have been deposited in the PRIDE database under accession code # PXD038726 The ChIP-MS and genome wide CRISPR/Cas9 negative selection screen data generated in this study are provided in the Supplementary Data file 1 and 2, respectively. In addition, source data (raw and processed data) for all data presented in graphs are provided as source data with this paper. Mice and cell lines are available from the corresponding author on request. Source data are provided with this paper.
References
Hotz, B. et al. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin. Cancer Res.: Off. J. Am. Assoc. Cancer Res. 13, 4769–4776 (2007).
Moody, S. E. et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell 8, 197–209 (2005).
De Craene, B. et al. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 65, 6237–6244 (2005).
Olmeda, D., Jorda, M., Peinado, H., Fabra, A. & Cano, A. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene 26, 1862–1874 (2007).
Peinado, H., Olmeda, D. & Cano, A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7, 415–428 (2007).
Kroepil, F. et al. Snail1 expression in colorectal cancer and its correlation with clinical and pathological parameters. BMC Cancer 13, 145 (2013).
Cano, A. et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 (2000).
Puisieux, A., Brabletz, T. & Caramel, J. Oncogenic roles of EMT-inducing transcription factors. Nat. Cell Biol. 16, 488–494 (2014).
Lamouille, S., Xu, J. & Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 15, 178–196 (2014).
Zhang, K. et al. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat. Cell Biol. 15, 677–687 (2013).
Ye, X. et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature 525, 256–260 (2015).
Ni, T. et al. Snail1-dependent p53 repression regulates expansion and activity of tumour-initiating cells in breast cancer. Nat. Cell Biol. 18, 1221–1232 (2016).
Zheng, X. et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527, 525–530 (2015).
Nieto, M. A., Huang, R. Y., Jackson, R. A. & Thiery, J.P. Emt: 2016. Cell 166, 21–45 (2016).
Vega, S. et al. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 18, 1131–1143 (2004).
De Craene, B. et al. Epidermal Snail expression drives skin cancer initiation and progression through enhanced cytoprotection, epidermal stem/progenitor cell expansion and enhanced metastatic potential. Cell Death Differ. 21, 310–320 (2014).
von Burstin, J. et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology 137, 371 e361–365 (2009).
Roy, H. K., Smyrk, T. C., Koetsier, J., Victor, T. A. & Wali, R. K. The transcriptional repressor snail is overexpressed in human colon cancer. Digestive Dis. Sci. 50, 42–46 (2005).
Bennecke, M. et al. Ink4a/Arf and oncogene-induced senescence prevent tumor progression during alternative colorectal tumorigenesis. Cancer cell 18, 135–146 (2010).
Cheung, A. F. et al. Complete deletion of Apc results in severe polyposis in mice. Oncogene 29, 1857–1864 (2010).
Rad, R. et al. A genetic progression model of Braf(V600E)-induced intestinal tumorigenesis reveals targets for therapeutic intervention. Cancer cell 24, 15–29 (2013).
Vogelstein, B. et al. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 319, 525–532 (1988).
Rustgi, A. K. BRAF: a driver of the serrated pathway in colon cancer. Cancer cell 24, 1–2 (2013).
Schneider, G., Schmidt-Supprian, M., Rad, R. & Saur, D. Tissue-specific tumorigenesis: context matters. Nat. Rev. Cancer 17, 239–253 (2017).
Tan, T. Z. et al. Epithelial-mesenchymal transition spectrum quantification and its efficacy in deciphering survival and drug responses of cancer patients. EMBO Mol. Med. 6, 1279–1293 (2014).
Stemmler, M. P., Eccles, R. L., Brabletz, S. & Brabletz, T. Non-redundant functions of EMT transcription factors. Nat. Cell Biol. 21, 102–112 (2019).
Batlle, E. et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2, 84–89 (2000).
Morton, J. P. et al. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc. Natl Acad. Sci. USA 107, 246–251 (2010).
Guerra, C. et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer cell 19, 728–739 (2011).
Collado, M. & Serrano, M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer 10, 51–57 (2010).
Caldwell, M. E. et al. Cellular features of senescence during the evolution of human and murine ductal pancreatic cancer. Oncogene 31, 1599–1608 (2012).
Mueller, S. et al. Evolutionary routes and KRAS dosage define pancreatic cancer phenotypes. Nature 554, 62–68 (2018).
Olive, K. P. et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860 (2004).
Bardeesy, N. et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc. Natl Acad. Sci. USA 103, 5947–5952 (2006).
Krimpenfort, P., Quon, K. C., Mooi, W. J., Loonstra, A. & Berns, A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413, 83–86 (2001).
Aguirre, A. J. et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 17, 3112–3126 (2003).
Symonds, H. et al. p53-Dependent apoptosis suppresses tumor growth and progression in vivo. Cell 78, 703–711 (1994).
Williams, B. O. et al. Cooperative tumorigenic effects of germline mutations in Rb and p53. Nat. Genet. 7, 480–p484 (1994).
Morgenbesser, S. D., Williams, B. O., Jacks, T. & DePinho, R. A. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature 371, 72–74 (1994).
Pan, H. et al. Key Roles for E2F1 in Signaling p53-dependent apoptosis and in cell division within developing tumors. Mol. Cell 2, 283–292 (1998).
Tsai, K. Y. et al. Mutation of E2f-1 Suppresses Apoptosis and Inappropriate S Phase Entry and Extends Survival of Rb-Deficient Mouse Embryos. Mol. Cell 2, 293–304 (1998).
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003).
Chen, H. Z., Tsai, S. Y. & Leone, G. Emerging roles of E2Fs in cancer: an exit from cell cycle control. Nat. Rev. Cancer 9, 785–797 (2009).
Ankers, J. M. et al. Dynamic NF-κB and E2F interactions control the priority and timing of inflammatory signalling and cell proliferation. eLife 5, e10473 (2016).
Ungefroren, H. et al. Differential roles of Smad2 and Smad3 in the regulation of TGF-β1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: control by Rac1. Mol. Cancer 10, 67 (2011).
Clark, E. A. et al. GR and LSD1/KDM1A-Targeted Gene Activation Requires Selective H3K4me2 Demethylation at Enhancers. Cell Rep. 27, 3522–3532.e3523 (2019).
Hou, X. et al. KDM1A and KDM3A promote tumor growth by upregulating cell cycle-associated genes in pancreatic cancer. Exp. Biol. Med. (Maywood) 246, 1869–1883 (2021).
Falcomatà, C. et al. Selective multi-kinase inhibition sensitizes mesenchymal pancreatic cancer to immune checkpoint blockade by remodeling the tumor microenvironment. Nat. Cancer 3, 318–336 (2022).
Connor, A. A. et al. Integration of genomic and transcriptional features in pancreatic cancer reveals increased cell cycle progression in metastases. Cancer Cell 35, 267–282.e267 (2019).
Nieto, M. A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 3, 155–166 (2002).
Krebs, A. M. et al. The EMT-activator Zeb1 is a key factor for cell plasticity and promotes metastasis in pancreatic cancer. Nat. Cell Biol. 19, 518–529 (2017).
Turner, N. C. et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 379, 1926–1936 (2018).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Hingorani, S. R. et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 (2003).
Jackson, E. L. et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 15, 3243–3248 (2001).
Nakhai, H. et al. Ptf1a is essential for the differentiation of GABAergic and glycinergic amacrine cells and horizontal cells in the mouse retina. Dev. (Camb., Engl.) 134, 1151–1160 (2007).
Kawaguchi, Y. et al. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat. Genet. 32, 128–134 (2002).
Hingorani, S. R. et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005).
Seidler, B. et al. A Cre-loxP-based mouse model for conditional somatic gene expression and knockdown in vivo by using avian retroviral vectors. Proc. Natl Acad. Sci. USA 105, 10137–10142 (2008).
Madison, B. B. et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 (2002).
Derksen, P. W. et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437–449 (2006).
Murray, S. A., Carver, E. A. & Gridley, T. Generation of a Snail1 (Snai1) conditional null allele. Genesis 44, 7–11 (2006).
Hughes, S. H., Greenhouse, J. J., Petropoulos, C. J. & Sutrave, P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J. Virol. 61, 3004–3012 (1987).
Schonhuber, N. et al. A next-generation dual-recombinase system for time- and host-specific targeting of pancreatic cancer. Nat. Med. 20, 1340–1347 (2014).
Eser, S. et al. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell 23, 406–420 (2013).
Falcomatà, C. et al. Genetic Screens Identify a Context-Specific PI3K/p27Kip1 Node Driving Extrahepatic Biliary Cancer. Cancer Discov. 11, 3158–3177 (2021).
Biederstädt, A. et al. SUMO pathway inhibition targets an aggressive pancreatic cancer subtype. Gut 69, 1472–1482 (2020).
Nagaki, K. et al. Chromatin immunoprecipitation reveals that the 180-bp satellite repeat is the key functional DNA element of Arabidopsis thaliana centromeres. Genetics 163, 1221–1225 (2003).
Hemmer, M. C. et al. E47 modulates hepatic glucocorticoid action. Nat. Commun. 10, 306 (2019).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Matson, J. P. et al. Rapid DNA replication origin licensing protects stem cell pluripotency. eLife 6, e30473 (2017).
Tetsu, O. & McCormick, F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 (1999).
Li, W. et al. MAGeCK enables robust identification of essential genes from genome-scale CRISPR/Cas9 knockout screens. Genome Biol. 15, 554 (2014).
Team, R. D. C., Vol. 2015 (R Foundation for Statistical Computing, Vienna, Austria; 2011).
Gentleman, R. C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Irizarry, R. A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 (2003).
Bolstad, B. M., Irizarry, R. A., Astrand, M. & Speed, T. P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinforma. (Oxf., Engl.) 19, 185–193 (2003).
Smyth, G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl Genet Mol. Biol. 3, Article3 (2004).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300 (1995).
Cunningham, F. et al. Ensembl 2015. Nucleic acids Res. 43, D662–D669 (2015).
Ward, J. H. Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58, 236–244 (1963).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinforma. (Oxf., Engl.) 30, 2114–2120 (2014).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinforma. (Oxf., Engl.) 29, 15–21 (2013).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Hruban, R. H. et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 66, 95–106 (2006).
Acknowledgements
We would like to thank Drs. Anton Berns, Jos Jonkers, Hassan Nakhei, Tyler Jacks, David Tuveson, Nabeel Bardeesy, Andrew Lowy, Deborah L Gumucio and Thomas Gridley for providing transgenic animals, Dr. Philippe Soriano for the Rosa26 targeting vector, U. Götz, J. Götzfried, M. Schiller and T. Schmid for excellent technical assistance and X. Zhang for bioinformatics analyses. This study was supported by Fritz Thyssen Stiftung (Project 10.11.2.162, to D.S.), the German Cancer Consortium (DKTK), the Laura-Bassi Award (TUM, to M.C.P.), the Otto Bayer Fellowship (Bayer Science & Education Foundation, to C.S.), Deutsche Forschungsgemeinschaft (DFG SA 1374/4-3; DFG SA 1374/6-1; DFG SCHO 1732/2-1; DFG SA 1374/8-1, Project-ID 515991405; DFG SA 1374/7-1, Project-ID 515571394; SFB 1321 Project-ID 329628492 P06 to D.S., P11 to D.S. and M.S.R., and S01 to K.S., D.S., R.R., and G.S.; SFB 1371 Project-ID 395357507 P12 to DS and AS; SFB 1479 Project ID: 441891347 S1 to M.B.; SFB 1160 Project Z02 to M.B.; SFB1453 Project ID 431984000 S1 to M.B.; and TRR167 Z01 to M.B.), the German Federal Ministry of Education and Research by MIRACUM within the Medical Informatics Funding Scheme (FKZ 01ZZ1801B to M.B. and EkoEstMed–FKZ 01ZZ2015 to G.A.), the Wilhelm Sander-Stiftung (2020.174.1 and 2017.091.2 to D.S.), and the European Research Council (ERC CoG No. 648521, to D.S.). The study was conducted with the support of the Ontario Institute for Cancer Research through funding provided by the Government of Ontario, Canada, for the RNAseq and survival data analyses of resected primary PDAC.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
M.C.P., C.S., C.F., C. Shan, D.R., S.K., C.K., M.Z., S.A.W., K.K.H., B.S., K.S., B.W., S.A.W., R.P, G.S., and D.S. performed research; M.T., A.S., M.S.R., G.A., M.B., R.R. and R.M.S. contributed new reagents and analytic tools; M.C.P., C.S., C.F., C. Shan, D.R., S.K., C.K., M.J., K.K.H, T.E., B.S., K. Steiger, B.W., M.S.R., G.A., M.B., R.R., G.S., and D.S. analysed data; and D.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Binhua Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paul, M.C., Schneeweis, C., Falcomatà, C. et al. Non-canonical functions of SNAIL drive context-specific cancer progression. Nat Commun 14, 1201 (2023). https://doi.org/10.1038/s41467-023-36505-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-36505-0
This article is cited by
-
Transcription factor ATMIN facilitates chemoresistance in nasopharyngeal carcinoma
Cell Death & Disease (2024)
-
Endothelial Robo4 suppresses endothelial-to-mesenchymal transition induced by irradiation and improves hematopoietic reconstitution
Cell Death & Disease (2024)
-
ID2 promotes tumor progression and metastasis in thyroid cancer
Endocrine (2024)
-
Combinatorial Cooperativity in miR200-Zeb Feedback Network can Control Epithelial–Mesenchymal Transition
Bulletin of Mathematical Biology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.